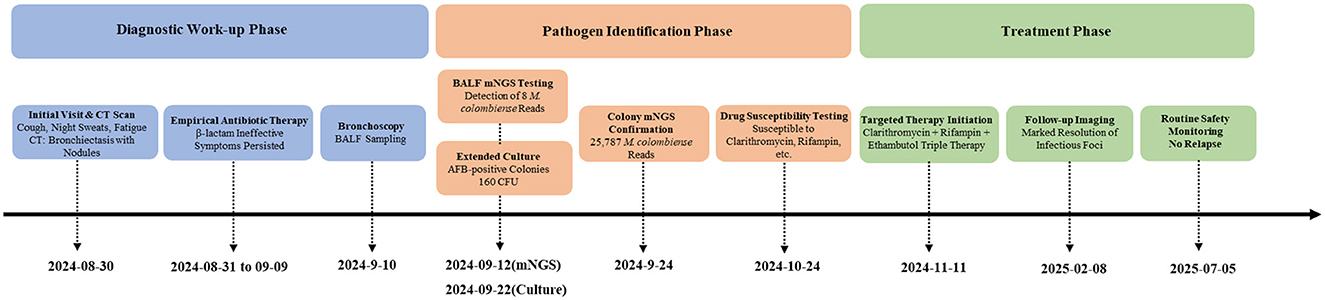
- 1Department of Clinical Laboratory, The General Hospital of Western Theater Command, Chengdu, Sichuan, China
- 2Department of Nuclear Medicine, The General Hospital of Western Theater Command, Chengdu, Sichuan, China
Mycobacterium colombiense, a rare slow-growing mycobacterium within the Mycobacterium avium complex (MAC), causes disseminated disease almost exclusively in immunocompromised hosts, with no prior reports of localized pulmonary infection in non-immunosuppressed individuals. A 47-year-old non-immunosuppressed male with bronchiectasis presented with progressive cough, night sweats, and fatigue. Computed tomography (CT) revealed bronchiectasis with nodules in the right middle and lower lobes. Empirical β-lactam therapy failed, and conventional bronchoalveolar lavage fluid (BALF) tests (smears, cultures, PCR) yielded no pathogens at 48 h. Although metagenomic next-generation sequencing (mNGS) of BALF detected a low number of M. colombiense sequences (eight reads), definitive confirmation was achieved through extended culture, which is considered the gold standard for the diagnosis of nontuberculous mycobacteria. This culture revealed acid-fast bacilli within 12 days (160 CFU), confirming the presence of viable M. colombiense. Subsequent mNGS of the isolated colonies further confirmed the species identity with high sequence reads (25,787 reads). Guideline-based triple therapy (guided by drug susceptibility testing and guidelines) with clarithromycin, rifampicin, and ethambutol achieved significant radiographic resolution at 24 weeks. This case demonstrates that M. colombiense pulmonary infection is diagnostically elusive and mimics non-specific respiratory syndromes. It defines the clinical features of this pathogen in non-immunosuppressed hosts and highlights the need for heightened surveillance for nontuberculous mycobacteria (NTM) in bronchiectasis patients, given the likelihood of underdiagnosis.
Introduction
Nontuberculous mycobacteria (NTM), encompassing all species beyond the Mycobacterium tuberculosis complex (MTBC) and Mycobacterium leprae, are environmentally ubiquitous organisms. Over 200 species have been identified to date (1), which act as opportunistic pathogens particularly threatening immunocompromised individuals, such as those with HIV infection (2–4) or organ transplant recipients (5). Although NTM can cause skin, lymph node, or disseminated infections, the lungs are the most common site of disease (6). NTM pulmonary disease (NTM-PD) presents a significant diagnostic challenge due to its non-specific symptoms and the difficulty in distinguishing true infection from colonization. Definitive diagnosis requires meeting clinical, microbiological, and radiological criteria as outlined in major international guidelines (7, 8). However, the low sensitivity and long turnaround time of traditional cultures frequently lead to misdiagnosis as tuberculosis or malignancy, delaying appropriate treatment (9, 10).
This diagnostic difficulty is compounded by a rising global incidence of NTM-PD, which has become a growing public health concern (11–14). The situation in China further illustrates this challenge, with a prevalence of 4.66%−6.3% for NTM infections among suspected tuberculosis cases (15, 16). The burden is higher in the south (up to 8.6%), where rapidly growing mycobacteria (RGM) dominate, unlike the north where the slowly growing Mycobacterium avium complex (MAC) is more common (15). The most prevalent species are M. intracellulare (40.5%) and M. abscessus (28.4%) (17), and immunocompromised hosts like HIV/AIDS patients bear a particularly high burden, with infection rates of 40%−46.7% largely due to MAC (18, 19). This complex epidemiology calls for heightened vigilance (15, 16, 20).
