Abstract
Aims:
Cardiac surgery leads to major post-operative changes in metabolism, but their exact nature and the underlying risk factors remains obscure. We aimed to characterize changes in plasma metabolites after coronary artery bypass grafting (CABG) to identify intra- and post-operative risk factors for global and specific alterations in plasma metabolites post-operatively.
Methods:
We performed a targeted metabolomic screen on plasma samples from patients undergoing on-pump CABG for coronary artery disease (CAD), collected 1 day before and 1, 3, and 7 days after surgery. We assessed correlations with parameters of intra-operative course (cardiopulmonary bypass time and aortic cross-clamping time), intensive care unit (ICU) care, (length of ICU stay, duration of mechanical ventilation, duration of epinephrine/dobutamine or norepinephrine therapy), and systemic inflammation.
Results:
Out of 1,019 detectable analytes, 970 passed the quality screen and were included in the analysis. With respect to d0, the greatest degree of change in metabolite populations occurred by d1, but substantial changes persisted through d7. Metabolites could be classified into those which were predominantly downregulated (e.g., triglycerides, bile acids, cholesterol esters, lysophosphatidylcholines, indoles and derivatives), up- or downregulated (e.g., phosphatidylinositol, phosphatidylethanolamines, phosphatidic acids, ceramides), or upregulated (free fatty acids, monoglycerides). Concentrations of food- and/or microbiota-derived metabolites (indole derivatives, trimethylamine N-oxide, trigonelline) were markedly reduced, particularly on d1 and d3. Changes in metabolite concentrations correlated most strongly with plasma C-reactive protein concentration (r = −0.67 to 0.59) and blood leukocyte count (−0.63 to 0.32) and less with intra-operative (−0.62 to 0.50) and ICU care (−0.52 to 0.38) parameters. Of note, neither C-reactive protein (CRP) nor leukocyte count correlated significantly with an intra-operative or ICU parameter.
Conclusions:
These results reveal pronounced changes in plasma metabolite populations after CABG, which likely result from the combined effects of surgical and post-operative stress, systemic inflammation, reduced dietary intake, and possibly changes in gut microflora.
Introduction
Despite advances in interventional therapies and medication, coronary artery bypass grafting (CABG) is still globally considered the gold standard for clinically significant coronary artery disease (CAD) (1). This complex surgical procedure is usually conducted with cardiopulmonary bypass (CPB) using the heart-lung-machine (HLM). The major role of HLM is to support heart and lung function, maintaining blood circulation and oxygenation while heart beat is stopped for the duration of surgery. This is achieved by de-routing the blood through the tubes and membranes of the HLM (2), where intimate contact between blood components (cellular and plasmatic fractions) and the non-endothelial surfaces of CPB and a subsequent post-CPB reperfusion injury could lead to aberrant blood cellular responses, i.e., by generating reactive oxygen and nitrogen species (3). Such responses are associated with increased post-surgical systemic inflammation with a high likelihood of organ dysfunction. Furthermore, traumatic incisions made during the surgical procedure are also considered key factors for acute systemic inflammation among these patients (4). Although both traumatic surgical injury and CPB have been known to induce conventional pathological factors including complements, endotoxin release, cytokines, oxygen-free radicals, and platelet-activating factors, changes in small-molecule metabolite populations are now being increasingly appreciated as manifestations of distinct inflammatory endotypes (5).
Metabolic signatures in CAD provide rapid and systematic identification of small-molecule metabolites and distinct lipid metabolites, which are considered as “fingerprint” of an individual, at the time of sampling. Indeed, altered cellular metabolism due to an underlying atherosclerotic pathological condition in CAD has been clearly evidenced by metabolic profiling in blood-based fluids (6). Plasma lipidomics includes profiling of sterol lipids, glycerolipids, glycerophospholipids, and sphingolipids, which are considered the most abundant lipids (7). Of note, both phosphatidylcholines (PC) and lysophosphatidylcholines (LPC) are important members of glycerophospholipids, where PC are integral molecules for (i) maintaining the structure of cell membrane and (ii) circulating lipoproteins and natural surfactants, and LPC is a hydrolyzed bioactive catabolite of PC. Both molecules play crucial roles in atherosclerosis development and progression (8).
Bile acids (BAs) are emerging as key metabolites in the context of cardiovascular health and disease, where they are originally synthesized in the liver and further metabolized by gut microbiota (9). These BAs play pivotal roles in lipid metabolism, as well as in cardiovascular physiology upon signaling through the bile salt receptor [the farnesoid-X receptor (FXR)] (10). The BA-FXR signaling axis is known for its anti-atherosclerotic properties, by regulating lipid profiles and vascular tension, and thereby showing protective effects during atherogenesis (11, 12). Nevertheless, dysregulated BA levels could also contribute to cardiovascular pathologies (13). This complexity is further underscored by research linking BAs with gut microbiota in the regulation of myocardial function (14). Despite this, the exact mechanisms remain poorly understood, with some studies indicating that altered bile acid profiles, particularly the balance between primary and secondary BAs, may influence cardiac outcomes in heart disease (15). Furthermore, clinical data suggest that serum concentration of BAs is inversely correlated with the presence of CAD and myocardial infarction (16), highlighting the dual role of BAs in both protection and injury of cardiac tissue.
Likewise, tryptophan (Trp) metabolism plays a significant role in cardiovascular physiology, where kynurenine, one of the Trp catabolites which breaks down further into several biologically active metabolites (17), is now considered a biomarker for coronary artery disease (18, 19). Furthermore, Trp is also readily catabolized by intestinal microbiota to produce indole and derivatives thereof, including indoleacetic acid (3-IAA), indolealdehyde (I3A), indolepropionic acid (3-IPA), and indole sulfate (Ind-SO4) (20). Of note, indole derivatives are ligands for the aryl hydrocarbon receptor (AhR), and AhR signaling plays important roles in maintaining vascular homeostasis (21). Most of these microbial-generated indole derivatives possess beneficial roles by (i) promoting tolerance; (ii) regulating mucosal integrity and gut permeability; (iii) promoting anticoagulant properties; (iv) ameliorating atherogenesis, showing their cardioprotective effects (22). In contrast, indole sulfate accumulates as uremic toxin and therefore has a detrimental role in patients with vascular disease comorbid with chronic kidney failure (23, 24). Elevated levels of indole sulfate are involved in the pathogenesis of aortic arterial stiffness among CAD patients (25). Though many studies show a causal relationship between blood metabolites and CAD progression, there are still major gaps in knowledge in identifying prominent metabolic signatures during surgery-related acute stress. In particular, there are no longitudinal studies assessing changes in peripheral blood metabolites after on-pump CABG surgery. Metabolites with significantly altered concentrations post-operative could potentially serve to predict postsurgical adverse events and CAD outcome.
We therefore investigated the impact of cardiac surgery and the consequences of using CPB, on plasma metabolite alterations among CAD patients. Here, we analyzed CAD patients before surgery and followed them for three time points post CABG, to observe a possible link between alterations in plasma metabolites and systemic acute inflammation. Furthermore, we focused on associations between metabolic changes and several perioperative parameters including (i) CPB utilized time; (ii) aortic cross clamping (ACC) time; (iii) mechanical ventilation (MV) time; (iv) epinephrine/dobutamine (E/dob) time; and (v) norepinephrine (NE) time. The results suggest that these metabolic indicators could serve as valuable molecular tools to identify disturbances in key metabolic pathways among CAD patients who have undergone CABG.
Materials and methods
Study cohort
This prospective study was approved by the Ethics Committee of Otto-von-Guericke-University Hospital (OVGU), Magdeburg, Germany (file no. 183/19), which is in accordance with the ethical principles of the Declaration of Helsinki. After giving informed consent, CAD patients number (N) = 24 from the Department of Cardiothoracic Surgery at OVGU were enrolled in 2023, all undergoing elective on-pump CABG. Among them, n = 3 (12.5%) had two vessel disease, and n = 21 (87.5%) had three vessel disease. The study did not include single vessel CAD patients. Inclusion criteria were (i) adults >18 years of age and (ii) elective on-pump CABG. Exclusion criteria were (i) intake of immunosuppressive medication, (ii) viral hepatitis or HIV infection, and (iii) anemia with hemoglobin < 7 mmol/L. All patients underwent the same standardized protocol for anesthesia (≥8 h no food and ≥2 h no beverage intake before surgery, general anesthesia with propofol, rocuronium and sufentanil), perfusion, and surgery. All patients resumed oral food intake on post-operative day 1 except for two patients who developed a complicated clinical course.
Study design
Blood samples were collected at the following time points: 1 day before surgery (pre-operative; d0, n = 24), 1 day post-surgery (post-operative; d1, n = 24), 3 days post-surgery (d3, n = 24), and either six (n = 10) or 7 days post-surgery (d7; n = 10). The 10 d6 CAD patients were sampled on day 6 because they were to be discharged to rehabilitation later that day. A two-group comparison between day 6 and 7 post-operative showed no significant separation in the principal component analysis (PCA) based on metabolic differences, and a differential abundance analysis [fold change (FC) > |1.5|, false discovery rate (FDR) < 0.05] did not reveal any significantly changed metabolites (Supplementary Figure S1). Thus, samples from day 6 and 7 post-operative were collectively analyzed as day 7 (d7, n = 20). On day 7, metabolite data were not available for four patients as one patient died on day 6 due to sudden cardiac death after transfer to general ward, and the blood sample for metabolomic studies was not taken from the other three patients due to internal error during sample collection. A PCA based on metabolite differences between d1 and d3 vs. d0 showed that the metabolite patterns of the four patients with missing samples on d7 did not differ from those with a complete set of samples on d7 (Supplementary Figure S2), and the missing data were therefore treated as missing at random and not replaced by imputation. However metabolic data from these 4 patients for day 1 and day 3 were included in the comparisons against day 0. The data were paired, and each post-operative sample (d1, d3, d7) was compared to the pre-operative sample (d0) from the same patient.
