- 1Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Gynecology Department, Jiangxi Provincial Maternal and Child Health Hospital, Nanchang, Jiangxi, China
- 3Oncology Department, Jiangxi Provincial Maternal and Child Health Hospital, Nanchang, Jiangxi, China
Objectives: Menopausal Hormone Replacement Therapy (MHT) is widely used by peri- and post-menopausal women to alleviate menopause-related symptoms and preventing bone loss, but the underlying mechanisms remain inadequately elucidated. Accumulating evidence suggested that gut microbiota was involved in the regulation of bone metabolic processes. The aim of this study was to characterize the alterations in gut microbiota profiles by MHT treatment in postmenopausal women and explore the relationship between gut microbiota and bone metabolism.
Methods: Fecal samples collected from a total of 31 postmenopausal women with or without MHT were subjected to 16S ribosomal RNA (rRNA) gene sequencing and short-chain fatty acid (SCFAs) analysis in this study. The serum levels of bone metabolic markers were determined via chemiluminescent immunoassays. Spearman correlation coefficient was utilizes to assess the correlation between genera and bone metabolism indexes.
Results: Postmenopausal women undergoing MHT exhibited lower serum procollagen type I N propeptide (P1NP) and C-terminal telopeptide of type I collagen (CTX-1). Significant differences in alpha diversity and beta diversity were observed in the microbial compositions between two groups (P < 0.05). Of the total 295 microbial taxa identified, 16 taxa displayed significant differential abundance, with Coprococcus, Eubacterium_ruminantium_group, Lachnospiraceae_UCG-010 being more enriched in MHT+, correlating with lower bone metabolic markers and higher estrogen level. Conversely, Escherichia-Shigella taxa was more abundant in MHT- group, positively correlating with high bone metabolic markers and lower estrogen level. SCFAs appeared to have a limited role in bone metabolism but were found to be associated with several genera, including Coprococcus, Adlercreutzia, Colidextribacter.
Conclusions: The findings of the study demonstrated that MHT has the potential to prevent osteoporosis through the alteration of the gut microbial composition in postmenopausal women and identified promising microbial taxonomic that may contribute to the protective effects of MHT on bone mass conservation. Comparing with most previous studies that focused on the gut microbiota profiles between individuals with different bone mass, our study emphasized the protective role of gut microbiota in MHT process while bone mineral content (BMC) has no significant difference.
Introduction
Osteoporosis is a progressive systemic skeletal disease characterized by reduced bone mass and microarchitectural alteration in bone tissue. Estrogen facilitates gut injury repair. Impairment of this function in low-estrogen states, such as postmenopause, may compromise intestinal barrier integrity, resulting in increased gut permeability. This promotes bacterial translocation—the dissemination of gut microbiota to extraintestinal sites via the bloodstream—potentially elevating the risk of metabolic and systemic diseases (1). The deficiency of endogenous estrogen after menopause leads women to bone loss, which increase the susceptibility to osteoporosis and bone fractures (2). As the lifespan of women extends in contemporary times, it is estimated that ~10% of the world's population and over 30% of postmenopausal women aged over 50 years suffered from osteoporosis according to statistics worldwide. Healthcare practitioners, including clinicians, dietitians, sports specialist are devoting much concerns to this issue.
Menopausal Hormone Replacement therapy (MHT) is a proven intervention for the prophylaxis of postmenopausal osteoporosis, which is recommended by International Menopause Society as a principle measures to improve the quality of life (3). The administration of MHT involves one to three categories of hormones: estrogens, the primary active constituent; progestogens, protecting endometrium against cancer in women with a uterus; and androgens when is needed. The underlying mechanisms by which MHT influences bone mass are complex, revealed factors include inflammation, immune response, intestinal mucosal barrier function and so on, while some are still unknown. Despite the numerous are there, the utilization of MHT is limited because of the potential risks for adverse events and uncertainty for prelonged hormone use. A more profound and comprehensive understanding of MHT is imperative.
Gut microbiota, known as the total microorganisms residing within the gastrointestinal tract, differs with gender, age, healthy statue and hormone level. Significantly, the gut microbiota contributes to numerous chronic diseases (4). Accumulating studies suggested that gut microbiota linked to bone metabolism and the maintenance of bone homeostasis, involving the formation by osteoblasts and resorption by osteoclasts through the so-called “gut microbiota-bone” axis (5). Previous studies have shown that postmenopausal osteoporosis is associated with diminished bacterial richness and diversity, as well as significant changes in abundance levels among phyla and genera in the microbial compositions (6, 7). Postmenopausal osteoporosis (PMO), also known as primary type I osteoporosis, is characterized by high transition rate, which is different from senile osteoporosis. Bone turnover markers (BTMs) serve as biomarkers for the diagnosis and evaluating the bone metabolic state (8). Therefore, it is of great significance to study PMOs separately and explore the relationship between gut microbiota and bone metabolism.
