Abstract
Background:
Hemoglobin is one of the most common laboratory tests for hospitalized patients, and both anemia and polycythemia are common comorbidities in severe acute exacerbation of chronic obstructive pulmonary disease (AECOPD). However, limited evidence focuses on the predictive value of anemia or polycythemia for in-hospital adverse outcomes of severe AECOPD.
Methods:
The patients hospitalized for severe AECOPD were prospectively enrolled from 10 medical centers in China. They were categorized into three groups: anemia, normal, and polycythemia, based on their hemoglobin levels on-admission. The adverse outcomes which included all-cause in-hospital mortality, invasive ventilation, and intensive care unit (ICU) admission.
Results:
A total of 9,660 AECOPD inpatients were included. The cohort identified a significant association between anemia and adverse outcomes when compared to the normal group (5.20% vs. 2.80%, p < 0.001), including In-hospital mortality (1.12% vs. 0.29%, p < 0.001), invasive ventilation (2.12% vs. 1.19%, p = 0.001), ICU admission (4.24% vs. 2.41%, p < 0.001). When hemoglobin was further categorized from <6 g/dL to ≥20 g/dL, and 12 to <16 g/dL was taken as reference, ORs for adverse outcomes increased with decreased hemoglobin in the overall cohort, hemoglobin<60 g/dL (OR = 7.714, 95% CI: 2.622 ~ 20.887), hemoglobin 6 to <9 g/dL (OR = 3.284, 95% CI: 2.142 ~ 4.93). Conversely, no significant relationship was observed between polycythemia and adverse outcomes when compared to the normal group. Additionally, compared with normal group, participants with anemia were found to be older and showed elevated levels of WBC, Neutrophil ratio, PCT, CRP, serum G test positive rate, GM test positive rate, BUN, creatinine and D-dimer.
Conclusion:
While there is no effect of polycythemia on adverse outcomes in severe AECOPD inpatients, anemia on-admission, particularly <9 g/dL, is associated with a heightened risk of adverse outcomes, which may serve as an effective biomarker of poor prognosis among inpatients with severe AECOPD.
Background
Chronic Obstructive Pulmonary Disease (COPD) is now one of the top three causes of death worldwide and 90% of these deaths occur in low- and middle-income countries (LMICs). Based on BOLD and other large scale epidemiological studies, it is estimated that the global prevalence of COPD is 10.3%. In China, COPD was accounted for a quarter of the global COPD population. From 1990 to 2019, the incidence and prevalence numbers of COPD increased by 61.2 and 67.8%. With the increasing prevalence of smoking in LMICs, and aging populations in high-income countries, the prevalence of COPD is expected to rise (1–3). The global burden of COPD is projected to rise in the coming decades due to persistent exposure to risk factors and population aging, COPD exacerbations account for the greatest proportion of the total COPD burden on the healthcare system (4, 5). Although a major public health challenge, COPD is both preventable and treatable. Annually, numerous patients are hospitalized or die from acute exacerbation of COPD (AECOPD). In order to improve adverse outcomes, it is critical to investigate comorbidities and prognostic factors in patients with AECOPD.
Clinical studies have identified multiple mortality risk factors in AECOPD, including comorbid heart failure, pulmonary consolidation, acidosis, eosinopenia, elevated D-dimer, increased blood urea nitrogen (BUN), low body mass index (BMI), and low diastolic blood pressure (6–11). Hemoglobin, a routine laboratory parameter for hospitalized patients, is often abnormal in AECOPD, anemia and polycythemia are both common. Polycythemia may correlate with pulmonary hypertension (12, 13), venous thromboembolism (14), and even mortality (15, 16). Anemia in stable COPD patients have also been confirmed to be associated with older, more cardiometabolic comorbidities, worse dyspnea, poorer quality of life, severe airflow obstruction, reduced exercise capacity, higher exacerbation risk, and increased mortality (17–19). Severe AECOPD carries a higher risk of mortality and healthcare resource utilization, but the evidence regarding the predictive value of adverse outcomes causing by anemia or polycythemia in hospitalized patients with severe AECOPD is limited. To elucidate the clinical implications is essential for improving severe AECOPD management and outcomes. Therefore, this study is dedicated to clarify the relationship between different ranges of hemoglobin and adverse outcomes in severe AECOPD inpatients.
Materials and methods
Study participants and design
MAGNET AECOPD (MAnaGement aNd advErse ouTcomes in inpatients with acute exacerbation of COPD) is a prospective observational study conducted across 10 clinical centers in China. It enrolled patients hospitalized for AECOPD in large tertiary general hospitals from September 2017 to July 2021. The primary objectives were to examine the management and adverse outcomes of AECOPD inpatients and to develop early warning models for these outcomes. This sub study specifically investigated the association between admission hemoglobin levels and in-hospital adverse outcomes. This study received approval from the institutional review boards of all participating academic medical centers.
The diagnosis of AECOPD was consistent across all 10 participating medical centers and required: a history of COPD, as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, and patients admitted to hospitals were defined as severe AECOPD. An acute worsening of respiratory symptoms necessitating additional treatment. The exclusion criteria were as follows: (1) patients aged < 40 years; (2) patients with missing hemoglobin data; (3) patients with any of the following diseases: hematopoietic system diseases, connective tissue disease, hepatic cirrhosis, chronic kidney disease, sepsis, pneumonia, bleeding history in 1 month before admission; The admission and treatment of patients were determined by the attending physician, and no additional direct intervention was performed.
