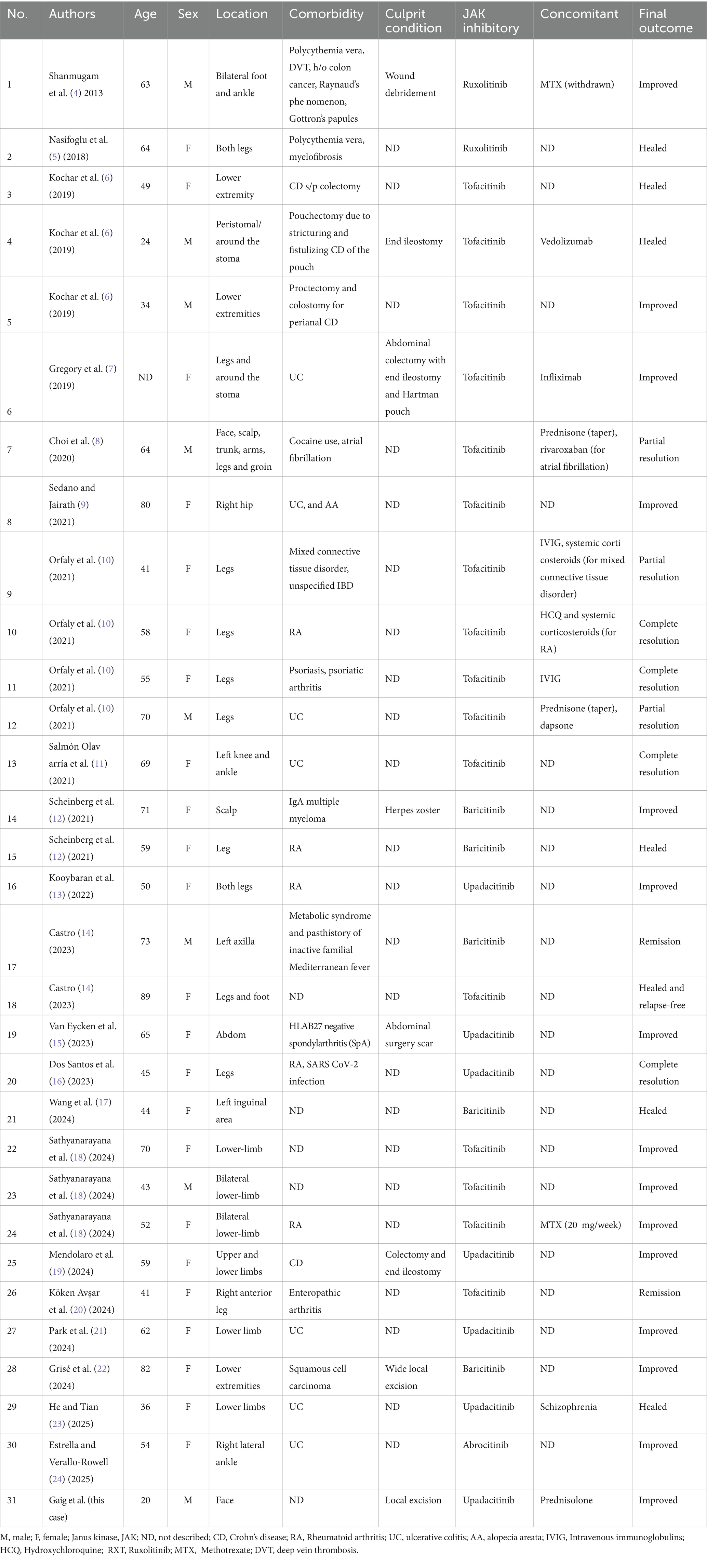
- 1Department of Dermatology, Traditional Chinese and Western Medicine Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Dermatology, Wuhan No. 1 Hospital, Wuhan, China
- 3Hubei Province & Key Laboratory of Skin Infection and Immunity, Wuhan, China
Pyoderma gangrenosum (PG) is a recurrent, painful, necrotizing ulcerative neutrophilic dermatosis. Facial PG (FPG) is a rare subtype of PG that is often misdiagnosed. A 20-year-old male with FPG showed improvement after treatment with a combination of prednisone and upadacitinib. Early diagnosis is crucial to avoid misdiagnosis.
