- 1Department of Radiology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Radiology, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, China
- 4Department of Acupuncture and Moxibustion, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
Giant gallbladder (GGB) is a rare condition that is generally associated with biliary obstruction. GGB induced by chemotherapeutic agents have not been previously reported. This article presents a case of GGB potentially related to adverse effects of anlotinib. The patient was a 74-year-old female who had been taking anlotinib regularly for 2 years following the diagnosis of a malignant lung tumor. The patient was admitted because of abdominal pain accompanied by vomiting and discomfort for 11 days. No fever was observed during onset, but inflammatory markers were abnormally elevated, accompanied by impaired liver function. On physical examination, a tender mass was palpable in the right inguinal region. Abdominal computed tomography showed an enlarged gallbladder (18.6 × 7.2 × 5.3 cm). Enhanced magnetic resonance imaging and magnetic resonance cholangiopancreatography ruled out biliary stones or space-occupying lesions, leading to a final diagnosis of acute cholecystitis with GGB. The patient received antibiotic treatment and anlotinib was immediately discontinued. After 1 week of anti-infective therapy, the abdominal pain resolved, vomiting ceased, liver function and inflammatory markers returned to normal ranges, and the gallbladder regressed to a normal size. To our knowledge, this is the first reported case of GGB associated with anlotinib use. Through a literature review, we conducted an in-depth discussion on the pathogenesis of GGB and the pharmacological effects of anlotinib, with the aim of sharing experiences and alerting clinicians to such adverse events.
Introduction
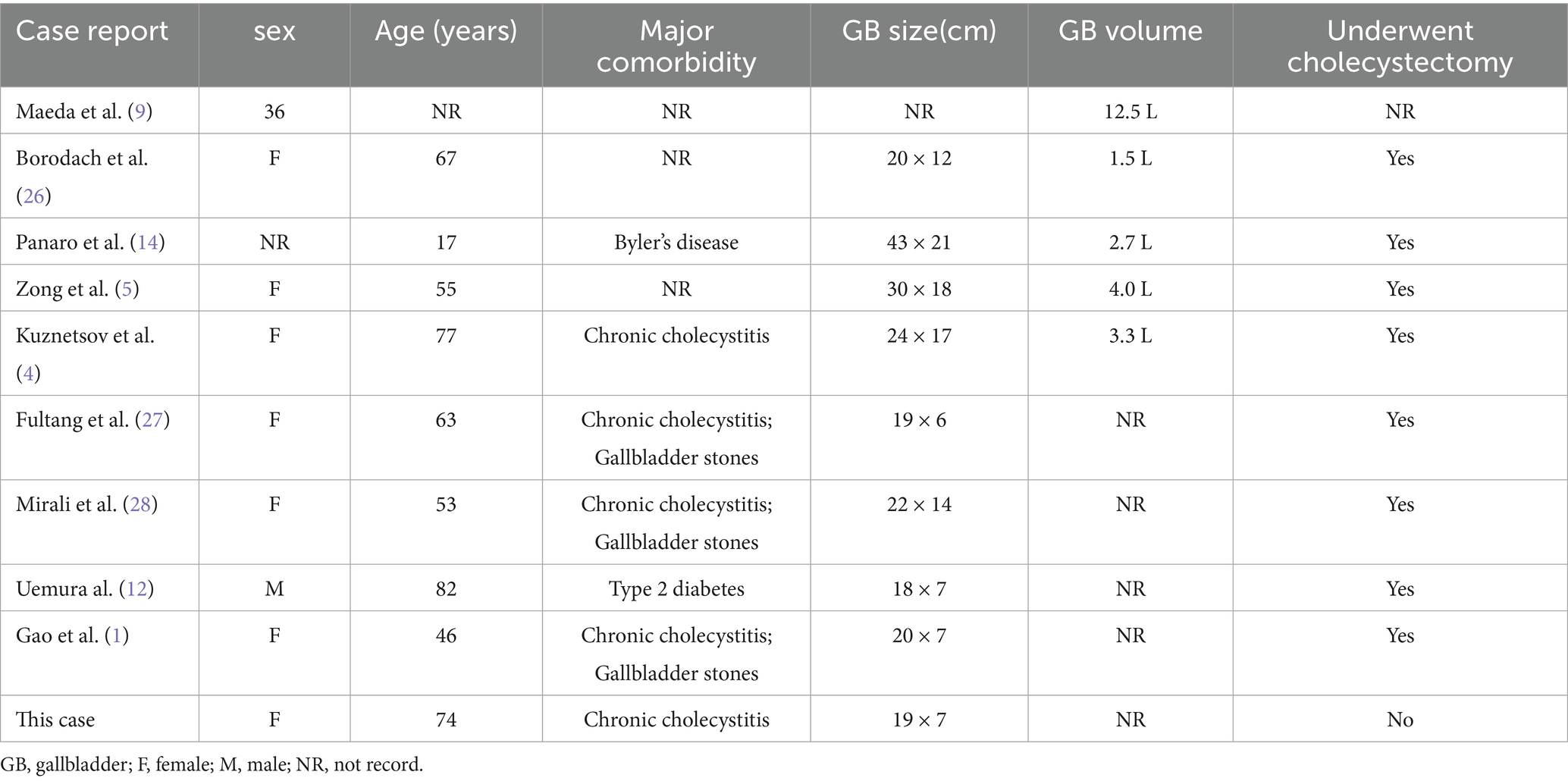
Cases of giant gallbladder (GGB) are exceedingly rare. A review of the literature spanning nearly 50 years yielded only 9 documented cases (Table 1). The pathogenesis underlying GGB formation remains unclear, although it is frequently caused by the obstruction of the biliary system by factors such as calculi, tumors, or parasites, which impede bile drainage and lead to near-unlimited gallbladder enlargement (1). To date, no case of anlotinib-induced GGB have been reported. However, occasional cases of gallbladder toxicity have been documented as adverse effects with other tyrosine kinase inhibitors (TKIs) such as sorafenib and sunitinib (2, 3).