Data collection and definitions
Sociodemographic information, ethnic group (minority or not), smoking status, BMI (weight divided by the square of height), and general vital signs including systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse, as well as respiratory rate (RR) were collected immediately upon admission. The comorbidities were also recorded in detail, hypertension, coronary heart disease (CHD), chronic heart failure (CHF), Cor pulmonale, obstructive sleep apnea (OSA), and diabetes were the most common comorbidities. Laboratory data, white blood cell (WBC), hemoglobin, neutrophil ratio, platelet, eosinophil ratio, procalcitonin (PCT), C-reactive protein (CRP), 1,3-β-d glucan test (G test), galactomannan test (GM test), blood urea nitrogen (BUN), creatinine, albumin, N-terminal pro-brain natriuretic peptide (NT-proBNP), D-dimer, were also collected subsequently. Blood gas analysis was also conducted to obtain the potential of hydrogen (PH), partial pressure of oxygen in arterial blood (PaO2), partial pressure of carbon dioxide in arterial blood (PaCO2), and lactic acid (LAC). Anemia was defined as peripheral blood hemoglobin concentration <11 g/dL in females, and <12 g/dL in males, while polycythemia was defined as hemoglobin ≥15 g/dL in females, and ≥16 g/dL in males. Patients were divided into three groups based on their hemoglobin levels: anemia, normal (11–14.9 g/dL in females, 12–15.9 g/dL in males). We firstly compare the differences in comorbidities and laboratory indicators among the three groups, and further to explore the relationships between hemoglobin level and adverse outcomes of AECOPD inpatients.
Adverse outcomes
The adverse outcomes included all cause in-hospital mortality, ICU admission, and invasive mechanical ventilation of AECOPD inpatients.
Statistical analyses
Statistical analysis of this study was conducted by R statistical software (version 4.0.5). Descriptive analysis of quantitative data was performed using mean ± standard deviation or median plus quartile interval; qualitative data was conducted using rates and proportions. Continuous variables were compared by T tests or rank sum tests; categorical variables were compared using chi-square test. Multiple imputations (20) based on 5 replications and chained equation approach were conducted to account for missing data using the Mice package in R software if the missing values were less than 20%, and variables with a missing rate of more than 20% were excluded. To further explore the association between adverse outcomes of severe AECOPD patients and hemoglobin groups, a multivariable logistic regression analysis was performed to adjust for confounding factors. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated for each patient group based on the multivariable analysis. A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 14,007 patients admitted for severe AECOPD were enrolled in the original registration study. Four thousand, three hundred forty-seven patients were excluded according to the exclusion criteria, and 9,660 severe AECOPD inpatients were eventually enrolled into our study. Among them, 2,406(24.91%) patients were diagnosed with anemia, 6,637(68.71%) patients had normal hemoglobin levels, and 617(6.39%) patients had polycythemia (Figure 1). Compared to the normal group, the anemia group was older (74.43 ± 9.49 vs. 71.09 ± 9.68 years, p < 0.001), had a lower proportion of women (15.54% vs. 20.57%, p < 0.001), and higher rates of hypertension (33.96% vs. 31.48%, p = 0.027), CHD (11.85% vs. 9.84%, p = 0.006), and diabetes (12.26% vs. 10.55%, p = 0.024), while the polycythemia group was younger (67.32 ± 10.46 vs. 71.09 ± 9.68 years, p < 0.001), had a higher proportion of women (24.15% vs. 20.57%, p = 0.041), and elevated rates of CHF (13.61% vs. 7.49%, p < 0.001), minority, Cor pulmonale (35.98% vs. 16.30%, p < 0.001), and OSA (2.11% vs. 0.60%, p < 0.001) (Table 1).
Figure 1

Flow chart of the study.
Table 1
| Variables | Normal (N = 6,637) | Anemia (N = 2,406) | P # -value | Polycythemia (N = 617) | P * -value |
|---|---|---|---|---|---|
| Characteristics | |||||
| Gender (female, %) | 1,365 (20.57) | 374 (15.54) | <0.001 | 149 (24.15) | 0.041 |
| Age (years) | 71.09 ± 9.68 | 74.43 ± 9.49 | <0.001 | 67.32 ± 10.46 | <0.001 |
| BMI (kg/m2) | 21.68 ± 3.01 | 20.97 ± 2.86 | <0.001 | 22.56 ± 3.36 | <0.001 |
| Minority | 165 (2.49) | 35 (1.45) | 0.004 | 59 (9.56) | <0.001 |
| Smoking status | 0.092 | 0.819 | |||
| Never | 2,461 (37.08) | 874 (36.33) | 233 (37.76) | ||
| Current smoker | 1,361 (20.51) | 455 (18.91) | 120 (19.45) | ||
| Former smoker | 2,815 (42.41) | 1,077 (44.76) | 264 (42.79) | ||
| Comorbidities | |||||
| Hypertension | 2,089 (31.48) | 817 (33.96) | 0.027 | 197 (31.93) | 0.852 |
| Coronary heart disease | 653 (9.84) | 285 (11.85) | 0.006 | 44 (7.13) | 0.035 |
| Chronic heart failure | 497 (7.49) | 200 (8.31) | 0.21 | 84 (13.61) | <0.001 |
| Cor pulmonale | 1,082 (16.30) | 365 (15.17) | 0.206 | 222 (35.98) | <0.001 |
| OSA | 40 (0.60) | 5 (0.21) | 0.029 | 13 (2.11) | <0.001 |
| Diabetes | 700 (10.55) | 295 (12.26) | 0.024 | 79 (12.80) | 0.096 |
| Symptoms and signs | |||||
| SBP (mmHg) | 132.98 ± 18.62 | 131.02 ± 19.86 | <0.001 | 130.69 ± 18.46 | 0.003 |
| DBP (mmHg) | 80.26 ± 11.96 | 76.74 ± 12.36 | <0.001 | 82.53 ± 11.83 | <0.001 |
| Pulse (times/min) | 88.92 ± 16.20 | 89.29 ± 17.03 | 0.347 | 88.28 ± 17.43 | 0.347 |
| Respiratory rate (times/min) | 20.78 ± 1.99 | 20.93 ± 2.21 | 0.002 | 20.78 ± 2.14 | 0.98 |
Baseline characteristics of the study population.
