- 1Clinical Medical College of North Sichuan Medical College, Nanchong, Sichuan, China
- 2Department of Anesthesiology, Guang'an People's Hospital, Guang'an, Sichuan, China
Arthroscopic rotator cuff repair (ARCR) is a commonly performed surgical intervention for rotator cuff injuries. The advent of ultrasound guidance has facilitated the widespread adoption of nerve blocks as an adjunct anesthetic and analgesic strategy in the perioperative phase of ARCR, offering notable benefits in maintaining patients' intraoperative hemodynamic stability and reducing opioid consumption. Despite the minimally invasive nature of ARCR, a subset of patients still experiences substantial pain, encompassing both acute pain and rebound pain following nerve block. Post-nerve block rebound pain is characterized by severe and disabling discomfort, which adversely impacts the patient experience and postoperative recovery. The mechanism underlying rebound pain after nerve block may involve aberrant excitation of relevant nerve fibers, the pharmacology of local anesthetics, nerve injury, local inflammatory factors, surgical anesthesia, and patient-specific variability. The review suggests that the incidence of post-nerve block rebound pain can be reduced, and postoperative pain management outcomes following ARCR can be improved, through interventions such as continuous nerve block, combined peripheral nerve block, administration of local anesthetic adjuvants, multimodal analgesia, and patient education.
1 Introduction
Rotator cuff injuries represent a common source of shoulder pain in middle-aged and elderly populations, primarily resulting from soft tissue damage to the tendons of the subscapularis, supraspinatus, infraspinatus, and teres minor muscles surrounding the glenohumeral joint. Clinically, these injuries are characterized by shoulder pain and restricted range of motion, potentially impacting daily function and quality of life (1, 2). In the general population, the overall prevalence of rotator cuff injuries is estimated at 20%−40%, rising to 50%−60% among individuals aged 60 years and above. Arthroscopic rotator cuff repair (ARCR) serves as a primary surgical intervention for rotator cuff injuries, offering effective pain relief and functional improvement (3). Despite its minimally invasive nature, ARCR is associated with varying degrees of postoperative pain, and the incidence of severe intraoperative or postoperative pain has been reported to be as high as 45% (4). In recent years, ultrasound-guided nerve blocks have become a common adjunct to anesthesia and analgesia during and after ARCR, owing to their superior analgesic efficacy and minimal systemic side effects. This technique offers effective perioperative analgesia, lowers opioid consumption and associated side effects, and may reduce hospital stay duration (5). However, significant pain may occur in some patients following the resolution of the nerve block, potentially due to rebound pain. Rebound pain, a significant complication after nerve block, is associated with a high incidence and may require increased postoperative opioid use, thereby diminishing the overall benefits of nerve block (6). This review aims to examine the definition, clinical characteristics, underlying mechanisms, influencing factors, and management strategies related to rebound pain following nerve block. The objective is to provide a framework and clinical guidance to mitigate rebound pain after an ARCR nerve block and optimize postoperative pain management protocols.
2 Material and methods
A literature search was performed in the PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) databases, with studies identified up to June 2025. The following search terms were employed: “arthroscopic rotator cuff repair,” “shoulder arthroscopy,” “rotator cuff injury,” “nerve block,” “rebound pain,” “postoperative pain,” “intermuscular groove brachial plexus nerve block,” “multimodal analgesia,” and “continuous nerve block.” Relevant literature, including reviews, clinical trials, randomized controlled trials, case reports, systematic reviews, and meta-analyses related to nerve block, rebound pain, postoperative pain, and analgesia following ARCR, was considered for inclusion. Irrelevant, duplicate, or controversial studies were excluded. The sources and content were independently screened by two researchers, and a total of 77 studies were ultimately selected for analysis.
3 Definition and clinical features of rebound pain after nerve block
The definition of rebound pain following nerve block remains without a universally accepted standard. In 2007, Williams first introduced the term “rebound pain” after nerve block to describe the acute and severe pain that occurs as the effects of the nerve block dissipate (7). In this study, rebound pain was operationalized as the measurable difference between the lowest pain score in the initial hours after block resolution, proposing the “rebound pain score” to quantify it, calculated as the highest pain score within 12 h of block resolution minus the lowest pain score before block resolution (7). Shi et al. (8) and Li et al. (9) used “rebound pain” as a translation of “flare pain.” Stone et al. (10) recommended a more neutral term, such as “pain on block resolution,” pending further clarification of its etiology and clinical implications. Given the current diversity of terminology used in academic literature to describe the acute pain phenomenon following the dissipation of nerve block effects, this review adopts “rebound pain” as the standardized term to maintain consistency and precision in its discussion. It is explicitly defined as acute, intense pain that emerges following the complete restoration of sensory function after nerve block. This definition and terminology will be uniformly applied throughout the text to ensure academic rigor and logical consistency.
Clinically, post-block rebound pain after ARCR typically occurs at night, begins 12–24 h post-nerve block, lasts 2–6 h, and progressively escalates, culminating in peak state nociceptive hypersensitivity. Patients often described the pain as a burning or dull ache in the shoulder. Individual variability in nerve block regression makes it difficult to determine the precise onset of rebound pain (11). Current research suggests a strong correlation between rebound pain intensity and the inflammatory repair process around the surgical site, indicating that it is a transient pain response distinct from chronic postoperative pain (12).
4 Mechanism of rebound pain after ARCR nerve block
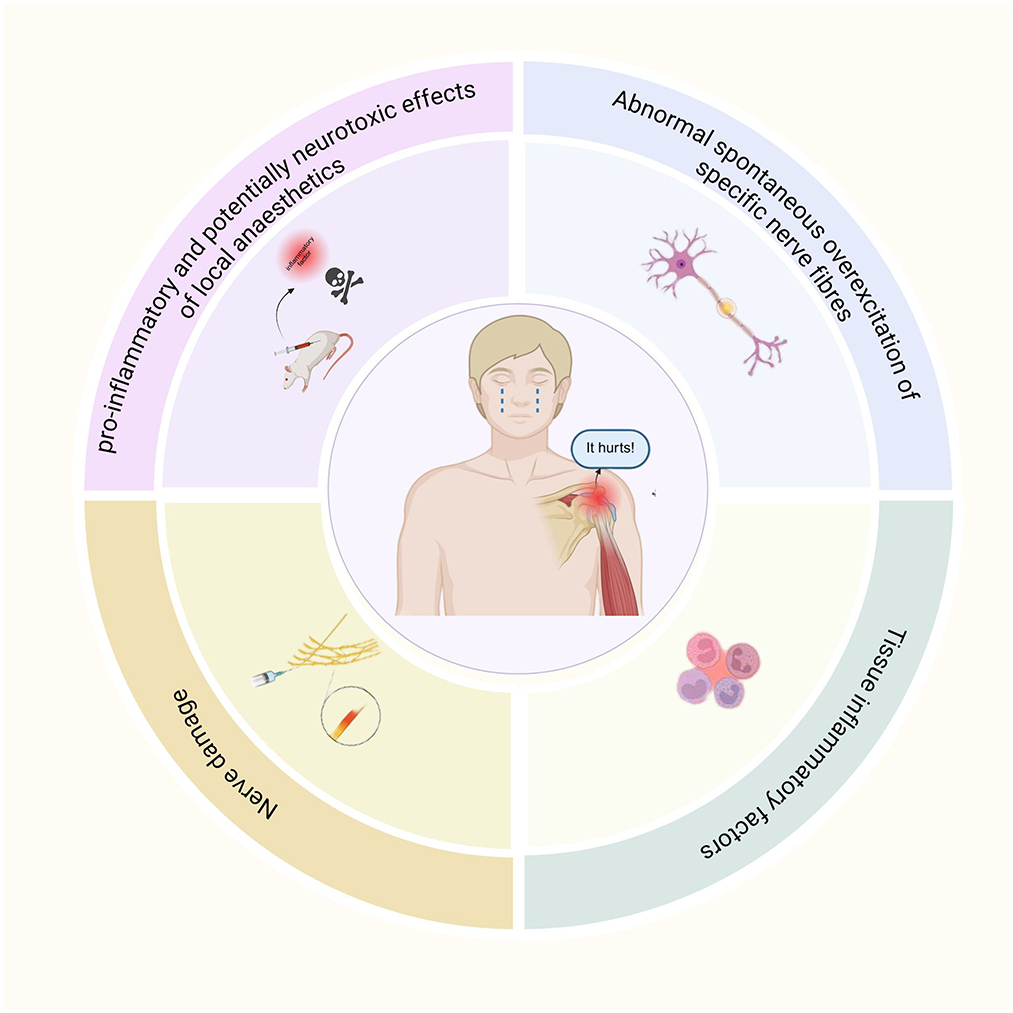
At present, the mechanism of rebound pain after nerve block remains incompletely understood; current evidence indicates that it may result from a complex interplay of multiple factors (Figure 1).
