Abstract
Background:
Postoperative nausea and vomiting (PONV) are prevalent complications following general anesthesia. The effectiveness of penehyclidine (PHC) in reducing PONV is still debated. To address this issue, we conducted this systematic review and meta-analysis to assess both the effectiveness and safety of PHC in preventing PONV after general anesthesia.
Methods:
To gather relevant studies on PHC use for preventing PONV, six electronic databases (PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, and Wanfang Database) and trial registries were searched. Placebo-controlled trials that explored the effect of PHC on PONV in patients undergoing general anesthesia were included. The primary outcome was the incidence of PONV. Adverse events were evaluated to explore the safety of PHC. This meta-analysis was carried out using Review Manager 5.3. Risk of bias for included studies was assessed using the Cochrane risk of bias tool 2.0. Quality of evidence was assessed using Grading of Recommendations Assessment, Development, and Evaluation. Heterogeneity was explored by subgroup analyses. Publication bias was evaluated by funnel plot analysis. Additionally, trial sequential analysis was used to reduce the risk of type I error.
Results:
This analysis included ten randomized controlled trials with 1,427 participants. The PHC group showed a significantly lower incidence of PONV compared to the control group (risk ratio = 0.48, 95% confidence interval [0.36, 0.65]; p < 0.05, I2 = 68%). A reduction in postoperative nausea, vomiting, and the need for rescue antiemetic therapy was also associated with PHC.
Conclusion:
Our research suggests that PHC might be a new option for preventing PONV after general anesthesia.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/recorddashboard, CRD42022355743.
Introduction
Postoperative nausea and vomiting (PONV) are prevalent complications following general anesthesia, with incidence rates ranging from 30 to 80%, depending on the type of surgery and patient population (1, 2). PONV causes significant patient discomfort and is linked to various adverse postoperative events, including reflux aspiration, electrolyte imbalance, esophageal injury, and wound dehiscence. In the Fourth Consensus Guidelines for the Management of PONV, volatile anesthetics, nitrous oxide, and postoperative opioids are identified as anesthetic risk factors (3).
The mechanism underlying PONV is multifaceted, involving various pathways and receptors, including cholinergic, dopaminergic, histaminergic, and serotonergic receptors (4, 5). Recent research has highlighted the central cholinergic system’s role in PONV, particularly the muscarinic 3 (M3) muscarinic acetylcholine receptor (6, 7). Current strategies for PONV prevention in high-risk patients are multimodal and often involve a combination of approaches targeting different pathways (3). Pharmacological prophylaxis remains the cornerstone and includes several classes of antiemetics: 5-HT3 (5-HT3) receptor antagonist (8), neurokinin-1 (NK-1) receptor antagonists (9), dopamine antagonists (10), corticosteroids (11), and anticholinergics (12). Non-pharmacological interventions, such as acupuncture (13) and ginger (14), have also been explored with varying levels of evidence supporting their use, often as adjuncts. Despite this array of options, the search for effective, well-tolerated, and cost-efficient preventive agents continues, especially those targeting specific mechanisms like the cholinergic pathway implicated in early PONV triggered by volatile anesthetics.
Penehyclidine (PHC) is a synthetic, long-acting anticholinergic agent developed in China. Pharmacodynamically, PHC acts as a competitive antagonist at both muscarinic and nicotinic acetylcholine receptors, but demonstrates high selectivity for muscarinic receptor subtypes, with the greatest affinity for M1 and M3 receptors, followed by M2, and significantly less for M4 and M5 subtypes (15). Its anti-nicotinic effect contributes to antagonism at neuromuscular junctions. Importantly, PHC readily crosses the blood–brain barrier, exerting significant central anticholinergic effects, which is highly relevant for targeting central pathways involved in PONV (16). Preliminary clinical studies have indeed suggested a beneficial effect of PHC in reducing PONV incidence (17, 18). However, this finding remains contentious. According to Ding et al., PHC did not significantly reduce the incidence or severity of PONV in laparoscopic bariatric surgery patients (19). Although PHC is currently approved in China for indications such as organophosphate poisoning and obstructive airway diseases, it is not approved by the U.S. Food and Drug Administration (FDA) for the prevention of PONV. Importantly, all clinical studies investigating PHC for PONV prevention to date, including those analyzed in this meta-analysis, constitute off-label use.
After identifying relevant randomized controlled trials (RCTs), we conducted a systematic review and meta-analysis with the primary objectives of evaluating the efficacy of PHC in preventing PONV following general anesthesia. Specifically, we aimed to: (1) assess the impact of PHC on the incidence of PONV compared to placebo or standard treatment; (2) evaluate the safety profile of PHC, focusing on adverse events.
Methods
PRISMA guidelines were followed in the conduct of our systematic review and meta-analysis. The research has been registered with the International Prospective Register of Systematic Reviews as CRD42022355743.
Systematic literature search
The search strategy for this systematic review and meta-analysis was comprehensive and systematic. We performed a systematic literature search across international databases (PubMed, Embase, Cochrane Library, and Web of science), Chinese databases (China Network Knowledge Infrastructure and Wanfang Database), and Trial registries (clinicaltrials.gov and the WHO International Clinical Trials Registry Platform) to identify RCTs related to PHC and PONV. The search terms included combinations of keywords such as ‘penehyclidine’, ‘postoperative nausea and vomiting’, ‘PONV’, ‘general anesthesia’, ‘antiemetic’, and ‘randomized controlled trial’. These terms were used in various combinations with Boolean operators (AND/OR) to ensure the identification of all relevant studies. We applied no language restrictions, and the search was limited to studies published up to July 31, 2025. All retrieved articles were screened for relevance, and duplicate studies were removed. The detailed search strategy for each database is available in the Supplementary materials. Additionally, we reviewed the references of the final eligible studies to identify any further relevant research.
Criteria for selection
The inclusion criteria for the studies were based on the “PICOS” framework:
-
(1) Participants (P): adult patients of any American Society of Anesthesiologists physical status undergoing general anesthesia. Studies in which all patients received a standardized baseline PONV prophylaxis regimen in both groups were included, provided the only systematic difference between groups was the administration of PHC or placebo;
-
(2) Intervention (I): trials specifying PHC dosage and timing;
-
(3) Comparison (C): saline;
-
(4) Outcome (O): trials evaluating the incidence of PONV as an outcome;
-
(5) Study Designs (S): RCTs.
The exclusion criteria were as follows:
-
(1) Patients not receiving general anesthesia;
-
(2) Studies lacking available outcomes;
-
(3) Incomplete research, such as conference abstracts or unfinished studies;
-
(4) Non-RCTs.
Extraction of data and outcomes
Initially, two independent reviewers screened for duplicate records. A review of the titles and abstracts of the trials was then conducted to determine whether they met the inclusion criteria. Following that, we reviewed the full texts of the remaining studies to determine final inclusion. The data extraction process was conducted independently by two reviewers using a standardized data extraction form. The form included sections for study design, participant characteristics, and intervention details. The extraction process was performed in a blinded manner, with both reviewers working independently to minimize bias. Any disagreements between reviewers were resolved through discussion or consultation with a third reviewer. For missing data, we made every effort to contact the corresponding authors of the included studies via email to request missing data.
