- 1Laboratory of Translational and Regenerative Medicine, The 2nd Affiliated Hospital of Shenzhen University, Medical School, Shenzhen University, Shenzhen, China
- 2School of Medicine, The Chinese University of Hong Kong Shenzhen, Shenzhen, China
- 3Department of Plastic and Reconstructive Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Diabetes is a global health problem, with diabetic wounds constituting one of its most severe complications. Advanced glycation end products (AGEs) and their receptor, the receptor for advanced glycation end products (RAGE), play a key role in the pathogenesis of diabetic wounds. Accumulated AGEs bind to RAGE, activating various inflammatory and oxidative stress pathways such as NF-κB, PI3K-AKT, and JAK–STAT signaling, impairing normal wound healing. This review describes mechanisms by which the AGEs-RAGE axis disrupts vascular function, immune regulation, and cellular regeneration, thereby driving the formation of chronic non-healing wounds. Furthermore, we discuss emerging therapeutic strategies targeting the AGEs-RAGE axis, such as selective RAGE inhibitors, monoclonal antibodies, gene-based interventions, and AGE scavengers, highlighting their potential to enhance the treatment of diabetic chronic wounds.
1 Introduction
The escalating diabetic patients imposes a substantial socioeconomic burden worldwide. Global prevalence of diabetes was estimated at 10.5% (536.6 million) in 2021, with a projected rise to 12.2% (783.2 million) by 2045 (1). Diabetes-related complications represent the primary cause of disability and mortality among diabetic patients. Among these chronic complications, diabetic wounds stand out as one of the most life-threatening conditions, demonstrating strong associations with hospitalization, limb amputation, and increased mortality rates (2). Epidemiological data reveal that approximately 18.6 million people with diabetes develop a foot ulcer (the most common type of diabetic wound) each year (3). Currently, no definitive therapeutic approach exists for the effective repair of diabetic wounds. A comprehensive understanding of the pathogenic mechanisms underlying diabetic wound formation may reveal novel therapeutic targets for this debilitating condition.
Wound healing is a highly orchestrated and complex process. Normal wounds (or acute wounds) healing typically contains four consecutive and overlapping stages: (a) Hemostasis stage: Vascular constriction occurs, with rapid recruitment of platelets and clotting factors to seal the wound; (b) Inflammation stage: Innate immune cells, such as neutrophils and macrophages, migrate to the wound in response to inflammatory cytokines, pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), and eliminate pathogens and necrotic cells; (c) Proliferation stage: As inflammation resolves, endothelial cells, fibroblasts, and keratinocytes proliferate and migrate from the wound margins, forming granulation tissue and regenerating the epidermis for wound closure; (d) Remodeling phase: The structure and composition of granulation tissue was remodeled. Some newly generated vessels atrophy, and collagen continues to deposit, ultimately leading to the formation of scars (4).
In diabetic wounds, pathological factors such as bacterial infections, oxidative stress, local ischemia, persistent inflammation, and skin nerve atrophy collectively disrupt the normal healing cascade, resulting in chronic non-healing wounds. Signaling pathways mediating inflammation and oxidative stress are closely associated with these pathological alterations (5). Advanced glycation end products (AGEs) are a class of heterogeneous glycated derivatives that exert biological effects primarily through binding to the Receptor for Advanced Glycation End Products (RAGE). This interaction activates various downstream pro-inflammatory and oxidative stress pathways, including the Nuclear Factor Kappa B (NF-κB), Phosphoinositide 3-kinase/Protein kinase B (PI3K/AKT), and Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signaling pathways. Numerous chronic, inflammatory diseases, such as neurodegenerative disorders, diabetes, atherosclerosis, and cancers, are associated with the persistent activity of the AGEs-RAGE axis, indicating its potential critical role in the pathogenesis of diabetic wounds (6, 7).
In this review, we introduce the structure and function of AGEs and their receptor RAGE. Furthermore, we emphasize the contribution of the AGEs-RAGE axis in diabetic wounds and discuss therapeutic strategies targeting this pathway, aiming to provide innovative perspectives for enhancing diabetic wound healing.
2 AGEs and RAGE
2.1 The formation and accumulation of AGEs
AGEs represent a highly heterogeneous class of compounds produced through a series of non-enzymatic reactions, known as the Maillard reaction (8). This reaction consists of three major steps: glycation, Amadori rearrangement, and non-enzymatic peptide cross-linking. Initially, reducing sugars react reversibly with the amino groups of proteins, lipids, and nucleic acids, forming early Schiff bases. In high-glucose systems, the rate of this reaction is significantly enhanced. Subsequently, the Schiff bases undergo slow, irreversible rearrangement to form Amadori products. Finally, these Amadori products undergo a series of complex reactions, including oxidation, dehydration, and cross-linking, which yield various types of AGEs (9, 10).
AGEs accumulate in the body via both exogenous intake and endogenous synthesis. Exogenous intake is the primary contributor to the total body burden of AGEs, predominantly derived from high-temperature-cooked foods, sugary beverages, and tobacco products (8). Following absorption, externally derived AGEs can bind to various proteins and tissues throughout the body. Endogenous AGEs mainly arise from post-translational glycation of proteins, exhibiting significant cellular toxicities (11). In healthy individuals, accumulated AGEs are efficiently cleared through multiple pathways. The ubiquitin–proteasome system and autophagy are responsible for the quality control of protein, selectively degrading damaged proteins to maintain cellular homeostasis (12). Moreover, low-molecular-weight AGEs can be excreted renally (13). Furthermore, the glyoxalase system helps reduce AGEs formation by catalyzing reducing sugar into non-toxic metabolites (14). However, under conditions such as chronic high-AGE diets or pathological states, such as aging, oxidative stress, hyperglycemia, and hyperlipidemia, the equilibrium between AGE formation and clearance becomes disrupted (15). Over time, increasing AGEs accumulate in tissues. Their presence triggers inflammatory responses and oxidative stress through persistent interaction with RAGE and additionally induces protein dysfunction through cross-linking-mediated structural alterations (15).
2.2 Structure and isoforms of RAGE
The RAGE gene is located within the histocompatibility complex (MHC) class III region on human chromosome 6 and contains 11 exons and 10 introns. The term “RAGE” generally refers to the full-length RAGE isoform, a transmembrane glycoprotein consisting of 404 amino acids with a molecular weight of approximately 50 kDa. It is recognized as the only isoform capable of transducing signals (16). Structurally, RAGE is divided into three distinct regions: the extracellular, transmembrane segment, and intracellular domain. The extracellular segment encompasses an immunoglobulin-like variable (V) domain and two constant-type domains (C1 and C2). The transmembrane segment includes a helical domain, and the intracellular domain contains a highly charged, disordered cytoplasmic tail. The V and C1 domains function as ligand-binding interfaces, characterized by a highly positively charged region that interacts with negatively charged regions of ligands (17–19). This binding mechanism is a hallmark of pattern recognition receptors (PRRs), enabling RAGE to engage with a broad spectrum of structurally diverse ligands, such as AGEs, high mobility group box 1 (HMGB1), S100 proteins, amyloid beta (Aβ), lipopolysaccharides (LPS), lysophosphatidic acid (LPA), nucleic acids, and complement protein C1q (20) (Figure 1).
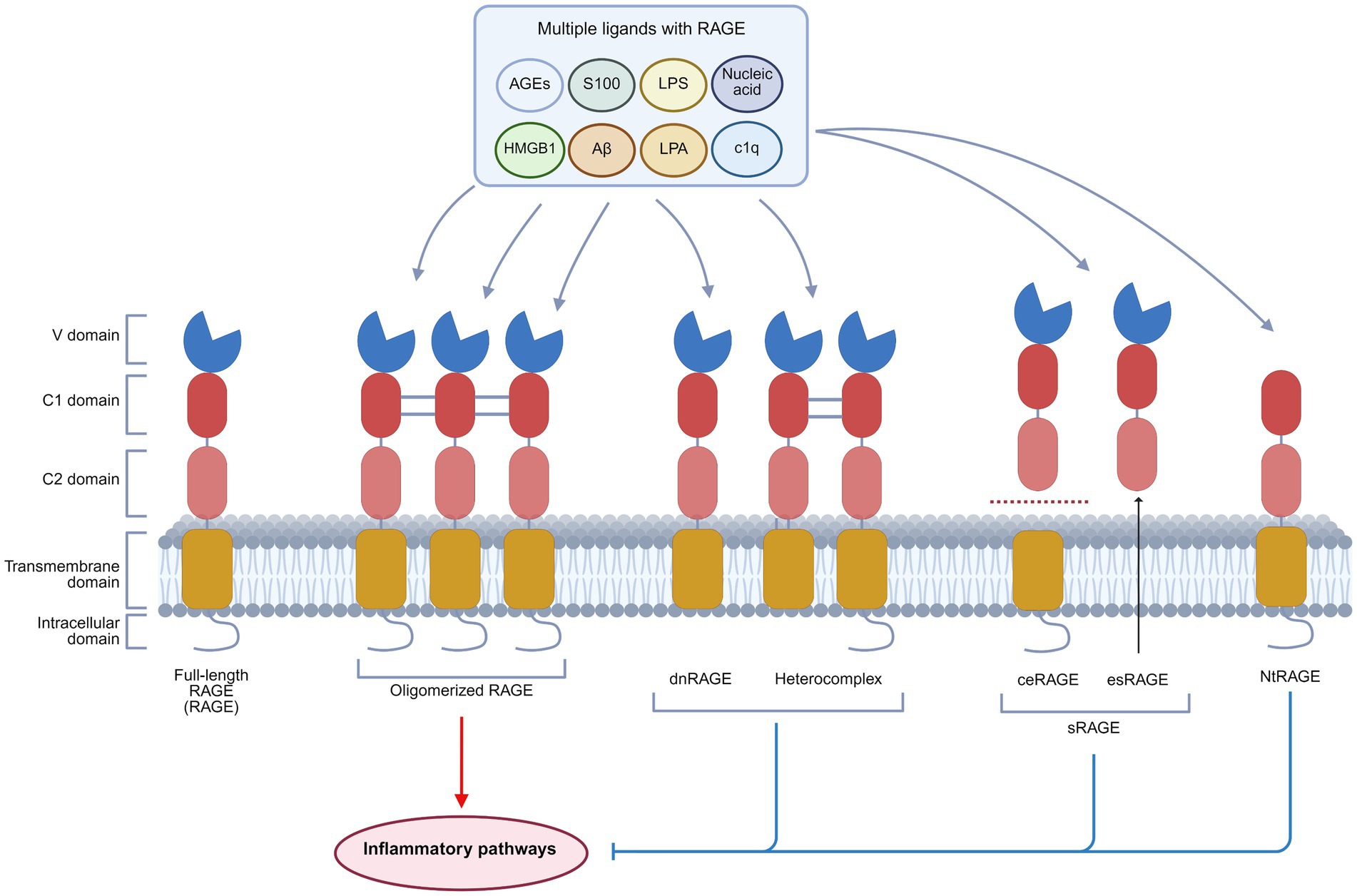
Figure 1. Basic structure and isoforms of RAGE. RAGE exists in four principal isoforms: Full-length RAGE, dnRAGE, sRAGE, and NtRAGE. Full-length RAGE consists of an extracellular domain (V, C1, and C2 domains), a transmembrane domain, and an intracellular domain. It is capable of binding multiple ligands, including AGEs, HMGB1, S100 proteins, Aβ, LPA, LPS, C1q, and nucleic acids, and requires oligomerization for signal transduction. dnRAGE, sRAGE, and NtRAGE lack complete structural domains. Upon ligand binding, they fail to activate downstream inflammatory pathways, thus functioning as signaling inhibitors. This figure was created in https://BioRender.com.
