- 1Department of Pulmonary and Critical Care Medicine, Xijing Hospital, Air Force Medical University, Xi'an, China
- 2School of Biomedical Engineering, Guangzhou Medical University, Guangzhou, China
- 3Department of Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
Objective: Preserved ratio impaired spirometry (PRISm) is a condition characterized by abnormal spirometry results despite a normal forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio. We hypothesized that electrical impedance tomography (EIT) would detect regional decreases in lung function in subjects with PRISm. The study aimed to explore the regional lung function differences between subjects with preserved ratio impaired spirometry (PRISm) and healthy volunteers.
Methods: In this observational study, subjects with respiratory symptoms visiting Xijing Hospital in May 2024 were rescreened for eligibility. Subjects meeting the PRISm criteria were included in the study. Electrical impedance tomography (EIT) and spirometry were conducted simultaneously. The same number of healthy subjects were studied, matching the gender, height, and weight in frequency. The regional lung function parameters were compared. A P-value of < 0.05 was considered statistically significant.
Results: A total of 16 subjects with PRISm, and 16 healthy volunteers were included in the study and evaluated. Functional EIT images provided information about the location of the dysfunctional lung regions. All evaluated EIT-based parameters were worse in the subjects with PRISm. Statistically significant differences were found in the regional obstructive ratio (rOR) and the median of the regional time constant τmed (p < 0.05). EIT-based parameters did not correlate with spirometry-based parameters, except for spirometry FEV1/FVC, which showed correlations with rOR (r = −0.53, p = 0.03) and τmed (r = −0.62, p = 0.01) in subjects with PRISm. Both τmed and rOR values were quite low in healthy volunteers, whereas substantial changes were observed in subjects with PRISm.
Conclusion: EIT-based regional lung function provides additional information to traditional spirometry, which visualizes and localizes regional lung function defects.
Introduction
Preserved ratio impaired spirometry (PRISm) is a condition characterized by abnormal spirometry results despite a normal FEV1/FVC ratio (the ratio of the forced expiratory volume in the first second to the forced vital capacity of the lungs). This condition is highly prevalent in several large cross-sectional and longitudinal studies (estimated at 5% to 20% globally) (1), with approximately 10%−40% of patients with PRISm tending to develop chronic obstructive pulmonary disease (COPD) (2), but no tools exist to predict who will deteriorate.
PRISm involves respiratory symptoms and reduced quality of life, despite normal spirometry values at early stages. This means that individuals with PRISm may not be classified as having impaired lung function based on traditional spirometry measures, which rely on the FEV1/FVC ratio and/or FEV1 predicted values. However, previous studies have found an association between decline in quality of life and onset of frequent exacerbations in subjects with normal or PRISm spirometry (3). The pathophysiology of PRISm is not fully understood (1). It may involve abnormalities in lung structure and function that are not detected by traditional spirometry. These abnormalities may include airway inflammation, small airway disease, and/or emphysema. These changes may lead to reduced lung function and increased susceptibility to respiratory infections and exacerbations.
Current treatment for PRISm is limited and often based on symptom management. Because the condition is not well-understood, there is a lack of specific therapies targeted at the underlying pathophysiology. However, lifestyle modifications such as smoking cessation, exercise, and weight management may be beneficial in improving lung function and reducing symptoms. Additionally, inhaled bronchodilators and corticosteroids may be prescribed to help manage symptoms and reduce exacerbations.
Given the high prevalence of PRISm and its association with increased risk of developing COPD, there is a need for more sensitive methods to identify individuals with impaired lung function. Spirometry cannot localize dysfunction, while CT lacks functional data (4). Research is ongoing to develop new diagnostic tools and therapies that can better address the underlying pathophysiology of PRISm and improve outcomes for patients with this condition.
