- 1School of Anesthesiology, Shandong Second Medical University, Weifang, Shandong, China
- 2Department of Anesthesiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Viscoelastic haemostasis assays (VHA) are increasingly used in clinical practice. These bedside whole-blood tests, commonly implemented through thromboelastography (TEG) and rotational thromboelastometry (ROTEM), are prized for their speed, accuracy, and accessibility, resulting in their increased usage in managing complex cases such as severe trauma, cardiac surgery, postpartum hemorrhage, and liver disease. Despite their widespread use, clear guidelines for regulating coagulation function in surgical patients through VHA remain undefined. This review searched the majority of the literature on VHA in the past decade and discussed the triggers and algorithms for hemostatic management guided by VHA in surgeries with a high risk of major bleeding. It also reviews the potential benefits of VHA over traditional coagulation tests (like prothrombin time and partial thromboplastin time) and clinical judgments, focusing on aspects such as reducing bleeding volume, decreasing use of allogeneic blood products, improving patient outcomes and mortality, and enhancing cost-effectiveness.
Background
Hemorrhage is one of the main causes of death and various complications in severe trauma and major surgeries (including cardiac surgery, organ transplantation, and obstetric delivery). 40% of trauma patient deaths and 10% of cardiac surgery patient deaths are due to hemorrhage, while maternal hemorrhage accounts for 30% of maternal mortality (1–3). Major bleeding can cause multiple pathophysiological responses such as tissue damage, endothelial irritation, immune system activation, platelet dysfunction, and coagulation disturbances, leading to bleeding-related coagulopathy and resulting in early and late hypercoagulability (4, 5). Ultimately, death occurs due to excessive blood loss, multi-organ dysfunction, or shock (6). Therefore, early identification and treatment of coagulopathy in patients with major bleeding or at risk is crucial for improving patient prognosis.
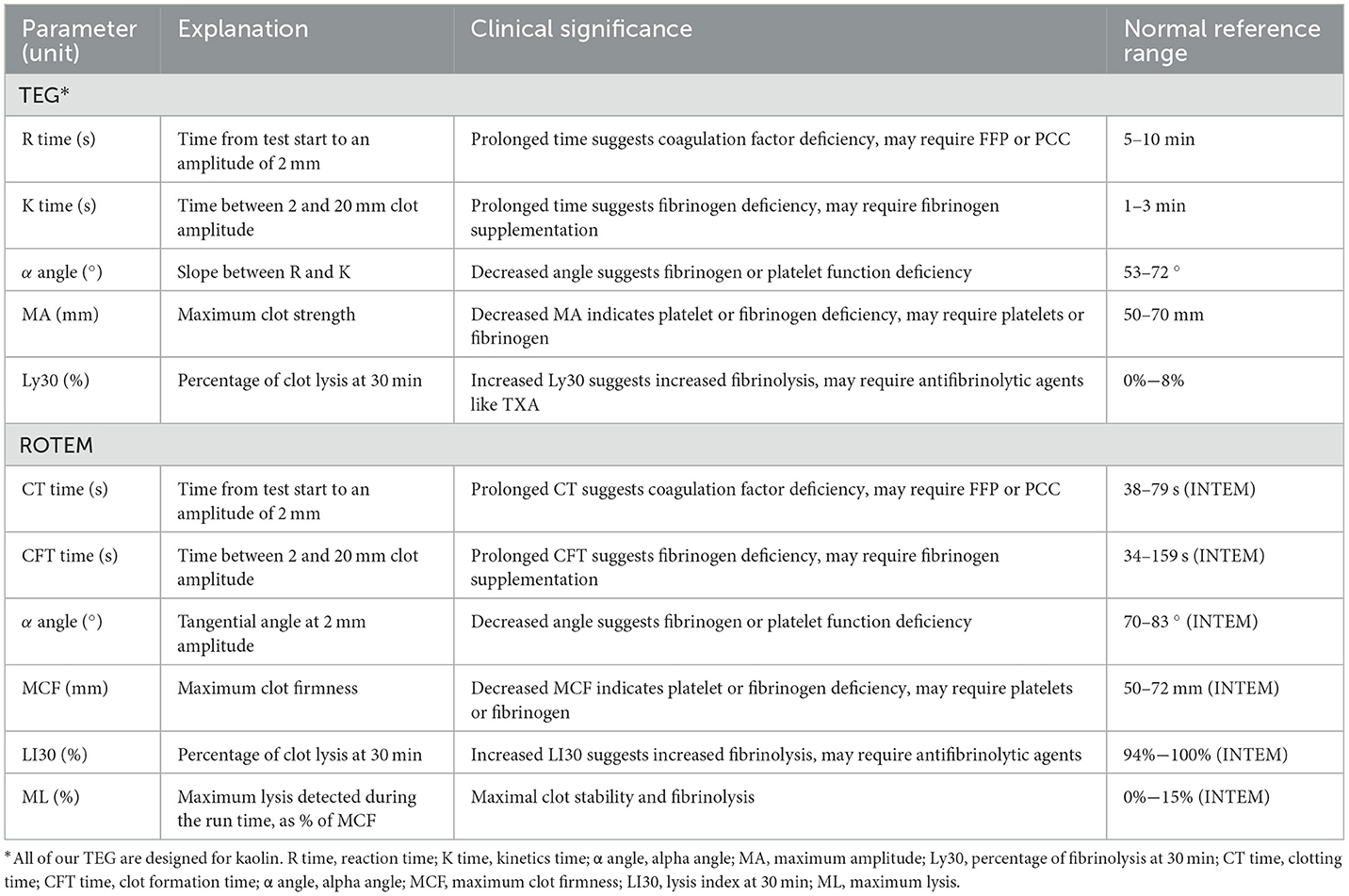
Conventional coagulation tests (CCT), including prothrombin time (PT), activated partial thromboplastin time and so on were primarily developed for the assessment of anticoagulant therapy and hemophilia (7). However, these tests cannot provide continuous, comprehensive, and accurate dynamic analyses of clot formation, stability, and the interactions among various blood components. Furthermore, their results can take 60 min or even longer to obtain, significantly affecting the diagnosis of coagulopathy in emergencies and may result in missing the optimal time for treatment (8–10). Viscoelastic hemostatic assays (VHA), such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM), are bedside whole-blood testing devices that can dynamically display the indicators, including clot initiation time, fibrinogen levels, platelet function (9, 11), and fibrinolytic activity. They can use just 0.3–0.4 ml blood to provide coagulation-related information for clinical transfusion guidance in as little as 10 min (10, 12, 13). Studies have shown that parameters like maximum clot firmness and clotting time in ROTEM or TEG can predict coagulopathy and major bleeding (14, 15) and that ROTEM is sensitive and specific for intraoperative platelet and fibrinogen levels (16) (Table 1).
A meta-analysis by Fahrendorff et al. (17) indicated that VHA-guided transfusion in various types of major bleeding surgeries can reduce total blood loss (Standardized Mean Difference (SMD) = 1.40, 95% CI: 2.57–0.23; p = 0.02), the transfusion of allogeneic red blood cells (RBCs; SMD = 0.64, 95% CI: 1.12–0.15; p = 0.01), and fresh frozen plasma (FFP; SMD = 1.98, 95% CI: 3.41–0.54; p = 0.01), but there was no significant statistical difference in all-cause mortality and platelet transfusion. Dias et al. (18) also noted that using VHA can reduce bleeding, decrease the use of plasma and platelets, shorten Intensive Care Unit stay, and increase ventilator-free days, but its impact on mortality remains controversial, which may be due to differences in types of surgery. Baksaas-Aasen et al. (6) demonstrated that in trauma patients receiving the massive hemorrhage protocols (MHPs)—which include empirical administration of tranexamic acid (TXA), transfusion of RBCs, plasma, and platelets (PLT) in a 1:1:1 ratio, and restricted crystalloid supplementation—there were no significant statistical differences in the allogeneic blood product transfusion and mortality when guided by VHA or CCT. Because the optimized balanced hemostatic treatment before grouping resulted in a lower-than-expected overall rate of coagulopathy in patients. Meanwhile, Santos et al. (19) believed that using VHA significantly reduces mortality compared to standard laboratory tests and/or clinical decisions (7.3 vs. 12.1%; RR = 0.64, p = 0.03). The aforementioned discrepancies may be primarily attributed to the fact that Baksaas-Aasen et al. employed a randomized controlled study design, whereas Santos et al. conducted a meta-analysis, with additional variations existing in surgical procedures and patient characteristics.