Blood and clinical parameters
Plasma samples were obtained from venous blood and used for metabolite profiling. Blood was centrifuged to sediment cells and debris, and aliquots of supernatants were frozen at −80 °C within 2 h. Additionally, the following laboratory parameters were measured at each time point (d0, d1, d3 and d7) at the central laboratory of OVGU hospital using standard protocols: leukocyte count, C-reactive protein (CRP), alanine aminotransferase (ALAT), creatine kinase (CK), creatinine, prothrombin time, international normalized ratio (INR), and activated partial thrombin time (aPTT). Furthermore, the recorded surgical parameters comprised number of vessel bypasses, cardiopulmonary bypass time (CPB time), and ACC time. The intensive care unit (ICU) parameters that were recorded comprised length of ICU stay, time of total MV, and length of catecholamine therapy, (E/Dob time and NE time).
Targeted metabolomic profiling
Metabolite concentrations were measured on a SCIEX 5500 QTrapTM mass spectrometer (SCIEX, Darmstadt, Germany) using the MxP Quant 500XL kit (Biocrates, Life Sciences AG, Innsbruck, Austria). The kit combines flow injection analysis tandem mass spectrometry (FIA-MS/MS) for lipids and liquid chromatography tandem mass spectrometry (LC-MS/MS) using Agilent 1290 Infinity II liquid chromatography (Santa Clara, CA, USA) coupled with a tandem mass spectrometer for small molecules. Depending on the sample to be analyzed, this kit allows the quantification of up to 1,019 metabolites, where analyte classes (number of metabolites) are described as follows: alkaloids (1), amine oxides (AO; 1), amino acids (AA; 20), amino acid related molecules (AAR; 30), bile acids (BA; 14), biogenic amines (BioAm; 9), carboxylic acids (CA; 7), cresols (1), fatty acids (FA; 12), hormones and related metabolites (4), indoles and derivatives (ID; 4), nucleobases and related metabolites (2), vitamins and cofactors (1), carbohydrates and related metabolites (1), acylcarnitines (AC; 40), lysophosphatidic acids (LPA; 8), phosphatidic acids (PA; 41), lysophosphatidylcholines (LPC; 12), phosphatidylcholines (PC; 76), lysophosphatidylethanolamines (LPE; 43), phosphatidylethanolamines (PE; 95), lysophophatidylglycerols (LPG; 10), phosphatidylglycerols (PG; 64), lysophosphatidylinositols (LPI; 16), phosphatidylinositols (PI; 53), lysophosphatidylserines (LPS; 12), phosphatidylserines (PS; 18), sphinganines and sphingosines (SPBP; 8), sphinganine and sphingosine phosphates (SPBP; 8), sphingomyelines (SM; 15), ceramides (Cer; 29), dihydroceramides (8), glycosylceramides (34), cholesterol esters (CE; 22), monoglycerides (MG; 12), diglycerides (DG; 44), and triglycerides (TG; 242). Metabolite extraction and all analytical assays were conducted in accordance with the protocols provided by the manufacturer (https://biocrates.com/mxp-quant-500-xl/, accessed on 02 Mai 2024). The WebIDQ™ software tool (Biocrates Life Science AG, Innsbruck, Austria) was used for peak integration and to calculate metabolite concentrations.
Quality screen
The samples were measured on three 96 well plates. Plate-to-plate variability was addressed by target normalization [median Quality Control level 2]. At least 4 QC2 were distributed and analyzed on each plate. To assess metabolic changes as accurately as possible, all metabolites were included in the analyses except for those with a constant value across all samples in the respective comparison. 51, 49 and 52 metabolites were excluded when comparing d1 vs. d0, d3 vs. d0 and d7 vs. d0, respectively. Supplementary Table S1 shows a complete list of included metabolites in each comparison. Any concentrations that were measured below limit of detection (LOD) were replaced with the pseudovalue LOD/2.
Statistical analyses
Due to non-normal distribution of the data, nonparametric statistical tests were employed. For PCA, data were log10 transformed to correct for heteroscedasticity and auto-scaled to make all variables equally important (26). Statistical Analysis of Metabolomics Data. University of North Carolina at Charlotte; Department of Bioinformatics & Genomics. Retrieved April 21, 2024, from https://www.uab.edu/proteomics/metabolomics/workshop/2014/statistical%20analysis.pdf. Mean Euclidian distances were calculated for each metabolite class based on the first two principal components of the PCAs in the respective comparison to assess the metabolite classes with the greatest degree of change. Differential abundance analysis was performed using the Wilcoxon rank sum test to assess significance of differences between groups based on paired samples. The Benjamini-Hochberg correction was implemented with a false discovery rate (FDR) of 0.05 to account for multiple testing. For paired analysis, fold change (FC) was determined by calculating the ratio between paired samples, resulting in one FC per pair. The means of these FC values, pair means, was then computed. A FC threshold of ≥|1.5| was set for differential abundance analysis. Spearman's rank correlation coefficient was used to assess correlations. PCA, differential abundance analysis, and correlation testing were performed using the open-source software MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/). Values for standard parameters that were < LOD were replaced by LOD/2: i.e., CRP LOD = 0.6 mg/L, was replaced with 0.3 mg/L.
Results
Recruitment and characteristics of CAD patients
Demographic and clinical characteristics of the patient cohort are summarized in Tables 1, 2. There was the expected preponderance of male patients and high proportion of patients with comorbidities which confer an increased cardiac risk (diabetes, overweight, chronic obstructive pulmonary disease, COPD; Table 1). Most patients presented for bypass surgery for three or more coronary vessels, and the recorded values for bypass time, need for vasopressors, and length of ICU stay fell into the expected range. Postoperatively, there was a transient increase in creatine kinase (indicating cardiac ischemia), but renal and liver function parameters remained stable (Table 2). There were no significant sex-specific differences except a higher blood leukocyte count in women on post-operative day 3 [12.1 (10.1–14.8) vs. 9.6 (7.13–13.5), p = 0.024]. Median CRP values rose greatly, manifesting the typical peak on day 3 and subsequent decline, but the median value of 59.5 mg/L on d7 indicated ongoing systemic inflammation. In contrast, the marked leukocytosis on d1 essentially normalized by d7. Taken together, these data suggest that the patient cohort has the typical clinical characteristics of individuals presenting for elective coronary bypass graft surgery and manifests the expected postoperative evolution of inflammatory parameters.
Table 1
| Demographic characteristics | |
|---|---|
| Gender female, n (%) | 8 (33.3) |
| Age at first blood collection, years [median (range)) | 66 (49–82) |
| BMI [median (range)] | 28.2 (23.1–39.7) |
| Acute coronary syndrome, n (%) | 4 (16.7) |
| LVEF, % [median (range)] | 55 (18–65) |
| Comorbidities | |
| Diabetes mellitus (I or II), n (%) | 12 (50) |
| Smoker, n (%) | 8 (33.3) |
| COPD, n (%) | 2 (8.0) |
| Chronic kidney disease (eGFR < 90 ml/min/1.73 m2), n (%) | 18 (75) |
| Atrial fibrillation, n (%) | 4 (16.7) |
| Surgical procedure | |
| Double bypass, n (%) | 2 (8.2) |
| Triple bypass, n (%) | 11 (45.8) |
| > triple bypass, n (%) | 11 (45.8) |
| Surgical parameters | |
| Cardiopulmonary bypass time, min [median (range)] | 83.5 (46–166) |
| Aortic cross clamping time, min [median (range)] | 51 (32–81) |
| Operating time, min [median (range)] | 200 (105–315) |
Baseline and intra-operative characteristics.
Table 2
| Parameter | d0 | d1 | d3 | d7 | Reference values |
|---|---|---|---|---|---|
| Median (range) | |||||
| Leukocyte count (Gpt/L) | 7.7 (5.8–93.8) | 13.4 (5.2–17.7) | 10.7 (7.1–19) | 9 (3.2–20) | 3.9–10.4 |
| CRP (mg/L) | 1.4 (0.3–67.1) | 32.7 (13.8–114.0) | 131.3 (58.6–242.5) | 59.5 (22.7–169.1) | < 5 |
| ALAT (μmol/s.L) | 0.5 (0.3–1.0) | 0.4 (0.3–2.5) | 0.5 (0.2–1.5) | 0.6 (0.0–1.6) | 0.17–0.58 |
| Creatin kinase (μmol/L) | 2.2 (0.5–10.5) | 8.2 (3.4–74.2) | 4.8 (0.8–35.4) | 2.1 (0.6–18.0) | < 0.40 |
| Creatinine (μmol/L) | 80.5 (60.0–232.0) | 73.5 (0.4–227.0) | 78 (57.0–252.0) | 79.5 (59.0–234.0) | 45–84 |
| CKD-EPI (ml/min/1.73 m2) | 1.4 (0.4–9.3) | 1.5 (0.4–23.3) | 1.4 (0.2–1.8) | 1.3 (0.2–1.9) | ≥1.5 |
| Quick (%) | 102.0 (76.0–120.0) | 84.0 (32.3–110.0) | 87.0 (73.0–104.0) | 83.5 (74.0–96.0) | >70 |
| INR | 1.0 (0.4–10.0) | 1.1 (1.0–1.4) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | < 1.15 |
| aPTT (s) | 28.7 (25.7–34.2) | 27.8 (19.4–32.9) | 28.8 (24.6–39.7) | 29.4 (23.3–58.0) | < 34.4 |
Hematologic and clinical chemistry parameters.
Postoperative plasma metabolite profiles show pronounced shifts over time
A PCA was performed to assess global differences in metabolite populations at each of the three post-operative time points (d1, d3, d7) with respect to the preoperative samples (d0). While substantial separation was already evident on d1, it increased further by d3 and persisted through d7. However, variance shifted from PC1 to PC2 on d7, indicating that it was driven, in part, by different metabolites than at the earlier time points. At all three time points, the greatest degree of variance with respect to d0 was due to alterations in triglyceride (TG) concentrations. For instance, the 20 analytes with the greatest contribution to variance in the first component (PC1, which contributed 17%−23.5% of overall variance) were TGs at all time points, indicating a considerable degree of coregulation within this analyte class (Figures 1A–C, Supplementary Table S2). Regarding PC2, PA and PG contributed most to variance at all time points, peaking on d3 and decreasing on d7. TG impacted on PC2 only on d1. MG and LPEs contributed to variance in PC2 only on d7, largely explaining the separation between d0 and d7 samples along PC2 at this time point.
