Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS), a severe T-cell-mediated hypersensitivity with mortality up to 10%, may progress to life-threatening multi-organ failure and culminate in multiple drug hypersensitivity (MDH). Clonorchiasis, a hepatobiliary parasitic endemic in China, manifests with nonspecific symptoms including fever, jaundice, and abdominal discomfort. We report an unique case of diclofenac-induced probable DRESS comorbid with acute clonorchiasis in which a praziquantel (PZQ)-related flare-up reaction occurred in a 42-year-old male. Following praziquantel administration, the patient exacerbated skin lesions, acute liver/kidney failure, likely triggered by Clonorchis sinensis lysis-released antigens amplifying IgE-mediated responses and PZQ-induced hepatic injury. Despite the reaction onset exceeding PZQ's peak concentration timeline, a type IV hypersensitivity reaction to PZQ cannot be ruled out. Therapeutic intervention with plasmapheresis, continuous renal replacement therapy, intravenous immunoglobulin, and systemic corticosteroids achieved clinical stabilization. One year later, the patient developed isolated hepatitis following administration of a structurally unrelated nonsteroidal anti-inflammatory drug. Combined with previous medical history, MDH was highly suspected. This case underscores the diagnostic complexity in distinguishing parasitic infections from DRESS through parasitological confirmation, herpesvirus reactivation profiling, validated DRESS criteria, and lesional skin histopathology. Therapeutically, stepwise immunomodulatory prioritization for DRESS control is essential, with PZQ therapy restricted to life-threatening parasitosis only after achieving immune stability, under intensive monitoring for hypersensitivity recrudescence and end-organ damage.
Context
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe T cell-mediated hypersensitivity reaction characterized by widespread cutaneous eruptions, fever, and multi-organ involvement, predominantly affecting the liver and kidneys (1). The latency period between exposure and symptoms is usually long (2–6 weeks) and may persist for weeks after drug discontinuation (1). Flare-up reaction may occur with abrupt steroid tapering, new drug exposures, or herpesvirus reactivations (2). Clonorchiasis, caused by Clonorchis sinensis (C. sinensis), is mainly prevalent in Asian countries and regions, especially China, and manifests with nonspecific symptoms including fever, jaundice, and abdominal discomfort (3). We report an unique case of diclofenac-induced probable DRESS comorbid with acute clonorchiasis in which a praziquantel (PZQ)-related flare-up reaction occurred in a 42-year-old male. This culminated in acute hepatic/renal failure and sepsis, providing critical insights for clinical management of differential diagnosis and treatment.
Case description
A 42-year-old male presented to our hospital with a 4-day history of fever accompanied by abdominal distention, jaundice, and erythematous rash on the day of admission. One month ago, he had taken diclofenac several times for low back pain. He denied previous drug allergies, and his medical history included raw freshwater fish consumption. Bilateral lymphadenopathy with the largest nodes measuring approximately 1.5 cm in diameter was palpable in the neck and submandibular angle. Admission laboratory studies revealed leukocytosis (18.21 × 109/L; normal range [NR]: 3.5–9.5 × 109/L) with marked eosinophilia (2.97 × 109/L; NR: 0.02–0.52 × 109/L). Hepatic dysfunction was evident in aspartate aminotransferase (AST) 415.8 U/L (NR: 15–40), alanine aminotransferase (ALT) 611.9 U/L (NR: 9–50), and total bilirubin 95 μmol/L (Figure 1). Viral hepatitis serologies, autoimmune hepatitis markers, and hemoculture were negative. Abdominal CT demonstrated cholecystitis, peritonitis, subcapsular and right abdominal effusion, and multiple enlarged lymph nodes in the hilar region and retroperitoneum. With an initial diagnosis of acute cholecystitis, he received ultrasound-guided gallbladder drainage and injection of diammonium glycyrrhizinate and adenosylmethionine butanedisulfonate. On day 4, the patient developed high-grade fever (39.5 °C) with a generalized erythematous rash and papular lesions involving >50% of body surface area, distributed across the extremities, trunk, and palms. The clinical presentation was interpreted as acute cholecystitis comorbid with diclofenac-induced cutaneous adverse reaction. Therapeutic interventions included oral methylprednisolone (12 mg/day) and intravenous dexamethasone (5 mg/day) to mitigate hypersensitivity manifestations.