1 Introduction
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis that causes pustules and ulcerations (1). Its diagnosis and appropriate management are often delayed because of its rarity and the presence of numerous clinical variants. Although corticosteroids remain the primary first-line treatment for severe forms of PG, the use of Janus kinase inhibitors (JAKis) is promising (2, 3). To date, 31 cases of PG treated with JAKi have been reported and are presented in Table 1 (4–24). In this case report, the authors describe a 20-year-old man with long-standing, non-healing, and painful facial ulcers that were not responsive to multiple antibiotics and serial wound debridement. Treatment with Upadacitinib hydrate and a tapering course of oral prednisolone was initiated. However, FPG is a rare dermatological pathology lacking characteristic distinguishing features, making treatment challenging (25).
2 Case report
We report the case of a 20-year-old man who presented with a 4-month history of painful facial skin ulcers on 18 March 2025 (Figures 1A,B). The lesion appeared after excision of facial cysts. The patient had a history of eczema and acne, with no family history of similar conditions and psychosocial issues. On 10 March 2025, a biopsy suggested an infectious granuloma; however, negative acid-fast and periodic acid–Schiff stains were negative (Figure 1I). During previous evaluations of the ulcers, next-generation sequencing suggested the presence of Streptococcus pneumoniae and Klebsiella pneumoniae; however, antimicrobial therapy was ineffective. A second biopsy was conducted on 27 March 2025. Histopathological analysis of a skin-biopsy specimen obtained from the lesion border revealed a diffuse mixed inflammatory cell infiltrate. Cultures and polymerase chain reaction tests for deep mycosis and mycobacterial infections were negative. No acid-fast bacilli, fungi, or bacteria were identified using acid-fast or periodic acid—Schiff stains. Test results for herpes simplex virus 1, herpes simplex virus 2, interferon-gamma release assays, syphilis, human immunodeficiency virus, and hepatitis B DNA all showed no abnormalities. Additionally, tests for rheumatologic conditions and immunodeficiency were negative. At the current presentation, physical examination revealed a skin ulcer with a violaceous border on the right lower portion of the face (Figures 1C–E). We made the diagnosis of facial pyoderma gangrenosum (FPG). The patient was treated with oral prednisolone (0.5 mg/kg, six tablets) monotherapy once daily for 2 weeks. However, as the ulcer enlarged, treatment was escalated to combination therapy with Upadacitinib (15 mg once daily) for 8 weeks. Treatment with Upadacitinib hydrate and a tapering course of oral prednisolone was initiated. The patient took six tablets of prednisone orally daily for 2 weeks, took four tablets for 2 weeks, and reduced