- Postgraduate School, Harbin Sport University, Harbin, China
Objective: In accordance with the 2024 Physical Activity Guidelines, this study aimed to evaluate the effects of exercise interventions on subjective sleep quality in older adults and to explore the potential dose–response relationship.
Methods: A systematic search was conducted in Web of Science, PubMed, Cochrane Library, Scopus, and Embase for randomized controlled trials (RCTs) published up to May 1, 2025. Meta-analysis was performed using R, with standardized mean differences (SMD) and 95% confidence intervals (95% CI) used to quantify effect sizes. Subgroup analysis, meta-regression, and nonlinear dose–response modeling were conducted.
Results: A total of 26 RCTs involving 2,189 elderly participants were included. The meta-analysis revealed that exercise interventions significantly improved subjective sleep quality [SMD = −2.46, 95% CI (−2.99, −1.93), p < 0.001]. The most pronounced effects were observed in interventions with session durations ≤30 min [WMD = −4.25, 95% CI (−5.49, −3.02)], low intensity [WMD = −2.79, 95% CI (−3.44, −2.14)], twice-weekly frequency [WMD = −2.52, 95% CI (−3.00, −2.04)], and intervention durations ≤8 weeks [WMD = −2.45, 95% CI (−2.99, −1.91)]. Meta-regression showed no significant linear associations between sleep outcomes and intervention duration, intensity, frequency, or length. A nonlinear “U-shaped” dose–response relationship was identified, with the optimal effect observed at approximately 527 MET·min/week [Hedges’ g = −0.82, 95% CI (−1.12, −0.52)].
Conclusion: Low-frequency, short-duration, and low-to-moderate intensity exercise interventions can effectively improve subjective sleep quality in older adults. Notably, even low-dose exercise can yield significant benefits.
Introduction
With the global trend of population aging intensifying, the growing burden of the elderly population on healthcare systems and socioeconomic development has made age-related diseases a pressing public health concern (1). Studies have shown that older adults frequently suffer from a range of chronic health conditions, among which sleep disturbances are among the most common, significantly impairing both physical and mental health as well as quality of life (2, 3). Compared to younger adults, older individuals are more prone to disruptions in sleep architecture, reductions in deep sleep, and disturbances in circadian rhythms, often manifested as difficulty falling asleep, early morning awakening, and frequent nocturnal awakenings (4).
Sleep disturbances are not only considered early indicators of frailty syndrome in older adults but are also closely associated with adverse outcomes such as hypertension, coronary heart disease, cognitive decline, depression, and increased mortality (5, 6). Although pharmacological interventions remain the primary approach to managing sleep disorders, the long-term use of hypnotic medications may lead to drug dependence, cognitive impairment, and a heightened risk of falls, rendering such treatments inadequate for meeting the needs of safe, sustainable, and effective rehabilitation in elderly populations (7). Therefore, the development of non-pharmacological interventions—particularly those involving safe and sustainable lifestyle modifications—is of critical importance for improving sleep health in older adults.
Growing evidence suggests that exercise, as a planned and repetitive form of physical activity, can effectively mitigate age-related health risks by enhancing muscular strength, improving cardiopulmonary function, and regulating metabolic and neuroendocrine processes (8). In the context of sleep, exercise interventions have been shown to improve subjective sleep quality, reduce sleep onset latency, and decrease nighttime awakenings, while also potentially reducing reliance on pharmacological treatments (2, 9, 10). However, there remains a lack of consensus regarding the optimal type, intensity, frequency, and duration of exercise for improving sleep, particularly among older adults. The formulation of safe, evidence-based, and personalized intervention strategies remains underexplored in this population. The Pittsburgh Sleep Quality Index (PSQI) was chosen as the primary outcome because it is widely validated in older adults, sensitive to intervention effects, and provides a multidimensional assessment of sleep quality beyond single indicators (11, 12).
To address this gap, the present study employed a systematic review and meta-analytic approach to synthesize existing randomized controlled trial (RCT) data, with the aim of evaluating the effects of exercise interventions on subjective sleep quality in older adults. Furthermore, the dose–response relationship between intervention parameters and sleep outcomes was investigated, in order to provide both theoretical and practical guidance for developing individualized exercise prescriptions tailored to the physiological characteristics of the elderly (13).
Methods
This systematic review was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14) and was prospectively registered in the PROSPERO database (Registration No. CRD42025636756).
Search strategy
A comprehensive literature search was performed across five electronic databases: PubMed, Web of Science, Scopus, Embase, and the Cochrane Library, covering all records from database inception to May 1, 2025. The search strategy for PubMed was as follows: (“sleep” [Title/Abstract] OR “sleep quality” [Title/Abstract] OR “slumber” [Title/Abstract] OR “rest” [Title/Abstract]) AND (“exercise” [Title/Abstract] OR “physical exercise” [Title/Abstract] OR “exercise training” [Title/Abstract] OR “physical activity” [Title/Abstract] OR “workout” [Title/Abstract]) AND (“older adults” [Title/Abstract] OR “elderly” [Title/Abstract] OR “aged adults” [Title/Abstract] OR “seniors” [Title/Abstract] OR “middle-aged and older adults” [Title/Abstract]) AND (randomized controlled trial [Filter]).
The detailed search strategy for each database is presented in Supplementary Appendix A. The literature screening process was conducted independently and in duplicate by two reviewers using a blinded method. Any disagreements were resolved by consultation with a third reviewer.
Inclusion criteria
1. Language: Only studies published in English were considered;
2. Participants: Studies involving older adults aged 60 years and above without medical conditions that severely impair sleep were included;
3. Study Design: Only randomized controlled trials (RCTs) were included;
4. Intervention: The experimental group received regular exercise interventions over a defined period, while the control group received no exercise intervention;
5. Type of Intervention: Various exercise modalities were accepted, including differences in content, intensity, duration, frequency, and intervention period.
Exclusion criteria
1. Review articles, conference abstracts, or case studies;
2. Animal studies;
3. Studies involving only a single exercise session rather than repeated or long-term interventions;
4. Duplicated publications, studies of poor methodological quality, or those for which the full text could not be obtained;
5. Studies that did not report the Pittsburgh Sleep Quality Index (PSQI) as an outcome measure.
Study selection and data extraction
Two reviewers independently screened the literature based on pre-specified inclusion and exclusion criteria. Initially, titles and abstracts were reviewed to exclude studies irrelevant to the research topic. Full-text screening was subsequently conducted for potentially eligible studies to determine their final inclusion. Any discrepancies during the selection process were resolved through discussion with a third reviewer.
Data extraction was conducted concurrently with the full-text review. The extracted information included the first author, year of publication, study location, study design, sample size, participant age, type of exercise, exercise intensity, frequency, duration per session, intervention period, and the primary outcome measure (Pittsburgh Sleep Quality Index, PSQI). In all included studies, the PSQI was assessed both at baseline and post-intervention to evaluate changes in subjective sleep quality. For the purpose of this meta-analysis, subjective sleep quality was operationalized as the global PSQI score, unless otherwise specified. Only the global score was used for analysis; individual PSQI components (e.g., total sleep time, sleep latency) were not examined separately due to inconsistent reporting across studies.
For multiple publications originating from the same trial, only one version was included to avoid duplicate data. The publication that best matched the research objectives and provided the most complete data—typically the most recent version—was prioritized, while others were excluded. To minimize subjectivity in the classification of exercise variables, the following criteria were applied:
1. Fixed values of intensity, frequency, and duration were recorded when explicitly reported;
2. For variables presented as ranges, the average value was calculated;
3. Exercise intensity, when reported using different scales (e.g., Borg 15-point scale or Borg 10-point scale), was standardized using the Borg CR10 scale for dose–response analysis (15). Specifically, the studies by Chen et al. (16); Chen et al. (17); and Siu et al. (18) reported the intensity directly using the CR10 scale; the studies by King (19); Sharif et al. (20); and Sternfeld et al. (21) used the Borg RPE15 scale and were therefore converted to CR10; all remaining studies reported exercise intensity as a percentage of maximum heart rate or oxygen uptake and were converted using percentage-based approximations.
Quality assessment
The risk of bias and methodological quality of included RCTs were assessed using the Cochrane Risk of Bias (RoB) tool. This tool evaluates seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other potential sources of bias.
Each domain was rated as “low risk,” “unclear risk,” or “high risk” of bias. The assessment was independently conducted by two reviewers based on the original reports. In cases of disagreement, a third reviewer participated in discussion to reach consensus, thereby ensuring objectivity and consistency in the evaluation process.
Statistical analysis
Meta-analyses were performed using R software. Subgroup analyses were conducted to examine the effects of different intervention characteristics. Heterogeneity was assessed using Cochran’s Q test, with a significance level set at α = 0.1. The degree of heterogeneity was quantified using the I2 statistic: I2 < 50% and p > 0.1 were interpreted as low heterogeneity, in which case a fixed-effects model was applied; I2 ≥ 50% and p < 0.1 indicated substantial heterogeneity, warranting the use of a random-effects model.
The primary outcome was treated as a continuous variable. For studies employing the same sleep quality scale, weighted mean differences (WMD) with 95% confidence intervals (CI) were calculated. For studies using different scales, standardized mean differences (SMD) with 95% CI were computed and used in sensitivity analyses.
To explore the relationship between intervention dose and sleep improvement, a nonlinear dose–response meta-analysis was conducted. A random-effects one-stage mixed-effects model was employed to simultaneously estimate the dose–response relationship across all studies. Restricted cubic spline models and maximum likelihood estimation were applied at fixed percentiles (5, 50, and 95%) to model the relationship between exercise dose (expressed in MET-minutes/week) and improvements in subjective sleep quality, without assuming a predefined functional form. Exercise dose was calculated as the product of session duration, frequency, and intensity. A reference dose of zero was used for estimating relative effect sizes under varying exercise exposure conditions.