Figure 1

Global and class-specific changes in metabolite populations at the three time points post-op. A principal component analysis (PCA) was performed using 968, 970 and 967 metabolites that passed quality assessment for the comparisons d1 vs. d0, d3 vs. d0 and d7 vs. d0, respectively (Supplementary Table S1). The degree of reprogramming within individual metabolite classes was additionally assessed by Euclidian distance analysis. Total patient numbers (N) = 24 with four time points/patient, where n = 24 (d0); 24 (d1); 24 (d3); 20 (d7). (A–C) PCA; (D) Euclidian distance analysis. A complete list of Euclidian distances across all metabolite classes and the abbreviations used is contained in Supplementary Table S3.
Euclidian distance analysis supported the above notion that the greatest changes in metabolite populations occurred by d1 and d3, whereby the greatest changes were seen in LPE, PE, TG, MG, PC, PA, LPC, and bile acids (Figure 1D). The latter stood out in that there was a marked increase in variance on d1, which decreased by d3 and essentially normalized by d7. Nonetheless, several metabolite classes showed the largest Euclidian distance on d7. These were characterized by, overall, moderate Euclidian distances on d1 and d3 (e.g., LPI, FA, LPS, Cer), underscoring the differences in metabolite populations on day 7 compared to d1 and d3.
A hierarchical clustering analysis based on the 100 most differentially abundant metabolites revealed a high degree of separation between the pre- and post-operative samples at all three time points, whereby all postoperative samples clustered in their own clade only on d1 (Figure 2, Supplementary Figure S3). There was an overall tendency toward lower analyte concentrations on d1, but MGs constituted a notable exception in that concentrations of the three MG among these 100 analytes were higher post-op. Furthermore, this analysis revealed a strong coregulation of metabolites within one class, es exemplified by the clades 1–4, marked by a red circle, containing mostly BA (clade 1), TG (clade 2), PC/LPC (clade 3), and PE (clade 4). Co-regulation within metabolite classes (particularly within PS, PI, PG, PE, PA, TG, LPC, CE, BA, and AA) is also evident in a matrix based on correlations among all metabolites (Supplementary Figure S4). This analysis also revealed between-class correlations, for instance of PI with PG and PA and of TG with PC, a subgroup of LPC, and DG.
Figure 2
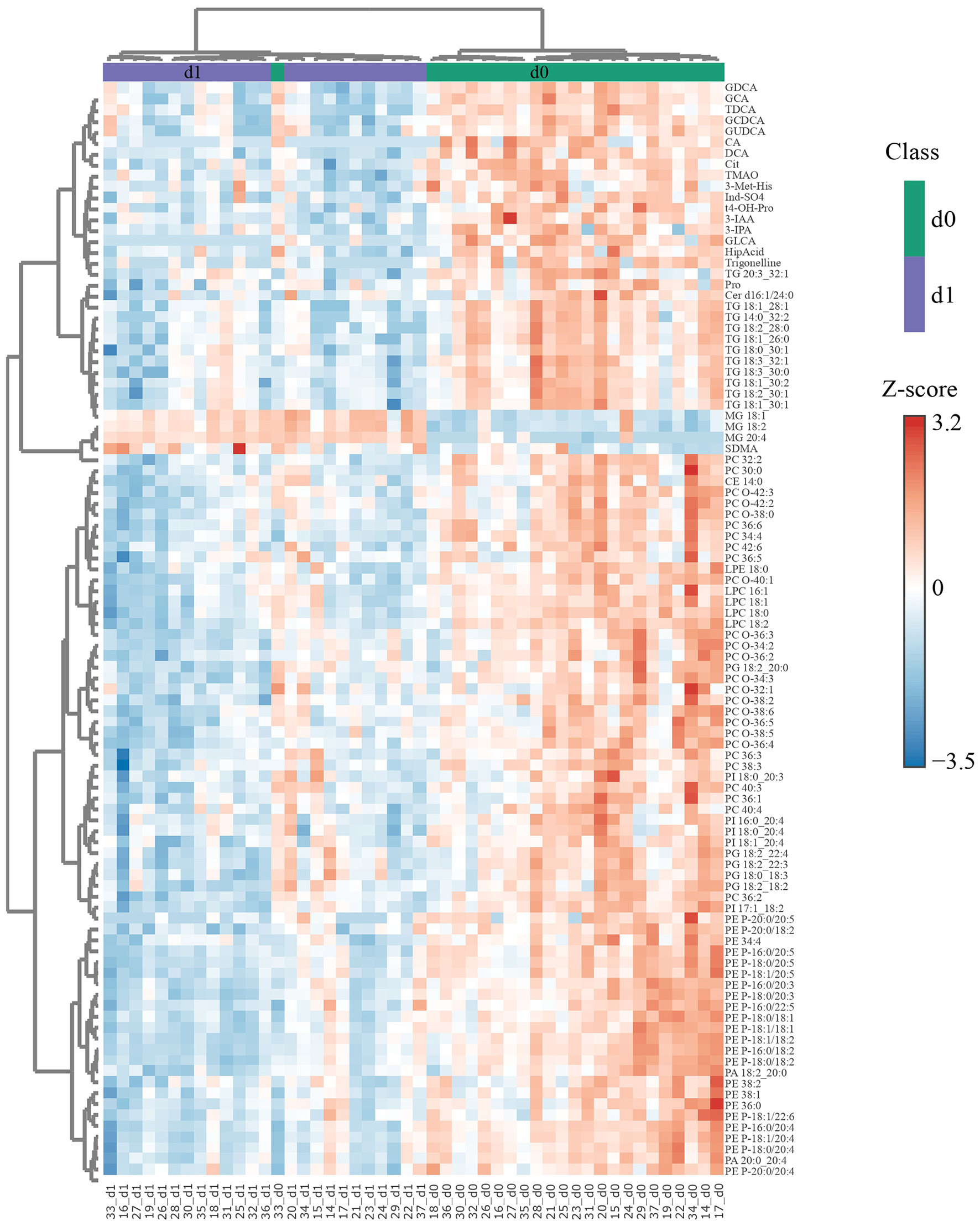
Differences in metabolite concentrations distinguish nearly perfectly between d0 and d1 samples. The 100 most significant (FDR obtained with t-test = 1.3e−9-5.86e−5) differentially abundant metabolites were selected by Euclidean distance for d1 with respect to d0 and subjected to unsupervised hierarchical biclustering analysis. Each colored cell in the heatmap corresponds to the group average concentration of the analyte with respect to the mean-centered and divided by standard deviation of the analyte (z-score). Y-axis = metabolite dendrogram, n = 24 (d0) and 24 (d1). The respective analyses for d3 vs. d0 and d7 vs. d0 are shown in Supplementary Figures S2a, b, respectively.
Two distinct kinetic patterns emerge from plasma metabolite abundance analysis
Results of a differential abundance (FC ≥|1.5|, FDR < 0.05) analysis are visualized in Figure 3 and summarized in Table 3. As seen in the volcano plots (Figures 3A–C) and Supplementary Figure S5 there was a trend toward higher p values and FC (log10) values from d1 to d7. The highest number of differentially abundant metabolites was observed on d3, but even on d7 there were still 154 differentially abundant metabolites. Overall, most of the differentially abundant metabolites were downregulated. However, the number of downregulated metabolites decreased throughout the time course, whereas the number of upregulated metabolites increased, which resulted in a slight preponderance of upregulated vs. downregulated metabolites by d7 (Table 3).
