- 1Department of Anesthesiology, Chengdu Wenjiang District People's Hospital, Chengdu, China
- 2School of Laboratory Medicine, North Sichuan Medical College, Nanchong, China
- 3Institute of Cardiovascular Diseases & Department of Cardiology, Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
High-flow nasal cannula (HFNC) oxygen therapy, a non-invasive respiratory support modality, has gained increasing attention for its advantages in perioperative care. This review outlines the basic components and key physiological effects of HFNC, including apneic oxygenation, positive end-expiratory pressure (PEEP), reduction of anatomical dead space, enhanced end-expiratory lung volume, accurate oxygen delivery, and active humidification. These mechanisms support its application across multiple perioperative phases, such as tracheal intubation, sedation for endoscopic procedures, upper airway surgeries, extubation, and recovery. HFNC has also shown promise in specific patient populations, including the obese, pregnant, and pediatric patients. Although its clinical benefits and safety profile are well-recognized, further studies are needed to clarify its indications, refine device settings, and explore its integration with other respiratory strategies. This review aims to summarize current clinical applications and recent developments of HFNC in anesthesia practice, providing both theoretical context and practical recommendations for its standardized use.
1 Introduction
HFNC therapy has gained prominence as a contemporary, non-invasive respiratory-support modality and is now embedded in routine critical-care and peri-operative practice. By delivering warmed, fully humidified gas at flow rates of 40–80 L min−1, HFNC maintains a constant, titratable fraction of inspired oxygen (FiO2) while generating a modest level of positive end-expiratory pressure (PEEP) (1, 2). In addition, the continuous flow flushes nasopharyngeal dead space, reduces work of breathing, and supports apnoeic oxygenation—the passive diffusion of oxygen in an apnoeic but patent airway—an effect of direct relevance to anesthetic induction and airway management (3–5). Compared with conventional low-flow oxygen interfaces such as standard nasal prongs and face masks, HFNC provides more reliable oxygen delivery, greater patient comfort, superior humidification, and an extended safe-apnoea window (6, 7). As a result, it has supplanted conventional oxygen therapy (COT) in many intensive-care settings, particularly for acute hypoxaemic respiratory failure, and is now advocated as first-line support in numerous guidelines (8–10). Peri-operatively, interest in HFNC has broadened rapidly. Reported benefits span the full anesthetic continuum: pre-oxygenation and induction of general anesthesia, management of the anticipated or unanticipated difficult airway, oxygenation during non-invasive endoscopic or airway procedures, facilitation of tubeless microlaryngeal surgery, support during emergence and extubation, and mitigation of early postoperative respiratory failure. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) refers to a specific clinical application of HFNC in which 100% oxygen is delivered at very high flow rates (often up to 90 L·min−1) to maximize apnoeic oxygenation during airway management. Although utilizing the same technology as HFNC, the term “THRIVE” emphasizes its strategic use in extending safe apnoea time and maintaining an unobstructed surgical field during upper-airway interventions (11, 12). Despite these advances, robust, standardized guidance for anesthetists on patient selection, FiO2/flow titration, monitoring of carbon-dioxide accumulation, and criteria for escalation or weaning remains limited. Practice therefore varies widely between centers. This narrative review (i) outlines the physiological mechanisms that underpin HFNC efficacy; (ii) collates and appraises contemporary evidence across core anesthetic milestones; and (iii) examines emerging data in special populations—including obese, obstetric, pediatric, and cardiac-surgical patients—where oxygen reserves are precarious and airway management challenging. By integrating mechanistic insight with clinical trial data, we aim to provide anesthesiologists with pragmatic, evidence-based recommendations to optimize peri-operative respiratory care and standardize HFNC use across diverse practice environments.
2 Structure and physiological effects of HFNC
2.1 Structural overview of HFNC
A standard HFNC set-up integrates four functional modules: (i) an air–oxygen blending unit, (ii) a heated humidifier, (iii) delivery circuits, and (iv) the nasal interface (13, 14). Air–oxygen blender. Ambient air and pressurized oxygen are mixed to the desired fraction of inspired oxygen before entering the turbine. FiO2 may be adjusted in two ways. The traditional float-type flowmeter alters oxygen flow and achieves an estimated FiO2 but lacks precise preset capability. Modern systems incorporate a micro-proportional valve coupled to an ultrasonic oxygen sensor, allowing programmable, closed-loop FiO2 control. Heated humidifier. The blended gas is conditioned to 31–37°C and fully saturated, protecting mucosal integrity and improving comfort. Gas-delivery circuit and cannula. Heated, humidified gas (8–80 L min−1, FiO2 0.21–1.00) is conveyed through insulated tubing to a soft, beveled nasal cannula secured with an adjustable head strap (14, 15). This configuration delivers a small level of positive end-expiratory pressure, maintains stable oxygen concentration, and enhances patient tolerance (Figure 1). Together, these components provide a high-flow, temperature- and humidity-controlled oxygen source that underpins the clinical advantages of HFNC therapy.
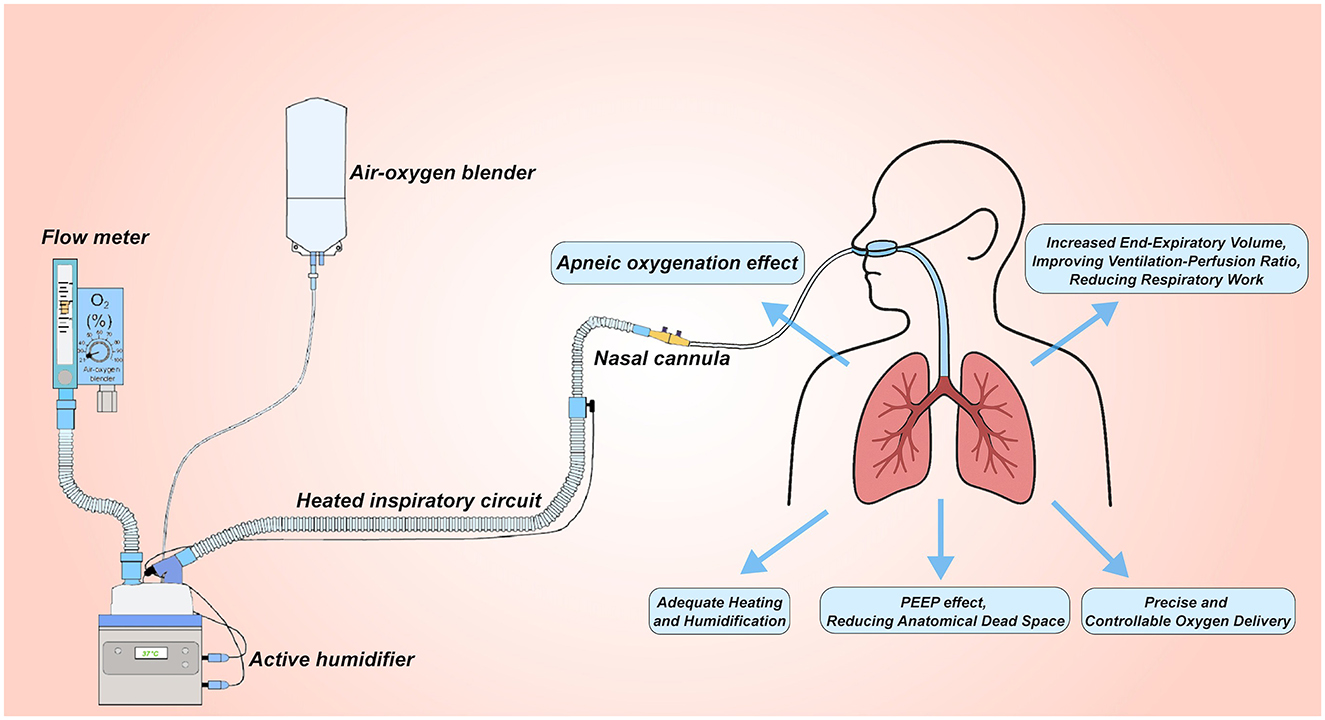
Figure 1. Setup for high-flow nasal cannula (HFNC) oxygen therapy. The system uses an air-oxygen blender to deliver controlled oxygen concentrations (FiO2) from 0.21 to 1.0 at up to 60 L/min flow. The gas is heated and humidified through an active humidifier, delivered via a heated inspiratory circuit, and administered through large-diameter nasal cannulas. This setup provides several benefits: (1) no-breathing oxygenation effect; (2) PEEP Effect, reducing anatomical dead space; (3) increased End-Expiratory Volume, improving ventilation-perfusion ratio and reducing respiratory work; (4) precise and controllable oxygen delivery; (5) adequate heating and humidification to optimize patient comfort and respiratory function.
