- 1Department of Emergency Medicine, American University of Beirut Medical Center, Beirut, Lebanon
- 2Department of Internal Medicine, Faculty of Medicine, Clinical Research Institute, American University of Beirut, Beirut, Lebanon
- 3College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
Background: Sepsis remains a significant global health burden and a leading cause of in-hospital mortality. Recent research has focused on the prognostic value of biomarker kinetics, particularly clearance rates and inflammatory markers such as neutrophil-to-lymphocyte (NLR) ratio. This study aimed to compare the utility of procalcitonin and lactate clearance in predicting in-hospital mortality among septic patients and to identify an optimal procalcitonin clearance (PCTc) cut-off to differentiate survivors from non-survivors.
Methods: This was a retrospective cohort study of adult patients who presented with sepsis or septic shock to a tertiary care ED in Lebanon between November 2018 and March 2024. Procalcitonin and lactate readings were recorded along with demographics, comorbidities and therapeutic interventions. PCTc and lactate clearance were calculated as percentage change between the first and second readings, and lactate clearance was considered positive if > 10%. The primary outcome was in-hospital mortality, and secondary outcomes included ED, ICU and hospital length of stay. ROC curve was used to assess prognostic accuracy of biomarkers and derive an optimal PCTc cutoff. Multivariable logistic regression was conducted to evaluate the association of in-hospital mortality with lactate and procalcitonin clearances.
Results: Five hundred seventy-four patients with sepsis and septic shock were included. Mean age was 71.4 ± 16.5 years with male predominance (55.4%). Optimal cutoff for PCTc was found to be 23.1% (94.0% sensitivity, 7.0% specificity). Patients were then stratified based on lactate and procalcitonin clearances above and below the cutoffs to compare baseline parameters, interventions and outcomes. Patients with lactate clearance > 10 had significantly lower rates of chronic kidney disease (p = 0.006), congestive heart failure (p = 0.02), and chronic obstructive pulmonary disease (p = 0.04). Only CRP showed a statistically significant difference with respect to PCTc. Therapeutic interventions were similar in both PCTc groups and lactate clearance groups except for 24-h IV fluid administration (p = 0.04). Mortality was significantly associated with lactate clearance > 10 (p = 0.045) but not with PCTc (p = 0.65). The area under the ROC curve was 0.40 (95% CI: 0.34–0.45, p = 0.56) for lactate clearance, 0.39 (95% CI: 0.33–0.45, p = 0.56) for PCTc and 0.51 (95% CI: 0.46–0.56, p = 0.67) for NLR, with a significant difference among the AUCs (p < 0.001). Multivariate analysis showed a borderline significant association of in-hospital mortality with lactate clearance (OR = 0.66, 95% CI 0.42–1.04, p = 0.07) but not with procalcitonin clearance (OR = 1.13, 95% CI 0.43–2.95, p = 0.81). Vasopressor use was associated with reduced odds of death, while steroid use was independently associated with increased mortality.
Conclusion: Lactate clearance with 10% cutoff is a better predictor of in-hospital mortality in patients presenting to the ED with sepsis or septic shock compared to PCTc. An optimal PCTc cutoff of 23.1% was identified; however, it did not reach statistical significance for survival. Future prospective studies are needed to better define optimal biomarker cutoffs and compare their predictive accuracy for in-hospital mortality.
Introduction
Sepsis remains a major global health burden and a leading cause of mortality with an estimated 48.9 million incident cases and 11 million sepsis-related deaths annually, accounting for approximately 19.7% of all global deaths (1, 2). Despite advances in early recognition and management, sepsis and septic shock continue to pose diagnostic and prognostic challenges in critical care (2–4). Early identification of high-risk patients and timely assessment of therapeutic response are essential for improving clinical outcomes (5–7). In this context, multiple biomarkers have been widely studied to aid in diagnosis, guide treatment, and monitor response to therapy (8, 9). Yet, no biomarker has been sufficiently useful for accurate diagnosis and outcome prediction, which could potentially guide clinical decision-making (7, 10). This led to exploring biomarker kinetics, serial changes in sepsis biomarkers rather than an isolated value, as a promising tool to identify clinically effective prognosticators of sepsis and septic shock hospitilizations (11).
Lactate is one of the most widely studied biomarkers. As a marker of tissue hypoperfusion and cellular metabolic dysfunction, it has been a well-established component of the sepsis definition, though its clinical utility in prognostication and resuscitation bundles has been deliberately challenged (12–16). While hyperlactatemia typically reflects an imbalance between oxygen delivery and demand with a shift toward anaerobic metabolism, there are other causes for elevated lactate such epinephrine use, malignancies, medication use such metformin and beta agonists (14, 17). This supports the notion that lactate clearance, defined as reduction in serum lactate levels over time, is a more reliable indicator of effective resuscitation in sepsis patients than isolated lactate values (14). Therefore, lactate clearance has emerged as a dynamic biomarker measure for guiding sepsis management and predicting survival. A multicenter study by Ryan et al concluded a value of 10% lactate clearance to be the strongest predictor of mortality in sepsis patients (18). Clearances < 10% were associated with worse outcomes, while more robust clearances were often interpreted as a sign of resolving hypoxia and effective resuscitative efforts (18, 19). Nguyen et al. demonstrated that early lactate clearance in patients with severe sepsis or septic shock is associated with improved morbidity and mortality outcomes (15, 17, 19). Similarly, meta-analyses of randomized controlled trials support lactate clearance-guided therapy, showing significant mortality reduction in septic patients (20–22). Moreover, fluid resuscitation protocols tailored according to lactate clearance have proven both effective and reliable, especially in guiding early interventions while persistently elevated lactate beyond 24 h is associated with mortality rates up to 89% (20–23).
Conversely, procalcitonin, a precursor of the hormone calcitonin, increases in response to severe systemic and bacterial infections, and rise in its levels has been associated with the severity of sepsis and septic shock (24, 25). With appropriate antimicrobial therapy, levels decline making procalcitonin a potentially valuable biomarker of clinical improvement and survival (24, 26). Multiple studies showed that dynamic changes in procalcitonin clearance (PCTc) were associated with survival prediction in sepsis patients admitted to the ICU (26–28).