These reseach primarily focused on VHA-guided transfusion algorithms; however, there is less emphasis on specific transfusion triggers. Most studies currently rely on institution-specific VHA protocols for guiding transfusion, and there is a lack of high-quality evidence calculating these specific thresholds. In this review, we have compiled and listed the various VHA algorithms reported in the literature, providing a reference for clinicians to understand the current practices and guide future standardization efforts.
Many regional and international guidelines recommend VHA use in surgeries with a high risk of major bleeding, such as trauma (20, 21), cardiac surgery, obstetrics (22), and liver disease (23). However, differences in VHA-guided transfusion algorithms across surgical contexts persist, largely due to the absence of standardized trigger thresholds. Agarwal et al. (24) highlighted that VHA use during and after cardiac surgery can reduce perioperative transfusions of allogeneic blood products, but the lack of standardized thresholds limits consistency across studies. Therefore, establishing standardized VHA algorithm triggers for different surgeries is essential to optimize transfusion management.
VHA in trauma
Trauma-induced coagulopathy (TIC) caused by trauma hemorrhage is one of the leading causes of death in patients affected by trauma and is independently associated with increased mortality (25). In severe traumatic patients, 20 to 30% of patients develop TIC within minutes after injury, leading to early mortality. It is now widely accepted in research that early identification and intervention of coagulopathy in severe trauma patients can reduce blood loss, decrease the need for allogeneic blood products, and improve patient prognosis (26). However, traditional post-trauma transfusion decisions rely primarily on clinical experience, lacking accuracy and generalizability, which leads to increased transfusion-related complications, blood product consumption, and mortality rates. Therefore, the introduction of VHA technology may provide valuable guidance for early trauma transfusion management and potentially help reduce coagulopathy-induced mortality (27). Although most studies of patients with traumatic hemorrhage have identified their respective transfusion trigger thresholds, there is still a lack of globally harmonized, high certainty of evidence of transfusion triggers' values for VHAs that can be widely used in clinical practice.
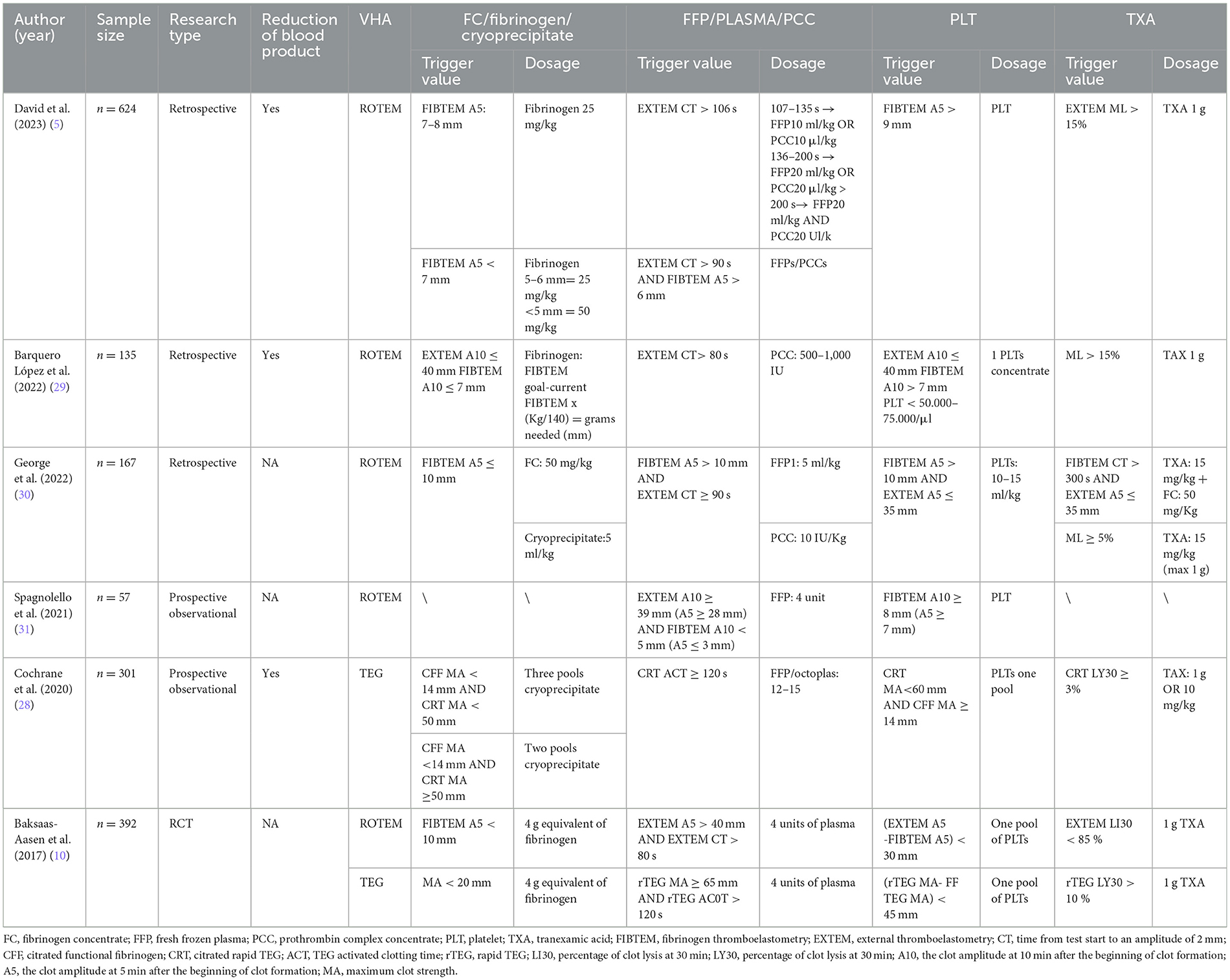
The trigger thresholds for VHA-guided blood management in trauma patients are presented in Table 2. ROTEM FIBTEM A5 ≤ 7–10 mm and TEG MA < 20 mm or citrated functional fibrinogen (CFF) MA < 14 mm are primarily used to guide the transfusion of fibrinogen, fibrinogen concentrate (FC), or cryoprecipitate (5, 10, 28). Additionally, FFP and Prothrombin complex concentrate (PCC) may be transfused when EXTEM CT > 80 s or CT > 90 s, and FIBTEM A5 ≥ 6–10 mm or rTEG ACT ≥ 120 s and MA > 65 mm (10, 29). Moreover, platelet transfusion in ROTEM is generally determined by EXTEM and FIBTEM indirectly, such as when EXTEM A5 < 35 mm and FIBTEM A5 > 9 or 10 mm (5, 30). Alternatively, it can be solely determined by FIBTEM A10/5 > 7–9 mm. In TEG, it is determined by the MA values. Furthermore, in ROTEM, TXA administration is guided by EXTEM lysis index at 30 min (LI30) < 85%, maximum lysis (ML) >15%, or FIBTEM CT > 300 s combined with EXTEM A5 < 35 mm or rTEG fibrinolysis rate (LY30) >10% (10, 29).
A retrospective study by David et al. (5) shows that, compared to CCT transfusion algorithms, using ROTEM-guided transfusion algorithms resulted in more patients surviving without substantial blood product transfusion within 24 h, fewer patients required extensive blood product treatment (32 cases, 15% vs. 91 cases, 42%; p < 0.01); however, there was no significant impact on mortality rates. Conversely, the study by Cochrane et al. (28) showed that compared to CCT, patients guided by TEG algorithms had significantly reduced mortality rates at 24 h (13% vs. 5%; p = 0.006) and 30 days (25% vs. 11%; p = 0.002), and reduced blood product wastage (1.8 ± 2.1 vs. 1.1 ± 2.0; p = 0.002); however, there was no difference in blood product transfusion volumes. Baksaas-Aasen et al. conducted a randomized controlled study involving 396 patients, showing no overall differences in survival without massive transfusion and 28-day mortality rates between the VHA and CCT groups. However, among a predefined subgroup of 74 patients with traumatic brain injury (TBI), the VHA group had a higher proportion of survivals without massive transfusion within 24 h compared to the CCT group (OR = 2.12, 95% CI: 0.84–5.34) (6).
Although there is still a controversy regarding the use of VHA algorithms in trauma patients for the transfusion of allogeneic blood products and prognosis. However, VHA enables quicker and more comprehensive assessment of coagulation in trauma settings, facilitating earlier clinical intervention (31). Additionally, guidelines recommend using VHA algorithms to guide fluid resuscitation in patients affected by trauma (20, 21). Furthermore, using VHA for clinical blood management enables early diagnosis of TIC and prediction of blood product transfusion and mortality post-trauma (32). The use of VHA in trauma patients not only optimizes transfusion algorithms, but also provides accurate, clinically generalizable transfusion trigger thresholds that offer a viable way to address traumatic coagulopathies.