the dosage by one tablet per week until the medication was discontinued. By 3 April 2025, the majority of the ulcers on the left mandible and the left neck had healed. The skin lesion started to subside 1 month after the start of treatment. Our case responded well to Upadacitinib without adverse events. After 4 months, the patient reported complete resolution of the lesions (Figures 1F–H). At present, the patient is still under our follow-up schedule.
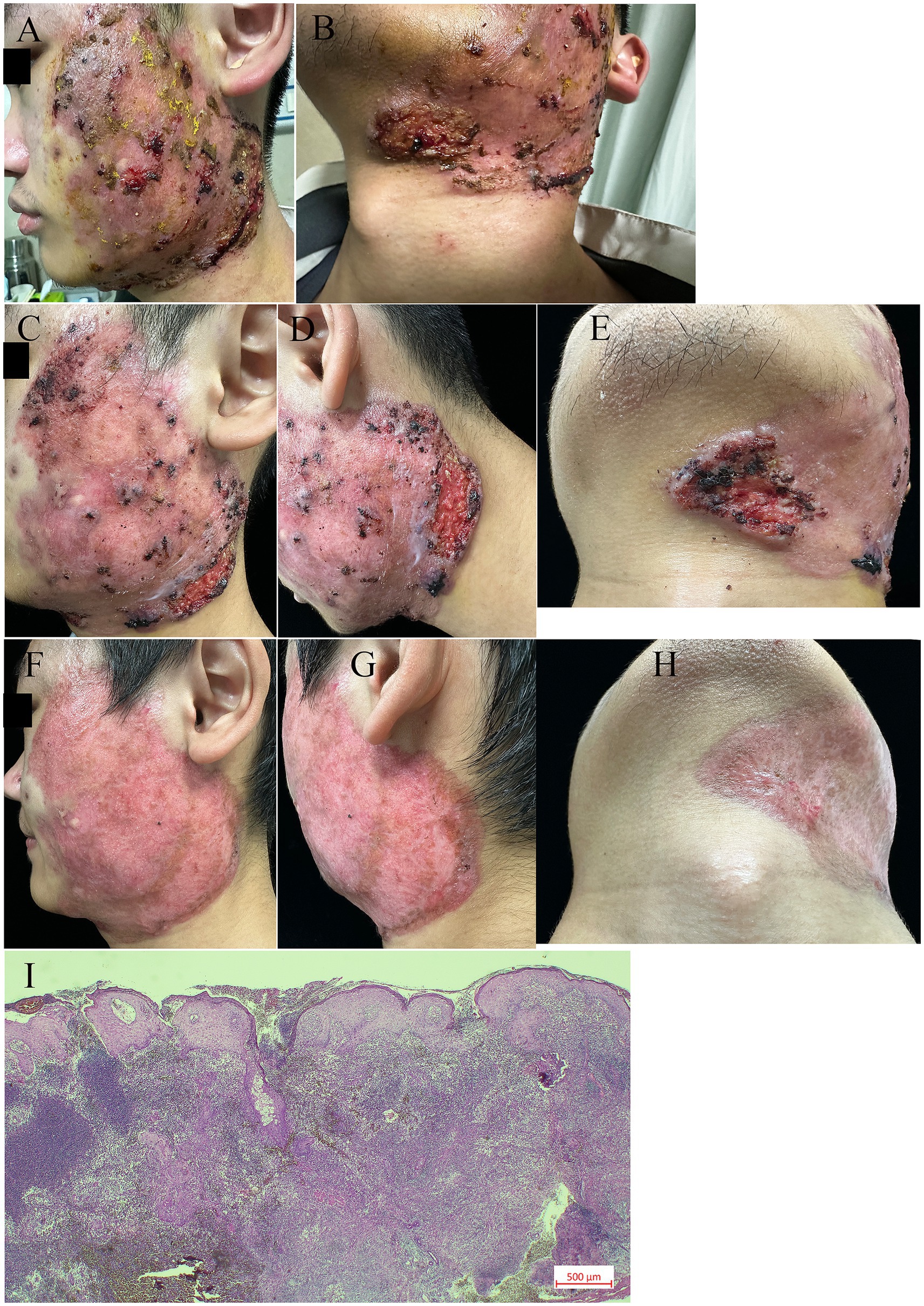
Figure 1. Clinical and histological features. (A–B) A well-demarcated dark red plaque, with local cysts present, was observed on the left side of the face. Multiple ulcers were noted on the lower edge of the red plaque, particularly near the left mandibular angle. These ulcers were surrounded by erythema, showing undermined borders, destroyed margins, and tenderness upon palpation. (C–E) Clinical improvement was documented at three days after the initiation of prednisolone treatment. (F–H) Clinical images of the left face after 24 days of pyoderma gangrenosum treatment with prednisolone and upadacitinib. (I) Biopsy showing diffuse mixed inflammatory cell infiltration (original magnification 10×).
3 Discussion
PG is a debilitating skin disease marked by idiopathic neutrophil infiltration that causes the destruction of tissue and ulceration (24). Epidemiological studies indicate that the average age of PG onset is in the mid-40s, with an incidence of a few cases per million person-years. PG involves dysregulation of both innate and adaptive immunity (1), leading to a neutrophil-rich autoinflammatory process with the elevation of multiple cytokines (15, 26). Some of these cytokines act through the JAK/STAT pathway (3). The importance of the JAK/STAT pathway in PG has also been demonstrated through immunohistochemistry in skin biopsy specimens (27). The predisposition of PG is not well understood. Drug induction and the postoperative period are two potential triggers. In our case, the cause of FPG is due to the excision of left-sided facial cysts.
FPG is a rare subtype of PG (25). PG needs to be differentiated from infections, such as mycobacterial cellulitis, syphilitic granulomatous ulcers, and scrofuloderma; lupus vulgaris; malignancies; and vasculitis (2). It is often associated with various other immune-mediated diseases, most commonly inflammatory bowel disease and rheumatoid arthritis (28). It may be associated with systemic inflammatory conditions, including inflammatory bowel disease (IBD), rheumatoid arthritis, or vasculitis, as well as leukemia or hepatitis. It may also be present in the setting of autoinflammatory syndromes, such as pyogenic arthritis, PG, and acne; PG, acne, and suppurative hidradenitis; and pyogenic arthritis, PG, acne, and suppurative hidradenitis, and in a small proportion of synovitis, acne, pustulosis, hyperostosis, and osteitis cases (28).