Electrical impedance tomography (EIT) is a non-invasive imaging modality to assess spatial and temporal ventilation distribution (5). A flexible belt with 16–32 electrodes is placed onto the chest. Insensible alternating currents are injected between electrode pairs. Subsequently, the surface voltages are measured and used to reconstruct the electrical impedance changes within the thorax. Lung volume changes are highly correlated with impedance changes (6). Chest EIT applications are mainly in the field of intensive care unit (ICU) [e.g., (7, 8)]. However, since flow is the derivative of volume changes, regional vital capacity and airway flow limitations can be assessed by EIT as well. EIT provides regional lung function information that cannot be captured by other techniques. Such information has been used to demonstrate differences between healthy subjects and patients with asthma (9), COPD (10), cystic fibrosis (11), amyotrophic lateral sclerosis (12), etc. Its application to PRISm, however, has not been well-studied. We hypothesized that EIT would detect regional decreases in lung function in PRISm subjects. The primary aim of this study was to determine whether EIT can characterize distinct patterns of regional lung dysfunction in subjects with PRISm that are not apparent from traditional spirometry. To address this aim, we pursued the following specific objectives:
To compare EIT-derived parameters of regional lung function between PRISm subjects and healthy volunteers.
To visualize the spatial distribution of pulmonary dysfunction in PRISm subjects through functional EIT imaging to identify common patterns.
Methods
This cross-sectional study was approved by the local ethics committee (No. KY20242044). Informed consent was signed by the subjects before the procedure. In May 2024, subjects with respiratory symptoms who visited our hospital were prospectively screened. Pulmonary Function Test (PFT) was conducted using MasterScreen (Jaeger, CareFusion GmbH, Hoechberg, Germany) according to the American Thoracic Society - European Respiratory Society (ATS-ERS) guidelines (13). EIT was conducted simultaneously with the FVC maneuver (VenTom-200, MidasMED Biomedical Technology, Shenzhen, China). A belt with 16 equidistantly fixed electrodes was placed around the chest in one transverse plane at the level of the 5th intercostal spaces at the parasternal line. For female subjects, if the 5th intercostal space was not accessible, the electrode belt was placed above the breast (~4th intercostal space) (14). Further details of the measurement procedure were described previously (15). The inclusion criteria were: individuals aged between 20 and 80 years; individuals with FEV1/FVC >0.7 after bronchodilator reversibility; individuals with FEV1 % predicted < 80%; and individuals willing to participate in the study. The exclusion criteria were: in hospitalization; malignant tumors, autoimmune diseases, systemic infectious diseases, and dysfunction of vital organs; pleural diseases or chest deformities; any contraindications to pulmonary function test (FVC maneuver); or EIT measurement (e.g., pacemaker, automatic implantable cardioverter defibrillator, and implantable pumps). The same number of healthy subjects were studied, matched by gender, height, and weight frequency. Inclusion criteria for healthy volunteers were: individuals aged between 20 and 80 years; no respiratory symptoms; FEV1/FVC >0.7 and FEV1 % predicted >80%; and willingness to participate in the study.
Subjects' demographics and history of smoking were recorded. EIT data were recorded at 20 Hz and reconstructed with reference to the lowest point of impedance. Regional FEV1 and FVC were calculated for each pixel within the lung areas (16). A functional EIT image was constructed as pixel-wise FEV1, FVC, FEV1/FVC, forced inspiratory vital capacity [FIVC defined in ATS/ERS 2019 (13)], mean flow rate 25 to 75% (MEF between 25 and 75% of FVC), T75 (time required for exhaling 75% of FVC), and time constant (which is described in detail in the next paragraph).
After defining the obstructed pixels (Equation 1), “regional obstructive ratio” rOR was calculated based on the size of obstructed regions in relation to the entire lung area (Equations 2, 3).
The rOR represents the area where FEV1/FVC is smaller than 0.7. The threshold “0.7” was selected according to the definition of COPD. The higher the value, the more area shows an airway obstruction pattern. In addition, similar to the global inhomogeneity (GI) index proposed in a previous study (17), the GI of functional FEV1 and FEV1/FVC images was calculated (Equations 4).
where the lung was the area identified as described previously (18). FEV1 could be substituted with pixel-wise FEV1/FVC. The higher the GI value, the more inhomogeneous the functional image.
Furthermore, time constant map was calculated, as described in previous studies (19–21). In brief, for every pixel within the lung region, the regional time constant was calculated by fitting the following exponential equation:
where Z(t) is the relative impedance for a pixel within the lung at time point t, Z0 is the impedance at the start of expiration, t represents the time from the end-inspiration to the end-expiration, τ denotes the regional time constant, and c the end-expiratory volume. Patients with obstructive lung disease require a longer time to empty the lungs, which thus leads to a longer time constant. Instead of calculating it for every tidal breath as proposed earlier, the time constant map (functional EIT with pixels showing τ) was calculated from the FVC data in the present study. The median of regional τ (τmed) and interquartile range (τiqr) were calculated.