M. colombiense is a slow-growing NTM first isolated in 2006 from blood and sputum samples of HIV-positive patients in Colombia (21). It was subsequently identified as a distinct member of MAC (22). MAC is a clinically significant group of environmentally ubiquitous NTMs that includes species such as M. avium and M. intracellulare and is known to cause opportunistic infections primarily in immunocompromised individuals or those with underlying lung conditions (23). However, isolation of M. colombiense from clinical samples is uncommon. Existing research on this pathogen has predominantly focused on immunodeficient populations, with very limited evidence regarding its pathogenicity in non-immunosuppressed hosts.
Against this background, we present the first documented case of M. colombiense pulmonary infection in a non-immunosuppressed individual with bronchiectasis. A definitive diagnosis was established through standard culture procedures, which remain the cornerstone of NTM identification. Although mNGS provided rapid preliminary detection, culture isolation was critical for confirming M. colombiense as the causative agent. Subsequent drug susceptibility testing (DST) guided targeted antimicrobial therapy, facilitating prompt and effective treatment.
Case presentation
On August 30, 2024, a 47-year-old male healthcare worker presented to our Infectious Diseases and Respiratory Department with a three-month history of persistent cough, night sweats, and progressive fatigue. He expressed particular concern about the worsening fatigue, which was impairing his daily work, and the night sweats, as he was troubled by the possibility of a protracted respiratory infection or other underlying systemic illness. His symptoms had an insidious onset and gradually worsened. Although a previous episode of acute sinusitis two months earlier had resolved, the cough, night sweats, and fatigue persisted. The patient denied any history of chronic diseases, immunodeficiency, exposure to endemic diseases, genetic risks, or known immunosuppression.
Physical examination was notable for the following findings. Vital signs were normal. The patient was alert and oriented with a lean build (BMI 20.60 kg/m2). On auscultation, scattered moist rales were audible in the bilateral lower lung fields, though no pleural friction rub was detected. Cardiovascular examination revealed normal heart sounds with a regular rhythm and no murmurs.
Laboratory investigations were conducted to assess the patient's systemic and immune status. Results of the complete blood count, liver and kidney function tests were all within normal ranges. Similarly, immune markers (T-cell subsets and immunoglobulins) and inflammatory markers were unremarkable. Serologic screening for HIV, syphilis, and autoimmune diseases returned negative results.
Chest CT imaging, however, demonstrated significant pathology, revealing bronchiectasis in the medial segment of the right middle lobe and the right lower lobe. These areas were accompanied by multiple surrounding patchy opacities and nodules, suggestive of bronchiectasis with superimposed infection (Figures 1 A1–A3).
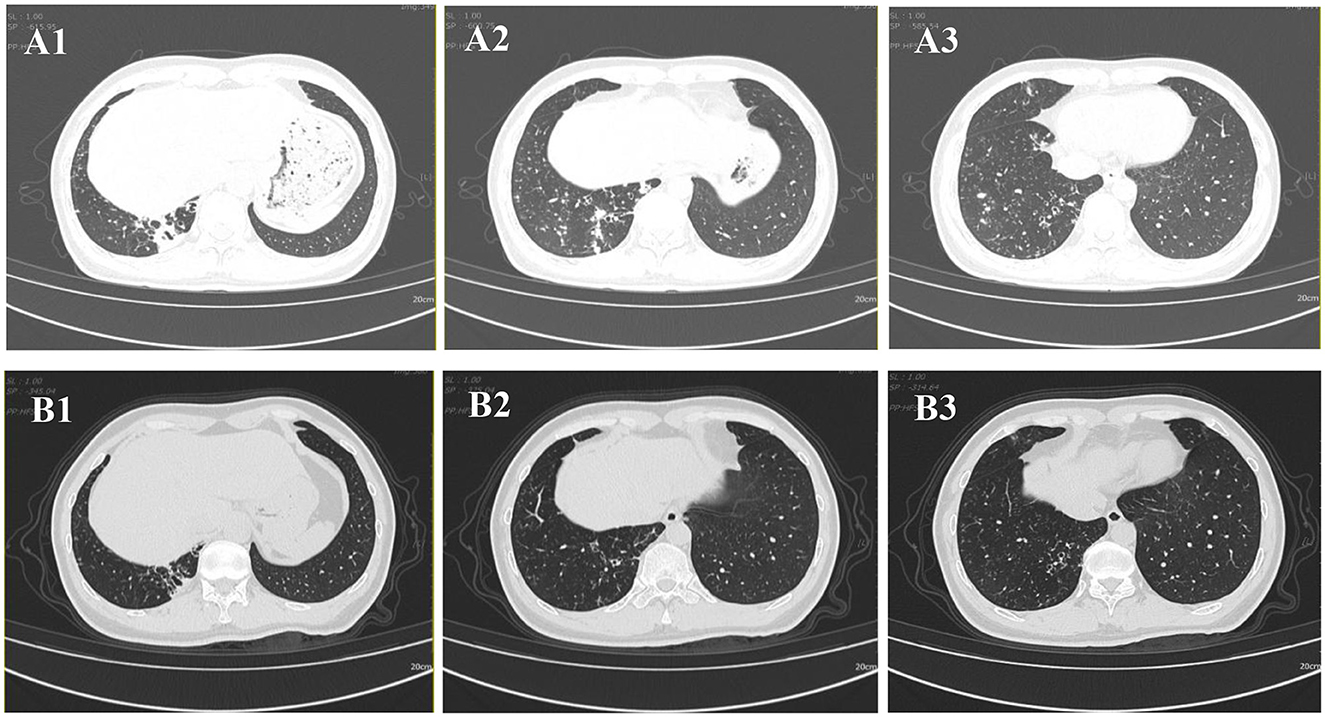
Figure 1. Chest CT images. (A1–A3) CT scan obtained on 2024-08-30 demonstrates bronchiectasis in the medial segment of the right middle lobe and the right lower lobe, accompanied by surrounding multiple patchy and nodular opacities. (B1–B3) Follow-up CT scan obtained on 2025-02-08 shows interval resolution of the peribronchial infectious foci in the right lower lobe.