Results
Search results
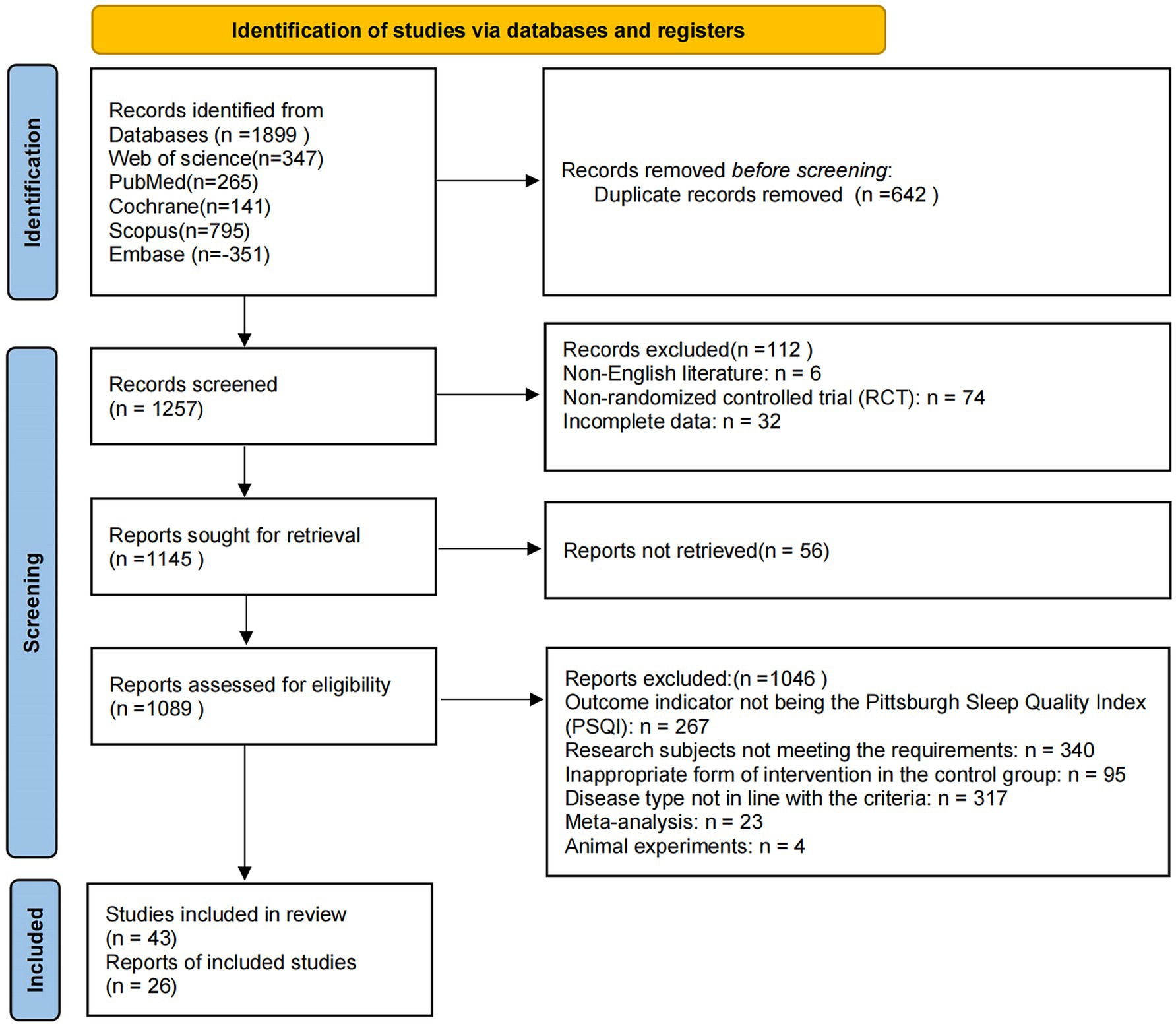
A systematic search of five databases (PubMed, Web of Science, Cochrane Library, Scopus, and Embase) yielded a total of 1,899 relevant records. After removing 642 duplicates, 1,257 studies remained for title and abstract screening. During this stage, 112 articles were excluded, including 6 non-English publications, 74 non-randomized controlled trials, and 32 with incomplete data. A total of 1,145 articles were retrieved for full-text review, of which 56 could not be obtained. Among the 1,089 reports assessed for eligibility, 1,046 were excluded for the following reasons: outcome indicators not involving the Pittsburgh Sleep Quality Index (PSQI; n = 267), study participants not meeting inclusion criteria (n = 340), inappropriate form of intervention in the control group (n = 95), disease type not in line with inclusion criteria (n = 317), meta-analysis articles (n = 23), and animal experiments (n = 4).
Following full-text assessment, 26 randomized controlled trials were identified as eligible and included in the systematic review and meta-analysis (17–42). The study selection process is illustrated in Figure 1. In this study, “subjective sleep quality” was consistently defined as the global PSQI score, unless otherwise specified. Only the global score of the PSQI was used for the meta-analysis, and individual components such as total sleep time or sleep latency were not analyzed separately due to inconsistent reporting across studies.
Characteristics of included studies
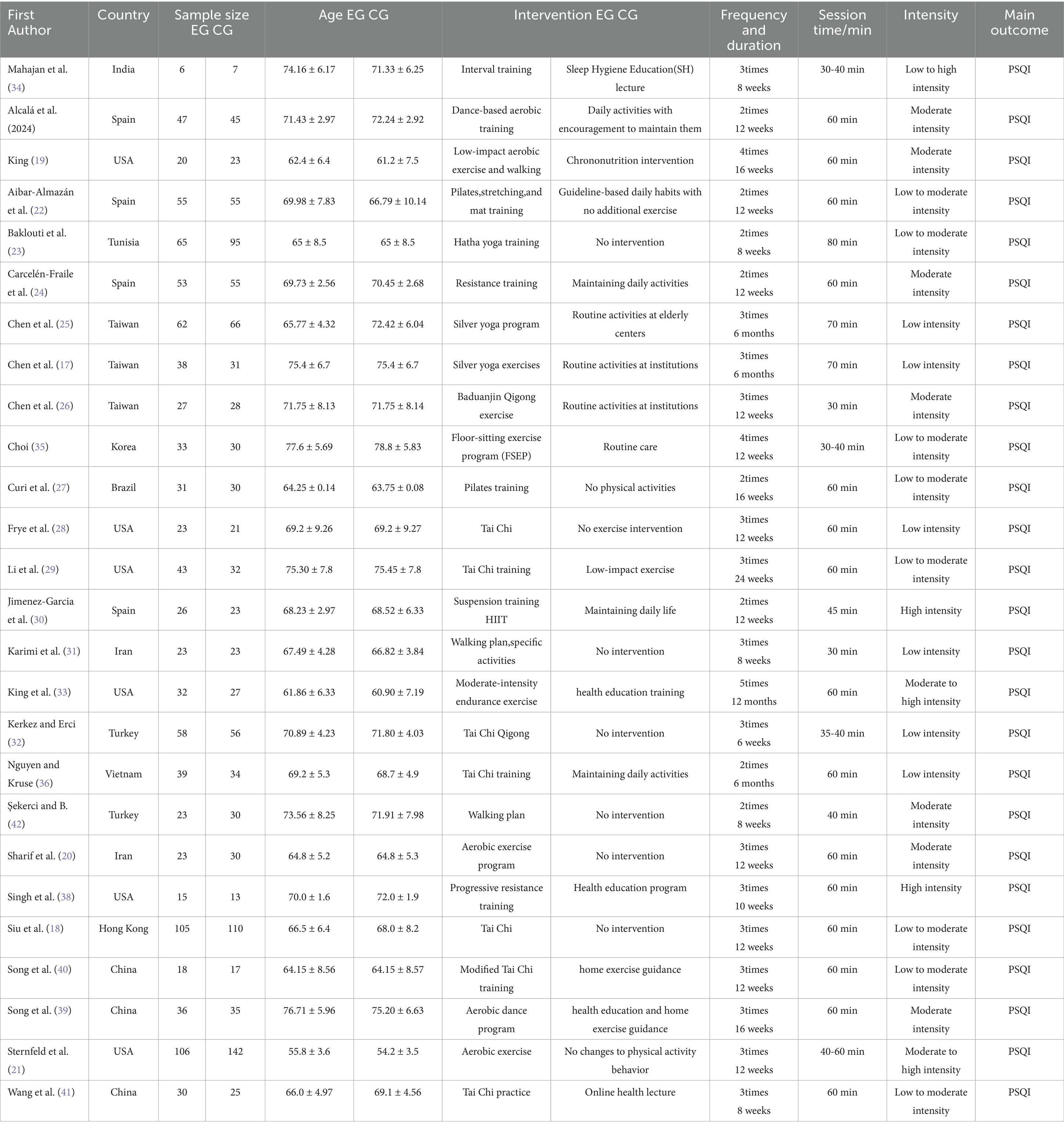
A total of 26 randomized controlled trials (17–42) involving a total of 2,189 older adults aged between 60 and 90 years were included in the analysis.
In terms of exercise type, several studies employed multicomponent programs integrating aerobic, resistance, flexibility, and balance training (19, 22–24, 30, 37, 41), while the remaining trials adopted single-modality interventions, such as: Tai Chi: (20, 26, 29); Yoga, Pilates, or stretching: (17, 34); Aerobic walking or dance: (21, 27, 35, 36); Progressive resistance training: (31, 38).
With respect to exercise intensity, most multicomponent programs and aerobic walking interventions were conducted at a moderate intensity; Tai Chi, yoga, and stretching were generally considered low-intensity mind–body exercises; whereas Singh et al. (38) and Karimi et al. (31) implemented high-intensity progressive resistance training protocols.
Regarding intervention duration, the included studies ranged from 6 weeks to 12 months, with the majority lasting 12 to 24 weeks. Training frequency was typically set at 2–5 sessions per week across studies.
Overall, the included trials encompassed exercise modes ranging from low to high intensity and intervention durations from short to long term. Detailed information on exercise types, intensity, frequency, and duration is presented in Table 1.
Quality assessment results
All 26 included studies (17–42) were evaluated using the Cochrane Risk of Bias assessment tool.
The results indicated that most studies exhibited a low risk of bias in domains such as random sequence generation, blinding of outcome assessors, and handling of incomplete outcome data, suggesting a generally high methodological quality. However, some studies provided unclear or insufficient descriptions regarding the blinding of participants and personnel, indicating a potential risk of performance bias in these domains. Detailed results of the quality assessment are presented in Figure 2.