RAGE exists in four main isoforms: the transmembrane signaling receptor (full-length RAGE, RAGE), the dominant negative form (dnRAGE), the circulating soluble forms (sRAGE), and the N-truncated form (NtRAGE) (21). RAGE can form oligomers on the plasma membrane, and the preassembly has substantial implications for its activation mechanism. This process is driven by heparan sulfate scaffolds on the cell surface, which facilitate RAGE oligomerization into signaling-competent complexes (22, 23). The dnRAGE isoform, which lacks the cytoplasmic domain, disrupts signal transduction and may form nonfunctional heterocomplexes with full-length RAGE (23–25). sRAGE contains cleaved RAGE (ceRAGE) and endogenous secreted RAGE (esRAGE). Lacking transmembrane and intracellular segments, sRAGE circulates in the serum and competitively binds RAGE ligands to inhibit the activation of RAGE (26). Moreover, sRAGE is considered a biomarker for inflammatory diseases (27). The NtRAGE isoform, which lacks the V domain, functions as a decoy receptor to modulate the RAGE signaling activity (28, 29) (Figure 1).
2.3 Biological function and distribution of RAGE
The primary biological function of RAGE is to act as a receptor that transduces ligand signals, initiating a cascade of downstream biochemical reactions. The cytoplasmic tail of RAGE lacks intrinsic kinase activity and therefore depends on adaptor proteins, such as the Formin homology 1 domain of diaphanous-1 (DIAPH1), DNAX-activating Protein 10 (DAP10), and Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) to initiate intracellular signaling (30–32). These adaptors subsequently activate multiple signaling pathways, including Ras/mitogen-activated extracellular signal-regulated kinase (MEK)/extracellular regulated protein kinases (ERK), mitogen-activated protein kinase (MAPK)/P38, JAK/STAT, PI3K/AKT, and cell division cycle 42 (Cdc42)/Rac family small GTPase 1 (Rac1) (20, 33). Downstream transcription factors, including NF-κB, STAT3, activator protein 1 (AP1), and early growth response 1 (Egr1), promote the secretion of inflammatory cytokines like interleukin (IL)-1, IL-6, tumor necrosis factor, interferon, and chemokines, and thereby regulate cell proliferation and migration, and induce apoptosis (33). Moreover, RAGE activation facilitates the production of reactive oxygen species (ROS) by inflammatory signaling pathways and the enhancement of the activity of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (Figure 2).
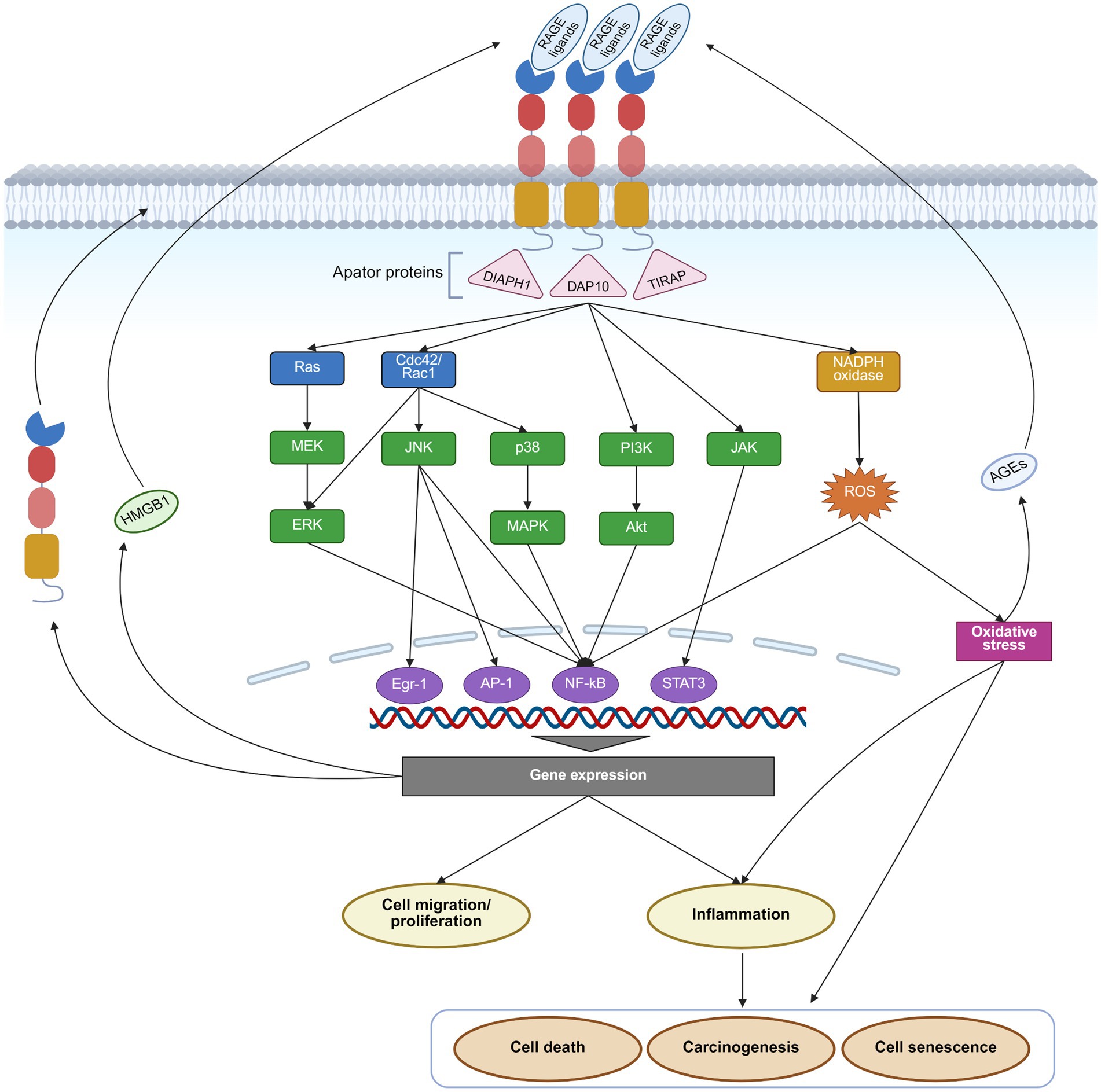
Figure 2. RAGE pathway and its effects. Upon binding its ligands, RAGE activates Ras/MEK/ERK, p38/MAPK, JAK/STAT, PI3K/AKT, and Cdc42/Rac1 signaling pathways, along with NADPH oxidase through adaptor proteins such as DIAPH1, DAP10, and TIRAP. These downstream pathways subsequently activate transcription factors such as NF-κB, AP1, Egr1, and STAT3, driving gene expression and triggering a cascade of effects, including inflammation, cell migration, proliferation, senescence, death, and carcinogenesis. Moreover, activation of the RAGE pathway enhances the expression of RAGE itself and several of its ligands, like AGEs and HMGB1, creating a positive feedback loop that further amplifies RAGE signaling. This figure was created in https://BioRender.com.
RAGE is widely expressed across multiple cell types. Under physiological conditions, RAGE is expressed at low levels in several cell types, including monocytes/macrophages, T-lymphocytes, epithelial cells, endothelial cells, dendritic cells, fibroblasts, smooth muscle cells, neuronal cells, glial cells, and chondrocytes (34–37). On the contrary, in pathophysiological settings, such as diabetes, chronic inflammation, cancer, or neurodegenerative disorders, RAGE expression is increased drastically (38). This upregulation is mediated by an NF-κB-dependent positive feedback mechanism. Specifically, ligand-induced RAGE activation potently stimulates NF-κB, which then binds to the RAGE promoter to enhance its transcription, thereby creating a vicious cycle that amplifies RAGE signaling (39). Furthermore, inflammatory response and oxidative stress promote the production of RAGE ligands, including AGEs and HMGB1, further amplifying the effects of the RAGE pathway (40, 41) (Figure 2). Therapeutic strategies aimed at suppressing inflammation and oxidative stress, clearing RAGE ligands, or directly inhibiting RAGE may present viable strategies to disrupt this detrimental positive feedback loop (42–45). For instance, clearance can be achieved by sRAGE or ligand scavengers like aminoguanidine, paquinimod, and glycyrrhizin (42, 44–46).
RAGE acts as a double-edged modulator in maintaining homeostasis. It exerts indispensable physiological roles while also driving pathological damage when dysregulated. During embryonic development, RAGE supports alveolar epithelial cell differentiation and the growth and migration of cerebral neurons (47, 48). As a PRR, it binds microbial ligands, initiates innate immune responses, and promotes immune cells to eliminate pathogens and apoptotic cells (49, 50). Moreover, moderate RAGE activation-induced recruitment of immune cells contributes to the regeneration of skeletal muscles and peripheral nerves (51, 52). However, in pathological conditions, excessive activation of RAGE results in chronic inflammation, excessive oxidative stress, and cellular dysfunction, which collectively contribute to disease progression (53). Aberrant RAGE signaling is implicated in various chronic inflammatory diseases, including diabetes, atherosclerosis, nephropathy, pathological scar, neurodegenerative disorders, and cancer (54–57). Notably, RAGE also demonstrates non-receptor effects. When phosphorylated by ATM kinase, RAGE translocates to the nucleus and participates in DNA double-strand break repair, thereby helping prevent cellular senescence, tumorigenesis, and fibrosis (58). Furthermore, RAGE on endothelial cell surfaces can act as a ligand, mediating leukocyte adhesion and recruitment (59).
3 The effects of AGEs-RAGE axis in diabetic wounds
3.1 Peripheral neuropathy
Peripheral neuropathy, including the impairment of sensory and autonomic nerves, is a common complication among diabetic patients (60).
Sensory nerves extend into the epidermal layer, where they detect and transmit external stimuli to the central nervous system to initiate defensive responses. Beyond their sensory role, these nerves regulate skin growth and maintain homeostasis by secreting neurotrophic factors and neuropeptides (61). However, AGEs–RAGE activation induces inflammation and oxidative stress by the NF-κB-p65 pathway, which inhibits nerve growth and disrupts their secretory capacity (62, 63). Furthermore, AGEs–RAGE signaling promotes the activation of pro-inflammatory M1 macrophages, leading to inflammatory infiltration that contributes to neuronal insulin resistance and atrophy (64). Such damage elevates mechanical and thermal pain thresholds, reducing the body’s ability to respond effectively to external stimuli. Additionally, the loss of neurotrophic support adversely affects the renewal and functionality of keratinocytes, fibroblasts, and immune cells, thereby compromising skin repair (61, 65).
Long-term activation of RAGE impairs the autonomic nervous system as well (66). Autonomic nerves in the skin primarily regulate vascular smooth muscles and sweat glands. Vasomotor dysfunction caused by autonomic neuropathy disrupts normal blood flow distribution, impairing skin perfusion. Atrophy of the autonomic nerves innervating the sweat glands reduces perspiration, causing skin dryness and increasing susceptibility to wound formation (67) (Figure 3).