2.2 The physiological effects of HFNC
2.2.1 Oxygenation effect without respiratory effort
HFNC therapy supplies a heated, humidified, high-flow oxygen mixture via a bespoke nasal interface, generating a modest but continuous positive airway pressure. The resulting alveolar–capillary oxygen gradient supports passive diffusion and sustains arterial oxygenation even in the absence of ventilatory effort (3). By prolonging the “safe apnoea” window—defined as the interval during which peripheral oxygen saturation (SpO2) remains ≥90% without ventilation (16)—HFNC has become an important adjunct during rapid-sequence induction, anticipated difficult airway management, and emergency intubation (4, 17). Patel et al. (18) applied THRIVE, a high-flow variant, in 25 patients with challenging airways undergoing oropharyngeal or laryngeal surgery. Median safe apnoea time reached 14 min (interquartile range, 9–19 min), illustrating HFNC's ability to maintain oxygenation while clinicians secure the airway.
2.2.2 PEEP effect and reduction of anatomical dead space
PEEP is the residual pressure maintained in the airway at end-expiration during mechanical ventilation. By preventing alveolar collapse, PEEP promotes recruitment of previously nonaerated lung units, thereby enhancing oxygenation and overall pulmonary mechanics (19, 20). Appropriately titrated PEEP helps counter atelectasis and hypoxaemia throughout anesthetic induction, intra-operative ventilation, and postoperative respiratory care (21–23). HFNC therapy produces a comparable pressure effect. In healthy volunteers, closed-mouth breathing with HFNC flow rates of 10, 20, 40, and 60 L min−1 generated mean airway pressures of 1.7, 2.9, 5.5, and 7.4 cm H2O, respectively—a near-linear flow–pressure relationship; opening the mouth lowered each value by roughly 1 cm H2O (24). Continuous high-flow insufflation also flushes the nasopharyngeal dead space, expelling retained CO2, limiting rebreathing, and raising the effective fraction of inspired oxygen. These combined effects improve alveolar ventilation and gas exchange, with efficacy proportional to both flow magnitude and duration of use (3, 25, 26).
2.2.3 Increase in end-expiratory volume, improvement of ventilation-perfusion ratio, and reduction of work of breathing (WOB)
WOB denotes the metabolic energy consumed by the respiratory muscles. Measures that lower breathing frequency and enhance thoraco-abdominal synchrony help curb this demand. The modest positive end-expiratory pressure generated by HFNC offsets intrinsic PEEP and recruits atelectatic units, thereby unloading inspiratory effort (27, 28). Concurrently, HFNC enlarges end-expiratory lung volume (EELV) and global lung impedance, the latter is used as a surrogate for end-expiratory lung volume (EELV), in mechanically ventilated patients, stepwise PEEP increase causes proportional increases in both EELV and end-expiratory lung impedance, with a demonstrated high linear correlation (R2 ≈ 0.95) (29). Thus, lung impedance provides a reliable bedside indicator of changes in lung volume (30). Electrical-impedance tomography confirmed flow-dependent rises in EELV in both supine and prone positions; the increase was evenly distributed prone but localized to dependent (ventral) zones supine (31). By recruiting alveoli, smoothing regional ventilation, and reducing inspiratory effort, HFNC enhances ventilation–perfusion matching. Compared with non-invasive ventilation or conventional oxygen therapy, it yields greater tidal volumes, higher dynamic compliance, and more complete re-expansion of collapsed lung (32, 33), while reducing airway resistance and inspiratory workload (32, 34). In a crossover study of 22 recently extubated ICU patients, 1 h of prophylactic HFNC lessened respiratory load, increased EELV, and improved PaO2/FiO2 vs. standard oxygen, without affecting hemodynamics or cardiac biomarkers (35). Collectively, these findings highlight HFNC's capacity to meaningfully reduce WOB and optimize gas exchange during the peri-extubation period.
2.2.4 Controlled and precise oxygen delivery
HFNC systems can provide up to 70–80 L min−1 of warmed, humidified gas, while an integrated air–oxygen blender allows fraction of inspired oxygen adjustment from 0.21 to 1.00, independent of flow setting (36). To avoid dilution by entrained room air, the delivered flow must at least match the patient's peak inspiratory demand (37). FiO2 accuracy also hinges on oral closure: nasal breathing with the mouth shut minimizes ambient mixing and preserves the prescribed concentration, whereas mouth opening lowers the effective FiO2 (38).
2.2.5 Adequate heating and humidification
Conventional oxygen devices delivering flows ≥ 6 L min−1 often desiccate and irritate the nasal mucosa, provoking discomfort and poor tolerance. Even brief exposure to inadequately humidified gas has been shown to impair airway-epithelial function (39). Optimal ciliary activity, which facilitates mucociliary clearance, requires an inspired-gas temperature of approximately 37°C (40). Contemporary HFNC systems incorporate active heating and humidification, supplying gas at up to 37°C with an absolute humidity of 44 mg H2O L−1 (100 % relative humidity). This conditioning maintains secretion mobility and supports efficient ciliary transport (41, 42), a particular advantage in patients with copious airway secretions where effective clearance is crucial (see Figure 1).
3 Clinical applications of HFNC in anesthesia
HFNC therapy is now used across a spectrum of peri-operative scenarios, including management of acute hypoxaemic respiratory failure, post-extubation support, pre-oxygenation before tracheal intubation or bronchoscopy, rescue of early postoperative respiratory compromise, and the intrahospital transfer of critically ill patients (43–45). HFNC is unsuitable when upper-airway obstruction or severe anatomical distortion precludes effective flow delivery, or in situations of life-threatening hypoxaemia, marked haemodynamic instability, facial or skull-base trauma, and untreated pneumothorax. Caution is also advised in patients with depressed consciousness, congenital cardiac shunts, acute bronchospasm, or chronic hypercapnic respiratory failure (14, 46, 47).
3.1 HFNC in tracheal intubation
Tracheal intubation is ubiquitous in anesthetic practice, yet the apnoea that accompanies induction eliminates spontaneous ventilation and jeopardizes continuous oxygen delivery. The danger is amplified when airway management proves difficult, as prolonged intubation attempts markedly heighten the risk of hypoxaemia. Although pre-oxygenation is routinely employed to buffer oxygen saturation (SpO2) during laryngoscopy, its efficacy is variable, and critically ill patients often fail to reach optimal end-tidal oxygen levels (≈ 90 %) with traditional techniques (48). HFNC therapy—capable of maintaining oxygenation throughout the apnoeic interval—therefore offers a compelling alternative to conventional pre-oxygenation strategies.
3.1.1 HFNC in preoxygenation
HFNC therapy delivers a continuous, heated, humidified oxygen stream at high flow, permitting apnoeic oxygenation as long as the airway remains patent. The sustained oxygen–carbon dioxide diffusion gradient allows alveolar O2 uptake to exceed the rate of progressive CO2 retention, thereby extending the “safe-apnoea” window and supporting oxygenation during intubation attempts (49). Traditional pre-oxygenation relies on a well-sealed face mask (FM) attached to the breathing circuit (flow > 6 L min−1) with either 3 min of quiet breathing or ≥ 4 vital-capacity breaths (50). Mask leak or poor tolerance, however, can undermine effectiveness and heighten the risk of peri-induction hypoxaemia. HFNC circumvents these limitations by supplying high-flow oxygen through nasal prongs, independent of mask seal. A meta-analysis of 14 randomized controlled trials (n = 1,012) showed that HFNC pre-oxygenation improved arterial PaO2 (mean difference ≈ 57 mm Hg) and lengthened safe-apnoea time (≈87 s) relative to FM, without altering minimum SpO2, PaCO2/EtCO2, end-tidal oxygen, or desaturation slope (51). In emergency intubations, 3 min of THRIVE (60 L min−1) similarly lowered hypoxaemia incidence in high-risk patients (52). Collectively, these data support using HFNC both for pre-oxygenation and continuous oxygen delivery throughout laryngoscopy, especially in anticipated difficult airways, thereby enhancing intubation safety (53).