The primary outcome of this study was the incidence of PONV. The temporal definition for PONV was set at 24 h postoperatively. However, if a study reported the incidence of PONV solely within a different timeframe (e.g., 48 h), it was still included in the pooled analysis. Secondary outcomes included severe PONV incidence, postoperative nausea (PON), postoperative vomiting (POV), dry mouth, headache, dizziness, urinary retention, fever, the number of patients needing rescue antiemetics, and post-anesthesia care unit (PACU) length of stay. The definition of severe PONV was not consistent across the included trials. To respect the original study designs and avoid introducing bias by imposing an arbitrary uniform definition, we extracted the outcome ‘severe PONV’ as it was defined by the authors of each primary study. Common definitions included multiple episodes of vomiting within a specified timeframe (19, 20) or severe nausea measured by a numerical analog scale score exceeding a certain threshold (17, 21).
Evaluation of the quality and the risk
We assessed the risk of bias in the included studies utilizing the Cochrane risk-of-Bias tool for RCTs 2.0 (ROB 2.0), which contained six types of bias. Each trial was categorized as having a high, some concerns, or low risk of bias. Furthermore, we employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to gauge the confidence in the evidence, classifying it into one of the four levels.
Statistical analysis
This study was conducted by using Review Manager (Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. Copenhagen). For dichotomous outcomes, we calculated risk ratios (RR) along with 95% confidence intervals (CIs). For continuous outcomes, we determined mean differences (MD) and their corresponding 95% CIs. Continuous data reported as medians with interquartile ranges were converted to means and standard deviations following established methods (22, 23). Statistical significance was set at a p-value <0.05. Heterogeneity among studies was evaluated using the I2 statistic, with I2 > 50% indicating substantial heterogeneity. Statistical significance was defined as a p-value <0.05. Heterogeneity across studies was assessed using the I2 statistic, where I2 > 50% signaled substantial heterogeneity. For studies showing low I2 values, a random-effects model was applied due to notable clinical variability. We performed pre-specified subgroup analyses to investigate whether the effect of PHC on preventing PONV varied by type of anesthesia (TIVA, total intravenous anesthesia vs. Combined, combined intravenous and inhalation anesthesia), dosage of PHC (high- dosage, > 0.5 mg vs. low- dosage, ≤ 0.5 mg), and timing of PHC administration (before induction vs. after induction). For studies that calculated the PHC dosage based on body weight, a dosage of ≤ 0.01 mg/kg was defined as the low- dosage group, while the other was defined as the high- dosage group. To assess the robustness of our finding, we conducted sensitivity analyses by excluded studies with risk of bias to explore the impact of risk of bias on the primary outcome. In addition, we conducted a leave-one-out analysis to assess the stability of the main results. We performed a funnel plot analysis to visually assess the potential for such bias in the included studies.
Trial sequential analysis (TSA) was conducted using TSA software (version 0.9.5.10 beta) to control the risk of type I error that may arise from repeated testing when accumulating data (24). We set the type I error rate at 5% and the type II error rate at 20% (i.e., 80% statistical power). The required information size (RIS) was calculated based on the incidence of PONV in the control group derived from the included studies, a relative risk reduction (RRR) of 38.5% (25). Specifically, if the cumulative Z-curve crossed the TSA monitoring boundary, it would indicate that the evidence is sufficient to draw a conclusion and further studies are unnecessary. Conversely, if the Z-curve did not cross the boundary but entered the conventional significance area, the result might be a false positive, and more studies would be needed. If the Z-curve crossed the RIS line without crossing the boundary, it would suggest that the intervention is ineffective even with sufficient information size. This approach adheres to current recommendations for trial sequential analysis.
Results
Search results
Following our search strategy, we initially identified 218 potentially relevant studies. After removing 53 duplicate publications and excluding 152 studies based on abstract and title reviews, we assessed the full texts of the remaining 13 studies to determine their eligibility. Out of these, 3 trials were excluded for the following reasons: one was a conference abstract, one was not an RCT, and one lacked available outcome. Ultimately, 10 studies met the inclusion criteria and were included in the meta-analysis (17–21, 26–30). A detailed account of the literature screening process is illustrated in Figure 1.
Figure 1

The inclusion process of the literature search.
Study characteristics
The publication years between 2008 and 2024, and the sample size was ranged from 40 to 353. The type of surgeries included laparoscopic bariatric surgery, thyroidectomy, strabismus surgery, laparoscopic cholecystectomy, microvascular decompression, bimaxillary surgery and gynecological laparoscopic surgery. Three studies routinely used neostigmine to antagonize neuromuscular blocking agents after surgery (18, 20, 21). Detailed information for included studies is presented in Table 1.
Table 1
| Study | Sample size | Type of surgery | Anesthesia induction | Anesthesia maintenance | Penehyclidine group | Control group |
|---|---|---|---|---|---|---|
| Ding 2023 (19) | P: 221 C: 113 |
Laparoscopic bariatric surgery | Dexamethasone 10 mg, midazolam 0.05 mg/kg, propofol 1.5–2.5 mg/kg, fentanyl 4–6 μg/kg, rocuronium 0.9 mg/kg or cis-atracurium 0.15 mg/kg. | Propofol 100–200 μg/kg/min, remifentanil 0.05–0.15 μg/kg/min, rocuronium 5–10 μg/kg/min or cis-atracurium 1–3 μg/ kg/min. | A single intravenous dose of 0.5 mg after anesthesia induction. | Same volume of saline. |
| Li 2021 (27) | P: 45 C: 45 |
Thyroidectomy | Midazolam 0.05 mg/kg, propofol 2 mg/kg, fentanyl 5 μg/kg, and cisatracurium 0.15 mg/kg. | Propofol 66–200 μg/kg/min, remifentanil 0.05–0.15 μg/kg/min, cisatracurium 1–3 μg/kg/min. | A single intravenous dose of 0.5 mg after anesthesia induction. | Same volume of saline. |
| Lu 2022 (21) | P: 50 C: 50 |
Total thyroidectomy |
Propofol 1.5–2.5 mg/kg and fentanyl 2 μg/kg, and cisatracurium 0.15 mg/kg. | Propofol 60–200 μg/kg/min, and remifentanil 0.1–0.15 μg/kg/min. | A single intravenous dose of 0.5 mg after anesthesia induction. | Same volume of saline. |
| Sun 2021 (20) | P: 114 C: 104 |
Strabismus surgery | Propofol 1.5–2.5 mg/kg, fentanyl 5.0 μg/kg, cisatracurium 0.15 mg/kg. | Propofol 60–200 μg/kg/min, remifentanil 0.1–0.15 μg/kg/min. | A single intravenous dose of 0.01 mg/kg after anesthesia induction. | Same volume of saline. |
| Wang 2008 (28) | P: 20 C: 20 |
Microvascular decompression | Not mentioned. | Fentanyl, propofol, and isoflurane | A dose of 0.1 mg before anesthesia induction. | Same volume of saline. |
| Wang 2022 (22) | P1: 117 P2: 118 C: 118 |
Bimaxillary surgery | Sufentanil/remifentanil, propofol, and rocuronium/cis-atracurium. | Propofol and remifentanil/ sufentanil, with or without inhalational sevoflurane and/or nitrous oxide or dexmedetomidine infusion. | P1: a dose of 0.5 mg before anesthesia induction. P2: a dose of 0.25 mg before anesthesia induction; a dose of 0.25 mg was added to the intravenous analgesia pump. |
Same volume of saline. |
| Yang 2011 (29) | P: 30 C: 30 |
Laparoscopic cholecystectomy | Midazolam 0.05 mg/kg, propofol 1.5 mg/kg, atracurium 0.6 mg/kg, and fentanyl 2 μg/kg. | Fentanyl and atracurium. | A dose of 1 mg before anesthesia induction. | Same volume of saline. |
| Zhang 2012 (18) | P: 40 C: 40 |
Gynecological laparoscopic surgery | Midazolam 0.08 mg/kg, fentanyl 5 lg/kg, etomidate 0.3 mg/kg, cisatracurium 0.2 mg/kg. | Propofol 3–4 lg/ml, remifentanil 3 ng/mL; muscle relaxation 0.08 mg/kg/min. | A dose of (0.01 mg/kg, maximal total dose, 1 mg) before anesthesia induction. | Same volume of saline. |
| Zhang 2010 (30) | P: 30 C: 30 |
Laparoscopic cholecystectomy | Midazolam 0.06 mg/kg, fentanyl 3 μg/kg, atracurium 0.6 mg/kg, and etomidate 0.3 mg/kg. | Propofol and remifentanil | A dose of (0.02 mg/kg) before anesthesia induction. | Same volume of saline. |
| Zhao 2024 (26) | P: 46 C: 46 |
Gynecological laparoscopic surgery | Midazolam 0.04 mg/kg, sufentanil 0.5 μg/kg, etomidate 0.3 mg/kg, rocuronium 0.8 mg/kg. | Sevoflurane 1%, remifentanil 0.1–0.3 μg/kg/min, propofol 2–5 mg/kg/h. | A bolus of 0.01 mg/kg after anesthesia induction. | Same volume of saline. |
The details of included studies.