Data are presented as the number of patients (%), mean ± standard deviation. Those with P value < 0.05 were highlighted using the bold font. P#: The p-value of analysis between the anemia group and the normal hemoglobin group. P*: The p-value of analysis between the polycythemia group and the normal hemoglobin group.
BMI, body mass index; OSA, obstructive sleep apnea; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Interestingly, in terms of laboratory indicators, the inflammatory markers like WBC, neutrophil ratio, PCT, CRP, and positivity rates for serum G and GM tests were higher in the anemia group compared to the normal group (p < 0.05). But the polycythemia group only had lower CRP (14.84 ± 28.71 vs. 19.42 ± 35.05 mg/dL, p = 0.002) but no significant differences in other inflammatory markers. As for blood gas, the polycythemia group had lower PaO2 (81.34 ± 28.95 vs. 87.85 ± 26.74 mmHg, p < 0.001) and higher PaCO2 (48.38 ± 13.23 vs. 45.47 ± 10.57 mmHg, p < 0.001), while the anemia group had higher PaO2 (92.03 ± 30.03 vs. 87.85 ± 26.74 mmHg, p < 0.001) (Table 2).
Table 2
| Variables | Normal (N = 6,637) | Anemia (N = 2,406) | P #value | Polycythemia (N = 617) | P *value |
|---|---|---|---|---|---|
| WBC (109/L) | 8.34 ± 3.73 | 8.55 ± 4.46 | 0.022 | 8.32 ± 4.05 | 0.904 |
| Neutrophil ratio (%) | 72.76 ± 12.61 | 74.74 ± 12.69 | <0.001 | 72.92 ± 12.58 | 0.763 |
| Platelet (109/L) | 204.69 ± 80.50 | 231.43 ± 20.26 | <0.001 | 166.96 ± 62.49 | <0.001 |
| Eosinophil ratio (%) | 1.20(0.20,2.80) | 1.0(0.10,2.80) | 0.2 | 0.9(0.20,2.30) | 0.03 |
| PCT (mg/mL) | 0.29 ± 3.46 | 0.80 ± 7.61 | <0.001 | 0.16 ± 1.14 | 0.374 |
| CRP (mg/dl) | 19.42 ± 35.05 | 29.60 ± 65.05 | <0.001 | 14.84 ± 28.71 | 0.002 |
| G test (Positive, %) | 103 (1.55) | 65 (2.70) | <0.001 | 5 (0.81) | 0.2 |
| GM test (Positive, %) | 70 (1.05) | 41 (1.70) | 0.018 | 6 (0.97) | 1 |
| PH | 7.40 ± 0.04 | 7.41 ± 0.05 | <0.001 | 7.39 ± 0.05 | <0.001 |
| PaO2 (mmHg) | 87.85 ± 26.74 | 92.03 ± 30.03 | <0.001 | 81.34 ± 28.95 | <0.001 |
| PaCO2 (mmHg) | 45.47 ± 10.57 | 45.01 ± 10.94 | 0.067 | 48.38 ± 13.23 | <0.001 |
| LAC (mmol/L) | 1.56 ± 0.47 | 1.55 ± 0.58 | 0.58 | 1.62 ± 0.56 | 0.001 |
| Albumin (g/dl) | 37.90 ± 4.90 | 34.63 ± 5.25 | <0.001 | 38.40 ± 5.57 | 0.015 |
| BUN (mmol/L) | 6.15 ± 10.09 | 7.55 ± 22.48 | <0.001 | 6.59 ± 3.61 | 0.28 |
| Creatinine (μmol/L) | 89.25 ± 71.92 | 97.62 ± 80.31 | <0.001 | 98.88 ± 90.85 | 0.002 |
| NT-proBNP (ng/dl) | 4,775.91 ± 186,400.01 | 3,113.92 ± 45,551.26 | 0.665 | 2,709.26 ± 20,142.72 | 0.783 |
| D-dimer (mg/dl FEU) | 1.26 ± 11.92 | 1.94 ± 6.12 | 0.007 | 1.63 ± 8.74 | 0.455 |
Laboratory tests of normal, anemia, polycythemia groups.
Data are presented as the number of patients (%), mean ± standard deviation, or median (Q1, Q3). Those with P-value < 0.05 were highlighted using the bold font. P#: The p-value of analysis between the anemia group and the normal hemoglobin group. P*: The p-value of analysis between the polycythemia group and the normal hemoglobin group.
WBC, white blood cell; PCT, procalcitonin; CRP, C-reactive protein; G test, 1,3-β-d glucan test; GM test, galactomannan test; PH, potential of hydrogen; PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood; LAC, lactic acid; BUN, blood urea nitrogen; NT-proBNP, N-terminal pro-brain natriuretic peptide.
During the hospital stay, 332(3.45%) patients suffered adverse outcomes, among them, 46 patients dead in hospital, 144 patients performed invasive ventilation, and 280 patients were admitted to the ICU. The incidence of adverse events was significantly higher in the anemia group when compared to normal group (5.20% vs. 2.80%, p < 0.001), no matter in terms of the in-hospital mortality, invasive ventilation, or ICU admission. The polycythemia group had a 3.40% adverse outcome rate, not significantly different from the normal group (2.80%, p = 0.465). Notably, despite the polycythemia group demonstrating the highest proportion of mechanical ventilation at 2.27%, no in-hospital mortality happened in polycythemia group (Table 3 and Figure 2).
