- Department of Internal Medicine, The Catholic University of Korea, St. Vincent’s Hospital, Suwon, Republic of Korea
Background: Eradication of Helicobacter pylori alone will not eliminate the risk of gastric cancer. There was no conclusive evidence that eradication therapy reduced the incidence of gastric cancer in patients with atrophic gastritis or intestinal metaplasia.
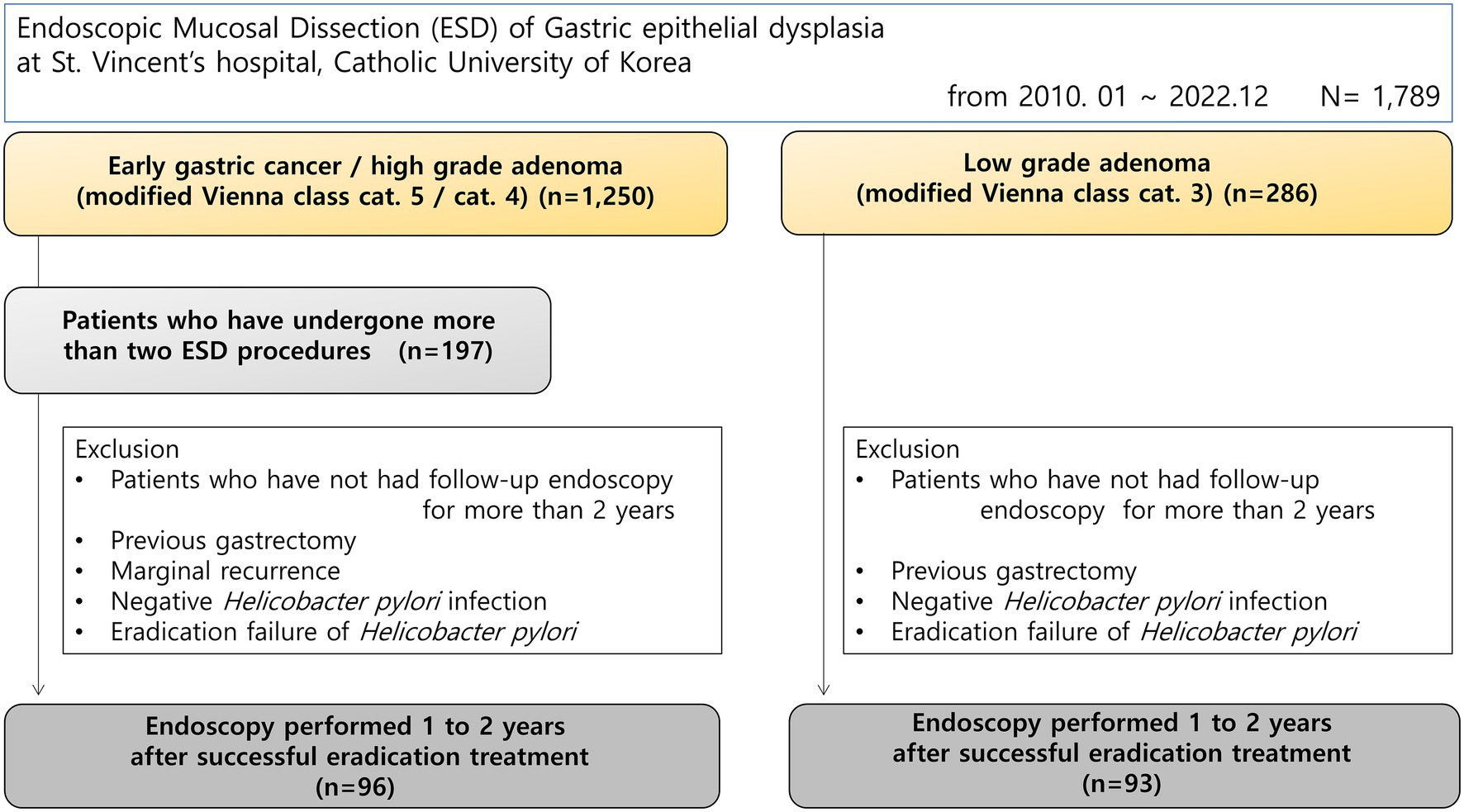
Materials and methods: This study was conducted from March 2010 to September 2022, and enrolled 1,789 consecutive patients who underwent endoscopic submucosal dissection for gastric mucosal dysplasia. Patients who underwent two or more procedures for simultaneous or metachronous gastric epithelial dysplasia as high-risk groups for gastric cancer, and were confirmed to have H. pylori infection at the time of the procedure were included. Patients who received eradication treatment and underwent endoscopic follow-up for at least 2 years were included. The control group included patients who underwent endoscopic treatment for simple low-grade dysplasia. We investigated whether endoscopy was performed between 1 and 2 years after successful eradication of H. pylori. The endoscopic findings investigated were diffuse atrophy (open type), fundal atrophy, map-like redness, patch redness, erosions (body/antrum), hematin, endoscopic reflux esophagitis, hematin, and duodenitis.