Microbiota plays an important role in sex steroid deficiency-associated bone loss (9). The relationship between bone metabolism and gut microbiota in MHT for postmenopausal women remains underexplored. Most previous studies have focused on comparing the gut microbiota profiles between individuals with different bone mass. In this study, we intended to investigate the relationship between MHT and gut microbiota in postmenopausal women with similar BMC, emphasize on bone metabolism from the perspective of the microbiota, while gut microbiota would be a new target for the prevention and treatment of PMO.
Materials and methods
Participants recruitment
The project was permitted by ethical committee of Jiangxi provincial maternal and children hospital before carrying out (EC-KY-2024098), written informed consent was obtained from all participants before enrollment. Participants were recruited from Jiangxi provincial maternal and children hospital and all residing in Jiangxi province. All participants had similar dietary habits. Women who were 45–59 years old and had experienced natural menopause for more than 1 year were eligible for inclusion in this study. Exclusion criteria comprised individuals with diabetes, hypertention, hyperlipidemia, obesity (BMI > 24), astrictions, diarrhea, bone disease, bone trauma within 1 year, malignancies, infectious diseases, endocrine disorders, chronic conditions with a history of long-term drug use, anti-depressant administration, any medications related to bone metabolism, or those who had used any prebiotics, probiotics, or antibiotic treatments within the previous 3 months. None of them had smoking history. The participants were divided into two groups: postmenopausal women who had taken hormone-replacement therapy for a duration exceeding 6 months (MHT+ group); postmenopausal women who had not received any form of hormone therapy for more than 6 months (MHT– group), Ultimately, 31 individuals who met all of these criteria and had used or not used MHT for more than half a year were enrolled in our study finally (n = 17 in the MHT+ group; n = 14 in the MHT– group) (Figure 1). Detailed information on hormone therapy, usage of alcohol, recreational drugs, travel history, nutritional supplements, physical exercising, menstrual and pregnancy histories were collected by questionnaire. All data were identified prior to analysis and all tests were completed during one morning.
Clinical measurements
Height (m), weight (kg), circumferences (m) of the waist and hips, blood pressure were recorded for every participant. Body Mass Index (BMI) was calculated as weight/height2. A Dual-energy X-ray Absorptiometry (DXA) scanner (NORLAND, XR-600, US) was utilized for measuring Bone Mineral Density (BMD) (g/cm2) for the lumbar spine (LS, L1-4) and femoral neck (FN). The coefficients of variation (CV), as the precision indicator, were 0.9% and 1.4% for the spine and hip BMD measurements, respectively. BMD were recorded as the ratio of bone mineral content (BMC) (g) to bone area (cm2), and these data are presented as g/cm2. Biomarkers of Bone turnover (BTMs) are affected by circadian rhythmicity, with peak values occurring in the early morning and nadirs in the early afternoon and evening. Therefore, all venous blood samples were rigorously collected at similar time points in the morning to minimize these fluctuations. Fasting serum levels of BTMs, including osteocalcin (OC), serum C-terminal telopeptide of type I collagen (CTX-1), serum procollagen type I N propeptide (P1NP), parathyroid hormone (PTH), calcitonin (CT), and serum 25-hydroxyvitamin D3 (25(OH)VD3), were quantified with an automated Roche Osteoporosis Int electrochemiluminescence system (Roche Diagnostics GmbH, Germany). The CVs for quality control was determined to be within 4.01%−3.57% for osteocalcin, 4.04%−3.57% for CTX-1, 4.16%−4.01% for P1NP, 6.68%−4.42% for PTH, 4.53%−1.98% for CT and 3.21%−1.5% for 25(OH)VD3, for the high and low level controls, respectively. E2, Po, and T were quantified using a chemiluminescence immunoassay analyzer (DXI 800, Beckman Coulter, USA). The CVs for quality control were determined to be within 2.95%−2.01% for E2, 4.80%−6.21% for Po, 3.01%−3.93% for T, for the high and low level controls, respectively.
Feces sample collection, 16S ribosomal RNA gene sequencing and data analysis
Human fecal samples was collected in sterile plastic containers, then the fecal samples were subsequently transferred for storage at −80 °C until use. Total microbial genomic DNA was extracted using the FastPure Stool DNA Isolation Kit (MJYH, shanghai, China) according to the manufacturer's instructions. The integrity and concentration of the extracted DNA were assessed by 1% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific Inc., USA) and kept at −80 °C prior to further use. The extracted DNA was served as a template for amplifying the hypervariable V3-V4 regions of the bacterial 16S rRNA gene, utilizing the primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG3′) and 806R (5′-GGACTACHVGGGTWTCTAAT3′) by an T100 Thermal Cycler (BIO-RAD, USA). The PCR reaction mixture consisted of 4 μl of 5 × Fast Pfu buffer, 2 μl of 2.5 mM dNTPs, 0.8 μl of each primer (5 μm), 0.4 μl of Fast Pfu polymerase, 10 ng of template DNA, and ddH2O to achieve a final volume of 20 μl. PCR amplification cycling conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 27 cycles of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °Cfor 45 s, and single extension at 72 °C for 10 min, and end at 4 °C. All samples were amplified in triplicate. The PCR product was extracted from 2% agarose gel and purified using the AxyPrepDNA Gel Extraction Kit (AXYGEN). Fluorescence quantification was performed with QuantiFluorTM-ST blue flourescence quantification system (Promege, USA).
Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina NextSeq 2000 PE300 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
The DNA library was constructed by TruSeqTM DNA Sample Prep Kit. After demultiplexing, the resulting sequences were quality filtered with fastp (v0.19.6) and subsequently merged using FLASH (v1.2.11). The high-quality sequences were denoised using DADA2 plugin in the Qiime2 (version 2020.2) pipeline with recommended parameters, which obtains single nucleotide resolution based on error profiles within samples. DADA2 denoised sequences are commonly referred to as amplicon sequence variants (ASVs). To mitigate the effects of sequencing depth on alpha and beta diversity measure, the number of sequence from each sample was rarefied to 20,000, resulting in an average Good's coverage of 97.90%. Taxonomic assignment of ASVs was performed using the Naive Bayes consensus taxonomy classifier implemented in Qiime2 and the SILVA 16S rRNA database (v138).
Bioinformatic analysis of the gut microbiota was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com). Analysis of ASVs information included rarefaction analysis and estimation of alpha diversities, such as Ace, Chao, Sobs, and Coverage indices, which suggests the richness of observed species. These analyses were performed using Mothur (version v.1.30.2). Beta diversity, which estimates the similarity in community structure across samples, was evaluated through principal coordinate analysis (PCoA) based on Bray-Curtis distance using Vegan v2.4.3 package. Linear discriminant analysis (LDA) effect size (LEfSe) (http://huttenhower.sph.harvard.edu/LEfSe) was employed to identify significant differences in ASVs between two groups. The heatmap for key ASVs was visualized using MATLAB 2019b (The MathWorks, Inc., Natick, MA, USA). The network of correlation results was generated using Cytoscape v3.7.2.
SCFAs analysis
Samples were transferred into 2 ml EP tubes and extracted with 1 ml H2O, followed by vortex mixing for 10 s. The samples were then homogenized in ball mill for 4 min at 40 Hz, then ultrasound treated for 5 min (incubated in ice water. This process was repeated 3 times. Tubes were centrifuged for 20 min at 5,000 rpm and 4 °C. The supernatant (0.8 ml) was transferred into a new 2 ml EP tubes; To this, 0.1 ml 50% H2SO4 and 0.8 ml of extracting solution (25 mg/L stock in methyl tert-butyl ether) were added as an internal standard, followed by vortex mixing for 10 s, oscillations in 10 min, and then ultrasonic treatment for additional 10 min (incubated in ice water). Tubes were then centrifuged for 15 min at 10,000 rpm and 4 °C. The samples were stored at −20 °C for 30 min before the supernatant was transferred into a fresh 2 ml glass vial for subsequent GC-MS analysis via SHIMADZU GC2030-QP2020 NX gas chromatography-mass spectrometer.
Statistical analysis
All data are represented as mean ± standard deviation (SD). Statistical analyses were performed using SPSS 22.0 for Windows (SPSS Inc.). One-way Analysis of Variance (ANOVA) was used for comparisons between groups; The significance of differences in the measured α-diversities and β-diversity between two groups were assessed by Wilcoxon Rank-Sum Test with ggbox in MicrobiotaProcess. A threshold of LDA ≥ 2 was applied for both taxonomic and functional analyses to ensure a stringent detection of relevant group differences. Correlations and associations analyses between key ASVs and clinical parameters were calculated based on Spearman's rank correlation coefficients. The Benjamini and Hochberg method was adopted to calculate false discovery rate (FDR), thereby adjusting the significance of the correlations. A value of P < 0.05 was considered to indicate statistically significant difference.