The data processing and statistical analysis were conducted using MATLAB R2023a (The MathWorks Inc., Natick, USA). The Lilliefors test was used for normality testing. Normally distributed data were expressed as mean ± standard deviation. An unpaired Student's t-test was used to compare the differences between spirometry- and EIT-based parameters between healthy subjects and PRISm subjects. For non-normally distribution, data were expressed as median (interquartile range). A two-sided Wilcoxon rank-sum test was used instead. Pearson's linear correlation coefficients were calculated between EIT-based parameters and spirometry-based parameters. A p-value of < 0.05 was considered statistically significant.
Results
A total of 21 subjects were screened, and 16 matched the PRISm definition. Subjects' demographics and their lung function measured with spirometry are summarized in Table 1. Figures 1 and 2 show the various functional EIT images visualizing the lung function parameters in one healthy subject (Figure 1) and one PRISm subject (Figure 2). The levels of FEV1, FVC, and FIVC were similar in both lungs in the healthy volunteers (Figures 1A, B and F). The ratio of FEV1/FVC was very close to 1 (as indicated in green in Figure 1C). T75 and the time constant were quite low (Figure 1E and G) and mainly distributed approximately 0.4 s (Figure 1H). On the contrary, the subject with PRISm had flow limitation mainly in his left lung, as indicated by a decrease in FEV1 (Figure 2A), FVC (Figure 2B), FEV1/FVC (Figure 2C), MEF25-75 (Figure 2D), and FIVC (Figure 2F), and an increase in T75 (Figure 2E) and time constant (Figure 2G). To be specific, the main pulmonary dysfunction was located in the left dorsal region, as highlighted in Figures 2C, E and G.
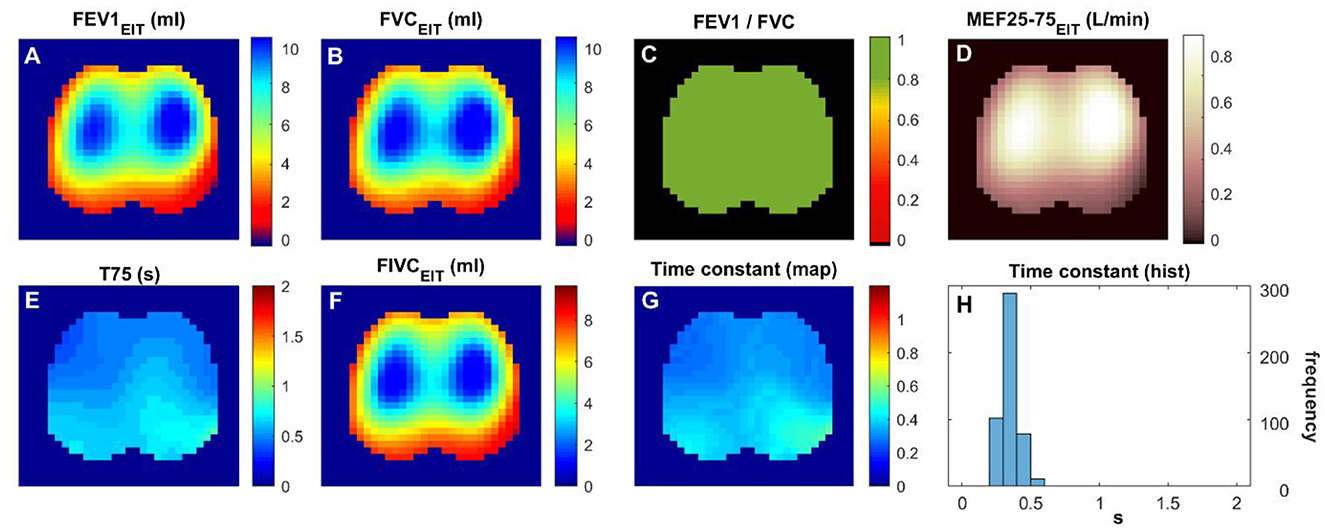
Figure 1. Functional EIT images visualizing the regional lung function of a healthy volunteer. (A–H) FEV1/FVC: the ratio of forced expiratory volume in the first second to forced vital capacity. Time constant: time related to the exponential decay of lung volume during expiration. The values in the pixels are indicated by the color bar on the right side of the sub-figures. FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; FIVC, forced inspiratory vital capacity; MEF 25–75, mean flow rate between 25 and 75% of FVC; T75, time required for exhaling 75% of FVC.
