Abstract
Mixed pain, defined by the concurrent involvement of nociceptive, neuropathic, and sometimes nociplastic mechanisms, poses a significant diagnostic and therapeutic challenge within modern pain medicine. This complex pain phenotype is increasingly recognized as a prevalent and burdensome clinical entity, yet it remains substantially underdiagnosed and sub-optimally managed across diverse healthcare settings. Epidemiological data indicate that mixed pain affects a substantial proportion of patients with chronic pain syndromes and is consistently associated with more severe symptomatology, prolonged pain duration, functional impairment, diminished quality of life, and escalated healthcare resource utilization compared to pain of a single mechanism. In response to this unmet clinical need, the present recommendations aim to provide a structured, evidence-informed framework for the diagnosis and management of mixed pain. Developed through a rigorous process involving systematic literature review and multidisciplinary expert consensus, this document emphasizes the importance of mechanism-based therapeutic strategies tailored to the individual patient’s pain profile. Central to the approach is the implementation of multimodal and interdisciplinary care models that address the biological, psychological, and functional dimensions of mixed pain. These recommendations are intended for a broad spectrum of healthcare professionals, including primary care physicians, pain specialists, neurologists, oncologists, physiatrists, nurses, pharmacists, physical and occupational therapists, and clinical psychologists. The target population encompasses patients affected by mixed pain conditions such as chronic low back pain with radiculopathy, cancer-related pain, persistent post-surgical pain, and osteoarthritis complicated by central sensitization. By facilitating accurate diagnosis and integrated treatment planning, these recommendations seek to advance clinical practice, reduce the burden of mixed pain, and enhance patient-centered outcomes. This guidance aims to transform mixed pain care by promoting mechanism-based, multidisciplinary strategies with direct clinical applicability.
1 Introduction
Mixed pain represents a complex clinical phenomenon, defined by the concurrent presence of nociceptive and neuropathic pain mechanisms within a single pain condition (1). Nociceptive pain arises from tissue injury or inflammation with activation of nociceptors and microglia (2), while neuropathic pain results from lesions or diseases of the somatosensory nervous system (3). Additionally, nociplastic pain (a mechanism involving altered nociceptive processing without clear evidence of tissue or nerve damage) has been identified as a further contributor in mixed pain states (4, 5). The coexistence of these mechanisms leads to a heterogeneous symptom profile, often blending dull, aching sensations with burning or electric shock-like features.
Mixed pain is prevalent across numerous acute and chronic conditions, such as low back pain with nerve root involvement or spinal stenosis (6), cancer pain associated with tumor invasion and nerve compression (7), post-surgical pain syndromes (e.g., post-mastectomy, post-thoracotomy) (8, 9), and musculoskeletal diseases with central sensitization (e.g., osteoarthritis, rheumatoid arthritis) (10, 11). However, despite its high prevalence, mixed pain remains frequently underdiagnosed, contributing to suboptimal treatment outcomes (12). This diagnostic challenge reflects the overlap of nociceptive and neuropathic features, often obscuring the underlying mechanisms. Screening tools such as the painDETECT questionnaire facilitate recognition of neuropathic components and aid in the identification of mixed pain, with validation for adult (13) and pediatric (14) populations.
Management of mixed pain is inherently challenging due to its mechanistic heterogeneity, variable clinical presentation, and partial responsiveness to unimodal treatments (1, 12–15). NSAIDs may alleviate inflammation-driven nociceptive pain, while having limited efficacy for neuropathic components (16); conversely, agents such as gabapentinoids or antidepressants target neuropathic mechanisms but may not address nociceptive or inflammatory pain (17). Therefore, multimodal and mechanism-based therapeutic strategies, combining pharmacologic interventions (NSAIDs, opioids, gabapentinoids, antidepressants) with non-pharmacologic modalities (physical rehabilitation, psychological support, neuromodulation and interventional procedures), are recommended.
Emerging evidence underscores the role of central sensitization in mixed pain; wherein prolonged nociceptive input induces alterations in central pain processing (18, 19). This supports treatment approaches that address both peripheral and central pain pathways. The IASP definition of pain captures its multidimensional nature, with mixed pain providing a clear clinical example (20). Recent IASP and ICD-11 classifications promote improved categorization of mixed pain, facilitating accurate diagnosis and individualized treatment planning (21). Given the multidimensional nature of pain, the biopsychosocial model provides an essential framework for understanding mixed pain. This model recognizes that biological drivers (e.g., nociceptive, neuropathic, and nociplastic mechanisms) interact with psychological processes (e.g., mood, coping strategies, catastrophizing) and social factors (e.g., work, family, cultural expectations) to shape the pain experience and influence outcomes. Mixed pain is associated with greater intensity, longer duration, impaired quality of life, increased healthcare utilization, and higher disability rates relative to pure nociceptive or neuropathic pain states (22). In this review, “multimodal” refers to the coordinated use of pharmacological, interventional, physical, and psychological therapies to achieve synergistic pain relief while minimizing reliance on any single treatment, whereas “mechanism-based” denotes tailoring interventions to the underlying drivers of mixed pain—nociceptive, neuropathic, or nociplastic—by selecting therapies such as anti-inflammatories, gabapentinoids, or cognitive-behavioral strategies that specifically target these mechanisms. The present recommendations aim to provide an evidence-based, clinically relevant framework for the diagnosis and management of mixed pain, supporting healthcare providers across disciplines in delivering effective, patient-centered care.
1.1 Scope and target audience
The main aim of the research was to provide a structured, evidence-based framework for the diagnosis and management of mixed pain. The guidance is organized around key clinical questions developed using the PICO (Population, Intervention, Comparator, Outcomes) methodology. It addresses: (1) diagnostic strategies, comparing validated screening tools combined with clinical assessment versus clinical assessment alone; (2) pharmacological management, comparing multimodal pharmacotherapy to monotherapy; and (3) interdisciplinary care, contrasting integrated pain management programs with single-discipline approaches. Outcomes of interest include diagnostic accuracy, treatment appropriateness, pain relief, functional improvement, quality of life, and healthcare resource utilization.
The document is intended for implementation across diverse healthcare settings (primary care, pain specialty clinics, hospitals, rehabilitation centers, and multidisciplinary programs) by a broad range of clinicians, including physicians, advanced practice providers, pharmacists, rehabilitation therapists, and mental health professionals. The framework promotes adaptable, patient-centered, and high-quality care.
2 Methods
These clinical practice recommendations were developed using a modified consensus approach, recognizing the limited availability of high-quality randomized controlled trials specifically addressing mixed pain management. The development process integrated best available evidence with expert clinical consensus and real-world practice considerations. The following scientific societies were involved in the development of these clinical recommendations:
-
Fondazione Paolo Procacci (FPP)
-
African Society of Regional Anesthesia (AFSRA)
-
European Society of Regional Anaesthesia & Pain Therapy (ESRA)
-
Federación Latinoamericana de Asociaciones para el Estudio del Dolor (FEDELAT)
2.1 Development group and process
A multidisciplinary expert panel was assembled, including different specialists, including methodological experts. The panel acknowledged that traditional guideline methods based on high-quality comparative trials were unfeasible due to limited mixed pain research. A pragmatic evidence review was adopted. Patient representatives were engaged during the initial project design to provide perspectives from individuals with lived experience of mixed pain. However, consensus could not be formally established, primarily due to the highly technical nature of the methodological processes involved in developing these recommendations. We emphasize that future initiatives should incorporate structured patient involvement, particularly in the prioritization of research questions and in the translation of recommendations into practical clinical pathways.
These clinical practice recommendations address the diagnosis and management of mixed pain through a systematic approach based on clearly defined clinical questions formulated using the PICO (Population, Intervention, Comparator, Outcomes) framework. The first clinical question examines diagnostic assessment approaches in adults with suspected mixed pain conditions, comparing validated screening tools (DN4, PainDETECT, LANSS) plus comprehensive clinical assessment against clinical assessment alone, with outcomes focusing on diagnostic accuracy, appropriate treatment selection, and time to diagnosis. The second question evaluates pharmacological management by comparing multimodal pharmacotherapy combinations against monotherapy approaches in adults with diagnosed mixed pain, measuring outcomes of pain reduction (≥30% improvement), functional improvement, quality of life scores, and adverse events. The third question investigates interdisciplinary care approaches, comparing comprehensive pain management programs with single-discipline care approaches in adults with chronic mixed pain, evaluating outcomes including pain intensity scores, functional status measures, healthcare utilization, and patient satisfaction.
Literature search strategy: A systemic comprehensive search (1990–2024) was performed in MEDLINE/PubMed, Cochrane CENTRAL, Embase, CINAHL, PsycINFO, and Web of Science, using terms such as “mixed pain,” “neuropathic pain,” “nociceptive pain,” “central sensitization,” “multimodal analgesia,” “pain assessment,” “pain management” and “chronic pain.”
2.2 Evidence inclusion
Given the limited clinical trials on mixed pain as a distinct entity, the evidence base was broadened to include RCTs in relevant populations, high-quality observational studies, systematic reviews, and meta-analyses on multimodal strategies. Where direct evidence was lacking, expert consensus, position statements, and real-world clinical data informed the recommendations.
2.3 Evidence quality assessment
Given the previously mentioned scarcity of trials, a modified framework was used to assess evidence quality in place of the traditional GRADE method. This approach prioritized study design, methodological rigor, applicability to mixed pain, consistency of outcomes, clinical relevance, safety, and feasibility. Expert consensus supplemented gaps in empirical data to ensure evidence-informed, practical recommendations. Due to the limited presence of articles specifically addressing mixed pain, GRADE was not feasible, as it is normally applied to bodies of evidence dominated by RCTs. To address gaps where direct evidence was lacking, we integrated a structured expert consensus (modified Delphi).
2.4 Recommendation development process
2.4.1 Consensus method
Recommendations were developed through structured expert consensus using a modified Delphi approach, recognizing that traditional evidence-based recommendations were not feasible given the current state of research. The complete methodology used for consensus development and results of the voting process are reported as Supplementary material.
2.4.2 Recommendation categories
Given the absence of RCTs specific to mixed pain, recommendation strength was determined by integrating expert consensus levels from our Delphi process with available clinical evidence:
Strong recommendations: Assigned when ALL of the following criteria were met:
-
Expert consensus ≥85% in the Delphi process (achieved by 11 of our 16 statements)
-
Consistent evidence from observational studies or established efficacy in component pain mechanisms (nociceptive and/or neuropathic)
-
Favorable benefit-risk profile documented in real-world clinical practice
-
Feasibility of implementation across diverse healthcare settings
Conditional recommendations: Assigned when any of the following applied:
-
Expert consensus 70%–84% in the Delphi process (achieved by 5 of our 16 statements)
-
Limited to extrapolated evidence from single pain mechanisms
-
Moderate consensus despite theoretical benefit (as seen with imaging/electrophysiology, dual-mechanism opioids, and topical agents, all achieving 80% consensus)
-
Variable benefit-risk ratios across patient subgroups or healthcare settings
Evidence levels were assigned as follows: High evidence required systematic reviews demonstrating effectiveness in both nociceptive and neuropathic pain populations, large registry data (>1,000 patients) with mixed pain phenotypes, or established guidelines with >10 years implementation; Moderate evidence required strong evidence in either pain mechanism with biological plausibility for mixed pain, or multiple observational studies (≥3) in heterogeneous pain populations; Low evidence included extrapolated data from related conditions, expert consensus, or limited observational studies; Very low evidence was restricted to case series or theoretical frameworks.
2.4.3 Limitations and transparency
The panel acknowledges key limitations: limited RCTs specifically addressing mixed pain necessitated reliance on indirect evidence and expert opinion; heterogeneity of mixed pain complicates universal recommendations; the evolving nature of mixed pain research may impact future guidance; and practical implementation may vary across healthcare systems, requiring local adaptation.
3 Results
3.1 Pathophysiology and mechanisms
Understanding the pathophysiology of mixed pain is essential for accurate diagnosis and personalized treatment. Mixed pain syndromes are characterized by the concurrent activation of nociceptive (2, 23) and neuropathic (3, 24) pathways, which may operate independently or interact synergistically at peripheral and central levels. This overlap enhances nociceptive transmission and complicates both pharmacological and interventional management (25). Recognizing these mechanisms is critical for optimizing multimodal pain strategies.
3.1.1 Nociceptive component
The nociceptive component arises from inflammation or tissue injury, leading to activation of peripheral nociceptors (23). Pain signals transmitted via Aδ and C fibers typically produce localized, aching, or throbbing sensations (26), reflecting a physiological response to noxious stimuli. This mechanism predominates in conditions such as osteoarthritis and post-surgical pain.