Results
Characteristics of the participants involved in this study
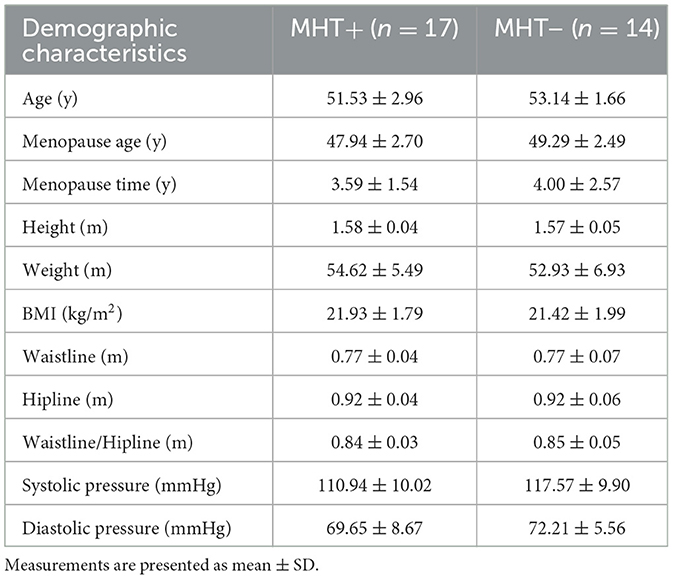
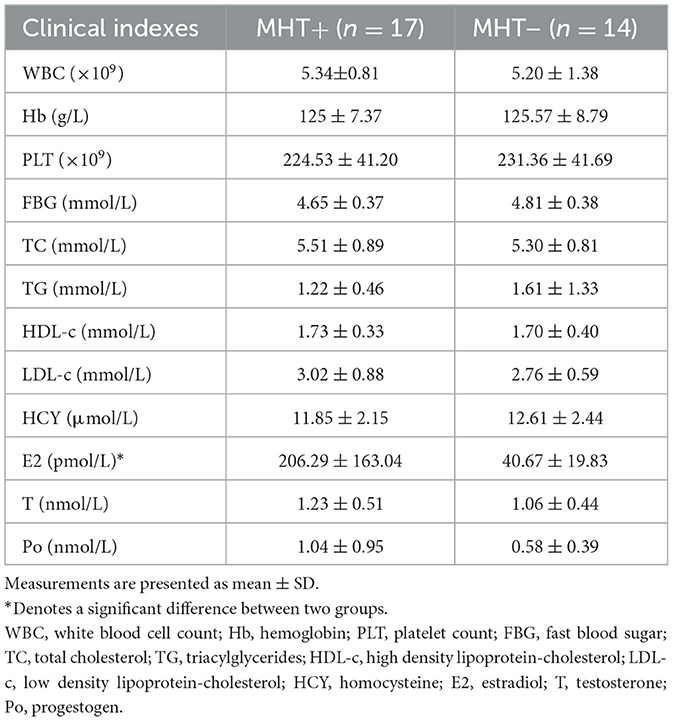
In this study, clinical information and biological samples from a cohort of 31 individuals were gathered and subjected to comprehensive analysis. The participants were divided into two groups: MHT+ group (n = 17) and MHT– group (n = 14), as detailed in Table 1. Within the MHT+ cohort, all subjects were administered estrogen, either orally through Tibolone (n = 2), or transdermally via estradiol gel (n = 2), or orally through femostone (1/10 mg) (n = 13) in combination with progestogen, the duration of MHT was 0.5 to 7 years (2.23 ± 1.88 years). No significant discrepancies were observed between the two groups with respect to age, BMI, blood pressure, smoking and alcohol consumption, the presence of common chronic diseases and medication usage. Furthermore, no significant differences were detected in circulating levels of hemoglobin (Hb), white blood cells count (WBC), platelet count (PLT), fasting blood glucose (FBG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), total cholesterol (TC), triglycerides (TG), and homocysteine (HCY) between the two groups.
Regarding sex steroid hormones, the serum levels of progestogen (Po) and testosterone (T) exhibited no significant difference between the two groups. However, the serum levels of estradiol (E2) were markedly higher in the MHT+ group relative to MHT– group, which was as expected. It is noteworthy that dydrogesterone, a progestogen widely used in MHT, does not show up in blood progestogen test. This explains why the serum progestogen levels in MHT+ were not found to be higher than those in the MHT- group despite their supplementation (see Table 2).
Bone metabolic state
DXA scanner was employed to assess bone mineral content (BMC), bone mineral density (BMD). T-score, which serves as the primary diagnostic criterion for bone osteoporosis according to WHO criteria, is derived from BMD value. When comparing the two groups, no significant disparities were noted in the BMC of LS and FN; however, the BMD was significantly higher in MHT+ group compared to the MHT- group contributed to the protective effects of MHT.
Estrogen deficiency-induced osteoporosis is characterized by heightened bone turnover activity. In our study, markers such as CTX and PINP, which are indicative of osteogenic and osteoclastic/absorptive activity, were found to be significantly more elevated in the MHT- group compared to the MHT+ group. This suggests that individuals in MHT- group were in a state of high bone turnover. No significant differences were observed with respect to PTH, CT, Vitamin D levels between two groups (see Table 3).
Diversity comparison of gut microbiota from MHT+ and MHT– groups
To ascertain the adequacy of the sequencing data and to characterize bacterial diversity, rarefaction analysis was performed, which revealed that each sample reached a plateau phase following a progressive increase (Figure 2A). The curve indicates that the sequencing data were robust sufficiently and could reflect the majority of the microbial composition in the samples reliably.