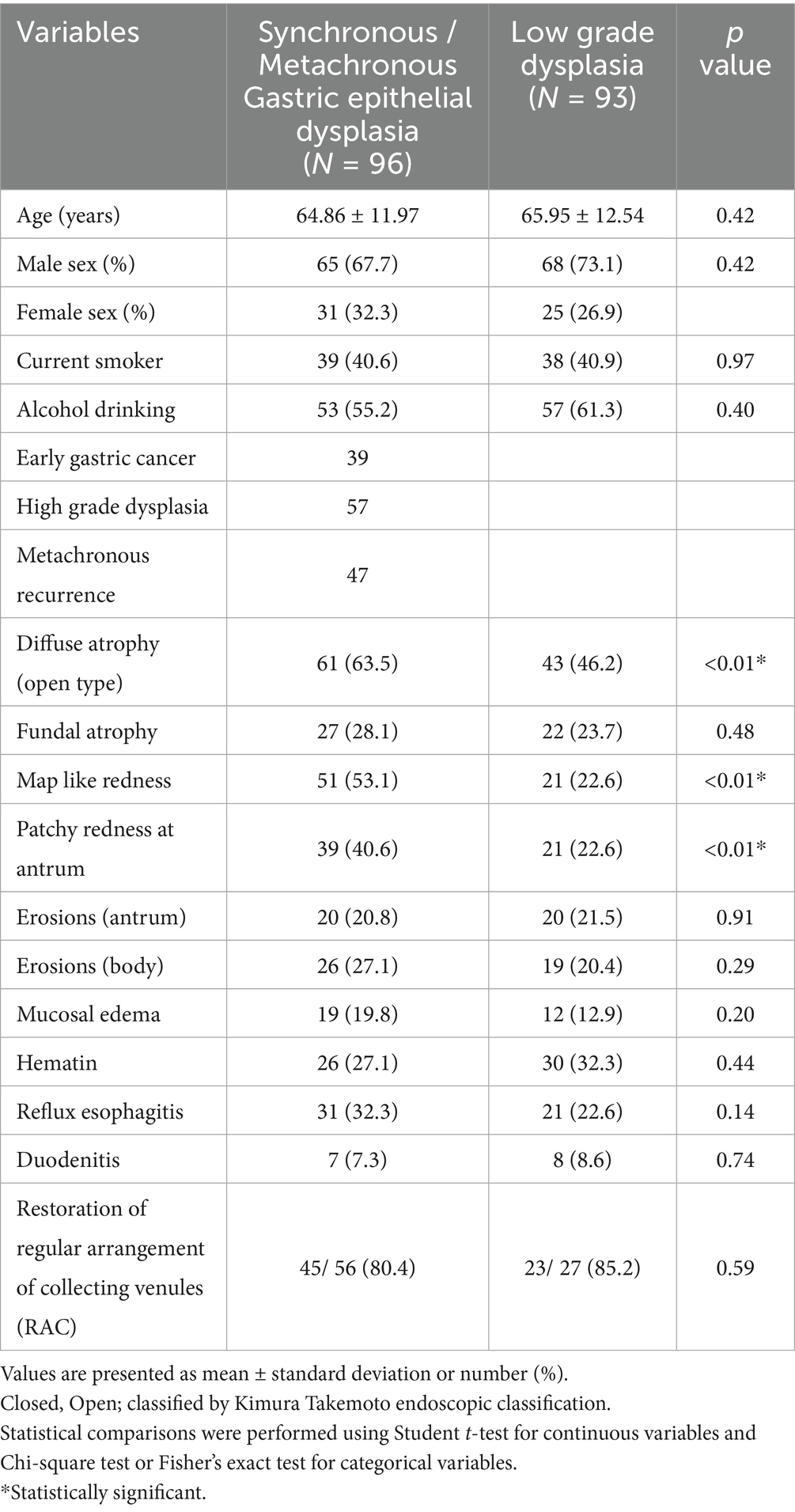
Results: In univariate analysis, diffuse atrophy, map-like redness, and patchy redness at antrum after H. pylori eradication were statistically different. In multivariate analysis, map-like redness [p < 0.01, 95% confidence interval (CI) 1.83–7.59] and patchy redness at antrum (p = 0.02, 95% CI 1.11–4.74) observed were statistically more frequent in patients with synchronous-metachronous gastric dysplasia.
Conclusion: Map-like redness and patchy redness are newly identified endoscopic risk factors for gastric cancer in patients treated for H. pylori eradication.
Introduction
Gastric cancer remains a critical global health burden, particularly in high-incidence regions like Korea, ranking among the leading causes of cancer-related mortality worldwide. The identification of H. pylori as the primary risk factor revolutionized understanding of gastric carcinogenesis. H. pylori is a Gram-negative, spiral-shaped bacterium transmitted primarily through fecal-oral and oral-oral routes, often during childhood, and uniquely adapted to colonize the hostile acidic environment of the human stomach through its production of urease (1). This chronic infection affects approximately half of the world’s population and is associated with a wide spectrum of gastroduodenal diseases, including peptic ulcer disease, MALT lymphoma, and gastric adenocarcinoma (2). The International Agency for Research on Cancer (IARC) classified H. pylori as a definitive Class I carcinogen in 1994 (3), solidifying its role in initiating the multi-step histological progression known as the Correa cascade (4).
Helicobacter pylori infection is closely related to the development of gastric cancer. Therefore, it is thought that H. pylori eradication treatment may reduce the risk of stomach cancer, but it is not clear how much eradication can reduce the risk. Previous studies have shown that it does not reduce the mortality associated with stomach cancer (5), but this is still unclear. A recently published meta-review has searched for randomized controlled trials (RCTs) and performed a final analysis of a total of 10 RCTs. The analysis focused on seven studies involving 8,323 healthy people and found that eradication treatment significantly reduced the risk of gastric cancer (RR = 0.54; 95% CI 0.40–0.72; NNT = 72) and gastric cancer mortality (RR = 0.61; 95% CI 0.40–0.92; NNT = 135) in healthy people (6). However, when analyzing 7,079 subjects from five studies that presented overall mortality, overall mortality was not reduced (RR = 0.97; 95% CI 0.85–1.12) by H. pylori eradication. To summarize the main results, eradication therapy was reported to reduce the risk of gastric cancer by 34% in patients with asymptomatic infection (RR = 0.66; 95% CI 0.46–0.95), but unfortunately, there was no conclusive evidence showing that eradication therapy reduced the incidence of gastric cancer in patients with atrophic gastritis or intestinal metaplasia (5).
We emphasize that gastric cancer screening endoscopy is necessary because eradication therapy does not prevent all gastric cancers. Although it decreases somewhat after eradication, most atrophic gastritis remains, and it has been predicted that genetic abnormalities will occur due to its high regenerative activity and rapid cell turnover, which will lead to dysplasia and subsequent cancer. A recent retrospective cohort study of 2,737 patients who underwent annual endoscopic follow-up after treatment of H. pylori infection reported that the longer the follow-up period, the greater the risk of developing diffuse gastric cancer in patients with mild to moderate atrophy at baseline (7). In the study, the incidence of gastric cancer was 0.35% per year after H. pylori eradication. Based on these results, there was an opinion that eradication treatment of H. pylori would not have a preventive effect on gastric cancer after chronic gastritis progressed (point-of-no return theory) (8). Therefore, the importance of endoscopic follow-up examinations is increasing, and methods to select high-risk groups or set alarm findings and induce careful observation based on specific endoscopic findings appear to be necessary. As eradication treatment has been ongoing for more than 30 years, most gastric cancers are now likely to occur in the post-eradication state. This study aimed to find meaningful results by gathering characteristic mucosal responses after successful H. pylori eradication treatment in high-risk patients with gastric cancer who presented with synchronous/metachronous lesions.