4.1 Abnormal spontaneous overexcitation of specific nerve fibers
Rebound hyperalgesia following nerve block may result from abnormal spontaneous excitation in specific nerve fibers. A study demonstrated that sciatic nerve block with ropivacaine in a rat model induced transient thermal hyperalgesia after analgesia, without persistent changes in mechanical sensitization or evidence of nerve injury during a 14-day observation period (13). This observation suggests that rebound pain shares features with neuropathic pain, potentially driven by spontaneous hyperexcitability of abnormal nerve fibers and heightened injury receptor excitability in the absence of mechanical nerve injury. Anatomically, patients experiencing rebound pain are typically in a denervated state. Following a primary lesion affecting a neuron's cell body or postganglionic axon, the postsynaptic membrane of adjacent neurons may generate spontaneous discharges when exposed to local transmitters, a process referred to as “denervation hypersensitivity” (14).
4.2 Pro-inflammatory and potentially neurotoxic effects of local anesthetics
In clinical practice, amide-linked lidocaine hydrochloride, long-acting bupivacaine hydrochloride, and ropivacaine are widely used for anesthesia and analgesic nerve blocks in ARCR. In a rat model, perisciatic injection of ropivacaine caused degeneration of myelinated nerve fibers and axons, accompanied by increased levels of the inflammatory cytokines interleukin-6 (IL-6) and interleukin-1β (IL-1β) (15). This finding suggests that, although nerve blockade reduces nociceptive signaling, local inflammatory responses persist and may even be exacerbated. Clinical investigations have demonstrated that amide and ester local anesthetics can induce neurotoxicity through mechanisms such as DNA damage, mitochondrial dysfunction, and neuronal apoptosis (16). Furthermore, bupivacaine has been shown to increase the production of reactive oxygen species (ROS) and upregulate cellular autophagy (17). The combined effects of these mechanisms may play a critical role in neurotoxicity and could contribute to rebound pain following nerve block. Gordon et al. (18) further indicated that local application of bupivacaine and lidocaine produced pro-inflammatory effects after tissue injury, leading to upregulation of cyclooxygenase-2 (COX-2) gene expression and the increased secretion of prostaglandin E2 (PGE2), thereby promoting inflammatory pain. Conversely, Martin et al. suggested that local anesthetics may also exert anti-inflammatory effects (19). In that study, patients undergoing knee arthroplasty were divided into two groups. The control group received intravenous morphine for patient-controlled analgesia after general anesthesia, whereas the experimental group received a continuous femoral nerve block with 0.2% ropivacaine. Results showed that the experimental group exhibited significantly smaller increases in local skin temperature and knee joint circumference compared to the control group. These findings suggest that local anesthetics may exert anti-inflammatory effects through the nerve block pathway. This dual role highlights the complexity of local anesthetics in rebound pain mechanisms. Therefore, further studies are warranted to clarify the relationship between rebound pain and the pro-inflammatory effects of local anesthetics.
4.3 Nerve damage
Peripheral nerve block procedures and surgical interventions pose a potential of iatrogenic nerve injury. Ultrasound-guided nerve blocks provide greater safety and accuracy compared with traditional techniques. However, factors such as anatomical variations (e.g., aberrant nerve courses), excessive subcutaneous fat in obese patients affecting image resolution, operator inexperience, and other variables may contribute to nerve damage. Neurovascular compromise during nerve block may also lead to peripheral nerve injury, potentially caused by direct trauma, vascular occlusion, or intraneural hematoma (20). Anatomical studies have shown substantial variability, up to 50% or more, in the composition, branching, and alignment of the brachial plexus, with reported nerve injury rates as high as 3.16% for interscalene brachial plexus blocks (21, 22). Consequently, to reduce the risk of iatrogenic nerve injury, anesthesia and analgesic nerve block for ARCR are best performed under real-time ultrasound guidance, supplemented by nerve stimulation. During ARCR, prolonged positioning (e.g., lateral or beach chair position) and surgical manipulation may cause local nerve compression. Furthermore, patients with pre-existing peripheral neuropathy are at higher risk of nerve injury (23).
4.4 Tissue inflammatory factors
Rotator cuff injuries commonly result from acute trauma or chronic strain. Preoperatively, chronic ischemia and hypoxia of periarticular tendons, fascia, and other soft tissues are often present, leading to aseptic inflammation. Intraoperatively, mechanical friction from arthroscopic instruments, tissue edema, the inflammatory response induced by the saline infusion, and the local foreign body reaction to suture anchors may contribute to local tissue injury. These processes initiate a local inflammatory cascade, involving the activation of multiple inflammatory mediators and peripheral nociceptors (6).
Beyond peripheral mechanisms, central immune responses such as microglial activation also play a critical role in rebound pain. Yamada et al. (24) demonstrated that peripheral nerve block can exacerbate the acute inflammatory response following surgical incisions and other trauma. They observed neutrophil and macrophage infiltration and upregulation of PGE2 and tumor necrosis factor-α (TNF-α) near the incision site. Thus, during the recovery phase of a nerve block, marked infiltration of inflammatory cells and elevated inflammatory mediators in the surgical incision area amplify the afferent impulses of nociceptive signals. Microglia, as key components of the intrinsic immune system of the central nervous system, are the earliest responders to nerve injury (25). They rapidly react by altering their phenotype and function, secreting multiple inflammatory mediators, and establishing close interactions with neuronal cells, thereby promoting the development of pain (26).