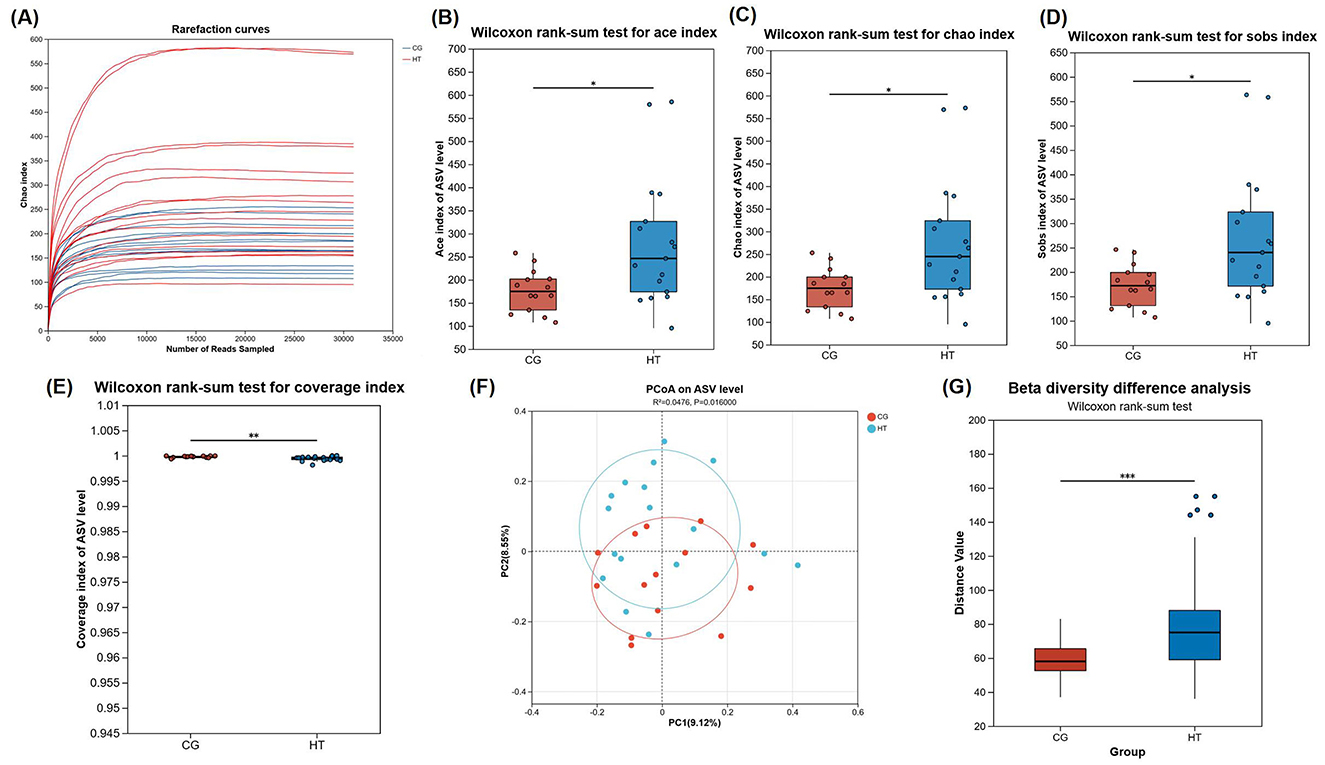
Figure 2. Diversity comparison of gut microbiota from MHT+ and MHT-groups. (A) Rarefaction curves. (B) Ace index. (C) Chao index. (D) coverage index. (E) Sobs index. (F) Bray-Curtis PCoA analysis. (G) Beta diversity difference analysis. CG, control group (MHT–); HT, hormone treatment (MHT+). *0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01, ***P ≤ 0.001.
To determine if there were disparities between groups, microbial alpha diversity and beta diversity were conducted. Our results indicated a statistically significant enhancement in alpha diversity within the MHT+ group when compared to the MHT- group, as evidenced by their Ace, Chao, Sobs, and Coverage indexes (Figures 2B–E). Furthermore, a Bray-Curtis principal coordinate analysis (PCoA) was performed to examine beta diversity. The resultant PCoA plot showed an obvious separation between two groups, indicative of a pronounced disparity in the structure of the intestinal microbial communities (Figures 2F, G).
Gut microbiota composition from MHT+ and MHT– groups
At the genus level, in addition to the 171 common species, the MHT+ group exhibited a significantly higher number of unique species (122) compared to the MHT– group (22). In order to explore potential discrepancies in gut microbiota composition based on women's hormone-replacement therapy status, we conducted a comparative analysis of the gut microbiota composition between the two groups at both the phylum, and genus level. Firmicutes was the most prevalent phylum in the microbiome of both groups. In MHT+ group, Actinobacteriota was the second most prevalent phylum, and followed by Bacteroidota and Proteobacteria. Conversely, in the MHT- group, Proteobacteria was the second most prevalent phylum, followed by Actinobacteriota and Bacteroidota. A notable increase in the abundance of Verrucomicrobiota and Desulfobacterota at the phylum level was observed in the MHT+ group relative to the MHT- group. The relative abundances of Proteobacteria was higher in the MHT– than MHT+, but not significant statistically. At the genus level, the relative abundances of Escherichia-Shigella was significantly higher in the MHT– group, whereas norank_o_Clostridia_UCG-014, Coprococcus, Eubacterium_ruminantium_group, norank_f_Oscillospiraceae, Eubacterium_eligens_group, Family_XIII_AD3011_group, Eubacterium_siraeum_group, UBA 1819, Lachnospiraceae_UCG-010 were significantly more abundant in the MHT+ group (Figures 3A–C). Further, linear discriminant analysis effect size (LEfSe) demonstrated an obvious difference existing between the two groups. An increased abundance of Escherichia-Shigella, Veillonelales-Selenomonadales, Megamonas, Oribacterium, norank_f_Saccharimonadaceae was observed in the MHT– group, while Clostridia, norank_o_Clostridia, Coprococcus, Verrucomicrobiota, Coriobacteriales_Incertae_Sedis, Eubacterium_ruminantium_group, Eubacterium_siraeum_group, norank_f_Oscillospiraceae were more abundant in the MHT+ group (Figures 3D, E).