While both lactate and procalcitonin have individually been shown to correlate with sepsis severity and mortality, there is limited evidence directly comparing their clearance kinetics as predictors of survival. It remains uncertain which biomarker more reliably reflects the resolution of sepsis and better predicts short-term outcomes such as in-hospital mortality. A comparative evaluation with other established prognostic biomarkers in sepsis, such as C-reactive protein (CRP), neutrophil count, and the neutrophil-to-lymphocyte ratio (NLR), is also warranted. Notably, NLR has gained attention as a simple, inexpensive, and readily available indicator of systemic inflammation, reflecting the imbalance between innate and adaptive immunity and demonstrating promising utility in sepsis prognostication (29). Moreover, while the optimal cut-off value of lactate clearance is primarily reported to be 10%, that of procalcitonin remains poorly defined. Therefore, the study aimed to calculate an optimal cut-off value for PCTc that could differentiate survivors from non-survivors, compare PCTc with lactate clearance to identify which better correlates with in-hospital mortality, and evaluate both clearances in relation to inflammatory markers such as CRP and NLR.
Materials and methods
Study objective
The objective of this study was to evaluate the prognostic ability of lactate clearance versus procalcitonin clearance to predict in-hospital mortality and to derive a cut off value for procalcitonin clearance.
Study design
This retrospective cohort study included patients presenting to the Emergency Department (ED) of a tertiary care center in Lebanon with sepsis or septic shock. The study aimed to better define the prognostic utility of procalcitonin and lactate clearance and to compare their performance with each other and with other established biomarkers, including NLR. The study was approved by the Institutional Review Board (IRB) of the American University of Beirut (AUB) with protocol number BIO-2024-0115.
Patient population
Patient charts were reviewed between November 2021 and March 2024. Adult patients (age ≥ 18 years) diagnosed with sepsis or septic shock with measured procalcitonin and lactate levels in the ED were included. Patients < 18 years were excluded alongside pregnant, transfer or trauma patients, patients presenting with cardiac arrest and those who did not meet the criteria for sepsis or septic shock.
Data collection
Variables extracted from patient charts included demographics, comorbidities, vital signs and workup done in the ED. Lab tests collected were Complete Blood Count (CBC), Creatinine, Electrolytes, Troponin, INR, Lactate, Procalcitonin, CRP, Albumin and Arterial Blood Gases (ABGs). Other variables retrieved were need for mechanical ventilation, infection site (Lung, Urine, Intravascular Catheter, Gastrointestinal (GI), Skin, Gallbladder, Surgical Site, Blood, Peritoneum, Pyelonephritis), vasopressor use, steroids use, and total fluids received in the ED and at 24 h since admission.
Outcomes
The primary outcome was mortality with respect to lactate clearance versus procalcitonin clearance, while compared to NLR. Secondary outcomes included ED length of stay (LOS), ICU LOS and hospital LOS.
Biomarker clearance measurements
Procalcitonin clearance (PCTc) was defined using the following equation (30):
The first procalcitonin reading is reported if it was taken within the first 24 h. A positive result number indicated clearance of procalcitonin, while a negative value denoted an increase in level of procalcitonin afted initial presentation to the ED.
Lactate clearance is calculated using the following formula (18):
Lactate clearance cutoff was taken at 10% change between the 1st and 2nd readings reported in our database. Lactate clearance was considered positive if clearance is > 10%, and negative if it is ≤ 10 (18).
To further elaborate on our analysis, we computed NLR from our data and compared its performance to both lactate and procalcitonin clearances in predicting in-hospital mortality.
Statistical analysis
Analyses were performed using Statistical Package for the Social Sciences (SPSS) version 29 (IBM Corp., Armonk, NY, United States). Data was described using frequencies, percentages, means, standard deviations, medians and interquartile ranges. Shapiro-Wilk test of normality was done to identify highly skewed non-normal variables. Pearson’s chi-square and Student’s t-test were used to analyze parametric categorical and continuous variables resepctively. Meanwhile, Mann-Whitney and Fisher’s tests were performed for non-parametric continuous and categorical variables, respectively. Statistical significance was defined as a p-value < 0.05 for both tests. Baseline characteristics, lab values, therapy and outcome measures of interest were compared between patients with lactate and procalcitonin clearances below and above the cutoff values. The accuracy of lactate and procalcitonin clearances for predicting in-hospital mortality were assessed using the area under the receiver operating characteristic curve (AUC and ROC) across multiple clinical subgroups including lactate < 2 mmol/L or ≥ 2 mmol/L, albumin < 30 g/L or ≥ 30 g/L, patient subgroups (CKD, Heart failure, malignancy), infection site (lung, urine GI, skin, gallbladder), diabetes and age < 65. An AUC was also derived for NLR and compared to that of lactate clearance and PCTc. Furthermore, the ROC curve was used to determine the optimal cut-off value for PCTc at its maximum sensitivity and specificity, and likelihood ratios were subsequently calculated. Finally, multivariable logistic regression was conducted to study the association of mortality with lactate clearance and procalcitonin clearance after adjusting for clinically and statistically significant variables.