5 Risk factors for rebound pain after ARCR nerve blocks
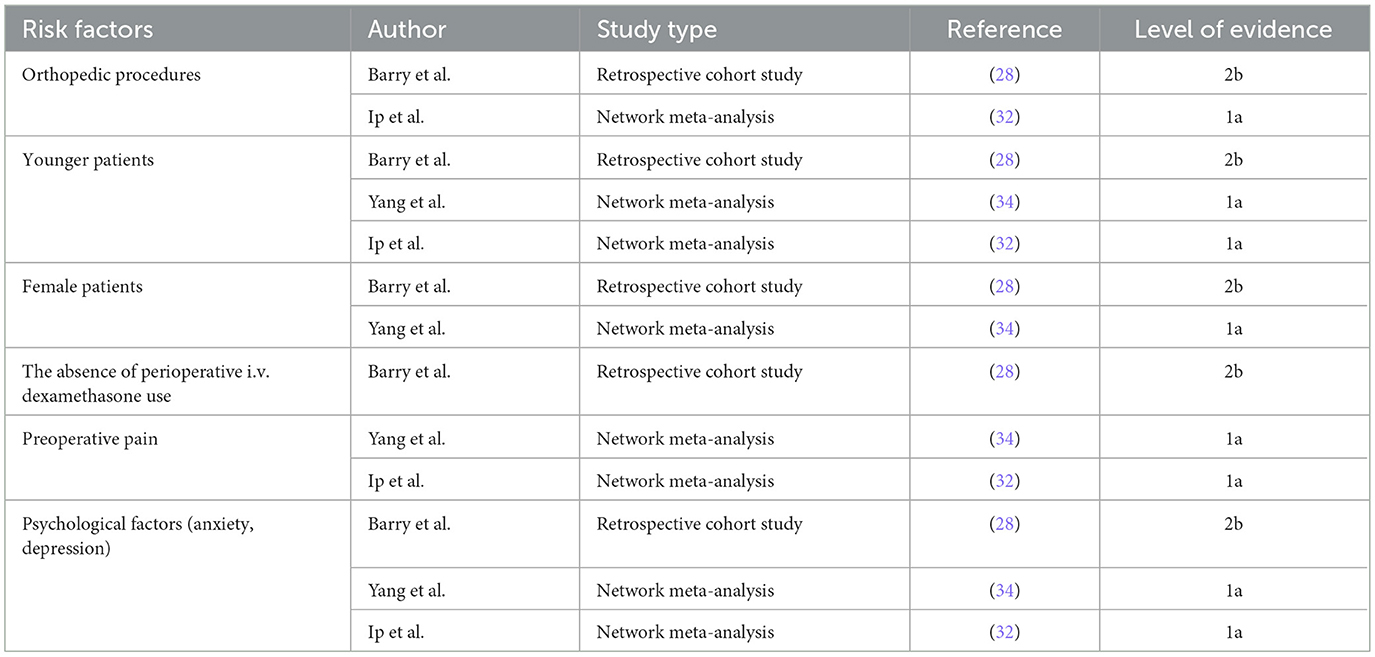
5.1 Surgical anesthesia factors
ARCR, a procedure within the realm of upper extremity joint orthopedics, typically uses regional nerve block, general anesthesia, or a combination thereof for anesthetic and analgesic management. The increasing use of ultrasound guidance, along with the demand for preemptive analgesia, patient comfort, and effective postoperative pain control, has promoted the widespread adoption of combined regional nerve block and general anesthesia (27). Barry et al. (28) reported that rebound pain was 6.5 times more common after orthopedic procedures than after soft tissue surgeries, with the knee and shoulder being particularly susceptible. De Boer et al. (29) found that rotator cuff repair was associated with a higher incidence of severe pain compared with other shoulder surgeries. Furthermore, postoperative rebound pain occurs more often after upper extremity surgery than after lower extremity procedures (30). In a study of patients undergoing internal fixation of distal radius fractures, those who received an interscalene brachial plexus block exhibited significantly higher pain scores at 12 h and 24 h postoperatively compared with those who received general anesthesia, suggesting that severe rebound pain may have occurred during recovery from the brachial plexus block (31).
5.2 Patient factors
Rebound pain has been associated with age, gender, preoperative pain level, and psychological factors (11, 32). Barry et al. (28) observed that approximately half of the patients undergoing ambulatory surgery with nerve block developed rebound pain, and their findings suggested that it was linked to patient age, gender, and other factors. The incidence and intensity of rebound pain were higher in younger patients than in older patients. This difference may be explained by diminished postoperative nociception in older patients, which contributes to greater pain tolerance and slower peripheral nerve conduction (33). Yang et al. (34) demonstrated that female patients were more susceptible to rebound pain. Female patients required higher doses of analgesic medication than males to achieve adequate postoperative analgesia. This phenomenon may be related to psychosocial complexity and sex-based differences in pain perception and experience. Furthermore, Riley et al. (35) found that patients with preoperative pain were more likely to develop postoperative rebound pain, suggesting a potential link to central and peripheral sensitization induced by preoperative pain. López-Millán et al. (36) proposed that preoperative chronic moderate-to-severe shoulder pain is a risk factor for postoperative shoulder pain, as these patients have a significantly higher risk of severe postoperative pain compared with the general population. Patients undergoing ARCR often present with preoperative pain, and those with chronic rotator cuff injuries usually experience a longer duration of symptoms.
In conclusion, patients undergoing ARCR with nerve block are at an increased risk of postoperative rebound pain. This association warrants confirmation in multicenter clinical studies with larger sample sizes. Risk factors are summarized in Table 1.
6 Preventive measures for rebound pain after nerve block for ARCR
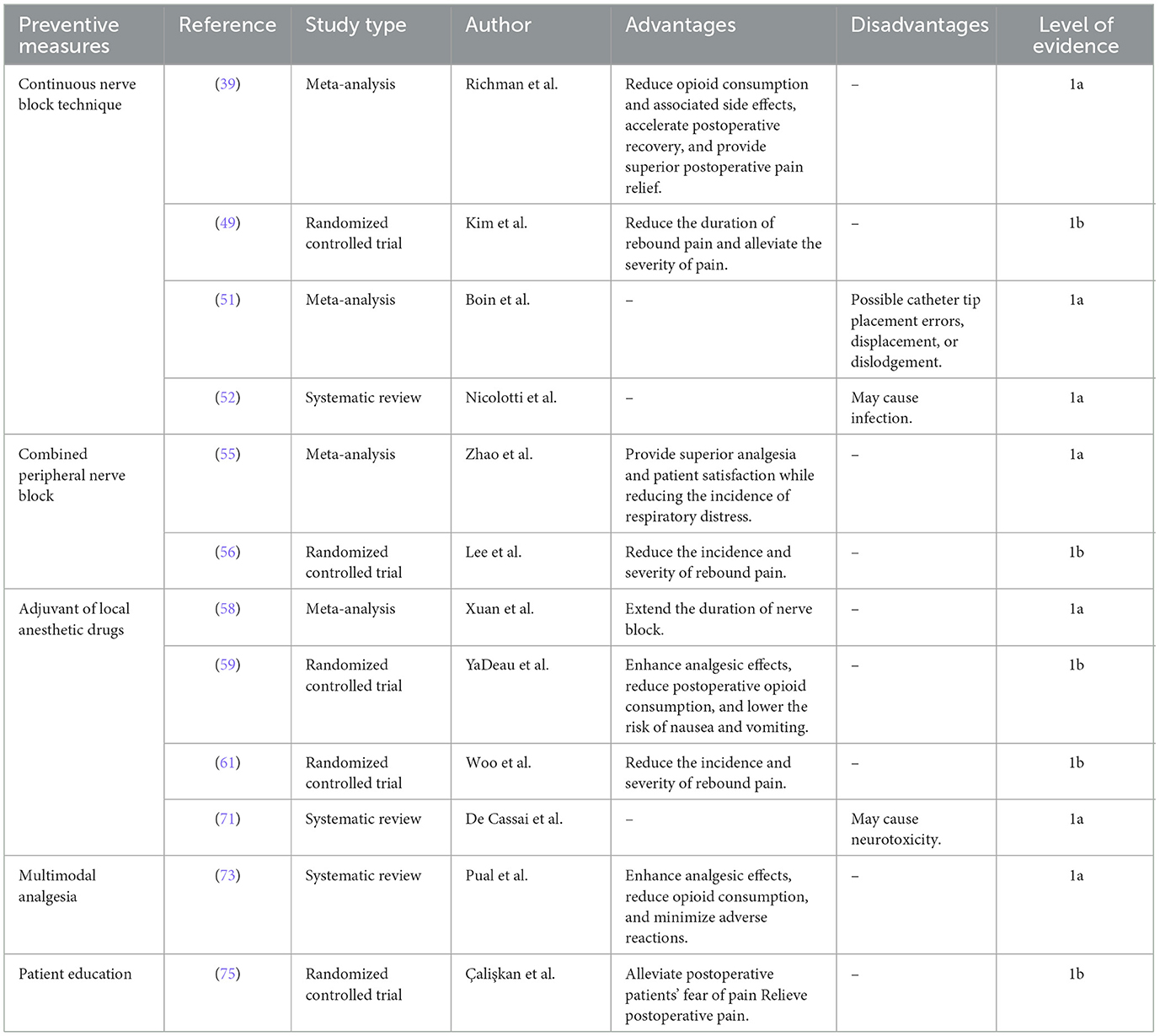
Given the mechanisms and risk factors of rebound pain following nerve block, preemptive strategies are essential. The management of rebound pain represents a multifaceted perioperative challenge that requires collaborative efforts among anesthesiologists, surgeons, and patients and involves the following interventions (Table 2).