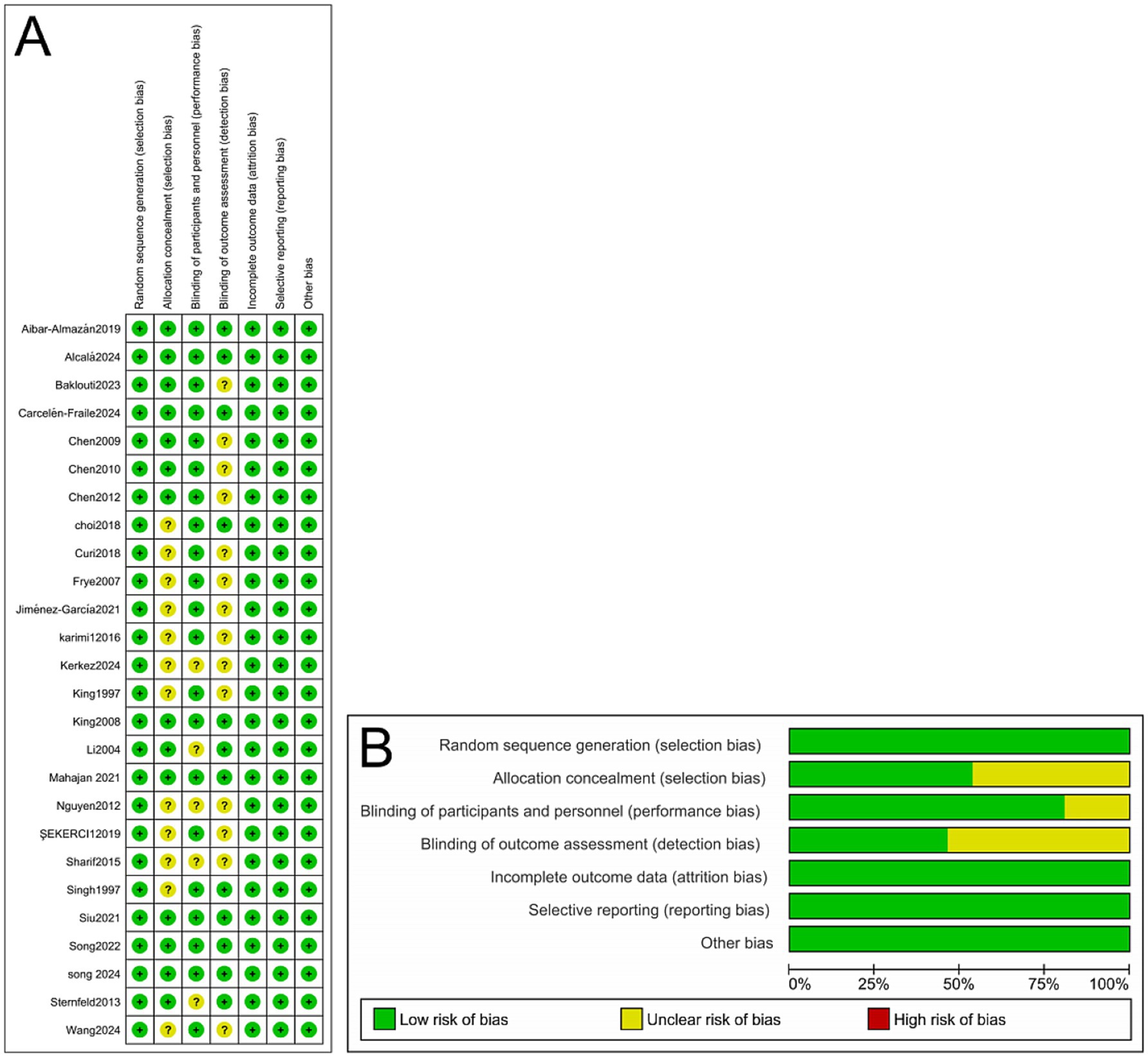
Figure 2. (A) Methodological quality assessment of 26 included RCTs. (B) Risk-of-bias summary and risk-of-bias graph for 26 RCTs.
Meta-analysis results
Effect of exercise on sleep in older adults
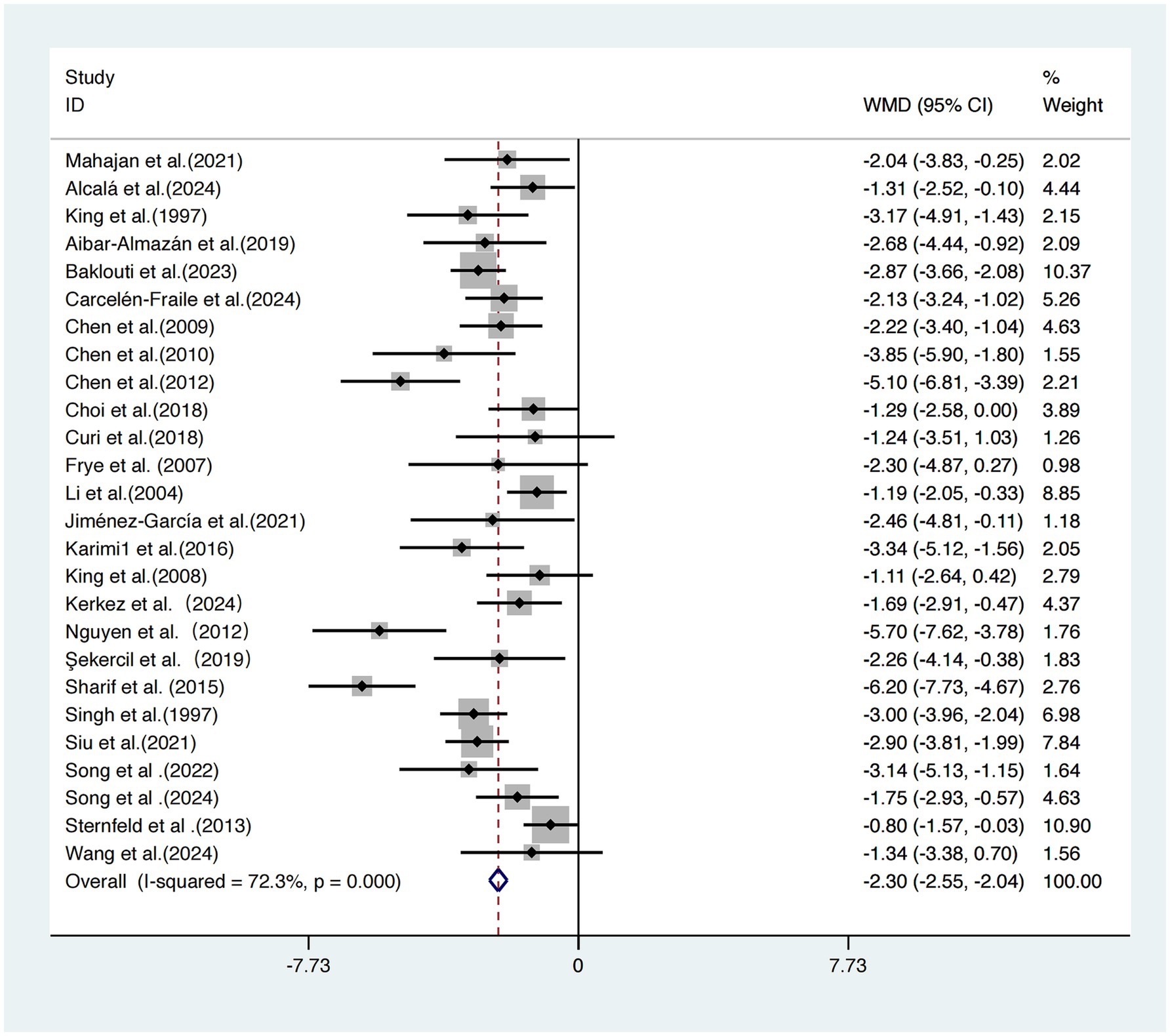
This meta-analysis included 26 studies (17–42) evaluating the effects of exercise interventions on subjective sleep quality in older adults.
Due to significant heterogeneity across studies (I2 = 72.3%, τ2 = 1.26, p < 0.001), a random-effects model was employed. The pooled results showed that exercise interventions significantly improved subjective sleep quality compared to control conditions, with a statistically significant effect size standardized mean difference [SMD = −2.30, 95% CI (−2.55, −2.04), p < 0.001] (Figure 3).
Publication bias
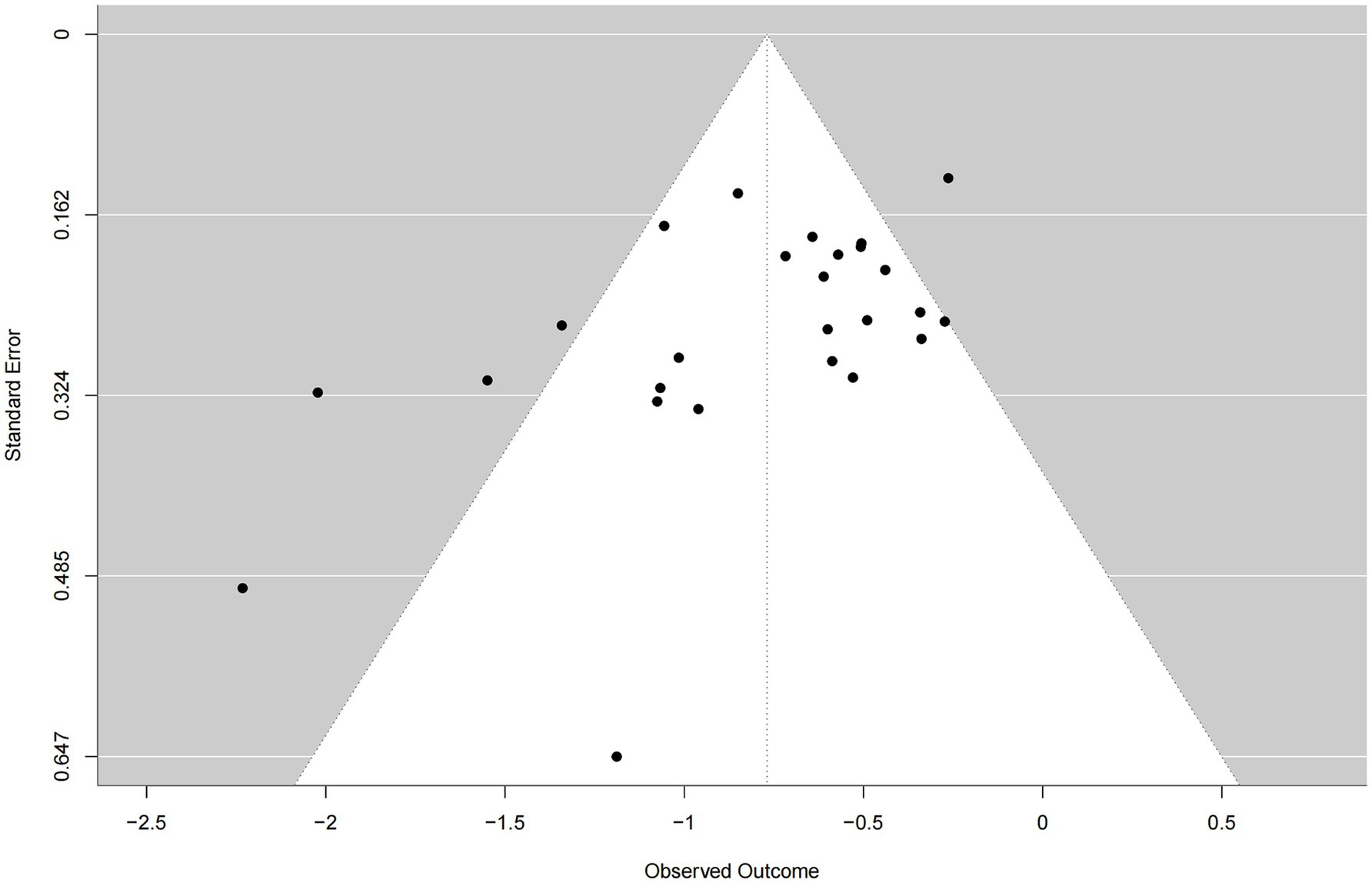
The funnel plot demonstrated an approximately symmetrical distribution of studies around the pooled effect estimate and showed convergence from the bottom to the top, suggesting no substantial evidence of publication bias in this meta-analysis (Figure 4).