Results
Our cohort included a total of 574 sepsis and septic shock patients with 295 (51.4%) survivors and 279 (48.6%) non-survivors. The mean age of the cohort was 71.4 ± 16.5 years with a male predominance (55.4%), and no significant difference observed between survivors and non-survivors in age or sex (p = 0.49 and p = 0.81 respectively). The most common comorbidities were hypertension (HTN) (57.3%), malignancy (49.7%), and diabetes mellitus (DM) (36.1%). Non-survivors were more likely to have congestive heart failure (CHF) (29.4 vs. 21.4%, p = 0.03) and malignancy (54.1 vs. 45.4%, p = 0.04), while no significant differences were observed for other comorbidities, including chronic kidney disease (CKD), HTN, DM, and chronic obstructive pulmonary disease (COPD). Non-survivors had significantly higher systolic blood pressure (SBP) (111.6 ± 27.3 vs. 105.8 ± 26.5, p = 0.01), higher CRP levels (155.6 ± 115.1 mg/L vs. 120.6 ± 98.0 mg/L, p < 0.001) and lower hemoglobin levels (10.8 ± 2.3 g/dL vs. 11.3 ± 3.7 g/dL, p = 0.04). Lactate clearance was notably lower in non-survivors (−0.23 ± 1.42) compared with survivors (0.18 ± 0.57, p < 0.002), whereas neither procalcitonin clearance nor NLR showed any significant difference (p = 0.13 and p = 0.92 respectively). Vasopressor use was significantly higher in survivors (50.2 vs. 35.5%, p < 0.001), while steroids were more frequently used in non-survivors (35.5 vs. 21.7%, p < 0.001). Non-survivors also had longer ED, ICU, and hospital stays (p = 0.01 and p < 0.001 respectively) (Table 1).

Table 1. Baseline characteristics of patients presenting to the emergency department with sepsis or septic shock.
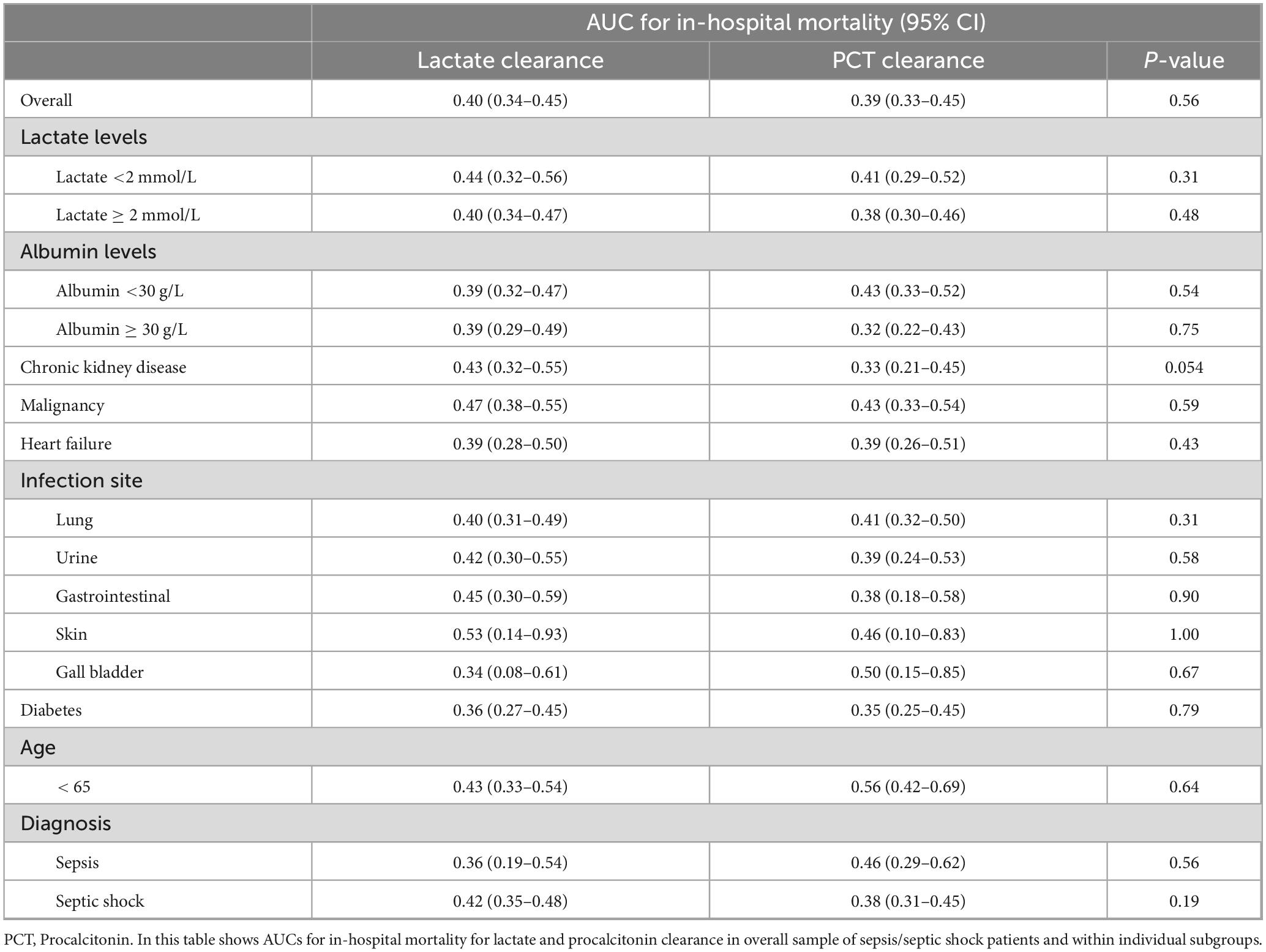
PCTc cutoff was found to be 23.1%, with a sensitivity of 94.0%, specificity of 7.0%, LR + = 1.01 and LR− = 0.86, using data from our generated sepsis database (Figure 1). Using lactate and procalcitonin clearance cut-offs, we stratified our study sample to compare baseline characteristics, ED parameters and outcomes. Age and sex distributions were similar across groups stratified by lactate clearance (p = 0.48 and p = 0.96, respectively) and by procalcitonin clearance (p = 0.76 and p = 0.59, respectively). Patients with lactate clearance > 10 had significantly lower rates of CKD (20.5 vs. 33.1%, p = 0.006), CHF (23.6 vs. 34.6%, p = 0.02), and COPD (9.4 vs. 16.5%, p = 0.04), while other comorbidities were similarly distributed between the 2 lactate clearance groups. However, none of the recorded comorbidities showed significant difference with respect to PCT clearance. Moreover, the most common infection sites in our sample were lung, urinary tract infections and gastrointestinal infections (43.6, 22.6, and 15.2%, respectively). However, infection site was not significantly different in either of the clearance groups (lactate clearance, p = 0.29; PCTc, p = 0.98) (Table 2).