3.1.2 HFNC in difficult airway management
Maintaining spontaneous ventilation during fiber-optic or video-assisted laryngoscopy is central to the management of anticipated difficult airways (42). A HFNC can deliver continuous, heated, humidified oxygen through the nares without obstructing the oral route, thereby sustaining arterial oxygenation while the bronchoscope or tracheal tube is advanced. This uninterrupted flow optimizes intubation conditions and may mitigate peri-procedural hypoxaemia. In 2015, Patel and Nouraei applied THRIVE in a cohort of high-risk difficult airway patients, achieving a median apnoea time of 14 min after induction without ventilation and without any occurrence of SpO2 < 90%, thereby establishing its physiological and clinical feasibility in this setting (18). Subsequently, a systematic review of 14 randomized controlled trials (n = 1,012) demonstrated that, compared with conventional facemask oxygenation, HFNC during anesthetic induction significantly increased PaO2 and prolonged the safe apnoea time by ~87 s (51). In intraoperative scenarios involving anticipated difficult airways, the PREOPTI-DAM randomized trial found no statistically significant difference between HFNC (4-min preoxygenation) and facemask oxygenation in the primary composite endpoint (SpO2 ≤ 94% or the need for facemask ventilation); however, patients reported better comfort, suggesting HFNC is at least non-inferior to facemask oxygenation while providing the added convenience of continuous oxygen delivery throughout the intubation process (54). In critically ill patients undergoing preoxygenation and intubation, multicentre ICU RCTs and meta-analyses have shown that HFNC may reduce the incidence of intubation-related adverse events and severe hypoxaemia, although improvements in nadir SpO2 vary depending on the patient population and baseline oxygenation status; combining HFNC with NIV may further enhance efficacy (55). For awake tracheal intubation (ATI), the main advantages of HFNC are its ability to maintain oxygenation without interrupting airway access: it does not obstruct oral or nasal routes, allowing adequate topical anesthesia and manipulation of fiber-optic bronchoscopes or video laryngoscopes while sustaining high SpO2 levels throughout the procedure (56, 57). Both retrospective and prospective studies have demonstrated that, compared with low-flow oxygen, HFNC during ATI improves the lowest SpO2 achieved and reduces the need for multiple intubation attempts. The 2022 ASA Difficult Airway Guidelines explicitly recommend continuous oxygen delivery—including via nasal routes—before, during, and after intubation, supporting its use in ATI (58). Practical recommendations include administering FiO2 1.0 at a flow rate ≥40–60 L/min for at least 3–4 min before induction, maintaining HFNC until the tracheal tube is secured, keeping the mouth closed and elevating the mandible to optimize pharyngeal pressure, and initiating awake-phase HFNC at 20–40 L/min for improved tolerance with subsequent titration as required. It should be noted that HFNC primarily addresses hypoxaemia but is ineffective for CO2 clearance; prolonged apnoea may lead to hypercapnia and acidosis. Therefore, the duration of apnoea should be limited, ventilation should be closely monitored, and the combination with NIV or other ventilation strategies should be considered on an individual basis (14).
3.1.3 HFNC in rapid sequence induction (RSI) for high-risk patients
RSI is often selected for patients with a full stomach, gastrointestinal obstruction, or other aspiration risks to minimize the interval between loss of protective reflexes and tracheal intubation. RSI aims to reduce aspiration risk by minimizing the time between loss of airway reflexes and securing the airway via tracheal tube placement (59). When applied during both pre-oxygenation and the subsequent apnoeic phase, HFNC therapy can enhance arterial oxygenation and lengthen the safe-apnoea window, although continuous flow may contribute to gastric insufflation and, theoretically, reflux. In older adults (≥65 yr) undergoing emergency laparotomy for bowel obstruction, pre-oxygenation with transnasal humidified rapid-insufflation ventilatory exchange (THRIVE: FiO2 1.0, 40 L min−1, closed mouth, 5 min) raised PaO2 to 446 ± 84 mm Hg vs. 262 ± 31 mm Hg with face-mask (FM) pre-oxygenation (FiO2 1.0, 6 L min−1) and doubled median safe-apnoea time (480 vs. 240 s) (60). Similar findings were reported by Mir et al. in 40 emergency surgical patients: THRIVE extended apnoea duration (248 ± 71 vs. 123 ± 55 s) without altering PaO2, PaCO2 or pH, and no participant in either group desaturated below SpO2 90% (61). In a prospective, unblinded randomized trial, minimum SpO2 1 min post-intubation was comparable between THRIVE and FM cohorts; however, 18% of FM patients fell below SpO2 96%, whereas all THRIVE recipients remained ≥ 96% (62).
In conclusion, HFNC can serve as an effective preoxygenation method before tracheal intubation and provide continuous oxygenation support during the intubation process, particularly in patients with difficult airways. It demonstrates significant advantages in prolonging the safe apnea time, optimizing intubation conditions, and reducing the incidence of hypoxemia.
3.2 Application of HFNC in painless endoscopic procedures
Sedation, partial airway obstruction and suppression of respiratory drive predispose patients to peri-procedural hypoxaemia, with oxygen saturation frequently falling below 90% despite supplemental oxygen (63, 64). Non-invasive ventilation can mitigate this risk, yet poor tolerance of a tight face-mask and technical difficulty introducing the endoscope limit its routine use (65). By contrast, HFNC therapy leaves the oral route unobstructed, generates low-level positive end-expiratory pressure and improves alveolar ventilation (25), making it an attractive alternative during painless endoscopy. In elderly patients (≥65 yr) undergoing sedated gastroscopy, HFNC set at 60 L min−1 (FiO2 0.6) reduced hypoxaemia from 22.6 to 3.2% compared with a conventional nasal cannula delivering 6 L min−1 (Figure 2A) (66). A larger, multicentre trial of nearly 2 000 ASA I–II patients confirmed these findings: HFNC (30–60 L min−1) abolished moderate and severe desaturation events that occurred with low-flow oxygen (67). A meta-analysis encompassing 2,998 subjects likewise demonstrated consistent reductions in hypoxaemia, severe desaturation and procedure interruptions across all endoscopy types and age groups (68). Evidence in bronchoscopy mirrors these benefits. During broncho-alveolar lavage, HFNC maintained PaO2, preserved end-expiratory lung impedance and lowered hypoxaemic episodes (11 vs. 56%) relative to standard oxygen therapy in a randomized study of out-patients (69). In a larger trial of 600 patients with chronic obstructive pulmonary disease, high-flow oxygen (60–80 L min−1, FiO2 0.6–0.8) halved both cumulative hypoxaemia time and the number of desaturation events vs. low-flow therapy, without compromising comfort (odds ratio 5.1; 95 % CI 3.2–8.2; p < 0.001) (70). Collectively, these studies highlight the substantial potential of HFNC in painless endoscopic procedures. However, several challenges remain. Firstly, there is currently no standardized recommendation for optimal flow settings in different procedural contexts. Secondly, large-scale, multicenter trials are needed to further validate the long-term clinical benefits of HFNC. With continued technological advancement and accumulating evidence, HFNC is poised to become a standardized oxygenation modality in painless endoscopy.
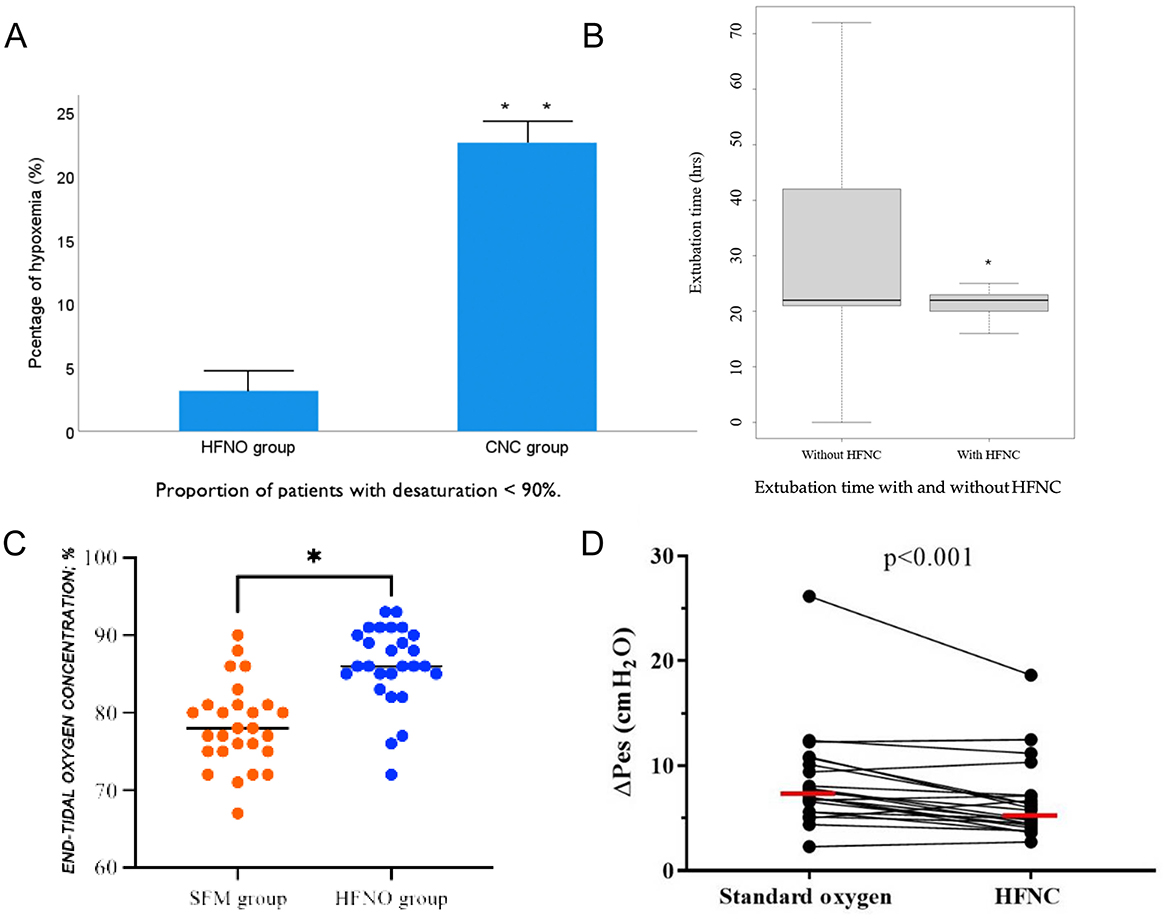
Figure 2. (A) HFNO significantly reduces the risk of post-extubation hypoxemia, especially in patients sensitive to oxygen levels (66). (B) HFNO significantly shortens extubation time and results in more consistent and stable extubation durations (75). (C) HFNO significantly increases end-tidal oxygen concentration (EtO2), reducing the risk of hypoxemia in high-risk parturients (90). (D) HFNC oxygen therapy after extubation reduces the work of breathing, suggesting advantages in lowering metabolic load and respiratory effort. The horizontal red line represents the median (35). *Indicates statistical significance (*p < 0.05, **p < 0.01).