Case description
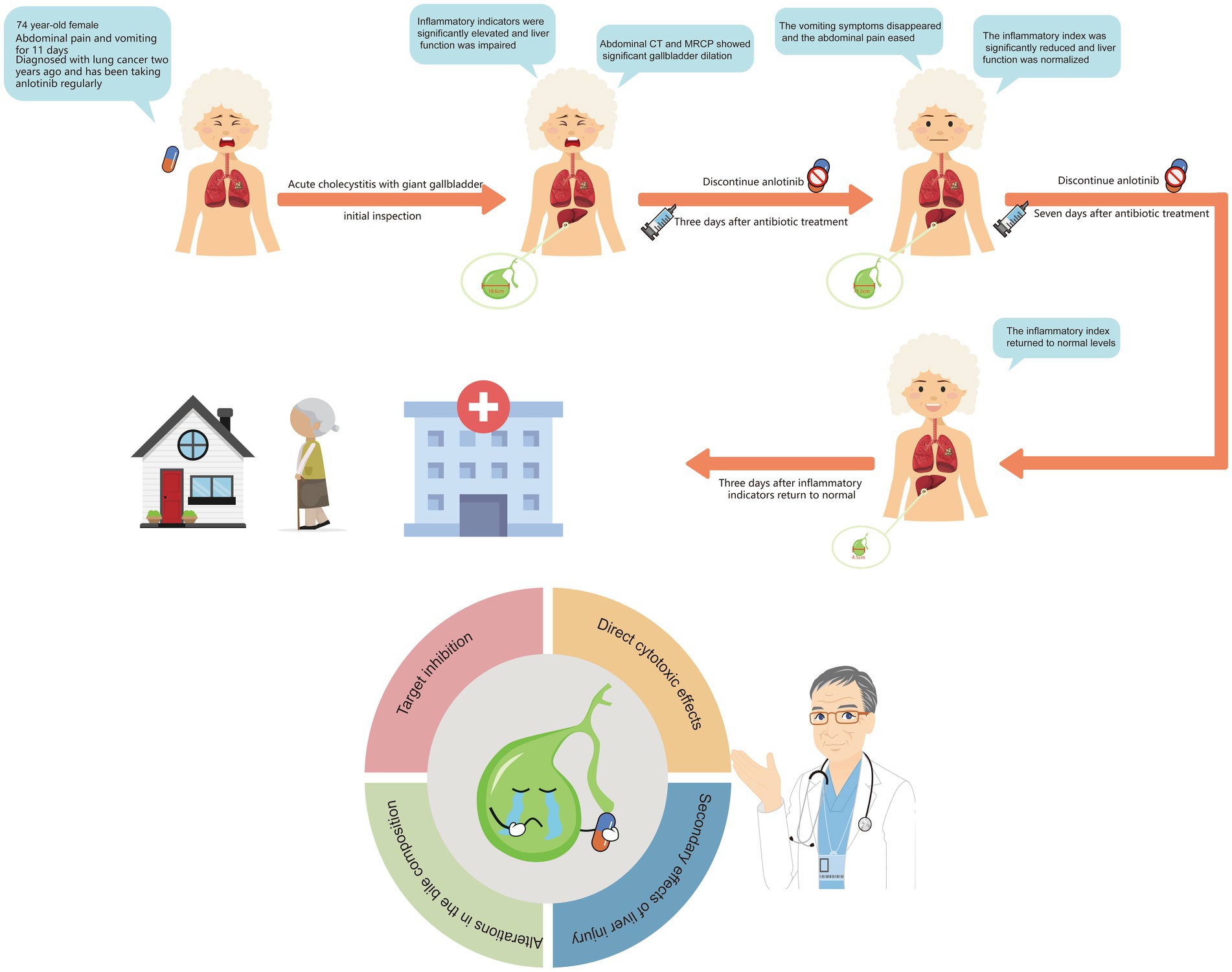
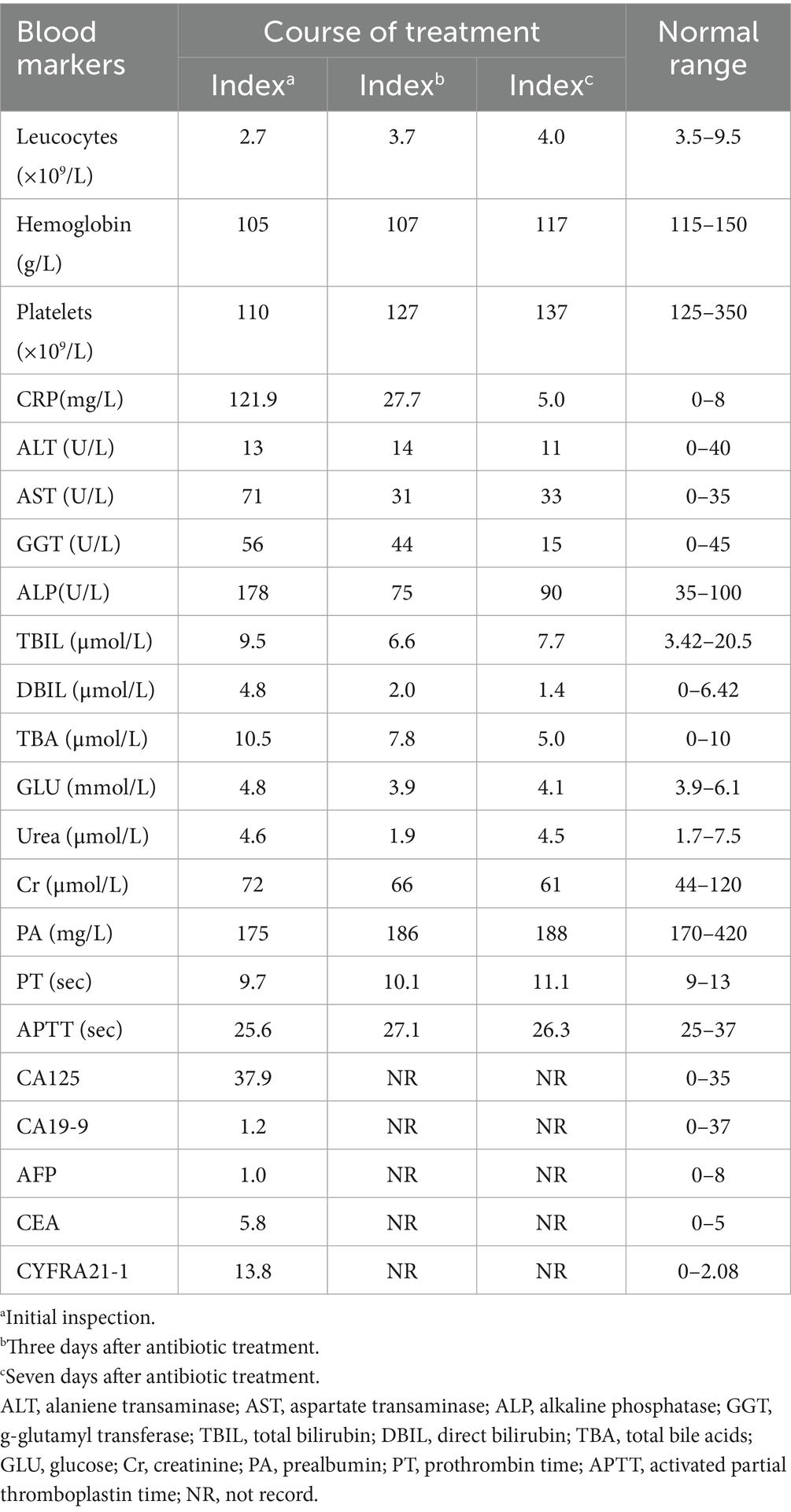
A 74-year-old female presented to our hospital with abdominal pain and vomiting for 11 days. The patient experienced mild-to-moderate abdominal pain without fever. Blood tests revealed a white blood cell count of 2.7*10^9/L within the normal range, with low hemoglobin and platelet levels. The monocyte percentage was 10.4%, absolute neutrophil count was 1.2*10^9/L, and C-reactive protein level was 121.9 mg/L. The levels of total bile acids (10.5 μmol/L), aspartate aminotransferase (71 U/L), alanine aminotransferase (56 U/L), and alkaline phosphatase (178 U/L) were elevated. Serum carcinoembryonic antigen (5.8 ng/mL), cytokeratin 19 fragment (13.8 ng/mL), and carbohydrate antigen 12–5 (12.5 ng/mL) were elevated, while carbohydrate antigen 19–9 remained within normal limits (see Table 2). The patient had been diagnosed with a pulmonary malignancy 2 years prior and had been regularly taking anlotinib (1 tablet daily). The patient denied a history of internal diseases such as hypertension, diabetes, heart disease, or kidney disease. Contrast-enhanced abdominal computed tomography (CT) scan showed significant gallbladder dilation (18.6 × 7.2 × 5.3 cm) with slight thickening and irregularity of the gallbladder wall. Additionally, magnetic resonance cholangiopancreatography and contrast-enhanced magnetic resonance imaging revealed mild dilation of the hepatic bile duct and cystic duct, while ruling out biliary stone impaction or tumor-like lesions in the biliary system. The final diagnosis was acute cholecystitis complicated with GGB (Figure 1).