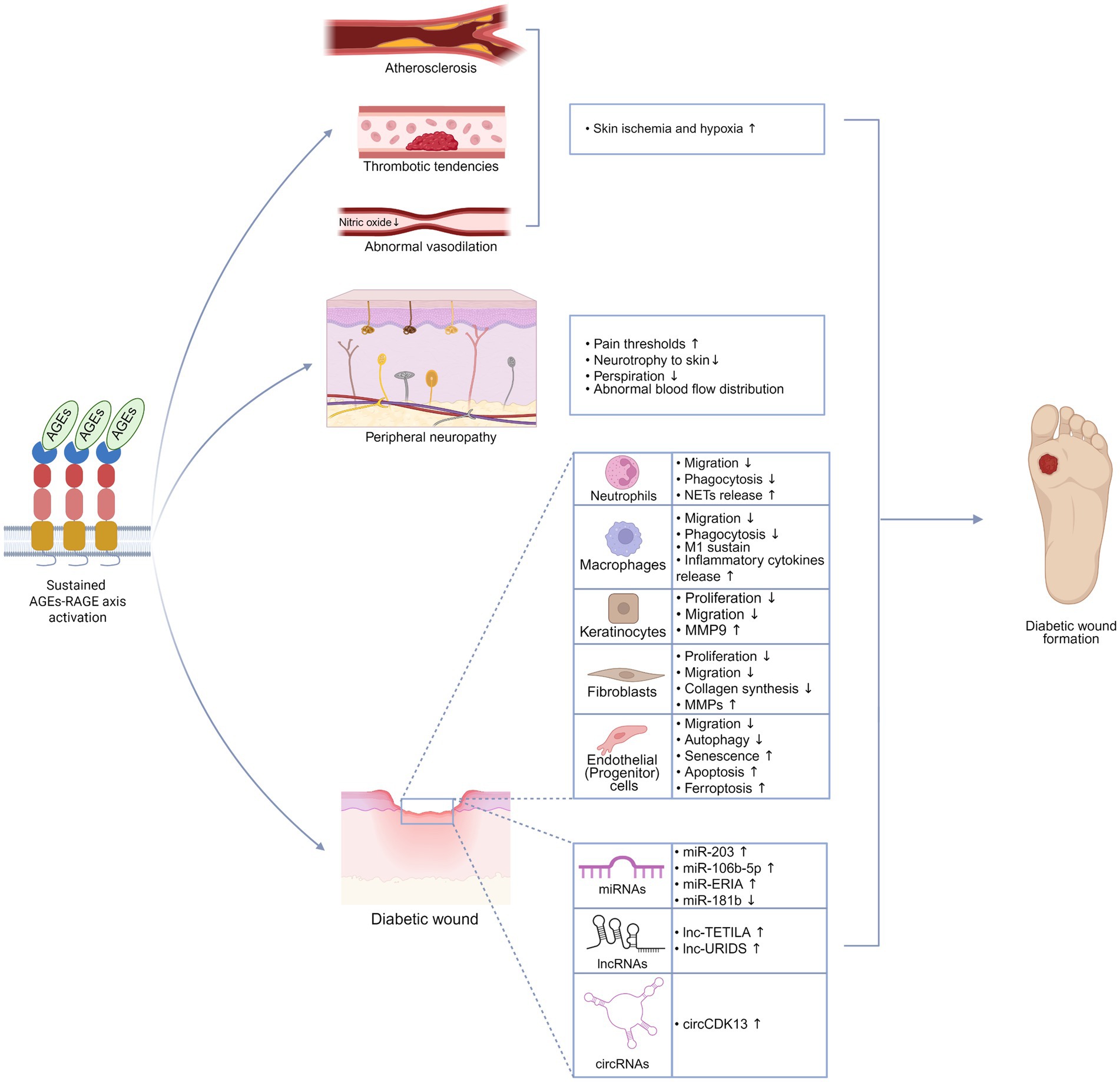
Figure 3. AGEs-RAGE axis promotes the formation of diabetic wounds. Sustained activation of the AGEs-RAGE axis drives a range of pathological alterations that accelerate the formation of diabetic wounds, including atherosclerosis, thrombotic tendencies, impaired vasodilation, peripheral neuropathy, dysfunction of repair cells, and abnormal expression of ncRNAs. This figure was created in https://BioRender.com.
3.2 Vascular lesions
Local skin ischemia and hypoxia, primarily resulting from vascular abnormalities such as atherosclerosis, endothelial dysfunction, and thrombotic tendencies, play a central role in the impaired healing of diabetic wounds. Activation of the RAGE pathway promotes the development of these pathological changes (68) (Figure 3).
Atherosclerosis is characterized by lipid deposition, fibrous proliferation, and calcification within the arterial intima, eventually leading to plaque formation and vascular narrowing. Atherosclerosis is prevalent in the lower extremities of diabetic patients and primarily affects the mid-sized arteries, such as the posterior and anterior tibial arteries (69). Therefore, diabetic wounds frequently manifest in the distal lower extremities, especially in the feet. The initial steps of atherosclerosis involve the accumulation of low-density lipoproteins (LDLs) beneath the arterial intima, where they are oxidized into oxidized LDLs (ox-LDLs) (70). AGEs promote LDL transcytosis across endothelial cells via the RAGE/NF-κB/Caveolin-1 axis, facilitating LDL deposition within the intima (71). Moreover, the oxidative stress induced by the AGEs-RAGE axis accelerates the oxidation of LDLs. Subsequently, macrophages migrate into the intima, engulf ox-LDLs, and transform into foam cells. AGEs–RAGE signaling upregulate the expression of cluster of differentiation 36 and Cyclin-dependent Kinase 5, promoting macrophage migration and enhancing the capacity to engulf ox-LDLs (72, 73). Furthermore, the AGEs-RAGE axis induces the expression of adhesion proteins such as intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin in endothelial cells, promoting the sustained retention of foam cells within the intima (74). Foam cells release substantial amounts of inflammatory factors that stimulate the migration, proliferation, and extracellular matrix (ECM) synthesis of vascular smooth muscle cells (VSMCs), leading to arterial intima thickening. Eventually, the plaque’s core consists of lipids, macrophages, and VSMCs (75). Progressive plaque enlargement reduces distal blood perfusion, directly contributing to wound formation and impairing wound healing.
Endothelial dysfunction, marked by impaired nitric oxide (NO)-mediated vasodilation, significantly influences blood supply to distal small vessels and exacerbates the narrowing of atherosclerotic vessels (76). NO production depends on endothelial nitric oxide synthase (eNOS). ROS induced through the AGEs-RAGE axis reduces the nuclear translocation of transcription factor EB, suppressing autophagic flux. This disruption contributes to eNOS uncoupling, reducing NO bioavailability and causing endothelial dysfunction (77, 78).
Thrombotic tendencies arise from increased platelet counts and hyperactivity, which can worsen atherosclerosis and occlude small blood vessels. RAGE expression is elevated in the platelets of diabetic and aging patients. The AGEs-RAGE axis enhances the activity and aggregation capacity of platelets, promoting a pre-thrombotic state (79, 80). Furthermore, the AGEs-RAGE axis upregulates endothelin-1 expression, which mediates vascular endothelial dysfunction and promotes thrombosis (81).
3.3 Dysfunction of cells for wound repair
3.3.1 Neutrophils
Neutrophils are the first immune cells to arrive at wounds, primarily tasked with killing microbes. Overactivation of RAGE by AGEs disrupts the neutrophil extravasation and compromises their pathogen-killing abilities (82). Increased oxidative stress is one of the major factors leading to diabetic wounds. RAGEs mediate the generation of ROS in neutrophils via activation of NADPH oxidase (83). Neutrophils can release neutrophil extracellular traps (NETs) to capture bacteria and restrict the progression of infection. However, NETs contain various proteases, and their increase enhances the digestion of wound ECM, thereby promoting the formation of unhealing wounds (84) (Figure 3). The AGEs-RAGE axis enhances MEK/p38-MAPK/TGF-beta activated kinase 1 signaling, inducing citrullination of histone H3 at its R2 residue, thereby promoting the formation of NETs (85).
3.3.2 Macrophages
Macrophages are the orchestrators of wound inflammation (86). In later stages of normal wound repair, macrophages transition from a pro-inflammatory M1 phenotype to a pro-reparative M2 phenotype, a shift critical for wound healing. RAGE activation, however, induces macrophages to retain a pro-inflammatory M1 phenotype by NF-κB signaling and ROS (87–89). Pyroptosis is a form of programmed cell death that leads to the release of intracellular pro-inflammatory substances. The AGEs-RAGE axis may exacerbate wound inflammation and impair wound healing by inducing the Caspase-11-dependent pyroptosis-related protein (90). Macrophages are also responsible for eliminating pathogens and cellular debris. Similar to neutrophils, their migratory and phagocytic functions are impaired by the AGEs-RAGE axis (Figure 3).
3.3.3 Keratinocytes
Wound closure depends on re-epithelialization driven by keratinocyte migration and proliferation. The AGEs-RAGE axis inhibits keratinocyte proliferation, migration, epidermal lipid synthesis, and the expression of epidermal antimicrobial peptides by promoting inflammation and oxidative stress, thereby impairing epidermal regeneration and barrier function (91). Keratinocytes are a major source of MMP9. MMP9 is regarded as a promising therapeutic target, given its significant expression in diabetic wounds compared to other MMPs (92). MMP9 not only hinders wound healing through ECM degradation but also induces keratinocyte apoptosis via the FasL/Fas pathway (93). The AGEs-RAGE axis enhances MMP9 expression by promoting the demethylation of its gene promoter in keratinocytes (94). Furthermore, RAGE activation in keratinocytes leads to the abnormal secretion of Notch ligands. Overactivated Notch1 signaling pathway, in turn, induces NF-κB to promote MMP9 expression (95) (Figure 3).
3.3.4 Fibroblasts
Dermal is the primary structural component of skin, and its repair largely relies on the proliferation, differentiation, and ECM remodeling by fibroblasts. Accumulated AGEs form stable cross-links with the main component of the ECM, collagen fibers, disrupting the normal collagen structure and leading to increased nano-stiffness and reduced hydration in the ECM (96–98). These cross-linked AGEs persistently interact with RAGE, compromising various fibroblast functions, including proliferation, migration, and collagen synthesis, while increasing secretion of MMPs (99). Moreover, a stiff ECM exacerbates chronic inflammation and accelerates cellular senescence (100). Senescent fibroblasts exhibit reduced contractility and impaired collagen secretion, adopting a senescence-associated secretory phenotype that releases inflammatory cytokines and MMPs (101) (Figure 3). These alterations further degrade the ECM and prolong inflammation.
3.3.5 Endothelial cells
Angiogenesis driven by endothelial cells is crucial to wound healing. The RAGE activated by high levels of AGEs inhibits endothelial cell migration and promotes various forms of cellular dysfunction, including autophagy abnormalities, senescence, apoptosis, and ferroptosis (102, 103). Vascular endothelial growth factor (VEGF), a key angiogenic stimulant that promotes endothelial cell proliferation and migration, is downregulated in AGE-treated wounds via the RAGE pathway (104). Bone marrow-derived endothelial progenitor cells (EPCs), which serve as reservoirs for vascular repair, are also compromised by RAGE activation. The AGEs-RAGE axis disrupts autophagy and induces apoptosis in EPCs, thereby diminishing their ability to support angiogenesis and vascular repair (105, 106) (Figure 3).
3.4 Abnormal expression of ncRNAs
Non-coding RNAs (ncRNAs), including microRNAs (miRNAs, miR), long non-coding RNAs (lncRNA, lnc), and circular RNAs (circRNAs, circ), serve as critical regulators of gene expression through epigenetic, transcriptional, and post-transcriptional mechanisms (107). The AGE-RAGE axis induces aberrant expression of specific ncRNAs, leading to impaired cellular homeostasis and tissue repair processes. Examples include miR-203 and lnc-TETILA in keratinocytes (108, 109), lnc-URIDS and circCDK13 in fibroblasts (110), as well as miR-181b and miR-106b-5p in endothelial cells (111, 112) (Figure 3). Extracellular vehicles (EVs) function as crucial mediators of intercellular communication by transporting diverse cargo, including nucleic acids, proteins, and lipids (113). The AGEs-RAGE axis promotes specific ncRNAs expression that becomes packaged into EVs, subsequently influencing recipient cell behavior. Recent studies elucidated that AGEs promote macrophage polarization toward the pro-inflammatory M1 phenotype. These activated macrophages secrete EVs enriched with miR-ERIA, which targets helicase with zinc finger 2 in vascular endothelial cells, ultimately impairing endothelial migration and tube formation capacity (114). Further investigation of ncRNAs modulated by the AGEs-RAGE axis may provide potential therapeutic targets for enhancing diabetic wound healing.
4 Strategies targeting the AGEs-RAGE axis for diabetic wound repair
RAGE-induced chronic inflammation plays a critical pathogenic role in the development of diabetic wounds, positioning the AGEs-RAGE axis as a promising therapeutic target. Several strategies have been developed to directly inhibit this axis, including specific small molecule inhibitors, gene therapy targeting RAGE, monoclonal antibodies, AGE scavenger, and sRAGE. Here, we focus on the AGEs-RAGE axis-targeted interventions that have shown efficacy in diabetic wound healing (Table 1).
