- 1Key Laboratory of Medical Molecular Virology (MOE/NHC/CAMS), School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai, China
- 2Department of Nursing, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Key Laboratory of Embryo Original Diseases, Shanghai, China
- 4Department of Gynaecology, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 6Clinical Research Center, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 7Shanghai Institute of Virology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Objective: The combination of low-dose aspirin (LDA) and low-molecular-weight heparin (LMWH) is the standard of care for obstetric antiphospholipid syndrome (OAPS), significantly improving live birth rates. However, whether this regimen fully normalizes the pregnancy course and mitigates risks for both the mother and the neonate remains unclear. This study aimed to systematically evaluate whether significant maternal and neonatal morbidity persists in OAPS patients despite successful treatment and live birth.
Methods: This retrospective cohort study included 256 OAPS patients, including 166 criteria OAPS patients (C-OAPS—patients who fulfilled both the clinical and laboratory criteria of the Sydney criteria) and 90 non-criteria OAPS patients (NC-OAPS—patients who fulfilled only the clinical or only the laboratory criteria of the Sydney criteria) who achieved live birth, along with 768 matched healthy controls. We compared basic characteristics, laboratory parameters, and perinatal outcomes between the groups.
Results: Compared to healthy controls (n = 768), treated OAPS patients (n = 256) exhibited a persistent hypercoagulable state (elevated D-dimer and fibrin degradation product (FDP), p < 0.01) and a higher incidence of anemia (p < 0.001). Their neonates had significantly lower birth weight (p = 0.006) and elevated risks of neonatal infection (adjusted OR = 3.12, p = 0.004) and hyperbilirubinemia (adjusted OR = 2.06, p = 0.024), with the infection risk remaining significant in full-term infants. A subgroup analysis revealed no significant differences in obstetric history, maternal complications, comorbidities, and outcomes between the C-OAPS and NC-OAPS groups.
Conclusion: Despite standard treatment, OAPS patients who deliver successfully remain at an increased risk for persistent maternal hypercoagulability and adverse neonatal outcomes. These findings underscore the need for a paradigm shift in management—from merely ensuring live birth to safeguarding neonatal health through proactive, multidisciplinary perinatal care.
1 Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by clinical manifestations, including thrombotic events and/or obstetric complications, and it is accompanied by the presence of antiphospholipid antibodies (aPLs) (1). The 2006 Sydney International Consensus provides an extensive overview of adverse obstetric outcomes linked to recurrent first-trimester miscarriage, fetal loss, stillbirth, early and severe pre-eclampsia, or preterm birth (before 34 weeks of gestation) (2). In patients with a history of thrombosis, these complications are classified as obstetric APS (OAPS) and are primarily attributed to placental dysfunction (3). This is partly due to the detrimental effects of aPLs throughout the stages from implantation and placentation to delivery (4).
Recent research has indicated that placental inflammatory responses, including complement activation and subsequent endothelial damage in patients with OAPS, may impair the invasive function of placental trophoblasts (5). Additionally, the formation of neutrophil extracellular traps (6), release of interleukin-8 (7), upregulation of the target of rapamycin complex on endothelial cells (8), and an imbalance in angiogenic factors (9) do not culminate in thrombosis. While the administration of glucocorticoids appears promising in mitigating inflammatory response (10), the current gold standard in clinical treatment remains the combination of low-dose aspirin (LDA) and low-molecular-weight heparin (LMWH) (11). This treatment regimen has significantly increased the rate of successful deliveries in patients with OAPS; however, potential maternal placental pathology and effects on the offspring cannot be ruled out, due to the persistence of aPLs.
However, whether this treatment fully normalizes the course of pregnancy and eliminates risks for both the mother and neonate remains a subject of inquiry. A focus solely on live birth may overlook significant maternal and neonatal morbidity that persists despite treatment. For instance, some reports suggest an association between OAPS and adverse outcomes such as fetal growth restriction, preterm birth, and placental pathology even in treated pregnancies (9). Furthermore, a notable proportion of patients with clinical features highly suggestive of OAPS tested negative for the criteria OAPS (C-OAPS), a category often referred to as seronegative or non-criteria OAPS (NC-OAPS) (12), presenting a diagnostic and management challenge. According to the 2023 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) classification criteria for antiphospholipid syndrome, these patients are likely to be classified into an under-recognized group. Although the new standards significantly enhance specificity and introduce a more detailed clinical and laboratory stratification weighting system, their strict inclusion criteria—requiring a cumulative score of at least 3 in both clinical and laboratory domains—may result in the exclusion of some patients with typical obstetric clinical manifestations whose laboratory tests do not meet the threshold from the classification (13).
Given these considerations, this study was conducted to systematically evaluate the pregnancy characteristics and outcomes in a cohort of OAPS patients who achieved a live birth following standard treatment. We aimed to retrospectively analyze and compare basic maternal characteristics, laboratory parameters, and perinatal outcomes between these treated OAPS patients and matched healthy controls. The objective of this study was to assess the potential persistent effects of OAPS on pregnancy and to provide a detailed clinical profile that may inform future management strategies and research directions.