6.1 Continuous nerve block technique
The shoulder joint receives innervation from the cervical and brachial plexus. Cutaneous sensation of the shoulder is mainly mediated by the supraclavicular, axillary, and lateral brachial cutaneous nerves. The joint capsule is predominantly innervated by the axillary and suprascapular nerves (37). Commonly used nerve blocks in the perioperative management of shoulder surgery include brachial plexus block, superior trunk block, suprascapular nerve block, and axillary nerve block. These nerve block techniques offer effective analgesia for ARCR. However, the duration of analgesia from a single nerve block is limited. Continuous nerve block techniques can prolong postoperative analgesia, thereby reducing opioid requirements and mitigating rebound pain after nerve block administration (38, 39).
6.1.1 Continuous interscalene brachial plexus block
The brachial plexus, comprised of the C5–C8 nerve roots and the anterior ramus of T1, is the target of the interscalene brachial plexus block (40). This technique effectively blocks the subscapular, axillary, and lateral thoracic nerves, thereby covering the innervation of the shoulder joint and surrounding structures. As a result, comprehensive anesthesia of the shoulder joint capsule, subacromial bursa, coracoclavicular ligaments, and cutaneous sensation of the shoulder is achieved, providing adequate analgesia for shoulder surgery (41). Interscalene brachial plexus block, often combined with general anesthesia is a preferred regional anesthetic technique for shoulder arthroscopy, as it reduces anesthetic drug requirements and provides significant benefits in postoperative analgesia and opioid sparing (42). Kim et al. (43) investigated the analgesic effects of single-injection vs. continuous-infusion interscalene brachial plexus block with an indwelling catheter in patients undergoing ARCR. The single-injection group demonstrated significant early postoperative analgesia, with lower pain scores at 6 h, followed by a subsequent increase after 12 h. Conversely, the continuous-infusion group exhibited progressively decreasing pain scores over 24 h. These findings suggest that single-injection interscalene brachial plexus block may be associated with rebound pain, likely due to the resolution of complete neural blockade.
Early postoperative mobilization of the affected limb is crucial to prevent joint stiffness and to promote functional recovery. While continuous interscalene brachial plexus block provides effective analgesia after shoulder arthroscopy, prolonged motor blockade may hinder mobility and delay recovery. Furthermore, local anesthetic may spread to the phrenic nerve region, posing a risk of phrenic nerve dysfunction, rendering this technique unsuitable for patients with respiratory compromise (44).
6.1.2 Continuous upper trunk nerve block
Burckett-St. Laurent et al. (45) proposed the upper trunk nerve block to reduce phrenic nerve impairment associated with conventional brachial plexus block. The upper trunk of the brachial plexus is formed by the union of the C5 and C6 nerve roots. Peripheral nerves supplying the shoulder joint arise distally from the upper trunk, and local anesthetic injection around the upper trunk can therefore provide shoulder analgesia. Kang et al. (46) confirmed that the analgesic efficacy of the superior trunk nerve block is comparable to that of the interscalene brachial plexus block, while better preserving phrenic nerve function. Kim et al. (47) further demonstrated that continuous upper trunk block provides superior postoperative analgesia compared with continuous suprascapular nerve block in patients undergoing shoulder arthroscopy. However, further investigation is warranted to clarify the impact of continuous upper trunk block on rebound pain following ARCR nerve block.
6.1.3 Continuous suprascapular nerve block
The suprascapular nerve, which originates from the upper trunk of the brachial plexus, provides essential innervation to the shoulder joint (48). Suprascapular nerve block carries minimal intraoperative and postoperative risks, making it a common technique in shoulder arthroscopy. Kim et al. (49) introduced a novel postoperative pain management strategy involving arthroscopic placement of a catheter around the suprascapular nerve, followed by continuous ropivacaine infusion. This study hypothesized that continuous analgesia via an indwelling catheter could effectively control postoperative pain, mitigate rebound pain, and reduce opioid use. Results demonstrated a significant difference in postoperative visual analog scale (VAS) scores between the single brachial plexus block group and the indwelling suprascapular nerve catheter group from 8 to 40 h postoperatively. The latter group exhibited earlier rebound pain onset, shorter duration, and lower mean pain severity. These findings suggest that continuous suprascapular nerve block may provide superior postoperative pain control compared with single brachial plexus block. Furthermore, suprascapular nerve block does not significantly impair diaphragmatic function, rendering it suitable for patients with respiratory insufficiency.
Despite the potential benefits of continuous nerve block analgesia, catheter placement poses challenges, such as incorrect tip placement, displacement, dislodgement, and puncture-site infection, which may limit its widespread clinical application (50–52).
6.2 Combined peripheral nerve block
Compared with a single nerve block technique, combined peripheral nerve blocks reduce the incidence of postoperative rebound pain in patients undergoing ARCR. The suprascapular nerve innervates 70% of the shoulder joint, whereas the axillary nerve supplies sensation to the lateral shoulder. Thus, a combined suprascapular and axillary nerve block represents a viable alternative to brachial plexus block (4, 53). Although either suprascapular nerve block or brachial plexus block alone may lead to rebound pain, the combined block effectively reduces this occurrence. Lee et al. (54) demonstrated that a combined suprascapular and axillary nerve block provided superior analgesia and reduced rebound pain after shoulder arthroscopy compared with suprascapular nerve block alone. Additionally, Zhao et al. (55) reported that during arthroscopic surgery, combined suprascapular and axillary nerve blocks resulted in greater pain relief and higher patient satisfaction within 24 h postoperatively than suprascapular nerve block alone. Furthermore, compared with brachial plexus block, the incidence of dyspnoea was significantly lower. Another study by Lee et al. (56) showed that the combined use of ultrasound-guided brachial plexus block and arthroscopically guided suprascapular nerve block during shoulder arthroscopy reduced the incidence of rebound pain, delayed its onset, and decreased its severity compared with brachial plexus block alone. A study on shoulder arthroplasty further revealed that patients receiving brachial plexus block combined with intraoperative periarticular injections had significantly lower 24 h postoperative opioid consumption and pain scores compared to those receiving brachial plexus block alone (57).
6.3 Adjuvant of local anesthetic drugs
To optimize the efficacy of ARCR peripheral nerve block, adjuvants can be co- administered with local anesthetic via perineural injection to enhance analgesia, prolong block duration, and reduce postoperative opioid requirements, thereby decreasing the incidence of postoperative nausea and vomiting (5, 58, 59).