Figure 3
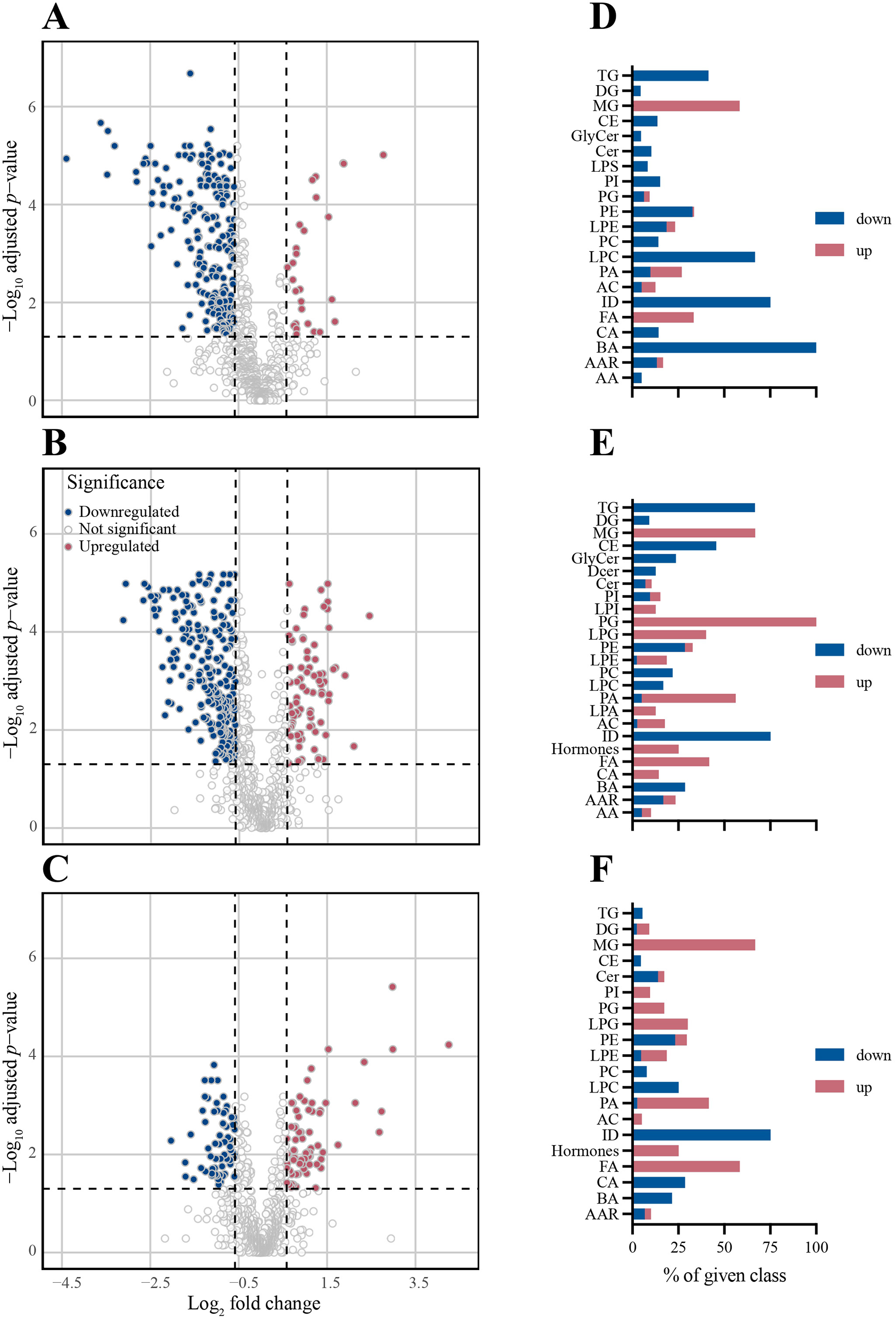
Differential abundance analysis based on the same metabolites as Figure 1 reveals a shift from down- to upregulation during post-operative recovery. Differentially abundant (FC ≥|1.5|, FDR < 0.05 Wilcoxon rank-sum test) metabolites with respect to d0 are indicated for each time point post-op. For each day, a volcano plot indicating differential abundance of individual metabolites and a bar chart indicating the direction of abundance change in each metabolite class are shown. Blue bars = downregulation; red bars = upregulation. (A, D) d1. (B, E) d3. (C, F) d7 and n = 24 (d0); 24 (d1); 24 (d3); 20 (d7).
Table 3
| Day post-op | Differentially abundant metabolites | ||
|---|---|---|---|
| Total no. | No. (%) up | No. (%) down | |
| d1 | 262 | 34 (13%) | 228 (87%) |
| d3 | 334 | 82 (25%) | 252 (75%) |
| d7 | 154 | 82 (53%) | 72 (47%) |
Number (%) of differentially abundant metabolites post-op.
Metabolite classes could be grouped into two kinetic patterns: those that were consistently downregulated (TG, BA, CE, ID) and those that were consistently upregulated (FA and MG; Figures 3D–F, 4, Supplementary Figures S6a, b). Notably, all BA were downregulated on d1 and 35.7% remained downregulated more than 1.5-fold on d3 and d7 (Figure 4B). Likewise, LPC (Supplementary Figure S6a) and ID (Figure 4E) were consistently downregulated. Downregulation of TG was most pronounced on d3 (66%), but nearly normalized by d7 (Figure 4C). Substantial persistent upregulation was observed with FA and MG (Figures 4F, G). Of the metabolite groups with few or only a single member, substantial downregulation of three metabolites was noted (Figure 4H). (i) Concentrations of the alkaloid trigenolline were very low on d1 and reached only about 25% of pre-op levels by d7. Trigenolline is found in foods, notably in coffee beans, where it makes up about 1% and can also originate from gut microflora (27). (ii) Similar downregulation was observed with trimethylamine N-oxide (TMAO), which can originate from gut microbiota or hepatic metabolism of trimethylamine. TMAO is closely related to the so called “western diet,” which is rich in choline and carnitine, and is associated with cardiovascular disease (28). (iii) 3-IAA is a Trp metabolite which is a common growth hormone in plants, but also an intermediate of Trp catabolism in humans. Thus, the initially low, but then rising plasma levels of trigenolline and TMAO likely reflect absence of oral alimentation on d1, and the gradual resumption of an oral diet thereafter. There was a mild-to-moderate increase in cortisol and consecutive decrease in dehydroepiandrosterone sulfate (DHEAS) post-operatively (indicating a physiologic stress response), whereas cortisone levels did not increase.
Figure 4
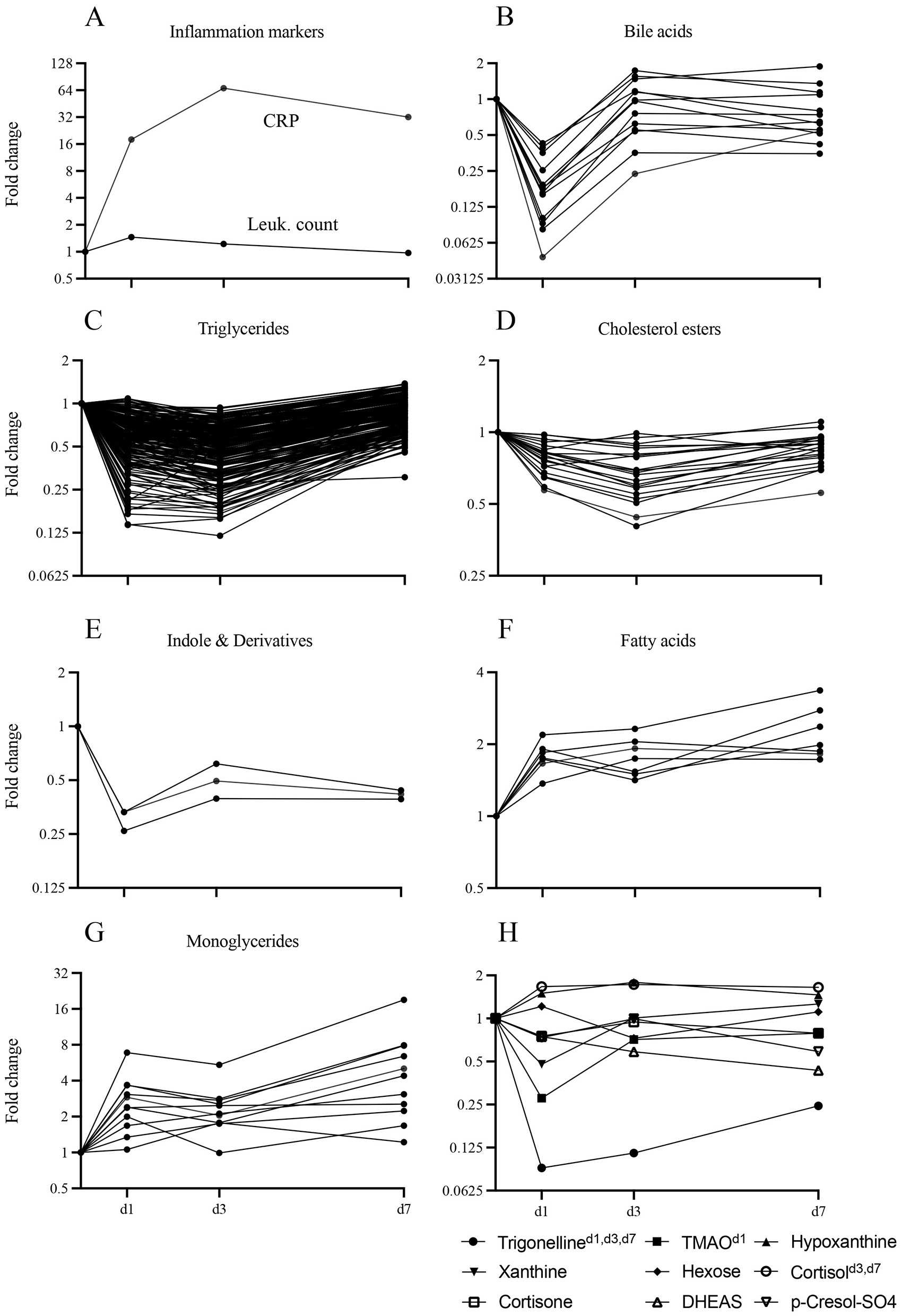
Kinetics of concentration changes in selected metabolite classes and blood inflammatory parameters throughout the time course. Concentration dynamics are expressed as fold change with respect to d0. The y-axis label “Fold change” also applies to panels (B, D, F, H). (A) Blood CRP and leukocyte count. (B–H) Plasma metabolites. (B–E) Selected predominantly downregulated metabolite classes (bile acids n = 13, triglycerides n = 238, cholesterol esters n = 22, indoles and derivatives n = 3). (F, G) Selected predominantly upregulated metabolite classes (fatty acids n = 8, monoglycerides n = 11). (H) Selected individual metabolites of interest [cortisol, cortisone, dehydroepiandrosterone sulfate (DHEAS), hexoses, hypoxanthine, p-cresol-SO4, trigonelline, trimethylamine N-oxide (TMAO)]. Days on which fold change was significant with respect to d0 are indicated as superscripts. n = 24 (d0); 24 (d1); 24 (d3); 20 (d7).
Correlations between metabolites and clinical parameters reflects strong systemic inflammation
We then aimed to identify clinical parameters that are most closely associated with changes in metabolite abundance and can be considered the main drivers of the marked metabolic alterations post-op. Among the intra-operative variables, the most significant correlations with metabolites at all three time points were observed for NE time, CPB time, and E/Dob time (Figure 5A, Supplementary Figure S8, Table 4). However, most significant correlation coefficients were of only modest strength, ranging between 0.25–0.5. On the other hand, substantially stronger correlations were observed between the markers of inflammation (CRP and leucocyte count) and metabolites, indicating that systemic inflammation is a strong, but certainly not the only, driver of the observed metabolite alterations. We then tested whether we could identify intra-operative parameters responsible for the observed systemic inflammation. However, the only significant correlation with inflammation was between ALAT and leukocytes (r= −0.41, p = 0.0006; Figure 5B). Furthermore, Spearman rank correlation analysis revealed a significant association between ICU stay and a single metabolite (PI 16:1_18:2; ρ = −0.72, FDR = 0.033) on postoperative day 3. No consistent associations were found between metabolites and ICU stay at other time points (data not shown).