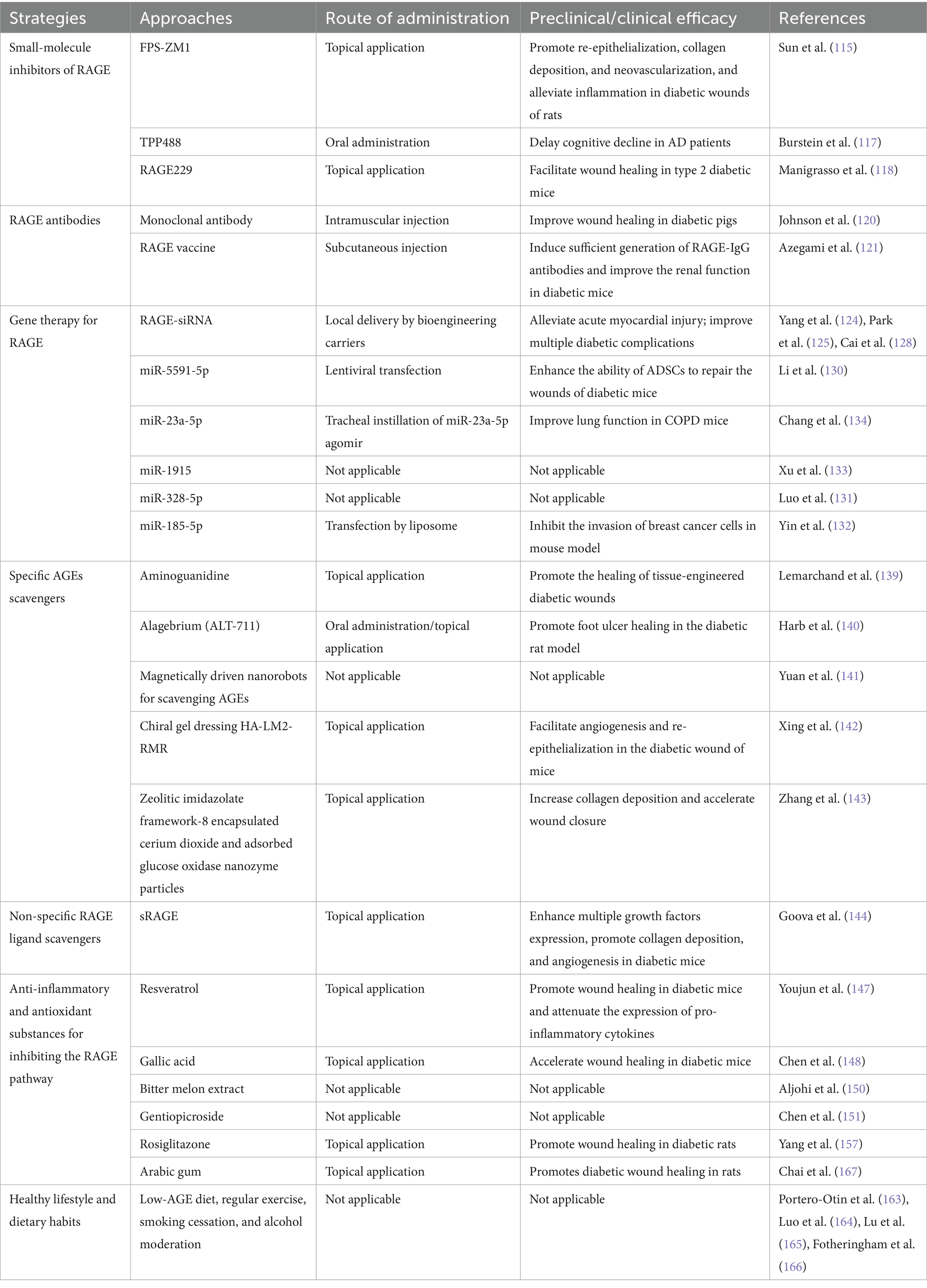
Table 1. Interventions for the AGEs-RAGE axis and their preclinical/clinical efficacy in diabetic wounds.
4.1 Small molecule RAGE inhibitors
Several small-molecule inhibitors targeting the extracellular or intracellular domains of RAGE have been developed.
FPS-ZM1 is a non-toxic tertiary amide compound that binds to the V-domain of RAGE to block its interaction with ligands. Recently, Sun et al. (115) designed a cobalt (Co)-based metal–organic framework loaded with FPS-ZM1 nanoparticles. This complex material, through localized release of FPS-ZM1 and Co-ions, promoted re-epithelialization, collagen deposition, and neovascularization, along with alleviating inflammation in diabetic wounds in rats. TTP488/Azeliragon is another inhibitor binding to the extracellular domain of RAGE, effectively preventing the interaction of RAGE with various ligands, including AGEs, HMGB1, S100, and Aβ (116). TTP488 is currently the only RAGE inhibitor undergoing clinical trials. In a Phase IIb clinical trial for mild-to-moderate Alzheimer’s disease patients, an 18-month treatment with TTP488 significantly delays cognitive decline compared to placebo (117). Further exploration of its application for wound treatment holds potential appeal.
RAGE229 is a novel small molecular inhibitor to block the interaction between the intracellular domain of RAGE and DIAPH1. Topical application of RAGE229 has been demonstrated to significantly accelerate wound healing in type 2 diabetic mice (118).
4.2 RAGE antibodies
Monoclonal antibodies targeting RAGE block signal transduction by directly binding to the extracellular domain of the receptor. Johnson et al. (119) developed a monoclonal IgG antibody against RAGE, named CR-3. In porcine models of diabetic wounds, the intramuscular injection of CR-3 at a dosage of 1 mg/kg every 10 days enhanced wound healing than non-immune IgG treatment (120). Interestingly, CR-3-loaded decellularized porcine skin patches showed no difference compared to the normal patches, likely due to poor retention or inadequate local concentration of the macromolecular antibody within the patch material (120). However, it is important to emphasize that the high cost of monoclonal antibody production poses a challenge to their clinical application in diabetic wounds. In contrast, vaccines may represent a more cost-effective approach. A vaccine designed to stimulate the production of RAGE antibodies has been developed. These antibodies selectively bind to the AGE-specific site on RAGE (amino acids 38–44), blocking AGE-driven signaling without affecting other RAGE ligands, resulting in partial inhibition of the pathway (121). In diabetic mice, three rounds of immunization with this vaccine successfully induced sufficient titers of specific IgG antibodies for at least 38 weeks. Treated mice exhibited improvements in renal function and histopathology (121), but the effects on diabetic wound healing have yet to be investigated. In addition, the long-term safety and efficacy profile of this vaccination strategy requires further evaluation.
4.3 Gene therapy for RAGE
Reducing the expression of RAGE through gene therapy is a promising approach (122). Small interfering RNA (siRNA) is a short double-stranded RNA that integrates into the RNA-induced silencing complex, targeting complementary messenger RNA (mRNA) for degradation to suppress gene expression (123). The knockdown of RAGE using siRNA has shown significant efficacy in animal models of diabetic wounds and pathological scars (57, 88). To enhance delivery efficiency and mitigate the concern of gene integration associated with viral vector delivery, several non-viral vectors for delivering RAGE siRNA have been developed, including deoxycholic acid-modified polyethyleneimine, bio-reducible polyethyleneimine, and EVs (124–126). Tetrahedral framework nucleic acid (tFNA) is an innovative carrier composed of four single-stranded DNA molecules, exhibiting potent cell membrane penetrability, high biosafety, and nucleases resistance (127). Cai et al. (128) recently developed a tFNA-based siRNA delivery system targeting RAGE, which effectively ameliorated various diabetic complications in animal models. miRNA is another type of small, single-stranded RNAs that regulate mRNA translation by binding to complementary sequences (129). miR-5591-5p has been identified to enhance the wound healing capacity of adipose tissue-derived stem cells in diabetic conditions by targeting RAGE mRNA (130). Moreover, miR-23a-5p, miR-1915, miR-328-5p, and miR-185-5p can also regulate RAGE mRNA (131–134). Further investigation into alternative gene silencing techniques, such as short hairpin RNA (shRNA), antisense oligonucleotides, and CRISPR/Cas9, in conjunction with other innovative delivery systems, including liposomes, hydrogels, microneedles, and micelles, would be advantageous in broadening therapeutic approaches for RAGE gene therapy (135).
Targeting the RAGE exhibits substantial potential in diabetic wound healing. However, given RAGE’s critical roles in physiological processes, complete blockade via vaccines or gene manipulation may impair its homeostatic functions. Increased abscess formation and delayed wound healing are observed in the RAGE knockout mice (136). Therefore, more experiments are needed to discover the potential adverse effects of long-term blockade of the RAGE pathway. Localized delivery and transient inhibition may contribute to mitigating such risks (137).
4.4 Targeting AGEs
In addition to directly targeting RAGE, another effective strategy for limiting RAGE pathway activation is enhancing the clearance of its ligands.
Several specific approaches targeting AGEs clearance have been identified to promote the healing of diabetic wounds. Aminoguanidine is a classic AGE scavenger that reduces the production of AGEs by neutralizing carbonyl compounds (138). It has been shown to reverse AGE-induced non-healing of wounds in tissue-engineered models (139). Alagebrium (ALT-711) is an AGE crosslink breaker. Systemic or local treatment with Alagebrium improves wound healing in diabetic rat models (140). Some novel bioactive materials are designed with binding sites for AGEs to facilitate their clearance (141–143). For example, a gel formed from L-phenylalanine and cationic hexapeptide co-assembled helical nanofibers cross-linked with hyaluronic acid possesses abundant chiral and cationic sites, effectively reducing AGEs through stereoselective interactions (142).
sRAGE non-specifically binds to RAGE ligands, obstructing their interaction with RAGE and consequently inhibiting RAGE signaling. Localized application of sRAGE significantly reduces inflammation in diabetic wounds while also enhancing the expression of multiple growth factors, promoting collagen deposition, and accelerating angiogenesis (144). However, sRAGE is prone to degradation in the protease-rich environment of wounds. Kang et al. (145) developed a recombinant fusion protein containing the V-domain of RAGE (vRAGE) linked to elastin-like polypeptides (ELPs). The vRAGE domain retains the ability to bind RAGE ligands, while the ELP domain enables temperature-dependent self-assembly into coacervates at around 30–31 °C, thereby shielding vRAGE from degradation and facilitating its sustained release. This fusion protein remains stable in elastase in vitro for up to 7 days and has demonstrated efficacy in promoting wound healing in diabetic mice.
4.5 Other approaches for the AGEs-RAGE axis inhibition
4.5.1 Pharmacological regulation
Another method for inhibiting the AGEs-RAGE axis involves natural or synthetic anti-inflammatory and antioxidant drugs along with diabetic management agents.
Polyphenolic compounds can scavenge ROS and inhibit the nuclear translocation of NF-κB to reduce inflammation (146). These effects significantly disrupt RAGE signaling and suppress RAGE expression. Polyphenolic compounds, including resveratrol and gallic acid, have been found to promote diabetic wound healing by inhibiting the AGEs-RAGE axis (147, 148). Steroidal saponins also suppress the RAGE pathway through their anti-inflammatory and antioxidant properties (149). Bitter melon extract, rich in steroidal saponins, effectively counteracts the inhibition of angiogenesis caused by the AGEs-RAGE axis in diabetic wounds (150). Gentiopicroside, a cleaved-ring enol ether terpene glycoside, was recently discovered to protect against glycation-induced damage in skin fibroblasts by inhibiting the AGEs-RAGE axis (151). Furthermore, anti-inflammatory and antioxidant substances such as flavonoids, natural polysaccharides, ascorbic acid, and tocopherol are also associated with the inhibition of the RAGE pathway (152–155). However, additional research is required to validate their therapeutic effects on diabetic wounds.
The classic anti-diabetic agent rosiglitazone inhibits the harmful effects of the AGEs-RAGE axis by activating the peroxisome proliferator-activated receptor gamma pathway (156). Topical application of a hydrogel dressing loaded with nanoparticles containing rosiglitazone and S-nitrosoglutathione markedly promotes wound healing in diabetic rats (157). Moreover, metformin, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 analogs, primarily used for type 2 diabetes management, may also facilitate diabetic wound closure by suppressing RAGE-mediated inflammation (158–162).
4.5.2 Lifestyle interventions
Lifestyle and dietary habits significantly influence the levels of AGEs. AGEs are particularly abundant in red meat, dairy products, processed foods, high-sugar diets, and foods prepared using high-temperature cooking methods such as frying, grilling, and roasting. In contrast, fruits, vegetables, whole grains, and foods prepared using moist-heat cooking techniques (poaching, steaming, and stewing) contain lower AGEs (163). Beyond diet, regular physical activity, smoking cessation, and moderate alcohol consumption constitute important approaches for reducing AGE accumulation in vivo (164–166). These lifestyle improvements may serve as effective adjuncts in the prevention and treatment of diabetic wounds.