Figure 1

The results of partial laboratory studies and major treatment. Because laboratory results were not measured before and after praziquantel administration (the arrow), the dashed line was shown between Day 9 and 12 for unclear trend. WBC, white blood cells; EOS, eosinophil; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transpeptidase; T-Bil, total bilirubin; Cr, creatinine; PEX, plasmapheresis; CRRT, continuous renal replacement therapy; IVIG, intravenous immunoglobulin.
On day 11, the patient exhibited partial resolution of cutaneous manifestations with generalized scattered desquamation (Figure 2A), though persistent fever and scleral icterus remained. Laboratory investigations revealed marked elevation of hepatic enzymes, eosinophilia, and hyper-immunoglobulinemia E (>2,500 IU/mL). Morphological abnormalities of peripheral blood cells showed 44% eosinophil and 2% atypical lymphocyte. Bile microscopy revealed numerous Clonorchis sinensis eggs. Negative serology for Paragonimus westermani, Angiostrongylus cantonensis, Schistosoma japonicum, and Plasmodium spp. IgG antibodies. A definitive diagnosis of clonorchiasis was established, prompting initiation of PZQ (total dose 210 mg/kg, 3 times a day for 3 days).
Figure 2
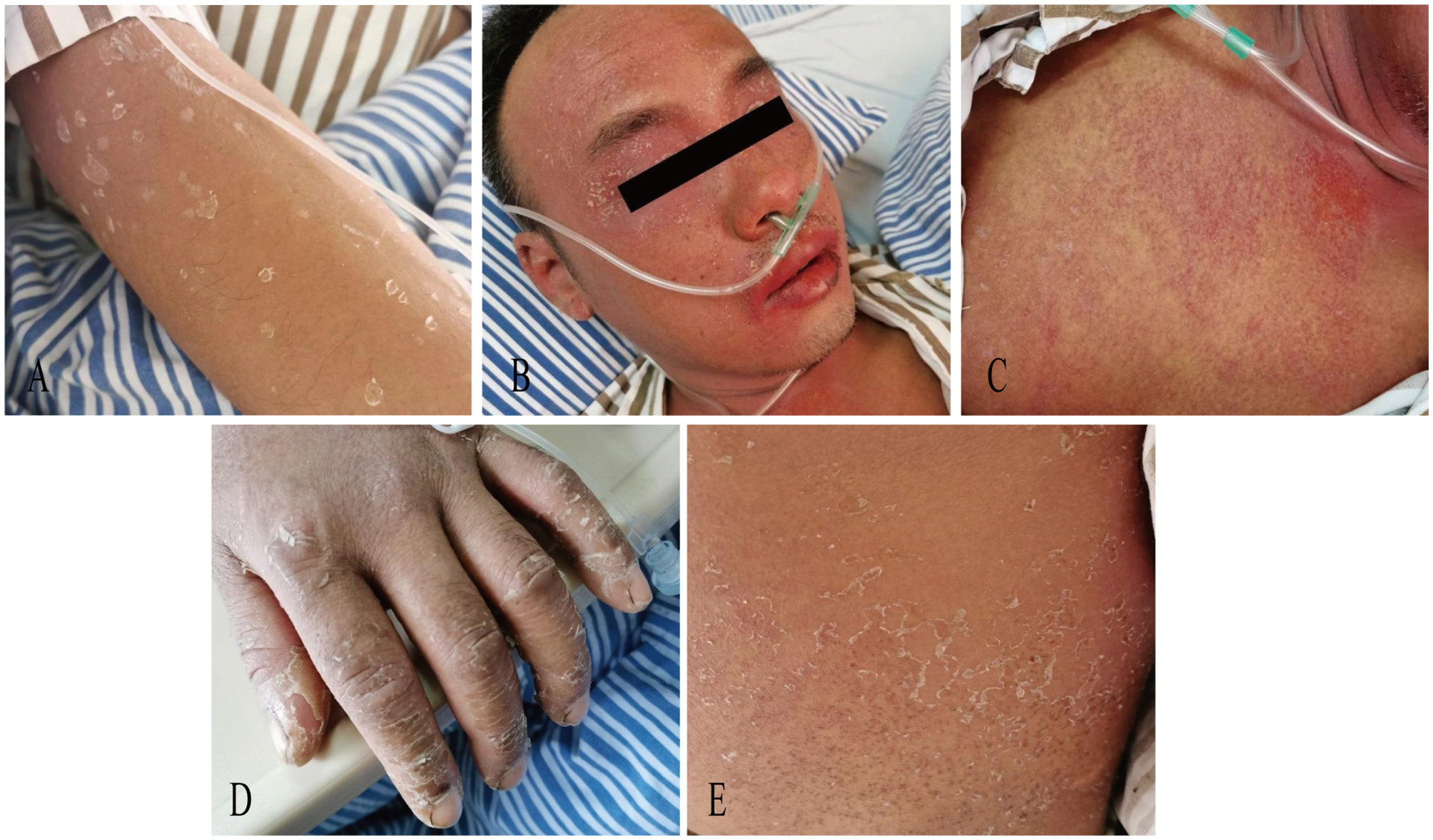
Photograph of scattered desquamation (A) on Day11, orofacial angioedema with perioral erosions (B) and a generalized erythematous rash (C) on Day12, desquamation with hyperpigmentation (D) and truncal pinpoint papules (E) on Day 19.