Dose–response relationship between exercise and sleep improvement in older adults
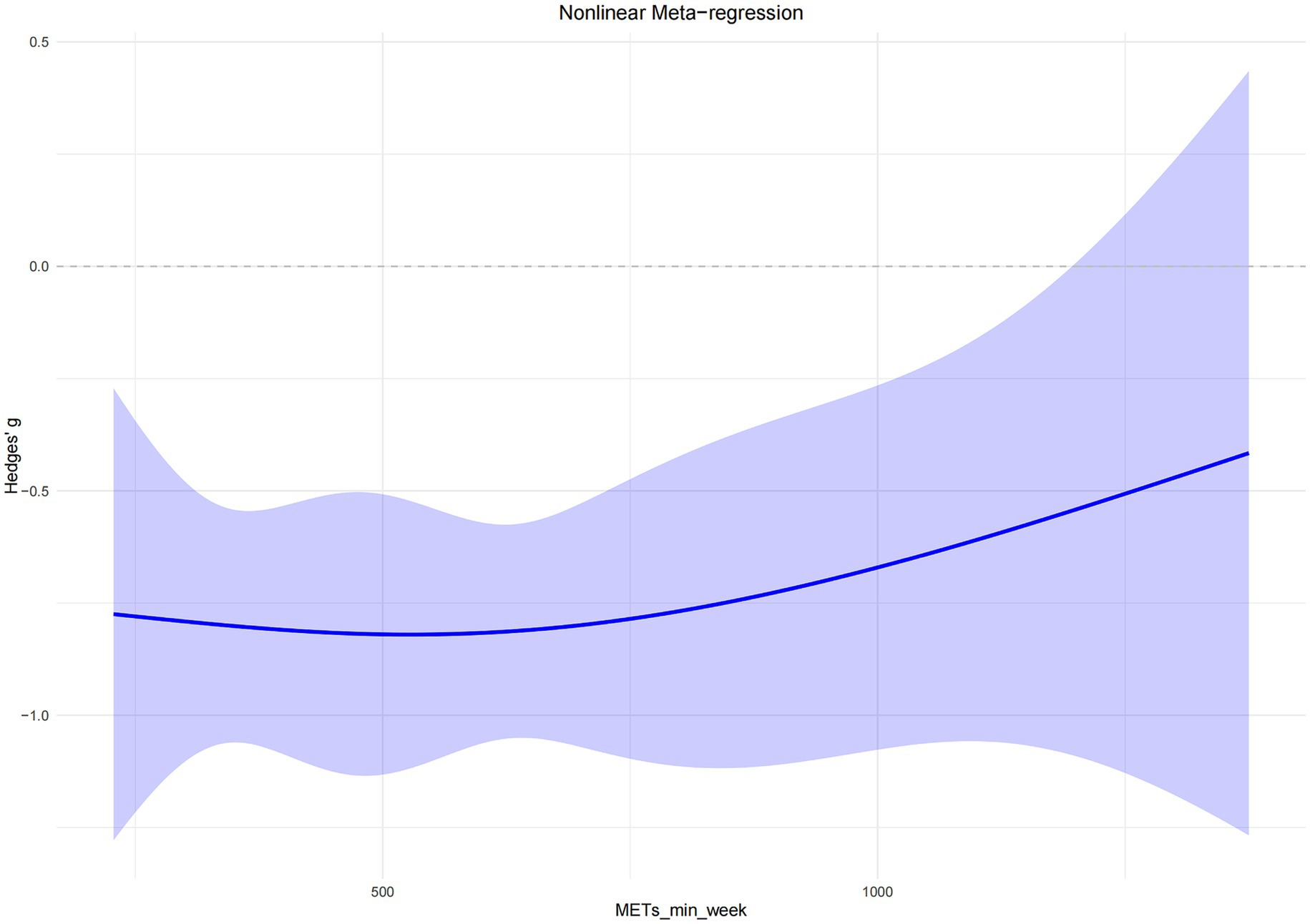
The overall dose–response curve between exercise dose and sleep improvement in older adults exhibited an inverted U-shaped pattern. In the low-dose range (approximately 200–500 MET-min/week), the intervention effect increased progressively with the amount of exercise. The greatest effect was observed at approximately 527 MET-min/week, representing the lowest point of the curve (Hedges’ g = −0.82), with a predicted effect estimate of −0.82 (95% CI: −1.12, −0.52). As exercise volume continued to increase beyond this point, the effect began to attenuate slightly, as indicated by the upward slope of the curve (Figure 5).
Subgroup analysis
Intervention duration per session
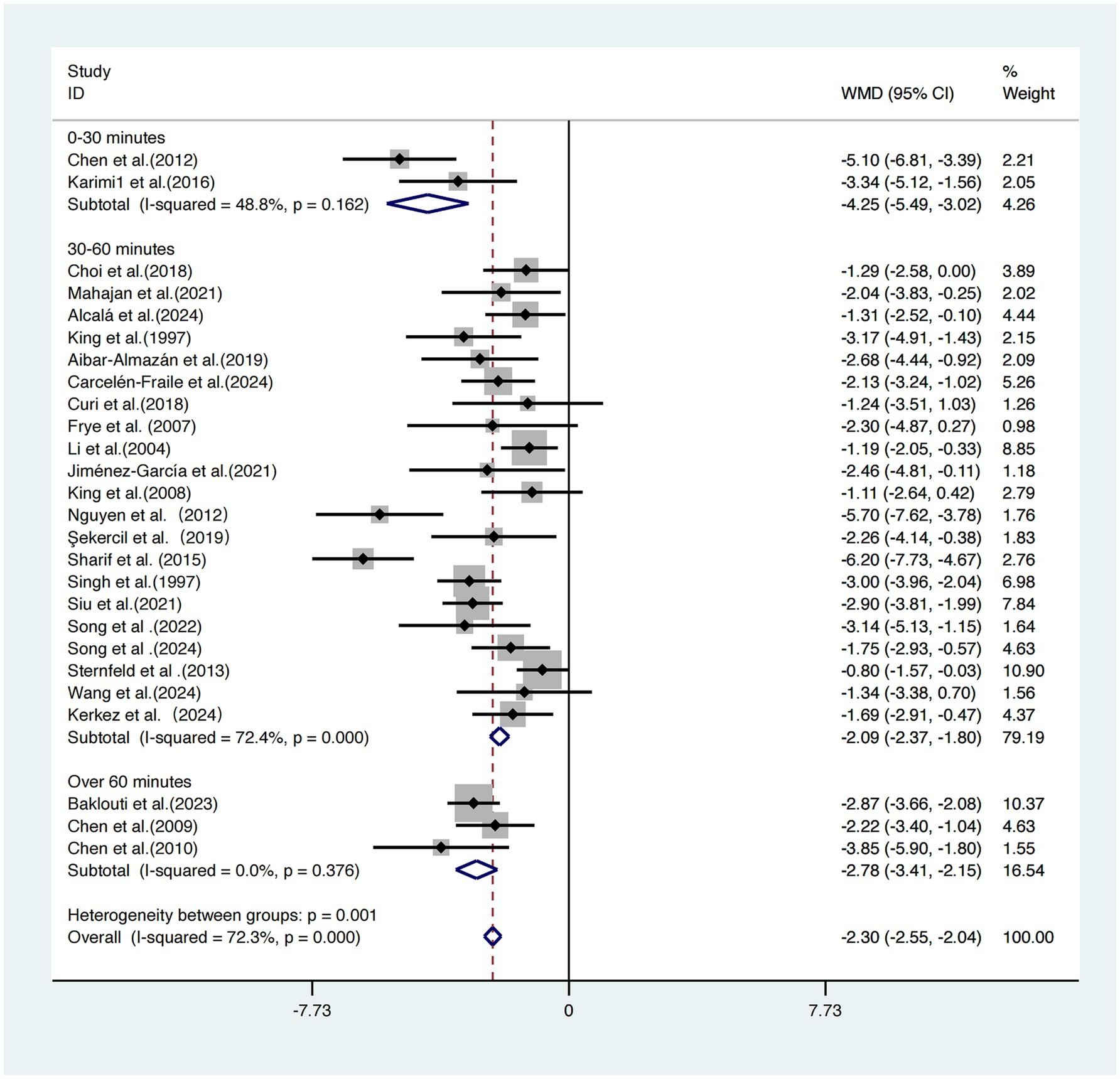
Based on the duration of each exercise session, the 26 included studies (17–42) were categorized into three subgroups for analysis.
The subgroup receiving 0–30-min sessions demonstrated the most pronounced intervention effect, with a weighted mean difference WMDs of −4.25 (95% CI: −5.49, −3.02), and moderate heterogeneity (I2 = 48.8%, p = 0.162), indicating relatively stable and consistent results.
The 30–60-min group showed a WMD of −2.09 (95% CI: −2.37, −1.80), but with high heterogeneity (I2 = 72.4%, p < 0.001), suggesting greater uncertainty in the results.
The >60-min group reported a WMD of −2.78 (95% CI: −3.41, −2.15) and exhibited the lowest heterogeneity (I2 = 0.0%, p = 0.376).
A statistically significant difference was found across subgroups (p = 0.001), indicating that session duration is a key moderator of intervention effectiveness.
Considering the magnitude of the effect size, the width of the confidence intervals, and heterogeneity levels, exercise sessions lasting 0–30 min appear to offer a relatively optimal balance between efficacy and practical feasibility (Figure 6).