Figure 5

Correlations between intra-operative parameters, inflammation markers, and metabolites, based on Spearman rank coefficients. (A) Correlation with metabolites, pooled analysis of data from all three time points combined. Each circle indicates one metabolite. Labeled metabolites represent the ones with highest correlation coefficient. Gray fill color = p > 0.05. (B) Correlations among intra-operative parameters (n = 5), inflammation markers (n = 2), and clinical chemistry parameters (n = 6). * = p < 0.05. p values based on FDR obtained with t-test. n = 24 (d0); 24 (d1); 24 (d3); 20 (d7).
Table 4
| Parametera | d1 | d3 | d7 | Sum |
|---|---|---|---|---|
| CRP | 152 | 32 | 54 | 238 |
| Leukocyte count | 66 | 123 | 29 | 218 |
| E/Dob time | 72 | 63 | 46 | 181 |
| NE time | 85 | 37 | 46 | 168 |
| CPB time | 56 | 64 | 47 | 167 |
| MV time | 53 | 21 | 27 | 101 |
| ACC time | 34 | 20 | 27 | 81 |
| All | 518 | 360 | 276 | 1,154 |
Number of significant correlations (p ≤ 0.05) between metabolites and clinical parameters.
aArranged in descending order according to sum of significant correlations.
CRP, C-reactive protein; E, epinephrine; Dob, dobutamine; NE, norepinephrine; CPB, cardiopulmonary bypass; MV, mechanical ventilation; ACC, aortic cross clamping.
Discussion
The use of targeted metabolomics to profile longitudinal plasma metabolite changes in CAD patients undergoing CABG fills an important gap in the literature as our study is the first to show dynamic alterations of plasma metabolite changes across four clinically meaningful timepoints, spanning both acute and recovery phase after CABG, offering new insight into the trajectory of metabolic recovery and the influence of surgical and inflammatory stress. This distinguishing it from similar studies (29, 30), as most prior studies have either focused on static metabolic profiles in chronic CAD or have lacked a time-resolved approach. Although changes in metabolites were evident during the acute phase and normalized during the recovery phase, intriguingly, there were few metabolite classes, belonging to the lipid, bile acid, and Trp derivatives, which exhibited a steady kinetic pattern throughout our investigations. Notably, plasma TG levels were significantly downregulated and were associated with increases in both MG and FA, suggesting perioperative modulation in lipid metabolism. Such alterations in metabolites could occur due to several plausible factors, including surgical trauma-associated inflammatory milieu, fasting, catecholamine-induced lipolysis, and heparin treatment. Of note, disturbances in gut microbiota community also play a significant role in inducing changes in metabolite profiles (31).
Increased systemic inflammation is a well-known consequence of CABG, in the process of which macrophages with inflammatoryhigh phenotype tend to accumulate plasma-circulating TG, by either endocytosis or receptor-mediated internalization (32). Such TG-laden inflammatory macrophages exhibit reduced capacity for lipolysis, which further lowers the inflammatory attributes of macrophages associated with increased mitochondrial dysfunction, decreased ability to polarize, migrate (33), and phagocytose, and reduced prostaglandin E2 production (34). This suggests a negative feedback loop to minimize the inflammatory state of these macrophages upon intracellular TG accumulation and could explain the reduced post-operative plasma TG levels. On the other hand, TG, CE, and PC accumulate in intracellular lipid droplets as cells respond to stress (34–36). In our cohort, surgery-induced acute stress could promote lipid droplet turnover to compensate the energy and redox imbalances, where TG are constantly being hydrolyzed (via lipolysis or lipophagy) to convert into free FA and MG (37), as reflected by their consistent upregulation in postoperative CAD circulation. Of note, CE-mediated lipid droplet nucleation is facilitated by these intracellular accumulated TG, since reduced plasma CE were also evident in our cohort (38). Our findings align with those of Ding et al. (39) who reported a significant reduction in specific cholesterol ester species (CE18:0, CE16:0) in patients developing sepsis following cardiac surgery with CPB. Their lipidomic analysis aimed to characterize the molecular signature of sepsis in this context. However, interpretation is limited by the use of sequential organ failure assessment (SOFA) ≥2 as a sepsis criterion, which may not reliably distinguish infection from postoperative inflammation, and by the heterogeneity of the cohort, including prolonged CPB durations and ICU stays (39). Despite these limitations, the lipidomic alterations reported by Su et al. are consistent with our data and support the notion that CABG-associated inflammation and physiological stress drive shifts in plasma CE levels, contributing to disrupted postoperative lipid homeostasis. Another contributing factor is the state of fasting, where the entire patient cohort remained fasting ≥6 h prior to surgery until an oral diet was resumed on the first postoperative day. In addition to cardiac surgery stress (40), this nutrient stress also triggers the sympathetic nervous system (41) to secrete catecholamines (norepinephrine) that activate lipolysis to satisfy the cellular energy demand, by promoting the breakdown of stored TG into FA and MG.
Furthermore, every patient received high-dose intravenous (IV) unfractionated heparin (400 IU/kg body weight) before commencement of extracorporeal circulation, followed by low-dose prophylactic therapy until discharge. Since IV administered heparin rapidly increases lipid hydrolyzing enzymes (lipoprotein lipase), there could be a massive hydrolysis of TG into FA and MG (42). Likewise, the entire patient cohort were uniformly treated with opioids, as part of peri- and post-operative pain management medications. Opioid could be regarded as a significant driving factor for changing cellular metabolism (43). In this context it is plausible that opioid administration may contribute to altered lipid metabolism, promoting lipolytic activity. This hypothetical mechanism could, in part, facilitate the mobilization of FA (44) and MG from intracellular TG stores in response to increased cellular energy demands (45).
PC, as predominant class of plasma lipids, was consistently decreased in circulation, possibly due to their role in lipid droplet biogenesis (46) for high-energy supply during surgery-induced inflammation. Another explanation could be PC uptake via the CD36 scavenger receptor, increasing the cellular PC pool in differentiating macrophages, which may accelerate PC turnover and amplify inflammation (47). Additionally, monocytes, as macrophage precursors, feature a higher intracellular PC content, particularly with aging (48). These concepts hold true in our cohort settings that are representative of an aged population together with ongoing post-surgical inflammation. We therefore propose that an increased demand for intracellular PC content might reflect decreased circulating PC in the blood stream. A study of plasma lipid changes in community-acquired pneumonia (CAP) reported lower PC concentrations during acute presentation, which normalized with clinical recovery (49). These results agree well with ours, as acute CAP features pronounced systemic inflammation of a similar magnitude as our cohort. Interestingly, a reduced plasma PC/PE ratio has led to increased levels of NLRP3, an integral component of the inflammasome activation pathway, which promoted changes in left ventricle geometry among CAD
patients with insulin resistance (50). Since 50% of our CAD patients have diabetes as comorbidity, a notable decrease in plasma PC/PE ratio could possibly pose an increased risk to these patients in terms of cardiac remodeling. Similar to our findings, plasma levels of PC were also reduced in patients with other chronic inflammatory diseases, such as Alzheimer's disease (51) and diabetic nephropathy (52), where both plasma PC and PC-Os were inversely associated with renal-related clinical covariates [estimated glomerular filtration rate (eGFR) and Albuminuria], end-stage renal disease (ESRD) and all-cause mortality in diabetic nephropathy (52). Sometimes, choline-deficient diet could also explain the reasons behind a sudden drop in plasma PC levels (53, 54) among CAD patients. However, a significant decline in plasma lipids could explain an ongoing catabolism due to acute stress (55), induced by surgical trauma. Further, these circulating PC might have sequestered by binding to structurally-altered CRP (56) due to massive inflammation. Since PC is further cleaved by phospholipase A2 activity to generate LPC (8), we noticed substantially reduced plasma LPC in CAD patients, which could reflect a limited availability of its precursor molecule, PC, in the circulation. Similar to our findings, plasma LPC levels were decreased among patients with obesity and diabetes (57) as well as Alzheimer's patients with chronic inflammation (58). Since LPC act as a carrier molecule for polyunsaturated fatty acids (PUFAs) (58), a deficit of plasma LPC pool might limit supply of PUFA to vascular health, causing vascular dysfunction (59), increased inflammation and all-cause mortality (60).
Several reports have shown alterations in gut microbiota composition following cardiac surgery together with CPB usage (61, 62). Such gut dysbiosis led to decreased LPC generation which was, in particular, associated with reduced colonization of Bacteriodes population, as investigated in chronic pathologies such as Alzheimer's disease (63). Furthermore, we speculate that there was increased ferroptosis with decreased plasma LPC (63) in our patient cohort, as iron accumulation and lipid peroxidation have been reported following cardiac surgical procedures, including the usage of CPB (64, 65). However, the notion that ferroptosis directly mediates LPC depletion remains hypothetical and warrants further investigation in the context of our CAD cohort. In line with the above-mentioned beneficial role of PC and LPC, our study shows a negative correlation between PC and ether-linked phosphatidylcholine (PC-Os) as well as LPC and acute inflammatory markers (CRP and leucocyte count) and the use of CPB during surgery, highlighting a possible role of PC and LPC in regulating ongoing inflammation.
