Abstract
Spondyloarthritis (SpA) is a group of immuno-mediated diseases likely caused by a complex interaction between autoimmune and autoinflammatory immunological mechanisms, where a febrile clinical presentation can be an early manifestation. This retrospective study aims to assess the prevalence of inflammatory involvement of the sacroiliac joints (SIJs), pelvis, and lumbosacral spine in patients with axial-SpA and recurrent febrile presentation. MR examinations of 57 patients fulfilling the axial-SpA according to ASAS criteria and presenting with febrile symptoms were evaluated, compared to 30 patients with axial-SpA and no febrile symptoms. 20/57 patients in the axial-SpA group with recurrent fevers underwent a US examination of the SIJs. Structural damage and inflammatory alterations of the SIJs were highly prevalent in both groups. In patients with febrile syndrome, bone marrow edema (78.9%) and erosions (85.9%) were the most prevalent findings in the SIJs; SPARCC score was significantly higher in patients with typical axial-SpA onset (BME: 20.1 ± 12.28 vs. 6.15 ± 3.21; erosions: 22.16 ± 8.13 vs. 6.60 ± 3.08; p = 0.01). Among pelvic enthesitis, enthesitis of the pubic symphysis showed a significant difference in prevalence (p < 0.001). Significant differences were found for the prevalence of vertebral body corner sclerosis (p = 0.01), zygapophyseal capsulitis (p = 0.005), and interspinous enthesitis (p = 0.006). No significant correlation was found between ultrasound findings and MR inflammatory changes (p > 0.05). SIJs and spinal inflammatory alterations and pelvis enthesitis were highly prevalent in axial-SpA patients with and without recurrent fever. Enthesitis of the pubic symphysis, vertebral body corner sclerosis, zygapophyseal capsulitis, and interspinous enthesitis showed a significant difference in frequency between axial-SpA patients with and without fever attacks.
Highlights
Axial spondyloarthritis (SpA) may be associated with extra-articular inflammatory manifestations, including periodic febrile episodes. Febrile syndromes indicate mixed pathogenesis, autoimmune and autoinflammatory in axial SpA.
The radiological features of spondyloarthritis identified in patients with febrile episodes are largely comparable to those of patients without febrile episodes. However, the latter tend to exhibit a greater frequency of enthesitis of the pubic symphysis, vertebral body corner sclerosis, zygapophyseal capsulitis, and interspinous enthesitis.
Clinical statement: Patients with axial SpA and febrile presentation have a high incidence of inflammatory changes of the sacroiliac joints (SIJs), pelvis and lumbosacral spine on MR. The most commonly reported changes are subchondral bone marrow edema and joint erosions in SIJs.
Introduction
Axial spondyloarthritis (SpA) represents a group of chronic inflammatory joint disorders characterized by the axial skeleton’s involvement, with or without peripheral joints (1). Axial SpA encompasses a range of diseases, including ankylosing spondylitis, psoriatic arthritis, arthritis associated with inflammatory bowel diseases, reactive arthritis, and undifferentiated arthritis. It represents the second most prevalent form of chronic inflammatory arthritis, with an estimated prevalence of 0.5–1.5% in the Caucasian population (2). In addition to joint symptoms, axial SpA has been associated with extra-articular inflammatory manifestations such as cutaneous psoriasis, uveitis, or chronic inflammatory bowel diseases, including Crohn’s disease or ulcerative colitis (2).
Axial SpA has long been considered an immunological disorder with a mixed pattern of autoimmune and autoinflammatory disease (3). However, a greater role for autoinflammatory pathogenesis has recently been suggested by the emerging predominant involvement of innate immunity (4, 5): it is established that, although infrequently, axial SpA can present with febrile episodes, and fever is considered one of the most common symptoms of autoinflammatory disorders (6, 7). Currently, there is still limited data characterizing the subgroup of patients with axial SpA and recurrent febrile episodes, and this remains a subject of investigation from clinical, pathogenetic, and also radiological perspectives. Therefore, the present study was conducted to achieve a better knowledge of the magnetic resonance (MR) and ultrasound features of patients suffering from axial SpA and recurrent febrile attacks.
Materials and methods
Study design
The primary objective of this study was to retrospectively investigate the pattern and prevalence of MR and ultrasound features of axial SpA in a cohort of patients presenting with apparently unexplained recurrent fever, in whom axial SpA fulfilled the Assessment of SpondyloArthritis international Society (ASAS) criteria during the diagnostic workup (8, 9). An additional objective was to assess any correlation between ultrasound findings and MR-detected active inflammatory changes.
From April 2021 to November 2022, we prospectively identified and enrolled 57 patients (39F, 18 M, median age 42 years, IQR 14–75). The data were collected through the International AutoInflammatory Disease Alliance (AIDA) Network registry for Undifferentiated Systemic AutoInflammatory Diseases (USAIDs) (10, 11). This registry functions as an observational study, intending to compile information from patients receiving treatment in accordance with the established standard of care. All patients were newly diagnosed with non-radiographic axial SpA by an expert rheumatologist based on clinical assessment and the subsequent application of classification criteria, as part of the diagnostic process for undiagnosed fever. Patients included in the study had previously undergone X-rays of the pelvis and entire spine to exclude those with radiographic axial SpA.
Patients reported inflammatory low back pain with or without other extra-articular manifestations associated with axial SpA, and at least one not otherwise explainable febrile episode per year, evaluated and confirmed by physicians as a fever of no apparent cause. Subsequent magnetic resonance (MR) imaging of SIJs confirmed the presence of radiologic signs consistent with axial SpA: ASAS criteria (imaging arm) were met in all cases (8, 9, 12, 13). The control group comprised 30 patients (20F, 10 M, median age 41.5 years, IQR 16–70) with a new diagnosis of axial SpA with chronic inflammatory low back pain and no unexplained fever attacks. All patients were Caucasian and were not undergoing any specific therapy.
Exclusion criteria encompassed reactive SpA, infections, autoimmune and neoplastic diseases, and gastrointestinal and/or urinary infections in the 6 months before the onset of symptoms. Radiographic axial SpA were excluded a priori from the control group, as they were not present in the axial SpA group with febrile episodes. Inclusion criteria required the fulfillment of ASAS criteria, irrespective of the presence of recurrent fever attacks, along with the provision of informed consent to participate in the project, which was obtained from all patients, parents, or legal guardians (10, 11). The study also included patients under 16 years of age diagnosed with juvenile idiopathic arthritis involving the sacroiliac joints.