Intervention intensity
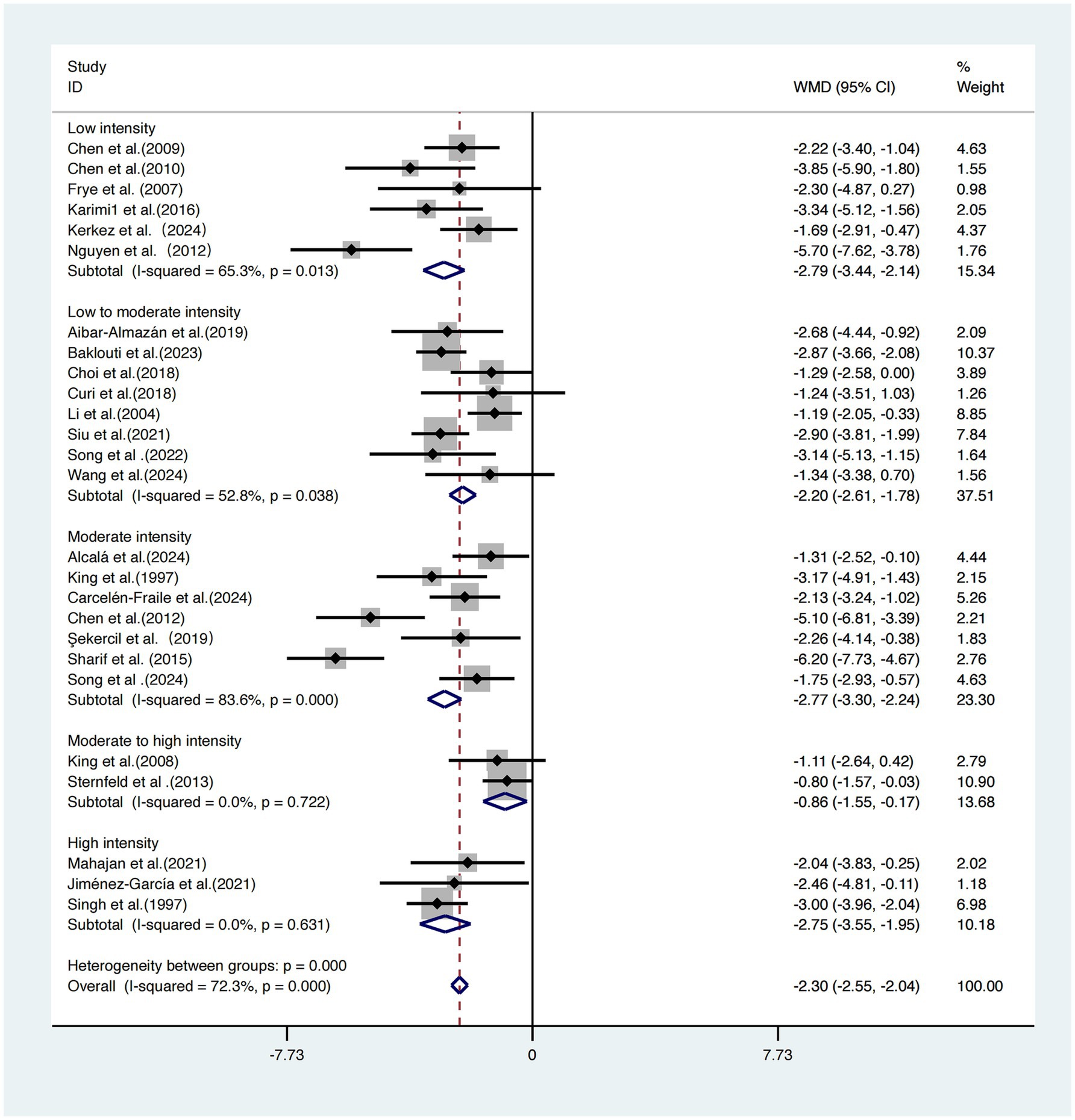
A subgroup analysis based on exercise intensity was conducted across the 26 included studies (17–42).
The subgroup with low-intensity interventions demonstrated the greatest effect size [WMD = −2.79, 95% CI (−3.44, −2.14)] with moderate heterogeneity (I2 = 65.3%). The moderate-intensity group yielded a similar effect size [WMD = −2.77, 95% CI (−3.30, −2.24)] but showed high heterogeneity (I2 = 83.6%). The low-to-moderate intensity group reported a WMD of −2.20 (95% CI: −2.61, −1.78) with moderate heterogeneity (I2 = 52.8%). The high-intensity group showed a WMD of −2.75 (95% CI: −3.55, −1.95) with no observed heterogeneity (I2 = 0.0%).
By contrast, the moderate-to-high intensity group demonstrated the weakest intervention effect [WMD = −0.86, 95% CI (−1.55, −0.17)]. The differences among subgroups were statistically significant (p = 0.000), indicating that intervention intensity plays a critical role in the effectiveness of sleep improvement (Figure 7).
Intervention frequency
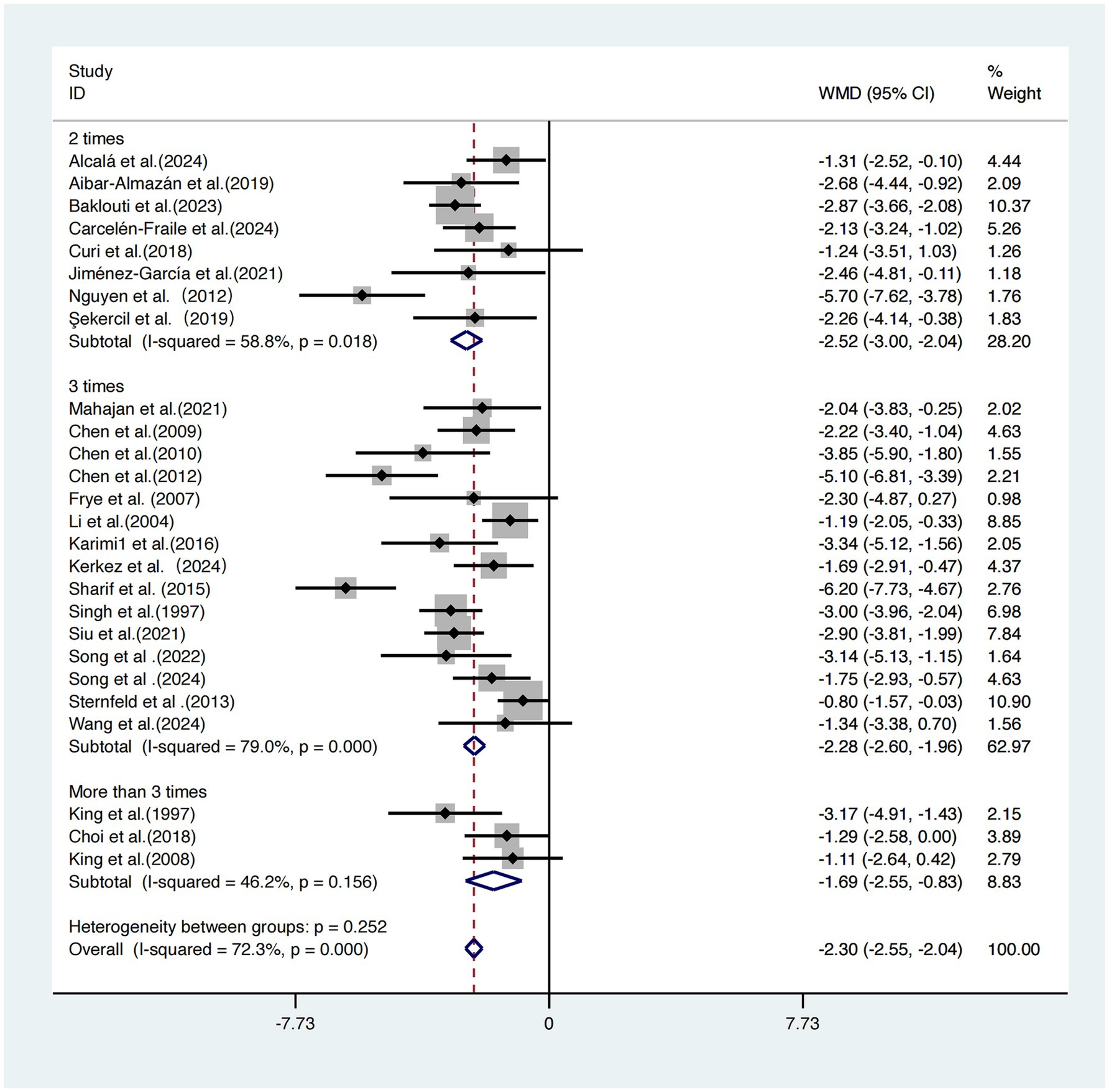
Subgroup analysis based on intervention frequency was performed on the same 26 studies (17–42).
The group exercising twice per week showed the greatest improvement [WMD = −2.52, 95% CI (−3.00, −2.04)]. The group exercising three times per week had a slightly smaller effect size [WMD = −2.28, 95% CI (−2.60, −1.96)]. The group exercising more than three times per week demonstrated the weakest effect [WMD = −1.69, 95% CI (−2.55, −0.83)].
Although effect sizes differed among subgroups, the differences were not statistically significant (p = 0.252), suggesting that intervention frequency may not be a key determinant of intervention efficacy (Figure 8).
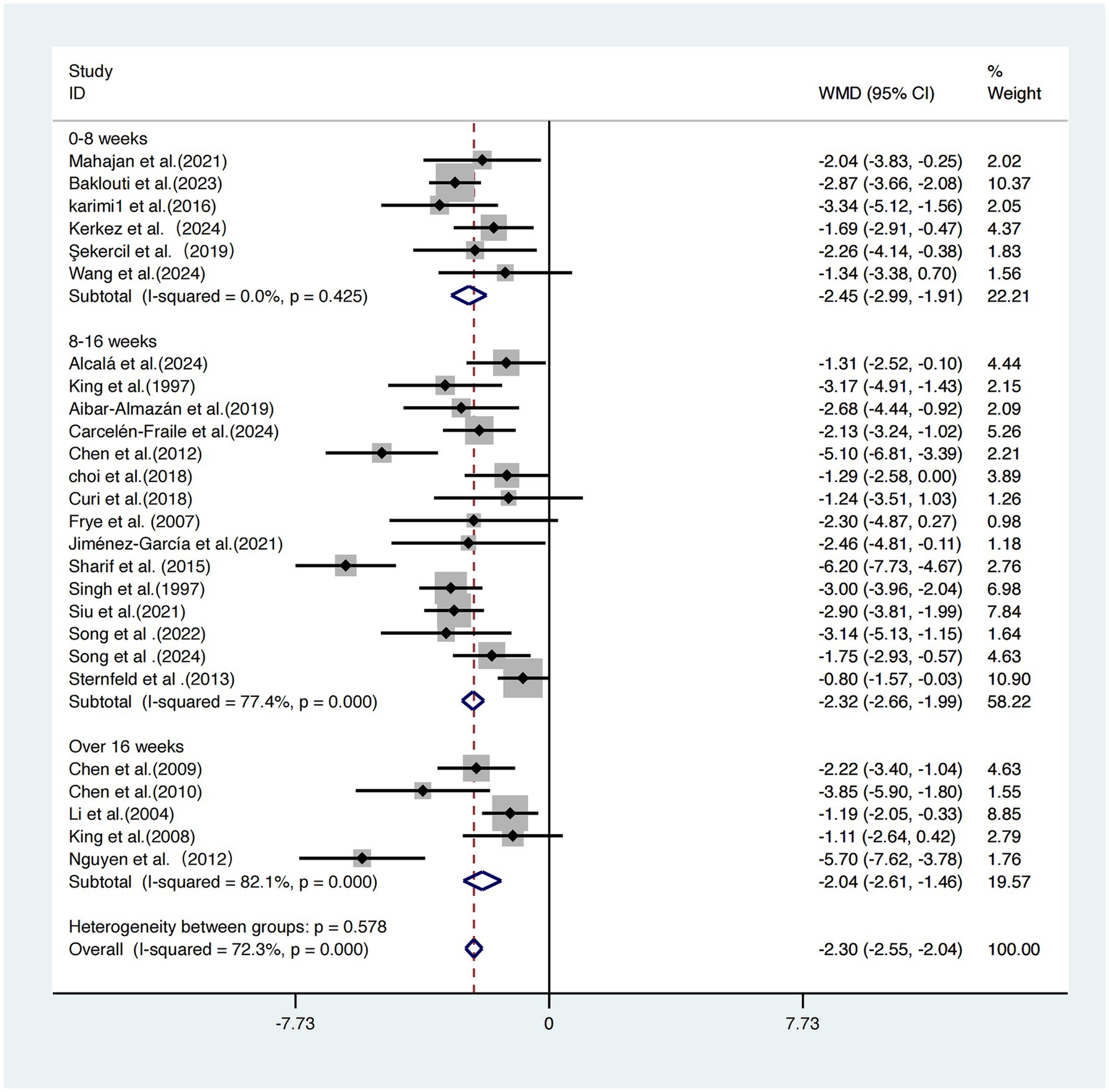
Intervention duration
The 26 included studies (17–42) were categorized into three groups based on total intervention duration: 0–8 weeks, 8–16 weeks, and >16 weeks.