3.1.2 Neuropathic component
Neuropathic pain results from direct injury or disease of the peripheral or central somatosensory system (27). Patients commonly describe burning, electric, shooting, or tingling sensations (24), reflecting aberrant neuronal excitability. Sensory disturbances can manifest as both “negative” symptoms (hypoesthesia, numbness) or “positive” symptoms, such as allodynia and hyperalgesia, which indicate altered pain modulation at spinal and supraspinal levels (28). Identifying these features is essential for accurate diagnosis and guiding multimodal therapy. These parameters were selected for the clinical recommendations because they represent core descriptors consistently reported in neuropathic pain populations, are embedded within the IASP NeuPSIG diagnostic criteria, and serve as reproducible markers in both clinical and research contexts. Their inclusion was therefore guided by their high clinical relevance and their utility in distinguishing neuropathic features from other pain mechanisms.
3.1.3 Nociplastic pain
Nociplastic pain, defined as arising from altered nociceptive processing without evident tissue or nerve injury, increasingly contributes to mixed pain states (29, 30). Frequently observed in fibromyalgia and chronic low back pain, it amplifies pain perception and complicates treatment. Despite some criticism of this concept (31), its recognition enhances the understanding and management of complex pain syndromes.
3.1.4 Central sensitization
Central sensitization, marked by heightened responsiveness of nociceptive neurons in the central nervous system (32), leads to amplified pain signals, increased intensity, pain spreading beyond the initial site, and persistence after tissue healing. Mechanisms include increased synaptic efficacy, reduced inhibition, and glial activation (33, 34). In chronic low back pain, osteoarthritis with sensitization, and post-surgical pain, central sensitization sustains and intensifies symptoms. Clinical signs include hyperalgesia and allodynia, which complicate management, necessitating multimodal approaches (35).
3.1.5 Examples of mixed pain conditions
As already explained, mixed pain involves nociceptive, neuropathic, nociplastic components, and central sensitization. Representative conditions include:
3.1.5.1 Low back pain with radiculopathy
Combines nociceptive musculoskeletal pain with neuropathic elements from nerve root compression (36), manifesting as localized back pain with radiating leg symptoms.
3.1.5.2 Cancer-related pain
Arises from tumor-induced tissue damage (nociceptive) and treatment-related neuropathy (e.g., chemotherapy-induced peripheral neuropathy - CIPN) (37, 38), necessitating multimodal management.
3.1.5.3 Post-surgical pain
Results from tissues and nerve trauma during surgery and subsequent inflammation (39), contributing to chronic post-surgical pain syndromes that require comprehensive treatment (40, 41).
3.1.5.4 Osteoarthritis with central sensitization
While traditionally nociceptive, OA pain may involve central sensitization, heightening pain disproportionate to joint damage (42). Recognizing these mixed mechanisms is critical for effective intervention targeting multiple pain pathways.
Recommendation 1
Mechanism-based classification. We strongly recommend that clinicians classify mixed pain based on the relative contributions—i.e., the estimated proportion of the overall pain experience—attributable to nociceptive, neuropathic, and nociplastic mechanisms, as determined by patient-reported symptoms, clinical examination findings, and validated screening tools, in order to guide targeted therapeutic approaches (Strong recommendation, Low evidence).
Recommendation 2
Central sensitization assessment. We suggest routine evaluation for clinical signs of central sensitization (hyperalgesia, allodynia, temporal summation) in patients with suspected mixed pain to inform treatment planning (Conditional recommendation, Low evidence).
3.2 Diagnostic approach
An accurate diagnostic approach to mixed pain requires systematic use of evidence-based assessment methods to identify the presence and relative contribution of different pain mechanisms. Based on a comprehensive evidence review, we present structured recommendations for diagnostic assessment, combining validated screening tools with clinical evaluation to optimize diagnostic accuracy and guide treatment selection.
3.2.1 Clinical assessment
3.2.1.1 Pain history
A thorough pain history should document onset, duration, intensity, temporal pattern, aggravating and relieving factors. Qualitative descriptors (e.g., stabbing, burning, aching) and associated symptoms such as numbness or weakness are critical for differential diagnosis and management.
3.2.1.2 Physical examination
Physical examination should include inspection, palpation, range of motion testing, and sensory evaluation (light touch, pinprick, thermal perception). Assessment of motor strength and deep tendon reflexes aids in detecting neurological or musculoskeletal impairments.
3.2.2 Screening tools
Screening tools are helpful for identifying neuropathic components within mixed pain. DN4 is a clinician-administered tool with high sensitivity and specificity for neuropathic pain (43). PainDETECT is a validated self-reported questionnaire for chronic back pain and other conditions (44). LANSS integrates patient-reported symptoms with bedside sensory testing, facilitating early diagnosis (45). These tools are widely endorsed for clinical use.
3.2.3 Imaging and electrophysiology
Imaging and electrophysiological studies help elucidate underlying causes. MRI effectively identifies structural abnormalities (disc herniation, spinal stenosis, nerve root compression) (46). EMG and NCS assess peripheral nerve function in radiculopathy, neuropathies, and plexopathies (47). Bone scintigraphy identifies inflammatory or neoplastic lesions; musculoskeletal ultrasound dynamically evaluates soft tissue structures without radiation and is recommended for diagnosing musculoskeletal conditions and assessing peripheral nerves allowing for detection of nerve entrapment, inflammation, and structural abnormalities (48, 49). However, imaging findings often lack correlation with clinical symptoms, particularly for facet joint or sacroiliac (SI) joint pain, which together account for over half of chronic spinal pain cases. Studies consistently show degenerative changes on MRI do not reliably predict facet joint pain (50).
Similarly, imaging for SI joint pain demonstrates limited diagnostic accuracy (CT: sensitivity 57.5%, specificity 69%; bone scans: sensitivity 100%, specificity 12.9%) (51). Degenerative findings on spinal imaging are also common in asymptomatic individuals, complicating interpretation (52).
3.2.4 Red and yellow flags
Red flags indicate serious pathology (infection, malignancy, neurological compromise) requiring urgent management (53). Yellow flags refer to psychosocial risk factors (depression, fear-avoidance, catastrophizing) that predict chronicity and poor outcomes, particularly in adolescents (54, 55).
3.2.5 Diagnosis by exclusion
A diagnosis of exclusion requires systematically ruling out referred pain, functional pain syndromes (e.g., fibromyalgia), and somatization disorders to ensure appropriate management (56, 57).
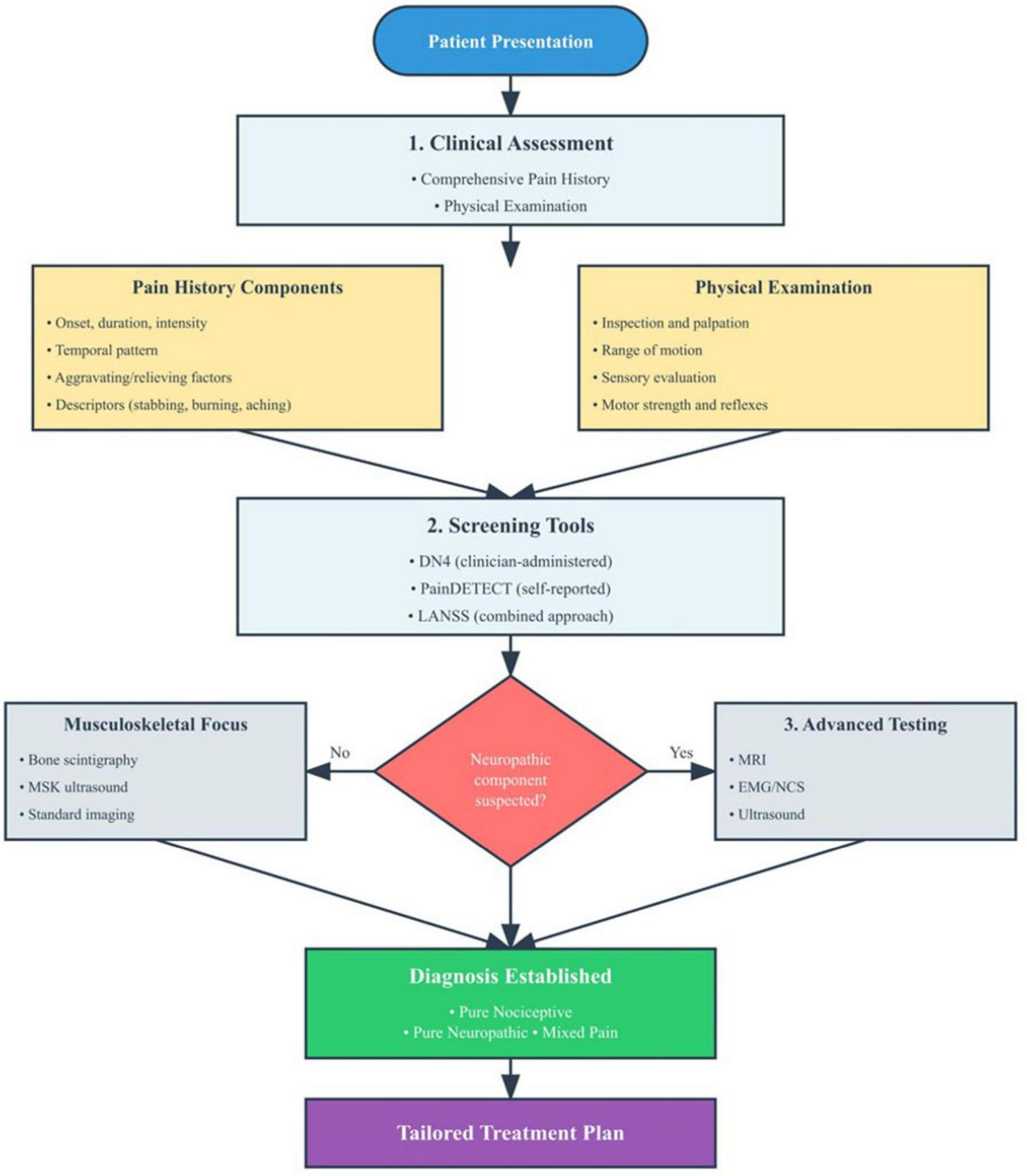
Figure 1 presents a diagnostic algorithm differentiating mixed pain from pure nociceptive or neuropathic pain syndromes, integrating clinical assessment, validated screening tools, and targeted investigations.
FIGURE 1

Diagnostic algorithm for the evaluation of mixed pain. The flowchart presents a structured approach beginning with comprehensive clinical assessment (pain history and physical examination), followed by application of validated screening tools (DN4, PainDETECT, LANSS) to identify neuropathic components. Based on screening results, patients undergo targeted investigations including advanced imaging (MRI), electrophysiological studies (EMG/NCS), or musculoskeletal-focused assessments. The diagnostic pathway culminates in classification as pure nociceptive, pure neuropathic, or mixed pain, enabling mechanism-based treatment selection. Key decision points and diagnostic modalities are highlighted to guide clinical practice.
Recommendation 3:
Use of validated screening tools: We strongly recommend that clinicians use validated screening tools, specifically the DN4 (Douleur Neuropathique en 4 Questions), PainDETECT, or LANSS (Leeds Assessment of Neuropathic Symptoms and Signs), in combination with comprehensive clinical assessment to identify neuropathic components in patients with suspected mixed pain (Strong recommendation, Moderate evidence).
Recommendation 4:
Comprehensive clinical assessment: We strongly recommend that diagnostic evaluation include a comprehensive clinical assessment, encompassing detailed pain history, physical examination with sensory testing, functional status, and systematic appraisal of psychosocial factors (e.g., emotional distress, fear-avoidance, catastrophizing, and social support). This biopsychosocial approach enables more accurate diagnosis and guides mechanism-based, patient-centered management strategies (Strong recommendation, Low evidence).
Recommendation 5:
Imaging and electrophysiology We suggest using advanced imaging (MRI) and electrophysiological studies (EMG/NCS) selectively based on clinical presentation and screening tool results rather than routinely in all mixed pain patients (Conditional recommendation, Low evidence).
Recommendation 6:
Red and yellow flag assessment We strongly recommend systematic screening for red flags (serious pathology) and yellow flags (psychosocial risk factors) during initial evaluation of all mixed pain patients (Strong recommendation, Moderate evidence)
3.3 Pharmacological management
Pharmacological management of mixed pain requires a paradigm shift from single-mechanism approaches toward evidence-based multimodal strategies targeting nociceptive, neuropathic, and nociplastic components. Treatment must be mechanism-based and multimodal (12, 58), as monotherapy rarely suffices. Combination therapies targeting multiple pain pathways often yield superior analgesia with acceptable safety profiles when appropriately implemented (59).
3.3.1 General principles
Management should be individualized, considering clinical phenotype, comorbidities, and drug tolerability. A “start low, go slow” approach minimizes adverse effects, especially in older adults or polypharmacy patients (60). Oral formulations are preferred for ease of use and safety, unless rapid or regional delivery is required (61). Regular reassessment is critical for optimizing efficacy and ensuring safety (62).
3.3.2 Drug classes
3.3.2.1 Non-steroidal anti-inflammatory drugs (NSAIDs)
Non-steroidal anti-inflammatory drugs inhibit cyclooxygenase (COX) enzymes, reducing prostaglandin synthesis and inflammatory nociceptive signaling (63). They effectively target inflammatory pain in musculoskeletal and arthritic conditions. Common agents include ibuprofen (64), ibuprofen arginine (41), naproxen (65), diclofenac (66), ketoprofen (67), dexketoprofen (68), and celecoxib (69). All carry gastrointestinal, renal, and cardiovascular risks, which increase with dose and duration.