Clinical characteristics of patients included in this study are reported in Table 1.
Table 1
| Clinical item | Axial SpA with recurrent fever | Typical onset Axial SpA without recurrent fever |
|---|---|---|
| n = 57 | n = 30 | |
| Sexa | ||
| Male | 18 (31.5) | 10 (33.3) |
| Female | 39 (68.5) | 20 (66.7) |
| Age | ||
| Mean | 42 | 41.5 |
| Median | 37 | 52.7 |
| Range | 14–75 | 16–70 |
| Articular disease duration, years | ||
| Mean | 5.21 | 11.8 |
| Median | 1.7 | 7.9 |
| Range | 0.3–36.6 | 0.3–61.6 |
| Items of ASAS criteriaa | ||
| Inflammatory low back pain | 57 (100) | 30 (100) |
| Arthritis | 23 (40.3) | 17 (56.7) |
| Enthesitis (heel) | 4 (7) | 3 (10) |
| Uveitis | 7 (12.2) | 4 (13.4) |
| Dactylitis | 5 (9) | 2 (6.7) |
| Psoriasis | 5 (9) | 12 (40) |
| Inflammatory Bowel Diseases | 3 (5.2) | 6 (20) |
| Response to NSAIDsb | 27 (47.3) | 19 (63.4) |
| Family history of SpA | 9 (15.8) | 10 (33.3) |
| Elevated C-reactive proteinc | 32 (56.1) | 16 (53.3) |
| HLA-B27 positivity | 5 (9) | 7 (23.3) |
Demographic characteristics of patients and frequency of the specific clinical and laboratory item included in the assessment of spondyloarthritis international society (ASAS) criteria.
SpA: spondyloarthritis; NSAIDs: non-steroidal anti-inflammatory drugs; HLA: human leukocyte antigen.
aData are the number of patients with the percentage in parentheses.
bAll the patients treated with NSAIDs experienced at least a partial improvement in the low back pain.
cC reactive protein assessed outside fever bouts.
The study received approval from the Ethics Committee of Azienda Ospedaliero Universitaria Senese, Siena, Italy (AIDA Project; Ref. N. 14951) as part of the AIDA Program (10, 11). The study protocol adhered to the principles of the Declaration of Helsinki.
MR evaluation
All patients underwent an MR examination on a 1.5 T scanner (Signa Twin Speed Hdxt; GE Healthcare, USA) with a dedicated protocol for the study of SIJs and hips. The acquired sequences included a coronal “panoramic” short-time inversion recovery (STIR) sequence for hip assessment, followed by sequences with a reduced field of view (FOV) for a detailed study of the SIJs. These sequences were obtained on the oblique coronal plane, parallel to the major axis of the sacrum, and on the oblique axial plane, perpendicular to the major axis of the sacrum, according to the European Society of Musculoskeletal Radiology (ESSR) recommendation (14). Both T1-weighted and STIR sequences were utilized. In 53 out of 87 cases (60.2%), the examination was extended by acquiring a T1 gradient-echo sequence with fat suppression on the coronal oblique plane (3D spoiled Gradient-Echo T1W with spectral inversion at lipidi, FSPGR, GE Healthcare) before and after intravenous contrast media (CM) administration (Gadoterate meglumine 0.2 mg/kg). The objective of this dynamic acquisition was to obtain post-contrast images of the SIJs, followed by a volumetric panoramic coronal acquisition of the pelvis and lumbar/sacral spine, in order to gather post-contrast information on the entheses and lumbar/sacral spine. Technical parameters of the MR protocol are detailed in Supplementary Table S1.
The MR analysis was retrospectively evaluated in agreement by two readers, a radiologist with expertise in rheumatological pathology, and a senior radiology trainee. Concerning SIJs, the analysis focused on identifying signs of acute and chronic inflammatory involvement according to criteria defined by the ASAS (12, 13). Acute inflammatory findings included subchondral bone edema (BME), enthesitis, capsulitis, and joint space hyperintensity. Structural lesions included joint erosions, subchondral bone sclerosis and fat metaplasia, backfill (the presence of fat metaplasia in joint erosions), bone bud, and joint ankylosis. Furthermore, signs of enthesitis in the pelvis (ischial tuberosities, pubic symphysis, greater trochanters) were investigated. The lumbar spine included in the FOV was assessed, considering acute or chronic lesions of the vertebral body (edema, fat metaplasia, and sclerosis of vertebral body corners, i.e., Romanus lesions, presence/absence of aseptic spondylodiscitis, i.e., Andersson lesions, enthesitis of the annulus fibrosus/syndesmophytes) and posterior elements (capsulitis/enthesitis of zygapophyseal joints and enthesitis of interspinous and supraspinous ligaments). BME in the SIJs was quantified using the MR score proposed by the Spondyloarthritis Research Consortium of Canada (SPARCC) (15). A score equal to or greater than 2 was considered the minimum indicative value of active sacroiliitis on MR, with a maximum score of 72. The SPARCC score adapted for structural lesions was also utilized to assess subchondral fat metaplasia, erosions, and subchondral bone sclerosis, with a maximum score of 40 (16).
Ultrasound evaluation
Twenty out of 57 patients in the axial SpA group with recurrent fevers had randomly undergone an ultrasound (US) examination of the SIJs. The patient was in the prone position, and the probe was positioned in a transverse scan over the median line of the sacrum, at the spinous apophysis of the first sacral vertebra. Subsequently, the probe was shifted over the top of the left first sacral foramen up to the margin of the ilium, where the probe was placed in an oblique position and the sound beam inclined about 20° downwards. The process was then repeated ab initio for the right SIJ. The first sacral foramen was always observed to avoid measurement of the pre-sacral arteries and veins, and all extensions of the SIJs were explored up to the second sacral foramen level. Any PW Doppler signal detected in the SIJs space was scored using a 3-point scale: 0 = absence of signal, 1 = isolated vessel, 2 = more than one vessel. The same vessels were also evaluated using quantitative PW Doppler, calculating the Resistive Index (RI = peak of systolic flow − end diastolic flow/peak systolic flow). At least two measurements were obtained for each vessel, and the mean value was recorded. An RI value of <0.60 was considered to be indicative of sacroiliitis, as previously performed in other studies (17–19).