The short-term intervention group (0–8 weeks) showed the most pronounced improvement in sleep quality [WMD = −2.45, 95% CI (−2.99, −1.91)], with no heterogeneity (I2 = 0.0%), suggesting highly consistent results. The moderate-term (8–16 weeks) and long-term (>16 weeks) groups yielded effect sizes of −2.32 and −2.04, respectively, but both exhibited high heterogeneity (I2 = 77.4 and 82.1%, respectively). Although all three subgroups demonstrated significant improvements in sleep outcomes, the between-group difference was not statistically significant (p = 0.578), indicating that total intervention duration may not be a critical factor influencing sleep improvement (Figure 9).
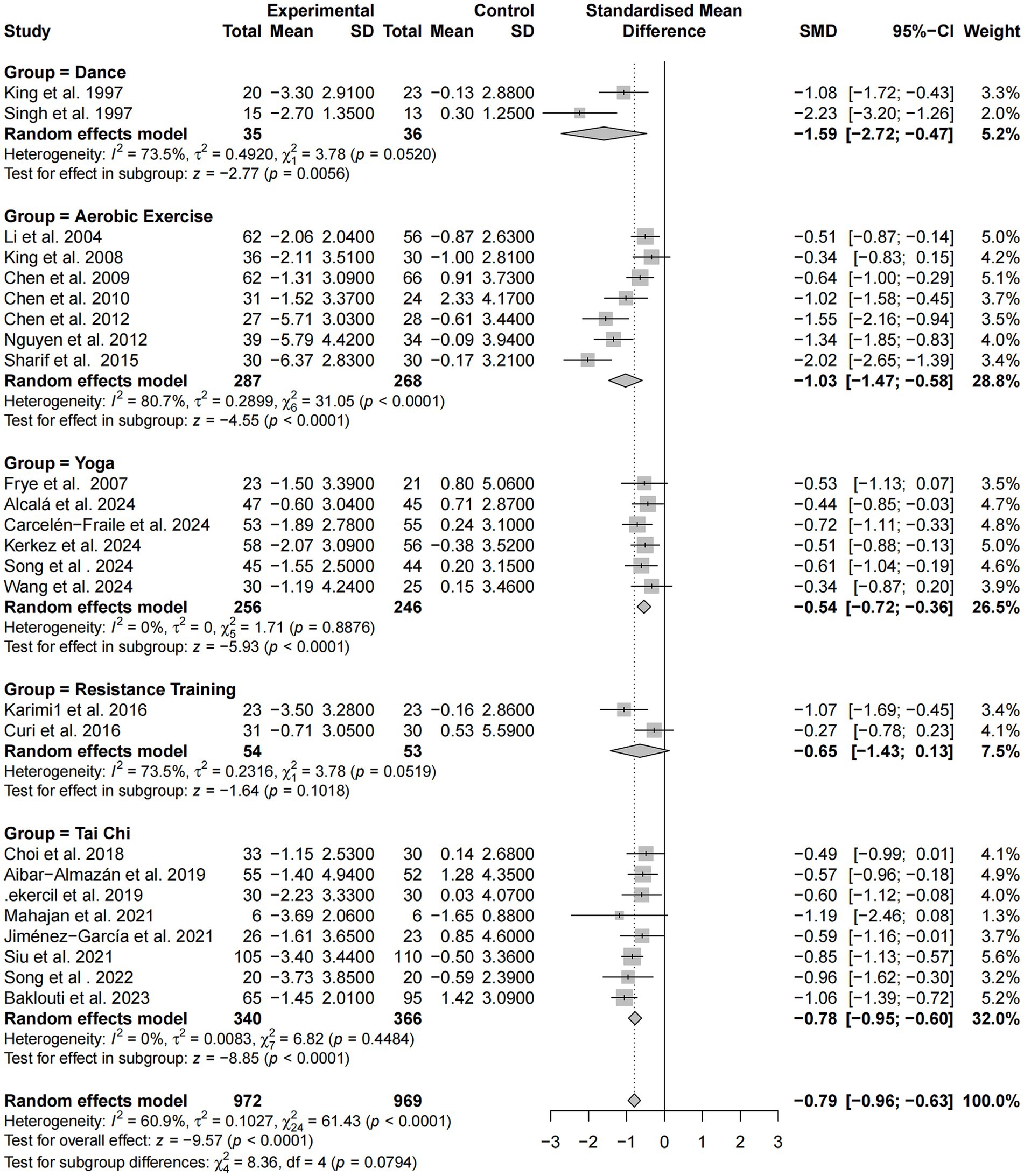
Intervention modality
A subgroup analysis based on exercise modality was performed using standardized mean differences (SMD) across the same 26 studies. Overall, exercise interventions produced a moderate improvement in sleep among older adults [SMD = −0.79, 95% CI (−0.96, −0.63), p < 0.0001], with moderate heterogeneity (I2 = 60.9%).
Among intervention types, dance-based programs showed the strongest effect (SMD = −1.59), albeit with substantial heterogeneity (I2 = 73.5%). Aerobic exercise also yielded a large effect (SMD = −1.03) with high heterogeneity (I2 = 80.7%). In contrast, yoga and tai chi interventions demonstrated stable and consistent effects, with effect sizes of −0.54 and −0.78, respectively, and no heterogeneity (I2 = 0.0%). Resistance training showed a modest effect (SMD = −0.65), but the confidence interval crossed zero, rendering the result statistically non-significant and heterogeneity relatively high (I2 = 73.5%).
Although the differences among subgroups approached statistical significance (p = 0.0794), the findings suggest that dance, aerobic exercise, and tai chi are particularly effective and replicable modalities for improving sleep quality, with dance-based interventions demonstrating the greatest potential benefit (Figure 10). It should be noted that SMD values were applied solely to the global PSQI score to harmonize differences in measurement scales across studies; no other sleep outcome measures were synthesized in this analysis.
Meta-regression analysis
The meta-regression analysis revealed no statistically significant linear relationships between intervention effect and any of the following variables: session duration (p = 0.933), exercise intensity (p = 0.867), intervention frequency (p = 0.849), or total intervention duration (p = 0.452; Figure 11).
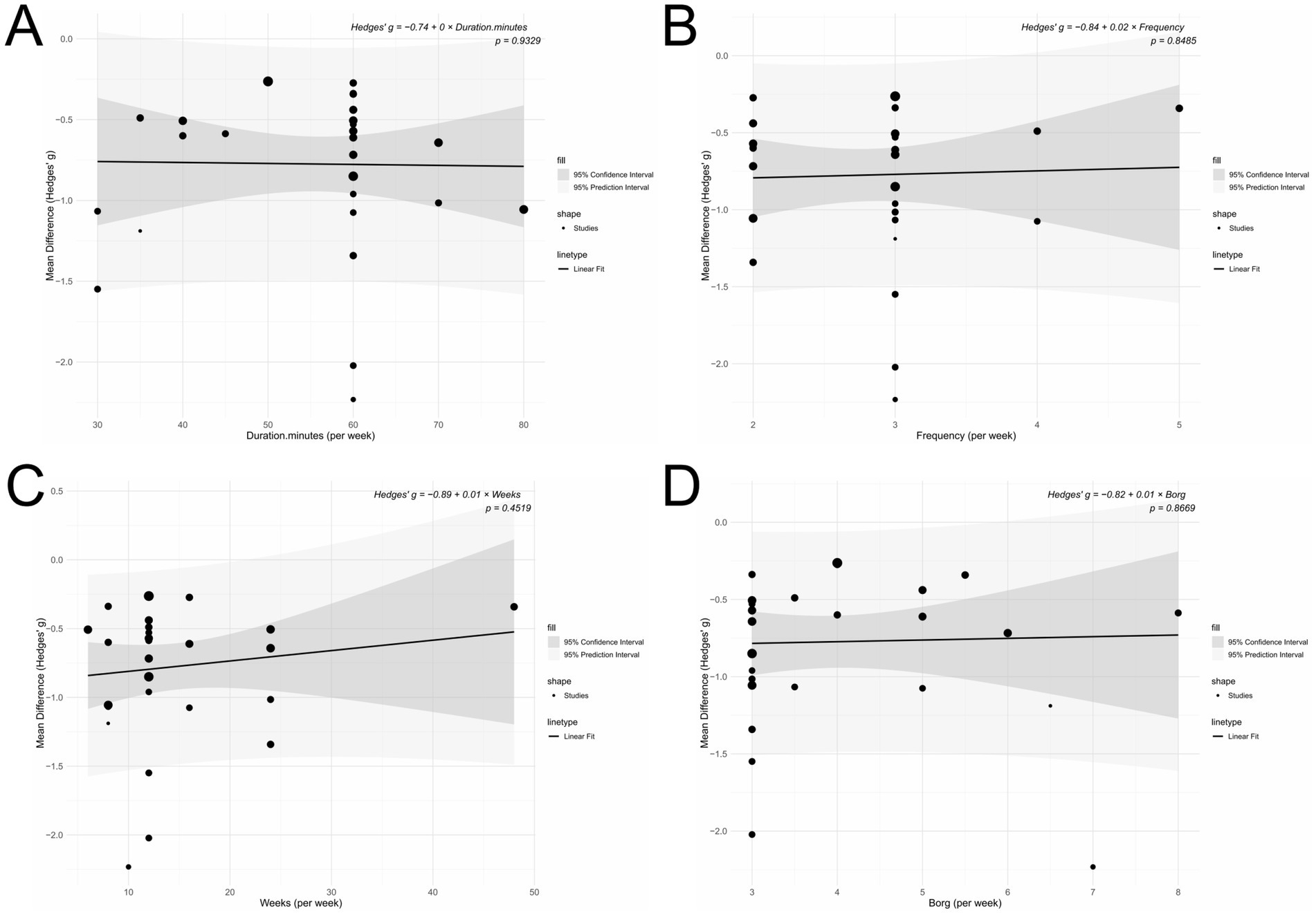
Figure 11. (A) Duration (minutes per week); (B) Frequency (per week); (C) Intervention period (weeks); (D) Intensity (Borg scale).