VHA in cardiothoracic surgery
Preoperative anticoagulant and antiplatelet drugs, cardiopulmonary bypass (CPB) surgery, anesthetic administration can all cause the patient's blood composition to become unstable during cardiac surgery (33). This can result in severe bleeding that requires reoperation and poses a serious risk to patient's life (24). There is a lack of quantitative metrics for the timing and amount of blood transfusions for cardiac surgery, where the VHA's transfusion trigger thresholds have a potential role.
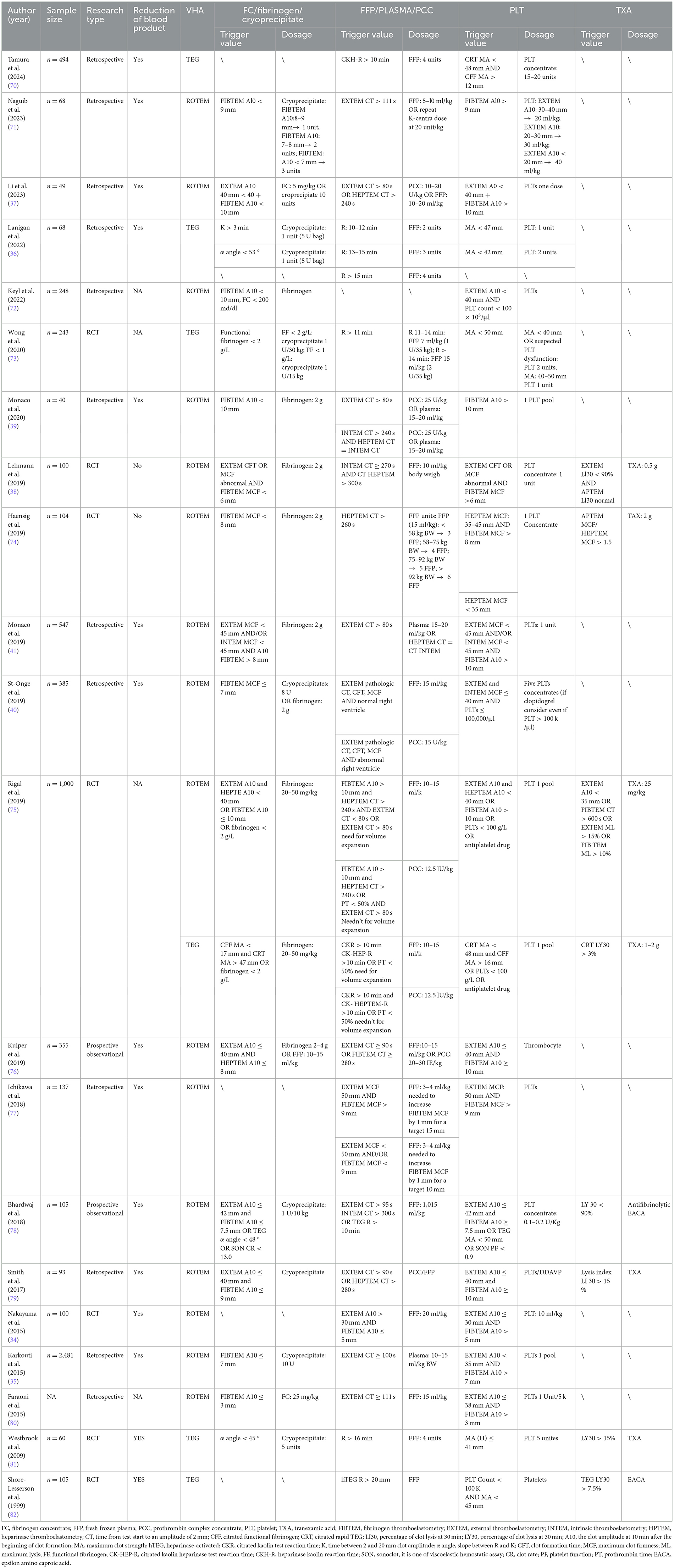
The algorithm for VHA-guided blood product transfusion in cardiac surgery is shown in Table 3. The transfusion of fibrinogen, FC, or cryoprecipitate is usually guided by ROTEM FIBTEM A10 ≤ 7–10 mm or maximum clot firmness (MCF) < 6–8 mm; in TEG, it is primarily determined by an α-angle of < 45–53 °, CFF MA < 12 mm, or MA < 17 mm in conjunction with citrated rapid TEG maximum amplitude (CRT MA) >47 mm. Additionally, when ROTEM EXTEM CT ≥ 80–111 s, HEPTEM CT > 240–280 s, or TEG reaction time (R) > 10 mins. FFP, plasma, or PCC can be transfused to prevent excessive depletion of coagulation factors. Platelet transfusion is primarily guided by minutes ROTEM EXTEM A10 ≤ 30–42 mm and FIBTEM A10 > 7–10 mm; HEPTEM MCF can also guide platelet transfusion (24). In TEG, intervention for platelet reduction is guided by an MA < 30–50 mm. TXA transfusion is primarily guided by ROTEM EXTEM LI30 < 90% or LI30 > 15% and TEG LY30 > 3, 7.5, or 15% to counteract hyperfibrinolysis.
Based on the aforementioned trigger thresholds, a randomized controlled trial by Nakayama et al. (34) demonstrated that ROTEM-guided management of blood products post-bypass surgery, compared to an algorithm based on ACT and platelet count (conventional laboratory measurements), reduced postoperative bleeding at 12 h (9 vs. 16 ml/kg, p = 0.001), the need for packed RBCs transfusions (11 vs. 23 ml/kg, p = 0.005), and intensive care unit stay duration (60 vs. 71 h, p = 0.014). A retrospective cohort study of 2,481 patients by Karkouti et al. (35) showed that the use of ROTEM was significantly associated with reduced transfusion rates of RBCs (OR = 0.50; 95% CI: 0.32–0.77; p = 0.002), platelets (OR = 0.22; 95% CI: 0.13–0.37; p = 0.002), and plasma (OR = 0.20; 95% CI: 0.12–0.34; p = 0.002). Similarly, a retrospective study by Lanigan et al. (36) that analyzed 68 patients who underwent left ventricular assist device implantation or heart transplantation showed reductions in absolute units of FFP, platelets, and cryoprecipitate by 40.2, 47.5, and 63.3%, respectively, along with significant decreases in transfusion rates and extubation time. However, Li et al. (37) and Lehmann et al. (38) showed that VHA-guided blood management did not significantly reduce the bleeding and transfusion of blood products like RBCs and FFP in cardiac surgery.
For major arterial surgeries, Monaco et al. (39) conducted a retrospective study on 40 patients who selectively underwent aortic arch replacement with the frozen elephant trunk technique; the results showed that ROTEM-guided treatment reduced the infusion of 1,345 ml of plasma and 0.91 units of platelets vs. conventional coagulation tests. A retrospective analysis by St-Onge et al. (40) on 385 patients undergoing aortic surgery using ROTEM showed a reduction in RBCs transfusion units [1.0 (0.0–4.0) units vs. 0.0 (0.0–2.0) units, p = 0.03] and a decrease in FFP transfusion [0.0 (0.0–4.0) units vs. 0.0 (0.0–2.0) units, p = 0.04], and was significantly associated with reduced massive transfusions (p = 0.04) and shorter Intensive Care Unit stays (p < 0.01). Additionally, a retrospective analysis by Monaco et al. (41) on 547 patients undergoing open repair of thoracoabdominal aortic aneurysms showed that patients managed with ROTEM rather than conventional coagulation tests had reduced transfusion of RBCs and FFP, with significantly fewer patients receiving FFP (p < 0.001); furthermore, the incidence of pulmonary complications (44 vs. 83%; p = 0.01) and medical costs were also reduced. Therefore, using VHA in major vascular surgeries can reduce the transfusion of blood products, decrease postoperative complications, and improve patient outcomes.
VHA in postpartum hemorrhage
Postpartum hemorrhage (PPH) is one of the most dangerous postpartum complications, which can be caused by uterine atony, retained placenta, and birth canal lacerations (42). During pregnancy, the increase in coagulation factors disrupts the balance of bleeding, clotting, and fibrinolysis in the blood system (43), leading to varying degrees of bleeding and postpartum thromboembolic events (44), therefore, timely and accurate identification of coagulation status and transfusion intervention in patients with PPH is very important. Studies have shown that VHA is advantageous in diagnosing coagulopathy, monitoring fibrinolysis in women with obstetric hemorrhage, predicting PPH, and reducing transfusions (45), but most studies have low certainty of evidence and are biased to be widely used in clinical practice.