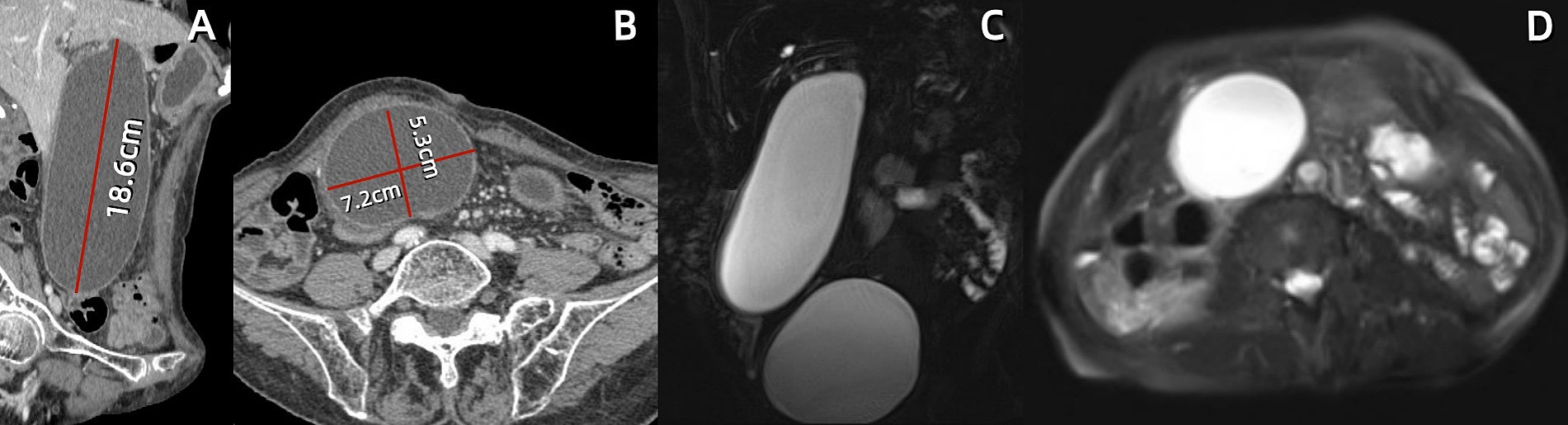
Figure 1. Abdominal contrast-enhanced computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) at first hospitalization. (A,B) Sagittal and axial CT scans reveal a markedly enlarged gallbladder measuring approximately 18.7 × 7.2 × 6.1 cm, with the fundus extending deep into the right iliac fossa. (C,D) Coronal MRCP and axial T2-weighted images demonstrate a massive gallbladder mass beneath the distended urinary bladder.
Given the patient’s impaired liver function and abnormally elevated inflammatory marker levels, anlotinib was discontinued and antibiotic therapy was initiated. Three days after intravenous administration of cefoperazone sodium and sulbactam (2 g/q12 h), the patient’s vomiting symptoms resolved, abdominal pain was alleviated, and blood tests revealed a C-reactive protein (CRP) level of 27.7 mg/L. Total bile acid, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels decreased to within normal ranges. Abdominal CT revealed a significant reduction in gallbladder size (11.2 × 5.9 × 4.4 cm), with no dilation observed in the intrahepatic bile ducts or cystic duct (Figures 2A,B). Following 1 week of antibiotic treatment, the patient’s abdominal pain symptoms nearly disappeared, and CRP levels decreased to the normal range (5.0 mg/L). CT confirmed that the gallbladder had returned to its normal size (Figures 2C,D). The patient was discharged after 3 days. Follow-up examination 2 weeks later in the general surgery clinic showed good recovery, and abdominal ultrasound examination did not show recurrence of gallbladder swelling. The strategy of trying to replace new anlotinib was adopted.
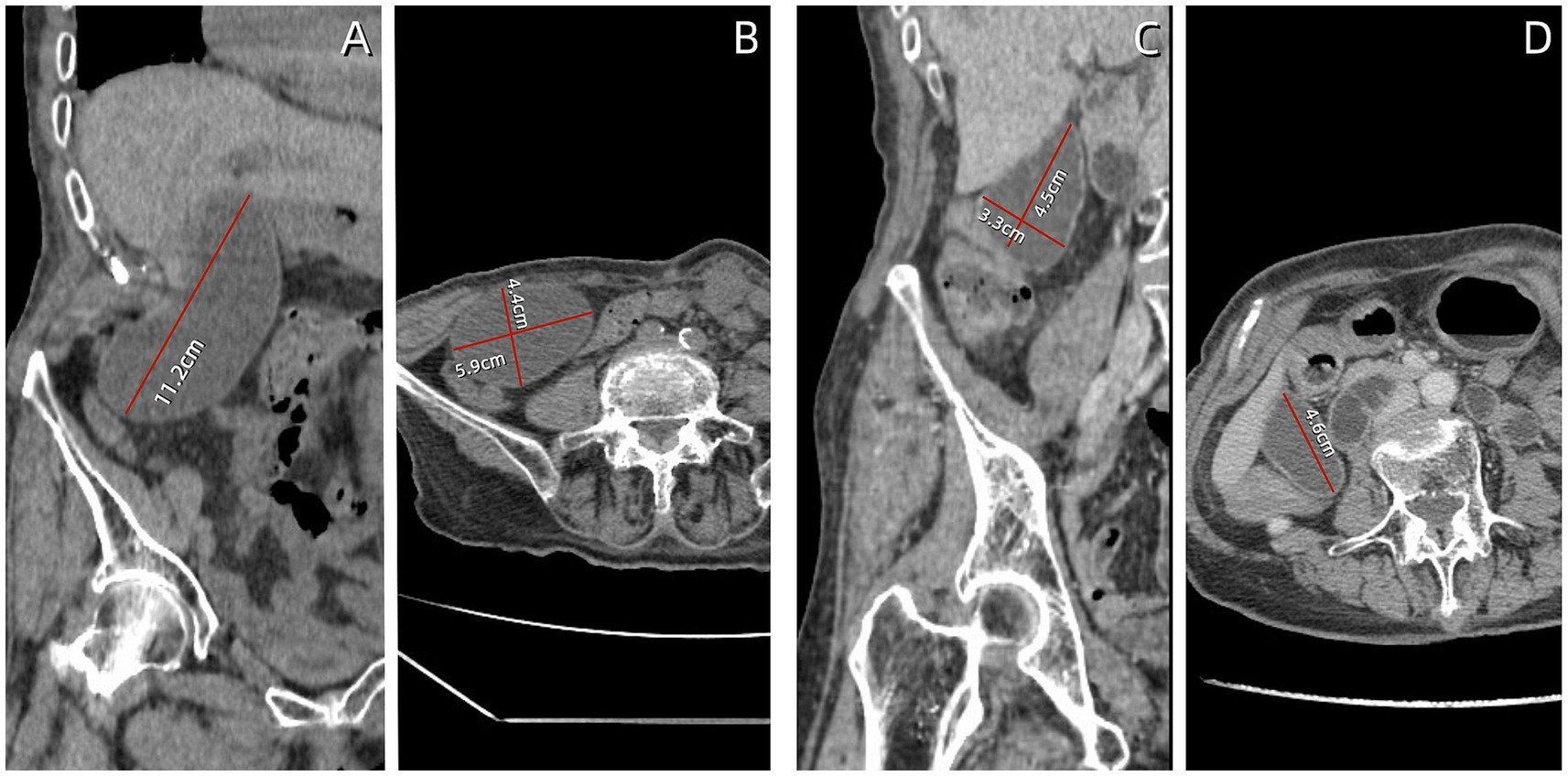
Figure 2. Follow-up abdominal computed tomography (CT) findings. (A,B) After 3 days of antibiotic treatment, the gallbladder volume decreased, measuring approximately 11.2 × 5.9 × 4.4 cm. (C,D) After 7 days of antibiotic treatment, the gallbladder returned to normal size, measuring approximately 4.6 × 4.5 × 3.3 cm.
Discussion
Generally, the gallbladder capacity of a normal adult does not exceed 60 mL. In clinical practice, it is relatively common for various pathological conditions to cause gallbladder enlargement of 250–350 mL (4). However, reports on extreme gallbladder sizes are rare. Due to the lack of clear clinical or histological definitions, some scholars define a gallbladder with a diameter >14 cm and a capacity ≥1.5 L as GGB (5). The pathogenesis of GGB remains unclear. We reviewed multiple literature sources that conducted in-depth research on the occurrence mechanisms of GGB. The Taxonera biliary valve mechanism is the mainstream