5 Conclusion and future perspective
The AGEs-RAGE axis serves as a crucial mediator in diabetic wound pathogenesis by driving chronic inflammation, oxidative stress, and cellular dysfunction through sustained activation of multiple inflammatory and oxidative stress pathways. Current intervention strategies, including RAGE inhibitors, gene silencing, AGE scavengers, various anti-inflammatory and antioxidant drugs, and several anti-diabetic agents, demonstrate preclinical efficacy in restoring angiogenesis, resolving inflammation, and promoting tissue repair. Dietary and lifestyle interventions further complement these approaches by reducing systemic AGE accumulation. However, the transition from preclinical success to clinical application remains a critical challenge, necessitating rigorous trials to validate safety and efficacy in diverse diabetic populations.
Future research should prioritize cost-effective, targeted therapies, such as innovative delivery systems and multimodal combinatorial regimens. Notably, the rapid advancements in artificial intelligence in recent years are expected to accelerate the discovery and development of more effective AGEs-RAGE axis-targeted drugs. Interdisciplinary collaboration and public health initiatives are essential to bridge mechanistic insights with transformative clinical outcomes, ultimately improving the quality of life for patients with diabetic wounds.
Author contributions
HL: Writing – original draft, Methodology, Conceptualization. YY: Methodology, Conceptualization, Writing – original draft. XW: Investigation, Writing – original draft, Methodology. MC: Writing – review & editing, Formal analysis, Data curation. LZ: Software, Writing – original draft. SC: Resources, Funding acquisition, Validation, Formal analysis, Conceptualization, Writing – review & editing, Supervision. XP: Conceptualization, Project administration, Writing – review & editing, Funding acquisition. YP: Supervision, Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 82572914, 82072163), Shenzhen Science and Technology Program (No. JCYJ20240813115701003), Health and Medical Scientific Research Project of Shenzhen Bao’an Medical Association (No. BAYXH2023005), Sanming Project of medicine in Shenzhen (SZSM202106019), and the 2024 High-quality Development Research Project of Shenzhen Bao’an Public Hospital (No. BAGZL2024025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun, H, Saeedi, P, Karuranga, S, Pinkepank, M, Ogurtsova, K, Duncan, BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Falanga, V, Isseroff, RR, Soulika, AM, Romanelli, M, Margolis, D, Kapp, S, et al. Chronic wounds. Nat Rev Dis Primers. (2022) 8:50. doi: 10.1038/s41572-022-00377-3
3. Armstrong, DG, Tan, TW, Boulton, AJM, and Bus, SA. Diabetic foot ulcers: a review. JAMA. (2023) 330:62–75. doi: 10.1001/jama.2023.10578
4. Pena, OA, and Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. (2024) 25:599–616. doi: 10.1038/s41580-024-00715-1
5. Deng, H, Li, B, Shen, Q, Zhang, C, Kuang, L, Chen, R, et al. Mechanisms of diabetic foot ulceration: a review. J Diabetes. (2023) 15:299–312. doi: 10.1111/1753-0407.13372
6. Zhou, M, Zhang, Y, Shi, L, Li, L, Zhang, D, Gong, Z, et al. Activation and modulation of the AGEs-RAGE axis: implications for inflammatory pathologies and therapeutic interventions - a review. Pharmacol Res. (2024) 206:107282. doi: 10.1016/j.phrs.2024.107282
7. Vitorakis, N, and Piperi, C. Pivotal role of AGE-RAGE axis in brain aging with current interventions. Ageing Res Rev. (2024) 100:102429. doi: 10.1016/j.arr.2024.102429
8. Khalid, M, Petroianu, G, and Adem, A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules. (2022) 12:542. doi: 10.3390/biom12040542
9. Li, L, Zhuang, Y, Zou, X, Chen, M, Cui, B, Jiao, Y, et al. Advanced glycation end products: a comprehensive review of their detection and occurrence in food. Foods. (2023) 12:2103. doi: 10.3390/foods12112103
10. Shi, B, Guo, X, Liu, H, Jiang, K, Liu, L, Yan, N, et al. Dissecting Maillard reaction production in fried foods: formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. (2024) 438:137994. doi: 10.1016/j.foodchem.2023.137994
11. Takeuchi, M, Sakasai-Sakai, A, Takata, T, Takino, JI, and Koriyama, Y. Effects of toxic AGEs (TAGE) on human health. Cells. (2022) 11:2178. doi: 10.3390/cells11142178
12. Chen, CY, Zhang, JQ, Li, L, Guo, MM, He, YF, Dong, YM, et al. Advanced glycation end products in the skin: molecular mechanisms, methods of measurement, and inhibitory pathways. Front Med (Lausanne). (2022) 9:837222. doi: 10.3389/fmed.2022.837222
13. Ma, Y, Wang, X, Lin, S, King, L, and Liu, L. The potential role of advanced glycation end products in the development of kidney disease. Nutrients. (2025) 17:758. doi: 10.3390/nu17050758
14. Schalkwijk, CG, and Stehouwer, CDA. Methylglyoxal, a highly reactive Dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. (2020) 100:407–61. doi: 10.1152/physrev.00001.2019
15. Zheng, W, Li, H, Go, Y, Chan, XHF, Huang, Q, and Wu, J. Research advances on the damage mechanism of skin glycation and related inhibitors. Nutrients. (2022) 14:4588. doi: 10.3390/nu14214588
16. Fritz, G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. (2011) 36:625–32. doi: 10.1016/j.tibs.2011.08.008
17. Xie, J, Reverdatto, S, Frolov, A, Hoffmann, R, Burz, DS, and Shekhtman, A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J Biol Chem. (2008) 283:27255–69. doi: 10.1074/jbc.M801622200
18. Koch, M, Chitayat, S, Dattilo, BM, Schiefner, A, Diez, J, Chazin, WJ, et al. Structural basis for ligand recognition and activation of RAGE. Structure. (2010) 18:1342–52. doi: 10.1016/j.str.2010.05.017
19. Park, H, Adsit, FG, and Boyington, JC. The 1.5 a crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem. (2010) 285:40762–70. doi: 10.1074/jbc.M110.169276
20. Dong, H, Zhang, Y, Huang, Y, and Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front Immunol. (2022) 13:931473. doi: 10.3389/fimmu.2022.931473
21. Hudson, BI, Carter, AM, Harja, E, Kalea, AZ, Arriero, M, Yang, H, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB J. (2008) 22:1572–80. doi: 10.1096/fj.07-9909com
22. Moysa, A, Hammerschmid, D, Szczepanowski, RH, Sobott, F, and Dadlez, M. Enhanced oligomerization of full-length RAGE by synergy of the interaction of its domains. Sci Rep. (2019) 9:20332. doi: 10.1038/s41598-019-56993-9
23. Li, M, Ong, CY, Langouet-Astrie, CJ, Tan, L, Verma, A, Yang, Y, et al. Heparan sulfate-dependent RAGE oligomerization is indispensable for pathophysiological functions of RAGE. eLife. (2022) 11:11. doi: 10.7554/eLife.71403
24. Xue, J, Manigrasso, M, Scalabrin, M, Rai, V, Reverdatto, S, Burz, DS, et al. Change in the molecular dimension of a RAGE-ligand complex triggers RAGE signaling. Structure. (2016) 24:1509–22. doi: 10.1016/j.str.2016.06.021
25. Jules, J, Maiguel, D, and Hudson, BI. Alternative splicing of the RAGE cytoplasmic domain regulates cell signaling and function. PLoS One. (2013) 8:e78267. doi: 10.1371/journal.pone.0078267
26. Schmidt, AM. Soluble RAGEs - prospects for treating & tracking metabolic and inflammatory disease. Vasc Pharmacol. (2015) 72:1–8. doi: 10.1016/j.vph.2015.06.011
27. Erusalimsky, JD. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. (2021) 42:101958. doi: 10.1016/j.redox.2021.101958
28. Prasad, K. Age-rage stress and coronary artery disease. Int J Angiol. (2021) 30:4–14. doi: 10.1055/s-0040-1721813
29. Yonekura, H, Yamamoto, Y, Sakurai, S, Petrova, RG, Abedin, MJ, Li, H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. (2003) 370:1097–109. doi: 10.1042/bj20021371
30. Sakaguchi, M, Murata, H, Aoyama, Y, Hibino, T, Putranto, EW, Ruma, IM, et al. DNAX-activating protein 10 (DAP10) membrane adaptor associates with receptor for advanced glycation end products (RAGE) and modulates the RAGE-triggered signaling pathway in human keratinocytes. J Biol Chem. (2014) 289:23389–402. doi: 10.1074/jbc.M114.573071
31. Sakaguchi, M, Murata, H, Yamamoto, K, Ono, T, Sakaguchi, Y, Motoyama, A, et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. (2011) 6:e23132. doi: 10.1371/journal.pone.0023132
32. Hudson, BI, Kalea, AZ, Del Mar Arriero, M, Harja, E, Boulanger, E, D'Agati, V, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. (2008) 283:34457–68. doi: 10.1074/jbc.M801465200
33. Hudson, BI, and Lippman, ME. Targeting RAGE signaling in inflammatory disease. Annu Rev Med. (2018) 69:349–64. doi: 10.1146/annurev-med-041316-085215
34. Teissier, T, and Boulanger, E. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology. (2019) 20:279–301. doi: 10.1007/s10522-019-09808-3
35. Zhang, X, Jiang, J, Xu, J, Chen, J, Gu, Y, and Wu, G. Liraglutide, a glucagon-like peptide-1 receptor agonist, ameliorates inflammation and apoptosis via inhibition of receptor for advanced glycation end products signaling in AGEs induced chondrocytes. BMC Musculoskelet Disord. (2024) 25:601. doi: 10.1186/s12891-024-07640-6
36. Zhao, L, Li, Y, Xu, T, Lv, Q, Bi, X, Liu, X, et al. Dendritic cell-mediated chronic low-grade inflammation is regulated by the RAGE-TLR4-PKCbeta(1) signaling pathway in diabetic atherosclerosis. Mol Med. (2022) 28:4. doi: 10.1186/s10020-022-00431-6
37. Kong, D, Liu, J, Lu, J, Zeng, C, Chen, H, Duan, Z, et al. HMGB2 release promotes pulmonary hypertension and predicts severity and mortality of patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. (2024) 44:e172–95. doi: 10.1161/ATVBAHA.123.319916
38. Cross, K, Vetter, SW, Alam, Y, Hasan, MZ, Nath, AD, and Leclerc, E. Role of the receptor for advanced glycation end products (RAGE) and its ligands in inflammatory responses. Biomolecules. (2024) 14:1550. doi: 10.3390/biom14121550
39. Li, J, and Schmidt, AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. (1997) 272:16498–506. doi: 10.1074/jbc.272.26.16498
40. Chen, Y, Meng, Z, Li, Y, Liu, S, Hu, P, and Luo, E. Advanced glycation end products and reactive oxygen species: uncovering the potential role of ferroptosis in diabetic complications. Mol Med. (2024) 30:141. doi: 10.1186/s10020-024-00905-9