Discussion
Synthesis of evidence
Grounded in the 2024 Guidelines for Elderly Health, this study investigated the dose–response relationship between physical exercise and improvements in sleep among older adults using metabolic equivalents (METs). The results provide an evidence-based foundation for designing personalized sleep intervention prescriptions. The findings demonstrated that exercise interventions significantly improved subjective sleep quality in older adults. Among various intervention types, dance-based programs, aerobic exercise, and Tai Chi were particularly effective, demonstrating large and consistent improvements in subjective sleep quality. Although this study shows that the subgroup with low-intensity intervention had the greatest effect, it also indicates that moderate-intensity exercise yields significant benefits. This result is in line with the World Health Organization (WHO) guidelines of 2020 (43). Supporting literature indicates that 150–300 min of moderate-intensity exercise per week is sufficient to substantially enhance subjective sleep quality in the elderly (13).
The nonlinear dose–response model revealed an inverted U-shaped relationship, with the maximal intervention effect occurring at approximately 527 MET-min/week. Beyond this threshold, the benefit plateaued, indicating that moderate exercise doses are sufficient to produce substantial improvements in sleep quality.
Importantly, the findings further suggested that even lower exercise doses—approximately 300 MET-min/week—could yield significant improvements in subjective sleep quality, despite falling below the WHO’s recommendation of 600 MET-min/week. This implies that near-optimal effects can be achieved within a critical dosage range, minimizing unnecessary exercise burden. Such an approach is particularly well-suited for older adults with lower physical capacity or limited exercise tolerance. Zang et al. (15) also reported that just 300 MET-min/week of low-to-moderate intensity exercise could significantly alleviate frailty symptoms in older adults, with optimal benefits observed within the 600–1,200 MET-min/week range. This underscores that moderate exercise loads are both sustainable and adaptable, offering high safety and better long-term adherence among older populations—leading to consistent health outcomes (15).
Subgroup analyses revealed that intervention frequency and duration did not significantly influence the effectiveness of sleep improvement, suggesting these parameters may be less critical. However, trends were observed regarding exercise intensity and session duration: lower-intensity exercise and sessions lasting less than 30 min tended to produce more stable and sustainable improvements. These findings align with previous studies by Reid et al. (44), and Tseng et al. (45), which emphasized that moderate-intensity activities are more manageable for older adults and are associated with better compliance and outcomes. Reid et al. (44) demonstrated that moderate aerobic exercise improved insomnia symptoms in the elderly more effectively than high-intensity activity, which, due to the associated physiological demands (e.g., elevated heart rate, breathlessness), may reduce adherence.
It is particularly noteworthy that although high-intensity, long-duration exercise has shown potential benefits in some trials, older adults are more vulnerable to fatigue and fall-related injuries under such regimens. This has been explicitly noted by You et al. (46) and Crane et al. (47). Therefore, low-to-moderate intensity exercise with moderate session duration appears to be the most suitable approach for promoting sustained engagement and improving subjective sleep quality among older adults. Such interventions are not only safer but also better aligned with the physiological characteristics and health needs of this population.
Intervention strategies should prioritize safety while encouraging participation in enjoyable, gentle, and varied low-to-moderate intensity activities to enhance adherence and achieve long-term improvements in sleep and overall health. Flexibility in intervention design is particularly important for older adults, as it reduces fatigue, minimizes the risk of exercise-related injuries, and supports sustainable participation. Given the high prevalence of sleep disorders among elderly populations, rehabilitation specialists, exercise prescription developers, and researchers should develop personalized, moderate-intensity, and easy-to-follow programs—guided by the 2024 Guidelines for Elderly Health—to improve sleep quality and enhance overall well-being in a safe and adherent manner.
Clinical implications of the dose–response analysis
This study highlights the critical role of exercise interventions in improving subjective sleep quality among older adults, demonstrating that even low-dose exercise programs can yield significant benefits. In clinical practice, many older individuals are unable to meet the World Health Organization’s recommended levels of physical activity due to physical limitations or mobility impairments. Against this backdrop, low-dose and low-intensity exercise interventions are especially important. Such approaches not only promote gradual adaptation to physical activity but also reduce the risk of excessive fatigue or falls. A progressive, stepwise intervention model can enhance physical function while ensuring safety, thereby improving both sleep quality and overall well-being in older adults (48).
Importantly, beyond statistical significance, the magnitude of improvement observed in the present meta-analysis also demonstrated clinical relevance. The weighted mean differences (WMD) for PSQI exceeded the minimal clinically important difference (MCID), which is generally defined as a reduction of approximately 3 points. This indicates that the improvements in sleep quality observed here are not only statistically robust but also meaningful from a clinical perspective, with tangible benefits for older adults experiencing sleep difficulties.
The findings further reinforce the importance of tailoring exercise prescriptions to individual needs. Intervention programs should be designed based on the elderly individual’s health status, preferences, and living environment (13). This person-centered approach supports the evolution of sleep improvement strategies toward greater precision and inclusivity across diverse populations (49).
Although evidence-based medicine has advanced significantly in the field of exercise and aging, substantial research gaps remain in understanding the mechanisms, optimal intensity thresholds, and intervention modalities for sleep disorders. Future studies are needed to explore the adaptability and differential effects of various exercise types, intensities, and frequencies across subpopulations with varying covariates. Dose–response analysis offers valuable insights for optimizing intervention strategies and enhancing their effectiveness, and this study seeks to provide a methodological reference for clinical practitioners and rehabilitation researchers in the field of sleep health.
Strengths and limitations
This study systematically evaluated the relationship between exercise dose and improvements in subjective sleep quality among older adults, and proposed a theoretical framework for personalized exercise prescriptions. The research process adhered strictly to established meta-analysis guidelines, with comprehensive literature searches conducted across multiple authoritative databases to ensure scientific rigor and representativeness. Through standardized screening procedures and stringent quality control measures, the risk of selection bias was minimized, enhancing the credibility and robustness of the conclusions.
The findings offer a practical pathway for older adults with limited physical capacity and provide evidence-based support for developing more accessible intervention strategies for sleep disorders. Moreover, the study lays a theoretical foundation for future individualized and precision-oriented rehabilitation programs, with significant potential for clinical translation and public health impact.
Nevertheless, several limitations should be acknowledged. First, the inclusion of English-language publications only may introduce language bias and limit the global generalizability of the findings. Second, although subgroup analyses were conducted, the number of studies included in some subgroups was small, potentially reducing statistical power and result stability. Third, most of the included studies lacked long-term follow-up data post-intervention, restricting our ability to assess the sustainability and long-term efficacy of exercise interventions. Additionally, many original studies failed to report adherence to the exercise protocol and intervention fidelity, which may affect the accuracy of dose–response estimations.
Future research should aim to improve study quality and reporting completeness, extend follow-up durations to evaluate long-term effects, and incorporate covariate-adjusted subgroup analyses and mechanistic investigations. Such efforts are essential for strengthening the scientific basis and practical guidance of exercise interventions tailored to older populations.
Conclusion
This study demonstrates that exercise interventions can significantly improve subjective sleep quality in older adults. The most pronounced effects were observed under conditions involving a frequency of 2–3 sessions per week, with each session lasting no more than 30 min and performed at low to moderate intensity. Notably, even low-dose interventions totaling approximately 300 MET-minutes per week were associated with substantial improvements, offering a practical and accessible pathway for older adults with limited physical capacity.
Given concerns related to safety and adherence, high-intensity or high-frequency exercise regimens are not recommended for this population. Future research should focus on elucidating the mechanisms underlying the long-term maintenance of intervention effects, and on optimizing exercise type, intensity, and frequency in an integrated manner. These efforts will be essential for developing more precise and sustainable exercise strategies tailored to the needs of older adults, and for advancing both theoretical and empirical support in the field of geriatric sleep health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YL: Data curation, Software, Supervision, Writing – original draft, Writing – review & editing. WQ: Data curation, Software, Writing – original draft, Writing – review & editing. ZG: Data curation, Writing – original draft, Writing – review & editing. XW: Supervision, Validation, Writing – original draft. YW: Supervision, Validation, Writing – original draft. HM: Supervision, Validation, Writing – original draft. JK: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to all the authors for their contributions during the completion of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1664567/full#supplementary-material
References
1. Rudnicka, E, Napierala, P, Podfigurna, A, Meczekalski, B, Smolarczyk, R, and Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas. (2020) 139:6–11. doi: 10.1016/j.maturitas.2020.05.018
2. Khaleghi, MM, and Ahmadi, F. Effect of different exercises on sleep quality in elderly women: a systematic review. Comparative Exercise Physiol. (2024) 20:219–30. doi: 10.1163/17552559-00001049
3. Ohayon, M, Wickwire, EM, Hirshkowitz, M, Albert, SM, Avidan, A, Daly, FJ, et al. National Sleep Foundation's sleep quality recommendations: first report. Sleep Health. (2017) 3:6–19. doi: 10.1016/j.sleh.2016.11.006
4. Zdanys, KF, and Steffens, DC. Sleep disturbances in the elderly. Psychiatr Clin N Am. (2015) 38:723–41. doi: 10.1016/j.psc.2015.07.010
5. Blanc, J, Seixas, A, Donley, T, Bubu, OM, Williams, N, and Jean-Louis, G. Resilience factors, race/ethnicity and sleep disturbance among diverse older females with hypertension. J Affect Disord. (2020) 271:255–61. doi: 10.1016/j.jad.2020.03.148
6. Lu, ZX, Sang, N, Liu, RC, Li, BH, Zhang, MY, Zhang, MH, et al. The causal relationship between sleep disturbances and the risk of frailty: a two-sample Mendelian randomization study. Eur J Ageing. (2024) 21:9. doi: 10.1007/s10433-024-00804-2
7. Andrade, C. Sedative hypnotics and the risk of falls and fractures in the elderly. J Clin Psychiatry. (2018) 79:19106. doi: 10.4088/JCP.18f12340
8. Feinsilver, SH, and Hernandez, AB. Sleep in the elderly: unanswered questions. Clin Geriatr Med. (2017) 33:579–96. doi: 10.1016/j.cger.2017.06.009
9. Ahmadi, F, Khaleghi, M, and Zar, A. The therapeutic effect of different exercises on premenstrual syndrome (PMS): a systematic review. Comparative Exercise Physiol. (2025) 21:17–32.