theory explaining mechanical obstruction-induced GGB. Chronic biliary obstruction caused by stones or tumors, particularly in patients with progressive diseases, such as malignant tumors, leads to long-term elevated intraductal pressure, resulting in gallbladder enlargement (6). Some scholars argue that, while gallstones can cause intermittent obstruction, they are insufficient to chronically sustain elevated intraductal pressure. Moreover, long-standing gallstones may lead to gallbladder atrophy and fibrosis, which limits enlargement (7, 8). Beyond obstructive factors, Kuznetsov proposed that other non-obstructive factors may alter the gallbladder drainage capacity, such as neurological, hydraulic, or combined congenital organ dysfunction. These cases often involve younger patients (4). For instance, Maeda reported a case of a 36-year-old GGB patient primarily presenting with progressive gallbladder enlargement without clinical complications, ultimately diagnosed with focal ganglion cell deficiency in the gallbladder neck (9). In addition to congenital gallbladder abnormalities, patients with type II diabetes exhibit significantly reduced gallbladder wall receptor sensitivity to cholecystokinin due to obesity, coupled with excessive somatostatin secretion. This dual mechanism suppresses normal production of cholecystokinin and cholecystokinin-like substances (cholagogues), thereby impairing gallbladder contraction (10, 11). Furthermore, some circumstances may be paraphysiological such as elevated progesterone levels during pregnancy or hormone replacement therapy which can damage gallbladder contractility. These conditions delay bile emptying, alter bile composition, and promote the precipitation of cholesterol and bilirubin calcium crystals, which facilitate gallstone formation. Chronic irritation from these stones may ultimately cause gallbladder enlargement and even acute pancreatitis (12, 13). A case of GGB associated with a genetic mutation was reported by Panaro et al. in 2012, which involved a 17-year-old adolescent preparing for liver transplantation because of chronic liver disease. Genetic testing revealed a mutation in the ABCB11 gene, confirming the diagnosis of progressive familial intrahepatic cholestasis type 2 (14). Mutations in the ABCB11 gene impair the activity of the bile salt export pump, resulting in bile retention in the biliary tract. This is the largest reported case of GGB, with the gallbladder reaching an astonishing size of 43 × 21 × 20 cm. Unlike the etiological analysis of the GGB cases reported above, we reported the first case of GGB caused by adverse reactions to chemotherapy drugs.