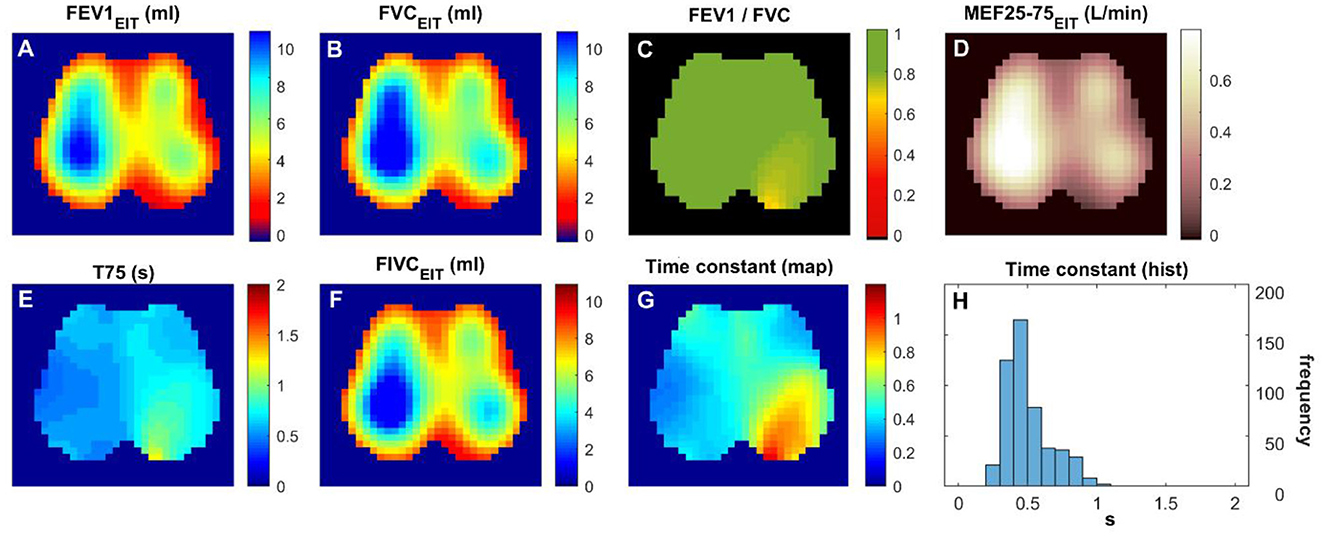
Figure 2. Functional EIT images visualizing the regional lung function of a subject with PRISm. (A–H) FEV1/FVC: the ratio of forced expiratory volume in the first second to forced vital capacity. Time constant: time related to the exponential decay of lung volume during expiration. The values in the pixels are indicated by the color bar on the right side of the sub-figures. FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; FIVC, forced inspiratory vital capacity; MEF 25–75, mean flow rate between 25 and 75% of FVC; T75, time required for exhaling 75% of FVC.
The EIT-based parameters were compared and summarized in Table 2. All the evaluated parameters were worse in the subjects with PRISm; however, statistical significances were only found in three parameters (i.e., rOR, τmed, and τiqr). EIT-based parameters were not correlated with spirometry-based parameters, except spirometry FEV1/FVC with rOR (r = −0.53, p = 0.03) and with τmed (r = −0.62, p = 0.01) in subjects with PRISm. Spirometry FEV1/FVC against τmed and rOR is plotted in Figure 3. In healthy volunteers, τmed and rOR values were quite low, and, therefore, not statistically correlated with spirometry parameters.