41. Hou, C, Lu, M, Lei, Z, Dai, S, Chen, W, Du, S, et al. HMGB1 positive feedback loop between Cancer cells and tumor-associated macrophages promotes osteosarcoma migration and invasion. Lab Investig. (2023) 103:100054. doi: 10.1016/j.labinv.2022.100054
42. Kim, CH, Kang, HY, Kim, G, Park, J, Nam, BY, Park, JT, et al. Soluble receptors for advanced glycation end-products prevent unilateral ureteral obstruction-induced renal fibrosis. Front Pharmacol. (2023) 14:1172269. doi: 10.3389/fphar.2023.1172269
43. Li, S, Wang, Y, Sun, X, Lu, L, Yong, Y, Kong, X, et al. Suppressing neuroinflammation by Shenfu injection against ischemic stroke in mice via inhibiting RAGE-PI3K-Akt pathway. Phytomedicine. (2025) 144:156940. doi: 10.1016/j.phymed.2025.156940
44. Shou, C, Sun, Y, Zhang, Q, Zhang, W, Yan, Q, Xu, T, et al. S100A9 inhibition mitigates acute pancreatitis by suppressing RAGE expression and subsequently ameliorating inflammation. Inflammation. (2025) 48:2355–66. doi: 10.1007/s10753-024-02194-0
45. Mees, MA, Boone, F, Bouwen, T, Vanaerschot, F, Titeca, C, Vikkula, HK, et al. Glycyrrhizin-based hydrogels accelerate wound healing of normoglycemic and diabetic mouse skin. Pharmaceutics. (2022) 15:27. doi: 10.3390/pharmaceutics15010027
46. Deng, G, Zhang, L, Wang, C, Wang, S, Xu, J, Dong, J, et al. AGES-RAGE axis causes endothelial-to-mesenchymal transition in early calcific aortic valve disease via TGF-beta1 and BMPR2 signaling. Exp Gerontol. (2020) 141:111088. doi: 10.1016/j.exger.2020.111088
47. Chou, DK, Zhang, J, Smith, FI, McCaffery, P, and Jungalwala, FB. Developmental expression of receptor for advanced glycation end products (RAGE), amphoterin and sulfoglucuronyl (HNK-1) carbohydrate in mouse cerebellum and their role in neurite outgrowth and cell migration. J Neurochem. (2004) 90:1389–401. doi: 10.1111/j.1471-4159.2004.02609.x
48. Stogsdill, JA, Stogsdill, MP, Porter, JL, Hancock, JM, Robinson, AB, and Reynolds, PR. Embryonic overexpression of receptors for advanced glycation end-products by alveolar epithelium induces an imbalance between proliferation and apoptosis. Am J Respir Cell Mol Biol. (2012) 47:60–6. doi: 10.1165/rcmb.2011-0385OC
49. Tadie, JM, Bae, HB, Banerjee, S, Zmijewski, JW, and Abraham, E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am J Physiol Cell Physiol. (2012) 302:C249–56. doi: 10.1152/ajpcell.00302.2011
50. He, M, Kubo, H, Morimoto, K, Fujino, N, Suzuki, T, Takahasi, T, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. (2011) 12:358–64. doi: 10.1038/embor.2011.28
51. Saleh, A, Smith, DR, Tessler, L, Mateo, AR, Martens, C, Schartner, E, et al. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol. (2013) 249:149–59. doi: 10.1016/j.expneurol.2013.08.018
52. Riuzzi, F, Beccafico, S, Sagheddu, R, Chiappalupi, S, Giambanco, I, Bereshchenko, O, et al. Levels of S100B protein drive the reparative process in acute muscle injury and muscular dystrophy. Sci Rep. (2017) 7:12537. doi: 10.1038/s41598-017-12880-9
53. Guarneri, F, Custurone, P, Papaianni, V, and Gangemi, S. Involvement of RAGE and oxidative stress in inflammatory and infectious skin diseases. Antioxidants (Basel). (2021) 10:82. doi: 10.3390/antiox10010082
54. Rojas, A, Lindner, C, Schneider, I, Gonzalez, I, and Uribarri, J. The RAGE Axis: a relevant inflammatory hub in human diseases. Biomolecules. (2024) 14:412. doi: 10.3390/biom14040412
55. Wang, B, Jiang, T, Qi, Y, Luo, S, Xia, Y, Lang, B, et al. AGE-RAGE Axis and cardiovascular diseases: pathophysiologic mechanisms and prospects for clinical applications. Cardiovasc Drugs Ther. (2024). doi: 10.1007/s10557-024-07639-0
56. Garza-Campos, A, Prieto-Correa, JR, Dominguez-Rosales, JA, and Hernandez-Nazara, ZH. Implications of receptor for advanced glycation end products for progression from obesity to diabetes and from diabetes to cancer. World J Diabetes. (2023) 14:977–94. doi: 10.4239/wjd.v14.i7.977
57. Zhao, J, Yu, J, Xu, Y, Chen, L, Zhou, F, Zhai, Q, et al. Epidermal HMGB1 activates dermal fibroblasts and causes hypertrophic scar formation in reduced hydration. J Invest Dermatol. (2018) 138:2322–32. doi: 10.1016/j.jid.2018.04.036
58. Kumar, V, Fleming, T, Terjung, S, Gorzelanny, C, Gebhardt, C, Agrawal, R, et al. Homeostatic nuclear RAGE-ATM interaction is essential for efficient DNA repair. Nucleic Acids Res. (2017) 45:10595–613. doi: 10.1093/nar/gkx705
59. Frommhold, D, Kamphues, A, Hepper, I, Pruenster, M, Lukic, IK, Socher, I, et al. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood. (2010) 116:841–9. doi: 10.1182/blood-2009-09-244293
60. Peltier, A, Goutman, SA, and Callaghan, BC. Painful diabetic neuropathy. BMJ. (2014) 348:g1799. doi: 10.1136/bmj.g1799
61. Nowak, NC, Menichella, DM, Miller, R, and Paller, AS. Cutaneous innervation in impaired diabetic wound healing. Transl Res. (2021) 236:87–108. doi: 10.1016/j.trsl.2021.05.003
62. Thakur, V, Sadanandan, J, and Chattopadhyay, M. High-mobility group box 1 protein signaling in painful diabetic neuropathy. Int J Mol Sci. (2020) 21:881. doi: 10.3390/ijms21030881
63. Abdelkader, NF, Ibrahim, SM, Moustafa, PE, and Elbaset, MA. Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-kappaB/Nrf2 and TGF-beta/PKC/TRPV1 signaling pathways. Biomed Pharmacother. (2022) 145:112395. doi: 10.1016/j.biopha.2021.112395
64. Osonoi, S, Mizukami, H, Takeuchi, Y, Sugawa, H, Ogasawara, S, Takaku, S, et al. RAGE activation in macrophages and development of experimental diabetic polyneuropathy. JCI Insight. (2022) 7:e160555. doi: 10.1172/jci.insight.160555
65. Lu, YZ, Nayer, B, Singh, SK, Alshoubaki, YK, Yuan, E, Park, AJ, et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature. (2024) 628:604–11. doi: 10.1038/s41586-024-07237-y
66. Xu, ML, Yu, XJ, Zhao, JQ, Du, Y, Xia, WJ, Su, Q, et al. Calcitriol ameliorated autonomic dysfunction and hypertension by down-regulating inflammation and oxidative stress in the paraventricular nucleus of SHR. Toxicol Appl Pharmacol. (2020) 394:114950. doi: 10.1016/j.taap.2020.114950
67. Ivanov, E, Akhmetshina, M, Erdiakov, A, and Gavrilova, S. Sympathetic system in wound healing: multistage control in Normal and diabetic skin. Int J Mol Sci. (2023) 24:2045. doi: 10.3390/ijms24032045
68. Forsythe, RO, Brownrigg, J, and Hinchliffe, RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes Metab. (2015) 17:435–44. doi: 10.1111/dom.12422
69. Bandyk, DF. The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg. (2018) 31:43–8. doi: 10.1053/j.semvascsurg.2019.02.001
70. Munno, M, Mallia, A, Greco, A, Modafferi, G, Banfi, C, and Eligini, S. Radical oxygen species, oxidized low-density lipoproteins, and lectin-like oxidized low-density lipoprotein receptor 1: a vicious circle in atherosclerotic process. Antioxidants (Basel). (2024) 13:583. doi: 10.3390/antiox13050583
71. Shu, M, Cheng, W, Jia, X, Bai, X, Zhao, Y, Lu, Y, et al. AGEs promote atherosclerosis by increasing LDL transcytosis across endothelial cells via RAGE/NF-kappaB/caveolin-1 pathway. Mol Med. (2023) 29:113. doi: 10.1186/s10020-023-00715-5
72. Yashima, H, Terasaki, M, Sotokawauchi, A, Matsui, T, Mori, Y, Saito, T, et al. AGE-RAGE Axis stimulates oxidized LDL uptake into macrophages through cyclin-dependent kinase 5-CD36 pathway via oxidative stress generation. Int J Mol Sci. (2020) 21:9263. doi: 10.3390/ijms21239263
73. Terasaki, M, Yashima, H, Mori, Y, Saito, T, Inoue, N, Matsui, T, et al. Glucose-dependent Insulinotropic polypeptide inhibits AGE-induced NADPH oxidase-derived oxidative stress generation and foam cell formation in macrophages partly via AMPK activation. Int J Mol Sci. (2024) 25:9724. doi: 10.3390/ijms25179724
74. Tatmatsu-Rocha, JC, and Mendes-Costa, LS. Inflammatory markers, oxidative stress, and mitochondrial dynamics: repercussions on coronary artery disease in diabetes. World J Diabetes. (2024) 15:1853–7. doi: 10.4239/wjd.v15.i9.1853
75. Orekhov, AN. LDL and foam cell formation as the basis of atherogenesis. Curr Opin Lipidol. (2018) 29:279–84. doi: 10.1097/MOL.0000000000000525
76. Sena, CM, Pereira, AM, and Seica, R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. (2013) 1832:2216–31. doi: 10.1016/j.bbadis.2013.08.006
77. Jing, C, Zhang, G, Liu, Z, Xu, Q, Li, C, Cheng, G, et al. Peroxidasin promotes diabetic vascular endothelial dysfunction induced by advanced glycation end products via NOX2/HOCl/Akt/eNOS pathway. Redox Biol. (2021) 45:102031. doi: 10.1016/j.redox.2021.102031
78. Zhao, L, Zhang, CL, He, L, Chen, Q, Liu, L, Kang, L, et al. Restoration of Autophagic flux improves endothelial function in diabetes through lowering mitochondrial ROS-mediated eNOS Monomerization. Diabetes. (2022) 71:1099–114. doi: 10.2337/db21-0660
79. Recabarren-Leiva, D, Burgos, CF, Hernandez, B, Garcia-Garcia, FJ, Castro, RI, Guzman, L, et al. Effects of the age/rage axis in the platelet activation. Int J Biol Macromol. (2021) 166:1149–61. doi: 10.1016/j.ijbiomac.2020.10.270
80. Arriagada-Petersen, C, Fernandez, P, Gomez, M, Ravello, N, Palomo, I, Fuentes, E, et al. Effect of advanced glycation end products on platelet activation and aggregation: a comparative study of the role of glyoxal and methylglyoxal. Platelets. (2021) 32:507–15. doi: 10.1080/09537104.2020.1767770
81. Zhang, Y, Liu, J, Jia, W, Tian, X, Jiang, P, Cheng, Z, et al. AGEs/RAGE blockade downregulates Endothenin-1 (ET-1), mitigating human umbilical vein endothelial cells (HUVEC) injury in deep vein thrombosis (DVT). Bioengineered. (2021) 12:1360–8. doi: 10.1080/21655979.2021.1917980
82. Kang, Y, Zheng, C, Ye, J, Song, F, Wang, X, Liu, Y, et al. Effects of advanced glycation end products on neutrophil migration and aggregation in diabetic wounds. Aging (Albany NY). (2021) 13:12143–59. doi: 10.18632/aging.202924
83. Mikhalchik, EV, Ivanov, VA, Borodina, IV, Pobeguts, OV, Smirnov, IP, Gorudko, IV, et al. Neutrophil activation by mineral microparticles coated with methylglyoxal-glycated albumin. Int J Mol Sci. (2022) 23:7840. doi: 10.3390/ijms23147840
84. Zhu, S, Yu, Y, Ren, Y, Xu, L, Wang, H, Ling, X, et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. (2021) 12:984. doi: 10.1038/s41419-021-04294-3