Changes in plasma bile acids were also noticed in our CAD cohort, where BA metabolites were consistently downregulated, especially on postoperative day 1 and 3. This could reflect limited generation of BA metabolites due to gut dysbiosis (66), which was also evident in patients with other chronic inflammation like ulcerative colitis (66). Hence, to this extent, there is a high likelihood for a surgical trauma-induced gut dysbiosis in decreasing circulating BA metabolites postoperatively CAD. In fact, increased BA concentrations in the circulation have been reported to modulate blood monocyte function upon specific activation of bile acid receptor (TGR5). These monocytes exhibit decreased phagocytic capacity and reduced production of pro-inflammatory cytokines (67) during bacterial infection. Nevertheless, bile receptor TGR5 agonism induces nitric oxide (NO) production and hampers the adhesion of circulating monocyte to vascular endothelium (67). In line with these reports, we supposed whether bile acid synthesis was reduced or their bioavailability was diminished due to their consumption at cell-expressed receptor binding sites in our cohort. But, in our previous study (68), we demonstrated IL-6high producing blood mononuclear cells among postoperative CAD patients, explaining the existence of functional monocytes and therefore, the possibility for decreased BA synthesis. Thus, a deficit in bile acid production could pose an increased risk for amplified inflammation in our postoperative cohort. Intriguingly, BA was positively correlated with CRP in these patients, suggesting a counteracting role of bile acids to reduce ongoing inflammation. This notion was supported by a report showing that increased CRP levels in lipopolysaccharide-induced endotoxemia among healthy volunteers correlate with a rise in total BA concentration (69) where these increased circulating BA metabolites exhibited immunosuppressive properties. However, a functional relationship between CRP and circulating BA would be interesting to investigate in our CAD cohort. Interestingly, a study on the effect of fasting and refeeding on plasma BA metabolites in mice demonstrated that both primary and secondary BA were decreased during fasting and this condition was reversed upon refeeding. These alterations were largely associated with decreased abundance of BA-metabolizing Lactobacillus and Bifidobacterium in gut. In addition, the genes involved in hepatic BA synthesis such as cholesterol 7 alpha-hydroxylase (CYP7A1), Oxysterol 7 alpha-hydroxylase (CYP7B1), and aldo-keto reductase family 1 member d 1 (AKR1D1) were repressed during the fasting state. However, BA metabolizing microbiotas as well as hepatic BA synthesizing genes were expressed during re-feeding (70). A similar mechanism might underlie the reduced plasma BA levels in our CAD patients, who were also in the state of fasting and gradual refeeding during the peri-and post-operative period. Of note, multiomic study involving metabolomics as well as metagenomics.
Next, a consistent downregulated pattern of indole derivatives (indole, 3-IAA, 3-IPA and Ind-SO4) was noticed. These molecules are generated as Trp catabolites by the gut microbial community (22), are absorbed by intestinal epithelium and enter the blood stream. They are particularly known to fortify intestinal epithelial barrier function, by increasing the expression of tight junctional proteins, and thereby to prevent leaky gut (22). Post-surgical gut microbial dysbiosis could explain the reason for reduced plasma indole derivatives in our cohort. To support our findings, significantly reduced levels of indole derivatives, in particular 3-IAA and 3-IPA, have been reported in patients with severe atherosclerosis (71), indicating their anti-inflammatory role (72). Additionally, 3-IPA is known to enhance systemic homeostasis with the gut-multiorgan axis during acute systemic inflammation (73). Although several indole derivatives promote inflammation-attenuating host responses, indole sulfate is considered to be the notable exception, which is a uremic toxin and accumulates in serum of chronic kidney disease patients (74). In line with this and despite reduced levels of indole sulfate in our cohort, a positive correlation between indole sulfate and CPB time was noted, reflecting its possible role in oxidative stress. In addition to this, another Trp catabolite (kynurenine) and trigonelline were positively associated with CPB time. Due to their immune suppressive qualities, both kynurenine and trigonelline could be involved in regulating the stressful inflammatory milieu during cardiac surgery. However, specific functions of these metabolites should be investigated. Besides, growing evidence suggests that gut microbiota can translocate following intestinal ischemia-reperfusion injury during surgery, leading to disruptions in host microbial ecology. To explore this, a study protocol has been developed to investigate microbial and metabolic profiles in patients with CAD undergoing CABG, aiming to understand fever of unknown origin and to improve sepsis stratification (75). This integrative metagenomic and metabolomic approach seeks to clarify how gut microbial shifts drive systemic metabolic changes and postoperative fever (75). This further highlights that reduced microbial diversity and their translocation may increase sepsis risk in this CAD population.
Lastly, the observation that inflammatory markers correlated more strongly with metabolite changes than intraoperative parameters supports the notion that systemic inflammation, rather than the surgical procedure itself, is the primary driver of metabolic disruption, which has potential implications for anti-inflammatory strategies in postoperative care. Henceforth, this study reveals time-resolved post-surgical changes in plasma metabolites that are tightly associated with surgical stress, inflammation, and gut dysbiosis, among CAD patients. Decreased PC, LPC, and CE, alongside increased FA and MG, indicate catabolic stress, mitochondrial dysfunction, and lipolysis, possibly in patients with type 2 diabetes mellitus (T2DM) or obesity. These identified metabolite trajectories could serve as molecular signatures for identifying patients at high risk of adverse perioperative outcomes. As clinical interventions, pre-operative and early postoperative nutritional support, particularly choline-enriched diets, may attenuate the observed declines in plasma PC and LPC, supporting vascular and metabolic homeostasis (76) and potentially improving patient recovery trajectories. Furthermore, sustained decreases in indole derivatives and bile acids suggest gut barrier dysfunction and microbial dysbiosis. Targeted, time-specific administration of probiotics or synbiotics, particularly those promoting Lactobacillus and Bifidobacterium, may restore microbial-derived metabolites (BA, indoles), modulate host inflammatory responses, and support intestinal barrier integrity (77). Further development of a perioperative metabolic risk score, intergrating lipidomic and microbiota-related metabolite markers (e.g., PC, LPC, FA, BA, indole derivatives), may enable improved identification of patients needing intensive monitoring, targeted nutritional support, or anti-inflammatory therapies.
However, this study has several limitations, including a relatively small sample size and missing data for four patients, particularly on day 7, which may reduce statistical power and increase the risk of type I/II errors. Due to the lack of experimental mechanistic link for the observed metabolites, our findings should be considered hypothesis-generating rather than conclusive. Therefore, both functional as well as validation studies in a larger CAD population with similar clinical characteristics are warranted to confirm these metabolic signatures to enhance the robustness of our findings and to intergrate them into a predictive algorithms or point-of-care testing risk assessments.
Conclusions
Taken together, our current study has demonstrated discrete changes in plasma metabolite populations during the postoperative period. Although we did not observe any distinct pattern of metabolite alterations observed with CPB usage, we presume that systemic acute inflammation, rather than surgical procedure itself, plays a significant role in aberrant regulation of metabolites. According to the observation, an excessive generation of free fatty acids in our cohort could pose an increased severity to coronary atherosclerosis (78), especially among those who are comorbid with T2DM, and obesity (79), by inducing endothelial dysfunctions and defects in insulin signaling (80) and sensitivity (81). Further PC supplementation as prophylactic measures might be an ideal option for CAD patients prior to cardiac surgery in order to compensate for reduced postoperative PC and LPC levels. A targeted investigation is needed to obtain data on gut microbial communities in CAD patients before and after surgery, especially during fasting and refeeding. A better understanding of depleted microbial taxa is essential to enrich the surgery-scheduled CAD patients with probiotics containing the target microbiota. Such microbial recolonization might benefit these patients to regulate ongoing acute inflammation by maintaining the levels of BA and indole derivatives, which were hampered in postoperative CAD patients. Hence our findings on biomarker potential of these metabolic signatures could ultimately lead to the discovery of biomarkers which will aid clinical decision making regarding perioperative care, nutritional supports strategies or individualized recovery monitoring among CAD patients after CABG surgery.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This prospective study was approved by the Ethics Committee of Otto-von-Guericke-University Hospital (OvGU; file no. 183/19), in Magdeburg, Germany. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MW: Conceptualization, Formal analysis, Methodology, Writing – original draft. SS: Data curation, Formal analysis, Writing – review & editing. SV: Methodology, Visualization, Writing – review & editing. GA: Methodology, Writing – review & editing. FW: Data curation, Formal analysis, Writing – review & editing. JW: Writing – review & editing. FP: Conceptualization, Funding acquisition, Project administration, Software, Supervision, Visualization, Writing – review & editing. PV: Conceptualization, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Frank Pessler is supported by internal funds of the Helmholtz Centre for Infection Research, Braunschweig, Germany. Frieder Neu is supported by Latvian State Scholarship, No.4.-10.3/3470. Max Wacker and Priya Veluswamy are supported by internal research funds of the Otto-von-Guericke University Hospital, Magdeburg, Germany.
Acknowledgments
We extend our thanks to Ms. Elena Denks for her excellent technical assistance, Esther Meyer for collecting patient blood and organizing screening for study participants. This paper has been uploaded to MedRxiv server as a preprint medRxiv 2025.04.11.25325708; doi: 10.1101/2025.04.11.25325708.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1673132/full#supplementary-material
Abbreviations
ALAT, alanine aminotransferase; BA, bile acids; CAD, coronary artery disease; CABG, coronary artery bypass graft; CE, cholesterol esters; CPB, cardiopulmonary bypass; CRP, C-reactive protein; E/Dob, epinephrine/dobutamine; FA, fatty acid; HLM, heart lung machine; ICU, intensive care unit; ID, indole derivatives; LOD, limit of detection; LPC, lyso phosphatidylcholine; MG, monoglycerides; MV, mean ventilation time; NE, norepinephrine; PCA, principal component analysis; PC, phosphatidylcholine; PUFA, polyunsaturated fatty acids; TG, triglycerides; TMAO, trimethylamine N-oxide; TRP, tryptophan; 3-IAA, 3-indole acetic acid; 3-IPA, 3-indole propionic acid.
References
1.
Caldonazo T Kirov H Riedel LL Gaudino M Doenst T . Comparing CABG and PCI across the globe based on current regional registry evidence. Sci Rep. (2022) 12:22164. 10.1038/s41598-022-25853-4
2.