Consequently, the initial diagnosis was bronchiectasis with infection. To identify the causative pathogen, a sputum sample was obtained and sent for microbiological analysis. While microscopy revealed 1± Gram-positive cocci, the 48-h culture only yielded normal respiratory microbiota. Initial management included rest, nutritional support, antitussive medication (dextromethorphan), and empirical antibiotic therapy with piperacillin/tazobactam. However, by day 11 of treatment (September 10, 2024), the patient's symptoms showed no improvement and were progressively worsening. Fiberoptic bronchoscopy was subsequently performed. Analysis of BALF revealed: (1) Smear microscopy (Ziehl-Neelsen staining and Gram stain): negative; (2) Culture at 48 h: no bacterial or fungal growth; (3) Fluorescent PCR for Mycobacterium tuberculosis DNA: negative. This test was performed using a commercial kit (Singclean Medical, China) for nucleic acid extraction and PCR amplification (Mycobacterium tuberculosis complex nucleic acid detection kit) on a real-time PCR system (TIANKONG Gentier 96E, China). These results preliminarily excluded common bacterial, fungal, and tuberculous infections.
On September 12, 2024, mNGS analysis of BALF detected nucleic acid sequences of M. colombiense (eight reads), suggesting a possible NTM infection. This molecular finding prompted the extension of an ongoing BALF culture that had been initiated earlier. The culture had been inoculated onto blood agar and incubated at 37 °C in a 5% CO2 incubator. Given that M. colombiense is a slow-growing mycobacterium, and encouraged by the mNGS signal, we decided to prolong the incubation period beyond the initial 48 h. To prevent desiccation and contamination, the gap between the lid and base of the Petri dish was sealed along the edge with transparent tape, and colony growth was observed every three days. After extending incubation to a total of 12 days (until September 22, 2024), pale yellow, umbonated colonies (approximately 0.2 mm in diameter; Figure 2 C1) became visible, with an estimated count of 160 CFU. Gram staining of these colonies yielded negative results, while acid-fast staining (Ziehl–Neelsen) confirmed the presence of short-chain acid-fast bacilli (Figure 2 C2).
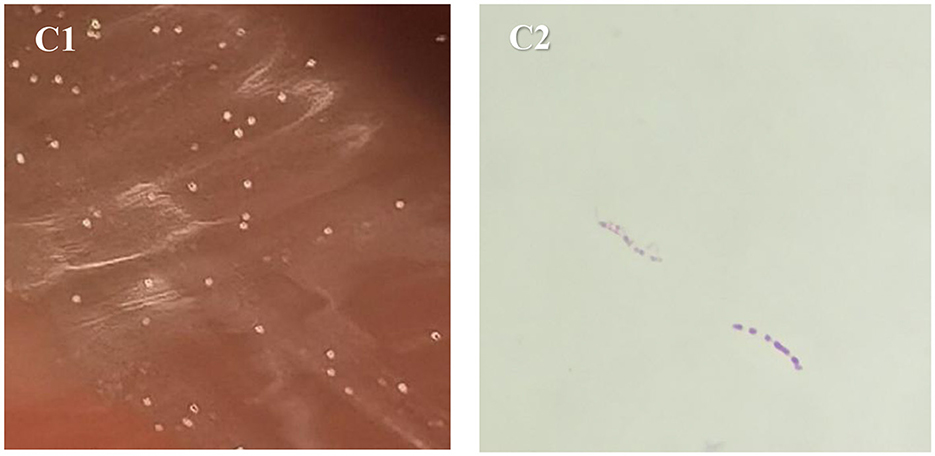
Figure 2. Morphological characteristics of M. colombiense. (C1) Culture results of blood agar on 2024-09-22; (C2) acid-fast-stained bacteria on 2024-09-22.
However, two independent matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analyses using the Clin-TOF II system (Bioyang, China) yielded inconsistent species identifications with low confidence scores: the first identified Mycobacterium tuberculosis (score 25), and the second identified Mycobacterium bovid (score 30). Both results showed poor reproducibility, confirming the unreliability of MS-based identification for this isolate.