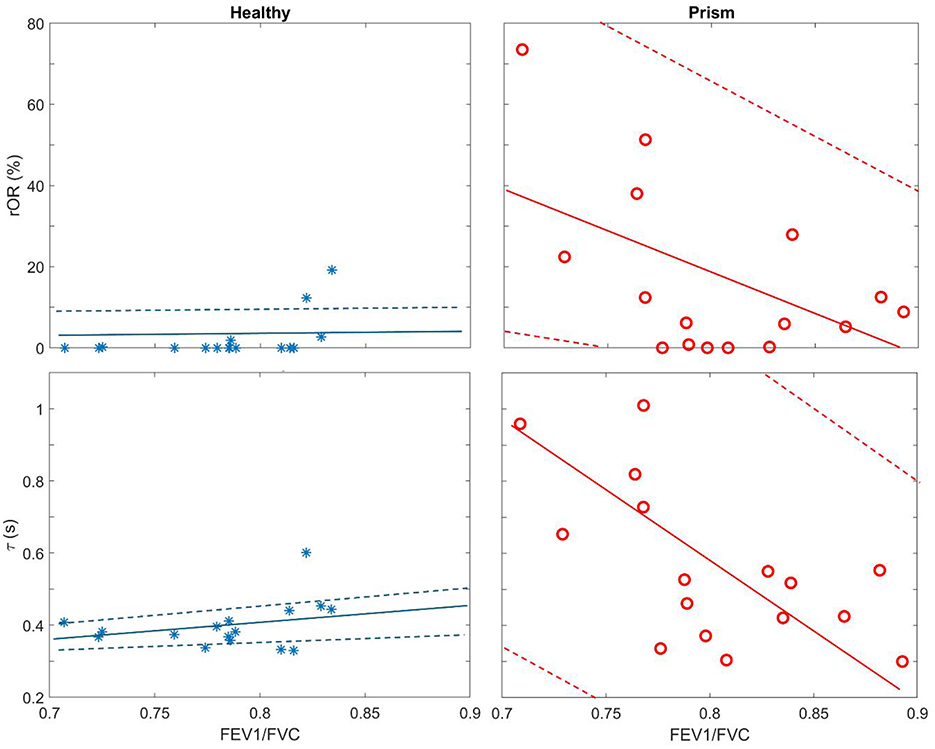
Figure 3. Scatter plots of regional obstructive ratio (rOR) against spirometry FEV1/FVC (Top row) and median of regional time constant (τmed) against spirometry FEV1/FVC (Bottom row). Blue stars, healthy volunteers; red circles, subjects with PRISm. Solid lines are the regression lines, and dashed lines are confidence intervals.
Discussion
In the present study, we demonstrated the ability of EIT-based regional lung function parameters to assess lung status in subjects with PRISm. Regional lung function differed between healthy subjects and those with PRISm. Given the small variation among healthy subjects and the full range observed in PRISm, EIT-based parameters might be more specific in detecting physiological abnormalities compared to traditional spirometry, allowing visualization and localization of the dysfunction areas. With such information, EIT might stratify PRISm subtypes (e.g., small airway vs. parenchymal disease), which was not explored in the current study. How this regional information may reform clinical practice requires further investigation.
In previous studies, regional lung function was found to be different between healthy subjects and subjects with asthma and COPD (9, 10, 22, 23). We also demonstrated the feasibility of training subjects for respiratory physiotherapy with the visual feedback provided by EIT (24, 25). Since subjects with PRISm are at risk of developing airflow obstruction over time, early and accurate identification of those at risk may help prevent disease progression. As observed in Figure 3, even in healthy volunteers, spirometry-based FEV1/FVC varied from 0.7 to 0.85, which overlaps with the range seen in PRISm. These results suggested that the specificity of spirometry in identifying PRISm might be limited. On the other hand, the variations of the EIT-based rOR and τmed were relatively small in healthy subjects (Figure 3 left) but became large in PRISm (Figure 3 right). One dilemma is that the definition of PRISm is based on spirometry. Therefore, the superiority of specificity in PRISm identification with EIT cannot be verified. Nevertheless, EIT-based regional lung function evaluation provides various visualized functional images (Figures 1, 2) with certain spatial resolution, which helps the clinical users to identify the location of dysfunction. Currently, to the best of our knowledge, no other established techniques could provide similar information at the bedside. Some might argue that computed tomography (CT) or spirometry provides structural and functional information about the respiratory system. Indeed, we could “guess” the lung function and status with the information provided by CT scans, but it is not functional. Spirometry provides global lung function without any regional information. Forced oscillation technique could be one of the promising techniques (26). Although it distinguishes information from large airways to small airways, the location of the dysfunction is not captured.