Statistics
Descriptive statistics methods were applied for the analysis of MR and US data. Statistical analyses were performed using non-parametric tests. The differences in the two study populations were analysed by applying the Chi2-test and Mann–Whitney U-test. Q-Q, and p–p plots were used to assess the normal distribution of the variables. Spearman’s Rho correlation coefficient was used to determine the correlation between quantitative variables with a non-normal distribution, and Pearson’s correlation coefficient for variables with a normal distribution. A p < 0.05 was considered statistically significant. All statistical analyses were performed in IBM Statistical Package for the Social Sciences (SPSS) version 22.0.
Results
A comparison of MR characteristics of the axial SpA patients with recurrent fever and controls is presented in Tables 2–4. Structural damage and inflammatory alterations of SIJs were present in a large percentage of patients with both recurrent febrile presentation and typical presentation, although the prevalence was higher in the latter group (Figure 1).
Table 2
| MR findings | Axial SpA with recurrent fever | Typical onset Axial SpA without recurrent fever | p |
|---|---|---|---|
| BME | 45 (78.9) | 30 (100) | 0.007 |
| SIJs Enthesitis | 38 (66.6) | 26 (86.7) | 0.057 |
| SIJs Capsulitis | 32 (56.1) | 23 (76.7) | 0.072 |
| Joint space hyperintensity | 10 (17.5) | 9 (30) | 0.181 |
| Erosions | 49 (85.9) | 30 (100) | 0.03 |
| Subchondral bone sclerosis | 29 (50.9) | 26 (86.7) | 0.01 |
| Subchondral fat metaplasia | 40 (70.1) | 29 (96.7) | 0.004 |
| Backfill | 7 (12.3) | 19 (63.3) | <0.001 |
| Bone Bud | 7 (12.3) | 6 (20) | 0.337 |
| Joint ankylosis | 1 (1.7) | 3 (10) | 0.081 |
Frequency of acute and chronic inflammatory MR changes of the SIJs.
BME, bone marrow edema; SIJs, sacroiliac joints; MR, Magnetic Resoncance; SpA, spondyloarthritis.
Data are the number of patients with the percentage in parentheses.
Table 3
| Enthesitis site | Axial SpA with recurrent fever | Typical onset Axial SpA without recurrent fever | p |
|---|---|---|---|
| Greater trochanters | 42 (73.7) | 27 (90) | 0.96 |
| Ischial tuberosities | 23 (40.4) | 17 (56.7) | 0.167 |
| Pubic symphysis | 4 (7) | 14 (46.7) | <0.001 |
Frequency of pelvic enthesitis at magnetic resonance examination.
SpA, spondyloarthritis.
Data are the number of patients with the percentage in parentheses.
Table 4
| MR findings | Axial SpA with recurrent fever | Typical onset Axial SpA without recurrent fever | p |
|---|---|---|---|
| Vertebral body corner BME | 7 (12.3) | 11 (36.7) | 0.08 |
| Andersson lesion | 5 (8.7) | 7 (23.4) | 0.061 |
| Vertebral body corner sclerosis | 4 (7) | 11 (36.7) | <0.01 |
| Vertebral body corner fat metaplasia | 1 (1.7) | 2 (6.7) | 0.41 |
| Enthesitis of the annulus fibrosus/syndesmophytes | 2 (3.5) | 3 (10) | 0.261 |
| Zygapophyseal joints capsulitis | 36 (63.1) | 23 (86.7) | 0.005 |