To definitively identify the pathogen, mNGS was performed directly on isolated colonies. Sequencing was conducted on an Illumina Nextel 550AR instrument. Following host depletion, libraries were prepared from the extracted DNA using the NextEra XT DNA Library Preparation Kit (Illumina, USA) according to the manufacturer's instructions. Microbial sequences were aligned against a curated database of approximately 13,000 bacterial, fungal, and parasitic genomes using SNAP. This analysis yielded 25,787 sequences uniquely mapping to M. colombiense, confirming species-level identification.
According to the Clinical Practice Guidelines for NTM-PD (7), diagnosis requires meeting all of the following criteria: (1) Pulmonary and/or systemic symptoms; (2) Radiographic findings consistent with NTM-PD (e.g., bronchiectasis with nodules on CT); (3) Exclusion of other pulmonary diseases; (4) Positive culture from at least one BALF sample. This patient fulfilled all criteria: CT showed bronchiectasis with infection, BALF culture confirmed M. colombiense, and symptoms persisted despite prior therapy. Therefore, he was definitively diagnosed with NTM-PD caused by M. colombiense. The diagnostic workflow that led from initial presentation to targeted therapy is outlined in Figure 3.
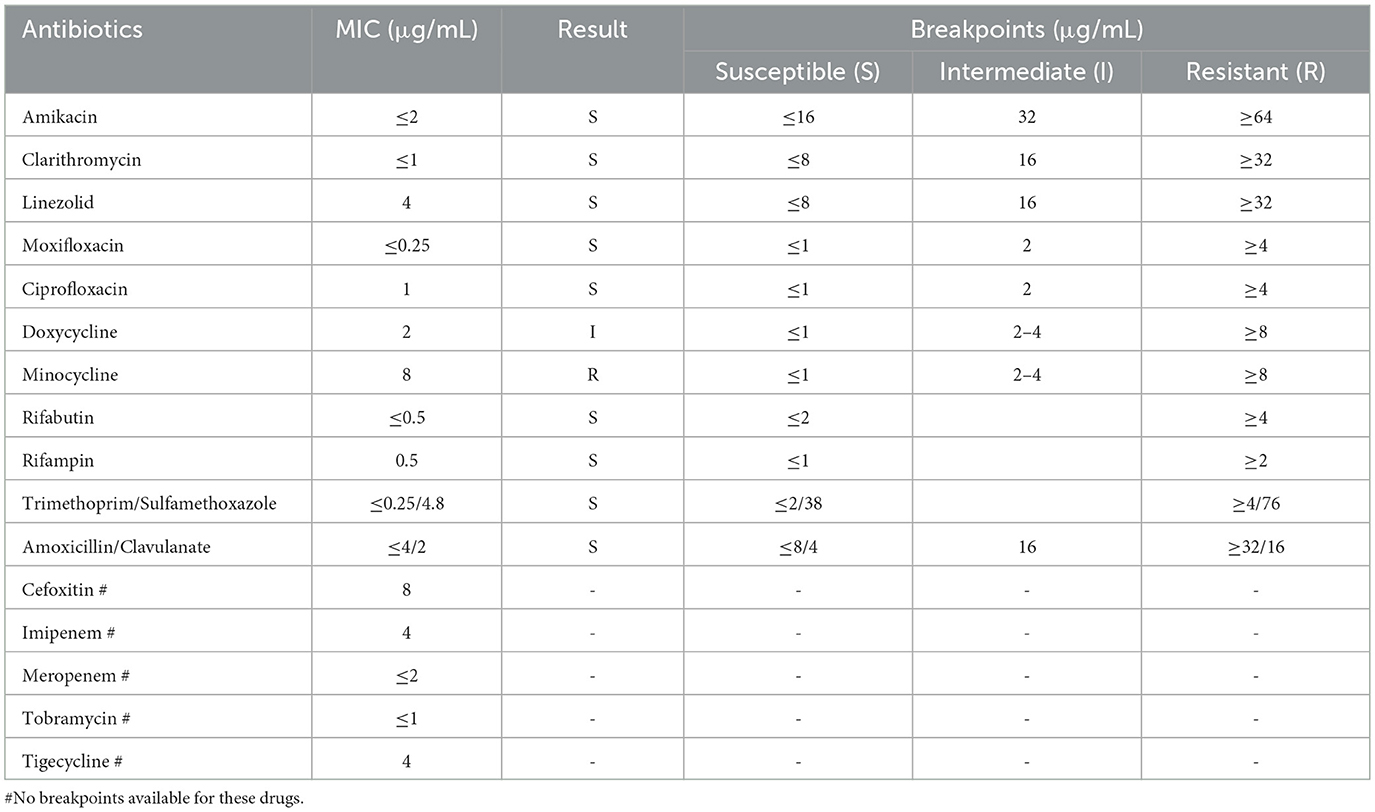
DST was performed on the isolated and purified strain using the broth microdilution method with a panel (Baso, Zhuhai, China), strictly according to the manufacturer's instructions. Results were interpreted following the criteria set forth in the Clinical and Laboratory Standards Institute (CLSI) guidelines M62, 1st ed and M24 3rd ed. Results indicated susceptibility to clarithromycin, rifampicin, rifabutin, and amikacin, intermediate susceptibility to doxycycline, and resistance to minocycline (Table 1).