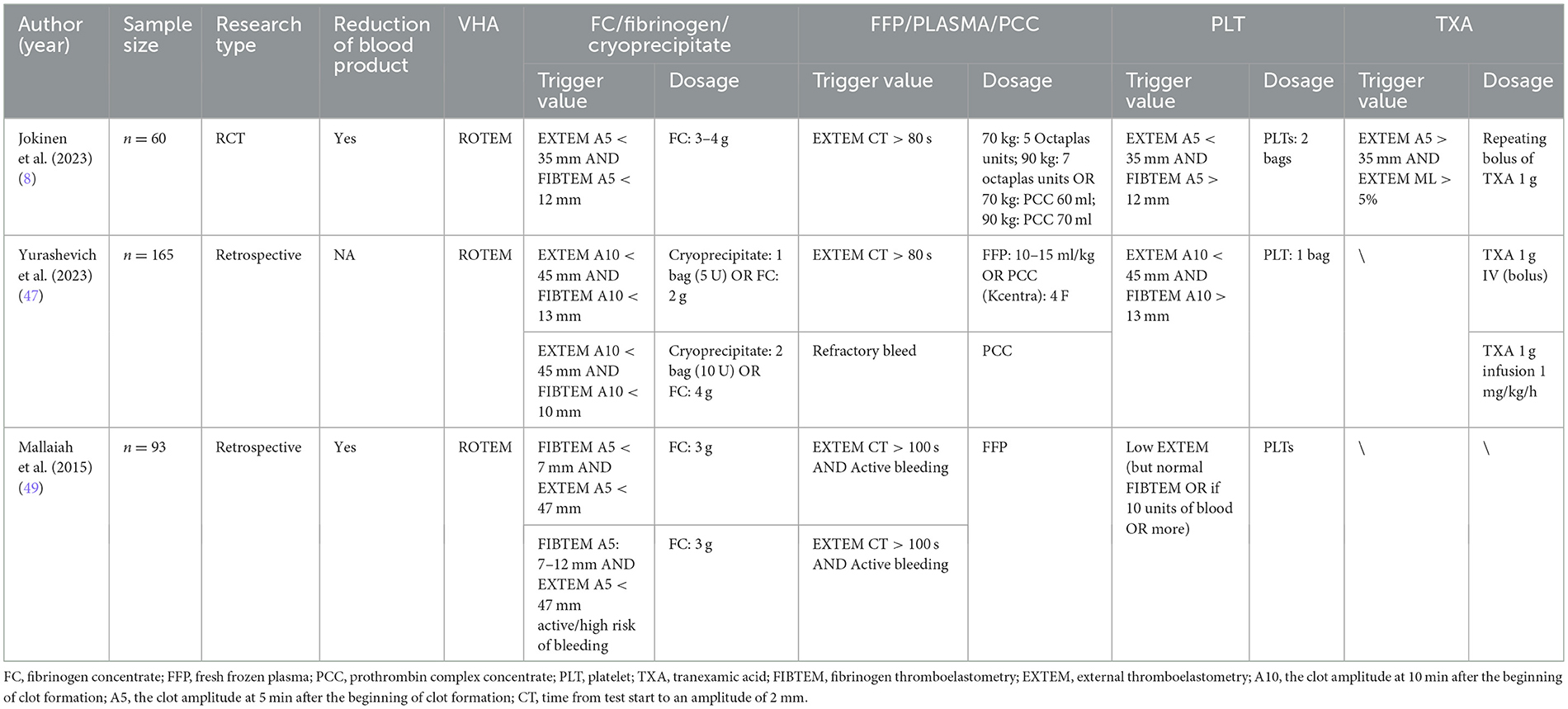
The algorithm for VHA-guided blood management in patients with PPH is shown in Table 4. Researchers initiate fibrinogen and other transfusions when ROTEM FIBTEM A5 is < 7–12 mm, alone or in combination with EXTEM A5 < 35 or 47 mm; in TEG, primarily when (CFF) MA is < 14–19 mm, A10 is ≤ 17 mm, or the α-angle is < 45 (45, 46). When EXTEM CT ≥ 75–100 s or TEG R ≥ 9–12 mins, FFP or PCC transfusion is administered. In addition, platelet transfusion is performed when EXTEM A5 < 35 mm or A10 < 47 mm and FIBTEM A5 > 11–13 mm, TEG MA < 48–57 mm (45, 46). For TXA, the trigger threshold is rarely mentioned because patients with PPH are generally given a standard intravenous infusion of 1 g of TXA.
In the randomized controlled trial by Jokinen et al. (8) where 54 women with PPH were divided into a ROTEM-guided group and a conventional treatment group, the results showed that the ROTEM-guided transfusion algorithm reduced plasma usage (0–0 vs. 0–2, p = 0.030) and total blood loss [2,500 ml (2,100–3,000) vs. 3,000 ml (2,200–3,100), p = 0.033]. However, there are few studies on using VHA to manage RBCs or PLT transfusion in PPH, with most research focusing on using VHA to guide FC or FFP.
A retrospective study by Yurashevich et al. (47) demonstrated that reduced fibrinogen levels are independently associated with severe hemorrhage. Similarly, Agarwal et al. (48) showed that fibrinogen levels in obstetrics can predict massive hemorrhage, with fibrinogen being the first to decrease during massive hemorrhage. Therefore, an early identification of reduced fibrinogen levels is crucial. Mallaiah et al. (49) demonstrated that a reduction in ROTEM FIBTEM A5 and EXTEM A5 indicates a decrease in fibrinogen and can guide the use of FC. McNamara et al. (50) analyzed 4 years of observational data, showing that FIBTEM A5 < 7, or 7–12 mm, indicates a risk of ongoing or severe bleeding, and using the VHA algorithm can reduce the number of blood product units transfused (p < 0.0001) and the total volume (p = 0.0007), as well as decrease transfusion-associated circulatory overload (p = 0.002). Additionally, an observational study by Collins et al. (51) on 605 postpartum women demonstrated that using FFP to control FIBTEM A5 < 15 mm is feasible in actual clinical practice. Therefore, in obstetrics, the VHA algorithm is widely used to guide the use of FC, aiming to prevent and reduce bleeding.
VHA in liver surgery
Liver transplantation has become the primary treatment for end-stage liver disease (52). In advanced cirrhosis and post-liver transplantation, reduced clotting factors establish a new but unstable hemostatic equilibrium, creating dual risks: increased thrombosis resulting from this delicate balance and bleeding risk caused by portal hypertension. Therefore, managing each component of the blood system is necessary to prevent major bleeding events. While the use of VHA is common in liver surgery, there is still a controversy regarding the trigger thresholds for using VHA in cases of major bleeding in liver surgery, as well as its impact on reducing transfusions and improving patient prognosis and mortality. This is due to factors such as the inherent complexity of coagulation in liver surgery patients, differences in patient types, and the generally low quality of evidence in related studies.