2 Materials and methods
2.1 Study population
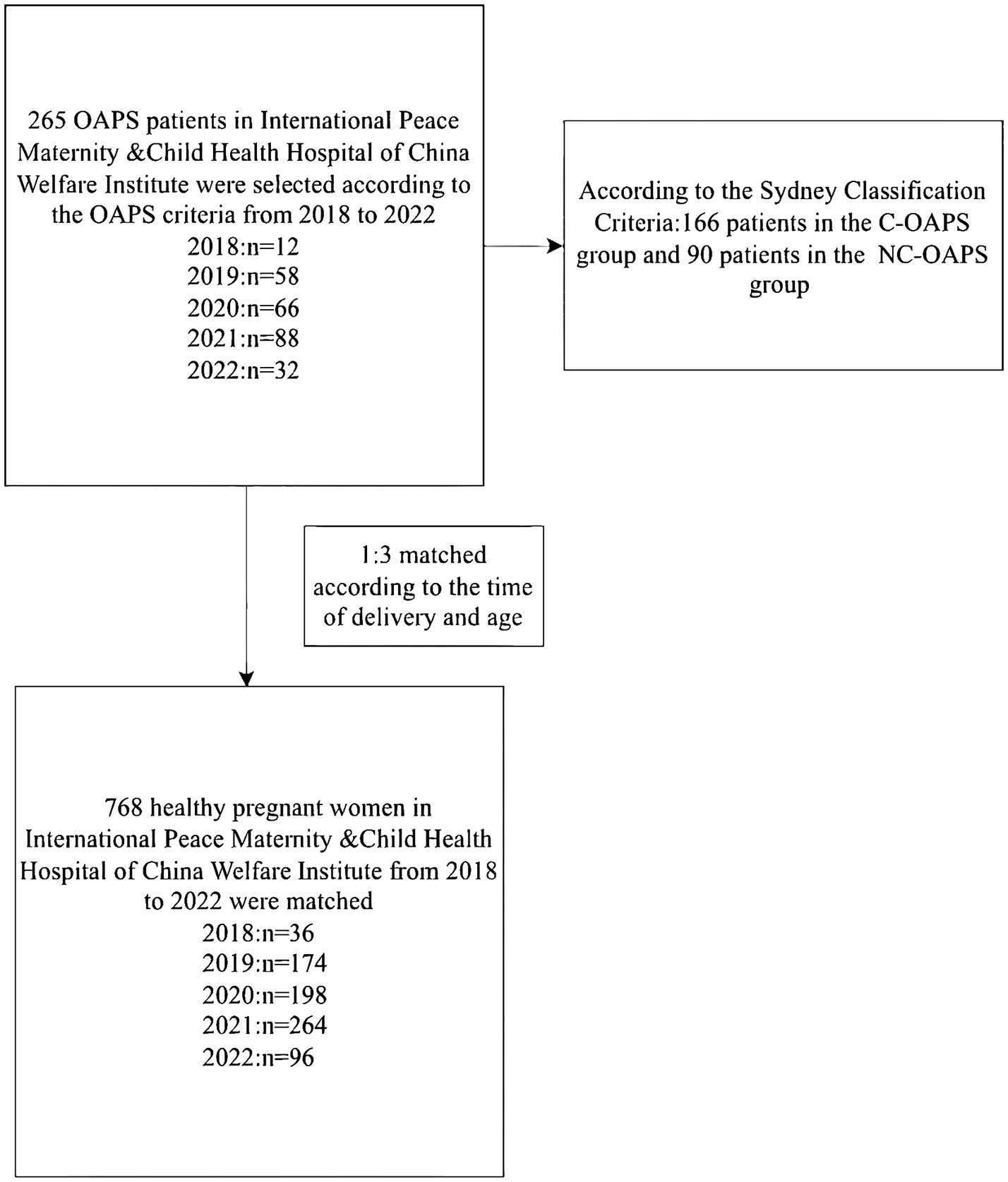
All medical histories for this study were recorded at the International Peace Maternity and Child Health Hospital of the China Welfare Institute from 2018 to 2022. All research participants had delivered successfully (see Figure 1).
2.2 The inclusion and exclusion criteria of the patients with OAPS
2.2.1 Clinical inclusion criteria for C-OAPS and NC-OAPS
Patients with OAPS are classified as either the C-OAPS group or the NC-OAPS group according to the Sydney criteria.
C-OAPS required at least one clinical criterion plus one laboratory criterion. Conversely, those who fulfilled the criteria in only one aspect—that is, patients who fulfilled the clinical criteria but did not fulfill the laboratory criteria or those who fulfilled the laboratory criteria but did not fulfill the clinical criteria—were classified as having non-criteria OAPS (NC-OAPS) (14). In accordance with the prevailing clinical guidelines, all enrolled OAPS patients were managed with a combination of low-dose aspirin and prophylactic low-molecular-weight heparin throughout pregnancy (from the first confirmation of pregnancy until at least 6 weeks postpartum) to improve obstetric outcomes (15).
(1) The clinical criteria were unexplained fetal death ≥10 weeks, premature birth <34 weeks due to placental dysfunction, or ≥3 consecutive miscarriages <10 weeks.
(2) The laboratory criteria were lupus anticoagulant (LA), medium−/high-titer immunoglobulin G (IgG)/immunoglobulin M (IgM) anticardiolipin (aCL), or anti-β2-glycoprotein-I (anti-β2GPI) antibodies—mandated positivity on two occasions at least 12 weeks apart.
2.2.2 Exclusion criteria
The clinical exclusion criteria were as follows:
(1) Patients with active hepatitis B virus, hepatitis C virus, human immunodeficiency virus, syphilis, or tuberculosis infection;
(2) Those with a history of smoking or drinking;
(3) Those who use drugs during pregnancy which can seriously affect maternal and infant outcomes;
(4) Those who underwent induced abortion due to family planning or personal request;
(5) Those with a history of significant diseases, such as severe lesions of vital organs, during pregnancy;
(6) Those with a history of previous thrombosis or pregnancy complicated by thrombosis;
(7) Those without routine prenatal examinations and whose data were incomplete; and
(8) Those with multiple gestations.
2.3 The inclusion and exclusion criteria of the healthy pregnant women
Case and control groups were matched at a 1:3 ratio according to the time of delivery and age to reduce confounding factors and selection bias.
2.3.1 Inclusion criteria for healthy pregnant women
The inclusion criteria for healthy pregnant women were as follows:
(1) Previously healthy pregnant women who gave birth during the same period as the case group;
(2) Women who did not meet any of the OAPS and non-criteria OAPS diagnostic criteria;
(3) Those with no history or family history of autoimmune disease; and
(4) Those who have not used drugs such as corticosteroids and immunosuppressants.
2.3.2 Exclusion criteria for healthy pregnant women
The exclusion criteria were the same as the OAPS patients.
2.4 Clinical data collection
Our study collected three types of information: basic information, laboratory examinations, and clinical characteristics during the perinatal period.
2.4.1 Basic information
Basic information included age, pre-pregnancy body mass index (BMI), weight gain during pregnancy, days of latest pregnancy, number of deliveries, number of pregnancies, history of preterm labor, history of stillbirth, and number of miscarriages.