Table 3
| Variables | Normal (N = 6,637) | Anemia (N = 2,406) | P #-value | Polycythemia (N = 617) | P *-value |
|---|---|---|---|---|---|
| Adverse outcome (%) | 186 (2.80) | 125 (5.20) | <0.001 | 21 (3.40) | 0.465 |
| In-hospital mortality (%) | 19(0.29) | 27(1.12) | <0.001 | 0(0.0) | 0.4 |
| Invasive ventilation (%) | 79(1.19) | 51(2.12) | 0.001 | 14(2.27) | 0.04 |
| ICU admission (%) | 160(2.41) | 102(4.24) | <0.001 | 18(2.92) | 0.5 |
Adverse outcomes of normal, anemia, polycythemia groups.
ICU, intensive care unit. P#: The p-value of analysis between the anemia group and the normal hemoglobin group. P*: The p-value of analysis between the polycythemia group and the normal hemoglobin group. Those with P value < 0.05 were highlighted using the bold font.
Figure 2
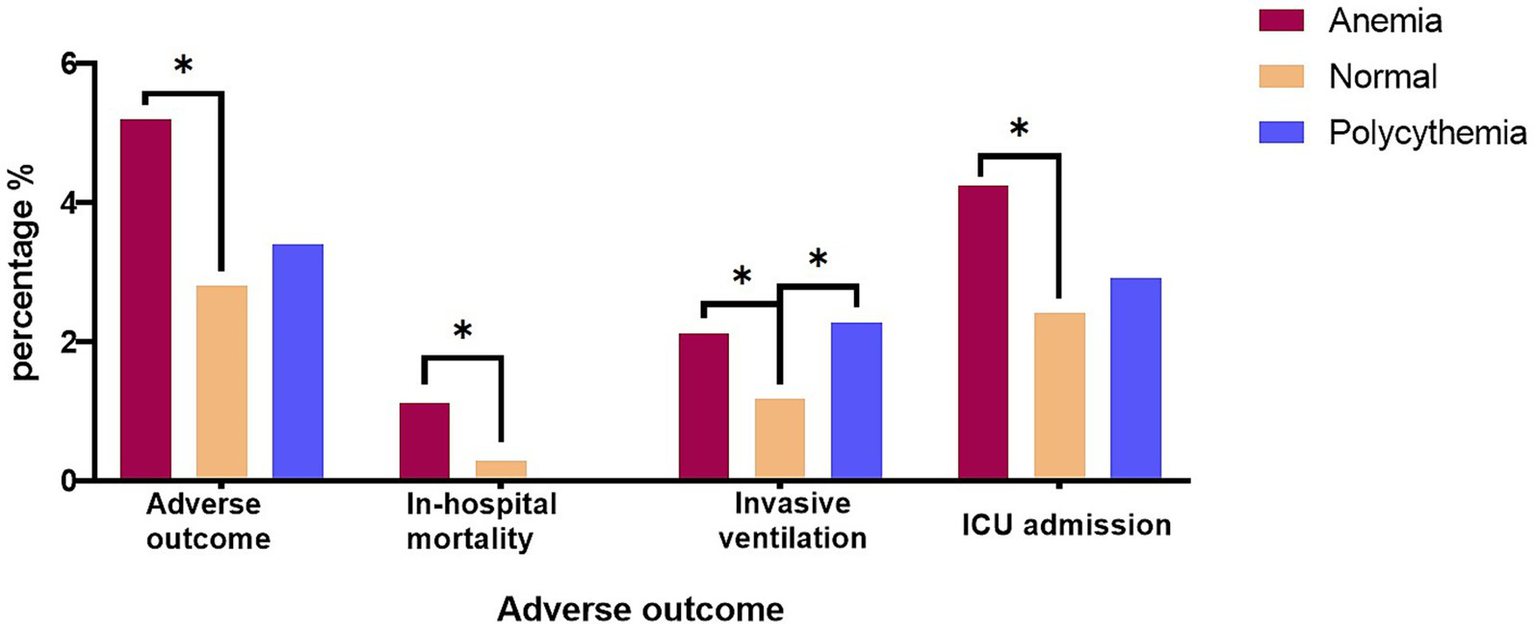
The adverse outcome among the three groups with In-hospital mortality, Invasive mechanical ventilation, and ICU admission, respectively. (*p-value < 0.05); ICU, intensive care unit.
The univariate and multivariate analyses revealed that hypertension, CHF, Cor pulmonale, low DBP, elevated WBC count, neutrophil ratio, serum creatinine, CRP, and PCT, decreased albumin and platelets were associated with adverse outcomes. Interestingly, anemia, but not polycythemia, showed significant correlation with adverse outcomes (p = 0.003) (Supplementary Tables 1, 2).
In order to further detect the different hemoglobin levels and the risk of adverse outcomes, stratification of hemoglobin levels from <6 g/dL to ≥20 g/dL were compared with the reference category (12–15.9 g/dL) respectively (Table 4). It is worth noting that the risk of adverse events significantly increases when hemoglobin below 9 g/dL (OR = 3.284, p < 0.001), especially in severe anemia (hemoglobin <6 g/dL, OR 7.714, p < 0.001) (Table 4 and Figure 3).