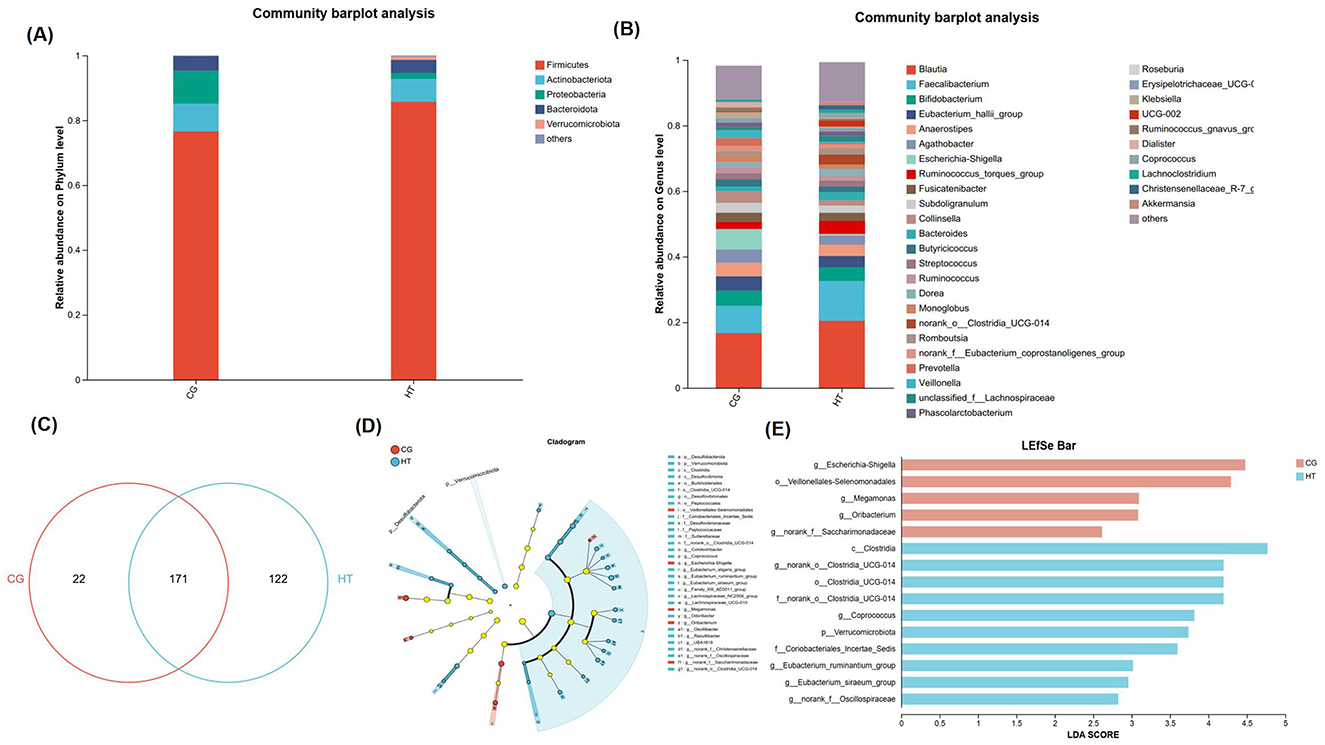
Figure 3. Gut microbiota composition from MHT+ and MHT- groups. (A) Gut microbiota at phylum level. (B) Gut microbiota at genus level. (C) Venn diagram for species composition analysis. (D) Bar chart of LDA value distribution in linear discriminant analysis effect size (LEfSe). (E) Evolutionary branch diagram of differential intestinal microbiota. CG, control group (MHT–); HT, hormone treatment (MHT+).
Correlations between the bacteria and clinical indexes
The relationships among various bacterial species, BTMs, and other clinical parameters were assessed by Spearman correlation analysis. The findings revealed that Clostridia_UCG-014 and Coprococcus exhibited a significant negative correlation with CTX and P1NP, suggesting a potential protective role in bone health. The correlation strength was moderate (−0.06 < R < −0.04). Additionally, these genera showed a positive correlation with serum E2 levels and BMC as well as BMD at the LS and FN, although not statistically significant. Other genera including Lachnospiraceae_NC2004_group and Eubacterium eligens group demonstrated similar trends in their correlations with these indexes, albeit with weaker correlation strength.