| Interspinous/supraspinous enthesitis | 41 (71.9) | 29 (96.7) | 0.006 |
Frequency of acute and chronic inflammatory changes of the spine at magnetic resonance examination.
MR, Magnetic Resonance; SpA, spondyloarthritis; BME, bone marrow edema. Data are the number of patients with the percentage in parentheses.
Figure 1
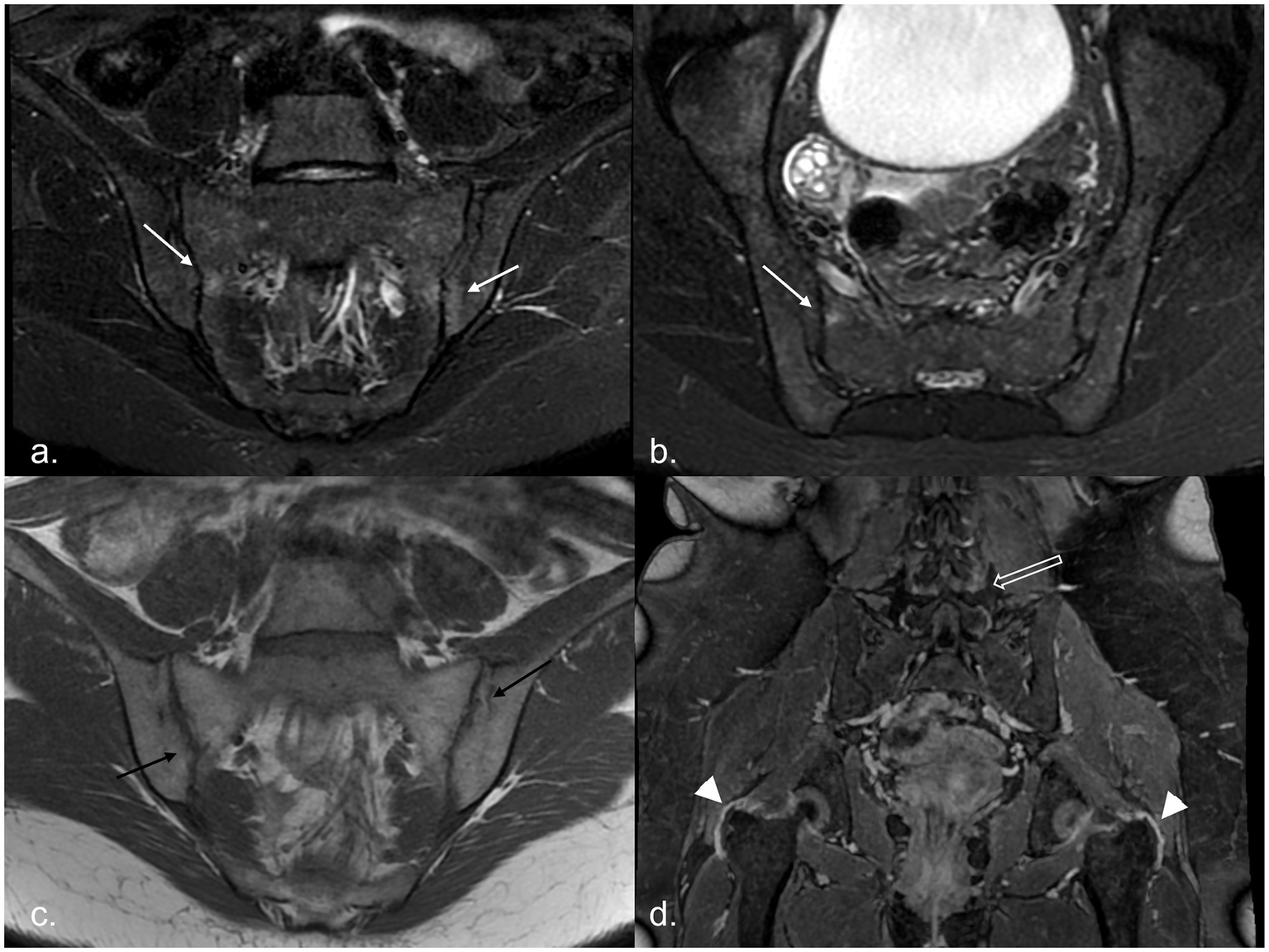
MR examination of a 20-year-old woman with recurrent fever: it shows areas of subchondral BME on the right sacral and left iliac sides of the sacroiliac joints [white arrows, panel (a) in the oblique coronal plane and panel (b) in the oblique axial plane], associated with multiple bilateral joint erosions [black arrows in panel (c)]. After administration of CM, in the 3D panoramic sequence for the hip, trochanteric enthesitis (arrowhead) and facet capsulitis (empty white arrow) are evident [panel (d)]. BME, bone marrow edema; CM, contrast media.
In patients presenting with febrile syndrome, 41 of 57 (71.9%) had inflammatory and structural changes of the SIJs, 5 of 57 (9%) had inflammatory changes only, and 11 of 57 (19.1%) had structural changes only. Of the 46 patients with active inflammation, 45 exhibited BME, while one showed other signs of active disease on contrast-enhanced imaging (joint space enhancement, capsulitis, and synovitis) alongside structural damage (erosions and sclerosis). In the remaining 11 patients without inflammatory changes, structural damage highly suggestive of inflammatory sacroiliitis (ankylosis, bone bud formation, marked sclerosis, and erosions) was observed. Among enthesitis of the pelvis, only enthesitis of the pubic symphysis showed a significant difference in prevalence (p < 0.001), with a lower frequency among patients without fever attacks. The only significant difference in the prevalence of spinal involvement was for sclerosis of vertebral body corners (p = 0.01), zygapophyseal capsulitis (p = 0.005), and interspinous enthesitis (p = 0.006), which were significantly more frequent among axial SpA patients without fever attacks. The number of MR changes observed with the use of CM is shown in Figure 2: a significant negative correlation with CM use, even if weak, was found only for SIJs erosions (Spearman’s Rho −0.255, p = 0.017), bone bud (Spearman’s Rho −0.259, p = 0.015), and interspinous enthesitis (Spearman’s Rho −0.276, p = 0.010); a negative correlation toward significance with CM use was found for joint space hyperintensity (Spearman’s Rho −0.204, p = 0.058).
Figure 2
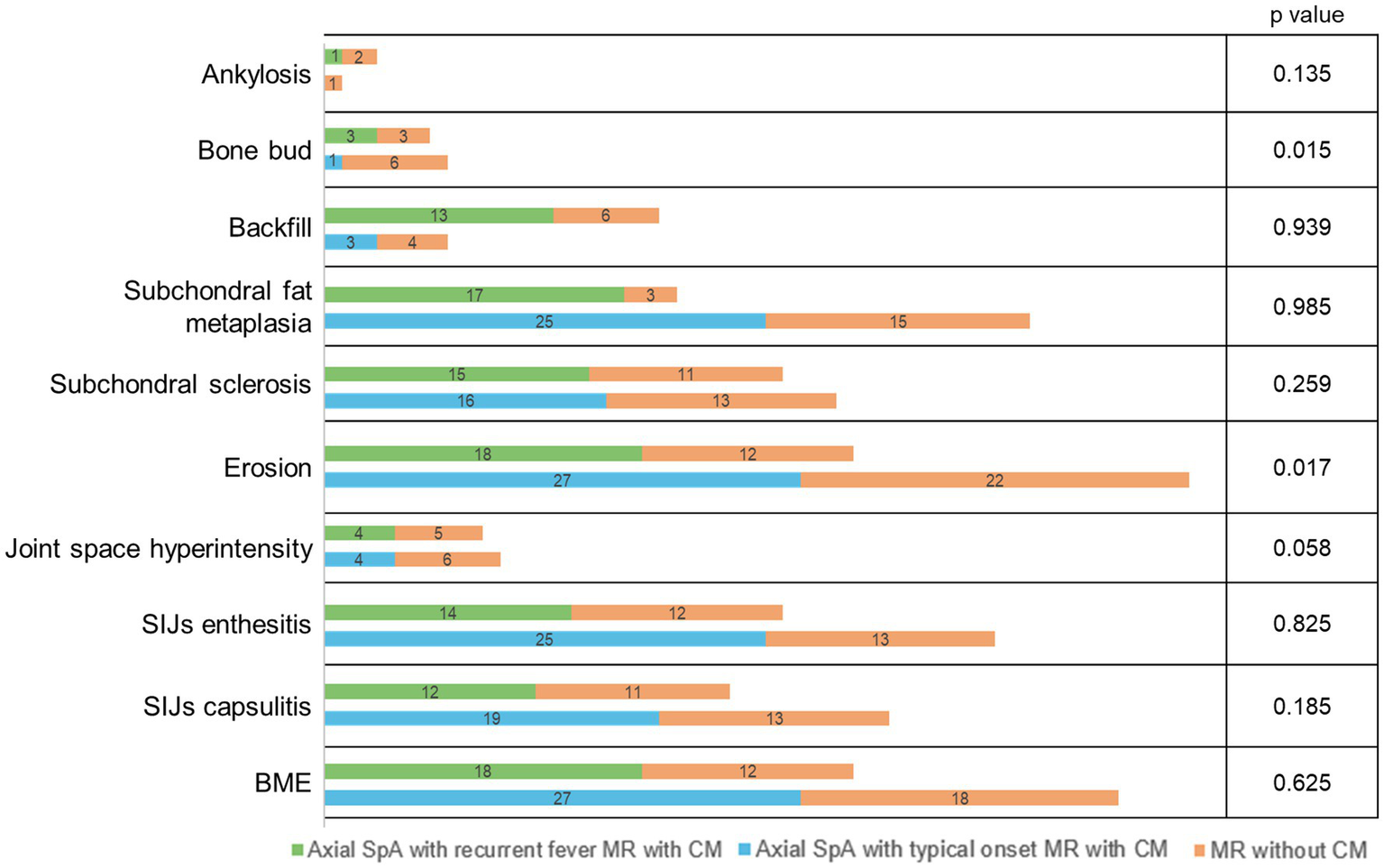
Number of patients for MR findings concerning the use of CM in axial SpA with recurrent fever and axial SpA with typical onset, respectively. p-values indicate the correlation between CM use and MR findings in the total population. SpA, spondyloarthritis; SIJs, sacroiliac joints; BME, bone marrow edema; CM, contrast media.