3.3.2.2 Acetaminophen (paracetamol)
Acetaminophen is effective for mild-to-moderate nociceptive pain and well tolerated in multimodal regimens through multiple mechanisms, including central COX inhibition, serotonergic modulation, action through cannabinoid channels, effects on TRPV1 channels, and the action of its metabolite AM404 (70).
3.3.2.3 Opioids
Opioids provide effective analgesia for moderate-to-severe mixed pain, acting via μ-opioid receptor agonism on spinal and supraspinal pathways. They also influence neuropathic pathways, proving useful in cancer, postoperative, and refractory chronic mixed pain (61). Common agents include morphine, oxycodone, buprenorphine, tramadol, and tapentadol. Tramadol and tapentadol combine opioid action with monoaminergic reuptake inhibition, enhancing efficacy for neuropathic pain (71, 72). Risks include tolerance, dependence, hyperalgesia, and misuse, necessitating careful monitoring (73). Of note, opioids have immunosuppressive effects, including natural killer (NK) cell inhibition, raising infection risks and potentially promoting tumor progression (74).
3.3.2.4 Anticonvulsants
Gabapentin and pregabalin modulate voltage-gated calcium channels via α2δ subunit binding, reducing excitatory neurotransmission in neuropathic pathways (75). They alleviate symptoms such as burning and electric pain (61), and are used in post-surgical, cancer-related, and musculoskeletal mixed pain. Side effects include sedation, dizziness, and edema, requiring cautious titration (28).
3.3.2.5 Antidepressants
Serotonin-norepinephrine reuptake inhibitors (SNRIs) (e.g., duloxetine) and tricyclic antidepressants (TCAs) (e.g., amitriptyline, nortriptyline) inhibit serotonin and norepinephrine reuptake, enhancing descending inhibitory pathways (61). Duloxetine is effective in chronic low back pain; TCAs are used for neuropathic syndromes (76). TCAs pose anticholinergic and cardiac risks, particularly in elderly patients, requiring ECG monitoring (77).
3.3.2.6 Topical agents
Topical agents target localized neuropathic pain. Lidocaine patches block sodium channels, reducing ectopic discharges (60). Capsaicin creams and patches desensitize TRPV1 receptors, reducing nociceptor activity (78). These options are advantageous in elderly or polypharmacy patients due to minimal systemic effects (79).
3.3.2.7 NMDA receptor antagonists
NMDA antagonists are useful for refractory mixed pain with central sensitization. Low-dose ketamine infusions reduce central hyperexcitability in post-surgical or cancer pain (80). Dextromethorphan has potential for neuropathic pain but is limited by psychomimetic effects and variable efficacy (81). These agents are reserved for specialized care (82).
3.3.2.8 Cannabinoids
Cannabinoids demonstrate therapeutic potential for mixed pain conditions, particularly those involving neuropathic and cancer-related components. Clinical evidence reveals variable analgesic efficacy, with studies showing modest pain relief benefits alongside improvements in sleep quality and functional outcomes (83). However, significant safety considerations must be weighed, including risks of cognitive dysfunction, psychological disturbances, and potential for cannabis use disorder, particularly with chronic THC exposure (84, 85). The endocannabinoid system’s interaction with both nociceptive and neuropathic pain pathways through CB1 and CB2 receptor modulation provides a mechanistic rationale for multimodal pain management, though optimal dosing protocols and long-term safety profiles remain incompletely characterized (86). Cannabis-based therapies should be considered within individualized treatment algorithms, with careful patient selection, regular monitoring for adverse effects and dependency, and implementation only within appropriate legal frameworks where permitted.
3.3.3 Rational polypharmacy
Rational polypharmacy is central to mixed pain management. Combining agents with complementary actions (e.g., opioids with anticonvulsants, NSAIDs with antidepressants) enhances analgesia while minimizing individual drug doses and side effects (87, 88). Topical plus systemic therapy offers targeted pain control with fewer systemic risks, especially valuable in elderly or complex patients (89). This aligns with guidelines advocating personalized, mechanism-based care for complex pain syndromes (61).
3.3.4 Fixed-dose combinations (FDCs)
Fixed-dose combinations (FDCs) offer practical, evidence-based options for mixed pain, particularly in musculoskeletal disorders (68, 90). Combinations such as tramadol/paracetamol deliver synergistic analgesia (μ-opioid modulation with prostaglandin inhibition) at lower doses with favorable safety (91). FDCs improve adherence, reduce pill burden, and minimize dosing errors (92), supporting their inclusion in multimodal pain strategies. Figure 2 illustrates the decision-making process for implementing evidence-based pharmacologic interventions in mixed pain, integrating the principles outlined in Recommendations 7–11.
FIGURE 2
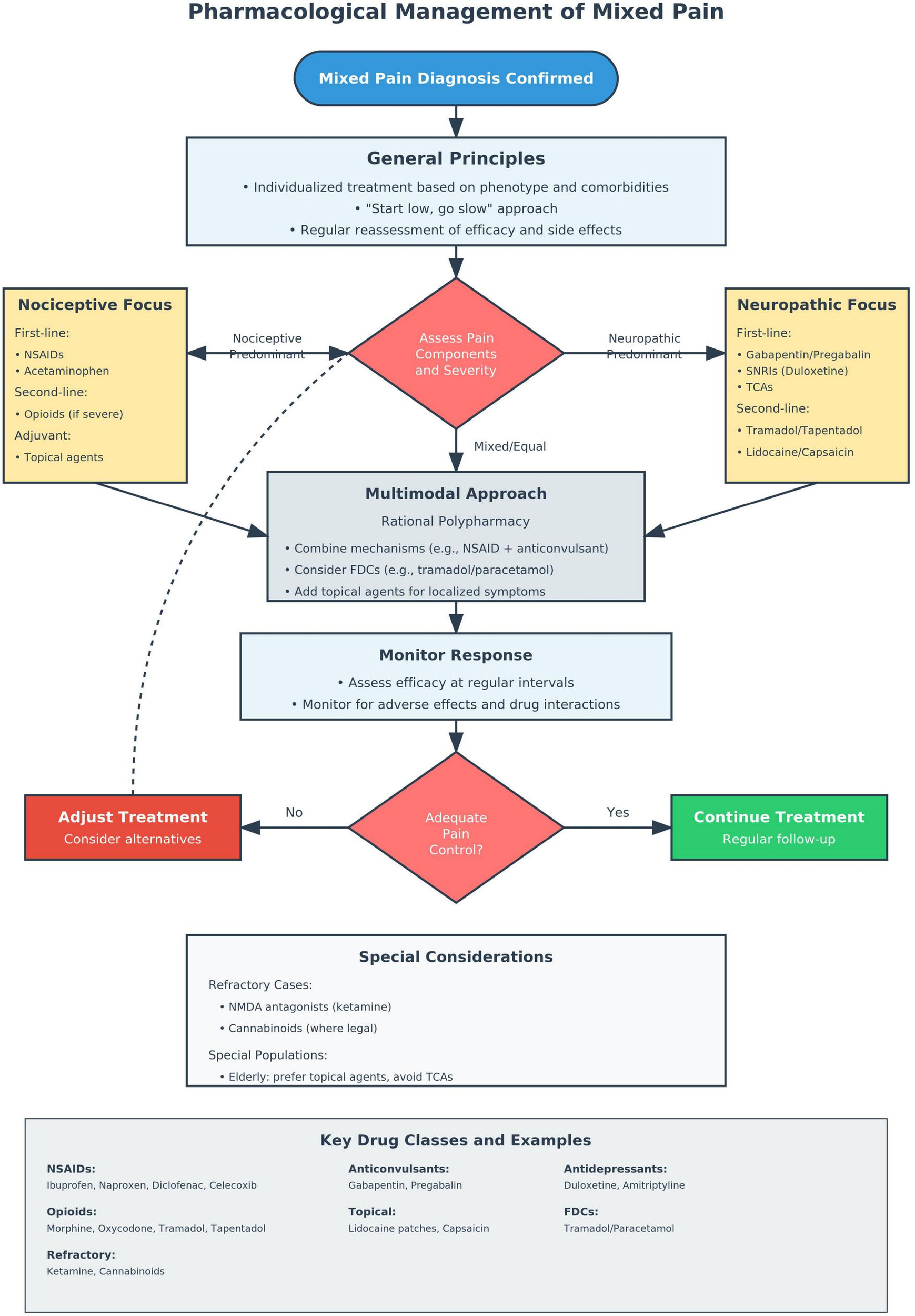
Pharmacological management algorithm for mixed pain.
First-line pharmacological combinations for mixed pain and their contraindications are resumed in Table 1.
TABLE 1
| Pain type | Recommended first-line combination | Dosage notes |
| Nociceptive-dominant mixed pain | • Ibuprofen 400 mg TID + Gabapentin 300 mg TID, titrate to 1800 mg/day • Acetaminophen 1 g TID + Duloxetine 30 mg daily, increase to 60 mg |
Titrate gabapentin gradually to improve tolerability. Monitor for renal function with NSAIDs. |
| Neuropathic-dominant mixed pain | • Pregabalin 75 mg BID, titrate to 300 mg BID + Topical lidocaine 5% • Duloxetine 30–60 mg daily + Tramadol 50 mg QID, max 400 mg/day |
Use caution with tramadol in elderly and serotonergic drugs. Titrate pregabalin slowly. |
First-line pharmacological combinations for mixed pain and their contraindications.
Recommendation 7:
Multimodal pharmacotherapy approach We strongly recommend multimodal pharmacotherapy combining agents with different mechanisms of action rather than monotherapy for mixed pain management (Strong recommendation, Moderate evidence).
Recommendation 8:
First-line Combination Therapy
We suggest combining NSAIDs or acetaminophen with gabapentinoids (gabapentin or pregabalin) or serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine as first-line therapy for most mixed pain conditions (Conditional recommendation, Low evidence).
Recommendation 9:
We suggest considering opioids with dual mechanisms of action, specifically tramadol or tapentadol, over traditional opioids when opioid therapy is indicated for mixed pain management (Conditional recommendation, Low evidence).
Recommendation 10:
Topical agent We suggest prioritizing topical agents (lidocaine patches, capsaicin) for localized mixed pain, particularly in elderly patients or those with multiple comorbidities (Conditional recommendation, Moderate evidence).
3.4 Non-pharmacological interventions
Pharmacological treatments alone are often inadequate for mixed pain management. Evidence supports multimodal approaches integrating non-pharmacological therapies—such as physical rehabilitation, psychological interventions (e.g., cognitive-behavioral therapy), and complementary modalities (e.g., art or music therapy)—to address nociceptive, neuropathic, and psychosocial components (40, 93, 94). These strategies address multiple pain dimensions, enhancing function, emotional well-being, and adherence (95). Multimodal management shows superior efficacy over pharmacotherapy alone in reducing pain and improving quality of life in acute and chronic pain (96, 97), and is now emphasized in guidelines for personalized, mechanism-based care.
3.4.1 Physical therapy
Physical therapy is a key component of multimodal mixed pain management, targeting nociceptive and neuropathic mechanisms to reduce pain, restore function, and prevent deconditioning. Techniques include manual therapy, stretching, strengthening, postural correction, and neuromuscular re-education (98), particularly effective in chronic low back pain, postoperative pain, and fibromyalgia (99). Additionally, Transcutaneous Electrical Nerve Stimulation (TENS) serves as an adjunct by stimulating large-diameter afferent fibers and modulating central pain transmission via gate-control mechanisms (100). TENS is particularly valuable for patients unsuitable for systemic pharmacotherapy due to comorbidities or polypharmacy.
3.4.2 Psychological interventions
Psychological interventions are essential in managing mixed pain, particularly chronic cases where cognitive, emotional, and behavioral factors exacerbate symptoms. In the context of mixed pain, maladaptive coping patterns include behaviors and cognitions such as pain catastrophizing (exaggerated negative orientation toward pain stimuli), fear-avoidance (avoiding movement or activity due to fear of worsening pain), low self-efficacy, and passive coping strategies (e.g., over-reliance on medications without active self-management). These patterns are consistently associated with greater pain intensity, disability, psychological distress, and poorer treatment outcomes. Conversely, adaptive strategies such as active coping, acceptance, and problem-focused behaviors are linked to better adjustment and functional improvement. Recognizing maladaptive coping early and addressing them through interventions such as cognitive-behavioral therapy (CBT), acceptance and commitment therapy (ACT), and mindfulness-based interventions is therefore critical to optimize long-term outcomes. CBT effectively reduces pain intensity, improves function, and mitigates fear-avoidance (101). ACT enhances psychological flexibility and coping (102), while Mindfulness-Based Stress Reduction (MBSR) alleviates distress and improves emotional regulation (103). These approaches are especially indicated for patients with high distress, fear-avoidance, or comorbid depression and anxiety, which amplify both nociceptive and neuropathic components in mixed pain syndromes.