Table 4
| Hemoglobin level | Adverse outcome | |
|---|---|---|
| OR (95% CI) | P-value | |
| <6 g/dl | 7.714(2.622 ~ 20.887) | <0.001 |
| 6 to <9 g/dl | 3.284(2.142 ~ 4.93) | <0.001 |
| 9 to <12 g/dl | 1.119(0.843 ~ 1.475) | 0.432 |
| 12 to15.9 g/dl | reference | |
| 16 to <18 g/dL | 1.074 (0.602 ~ 1.789) | 0.798 |
| 18 to <20 g/dl | 1.824(0.731 ~ 3.914) | 0.155 |
| ≥20 g/dl | 2.282(0.352 ~ 8.336) | 0.283 |
Associations between the hemoglobin level with adverse outcome in the AECOPD inpatients by multivariable logistic regression.
P-value < 0.05 were highlighted using the bold font.
Figure 3
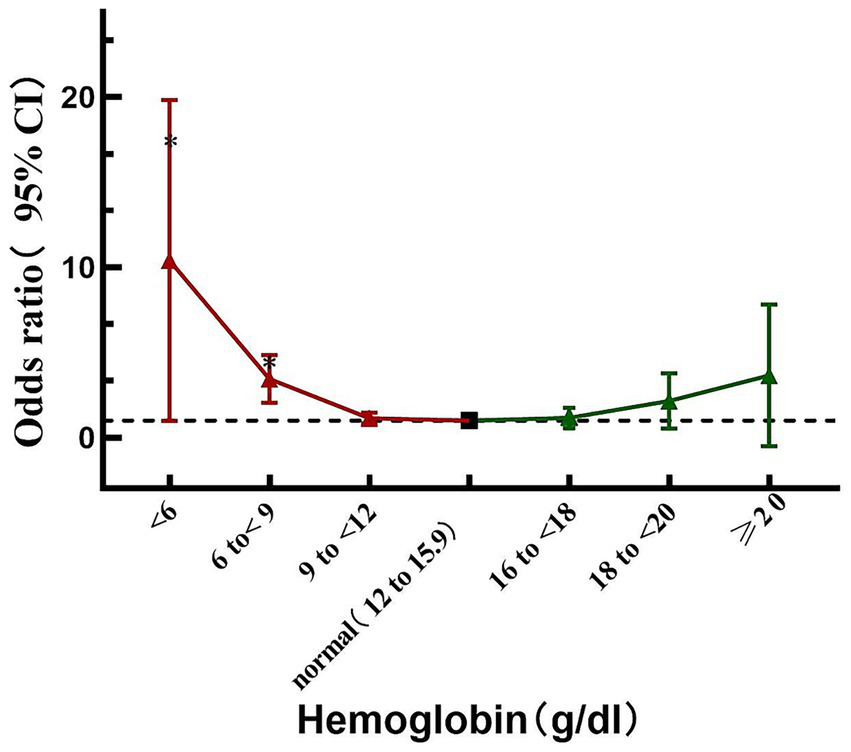
Relationship between Hemoglobin and the risk of adverse outcome; the black thick dot refers to the reference (i.e., 12 to <15.9 g/dL; the red triangle refers to the hemoglobin lower than the reference; the green triangle refers to the Hemoglobin higher than the reference range); *p < 0.05.
Discussion
This large multicenter cohort study found that severe AECOPD patients with anemia had higher risk of adverse outcomes during hospitalization, including in-hospital mortality, ICU admission, and invasive mechanical ventilation, than those with normal hemoglobin. Further stratified analysis showed that moderate and severe anemia patients had significantly higher odds ratios for adverse outcomes, while polycythemia did not exhibit this trend. This study for the first time to explore the relationship between hemoglobin levels and the risk of adverse outcomes during hospitalization for severe AECOPD.
Data from a large cohort (COPDGene cohort) indicated 9.2% of men and 3.5% of women had secondary polycythemia (21), but anemia is more frequently in people with COPD, with a reported prevalence of 7.5 to 34% (22). We found that 24.91% of the subjects with AECOPD had anemia, 6.39% of the subjects with AECOPD had polycythemia, similar to Nielsen’s found (35.2% of AECOPD patients had anemia and 6.8% had polycythemia) (23).
Polycythemia was associated with higher incidence of pulmonary embolism in COPD, and polycythemia in COPD patients was associated with more frequent re-hospitalization per year, more frequent rate of arrhythmia, a longer hospital stay and increased rate of mechanical ventilation (including noninvasive) and mortality, compared with COPD patients without polycythemia (15, 24). However, their studies main points to note polycythemia and long-term outcomes of COPD, the impact of polycythemia on the short-term outcomes of AECOPD has not been extensively explored. Recently, Nielsen et al. (23) revealed that readmission or mortality in AECOPD with polycythemia did not differ from the subjects with normal hemoglobin. However, the inclusion criteria for their study required subjects to be admitted with AECOPD as the primary diagnosis, or respiratory failure or pneumonia as the primary diagnosis alongside COPD as a secondary diagnosis. Our research, only enrolled severe AECOPD, still found that polycythemia does not significantly increase the risk of adverse outcomes (in-hospital mortality, invasive ventilation, ICU admission) when compared with normal hemoglobin. In fact, the SPIROMICS study (25) has pointed that polycythemia was associated with lower incidence of severe AECOPD than normal hemoglobin. The adverse outcomes of AECOPD may stem from uncorrected hypoxemia, a known predictor of mortality in COPD and long-term oxygen therapy (LTOT) has been proved can mitigate hypoxemia, reducing adverse outcomes (26, 27). Furthermore, in our study, patients with polycythemia were younger, had a better nutritional status (higher BMI and albumin), lower inflammatory markers (CRP, PCT, G test, GM test), which might also be one of the reasons why they had a lower risk of adverse outcomes. Hardly any published articles have confirmed that polycythemia is associated with the low-inflammatory state of COPD, further study is need to explore this phenomenon.
