Abstract
Purpose:
Negative pressure closed thoracic drainage improves drainage efficiency, accelerates the removal of fluid and air, and facilitates lung re-expansion. Conventional wall negative pressure systems restrict patient mobility, while digital drainage devices are associated with high costs that hinder widespread clinical adoption. Hence, there is an urgent need for a cost-effective, practical, and portable negative pressure drainage system.
Methods:
Based on the principles outlined in the Chinese national patent (ZL 202122505281.4), we innovatively integrated a micro-pump and power system into a compact, portable negative pressure generator, which was then connected to the pressure-regulating chamber of a conventional three-chamber closed drainage bottle. This integration resulted in a novel portable closed thoracic drainage device with active negative pressure control. The device underwent in vitro testing followed by preliminary proof-of-concept evaluation.
Results:
The test results indicate that the novel closed thoracic drainage device can achieve a maximum negative pressure of approximately 20 cm H₂O and a maximum airflow rate of 15 L/min. The novel device was initially used in three patients.
Conclusion:
A novel type of negative pressure closed thoracic drainage device has been successfully developed and a preliminary concept verification has been carried out. This device offers the advantages of cost-effectiveness and portability, demonstrating potential for wide application in postoperative thoracic drainage following lung surgery.
Introduction
The primary function of closed thoracic drainage is to effectively remove air and fluid from the pleural cavity, thereby facilitating lung re-expansion. As a result, it has become an essential component of perioperative management in thoracic surgery (1). Following lung surgery, closed thoracic drainage is generally categorized into two types: simple water-seal drainage and negative pressure drainage (2). Although clinical practice regarding postoperative thoracic drainage remains controversial, accumulating evidence supports the advantages of negative pressure drainage, including earlier chest tube removal and shorter postoperative hospital stays (3–5). In 2017, postoperative negative pressure drainage following lung surgery was incorporated into translational medicine clinical guidelines. However, conventional three-chamber closed thoracic drainage systems connected to wall suction units restrict patient mobility (Figure 1). Digital drainage systems, although advanced, are costly and require changes to established surgeon practices, limiting their widespread clinical adoption (6, 7). Inspired by a breast pump used at home, we previously proposed a novel portable negative pressure closed thoracic drainage device to overcome the limitations of traditional wall-mounted systems and explore its potential benefits (6). In this study, we integrated a micro-pump and power supply system into a modified closed thoracic drainage setup by connecting it to the pressure-regulating chamber of a standard three-chamber drainage bottle, thereby developing a novel type of split-design negative pressure thoracic drainage device. This device is cost-effective, practical, and portable. Its initial clinical application has been undertaken in a preliminary setting.
Figure 1

Wall suction drainage system.
Methods
Device description and preliminary use cases.
Device description
The novel closed chest drainage device consists of a three-chamber closed drainage bottle, a micro air pump and a power bank (Figure 2). Comparison of different negative pressure drainage systems is presented in Table 1.
Figure 2
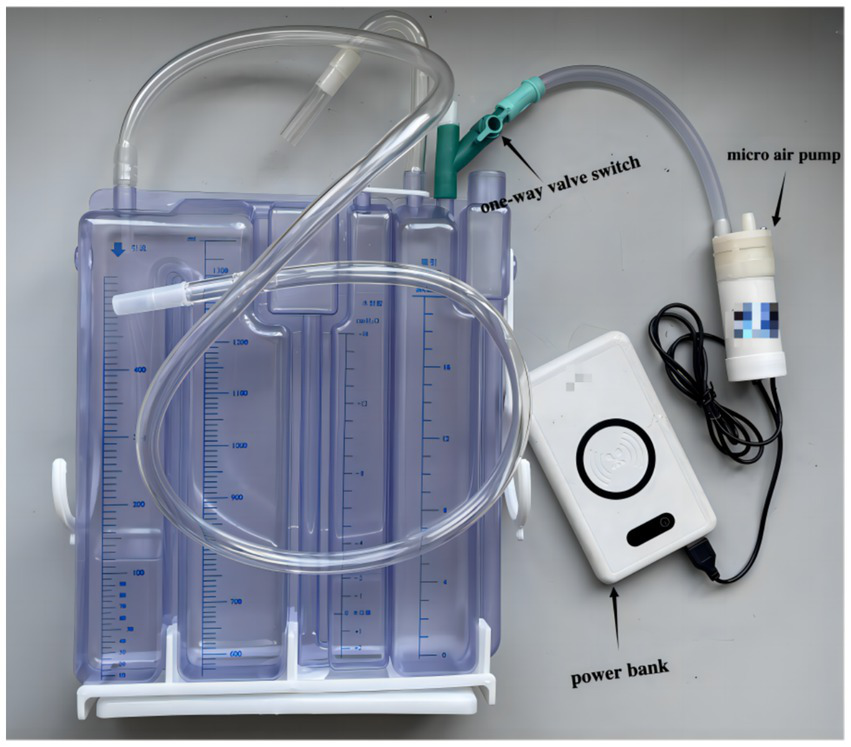
The novel closed chest drainage device.