6.3.1 Dexamethasone
Dexamethasone, a long-acting glucocorticoid, is frequently used as an adjuvant to local anesthetics in clinical practice. Its use enhances analgesia, prolongs block duration, and reduces adverse effects associated with local anesthetics. Although the precise mechanism by which dexamethasone attenuates rebound pain remains unclear, its efficacy as an adjuvant has been well established. Wei et al. (60) reported that among single adjuvants to local anesthesia, dexamethasone was superior in prolonging analgesic duration and reducing opioid requirements. Woo et al. (61) conducted a study that patients received a single brachial plexus block before shoulder arthroscopy to evaluate postoperative analgesic efficacy of ropivacaine alone vs. ropivacaine combined with dexamethasone. The study found that the ropivacaine plus dexamethasone group had a more gradual block resolution, significantly smaller increases in numeric rating scale (NRS) pain scores (4.5 ± 2.4 vs. 6.9 ± 2.2), and a lower incidence of rebound pain (37.1% vs. 82.9%) compared with the ropivacaine group. Kim et al. (62) investigated the analgesic effects of perineural dexamethasone vs. continuous analgesia with an indwelling catheter in ARCR patients. The dexamethasone group demonstrated superior analgesia within the first 6 postoperative hours, but no significant difference in visual analog scale (VAS) scores thereafter. Furthermore, the dexamethasone group required fewer postoperative analgesic and less fentanyl. These findings suggest that dexamethasone, when added to local anesthetics, can prolong nerve block duration, reduce the need for postoperative analgesic requirements, and improve pain control after ARCR. The observed effects may be attributed to dexamethasone's peripheral anti-inflammatory properties or its interaction with peripheral glucocorticoid receptors, thereby inhibiting C-fiber activity and potassium channel function (61, 63). Lee et al. (64) compared the effects of adding 5 mg dexamethasone to local anesthetics with those of intravenous administration of 5 mg dexamethasone in patients undergoing interscalene brachial plexus block. The intravenous group experienced rebound pain later than the peripheral nerve injection group and demonstrated a greater reduction in the total degree of pain exacerbation and the incidence of rebound pain. These results align with the findings of Yang et al. (65), indicating that both intravenous and perineural dexamethasone administration can effectively reduce rebound pain.
6.3.2 Dexmedetomidine
Dexmedetomidine, a potent and selective α2-adrenergic agonist, has shown efficacy as an adjuvant to local anesthetics. Its mechanism of action involves binding to peripheral α2-adrenergic receptors, thereby inhibiting the release of norepinephrine from nerve terminals. This action subsequently prevents the initiation of action potentials in nerve fibers and inhibits the transmission of pain signals (15). Hwang et al. (66) compared ropivacaine plus dexmedetomidine with ropivacaine alone for interscalene brachial plexus block in patients undergoing ARCR. The study demonstrated that the dexmedetomidine group had significantly lower VAS scores within 18 postoperative hours and a significant delay in the onset of rebound pain. Additionally, plasma levels of inflammatory cytokines IL-6 and interleukin-8 (IL-8) were significantly lower in the dexmedetomidine group than in the ropivacaine-only group within 48 postoperative hours, suggesting that dexmedetomidine may suppress the systemic inflammatory response. In a study by Huan et al. (67), patients undergoing shoulder arthroscopy were randomized into two groups. One group received an interscalene brachial plexus block with dexmedetomidine and ropivacaine, whereas the control group received ropivacaine alone. The results indicated that the dexmedetomidine-ropivacaine combination significantly reduced rebound pain, prolonged the analgesia duration, and improved postoperative pain control.
6.3.3 Ketamine
Ketamine, a non-selective N-methyl-D-aspartate (NMDA) receptor antagonist, has analgesic, antinociceptive, and anti-inflammatory properties. A study on anterior cruciate ligament reconstruction (ACLR) compared intravenous and perineural nerve injections of ketamine. Both methods prolonged block duration and reduced the incidence of rebound pain, although the intravenous group showed a higher rate of postoperative hallucinations (68). Zhu et al. (69) demonstrated that ropivacaine combined with ketamine, administered via brachial plexus nerve block in patients with upper limb fractures, effectively reduced the incidence of rebound pain, accelerated block onset, and prolonged block duration. Conversely, the Touil et al. (70) study found intraoperative intravenous ketamine, administered after preoperative axillary brachial plexus block, did not affect the incidence or intensity of rebound pain. These findings suggest that ketamine's impact on postoperative rebound pain depends on the route of administration. Further studies are needed to clarify its efficacy in mitigating rebound pain after ARCR nerve block.
Local anesthetic adjuvants play a significant role in clinical practice, as they enhance nerve block efficacy, prolong block duration, and reduce opioid requirements. However, the combination of local anesthetic adjuvants with local anesthetic drugs may increase the risk of neurotoxicity. Research by De Cassai et al. (71) suggested that high-dose administration of local anesthetic adjuvants in patients with neuropathy may pose risks of neurotoxicity. Therefore, when local anesthetic adjuvants are combined with local anesthetic agents, the potential neurotoxic risks should be carefully evaluated, and administration should be undertaken with caution.
6.3.4 Multimodal analgesia
Multimodal analgesia is a fundamental approach in clinical pain management, in which multiple analgesic agents or techniques with distinct mechanisms are administered concurrently to enhance efficacy and reduce adverse effects (72). In the context of postoperative pain management in ARCR, multimodal analgesia that incorporates nerve blocks is essential. This typically involves a combination of oral analgesics, incisional local blocks, and non-pharmacological and reduce opioid requirements. Studies by Paul et al. (73) demonstrated that multimodal analgesia strategies reduce postoperative pain and opioid usage.
Currently used oral analgesics include acetaminophen, non-steroidal anti- inflammatory drugs (NSAIDs), calcium channel modulators (pregaba lin, gaba pentin), and tramadol. The PROSPECT guidelines based on rotator cuff repair recommend regular perioperative use of paracetamol and NSAIDs in the absence of contraindications. Meanwhile, intravenous dexamethasone prolongs the duration of interscalene brachial plexus block analgesia, reduces the need for additional analgesics, and has antiemetic properties. Opioids are reserved for postoperative rescue analgesia drugs (42).
Park et al. (74) conducted a study involving axillary nerve blocks performed without imaging guidance in ARCR patients, evaluating the impact of multimodal shoulder injections on postoperative rebound pain. The findings indicated that multimodal shoulder injections significantly reduced rebound pain after brachial plexus block. Specifically, this approach was associated with a significant reduction in postoperative pain scores and opioid consumption.
6.4 Patient education
The precise pathophysiologic mechanism underlying rebound pain after nerve block remains unclear, which hinders the development of effective preventative strategies. Prior to nerve block administration, patients should receive comprehensive information about the procedure's benefits and limitations, including the potential for moderate to severe pain after block resolution. Enhancing patient understanding of pain perception may improve their capacity to manage discomfort after block resolution. Galos et al. (31) proposed that preoperative pain education and counseling may improve patient awareness of rebound pain. They recommended that high-risk patients use analgesic medications proactively before block resolution to optimize prevention and control. In a randomized controlled trial, Çalişkan et al. (75) found that preoperative pain management education for orthopedic surgery patients effectively reduced fear of pain and alleviated discomfort. Shih et al. (76) suggested that preoperative education combined with postoperative follow-up may enhance' comprehension and recall of pain management strategies, improve adherence, reduce perioperative anxiety and uncertainty, and potentially mitigate the incidence of rebound pain. Furthermore, incorporating techniques such as meditation and relaxation training may reduce anxiety and improve pain perception (77).