As guidelines mandate treatment over observation for patients fulfilling the diagnostic criteria of NTM-PD with progressive symptoms (7), targeted therapy was commenced on November 11, 2024, due to the patient's clinical deterioration. The regimen consisted of clarithromycin (1.0 g daily), rifampicin (0.45 g daily), and ethambutol (0.75 g daily). Monthly complete blood counts and liver/kidney function tests were performed to monitor for adverse drug effects.
Following targeted therapy, the patient's clinical symptoms improved significantly. A follow-up chest CT on February 8, 2025 (Figure 1 B1–B3), showed marked resolution of the bronchiectasis with nodules compared to baseline (August 30, 2024). Subsequent follow-up at 24 weeks (until July 2025) confirmed sustained clinical stability without relapse and no reported drug-related adverse events.
Discussion
This case provides the first documented evidence that pulmonary infection caused by M. colombiense can occur in non-immunosuppressed hosts, highlighting the pathogen's rare ability to breach the immune defenses of such individuals. Previous reports describe this bacterium almost exclusively affecting immunocompromised populations (e.g., HIV-positive individuals, organ transplant recipients), predominantly causing disseminated disease (involving ≥2 non-contiguous organ systems) (24). In stark contrast, our patient had no evidence of immunodeficiency, and the disease was confined to the lungs (bronchiectasis with infection in the right middle and lower lobes). This challenges the prevailing notion that “M. colombiense infection necessitates underlying systemic immunodeficiency.”
It is well-established that MAC infections are primarily acquired from environmental sources through inhalation, ingestion, or skin contact, manifesting as pulmonary disease, lymphadenitis, skin and soft tissue infections, or disseminated disease (7, 25–27). Risk factors for NTM-PD can be broadly categorized into three groups: host-related factors, drug-induced immunosuppression, and environmental exposure. Among these, host factors play a particularly critical role. Pre-existing pulmonary diseases—such as previous tuberculosis, bronchiectasis, chronic obstructive pulmonary disease (COPD), among others—significantly increase the risk of developing NTM-PD (23, 28–31). The underlying mechanism lies in the fact that these conditions often lead to impaired bronchial mucociliary clearance, thereby compromising local pulmonary defense. Recent studies further indicate that even in the absence of overt systemic disease or an immunosuppressed state, such localized impairment of mucociliary clearance alone can constitute an important risk factor for NTM infection (32–34). As an environmental mycobacterium, M. colombiense exploits defects in local host defenses to establish infection. In this case, although the host was not immunosuppressed, the presence of bronchiectasis likely facilitated M. colombiense infection by disrupting the mucosal barrier and compromising ciliary clearance. This highlights the critical need for heightened clinical vigilance for NTM infection in patients with structural lung diseases such as bronchiectasis, irrespective of their systemic immune status.
Cases of M. colombiense infection are rarely reported, likely due to historical diagnostic challenges in accurately distinguishing between MAC subspecies (3). The definitive diagnosis in this case relied on the synergistic application of mNGS and culture techniques. Although mNGS of BALF initially provided a crucial clue with a low sequence count (eight reads), it was insufficient for definitive diagnosis. Subsequent culture successfully isolated colonies within 12 days, a notably shorter duration than previously reported for respiratory specimens (4, 5, 9). Furthermore, MALDI-TOF MS failed to identify the species, likely due to the absence of reference spectra for M. colombiense in the database. Ultimately, definitive species-level identification was achieved by applying mNGS directly to the cultured isolate, which yielded 25,787 M. colombiense sequences. This synergistic approach of culture and mNGS was therefore critical for confirming the infection.
Drug therapy for NTM disease requires careful consideration due to the diversity of NTM species and their varying drug susceptibility profiles. Current guidelines strongly recommend performing in vitro DST before initiating treatment (35). The regimen should include at least two drugs likely or known to be effective against the specific NTM isolate; severe cases may require 4–6 effective drugs, and monotherapy must be avoided (36, 37). Our DST results demonstrated susceptibility to clarithromycin and rifampicin but resistance to minocycline. The subsequent successful clinical and radiological response to a DST-guided triple-drug regimen (clarithromycin, rifampicin, and ethambutol) further validates this approach (Figure 1 B1–B3 vs. Figure 1 A1–A3), and is consistent with treatment outcomes described in other susceptible cases (5, 24). However, a critical comparison with the broader literature reveals noteworthy heterogeneity in the drug susceptibility profiles of M. colombiense. Most notably, a contrasting case from Brazil involved an isolate exhibiting high-level resistance to both clarithromycin and rifampicin (9), which culminated in a fatal outcome despite therapy. This discrepancy underscores that susceptibility to first-line macrolides and rifamycins should not be universally assumed. Therefore, the variations in susceptibility profiles among M. colombiense isolates highlight the importance of DST (38). Guided treatment based on individual DST results remains essential for effective clinical management.