Table 2. Characteristics of patients with sepsis or septic shock stratified by lactate and procalcitonin clearances.
None of the reported vitals reached statistical significance in either the lactate clearance or the PCTc counterpart. In terms of laboratory values, there were no significant differences in relation to lactate clearance, while only CRP showed a statistically significant difference with respect to PCTc (p < 0.001) (Table 3).
Both vasopressor use and steroid use were not significantly different between the 2 lactate clearance groups (p = 0.36 and p = 0.31, respectively). Similarly, they did not differ with respect to PCTc (p = 0.19 and p = 0.29, respectively). On the other hand, patients with high lactate clearance > 10 received more IV fluids in the first 24 h of ED presentation (p = 0.04) compared to patients with a lower lactate clearance. In the PCTc arm, fluid resuscitation did not show a statistically significant difference between the 2 groups. Moreover, ED, ICU, and hospital lengths of stay did not differ significantly with either lactate clearance or procalcitonin clearance. Finally, 56.4% of patients with lactate clearance ≤ 10 died within their hospital stay compared to 45.7% with lactate clearance > 10, with a significant association between lactate clearance and survival (p = 0.045). Meanwhile, no significant difference in mortality rates was shown between PCTc groups below and above the cutoff (45.0 vs. 50.2%, p = 0.65) (Table 4).
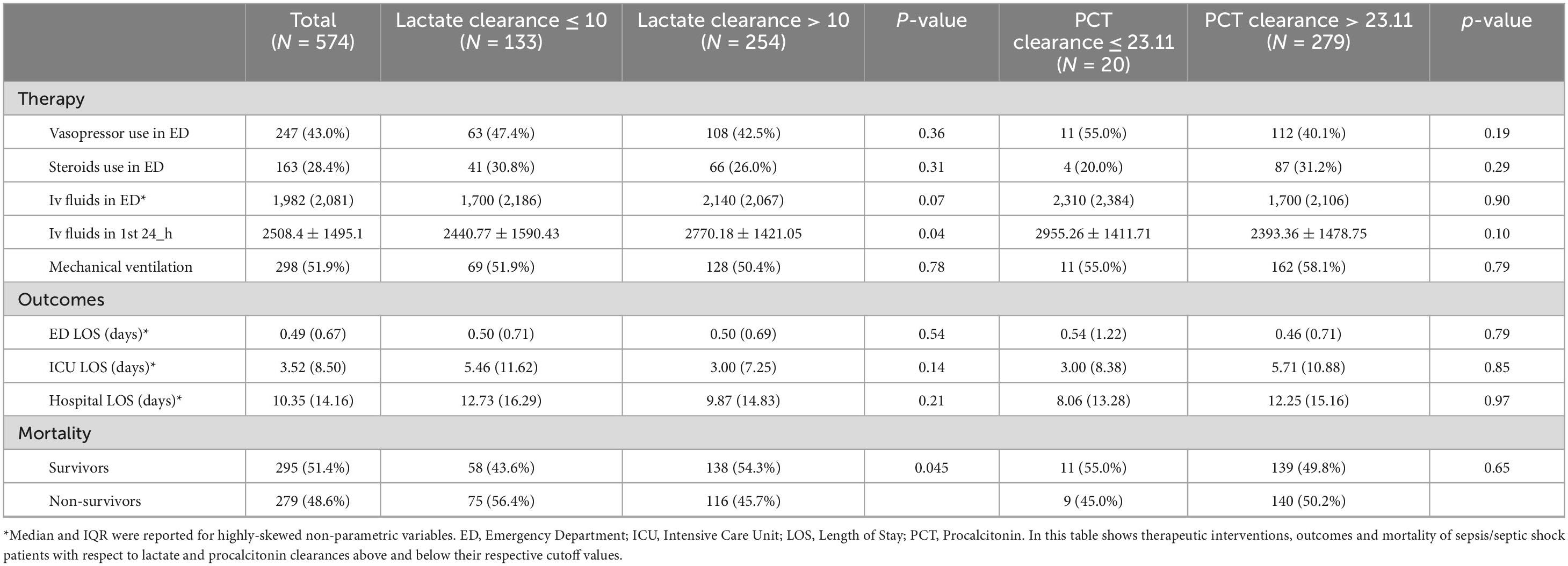
Table 4. Therapeutic interventions, outcomes and mortality in lactate and procalcitonin clearance groups.
Both lactate clearance and PCTc demonstrated poor discriminatory ability for in-hospital mortality, with overall AUCs of 0.40 (95% CI: 0.34–0.45) and 0.39 (95% CI: 0.33–0.45), respectively (p = 0.56). Subgroup analyses showed similarly low predictive performance across different lactate and albumin levels, with AUCs below 0.5 and no significant differences between groups. Among comorbidities, AUCs remained low, though in patients with chronic kidney disease, lactate clearance reached an AUC of 0.43 compared to 0.33 for PCTc, with a near-significant p-value of 0.054. In malignancy, lactate clearance reached an AUC of 0.47, but the comparison remained non-significant between groups (p = 0.59). Regarding infection sites, skin infections had a slightly higher AUC of 0.53 for lactate clearance, but without statistical significance (p = 1.00); other infection sites showed low AUCs. In patients under 65 years, PCTc reached an AUC of 0.56 without significant difference (p = 0.64). For sepsis and septic shock patients, lactate clearance AUCs were 0.36 and 0.42, respectively (p = 0.56), and procalcitonin clearance AUCs were 0.46 and 0.38, respectively (p = 0.19) (Table 5). The AUC of NLR was similarly weak, with a value of 0.51 (95% CI: 0.46–0.56) and a p-value of 0.67 for mortality. When comparing all three parameters, a statistically significant difference was observed among the AUCs (p < 0.001). Pairwise comparisons also showed significant differences between NLR and lactate clearance (p = 0.005) and between NLR and PCT clearance (p = 0.04) (Figure 1).