In contrast, Escherichia-Shigella, which was significantly associated with infectious diseases, displayed a negative correlation with serum E2 and BMC, BMD at LS and FN, while positively correlating with CTX and P1NP (although not significantly). Another genera, Odoribacter, was strongly and negatively correlated with CTX and P1NP (P < 0.05), and correlated with LS BMD and FN BMD positively. A similar trend was observed for Colidextribacter. With the exception of Escherichia-Shigella, most of these genera also exhibited a strong association with serum E2 levels, regardless of statistical significance (Figure 4A) (Supplementary materials 1, 2).
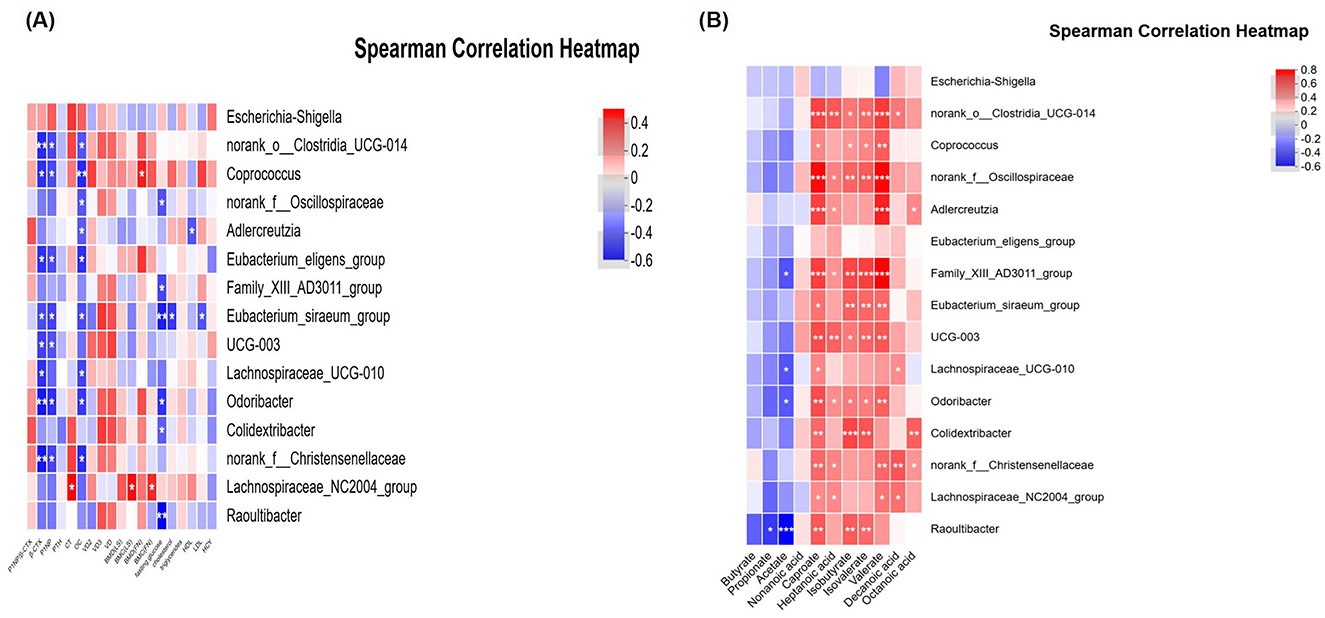
Figure 4. Correlations between the bacteria and other indexes. (A) Relationship between intestinal flora and clinical indexes. (B) Relationship between intestinal flora and SCFAs. CG, control group (MHT-); HT, hormone treatment (MHT+). *0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01, ***P ≤ 0.001.
SCFAs profiles and correlations with bacteria
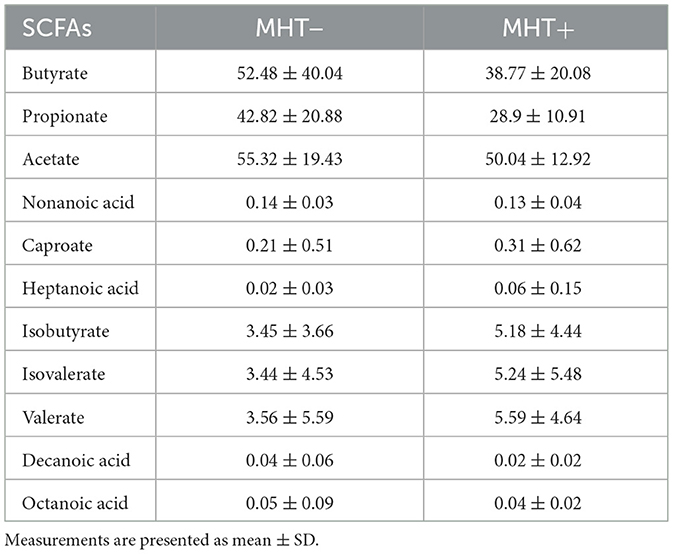
No significant differences in SCFAs were observed between the two groups, suggesting a limited role in bone metabolism (see Table 4). Several genera, including Clostridia_UCG-014, Coprococcus, Oscillospiraceae, Family_XIII_AD3011_group, Eubacterium_siraeum_group, UCG-003, Lachnospiraceae_UCG-010, Colidextribacter, Christensenellaceae, and Lachnospiraceae_NC2004_group (all belonging to the phylum Firmicutes), as well as Adlercreutzia and Raoultibacter (phylum Actinobacteriota), and Odoribacter (phylum Bacteroidota), were found to be positively and moderately associated with various SCFAs (P < 0.05). In contrast, Escherichia-Shigella demonstrated a negative correlation with the majority of SCFAs, although this association did not reach statistical significance (see Figure 4B) (Supplementary materials 3, 4).