Diagnosing PG is challenging since there are no pathognomonic laboratory parameters or histopathological features (13). In 2018, a new Delphi consensus was published on the diagnostic criteria for PG, stating that diagnosis could be made by using one major and several minor criteria. The major criterion was neutrophilic infiltration at the ulcer edge on biopsy. The eight minor criteria are 1) the exclusion of infection; 2) a positive pathergy test; 3) a history of IBD or inflammatory arthritis; 4) the evolution of pustules, papules, or vesicles into ulcers within four days; 5) erythema, undermined borders, and tenderness around ulcers; 6) multiple ulcers with at least one location on the extensor surface of the lower leg; 7) cribriform or “wrinkled paper” scars at the site of healed ulcers; and 8) a reduction in ulcer size within one month after treatment with immunosuppressive drugs (29).
Treatment of PG typically starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by the addition of more slow-acting immunosuppressive drugs with superior adverse event profiles, including biologics, intravenous immunoglobulin (30), and JAK inhibitors (3, 28, 31). Our case and analysis of the previously published cases demonstrate JAKi as an effective treatment option for PG (3) (Table 1). Tofacitinib (a non-selective JAK inhibitor), ruxolitinib (JAK-1/2 inhibitor), and Upadacitinib (JAK-1 inhibitor) have been reported to be successful in treating PG in a handful of reported cases (10). Patients responded in a relatively brief period of time with few reported adverse events (22).
Patients documented to be treated with JAKis (Table 1) had a mean age of 55.2 years (range: 20–89 years) and consisted of 25.8% males and 74.2% females (Table 1). PG presented as ulcerations on the lower extremities in approximately 74% (23/31) of cases. Treatments were categorized as JAKi treatment combined with concomitant medications (11/31, 35.5%) or JAKi monotherapy without concomitant medications (20/31, 64.5%). Among JAKis, the most commonly used was tofacitinib (8/20, 40.0%), followed by baricitinib (5/20, 25%) and upadacitinib (5/20, 25%). Among JAKis used with concomitant medication, tofacitinib (8/11, 72.7%) was the most common, followed by upadacitinib (2/11, 18.2%) and ruxolitinib (1/11, 9.1%). The most common concomitant medication used was systemic corticosteroids (6/11, 54.5%).
4 Conclusion
While the management of PG is challenging because of the lack of standardized evidence-based treatments, notable advancements are being made in its identification and management. In our case, PG initially stabilized and subsequently decreased in severity under treatment with a JAKi and a tapering course of oral prednisolone.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
GG: Funding acquisition, Writing – original draft. LZ: Writing – review & editing. MH: Writing – review & editing. FS: Writing – review & editing. JC: Conceptualization, Validation, Writing – review & editing. JD: Writing – review & editing, Conceptualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Wuhan (2025020701020244) and the Outstanding Doctor Fund Project of Wuhan No. 1 Hospital (2024D013).