Concerning the SPARCC score for SIJs, the distribution of values is shown in Figure 3 and Supplementary Table S2: the SPARCC score for BME and erosions is significantly higher in the axial SpA patients with typical onset (BME: mean value 20.1 ± 12.28 vs. 6.15 ± 3.21; erosions: 22.16 ± 8.13 vs. 6.60 ± 3.08, respectively). Regarding the ultrasound evaluation of the SIJs, the PW Doppler vascular signal was classified as 0 in one patient (5%), 1 in nine patients (45%), and 2 in ten patients (50%). The mean RI value was 0.49 ± 0.18 (range 0–0.66).
Figure 3
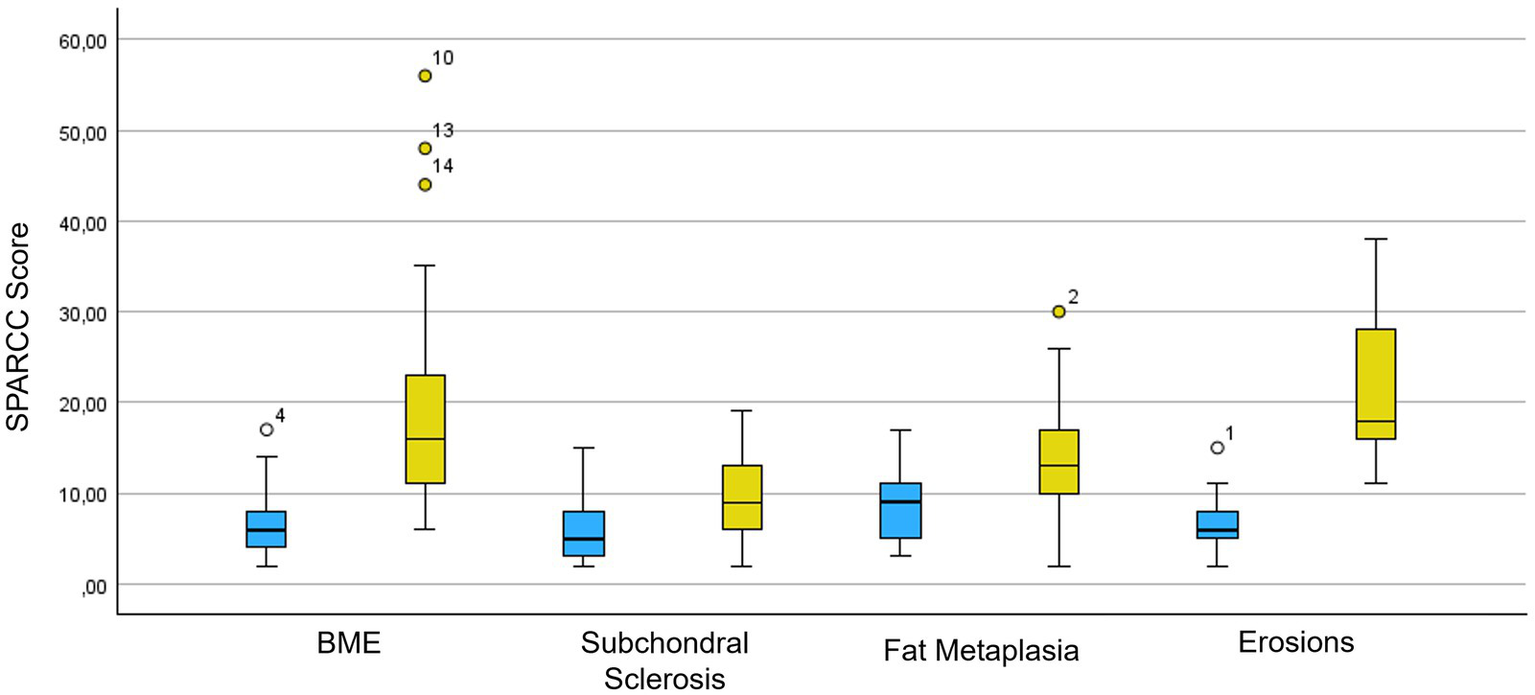
SPARCC score box plot for acute and chronic inflammatory MR changes of the SIJs. In blue is the population of axial SpA patients with recurrent fever syndromes and in yellow is the population of axial SpA patients with typical presentation. SPARCC, SPondyloArthritis Research Consortium of Canada; BME, bone marrow edema.