Two previous studies examined the EIT-based regional lung function in PRISm (27, 28). While Li et al. reported spatial heterogeneity and expiratory time (T-75), they did not calculate time constants, which provide direct physiological insights into lung mechanics (e.g., compliance and resistance) (28). Similarly, Zhao et al. focused on obstructive ratios (rOR) and symptom correlation but omitted temporal parameters entirely (27). In addition, we used frequency matching for gender, height, and weight between PRISm and healthy groups, reducing confounding biases.
All the evaluated EIT-based parameters were worse in the subjects with PRISm than in the healthy subjects (Table 2). Due to the limited number of subjects in each group, only three parameters showed statistical significance. Another cause that led to this result could be the inclusion criteria for the healthy group. Although we have matched the gender, height, and weight, we did not match the age and did not exclude subjects with a smoking history. Age is a known determinant of lung function and could confound the observed differences. The current study did not match the age, which was a limitation. Up to now, no study has investigated the regional lung function in various age groups. It is unknown how age difference impacts the EIT-based regional lung function assessment. Smoking history in the control group may introduce bias. A previous study suggests that even subjects with a >10 pack-year smoking history might not show defects in spirometry, EIT-based regional lung function, or CT assessment (29). Nevertheless, we cannot rule out the potential bias, which might have contributed to statistical insignificance in some parameters. Two subjects in the healthy group showed raised rOR, and one subject showed raised τmed (Figure 3 left), suggesting that subjects with normal spirometry might not be “normal” in EIT. Further confirmation with other techniques, e.g., CT or ultrasound, should be conducted, which was not performed in the current study. The EIT-derived parameters (especially rOR and τmed) are relatively new. Further research in this area is needed to validate and consolidate their clinical relevance.
In addition to the above-mentioned limitations, no sample size was calculated prior to the study. At the time of the study, no literature provided relevant information. In this preliminary pilot study, only a convenience sample was collected, which resulted in low statistical power and some parameters not reaching statistical significance. Nevertheless, this study provides a standpoint for the design of further studies that may demonstrate the clinical benefits when using regional lung function information in PRISm (or suspected PRISm). The association between EIT-based regional lung function and patient-reported outcomes regarding patient symptoms, quality of life, or exacerbation risk would strengthen clinical relevance. Unfortunately, it was not a clinical routine to complete the questionnaires, and we did not include these tools in our study design. We recognize that healthy volunteers may not fully represent the general population in addition to age and smoking history which we acknowledged. Future studies could incorporate population-based controls or inverse probability weighting.
Conclusion
EIT-based regional lung function provides additional information to traditional spirometry, which visualizes and localizes regional lung function defects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the Xijing Hospital of Air Force Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Data curation, Writing – original draft. SQ: Writing – review & editing. MK: Writing – review & editing, Data curation. LH: Data curation, Writing – original draft. XT: Funding acquisition, Conceptualization, Project administration, Supervision, Writing – review & editing. ZZ: Supervision, Methodology, Writing – review & editing, Formal analysis, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was partially supported by the National Natural Science Foundation of China (82470088), the Innovative Project of Universities in Guangdong Province (2023KCXTD026), the Program of ShanXi Province Science Foundation (No. 2023-JC-YB-653), the Xijing Hospital Promoting Project (No. XJZT24CY20), and the Joint Founding Project of Innovation Research Institution Xijing Hospital (LHJJ24YG03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. (2021) 326:2287–98. doi: 10.1001/jama.2021.20939
2. Wijnant SRA, De Roos E, Kavousi M, Stricker BH, Terzikhan N, Lahousse L, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam study. Eur Respir J. (2020) 55:1901217. doi: 10.1183/13993003.01217-2019
3. Parekh TM, Bhatia S, Cherrington A, Kim YI, Lambert A, Iyer A, et al. Factors influencing decline in quality of life in smokers without airflow obstruction: the COPDGene study. Respir Med. (2020) 161:105820. doi: 10.1016/j.rmed.2019.105820
4. Global Initiative for Chronic Obstructive Lung Disease. Global Initiative for Chronic Obstructive Lung Disease-GOLD. (2025). Available online at: https://goldcopd.org/ (Accessed September 30, 2025).