One day later, the patient developed a generalized erythematous rash with orofacial angioedema, perioral erosions, xerostomia, oliguria, and progressive jaundice (Figures 2B, C). Hepatorenal dysfunction demonstrated ALT of 5179 U/L (NR: 9–50), AST of 6,689.3 U/L (NR: 15–40), and total bilirubin of 402.6 μmol/L. The creatinine was 229.4 μmol/L. Systemic inflammation revealed C-reactive protein of 148 mg/L and procalcitonin of 2.3 ng/mL. The human herpesvirus-6 (HHV-6) and Epstein-Barr virus (EBV) serology were negative. The clinical constellation met RegiSCAR criteria for probable DRESS (Table 1) (4), with concurrent acute liver/kidney failure, and sepsis (Sequential Organ Failure Assessment (SOFA) score of 5 points). Therapeutic interventions included plasmapheresis (PEX) for 2 sessions, continuous renal replacement therapy (CRRT) for 40.9 h, intravenous immunoglobulin (IVIG) 50 ml for 6 days, and IV intravenous methylprednisolone (80 mg/day for 3 days, tapered to 40 mg for 4 days). On day 19, cutaneous findings evolved to generalized desquamation with hyperpigmentation and truncal pinpoint papules (Figures 2D, E). Oral Prednisolone tapering occurred gradually with improvement of symptoms and laboratory tests for three weeks.
Table 1
| RegiSCAR group study criteria | Yes | No | Unknown | Before | After |
|---|---|---|---|---|---|
| Fever ≥ 38.5 °C | 0 | −1 | 0 | 0 | 0 |
| Enlarged lymph nodes (≥2 sites, >1 cm) | 1 | 0 | 0 | 1 | 1 |
| Eosinophilia | 0 | 0 | 0 | 0* | 0* |
| 0.7–1.499 × 109/L (or 10%−19.9% if leukocytes < 4.0 × 109/L) | 1 | ||||
| ≥1.5 × 109/L (or ≥ 20% if leukocytes < 4.0 × 109/L) | 2 | ||||
| Atypical lymphocytes | 1 | 0 | 0 | 1 | 1 |
| Skin rash extent >50% BSA | 1 | 0 | 0 | 1 | 1 |
| At least 2 of: edema, infiltration, purpura, scaling | 1 | −1 | 0 | 1 | 1 |
| Biopsy suggesting DRESS | 0 | −1 | 0 | −1 | −1 |
| Internal organ involved | 0 | 0 | 0* | 1* | |
| 1 | 1 | ||||
| ≥2 | 2 | ||||
| Resolution ≥15 days | 0 | −1 | 1 | 1 | 1 |
| Evaluation of other potential causes | 1 | 0 | 0 | 0 | 0 |
| Total | 4 | 5 |
RegiSCAR scoring for DRESS syndrome (4).
Before: before PZQ administration; After: after PZQ administration; Total: 2 to 3 (possible case), 4 to 5 (probable case), and > 5 (definitive case); *Points for eosinophilia and hepatic involvement were not assigned, as these findings could be attributed to either DRESS syndrome or the concomitant acute clonorchiasis. A conservative scoring approach was adopted, classifying them as “unknown” etiology.