P, penehyclidine; C, control; PONV, postoperative nausea and vomiting.
Risk of bias
Risk of bias assessment for individual studies is shown in Figure 2. Four trials had the high risk of randomization process, and two trials had some concerns risk of randomization process. Three trials had some concerns risk of deviations from intended interventions. Of the included trials, four were classified as low risk of bias, two raised some concerns, and four were considered high risk.
Figure 2
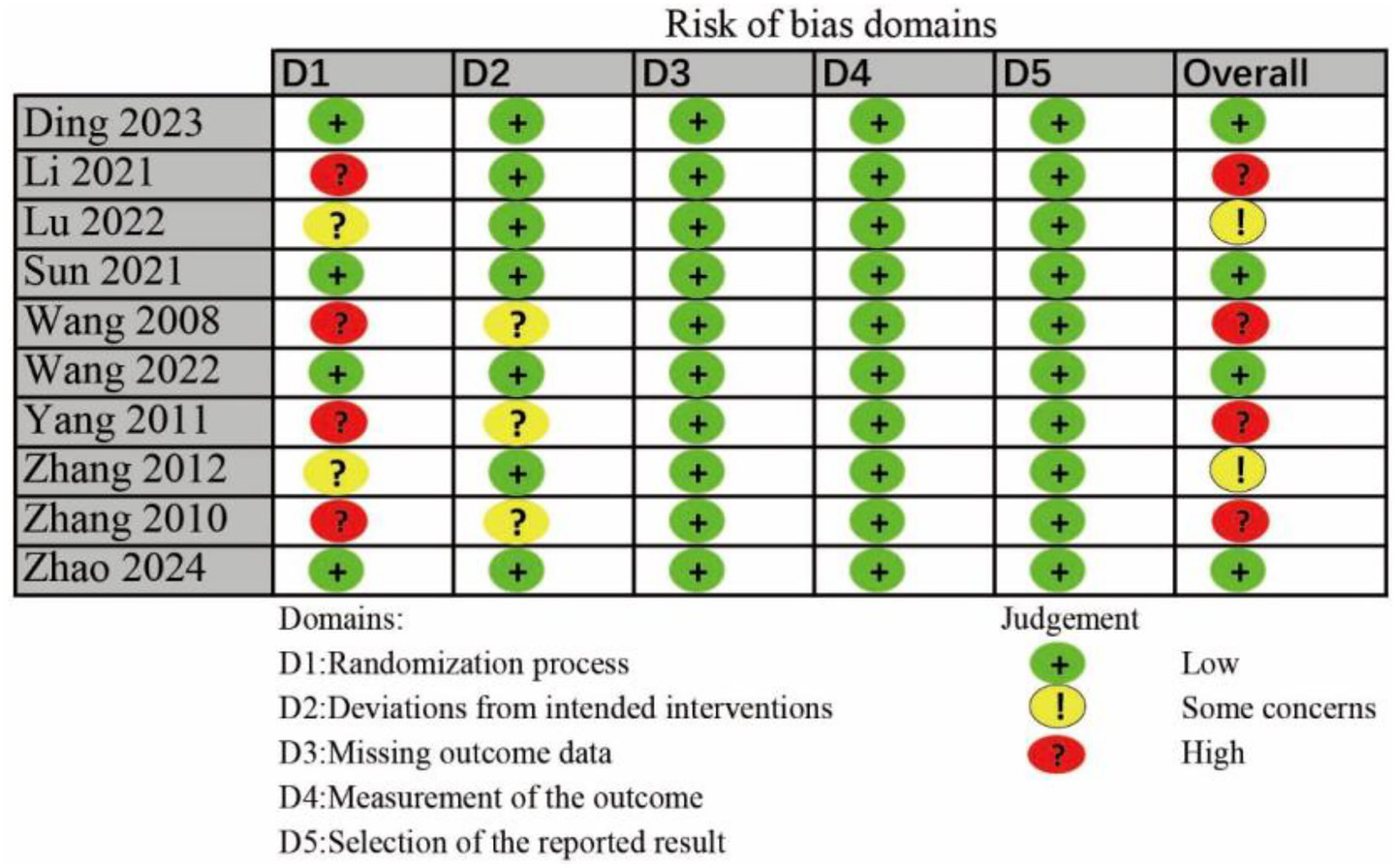
The risk bias assessment of all included studies.
Outcomes
Primary outcome
The incidence of PONV
All the trials included reported on the incidence of PONV. The forest plot indicated a significant reduction in PONV rates for the PHC group (RR = 0.48, 95% CI [0.36, 0.65], p < 0.05, I2 = 68%, Figure 3), highlighting substantial heterogeneity among the studies. Notably, the trial by Ding et al. was identified as a major contributor to this variability. After excluding this study, we re-conducted the meta-analysis, which yielded similar results with reduced heterogeneity (Supplementary Figure 1). Further subgroup analyses also yielded results consistent with the overall finding (Supplementary Figures 2–4). Sensitivity analyses suggest that the risk of bias did not dramatically alter the overall effect estimate. Furthermore, we further evaluated the effect of PHC on preventing PONV in studies that routinely used neostigmine for neuromuscular blockade reversal after surgery, and consistent result was obtained (RR = 0.42, 95% CI [0.23, 0.77], p < 0.05, I2 = 41%). In addition, the results of the leave-one-out analysis confirmed that the primary outcome was stable.