Materials and methods
This retrospective cohort study was conducted at the Division of Gastroenterology, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea. We reviewed the medical records and endoscopic databases of consecutive patients who underwent endoscopic submucosal dissection (ESD) for gastric epithelial dysplasia between March 2010 and December 2022. During the study period, a total of 1,789 patients underwent ESD for gastric dysplasia at our institution. These patients were categorized these groups based on histological diagnosis: (1) early gastric cancer or high-grade dysplasia (modified Vienna classification categories 4 or 5, n = 1,250), and (2) low-grade dysplasia (modified Vienna classification category 3, n = 286) (9). An additional 253 patients had other diagnoses or incomplete classification data. From the early gastric cancer/high-grade dysplasia group (n = 1,250), we identified 197 patients who underwent two or more ESD procedures for synchronous or metachronous lesions, representing a high-risk population for gastric cancer. Only patients with confirmed H. pylori infection at the time of the procedure, who received eradication treatment and were followed endoscopically for at least 2 years were included. Patients with recurrence in the periphery of the previous procedure were excluded. The control group included patients who received endoscopic treatment for simple low-grade dysplasia, were confirmed to have H. pylori infection at the time of procedure, and were followed for at least 2 years (Figure 1). Patients who had gastrectomy surgery, negative H. pylori infection status, eradication refusal or failure were also excluded. This study was approved by the Institutional Review Board of St. Vincent’s Hospital, Catholic University of Korea (approval number: VC24RISI0243).
Definition of Helicobacter pylori infection and eradication
The diagnosis of H. pylori infection was based on two or more positive results from tests including the rapid urease test (RUT), polymerase chain reaction (PCR), urea breath test (UBT), histologic evidence by silver staining, and a serologic test. Two biopsies were used for RUT (CLO® test; Kimberly-Clark, UT, USA). DNAs were extracted from specimens using QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol, and stored at −20 °C until use. PCR (Seeplex® ClaR-H. pylori ACE Detection; Seegene Institute of Life Science, Seoul, Korea) was performed to identify point mutation-containing gene fragments, according to the manufacturer’s recommendations. A 13C-UBT was performed with a Pranactin-Citric drug product, a component of the BreathTek UBT Kit (Korea Otsuka Pharmaceutical Co. Ltd., Seoul, Korea). Three grams of reconstituted Pranactin-Citric containing 75 mg of 13C-urea was ingested by the patient. Breath samples before and 20 min after the administration of 13C-urea were collected after a mouthwash. The 13C/12C ratio in the breath samples was measured with an infrared spectrophotometer (UBiT-IR300; Korea Otsuka Pharmaceutical Co. Ltd.). Changes in 13C value over baseline were expressed as Δ13C, and a positive result was defined as an increase of >2.5%. The presence of serum immunoglobulin G antibodies against H. pylori (Enzygnost®, Dade Behring, Marburg, Germany) was determined. An antibody titer >15 U/mL was classified as H. pylori seropositive, whereas a titer <10 U/mL was considered as H. pylori seronegative accordance with the manufacturer’s instructions. All these tests were performed at the Department of Laboratory Medicine, St. Vincent’s Hospital, as part of routine clinical practice during the study period.
Eradication therapy was performed according to the Korean guideline for H. pylori infection (2020 revision) (10), and eradication success was confirmed by UBT at least 4 weeks after completion of therapy. If H. pylori test was positive within 1 year after eradication (recrudescence) or after 1 year (reinfection) during follow-up period, either patient was excluded.
Definition of synchronous and metachronous gastric epithelial dysplasia
In this study, patients were classified as marginal, synchronous, or metachronous lesions. A synchronous lesion was defined as a lesion found within 12 months other than a concurrent gastric epithelial lesion found on ESD. A metachronous lesion was defined as a recurrence more than 12 months after the initial ESD in a lesion that was not connected to the primary lesion and was located more than 1 cm away (11).
Evaluation of endoscopy
Esophagoduodenoscopy was performed using a GIF-XQ 260 (Olympus Medical, Inc. Seoul, Korea). We investigated whether endoscopy was performed between 1 and 2 years after successful eradication of H. pylori. Endoscopic examinations reviewed all images in picture archiving and communication system (PACS) by two different endoscopists (Chung WC; yearly >1,000 diagnostic endoscopies, 20 years, Lim NR; yearly 1,000 diagnostic endoscopies, 5 years). Disagreement between the two examiners made an exclusion, and we evaluated specific endoscopic findings – diffuse atrophy (open type), fundal atrophy, map-like redness, patchy redness at antrum, erosions (body/ antrum), mucosal edema, hematin, reflux esophagitis, and duodenitis (12, 13). We also checked the restoration of regular arrangement of collecting venule (RAC) pattern (14). Figure 2 showed clinical photos of endoscopic findings.
1. Diffuse atrophy (open type)—the classification of diffuse atrophic gastritis was defined as open type if it included gastric cardia according to the Kimura-Takemoto classification (Figure 2A) (15).
2. Fundal atrophy—in cases where atrophy of the gastric glands progresses from the body side to extensive fundal atrophy is observed (Figure 2B).
3. Map-like redness—in gastric atrophy, the improvement pattern is different between the area with only inflammation and the area with atrophy after H. pylori eradication treatment. In this case, map-like redness is observed in the gastric body (Figure 2C) (16, 17).
4. Patchy redness at antrum—the patchy redness seen after H. pylori eradication often has a clear border and a slightly sunken form, and sometimes it is accompanied by extensive map-like erythema in the gastric body. It is also associated with intestinal metaplasia (Figure 2D) (16).
5. Mucosal edema—soft, thick, and distended mucosa (Figure 2E).
6. Erosion (body/antrum) (Figure 2F).
7. Reflux esophagitis—patchy, striated, or circular epithelial defects in the mucosa in the distal esophagus. We also included erythema, blurred junction between squamous epithelium and columnar epithelium, and increased vascular marking in the distal esophagus as minor changes (Figure 2G).
8. Duodenitis—erythematous or exudative change with erosions in the bulb or post bulbar area (Figure 2H).
9. Hematin—red or dark ecchymosis spots or flecks (Figure 2I).
10. Restoration of regular arrangement of collecting venules (RAC). When the gastric mucosa of the gastric body without atrophy is observed using a magnifying endoscope and narrow band image, collecting venules appear as numerous, uniformly sized, fine red dots at regular intervals in the gastric body. This finding is called a RAC (Figure 2J) (14).