2.4.2 Laboratory examination
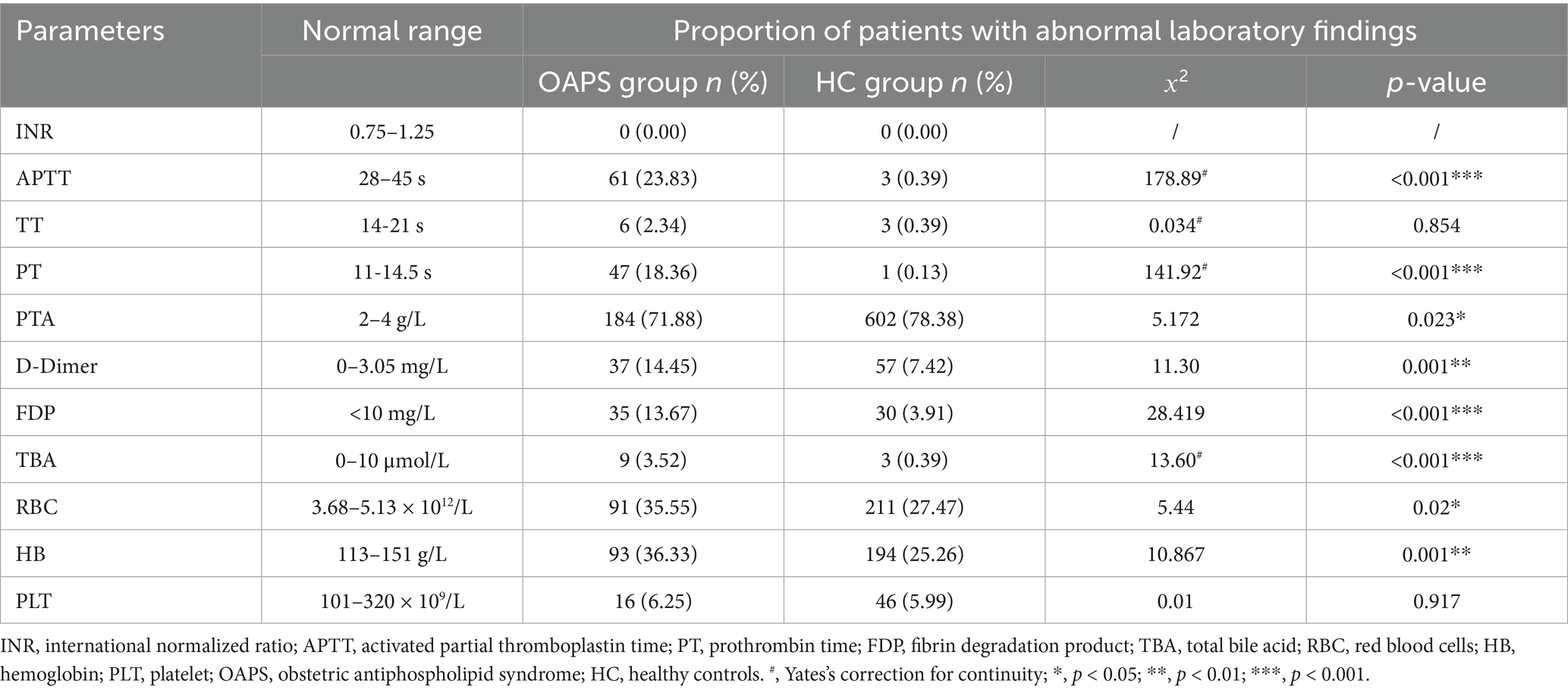
Upon admission to the hospital for delivery, blood samples were collected. Relevant experimental indices, including international normalized ratio (INR), activated partial thromboplastin time (APTT), thromboplastin time (TT), prothrombin time (PT), D-dimer, FDP, total bile acid (TBA), red blood cells (RBCs), hemoglobin (HB), and platelets (PLTs), were tested.
2.4.3 Detection of antiphospholipid antibodies: main instruments and reagents
Our laboratory used the enzyme-linked immunosorbent assay (ELISA) as the core detection technology, utilizing the fully automated EUROIMMUN analyzer and corresponding test kits to perform quantitative detection of antiphospholipid antibodies in clinical serum samples.
2.4.3.1 Main instruments
The main instruments were the Fully Automated Fluorescence Immunoassay Analyzer SPRINTER XL from EUROIMMUN.
2.4.3.2 Reagent kits (all from EUROIMMUN)
The reagent kits included the Anti-Cardiolipin Antibody IgA Test Kit (product code: EA 1621-9601A), Anti-Cardiolipin Antibody IgG Test Kit (product code: EA 1621-9601G), Anti-Cardiolipin Antibody IgM Test Kit (product code: EA 1621-9601 M), Anti-β2-Glycoprotein I Antibody IgG Test Kit (product code: EA 1632-9601G), and Anti-β2-Glycoprotein I Antibody IgM Test Kit (product code: EA 1632-9601 M).
2.4.4 Clinical characteristics of the perinatal period
To accurately gauge the severity of obstetric complications, the study adhered to the Sydney International Consensus, which categorizes complications into three primary groups:
(1) Adverse pregnancy outcomes: intrauterine distress, fetal growth restriction (FGR), and postpartum hemorrhage;
(2) Maternal complications and comorbidities: diabetes mellitus in pregnancy (pre-pregnancy diabetes mellitus and gestational diabetes mellitus [GDM]), hypertensive disorders of pregnancy (gestational hypertension [GH] and pre-eclampsia/eclampsia), thyroid disorders of pregnancy, intrahepatic cholestasis in pregnancy, hematologic disorders of pregnancy (anemia), placental abnormalities (placental adhesion, placental lakes, placental abruption, and placental implantation), abnormalities (abnormal amount and color of amniotic fluid), pregnancy-related sexually transmitted diseases (mycoplasma infection), and pregnancy-related streptococcal infection;
(3) Neonatal complications: preterm labor, low birth weight, neonatal asphyxia, neonatal infections, and neonatal hyperbilirubinemia.
2.5 Statistical methods
Values are expressed as mean (±S.D.) and numbers and percentages for qualitative variables. Student’s t-test was used to compare continuous variable data following a normal distribution, while Mann–Whitney Wilcoxon’s test was used for continuous variable data not following a normal distribution. The chi-squared test, Yates’s correction for continuity, and Fisher’s exact test were applied to compare categorical variables. For comparisons of continuous variables that were not normally distributed across the three independent groups, the Kruskal–Wallis H test was used. Post-hoc pairwise comparisons were then conducted using Dunn’s test, applying a Bonferroni correction for multiple comparisons. A difference was considered statistically significant at a p-value of <0.05. To further evaluate the independent association between obstetric antiphospholipid syndrome (OAPS) and maternal/neonatal outcomes, binary logistic regression analyses were performed. Outcomes that showed significant differences (p < 0.05) in the univariate analyses were included in the multivariate models. The regression models were adjusted for potential confounding factors identified from clinical relevance and univariate results; the results are expressed as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). The statistical software SPSS 23.0 (IBM, United States) was used for dataset analyses.