3.3 Application of HFNC in patients undergoing laryngeal surgery
The role of HFNC therapy during laryngeal procedures is under active evaluation. In cases that require a shared airway—such as vocal-cord biopsy, tracheoscopy and dilation of subglottic stenosis—HFNC may obviate the need for tracheal intubation or jet ventilation and extend the apnoeic oxygenation window (71, 72). In 19 ASA I–II adults (body-mass index < 30 kg m−2) undergoing microlaryngoscopic resection of vocal-cord lesions, HFNC alone (60 L min−1; FiO2 0.95) provided a mean apnoea time of 21.5 min (maximum 35 min). Peripheral oxygen saturation remained ≥ 90% in 18 patients; one transiently fell to 88%. Arterial carbon-dioxide tension rose linearly (mean 1.68 ± 0.12 mm Hg min−1) to a peak of 79.4 mmHg (73). A separate randomized study of ASA I–II patients undergoing short laryngeal procedures used transnasal humidified rapid-insufflation ventilatory exchange (THRIVE: 100% O2, 40–70 L min−1). Mean safe apnoea time was 22.5 min, with no patient desaturating below 91%. PaCO2 and end-tidal CO2 increased by 0.24 and 0.12 kPa min−1, respectively—slower than the rise expected during conventional apnoea (71). These findings suggest that, in non-obese patients without major co-morbidities, THRIVE can maintain oxygenation for up to 30 min during laryngeal surgery. Nevertheless, vigilant monitoring of PaCO2 and arterial pH is advisable to mitigate hypercapnia and acidaemia.
3.4 Application of HFNC in extubation
Post-extubation complications such as airway obstruction, respiratory distress, and hypoxemia are associated with increased pulmonary infection risk and prolonged hospitalization (74). HFNC therapy has been shown to reduce the need for reintubation in both postoperative and medically complex patient cohorts. HFNC offers continuous oxygen delivery, increases end-expiratory lung volume, improves alveolar ventilation and lung compliance, and promotes re-expansion of collapsed alveoli. These physiological effects optimize ventilation–perfusion matching and enhance oxygenation (25). Additionally, active warming and humidification preserve mucosal integrity and facilitate secretion clearance by enhancing ciliary function. In patients with obstructive sleep apnea (OSA), HFNC—with or without an airway exchange catheter (AEC)—was associated with shorter time to extubation and fewer hypoxemic episodes, without major adverse events (75) (Figure 2B). A multicenter randomized controlled trial comparing HFNC (50 L·min−1, FiO2 0.5) with bilevel positive airway pressure (BiPAP: pressure support 8 cm H2O, PEEP 4 cm H2O, FiO2 0.5, ≥4 h/day) in 830 patients with acute respiratory failure after cardiothoracic surgery found no significant differences in treatment failure or ICU mortality between groups, supporting HFNC as a non-inferior and better-tolerated alternative (Figure 2D) (76). A meta-analysis of 11 RCTs involving 2,201 patients further demonstrated that HFNC initiated immediately postoperatively significantly reduced reintubation rates and the need for escalation of respiratory support compared to COT, particularly in high-risk and obese patients (77). Another RCT showed that early application of HFNC after extubation led to better oxygenation, improved patient comfort, and fewer pressure-related mucosal injuries compared to standard face mask oxygen therapy (78). Collectively, these findings support HFNC as a safe and effective post-extubation strategy. Its clinical benefits—comparable to those of non-invasive ventilation (NIV)—include reduced respiratory effort, improved oxygenation, and enhanced patient tolerance, particularly in high-risk populations such as those undergoing cardiothoracic surgery. In appropriately selected patients, HFNC may be considered a more comfortable and equally effective alternative to facemask NIV, although evidence regarding helmet NIV is still lacking.
4 Application of HFNC in anesthesia for special populations
4.1 Application of HFNC in anesthesia for obese patients
Obesity is a well-recognized risk factor for perioperative hypoxemia and difficult airway management, attributed to anatomical and physiological alterations such as excessive adipose deposition, diaphragmatic elevation, reduced lung compliance, and decreased functional residual capacity (79). In patients with morbid obesity—particularly those with obstructive sleep apnoea–hypopnoea syndrome (OSAHS)—NIV, including CPAP or BiPAP, is widely regarded as the most effective strategy for optimizing pre-oxygenation and prolonging safe apnoea time (80). Nevertheless, HFNC therapy offers rapid and sustained oxygen delivery, augments oxygen reserves, and extends the safe apnoea window, thereby lowering the risk of peri-intubation and intraoperative desaturation in selected patients, especially when NIV is not feasible or poorly tolerated (81). A 2022 meta-analysis by Zhou et al. included 12 randomized controlled trials (RCTs) involving 798 obese patients and found that, compared to COT, HFNC significantly reduced the incidence of hypoxemia, increased lowest SpO2 values, decreased the need for additional respiratory support, and shortened hospital stay (82). Another meta-analysis of six RCTs (n = 351) comparing HFNC and face-mask (FM) oxygenation during general anesthesia found no significant differences in the occurrence of pre-intubation SpO2 < 92% or minimum SpO2; however, HFNC notably prolonged the duration of safe apnoea (83). In a cohort of 25 obese patients (BMI > 30 kg m−2) with difficult airways undergoing upper airway surgery, the use of transnasal THRIVE extended the mean apnoea time to 14 min, offering clinicians additional time for airway intervention (18). A separate case report described the use of HFNC in a morbidly obese patient (BMI 90 kg m−2) with COVID-19 pneumonia and obstructive sleep apnea. The patient, classified as ASA IV-E, underwent emergency laparotomy after preoxygenation with HFNC, which facilitated successful induction and intubation; alternating HFNC and BiPAP in the ICU subsequently maintained SpO2 at 91–92% (FiO2 0.6) (84). Further evidence from bariatric surgery populations demonstrates that HFNC enhances arterial oxygenation during preoxygenation. Compared to FM and continuous positive airway pressure (CPAP), HFNC achieved higher PaO2 values after 3 min of preoxygenation. Prolonging the preoxygenation period beyond 5 min did not result in further PaO2 gains (85).
In summary, HFNC provides distinct advantages in the perioperative care of obese patients—including improved pre-intubation oxygenation, reduced reintubation risk, extended apnoea time, and greater patient tolerance—supporting its role as a preferred oxygenation strategy in this high-risk population.