Table 1
| Device type | Cost | Portability | Leak monitoring | Data logging | Noise | Clinical use |
|---|---|---|---|---|---|---|
| Wall suction | Low | No | No | No | Yes | Quiet widely used |
| Digital system | High ($2000–$3,000) | Yes | Yes | Yes | No | Quiet limited by cost |
| Novel device | Very low ($20) | Yes | No | No | Yes | Prototype stage |
Comparison of negative pressure systems.
Three-chamber closed drainage bottle
Made in China; About 6 dollars; The three-chamber closed drainage bottle has a capacity cavity, a water sealing cavity, and a pressure regulating cavity. The maximum height of the water column in the pressure regulating chamber is 20 cm; The biggest chest fluid storage capacity is more than 1,300 mL. The pressure regulating chamber has a one-way valve, which can be used to adjust the flow rate of gas by turning the valve switch.
Micro air pump
Made in China; About 6 dollars; Micro air pump has an air inlet and outlet, The inner diameter of the pipe to 8 mm; Flow 15 L/ min; USB input type. There is a start-stop switch at the top.
Power bank
Made in China; about 8 dollars; capacity 20,000 ma; USB output type.
Preliminary use cases
The novel device has undergone proof-of-concept testing; however, the findings are limited by the small sample size due to the limited number of clinical cases. Three patients who underwent lobectomy, segmentectomy, and wedge resection, respectively, were enrolled in the study. Baseline clinical information was collected preoperatively (Table 2). The novel drainage device was implemented immediately after surgery, with negative pressure adjusted to −20 cm H₂O to maintain 3–5 bubbles per second in the pressure-regulating chamber via the single-valve switch. Negative pressure suction was maintained for 24 to 48 h or until no bubbles appeared in the water-seal chamber during coughing. Power supply was ensured by replacing the portable power bank during use; wall suction was used when patients remained in bed. Continuous drainage was maintained if air bubbles were observed in the water-seal chamber upon coughing. Postoperatively, all patients received routine intravenous infusion of non-steroidal analgesics twice daily. Additional analgesic injections were administered as needed for breakthrough pain. Chest tube removal was performed after 24 h of bubble-free drainage during coughing and when pleural fluid became lightly serosanguineous. A routine chest X-ray was obtained 24 h after tube removal. One week after discharge, patients returned for follow-up chest X-ray to assess for residual pneumothorax or pleural effusion, with symptomatic management provided if necessary. Daily and total pleural fluid volumes were recorded, along with the timing of chest tube removal and hospital discharge.
Table 2
| Gender | Age(y) | Smoking | FEV1(%) | Diameter of tumor(cm) | Type of Surgery | pathology |
|---|---|---|---|---|---|---|
| Female | 56 | No | 86 | 0.7 | Uniportal VATS wedge resection of the right upper lobe + lymph node sampling | Benign |
| Male | 65 | No | 96.7 | 0.8 | Uniportal VATS lingual resection of the left upper lobe + pleural adhesion release + lymph node dissection | Adenocarcinoma |
| Female | 74 | No | 72.5 | 1.0 | Uniportal VATS wedge resection of right upper lobe + right middle lobe resection + lymph node dissection + pleura release | Adenocarcinoma |
Basic information of the three patients.