10. Varrasse, M, Li, J, and Gooneratne, N. Exercise and sleep in community-dwelling older adults. Curr Sleep Med Rep. (2015) 1:232–40. doi: 10.1007/s40675-015-0028-6
11. Black, DS, O'Reilly, GA, Olmstead, R, Breen, EC, and Irwin, MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med. (2015) 175:494–501. doi: 10.1001/jamainternmed.2014.8081
12. Leroy, V, Ayers, E, Adhikari, D, and Verghese, J. Association of Sleep Disturbances with Prevalent and Incident Motoric Cognitive Risk Syndrome in community-residing older adults. Neurology. (2024) 103:e210054. doi: 10.1212/WNL.0000000000210054
13. Yang, PY, Ho, KH, Chen, HC, and Chien, MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Aust J Phys. (2012) 58:157–63. doi: 10.1016/S1836-9553(12)70106-6
14. Chandler, J, Cumpston, M, Li, T, Page, MJ, and Welch, V. Cochrane handbook for systematic reviews of interventions, vol. 4. Hoboken: Wiley (2019).
15. Zang, W, Fang, M, Meng, L, Kong, L, Xiao, N, Xue, J, et al. Exercise prescription prescriptions for frailty improvement in older adults: an evidence-based approach based on the 2024 older adult compendium. Arch Gerontol Geriatr. (2025) 130:105717. doi: 10.1016/j.archger.2024.105717
16. Chen, Z, Hu, Q, Xie, Q, Wu, S, Pang, Q, Liu, M, et al. Effects of treadmill exercise on motor and cognitive function recovery of MCAO mice through the Caveolin-1/VEGF signaling pathway in ischemic penumbra. Neurochem Res. (2019) 44:930–46. doi: 10.1007/s11064-019-02728-1
17. Chen, K-M, Lin, M-H, Fan, J-T, Lin, H-S, and Li, C-H. Effects of yoga on sleep quality and depression in elders in assisted living facilities. J Nurs Res. (2010) 18:53–61. doi: 10.1097/JNR.0b013e3181ce5189
18. Siu, PM, Yu, AP, Tam, BT, Chin, EC, Yu, DS, Chung, K-F, et al. Effects of tai chi or exercise on sleep in older adults with insomnia. JAMA Netw Open. (2021) 4:e2037199. doi: 10.1001/jamanetworkopen.2020.37199
19. King, AC. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. (1997) 277:32–7.
20. Sharif, F, Jahanbin, I, and Keshavarzi, S. The effect of aerobic exercise on quantity and quality of sleep among elderly people referring to health centers of Lar City, southern of Iran: A randomized controlled clinical trial. Curr Aging Sci. (2015) 8:248–55. doi: 10.2174/1874609808666150727113127
21. Sternfeld, B, Guthrie, KA, Ensrud, KE, LaCroix, AZ, Larson, JC, Dunn, AL, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. (2014) 21:330–8. doi: 10.1097/GME.0b013e31829e4089
22. Aibar-Almazán, A, Hita-Contreras, F, Cruz-Díaz, D, de la Torre-Cruz, M, Jiménez-García, JD, and Martínez-Amat, A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: A randomized controlled trial. Maturitas. (2019) 124:62–7. doi: 10.1016/j.maturitas.2019.03.019
23. Baklouti, S, Fekih-Romdhane, F, Guelmami, N, Bonsaksen, T, Baklouti, H, Aloui, A, et al. The effect of web-based hatha yoga on psychological distress and sleep quality in older adults: A randomized controlled trial. Complement Ther Clin Pract. (2023) 50:101715. doi: 10.1016/j.ctcp.2022.101715
24. Carcelén-Fraile, MDC, Déniz-Ramírez, NDP, Sabina-Campos, J, Aibar-Almazán, A, Rivas-Campo, Y, González-Martín, AM, et al. Exercise and nutrition in the mental health of the older adult population: A randomized controlled clinical trial. Nutrients. (2024) 16:1741. doi: 10.3390/nu16111741
25. Chen, KM, Chen, MH, Chao, HC, Hung, HM, Lin, HS, and Li, CH. Sleep quality, depression state, and health status of older adults after silver yoga exercises: cluster randomized trial. Int J Nurs Stud. (2009) 46:154–63. doi: 10.1016/j.ijnurstu.2008.09.005
26. Chen, MC, Liu, HE, Huang, HY, and Chiou, AF. The effect of a simple traditional exercise programme (Baduanjin exercise) on sleep quality of older adults: a randomized controlled trial. Int J Nurs Stud. (2012) 49:265–73. doi: 10.1016/j.ijnurstu.2011.09.009
27. Curi, VS, Vilaça, J, Haas, AN, and Fernandes, HM. Effects of 16-weeks of Pilates on health perception and sleep quality among elderly women. Arch Gerontol Geriatr. (2018) 74:118–22. doi: 10.1016/j.archger.2017.10.012
28. Frye, B, Scheinthal, S, Kemarskaya, T, and Pruchno, R. Tai chi and low impact exercise: effects on the physical functioning and psychological well-being of older people. J Appl Gerontol. (2007) 26:433–53. doi: 10.1177/0733464807306915
29. Li, F, Harmer, P, Irbe, D, Tearse, RG, and Weimer, C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. (2004) 52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x
30. Jimenez-Garcia, JD, Hita-Contreras, F, de la Torre-Cruz, MJ, Aibar-Almazan, A, Achalandabaso-Ochoa, A, Fabrega-Cuadros, R, et al. Effects of HIIT and MIIT suspension training programs on sleep quality and fatigue in older adults: randomized controlled clinical trial. Int J Environ Res Public Health. (2021) 18:1211. doi: 10.3390/ijerph18031211
31. Karimi, S, Soroush, A, Towhidi, F, Makhsosi, BR, Karimi, M, Jamehshorani, S, et al. Surveying the effects of an exercise program on the sleep quality of elderly males. Clin Interv Aging. (2016) 11:997–1002. doi: 10.2147/CIA.S106808
32. Kerkez, M, and Erci, B. The effect of moving meditation exercise on depression and sleep quality of the elderly: A randomized controlled study. Holist Nurs Pract. (2024) 38:41–9. doi: 10.1097/HNP.0000000000000627
33. King, AC, Woo, S, Castro, CM, Ahn, DK, Vitiello, MV, Woodward, SH, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. (2008) 63A:997–1004. doi: 10.1093/gerona/63.9.997
34. Mahajan, A, Mahajan, S, and Tilekar, S. A pilot randomized controlled trial of interval training and sleep hygiene for improving sleep in older adults. J Aging Phys Act. (2021) 29:993–1002. doi: 10.1123/japa.2020-0207
35. Choi, MJ, and Sohng, KY. The effects of floor-seated exercise program on physical fitness, depression, and sleep in older adults: a cluster randomized controlled trial. Int J Gerontol. (2018) 12:116–21. doi: 10.1016/j.ijge.2017.06.003
36. Nguyen, MH, and Kruse, A. A randomized controlled trial of tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin Interv Aging. (2012) 7:185–90. doi: 10.2147/CIA.S32600
37. Sánchez-Alcalá, M, Aibar-Almazán, A, Hita-Contreras, F, Castellote-Caballero, Y, Carcelén-Fraile, MdC, Infante-Guedes, A, et al. Effects of dance-based aerobic training on mental health and quality of life in older adults with mild cognitive impairment. J Pers Med. (2024) 14:844. doi: 10.3390/jpm14080844
38. Singh, NA, Clements, KM, and Fiatarone, MA. A randomized controlled trial of the effect of exercise on sleep. Sleep Breath. (1997) 20:95–101.
39. Song, D, Yu, D, Liu, T, and Wang, J. Effect of an aerobic dancing program on sleep quality for older adults with mild cognitive impairment and poor sleep: a randomized controlled trial. J Am Med Dir Assoc. (2024) 25:494–9. doi: 10.1016/j.jamda.2023.09.020
40. Song, J, Wei, L, Cheng, K, Lin, Q, Xia, P, Wang, X, et al. The effect of modified tai chi exercises on the physical function and quality of life in elderly women with knee osteoarthritis. Front Aging Neurosci. (2022) 14:860762. doi: 10.3389/fnagi.2022.860762
41. Wang, C, Jiang, T, Li, H, Cao, G, and Zhang, G. The effects of tai chi exercise on sleep quality among the elderly: a study based on polysomnographic monitoring. Front Neurol. (2024) 15:1304463. doi: 10.3389/fneur.2024.1304463
42. Gümüş Şekerci, Y, and Kir Bicer, E. The effect of walking exercise on quality of life and sleep in elderly individuals: randomized controlled study. Turk J Geriatr. (2019) 22:443–53. doi: 10.31086/tjgeri.2020.123
43. Bull, FC, Al-Ansari, SS, Biddle, S, Borodulin, K, Buman, MP, Cardon, G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
44. Reid, KJ, Baron, KG, Lu, B, Naylor, E, Wolfe, L, and Zee, PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. (2010) 11:934–40. doi: 10.1016/j.sleep.2010.04.014
45. Tseng, TH, Chen, HC, Wang, LY, and Chien, MY. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: a randomized controlled trial. J Clin Sleep Med. (2020) 16:1483–92. doi: 10.5664/jcsm.8560
46. You, Y, Liu, J, Tang, M, Wang, D, and Ma, X. Effects of tai chi exercise on improving walking function and posture control in elderly patients with knee osteoarthritis: A systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e25655. doi: 10.1097/MD.0000000000025655
47. Crane, JD, Macneil, LG, and Tarnopolsky, MA. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci. (2013) 68:631–8. doi: 10.1093/gerona/gls237
48. Li, N, Xu, G, Chen, G, and Zheng, X. Sleep quality among Chinese elderly people: A population-based study. Arch Gerontol Geriatr. (2020) 87:103968. doi: 10.1016/j.archger.2019.103968
Keywords: physical exercise, sleep, older adults, subgroup analysis, meta-analysis
Citation: Li Y, Quan W, Gao Z, Wang X, Wang Y, Meng H and Kang J (2025) Effects of exercise interventions on subjective sleep quality in older adults: a systematic review and meta-analysis of studies using the Pittsburgh sleep quality index. Front. Med. 12:1664567. doi: 10.3389/fmed.2025.1664567
Edited by:
Xuewen Wang, University of South Carolina, United StatesReviewed by:
Chao Yang, University of Texas MD Anderson Cancer Center, United StatesMohammad Mehdi Khaleghi, Persian Gulf University, Iran
Copyright © 2025 Li, Quan, Gao, Wang, Wang, Meng and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianxin Kang, a2FuZ2ppYW54aW5AaHJiaXBlLmVkdS5jbg==
 Yafan Li
Yafan Li Wei Quan
Wei Quan Ziqi Gao
Ziqi Gao Xinyi Wang
Xinyi Wang Yaxin Wang
Yaxin Wang Hongnan Meng
Hongnan Meng Jianxin Kang
Jianxin Kang