4.2 Application of HFNC in obstetric anesthesia
Pregnancy-associated reductions in functional residual capacity, coupled with heightened oxygen consumption, place parturients at particular risk of rapid desaturation and difficult airway management. During rapid-sequence induction (RSI), up to 17% of women experience SpO2 < 90% (86). Maternal hypoxaemia jeopardizes fetal oxygenation and may precipitate acidaemia, underscoring the need for prompt, reliable oxygen delivery throughout induction and intubation (87, 88). HFNC provides a continuous, heated, humidified oxygen stream that augments alveolar ventilation, improves lung compliance, and expands oxygen reserves during induction, laryngoscopy, and extubation. Its safety in pregnant patients with acute respiratory failure has been confirmed (89). In a prospective, dual-center trial of parturients (BMI > 30 kg m−2) undergoing cesarean delivery under general anesthesia, HFNC significantly increased oxygen reserve and extended safe-apnoea time, thereby reducing peri-intubation hypoxaemia (Figure 2C) (90). Comparative data further support these advantages. In term pregnancies, 3 min of HFNC at 70 L min−1 achieved an end-tidal oxygen concentration ≥ 90 % in 71 % of subjects vs. 44 % with face-mask (FM) oxygenation at 10 L min−1 (91). Similarly, HFNC during RSI for cesarean section improved immediate post-intubation PaO2 relative to FM, without affecting nadir SpO2 or neonatal outcomes (92). Collectively, these studies indicate that HFNC is a safe, effective, and aspiration-neutral technique for obstetric airway management, offering superior oxygenation and a longer margin of safety compared with conventional methods.
4.3 Application of HFNC in pediatric anesthesia
HFNC therapy has gained widespread use in children and neonates due to its ease of setup, superior comfort, and non-invasive delivery profile (93). Flow rates in pediatric HFNC are typically weight- and age-adjusted (94). Compared with adults, children have lower functional oxygen reserves and higher metabolic oxygen demands, placing them at greater risk of hypoxemia during sedation, airway instrumentation, or apnea. HFNC enhances oxygenation, improves ventilation efficiency, and extends safe apneic duration, making it increasingly valuable in pediatric anesthesia for difficult airway management, post-extubation oxygen therapy, and sedation procedures (4). In a single-center randomized controlled trial (RCT), 120 children (aged 2–7 years, 10–30 kg) undergoing outpatient oral surgery under deep sedation were randomized to receive either HFNC with propofol intravenous anesthesia (HFNC-IV) or laryngeal mask airway with propofol (LMA-IV) (95). HFNC was administered at 2 L· kg−1·min−1. Results showed comparable oxygenation (SpO2 > 97%) and ventilation (TcCO2 mean difference = −1.4 mmHg) between groups, with better surgical field visibility in the HFNC group and no significant differences in adverse event rates. This study supports HFNC as an effective alternative to LMA in pediatric sedation, offering adequate oxygenation and superior procedural access under conditions of spontaneous ventilation. Another RCT evaluated THRIVE in 48 healthy children (0–10 years) undergoing elective procedures under general anesthesia with neuromuscular blockade (96). HFNC flow rates were stratified by weight (5–15 kg: 2 L·kg−1·min−1; 15–30 kg: 35 L·min−1; 30–50 kg: 40 L·min−1; >50 kg: 50 L·min−1). Compared to controls, THRIVE significantly prolonged safe apnea time (by 80–170 s across weight groups) and maintained SpO2 at 99.6%, with no difference in CO2 accumulation rates (2.4 mmHg·min−1). These findings highlight THRIVE's ability to extend apneic tolerance in healthy pediatric patients, though it does not enhance CO2 clearance. In a separate study of preterm infants undergoing glottic stenosis dilation under general anesthesia, HFNC was shown to extend apneic time, providing a valuable oxygenation bridge during complex neonatal airway procedures (97). However, findings are not universally positive. A non-blinded, single-center RCT comparing HFNC with COT during pediatric upper gastrointestinal endoscopy reported no significant improvements in respiratory stability, including hypoxemia, hypercapnia, or apnea (98). Taken together, the available evidence suggests that HFNC and THRIVE offer safe and effective oxygenation support in pediatric and neonatal populations, especially in procedures requiring spontaneous ventilation or shared airway access. However, clinical benefits may vary by procedure type and patient risk profile, warranting further research in specific pediatric subgroups.
HFNC therapy is gaining traction as a non-invasive respiratory support strategy after pediatric cardiac procedures, where rapid desaturation and increased work of breathing are common. Testa et al. (99) observed that, within 48 h of extubation, HFNC did not alter PaCO2 relative to COT but significantly improved PaO2 and PaO2/FiO2 ratios. In ten infants studied by Itagaki and colleagues (median age 7 months), a flow of 2 L kg−1 min−1—though not 1 L kg−1 min−1—reduced respiratory rate, minute ventilation and thoraco-abdominal asynchrony, indicating lower inspiratory workload (100). During catheter-based interventions in 200 children with non-cyanotic congenital heart disease, weight-adjusted HFNC (2 or 35–50 L min−1; FiO2 0.40) raised the minimum SpO2 and curtailed rescue ventilation needs compared with a 5 L min−1 face-mask; hypoxaemia occurred in 7.1% of controls and in none of the HFNC group, without extra gastric or haemodynamic complications (101). Post-extubation, Kumar et al. (102) reported superior oxygenation with HFNC vs. non-invasive ventilation, with similar re-intubation rates and CO2 clearance. More recently, early HFNC use after cardiopulmonary bypass was associated with less atelectasis and fewer early re-intubations (103). Collectively, these findings suggest HFNC can enhance oxygenation, reduce respiratory effort, and provide well-tolerated support in children with congenital heart disease, although careful haemodynamic monitoring remains prudent in complex cardiac physiology.
5 Contraindications to HFNC therapy
Although HFNC therapy offers multiple clinical advantages, its use must be carefully considered in specific scenarios due to established contraindications. Absolute contraindications include complete upper airway obstruction, basilar skull or nasal bone fractures, and patient refusal—primarily reflecting anatomical limitations or ethical concerns. Relative contraindications encompass poor patient cooperation, procedures involving electrosurgical or laser use in the airway, elevated aspiration risk, and significant carbon dioxide retention. In these cases, individualized risk-benefit assessments are essential. Its use is limited in the settings outlined below (Tables 1, 2).
These contraindications reflect the underlying physiology and safety profile of HFNC. Absolute contraindications involve fixed anatomical or ethical barriers, whereas relative contraindications require individual risk–benefit assessment, taking into account surgical environment, aspiration risk, and the device's capacity for CO2 elimination.
6 Conclusion and outlook
HFNC oxygen therapy, as an emerging non-invasive respiratory support modality, has gained widespread clinical application owing to its advantages in improving oxygenation, reducing the WOB, and enhancing patient tolerance. Its efficacy is particularly notable in the management of mild to moderate hypoxemic respiratory failure. In anesthetic practice, HFNC offers a promising oxygenation support strategy during critical perioperative stages, such as difficult airway management, non-invasive airway interventions, postoperative recovery, and anesthesia for special populations, demonstrating strong clinical potential. However, the standardized use of HFNC still faces several challenges. There remains a lack of consensus and robust evidence regarding its indications, optimal parameter settings, and management strategies for high-risk populations. In addition, device capabilities require further refinement, particularly in areas such as pressure monitoring, automatic regulation, and integration with other respiratory support systems. Future research should focus on large-scale, prospective, multicenter clinical trials to clarify the clinical value of HFNC in various intraoperative and postoperative scenarios. At the same time, technological innovation should be pursued to improve device intelligence and system integration. In conclusion, as a bridging technology between COT and NIV, HFNC holds significant promise for optimizing perioperative respiratory management and improving patient outcomes, with substantial value for future research and clinical application.
Author contributions
HL: Writing – original draft, Writing – review & editing. PQ: Data curation, Methodology, Writing – review & editing. QL: Supervision, Writing – review & editing. FX: Methodology, Writing – review & editing. YY: Funding acquisition, Writing – review & editing. LX: Funding acquisition, Resources, Visualization, Writing – review & editing. HZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Health Commission of Sichuan Province Medical Science and Technology Program (24QNMP067), the Medical Research Subject of Chengdu Health Commission (2024083), and the Medical Research Project of Sichuan Medical Association (S2024086).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li J, Albuainain FA, Tan W, Scott JB, Roca O, Mauri T, et al. The effects of flow settings during high-flow nasal cannula support for adult subjects: a systematic review. Crit Care. (2023) 27:78. doi: 10.1186/s13054-023-04361-5
2. Huh JW, Seo WJ, Ahn JH, Lee SY, Suh HJ, Seo GJ, et al. Physiological effects of bi-level high-flow nasal cannula in healthy individuals: a proof of concept trial. Front Med. (2025) 12:1538832. doi: 10.3389/fmed.2025.1538832
3. Masclans JR, Pérez-Terán P, Roca O. The role of high flow oxygen therapy in acute respiratory failure. Med Intens. (2015) 39:505–15. doi: 10.1016/j.medine.2015.05.004
4. Kleine-Brueggeney M, Grosshauser M, Greif R. Apneic oxygenation in pediatric anesthesia. Curr Opin Anaesthesiol. (2022) 35:361–6. doi: 10.1097/ACO.0000000000001127
5. Fayed M, Maroun W, Patel N, Galusca D. Apneic oxygenation: a summarized review and stepwise approach. Cureus. (2023) 15:e50916. doi: 10.7759/cureus.50916
6. Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M, et al. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. (2008) 63:938–40. doi: 10.1111/j.1365-2044.2008.05536.x
7. Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. (2005) 50:604–9.