Several studies have confirmed that anemia is significantly associated with COPD exacerbations and reduced quality of life, hospitalization costs and duration, readmission, as well as mortality (18, 23, 28–30). Similar to the previous research results, our study found that anemia increases the risk of adverse outcomes among in-hospital severe AECOPD when compared to the normal hemoglobin. Previous studies have focused on COPD exacerbations, readmission and long-term prognosis, but adverse outcomes during hospitalization, which determine whether patients can recover and be discharged, have received little attention. For the first time, we explored the relationship between different hemoglobin stratification in hospitalized severe AECOPD and adverse clinical outcomes, when hemoglobin< 9 g/dL, the risk of adverse events significantly increases. In our study, anemia patients are more likely to have a higher proportion of comorbidities such as coronary heart disease and diabetes and diabetes, these diseases may deteriorate simultaneously during the COPD exacerbation and can also lead to a certain risk of adverse outcomes. Then, patients with anemia are older, and have lower BMI, worse albumin levels, renal function, and blood pressure, in other words, they have a greater risk of malnutrition and frailty, which may explain their higher risk of infection, for they have higher GM or G test positivity rates, and elevated inflammatory markers (CRP, PCT, WBC). Markoulaki et al. (31) also confirmed a negative association between hemoglobin and interleukin-6 (IL-6) during acute phase of COPD exacerbation, the other studies also observed lower hemoglobin levels alongside higher CRP in AECOPD patients (17, 32). Anemia caused by inflammation, infection and malnutrition forms a vicious cycle, which aggravates each other and eventually leads to worse adverse outcomes.
In fact, systemic inflammation plays a key role in anemia development among COPD patients (33–35). These patients demonstrate elevated inflammatory markers including CRP, tumor necrosis factor alpha (TNF-α), and IL-6. CRP production (primarily regulated by IL-6 and indirectly by TNF-α) and TNF-α’s direct suppression of erythrocyte progenitor cell proliferation both contribute to anemia. Additionally, IL-6 upregulates hepcidin, impairing iron absorption and release, which leads to functional iron deficiency and further exacerbates anemia (34, 36, 37). Elevated PCT typically suggests bacterial infection, which may cause hemolytic anemia through toxin production, immune activation, or direct erythrocyte damage. COPD patients also frequently exhibit nutritional deficiencies (particularly albumin, iron, vitamin B12, and folate), further predisposing to anemia (35). Ultimately, impaired erythropoiesis, reduced erythropoietin production, shortened red blood cell survival, and iron dysregulation collectively cause anemia (38–40). Markoulaki et al. (31) found when AECOPD transitions to resolution and stable phases, the hemoglobin level rises while the inflammatory indicators also decline significantly. Our study comparing hemoglobin levels found that anemia, particularly moderate-to-severe cases, correlated with worse outcomes during hospitalization, that suggested clinicians the importance of correcting anemia in AECOPD patients and breaking the vicious cycle between anemia, inflammation, and malnutrition to reduce hospitalization costs and duration, and lower the risk of adverse events and ultimately improve the prognosis of patients.
This study has several strengths. First, as a multicenter, large-scale real-world study, it consecutively enrolled unselected hospitalized AECOPD patients and comprehensively collected data including demographics, comorbidities, and laboratory results. Second, while previous research primarily focused on stable COPD, we specifically examined acute exacerbations, which impose greater economic and health burdens. Finally, we performed multiple hemoglobin subgroup analyses to confirm the association between moderate-to-severe anemia and adverse outcomes. However, some limitations exist. The study lacked serum iron and ferritin measurements, preventing assessment of iron-deficiency anemia prevalence. Additionally, without serial hemoglobin data, we could not evaluate whether anemia correction improves prognosis. Finally, this study only included people with severe AECOPD and lacked data on those with mild and moderate AECOPD, we failed to analyze whether the clinical outcomes of all AECOPD patients were affected by anemia.
In conclusion, anemia (particularly <9 g/dL,) on-admission, not polycythemia, was associated with an increased risk of adverse outcomes, which may serve as an effective biomarker of poor short-term prognosis among inpatients with severe AECOPD. This finding may remind the clinicians should attach more importance to further exploration and correction of the causes of anemia and correcting anemia itself in patients with AECOPD.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (2019–1,056). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SW: Writing – review & editing, Writing – original draft. QY: Conceptualization, Writing – review & editing. YL: Writing – review & editing, Formal analysis. HW: Formal analysis, Writing – review & editing. HG: Writing – review & editing, Methodology. HL: Methodology, Writing – review & editing. XL: Writing – review & editing, Methodology. JZ: Methodology, Writing – review & editing. PP: Writing – review & editing, Methodology. MY: Writing – review & editing, Methodology. LC: Methodology, Visualization, Writing – review & editing. HuZ: Writing – review & editing, Methodology. YT: Writing – review & editing, Supervision. HaZ: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82170013), the Sichuan Science and Technology Program (2022YFS0262) and the National Key Research Program of China (2016YFC1304202).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1674268/full#supplementary-material
References
1.
Meghji J Mortimer K Agusti A Allwood BW Asher I Bateman ED et al . Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet. (2021) 397:928–40. doi: 10.1016/S0140-6736(21)00458-X
2.
Halpin DMG Celli BR Criner GJ Frith P Lopez Varela MV Salvi S et al . The GOLD summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int J Tuberc Lung Dis. (2019) 23:1131–41. doi: 10.5588/ijtld.19.0397
3.