3 Results
A total of 1,024 women were included in this study: 256 OAPS cases and 768 healthy controls (HCs). The OAPS group was divided into 166 patients in the typical OAPS group and 90 patients in the atypical NC-OAPS group, according to the Sydney Classification Criteria. All of the participating women had delivered successfully following treatment during the most recent pregnancy in the hospital. We also randomly selected 768 healthy pregnant women admitted to the obstetrics department during the same period, who were matched at a 1:3 ratio to the OAPS group based on the time of delivery and age.
3.1 Basic information and reproductive history of the OAPS and healthy control groups
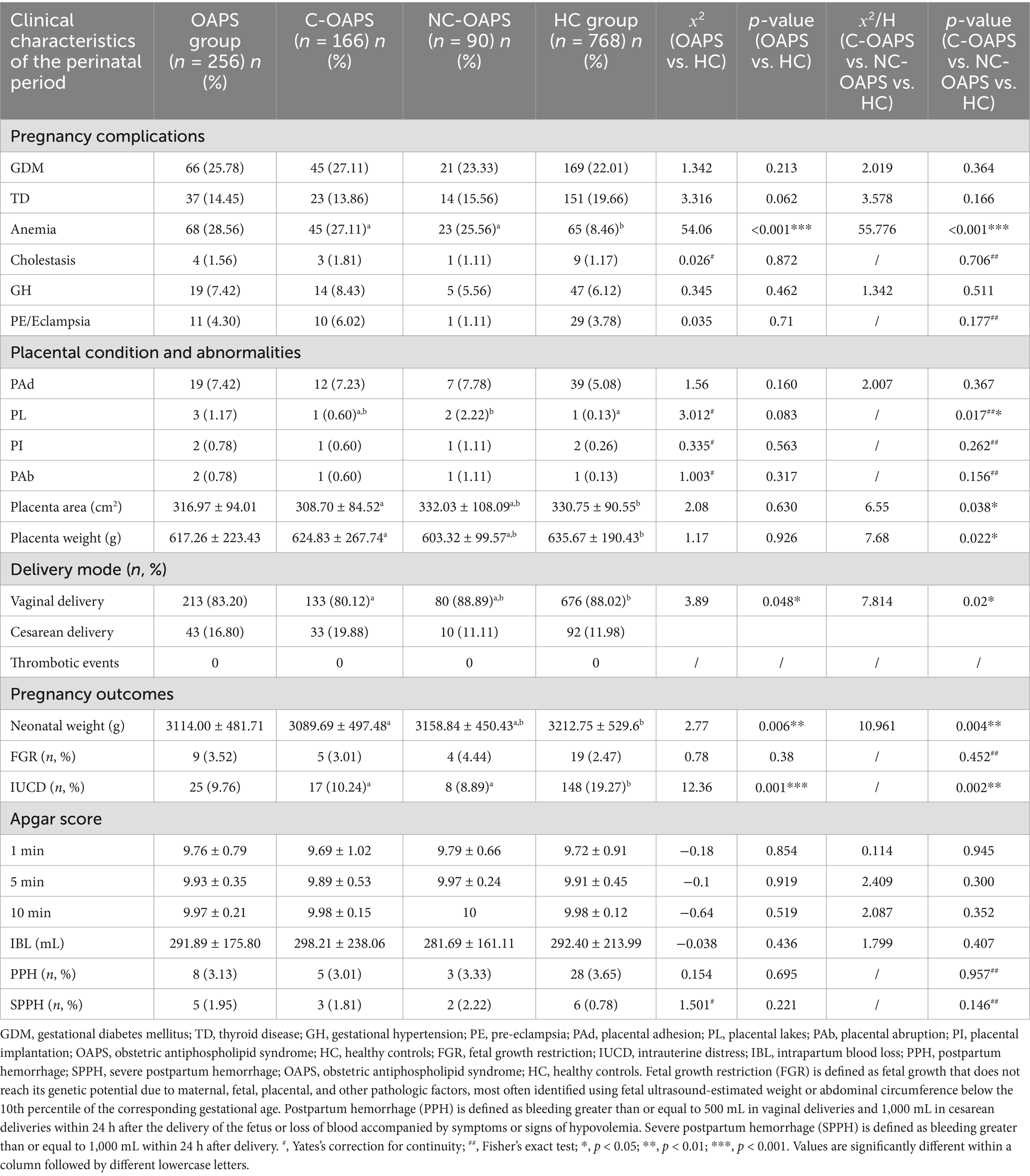
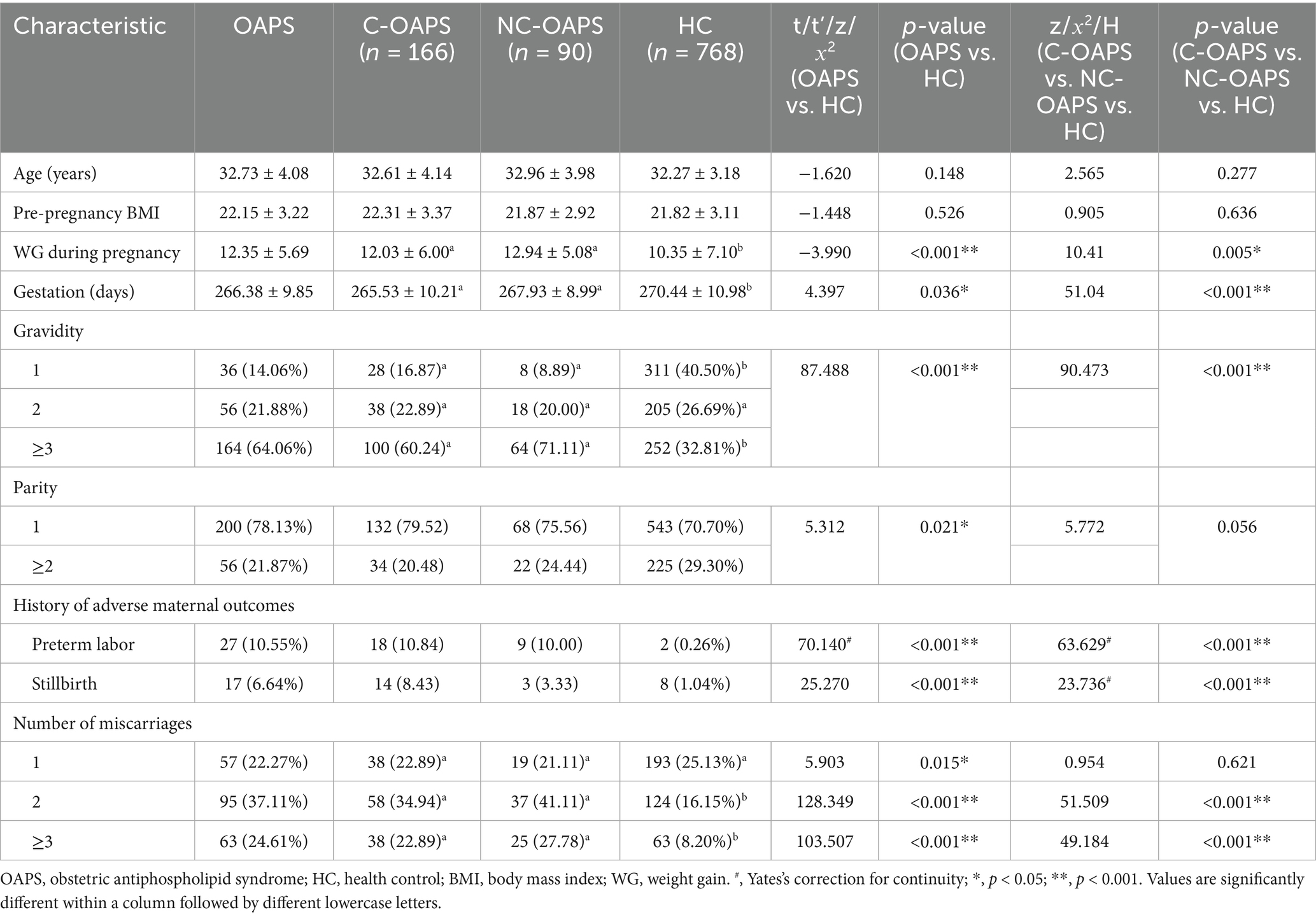
In this study, significant differences were observed in gestational weight gain and gestational duration between the OAPS and HC groups. The OAPS group exhibited a higher mean weight gain (12.35 ± 5.69 kg) than the HC group (10.35 ± 7.10 kg, p < 0.001), as well as a shorter mean gestational period (266.38 ± 9.85 days versus 270.44 ± 10.98 days, p < 0.05). Additionally, marked disparities in gravidity and parity were evident between the groups. Compared to the HC group (32.81%), the OAPS group had a higher incidence of pregnancies with three or more instances of gravidity (64.06%) and a lower incidence of two or more instances of parity (21.87% versus 29.30%). Furthermore, a significant difference was observed in adverse maternal history: the incidence of preterm deliveries was substantially higher in the OAPS group (10.55%) than in the HC group (0.26%), and the rate of stillbirths was higher in the OAPS group (6.64%) than in the HC group (1.04%). Moreover, the OAPS group exhibited a higher frequency of miscarriages, with two and three or more occurrences corresponding to percentages of 37.11% versus 16.15 and 24.61% versus 8.20%, respectively. There were no differences between the C-OAPS and NC-OAPS groups (refer to Table 4).
3.2 Results of laboratory test parameters
The incidence of APTT and PT abnormalities was significantly greater in the OAPS group than in the HC group (p < 0.001). Conversely, the incidence of PTA abnormalities was lower in the OAPS group than in the HC group (p = 0.023). Additionally, patients with OAPS exhibited higher rates of abnormalities in D-dimer, FDP, and TBA than the HC group (p < 0.001), with abnormality rates of 14.51% vs. 7.42, 13.67% vs. 3.91, and 3.52% vs. 0.39%, respectively. Furthermore, the incidence of RBC abnormalities was higher in the OAPS group than in the HC group (p = 0.02), consistent with the trend observed in the HB test (p = 0.001) (see Table 1).
3.3 Maternal complications, comorbidities, and outcomes
The prevalence of anemia was significantly greater in the OAPS group than in the HC group (p < 0.001). Furthermore, no significant difference was observed in the incidence of abnormal placental invasion and morphological anomalies between the OAPS group and the HC group (p > 0.05). Additionally, the rate of cesarean delivery was higher in the OAPS group than in the HC group (p = 0.048). Among the pregnancy outcomes, neonatal weight was significantly lower in the OAPS group than in the HC group (p = 0.006). Conversely, the incidence of intrauterine distress was significantly higher in the HC group than in the OAPS group (p = 0.001). There were no differences between the C-OAPS and NC-OAPS groups (Table 2).