Concerning the correlation between US findings and MR acute inflammatory findings in SIJs, no significant association was found between the PW Doppler scale and the presence/absence of BME (Spearman’s Rank Correlation Coefficient −0.132, p = 0.578) or the presence/absence of enthesitis (Spearman’s Rank Correlation Coefficient −0.168, p = 0.478). No significant correlation was found between the RI and the presence/absence of BME (Spearman’s Rank Correlation Coefficient −0.289, p = 0.217) or the presence/absence of SIJ enthesitis (Spearman’s Rank Correlation Coefficient 0.157, p = 0.508).
Discussion
The occurrence of fever in axial SpA has been described infrequently, and the exact aetiology of this clinical manifestation is unclear. One potential explanation is that the fever could be a manifestation of extra-articular inflammation; alternatively, axial SpA may be part of a more complex clinical picture (6, 7, 20). The clinical approach to autoinflammatory disease involves assessing all clinical elements that may explain the genesis and persistence of systemic inflammation. For this purpose, the creation of the international AIDA registry, dedicated to undifferentiated autoinflammatory diseases, allows for attention to be focused on new clinical and pathological entities in patients with recurrent febrile syndromes of unknown aetiology (10).
A total of 57 patients with chronic low back pain and recurrent febrile syndromes were identified in the AIDA registry: in all these patients, axial SpA could be diagnosed according to the ASAS criteria (imaging arm). The purpose of this study was to analyse the imaging characteristics compared to a population of patients with a typical presentation of axial SpA. Although with a lower prevalence compared to the typical axial SpA population, patients with febrile syndromes also exhibited a notable prevalence of acute inflammatory changes and structural damage to the SIJs, either isolated or combined (9 and 19.2% respectively, vs. 72.8%).
Consistent with the literature data, our study revealed that the most common changes observed in SIJs in axial SpA patients with recurrent fever were subchondral BME (78.9%) and joint erosions (85.9%) (13). The concomitant presence of subchondral BME and joint erosions has been demonstrated to have a 94% specificity in diagnosing sacroiliitis (21). The high prevalence of structural damage of the SIJs in our population is probably related to the relative delay of the diagnosis in the context of a non-specific clinical presentation (11). The inclusion of a panoramic coronal sequence of the hip in our MR protocol allowed the assessment of enthesitic involvement of the pelvis, which was observed with notable prevalence in both patient groups, predominantly at the level of the greater trochanters (73.7%). Pelvic enthesitis occurs in 5–20% of cases of axial SpA, particularly associated with forms of psoriatic arthritis (22, 23). Our data may be related to the study protocol, which includes a panoramic evaluation of the pelvis in all patients and not only those presenting with symptoms, as suggested in the literature: in fact, the study protocol outlined in the literature includes only oblique sequences oriented along the major axis of the SIJs (24).
Active inflammatory involvement of the lumbosacral spine was found, with a high prevalence of inflammatory lesions observed in the posterior elements, including interspinous enthesitis (71.9%) and zygapophyseal joint capsulitis (63.1%). In all cases, spinal inflammatory involvement was associated with signs of active or chronic inflammatory sacroiliitis, a scenario more commonly observed in patients with axial SpA. Hoffstetter et al. report concomitant SIJs and spinal involvement in patients with axial SpA in up to 62.2% of cases, and isolated spinal involvement in 9.8% of cases (25). Vertebral body involvement was less prevalent than posterior element involvement, probably due to an intrinsic limitation of our MR study protocol, which primarily focused on evaluating the pelvis without dedicated sequences for the lumbosacral spine. Consequently, the spine was primarily assessed using panoramic coronal sequences and 3D post-contrast sequences. However, Bochkova et al. reported a higher incidence of inflammatory involvement of the posterior elements of the spine compared to the vertebral body (90.9% vs. 27.2%) (26).
Regarding the use of CM, no significant diagnostic advantage has been demonstrated. In the literature, it is reported that CM sequences are more sensitive than fat-saturated fluid-sensitive sequences in evaluating acute inflammatory lesions of SIJs and are beneficial to ensure maximum diagnostic confidence when examining patients with early sacroiliitis (27–29). However, fat-saturated fluid-sensitive sequences alone are sufficient for establishing a reliable diagnosis (27, 30).
In a subset of patients with axial SpA group with recurrent fevers (20/57), US assessment of the SIJs was performed; however, no significant correlation with MRI findings was observed. US evaluation of the SIJs remains an unvalidated technique with limited clinical utility in axial SpA patients (30).
This study cannot elucidate the role of fever in the pathogenesis of SIJ and spinal lesions observed in axial SpA patients with recurrent fever. However, the different prevalence of inflammatory and structural lesions between patients with axial SpA with recurrent fever and those with typical onset could be explained by the difference in disease duration between the two groups (5.21 vs. 11.8 years).
The additional main limitation of our study is its retrospective and descriptive nature, as well as the restricted cohort of patients included in the analysis. Although the ASAS criteria were developed for patients up to 45 years of age, they were also applied to patients over 45, as no other criteria could be used for preradiographic forms. These forms, having escaped early diagnosis and not progressing to a radiographic stage, were identified after the age of 45.