Figure 3
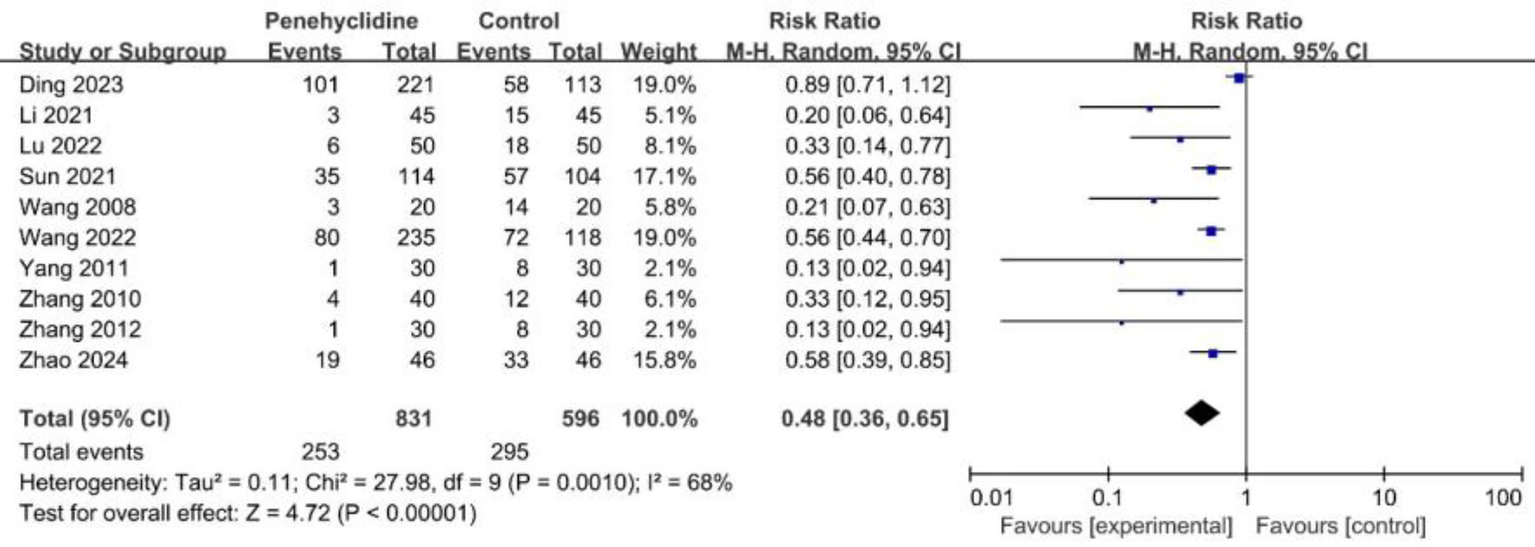
Forest plot of the incidence of PONV between penehyclidine and control groups. PONV, postoperative nausea and vomiting.
The incidence of PONV was 49.5% in the control group, and 30.4% in the PHC group, the absolute risk reduction (ARR) was 19.1%. The number needed to treat (NNT) to prevent one case of PONV was 5.2.
Secondary outcomes
PON occurrence
Three trials assessed the incidence of PON. The forest plot revealed a significantly lower incidence in the PHC group (RR = 0.59, 95% CI [0.35, 0.97], p < 0.05, I2 = 46%, Figure 4), indicating low heterogeneity among the studies.
Figure 4
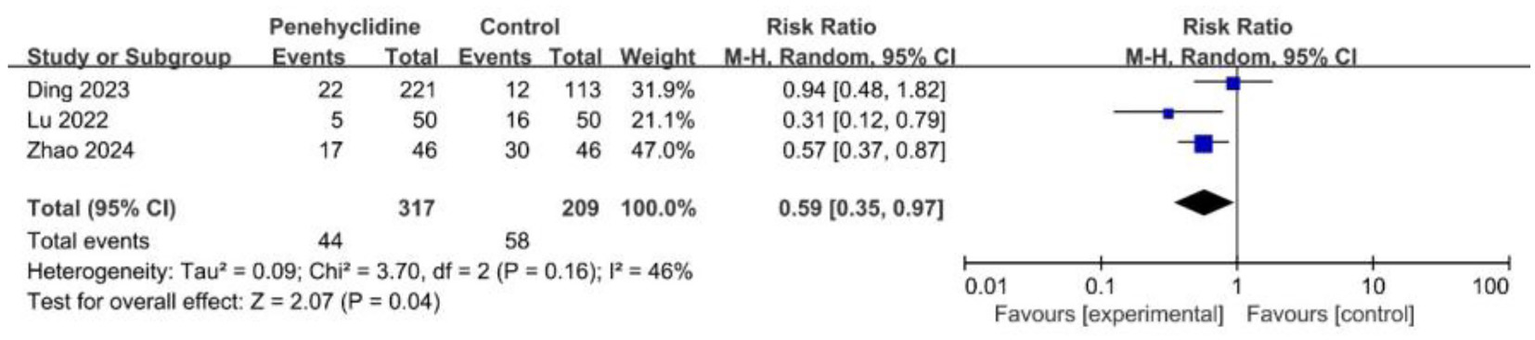
Forest plot of the incidence of PON between penehyclidine and control groups. PON, postoperative nausea.
POV occurrence
Four trials examined the incidence of POV. The forest plot demonstrated a significantly reduced incidence in the PHC group (RR = 0.41, 95% CI [0.19, 0.92], p < 0.05, I2 = 76%, Figure 5), reflecting high heterogeneity among the studies. Similarly, after excluding Ding et al.’s study, we re-conducted the meta-analysis, the result remained consistent, with reduced heterogeneity (Supplementary Figure 5).
Figure 5
![Forest plot showing the risk ratio for penetlyclidine versus control across four studies: Ding 2023, Lu 2022, Zhang 2012, and Zhao 2024. The risk ratios and 95% confidence intervals range from 0.17 to 0.88, with a total effect size of 0.41 [0.19, 0.92]. The plot indicates heterogeneity with I² = 76% and a P-value of 0.03 for overall effect. The diamond shape represents the combined effect size.](https://www.frontiersin.org/files/Articles/1676087/xml-images/fmed-12-1676087-g005.webp)
Forest plot of the incidence of POV between penehyclidine and control groups. POV, postoperative vomiting.
Severe PONV occurrence
Four trials reported on the incidence of severe PONV, the forest plot analysis revealed a consistent direction of effect and a significantly lower incidence in the PHC group (RR = 0.50, 95% CI [0.34, 0.74], p < 0.05, I2 = 13%; Supplementary Figure 6). The low statistical heterogeneity (I2 = 13%) suggests that the treatment effect of PHC may be robust across these different definitions of severity.
Rescue antiemetic occurrence
Six trials evaluated the incidence of required rescue antiemetics. The forest plot analysis revealed a significantly lower incidence in the PHC group (RR = 0.39, 95% CI [0.23, 0.66], p < 0.05, I2 = 70%, Figure 6), indicating high heterogeneity. Similarly, after excluding Ding et al.’s study and redoing the meta-analysis, the result remained consistent, with reduced heterogeneity (Supplementary Figure 7).
Figure 6
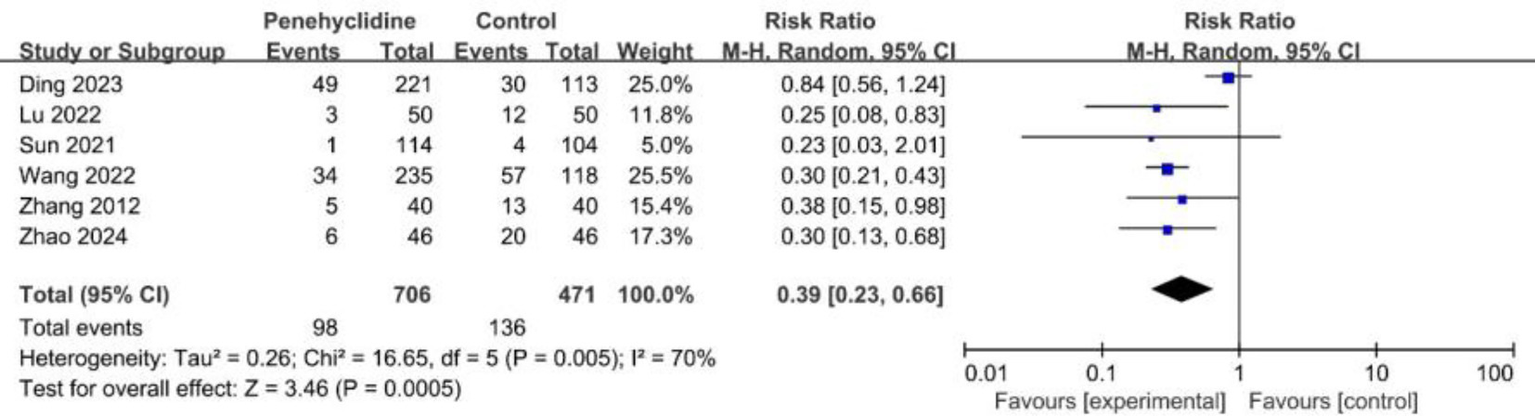
Forest plot of the incidence of required rescue antiemetic between penehyclidine and control groups.