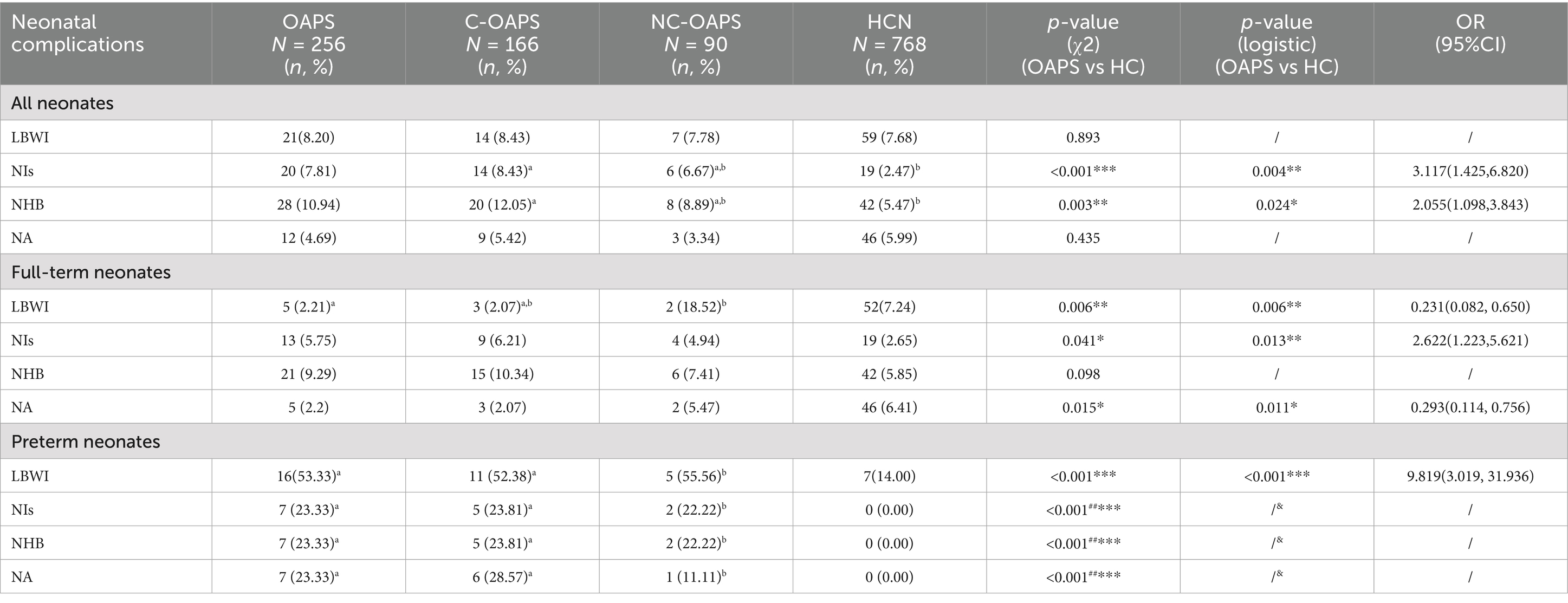
The analysis demonstrated that there was no statistically significant difference in the percentage of preterm neonates with low birth weight between the two groups (p = 0.893). However, the incidence of low birth weight was significantly higher among preterm neonates in the OAPS group than in the HC group (p = 0.004), while it was significantly lower among full-term neonates (p < 0.001). Regarding neonatal complications, the findings indicated that both the incidence of neonatal infections and the prevalence of neonatal hyperbilirubinemia were significantly higher in the OAPS group than in the HC group (p = 0.004, p = 0.024). For full-term infants, the incidence of neonatal infection and neonatal asphyxia was significantly greater in the OAPS group than in the HC group (p = 0.013, p = 0.011). Additionally, the occurrence of complications, including infection, asphyxia, and hyperbilirubinemia, was markedly higher in preterm infants in the OAPS group than in those in the HC group (p < 0.001) (refer to Table 3). There were no differences between the C-OAPS and NC-OAPS groups (Table 2).
4 Discussion
Compared to the HC group, the most salient clinical manifestation in the OAPS group was pathological pregnancy, particularly a history of preterm labor, stillbirth, and miscarriages, all of which were significantly more frequent than in the HC group. The underlying causes of pathological pregnancy may involve activating endothelial cells, monocytes, and platelets by aPLs, leading to a procoagulant state. This process may be further exacerbated by the direct action of the placental trophoblast, resulting in trophoblast cell destruction and apoptosis. This process can lead to a cascade of adverse outcomes, including reduction of hormones such as human chorionic gonadotropin, diminished capacity of trophoblast cells to invade and implant in the uterus, and suppression of trophoblast cell proliferation. These alterations may hinder the embryo’s attachment to the uterus and progression of the pregnancy (16). Although the standard treatment regimen of low-dose aspirin combined with low-molecular-weight heparin significantly improved the live birth rate in patients with OAPS (17), our research found that, even after standard treatment, OAPS mothers and fetuses still face a series of significant residual risks, including a hypercoagulable state of the mother, unique placental pathological changes, and adverse neonatal outcomes. This finding indicates that the current therapy has limitations in correcting the fundamental pathophysiological mechanisms of OAPS, highlighting the urgency of assessing its impact on pregnancy quality beyond the live birth rate.
Key laboratory findings from this study delineate distinct hematological and biochemical profiles in OAPS patients compared to healthy controls. The OAPS group exhibited a significantly higher incidence of abnormalities in activated partial thromboplastin time (APTT) and prothrombin time (PT), yet a lower incidence of abnormal prothrombin activity (PTA). This apparent prolonged conventional coagulation time alongside a lower rate of reduced PTA likely reflects the therapeutic effect of low-molecular-weight heparin (LMWH) (18). The observed alterations in these coagulation parameters are consistent with the documented pharmacologic profile of LMWH calcium, as previously reported (19). LMWH, primarily through the inhibition of factor Xa, can induce a modest prolongation of APTT and PT, representing a predictable and intended anticoagulant state rather than a marker of bleeding risk (20). Concurrently, the significant elevations in the mean values and abnormality rates of D-dimer and fibrin degradation products (FDP) in the OAPS group provide direct laboratory evidence of ongoing fibrin formation and degradation, indicating a persistent hypercoagulable state with secondary fibrinolysis. Furthermore, the observed increase in total bile acid (TBA) levels suggests the presence of intrahepatic cholestasis in a subset of patients, which may compound obstetric risks. The higher incidence of red blood cell (RBC) and hemoglobin (HB) abnormalities is consistent with the greater prevalence of anemia observed in the OAPS cohort, implying a potential impact of the disease on hematopoiesis or erythrocyte stability. Collectively, these laboratory findings define a distinct hematological profile in OAPS, characterized by a treated prothrombotic phenotype and associated systemic involvement, underscoring the need for comprehensive monitoring.
The observed lower incidence of intrauterine distress in the OAPS group can be logically explained by its standardized clinical management. The implementation of intensified antenatal surveillance in these high-risk pregnancies facilitated timely detection of complications, leading to earlier elective delivery and thereby reducing the incidence of frank distress. In addition, a comprehensive review of the extant literature indicates that the effects of OAPS on fetal and neonatal outcomes primarily encompass birth weight, fetal death, preterm delivery, FGR, and fetal acidosis (21). In this study, the birth weight of neonates in the OAPS group was significantly lower than that in the HC group. However, there was no statistically significant difference in the proportion of low birth weight. Additionally, the OAPS group exhibited a higher proportion of preterm births than the HC group. Previous research found that 127 patients with OAPS were at risk of preterm labor, and the incidence of “spontaneous preterm labor” was 16.5% (22). Consequently, the occurrence of preterm labor in pregnancies affected by OAPS encompasses two distinct categories: (1) medically indicated preterm labor associated with pre-eclampsia or fetal factors and (2) a latent predisposition to preterm labor. The underlying predisposition to preterm labor is well-documented. Furthermore, the study noted that neonatal infections and hyperbilirubinemia were more prevalent in the OAPS group than in the HC group. Previous research has indicated that preterm infants are more susceptible to diseases and hyperbilirubinemia compared to term infants (23). To further investigate this observation, a subgroup analysis was conducted. Crucially, the subgroup analysis confirmed that this elevated risk remained significant even after accounting for prematurity, as the infection rate was also considerably higher in term neonates from the OAPS group. This suggests that the predisposition to infection is not solely a consequence of preterm birth but is intrinsically linked to the OAPS condition itself. We propose that the altered intrauterine environment in OAPS—characterized by a persistent placental inflammatory state and immune dysregulation (24)—may impair fetal immune system programming or function, increasing susceptibility to postnatal infection. These findings underscore that the ramifications of OAPS transcend the endpoint of live birth, warranting a shift in clinical focus from merely “securing fetal survival” to “safeguarding neonatal health.” This necessitates closer collaboration between obstetricians and neonatologists to optimize perinatal management from pregnancy into the postpartum period.