Acknowledgments
The authors acknowledge the contributions of all the scientists in this area and apologize for failing to cite any study due to constraints of space.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yin, H, and Lu, L. Evolution of pyoderma gangrenosum. N Engl J Med. (2024) 390:e36. doi: 10.1056/nejmicm2311165
2. Keum, H, Zhivov, EV, and Ortega-Loayza, AG. Updates in innovation of the treatment of pyoderma gangrenosum. Expert Rev Clin Pharmacol. (2025) 18:29–39. doi: 10.1080/17512433.2024.2447776
3. Maronese, CA, Pimentel, MA, Li, MM, Genovese, G, Ortega-Loayza, AG, and Marzano, AV. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. (2022) 23:615–34. doi: 10.1007/s40257-022-00699-8
4. Shanmugam, VK, McNish, S, Shara, N, Hubley, KJ, Kallakury, B, Dunning, DM, et al. Chronic leg ulceration associated with polycythemia vera responding to Ruxolitinib (Jakafi®). J Foot Ankle Surg. (2013) 52:781–5. doi: 10.1053/j.jfas.2013.07.003
5. Nasifoglu, S, Heinrich, B, and Welzel, J. Successful therapy for pyoderma gangrenosum with a Janus kinase 2 inhibitor. Br J Dermatol. (2018) 179:504–5. doi: 10.1111/bjd.16468
6. Kochar, B, Herfarth, N, Mamie, C, Navarini, AA, Scharl, M, and Herfarth, HH. Tofacitinib for the treatment of pyoderma gangrenosum. Clin Gastroenterol Hepatol. (2019) 17:991–3. doi: 10.1016/j.cgh.2018.10.047
7. Gregory, MH, Ciorba, MA, Deepak, P, and Christophi, GP. Successful treatment of pyoderma gangrenosum with concomitant Tofacitinib and infliximab. Inflamm Bowel Dis. (2019) 25:e87–8. doi: 10.1093/ibd/izz015
8. Choi, AW, Abuav, R, Rabizadeh, SM, Ansari, R, and Marsch, AF. Recalcitrant and severe pyoderma gangrenosum attributable to levamisole-adulterated cocaine and treated successfully with oral tofacitinib. JAAD Case Rep. (2020) 6:939–41. doi: 10.1016/j.jdcr.2020.07.035
9. Sedano, R, and Jairath, V. Tofacitinib for the treatment of three immune-mediated conditions in one patient: ulcerative colitis, pyoderma gangrenosum, and alopecia areata. Inflamm Bowel Dis. (2021) 27:e65. doi: 10.1093/ibd/izab005
10. Orfaly, VE, Kovalenko, I, Tolkachjov, SN, Ortega-Loayza, AG, and Nunley, JR. Tofacitinib for the treatment of refractory pyoderma gangrenosum. Clin Exp Dermatol. (2021) 46:1082–5. doi: 10.1111/ced.14683
11. Salmón Olavarría, P, Rubio Iturria, S, and Nantes Castillejo, O. Tofacitinib, useful option for the treatment of pyoderma gangrenosum in an ulcerative colitis patient. Rev Esp Enferm Dig. (2021) 113:733–4. doi: 10.17235/reed.2021.7977/2021
12. Scheinberg, M, Machado, LA, Castro, LGM, Ferreira, SB, and Michalany, N. Successful treatment of ulcerated pyoderma gangrenosum with baricitinib, a novel JAK inhibitor. J Transl Autoimmun. (2021) 4:100099. doi: 10.1016/j.jtauto.2021.100099
13. Kooybaran, NR, Korsten, P, Schön, MP, and Mössner, R. Response of rheumatoid arthritis-associated pyoderma gangrenous to the JAK1 inhibitor upadacitinib. J Dtsch Dermatol Ges. (2022) 20:522–4. doi: 10.1111/ddg.14716
14. Castro, LGM. JAK inhibitors: a novel, safe, and efficacious therapy for pyoderma gangrenosum. Int J Dermatol. (2023) 62:1088–93. doi: 10.1111/ijd.16676
15. Van Eycken, L, Dens, A-C, De Vlam, K, Neerinckx, B, and De Haes, P. Resolution of therapy-resistant pyoderma gangrenosum with upadacitinib. JAAD Case Rep. (2023) 37:89–91. doi: 10.1016/j.jdcr.2023.05.016
16. Dos Santos, MR, Ianhez, M, Ribeiro, BN, De Queiroz, BB, and Miot, HA. Refractory pyoderma gangrenosum associated with rheumatoid arthritis successfully treated with upadacitinib comments on: “JAK inhibitors: a novel, safe, and efficacious therapy for pyoderma gangrenosum.”. Int J Dermatol. (2023) 62:e595–8. doi: 10.1111/ijd.16791