5. Frerichs I, Amato MB, van Kaam AH, Tingay DG, Zhao Z, Grychtol B, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. (2017) 72:83–93. doi: 10.1136/thoraxjnl-2016-208357
6. Frerichs I, Hinz J, Herrmann P, Weisser G, Hahn G, Dudykevych T, et al. Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J Appl Physiol (1985). (2002) 93:660–6. doi: 10.1152/japplphysiol.00081.2002
7. Hsu HJ, Chang HT, Zhao Z, Wang PH, Zhang JH, Chen YS, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve: a randomized trial in moderate to severe ARDS. Physiol Meas. (2021) 42:014002. doi: 10.1088/1361-6579/abd679
8. Becher T, Buchholz V, Hassel D, Meinel T, Schädler D, Frerichs I, et al. Individualization of PEEP and tidal volume in ARDS patients with electrical impedance tomography: a pilot feasibility study. Ann Intensive Care. (2021) 11:89. doi: 10.1186/s13613-021-00877-7
9. Frerichs I, Zhao Z, Becher T, Zabel P, Weiler N, Vogt B. Regional lung function determined by electrical impedance tomography during bronchodilator reversibility testing in patients with asthma. Physiol Meas. (2016) 37:698–712. doi: 10.1088/0967-3334/37/6/698
10. Vogt B, Zhao Z, Zabel P, Weiler N, Frerichs I. Regional lung response to bronchodilator reversibility testing determined by electrical impedance tomography in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. (2016) 311:L8–19. doi: 10.1152/ajplung.00463.2015
11. Zhao Z, Müller-Lisse U, Frerichs I, Fischer R, Möller K. Regional airway obstruction in cystic fibrosis determined by electrical impedance tomography in comparison with high resolution CT. Physiol Meas. (2013) 34:N107–14. doi: 10.1088/0967-3334/34/11/N107
12. Munir B, Murphy EK, Mallick A, Gutierrez H, Zhang F, Smith C, et al. Electrical impedance tomography as a quantitative measure of pulmonary function in ALS patients. Neurology. (2020) 94:1187. doi: 10.1212/WNL.94.15_supplement.1187
13. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update an official American thoracic society and european respiratory society technical statement. Am J Respir Crit Care Med. (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
14. Krueger-Ziolek S, Schullcke B, Kretschmer J, Müller-Lisse U, Möller K, Zhao Z. Positioning of electrode plane systematically influences EIT imaging. Physiol Meas. (2015) 36:1109–18. doi: 10.1088/0967-3334/36/6/1109
15. Yang L, Gao Z, Cao X, Sun S, Wang C, Wang H, et al. Electrical impedance tomography as a bedside assessment tool for COPD treatment during hospitalization. Front Physiol. (2024) 15:1352391. doi: 10.3389/fphys.2024.1352391
16. Vogt B, Pulletz S, Elke G, Zhao Z, Zabel P, Weiler N, et al. Spatial and temporal heterogeneity of regional lung ventilation determined by electrical impedance tomography during pulmonary function testing. J Appl Physiol (1985). (2012). 113:1154-1161. doi: 10.1152/japplphysiol.01630.2011
17. Zhao Z, Moller K, Steinmann D, Frerichs I, Guttmann J. Evaluation of an electrical impedance tomography-based global inhomogeneity index for pulmonary ventilation distribution. Intensive Care Med. (2009) 35:1900–6. doi: 10.1007/s00134-009-1589-y
18. Zhao Z, Steinmann D, Müller-Zivkovic D, Martin J, Frerichs I, Guttmann J, et al. A lung area estimation method for analysis of ventilation inhomogeneity based on electrical impedance tomography. J Xray Sci Technol. (2010) 18:171–82. doi: 10.3233/XST-2010-0252
19. Karagiannidis C, Waldmann AD, Róka PL, Schreiber T, Strassmann S, Windisch W, et al. Regional expiratory time constants in severe respiratory failure estimated by electrical impedance tomography: a feasibility study. Crit Care. (2018) 22:221. doi: 10.1186/s13054-018-2137-3
20. Pikkemaat R, Tenbrock K, Lehmann S, Leonhardt S. Electrical impedance tomography: new diagnostic possibilities using regional time constant maps. Appl Cardiopulm Pathophysiol. (2012) 16:212–25.