7 Conclusion
Nerve block is a frequently employed adjunct for anesthesia and analgesia in ARCR. However, a subset of patients experience significant pain after block resolution, a phenomenon that is frequently overlooked. This paper provides a comprehensive overview of the pathophysiological mechanisms, risk factors, and strategies for the prevention of rebound pain. However, it should be noted that, as a narrative review, this study has limitations, including the lack of a systematic literature search and the absence of quantitative data analysis. Although various strategies have been proposed to mitigate rebound pain, each has inherent limitations. Patients undergoing ARCR represent a high-risk group for rebound pain. Future research should focus on elucidating the underlying mechanisms and developing preventative and therapeutic interventions for ARCR patients. These efforts should include optimizing nerve block techniques, selecting appropriate local anesthetics and adjuvants, and designing multimodal analgesia protocols. Multicenter studies with large sample sizes are essential to reduce the incidence of rebound pain and improve postoperative pain management after ARCR.
Author contributions
XY: Data curation, Writing – original draft, Conceptualization. YY: Conceptualization, Writing – review & editing. SQ: Conceptualization, Supervision, Writing – review & editing. YC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to sincerely thank all the researchers who have contributed to our understanding of rebound pain, and whose research has helped to refine our management of rebound pain after arthroscopic rotator cuff repair, resulting in a more comfortable patient experience.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ARCR, Arthroscopic rotator cuff repair.
References
1. Tang H, Luo F, Fan H, Huang L, Liao S, Yu W, et al. Acupuncture and manual therapy for rotator cuff tears: a protocol for systematic review and meta analysis. Medicine. (2020) 99:e20377. doi: 10.1097/MD.0000000000020377
2. Lin JC, Weintraub N, Aragaki DR. Nonsurgical treatment for rotator cuff injury in the elderly. J Am Med Dir Assoc. (2008) 9:626–32. doi: 10.1016/j.jamda.2008.05.003
3. Mancini MR, Horinek JL, Phillips CJ, Denard PJ. Arthroscopic rotator cuff repair: a review of surgical techniques and outcomes. Clin Sports Med. (2023) 42:81–94. doi: 10.1016/j.csm.2022.08.004
4. Checcucci G, Allegra A, Bigazzi P, Gianesello L, Ceruso M, Gritti G. New technique for regional anesthesia for arthroscopic shoulder surgery based on a suprascapular nerve block and an axillary nerve block: an evaluation of the first results. Arthroscopy. (2008) 24:689–96. doi: 10.1016/j.arthro.2008.01.019
5. Muñoz-Leyva F, Cubillos J, Chin KJ. Managing rebound pain after regional anesthesia. Korean J Anesthesiol. (2020) 73:372–83. doi: 10.4097/kja.20436
6. Dada O, Gonzalez Zacarias A, Ongaigui C, Echeverria-Villalobos M, Kushelev M, Bergese SD, et al. Does rebound pain after peripheral nerve block for orthopedic surgery impact postoperative analgesia and opioid consumption? A narrative review. Int J Environ Res Public Health. (2019) 16:3257. doi: 10.3390/ijerph16183257
7. Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med. (2007) 32:186–92. doi: 10.1016/j.rapm.2006.10.011
8. Shi YC, Fang J, Cang J, Zhang XG. Clinical characteristics and influencing factors of rebound pain following ultrasound-guided peripheral nerve block. Chin J Clin Med. (2019) 26:420–4. doi: 10.12025/j.issn.1008-6358.2019.20190341
9. Li JJ, Feng Y, Sun DF. New progress in anesthesia for day surgery. West China Med J. (2022) 37:295–300.
10. Stone A, Lirk P, Vlassakov K. Rebound pain after peripheral nerve blockade-bad timing or rude awakening? Anesthesiol Clin. (2022) 40:445–54. doi: 10.1016/j.anclin.2022.03.002
11. Lavand'homme P. Rebound pain after regional anesthesia in the ambulatory patient. Curr Opin Anaesthesiol. (2018) 31:679–84. doi: 10.1097/ACO.0000000000000651
12. Sunderland S, Yarnold CH, Head SJ, Osborn JA, Purssell A, Peel JK, et al. Regional versus general anesthesia and the incidence of unplanned health care resource utilization for postoperative pain after wrist fracture surgery: results from a retrospective quality improvement project. Reg Anesth Pain Med. (2016) 4:22–7. doi: 10.1097/AAP.0000000000000325
13. Kolarczyk LM, Williams BA. Transient heat hyperalgesia during resolution of ropivacaine sciatic nerve block in the rat. Reg Anesth Pain Med. (2011) 36:220–4. doi: 10.1097/AAP.0b013e3182176f5a
14. Kleggetveit IP, Namer B, Schmidt R, Helås T, Rückel M, Ørstavik K, et al. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. (2012) 153:2040–7. doi: 10.1016/j.pain.2012.05.017
15. Kim BS, Choi JH, Baek SH, Lee DH. Effects of intraneural injection of dexmedetomidine in combination with ropivacaine in rat sciatic nerve block. Reg Anesth Pain Med. (2018) 43:378–84. doi: 10.1097/AAP.0000000000000745
16. Yu XJ, Zhao W, Li YJ, Li FX, Liu ZJ, Xu HL, et al. Neurotoxicity comparison of two types of local anaesthetics: amide-bupivacaine versus ester-procaine. Sci Rep. (2017) 7:45316. doi: 10.1038/srep45316
17. Zhao W, Liu Z, Yu X, Lai L, Li H, Liu Z, et al. iTRAQ proteomics analysis reveals that PI3K is highly associated with bupivacaine-induced neurotoxicity pathways. Proteomics. (2016) 16:564–75. doi: 10.1002/pmic.201500202
18. Gordon SM, Chuang BP, Wang XM, Hamza MA, Rowan JS, Brahim JS, et al. The differential effects of bupivacaine and lidocaine on prostaglandin E2 release, cyclooxygenase gene expression and pain in a clinical pain model. Anesth Analg. (2008) 106:321–7. doi: 10.1213/01.ane.0000296474.79437.23
19. Martin F, Martinez V, Mazoit JX, Bouhassira D, Cherif K, Gentili ME, et al. Antiinflammatory effect of peripheral nerve blocks after knee surgery: clinical and biologic evaluation. Anesthesiology. (2008) 109:484–90. doi: 10.1097/ALN.0b013e318182c2a1
20. Brull R, Hadzic A, Reina MA, Barrington MJ. Pathophysiology and Etiology of nerve injury following peripheral nerve blockade [published correction appears in Reg Anesth Pain Med. (2024) 49:e2. Reg Anesth Pain Med. (2015) 40:479–90. doi: 10.1097/AAP.0000000000000125
21. Johnson EO, Vekris M, Demesticha T, Soucacos PN. Neuroanatomy of the brachial plexus: normal and variant anatomy of its formation. Surg Radiol Anat. (2010) 32:291–7. doi: 10.1007/s00276-010-0646-0
22. Holbrook HS, Parker BR. Peripheral nerve injury following interscalene blocks: a systematic review to guide orthopedic surgeons. Orthopedics. (2018) 41:e598–606. doi: 10.3928/01477447-20180815-04