8. Pitre T, Zeraatkar D, Kachkovski GV, Leung G, Shligold E, Dowhanik S, et al. Noninvasive oxygenation strategies in adult patients with acute hypoxemic respiratory failure: a systematic review and network meta-analysis. Chest. (2023) 164:913–28. doi: 10.1016/j.chest.2023.04.022
9. Lewis SR, Baker PE, Parker R, Smith AF. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochr Database Syst Rev. (2021) 3:Cd010172. doi: 10.1002/14651858.CD010172.pub3
10. Venanzi A, Di Filippo P, Santagata C, Di Pillo S, Chiarelli F, Attanasi M. Heated humidified high-flow nasal cannula in children: state of the art. Biomedicines. (2022) 10:2353. doi: 10.3390/biomedicines10102353
11. Burra V, Putta G, Prasad SR, Manjunath V. A prospective study on use of thrive (transnasal humidified rapid insufflation ventilatory exchange) versus conventional nasal oxygenation following extubation of adult cardiac surgical patients. Ann Card Anaesth. (2021) 24:353–7. doi: 10.4103/aca.ACA_16_20
12. Toyota Y, Kondo T, Narasaki S, Miyoshi H, Tsutsumi YM. Prevention of oxygen desaturation in a patient with previous experience of severe hypoxia in modified electroconvulsive therapy by transnasal humidified rapid-insufflation ventilator exchange: a case report. Cureus. (2024) 16:e60564. doi: 10.7759/cureus.60564
13. Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. (2019) 64:735–42. doi: 10.4187/respcare.06718
14. Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intens Care. (2015) 3:15. doi: 10.1186/s40560-015-0084-5
15. Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. (2016) 61:529–41. doi: 10.4187/respcare.04577
16. Chua MT, Ng WM, Lu Q, Low MJW, Punyadasa A, Cove ME, et al. Pre- and apnoeic high-flow oxygenation for rapid sequence intubation in the emergency department (the Pre-AeRATE trial): a multicentre randomised controlled trial. Ann Acad Med Singapore. (2022) 51:149–60. doi: 10.47102/annals-acadmedsg.2021407
17. Sekhar P, Thiruvenkatarajan V, Sekhar V, van Wijk R, Barker T. Effectiveness of high-flow nasal cannula oxygen in apneic oxygenation during intubation in high-risk surgical patients: a systematic review protocol. JBI Evid Synth. (2023) 21:1896–902. doi: 10.11124/JBIES-22-00366
18. Patel A, Nouraei SA. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. (2015) 70:323–9. doi: 10.1111/anae.12923
19. Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, et al. Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med. (2003) 167:1620–6. doi: 10.1164/rccm.200205-435OC
20. Mols G, Priebe HJ, Guttmann J. Alveolar recruitment in acute lung injury. Br J Anaesth. (2006) 96:156–66. doi: 10.1093/bja/aei299
21. Covotta M, Claroni C, Torregiani G, Menga LS, Venti E, Gazzè G, et al. Recruitment-to-inflation ratio to assess response to PEEP during laparoscopic surgery: A physiologic study. J Clin Anesth. (2024) 98:111569. doi: 10.1016/j.jclinane.2024.111569
22. Treschan TA, Schaefer M, Kemper J, Bastin B, Kienbaum P, Pannen B, et al. Ventilation with high versus low peep levels during general anaesthesia for open abdominal surgery does not affect postoperative spirometry: a randomised clinical trial. Eur J Anaesthesiol. (2017) 34:534–43. doi: 10.1097/EJA.0000000000000626
23. Chiumello D, Coppola S, Fratti I, Leone M, Pastene B. Ventilation strategy during urological and gynaecological robotic-assisted surgery: a narrative review. Br J Anaesth. (2023) 131:764–74. doi: 10.1016/j.bja.2023.06.066
24. Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. (2007) 20:126–31. doi: 10.1016/j.aucc.2007.08.001
25. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. (2018) 15:145–55. doi: 10.1513/AnnalsATS.201707-548FR
26. Möller W, Feng S, Domanski U, Franke KJ, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol. (2017) 122:191–7. doi: 10.1152/japplphysiol.00584.2016
27. Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch Dis Childh Fetal Neonat Edi. (2014) 99:F315–320. doi: 10.1136/archdischild-2013-305855
28. Pham TM, O'Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. (2015) 50:713–20. doi: 10.1002/ppul.23060
29. Hinz J, Hahn G, Neumann P, Sydow M, Mohrenweiser P, Hellige G, et al. End-expiratory lung impedance change enables bedside monitoring of end-expiratory lung volume change. Intensive Care Med. (2003) 29:37–43. doi: 10.1007/s00134-002-1555-4
30. Yildirim S, Saygili SM, Süneçli O, Kirakli C. Comparison of the effects of open and closed aspiration on end-expiratory lung volume in acute respiratory distress syndrome. Korean J Anesthesiol. (2024) 77:115–21. doi: 10.4097/kja.23194
31. Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J, et al. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. (2013) 58:589–96. doi: 10.4187/respcare.02086
32. Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. (2017) 195:1207–15. doi: 10.1164/rccm.201605-0916OC
33. Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med. (2012) 40:1578–85. doi: 10.1097/CCM.0b013e3182451c40
34. Delorme M, Bouchard PA, Simon M, Simard S, Lellouche F. Effects of high-flow nasal cannula on the work of breathing in patients recovering from acute respiratory failure. Crit Care Med. (2017) 45:1981–8. doi: 10.1097/CCM.0000000000002693
35. Basoalto R, Damiani LF, Jalil Y, Bachmann MC, Oviedo V, Alegría L, et al. Physiological effects of high-flow nasal cannula oxygen therapy after extubation: a randomized crossover study. Ann Intens Care. (2023) 13:104. doi: 10.1186/s13613-023-01203-z
36. Grensemann J, Simon M, Wachs C, Kluge S. High-flow oxygen therapy-chances and risks. Der Pneumologe. (2022) 19:21–6. doi: 10.1007/s10405-021-00415-z
37. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. (2010) 55:408–13.