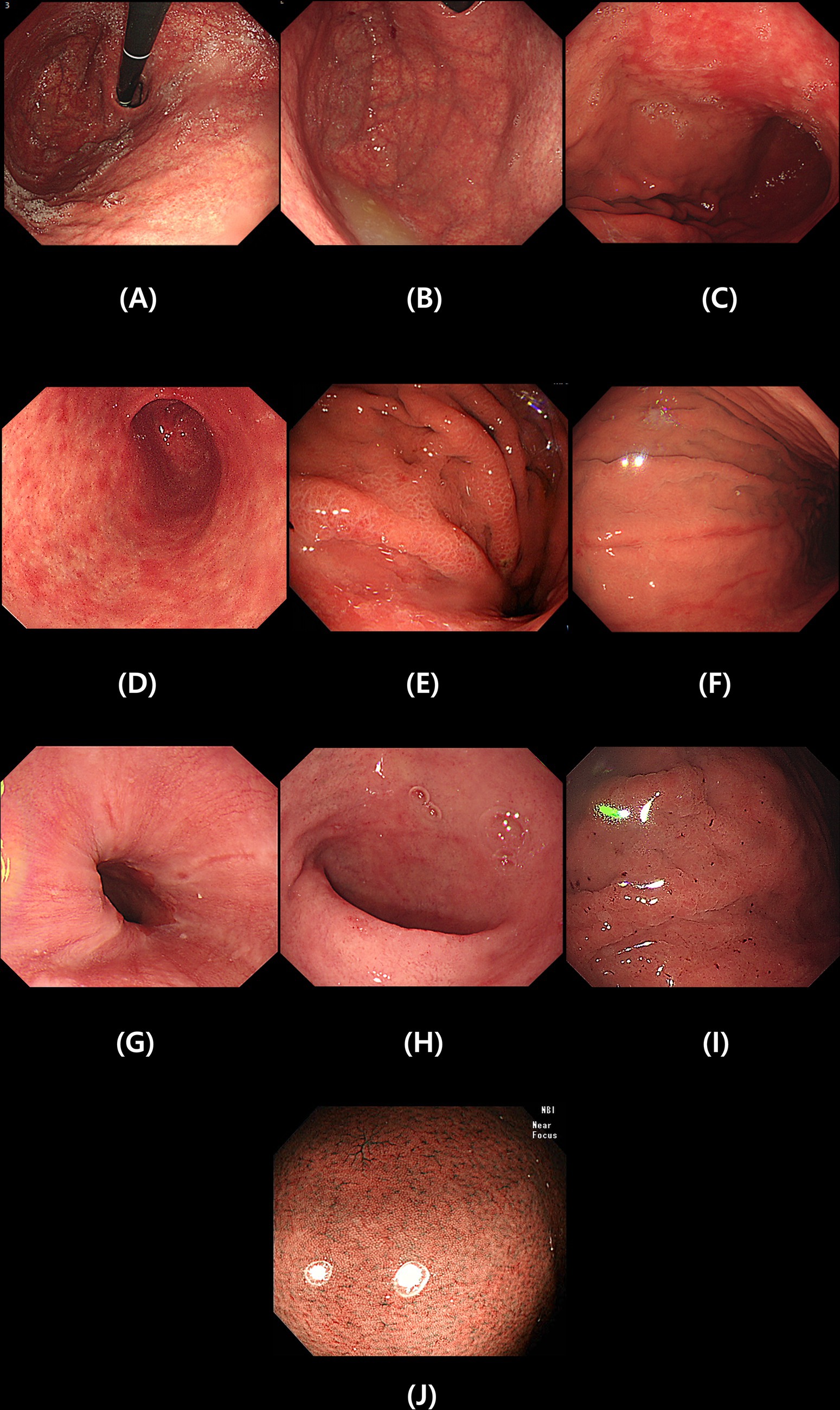
Figure 2. Specific endoscopic findings after Helicobacter pylori eradication. Diffuse atrophy (A), Fundal atrophy (B), Map-like redness (C), Patchy redness at antrum (D), Mucosal edema (E), Erosion (F), Reflux esophagitis (G), Duodenitis (H), Hematin (I) and Restoration of regular arrangement of collecting venules (J).
Statistics
All statistical analyses were performed with the SPSS program (SPSS version 25.0; IBM, Chicago, IL, USA). Values are expressed as mean ± standard deviation. Categorical variables were presented as numbers with percentages and were tested by a Chi-square test or Fisher’s exact test. Multivariate logistic regression was performed to identify independent risk factors. p-value <0.05 was considered significant.
Results
Between March 2010 and December 2022, 1,789 consecutive patients underwent endoscopic submucosal dissection (ESD) for gastric epithelial dysplasia at our institution. Based on histological diagnosis using the modified Vienna classification, patients were categorized as follows: 1,250 patients had early gastric cancer or high-grade dysplasia and 286 patients had low-grade dysplasia. Among patients with early gastric cancer of high-grade dysplasia, we identified patients who had synchronous or metachronous gastric dysplasia at high risk for gastric cancer and underwent mucosal dissection more than twice. 96 patients met all criteria and were enrolled in the study. Among 286 patients who underwent gastric mucosal resection or submucosal dissection for simple low-grade gastric epithelial dysplasia, the control group consisted of 93 patients diagnosed with H. pylori infection at the time of the procedure, successfully treated for eradication, and followed up for more than 2 years. However, patients who refused eradication treatment or did not achieve successful eradication even after third-line eradication treatment were excluded from the analysis.
There were no significant differences in the patients’ baseline age, gender, smoking history, or drinking history. In univariate analysis, diffuse atrophy, map-like redness, and patchy redness at antrum after H. pylori eradication were significantly different (Table 1). The frequency of recovery of RAC pattern was 80.4% in synchronous-metachronous gastric dysplasia group and 85.2% in control group, with no difference between the two groups. There was no statistically significant difference in the presence or absence of diffuse atrophy or fundal atrophy in synchronous-metachronous gastric dysplasia group compared to the control group, which were previously known as risk factors. In multivariate analysis, map-like redness [p < 0.01, 95% confidence interval (CI) 1.83–7.59] and patchy redness at antrum (p = 0.02, 95% CI 1.11–4.74) observed were found to be significantly more frequent in patients with synchronous-metachronous gastric dysplasia compared to control group (Table 2). Map like redness and patchy redness at antrum were often observed simultaneously. When these two items were combined into one multivariate analysis, the statistical difference was more evident (p < 0.01, 95% CI 2.248–8.918). When only the 45 patients classified as patients with isogenic dysplasia in this study were analyzed separately, map-like erythema (p < 0.01, 95% CI 1.69–10.89) and irregular erythema of the stomach (p = 0.01, 95% CI 1.34–8.53) were observed frequently in target patients. (Table 3).