23. Hewson DW, Bedforth NM, Hardman JG. Peripheral nerve injury arising in anaesthesia practice. Anaesthesia. (2018) 73:51–60. doi: 10.1111/anae.14140
24. Yamada T, Hasegawa-Moriyama M, Kurimoto T, Saito T, Kuwaki T, Kanmura Y. Peripheral nerve block facilitates acute inflammatory responses induced by surgical incision in mice. Reg Anesth Pain Med. (2016) 41:593–600. doi: 10.1097/AAP.0000000000000458
25. Shen YH, Zhang YC, Xiao B, Zhang J, Fang M, Yao CJ. Research progress on the role of neuro-immune communication in pain. Chin J Pain Med. (2023) 29:847–54. doi: 10.3969/j.issn.1006-9852.2023.11.008
26. Tansley S, Gu N, Guzmán AU, Cai W, Wong C, Lister KC, et al. Microglia-mediated degradation of perineuronal nets promotes pain. Chin J Pain Med. (2022) 377:80–6. doi: 10.1126/science.abl6773
27. Kim TY, Hwang JT. Regional nerve blocks for relieving postoperative pain in arthroscopic rotator cuff repair. Clin Shoulder Elb. (2022) 25:339–46. doi: 10.5397/cise.2022.01263
28. Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. (2021) 126:862–71. doi: 10.1016/j.bja.2020.10.035
29. de Boer FA, Schouten TTJ, Boekestein EP, van Eijk F, van Kampen PM, Bazuin R, et al. Risk factors for postoperative pain in the first three weeks after arthroscopic or open shoulder surgery. Orthop Traumatol Surg Res. (2019) 105:241–4. doi: 10.1016/j.otsr.2018.08.018
30. Yao JH, Pu SF, Du DP. Research progress on rebound pain after peripheral nerve lock. Chin J Pain Med. (2024) 30:453–7. doi: 10.3969/j.issn.1006-9852.2024.06.009
31. Galos DK, Taormina DP, Crespo A, Ding DY, Sapienza A, Jain S, et al. Does brachial plexus blockade result in improved pain scores after distal radius fracture fixation? A randomized trial. Clin Orthop Relat Res. (2016) 474:1247–54. doi: 10.1007/s11999-016-4735-1
32. Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. (2009) 111:657–77. doi: 10.1097/ALN.0b013e3181aae87a
33. van Dijk JFM, Zaslansky R, van Boekel RLM, Cheuk-Alam JM, Baart SJ, Huygen FJPM, et al. Postoperative pain and age: a retrospective cohort association study. Anesthesiology. (2021) 135:1104–19. doi: 10.1097/ALN.0000000000004000
34. Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. (2019) 9:e025091. doi: 10.1136/bmjopen-2018-025091
35. Riley M, Tassie B, Gawthorne J, Hadzic R, Stevens J. Increased opioid consumption after regional nerve blockade: association of fascia iliaca block with rebound pain in neck of femur fracture. Br J Anaesth. (2021) 127:e15–7. doi: 10.1016/j.bja.2021.03.034
36. López-Millán JM, Ruiz Iban MÁ, Díaz Heredia J, Roca Ruiz LJ. Preoperative management of patients with chronic moderate to severe shoulder pain to improve postoperative outcomes: a systematic review. Pain Med. (2025) 26:299–320. doi: 10.1093/pm/pnaf023
37. Bakhsh W, Nicandri G. Anatomy and physical examination of the shoulder. Sports Med Arthrosc Rev. (2018) 26:e10–22. doi: 10.1097/JSA.0000000000000202
38. Chelly JE, Ghisi D, Fanelli A. Continuous peripheral nerve blocks in acute pain management. Br J Anaesth. (2010) 105:i86–96. doi: 10.1093/bja/aeq322
39. Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. (2006) 102:248–57. doi: 10.1213/01.ANE.0000181289.09675.7D
40. Kimura S, Amatani H, Nakai H, Miyauchi R, Nagaoka T, Abe M, et al. A novel case of multiple variations in the brachial plexus with the middle trunk originating from the C7 and C8. Anat Sci Int. (2020) 95:559–63. doi: 10.1007/s12565-020-00541-3
41. Price DJ. The shoulder block: a new alternative to interscalene brachial plexus blockade for the control of postoperative shoulder pain. Anaesth Intensive Care. (2007) 35:575–81. doi: 10.1177/0310057X0703500418
42. Toma O, Persoons B, Pogatzki-Zahn E, Van de Velde M, Joshi GP. PROSPECT Working Group collaborators. PROSPECT guideline for rotator cuff repair surgery: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. (2019) 74:1320–31. doi: 10.1111/anae.14796
43. Kim JH, Koh HJ, Kim DK, Lee HJ, Kwon KH, Lee KY, et al. Interscalene brachial plexus bolus block versus patient-controlled interscalene indwelling catheter analgesia for the first 48 hours after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. (2018) 27:1243–50. doi: 10.1016/j.jse.2018.02.048
44. Verelst P, van Zundert A. Respiratory impact of analgesic strategies for shoulder surgery. Reg Anesth Pain Med. (2013) 38:50–3. doi: 10.1097/AAP.0b013e318272195d
45. Burckett-St Laurent D, Chan V, Chin KJ. Refining the ultrasound-guided interscalene brachial plexus block: the superior trunk approach. Can J Anaesth. (2014) 61:1098–102. doi: 10.1007/s12630-014-0237-3
46. Kang R, Jeong JS, Chin KJ, Yoo JC, Lee JH, Choi SJ, et al. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology. (2019) 131:1316–26. doi: 10.1097/ALN.0000000000002919
47. Kim HJ, Koh KH, Park JI, Kim YJ, Kim MJ, Kim H, et al. Comparison of the analgesic efficacy between arthroscopically placed continuous suprascapular nerve block and ultrasound-guided continuous superior trunk block: a double-blinded randomized controlled trial. Anesthesiology. (2023) 139:591–601. doi: 10.1097/ALN.0000000000004691
48. Heron M, Dattani R, Smith R. Interscalene vs suprascapular nerve block for shoulder surgery. Br J Hosp Med. (2016) 77:494. doi: 10.12968/hmed.2016.77.8.494
49. Kim JY, Kang MW, Lee HW, Noh KC. Suprascapular nerve block is an effective pain control method in patients undergoing arthroscopic rotator cuff repair: a randomized controlled trial. Orthop J Sports Med. (2021) 9:2325967120970906. doi: 10.1177/2325967120970906
50. Hauritz RW, Hannig KE, Balocco AL, Peeters G, Hadzic A, Børglum J, et al. Peripheral nerve catheters: a critical review of the efficacy. Best Pract Res Clin Anaesthesiol. (2019) 33:325–39. doi: 10.1016/j.bpa.2019.07.015
51. Boin MA, Mehta D, Dankert J, Umeh UO, Zuckerman JD, Virk MS. Anesthesia in total shoulder arthroplasty: a systematic review and meta-analysis. JBJS Rev. (2021) 9:e21.00115. doi: 10.2106/JBJS.RVW.21.00115
52. Nicolotti D, Iotti E, Fanelli G, Compagnone C. Perineural catheter infection: a systematic review of the literature. J Clin Anesth. (2016) 35:123–8. doi: 10.1016/j.jclinane.2016.07.025
53. Lee SM, Park SE, Nam YS, Han SH, Lee KJ, Kwon MJ, et al. Analgesic effectiveness of nerve block in shoulder arthroscopy: comparison between interscalene, suprascapular and axillary nerve blocks. Knee Surg Sports Traumatol Arthrosc. (2012) 20:2573–8. doi: 10.1007/s00167-012-1950-5
54. Lee JJ, Kim DY, Hwang JT, Lee SS, Hwang SM, Kim GH, et al. Effect of ultrasonographically guided axillary nerve block combined with suprascapular nerve block in arthroscopic rotator cuff repair: a randomized controlled trial. Arthroscopy. (2014) 30:906–14. doi: 10.1016/j.arthro.2014.03.014