Finally, in the multivariate analysis and after adjusting for potential confounders, lactate clearance showed a borderline association with in-hospital mortality (OR = 0.66, 95% CI 0.42–1.04, p = 0.07), while no association was established with procalcitonin clearance (OR = 1.13, 95% CI 0.43–2.95, p = 0.81). In both the lactate and procalcitonin models, vasopressor use was associated with reduced odds of death (OR = 0.46, 95% CI 0.29–0.71, p < 0.001; OR = 0.54, 95% CI 0.33–0.90, p = 0.02, respectively), while steroid use was independently associated with increased mortality (OR = 1.93, 95% CI 1.17–3.17, p = 0.01; OR = 2.02, 95% CI 1.19–3.43, p = 0.009, respectively) (Table 6).

Table 6. Odds ratio of mortality in patients with sepsis and septic shock with respect to lactate and procalcitonin clearance.
Discussion
Our study looked at a cohort of patients with sepsis and septic shock to evaluate the prognostic ability of procalcitonin and lactate clearances for in-hospital mortality. We found a PCTc of 23.1% (sensitivity, 94.0%; specificity 7.0%) to be the optimal cut-off to differentiate survivors from non-survivors. Other studies reported cutoffs ranging from 30 to 70% at different time intervals (24, 31–33). Results revealed 50.2% mortality with higher PCTc and 56.4% mortality with lower lactate clearance. However, only lactate clearance was significantly associated with in-hospital mortality (p = 0.045).
Our findings align with prior literature in affirming the prognostic utility of lactate clearance in sepsis. Several studies and meta-analyses have reported strong associations between higher lactate clearance and reduced mortality in sepsis patients (18–21, 23, 34). In particular, a 10% lactate clearance cutoff was shown to be significantly associated with better outcomes. This corroborates prior evidence showing similar survival prediction with a 10% lactate clearance cut-off (18, 19). A study on neonatal sepsis showed higher mortality among septic neonates with < 10% lactate clearance (35). Conversely, Lee et al. identified an optimal lactate clearance cut-off of 24.4% for predicting 30-day mortality and concluded that 6-h lactate levels were superior to 6-h lactate clearance in mortality prediction, a result reproduced by Ryoo et al in their study on lactate clearance in patients with septic shock (36, 37). Another study also found that lactate clearance < 20% was associated with increased in-hospital mortality while that < 10% was not (38). Despite this present controversy on its optimal cutoff, lactate clearance remains a reliable predictor of sepsis-induced mortality.
Procalcitonin clearance showed no significant association with survival of our sepsis and septic shock cohort (p = 0.65). A lack of association was also demonstrated in the study by Karlsson et al., which found that a procalcitonin decrease of more than 50% within 72 h was not independently associated with in-hospital mortality (39). In pediatric sepsis, one study found that 48-h PCT did not predict early clinical stability (40), contrasting with another showing reduced 24-h PCT clearance independently associated with higher mortality (41). Thus, evidence on reliability of PCTc as a predictor of mortality remains conflicting. Several studies supported it as a prognosticator of severe sepsis and septic shock (24, 26–28, 31, 32). A 2017 multicenter study concluded that PCT kinetics in the first 4 days were predictive for survival of patients diagnosed with severe sepsis or septic shock (42). Additionally, two systematic review and meta-analysis revealed a significant association between procalcitonin non-clearance and mortality despite significant heterogeneity across included studies (43, 44). Notably, a recent study explored a prognostic advantage of combining PCTc with blood parameters, such as white cell count, platelet count and CRP, for predicting mortality in cancer patients with sepsis (45). In our study, some patients did not have a second procalcitonin level recorded within the first 24 h of admission, resulting in a small sample size. This may have biased the analysis toward a smaller subset of patients who had two documented readings and were thus included in the final analysis.
Our results also showed patients with lower lactate clearance but not PCTc were more likely to have comorbidities including CKD and CHF. Similarly, non-survivors exhibited a higher overall comorbidity burden, with increased rates of CHF and malignancy. Multiple studies highlighted that patients admitted for sepsis or septic shock with concomitant malignancy or heart failure had higher rates of in-hospital mortality (46–49). We believe this reflects an intrinsic relationship between having comorbidities, the ability to clear lactate and mortality. Interestingly, a study by Thomas-Rüddel et al. reported that mortality attributable to sepsis alone ranged from 6 to 12%, whereas mortality involving both sepsis and underlying comorbidities rose to 54–76% (50).
CRP levels were significantly higher in patients with high PCTc, similar to prior findings (51) Serial decrease in PCT remains a better predictor of survival compared to CRP, however, this could hint to the potential benefit of combining both parameters for a more accurate risk assessment and outcome prediction (45, 51).
The neutrophil-to-lymphocyte ratio (NLR) has emerged as an inexpensive and readily obtainable biomarker for prognostication in infectious clinical settings, including sepsis, pneumonia, and COVID-19 (29). Several studies have shown that elevated NLR strongly predicts 30-day mortality in acutely ill elderly patients and in those with community-acquired pneumonia (CAP), a common precursor of sepsis, highlighting its association with disease severity and overall mortality (52, 53). Notably, NLR has been shown to outperform traditional prognostic markers such as CURB-65, CRP, and individual leukocyte counts (52, 54). Furthermore, a meta-analysis including more than 11,000 patients with sepsis reported that higher baseline NLR values were significantly associated with worse outcomes (hazard ratio ≈ 1.75; 95% CI 1.56–1.97) (55).
Despite showing only modest prognostic value overall, NLR outperformed lactate and procalcitonin clearances in our analysis, achieving the largest area under the ROC curve and reinforcing its potential as a powerful biomarker for sepsis prognostication. The discrepancy in results can be potentially explained by unmeasured confounding factors and the heterogeneity of our patient population with variability in sepsis presentation across patients. Additionally, the relatively limited performance of NLR could be partly due to the absence of serial measurements. These limitations underscore the need for future studies to assess NLR as a dynamic marker, as illustrated by Lee H. et al., showing that incremental changes in NLR significantly predicted 30-day mortality in patients with CAP (56).