Safety outcomes
Six trials evaluated the incidence of dry mouth. The forest plot analysis showed a significantly higher incidence in the PHC group (RR = 2.46, 95% CI [1.75, 3.46], p < 0.05, I2 = 18%, Figure 7), with low heterogeneity among the studies. Three trials reported the incidence of headache, and the forest plot analysis showed no significant difference between two groups (RR = 0.91, 95% CI [0.52, 1.60], p = 0.75, I2 = 0%, Supplementary Figure 8). Also, forest plot analyses showed no significant difference about the incidence of dizziness, urinary retention, fever between two groups (Supplementary Figures 9–11).
Figure 7
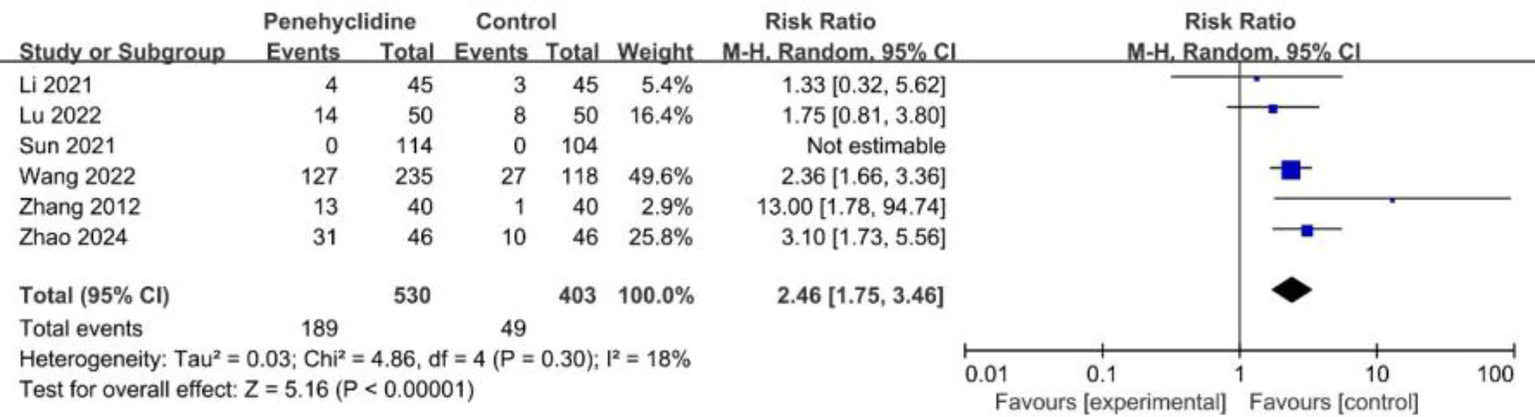
Forest plot of the incidence of dry mouth between penehyclidine and control groups.
PACU stay
Three trials assessed the length of stay in the PACU. The forest plot analysis showed no significant difference between two groups (Supplementary Figure 12).
Publication bias
A funnel plot was generated to visually assess potential publication bias (Supplementary Figure 13). While the plot appears largely asymmetrical, we note that the small number of included studies (n = 10) limits the interpretability of the plot (31).
GRADE result
Table 2 shows the summary of the GRADE assessment.
Table 2
| Outcome | Included studies (n) | Patients (n) | RR/MD | 95% CI | I 2 | Quality of evidence | Reasons |
|---|---|---|---|---|---|---|---|
| Incidence of PONV | 10 | 1,427 | 0.48 | (0.36, 0.65) | 68% | ⨁⨁◯◯ LOW |
“Imprecision” and “Other considerations” were downgraded to “serious.” |
| Incidence of PON | 3 | 526 | 0.59 | (0.35, 0.97) | 46% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| Incidence of POV | 4 | 606 | 0.41 | (0.19, 0.92) | 76% | ⨁⨁◯◯ LOW |
“Imprecision” and “Other considerations” was downgraded to “serious.” |
| Incidence of severe PONV | 4 | 1,005 | 0.50 | (0.34, 0.74) | 13% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| Incidence of rescue antiemetic | 6 | 1,177 | 0.39 | (0.23, 0.66) | 70% | ⨁⨁◯◯ LOW |
“Imprecision” and “Other considerations” were downgraded to “serious.” |
| Incidence of dry mouth | 6 | 933 | 2.46 | (1.75, 3.46) | 18% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| Incidence of headache | 3 | 272 | 0.91 | (0.52, 1.60) | 0% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| Incidence of dizziness | 4 | 635 | 1.10 | (0.72, 1.67) | 0% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| Incidence of urinary retention | 2 | 445 | 0.79 | (0.25, 2.49) | 0% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| Incidence of fever | 2 | 445 | 0.90 | (0.76, 1.07) | 0% | ⨁⨁⨁◯ MODERATE |
“Other considerations” was downgraded to “serious.” |
| PACU stay | 3 | 644 | 1.45 | (−2.62, 5.51) | 0% | ⨁⨁◯◯ LOW |
“Inconsistency” and “Other considerations” were downgraded to “serious.” |
Summary for GRADE assessment.
PONV, postoperative nausea and vomiting; PON, postoperative nausea; POV, postoperative vomiting; PACU, post-anesthesia care unit; RR, risk ratio; MD, mean difference; CI, confidence interval.
TSA result
The TSA for the primary outcome (incidence of PONV) is presented in Figure 8. The cumulative Z-curve crossed both the conventional significance boundary, the TSA monitoring boundary, and the RIS line. This indicates that the current evidence is might sufficient to conclude that PHC significantly reduces the incidence of PONV, and the risk of a false positive result is low.