85. Tatsiy, O, de Carvalho Oliveira, V, Mosha, HT, and McDonald, PP. Early and late processes driving NET formation, and the autocrine/paracrine role of endogenous RAGE ligands. Front Immunol. (2021) 12:675315. doi: 10.3389/fimmu.2021.675315
86. Al Sadoun, H. Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells. (2022) 11:2430. doi: 10.3390/cells11152430
87. Guo, Y, Lin, C, Xu, P, Wu, S, Fu, X, Xia, W, et al. AGEs induced autophagy impairs cutaneous wound healing via stimulating macrophage polarization to M1 in diabetes. Sci Rep. (2016) 6:36416. doi: 10.1038/srep36416
88. Wang, Q, Zhu, G, Cao, X, Dong, J, Song, F, and Niu, Y. Blocking AGE-RAGE signaling improved functional disorders of macrophages in diabetic wound. J Diabetes Res. (2017) 2017:1–10. doi: 10.1155/2017/1428537
89. Juranek, JK, Geddis, MS, Song, F, Zhang, J, Garcia, J, Rosario, R, et al. RAGE deficiency improves postinjury sciatic nerve regeneration in type 1 diabetic mice. Diabetes. (2013) 62:931–43. doi: 10.2337/db12-0632
90. Zhao, Y, Li, K, Wang, L, Kuang, G, Xie, K, and Lin, S. Dexmedetomidine mitigates acute lung injury by enhancing M2 macrophage polarization and inhibiting RAGE/Caspase-11-mediated Pyroptosis. Front Biosci (Landmark Ed). (2024) 29:409. doi: 10.31083/j.fbl2912409
91. Kim, JH, Yoon, NY, Kim, DH, Jung, M, Jun, M, Park, HY, et al. Impaired permeability and antimicrobial barriers in type 2 diabetes skin are linked to increased serum levels of advanced glycation end-product. Exp Dermatol. (2018) 27:815–23. doi: 10.1111/exd.13466
92. Liu, Y, Min, D, Bolton, T, Nube, V, Twigg, SM, Yue, DK, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. (2009) 32:117–9. doi: 10.2337/dc08-0763
93. Liang, Y, Yang, C, Lin, Y, Parviz, Y, Sun, K, Wang, W, et al. Matrix metalloproteinase 9 induces keratinocyte apoptosis through FasL/Fas pathway in diabetic wound. Apoptosis. (2019) 24:542–51. doi: 10.1007/s10495-019-01536-w
94. Lu, W, Li, J, Ren, M, Zeng, Y, Zhu, P, Lin, L, et al. Role of the mevalonate pathway in specific CpG site demethylation on AGEs-induced MMP9 expression and activation in keratinocytes. Mol Cell Endocrinol. (2015) 411:121–9. doi: 10.1016/j.mce.2015.04.019
95. Zhu, P, Chen, C, Wu, D, Chen, G, Tan, R, and Ran, J. AGEs-induced MMP-9 activation mediated by Notch1 signaling is involved in impaired wound healing in diabetic rats. Diabetes Res Clin Pract. (2022) 186:109831. doi: 10.1016/j.diabres.2022.109831
96. Rufin, M, Nalbach, M, Rakus, M, Fuchs, M, Poik, M, Schitter, G, et al. Methylglyoxal alters collagen fibril nanostiffness and surface potential. Acta Biomater. (2024) 189:208–16. doi: 10.1016/j.actbio.2024.08.039
97. He, T, Fisher, GJ, Kim, AJ, and Quan, T. Age-related changes in dermal collagen physical properties in human skin. PLoS One. (2023) 18:e0292791. doi: 10.1371/journal.pone.0292791
98. Guillon, C, Ferraro, S, Clement, S, Bouschbacher, M, Sigaudo-Roussel, D, and Bonod, C. Glycation by glyoxal leads to profound changes in the behavior of dermal fibroblasts. BMJ Open Diabetes Res Care. (2021) 9:e002091. doi: 10.1136/bmjdrc-2020-002091
99. Bian, X, Li, B, Yang, J, Ma, K, Sun, M, Zhang, C, et al. Regenerative and protective effects of dMSC-sEVs on high-glucose-induced senescent fibroblasts by suppressing RAGE pathway and activating Smad pathway. Stem Cell Res Ther. (2020) 11:166. doi: 10.1186/s13287-020-01681-z
100. Zgutka, K, Tkacz, M, Tomasiak, P, and Tarnowski, M. A role for advanced glycation end products in molecular ageing. Int J Mol Sci. (2023) 24:9881. doi: 10.3390/ijms24129881
101. Gauthier, V, Kyriazi, M, Nefla, M, Pucino, V, Raza, K, Buckley, CD, et al. Fibroblast heterogeneity: keystone of tissue homeostasis and pathology in inflammation and ageing. Front Immunol. (2023) 14:1137659. doi: 10.3389/fimmu.2023.1137659
102. Qin, S, Bie, F, Chen, S, Xu, Y, Chen, L, Shu, B, et al. Targeting S100A12 to improve angiogenesis and accelerate diabetic wound healing. Inflammation. (2024) 48:633–48. doi: 10.1007/s10753-024-02073-8
103. Li, X, Wang, T, Tao, Y, Wang, X, Li, L, and Liu, J. Inhibition of USP7 suppresses advanced glycation end-induced cell cycle arrest and senescence of human umbilical vein endothelial cells through ubiquitination of p53. Acta Biochim Biophys Sin Shanghai. (2022) 54:311–20. doi: 10.3724/abbs.2022003
104. Fei, J, Ling, YM, Zeng, MJ, and Zhang, KW. Shixiang plaster, a traditional Chinese medicine, promotes healing in a rat model of diabetic ulcer through the receptor for advanced glycation end products (RAGE)/nuclear factor kappa B (NF-kappaB) and vascular endothelial growth factor (VEGF)/vascular cell adhesion molecule-1 (VCAM-1)/endothelial nitric oxide synthase (eNOS) signaling pathways. Med Sci Monit. (2019) 25:9446–57. doi: 10.12659/MSM.918268
105. Jin, H, Zhang, Z, Wang, C, Tang, Q, Wang, J, Bai, X, et al. Melatonin protects endothelial progenitor cells against AGE-induced apoptosis via autophagy flux stimulation and promotes wound healing in diabetic mice. Exp Mol Med. (2018) 50:1–15. doi: 10.1038/s12276-018-0177-z
106. Chen, J, Jing, J, Yu, S, Song, M, Tan, H, Cui, B, et al. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am J Transl Res. (2016) 8:2169–78.
107. Chen, LL, and Kim, VN. Small and long non-coding RNAs: past, present, and future. Cell. (2024) 187:6451–85. doi: 10.1016/j.cell.2024.10.024
108. Zhou, L, Ren, M, Zeng, T, Wang, W, Wang, X, Hu, M, et al. TET2-interacting long noncoding RNA promotes active DNA demethylation of the MMP-9 promoter in diabetic wound healing. Cell Death Dis. (2019) 10:813. doi: 10.1038/s41419-019-2047-6
109. Yuan, L, Sun, Y, Xu, M, Zeng, F, and Xiong, X. MiR-203 acts as an inhibitor for epithelial-mesenchymal transition process in diabetic foot ulcers via targeting interleukin-8. Neuroimmunomodulation. (2019) 26:239–49. doi: 10.1159/000503087
110. Hu, M, Wu, Y, Yang, C, Wang, X, Wang, W, Zhou, L, et al. Novel long noncoding RNA lnc-URIDS delays diabetic wound healing by targeting Plod1. Diabetes. (2020) 69:2144–56. doi: 10.2337/db20-0147
111. Zeng, T, Wang, X, Wang, W, Feng, Q, Lao, G, Liang, Y, et al. Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin Sci (Lond). (2019) 133:CS20190008. doi: 10.1042/CS20190008
112. Cheng, CK, Shang, W, Liu, J, Cheang, WS, Wang, Y, Xiang, L, et al. Activation of AMPK/miR-181b Axis alleviates endothelial dysfunction and vascular inflammation in diabetic mice. Antioxidants (Basel). (2022) 11:1137. doi: 10.3390/antiox11061137
113. van Niel, G, Carter, DRF, Clayton, A, Lambert, DW, Raposo, G, and Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. (2022) 23:369–82. doi: 10.1038/s41580-022-00460-3
114. Zeng, T, Sun, K, Mai, L, Hong, X, He, X, Lin, W, et al. Extracellular vesicle-associated miR-ERIA exerts the antiangiogenic effect of macrophages in diabetic wound healing. Diabetes. (2025) 74:596–610. doi: 10.2337/db24-0701
115. Sun, Y, Bao, B, Zhu, Y, Shen, J, Liu, X, Gao, T, et al. An FPS-ZM1-encapsulated zeolitic imidazolate framework as a dual proangiogenic drug delivery system for diabetic wound healing. Nano Res. (2022) 15:5216–29. doi: 10.1007/s12274-022-4106-z
116. Burstein, AH, Sabbagh, M, Andrews, R, Valcarce, C, Dunn, I, and Altstiel, L. Development of Azeliragon, an Oral small molecule antagonist of the receptor for advanced glycation Endproducts, for the potential slowing of loss of cognition in mild Alzheimer's disease. J Prev Alzheimers Dis. (2018) 5:149–54. doi: 10.14283/jpad.2018.18
117. Burstein, AH, Grimes, I, Galasko, DR, Aisen, PS, Sabbagh, M, and Mjalli, AM. Effect of TTP488 in patients with mild to moderate Alzheimer's disease. BMC Neurol. (2014) 14:12. doi: 10.1186/1471-2377-14-12
118. Manigrasso, MB, Rabbani, P, Egana-Gorrono, L, Quadri, N, Frye, L, Zhou, B, et al. Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci Transl Med. (2021) 13:eabf7084. doi: 10.1126/scitranslmed.abf7084
119. Johnson, LL, Johnson, J, Ober, R, Holland, A, Zhang, G, Backer, M, et al. Novel receptor for advanced glycation end products-blocking antibody to treat diabetic peripheral artery disease. J Am Heart Assoc. (2021) 10:e016696. doi: 10.1161/JAHA.120.016696
120. Johnson, JM, Takebe, Y, Zhang, G, Ober, R, McLuckie, A, Niedt, GW, et al. Blocking RAGE improves wound healing in diabetic pigs. Int Wound J. (2023) 20:678–86. doi: 10.1111/iwj.13909
121. Azegami, T, Nakayama, T, Hayashi, K, Hishikawa, A, Yoshimoto, N, Nakamichi, R, et al. Vaccination against receptor for advanced glycation end products attenuates the progression of diabetic kidney disease. Diabetes. (2021) 70:2147–58. doi: 10.2337/db20-1257
122. Karimzadeh, F, Soltani Fard, E, Nadi, A, Malekzadeh, R, Elahian, F, and Mirzaei, SA. Advances in skin gene therapy: utilizing innovative dressing scaffolds for wound healing, a comprehensive review. J Mater Chem B. (2024) 12:6033–62. doi: 10.1039/D4TB00966E
123. Hu, B, Zhong, L, Weng, Y, Peng, L, Huang, Y, Zhao, Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. (2020) 5:101. doi: 10.1038/s41392-020-0207-x
124. Yang, MJ, Ku, SH, Kim, D, Kim, WJ, Mok, H, Kim, SH, et al. Enhanced cytoplasmic delivery of RAGE siRNA using bioreducible Polyethylenimine-based Nanocarriers for myocardial gene therapy. Macromol Biosci. (2015) 15:1755–63. doi: 10.1002/mabi.201500213
125. Park, H, Ku, SH, Park, H, Hong, J, Kim, D, Choi, BR, et al. RAGE siRNA-mediated gene silencing provides cardioprotection against ventricular arrhythmias in acute ischemia and reperfusion. J Control Release. (2015) 217:315–26. doi: 10.1016/j.jconrel.2015.09.006
126. Kim, H, Mun, D, Kang, JY, Lee, SH, Yun, N, and Joung, B. Improved cardiac-specific delivery of RAGE siRNA within small extracellular vesicles engineered to express intense cardiac targeting peptide attenuates myocarditis. Mol Ther Nucleic Acids. (2021) 24:1024–32. doi: 10.1016/j.omtn.2021.04.018
127. Fan, Q, Sun, B, and Chao, J. Advancements in engineering tetrahedral framework nucleic acids for biomedical innovations. Small Methods. (2024):e2401360. doi: 10.1002/smtd.202401360