Combining dynamic biomarkers with established scoring systems such as SOFA, APACHE II, and qSOFA enhances prognostic accuracy in sepsis and septic shock. For example, Li et al. demonstrated that combination of NLR with SOFA outperformed each individually and exhibited predictive performance comparable to APACHE II, while models incorporating lactate or PCT clearance with qSOFA or SOFA were shown to outperform either measure alone in multiple studies, improving early identification of high-risk patients (57–61). By leveraging both laboratory and clinical parameters, these multimodal approaches offer a robust tool for mortality prediction and risk stratification. Future studies should explore combining lactate and PCT clearance with NLR and other dynamic scores to further enhance predictive performance and guide individualized care.
Finally, our results indicated that vasopressor use was significantly higher among survivors and independently associated with decreased odds of in-hospital death. We find this result plausible because rapid restoration of mean arterial pressure (MAP) improves organ perfusion and reduces the need for excessive fluid resuscitation, thereby lowering the risk of fluid overload and improving outcomes. Growing evidence further supports the prognostic benefit of early vasopressor use in septic patients. For example, a randomized phase II trial in patients with sepsis-associated hypotension demonstrated significantly improved shock control at 6 h with early low-dose norepinephrine, though without a significant mortality difference (62). Subsequent systematic reviews and meta-analyses indicated that vasopressor initiation within 1–6 h of shock onset is associated with lower short-term mortality, faster attainment of target MAP and reduced fluid volume (63, 64). Therefore, our findings align with evidence suggesting improved outcomes with earlier, protocolized vasopressor use, yet prospective studies are warranted to further evaluate this intervention, given the inherent confounding bias of retrospective analyses. Conversely, steroid use was significantly higher among non-survivors and associated with increased odds of mortality. While this could likely reflect a confounding state with corticosteroids often reserved for patients with refractory shock or a severe disease course, a systemic review of 61 RCTs demonstrated a slightly reduced 28-day mortality with steroid use, though it had little to no effect on long-term mortality (65). Other trials have demonstrated faster shock resolution with steroid use but did not show a clear mortality benefit, with potential adverse effects contributing to worse outcomes in high-risk patients (66, 67).
Importantly, our study adds to the literature by directly comparing lactate and procalcitonin clearances in a real-world ED population of sepsis and septic shock patients. Lactate clearance appeared to be a better predictor for in-hospital mortality than procalcitonin clearance. Moreover, our subgroup analyses across comorbidities, age groups, and infection sources provide further insight into the predictive performance of both biomarkers, helping to address the gap in understanding which biomarker perform better across heterogeneous sepsis presentations. By confirming lactate clearance threshold that better correlates with mortality and evaluating its performance in key clinical subgroups, our study contributes practical data to help refine biomarker-based resuscitation strategies.
The study presents several important limitations. First, the timing of lactate measurement was not standardized and patients without serial biomarker measurements were excluded, which may have introduced variability and selection bias. Second, there is a risk of survivor bias, as patients who died early may not have had the opportunity to demonstrate clearance, possibly overestimating the association between low clearance and mortality. Missing data on important parameters such as PaO2, FiO2 and platelets limited our ability to compare clearances with established biomarkers to determine the better mortality predictors and strengthen our analysis. Additionally, the retrospective, single-center design and exclusion of patients lacking Sepsis-3 criteria data (245 of the initial 819 patient dataset) may limit generalizability and applicability to less severe sepsis, while the moderate sample size could have reduced statistical power for some subgroup analyses. Future prospective, multicenter studies with standardized biomarker timing and validated clinical severity scoring are warranted to confirm and expand upon these results.
Conclusion
When comparing procalcitonin and lactate clearances, the latter appears to be a more reliable predictor of mortality in patients presenting to the emergency department with sepsis or septic shock. While an optimal procalcitonin clearance cutoff of 23.1% was identified for survival, it did not reach statistical significance as a predictor of mortality. In contrast, a lactate clearance of 10% continues to demonstrate prognostic value, despite some controversy in the literature. Our findings also support a prognostic potential for NLR. Future studies are needed to better define optimal cutoffs for dynamic biomarkers and investigate models that integrate biomarkers with clinical scoring systems to improve risk stratification and mortality prediction in sepsis and septic shock.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of the American University of Beirut (AUB). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective study design and absence of any direct contact with participants that could affect their rights or welfare.
Author contributions
RD: Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. RB: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NB: Writing – original draft. RS: Writing – original draft. MM: Formal analysis, Writing – original draft. HT: Formal analysis, Validation, Writing – original draft. GA: Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11.
3. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. (2014) 311:1308–16.
4. Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. (2014) 2:380–6. doi: 10.1016/S2213-2600(14)70061-X
5. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. (2017) 43:304–77.
6. Keegan J, Wira CR III. Early identification and management of patients with severe sepsis and septic shock in the emergency department. Emerg Med Clin. (2014) 32:759–76.
7. Cohen J, Vincent J-L, Adhikari NKJ, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Inf Dis. (2015) 15:581–614.
8. Lee JH, Kim S-H, Jang JH, Park JH, Jo KM, No T-H, et al. Clinical usefulness of biomarkers for diagnosis and prediction of prognosis in sepsis and septic shock. Medicine. (2022) 101:e31895.
9. Orfanu A, Aramă V, Popescu C, Tilişcan C, Streinu-Cercel A., Aramă ŞS. The dynamical assessment of inflammatory biomarkers in predicting the outcome of septic patients and the response to antimicrobial therapy. J Crit Care Med (Targu Mures). (2020) 6:25–31.
11. Bahloul M, Bradii S, Turki M, Bouchaala K, Ben Hamida C, Chelly H, et al. The value of sepsis biomarkers and their kinetics in the prognosis of septic shock due to bacterial infections. Anaesthesiol Intensive Ther. (2021) 53:312–8.
12. Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. (2007) 33:1892–9.