21. Strodthoff C, Kähkönen T, Bayford RH, Becher T, Frerichs I, Kallio M. Bronchodilator effect on regional lung function in pediatric viral lower respiratory tract infections. Physiol Meas. (2022) 43:104001. doi: 10.1088/1361-6579/ac9450
22. Vogt B, Löhr S, Zhao Z, Falkenberg C, Ankermann T, Weiler N, et al. Regional lung function testing in children using electrical impedance tomography. Pediatr Pulmonol. (2018) 53:293–301. doi: 10.1002/ppul.23912
23. Vogt B, Deuß K, Hennig V, Zhao Z, Lautenschläger I, Weiler N, et al. Regional lung function in nonsmokers and asymptomatic current and former smokers. ERJ Open Res. (2019) 5:00240-2018. doi: 10.1183/23120541.00240-2018
24. Li Q, Li Y, Niu G, Li M, Deng J, Möller K, et al. Chest physiotherapy guided by electrical impedance tomography in high-dependency unit patients with pulmonary diseases: an introduction of methodology and feasibility. Crit Care. (2023) 27:24. doi: 10.1186/s13054-023-04308-w
25. Yang L, Gao Z, Cao X, Wang C, Wang H, Dai J, et al. Visualizing pursed lips breathing of patients with chronic obstructive pulmonary disease through evaluation of global and regional ventilation using electrical impedance tomography. Physiol Meas. (2024) 45:2724–41. doi: 10.1088/1361-6579/ad33a1
26. Tirelli C, Mira S, Italia M, Maggioni S, Intravaia C, Zava M, et al. Applications of forced oscillatory technique in obstructive and restrictive pulmonary diseases: a concise state of the art. J Clin Med. (2025) 14:5718. doi: 10.3390/jcm14165718
27. Zhao Z, Li J, He R, Möller K, Frerichs I, Ge H. Regional pulmonary function testing: a new tool for screening subjects with preserved ratio impaired spirometry? ERJ Open Res. (2025) 11:01364–2024. doi: 10.1183/23120541.01364-2024
28. Li J, Zhao Z, He R, Xie Y, Xu Z, Ni C, et al. Regional lung function assessment using electrical impedance tomography in COPD, PRISm, and normal spirometry subjects: insights into early diagnostic potential. BMC Pulm Med. (2025) 25:215. doi: 10.1186/s12890-025-03668-z
Keywords: PRISm, electrical impedance tomography (EIT), regional lung function, visualize, localize
Citation: Zhang J, Qu S, Kong M, Han L, Ti X and Zhao Z (2025) Electrical impedance tomography reveals regional lung dysfunction in adults with preserved ratio impaired spirometry: an observational cross-sectional study. Front. Med. 12:1678253. doi: 10.3389/fmed.2025.1678253
Received: 02 August 2025; Accepted: 23 September 2025;
Published: 15 October 2025.
Edited by:
Russell W Chan, E-SENSE Innovation & Technology Ltd., Hong Kong SAR, ChinaReviewed by:
Rajesh Kathrotia, All India Institute of Medical Sciences, Rajkot, IndiaArbab Husain, Mangalayatan University, India
Viral Ishvarlal Champaneri, Zydus Medical College and Hospital, India
Copyright © 2025 Zhang, Qu, Kong, Han, Ti and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyu Ti, dGlfeGlueXVmbW11QDEyNi5jb20=; Zhanqi Zhao, emhhbnFpemhhb0BnemhtdS5lZHUuY24=
†These authors share first authorship
 Junsong Zhang
Junsong Zhang Shuoyao Qu
Shuoyao Qu Mingyi Kong2
Mingyi Kong2 Xinyu Ti
Xinyu Ti Zhanqi Zhao
Zhanqi Zhao