17. Wang, Z, Li, T, Gong, L, Song, Z, and Piao, Y. Successful treatment of multiple site involvement pyoderma gangrenosum with baricitinib. Int J Dermatol. (2024) 63:1444–6. doi: 10.1111/ijd.17200
18. Sathyanarayana, VA, Roy, D, Nagaraju, B, and Rao, VKR. Tofacitinib in pyoderma gangrenosum—a case series. Int J Rheum Dis. (2024) 27:e14810. doi: 10.1111/1756-185x.14810
19. Mendolaro, M, Morello, E, Salacone, P, and Rocca, R. A case of refractory severe pyoderma gangrenosum successfully treated with upadacitinib. Dig Liver Dis. (2024) 56:1248. doi: 10.1016/j.dld.2024.03.016
20. Köken Avşar, A, Demirci Yıldırım, T, and Sarı, İ. Tofacitinib therapy for severe pyoderma gangrenosum in a patient with enteropathic arthritis: a case-based review. Rheumatol Int. (2024) 44:2227–37. doi: 10.1007/s00296-024-05560-1
21. Park, S, St Pierre, J, Onajin, O, and Rubin, DT. Successful treatment of severe pyoderma gangrenosum and ulcerative colitis with Upadacitinib. ACG Case Rep J. (2024) 11:e01531. doi: 10.14309/crj.0000000000001531
22. Grisé, A, Valere, L-C, Weinstein, D, and Sami, N. Janus kinase inhibitors in the treatment of pyoderma gangrenosum: case report and review. Arch Dermatol Res. (2024) 316:238. doi: 10.1007/s00403-024-02958-6
23. He, S-D, and Tian, Y. Upadacitinib for ulcerative colitis and pyoderma gangrenosum in a patient with schizophrenia on long-term risperidone: a case report. World J Gastroenterol. (2025) 31:104038. doi: 10.3748/wjg.v31.i20.104038
24. Estrella, MME, and Verallo-Rowell, VM. Pyoderma gangrenosum treated with oral abrocitinib in a 54-year-old woman: a case report. JAAD Case Rep. (2025) 60:4–6. doi: 10.1016/j.jdcr.2025.02.022
25. Kaur, M, Anthony, MR, Yamakoshi, C, Schildmeyer, A, Mallela, T, Diaz, MJ, et al. Clinical characteristics, treatments, and outcomes of pyoderma Gangrenosum of the face: a systematic review. Int Wound J. (2025) 22:e70334. doi: 10.1111/iwj.70334
26. Becker, SL, Vague, M, and Ortega-Loayza, AG. Insights into the pathogenesis of pyoderma Gangrenosum. J Invest Dermatol. (2025) 145:1305–22. doi: 10.1016/j.jid.2024.09.023
27. Ortega-Loayza, AG, Friedman, MA, Reese, AM, Liu, Y, Greiling, TM, Cassidy, PB, et al. Molecular and cellular characterization of pyoderma gangrenosum: implications for the use of gene expression. J Invest Dermatol. (2022) 142:1217–1220.e14. doi: 10.1016/j.jid.2021.08.431
28. Maverakis, E, Marzano, AV, Le, ST, Callen, JP, Brüggen, M-C, Guenova, E, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. (2020) 6:6. doi: 10.1038/s41572-020-0213-x
29. Maverakis, E, Ma, C, Shinkai, K, Fiorentino, D, Callen, JP, Wollina, U, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. (2018) 154:461–6. doi: 10.1001/jamadermatol.2017.5980
30. Ronicke, M, Sollfrank, L, Vitus, MV, Walter, LJ, Krieter, M, Moelleken, M, et al. Intravenous immunoglobulin therapy for pyoderma gangrenosum: a multicenter retrospective analysis in 81 patients. Am J Clin Dermatol. (2025) 26:139–46. doi: 10.1007/s40257-024-00904-w
Keywords: facial ulcers, pyoderma gangrenosum, Janus kinase inhibitor, Upadacitinib, neutrophilic dermatosis
Citation: Ge G, Zhan L, Huang M, Su F, Chen J and Dong J (2025) Case Report: Treatment of facial pyoderma gangrenosum with Upadacitinib. Front. Med. 12:1665013. doi: 10.3389/fmed.2025.1665013
Edited by:
Giusto Trevisan, University of Trieste, ItalyReviewed by:
Silvana Trincone, Maurizio Bufalini Hospital, ItalyMassimo Lucchi, Centro Studi Malattie Vascolari JF MERLEN, Italy
Copyright © 2025 Ge, Zhan, Huang, Su, Chen and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinbo Chen, Y2hlbjk5OWpiQDE2My5jb20=; Jing Dong, cWlhbmxpY2FvMTk4MkAxNjMuY29t
 Gai Ge
Gai Ge Lirui Zhan1,2
Lirui Zhan1,2 Jinbo Chen
Jinbo Chen