3.4.3 Interventional pain management
Interventional pain management is pivotal in mixed pain treatment, particularly for refractory cases or as adjunctive therapy. Nerve blocks (e.g., epidural steroids, peripheral nerve blocks) provide targeted relief of nociceptive and neuropathic components (104). Radiofrequency ablation of facet joints or dorsal root ganglia offers sustained reduction in segmental pain transmission, notably in spinal syndromes (105). Neuromodulation, such as spinal cord stimulation or non-invasive cortical stimulation (rTMS) benefits complex mixed pain, including persistent spinal pain syndrome and diabetic neuropathy (106). In advanced cancer pain, intraspinal drug delivery enables potent analgesia with fewer systemic effects (107). These techniques are most effective when integrated within a comprehensive, multidisciplinary treatment strategy tailored to the pain mechanisms involved. Nevertheless, the evidence base for interventional therapies in mixed pain syndromes remains limited, preventing their designation as standard care or their incorporation into evidence-based clinical recommendations.
3.4.4 Complementary and integrative medicine
Complementary and integrative medicine (CIM) provides effective adjunctive strategies for managing mixed pain by targeting nociceptive and neuropathic components through non-pharmacological means. Acupuncture modulates endogenous opioids and central sensitization, reducing pain in chronic low back pain and neuropathy (108). Massage therapy alleviates musculoskeletal pain, improves circulation, and reduces muscle tension (109). Chiropractic manipulation, particularly spinal adjustments, addresses mechanical nociceptive pain and enhances biomechanical alignment (110). Biofeedback and relaxation techniques reduce pain-related distress, improve autonomic regulation, and support coping in persistent pain (111). When incorporated into a multidisciplinary treatment plan, these modalities enhance clinical outcomes and may decrease dependence on pharmacologic therapies. However, the current evidence supporting complementary and integrative medicine in mixed pain remains limited. While modalities such as acupuncture, massage therapy, and mindfulness-based interventions show promise in improving pain and quality of life, these findings are often based on small-scale or heterogeneous studies with variable methodological quality. Large, well-designed randomized controlled trials focusing specifically on mixed pain are urgently needed to establish efficacy, clarify mechanisms of action, and determine how these therapies can be optimally integrated within multimodal, interdisciplinary care frameworks.
3.4.5 Occupational therapy
Occupational therapy contributes to comprehensive mixed pain management by promoting function, independence, and engagement in daily activities. Interventions—ergonomics, joint protection, and assistive devices—reduce biomechanical stress and disability (112). Addressing physical and psychosocial aspects, it improves coping and life participation, particularly in chronic or musculoskeletal mixed pain (113).
3.4.6 Patient education
Patient education is essential in mixed pain management, fostering understanding of its multifactorial nature, setting realistic goals (e.g., pain reduction), and promoting self-management through activity pacing and lifestyle modifications. Education enhances adherence and supports patient-centered care (114). Figure 3 illustrates coordinated, multimodal non-pharmacological strategies for addressing mixed pain complexity.
FIGURE 3
Non-pharmacological management algorithm for mixed pain. The flowchart presents a comprehensive decision-making framework for implementing non-pharmacological interventions in patients with confirmed mixed pain diagnosis. Following initial comprehensive assessment of functional capacity, psychological status, and patient-specific factors, the algorithm guides selection from six evidence-based intervention categories: physical therapy (manual therapy, exercise programs, TENS), psychological interventions (CBT, ACT, MBSR), interventional procedures for refractory cases, complementary and integrative medicine approaches, occupational therapy, and patient education.
Recommendation 11:
Multimodal non-pharmacological approach We strongly recommend adopting multimodal approaches that combine pharmacological, interventional, and rehabilitative therapies to optimize outcomes and minimize adverse effects in mixed pain management (Strong recommendation, High evidence).
Recommendation 12:
Early psychological intervention and patient education We suggest early integration of psychological interventions (e.g., CBT, ACT, MBSR) for patients with mixed pain, particularly those with high pain-related distress or maladaptive coping patterns (Conditional recommendation, Moderate evidence). We strongly recommend providing comprehensive patient education about the nature of mixed pain, realistic treatment goals, and self-management strategies as a fundamental component of care (Strong recommendation, Low evidence).
3.5 Interdisciplinary care and rehabilitation
Mixed pain requires a comprehensive approach due to its multifactorial biopsychosocial nature. Effective management involves coordinated input from physicians, psychologists, physiotherapists, and allied health professionals to optimize function and quality of life. Interdisciplinary care improves pain intensity, emotional well-being, and disability in complex pain presentations, underscoring its value in modern pain medicine (115, 116).
3.5.1 Core team members
A comprehensive approach integrates diverse expertise to enhance biopsychosocial outcomes.
3.5.1.1 Physicians (general practitioners, pain specialists, neurologists, oncologists, rheumatologists) conduct clinical and neurophysiological assessments and coordinate mechanism-based therapies (117).
3.5.1.2 Nurses, including pain nurses and case managers, support care coordination, deliver education, and enhance adherence (118).
3.5.1.3 Pharmacists optimize polypharmacy management, reducing drug interactions and improving regimen safety (119).
3.5.1.4 Physiotherapists implement exercise and manual therapies to restore mobility and reduce sensitization (120).
3.5.1.5 Occupational Therapists promote independence through adaptive strategies and task modification (121).
3.5.1.6 Psychologists provide cognitive-behavioral and psychotherapeutic interventions to address distress and maladaptive coping (122).
3.5.1.7 Social Workers address socioeconomic barriers, connect patients to services, and advocate for resources (123).
Evidence shows that interdisciplinary programs involving at least three disciplines, with coordinated or virtual collaboration, yield superior outcomes in pain relief, function, and satisfaction compared to monodisciplinary care (124).
3.5.2 Multidisciplinary vs. interdisciplinary
Interdisciplinary management surpasses multidisciplinary models. While multidisciplinary teams operate in parallel, interdisciplinary care employs integrated plans, shared decision-making, and unified goals. Evidence demonstrates that interdisciplinary multimodal pain treatment:
reduces pain and improves function and quality of life, with sustained benefits (125);
lowers healthcare utilization, decreasing reliance on specialty services and diagnostics (126);
increases return-to-work rates through integrated biopsychosocial programs (127).
These findings support interdisciplinary rehabilitation as superior for mitigating mixed pain burden.
3.5.3 Rehabilitation strategies
Effective rehabilitation combines structured, evidence-based interventions:
-
Goal setting with SMART (specific, measurable, achievable, relevant, time-bound) goals enhances engagement and tracks progress (128).
-
Functional restoration prioritizes meaningful activity participation over complete pain elimination (129).
-
Gradual activity pacing via quota-based models balances activity and rest, reducing boom-bust cycles and improving quality of life (130).
-
Pain self-management programs, integrating education, activity, and psychological support, improve independence, mental health, and quality of life (131).
These strategies reinforce a biopsychosocial model for mixed pain, promoting resilience, patient agency, and functional recovery beyond symptom control.
Recommendation 13:
Interdisciplinary team composition and regular team communication. We strongly recommend that interdisciplinary teams for mixed pain include at minimum: physician, nurse, pharmacist, physiotherapist, and psychologist, with additional specialists as clinically indicated (Strong recommendation, High evidence). We suggest establishing regular interdisciplinary team meetings and communication protocols to ensure coordinated care and treatment plan optimization (Conditional recommendation, Low evidence).
3.6 Monitoring and outcome assessment
Long-term management of mixed pain requires ongoing, systematic evaluation of therapeutic efficacy and patient safety. Regular outcome monitoring ensures treatments remain aligned with SMART patient goals while facilitating timely therapeutic adjustments (128). Structured follow-up (encompassing pain intensity, functional status, side effects, and patient-reported outcomes) is critical to optimizing benefit-risk balance and sustaining functional gains over time (132).
3.6.1 Key domains to assess
In mixed pain, comprehensive assessment extends beyond pain intensity, encompassing multiple biopsychosocial dimensions. Intensity is captured by validated tools such as the Numerical Rating Scale (NRS) and Visual Analog Scale (VAS), with proven responsiveness in chronic low back pain (133). Pain interference, reflecting daily activity limitations, is assessed with the Brief Pain Inventory (BPI), which correlates with disability outcomes (134).
Functional ability is evaluated using disease-specific tools like the Oswestry Disability Index (ODI) and Roland–Morris Disability Questionnaire, validated for chronic pain (133). Psychosocial factors, including catastrophizing, anxiety, and depression, are assessed with instruments such as the Pain Catastrophizing Scale and Hospital Anxiety and Depression Scale (HADS), key for identifying at-risk patients (135). Moreover, the Patient Global Impression of Change (PGIC) can be used as a patient-reported outcome measure that assesses the patient’s overall perception of improvement or deterioration in their condition since the beginning of treatment.
Health-related quality of life is measured using generic instruments like EQ-5D and SF-36. Incorporating patient-reported outcomes (PROs) enhances understanding of subjective treatment responses and complements clinician assessments (133). A multidimensional evaluation strategy (spanning intensity, interference, function, psychosocial status, quality of life, and PROs) aligns with best practices in mixed pain management.
3.6.2 Monitoring tools
Effective monitoring employs various tools to capture the complex nature of mixed pain. Electronic pain diaries enable real-time tracking of fluctuations and responses, enhancing patient awareness and communication. Diary use improves pain intensity, mood, and function (136).
Electronic Health Records (EHRs) support systematic documentation of progress, adjustments, and care coordination, improving evaluation and treatment quality (137, 138). Wearable devices provide objective data on activity and sleep patterns, offering insight into pain-sleep relationships (139). Advanced wearables (e.g., polysomnography, AI-driven tools) enable high-resolution, longitudinal monitoring in real-world settings. Clinicians should inform patients thoroughly when applying wearable technologies (140).
Artificial intelligence may further enhance monitoring, though ethical considerations remain (141, 142). Collectively, these tools promote adaptive, patient-centered adjustments and proactive pain management.
3.6.3 Red flags in monitoring
Vigilant monitoring identifies red flags signaling inadequate or unsafe therapy. One key concern is escalating opioid doses without functional improvement, warranting reassessment if exceeding ≥50 morphine milligram equivalents daily (143). Rising psychological distress (linked to worse outcomes on long-term opioids) also requires attention (144).
Functional decline despite treatment or signs of medication misuse (early refills, dose escalation, abnormal toxicology screens) indicate potential opioid use disorder and necessitate intervention (145).
Monitoring these domains with validated tools, clinical judgment, and prescription tracking ensures safe, effective mixed pain management and improved outcomes.
Recommendation 14
Multidimensional assessment tools We strongly recommend using validated multidimensional assessment tools (Brief Pain Inventory, Oswestry Disability Index, Pain Catastrophizing Scale) for comprehensive outcome monitoring (Strong recommendation, Low evidence). Moreover, we suggest implementing structured follow-up schedules with assessment intervals appropriate to treatment intensity and patient risk profile (Conditional recommendation, Low evidence).
3.7 Special populations
Mixed pain manifests differently across patient populations due to varying biological, psychosocial, and contextual factors, necessitating tailored therapeutic approaches. Psychosocial influences modulate pain perception and treatment responsiveness, underscoring the need for individualized care strategies (146).
3.7.1 Elderly patients
Mixed pain in older adults commonly arises from musculoskeletal conditions (e.g., osteoarthritis) combined with neuropathic elements such as diabetic polyneuropathy. Approximately 40%–65% report musculoskeletal pain, and up to one-third experience neuropathic symptoms (147). Management must account for increased risks of adverse drug events and pharmacokinetic changes due to aging and polypharmacy. Guidelines recommend simplified regimens, lower dosages, and topical agents to minimize systemic risks (148). Cognitive impairment and frailty, which affect symptom reporting and treatment tolerability, must also be assessed routinely (149). Age-related pharmacokinetic and pharmacodynamic changes increase risks of adverse effects and drug–drug interactions. A “start low, go slow” principle is critical. In patients >75 years, topical formulations (lidocaine, capsaicin, NSAID patches) should be prioritized to minimize systemic exposure. Polypharmacy must be carefully reviewed to avoid drug interactions and adverse outcomes. Non-pharmacological therapies such as physiotherapy, occupational therapy, and balance training are essential to maintain function, prevent falls, and improve quality of life. Invasive procedures may be considered selectively but should take into account comorbidities, frailty, and anticoagulation status. A tailored, patient-centered model improves safety and outcomes in this vulnerable group.
3.7.2 Pediatric patients
Mixed pain in children (arising from sickle cell crises, postoperative recovery, or oncology treatment) is frequently underrecognized. A 2023 systematic review in sickle cell disease showed that psychological interventions (e.g., cognitive-behavioral therapy, biofeedback) effectively reduce pain frequency and intensity, addressing neuropathic and nociceptive components (150). Inpatient therapies such as virtual reality and yoga, and outpatient approaches like massage, offer further benefit (151). Integrating these strategies into family-centered care models aligns interventions with patient and family needs, improving adherence and outcomes. Evidence on mixed pain in children is limited, and treatment should emphasize conservative and multimodal approaches. Pharmacological therapy must be guided by strict age- and weight-based dosing: paracetamol and NSAIDs may be used as first-line, while adjuvants (gabapentinoids, antidepressants) should only be prescribed under specialist supervision due to limited pediatric safety data. Non-pharmacological approaches—including cognitive-behavioral therapy, school reintegration support, and family-centered interventions—are particularly important, as children are highly vulnerable to psychosocial drivers of pain chronification. Interventional techniques should be reserved for highly selected cases and delivered in specialized centers.