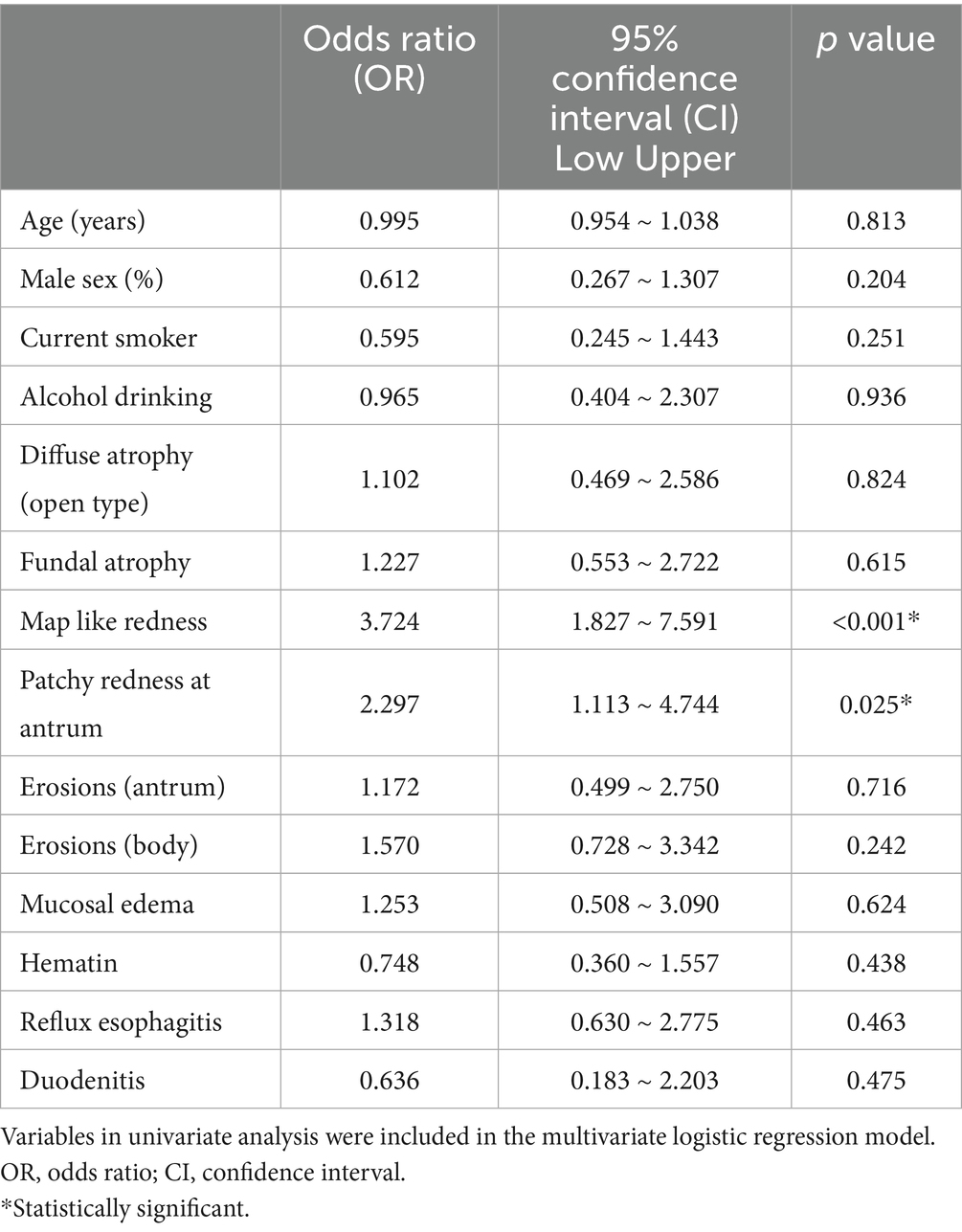
Table 2. Results of a multivariate analysis comparing mucosal changes after Helicobacter pylori eradication in patients with simultaneous / metachronous gastric epithelial dysplasia with those in patients with low-grade dysplasia.