The patient in this report is an elderly female with malignant lung tumor who had been taking anlotinib orally for 2 years on a regular basis, with no history of internal diseases such as hypertension, diabetes, heart disease, or kidney disease. Laboratory tests revealed impaired liver function and abnormally elevated levels of blood inflammatory markers. Imaging examinations revealed acute cholecystitis with GGB, clearly ruling out biliary tract stones and space-occupying lesions causing biliary obstruction. After discontinuing the chemotherapy drug and administering antibiotic treatment, the gallbladder returned to its normal size, and the patient’s liver function and inflammatory markers normalized. The Naranjo scale was used to assess whether the patient’s acute cholecystitis with GGB was caused by anlotinib. This scale is primarily used to evaluate the likelihood of drug-related adverse events (15). Our patient scored five points, indicating that the occurrence of acute cholecystitis with GGB was likely associated with the use of anlotinib (Table 3). Anlotinib is a multi-target tyrosine kinase inhibitor (TKI) primarily used to treat non-small cell lung cancer. It exerts anti-tumor effects by inhibiting the expression of vascular endothelial growth factor, fibroblast growth factor, and stem cell factor receptors, thereby suppressing tumor angiogenesis and tumor cell proliferation (16). TKI directly inhibits the activity of activated enzymes and changes the expression spectrum of miRNA in cells. MiRNA is the core regulator of angiogenesis, and some miRNA participate in angiogenesis by directly targeting proangiogenic factors or receptors (17). For example, miR-29 inhibits angiogenesis by downregulating VEGF overexpression, while miR − 195 promotes angiogenesis and metastasis by enhancing the expression of VEGF and its metastasis-promoting factors. Beyond directly targeting VEGF, certain miRNAs guide mature endothelial cells to migrate into hypoxic environments by inhibiting VEGF-inducible factors (such as miR-22, miR − 107, miR-519c, and miR − 145 in the HIF-1 pathway). This ultimately achieves tumor growth inhibition (18–21). Inevitably, while inhibiting tumor angiogenesis, anlotinib also suppresses blood vessel growth in healthy organs such as the gallbladder (22). Although there is currently no definitive evidence or widespread reports indicating that anlotinib causes inflammatory changes in the gallbladder, there are numerous reports of cholecystitis or gallbladder abnormalities associated with other TKI, such as sorafenib and sunitinib (3, 23). The pharmacological mechanisms underlying TKI-induced gallbladder toxicity are complex and not yet fully elucidated; however, they are generally considered to result from multifactorial effects, primarily involving the following aspects: 1. Target inhibition: One of the main targets of many TKIs (e.g., sunitinib and sorafenib) is the vascular endothelial growth factor receptor. Vascular endothelial growth factor receptor inhibition leads to impaired vascular endothelial cell function, affecting blood supply to the gallbladder wall (microcirculatory disturbance). Gallbladder wall ischemia compromises gallbladder smooth muscle function, resulting in weakened contractility and delayed emptying. 2. Alterations in the bile composition: TKIs may interfere with bile production and secretion by inhibiting bile acid transporter enzymes in the hepatocytes. The abnormal elevation in bile acid concentration can exert direct chemical irritation and toxic effects on the gallbladder mucosa, thereby inducing inflammation. 3. Direct cytotoxic effects: Some TKIs or their metabolites may exert direct toxic effects on the gallbladder mucosal epithelial cells, compromising the integrity of the mucosal barrier. Cellular damage releases inflammatory mediators, triggering local inflammatory responses that attract inflammatory cell infiltration, resulting in gallbladder wall edema, thickening, and inflammation. 4. Secondary effects of liver injury: TKI-induced drug-related liver injury directly affects bile production and excretion, leading to intrahepatic cholestasis. This increases the viscosity of bile entering the gallbladder and may cause reflux, which affects gallbladder function, indirectly promoting cholecystitis and gallbladder enlargement (16, 24, 25).
Currently, there are no management guidelines for giant gallbladder (GGB) both domestically and internationally. The clinical management principles for GGB generally involve first identifying the underlying causes of extreme gallbladder enlargement, followed by standardized treatment according to corresponding etiology guidelines. Regarding etiology, we have reviewed and summarized the latest expert consensus and management guidelines from domestic and international sources, supplemented by Table 4. Laparoscopic cholecystectomy (LC) is considered the preferred surgical option for benign GGB cases with identifiable etiologies. Notably, antibiotic therapy serves as an effective supportive treatment for first-time GGB patients and helps control postoperative infections.
This case report has certain limitations. First, due to the patient’s poor physical condition, they refused cholecystectomy, preventing us from obtaining histological results for in-depth exploration of the specific pathological mechanisms of anlotinib-induced gallbladder toxicity. We will conduct extended research through long-term follow-up observations and collect similar cases from other hospitals or regions for comprehensive analysis. Second, the case did not undergo multidisciplinary consultation to formulate the optimal treatment plan. In future, we will integrate resources from oncology, hepatobiliary surgery, gastroenterology, and radiology departments to establish a personalized multidisciplinary diagnosis and treatment system. Meanwhile, we will advance basic and translational medical research through animal models or organoid technology to precisely decipher the molecular mechanisms of anlotinib-induced gallbladder injury, identify key signaling pathways, and provide crucial evidence for targeted preventive drug development.
In summary, during the treatment of malignant tumors with anlotinib, when patients present with persistent gallbladder enlargement accompanied by cholecystitis, it is necessary to first clarify the true etiology of extreme gallbladder enlargement. Once biliary obstruction is ruled out as a causative factor of GGB, anlotinib can be discontinued while administering antibiotic therapy. After the gallbladder size and inflammatory marker levels return to the normal ranges, anlotinib treatment can be resumed. The entire process typically requires only a few days for completion.