13. Bakker J, Postelnicu R, Mukherjee V. Lactate: where are we now? Crit Care Clin. (2020) 36:115–24.
14. James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. (1999) 354:505–8.
15. Gore DC, Jahoor F, Hibbert JM, DeMaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production. Not deficits in tissue oxygen availability. Ann Surg. (1996) 224:97–102. doi: 10.1097/00000658-199607000-00015
16. Gernardin G, Pradier C, Tiger F, Deloffre P, Mattei M. Blood pressure and arterial lactate level are early indicators of short-term survival in human septic shock. Intensive Care Med. (1996) 22:17–25. doi: 10.1007/BF01728326
17. Vincent J-L, Dufaye P, Berré J, Leeman M, Degaute J-P, Kahn RJ. Serial lactate determinations during circulatory shock. Crit Care Med. (1983) 11:449–51.
18. Arnold RC, Shapiro NI, Jones AE, Schorr C, Pope J, Casner E, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. (2009) 32:35–9. doi: 10.1097/shk.0b013e3181971d47
19. Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. (2004) 32:1637–42.
20. Gu W-J, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med. (2015) 41:1862–3.
21. Ding XF, Yang ZY, Xu ZT, Li LF, Yuan B, Guo LN, et al. Early goal-directed and lactate-guided therapy in adult patients with severe sepsis and septic shock: a meta-analysis of randomized controlled trials. J Transl Med. (2018) 16:331.
22. Yu B, Tian HY, Hu ZJ, Zhao C, Liu LX, Zhang Y, et al. [Comparison of the effect of fluid resuscitation as guided either by lactate clearance rate or by central venous oxygen saturation in patients with sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2013) 25:578–83.
23. Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. (1964) 143:1457–9. doi: 10.1126/science.143.3613.1457
24. Ruiz-Rodríguez JC, Caballero J, Ruiz-Sanmartin A, Ribas VJ, Pérez M, Bóveda JL, et al. Usefulness of procalcitonin clearance as a prognostic biomarker in septic shock. A prospective pilot study. Med Intensiva (English Ed.). (2012) 36:475–80.
25. Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. (2006) 34:2596–602.
26. Huang MY, Chen CY, Chien JH, Wu KH, Chang YJ, Wu KH, et al. Serum procalcitonin and procalcitonin clearance as a prognostic biomarker in patients with severe sepsis and septic shock. Biomed Res Int. (2016) 2016:1758501.
27. Guan J, Lin Z, Lue H. Dynamic change of procalcitonin, rather than concentration itself, is predictive of survival in septic shock patients when beyond 10 ng/mL. Shock. (2011) 36:570–4. doi: 10.1097/SHK.0b013e31823533f9
28. Azevedo JR, Torres OJ, Czeczko NG, Tuon FF, Nassif PA, Souza GD. Procalcitonin as a prognostic biomarker of severe sepsis and septic shock. Rev Col Bras Cir. (2012) 39:456–61.
29. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. (2022) 23:3636.
30. Vitorio D, Nassar AP Jr., Caruso P. Procalcitonin clearance and prognosis in sepsis: are there really an optimal cutoff and time interval? Crit Care Med. (2017) 45:e1097–8.
31. Suberviola B, Castellanos-Ortega A, González-Castro A, García-Astudillo LA, Fernández-Miret B. [Prognostic value of procalcitonin. C-reactive protein and leukocytes in septic shock]. Med Intensiva. (2012) 36:177–84.
32. de Azevedo JRA, Torres OJM, Beraldi RA, Ribas CAPM, Malafaia O. Prognostic evaluation of severe sepsis and septic shock: procalcitonin clearance vs Δ sequential organ failure assessment. J Crit Care. (2015) 30:219.e9–12.
33. Mat Nor MB, Md Ralib A. Procalcitonin clearance for early prediction of survival in critically ill patients with severe sepsis. Crit Care Res Pract. (2014) 2014:819034.
34. Vincent JL, Quintairos ESA, Couto L Jr., Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. (2016) 20:257.
35. Chaudhry S, Haroon F, Irfan Waheed KA, Victor G, Shahzad M, Fatima B. Blood lactate levels and lactate clearance as predictors of mortality in neonatal sepsis. J Ayub Med Coll Abbottabad. (2022) 34:438–41. doi: 10.55519/JAMC-03-9087
36. Lee SG, Song J, Park DW, Moon S, Cho HJ, Kim JY, et al. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: a retrospective cohort study according to the sepsis-3 definitions. Medicine (Baltimore). (2021) 100:e24835. doi: 10.1097/MD.0000000000024835
37. Ryoo SM, Lee J, Lee Y-S, Lee JH, Lim KS, Huh JW, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med. (2018) 46:e489–95. doi: 10.1097/CCM.0000000000003030
38. Lokhandwala S, Andersen LW, Nair S, Patel P, Cocchi MN, Donnino MW. Absolute lactate value vs relative reduction as a predictor of mortality in severe sepsis and septic shock. J Crit Care. (2017) 37:179–84. doi: 10.1016/j.jcrc.2016.09.023
39. Karlsson S, Heikkinen M, Pettilä V, Alila S, Väisänen S, Pulkki K, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care. (2010) 14:R205.
40. Rubbab B, Davila S, Moreland J, Firmani S, Most Z. Does serial procalcitonin monitoring predict clinical outcomes in children with sepsis? A diagnostic stewardship study. Antimicrob Steward Healthc Epidemiol. (2025) 5:e124. doi: 10.1017/ash.2025.10032
41. Uppala R, Sitthikarnkha P, Kriengwatanasiri A, Techasatian L, Saengnipanthkul S, Niamsanit S, et al. Serum procalcitonin and procalcitonin clearance as a prognostic biomarker of sepsis in a pediatric critical care setting: a tertiary care experience 2016–2021. PLoS One. (2025) 20:e0324980. doi: 10.1371/journal.pone.0324980
42. Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin MOnitoring SEpsis (MOSES) study. Crit Care Med. (2017) 45:781–9. doi: 10.1097/CCM.0000000000002321
43. Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One. (2015) 10:e0129450.