Figure 8
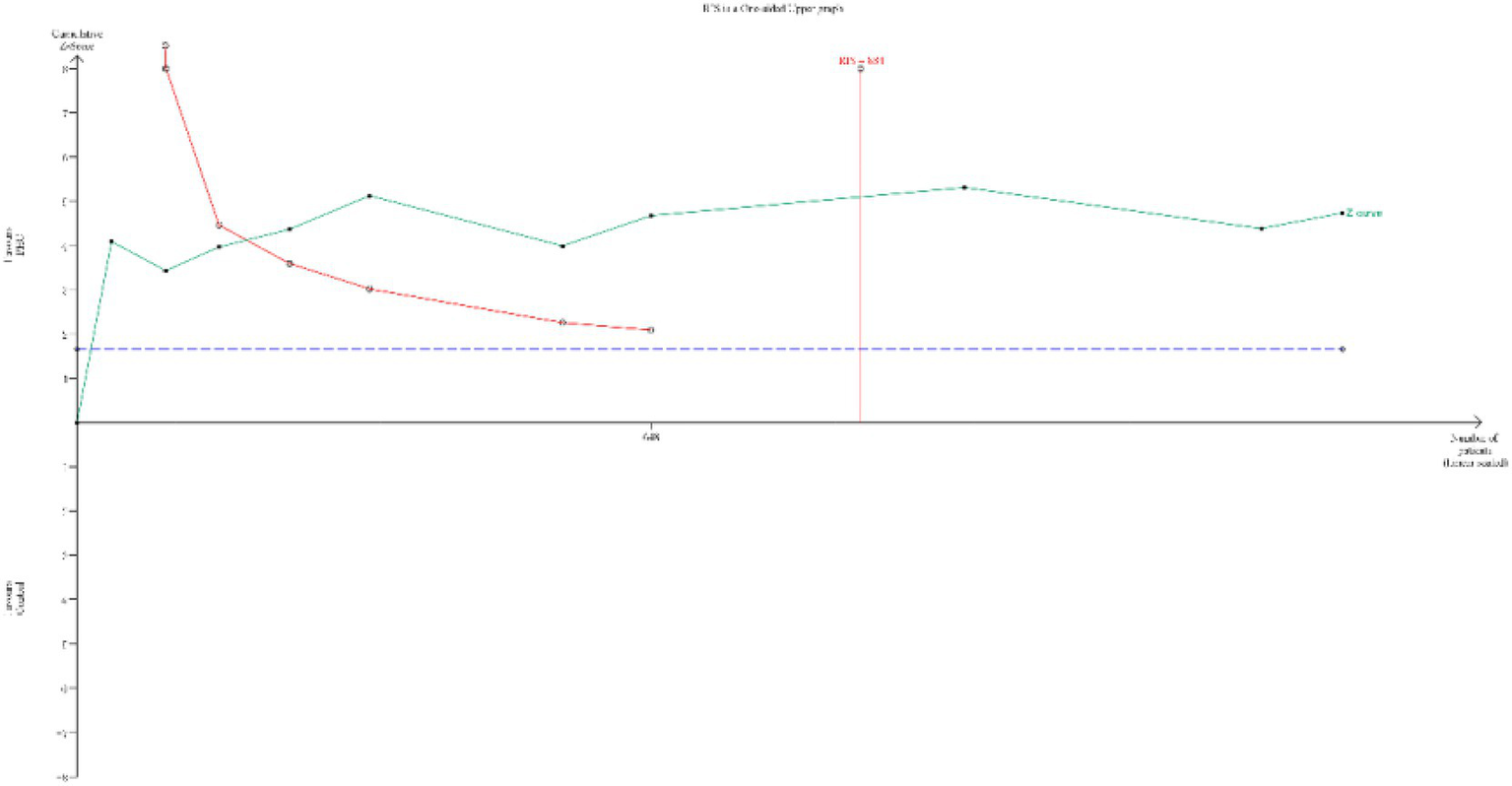
TSA for the incidence of PONV. (TSA, trial sequential analysis; PONV, postoperative nausea and vomiting) The cumulative Z-curve (green line) crossed the conventional test boundary (purple dotted line), TSA monitoring boundary (left red line) and required information size (right red line), which indicated that the evidence is sufficient.
Discussion
To our knowledge, this meta-analysis is the first to examine both the safety and efficacy of PHC for preventing PONV after general anesthesia. Our results indicated PHC significantly reduced the incidence of PONV, as well as PON, POV, and severe PONV.
In our meta-analysis, we found that PHC significantly reduced the incidence of PONV, aligning with previous studies that explored PHC’s efficacy in reducing PONV in patients undergoing various types of surgery. For example, studies by Wang et al. (17) and Zhao et al. (26) also demonstrated that PHC effectively decreased PONV in patients undergoing gynecological and bariatric surgeries. Our pooled analysis of ten RCTs involving 1,427 participants provides a more robust evaluation of PHC’s effectiveness by integrating data from multiple settings and surgery types.
However, we observed differences in the results when comparing our findings to those of Ding et al. (19), who reported that PHC did not significantly reduce the incidence or severity of PONV in laparoscopic bariatric surgery patients. Also, this trial introduced significant heterogeneity into the results of this study. In the study, they enrolled patients who underwent laparoscopic bariatric surgery. The BMI was 38 (7) in both control group and PHC group. Several factors may account for this outcome. First, the trial included obese patients with high vagal tone, and the dosage of PHC administered may have been insufficient to effectively inhibit the activation of enteric vagus nerve afferent pathways (32). Second, the pharmacokinetics of PHC in obese patients may differ due to variations in drug distribution volume, which warrants further investigation (33). Third, during laparoscopic bariatric surgery, gastric denervation might occur, potentially diminishing the efficacy of PHC in alleviating gastrointestinal smooth muscle spasms.
The use of opioids and inhaled anesthetics during the perioperative period is strongly linked to a higher incidence of PONV (3). Opioid-induced PONV is typically dose-dependent (34, 35), and the use of opioids for postoperative analgesia prolongs the duration of PONV (36). Volatile anesthetics are a major cause of PONV in the early postoperative period (within 6 h), exhibiting a dose-dependent effect (37). Previous studies have suggested that various interventions can help reduce the incidence of PONV, including total intravenous anesthesia (21), opioid-free general anesthesia (38), and the combination of general anesthesia with regional anesthesia (39).
In our meta-analysis, the pooled RR for PONV with PHC prophylaxis was 0.48, indicating a statistically significant reduction. Also, the result of subgroup analysis indicated that PHC’s efficacy persisted. An interesting pharmacological interaction worth noting is that PHC, as an anticholinergic agent, might not only prevent PONV directly but also counteract the nausea-inducing effect of neostigmine, which is commonly used to reverse neuromuscular blockade. This potential dual mechanism could partly explain its efficacy, but our analysis could not definitively separate this effect due to insufficient reporting of neostigmine use. Currently, 5-HT3 receptor antagonists are the most frequently used medications for preventing PONV (8). However, the risk of QT interval prolongation linked to 5-HT3 receptor antagonists is gaining heightened scrutiny (40). PHC is commonly administered via intravenous injection or intramuscular injection. It has a half-life of approximately 10 h and is typically given as a single perioperative dose. Currently, there is no available oral formulation of PHC (15). The prolonged action of PHC permits its once-only administration during surgery, which may help reduce the workload of nursing staff and simplify perioperative antiemetic protocols. In contrast, 5-HT₃ antagonists, particularly newer agents like palonosetron, tend to be more costly. Furthermore, the need for repeated dose or additional rescue antiemetics may increase the overall cost burden of traditional treatments. Limited pharmacoeconomic evaluations suggest that PHC may offer a favorable cost profile, especially in resource-limited settings. However, further direct cost-comparison studies are warranted to validate these findings.
The pathophysiological mechanism of PONV is closely related to muscarinic receptors (41). The vestibular system has many M1 receptors, and anticholinergics inhibit cholinergic transmission between the vestibular nuclei and the central nervous system, as well as between the medullary reticular formation and the vomiting center (42). Studies have indicated that M3 and M5 acetylcholine receptors may help reduce the risk of PONV by mitigating motion sickness (43). PHC is a new long-acting anticholinergic, exhibits both anti-muscarinic and anti-nicotine properties, providing robust central and peripheral anticholinergic effects (44). It shows strong selectivity for the muscarinic M1 and M3 subtypes of acetylcholine receptors (15). Given its pharmacological profile, PHC has been increasingly investigated for PONV prevention, with prior studies demonstrating promising results (17, 21). However, strong comprehensive evidence is still lacking. Furthermore, PHC is relatively inexpensive and requires only single-dose administration, which may improve cost-effectiveness and compliance in clinical settings. Given these factors, we believe that the exploration of PHC as either an adjunct or alternative to existing PONV prevention strategies is clinically and scientifically justified.