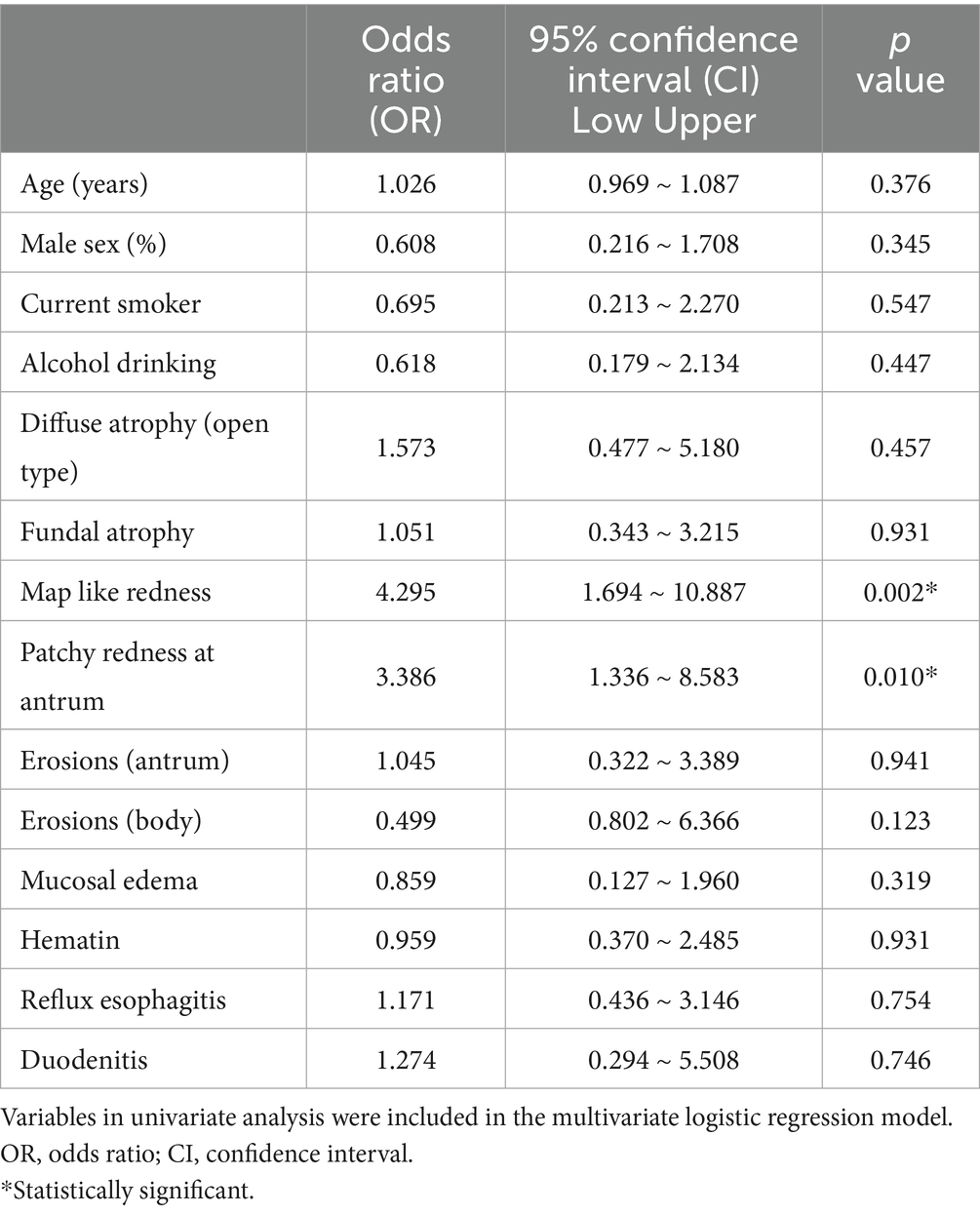
Table 3. Results of a multivariate analysis comparing mucosal changes after Helicobacter pylori eradication in patients with metachronous gastric epithelial dysplasia (n = 45) with those in patients with low-grade dysplasia.
Discussion
This study identified map like redness and patchy redness at antrum observed after H. pylori eradication as novel endoscopic features associated with synchronous or metachronous gastric dysplasia in high-risk patients. These findings were statistically significant after adjusting for known risk factors including the extent of atrophic gastritis. The odds ratios were substantial, with a 3.7-fold increased risk for map like redness and a 2.3-fold increased risk for patchy redness at antrum. Importantly, when either finding was present, the odds ratio increased to 4.5, suggesting these markers can effectively identify a high-risk population requiring intensive surveillance.
This persistent cancer risk aligns with existing clinical evidence. When eradication therapy is performed, it has been reported that the effect of eradication therapy in suppressing the occurrence of cancer can be maximized in the absence of precancerous lesions (atrophic gastritis, intestinal metaplasia, or dysplasia) (8). Atrophic gastritis is a chronic inflammatory change in the gastric mucosa, which occurs in association with H. pylori infection, various environmental factors, and autoimmune responses to gastric glandular tissue. The decrease in the gastric glandular tissues in the gastric body due to this atrophic change causes hypoacidity, and the resulting change in the gastric environment causes a decrease in ascorbic acid in the stomach and bacterial overgrowth, which induces the production of N-nitroso compounds, an important mechanism for the occurrence of gastric cancer (4). However, the reversibility of advanced precancerous lesions remains inconsistent. While some studies observed an improvement in atrophic changes and intestinal metaplasia after H. pylori eradication, others reported no changes or even significant progression of intestinal metaplasia, suggesting limited efficacy (18, 19). A clinical study conducted in Japan followed up patients with atrophic gastritis infected with H. pylori for 5 years after eradication treatment (20). The results showed that atrophic changes and intestinal metaplasia improved in both the gastric body and the gastric antrum, but this was highly correlated with high blood levels of pepsinogen I. Since there is a lack of specific tests or findings that can determine the degree of atrophic gastritis or intestinal metaplasia before eradication, there is a possibility that atrophic gastritis or intestinal metaplasia that has already progressed significantly may continue to progress along with genetic changes even after eradication.
The molecular mechanisms underlying this persistent cancer risk can be understood through the stepwise transformation process of the Correa cascade (4). H. pylori infection leads to a dysregulated immune response characterized by the infiltration of inflammatory cells in the tumor microenvironment, thus secrete numerous inflammatory cytokines, chemokines, and reactive oxygen species/reactive nitrogen species, resulting in apoptosis, genomic instability, and the development of gastric cancer (21–23). These chronic inflammatory processes induce widespread epigenetic modifications, most notably DNA hypermethylation of tumor suppressor genes. As inflammation progresses to atrophic gastritis, glandular loss leads to hypochlorhydria and bacterial overgrowth, which facilitates the production of N-nitroso compounds. These molecular damage is imprinted in the genome of premalignant lesion (intestinal metaplasia and dysplasia), which represents a “point of no return” (24). Therefore, while eradication eliminates the bacterial initiator, the accumulated non-reversible damage in the mucosa—reflected by persistent endoscopic findings such as map-like redness and patchy redness—remains the core driver of long-term risk for gastric adenocarcinoma.
The Operative Link on Gastritis Assessment (OLGA) system was designed to identify the degree of atrophic gastritis and intestinal metaplasia that persists after H. pylori eradication (25, 26). This system calculates the risk of gastric cancer by adding endoscopic findings to the histological semiquantitative evaluation of the existing Sydney system and classifies the risk of gastric cancer by considering the location and degree of inflammation in the antrum and body. Low OLGA stages (0, I, and II) reflect low gastric cancer risk, and high stages (III and IV) are high gastric cancer risks. The existing OLGA system included too many high-risk groups in areas with high H. pylori prevalence, and a more selective system was required. High interobserver agreement was reported for the Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM), which utilizes the degree of intestinal metaplasia (27). However, these histology-based systems face practical challenges in routine clinical practice. They require a minimum of 5 tissue biopsies and a maximum of 12 specimens must be collected basically, posing a significant burden in practice. In regions with high H. pylori prevalence, a large proportion of patients fall into high-risk categories, limiting the systems’ discriminatory value (28). Moreover, the relatively small number of prospective studies reporting that usefulness of systems is limited (29, 30). Clinicians have called for the development of methods to grade and assess the risk of gastric cancer based solely on endoscopic findings without requiring a biopsy.