Results
The test results indicate that the novel closed thoracic drainage device can achieve a maximum negative pressure of approximately 20 cm H₂O and a maximum airflow rate of 15 L/min. The average duration of postoperative air leakage was 1 day, the mean time to chest tube removal was 3.7 days, and the average postoperative hospital stay was 4.7 days (Figure 3A). The mean daily postoperative chest drainage volume was 124 mL (Figure 3B). The device was successfully applied in all three patients, who reported no discomfort, recovered well, and were discharged uneventfully. One week after discharge, follow-up chest X-rays showed no evidence of pleural effusion or pneumothorax in any of the patients.
Figure 3
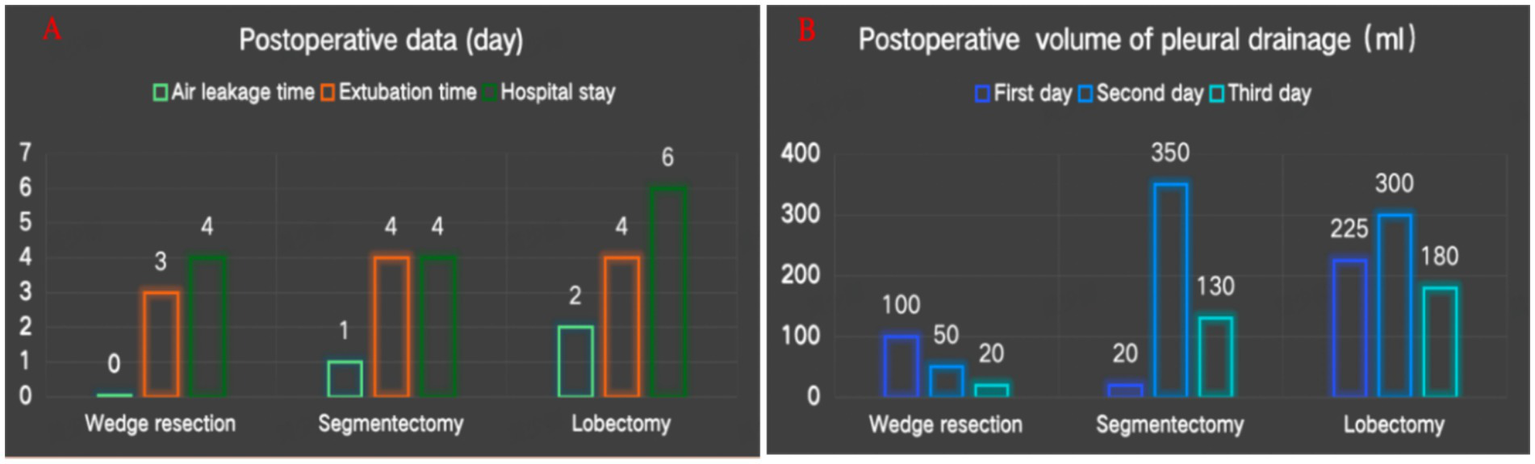
(A) Postoperative data. (B) Postoperative volume of pleural drainage.
Discussion
In the perioperative management of chest tubes during pulmonary surgery, closed thoracic drainage devices play a crucial role, and negative pressure drainage has been shown to facilitate postoperative recovery (3–5). Traditional closed thoracic drainage systems include single-chamber, double-chamber, and triple-chamber drainage bottles. Based on their operating principles, these devices can be classified into wet and dry closed thoracic drainage systems (8). Conventionally, standard triple-chamber closed drainage bottles require connection to wall suction to generate negative pressure, which often restricts patient mobility in the postoperative period. Although dry digital drainage systems overcome this limitation, they are costly—typically priced between $2,000 and $3,000—and may disrupt established surgeon workflows. Moreover, despite their ability to monitor air leaks, digital systems do not consistently lead to earlier chest tube removal, suggesting limited surgeon confidence in their reliability for air leak assessment (9). Recently, Le et al. introduced a hybrid closed thoracic drainage device that combines a simple water seal with a digital system, indicating its potential for managing complex persistent pneumothorax; however, cost information was not reported (10). We initially proposed the concept of a closed thoracic drainage bottle integrated with a miniature air pump for negative pressure drainage, as described in a Chinese national patent (ZL 202122505281.4). Subsequently, in our prior study, we refined this concept into an integrated portable negative pressure drainage device and discussed its advantages and limitations (6, 7). In the present study, we innovatively assembled a miniature air pump and power system and connected them to the pressure-regulating chamber of a conventional triple-chamber closed drainage bottle, thereby developing a novel split-type negative pressure triple-chamber drainage device (Figure 4). A preliminary proof-of-concept evaluation was also conducted.