Furthermore, our analysis provides a comprehensive overview of the safety profile of penehyclidine. The most commonly reported adverse event, dry mouth, was predictable based on its anticholinergic mechanism and was generally mild and self-limiting. When directly compared to standard antiemetics based on our study, PHC demonstrates comparable efficacy to first-line agents like ondansetron and dexamethasone in reducing PONV incidence. This favorable benefit–risk balance, where significant PONV reduction outweighs manageable side effects, supports a dual role for PHC within a multimodal PONV prophylaxis strategy. It can serve not only as a viable alternative for patients who are intolerant or have contraindications to conventional antiemetics but also as a potent additive component, potentially enhancing efficacy when combined with other agents through its different mechanism of action.
One notable limitation of this meta-analysis is that all included studies were conducted in China. This geographic concentration may limit the generalizability of our findings to other populations. Ethnic and genetic variability may influence the pharmacokinetics and pharmacodynamics of PHC. For instance, variations in cytochrome P450 enzyme expression or muscarinic receptor polymorphisms across different populations could potentially impact drug metabolism, efficacy, and safety profiles (45). Therefore, the TSA suggested the evidence may be sufficient, but the geographical concentration and methodological limitations of the included trials indicate that larger, more rigorous multi-center trials, particularly outside of China, would be beneficial to confirm these findings and enhance their generalizability.
It is important to note that PHC is not currently approved by the FDA for the prevention of PONV. While our findings suggest that PHC may be a promising agent for the prevention of PONV, the current body of evidence remains limited in scope and geographic diversity. For countries where it is not yet approved, this study positions PHC as a promising candidate for broader clinical evaluation and formulary inclusion. Its proven efficacy and acceptable safety profile suggest that it could valuably expand the armamentarium against PONV, particularly for high-risk patients or in settings where existing options are limited or ineffective. Future head-to-head trials against established antiemetics and cost-effectiveness analyses would be invaluable to further solidify its global role.
This meta-analysis has several limitations that need to be addressed. First, despite including 1,427 participants, only ten eligible trials were analyzed, resulting in a relatively small sample size. Second, all included studies were conducted in China, which may limit the generalizability of the findings to populations of different racial or ethnic backgrounds. Third, insufficient data prevented us from performing subgroup analyses for various types of surgeries and patient characteristics. Fourth, our meta-analysis has analyzed only some adverse events; however, comprehensive data on other potential side effects (such as blurred vision and heart rate changes) were not consistently reported.
Conclusion
In conclusion, the findings of this meta-analysis suggest that PHC may have potential in reducing the incidence of PONV. There is a clear need for further high-quality, multicenter RCTs, ideally conducted across diverse patient populations and in alignment with international regulatory standards. Such studies are essential to confirm the efficacy and safety of PHC for PONV prevention and to determine its potential role in routine clinical practice.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
J-CL: Writing – review & editing, Conceptualization, Writing – original draft. YC: Writing – review & editing, Formal analysis, Data curation. LL: Methodology, Formal analysis, Data curation, Writing – review & editing. Q-hS: Funding acquisition, Writing – original draft, Formal analysis, Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Jiaxing Supporting Disciplines - Anesthesiology (2023-ZC-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1676087/full#supplementary-material
References
1.
Apfel CC Läärä E Koivuranta M Greim CA Roewer N . A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. (1999) 91:693–700. doi: 10.1097/00000542-199909000-00022
2.
Apfel CC Korttila K Abdalla M Kerger H Turan A Vedder I et al . A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. (2004) 350:2441–51. doi: 10.1056/NEJMoa032196
3.
Gan TJ Belani KG Bergese S Chung F Diemunsch P Habib AS et al . Fourth consensus guidelines for the Management of Postoperative Nausea and Vomiting. Anesth Analg. (2020) 131:411–48. doi: 10.1213/ANE.0000000000004833
4.
Horn CC Wallisch WJ Homanics GE Williams JP . Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol. (2014) 722:55–66. doi: 10.1016/j.ejphar.2013.10.037
5.
Nielsen M Olsen NV . Genetic polymorphisms in the cytochrome P450 system and efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Br J Anaesth. (2008) 101:441–5. doi: 10.1093/bja/aen246
6.
Janicki PK Vealey R Liu J Escajeda J Postula M Welker K . Genome-wide association study using pooled DNA to identify candidate markers mediating susceptibility to postoperative nausea and vomiting. Anesthesiology. (2011) 115:54–64. doi: 10.1097/ALN.0b013e31821810c7
7.
Klenke S de Vries GJ Schiefer L Seyffert N Bachmann HS Peters J et al . CHRM3 rs2165870 polymorphism is independently associated with postoperative nausea and vomiting, but combined prophylaxis is effective. Br J Anaesth. (2018) 121:58–65. doi: 10.1016/j.bja.2018.02.025
8.
Bunce KT Tyers MB . The role of 5-HT in postoperative nausea and vomiting. Br J Anaesth. (1992) 69:60s–2s. doi: 10.1093/bja/69.supplement_1.60S
9.
Jin Z Daksla N Gan TJ . Neurokinin-1 antagonists for postoperative nausea and vomiting. Drugs. (2021) 81:1171–9. doi: 10.1007/s40265-021-01532-y
10.
Wolfe RC Bequette J . Dopamine receptor antagonists for the prevention and treatment of postoperative nausea and vomiting. J Perianesth Nurs. (2021) 36:199–202. doi: 10.1016/j.jopan.2020.12.009
11.
Wu L Si H Li M Zeng Y Wu Y Liu Y et al . The optimal dosage, route and timing of glucocorticoids administration for improving knee function, pain and inflammation in primary total knee arthroplasty: a systematic review and network meta-analysis of 34 randomized trials. International journal of surgery (London, England). (2020) 82:182–91. doi: 10.1016/j.ijsu.2020.07.065
12.
Chhibber AK Lustik SJ Thakur R Francisco DR Fickling KB . Effects of anticholinergics on postoperative vomiting, recovery, and hospital stay in children undergoing tonsillectomy with or without adenoidectomy. Anesthesiology. (1999) 90:697–700. doi: 10.1097/00000542-199903000-00010
13.
Yan S Xu M Zou X Xiong Z Li H Yang J et al . Acupuncture combined with ondansetron for prevention of postoperative nausea and vomiting in high-risk patients undergoing laparoscopic gynaecological surgery: a randomised controlled trial. United European Gastroenterol J. (2023) 11:564–75. doi: 10.1002/ueg2.12421
14.
Ernst E Pittler MH . Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. (2000) 84:367–71. doi: 10.1093/oxfordjournals.bja.a013442
15.
Wang Y Gao Y Ma J . Pleiotropic effects and pharmacological properties of penehyclidine hydrochloride. Drug Des Devel Ther. (2018) 12:3289–99. doi: 10.2147/DDDT.S177435
16.
Griffiths JD Gyte GM Popham PA Williams K Paranjothy S Broughton HK et al . Interventions for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section. Cochrane Database Syst Rev. (2021) 5:Cd007579. doi: 10.1002/14651858.CD007579.pub3
17.