In this respect, the Kyoto classification system of gastritis is very attractive to clinicians because it allows them to make judgments based only on endoscopic findings without tissue examination (12). However, the usefulness of the risk of gastric cancer according to the Kyoto classification of gastritis needs to be continuously verified, and the score or items may need to be changed depending on the results. In addition, this classification system is a system that judges at the time of performing an endoscopy and hopes for an immediate solution, and a considerable period of training may be required to understand this and apply this system during an endoscopy (31, 32), so there may be controversy over its usefulness. To alleviate these concerns, it is necessary to identify high-risk groups in the field of endoscopy by presenting more sensitive, strong, and rational opinions, and then induce close observation and tissue examination.
After H. pylori eradication treatment, endoscopic findings of the gastric mucosa generally improve, which is a change that occurs as chronic inflammation decreases, and histologically, polynuclear cell infiltration and mononuclear cell infiltration improve. This improvement in the inflammatory response appears over a long period of time, from immediately after eradication treatment to several years (33). After successful eradication, punctate erythema, diffuse erythema, mucosal edema, hypertrophy of the gastric folds, and turbid mucus often improve (34). It is also known that the uniform vascular pattern reappears in the background mucosa and the border of atrophy becomes unclear. Among the findings observed after eradication, map like redness is a highly specific lesion. Histologically, it corresponds to intestinal metaplasia. The mechanism by which map like redness appears can be explained by the disappearance of diffuse erythema after successful eradication treatment and the enhancement of the contrast between non-atrophic mucosa and atrophic mucosa (16).
This study is to investigate whether there were characteristic mucosal changes after eradication in high-risk groups related to the occurrence of gastric cancer. Therefore, we focused on the unique mucosal changes that appear after eradication in patients with simultaneous or metachronous early gastric cancer or precancerous lesions, and among them, map like redness and patchy redness at antrum were significant findings. It is noteworthy that the odds ratio was approximately 4.48, when even one of these two lesions appeared. When limited to metachronous lesions, the values were 4.30 and 3.39, respectively, which suggests that cases with these findings can be considered a high-risk group.
Several limitations warrant acknowledgment. First, this was a single-center retrospective study, which may limit generalizability to other populations and healthcare settings. Our findings require validation in independent, preferably multi-center prospective cohorts. Second, we did not systematically perform biopsies according to standardized protocols to correlate endoscopic findings with histological severity of atrophy and intestinal metaplasia. Such correlation would strengthen understanding of the biological basis of map-like redness and patchy redness and their relationship to underlying mucosal pathology. Third, inter-observer variability for recognizing map-like redness and patchy redness was not formally assessed, although we addressed this by excluding cases with disagreement between reviewers. Fourth, the relatively short follow-up period may not capture all cases of metachronous gastric cancer, as some cancers may develop after longer intervals. Fifth, both study and control groups consisted of patients with dysplasia who had undergone ESD, representing a selected risk population. This design allowed us to compare very high-risk patients (synchronous/metachronous lesions) with relatively low to moderate risk patients (single low-grade dysplasia), but limits assessment of how these markers perform in screening populations. Finally, we did not assess the severity, extent, or specific anatomical distribution patterns of map-like redness and patchy redness, which might provide additional prognostic information.
In conclusion, this study, based on 12 years of data from a substantial patient cohort, demonstrates that map-like redness and patchy redness at antrum observed after H. pylori eradication are independent endoscopic risk factors for synchronous or metachronous gastric dysplasia in high-risk patients. These findings can be readily recognized during routine endoscopy without requiring biopsies, making them practical tools for risk stratification in clinical practice. Patients exhibiting these endoscopic features may benefit from intensified surveillance strategies including shorter examination intervals and more meticulous evaluation. Our findings address an important clinical need: identifying which patients remain at elevated gastric cancer risk despite successful H. pylori eradication. As eradication therapy becomes increasingly widespread and most future gastric cancers will occur in post-eradication patients, practical methods for risk stratification become critical. Map-like redness and patchy redness represent easily recognizable markers that can guide personalized surveillance strategies. However, these findings require validation in independent, prospective, multi-center cohorts before definitive clinical recommendations can be established. With such validation, these endoscopic findings could be incorporated into clinical guidelines to optimize post-eradication surveillance and improve early detection of gastric cancer in high-risk populations.
Data availability statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Ethics statement
The studies involving humans were approved by Institutional Review Board of St. Vincent’s Hospital, Catholic University of Korea. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NL: Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. WC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burucoa, C, and Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter. (2017) 22:e12403. doi: 10.1111/hel.12403
2. Uemura, N, Okamoto, S, Yamamoto, S, Matsumura, N, Yamaguchi, S, Yamakido, M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. (2001) 345:784–9. doi: 10.1056/NEJMoa001999
3. IARC Monograph with institutional authorship (IARC Working Group). Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. (1994) 61:1–241.