Given these persistent challenges in management, the recent introduction of the ACR/EULAR 2023 classification criteria for APS marks a significant step forward. These updated criteria refine the original Sydney standards by incorporating a weighted scoring system and expanded serological markers, thereby enhancing specificity for research (13). However, it is important to distinguish between classification criteria, designed to ensure cohort homogeneity, and the broader clinical spectrum of the disease. The stringent nature of such criteria may inevitably exclude some patients with highly suggestive clinical presentations—a group often identified as NC-OAPS. Our study found that there was no significant difference between the NC-OAPS group and the C-OAPS group, not only in obstetric history but also in terms of maternal complications, comorbidities, and outcomes (Tables 2–4). This key finding demonstrates that patients in the NC-OAPS group experience a clinical burden of disease comparable to that of patients who meet the formal criteria. By intentionally including NC-OAPS patients, our study aims to characterize the outcomes and pathophysiological features across the entire clinical phenotype managed as OAPS, thus providing insights that may inform future diagnostic and therapeutic strategies.
For patients diagnosed with OAPS, particularly those with recurrent pregnancy loss, combination therapy with low-dose aspirin and low-molecular-weight heparin remains the cornerstone of management and is effective in improving live birth rates (25). However, our findings underscore that this standard regimen does not fully eliminate significant residual risks, including maternal hypercoagulability, placental pathology, and adverse neonatal outcomes. Therefore, meticulous monitoring for maternal and neonatal complications is imperative, and management must be highly individualized. Future care for OAPS necessitates a paradigm shift, extending beyond anticoagulation. A proactive, multidisciplinary approach—involving obstetricians, rheumatologists, and often neonatologists—is essential throughout the pre-pregnancy, antenatal, and postpartum periods. The overarching goal must transition from merely “ensuring survival” to comprehensively “optimizing health.” Achieving this requires a dual strategy: advancing interventions to target fundamental pathophysiological mechanisms earlier in the disease course and deepening clinical vigilance toward placental function and long-term neonatal wellbeing. This redefined focus should guide the next generation of research and clinical innovation in OAPS.
5 Conclusion
This study, focusing on OAPS patients who achieved successful delivery, shows that despite standard treatment with low-dose aspirin and LMWH, these women continue to exhibit a distinct and complex clinical profile. Key findings include a persistent maternal hypercoagulable state, evidenced by elevated D-dimer and FDP and an increased risk of adverse neonatal outcomes, such as lower birth weight, preterm delivery, and a heightened susceptibility to neonatal infection and hyperbilirubinemia. These findings collectively indicate that the central clinical challenge in OAPS is shifting from achieving live birth to safeguarding the quality of pregnancy and neonatal health. Consequently, a paradigm shift in management is warranted—extending beyond current anticoagulation strategies to embrace a proactive, multidisciplinary approach focused on pre-pregnancy counseling, intensified placental and fetal surveillance, and long-term neonatal care, with the ultimate goal of optimizing overall maternal and offspring health outcomes.
6 Limitations
Several limitations of this study should be acknowledged. First, its retrospective and single-center design may introduce selection bias and limit the generalizability of the findings. The sample size, though substantial, may still be insufficient to detect significant differences in rarer complications. Second, the study population consisted of patients who ultimately achieved a successful delivery, which may not fully represent the entire spectrum of OAPS severity, particularly those with the majority of refractory cases. Furthermore, the absence of detailed placental histopathological analysis for all participants limits our ability to correlate clinical outcomes with specific underlying placental lesions. Third, the subgroup analyses based on antibody profiles were limited by small sample sizes, which may have obscured statistically significant differences and precluded a more robust comparison. Finally, potential unmeasured confounding factors, despite our matching efforts, could have influenced the observed associations. Future prospective, multi-center studies with larger cohorts and systematic placental examination are needed to validate these findings and further elucidate the pathophysiological mechanisms linking OAPS to adverse outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the China Welfare Institute International Peace Maternity and Child Health Hospital (No: (GKLW)2020-16). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LR: Investigation, Data curation, Validation, Writing – original draft, Methodology. JL: Validation, Software, Writing – original draft. HL: Supervision, Writing – review & editing. LX: Writing – review & editing. DY: Software, Writing – review & editing. WH: Writing – review & editing, Supervision. LC: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by the Shanghai Association of Rehabilitation Medicine under grant 2024JGYQ09. Open Research Fund of Shanghai Key Laboratory of Embryo Original Diseases [Grant No. SHELAB2024ZD03].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1660134/full#supplementary-material
References
1. Ruiz-Irastorza, G, Crowther, M, Branch, W, and Khamashta, MA. Antiphospholipid syndrome. Lancet. (2010) 376:1498–509. doi: 10.1016/S0140-6736(10)60709-X,
2. Miyakis, S, Lockshin, MD, Atsumi, T, Branch, DW, Brey, RL, and Cervera, R. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. (2006) 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x,