Figure 4
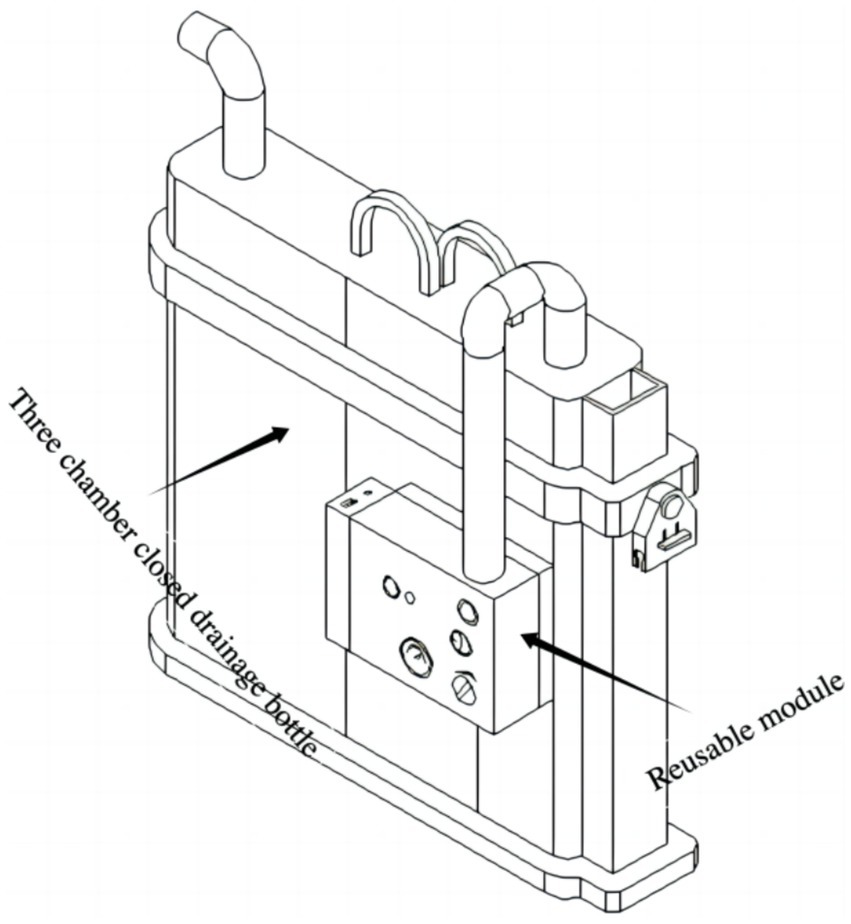
The novel closed chest drainage device.
The design offers several key advantages. First, it builds upon the clinically validated triple-chamber drainage bottle; thus, if the active negative pressure system fails, the device reverts to standard passive drainage function, enhancing both safety and clinical feasibility. Second, the device preserves established clinical practices—for example, surgeons continue to assess air leaks by observing bubble formation in the water-seal chamber—minimizing the need for behavioral adaptation and facilitating clinical adoption. Third, the modular (split) design allows for future cost reduction: the triple-chamber bottle can be disposable, while the negative pressure generation module (pump and power supply) can be reused. This low-cost, reusable model significantly enhances the potential for large-scale clinical implementation.
However, this study has limitations. The device cannot objectively quantify air leak parameters, lacks data traceability, has a limited maximum negative pressure capacity, and generates operational noise. Additionally, the current findings are based on a small sample size, limiting generalizability.
Despite these drawbacks, clinical experience suggests that reliable negative pressure support—rather than digital monitoring—is the primary need for clinicians and patients, particularly given that clinical decisions are typically made during routine morning rounds.
Looking ahead, we plan to conduct larger-scale clinical trials to further evaluate the device’s performance and develop a remote monitoring system incorporating a sensor module capable of collecting and transmitting clinical data in real time, enabling continuous assessment of patient recovery and supporting personalized care planning.