Global Strategy for Prevention, Diagnosis and Management of COPD : (2024) Report[EB/OL]. Available online at: https://goldcopd.org/2024-gold-report/ (Accessed December 11, 2023).
4.
Marshall DC Al Omari O Goodall R Shalhoub J Adcock IM Chung KF et al . Trends in prevalence, mortality, and disability-adjusted life-years relating to chronic obstructive pulmonary disease in Europe: an observational study of the global burden of disease database, 2001-2019. BMC Pulm Med. (2022) 22:289. doi: 10.1186/s12890-022-02074-z
5.
Adeloye D Song P Zhu Y Campbell H Sheikh A Rudan I et al . Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. (2022) 10:447–58. doi: 10.1016/S2213-2600(21)00511-7
6.
Yu X Zhu GP Cai TF Zheng JY . Establishment of risk prediction model and risk score for in-hospital mortality in patients with AECOPD. Clin Respir J. (2020) 14:1090–8. doi: 10.1111/crj.13246
7.
Vallabhajosyula S Haddad TM Sundaragiri PR Ahmed AA Nawaz MS Rayes HAA et al . Role of B-type natriuretic peptide in predicting in-hospital outcomes in acute exacerbation of chronic obstructive pulmonary disease with preserved left ventricular function: a 5-year retrospective analysis. J Intensive Care Med. (2018) 33:635–44. doi: 10.1177/0885066616682232
8.
Prajapati A Sharma Y Thapa S Devkota S . Decaf score in predicting outcomes of acute exacerbation of chronic obstructive pulmonary disease: an observational study. JNMA J Nepal Med Assoc. (2025) 63:144–8. doi: 10.31729/jnma.8903
9.
Zhang J Qin Y Zhou C Luo Y Wei H Ge H et al . Elevated BUN upon admission as a predictor of in-hospital mortality among patients with acute exacerbation of COPD: a secondary analysis of multicenter cohort study. Int J Chron Obstruct Pulmon Dis. (2023) 18:1445–55. doi: 10.2147/COPD.S412106
10.
Zhou C Yi Q Luo Y Wei H Ge H Liu H et al . Low diastolic blood pressure and adverse outcomes in inpatients with acute exacerbation of chronic obstructive pulmonary disease: a multicenter cohort study. Chin Med J. (2023) 136:941–50. doi: 10.1097/CM9.0000000000002666
11.
Pu J Yi Q Luo Y Wei H Ge H Liu H et al . Blood eosinophils and clinical outcomes in inpatients with acute exacerbation of chronic obstructive pulmonary disease: a prospective cohort study. Int J Chron Obstruct Pulmon Dis. (2023) 18:169–79. doi: 10.2147/COPD.S396311
12.
Nakamura A Kasamatsu N Hashizume I Shirai T Hanzawa S Momiki S et al . Effects of hemoglobin on pulmonary arterial pressure and pulmonary vascular resistance in patients with chronic emphysema. Respiration. (2000) 67:502–6. doi: 10.1159/000067463
13.
Edmonston DL Matsouaka R Shah SH Rajagopal S Wolf M . Noninvasive risk score to screen for pulmonary hypertension with elevated pulmonary vascular resistance in diseases of chronic volume overload. Am J Cardiol. (2021) 159:113–20. doi: 10.1016/j.amjcard.2021.08.016
14.
Samareh Fekri M Torabi M Azizi Shoul S Mirzaee M . Prevalence and predictors associated with severe pulmonary hypertension in COPD. Am J Emerg Med. (2018) 36:277–80. doi: 10.1016/j.ajem.2017.08.014
15.
Li J Xiong Y Li S Ye Q Han Y Zhang X et al . Prevalence and risk factors of pulmonary embolism in COPD patients complicated with secondary polycythemia. Int J Chron Obstruct Pulmon Dis. (2024) 19:2371–85. doi: 10.2147/COPD.S481905
16.
Xu L Chen Y Xie Z He Q Chen S Wang W et al . High hemoglobin is associated with increased in-hospital death in patients with chronic obstructive pulmonary disease and chronic kidney disease: a retrospective multicenter population-based study. BMC Pulm Med. (2019) 19:174. doi: 10.1186/s12890-019-0933-4
17.
Putcha N Fawzy A Paul GG Lambert AA Psoter KJ Sidhaye VK et al . Anemia and adverse outcomes in a chronic obstructive pulmonary disease population with a high burden of comorbidities. An analysis from SPIROMICS. Ann Am Thorac Soc. (2018) 15:710–7. doi: 10.1513/AnnalsATS.201708-687OC
18.
Balasubramanian A Henderson RJ Putcha N Fawzy A Raju S Hansel NN et al . Haemoglobin as a biomarker for clinical outcomes in chronic obstructive pulmonary disease. ERJ Open Res. (2021) 7:00068-02021. doi: 10.1183/23120541.00068-2021
19.
Garcia-Pachon E Padilla-Navas I . The impact of Anemia on long-term mortality in hospitalized patients with exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2024) 19:2229–37. doi: 10.2147/COPD.S469627
20.
Sterne JA White IR Carlin JB Spratt M Royston P Kenward MG et al . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. (2009) 338:b2393. doi: 10.1136/bmj.b2393
21.
Zhang J DeMeo DL Silverman EK Make BJ Wade RC Wells JM et al . Secondary polycythemia in chronic obstructive pulmonary disease: prevalence and risk factors. BMC Pulm Med. (2021) 21:235. doi: 10.1186/s12890-021-01585-5
22.
Yohannes AM Ershler WB . Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care. (2011) 56:644–52. doi: 10.4187/respcare.01002
23.