3. Alijotas-Reig, J, and Ferrer-Oliveras, RE.S. Grp. The European registry on obstetric antiphospholipid syndrome (EUROAPS): a preliminary first year report. Lupus. (2012) 21:766–8. doi: 10.1177/0961203312440058,
4. Out, HJ, Kooijman, CD, Bruinse, HW, and Derksen, RHWM. Histopathological findings in placentae from patients with intra-uterine fetal death and anti-phospholipid antibodies. Eur J Obstet Gynecol Reprod Biol. (1991) 41:179–86. doi: 10.1016/0028-2243(91)90021-C,
5. Agostinis, C, Biffi, S, Garrovo, C, Durigutto, P, Lorenzon, A, and Bek, A. In vivo distribution of beta2 glycoprotein I under various pathophysiologic conditions. Blood. (2011) 118:4231–8. doi: 10.1182/blood-2011-01-333617
6. Marder, W, Knight, JS, Kaplan, MJ, Somers, EC, Zhang, X, O'Dell, AA, et al. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med. (2016) 3:e000134. doi: 10.1136/lupus-2015-000134,
7. Gladigau, G, Haselmayer, P, Scharrer, I, Munder, M, Prinz, N, Lackner, K, et al. A role for toll-like receptor mediated signals in neutrophils in the pathogenesis of the anti-phospholipid syndrome. PLoS One. (2012) 7:e42176. doi: 10.1371/journal.pone.0042176,
8. Canaud, G, Bienaimé, F, Tabarin, F, Bataillon, G, Seilhean, D, Noël, LH, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. (2014) 371:303–12. doi: 10.1056/NEJMoa1312890,
9. Girardi, G, Yarilin, D, Thurman, JM, Holers, VM, and Salmon, JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. (2006) 203:2165–75. doi: 10.1084/jem.20061022,
10. Ye, SL, Gu, XK, Tao, LY, Cong, JM, and Wang, YQ. Efficacy of different treatment regimens for antiphospholipid syndrome-related recurrent spontaneous abortion. Chin Med J. (2017) 130:1395–9. doi: 10.4103/0366-6999.207471,
11. Hoppe, B, Burmester, GR, and Dorner, T. Heparin or aspirin or both in the treatment of recurrent abortions in women with antiphospholipid antibody (syndrome). Curr Opin Rheumatol. (2011) 23:299–304. doi: 10.1097/BOR.0b013e328344c3f7,
12. Alijotas-Reig, J, Esteve-Valverde, E, Ferrer-Oliveras, R, Sáez-Comet, L, Lefkou, E, Mekinian, A, et al. Comparative study of obstetric antiphospholipid syndrome (OAPS) and non-criteria obstetric APS (NC-OAPS): report of 1640 cases from the EUROAPS registry. Rheumatology. (2020) 59:1306–14. doi: 10.1093/rheumatology/kez419,
13. Barbhaiya, M, Zuily, S, Naden, R, Hendry, A, Manneville, F, Amigo, M‐C, et al. The 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol. (2023) 75:1687–702. doi: 10.1002/art.42624
14. Alijotas-Reig, J, Esteve-Valverde, E, Ferrer-Oliveras, R, Sáez-Comet, L, Lefkou, E, Mekinian, A, et al. The European registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev. (2019) 18:406–14. doi: 10.1016/j.autrev.2018.12.006,
15. Tektonidou, MG, Andreoli, L, Limper, M, Amoura, Z, Cervera, R, Costedoat-Chalumeau, N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. (2019) 78:1296–304. doi: 10.1136/annrheumdis-2019-215213,
16. Bouvier, S, Cochery-Nouvellon, E, Lavigne-Lissalde, G, Mercier, E, Marchetti, T, and Balducchi, JP. Comparative incidence of pregnancy outcomes in treated obstetric 388 antiphospholipid syndrome: the NOH-APS observational study. Blood. (2014) 123:404–13. doi: 10.1182/blood-2013-08-522623
17. Deng, T, Liao, X BS, and Zhu, S. Recent advances in treatment of recurrent spontaneous abortion. Obstet Gynecol Surv. (2022) 77:355–66. doi: 10.1097/OGX.0000000000001033,
18. Qi, X, Li, X, Han, Y, Lei, L, and Guo, H. Diagnostic value of blood coagulation index in obstetric antiphospholipid syndrome and its subtypes. Med Clin (Barc). (2025) 165:106997. doi: 10.1016/j.medcli.2025.106997,
19. Qiuling, X, and Hongli, K. Clinical efficacy of low molecular weight heparin combined with expectant therapy in the treatment of preeclampsia. Clin Res. (2023) 31:4.
20. Hirsh, J, Dalen, JE, Deykin, D, and Poller, L. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. (1992) 102:337S–51S. doi: 10.1378/chest.102.4_supplement.337s,
21. Castellanos Gutierrez, AS, Figueras, F, Espinosa, G, Youssef, L, Crispi, F, Santana, M, et al. Correlation of placental lesions in patients with systemic lupus erythematosus, antiphospholipid syndrome and non-criteria obstetric antiphospholipid syndrome and adverse perinatal outcomes. Placenta. (2023) 139:92–8. doi: 10.1016/j.placenta.2023.06.013,
22. Wenghui, S, Zi, Y, Hongxia, G, and Hailing, W. Effect of different intervention time and intervention method for preventing preterm birth in APS with pregnancy. Chin J Pract Gynecol Obstet. (2016) 32:973–8.
23. Sifeng, L, Hongliang, X, Dijin, G, Yufeng, Z, and Yanhui, Y. Efficacy and adverse effects of blue light irradiation supplemented by breastfeeding with breast milk fortification in the treatment of jaundice in preterm infants. Jilin Yixue. (2023) 44:44–7.
24. Raschi, E, Borghi, MO, Tedesco, F, and Meroni, PL. Antiphospholipid syndrome pathogenesis in 2023: an update of new mechanisms or just a reconsideration of the old ones? Rheumatology (Oxford). (2024) 63:Si4–si13. doi: 10.1093/rheumatology/kead603,
Keywords: obstetric antiphospholipid syndrome (OAPS), placental vasculopathy, non-canonical antiphospholipid antibodies, neonatal hyperbilirubinemia, pregnancy-related hypercoagulability, multidisciplinary perinatal management
Citation: Rao L, Lu J, Li H, Xu L, Yang D, Han W and Chen L (2025) Maternal and neonatal outcomes in obstetric antiphospholipid syndrome: a retrospective case-control study. Front. Med. 12:1660134. doi: 10.3389/fmed.2025.1660134
Edited by:
A. Seval Ozgu-Erdinc, University of Health Sciences Bilkent City Hospital, TürkiyeReviewed by:
Alban Deroux, Centre Hospitalier Universitaire de Grenoble, FranceAnca Huniadi, University of Oradea, Romania
Copyright © 2025 Rao, Lu, Li, Xu, Yang, Han and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wendong Han, aGFud2VuZG9uZ0BzanR1LmVkdS5jbg==; Li Chen, bGljaGVuX2JrQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Lin Rao1,2,3†
Lin Rao1,2,3† Jia Lu
Jia Lu Li Chen
Li Chen