Conclusion
To our knowledge, this is the first reported case of GGB associated with adverse reactions to anlotinib. By sharing the diagnosis and treatment experience of this case, we hope to provide new insights into the etiology of GGB and to alert clinicians to remain vigilant against such adverse events when using TKI-class chemotherapy drugs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Second Affiliated Hospital of Zhejiang Chinese Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Conceptualization, Methodology, Writing – original draft. XW: Formal analysis, Writing – review & editing. JL: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. YS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the school level scientific research project of Zhejiang Chinese Medicial University under grant no. 2022JKJNTZ34.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gao, Y, He, D, Feng, W, Yue, J, and Jian, Z. Laparoscopic cholecystectomy for giant gallbladder: a case report. Medicine. (2023) 102:e35429. doi: 10.1097/MD.0000000000035429
2. Wang, L, He, Z, Yang, S, Tang, H, Wu, Y, Li, S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res. (2019) 8:575–83. doi: 10.21037/tlcr.2019.09.21
3. Zhong, Q, and Liu, Z. Efficacy and safety of anlotinib in patients with advanced non-small cell lung cancer: a real-world study. Cancer Manag Res. (2021) 13:4115–28. doi: 10.2147/CMAR.S304838
4. Kuznetsov, AV, Borodach, AV, Fedin, EN, and Khromova, AD. Giant gallbladder: a case report and review of literature. Int J Surg Case Rep. (2014) 5:673–6. doi: 10.1016/j.ijscr.2014.08.005
5. Zong, L, Chen, P, Wang, L, He, C, Wang, G, Jiang, J, et al. A case of congenital giant gallbladder with massive hydrops mimicking celiac cyst. Oncol Lett. (2013) 5:226–8. doi: 10.3892/ol.2012.1010
6. Taxonera Samso, C, García Albarrán, J, Villacorta Patinoˇ, J, and Diaz-Rubia, GM. Vesícula gigante: Aportatión de un caso y revisión de la literatura. Rev Esp Enferm Apar Dig. (1983) 64:141–4.
7. Singal, R, Gupta, S, Singal, RP, Mittal, A, Sharma, S, and Khurana, R. Gall bladder perforation leads to liver abscess formation – role of ultrasonography. J Gastrointest Dig Syst. (2015) 5:279. doi: 10.4172/2161-069X.1000279
8. Woywodt, A, and Matteson, E. Should eponyms be abandoned? Yes BMJ. (2007) 335:424. doi: 10.1136/bmj.39308.342639.AD
9. Maeda, Y, Setoguchi, T, Yoshida, T, and Katsuki, T. A giant gallbladder. Gastroenterol Jpn. (1979) 14:621–4. doi: 10.1007/BF02773722
10. Fitzgerald, JE, White, MJ, and Lobo, DN. Courvoisier’s gallbladder: law or sign? World J Surg. (2009) 33:886–91. doi: 10.1007/s00268-008-9908-y
11. Calomino, N, Poto, GE, Carbone, L, Bagnacci, G, Piccioni, S, Andreucci, E, et al. Neuroendocrine tumors' patients treated with somatostatin analogue could complicate with emergency cholecystectomy. Ann Ital Chir. (2023) 94:518–22. Available at: https://pubmed.ncbi.nlm.nih.gov/38051513/
12. Uemura, S, Namikawa, T, Uchida, K, and Hanazaki, K. Gastrointestinal: Giant gallbladder. J Gastroenterol Hepatol. (2022) 37:2206. doi: 10.1111/jgh.15858
13. Veres, M, Flamind Oltean, S, Pascanu, S, Butiulca, M, Branea, OE, Lazar, AE, et al. Too late to reverse: an atypical postpartum case of acute necrotizing pancreatitis with refractory ARDS despite ECMO support. Life. (2025) 15:1347. doi: 10.3390/life15091347
14. Panaro, F, Chastaing, L, and Navarro, F. Hepatobiliary and pancreatic: Giant gallbladder associated with Byler’s disease. J Gastroenterol Hepatol. (2012) 27:620. doi: 10.1111/j.1440-1746.2012.07058.x
15. Naranjo, CA, Busto, U, Sellers, EM, Sandor, P, Ruiz, I, Roberts, EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. (1981) 30:239–45. doi: 10.1038/clpt.1981.154
16. Aihara, Y, Yoshiji, H, Yamazaki, M, Ikenaka, Y, Noguchi, R, Morioka, C, et al. A case of severe acalculous cholecystitis associated with sorafenib treatment for advanced hepatocellular carcinoma. World J Gastrointest Oncol. (2012) 4:115–8. doi: 10.4251/wjgo.v4.i5.115
17. Giuppi, M, La Salvia, A, Evangelista, J, and Ghidini, M. The role and expression of angiogenesis-related miRNAs in gastric Cancer. Biology (Basel). (2021) 10:146. doi: 10.3390/biology10020146
18. Yamakuchi, M, Yagi, S, Ito, T, and Lowenstein, CJ. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS One. (2011) 6:e20291. doi: 10.1371/journal.pone.0020291
19. Yamakuchi, M, Lotterman, CD, Bao, C, Hruban, RH, Karim, B, Mendell, JT, et al. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci USA. (2010) 107:6334–9. doi: 10.1073/pnas.0911082107