In conclusion, a high prevalence of active and chronic inflammatory changes in the SIJs, enthesitic involvement of the pelvis, and inflammatory changes in the lumbar spine were observed in patients with axial SpA presenting with recurrent febrile syndromes. Axial SpA should be considered among the possible differential diagnoses in patients presenting recurrent febrile syndromes of unknown aetiology and inflammatory back pain, as demonstrated in our patient cohort. Enthesitis of the pubic symphysis, vertebral body corner sclerosis, zygapophyseal capsulitis, and interspinous enthesitis were the only radiologic lesions that showed a significant difference in frequency between axial SpA patients with and without fever attacks.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Azienda Ospedaliero Universitaria Senese, Siena, Italy (AIDA Project; Ref. N. 14951) as part of the AIDA Program. The study protocol conformed to the tenets of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NDM: Writing – original draft, Writing – review & editing. CS: Writing – review & editing, Investigation. AV: Formal analysis, Validation, Writing – review & editing, Supervision. VC: Writing – review & editing, Data curation. AP: Investigation, Writing – review & editing. VDM: Writing – review & editing. MDS: Investigation, Writing – review & editing. MF: Writing – review & editing, Investigation. GB: Methodology, Writing – review & editing, Formal analysis. PR: Writing – review & editing, Visualization. PC: Writing – review & editing, Visualization. ASP: Writing – review & editing, Visualization. ST: Writing – review & editing, Validation. HME: Writing – review & editing, Visualization. EEO: Visualization, Writing – review & editing. AC: Writing – review & editing, Validation. LL: Writing – review & editing, Visualization. FV: Visualization, Writing – review & editing. JS: Visualization, Writing – review & editing. GAG-B: Writing – review & editing, Validation. PAK-C: Writing – review & editing, Visualization. EM-N: Writing – review & editing, Visualization. MT: Writing – review & editing, Validation. BYW: Visualization, Writing – review & editing. SE: Validation, Writing – review & editing. MAE: Writing – review & editing, Visualization. BÖU: Writing – review & editing, Visualization. PB: Writing – review & editing, Validation. SP: Writing – review & editing, Visualization. AG: Visualization, Writing – review & editing. SG: Investigation, Visualization, Writing – review & editing. CG: Visualization, Investigation, Writing – review & editing. SB: Writing – review & editing, Investigation. SH: Validation, Writing – review & editing. HE: Writing – review & editing, Visualization. AT: Writing – review & editing, Validation. GR: Validation, Writing – review & editing. EW-S: Writing – review & editing, Validation. JH-R: Validation, Writing – review & editing. AB: Writing – review & editing, Software, Data curation. AH-A: Validation, Writing – review & editing. CF: Validation, Writing – review & editing. BF: Writing – review & editing, Validation. LC: Conceptualization, Writing – review & editing, Validation, Supervision. MAM: Supervision, Validation, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1662890/full#supplementary-material
Abbreviations
AIDA, AutoInflammatory Disease Alliance; ASAS, Assessment of SpondyloArthritis international Society; BME, bone marrow edema; CM, contrast media; ESSR, European Society of Musculoskeletal Radiology; FOV, field of view; PFR, Pulse Repetition Frequency; PW, Pulse Wave; RI, Resistive Index; SIJs, sacroiliac joints; SpA, spondyloarthritis; SPARCC, Spondyloarthritis Research Consortium of Canada; STIR, short-time inversion recovery; USAIDs, Undifferentiated Systemic AutoInflammatory Diseases.
References
1.
Dougados M Baeten D . Spondyloarthritis. Lancet. (2011) 377:2127–37. doi: 10.1016/S0140-6736(11)60071-8
2.
Van Tubergen A . The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol. (2015) 11:110–8. doi: 10.1038/nrrheum.2014.181
3.
McGonagle D McDermott MF . A proposed classification of the immunological diseases. PLoS Med. (2006) 3:e297. doi: 10.1371/journal.pmed.0030297
4.
Generali E Bose T Selmi C Voncken JW Damoiseaux JGMC . Nature versus nurture in the spectrum of rheumatic diseases: classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun Rev. (2018) 17:935–41. doi: 10.1016/j.autrev.2018.04.002
5.
Rigante D Vitale A Lucherini OM Cantarini L . The hereditary autoinflammatory disorders uncovered. Autoimmun Rev. (2014) 13:892–900. doi: 10.1016/j.autrev.2014.08.001
6.
Byun SJ Bae WH Jung SM Lee SW Park YB Song JJ . Fever as an initial manifestation of spondyloarthritis: a retrospective study. PLoS One. (2017) 12:e0184323. doi: 10.1371/journal.pone.0184323
7.
Rigante D Lopalco G Vitale A Lucherini OM Caso F De Clemente C et al . Untangling the web of systemic autoinflammatory diseases. Mediat Inflamm. (2014) 2014:948154. doi: 10.1155/2014/948154
8.
Rudwaleit M Landewé R van der Heijde D Listing J Brandt J Braun J et al . The development of assessment of SpondyloArthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. (2009) 68:770–6. doi: 10.1136/ard.2009.108217
9.
Rudwaleit M van der Heijde D Landewé R Listing J Akkoc N Brandt J et al . The development of assessment of SpondyloArthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. (2019) 78:e59. doi: 10.1136/ard.2009.108233corr1
10.
Della Casa F Vitale A Lopalco G Ruscitti P Ciccia F Emmi G et al . Development and implementation of the AIDA international registry for patients with undifferentiated systemic autoinflammatory diseases. Front Med (Lausanne). (2022) 9:908501. doi: 10.3389/fmed.2022.908501
11.
Vitale A Caggiano V Silva I Oliveira DG Ruscitti P Ciccia F et al . Axial spondyloarthritis in patients with recurrent fever attacks: data from the AIDA network registry for undifferentiated autoinflammatory diseases (USAIDs). Front Med. (2023) 10:1195995. doi: 10.3389/fmed.2023.1195995
12.
Lambert RG Bakker PA van der Heijde D Weber U Rudwaleit M Hermann KG et al . Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. (2016)
13.
Maksymowych WP Lambert RG Østergaard M Pedersen SJ Machado PM Weber U et al . MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis. (2019)
14.
Vereecke E Diekhoff T Eshed I Herregods N Morbée L Jaremko JL et al . ESR essentials: imaging of sacroiliitis-practice recommendations by ESSR. Eur Radiol. (2024) 34:5773–82. doi: 10.1007/s00330-024-10653-3
15.
Maksymowych WP Inman RD Salonen D Dhillon SS Williams M Stone M et al . Spondyloarthritis research consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. (2005) 53:703–9. doi: 10.1002/art.21445
16.
Maksymowych WP Wichuk S Chiowchanwisawakit P Lambert RG Pedersen SJ . Development and preliminary validation of the Spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol. (2015) 42:79–86. doi: 10.3899/jrheum.140519
17.
Rosa JE Ruta S Bravo M Pompermayer L Marin J Ferreyra-Garrot L et al . Value of color doppler ultrasound assessment of sacroiliac joints in patients with inflammatory low back pain. J Rheumatol. (2019) 46:694–700. doi: 10.3899/jrheum.180550
18.