The management of suspected M. colombiense infection in resource-limited settings, where access to mNGS is unavailable, must rely on a structured, stepwise approach. Diagnosis should be pursued through sustained mycobacterial culture and acid-fast staining of respiratory specimens, which remain the fundamental and accessible methods for confirming NTM infection (39, 40). For treatment, initiating an empiric macrolide-based multidrug regimen following standard MAC guidelines is the cornerstone of management. The combination of clarithromycin, ethambutol, and a rifamycin is the recommended first-line therapy, with the addition of amikacin considered in severe or refractory cases (10, 41). While clarithromycin serves as the cornerstone with a relatively low resistance rate, the potential for emergent resistance necessitates monitoring, ideally via phenotypic DST when available (42, 43). In the absence of routine DST, therapeutic efficacy must be meticulously evaluated through clinical assessment and serial radiographic changes, with regular patient follow-up to prevent relapse (41, 44). This integrated strategy of conventional microbiology, guideline-based empiric therapy, and vigilant clinical-radiographic monitoring provides a viable framework for the effective management of M. colombiense infection where advanced resources are constrained.
Given these diagnostic hurdles, the incidence of M. colombiense is likely underestimated. Thus, in bronchiectasis patients with infection refractory to conventional antibiotics, NTM infection should be suspected and appropriate diagnostic tests pursued, even in non-immunosuppressed hosts. However, this study is limited by its nature as a single-case report. Therefore, it cannot provide a systematic assessment of the epidemiology, full spectrum of clinical manifestations, or long-term prognosis of M. colombiense infections in non-immunosuppressed hosts. Future multi-center, prospective studies are needed to better characterize this pathogen and optimize its clinical management.
Conclusions
In conclusion, infections caused by M. colombiense are extremely rare. Compared to existing studies focusing on immunocompromised hosts, our case highlights that M. colombiense pulmonary infection can occur even in patients without demonstrable immunodeficiency. Therefore, both mNGS and optimized cultures are indispensable, serving as complementary diagnostic modalities for the effective identification and management of challenging NTM infections. Given the current limitations in diagnostic, surveillance, and reporting systems for M. colombiense infection, its true prevalence is likely underestimated, and the condition is prone to misdiagnosis. Clinicians should maintain vigilance for M. colombiense as a potential causative agent in bronchiectasis patients with refractory infections.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JT: Writing – original draft, Data curation, Conceptualization. LL: Formal analysis, Visualization, Validation, Writing – review & editing. LW: Writing – review & editing, Validation. YQ: Validation, Supervision, Writing – review & editing. ZS: Writing – original draft, Investigation, Resources. QW: Writing – original draft, Validation. YL: Writing – review & editing, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the Research Project of the General Hospital of the Western Theater Command (2021-XZYG-B11) and the Medical Research Project of Chengdu (2024281 and 2024544).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pavlik I, Ulmann V, Hubelova D, Weston RT. Nontuberculous mycobacteria as sapronoses: a review. Microorganisms (2022) 10:1345. doi: 10.3390/microorganisms10071345
2. Pena E, Machado D, Viveiros M, Jordão S, A. case report of disseminated Mycobacterium colombiense infection in an HIV patient. Int J Mycobacteriol. (2019) 8:295–7. doi: 10.4103/ijmy.ijmy_100_19
3. Yu X, Jiang W. Mycobacterium colombiense and Mycobacterium avium complex causing severe pneumonia in a patient with HIV identified by a novel molecular-based method. Infect Drug Resist. (2021) 14:11–6. doi: 10.2147/IDR.S282190
4. Guo Y, Li X, Xiao Q, Yang J, Tao R, Xu L, et al. Mycobacterium colombiense pneumonia in HIV-infected patients: three case reports and a literature review. Infect Drug Resist. (2023) 16:7767–73. doi: 10.2147/IDR.S441083
5. Gosal J, Lee BC, A. case report of fatal disseminated Mycobacterium colombiense infection in a renal transplant recipient. Transp infect Dis. (2018) 20:e12890. doi: 10.1111/tid.12890