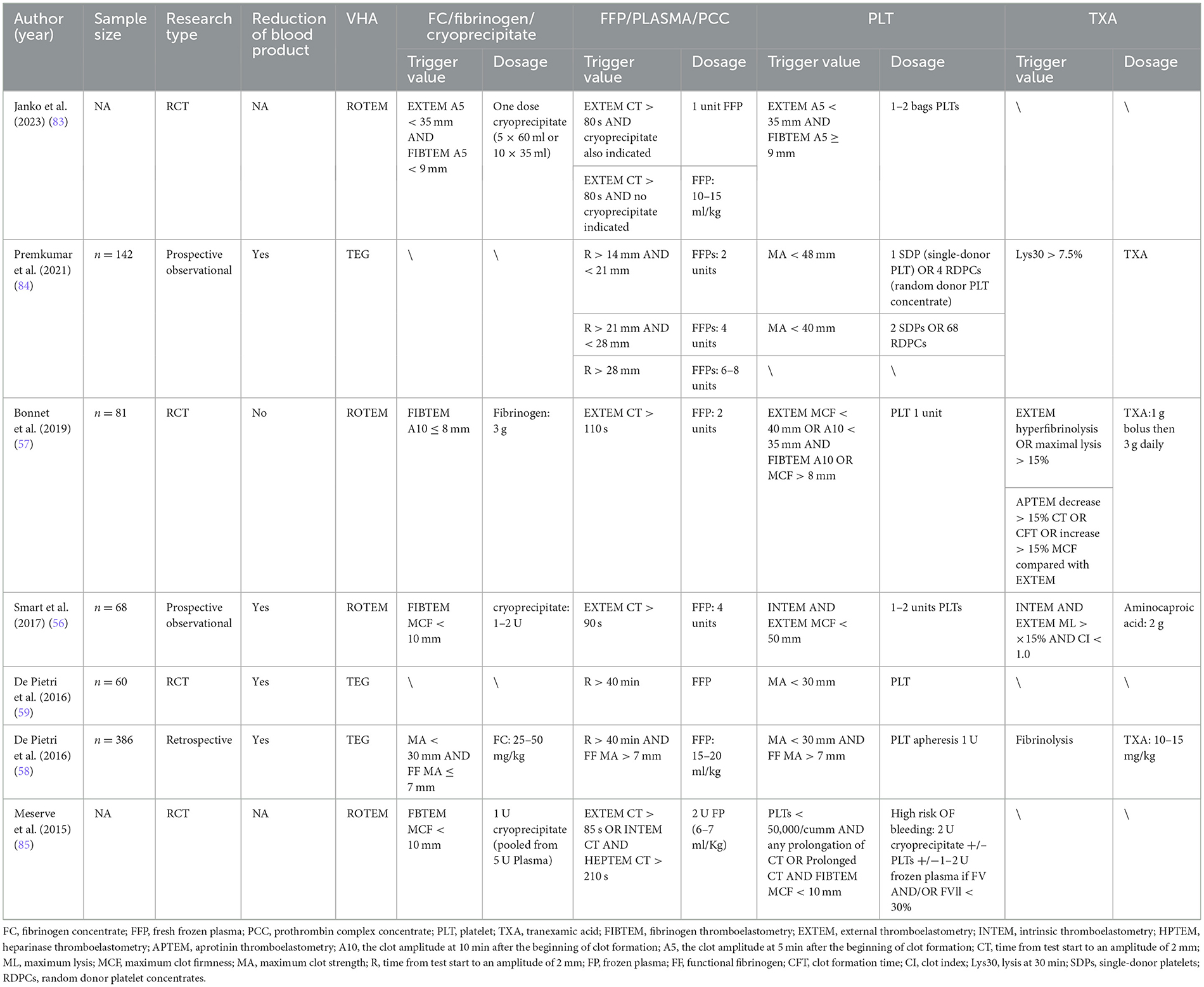
Table 5 outlines the algorithm for VHA-guided blood management in patients with severe bleeding and those at risk of severe bleeding due to liver surgery. The management is mainly guided by ROTEM FIBTEM A10 ≤ 8 mm alone or combined with EXTEM A5 or A10; TEG MA < 30 mm and fibrinogen functional MA (FF MA) ≤ 7 mm, as well as TEG α angle < 45 °, to guide the transfusion of fibrinogen, FC and cryoprecipitate, to achieve fibrinogen supplementation purposes (53, 54). FFP or PCC transfusion is indicated when EXTEM CT > 100 s or > 80–90 s, or combined with INTEM CT and HEPTEM CT, or TEG R > 14–40 min. In ROTEM, platelet transfusion is triggered by EXTEM A10, A5, and MFC combined with FIBTEM A10 > 8 or 9 mm. TEG indicates platelet transfusion when MA < 30 or 40–48 mm. Additionally, TXA infusion primarily focuses on TEG Ly30 > 7.5% or ROTEM ML, CTF, or MCF.
The use of VHA for blood management and treatment in liver transplantation is widespread (53, 55). A randomized controlled trial by Smart et al. (56) involving 64 orthotopic liver transplantation (OLT) patients showed that ROTEM-guided therapy, compared to conventional coagulation tests, reduced intraoperative blood loss (2.0 vs. 3.0 L, p = 0.04) and FFP transfusions (4 vs. 6.5 units, p = 0.015), but increased cryoprecipitate use (73% vs. 56%, p = 0.033). Bonnet et al.'s (57) randomized study of 81 OLT patients found that replacing standard coagulation test algorithm with ROTEM reduced transfusion requirements and FFP use, though fibrinogen transfusion increased (72.5 vs. 29.3%, p < 0.001). Similarly, De Pietri et al.'s (58) retrospective study of 386 liver transplant patients showed that FF-TEG testing, rather than the previously used TEG-based algorithm, significantly reduced blood, FFP, and platelet transfusions while increasing fibrinogen administration. This suggests that VHA can reduce the use of blood products other than fibrinogen.
Patients with advanced liver cirrhosis face the risk of coagulation system disorders, which can lead to life-threatening bleeding during interventional treatments. Therefore, VHA algorithms are particularly necessary to guide pre-intervention blood management in these patients. The study by De Pietri et al. (59) demonstrated that using TEG to guide transfusions before interventional treatment for liver cirrhosis can reduce the use of FFP and PLT. Maria et al. (60) support this viewpoint, and their randomized controlled study using the ROTEM algorithm on 60 patients undergoing interventional treatment for liver cirrhosis demonstrated not only a reduction in the use of FFP and PLT but also a decrease in overall blood product utilization (46.7 vs. 100%, p < 0.001), highlighting its cost-effectiveness. Therefore, before interventional surgery for liver cirrhosis, clotting function management guided by VHA algorithms should be conducted to optimize treatment protocols.
VHA in pediatric surgery
Due to underdeveloped immune and coagulation systems, inadequate temperature regulation, and lower tolerance to post-major surgery stress, pediatric patients are more prone to complications such as bleeding after a major surgery. Therefore, many researchers apply VHA in pediatric patients to enhance the management of post-major surgery bleeding. Nevertheless, the clinical use of VHA for guiding transfusion trigger thresholds in pediatric patients undergoing major surgery is still uncommon, and the level of evidence from relevant studies is insufficient to be used for generalization, with each study's trigger thresholds having their own characteristics, making it difficult to unify them.
A randomized controlled study by Haas et al. (61) using fibrinogen concentrate (FC) under the ROTEM algorithm to reduce RBCs transfusion demonstrated that triggering FC use with ROTEM FIBTEM < 13 mm can reduce bleeding and transfusion requirements in pediatric patients undergoing craniosynostosis surgery. A randomized controlled study by Zhang et al. (62) on 83 pediatric patients undergoing resective epilepsy surgery showed that the prophylactic use of TXA combined with the TEG algorithm significantly reduced the transfusion rates by 34.7% (p = 0.001), primarily through a decrease in plasma transfusions, though there was an increase in FC transfusion rates. Similarly, Raffaeli et al. (63) also believe that TEG can reduce the use of FFP, and experimental verification showed that TEG use in neonatal surgery decreased the use of FFP (31 vs. 60%, p < 0.001). However, the algorithm for using VHA in pediatric patients is still not fully developed, and further research and precision are needed regarding the trigger thresholds for VHA. Nevertheless, it is undeniable that its use in pediatric patients has optimized intraoperative blood management.
Conclusions
In various fields such as trauma, cardiac, postpartum hemorrhage, hepatic, and pediatrics, there is a lack of tools for timely and accurate assessment and transfusion intervention in patients with massive transfusion risk, thus making VHA a clinically worthwhile transfusion algorithm. Most studies indicate that VHA transfusion trigger thresholds exhibit significant heterogeneity due to different populations and research methodologies. Therefore, establishing different VHA trigger thresholds for different populations holds epoch-making significance for the development of viscoelastic hemostatic technology, the resolution of clinical problems, and clinical research.
While the trigger thresholds of VHA algorithms vary across different types of surgeries, there are still commonalities TEG and ROTEM parameters within the same population. The transfusion of fibrinogen, FC, and cryoprecipitate is primarily determined by ROTEM FIBTEM A5, A10, or MCF, TEG α-angle, or MA. FFP and PCC transfusions are guided mainly by ROTEM EXTEM CT and TEG R. Additionally, platelet transfusions are mostly guided by ROTEM EXTEM combined with FIBTEM A5, A10, or MCF, or TEG MA. The administration of TXA is primarily determined by ROTEM ML, LI30, or TEG Ly30.