One year later, he was hospitalized again because of drug-induced liver injury after taking nimesulide and dexketoprofen tromethamine tablets. Laboratory studies revealed ALT of 3,056.7 U/L, AST of 2,178.1 U/L, eosinophilia of 17.5%, and immunoglobulin E (IgE) of 839.2IU/ml with elevated tumor necrosis factor α and interleukin 6. Following injection of magnesium isoglycyrrhizinate and oral prednisone acetate tablets, the patient's condition improved. Although the patient did not present with hypersensitivity-associated skin eruptions during the latter hospital stay, we suspect multiple drug hypersensitivity (MDH) triggered by structurally unrelated NSAIDs based on clinical history. The final hospitalization manifested as isolated drug-induced liver injury. We recommended further diagnostic investigations including Patch Tests and Lymphocyte Transformation Test (LTT) to confirm MDH, but the patient declined. Consequently, we strongly advise patients to avoid NSAIDs whenever possible. If NSAID use is unavoidable, high-affinity NSAIDs at minimal effective doses such as celecoxib or etoricoxib should be prioritized.
Discussion
This case highlights the importance of early DRESS recognition and the risk of using PZQ in patients with hypersensitivity reactions. Given the significant overlap in laboratory (elevated eosinophils, hepatic dysfunction, and hyper-IgE) and clinical presentations between clonorchiasis and DRESS, we calculated the RegiSCAR score conservatively. Although marked eosinophilia and hepatic dysfunction are classic for DRESS, these parameters were classified as “unknown” regarding their etiology, and points were not awarded for them in the scoring system. Despite this, the total score still met the threshold for a “probable” case of DRESS, underscoring the strength of the clinical diagnosis even amidst significant diagnostic challenges. In this case, clonorchiasis was confirmed through microscopic identification of C. sinensis eggs in both stool and bile specimens. While low-dose corticosteroids therapy led to partial resolution of cutaneous manifestations, the rarity and variability of DRESS likely contributed to the clinicians' hesitation in pursuing further investigations for severe drug hypersensitivity or alternative etiologies. Pathophysiologically, clonorchiasis primarily manifests through direct mechanical obstruction and inflammatory infiltration of bile ducts by adult flukes, typically resulting in obstructive jaundice. This case exhibited mixed-type liver injury (R ratio[ALT/ULN ÷ ALP/ULN] of 2–5) rather than pure cholestasis, accompanied by progressive hepatic synthetic decline evidenced by hypoalbuminemia and coagulopathy. In addition, the combination of markedly elevated levels of high-sensitivity C-reactive protein, eosinophils, and the extent of body surface area involvement can be used for early DRESS recognition (5). Furthermore, classic features such as facial edema and the presence of atypical lymphocytes are also pivotal clues for diagnosis (4).
The prevailing hypothesis on the pathogenesis of DRESS posits a multifactorial interplay between drug-specific immune activation and dysregulated antiviral responses (6). When exposed to a drug, its metabolites with T-cell receptors and human leukocyte antigen complexes bypassing any prior metabolic processing or protein binding to initiate T-cell clonal expansion, thereby escalating hypersensitivity reactions. The cytokines such as IL-4, IL-5, and IL-13 are released causing eosinophilia and the pro-inflammatory cytokines like IFN-γ, TNF, IL-6, and IL-15 are increased promoting systemic inflammation. After PZQ administration, the patient experienced flare-up reaction of DRESS, characterized by exacerbated cutaneous manifestations, acute liver failure, and acute kidney failure. In this patients, the HSV and EBV serology of the patients were negative. Critically, the abrupt lysis of Clonorchis sinensis may release parasite derived antigens following PZQ administration, which may act as pathogen-associated molecular patterns to amplify mast cell degranulation and IgE-mediated anaphylactoid responses (7). Furthermore, PZQ is mainly metabolized in the liver and may aggravate drug-induced hepatic injury. Notably, the time for flare-up reaction has already exceeded the time when PZQ reached its maximum concentration, but a type IV hypersensitivity reaction to PZQ cannot be ruled out. Unfortunately, the patient did not consent to the lymphocyte transformation test. Combination therapy with systemic glucocorticoids, PEX, CRRT, and IVIG achieved clinical stabilization. This antigenic surge, coupled with pre-existing drug hypersensitivity, likely synergized to potentiate multi-organ involvement in this patient.