Zamzami B Alghamdi AA . Cardiopulmonary bypass. In:YelbuzTMBin-MoallimMAHusainWJMAlakeelYSKabbaniMSAlghamdiAA, editors. Manual of Pediatric Cardiac Care: Volume II. Singapore: Springer Nature (2024). p. 33–5. 10.1007/978-981-99-5683-8_7
3.
Lesouhaitier M Belicard F Tadié J-M . Cardiopulmonary bypass and VA-ECMO induced immune dysfunction: common features and differences, a narrative review. Crit Care. (2024) 28:300. 10.1186/s13054-024-05058-z
4.
Ni Choileain N Redmond HP . Cell response to surgery. Arch Surg. (2006) 141:1132–40. 10.1001/archsurg.141.11.1132
5.
Viswan A Ghosh P Gupta D Azim A Sinha N . Distinct metabolic endotype mirroring acute respiratory distress syndrome (ARDS) subphenotype and its heterogeneous biology. Sci Rep. (2019) 9:2108. 10.1038/s41598-019-39017-4
6.
Zhu Q Wu Y Mai J Guo G Meng J Fang X et al . Comprehensive metabolic profiling of inflammation indicated key roles of glycerophospholipid and arginine metabolism in coronary artery disease. Front Immunol. (2022) 13:829425. 10.3389/fimmu.2022.829425
7.
Quehenberger O Armando AM Brown AH Milne SB Myers DS Merrill AH et al . Lipidomics reveals a remarkable diversity of lipids in human plasma1[S]. J Lipid Res. (2010) 51:3299–305. 10.1194/jlr.M009449
8.
Law SH Chan ML Marathe GK Parveen F Chen CH Ke LY . An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci. (2019) 20:1149. 10.3390/ijms20051149
9.
Wahlström A Sayin SI Marschall HU Bäckhed F . Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. 10.1016/j.cmet.2016.05.005
10.
Fleishman JS Kumar S . Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. (2024) 9:97. 10.1038/s41392-024-01811-6
11.
Li YTY Swales KE Thomas GJ Warner TD Bishop-Bailey D . Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. (2007) 27:2606–11. 10.1161/ATVBAHA.107.152694
12.
Porez G Prawitt J Gross B Staels B . Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease: thematic review series: new lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J Lipid Res. (2012) 53:1723–37. 10.1194/jlr.R024794
13.
de Vries HD Eijgenraam TR Bloks VW Mulder NL van Zutphen T Silljé HHW et al . Elevated plasma bile acids coincide with cardiac stress and inflammation in young Cyp2c70–/– mice. Pediatr Res. (2024) 97:2145–52. 10.1038/s41390-024-03596-4>
14.
Yntema T Koonen DPY Kuipers F . Emerging roles of gut microbial modulation of bile acid composition in the etiology of cardiovascular diseases. Nutrients. (2023) 15:1850. 10.3390/nu15081850
15.
Mayerhofer CCK Ueland T Broch K Vincent RP Cross GF Dahl CP et al . Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. (2017) 23:666–71. 10.1016/j.cardfail.2017.06.007
16.
Feng X Zhai G Yang J Liu Y Zhou Y Guo Q . Myocardial infarction and coronary artery disease in menopausal women with type 2 diabetes mellitus negatively correlate with total serum bile acids. Front Endocrinol. (2021) 12:754006. 10.3389/fendo.2021.754006
17.
Jrad-Lamine A Henry-Berger J Gourbeyre P Damon-Soubeyrand C Lenoir A Combaret L et al . Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J Biol Chem. (2011) 286:8030–42. 10.1074/jbc.M110.172114
18.
Pedersen ER Svingen GF Schartum-Hansen H Ueland PM Ebbing M Nordrehaug JE et al . Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J. (2013) 34:2689–96. 10.1093/eurheartj/eht264
19.
Zuo H Ueland PM Ulvik A Eussen SJ Vollset SE Nygård O et al . Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: the hordaland health study. Am J Epidemiol. (2016) 183:249–58. 10.1093/aje/kwv242
20.
Sinha AK Laursen MF Brinck JE Rybtke ML Hjørne AP Procházková N et al . Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat Microbiol. (2024) 9:1964–78. 10.1038/s41564-024-01737-3
21.
Veluswamy P Wippermann J Wacker M . Feeding the vasculature with cruciferous vegetables: a secret for organ protection. Signal Transduct Target Ther. (2024) 9:36. 10.1038/s41392-024-01747-x
22.
Ye X Li H Anjum K Zhong X Miao S Zheng G et al . Dual role of indoles derived from intestinal microbiota on human health. Front Immunol. (2022) 13:903526. 10.3389/fimmu.2022.903526
23.
Gao H Liu S . Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci. (2017) 185:23–9. 10.1016/j.lfs.2017.07.027
24.
Barreto FC Barreto DV Liabeuf S Meert N Glorieux G Temmar M et al . Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol Oct. (2009) 4:1551–8. 10.2215/CJN.03980609
25.
Lin T-J Hsu B-G Wang J-H Lai Y-H Dongoran RA Liu C-H . Serum indoxyl sulfate as a potential biomarker of aortic arterial stiffness in coronary artery disease. Nutr Metab Cardiovasc Dis. (2020) 30:2320–7. 10.1016/j.numecd.2020.07.035
26.
van den Berg RA Hoefsloot HC Westerhuis JA Smilde AK van der Werf MJ . Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. (2006) 7:142. 10.1186/1471-2164-7-142
27.
Li KJ Brouwer-Brolsma EM Burton-Pimentel KJ Vergeres G Feskens EJM . A systematic review to identify biomarkers of intake for fermented food products. Genes Nutr. (2021) 16:5. 10.1186/s12263-021-00686-4
28.
Wang Z Kaplan RC Burk RD Qi Q . The oral microbiota, microbial metabolites, and immuno-inflammatory mechanisms in cardiovascular disease. Int J Mol Sci. (2024) 25:12337. 10.3390/ijms252212337
29.
Kirov H Schwarzer M Neugebauer S Faerber G Diab M Doenst T . Metabolomic profiling in patients undergoing off-pump or on-pump coronary artery bypass surgery. BMC Cardiovasc Disord. (2017) 17:93. 10.1186/s12872-017-0518-1
30.
Bojko B Wasowicz M Pawliszyn J . Metabolic profiling of plasma from cardiac surgical patients concurrently administered with tranexamic acid: DI-SPME-LC-MS analysis. J Pharm Anal. (2014) 4:6–13. 10.1016/j.jpha.2013.03.002
31.
Hou K Wu ZX Chen XY Wang JQ Zhang D Xiao C et al . Microbiota in health and diseases. Signal Transduct Target Ther. (2022) 7:135. 10.1038/s41392-022-00974-4
32.
Deng L Kersten S Stienstra R . Triacylglycerol uptake and handling by macrophages: from fatty acids to lipoproteins. Prog Lipid Res. (2023) 92:101250. 10.1016/j.plipres.2023.101250
33.
Aflaki E Balenga NA Luschnig-Schratl P Wolinski H Povoden S Chandak PG et al . Impaired rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis. Cell Mol Life Sci. (2011) 68:3933–47. 10.1007/s00018-011-0688-4
34.
van Dierendonck XAMH Vrieling F Smeehuijzen L Deng L Boogaard JP Croes CA et al . Triglyceride breakdown from lipid droplets regulates the inflammatory response in macrophages. Proc Natl Acad Sci U S A. (2022) 119:e2114739119. 10.1073/pnas.2114739119
35.
Goldberg IJ Reue K Abumrad NA Bickel PE Cohen S Fisher EA et al . Deciphering the role of lipid droplets in cardiovascular disease. Circulation. (2018) 138:305–15. 10.1161/CIRCULATIONAHA.118.033704
36.
Kuo A Lee MY Sessa WC . Lipid droplet biogenesis and function in the endothelium. Circ Res. (2017) 120:1289–97. 10.1161/CIRCRESAHA.116.310498
37.
Borgström B Tryding N Westöö G . On the extent of hydrolysis of triglyceride ester bonds in the lumen of human small intestine during digestion. Acta Physiol Scand. (1957) 40:241–7. 10.1111/j.1748-1716.1957.tb01493.x
38.
Dumesnil C Vanharanta L Prasanna X Omrane M Carpentier M Bhapkar A et al . Cholesterol esters form supercooled lipid droplets whose nucleation is facilitated by triacylglycerols. Nat Commun. (2023) 14:915. 10.1038/s41467-023-36375-6
39.
Ding W Xu S Zhou B Zhou R Liu P Hui X et al . Dynamic plasma lipidomic analysis revealed cholesterol ester and amides associated with sepsis development in critically ill patients after cardiovascular surgery with cardiopulmonary bypass. J Pers Med. (2022) 12:1838. 10.3390/jpm12111838
40.
Engelman RM Haag B Lemeshow S Angelo A Rousou JH . Mechanism of plasma catecholamine increases during coronary artery bypass and valve procedures. J Thorac Cardiovasc Surg. (1983) 86:608–15. 10.1016/S0022-5223(19)39130-5
41.
Landsberg L Young JB . Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. (1978) 298:1295–301. 10.1056/NEJM197806082982306
42.
Yang JY Kim TK Koo BS Park BH Park JW . Change of plasma lipoproteins by heparin-released lipoprotein lipase. Exp Mol Med. (1999) 31:60–4. 10.1038/emm.1999.10
43.
Tarazi D Maynes JT . Impact of opioids on cellular metabolism: implications for metabolic pathways involved in cancer. Pharmaceutics. (2023) 15:2225. 10.3390/pharmaceutics15092225
44.
Wong SC Yeung YG Yeung D . Acute and chronic effects of morphine on lipolysis in rat epididymal fat pads. Biochem Pharmacol. (1977) 26:143–7. 10.1016/0006-2952(77)90387-2
45.
Cone AL Wu KK Kravitz AV Norris AJ . Kappa opioid receptor activation increases thermogenic energy expenditure which drives increased feeding. iScience. (2023) 26:107241. 10.1016/j.isci.2023.107241
46.