55. Zhao J, Xu N, Li J, Liang G, Zeng L, Luo M, et al. Efficacy and safety of suprascapular nerve block combined with axillary nerve block for arthroscopic shoulder surgery: A systematic review and meta-analysis of randomized controlled trials. Int J Surg. (2021) 94:106111. doi: 10.1016/j.ijsu.2021.106111
56. Lee JJ, Hwang JT, Kim DY, Lee SS, Hwang SM, Lee NR, et al. Effects of arthroscopy-guided suprascapular nerve block combined with ultrasound-guided interscalene brachial plexus block for arthroscopic rotator cuff repair: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. (2017) 25:2121–8. doi: 10.1007/s00167-016-4198-7
57. Vijittrakarnrung C, Freshman R, Anigwe C, Lansdown DA, Feeley BT, Ma CB. Periarticular injection in addition to interscalene nerve block can decrease opioid consumption and pain following total shoulder arthroplasty: a comparison cohort study. J Shoulder Elbow Surg. (2023) 32:e597–607. doi: 10.1016/j.jse.2023.05.009
58. Xuan C, Yan W, Wang D, Li C, Ma H, Mueller A, et al. The facilitatory effects of adjuvant pharmaceutics to prolong the duration of local anesthetic for peripheral nerve block: a systematic review and network meta-analysis. Anesth Analg. (2021) 133:620–9. doi: 10.1213/ANE.0000000000005640
59. YaDeau JT, Paroli L, Fields KG, Kahn RL, LaSala VR, Jules-Elysee KM, et al. Addition of dexamethasone and buprenorphine to bupivacaine sciatic nerve block: a randomized controlled trial. Reg Anesth Pain Med. (2015) 40:321–9. doi: 10.1097/AAP.0000000000000254
60. Wei XM, Liu Z, Lv LC, Wu GH, Sun PY, Gu CP, et al. Comparison of dexmedetomidine and dexamethasone as adjuvants to the ultrasound-guided interscalene nerve block in arthroscopic shoulder surgery: a systematic review and Bayesian network meta-analysis of randomized controlled trials. Front Med. (2023) 10:1159216. doi: 10.3389/fmed.2023.1159216
61. Woo JH, Lee HJ, Oh HW, Lee JW, Baik HJ, Kim YJ. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: a randomized controlled trial. Reg Anesth Pain Med. (2021) 46:965–70. doi: 10.1136/rapm-2021-102795
62. Kim YS, Park Y, Koh HJ. Is there a difference between perineural dexamethasone with single-shot interscalene block (SSIB) and interscalene indwelling catheter analgesia (IICA) for early pain after arthroscopic rotator cuff repair? A pilot study. J Clin Med. (2022) 11:3409. doi: 10.3390/jcm11123409
63. Mao Y, Zuo Y, Mei B, Chen L, Liu X, Zhang Z, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial. J Pain Res. (2018) 11:1811–9. doi: 10.2147/JPR.S164225
64. Lee HJ, Woo JH, Chae JS, Kim YJ, Shin SJ. Intravenous versus perineural dexamethasone for reducing rebound pain after interscalene brachial plexus block: a randomized controlled trial. J Korean Med Sci. (2023) 38:e183. doi: 10.3346/jkms.2023.38.e183
65. Yang ZS, Lai HC, Jhou HJ, Chan WH, Chen PH. Rebound pain prevention after peripheral nerve block: a network meta-analysis comparing intravenous, perineural dexamethasone, and control. J Clin Anesth. (2024) 99:111657. doi: 10.1016/j.jclinane.2024.111657
66. Hwang JT, Jang JS, Lee JJ, Song DK, Lee HN, Kim DY, et al. Dexmedetomidine combined with interscalene brachial plexus block has a synergistic effect on relieving postoperative pain after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. (2020) 28:2343–53. doi: 10.1007/s00167-019-05799-3
67. Huan X, Zhang T, Zhou M, Wang L. Efficacy of perineural dexmedetomidine in ultrasound-guided interscalene block on rebound pain after shoulder arthroscopy. Clin J Pain. (2025) 41:e1267. doi: 10.1097/AJP.0000000000001267
68. Zhu T, Gao Y, Xu X, Fu S, Lin W, Sun J. Effect of ketamine added to ropivacaine in nerve block for postoperative pain management in patients undergoing anterior cruciate ligament reconstruction: a randomized trial. Clin Ther. (2020) 42:882–91. doi: 10.1016/j.clinthera.2020.03.004
69. Zhu S, Wang D, Gao H, Heng L, Shui W, Zhu S. Clinical value of esketamine combined with ropivacaine in rebound pain after brachial plexus block in patients with upper limb fractures. Front Surg. (2024) 11:1470205. doi: 10.3389/fsurg.2024.1470205
70. Touil N, Pavlopoulou A, Barbier O, Libouton X, Lavand'homme P. Evaluation of intraoperative ketamine on the prevention of severe rebound pain upon cessation of peripheral nerve block: a prospective randomised, double-blind, placebo-controlled study. Br J Anaesth. (2022) 128:734–41. doi: 10.1016/j.bja.2021.11.043
71. De Cassai A, Santonastaso DP, Coppolino F, D'Errico C, Melegari G, Dost B, et al. Perineural dexamethasone: neurotoxicity or neuroprotection? A systematic review of preclinical evidence. J Anesth Analg Crit Care. (2025) 5:50. doi: 10.1186/s44158-025-00271-w
72. Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. (2014) 134:85S−93S. doi: 10.1097/PRS.0000000000000671
73. Paul RW, Szukics PF, Brutico J, Tjoumakaris FP, Freedman KB. Postoperative multimodal pain management and opioid consumption in arthroscopy clinical trials: a systematic review. Arthrosc Sports Med Rehabil. (2021) 4:e721–46. doi: 10.1016/j.asmr.2021.09.011
74. Park KB, Cho HO, Kim MS, Jeon YD. Rebound pain after arthroscopic cuff repair with interscalene brachial plexus block anesthesia is reduced by surgeon-administered multimodal shoulder injections: a prospective randomized controlled trial. Arthroscopy. (2025) 41:1291–8. doi: 10.1016/j.arthro.2024.07.029
75. Çalişkan F, Seller A, Gerçek M. The effect of patient education on pain level and fear of pain in orthopedic surgery: a randomized controlled trial. J Perianesth Nurs. (2025) 40:612–8. doi: 10.1016/j.jopan.2024.07.015
76. Shih YW, Tsai HY, Lin FS, Lin YH, Chiang CY, Lu ZL, et al. Effects of positive and negative expectations on human pain perception engage separate but interrelated and dependently regulated cerebral mechanisms. J Neurosci. (2019) 39:1261–74. doi: 10.1523/JNEUROSCI.2154-18.2018
Keywords: arthroscopic rotator cuff repair, nerve block, rebound pain, postoperative pain, postoperative analgesia
Citation: Yang X, Yang Y, Qin S and Chen Y (2025) Research progress of rebound pain after nerve block in arthroscopic rotator cuff repair. Front. Med. 12:1659133. doi: 10.3389/fmed.2025.1659133
Received: 03 July 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Turan Kizkapan, Bahçeşehir University, TürkiyeArjun Ballal, K S Hegde Medical Academy, India
Copyright © 2025 Yang, Yang, Qin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sulan Qin, c3VsYW5xNzdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xue Yang
Xue Yang Yujiao Yang1†
Yujiao Yang1†