38. McCollum ED, Mvalo T. Bubble continuous positive airway pressure for children with pneumonia and hypoxaemia in Ethiopia. Lancet Global Health. (2024) 12:e721–2. doi: 10.1016/S2214-109X(24)00099-8
39. Kilgour E, Rankin N, Ryan S, Pack R. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. (2004) 30:1491–4. doi: 10.1007/s00134-004-2235-3
40. Chidekel A, Zhu Y, Wang J, Mosko JJ, Rodriguez E, Shaffer TH, et al. The effects of gas humidification with high-flow nasal cannula on cultured human airway epithelial cells. Pulm Med. (2012) 2012:380686. doi: 10.1155/2012/380686
41. Vitaliti G, Vitaliti MC, Finocchiaro MC, Stefano VA, Di Pavone P, Matin N, et al. Randomized comparison of helmet CPAP versus high-flow nasal cannula oxygen in pediatric respiratory distress. Respir Care. (2017) 62:1036–42. doi: 10.4187/respcare.05384
42. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG, et al. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. (2017) 189:66–71.e63. doi: 10.1016/j.jpeds.2017.06.006
43. Lodeserto FJ, Lettich TM, Rezaie SR. High-flow nasal cannula: mechanisms of action and adult and pediatric indications. Cureus. (2018) 10:e3639. doi: 10.7759/cureus.3639
44. Mikalsen IB, Davis P, Øymar K. High flow nasal cannula in children: a literature review. Scand J Trauma Resusc Emerg Med. (2016) 24:93. doi: 10.1186/s13049-016-0278-4
45. Ischaki E, Pantazopoulos I, Zakynthinos S. Nasal high flow therapy: a novel treatment rather than a more expensive oxygen device. Eur Respir Rev. (2017) 26:2017. doi: 10.1183/16000617.0028-2017
46. Mukherjee D, Mukherjee R. High-flow nasal cannula oxygen therapy in the management of respiratory failure: a review. Cureus. (2023) 15:e50738. doi: 10.7759/cureus.50738
47. Kwon JW. High-flow nasal cannula oxygen therapy in children: a clinical review. Clin Exp Pediatr. (2020) 63:3–7. doi: 10.3345/kjp.2019.00626
48. Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. (2005) 33:2672–5. doi: 10.1097/01.CCM.0000187131.67594.9E
49. Zhang J, Lin L, Pan K, Zhou J, Huang X. High-flow nasal cannula therapy for adult patients. J Int Med Res. (2016) 44:1200–11. doi: 10.1177/0300060516664621
50. Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesth Analg. (2017) 124:507–17. doi: 10.1213/ANE.0000000000001589
51. Song JL, Sun Y, Shi YB, Liu XY, Su ZB. Comparison of the effectiveness of high-flow nasal oxygen vs. standard facemask oxygenation for pre- and apneic oxygenation during anesthesia induction: a systematic review and meta-analysis. BMC Anesthesiol. (2022) 22:100. doi: 10.1186/s12871-022-01615-7
52. Doyle AJ, Stolady D, Mariyaselvam M, Wijewardena G, Gent E, Blunt M, et al. Preoxygenation and apneic oxygenation using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange for emergency intubation. J Crit Care. (2016) 36:8–12. doi: 10.1016/j.jcrc.2016.06.011
53. Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. (2015) 115:827–48. doi: 10.1093/bja/aev371
54. Vourc'h M, Huard D, Le Penndu M, Deransy R, Surbled M, Malidin M, et al. Asehnoune. High-flow oxygen therapy versus facemask preoxygenation in anticipated difficult airway management (PREOPTI-DAM): an open-label, single-centre, randomised controlled phase 3 trial. EClinicalMedicine. (2023) 60:101998. doi: 10.1016/j.eclinm.2023.101998
55. Cabrini L, Landoni G, Baiardo Redaelli M, Saleh O, Votta CD, Fominskiy E, et al. Tracheal intubation in critically ill patients: a comprehensive systematic review of randomized trials. Crit Care. (2018) 22:6. doi: 10.1186/s13054-017-1927-3
56. Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth. (2015) 115:629–32. doi: 10.1093/bja/aev262
57. Kim HJ, Kim MS, Kim SY, Min IK, Park WK, Song SH, et al. A propensity score-adjusted analysis of efficacy of high-flow nasal oxygen during awake tracheal intubation. Sci Rep. (2022) 12:11306. doi: 10.1038/s41598-022-15608-6
58. Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American Society of anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. (2022) 136:31–81. doi: 10.1097/ALN.0000000000004002
59. Collins J, O'Sullivan E. Rapid sequence induction and intubation. BJA Educ. (2022) 22:484–90. doi: 10.1016/j.bjae.2022.09.001
60. Wang W, Zhang W, Lu Y, Xu Y, Zhang Y, Shi H, et al. Comparison of the effectiveness of transnasal humidified rapid insufflation ventilator exchange (THRIVE) with facemask pre-oxygenation in 40 patients ≥65 years of age undergoing general anaesthesia during gastrointestinal surgery for intestinal obstruction. Med Sci Monit. (2022) 28:e938168. doi: 10.12659/MSM.938168
61. Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. (2017) 72:439–43. doi: 10.1111/anae.13799
62. Lodenius Å, Piehl J, Östlund A, Ullman J, Jonsson Fagerlund M. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. (2018) 73:564–71. doi: 10.1111/anae.14215
63. Matsushima Y, Jones R, King E, Moysa G, Alton J. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. (1984) 86:184–8. doi: 10.1378/chest.86.2.184
64. Antonelli M, Conti G, Rocco M, Arcangeli A, Cavaliere F, Proietti R, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest. (2002) 121:1149–54. doi: 10.1378/chest.121.4.1149
65. Maitre B, Jaber S, Maggiore SM, Bergot E, Richard JC, Bakthiari H, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients: a randomized double-blind study using a new device. Am J Respir Crit Care Med. (2000) 162:1063–7. doi: 10.1164/ajrccm.162.3.9910117
66. Yin X, Xu W, Zhang J, Wang M, Chen Z, Liu S, et al. High-flow nasal oxygen versus conventional nasal cannula in preventing hypoxemia in elderly patients undergoing gastroscopy with sedation: a randomized controlled trial. Int J Med Sci. (2024) 21:914–20. doi: 10.7150/ijms.91607
67. Lin Y, Zhang X, Li L, Wei M, Zhao B, Wang X, et al. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointest Endosc. (2019) 90:591–601. doi: 10.1016/j.gie.2019.06.033
68. Hung KC, Chang YJ, Chen IW, Soong TC, Ho CN, Hsing CH, et al. Efficacy of high flow nasal oxygenation against hypoxemia in sedated patients receiving gastrointestinal endoscopic procedures: a systematic review and meta-analysis. J Clin Anesth. (2022) 77:110651. doi: 10.1016/j.jclinane.2022.110651
69. Longhini F, Pelaia C, Garofalo E, Bruni A, Placida R, Iaquinta C, et al. High-flow nasal cannula oxygen therapy for outpatients undergoing flexible bronchoscopy: a randomised controlled trial. Thorax. (2022) 77:58–64. doi: 10.1136/thoraxjnl-2021-217116
70. Darie AM, Grize L, Jahn K, Salina A, Röcken J, Herrmann MJ, et al. Preventing oxygen desaturation during bronchoscopy in COPD patients using high-flow oxygen versus standard management: the randomised controlled PROSA 2 trial. Eur Respir J. (2025) 65:1568. doi: 10.1183/13993003.01586-2024
71. Gustafsson IM, Lodenius Å, Tunelli J, Ullman J, Jonsson Fagerlund M. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) - a physiological study. Br J Anaesth. (2017) 118:610–7. doi: 10.1093/bja/aex036
72. Yang SH, Wu CY, Tseng WH, Cherng WY, Hsiao TY, Cheng YJ, et al. Nonintubated laryngomicrosurgery with transnasal humidified rapid-insufflation ventilatory exchange: a case series. J Formos Med Assoc. (2019) 118:1138–43. doi: 10.1016/j.jfma.2018.11.009
73. Ma B, Liu F, Wang D, Zhong R, Lin K, Li S, et al. High-flow nasal cannula in nonlaser microlaryngoscopic surgery: a prospective study of 19 cases in a chinese population. BMC Anesthesiol. (2022) 22:81. doi: 10.1186/s12871-022-01627-3
74. Haines KL, Agarwal S. Postoperative pulmonary complications-a multifactorial outcome. JAMA Surg. (2017) 152:166–7. doi: 10.1001/jamasurg.2016.4102
75. Recchia A, Cascella M, Copetti M, Barile A, Bignami EG, D'Ecclesia A, et al. Assessing the efficacy and safety of extubation protocols in the intensive care unit following transoral robotic surgery for obstructive sleep apnea syndrome: a retrospective cohort study. J Clin Med. (2024) 13:1501. doi: 10.20944/preprints202410.1501.v1
76. Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. (2015) 313:2331–9. doi: 10.1001/jama.2015.5213
77. Chaudhuri D, Granton D, Wang DX, Burns KEA, Helviz Y, Einav S, et al. High-flow nasal cannula in the immediate postoperative period: a systematic review and meta-analysis. Chest. (2020) 158:1934–46. doi: 10.1016/j.chest.2020.06.038
78. Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. (2016) 316:1565–74. doi: 10.1001/jama.2016.14194
79. Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga M, et al. Obesity hypoventilation syndrome. Eur Respir Rev. (2019) 28:97. doi: 10.1183/16000617.0097-2018
80. Chiang TL, Tam KW, Chen JT, Wong CS, Yeh CT, Huang TY, et al. Non-invasive ventilation for preoxygenation before general anesthesia: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. (2022) 22:306. doi: 10.1186/s12871-022-01842-y
81. Vourc'h M, Baud G, Feuillet F, Blanchard C, Mirallie E, Guitton C, et al. High-flow nasal cannulae versus non-invasive ventilation for preoxygenation of obese patients: The PREOPTIPOP Randomized Trial. EClinicalMedicine. (2019) 13:112–9. doi: 10.1016/j.eclinm.2019.05.014
82. Zhou R, Wang HT, Gu W. Efficacy of high-flow nasal cannula versus conventional oxygen therapy in obese patients during the perioperative period: a systematic review and meta-analysis. Can Respir J. (2022) 2022:4415313. doi: 10.1155/2022/4415313
83. Bright MR, Harley WA, Velli G, Zahir SF, Eley V. High-flow nasal cannula for apneic oxygenation in obese patients for elective surgery: a systematic review and meta-analysis. Anesth Analg. (2023) 136:483–93. doi: 10.1213/ANE.0000000000006304
84. Hajnour MS, Amlih HF, Shabr FFB. Efficacy of HFNO during airway management of a COVID pneumonia patient with super morbid obesity undergoing emergency laparotomy. Saudi J Anaesth. (2022) 16:368–70. doi: 10.4103/sja.sja_327_22
85. Heinrich S, Horbach T, Stubner B, Prottengeier J, Irouschek A, Schmidt J, et al. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J Obes Bariatrics. (2014) 1:7. doi: 10.13188/2377-9284.1000003
86. Wong CA, Mushambi M. Peri-intubation oxygenation for Caesarean delivery: is there an optimal technique? Br J Anaesth. (2022) 129:468–71. doi: 10.1016/j.bja.2022.07.014
87. Kinsella SM, Winton AL, Mushambi MC, Ramaswamy K, Swales H, Quinn AC, et al. Failed tracheal intubation during obstetric general anaesthesia: a literature review. Int J Obstet Anesth. (2015) 24:356–74. doi: 10.1016/j.ijoa.2015.06.008
88. Quinn AC, Kinsella SM, Gorton HJ, Knight M, Columb MO, Robson SC, et al. Neonatal outcomes after failed tracheal intubation during obstetric general anaesthesia for caesarean section: Secondary analysis of a UKOSS case-control study. Eur J Obstet Gynecol Reprod Biol. (2017) 217:181–2. doi: 10.1016/j.ejogrb.2017.08.035
89. Lee T, Shinn HK, Kim NE, Kim D. Application of high-flow nasal cannula oxygen therapy in patient with pulmonary edema following cesarean-section under combined spinal-epidural anesthesia: a case report. Medicine. (2023) 102:e34140. doi: 10.1097/MD.0000000000034140
90. Zhou SQ, Lian JF, Zhou Y, Cao XH, Ni X, Zhang XP, et al. Efficacy of high-flow nasal oxygenation during induction of general anaesthesia in parturients living with obesity: a two-centre, prospective, randomised clinical trial. Anaesthesia. (2025) 80:378–85. doi: 10.1111/anae.16492
91. Tan PCF, Peyton PJ, Deane A, Unterscheider J, Dennis AT. Pre-oxygenation using high flow humidified nasal oxygen or face mask oxygen in pregnant people - a prospective randomised controlled crossover non-inferiority study (The HINOP2 study). Int J Obstet Anesth. (2024) 60:104236. doi: 10.1016/j.ijoa.2024.104236
92. Zhou S, Zhou Y, Cao X, Ni X, Du W, Xu Z, et al. The efficacy of high flow nasal oxygenation for maintaining maternal oxygenation during rapid sequence induction in pregnancy: a prospective randomised clinical trial. Eur J Anaesthesiol. (2021) 38:1052–8. doi: 10.1097/EJA.0000000000001395
93. Huang Y, Zhao J, Hua X, Luo K, Shi Y, Lin Z, et al. Guidelines for high-flow nasal cannula oxygen therapy in neonates). J Evid Based Med. (2023) 16:394–413. doi: 10.1111/jebm.12546
94. Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. (2013) 39:247–57. doi: 10.1007/s00134-012-2743-5
95. Ran L, Huang G, Yao Y, Wu Y, Zhang C, Wang Y, et al. Efficacy of high-flow nasal oxygenation compared with laryngeal mask airway in children undergoing ambulatory oral surgery under deep sedation: a randomized controlled non-inferiority trial. Front Med. (2022) 9:1001213. doi: 10.3389/fmed.2022.1001213
96. Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A, et al. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. (2017) 118:232–8. doi: 10.1093/bja/aew401
97. Riva T, Seiler S, Stucki F, Greif R, Theiler L. High-flow nasal cannula therapy and apnea time in laryngeal surgery. Paediatr Anaesth. (2016) 26:1206–8. doi: 10.1111/pan.12992
98. Klotz D, Seifert V, Baumgartner J, Teufel U, Fuchs H. High-flow nasal cannula vs standard respiratory care in pediatric procedural sedation: a randomized controlled pilot trial. Pediatr Pulmonol. (2020) 55:2706–12. doi: 10.1002/ppul.24975
99. Testa G, Iodice F, Ricci Z, Vitale V, Razza FD, Haiberger R, et al. Comparative evaluation of high-flow nasal cannula and conventional oxygen therapy in paediatric cardiac surgical patients: a randomized controlled trial. Interact Cardiovasc Thorac Surg. (2014) 19:456–61. doi: 10.1093/icvts/ivu171
100. Itagaki T, Nakanishi N, Okuda N, Nakataki E, Onodera M, Oto J, et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in pediatric subjects after cardiac surgery. Respir Care. (2019) 64:10–6. doi: 10.4187/respcare.06193
101. Duan X, Wei N, Wei J, Zhu Y, Kang Y, He Y, et al. Effect of high-flow nasal cannula oxygen therapy on pediatric patients with congenital heart disease in procedural sedation: a prospective, randomized trial. J Cardiothorac Vasc Anesth. (2021) 35:2913–9. doi: 10.1053/j.jvca.2021.03.031
102. Kumar A, Joshi S, Tiwari N, Kumar V, Ramamurthy HR, Kumar G, et al. Comparative evaluation of high-flow nasal cannula oxygenation vs nasal intermittent ventilation in postoperative paediatric patients operated for acyanotic congenital cardiac defects. Med J. (2022) 78:454–62. doi: 10.1016/j.mjafi.2021.07.006
103. Öztürk E, Ozyilmaz I, Yücel ED, Recep BZT, Tanidir C, Hatemi IAC. Impact of high-flow nasal oxygen therapy on postoperative atelectasis and reintubation rate after paediatric cardiac surgery. Cardiol Young. (2024) 34:2178–81. doi: 10.1017/S1047951124025630
104. Polytarchou A, Moudaki A, Van de Perck E, Boudewyns A, Kaditis AG, Verhulst S, et al. An update on diagnosis and management of obstructive sleep apnoea in the first 2 years of life. Eur Respir Rev. (2024) 33:230121. doi: 10.1183/16000617.0121-2023
105. Carratalá JM, Diaz-Lobato S, Brouzet B, Más-Serrano P, Rocamora JLS, Castro AG, et al. Efficacy and safety of high-flow nasal cannula therapy in elderly patients with acute respiratory failure. Pulmonology. (2024) 30:437–44. doi: 10.1016/j.pulmoe.2023.01.004
106. Lyons C, Jonsson Fagerlund M. Airway fires during use of high-flow nasal oxygen: a cause for concern. Br J Anaesth. (2025) 134:893–7. doi: 10.1016/j.bja.2024.12.021
107. Qin J, Wang G, Liao Y, Shang W, Han D. High flow nasal therapy versus noninvasive ventilation for AECOPD with acute hypercapnic respiratory failure: a meta-analysis of randomized controlled trials. Ann Intensive Care. (2025) 15:64. doi: 10.1186/s13613-025-01480-w
108. Nagata K, Horie T, Chohnabayashi N, Jinta T, Tsugitomi R, Shiraki A, et al. Home high-flow nasal cannula oxygen therapy for stable hypercapnic COPD: a randomized clinical trial. Am J Respir Crit Care Med. (2022) 206:1326–35. doi: 10.1164/rccm.202201-0199OC
109. Tan D, Wang B, Cao P, Wang Y, Sun J, Geng P, et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: a randomized controlled non-inferiority trial. Crit Care. (2024) 28:250. doi: 10.1186/s13054-024-05040-9
Keywords: HFNC, physiological mechanisms, perioperative care, special populations, anesthesia practice
Citation: Liu H, Qu P, Liu Q, Xiao F, Yang Y, Xu L and Zhang H (2025) High-flow nasal cannula oxygen therapy: physiological basis and clinical applications in anesthesia. Front. Med. 12:1661569. doi: 10.3389/fmed.2025.1661569
Received: 08 July 2025; Accepted: 08 September 2025;
Published: 26 September 2025.
Edited by:
Luigi Vetrugno, Azienda Sanitaria Universitaria Friuli Centrale, ItalyReviewed by:
Irene Steinberg, University of Turin, ItalyTeresa López, University Hospital of Salamanca, Spain
Copyright © 2025 Liu, Qu, Liu, Xiao, Yang, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Zhang, MTM3MDgyMDY0NDNAMTYzLmNvbQ==; Liu Xu, MjY4NzM1ODM4OEBxcS5jb20=; Yanling Yang, NjM5Nzg5MzI3QHFxLmNvbQ==
†These authors have contributed equally to this work
 Hui Liu1†
Hui Liu1† Qian Liu
Qian Liu Liu Xu
Liu Xu Hongyan Zhang
Hongyan Zhang