Wang LK Cheng T Yang XD Xiong GL Li N Wang DX . Penehyclidine for prevention of postoperative nausea and vomiting following bimaxillary orthognathic surgery: a randomized, double-blind, controlled trial. J Anesth. (2022) 36:122–36. doi: 10.1007/s00540-021-03017-4
18.
Zhang Z Zhuang Y Ouyang F Zhang A Zeng B Gu M . Penehyclidine enhances the efficacy of tropisetron in prevention of PONV following gynecological laparoscopic surgery. J Anesth. (2012) 26:864–9. doi: 10.1007/s00540-012-1443-1
19.
Ding X Chen D Che J Xu S Liang H Gui B . Penehyclidine hydrochloride for treating postoperative nausea and vomiting after laparoscopic bariatric surgery: a double-blinded randomized controlled trial. BMC Anesthesiol. (2023) 23:135. doi: 10.1186/s12871-023-02078-0
20.
Sun J Cao X Lu T Li N Min X Ding Z . Penehyclidine mitigates postoperative nausea and vomiting and intraoperative oculocardiac reflex in patients undergoing strabismus surgery: a prospective, randomized, double-blind comparison. BMC Anesthesiol. (2021) 21:49. doi: 10.1186/s12871-021-01266-0
21.
Lu T Li R Sun J Chen J . Evaluation of penehyclidine for prevention of post operative nausea and vomitting in patients undergoing total thyroidectomy under total intravenous anaesthesia with propofol-remifentanil. BMC Anesthesiol. (2022) 22:317. doi: 10.1186/s12871-022-01857-5
22.
Luo D Wan X Liu J Tong T . Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
23.
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
24.
Wetterslev J Jakobsen JC Gluud C . Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. (2017) 17:39. doi: 10.1186/s12874-017-0315-7
25.
Brok J Thorlund K Gluud C Wetterslev J . Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. (2008) 61:763–9. doi: 10.1016/j.jclinepi.2007.10.007
26.
Zhao K Gao Y Zhang J Wang S Chen J Guo F et al . Penehyclidine for prevention of postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery under combined intravenous and inhalation anesthesia: a randomized, double-blind, placebo-controlled trial. Drug Design Develop Therapy. (2024) 18:685–97. doi: 10.2147/DDDT.S453327
27.
Li RD Hongquan Liu C Ding Z Chen J . Effect of penehyclidine hydrochloride on preventing postoperative nausea and vomiting after thyroid surgery. J Nanjing Med Univ. (2021) 41:609–11.
28.
Wang LH Bo L Li Q Cao Y Zhang Y Fang Y et al . The effect of penehyclidine hydrochloride on postoperative nausea and vomiting after microvascular decompression stereotactic and functional neurosurgery. J. Anesthesia (2008) 21:368–9.
29.
Yang CF Lijuan . The prophylactic effect of penehyclidine hydrochloride against postoperative nausea and vomiting. Sichuan Yi Xue Yuan Xue Bao. (2011) 32:1277–8.
30.
Zhang ZO Yangfan Liu Q Li S . Effect of penehyclidine hydrochloride in the prevention of nausea and vomiting after laparoscopic cholecystectomy. Hainan Yi Xue Yuan Xue Bao. (2010) 21:33–4.
31.
Sterne JA Sutton AJ Ioannidis JP Terrin N Jones DR Lau J et al . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed). (2011) 343:d4002. doi: 10.1136/bmj.d4002
32.
Geronikolou SA Albanopoulos K Chrousos G Cokkinos D . Evaluating the homeostasis assessment model insulin resistance and the cardiac autonomic system in bariatric surgery patients: a Meta-analysis. Adv Exp Med Biol. (2017) 988:249–59.
33.
Gouju J Legeay S . Pharmacokinetics of obese adults: not only an increase in weight. Biomed pharmaco. (2023) 166:115281. doi: 10.1016/j.biopha.2023.115281
34.
Hong D Flood P Diaz G . The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg. (2008) 107:1384–9. doi: 10.1213/ane.0b013e3181823efb
35.
Roberts GW Bekker TB Carlsen HH Moffatt CH Slattery PJ McClure AF . Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. (2005) 101:1343–8. doi: 10.1213/01.ANE.0000180204.64588.EC
36.
Apfel CC Philip BK Cakmakkaya OS Shilling A Shi YY Leslie JB et al . Who is at risk for postdischarge nausea and vomiting after ambulatory surgery?Anesthesiology. (2012) 117:475–86. doi: 10.1097/ALN.0b013e318267ef31
37.
Apfel CC Kranke P Katz MH Goepfert C Papenfuss T Rauch S et al . Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. (2002) 88:659–68. doi: 10.1093/bja/88.5.659
38.
Frauenknecht J Kirkham KR Jacot-Guillarmod A Albrecht E . Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. (2019) 74:651–62. doi: 10.1111/anae.14582
39.
Liu MJ Zhou XY Yao YB Shen X Wang R Shen QH . Postoperative analgesic efficacy of erector Spinae plane block in patients undergoing lumbar spinal surgery: a systematic review and Meta-analysis. Pain Ther. (2021) 10:333–47. doi: 10.1007/s40122-021-00256-x
40.
Quraishi SA Schuler GH Janicki PK . 5 HT(3)-receptor antagonists and cardiac repolarization time in patients expressing a novel genetic target associated with baseline QTc interval abnormalities. J Clin Anesth. (2011) 23:297–302. doi: 10.1016/j.jclinane.2010.11.003
41.
Dewinter G Teunkens A Vermeulen K Devroe S Van Hemelrijck J Meuleman C et al . Alizapride and ondansetron for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic gynaecological surgery: a double-blind, randomised, placebo-controlled noninferiority study. Eur J Anaesthesiol. (2016) 33:96–103. doi: 10.1097/EJA.0000000000000288
42.
Klenke S Frey UH . Genetic variability in postoperative nausea and vomiting: a systematic review. Eur J Anaesthesiol. (2020) 37:959–68. doi: 10.1097/EJA.0000000000001224
43.
Golding JF Stott JR . Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. Br J Clin Pharmacol. (1997) 43:633–7. doi: 10.1046/j.1365-2125.1997.00606.x
44.
Han XY Liu H Liu CH Wu B Chen LF Zhong BH et al . Synthesis of the optical isomers of a new anticholinergic drug, penehyclidine hydrochloride (8018). Bioorg Med Chem Lett. (2005) 15:1979–82. doi: 10.1016/j.bmcl.2005.02.071
45.
Mokhosoev IM Astakhov DV Terentiev AA Moldogazieva NT . Human cytochrome P450 cancer-related metabolic activities and gene polymorphisms: a review. Cells. (2024) 13:1958. doi: 10.3390/cells13231958
Summary
Keywords
penehyclidine, nausea and vomiting, meta-analysis, PONV, POV
Citation
Lu J-C, Chen Y, Lai L and Shen Q-h (2025) Penehyclidine in prevention of postoperative nausea and vomiting: a systematic review and meta-analysis of randomized controlled trials. Front. Med. 12:1676087. doi: 10.3389/fmed.2025.1676087
Received
05 August 2025
Accepted
17 September 2025
Published
30 September 2025
Volume
12 - 2025
Edited by
Redhwan Ahmed Al-Naggar, National University of Malaysia, Malaysia
Reviewed by
Hong Fu, Chongqing Emergency Medical Center, China
Theogene Twagirumugabe, University of Rwanda, Rwanda
Updates
Copyright
© 2025 Lu, Chen, Lai and Shen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-hong Shen, shenqihong1989@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.