3.7.3 Cancer patients
Cancer-related mixed pain combines tumor-induced nociceptive mechanisms (e.g., bone compression) with treatment-related neuropathic processes (e.g., chemotherapy-induced neuropathy). Approximately 20% of cancer patients exhibit this mixed phenotype (152). The traditional WHO analgesic ladder often proves insufficient, with 39% experiencing inadequate pain relief; mechanism-based adaptations significantly improve outcomes (153). Current guidelines recommend integrating adjuvant agents (e.g., anticonvulsants, antidepressants) and early interventional techniques to better address complex cancer-related mixed pain (154). This mechanism-based, multimodal analgesic approach better aligns with the evolving phenotypic complexity of cancer-related mixed pain and optimizes patient outcomes within a personalized palliative care framework.
3.7.4 Patients with psychiatric comorbidities
Psychiatric comorbidities (depression, anxiety, PTSD) amplify mixed pain through biopsychosocial pathways (155, 156). SNRIs and other psychotropic agents offer dual benefits for mood and pain symptoms (157). Integrated care involving pain specialists and mental health professionals optimizes pharmacological regimens, minimizes interaction risks, and addresses emotional drivers of pain, enhancing outcomes.
3.7.5 Socioeconomically disadvantaged populations
Disadvantaged individuals face systemic barriers, including poor access to specialized care, limited health literacy, and higher rates of untreated chronic pain. Lower health literacy is linked to poor adherence and suboptimal outcomes (158), with disproportionate burdens of unmanaged pain reflecting social inequities (159). Culturally tailored education, mobile health solutions, and equity-focused programs are needed to promote engagement and improve long-term outcomes in these populations.
Recommendation 15
Special population. We strongly recommend adapting mixed pain treatment strategies to patient age, including simplified regimens and topical therapies for elderly patients, and family-centered, non-pharmacological approaches for pediatric patients (Strong recommendation, Very low evidence). We strongly recommend mechanism-based multimodal approaches for cancer patients with mixed pain (Strong recommendation, Low evidence). We strongly recommend close collaboration between pain specialists and mental health professionals in patients with mixed pain and psychiatric comorbidities (Strong recommendation, Moderate evidence). Future research should address the major knowledge gaps on mixed pain in minority and vulnerable populations, who remain underrepresented in clinical trials and face barriers to access and care. Tackling these disparities is crucial to ensure inclusive, equitable, and generalizable recommendations.
4 Ethical and practical considerations
Ethical principles are fundamental to the management of mixed pain. Its multifactorial nature requires not only clinical expertise but also a robust ethical framework to ensure patient-centered care. Central is the principle of autonomy, which mandates respect for patients’ rights to informed decision-making. In mixed pain, where treatment often involves trade-offs between efficacy, side effects, and personal values, shared decision-making is essential. Active patient engagement strengthens therapeutic alliances, enhances adherence, and respects individual goals (160).
The principle of beneficence obliges clinicians to optimize outcomes through evidence-based multimodal interventions, integrating pharmacological and non-pharmacological strategies. However, this must be balanced by non-maleficence, given the risks associated with opioids, anticonvulsants, and antidepressants, which may cause sedation, dependence, or systemic harm. Ethical care requires careful benefit-risk assessment and vigilant monitoring to prevent iatrogenic complications (161).
Justice demands equitable access to effective pain care. Yet disparities persist, with underserved populations facing barriers to multidisciplinary services. Addressing such inequities is an ethical imperative, requiring fair allocation of resources (including specialist care, rehabilitation, and psychosocial support) to ensure that all patients can benefit from integrated pain management (162).
Informed consent is central to ethical care, particularly in mixed pain where long-term pharmacotherapy, invasive interventions, or off-label treatments are common. Patients must be fully informed about their condition, treatment options, risks, and uncertainties. Compassionate communication and documented shared decision-making are essential, particularly when managing therapies with significant risks such as opioids or neuromodulation (163).
Resource limitations often constrain optimal multimodal care. In settings where comprehensive approaches or advanced modalities are unavailable, ethical management requires judicious resource allocation, telemedicine integration, and advocacy for system reform to enhance equity. Clinicians must deliver care within existing constraints while advocating for broader structural improvements (164).
Professional competence is equally vital. The complexity of mixed pain may exceed the expertise of individual providers. Recognizing limits and ensuring timely referral to specialized centers or m teams is ethically warranted. Collaborative care models are essential to deliver comprehensive, evidence-based treatment across medical, psychological, and rehabilitative domains (165).
Finally, increasing reliance on digital tools (EHRs, remote monitoring, telehealth) raises concerns regarding privacy and data security. In fact, ethical practice demands adherence to privacy regulations, transparency about data use, and robust cybersecurity. Informed consent for digital data use is essential to maintain trust and uphold ethical standards in technologically supported care (166). When integrating telemedicine, digital tools, and wearable monitoring into mixed pain management, adherence to established data privacy and security regulations is essential. In the European Union, the General Data Protection Regulation (GDPR) provides the legal framework for processing personal health data, mandating explicit patient consent, data minimization, and secure storage and transfer of sensitive information. In the United States, the Health Insurance Portability and Accountability Act (HIPAA) sets the standards for protecting patient health information, with specific requirements for confidentiality, integrity, and data access control. Other jurisdictions may apply additional regulations, such as the Personal Information Protection and Electronic Documents Act (PIPEDA) in Canada or comparable national laws. For clinical implementation, it is therefore crucial that institutions deploying digital health solutions ensure compliance with the relevant regional legal framework, establish clear protocols for data handling and storage, and provide transparent information to patients about how their data are collected, processed, and protected. This safeguards patient rights while fostering trust in the use of digital technologies for pain care.
In sum, ethical management of mixed pain requires more than clinical skill: it demands a steadfast commitment to autonomy, beneficence, non-maleficence, and justice, underpinned by transparency, resource stewardship, professional integrity, and data governance. These principles form the foundation of ethical, patient-centered pain care.
Recommendation 16
Ethical and Equitable Mixed Pain Care
We strongly recommend that mixed pain management be grounded in ethical principles, including comprehensive informed consent and shared decision-making that respect patient autonomy and ensure patients are fully informed of the complex nature of their condition, available treatment options, associated risks, and realistic outcomes. In parallel, healthcare systems should implement equitable policies to guarantee access to interdisciplinary care across geographic and socioeconomic barriers (Strong recommendation, Low evidence). Furthermore, we suggest establishing and maintaining professional competency standards through minimum qualifications and ongoing education for all providers involved in mixed pain management (Conditional recommendation, Low evidence).
5 Conclusion and future directions
Mixed pain presents a complex, multidimensional challenge in clinical practice, requiring a shift from traditional single-modality treatments to comprehensive, individualized strategies. These clinical recommendations provide a systematic framework for diagnosing mixed pain, integrating validated assessment tools with clinical reasoning to accurately characterize pain profiles. Pharmacological management should be mechanism-based, often necessitating multimodal combinations for effective relief. Concurrently, non-pharmacological interventions (including physical rehabilitation, psychological therapies, and integrative modalities) are essential for functional restoration and enhancing resilience. Integrated care models offer the most effective approach for sustained relief and improved quality of life, fostering coordinated efforts across clinical domains. Ethical responsibility, continuous outcome evaluation, and equitable access to care remain essential pillars of best practice. Table 2 summarizes key clinical recommendations for the diagnosis and management of mixed pain, organized by thematic chapters. Each recommendation aligns with a specific clinical domain and embodies a mechanism-based, patient-centered strategy.
TABLE 2
| Recommendation | Topic | Key elements |
| 1 | Mechanism-based classification | Classify mixed pain based on relative contributions of nociceptive, neuropathic, and nociplastic components to guide targeted therapeutic approaches |
| 2 | Central sensitization assessment | Routine evaluation for clinical signs of central sensitization (hyperalgesia, allodynia, temporal summation) in patients with suspected mixed pain |
| 3 | Validated screening tools | Use validated screening tools (DN4, PainDETECT, LANSS) in combination with comprehensive clinical assessment to identify neuropathic components |
| 4 | Comprehensive clinical assessment | Include detailed pain history, physical examination with sensory testing, and systematic assessment of functional impact and psychosocial factors |
| 5 | Imaging and electrophysiology | Use advanced imaging (MRI) and electrophysiological studies (EMG/NCS) selectively based on clinical presentation rather than routinely |
| 6 | Red and yellow flag assessment | Systematic screening for red flags (serious pathology) and yellow flags (psychosocial risk factors) during initial evaluation |
| 7 | Multimodal pharmacotherapy | Use multimodal pharmacotherapy combining agents with different mechanisms of action rather than monotherapy |
| 8 | First-line combination therapy | Combine NSAIDs or acetaminophen with gabapentinoids (gabapentin/pregabalin) or SNRIs (duloxetine) as first-line therapy |
| 9 | Dual-mechanism opioids | Consider opioids with dual mechanisms (tramadol or tapentadol) over traditional opioids when opioid therapy is indicated |
| 10 | Topical agents | Prioritize topical agents (lidocaine patches, capsaicin) for localized mixed pain, particularly in elderly patients or those with multiple comorbidities |
| 11 | Multimodal non-pharmacological approach | Integrate multiple non-pharmacological interventions (physical therapy, psychological support, patient education) alongside pharmacological treatment |
| 12 | Early psychological intervention and education | Early integration of psychological interventions (CBT, ACT, MBSR) for patients with high pain-related distress; provide comprehensive patient education |
| 13 | Team composition and communication | Interdisciplinary teams should include minimum: physician, nurse, pharmacist, physiotherapist, psychologist; establish regular team meetings |
| 14 | Multidimensional assessment | Use validated multidimensional assessment tools (Brief Pain Inventory, Oswestry Disability Index, Pain Catastrophizing Scale); implement structured follow-up schedules |
| 15 | Age-specific and population-based adaptations | Modify treatment approaches based on patient age (simplified regimens for elderly, family-centered approaches for pediatric); mechanism-based multimodal approaches for cancer patients; close collaboration between pain specialists and mental health professionals for patients with psychiatric comorbidities |
| 16 | Ethical and equitable care | Ground management in ethical principles including comprehensive informed consent, shared decision-making, and equitable access policies; establish professional competency standards through minimum qualifications and ongoing education |
Summary of clinical recommendations for mixed pain management.
Future research should focus on developing objective diagnostic tools (e.g., biomarkers, advanced imaging) and advancing system-level innovations through digital health and personalized medicine. Ultimately, managing mixed pain demands a holistic, person-centered approach that mobilizes all resources to alleviate suffering and restore function.
Statements
Author contributions
GV: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. GF: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MN: Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. AG: Supervision, Writing – original draft, Writing – review & editing. CP: Supervision, Writing – original draft, Writing – review & editing. ES: Investigation, Supervision, Writing – original draft, Writing – review & editing. CG: Investigation, Supervision, Writing – original draft, Writing – review & editing. ADK: Investigation, Supervision, Writing – original draft, Writing – review & editing. LG-L: Investigation, Supervision, Writing – original draft, Writing – review & editing. ML: Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EM: Writing – review & editing. AK: Writing – review & editing. TV: Writing – review & editing. AA-A: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our sincere gratitude to the members of the Fondazione Paolo Procacci (FPP), the African Society for Regional Anesthesia (AFSRA), European Society of Regional Anaesthesia & Pain Therapy (ESRA) and Federación Latinoamericana de Asociaciones para el Estudio del Dolor (FEDELAT) for their valuable contributions, thoughtful discussions, and exchange of ideas. Their collaborative input and international perspective were instrumental in the development and refinement of these clinical practice recommendations. We are grateful to Fondazione Paolo Procacci for the support during the realization of the study and publication phases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1659490/full#supplementary-material
References
1.
Freynhagen R Parada H Calderon-Ospina C Chen J Rakhmawati Emril D Fernández-Villacorta F et al Current understanding of the mixed pain concept: a brief narrative review. Curr Med Res Opin. (2019) 35:1011–8. 10.1080/03007995.2018.1552042
2.
Shi S Gong X . The role of microglia in perioperative pain and pain treatment: recent advances in research.J Integr Neurosci. (2025) 24:22675. 10.31083/JIN22675
3.
Treede R Jensen T Campbell J Cruccu G Dostrovsky J Griffin J et al Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. (2008) 70:1630–5. 10.1212/01.wnl.0000282763.29778.59
4.
Danilov A Danilov A Badaeva A Kosareva A Popovskaya K Novikov V . State-of-the-art personalized therapy approaches for chronic non-specific low back pain: understanding the mechanisms and drivers.Pain Ther. (2025) 14:479–96. 10.1007/s40122-025-00706-w
5.