Castillo-Gallego C De Miguel E García-Arias M Plasencia C Lojo-Oliveira L Martín-Mola E . Color doppler and spectral doppler ultrasound detection of active sacroiliitis in spondyloarthritis compared to physical examination as gold standard. Rheumatol Int. (2017) 37:2043–7. doi: 10.1007/s00296-017-3813-3
19.
Falsetti P Conticini E Mazzei MA Baldi C Sota J Bardelli M et al . Power and spectral doppler ultrasound in suspected active sacroiliitis: a comparison with magnetic resonance imaging as gold standard. Rheumatology. (2021) 60:1338–45. doi: 10.1093/rheumatology/keaa546
20.
Seyahi E Karaaslan H Ugurlu S Yazici H . Fever in Behçet’s syndrome. Clin Exp Rheumatol. (2013) 31:64–7.
21.
Maksymowych WP Wichuk S Dougados M Jones H Szumski A Bukowski JF et al . MRI evidence of structural changes in the sacroiliac joints of patients with non-radiographic axial spondyloarthritis even in the absence of MRI inflammation. Arthritis Res Ther. (2017) 19:126. doi: 10.1186/s13075-017-1342-9
22.
Perrotta FM Scriffignano S Lubrano E . MRI assessment of extra-axial findings at pelvic sites in a Group of Axial-SpA patients. Rheumatol Ther. (2021) 8:1897–904. doi: 10.1007/s40744-021-00375-z
23.
Huang ZG Zhang XZ Hong W Wang GC Zhou HQ Lu X et al . The application of MR imaging in the detection of hip involvement in patients with ankylosing spondylitis. Eur J Radiol. (2013) 82:1487–93. doi: 10.1016/j.ejrad.2013.03.020
24.
Sudoł-Szopińska I Jurik AG Eshed I Lennart J Grainger A Østergaard M et al . Recommendations of the ESSR arthritis subcommittee for the use of magnetic resonance imaging in musculoskeletal rheumatic diseases. Semin Musculoskelet Radiol. (2015) 19:396–411. doi: 10.1055/s-0035-1564696
25.
Hoffstetter P Al Suwaidi MH Joist A Benditz A Fleck M Stroszczynski C et al . Magnetic resonance imaging of the axial skeleton in patients with Spondyloarthritis: distribution pattern of inflammatory and structural lesions. Clin Med Insights Arthritis Musculoskelet Disord. (2017) 10:117954411772808. doi: 10.1177/1179544117728081
26.
Bochkova AG Levshakova AV Bunchuk NV Braun J . Spinal inflammation lesions as detected by magnetic resonance imaging in patients with early ankylosing spondylitis are more often observed in posterior structures of the spine. Rheumatology (Oxford). (2010) 49:749–55. doi: 10.1093/rheumatology/kep419
27.
Althoff CE Feist E Burova E Eshed I Bollow M Hamm B et al . Magnetic resonance imaging of active sacroiliitis: do we really need gadolinium?Eur J Radiol. (2009) 71:232–6. doi: 10.1016/j.ejrad.2009.04.034
28.
Bredella MA Steinbach LS Morgan S Ward M Davis JC . MRI of the sacroiliac joints in patients with moderate to severe ankylosing spondylitis. AJR Am J Roentgenol. (2006) 187:1420–6. doi: 10.2214/AJR.05.1423
29.
Gentili F Cantarini L Fabbroni M Nigri A Mazzei FG Frediani B et al . Magnetic resonance imaging of the sacroiliac joints in SpA: with or without intravenous contrast media? A preliminary report. Radiol Med. (2019) 124:1142–50. doi: 10.1007/s11547-019-01016-w
30.
Mandl P Navarro-Compán V Terslev L Aegerter P van der Heijde D D'Agostino MA et al . EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis. (2015) 74:1327–39. doi: 10.1136/annrheumdis-2014-206971
Summary
Keywords
spondyloarthritis, autoinflammatory disease, fever attacks, magnetic resonance imaging, sacroiliitis, power doppler ultrasound
Citation
Di Meglio N, Sica C, Vitale A, Caggiano V, Perrella A, Di Martino V, Di Stefano M, Fratarcangeli M, Bagnacci G, Ruscitti P, Cipriani P, Shariat Panahi A, Tharwat S, Elberashi HM, Othman EE, Conforti A, Lucchetti L, Varini F, Sota J, Guaracha-Basañez GA, Kawakami-Campos PA, Martín-Nares E, Thabet M, Wissal BY, Erten S, Eksin MA, Özdemir Ulusoy B, Barone P, Papa S, Giugno A, Gentileschi S, Gaggiano C, Buonanno S, Hashad S, Etayari H, Tufan A, Ragab G, Wiesik-Szewczyk E, Hernández-Rodríguez J, Balistreri A, Hinojosa-Azaola A, Fabiani C, Frediani B, Cantarini L and Mazzei MA (2025) Imaging findings in patients with axial spondyloarthritis presenting with recurrent fever attacks: data from the international AIDA network spondyloarthritis registry. Front. Med. 12:1662890. doi: 10.3389/fmed.2025.1662890
Received
09 July 2025
Accepted
25 August 2025
Published
15 September 2025
Volume
12 - 2025
Edited by
Giuseppe Lopalco, University of Bari Aldo Moro, Italy
Reviewed by
Michele Maria Luchetti Gentiloni, Marche Polytechnic University, Italy
Fabio Massimo Perrotta, University of Molise, Italy
Updates
Copyright
© 2025 Di Meglio, Sica, Vitale, Caggiano, Perrella, Di Martino, Di Stefano, Fratarcangeli, Bagnacci, Ruscitti, Cipriani, Shariat Panahi, Tharwat, Elberashi, Othman, Conforti, Lucchetti, Varini, Sota, Guaracha-Basañez, Kawakami-Campos, Martín-Nares, Thabet, Wissal, Erten, Eksin, Özdemir Ulusoy, Barone, Papa, Giugno, Gentileschi, Gaggiano, Buonanno, Hashad, Etayari, Tufan, Ragab, Wiesik-Szewczyk, Hernández-Rodríguez, Balistreri, Hinojosa-Azaola, Fabiani, Frediani, Cantarini and Mazzei.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nunzia Di Meglio, dimeglionunzia@gmail.com; Maria Antonietta Mazzei, mazzei@unisi.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.