Krahmer N Guo Y Wilfling F Hilger M Lingrell S Heger K et al . Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP: phosphocholine cytidylyltransferase. Cell Metab. (2011) 14:504–15. 10.1016/j.cmet.2011.07.013
47.
Petkevicius K Virtue S Bidault G Jenkins B Çubuk C Morgantini C et al . Accelerated phosphatidylcholine turnover in macrophages promotes adipose tissue inflammation in obesity. Elife. (2019) 8:e47990. 10.7554/eLife.47990
48.
Saare M Tserel L Haljasmägi L Taalberg E Peet N Eimre M et al . Monocytes present age-related changes in phospholipid concentration and decreased energy metabolism. Aging Cell. (2020) 19:e13127. 10.1111/acel.13127
49.
Arshad H Alfonso JCL Franke R Michaelis K Araujo L Habib A et al . Decreased plasma phospholipid concentrations and increased acid sphingomyelinase activity are accurate biomarkers for community-acquired pneumonia. J Transl Med. (2019) 17:365. 10.1186/s12967-019-2112-z
50.
Vianello E Ambrogi F Kalousová M Badalyan J Dozio E Tacchini L et al . Circulating perturbation of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) is associated to cardiac remodeling and NLRP3 inflammasome in cardiovascular patients with insulin resistance risk. Exp Mol Pathol. (2024) 137:104895. 10.1016/j.yexmp.2024.104895
51.
Whiley L Sen A Heaton J Proitsi P García-Gómez D Leung R et al . Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging. (2014) 35:271–8. 10.1016/j.neurobiolaging.2013.08.001
52.
Tofte N Suvitaival T Ahonen L Winther SA Theilade S Frimodt-Møller M et al . Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci Rep. (2019) 9:16398. 10.1038/s41598-019-52916-w
53.
da Costa KA Niculescu MD Craciunescu CN Fischer LM Zeisel SH . Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. (2006) 84:88–94. 10.1093/ajcn/84.1.88
54.
Zeisel SH Costa KD Franklin PD Alexander EA Lamont JT Sheard NF et al . Choline, an essential nutrient for humans. FASEB J. (1991) 5:2093–8. 10.1096/fasebj.5.7.2010061
55.
Wu J Cyr A Gruen DS Lovelace TC Benos PV Das J et al . Lipidomic signatures align with inflammatory patterns and outcomes in critical illness. Nat Commun. (2022) 13:6789. 10.1038/s41467-022-34420-4
56.
Pathak A Singh SK Thewke DP Agrawal A . Conformationally altered C-reactive protein capable of binding to atherogenic lipoproteins reduces atherosclerosis. Front Immunol. (2020) 11:1780. 10.3389/fimmu.2020.01780
57.
Barber MN Risis S Yang C Meikle PJ Staples M Febbraio MA et al . Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE. (2012) 7:e41456. 10.1371/journal.pone.0041456
58.
Semba RD . Perspective: the potential role of circulating lysophosphatidylcholine in neuroprotection against Alzheimer disease. Adv Nutr. (2020) 11:760–72. 10.1093/advances/nmaa024
59.
Wiest EF Walsh-Wilcox MT Walker MK . Omega-3 polyunsaturated fatty acids protect against cigarette smoke-induced oxidative stress and vascular dysfunction. Toxicol Sci. (2017) 156:300–10. 10.1093/toxsci/kfw255
60.
Colussi G Catena C Novello M Bertin N Sechi LA . Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: relevance for cardiovascular outcomes. Nutr Metab Cardiovasc Dis. (2017) 27:191–200. 10.1016/j.numecd.2016.07.011
61.
Maekawa M Yoshitani K Yahagi M Asahara T Shishido Y Fukushima S et al . Association between postoperative changes in the gut microbiota and pseudopsia after cardiac surgery: prospective observational study. BMC Surg. (2020) 20:247. 10.1186/s12893-020-00907-4
62.
Zhang Y Luo W Zhao M Li Y Wu X . Advances in understanding the effects of cardiopulmonary bypass on gut microbiota during cardiac surgery. Int J Artif Organs. (2025) 48:51–63. 10.1177/03913988251313881
63.
Zha X Liu X Wei M Huang H Cao J Liu S et al . Microbiota-derived lysophosphatidylcholine alleviates Alzheimer's disease pathology via suppressing ferroptosis. Cell Metab. (2025) 37:169–86.e9. 10.1016/j.cmet.2024.10.006
64.
Mumby S Koh TW Pepper JR Gutteridge JMC . Risk of iron overload is decreased in beating heart coronary artery surgery compared to conventional bypass. Biochim Biophys Acta. (2001) 1537:204–10. 10.1016/S0925-4439(01)00070-9
65.
Hadjinikolaou L Alexiou C Cohen AS Standbridge RDL McColl AJ Richmond W . Early changes in plasma antioxidant and lipid peroxidation levels following coronary artery bypass surgery: a complex response. Eur J Cardiothorac Surg. (2003) 23:969–75. 10.1016/S1010-7940(03)00115-5
66.
Sinha SR Haileselassie Y Nguyen LP Tropini C Wang M Becker LS et al . Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe. (2020) 27:659–70.e5. 10.1016/j.chom.2020.01.021
67.
Leonhardt J Haider RS Sponholz C Leonhardt S Drube J Spengler K et al . Circulating bile acids in liver failure activate TGR5 and induce monocyte dysfunction. Cell Mol Gastroenterol Hepatol. (2021) 12:25–40. 10.1016/j.jcmgh.2021.01.011
68.
Wacker M Ball A Beer HD Schmitz I Borucki K Azizzadeh F et al . Immunophenotyping of monocyte migration markers and therapeutic effects of selenium on IL-6 and IL-1β cytokine axes of blood mononuclear cells in preoperative and postoperative coronary artery disease patients. Int J Mol Sci. (2023) 24:7198. 10.3390/ijms24087198
69.
Leonhardt J Dorresteijn MJ Neugebauer S Mihaylov D Kunze J Rubio I et al . Immunosuppressive effects of circulating bile acids in human endotoxemia and septic shock: patients with liver failure are at risk. Crit Care. (2023) 27:372. 10.1186/s13054-023-04620-5
70.
Zhang Y Qi H Wang L Hu C Gao A Wu Q et al . Fasting and refeeding triggers specific changes in bile acid profiles and gut microbiota. J Diabetes. (2023) 15:165–80. 10.1111/1753-0407.13356
71.
Cason CA Dolan KT Sharma G Tao M Kulkarni R Helenowski IB et al . Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. (2018) 68:1552–62.e7. 10.1016/j.jvs.2017.09.029
72.
Zhuang H Ren X Jiang F Zhou P . Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. (2023) 29:17. 10.1186/s10020-023-00614-9
73.
Jiang H Chen C Gao J . Extensive summary of the important roles of indole propionic acid, a gut microbial metabolite in host health and disease. Nutrients. (2022) 15:151. 10.3390/nu15010151
74.
Meijers BK Evenepoel P . The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. (2011) 26:759–61. 10.1093/ndt/gfq818
75.
Ding W Liu J Zhou X Miao Q Zheng H Zhou B et al . Clinical multi-omics study on the gut microbiota in critically ill patients after cardiovascular surgery combined with cardiopulmonary bypass with or without sepsis (MUL-GM-CSCPB study): a prospective study protocol. Front Med. (2020) 7:269. 10.3389/fmed.2020.00269
76.
Kansakar U Trimarco V Mone P Varzideh F Lombardi A Santulli G . Choline supplements: an update. Review. Front Endocrinol. (2023) 14:1148166. 10.3389/fendo.2023.1148166
77.
Yang Y-C Chang S-C Hung C-S Shen M-H Lai C-L Huang C-J . Gut-microbiota-derived metabolites and probiotic strategies in colorectal cancer: implications for disease modulation and precision therapy. Nutrients. (2025) 17:2501. 10.3390/nu17152501
78.
Neb H Roth V Roos J Bauer T Urbschat A Heinicke U et al . Analysis of fatty acid-derived lipids in critically ill patients after cardiac surgery yields novel pathophysiologically relevant mediators with possible relevance for systemic inflammatory reactions. Front Immunol. (2024) 15:1148806. 10.3389/fimmu.2024.1148806
79.
Xin Y Zhang J Fan Y Wang C . Serum free fatty acids are associated with severe coronary artery calcification, especially in diabetes: a retrospective study. BMC Cardiovasc Disord. (2021) 21:343. 10.1186/s12872-021-02152-w
80.
Ghosh A Gao L Thakur A Siu PM Lai CWK . Role of free fatty acids in endothelial dysfunction. J Biomed Sci. (2017) 24:50. 10.1186/s12929-017-0357-5
81.
Chueire VB Muscelli E . Effect of free fatty acids on insulin secretion, insulin sensitivity and incretin effect - a narrative review. Arch Endocrinol Metab. (2021) 65:24–31. 10.20945/2359-3997000000313
Summary
Keywords
bile acids, biomarkers, coronary artery disease, coronary artery bypass graft, free fatty acids, metabolomics, phosphatidylcholine, plasma metabolites
Citation
Neu F, Wacker M, Schuchardt S, Varghese S, Awad G, Waqas FH, Wippermann J, Pessler F and Veluswamy P (2025) Targeted metabolomic profiling reveals inflammation–associated longitudinal changes in plasma metabolites following on-pump coronary bypass surgery. Front. Med. 12:1673132. doi: 10.3389/fmed.2025.1673132
Received
25 July 2025
Accepted
29 September 2025
Published
30 October 2025
Volume
12 - 2025
Edited by
Stefano Cacciatore, International Centre for Genetic Engineering and Biotechnology (ICGEB), South Africa
Reviewed by
Longxiang Su, Peking Union Medical College Hospital (CAMS), China
Richa Dwivedi, Meharry Medical College, United States
Updates
Copyright
© 2025 Neu, Wacker, Schuchardt, Varghese, Awad, Waqas, Wippermann, Pessler and Veluswamy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank Pessler Frank.Pessler@helmholtz-hzi.de
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.