Fernández-de-Las-Peñas C Nijs J Cagnie B Gerwin R Plaza-Manzano G Valera-Calero J et al Myofascial pain syndrome: a nociceptive condition comorbid with neuropathic or nociplastic pain. Life. (2023) 13:694. 10.3390/life13030694
6.
Nyqvist L Åkerstedt J Thoreson O . Current trends in the medical treatment of neuropathic low back pain: a Swedish registry-based study of 1.7 million people.BMC Musculoskelet Disord. (2024) 25:486. 10.1186/s12891-024-07599-4
7.
Mulvey M Paley C Schuberth A King N Page A Neoh K . Neuropathic pain in cancer: What are the current guidelines?Curr Treat Options Oncol. (2024) 25:1193–202. 10.1007/s11864-024-01248-7
8.
Larsson I Ahm Sørensen J Bille C . The post-mastectomy pain syndrome-a systematic review of the treatment modalities.Breast J. (2017) 23:338–43. 10.1111/tbj.12739
9.
Makkad B Heinke T Sheriffdeen R Meng M Kachulis B Grant M et al Practice advisory for postoperative pain management of thoracic surgical patients: a report from the society of cardiovascular anesthesiologists. J Cardiothorac Vasc Anesth. (2025) 39:1306–24. 10.1053/j.jvca.2024.12.004
10.
Goldoni E Bittencourt J do Espirito Santo L Sousa E Faria J Alexandre D et al Neuropathic-like symptoms and central sensitization related signs and symptoms negatively affect the functional performance of patients with knee osteoarthritis - a cross-sectional study. Osteoarthr Cartil Open. (2023) 5:100358. 10.1016/j.ocarto.2023.100358
11.
Salaffi F Carotti M Farah S Ciccullo C Gigante A Bandinelli F et al A mediation appraisal of neuropathic-like symptoms, pain catastrophizing, and central sensitization-related signs in adults with knee osteoarthritis-a cross-sectional study. J Pers Med. (2025) 15:22. 10.3390/jpm15010022
12.
Leoni M Mercieri M Gazzeri R Cascella M Rekatsina M Viswanath O et al Trends in mixed pain research over three decades (1993-2024): a bibliometric analysis. Curr Pain Headache Rep. (2025) 29:65. 10.1007/s11916-025-01371-6
13.
Lapkin S Sima S Gan Z Diwan A . A confirmatory factor analysis of an electronic format painDETECT questionnaire for patients with low back pain.Curr Med Res Opin. (2024) 40:259–65. 10.1080/03007995.2023.2293570
14.
Hess C Van Orden A Mesaroli G Stinson J Borsook D Simons L . Application of PainDETECT in pediatric chronic pain: How well does it identify neuropathic pain and its characteristics?Pain Rep. (2023) 8:e1109. 10.1097/PR9.0000000000001109
15.
Freynhagen R Rey R Argoff C . When to consider “mixed pain”? The right questions can make a difference!Curr Med Res Opin. (2020) 36:2037–46. 10.1080/03007995.2020.1832058
16.
Magni A Agostoni P Bonezzi C Massazza G Menè P Savarino V et al Management of Osteoarthritis: expert Opinion on NSAIDs. Pain Ther. (2021) 10:783–808. 10.1007/s40122-021-00260-1
17.
Jayathilake N Phan T Kim J Lee K Park J . Modulating neuroplasticity for chronic pain relief: noninvasive neuromodulation as a promising approach.Exp Mol Med. (2025) 57:501–14. 10.1038/s12276-025-01409-0
18.
Nijs J George S Clauw D Fernández-de-Las-Peñas C Kosek E Ickmans K et al Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. (2021) 3:e383–92. 10.1016/S2665-9913(21)00032-1
19.
Tedeschi R Giorgi F Platano D Berti L . Classifying low back pain through pain mechanisms: a scoping review for physiotherapy practice.J Clin Med. (2025) 14:412. 10.3390/jcm14020412
20.
Raja S Carr D Cohen M Finnerup N Flor H Gibson S et al The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. 10.1097/j.pain.0000000000001939
21.
Treede R Rief W Barke A Aziz Q Bennett M Benoliel R et al Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. (2019) 160:19–27. 10.1097/j.pain.0000000000001384
22.
Fernandez-Fairen M Calderón-Ospina C Chen J Duarte Vega M Fernández-Villacorta F Gómez-García F et al A Latin American consensus meeting on the essentials of mixed pain. Curr Med Res Opin. (2023) 39:451–66. 10.1080/03007995.2023.2177401
23.
De Feo M Paladini A Ferri C Carducci A Del Pinto R Varrassi G et al Anti-inflammatory and anti-nociceptive effects of cocoa: a review on future perspectives in treatment of pain. Pain Ther. (2020) 9:231–40. 10.1007/s40122-020-00165-5
24.
Baron R . Mechanisms of disease: neuropathic pain–a clinical perspective.Nat Clin Pract Neurol. (2006) 2:95–106. 10.1038/ncpneuro0113
25.
Mangnus T Dirckx M Huygen F . Different types of pain in complex regional pain syndrome require a personalized treatment strategy.J Pain Res. (2023) 16:4379–91. 10.2147/JPR.S432209
26.
Olausson H Marshall A Nagi S Cole J . Slow touch and ultrafast pain fibres: revisiting peripheral nerve classification.Clin Neurophysiol. (2024) 163:255–62. 10.1016/j.clinph.2024.04.008
27.
Jensen T Baron R . Translation of symptoms and signs into mechanisms in neuropathic pain.Pain. (2003) 102:1–8. 10.1016/s0304-3959(03)00006-x
28.
Colloca L Ludman T Bouhassira D Baron R Dickenson A Yarnitsky D et al Neuropathic pain. Nat Rev Dis Primers. (2017) 3:17002. 10.1038/nrdp.2017.2
29.
Kosek E Cohen M Baron R Gebhart G Mico J Rice A et al Do we need a third mechanistic descriptor for chronic pain states? Pain. (2016) 157:1382–6. 10.1097/j.pain.0000000000000507
30.
Fitzcharles M Cohen S Clauw D Littlejohn G Usui C Häuser W . Nociplastic pain: towards an understanding of prevalent pain conditions.Lancet. (2021) 397:2098–110. 10.1016/S0140-6736(21)00392-5
31.
Granan L . We do not need a third mechanistic descriptor for chronic pain states! Not yet.Pain. (2017) 158:179. 10.1097/j.pain.0000000000000735
32.
Volcheck M Graham S Fleming K Mohabbat A Luedtke C . Central sensitization, chronic pain, and other symptoms: better understanding, better management.Cleve Clin J Med. (2023) 90:245–54. 10.3949/ccjm.90a.22019
33.
Woolf C . Central sensitization: implications for the diagnosis and treatment of pain.Pain. (2011) 152(3 Suppl):S2–15. 10.1016/j.pain.2010.09.030
34.
Tassou A Richebe P Rivat C . Mechanisms of chronic postsurgical pain.Reg Anesth Pain Med. (2025) 50:77–85. 10.1136/rapm-2024-105964
35.
Öz N Özer A Duruöz M . Central sensitization and its role in persistent pain among spondyloarthritis patients on biological treatments.Medicina. (2025) 61:319. 10.3390/medicina61020319
36.
Giglio M Farì G Preziosa A Corriero A Grasso S Varrassi G et al Low back pain and radiofrequency denervation of facet joint: beyond pain control-a video recording. Pain Ther. (2023) 12:879–84. 10.1007/s40122-023-00489-y
37.
Mitello L Marti F Mauro L Siano L Pucci A Tarantino C et al The usefulness of virtual reality in symptom management during chemotherapy in lung cancer patients: a quasi-experimental study. J Clin Med. (2024) 13:4374. 10.3390/jcm13154374
38.
Kouri M Rekatsina M Vadalouca A Viswanath O Varrassi G . Oral neuropathy associated with commonly used chemotherapeutic agents: a narrative review.Curr Pain Headache Rep. (2024) 28:1209–17. 10.1007/s11916-024-01305-8
39.
Charipova K Gress K Berger A Kassem H Schwartz R Herman J et al A comprehensive review and update of post-surgical cutaneous nerve entrapment. Curr Pain Headache Rep. (2021) 25:11. 10.1007/s11916-020-00924-1
40.
Malik A Elshazly T Pokuri K Apai C Rothkrug A Hasoon J et al Virtual reality for postoperative pain management: a review of current evidence. Curr Pain Headache Rep. (2024) 28:1307–19. 10.1007/s11916-024-01308-5
41.
Sarzi-Puttini P Perrot S Perez-Cajaraville J Fornasari D Radaelli F Varrassi G . Clinical benefits of ibuprofen arginine: a narrative review.Pain Ther. (2025) 14:891–912. 10.1007/s40122-025-00735-5
42.
Coaccioli S Sarzi-Puttini P Zis P Rinonapoli G Varrassi G . Osteoarthritis: new insight on its pathophysiology.J Clin Med. (2022) 11:6013. 10.3390/jcm11206013
43.
Bouhassira D Attal N Alchaar H Boureau F Brochet B Bruxelle J et al Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. (2005) 114:29–36. 10.1016/j.pain.2004.12.010
44.
Freynhagen R Baron R Gockel U Tölle T . painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain.Curr Med Res Opin. (2006) 22:1911–20. 10.1185/030079906X132488
45.
Bennett M . The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs.Pain. (2001) 92:147–57. 10.1016/s0304-3959(00)00482-6
46.
Modic M Ross J . Lumbar degenerative disk disease.Radiology. (2007) 245:43–61. 10.1148/radiol.2451051706
47.
Preston D Shapiro B. Electromyography and Neuromuscular Disorders E-Book: Clinical-Electrophysiologic-Ultrasound Correlations. Amsterdam: Elsevier Health Sciences (2020).
48.
Helyar V Mohan H Barwick T Livieratos L Gnanasegaran G Clarke S et al The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imaging. (2010) 37:706–13. 10.1007/s00259-009-1334-3
49.
Lorenzana D Platzgummer H Peyer A Krol A Eichenberger U . Diagnostic use of ultrasound for patients with neuropathic pain.J Clin Anesth. (2024) 96:111314. 10.1016/j.jclinane.2023.111314
50.
Occhigrossi F Carpenedo R Leoni M Varrassi G Chinè E Cascella M et al Delphi-based expert consensus statements for the management of percutaneous radiofrequency neurotomy in the treatment of lumbar facet joint syndrome. Pain Ther. (2023) 12:863–77. 10.1007/s40122-023-00512-2
51.
Tsoi C Zheng J Xu C Ng A Lo C Li G et al Sacroiliac joint imaging: a comprehensive review. Quant Imaging Med Surg. (2019) 9:318–35. 10.21037/qims.2019.02.09
52.
Brinjikji W Luetmer P Comstock B Bresnahan B Chen L Deyo R et al Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. (2015) 36:811–6. 10.3174/ajnr.A4173
53.
Henschke N Maher C Ostelo R de Vet H Macaskill P Irwig L . Red flags to screen for malignancy in patients with low-back pain.Cochrane Database Syst Rev. (2013) 2013:CD008686. 10.1002/14651858.CD008686.pub2
54.
Basiński K Zdun-Ryżewska A Majkowicz M . Psychosocial predictors of persistent low back pain in patients presenting to the emergency department.Am J Emerg Med. (2022) 51:85–91. 10.1016/j.ajem.2021.10.018
55.
Murray C Li R Kashikar-Zuck S Zhou C Palermo T . Adolescent predictors of young adult pain and health outcomes: results from a 6-year prospective follow-up study.Pain. (2025) 166:42–51. 10.1097/j.pain.0000000000003308
56.
Clauw D . Fibromyalgia: a clinical review.JAMA. (2014) 311:1547–55. 10.1001/jama.2014.3266
57.
Häuser W Ablin J Fitzcharles M Littlejohn G Luciano J Usui C et al Fibromyalgia. Nat Rev Dis Primers. (2015) 1:15022. 10.1038/nrdp.2015.22
58.
Guillén-Nuñez M Juárez-Lemus M Hernandez-Porras C Hernández-Rodríguez D . Current perspective in mixed pain.J Drug Deliv Therapeut. (2024) 14:170–3. 10.22270/jddt.v14i3.6451
59.
Wang J Doan L . Clinical pain management: Current practice and recent innovations in research.Cell Rep Med. (2024) 5:101786. 10.1016/j.xcrm.2024.101786
60.
Baron R Binder A Wasner G . Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment.Lancet Neurol. (2010) 9:807–19. 10.1016/S1474-4422(10)70143-5
61.
Finnerup N Attal N Haroutounian S McNicol E Baron R Dworkin R et al Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14:162–73. 10.1016/S1474-4422(14)70251-0
62.
Scholz J Finnerup N Attal N Aziz Q Baron R Bennett M et al The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. (2019) 160:53–9. 10.1097/j.pain.0000000000001365
63.