128. Cai, Z, Li, Y, Bai, L, Xu, J, Liu, Z, Zhang, T, et al. Tetrahedral framework nucleic acids based small interfering RNA targeting receptor for advanced glycation end products for diabetic complications treatment. ACS Nano. (2023) 17:22668–83. doi: 10.1021/acsnano.3c06999
129. McGeary, SE, Lin, KS, Shi, CY, Pham, TM, Bisaria, N, Kelley, GM, et al. The biochemical basis of microRNA targeting efficacy. Science. (2019) 366:eaav1741. doi: 10.1126/science.aav1741
130. Li, Q, Xia, S, Yin, Y, Guo, Y, Chen, F, and Jin, P. miR-5591-5p regulates the effect of ADSCs in repairing diabetic wound via targeting AGEs/AGER/JNK signaling axis. Cell Death Dis. (2018) 9:566. doi: 10.1038/s41419-018-0615-9
131. Luo, T, Yan, Y, He, Q, Ma, X, and Wang, W. miR-328-5p inhibits MDA-MB-231 breast cancer cell proliferation by targeting RAGE. Oncol Rep. (2018) 39:2906–14. doi: 10.3892/or.2018.6353
132. Yin, C, Zhang, G, Sun, R, Pan, X, Wang, X, Li, H, et al. miR-185-5p inhibits F-actin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol Med Rep. (2018) 18:2621–30. doi: 10.3892/mmr.2018.9294
133. Xu, XC, Zhang, WB, Li, CX, Gao, H, Pei, Q, Cao, BW, et al. Up-regulation of MiR-1915 inhibits proliferation, invasion, and migration of Helicobacter pylori-infected gastric Cancer cells via targeting RAGE. Yonsei Med J. (2019) 60:38–47. doi: 10.3349/ymj.2019.60.1.38
134. Chang, C, Huang, K, Xu, X, Duan, R, Yu, T, Chu, X, et al. MiR-23a-5p alleviates chronic obstructive pulmonary disease through targeted regulation of RAGE-ROS pathway. Respir Res. (2024) 25:93. doi: 10.1186/s12931-024-02736-y
135. Mariadoss, AVA, Sivakumar, AS, Lee, CH, and Kim, SJ. Diabetes mellitus and diabetic foot ulcer: etiology, biochemical and molecular based treatment strategies via gene and nanotherapy. Biomed Pharmacother. (2022) 151:113134. doi: 10.1016/j.biopha.2022.113134
136. Na, M, Mohammad, M, Fei, Y, Wang, W, Holdfeldt, A, Forsman, H, et al. Lack of receptor for advanced glycation end products leads to less severe staphylococcal skin infection but more skin abscesses and prolonged wound healing. J Infect Dis. (2018) 218:791–800. doi: 10.1093/infdis/jiy007
137. Vargason, AM, Anselmo, AC, and Mitragotri, S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. (2021) 5:951–67. doi: 10.1038/s41551-021-00698-w
138. Reddy, VP, Aryal, P, and Darkwah, EK. Advanced glycation end products in health and disease. Microorganisms. (2022) 10:1848. doi: 10.3390/microorganisms10091848
139. Lemarchand, M, Thouin, K, De Serres-Berard, T, Bellenfant, S, Cadau, S, and Berthod, F. In vitro glycation of a tissue-engineered wound healing model to mimic diabetic ulcers. Biotechnol Bioeng. (2023) 120:1657–66. doi: 10.1002/bit.28359
140. Harb, A, Elbatreek, MH, Elshahat, A, El-Akabawy, N, Barakat, W, and Elkomy, NM. Repurposing alagebrium for diabetic foot ulcer healing: impact on AGEs/NFkappaB/NOX1 signaling. Eur J Pharmacol. (2023) 959:176083. doi: 10.1016/j.ejphar.2023.176083
141. Yuan, XY, Meng, C, Liu, H, and Sun, B. Magnetically driven nanorobots based on peptides nanodots with tunable photoluminescence for rapid scavenging reactive alpha-dicarbonyl species and effective blocking of advanced glycation end products. Food Chem. (2023) 422:136252. doi: 10.1016/j.foodchem.2023.136252
142. Xing, C, Zhu, H, Dou, X, Gao, L, Baddi, S, Zou, Y, et al. Infected diabetic wound regeneration using peptide-modified chiral dressing to target revascularization. ACS Nano. (2023) 17:6275–91. doi: 10.1021/acsnano.2c10039
143. Zhang, X, Yang, Y, Su, J, Zhong, H, and Fang, L. Cascade Nanozyme-loaded Sprayable hydrogels for fibroblast rejuvenation and diabetic wound regeneration. ACS Appl Mater Interfaces. (2025) 17:20968–79. doi: 10.1021/acsami.5c02168
144. Goova, MT, Li, J, Kislinger, T, Qu, W, Lu, Y, Bucciarelli, LG, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. (2001) 159:513–25. doi: 10.1016/S0002-9440(10)61723-3
145. Kang, HJ, Kumar, S, D'Elia, A, Dash, B, Nanda, V, Hsia, HC, et al. Self-assembled elastin-like polypeptide fusion protein coacervates as competitive inhibitors of advanced glycation end-products enhance diabetic wound healing. J Control Release. (2021) 333:176–87. doi: 10.1016/j.jconrel.2021.03.032
146. Jin, Q, Liu, T, Qiao, Y, Liu, D, Yang, L, Mao, H, et al. Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Front Immunol. (2023) 14:1185317. doi: 10.3389/fimmu.2023.1185317
147. Youjun, D, Huang, Y, Lai, Y, Ma, Z, Wang, X, Chen, B, et al. Mechanisms of resveratrol against diabetic wound by network pharmacology and experimental validation. Ann Med. (2023) 55:2280811. doi: 10.1080/07853890.2023.2280811
148. Chen, SA, Chen, HM, Yao, YD, Hung, CF, Tu, CS, and Liang, YJ. Topical treatment with anti-oxidants and au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur J Pharm Sci. (2012) 47:875–83. doi: 10.1016/j.ejps.2012.08.018
149. Guan, L, Mao, Z, Yang, S, Wu, G, Chen, Y, Yin, L, et al. Dioscin alleviates Alzheimer's disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed Pharmacother. (2022) 152:113248. doi: 10.1016/j.biopha.2022.113248
150. Aljohi, A, Matou-Nasri, S, Liu, D, Al-Khafaji, N, Slevin, M, and Ahmed, N. Momordica charantia extracts protect against inhibition of endothelial angiogenesis by advanced glycation endproducts in vitro. Food Funct. (2018) 9:5728–39. doi: 10.1039/c8fo00297e
151. Chen, C, Liu, X, Li, L, Guo, M, He, Y, Dong, Y, et al. Study of the mechanism by gentiopicroside protects against skin fibroblast glycation damage via the RAGE pathway. Sci Rep. (2024) 14:4685. doi: 10.1038/s41598-024-55525-4
152. Liu, YC, Chen, SY, Chen, YY, Chang, HY, Chiang, IC, and Yen, GC. Polysaccharides extracted from common buckwheat (Fagopyrum esculentum) attenuate cognitive impairment via suppressing RAGE/p38/NF-kappaB signaling and dysbiosis in AlCl(3)-treated rats. Int J Biol Macromol. (2024) 276:133898. doi: 10.1016/j.ijbiomac.2024.133898
153. Zhang, N, Chen, P, Liang, X, Sun, J, Liu, Q, Guan, S, et al. Luteolin targets the AGE-RAGE signaling to mitigate inflammation and ferroptosis in chronic atrophic gastritis. Aging (Albany NY). (2024) 16:10918–30. doi: 10.18632/aging.205969
154. May, JM, Jayagopal, A, Qu, ZC, and Parker, WH. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem Biophys Res Commun. (2014) 452:112–7. doi: 10.1016/j.bbrc.2014.08.057
155. Chua, GHI, Phang, SCW, Wong, YO, Ho, LS, Palanisamy, UD, and Abdul Kadir, K. Vitamin E levels in ethnic communities in Malaysia and its relation to glucose tolerance, insulin resistance and advanced glycation end products: a cross-sectional study. Nutrients. (2020) 12:3659. doi: 10.3390/nu12123659
156. Wang, L, Yu, CJ, Liu, W, Cheng, LY, and Zhang, YN. Rosiglitazone protects neuroblastoma cells against advanced glycation end products-induced injury. Acta Pharmacol Sin. (2011) 32:991–8. doi: 10.1038/aps.2011.81
157. Yang, Y, Huang, S, Ma, Q, Li, N, Li, R, Wang, Y, et al. Combined therapeutic strategy based on blocking the deleterious effects of AGEs for accelerating diabetic wound healing. Regen Biomater. (2024) 11:rbae062. doi: 10.1093/rb/rbae062
158. Sourris, KC, Ding, Y, Maxwell, SS, Al-Sharea, A, Kantharidis, P, Mohan, M, et al. Glucagon-like peptide-1 receptor signaling modifies the extent of diabetic kidney disease through dampening the receptor for advanced glycation end products-induced inflammation. Kidney Int. (2024) 105:132–49. doi: 10.1016/j.kint.2023.09.029
159. Werkman, NCC, Driessen, JHM, Klungel, OH, Schaper, NS, Souverein, PC, Stehouwer, CDA, et al. Incretin-based therapy and the risk of diabetic foot ulcers and related events. Diabetes Obes Metab. (2024) 26:3764–80. doi: 10.1111/dom.15721
160. Gupta, S, and Sen, U. More than just an enzyme: dipeptidyl peptidase-4 (DPP-4) and its association with diabetic kidney remodelling. Pharmacol Res. (2019) 147:104391. doi: 10.1016/j.phrs.2019.104391
161. Song, H, Fu, F, Chen, Y, Yang, R, Luo, Z, Shao, J, et al. A poly(lipoic acid)-based elastomer adhesive with synergistic activity of microenvironment regulation and peripheral neuropathy repair facilitates infectious diabetic wound healing. Biomaterials. (2026) 324:123489. doi: 10.1016/j.biomaterials.2025.123489
162. Lin, R, Xu, B, Ye, Z, Gao, Y, Fang, H, Song, J, et al. Metformin attenuates diabetes-induced osteopenia in rats is associated with down-regulation of the RAGE-JAK2-STAT1 signal axis. J Orthop Translat. (2023) 40:37–48. doi: 10.1016/j.jot.2023.05.002
163. Portero-Otin, M, de la Maza, MP, and Uribarri, J. Dietary advanced glycation end products: their role in the insulin resistance of aging. Cells. (2023) 12:1684. doi: 10.3390/cells12131684
164. Luo, L, Li, R, Wang, G, Chen, J, Chen, L, Qin, LQ, et al. Age-dependent effects of a high-fat diet combined with dietary advanced glycation end products on cognitive function and protection with voluntary exercise. Food Funct. (2022) 13:4445–58. doi: 10.1039/D1FO03241K
165. Lu, T, Lahousse, L, Wijnant, S, Chen, J, Brusselle, GG, van Hoek, M, et al. The AGE-RAGE axis associates with chronic pulmonary diseases and smoking in the Rotterdam study. Respir Res. (2024) 25:85. doi: 10.1186/s12931-024-02698-1
166. Fotheringham, AK, Gallo, LA, Borg, DJ, and Forbes, JM. Advanced glycation end products (AGEs) and chronic kidney disease: does the modern diet AGE the kidney? Nutrients. (2022) 14:2675. doi: 10.3390/nu14132675
Keywords: advanced glycation end products, receptor for advanced glycation end products, inflammation, diabetes, wound healing, diabetic wound
Citation: Lin H, Yang Y, Wang X, Chung M, Zhang L, Cai S, Pan X and Pan Y (2025) Targeting the AGEs-RAGE axis: pathogenic mechanisms and therapeutic interventions in diabetic wound healing. Front. Med. 12:1667620. doi: 10.3389/fmed.2025.1667620
Edited by:
Haiyang Wu, Tianjin Medical University, ChinaReviewed by:
Alessia Faggian, University of Trento, ItalyMuhammed Okuyucu, Ondokuz Mayıs University, Türkiye
Copyright © 2025 Lin, Yang, Wang, Chung, Zhang, Cai, Pan and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sa Cai, Y2Fpc2FAc3p1LmVkdS5jbg==; Xiaohua Pan, Y3N3azIwMTVAMTI2LmNvbQ==; Yu Pan, cGFuLXl1QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Haohui Lin1†
Haohui Lin1† Yi Yang
Yi Yang Sa Cai
Sa Cai