20. Cha, ST, Chen, PS, Johansson, G, Chu, CY, Wang, MY, Jeng, YM, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis [published correction appears in cancer res. 2019; 79 (14): 3790]. Cancer Res. (2010) 70:2675–85. doi: 10.1158/0008-5472.CAN-09-2448
21. Zhang, H, Pu, J, Qi, T, Qi, M, Yang, C, Li, S, et al. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene. (2014) 33:387–97. doi: 10.1038/onc.2012.574
22. Sanda, M, Tamai, H, Deguchi, H, Mori, Y, Moribata, K, Shingaki, N, et al. Acalculous cholecystitis in a patient with hepatocellular carcinoma on sorafenib. ISRN Gastroenterol. (2011) 2011:201529. doi: 10.5402/2011/201529
23. Choi, SW, Lee, JM, Kim, DG, and Noh, MH. Acute acalculous cholecystitis associated with sunitinib treatment for renal cell carcinoma. Korean J Gastroenterol. (2020) 75:103–7. doi: 10.4166/kjg.2020.75.2.103
24. Di Stefano, M, Colombo, C, De Leo, S, Perrino, M, Viganò, M, Persani, L, et al. High prevalence and conservative management of acute cholecystitis during Lenvatinib for advanced thyroid cancer. Eur Thyroid J. (2021) 10:314–22. doi: 10.1159/000510369
25. Tirumani, SH, Krajewski, KM, Shinagare, AB, Jagannathan, JP, and Ramaiya, NH. Gallbladder complications associated with molecular targeted therapies: clinical and imaging features. Clin Imaging. (2014) 38:50–5. doi: 10.1016/j.clinimag.2013.08.012
26. Borodach, AV, Borodach, VA, and Kim, AN. Gigantskaya vodyanka zhelchnogo puzyrya. Novosibirsk: Sibirskiy Universitet; (2005). p. 62–63.
27. Fultang, J, Chinaka, U, and Ali, A. Giant gallbladder presenting as a right iliac Fossa mass removed by Mini-laparoscopic cholecystectomy. Cureus. (2019) 11:e5576. doi: 10.7759/cureus.5576
28. Mirali, H, Kamaoui, I, El Harroudi, T, Skiker, I, and Serji, B. Giant gallbladder revealed by chronic cholecystitis gallstone: a case report and review of the literature. Cureus. (2021) 13:e13906. doi: 10.7759/cureus.13906
29. de’Angelis, N, Catena, F, Memeo, R, Coccolini, F, Martínez-Pérez, A, Romeo, OM, et al. 2020 WSES guidelines for the detection and management of bile duct injury during cholecystectomy. World J Emerg Surg. (2021) 16:30. doi: 10.1186/s13017-021-00369-w
30. Okamoto, K, Suzuki, K, Takada, T, Strasberg, SM, Asbun, HJ, Endo, I, et al. Tokyo guidelines 2018: flowchart for the management of acute cholecystitis [published correction appears in J hepatobiliary Pancreat Sci. 2019; 26 (11): 534. Doi:10.1002/jhbp.686.]. J Hepatobiliary Pancreat Sci. (2018) 25:55–72. doi: 10.1002/jhbp.516
31. Vera, K, Pei, KY, Schuster, KM, and Davis, KA. Validation of a new American Association for the Surgery of Trauma (AAST) anatomic severity grading system for acute cholecystitis. J Trauma Acute Care Surg. (2018) 84:650–4. doi: 10.1097/TA.0000000000001762
32. Ansaloni, L, Pisano, M, Coccolini, F, Peitzmann, B, Fingerhut, A, Catena, F, et al. WSES guidelines on acute calculous cholecystitis [published correction appears in world]. World J Emerg Surg. (2016) 11:25. doi: 10.1186/s13017-016-0082-5
33. Shi, J, Li, K, and Chen, Y. Rare giant bile duct cyst with new-onset liver neoplasm nine years after surgery: a cast report. Chin J Hepatic Surg. (2025) 14:126–7. doi: 10.3877/cma.j.issn.2095-3232.2025001
34. Keplinger, KM, and Bloomston, M. Anatomy and embryology of the biliary tract. Surg Clin North Am. (2014) 94:203–17. doi: 10.1016/j.suc.2014.01.001
35. Paraf, F, Lemaigre, G, and Bedossa, P. Vésicule biliaire trilobée [Trilobed gallbladder]. Gastroenterol Clin Biol. (1987) 11:713–4.
36. Vogel, A, Bridgewater, J, Edeline, J, Kelley, RK, Klümpen, HJ, Malka, D, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:127–40. doi: 10.1016/j.annonc.2022.10.506
Keywords: giant gallbladder, chemotherapeutic drugs, anlotinib, case report, acute cholecystitis, adversereactions
Citation: Cheng J, Wang X, Liao J and Shen Y (2025) Case Report: A rare case of giant gallbladder associated with adverse reactions to anlotinib. Front. Med. 12:1665373. doi: 10.3389/fmed.2025.1665373
Edited by:
Xiaowei Wu, Dana–Farber Cancer Institute, United StatesReviewed by:
Natale Calomino, University of Siena, ItalyAnna La Salvia, National Institute of Health (ISS), Italy
Copyright © 2025 Cheng, Wang, Liao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyun Liao, bGp5OTQzNDI1OTk3QGhvdG1haWwuY29t; Yuping Shen, MjAyMTQwNDlAemNtdS5lZHUuY24=
 Jihao Cheng
Jihao Cheng Xin Wang
Xin Wang Jingyun Liao
Jingyun Liao Yuping Shen
Yuping Shen