Conclusion
To our knowledge, the first reported case of DRESS comorbid with clonorchiasis highlights two critical imperatives: (1) Systematic diagnostic differentiation through parasitological confirmation (microscopy/serology), herpesvirus reactivation profiling, validated DRESS criteria (RegiSCAR), and lesional skin histopathology; (2) Stepwise immunomodulatory prioritization for DRESS control, restricting PZQ therapy to life-threatening parasitosis only after achieving immune stability, with intensive monitoring for hypersensitivity recrudescence and end-organ damage.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Foshan Hospital of Traditional Chinese medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL: Writing – original draft. KH: Data curation, Funding acquisition, Writing – original draft. YC: Data curation, Formal analysis, Visualization, Writing – original draft. YH: Conceptualization, Visualization, Writing – original draft. YZ: Data curation, Formal analysis, Investigation, Writing – review & editing. MZ: Conceptualization, Formal analysis, Investigation, Writing – review & editing. KJ: Conceptualization, Formal analysis, Investigation, Supervision, Writing – review & editing. XM: Conceptualization, Formal analysis, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Wei BM Fox LP Kaffenberger BH Korman AM Micheletti RG Mostaghimi A et al . Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. Part I. Epidemiology, pathogenesis, clinicopathological features, and prognosis. J Am Acad Dermatol. (2024) 90:885–908. 10.1016/j.jaad.2023.02.072
2.
Jörg L Yerly D Helbling A Pichler W . The role of drug, dose, and the tolerance/intolerance of new drugs in multiple drug hypersensitivity syndrome. Allergy. (2020) 75:1178–87. 10.1111/all.14146
3.
Qian MB Keiser J Utzinger J Zhou XN . Clonorchiasis and opisthorchiasis: epidemiology, transmission, clinical features, morbidity, diagnosis, treatment, and control. Clin Microbiol Rev. (2024) 37:e923. 10.1128/cmr.00009-23
4.
Kardaun SH Sekula P Valeyrie-Allanore L Liss Y Chu CY Creamer D et al . Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. (2013) 169:1071–80. 10.1111/bjd.12501
5.
Choudhary R Vinay K Srivastava N Bishnoi A Kamat D Parsad D et al . Clinical, biochemical, and serologic predictors of drug reaction with eosinophilia and systemic symptoms syndrome: a prospective case–control study. J Am Acad Dermatol. (2021) 85:901–9. 10.1016/j.jaad.2021.03.075
6.
Miyagawa F Asada H . Current perspective regarding the immunopathogenesis of drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/ DRESS). Int J Mol Sci. (2021) 22:2147. 10.3390/ijms22042147
7.
Matsumoto J . Adverse effects of praziquantel treatment of Schistosoma japonicum infection: involvement of host anaphylactic reactions induced by parasite antigen release. Int. J. Parasitol. (2002) 32:461–71. 10.1016/S0020-7519(01)00368-X
Summary
Keywords
clonorchiasis, praziquantel, flare-up reaction, drug reaction with eosinophilia and systemic symptom, multiple drug hypersensitivity
Citation
Li W, Huang K, Chen Y, Huang Y, Zheng Y, Zhong M, Jiang K and Ma X (2025) Case Report: Praziquantel-induced flare-up reaction in a rare case of diclofenac-induced probable DRESS comorbid with acute clonorchiasis: diagnostic and therapeutic challenges. Front. Med. 12:1678509. doi: 10.3389/fmed.2025.1678509
Received
02 August 2025
Accepted
07 October 2025
Published
28 October 2025
Volume
12 - 2025
Edited by
Teresa Bellon, University Hospital La Paz Research Institute (IdiPAZ), Spain
Reviewed by
Rannakoe Lehloenya, University of Cape Town, South Africa
Chia-Yu Chu, National Taiwan University Hospital, Taiwan
Updates
Copyright
© 2025 Li, Huang, Chen, Huang, Zheng, Zhong, Jiang and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiping Jiang jkpingfs@126.comXiaojun Ma mxjzjl@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.