5. Ford, AC, Forman, D, Hunt, RH, Yuan, Y, and Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. (2014) 348:g3174. doi: 10.1136/bmj.g3174
6. Ford, AC, Yuan, Y, and Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. (2020) 69:2113–21. doi: 10.1136/gutjnl-2020-320839
7. Take, S, Mizuno, M, Ishiki, K, Kusumoto, C, Imada, T, Hamada, F, et al. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. (2020) 55:281–8. doi: 10.1007/s00535-019-01639-w
8. Chen, HN, Wang, Z, Li, X, and Zhou, ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. (2016) 19:166–75. doi: 10.1007/s10120-015-0462-7
9. Schlemper, RJ, Riddell, RH, Kato, Y, Borchard, F, Cooper, HS, Dawsey, SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. (2000) 47:251–5. doi: 10.1136/gut.47.2.251
10. Jung, HK, Kang, SJ, Lee, YC, Yang, HJ, Park, SY, Shin, CM, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea. Gut Liver. (2020, 2021) 15:168–95. doi: 10.5009/gnl20288
11. Nakajima, T, Oda, I, Gotoda, T, Hamanaka, H, Eguchi, T, Yokoi, C, et al. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. (2006) 9:93–8. doi: 10.1007/s10120-006-0372-9
12. Sugano, K, Tack, J, Kuipers, EJ, Graham, DY, el-Omar, EM, Miura, S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. (2015) 64:1353–67. doi: 10.1136/gutjnl-2015-309252
13. Lim, E, Jo, IH, Kim, Y-J, and Chung, WC. In situ diagnosis of Helicobacter pylori infection using the endoscopic Kyoto scoring system. Korean J Helicobacter Up Gastrointest Res. (2021) 21:322–32. doi: 10.7704/kjhugr.2021.0046
14. Yagi, K, Nakamura, A, and Sekine, A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. (2002) 17:39–45. doi: 10.1046/j.1440-1746.2002.02665.x
15. Kimura, K, and Takemoto, T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. (1969) 1:87–97. doi: 10.1055/s-0028-1098086
16. Nagata, N, Shimbo, T, Akiyama, J, Nakashima, R, Kim, HH, Yoshida, T, et al. Predictability of gastric intestinal metaplasia by mottled patchy erythema seen on endoscopy. Gastroenterology Res. (2011) 4:203–9. doi: 10.4021/gr357w
17. Matsumoto, S, Sugimoto, M, Terai, T, Maruyama, Y, Sahara, S, Kanaoka, S, et al. Map-like redness development after eradication therapy for Helicobacter pylori infection: prospective multicenter observational study. Helicobacter. (2024) 29:e13146. doi: 10.1111/hel.13146
18. Wang, J, Xu, L, Shi, R, Huang, X, Li, SWH, Huang, Z, et al. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. (2011) 83:253–60. doi: 10.1159/000280318
19. Aumpan, N, Vilaichone, RK, Pornthisarn, B, Chonprasertsuk, S, Siramolpiwat, S, Bhanthumkomol, P, et al. Predictors for regression and progression of intestinal metaplasia (IM): a large population-based study from low prevalence area of gastric cancer (IM-predictor trial). PLoS One. (2021) 16:e0255601. doi: 10.1371/journal.pone.0255601
20. Ito, M, Haruma, K, Kamada, T, Mihara, M, Kim, S, Kitadai, Y, et al. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. (2002) 16:1449–56. doi: 10.1046/j.1365-2036.2002.01311.x
21. Butcher, LD, den Hartog, G, Ernst, PB, and Crowe, SE. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell Mol Gastroenterol Hepatol. (2017) 3:316–22. doi: 10.1016/j.jcmgh.2017.02.002
22. Kim, JM, Kim, JS, Lee, JY, Kim, YJ, Youn, HJ, Kim, IY, et al. Vacuolating cytotoxin in Helicobacter pylori water-soluble proteins upregulates chemokine expression in human eosinophils via Ca2+ influx, mitochondrial reactive oxygen intermediates, and NF-kappaB activation. Infect Immun. (2007) 75:3373–81. doi: 10.1128/IAI.01940-06
23. Duan, Y, Xu, Y, Dou, Y, and Xu, D. Helicobacter pylori and gastric cancer: mechanisms and new perspectives. J Hematol Oncol. (2025) 18:10. doi: 10.1186/s13045-024-01654-2
24. Capparelli, R, and Iannelli, D. Epigenetics and Helicobacter pylori. Int J Mol Sci. (2022) 23:1759. doi: 10.3390/ijms23031759
25. Rugge, M, and Genta, RM. Staging and grading of chronic gastritis. Hum Pathol. (2005) 36:228–33. doi: 10.1016/j.humpath.2004.12.008
26. Rugge, M, Correa, P, Di Mario, F, El-Omar, E, Fiocca, R, Geboes, K, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. (2008) 40:650–8. doi: 10.1016/j.dld.2008.02.030
27. Capelle, LG, de Vries, AC, Haringsma, J, ter Borg, F, de Vries, RA, Bruno, MJ, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. (2010) 71:1150–8. doi: 10.1016/j.gie.2009.12.029
28. Nam, JH, Choi, IJ, Kook, MC, Lee, JY, Cho, SJ, Nam, SY, et al. OLGA and OLGIM stage distribution according to age and Helicobacter pylori status in the Korean population. Helicobacter. (2014) 19:81–9. doi: 10.1111/hel.12112
29. Esposito, G, Dilaghi, E, Cazzato, M, Pilozzi, E, Conti, L, Carabotti, M, et al. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig Liver Dis. (2021) 53:467–73. doi: 10.1016/j.dld.2020.10.038
30. Mansour-Ghanaei, F, Joukar, F, Yeganeh, S, Sadeghi, M, Daryakar, A, and Sepehrimanesh, M. OLGA- and OLGIM-based staging in the patients with gastritis and endoscopy indications. Turk J Gastroenterol. (2022) 33:95–102. doi: 10.5152/tjg.2021.201154
31. Quach, DT, and Hiyama, T. Assessment of endoscopic gastric atrophy according to the Kimura-Takemoto classification and its potential application in daily practice. Clin Endosc. (2019) 52:321–7. doi: 10.5946/ce.2019.072
32. Sugimoto, M, Ban, H, Ichikawa, H, Sahara, S, Otsuka, T, Inatomi, O, et al. Efficacy of the Kyoto classification of gastritis in identifying patients at high risk for gastric Cancer. Intern Med. (2017) 56:579–86. doi: 10.2169/internalmedicine.56.7775
33. Toyokawa, T, Suwaki, K, Miyake, Y, Nakatsu, M, and Ando, M. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J Gastroenterol Hepatol. (2010) 25:544–7. doi: 10.1111/j.1440-1746.2009.05995.x
Keywords: gastric cancer, Helicobacter pylori, eradication therapy, endoscopic submucosal dissection, metachronous gastric cancer
Citation: Lim NR and Chung WC (2025) Gastric mucosal change after the eradication of Helicobacter pylori in patients at high risk for gastric cancers. Front. Med. 12:1679012. doi: 10.3389/fmed.2025.1679012
Edited by:
Dan-Lucian Dumitrașcu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Mohammad Tahir Siddiqui, Indian Institute of Technology Delhi, IndiaBekir Kocazeybek, Istanbul University-Cerrahpasa, Türkiye
Copyright © 2025 Lim and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woo Chul Chung, andjaHVsa3JAY2F0aG9saWMuYWMua3I=
 Na Rae Lim
Na Rae Lim Woo Chul Chung
Woo Chul Chung