In conclusion, we have developed a novel portable negative pressure thoracic drainage device that preserves existing surgeon workflows and offers significant cost advantages, demonstrating strong potential for widespread clinical application.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the ethics committee of Ningbo No. 2 Hospital (ethical approval number: YJ-NBEY-KYSB-2024-089-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent to publish was obtained from the study participants.
Author contributions
SH: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. XS: Methodology, Writing – original draft. QS: Methodology, Writing – original draft. JL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article supported by Ningbo Health Technology Project (grant no. 2024Y10), Zhu Xiu Shan Talent Project of Ningbo No. 2 Hospital (grant no. 2023HMYQ07) and Ningbo Top Medical and Health Research Program (grant no. 2022030208).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Souza RC Morais LLS Ghefter MC Franceschini JP Pinto FCG . Comparison between use of a pleural drainage system with flutter valve and a conventional water-seal drainage system after lung resection: a randomized prospective study. Sao Paulo Med J. (2024) 142:e2023224. doi: 10.1590/1516-3180.2023.0224.R1.08022024
2.
Brunelli A Beretta E Cassivi SD Cerfolio RJ Detterbeck F Kiefer T et al . Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardiothorac Surg. (2011) 40:291–7. doi: 10.1016/j.ejcts.2011.05.020
3.
Holbek BL Christensen M Hansen HJ Kehlet H Peterson RH . The effect of low suction on digital drainage devices after lobectomy using video assisted thoracoscopic surgery: a randomized controlled trial. Eur J Cardiothorac Surg. (2019) 55:673–81. doi: 10.1093/ejcts/ezy361
4.
Mitsui S Tauchi S Uchida T Ohnishi H Shimokawa T Tobe S . Low suction on digital drainage devices promptly improves post-operative air leaks following lung resection operations: a retrospective study. J Cardiothorac Surg. (2021) 16:105. doi: 10.1186/s13019-021-01485-z
5.
Cui Z Zhang Y Xu C Ding C Chen J Li C et al . Comparison of the results of two chest tube managements during an enhanced recovery program after video-assisted thoracoscopic lobectomy: a randomized trial. Thoracic Cancer. (2019) 10:1993–9. doi: 10.1111/1759-7714.13183
6.
Huang S Song X Tong Z Shi Q Li J . Patent conversion of a novel closed chest drainage device. J Cardiothorac Surg. (2024) 19:431. doi: 10.1186/s13019-024-02873-x
7.
Huang S Song X Shi Q Li J . Chest tube management after pulmonary resection. Eur J Cardiothorac Surg. (2025) 67:ezaf190. doi: 10.1093/ejcts/ezaf190
8.
Toth JW Reed MF Ventola LK . Chest tube drainage devices. Semin Respir Crit Care Med. (2019) 40:386–93. doi: 10.1055/s-0039-1694769
9.
Embalabala A Mitzman B Crabtree T . Digital pleural versus analog drainage devices for postoperative management of patients after pulmonary resection. Eur J Cardiothorac Surg. (2025) 67:i31–40. doi: 10.1093/ejcts/ezae215
10.
Le UT Hümmler N Greiser F Tullius-Modlmeier R Benitz M Passlick B . Hybrid (digital/water seal) chest drainage system—an innovative device for patients with anticipated air leaks. Surg Innov. (2024) 31:185–94. doi: 10.1177/15533506241232618
Summary
Keywords
lung surgery, negative pressure drainage device, novel closed chest drainage device, digital drainage device, traditional closed chest drainage systems
Citation
Huang S, Song X, Shi Q and Li J (2025) A novel closed chest drainage device. Front. Med. 12:1675836. doi: 10.3389/fmed.2025.1675836
Received
30 July 2025
Accepted
03 October 2025
Published
17 October 2025
Volume
12 - 2025
Edited by
Alessandro Boscarelli, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), Italy
Reviewed by
Valentina Scheggi, Careggi University Hospital, Italy
Ariela Torres Cruz, Centro Universitário de Barra Mansa, Brazil
Updates
Copyright
© 2025 Huang, Song, Shi and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoqing Huang, huangshaoqing7@163.comJie Li, xueshan20042007@aliyun.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.