Nielsen BBS Springborg C-J Jacobsen PA Weinreich UM . Anemia and polycythemia in patients hospitalised with acute exacerbations of chronic obstructive pulmonary disease: prevalence, patient characteristics, and risk of readmission and mortality. Eur Clin Respir J. (2025) 12:6672. doi: 10.1080/20018525.2025.2546672
24.
Guo L Chughtai AR Jiang H Gao L Yang Y Yang Y et al . Relationship between polycythemia and in-hospital mortality in chronic obstructive pulmonary disease patients with low-risk pulmonary embolism. J Thorac Dis. (2016) 8:3119–31. doi: 10.21037/jtd.2016.11.31
25.
Fawzy A Woo H Balasubramanian A Barjaktarevic I Barr RG Bowler RP et al . Polycythemia is associated with lower incidence of severe COPD exacerbations in the SPIROMICS study. Chronic Obstr Pulm Dis. (2021) 8:326–35. doi: 10.15326/jcopdf.2021.0216
26.
Owens RL Derom E Ambrosino N . Supplemental oxygen and noninvasive ventilation. Eur Respir Rev. (2023) 32:220159. doi: 10.1183/16000617.0159-2022
27.
Vlahakos V Marathias K Lionaki S Loukides S Zakynthinos S Vlahakos D . The paradigm shift from polycythemia to anemia in COPD: the critical role of the renin-angiotensin system inhibitors. Expert Rev Respir Med. (2022) 16:391–8. doi: 10.1080/17476348.2022.2045958
28.
Sonani H Dhaduk K Dankhara N Viroliya K Desai CH Sonani AA et al . Anemia as a significant predictor of adverse outcomes in hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease: analysis of national (Nationwide) inpatient sample database. Cureus. (2023) 15:e34343. doi: 10.7759/cureus.34343
29.
Martinez-Rivera C Portillo K Munoz-Ferrer A Martinez-Ortiz ML Molins E Serra P et al . Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. COPD. (2012) 9:243–50. doi: 10.3109/15412555.2011.647131
30.
Ergan B Ergun R . Impact of anemia on short-term survival in severe COPD exacerbations: a cohort study. Int J Chron Obstruct Pulmon Dis. (2016) 11:1775–83. doi: 10.2147/COPD.S111758
31.
Markoulaki D Kostikas K Papatheodorou G Koutsokera A Alchanatis M Bakakos P et al . Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur J Intern Med. (2011) 22:103–7. doi: 10.1016/j.ejim.2010.07.010
32.
Nunez A Marras V Harlander M Mekov E Esquinas C Turel M et al . Association between routine blood biomarkers and clinical phenotypes and exacerbations in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2020) 15:681–90. doi: 10.2147/COPD.S240720
33.
Stauder R Valent P Theurl I . Anemia at older age: etiologies, clinical implications, and management. Blood. (2018) 131:505–14. doi: 10.1182/blood-2017-07-746446
34.
Tariq S Ismail D Thapa M Goriparthi L Pradeep R Khalid K et al . Chronic obstructive pulmonary disease and its effect on red blood cell indices. Cureus. (2023) 15:e36100. doi: 10.7759/cureus.36100
35.
Boutou AK Hopkinson NS Polkey MI . Anaemia in chronic obstructive pulmonary disease: an insight into its prevalence and pathophysiology. Clin Sci. (2015) 128:283–95. doi: 10.1042/CS20140344
36.
Weiss G Ganz T Goodnough LT . Anemia of inflammation. Blood. (2019) 133:40–50. doi: 10.1182/blood-2018-06-856500
37.
Nemeth E Ganz T . Hepcidin and Iron in health and disease. Annu Rev Med. (2023) 74:261–77. doi: 10.1146/annurev-med-043021-032816
38.
Patel MS McKie E Steiner MC Pascoe SJ Polkey MI . Anaemia and iron dysregulation: untapped therapeutic targets in chronic lung disease?BMJ Open Respir Res. (2019) 6:e000454. doi: 10.1136/bmjresp-2019-000454
39.
Lanser L Fuchs D Kurz K Weiss G . Physiology and inflammation driven pathophysiology of Iron homeostasis-mechanistic insights into Anemia of inflammation and its treatment. Nutrients. (2021) 13:3732. doi: 10.3390/nu13113732
40.
Pizzini A Aichner M Sonnweber T Tancevski I Weiss G Loffler-Ragg J . The significance of iron deficiency and anemia in a real-life COPD cohort. Int J Med Sci. (2020) 17:2232–9. doi: 10.7150/ijms.46163
Summary
Keywords
anemia, polycythemia, adverse outcome, acute exacerbation, chronic obstructive pulmonary disease
Citation
Wu S, Yi Q, Luo Y, Wei H, Ge H, Liu H, Li X, Zhang J, Pan P, Yi M, Cheng L, Zhou H, Tang Y and Zhou H (2025) Hemoglobin and clinical outcomes of in-hospital patients with severe acute exacerbation of chronic obstructive pulmonary disease: a multicenter cohort study. Front. Med. 12:1674268. doi: 10.3389/fmed.2025.1674268
Received
27 July 2025
Accepted
29 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Hsiao-Chi Chuang, Taipei Medical University, Taiwan
Reviewed by
Min Kwang Byun, Yonsei University, Republic of Korea
Mara Amalia Bălteanu, Titu Maiorescu University, Romania
Updates
Copyright
© 2025 Wu, Yi, Luo, Wei, Ge, Liu, Li, Zhang, Pan, Yi, Cheng, Zhou, Tang and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjiang Tang, lauler@163.com; Haixia Zhou, zhouhaixia@wchscu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.