Varrassi G Yeam C Rekatsina M Pergolizzi J Zis P Paladini A . The expanding role of the COX inhibitor/opioid receptor agonist combination in the management of pain.Drugs. (2020) 80:1443–53. 10.1007/s40265-020-01369-x
64.
Varrassi G Pergolizzi J Dowling P Paladini A . Ibuprofen safety at the golden anniversary: are all NSAIDs the Same? A narrative review.Adv Ther. (2020) 37:61–82. 10.1007/s12325-019-01144-9
65.
Sklenář A Zehnacker-Rentien A Kaminský J Rohlíček J Bouř P . Exploring naproxen cocrystals through solid-state vibrational circular dichroism.Chirality. (2025) 37:e70027. 10.1002/chir.70027
66.
Chen Z Chen B Li P Feng K . Efficacy and safety of different topical diclofenac formulations for the treatment of knee osteoarthritis: a meta-analysis of short-term and long-term treatment comparisons.BMC Musculoskelet Disord. (2025) 26:230. 10.1186/s12891-025-08465-7
67.
Varrassi G Alon E Bagnasco M Lanata L Mayoral-Rojals V Paladini A et al Towards an effective and safe treatment of inflammatory pain: a delphi-guided expert consensus. Adv Ther. (2019) 36:2618–37. 10.1007/s12325-019-01053-x
68.
Varrassi G Hanna M Coaccioli S Fabrizzi P Baldini S Kruljac I et al Dexketoprofen trometamol and tramadol hydrochloride fixed-dose combination in moderate to severe acute low back pain: a phase IV, randomized, parallel group, placebo, active-controlled study (DANTE). Pain Ther. (2024) 13:1007–22. 10.1007/s40122-024-00623-4
69.
Noor N LaChute C Root M Rogers J Richard M Varrassi G et al A comprehensive review of celecoxib oral solution for the acute treatment of migraine. Health Psychol Res. (2022) 10:34265. 10.52965/001c.34265
70.
Al-Kuraishy H Al-Gareeb A Albuhadily A Abd El-Maksoud E Shokr M Alexiou A et al Paracetamol: the potential therapeutic pathways defining its clinical use. Inflammopharmacology. (2025) 33:2907–18. 10.1007/s10787-025-01779-x
71.
Häuser W Morlion B Vowles K Bannister K Buchser E Casale R et al European* clinical practice recommendations on opioids for chronic noncancer pain - Part 1: Role of opioids in the management of chronic noncancer pain. Eur J Pain. (2021) 25:949–68. 10.1002/ejp.1736
72.
Tzschentke T Jahnel U Kogel B Christoph T Englberger W De Vry J et al Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today. (2009) 45:483–96. 10.1358/dot.2009.45.7.1395291
73.
Volkow N Blanco C . The changing opioid crisis: development, challenges and opportunities.Mol Psychiatry. (2021) 26:218–33. 10.1038/s41380-020-0661-4
74.
Sacerdote P . Opioid-induced immunosuppression.Curr Opin Support Palliat Care. (2008) 2:207–10. 10.1097/SPC.0b013e32830cba54
75.
Dworkin R O’Connor A Backonja M Farrar J Finnerup N Jensen T et al Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. (2007) 132:237–51. 10.1016/j.pain.2007.08.033
76.
Häuser W Urrútia G Tort S Uçeyler N Walitt B . Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome.Cochrane Database Syst Rev. (2013) 23:CD010292. 10.1002/14651858.CD010292
77.
Moore R Derry S Aldington D Cole P Wiffen P . Amitriptyline for neuropathic pain and fibromyalgia in adults.Cochrane Database Syst Rev. (2012) 12:CD008242. 10.1002/14651858.CD008242.pub2
78.
Anand P Bley K . Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch.Br J Anaesth. (2011) 107:490–502. 10.1093/bja/aer260
79.
Kocot-Kępska M Zajączkowska R Mika J Kopsky D Wordliczek J Dobrogowski J et al Topical treatments and their molecular/cellular mechanisms in patients with peripheral neuropathic pain-narrative review. Pharmaceutics. (2021) 13:450. 10.3390/pharmaceutics13040450
80.
Cohen S Bhatia A Buvanendran A Schwenk E Wasan A Hurley R et al Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. (2018) 43:521–46. 10.1097/AAP.0000000000000808
81.
Puşcaşu C Chiriţă C Negreş S Blebea N . Exploring the therapeutic potential of N-Methyl-D-aspartate receptor antagonists in neuropathic pain management.Int J Mol Sci. (2024) 25:11111. 10.3390/ijms252011111
82.
Noppers I Niesters M Aarts L Smith T Sarton E Dahan A . Ketamine for the treatment of chronic non-cancer pain.Expert Opin Pharmacother. (2010) 11:2417–29. 10.1517/14656566.2010.515978
83.
Mücke M Phillips T Radbruch L Petzke F Häuser W . Cannabis-based medicines for chronic neuropathic pain in adults.Cochrane Database Syst Rev. (2018) 3:CD012182. 10.1002/14651858.CD012182.pub2
84.
Häuser W Petzke F Fitzcharles M . Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management - An overview of systematic reviews.Eur J Pain. (2018) 22:455–70. 10.1002/ejp.1118
85.
Kritikos A Taylor B Lamuda P Pollack H Schneider J . Patterns of past month cannabis consumption and cannabis use disorder - Insights from a nationally representative survey.Drug Alcohol Depend. (2025) 272:112680. 10.1016/j.drugalcdep.2025.112680
86.
Al-Husinat L Obeidat S Azzam S Al-Gwairy Y Obeidat F Al Sharie S et al Role of cannabis in the management of chronic non-cancer pain: a narrative review. Clin Pract. (2025) 15:16. 10.3390/clinpract15010016
87.
Hanna M Perrot S Varrassi G . Critical appraisal of current acute lbp management and the role of a multimodal analgesia: a narrative review.Pain Ther. (2023) 12:377–98. 10.1007/s40122-023-00479-0
88.
Leoni M Mercieri M Viswanath O Cascella M Rekatsina M Pasqualucci A et al Neuropathic pain: a comprehensive bibliometric analysis of research trends, contributions, and future directions. Curr Pain Headache Rep. (2025) 29:73. 10.1007/s11916-025-01384-1
89.
Nalamachu S Mallick-Searle T Adler J Chan E Borgersen W Lissin D . Multimodal therapies for the treatment of neuropathic pain: the role of lidocaine patches in combination therapy: a narrative review.Pain Ther. (2025) 14:865–79. 10.1007/s40122-025-00733-7
90.
Varrassi G Farì G Mercieri M Luigi M Leoni G . Acute pain management: the role of multimodal analgesia with a focus on dexketoprofen and tramadol hydrochloride fixed-dose combination in acute low back pain.Signa Vitae. (2025) 21:1–10. 10.22514/sv.2025.078
91.
Gay-Escoda C Hanna M Montero A Dietrich T Milleri S Giergiel E et al Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study). BMJ Open. (2019) 9:e023715. 10.1136/bmjopen-2018-023715
92.
Pergolizzi J Varrassi G LeQuang J Breve F Magnusson P . Fixed dose versus loose dose: analgesic combinations.Cureus. (2023) 15:e33320. 10.7759/cureus.33320
93.
García-de-la-Banda-García R Cruz-Díaz D García-Vázquez J Martínez-Lentisco M León-Morillas F . Effects of virtual reality on adults diagnosed with chronic non-specific low back pain: a systematic review.Healthcare. (2025) 13:1328. 10.3390/healthcare13111328
94.
Carvajal-Parodi C Rossel P Rodríguez-Alvarado A Guede-Rojas F Ponce-González J . Effects of virtual reality-based interventions on pain catastrophizing in people with chronic pain: a systematic review and meta-analysis.J Clin Med. (2025) 14:3782. 10.3390/jcm14113782
95.
Gatchel R Reuben D Dagenais S Turk D Chou R Hershey A et al Research agenda for the prevention of pain and its impact: report of the work group on the prevention of acute and chronic pain of the federal pain research strategy. J Pain. (2018) 19:837–51. 10.1016/j.jpain.2018.02.015
96.
Chou R Gordon D de Leon-Casasola O Rosenberg J Bickler S Brennan T et al Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. (2016) 17:131–57. 10.1016/j.jpain.2015.12.008
97.
Chipps J . Psychological therapies for the management of chronic and recurrent pain in children and adolescents: a Cochrane review summary.Int J Nurs Stud. (2021) 113:103393. 10.1016/j.ijnurstu.2019.103393
98.
Hayden J Ellis J Ogilvie R Malmivaara A van Tulder M . Exercise therapy for chronic low back pain.Cochrane Database Syst Rev. (2021) 9:CD009790. 10.1002/14651858.CD009790.pub2
99.
Farì G Fiore S Varrassi G Bernetti A . Non-pharmacological therapies to treat musculoskeletal pain.Pain Manag. (2025) 15:297–9. 10.1080/17581869.2025.2509475
100.
Johnson M Paley C Jones G Mulvey M Wittkopf P . Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study).BMJ Open. (2022) 12:e051073. 10.1136/bmjopen-2021-051073
101.
O’Keeffe M O’Sullivan P Purtill H Bargary N O’Sullivan K . Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT).Br J Sports Med. (2020) 54:782–9. 10.1136/bjsports-2019-100780
102.
Veehof M Trompetter H Bohlmeijer E Schreurs K . Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review.Cogn Behav Ther. (2016) 45:5–31. 10.1080/16506073.2015.1098724
103.
Paschali M Lazaridou A Sadora J Papianou L Garland E Zgierska A et al Mindfulness-based interventions for chronic low back pain: a systematic review and meta-analysis. Clin J Pain. (2024) 40:105–13. 10.1097/AJP.0000000000001173
104.
Manchikanti L Knezevic E Knezevic N Sanapati M Kaye A Abdi S et al Effectiveness of facet joint nerve blocks in managing chronic axial spinal pain of facet joint origin: a systematic review and meta-analysis. Pain Phys. (2024) 27:E169–206. 10.36076/ppj.2024.27.E169
105.
Engle A Khanna R Abd-Elsayed A . Radiofrequency ablation for the cervical spine.Ann Palliat Med. (2024) 13:1047–55. 10.21037/apm-23-520
106.
Hasoon J Urits I Viswanath O Varrassi G Simopoulos T Kohan L et al Percutaneous spinal cord stimulation lead placement under deep sedation and general anesthesia. Pain Ther. (2021) 10:1719–30. 10.1007/s40122-021-00332-2
107.
Lawson E Wallace M . Current developments in intraspinal agents for cancer and noncancer pain.Curr Pain Headache Rep. (2010) 14:8–16. 10.1007/s11916-009-0092-z
108.
Vickers A Vertosick E Lewith G MacPherson H Foster N Sherman K et al Acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain. (2018) 19:455–74. 10.1016/j.jpain.2017.11.005
109.
Rizzo R Cashin A Wand B Ferraro M Sharma S Lee H et al Non-pharmacological and non-surgical treatments for low back pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev. (2025) 3:CD014691. 10.1002/14651858.CD014691.pub2
110.
Paige N Miake-Lye I Booth M Beroes J Mardian A Dougherty P et al Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systematic review and meta-analysis. JAMA. (2017) 317:1451–60. 10.1001/jama.2017.3086
111.
Calderone A Mazzurco Masi V De Luca R Gangemi A Bonanno M Floridia D et al The impact of biofeedback in enhancing chronic pain rehabilitation: a systematic review of mechanisms and outcomes. Heliyon. (2025) 11:e41917. 10.1016/j.heliyon.2025.e41917
112.
Gatchel R Okifuji A . Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain.J Pain. (2006) 7:779–93. 10.1016/j.jpain.2006.08.005
113.
Furtado R MacDermid J Walton D Nazari G Bobos P . Non-pharmacological interventions for veterans living with chronic pain: a scoping review and intervention map.Disabil Rehabil. (2025) [Online ahead of print]. 10.1080/09638288.2025.2474703
114.
Migliorini F Maffulli N Schäfer L Manocchio N Bossa M Foti C et al Impact of education in patients undergoing physiotherapy for lower back pain: a level I systematic review and meta-analysis. Eur J Trauma Emerg Surg. (2025) 51:113. 10.1007/s00068-025-02788-9
115.
Liechti S Tseli E Taeymans J Grooten W . Prognostic factors for quality of life after interdisciplinary pain rehabilitation in patients with chronic pain-a systematic review.Pain Med. (2023) 24:52–70. 10.1093/pm/pnac098
116.
Courtney R Schadegg M Curry L Johns W Walters-Threat L Tirado D et al Determining the uptake of best practices in pain interdisciplinary teams: a scoping review. J Public Health. (2025) 1–2. 10.1007/s10389-025-02505-1[Epub ahead of print].
117.
Staudt M . The multidisciplinary team in pain management.Neurosurg Clin N Am. (2022) 33:241–9. 10.1016/j.nec.2022.02.002
118.
Benes L Keefe F DeBar L . Treating persistent pain: a nurse co-led, interdisciplinary model for primary care.Pain Manag Nurs. (2022) 23:728–36. 10.1016/j.pmn.2022.07.004
119.