VHA is now used in various surgeries involving severe hemorrhage and potential coagulopathy; however, clinical adherence to VHA-guided blood product transfusion remains low. Therefore, while there are challenges in the current practical application of using VHA to assess patients' coagulopathy risk and guide clinical transfusion decisions, the future prospects are promising. Based on factors such as poor historical compliance with VHA guidance, many studies suffer from biases, omissions, insufficient sample sizes, and other issues, resulting in insufficient evidence strength for many VHA studies. Despite this, many guidelines still recommend using VHA to guide blood management and fluid resuscitation (20–23). Furthermore, an increasing number of researchers are exploring using VHA algorithms to guide transfusion in various types of major bleeding or coagulation disorder surgeries. These studies further refine and validate the VHA algorithms, demonstrating that VHA-guided management facilitates early diagnosis and prevention of coagulopathy, reduces bleeding and the use of blood products (RBCs, FFP, FC, and PLT), and can lower the incidence of transfusion-related complications to improve prognosis. To address these issues, we should conduct education and training on VHA usage, standardize clinical workflows, and reduce VHA instrument costs to achieve widespread adoption and increased adherence.
At the same time, viscoelastic hemostasis technology continues to evolve, with the emergence of new tools for dynamic measurement of coagulation status, including Sonoclot (64), ClotPro (65, 66), SEER (67) and Quantra QPlus systems (68), in addition to the commonly used TEG and ROTEM. They significantly reduce measurement time, broaden the range of applications, and increase the accuracy of measurements (69).
VHA-guided blood management facilitates the management and treatment of major bleeding, improves patient's quality of life, and conserves medical resources. However, research on VHA-guided transfusion algorithms is still far from sufficient. Alongside standardizing VHA utilization among clinicians, extensive prospective randomized multicenter studies and multidisciplinary collaboration are essential to explore whether universal thresholds can be established or whether VHA algorithm trigger thresholds can be optimized for different surgical types and individualized intraoperative and postoperative major bleeding scenarios, while demonstrating the clinical benefits of VHA algorithms. Furthermore, comparative studies of various VHA devices are needed to identify economical, rapid, and accurate VHA instruments with their corresponding thresholds for perioperative application.
Author contributions
Z-LX: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. HC: Investigation, Writing – original draft. D-WS: Methodology, Writing – review & editing. JX: Visualization, Writing – review & editing. Y-YY: Supervision, Writing – review & editing. GL: Visualization, Writing – review & editing. T-TW: Visualization, Writing – review & editing. QG: Methodology, Writing – review & editing. J-CZ: Visualization, Writing – review & editing. X-CT: Visualization, Writing – review & editing. MY: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (U24A200213, 82371217, and 82071227), the National Clinical Key Specialty Construction Project of China 2021 (2021-LCZDZK-01), the Key Research and Development Program of Zhejiang Province (2024C03091), and the Leading Health Talents of Zhejiang Province, Zhejiang Health Office No. 18 (2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Curry NS, Davenport R. Transfusion strategies for major haemorrhage in trauma. Br J Haematol. (2019) 184:508–23. doi: 10.1111/bjh.15737
2. Serraino GF, Murphy GJ. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth. (2017) 118:823–33. doi: 10.1093/bja/aex100
3. Shah A, Kerner V, Stanworth SJ, Agarwal S. Major haemorrhage: past, present and future. Anaesthesia. (2023) 78:93–104. doi: 10.1111/anae.15866
4. Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nat Rev Dis Prim. (2021) 7:30. doi: 10.1038/s41572-021-00264-3
5. David JS, James A, Orion M, Selves A, Bonnet M, Glasman P, et al. Thromboelastometry-guided haemostatic resuscitation in severely injured patients: a propensity score-matched study. Crit Care. (2023) 27:141. doi: 10.1186/s13054-023-04421-w
6. Baksaas-Aasen K, Gall LS, Stensballe J, Juffermans NP, Curry N, Maegele M, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Int Care Med. (2021) 47:49–59. doi: 10.1007/s00134-020-06266-1
7. Lv X, Mao Y, Qin Z. Evaluation for effects of severe acidosis on hemostasis in trauma patients using thrombelastography analyzer. Am J Emerg Med. (2018) 36:1332–40. doi: 10.1016/j.ajem.2017.12.037
8. Jokinen S. Thromboelastometry-guided treatment algorithm in postpartum haemorrhage: a randomised, controlled pilot trial. BJA. (2023) 130:165–74. doi: 10.1016/j.bja.2022.10.031
9. Sahli SD, Rössler J, Tscholl DW, Studt J-D, Spahn DR, Kaserer A. Point-of-care diagnostics in coagulation management. Sensors. (2020) 20:4254. doi: 10.3390/s20154254
10. Baksaas-Aasen K, Gall L, Eaglestone S, Rourke C, Juffermans NP, Goslings JC, et al. iTACTIC - implementing treatment algorithms for the correction of trauma-induced coagulopathy: study protocol for a multicentre, randomised controlled trial. Trials. (2017) 18:486. doi: 10.1186/s13063-017-2224-9
11. Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. (2014) 89:228–32. doi: 10.1002/ajh.23599
12. Cannata G, Mariotti Zani E, Argentiero A, Caminiti C, Perrone S, Esposito S. TEG((R)) and ROTEM((R)) traces: clinical applications of viscoelastic coagulation monitoring in neonatal intensive care unit. Diagnostics. (2021) 11:15. doi: 10.3390/diagnostics11091642
13. Emani S, Diallo FB, Dutta P, Matte GS, Nathan M, Emani SM, et al. Comparison of thromboelastography devices TEG((R))6S point of care device vs. TEG((R))5000 in pediatric patients undergoing cardiac surgery. J Extra Corpor Technol. (2022) 54:42–9. doi: 10.1051/ject/202254042
14. Soh S, Kwak Y-L, Song J-W, Yoo K-J, Kim H-J, Shim J-K. Rotational thromboelastometry predicts increased bleeding after off-pump coronary bypass surgery. Ann Thorac Surg. (2017) 104:1318–24. doi: 10.1016/j.athoracsur.2017.02.046
15. Tsantes AG, Papadopoulos DV, Trikoupis IG, Tsante KA, Mavrogenis AF, Koulouvaris P, et al. The prognostic performance of rotational thromboelastometry for excessive bleeding and increased transfusion requirements in hip fracture surgeries. Thromb Haemost. (2021) 122:895–904. doi: 10.1055/s-0041-1736617
16. Scott JP, Niebler RA, Stuth EAE, Newman DK, Tweddell JS, Bercovitz RS, et al. Rotational thromboelastometry rapidly predicts thrombocytopenia and hypofibrinogenemia during neonatal cardiopulmonary bypass. World J Pediatr Congenit Heart Surg. (2018) 9:424–33. doi: 10.1177/2150135118771318
17. Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. (2017) 25:39. doi: 10.1186/s13049-017-0378-9
18. Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost. (2019) 17:984–94. doi: 10.1111/jth.14447
19. Santos AS, Felix Oliveira AJ, Lage Barbosa MC, dos Santos Nogueira JL. Viscoelastic haemostatic assays in the perioperative period of surgical procedures: systematic review and meta-analysis. J Clin Anesthes. (2020) 64:109809. doi: 10.1016/j.jclinane.2020.109809
20. Bugaev N, Como JJ, Golani G, Freeman JJ, Sawhney JS, Vatsaas CJ, et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: practice management guideline from the eastern association for the surgery of trauma. J Trauma Acute Care Surg. (2020) 89:999–1017. doi: 10.1097/TA.0000000000002944
21. Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, et al. The european guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Crit Care. (2023) 27:80. doi: 10.1186/s13054-023-04327-7