6. Tang M, Zeng W, Qiu Y, Fang G, Pan M, Li W, et al. Clinical features of rare disseminated Mycobacterium colombiense infection in nine patients who are HIV-negative in Guangxi, China. Int J Infect Dis. (2023) 128:321–4. doi: 10.1016/j.ijid.2023.01.002
7. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respirat J (2020) 56:1. doi: 10.1183/13993003.00535-2020
8. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. (2007) 175:367–416. doi: 10.1164/rccm.200604-571ST
9. Barretto AR, Felício JS, Sales LHM, Yamada ES, Lopes ML, da Costa ARF, et al. fatal case of pulmonary infection by Mycobacterium colombiense in Para State, Amazon Region, Brazil. Diagn Microbiol Infect Dis. (2016) 85:344–6. doi: 10.1016/j.diagmicrobio.2016.02.011
10. Qin J, Tang G. Disseminated Mycobacterium colombiense infection mimicking malignancy: a case report. Heliyon. (2024) 10:e30567. doi: 10.1016/j.heliyon.2024.e30567
11. Dahl VN, Mølhave M, Fløe A, van Ingen J, Schön T, Lillebaek T, et al. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis. (2022) 125:120–31. doi: 10.1016/j.ijid.2022.10.013
12. Lange C, Böttger EC, Cambau E, Griffith DE, Guglielmetti L, van Ingen J, et al. Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases. Lancet Infect Dis. (2022) 22:e178–90. doi: 10.1016/S1473-3099(21)00586-7
13. Prevots DR, Marshall JE, Wagner D, Morimoto K. Global epidemiology of nontuberculous mycobacterial pulmonary disease: a review. Clin Chest Med. (2023) 44:675–721. doi: 10.1016/j.ccm.2023.08.012
14. Burzyńska W, Fol M, Druszczynska M. Growing challenges of lung infections with non-tuberculous mycobacteria in immunocompromised patients: epidemiology and treatment. Arch Immunol Ther Exp (Warsz). (2025) 73:1. doi: 10.2478/aite-2025-0005
15. Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect. (2016) 73:558–67. doi: 10.1016/j.jinf.2016.08.020
16. Zhou L, Xu D, Liu H, Wan K, Wang R, Yang Z. Trends in the prevalence and antibiotic resistance of non-tuberculous Mycobacteria in mainland China, 2000-2019: systematic review and meta-analysis. Front Public Health. (2020) 8:295. doi: 10.3389/fpubh.2020.00295
17. Duan H, Han X, Wang Q, Wang J, Wang J, Chu N, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in a Chinese tuberculosis tertiary care center. Sci Rep. (2016) 6:36299. doi: 10.1038/srep36299
18. Yuan J, Wang Y, Wang L, Wang H, Ren Y, Yang W. What do the clinical features of positive nontuberculous mycobacteria isolates from patients with HIV/AIDS in China reveal? A systematic review and meta-analysis. J Global Health. (2023) 13:04093. doi: 10.7189/jogh.13.04093
19. Liu L, Zhang R, Tang Y, Qi T, Song W, Wang Z, et al. The importance of non-tuberculous mycobacteria identification in Chinese patients infected with HIV. Biosci Trends. (2018) 12:515–6. doi: 10.5582/bst.2018.01254
20. Kang S, Schmidt JE, Chen I, Tiberi S. Treatment outcomes in NTM-PD in a high TB burden context. IJTLD Open. (2024) 1:547–55. doi: 10.5588/ijtldopen.24.0413
21. Murcia MI, Tortoli E, Menendez MC, Palenque E, Garcia MJ. Mycobacterium colombiense sp. nov., a novel member of the Mycobacterium avium complex and description of MAC-X as a new ITS genetic variant. Int J Syst Evolut Microbiol. (2006) 56:2049–54. doi: 10.1099/ijs.0.64190-0
22. Maya-Hoyos M, Leguizamón J, Mariño-Ramírez L, Soto CY. Sliding motility, biofilm formation, and glycopeptidolipid production in Mycobacterium colombiense strains. Biomed Res Int. (2015) 2015:419549. doi: 10.1155/2015/419549
23. Kumar K, Loebinger MR. Nontuberculous mycobacterial pulmonary disease: clinical epidemiologic features, risk factors, and diagnosis: the nontuberculous mycobacterial series. Chest. (2022) 161:637–46. doi: 10.1016/j.chest.2021.10.003
24. Matono T, Suzuki S, Yamate R, Nakamura K, Sakagami T. Diagnostic and therapeutic challenges in disseminated Mycobacterium colombiense infection caused by interferon-γ neutralizing autoantibodies. Open Forum Infect Dis. (2023) 10:ofad035. doi: 10.1093/ofid/ofad035
25. Falkinham JO. Current epidemiologic trends of the nontuberculous mycobacteria (NTM). Curr Environm Health Reports. (2016) 3:161–7. doi: 10.1007/s40572-016-0086-z
26. Horsburgh CR, Selik RM. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respirat Dis. (1989) 139:4–7. doi: 10.1164/ajrccm/139.1.4
27. Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clinic Proc. (2013) 88:38–45. doi: 10.1016/j.mayocp.2012.06.029
28. Furuuchi K, Morimoto K, Yoshiyama T, Tanaka Y, Fujiwara K, Okumura M, et al. Interrelational changes in the epidemiology and clinical features of nontuberculous mycobacterial pulmonary disease and tuberculosis in a referral hospital in Japan. Respir Med. (2019) 152:74–80. doi: 10.1016/j.rmed.2019.05.001