Goetschi A Meyer-Massetti C . Characterising pharmacists’ interventions in chronic non-cancer pain care: a scoping review.Int J Clin Pharm. (2024) 46:1010–23. 10.1007/s11096-024-01741-x
120.
Jamkar M Azeem Z Giri S Palekar T . Consensus-building on patient education for chronic non-specific low back pain: a qualitative study on patient physiotherapist perspectives.Musculoskeletal Care. (2025) 23:e70107. 10.1002/msc.70107
121.
Slee R Warren A Noonan M Henderson T . Occupational therapy in pain management: an exploration and description of current UK practice.Br J Occup Ther. (2025) 88:228–36. 10.1177/03080226241300837
122.
Szczotkowski D Meyer-Moock S Kohlmann T Deppe K Gärtner A Hoffmann G et al Evaluating an early interdisciplinary multimodal assessment for patients at risk of developing chronic pain: results of a multicentre RCT in Germany. Pain Ther. (2025) 14:1081–102. 10.1007/s40122-025-00729-3
123.
Larsson K Fredriksson R Sjogren Fugl-Meyer K . Health social workers’ assessments as part of a specialized pain rehabilitation: a clinical data-mining study.Soc Work Health Care. (2019) 58:936–51. 10.1080/00981389.2019.1679322
124.
Ghafouri N Bäckryd E Dragioti E Rivano Fischer M Ringqvist Å Gerdle B . Effects of interdisciplinary pain rehabilitation programs on neuropathic and non-neuropathic chronic pain conditions - a registry-based cohort study from Swedish Quality Registry for Pain Rehabilitation (SQRP).BMC Musculoskelet Disord. (2023) 24:357. 10.1186/s12891-023-06462-2
125.
Elbers S Wittink H Konings S Kaiser U Kleijnen J Pool J et al Longitudinal outcome evaluations of Interdisciplinary Multimodal Pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. (2022) 26:310–35. 10.1002/ejp.1875
126.
Murphy J Schatman M . Interdisciplinary chronic pain management: overview and lessons from the public sector.5th ed. In: BallantyneJFishmanSRathmellJeditors. Bonica’s Management of Pain.Netherlands: Wolters Kluwer (2019). p. 1709–16.
127.
Dragioti E Björk M Larsson B Gerdle BA . Meta-epidemiological appraisal of the effects of interdisciplinary multimodal pain therapy dosing for chronic low back pain.J Clin Med. (2019) 8:871. 10.3390/jcm8060871
128.
Flynn D Eaton L Langford D Ieronimakis N McQuinn H Burney R et al A SMART design to determine the optimal treatment of chronic pain among military personnel. Contemp Clin Trials. (2018) 73:68–74. 10.1016/j.cct.2018.08.008
129.
Jacob L Clouzeau A Ostertag A Petrover D Vergnol J Morchoisne O et al Response to functional restoration in non-specific chronic low back pain with Modic type 1 changes. Eur Spine J. (2025) 34:1095–106. 10.1007/s00586-025-08665-8
130.
Barakou I Hackett K Finch T Hettinga F . Self-regulation of effort for a better health-related quality of life: a multidimensional activity pacing model for chronic pain and fatigue management.Ann Med. (2023) 55:2270688. 10.1080/07853890.2023.2270688
131.
Turcotte S Lapointe M Shea C Rousseau J Masse J Higgins J et al Outcomes and characteristics of interdisciplinary self-management interventions for older adults living with chronic pain: insights from a scoping review. J Ageing Longev. (2024) 4:83–118. 10.3390/jal4020007
132.
Guven Kose S Kose H Celikel F Tulgar S De Cassai A Akkaya O et al Chronic pain: an update of clinical practices and advances in chronic pain management. Eurasian J Med. (2022) 54(Suppl1):57–61. 10.5152/eurasianjmed.2022.22307
133.
Takahashi M Kodama S Akahane M Yamamoto S Yonemoto T Seki H . The relationship between treatment satisfaction and medication understanding among patients taking a novel oral pain reliever: a questionnaire-based cross-sectional study.Pain Ther. (2025) 14:931–45. 10.1007/s40122-025-00709-7
134.
Poquet N Lin C . The Brief Pain Inventory (BPI).J Physiother. (2016) 62:52. 10.1016/j.jphys.2015.07.001
135.
Gebke K McCarberg B Shaw E Turk D Wright W Semel D . A practical guide to recognize, assess, treat and evaluate (RATE) primary care patients with chronic pain.Postgrad Med. (2023) 135:244–53. 10.1080/00325481.2021.2017201
136.
Charoenpol F Tontisirin N Leerapan B Seangrung R Finlayson R . Pain experiences and intrapersonal change among patients with chronic non-cancer pain after using a pain diary: a mixed-methods study.J Pain Res. (2019) 12:477–87. 10.2147/JPR.S186105
137.
Kim E Rubinstein S Nead K Wojcieszynski A Gabriel P Warner J . The evolving use of electronic health records (EHR) for research.Semin Radiat Oncol. (2019) 29:354–61. 10.1016/j.semradonc.2019.05.010
138.
Kawji Y Almoaswes H Bise C Kawji L Murphy A Reed T et al Electronic health record recording of patient pain: challenges and discrepancies. Curr Pain Headache Rep. (2023) 27:737–45. 10.1007/s11916-023-01170-x
139.
Kim S Zeitzer J Mackey S Darnall B . Revealing sleep and pain reciprocity with wearables and machine learning.Commun Med. (2025) 5:160. 10.1038/s43856-025-00886-8
140.
Pergolizzi J Jr. LeQuang J El-Tallawy S Varrassi G . What clinicians should tell patients about wearable devices and data privacy: a narrative review.Cureus. (2025) 17:e81167. 10.7759/cureus.81167
141.
Cascella M Leoni M Shariff M Varrassi G . Artificial intelligence-driven diagnostic processes and comprehensive multimodal models in pain medicine.J Pers Med. (2024) 14:983. 10.3390/jpm14090983
142.
Cascella M Shariff M Viswanath O Leoni M Varrassi G . Ethical considerations in the use of artificial intelligence in pain medicine.Curr Pain Headache Rep. (2025) 29:10. 10.1007/s11916-024-01330-7
143.
Dowell D Ragan K Jones C Baldwin G Chou R . CDC clinical practice guideline for prescribing opioids for Pain - United States, 2022.MMWR Recomm Rep. (2022) 71:1–95. 10.15585/mmwr.rr7103a1
144.
Leyde S Price C Colgan D Pike K Tsui J Merrill J . Mental health distress is associated with higher pain interference in patients with opioid use disorder stabilized on buprenorphine or methadone.Subst Use Addctn J. (2024) 45:423–33. 10.1177/29767342241227402
145.
Marshall B Bland M Hulla R Gatchel R . Considerations in addressing the opioid epidemic and chronic pain within the USA.Pain Manag. (2019) 9:131–8. 10.2217/pmt-2018-0070
146.
Kovačević I Pavić J Filipović B Ozimec Vulinec Š Ilić B Petek D . Integrated approach to chronic pain-the role of psychosocial factors and multidisciplinary treatment: a narrative review.Int J Environ Res Public Health. (2024) 21:1135. 10.3390/ijerph21091135
147.
Dagnino A Campos M . Chronic pain in the elderly: mechanisms and perspectives.Front Hum Neurosci. (2022) 16:736688. 10.3389/fnhum.2022.736688
148.
Schofield P Dunham M Martin D Bellamy G Francis S Sookhoo D et al Evidence-based clinical practice guidelines on the management of pain in older people - a summary report. Br J Pain. (2022) 16:6–13. 10.1177/2049463720976155
149.
Bell T Franz C Kremen W . Persistence of pain and cognitive impairment in older adults.J Am Geriatr Soc. (2022) 70:449–58. 10.1111/jgs.17542
150.
van Veelen S Vuong C Gerritsma J Eckhardt C Verbeek S Peters M et al Efficacy of non-pharmacological interventions to reduce pain in children with sickle cell disease: a systematic review. Pediatr Blood Cancer. (2023) 70:e30315. 10.1002/pbc.30315
151.
Williams H Tanabe P . Sickle cell disease: a review of nonpharmacological approaches for pain.J Pain Symptom Manage. (2016) 51:163–77. 10.1016/j.jpainsymman.2015.10.017
152.
Giglio M Varrassi G Puntillo F . Cancer-related pain: inside a new dynamic personalized approach. a narrative review.J Cancer Immunol. (2022) 4:17–21. 10.33696/cancerimmunol.4.060
153.
Pergolizzi J Raffa R . The WHO pain ladder: Do we need another step.Pract Pain Manag. (2014) 14:42–52.
154.
Ghosh A Berger A . Opioids, adjuvants, and interventional options for pain management of symptomatic metastases.Ann Palliat Med. (2014) 3:172–91. 10.3978/j.issn.2224-5820.2014.07.07
155.
Folch Ibáñez J Vargas Domingo M Coma Alemany J Callao Sánchez R Guitart Vela J . Effectiveness of vortioxetine in patients with major depressive disorder associated with chronic pain: an observational study in a Spanish Population.Pain Ther. (2024) 13:621–35. 10.1007/s40122-024-00597-3
156.
El-Tallawy S Pergolizzi J Ahmed R Kaki A Nagiub M LeQuang J et al Pain Management in the Post-COVID Era-an update: a narrative review. Pain Ther. (2023) 12:423–48. 10.1007/s40122-023-00486-1
157.
Ayub S Bachu A Jain L Parnia S Bhivandkar S Ahmed R et al Non-opioid psychiatric medications for chronic pain: systematic review and meta-analysis. Front Pain Res. (2024) 5:1398442. 10.3389/fpain.2024.1398442
158.
Kim K Yang Y Wang Z Chen J Barandouzi Z Hong H et al A systematic review of the association between health literacy and pain self-management. Patient Educ Couns. (2022) 105:1427–40. 10.1016/j.pec.2021.09.037
159.
Nguyen L Dawson J Brooks M Khan J Telusca N . Disparities in pain management.Anesthesiol Clin. (2023) 41:471–88. 10.1016/j.anclin.2023.03.008
160.
Scholl I Zill J Härter M Dirmaier J . An integrative model of patient-centeredness - a systematic review and concept analysis.PLoS One. (2014) 9:e107828. 10.1371/journal.pone.0107828
161.
Eccleston C Fisher E Thomas K Hearn L Derry S Stannard C et al Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. (2017) 11:CD010323. 10.1002/14651858.CD010323.pub3
162.
Meints S Edwards R . Evaluating psychosocial contributions to chronic pain outcomes.Prog Neuropsychopharmacol Biol Psychiatry. (2018) 87(Pt B):168–82. 10.1016/j.pnpbp.2018.01.017
163.
Karra R Holten-Rossing S Mohammed D Parmeggiani L Heine M Namnún O . Unmet needs in the management of functional impairment in patients with chronic pain: a multinational survey.Pain Manage. (2021) 11:303–14. 10.2217/pmt-2020-0098
164.
Booker S Merriwether E Powell-Roach K Jackson S . From stepping stones to scaling mountains: overcoming racialized disparities in pain management.Pain Manag. (2024) 14:5–12. 10.2217/pmt-2023-0098
165.
Lentz T Gonzalez-Smith J Huber K Goertz C Bleser W Saunders R . Overcoming barriers to the implementation of integrated musculoskeletal pain management programs: a multi-stakeholder qualitative study.J Pain. (2023) 24:860–73. 10.1016/j.jpain.2022.12.015
166.
Li D Hu Y Liu S Li G Lu C Yuan S et al The effect of using internet hospitals on the physician-patient relationship: patient perspective. Int J Med Inform. (2023) 174:105058. 10.1016/j.ijmedinf.2023.105058
Summary
Keywords
mixed pain, multimodal analgesia, neuropathic pain, nociceptive pain, nociplastic pain, interdisciplinary care, pain management, pain
Citation
Varrassi G, Farì G, Narvaez Tamayo MA, Gomez MP, Guerrero Liñeiro AM, Pereira CL, Samy Aziz E, Gharibo C, Kaye AD, Garcia-Larrea L, Moka E, Król A, Volk T, Al-Alwany AA and Leoni MLG (2025) Mixed pain: clinical practice recommendations. Front. Med. 12:1659490. doi: 10.3389/fmed.2025.1659490
Received
04 July 2025
Accepted
09 September 2025
Published
09 October 2025
Corrected
14 October 2025
Volume
12 - 2025
Edited by
Panjamurthy Kuppusamy, University of Maryland, Baltimore, United States
Reviewed by
Fahad S. Alshehri, Umm Al-Qura University, Saudi Arabia
Manasi Murthy Mittinty, The University of Melbourne, Australia
Updates
Copyright
© 2025 Varrassi, Farì, Narvaez Tamayo, Gomez, Guerrero Liñeiro, Pereira, Samy Aziz, Gharibo, Kaye, Garcia-Larrea, Moka, Król, Volk, Al-Alwany and Leoni.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giustino Varrassi, giuvarr@gmail.com
†ORCID: Giustino Varrassi, orcid.org/0000-0002-3822-2923
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.