22. Management ASoATFoPB. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on perioperative blood management. Anesthesiology. (2015) 122:241–75. doi: 10.1097/ALN.0000000000000463
23. Spahn DR. TEG®- or ROTEM®-based individualized goal-directed coagulation algorithms: don't wait - act now! Crit Care. (2014) 18:637. doi: 10.1186/s13054-014-0637-3
24. Agarwal S, Abdelmotieleb M. Viscoelastic testing in cardiac surgery. Transfusion. (2020) 60 Suppl 6:S52–60. doi: 10.1111/trf.16075
25. Winearls J, Reade M, Miles H, Bulmer A, Campbell D, Görlinger K, et al. Targeted coagulation management in severe trauma: the controversies and the evidence. Anesthes Analges. (2016) 123:910–24. doi: 10.1213/ANE.0000000000001516
26. Wirtz MR, Baumann HM, Klinkspoor JH, Goslings JC, Juffermans NP. Viscoelastic testing in trauma. Semin Thromb Hemost. (2017) 43:375–85. doi: 10.1055/s-0037-1598057
27. Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. (2016) 263:1051–9. doi: 10.1097/SLA.0000000000001608
28. Cochrane C, Chinna S, Um JY, Dias JD, Hartmann J, Bradley J, et al. Site-of-care viscoelastic assay in major trauma improves outcomes and is cost neutral compared with standard coagulation tests. Diagnostics. (2020) 10:486. doi: 10.3390/diagnostics10070486
29. Barquero López M, Martínez Cabañero J, Muñoz Valencia A, Sáez Ibarra C, De la Rosa Estadella M, Campos Serra A, et al. Dynamic use of fibrinogen under viscoelastic assessment results in reduced need for plasma and diminished overall transfusion requirements in severe trauma. J Trauma Acute Care Surg. (2022) 93:166–75. doi: 10.1097/TA.0000000000003624
30. George S, Wake E, Sweeny A, Campbell D, Winearls J. Rotational thromboelastometry in children presenting to an Australian major trauma centre: a retrospective cohort study. Emerg Med Australas. (2022) 34:590–8. doi: 10.1111/1742-6723.13939
31. Spagnolello O, Reed MJ, Blackstock C, Dauncey S, Timony-Nolan E, Innes C, et al. Introduction of a ROTEM protocol for the management of trauma-induced coagulopathy. Trauma. (2021) 23:308–21. doi: 10.1177/1460408620957919
32. Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NKJ. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care. (2014) 18:518. doi: 10.1186/s13054-014-0518-9
33. Alkadri J, Hu R, Jeffers MS, Ross J, McIsaac DI, McDonald B. Comparison of milrinone with dobutamine in patients undergoing cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. (2023) 70:1272–4. doi: 10.1007/s12630-023-02482-7
34. Nakayama Y, Nakajima Y, Tanaka KA, Sessler DI, Maeda S, Iida J, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth. (2015) 114:91–102. doi: 10.1093/bja/aeu339
35. Karkouti K, McCluskey SA, Callum J, Freedman J, Selby R, Timoumi T, et al. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery: a retrospective cohort study with interrupted time-series analysis. Anesthesiology. (2015) 122:560–70. doi: 10.1097/ALN.0000000000000556
36. Lanigan M, Siers D, Wilkey A, Barakat A, Shaffer A, John R, et al. The use of a viscoelastic-based transfusion algorithm significantly reduces non-red blood cell transfusion in patients undergoing left ventricular assist device placement or heart transplantation: a single-center observational study. J Cardiothorac Vasc Anesth. (2022) 36(8 Pt B):3038–46. doi: 10.1053/j.jvca.2022.03.017
37. Li KC, Coley MA, Chau A, Dotto A, McMillan A, Chiu H, et al. Rotational thromboelastometry-guided use of synthetic blood products in cardiac transplant patients: a retrospective before-after study. J Cardiothorac Vasc Anesth. (2023) 37:1121–8. doi: 10.1053/j.jvca.2023.02.042
38. Lehmann F, Rau J, Malcolm B, Sander M, von Heymann C, Moormann T, et al. Why does a point of care guided transfusion algorithm not improve blood loss and transfusion practice in patients undergoing high-risk cardiac surgery? A prospective randomized controlled pilot study. BMC Anesthesiol. (2019) 19:24. doi: 10.1186/s12871-019-0689-7
39. Monaco F, Nardelli P, Denaro G, De Luca M, Franco A, Bertoglio L, et al. First experience with a ROTEM-enhanced transfusion algorithm in patients undergoing aortic arch replacement with frozen elephant trunk technique. A theranostic approach to patient blood management. J Clin Anesth. (2020) 66:109910. doi: 10.1016/j.jclinane.2020.109910
40. St-Onge S, Lemoine É, Bouhout I, Rochon A, El-Hamamsy I, Lamarche Y, et al. Evaluation of the real-world impact of rotational thromboelastometry-guided transfusion protocol in patients undergoing proximal aortic surgery. J Thorac Cardiovasc Surg. (2019) 157:1045–54.e1044. doi: 10.1016/j.jtcvs.2018.07.043
41. Monaco F, Barucco G, Nardelli P, Licheri M, Notte C, De Luca M, et al. Editor's choice - a rotational thromboelastometry driven transfusion strategy reduces allogenic blood transfusion during open thoraco-abdominal aortic aneurysm repair: a propensity score matched study. Eur J Vasc Endovasc Surg. (2019) 58:13–22. doi: 10.1016/j.ejvs.2019.02.009
42. Collins P. Point-of-care coagulation testing for postpartum haemorrhage. Best Pract Res Clin Anaesthesiol. (2022) 36:383–98. doi: 10.1016/j.bpa.2022.08.002
43. Prevention and management of postpartum haemorrhage: green-top guideline no. 52. BJOG. (2024) 124:e10649. doi: 10.1111/1471-0528.14178
44. Bukhari S, Fatima S, Barakat AF, Fogerty AE, Weinberg I, Elgendy IY. Venous thromboembolism during pregnancy and postpartum period. Eur J Int Med. (2022) 97:8–17. doi: 10.1016/j.ejim.2021.12.013
45. Dias JD, Butwick AJ, Hartmann J, Waters JH. Viscoelastic haemostatic point-of-care assays in the management of postpartum haemorrhage: a narrative review. Anaesthesia. (2022) 77:700–11. doi: 10.1111/anae.15662
46. Frigo MG, Agostini V, Brizzi A, Ragusa A, Svelato A. Practical approach to transfusion management of post-partum haemorrhage. Transfus Med. (2021) 31:11–5. doi: 10.1111/tme.12755
47. Yurashevich M, Rosser M, Small M, Grotegut C, Kota N, Toffaletti J, et al. Evaluating the association between fibrinogen and rotational thromboelastometry and the progression to severe obstetric hemorrhage. Clin Appl Thromb Hemost. (2023) 29:10760296231175089. doi: 10.1177/10760296231175089
48. Agarwal S, Laycock HC. The debate ROTEMs on - the utility of point-of-care testing and fibrinogen concentrate in postpartum haemorrhage. Anaesthesia. (2020) 75:1247–51. doi: 10.1111/anae.15193
49. Mallaiah S, Barclay P, Harrod I, Chevannes C, Bhalla A. Introduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in major obstetric haemorrhage. Anaesthesia. (2015) 70:166–75. doi: 10.1111/anae.12859
50. McNamara H, Kenyon C, Smith R, Mallaiah S, Barclay P. Four years' experience of a ROTEM(®) -guided algorithm for treatment of coagulopathy in obstetric haemorrhage. Anaesthesia. (2019) 74:984–91. doi: 10.1111/anae.14628
51. Collins PW, Cannings-John R, Bruynseels D, Mallaiah S, Dick J, Elton C, et al. Viscoelastometry guided fresh frozen plasma infusion for postpartum haemorrhage: OBS2, an observational study. Br J Anaesth. (2017) 119:422–34. doi: 10.1093/bja/aex245
52. Ng KK, Lo CM. Liver transplantation in Asia: past, present and future. Ann Acad Med. (2009) 38:16. doi: 10.47102/annals-acadmedsg.V38N4p322
53. Sakai T. Viscoelastic testing in liver transplantation. Transfusion. (2020) 60 S61–9. doi: 10.1111/trf.16077
54. Roullet S, Freyburger G, Cruc M, Quinart A, Stecken L, Audy M, et al. Management of bleeding and transfusion during liver transplantation before and after the introduction of a rotational thromboelastometry-based algorithm. Liver Transplant. (2015) 21:169–79. doi: 10.1002/lt.24030
55. Von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Arch Surg. (1966) 92:16. doi: 10.1001/archsurg.1966.01320190073016
56. Smart L, Mumtaz K, Scharpf D, Gray NO, Traetow D, Black S, et al. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol. (2017) 16:916–23. doi: 10.5604/01.3001.0010.5283