44. Patnaik R, Azim A, Mishra P. Should serial monitoring of procalcitonin be done routinely in critically ill patients of ICU: a systematic review and meta-analysis. J Anaesthesiol Clin Pharmacol. (2020) 36:458–64. doi: 10.4103/joacp.JOACP_388_19
45. Zhu T, Tian B, Wang L. Predictive value of peripheral blood indicators plus procalcitonin clearance rate for mortality in cancer patients with sepsis. Am J Cancer Res. (2024) 14:5839–50. doi: 10.62347/NKOL2327
46. Kang C, Choi S, Jang EJ, Joo S, Jeong JH, Oh SY, et al. Prevalence and outcomes of chronic comorbid conditions in patients with sepsis in Korea: a nationwide cohort study from 2011 to 2016. BMC Infect Dis. (2024) 24:184. doi: 10.1186/s12879-024-09081-x
47. Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One. (2013) 8:e72476.
48. Abou Dagher G, Hajjar K, Khoury C, El Hajj N, Kanso M, Makki M, et al. Outcomes of patients with systolic heart failure presenting with sepsis to the emergency department of a tertiary hospital: a retrospective chart review study from Lebanon. BMJ Open. (2018) 8:e022185. doi: 10.1136/bmjopen-2018-022185
49. Jones TW, Smith SE, Van Tuyl JS, Newsome AS. Sepsis with preexisting heart failure: management of confounding clinical features. J Intensive Care Med. (2021) 36:989–1012.
50. Thomas-Rüddel DO, Fröhlich H, Schwarzkopf D, Bloos F, Riessen R. Sepsis and underlying comorbidities in intensive care unit patients: analysis of the cause of death by different clinicians-a pilot study. Med Klin Intensivmed Notfmed. (2024) 119:123–8. doi: 10.1007/s00063-023-01037-4
51. Claeys R, Vinken S, Spapen H, ver Elst K, Huyghens L, Gorus FK. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. In: CA Burtis, MM Müller editors. Advances in Critical Care Testing. Berlin, Heidelberg: Springer Berlin Heidelberg (2004). doi: 10.1097/00003246-200204000-00006
52. Cataudella E, Giraffa CM, Di Marca S, Pulvirenti A, Alaimo S, Pisano M, et al. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J Am Geriatr Soc. (2017) 65:1796–801.
53. Galardo G, Crisanti L, Gentile A, Cornacchia M, Iatomasi F, Egiddi I, et al. Neutrophil to lymphocyte ratio (NLR) and short-term mortality risk in elderly acute medical patients admitted to a University Hospital Emergency Department. Intern Emerg Med. (2025) 20:553–62.
54. de Jager CP, Wever PC, Gemen EF, Kusters R, van Gageldonk-Lafeber AB, van der Poll T, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. (2012) 7:e46561.
55. Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Ame J Emerg Med. (2020) 38:641–7.
56. Lee H, Kim I, Kang BH, Um S-J. Prognostic value of serial neutrophil-to-lymphocyte ratio measurements in hospitalized community-acquired pneumonia. PLoS One. (2021) 16:e0250067.
57. Li Y, Wang J, Wei B, Zhang X, Hu L, Ye X. Value of neutrophil:lymphocyte ratio combined with sequential organ failure assessment score in assessing the prognosis of sepsis patients. Int J Gen Med. (2022) 15:1901–8.
58. Zhao M, Duan M. [Lactic acid, lactate clearance and procalcitonin in assessing the severity and predicting prognosis in sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2020) 32:449–53. doi: 10.3760/cma.j.cn121430-20200129-00086
59. García de Guadiana-Romualdo L, Botella LA, Rodríguez Rojas C, Puche Candel A, Jimenez Sánchez R, Conesa Zamora P, et al. Mortality prediction model from combined serial lactate, procalcitonin and calprotectin levels in critically ill patients with sepsis: A retrospective study according to sepsis-3 definition. Medicina Intensiva. (2024) 48:629–38. doi: 10.1016/j.medine.2024.05.015
60. Tian K-X, Dong J-X, Du J, Huang X-B, Liu Y. Development and validation of a lactate and procalcitonin-based nomogram model for predicting prognosis in ICU sepsis patients. Front Dis Emerg Med. (2025) 3:1612093.
61. Park H, Lee J, Oh DK, Park MH, Lim C-M, Lee S-M, et al. Serial evaluation of the serum lactate level with the SOFA score to predict mortality in patients with sepsis. Sci Rep. (2023) 13:6351.
62. Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early use of norepinephrine in septic shock resuscitation (CENSER). A randomized trial. Am J Respir Crit Care Med. (2019) 199:1097–105. doi: 10.1164/rccm.201806-1034OC
63. Li Y, Li H, Zhang D. Timing of norepinephrine initiation in patients with septic shock: a systematic review and meta-analysis. Crit Care. (2020) 24:488.
64. Ye E, Ye H, Wang S, Fang X. Initiation timing of vasopressor in patients with septic shock: a systematic review and meta-analysis. Shock. (2023) 60:627–36.
65. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev. (2019) 12:Cd002243.
66. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. (2018) 378:797–808.
Keywords: sepsis, septic shock, procalcitonin, lactate, clearance, mortality
Citation: Diab R, Bou Chebl R, Barmo N, Siblini R, Makki M, Tamim H and Abou Dagher G (2025) Prognostic utility of procalcitonin and lactate clearance for in-hospital mortality in sepsis. Front. Med. 12:1679297. doi: 10.3389/fmed.2025.1679297
Received: 04 August 2025; Revised: 29 October 2025; Accepted: 06 November 2025;
Published: 20 November 2025.
Edited by:
Stefano Busani, University Hospital of Modena, ItalyReviewed by:
Jesús Javier Martínez García, Autonomous University of Sinaloa, MexicoLorenzo Malatino, Cannizzaro Hospital, Italy
Copyright © 2025 Diab, Bou Chebl, Barmo, Siblini, Makki, Tamim and Abou Dagher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilbert Abou Dagher, Z2E2NkBhdWIuZWR1Lmxi
†These authors have contributed equally to this work
 Razan Diab
Razan Diab Ralphe Bou Chebl1†
Ralphe Bou Chebl1† Nour Barmo
Nour Barmo Reem Siblini
Reem Siblini Hani Tamim
Hani Tamim Gilbert Abou Dagher
Gilbert Abou Dagher