29. Lu M, Saddi V, Britton PN, Selvadurai H, Robinson PD, Pandit C, et al. Disease caused by non-tuberculous mycobacteria in children with cystic fibrosis. Paediatr Respir Rev. (2019) 29:42–52. doi: 10.1016/j.prrv.2018.05.001
30. Adjemian J, Olivier KN, Prevots DR. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010-2014. Ann Am Thorac Soc. (2018) 15:817–26. doi: 10.1513/AnnalsATS.201709-727OC
31. Mencarini J, Cresci C, Simonetti MT, Truppa C, Camiciottoli G, Frilli ML, et al. Non-tuberculous mycobacteria: epidemiological pattern in a reference laboratory and risk factors associated with pulmonary disease. Epidemiol Infect. (2017) 145:515–22. doi: 10.1017/S0950268816002521
32. Retuerto-Guerrero M, López-Medrano R, de Freitas-González E, Rivero-Lezcano OM. Nontuberculous mycobacteria, mucociliary clearance, and bronchiectasis. Microorganisms (2024) 12:665. doi: 10.3390/microorganisms12040665
33. Wyrostkiewicz D, Opoka L, Filipczak D, Jankowska E, Skorupa W, Augustynowicz-Kopeć E, et al. Nontuberculous mycobacterial lung disease in the patients with cystic fibrosis-a challenging diagnostic problem. Diagnostics. (2022) 12:1514. doi: 10.3390/diagnostics12071514
34. Van Braeckel E, Bosteels C. Growing from common ground: nontuberculous mycobacteria and bronchiectasis. Eur Respirat Rev. (2024) 33:173. doi: 10.1183/16000617.0058-2024
35. Johnson TM, Byrd TF, Drummond WK, Childs-Kean LM, Mahoney MV, Pearson JC, et al. Contemporary pharmacotherapies for nontuberculosis mycobacterial infections: a narrative review. Infect Dis Thera. (2023) 12:343–65. doi: 10.1007/s40121-022-00750-5
36. Varley CD, Winthrop KL. Nontuberculous mycobacteria: diagnosis and therapy. Clin Chest Med. (2022) 43:89–98. doi: 10.1016/j.ccm.2021.11.007
37. Li XY, Chen H, Han XW, Peng XM Li DF, Zhou DH, et al. Unconventional treatment for an unusual cauda equina syndrome associated with nontuberculous mycobacteria after allogenic stem cell transplantation in a child with acute lymphoblastic leukemia. Pediatr Blood Cancer. (2023) 70:e29977. doi: 10.1002/pbc.29977
38. Maurer FP, Pohle P, Kernbach M, Sievert D, Hillemann D, Rupp J, et al. Differential drug susceptibility patterns of Mycobacterium chimaera and other members of the Mycobacterium avium-intracellulare complex. Clini Microbiol Infect. (2019) 25:379.e371–379.e377. doi: 10.1016/j.cmi.2018.06.010
39. Zuo Z, Zheng R, Li F, Xu A, Shi J. Coinfection of Cytomegalovirus, Pneumocystis jirovecii pneumonia, COVID-19, and Mycobacterium colombiense in an AIDS patient: a case report. Heliyon. (2024) 10:e31729. doi: 10.1016/j.heliyon.2024.e31729
40. Meireles SI, Cruz MV, Irffi GP, Testagrossa LA. Incidence of mycobacteria in pulmonary granulomatous lesions. Clinics. (2025) 80:100564. doi: 10.1016/j.clinsp.2024.100564
41. Zhou D, Xie Y, Xiao Y. Disseminated Mycobacterium colombiense infection associated with anti-interferon-γ autoantibody: a case report and literature review. Diagn Microbiol Infect Dis. (2026) 114:117087. doi: 10.1016/j.diagmicrobio.2025.117087
42. Sawaswong V, Wongjarit K, Petsong S, Yuliani Y, Somsukpiroh U, Faksri K, et al. Diversity and antimicrobial resistance profiles of Mycobacterium avium complex clinical isolates in Thailand based on whole genome comparative analysis. Sci Rep. (2025) 15:772. doi: 10.1038/s41598-024-84511-z
43. Ka Lip C, Go J, Binte Abu Bakar NA, Octavia S, Pin Lin RT, Teo JWP. Whole-genome phylogenetic analysis of Mycobacterium avium complex from clinical respiratory samples. Microbiol Spect. (2025) 13:e0160024. doi: 10.1128/spectrum.01600-24
Keywords: Mycobacterium colombiense, non-immunosuppressed host, mNGS, bronchiectasis, non-tuberculous mycobacteria, NTM
Citation: Tan J, Liu L, Wang L, Qu Y, Sun Z, Wang Q and Liu Y (2025) Case Report: First pulmonary infection caused by Mycobacterium colombiense in a non-immunosuppressed host with bronchiectasis: diagnosis facilitated by synergistic mNGS and culture. Front. Med. 12:1671968. doi: 10.3389/fmed.2025.1671968
Received: 23 July 2025; Accepted: 15 October 2025;
Published: 30 October 2025.
Edited by:
Octavio Rivero-Lezcano, Complejo Asistencial Universitario de León (CHLeon), SpainReviewed by:
Madhan Kumar, National JALMA Institute for Leprosy & Other Mycobacterial Diseases (ICMR), IndiaRamiro Lopez-Medrano, Complejo Hospitalario de León, Spain
Copyright © 2025 Tan, Liu, Wang, Qu, Sun, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Liu, bGl1eXVhbjE5ODIzMUAxNjMuY29t
†These authors have contributed equally to this work
 Jishan Tan1†
Jishan Tan1† Lu Liu
Lu Liu Yuan Liu
Yuan Liu