57. Bonnet A, Gilquin N, Steer N, Gazon M, Quattrone D, Pradat P, et al. The use of a thromboelastometry-based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: a randomised controlled study. Eur J Anaesthesiol. (2019) 36:825–33. doi: 10.1097/EJA.0000000000001084
58. De Pietri L, Ragusa F, Deleuterio A, Begliomini B, Serra V. Reduced transfusion during OLT by POC coagulation management and TEG functional fibrinogen: a retrospective observational study. Transplant Direct. (2016) 2:e49. doi: 10.1097/TXD.0000000000000559
59. De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. (2016) 63:566–73. doi: 10.1002/hep.28148
60. Maria A, Lal BB, Khanna R, Sood V, Mukund A, Bajpai M, et al. Rotational thromboelastometry-guided blood component use in cirrhotic children undergoing invasive procedures: randomized controlled trial. Liver Int. (2022) 42:2492–500. doi: 10.1111/liv.15398
61. Haas T, Spielmann N, Restin T, Seifert B, Henze G, Obwegeser J, et al. Higher fibrinogen concentrations for reduction of transfusion requirements during major paediatric surgery: a prospective randomised controlled trial. Br J Anaesth. (2015) 115:234–43. doi: 10.1093/bja/aev136
62. Zhang T, Feng H, Xiao W, Li J, Liu Q, Feng X, et al. Prophylactic administration of tranexamic acid combined with thromboelastography-guided hemostatic algorithm reduces allogeneic transfusion requirements during pediatric resective epilepsy surgery: a randomized controlled trial. Front Pharmacol. (2022) 13:916017. doi: 10.3389/fphar.2022.916017
63. Raffaeli G, Pesenti N, Cavallaro G, Cortesi V, Manzoni F, Amelio GS, et al. Optimizing fresh-frozen plasma transfusion in surgical neonates through thromboelastography: a quality improvement study. Eur J Pediatr. (2022) 181:2173–82. doi: 10.1007/s00431-022-04427-6
64. Hett DA, Walker D, Pilkington SN, Smith DC. Sonoclot analysis. Br J Anaesth. (1995) 75:771–6. doi: 10.1093/bja/75.6.771
65. Laukova K, Petrikova V, Poloniova L, Babulicova L, Wsolova L, Haas T. Determination of reference ranges for the ClotPro® thromboelastometry device in paediatric patients. Br J Anaesth. (2023) 130:183–90. doi: 10.1016/j.bja.2022.09.023
66. Yoshii R, Sawa T, Kawajiri H, Amaya F, Tanaka KA, Ogawa S. A comparison of the ClotPro system with rotational thromboelastometry in cardiac surgery: a prospective observational study. Sci Rep. (2022) 12:17269. doi: 10.1038/s41598-022-22119-x
67. Huffmyer JL, Fernandez LG, Haghighian C, Terkawi AS, Groves DS. Comparison of SEER sonorheometry with rotational thromboelastometry and laboratory parameters in cardiac surgery. Anesth Analg. (2016) 123:1390–9. doi: 10.1213/ANE.0000000000001507
68. Groves DS, Welsby IJ, Naik BI, Tanaka K, Hauck JN, Greenberg CS, et al. Multicenter evaluation of the quantra QPlus system in adult patients undergoing major surgical procedures. Anesth Analg. (2020) 130:899–909. doi: 10.1213/ANE.0000000000004659
69. Carll T. Viscoelastic testing methods. Adv Clin Chem. (2023) 117:1–52. doi: 10.1016/bs.acc.2023.09.001
70. Tamura T, Suzuki S, Fujii T, Hirai T, Imaizumi T, Kubo Y, et al. Thromboelastographic evaluation after cardiac surgery optimizes transfusion requirements in the intensive care unit: a single-center retrospective cohort study using an inverse probability weighting method. Gen Thorac Cardiovasc Surg. (2024) 72:15–23. doi: 10.1007/s11748-023-01941-8
71. Naguib AN, Carrillo SA, Corridore M, Bigelow AM, Walczak A, Tram NK, et al. A ROTEM-guided algorithm aimed to reduce blood product utilization during neonatal and infant cardiac surgery. J Extra Corpor Technol. (2023) 55:60–9. doi: 10.1051/ject/2023017
72. Keyl C, Bashota A, Beyersdorf F, Trenk D. Rotational thromboelastometry and conventional coagulation tests in patients undergoing major cardiac or aortic surgery: a retrospective single-center cohort study. J Thromb Thrombolysis. (2022) 53:149–57. doi: 10.1007/s11239-021-02519-y
73. Wong Q, Byrne KP, Robinson SC. Clinical agreement and interchangeability of TEG5000 and TEG6s during cardiac surgery. Anaesth Intensive Care. (2020) 48:43–52. doi: 10.1177/0310057X19897657
74. Haensig M, Kempfert J, Kempfert PM, Girdauskas E, Borger MA, Lehmann S. Thrombelastometry guided blood-component therapy after cardiac surgery: a randomized study. BMC Anesthesiol. (2019) 19:201. doi: 10.1186/s12871-019-0875-7
75. Rigal J-C, Boissier E, Lakhal K, Riche V-P, Durand-Zaleski I, Rozec B. Cost-effectiveness of point-of-care viscoelastic haemostatic assays in the management of bleeding during cardiac surgery: protocol for a prospective multicentre pragmatic study with stepped-wedge cluster randomised controlled design and 1-year follow-up (the IMOTEC study). BMJ Open. (2019) 9:e029751. doi: 10.1136/bmjopen-2019-029751
76. Kuiper GJAJM, van Egmond LT, Henskens YMC, Roekaerts PM, Maessen JG, Ten Cate H, et al. Shifts of transfusion demand in cardiac surgery after implementation of rotational thromboelastometry-guided transfusion protocols: analysis of the HEROES-CS (HEmostasis Registry of patiEntS in Cardiac Surgery) observational, prospective open cohort database. J Cardiothorac Vasc Anesth. (2019) 33:307–17. doi: 10.1053/j.jvca.2018.08.203
77. Ichikawa J, Marubuchi T, Nishiyama K, Kodaka M, Görlinger K, Ozaki M, et al. Introduction of thromboelastometry-guided administration of fresh-frozen plasma is associated with decreased allogeneic blood transfusions and post-operative blood loss in cardiopulmonary-bypass surgery. Blood Transfus. (2018) 16:244–52. doi: 10.2450/2017.0265-16
78. Bhardwaj V, Kapoor PM, Karanjkar AA, Chowdhury UK, Hote MP, Rajashekhar P. Congenital cyanotic cardiac surgery in children: is algorithm-based point-of-care testing essential to prevent bleeding? J Cardiac Crit Care. (2018) 2:84–90. doi: 10.1055/s-0039-1692118
79. Smith I, Pearse BL, Faulke DJ, Naidoo R, Nicotra L, Hopkins P, et al. Targeted bleeding management reduces the requirements for blood component therapy in lung transplant recipients. J Cardiothorac Vasc Anesth. (2017) 31:426–33. doi: 10.1053/j.jvca.2016.06.027
80. Faraoni D, Willems A, Romlin BS, Belisle S, Van der Linden P. Development of a specific algorithm to guide haemostatic therapy in children undergoing cardiac surgery: a single-centre retrospective study. Eur J Anaesthesiol. (2015) 32:320–29. doi: 10.1097/EJA.0000000000000179
81. Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. (2009) 18:277–88. doi: 10.1016/j.hlc.2008.08.016
82. Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analges. (1999) 88:312–9. doi: 10.1213/00000539-199902000-00016
83. Janko N, Majeed A, Kemp W, Hogan C, Nandurkar H, Roberts SK. Rotational thromboelastometry-guided blood component administration versus standard of care in patients with cirrhosis and coagulopathy undergoing invasive ProcEdures (RECIPE): study protocol for a randomised controlled trial. Trials. (2023) 24:516. doi: 10.1186/s13063-023-07552-1
84. Premkumar M, Mehtani R, Divyaveer S, Kajal K, Kulkarni AV, Ahmed S, et al. Clinical validation of global coagulation tests to guide blood component transfusions in cirrhosis and ACLF. J Clin Transl Hepatol. (2021) 9:210–19. doi: 10.14218/JCTH.2020.00121
Keywords: viscoelastic hemostatic assay, thromboelastography, rotational thromboelastometry, major bleeding, coagulopathy, transfusion
Citation: Xu Z-L, Cao H, Sun D-W, Xiao J, Yao Y-Y, Luo G, Wang T-T, Gao Q, Zou J-C, Tao X-C and Yan M (2025) Critical trigger thresholds for hemostatic management: a narrative review of viscoelastic hemostatic assay applications. Front. Med. 12:1658845. doi: 10.3389/fmed.2025.1658845
Received: 03 July 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Kimberly A. Thomas, Vitalant Research Institute, United StatesCopyright © 2025 Xu, Cao, Sun, Xiao, Yao, Luo, Wang, Gao, Zou, Tao and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Yan, enJ5YW5taW5Aemp1LmVkdS5jbg==; MTM3NTcxMTg2MzI=
 Zhi-Li Xu
Zhi-Li Xu Han Cao1,2
Han Cao1,2 Jie Xiao
Jie Xiao Yuan-Yuan Yao
Yuan-Yuan Yao Ge Luo
Ge Luo Ting-Ting Wang
Ting-Ting Wang Xin-Chen Tao
Xin-Chen Tao Min Yan
Min Yan