Discussion
The gut microbiota, by modulating the host's immune, metabolic, and endocrine systems, participates in various host physiological activities, exerting a broad spectrum of effects within the body and is considered a key factor influencing human health. As we know, intestinal epithelial cells are sealed by tight junction proteins (TJs) including ZO-1 and occludin, dysbiosis of gut microbiota can alter the expression and distribution of TJs, coupled with the disruption of the structural and functional integrity of the intestinal barrier. The permeability altering of the intestinal barrier facilitates the translocation of gut microbes and their metabolites, thereby triggering a local inflammatory state within the intestines. This inflammatory state, once entering the systemic circulation, can further amplify its impact, inducing systemic immune system imbalance and causing inflammatory responses in other tissues or organs, thereby leading to damage to distant target organs. This is one of the main mechanisms of the “gut-bone axis”, which is involved in aging (10, 11). Oxidative stress contributes to the development of osteoporosis by enhancing the breakdown of bone by osteoclasts and reducing the formation of new bone by osteoblasts. This process involves complex signaling pathways, including NF-κB, PI3K/Akt, and Wnt/beta-catenin (12, 13). Additionally, T cells, a type of immune cell, play a role in this process by influencing the balance between different T cell subsets (Th17 and Treg) and the production of various signaling molecules, or cytokines. Interestingly, consuming certain beneficial bacteria, known as probiotics, can help reduce inflammation throughout the body and in the bones, which is often linked to changes in how permeable the intestines are. This, in turn, helps to preserve bone density (14).
The gut microbiota can promote the absorption of calcium and vitamin D and produce serotonin and SCFAs, all of which can affect bone formation and resorption (15). In addition, extracellular vesicles (EVs) produced by the gut microbiota can cross the intestinal epithelial barrier to reach distant target tissues and regulate the function of distant target organs (16). This new mechanism has been found to be involved in the well-known protective effect of the beneficial bacterium including Akkermansia on osteoporosis (17, 18). Observations and comparisons of the gut microbiota in people with different bone mass and hormone levels have been reported (19), and the activation of estrogen and the gut microbiota interact with each other, suggesting a close connection among bone metabolism, hormones and the gut microbiota (9, 20, 21). The roles of sex steroids, gut fora and gut dynamics are also interrelated. Estrogens and androgens play important regulatory roles in gut motility and psychological conditions, possibly through modulating the gut-brain axis (22), while the acceleration or slowdown of intestinal motility will affect the reproduction of suitable bacterial species and change the composition and function of the microbiota. Meanwhile, dysbiosis of the gut microbiota and their metabolites can Induce Intestinal motility disorder, affect the contraction of intestinal smooth muscle and the transmission (23).
Hormone replacement therapy aims to protect bone mass and cardiovascular health by supplementing estrogen in postmenopausal women for a long term. Its protective effect on the cardiovascular system has been confirmed to be related to the gut microbiota (24), and changes in the gut microbiota also occur during the treatment of osteoporosis with various traditional Chinese and western medicines (25).
This study enrolled a homogeneous cohort of participants, exhibiting no statistically significant disparities in geographical demographics, dietary patterns, menopausal duration, chronological age, body mass index, fasting blood glucose, lipid profiles, blood pressure, and inflammatory status indicated by leukocyte cell count. This stringent participant selection criterion effectively minimized the influence of other factors on the gut microbiota. DXA assessments revealed a lack of difference in BMC and a small difference in BMD, which may attribute to the relatively short time since menopaus among the enrolled females (average menopausal duration was < 4 years). Nonetheless, the observed discrepancies in skeletal metabolism biomarkers, including P1NP, CTX, and OC, underscored that the bone metabolism status of women under different treatment conditions varies. MHT exerts a beneficial effect on bone density preservation through long-term administration. This further emphasized the focus of this study on the role of gut microbiota in the process of bone metabolism by MHT, while previous studies mostly reported comparisons of the microbiota after bone mass differences had occurred. This study demonstrated a significant impact of hormone replacement therapy on the intestinal microbiota of women, significantly increasing the diversity of the intestinal microbiota. The ratio of Bacteroidetes to Firmicutes is an important indicator of the balance of the human microbiota. From the composition structure of the microbiota, it can be seen that the ratio of the two groups, MHT+ and MHT–, is different, with a higher proportion of Firmicutes in the MHT+ group. LEfSe analysis indicated that the relative abundance of Verrucomicrobiota was higher in the MHT+ group. Akkermansia, which belongs to this phylum and has significant effects in immunity, anti-aging, and anti-cancer, indeed had a higher abundance in the MHT+ group, but the difference was not statistically significant.
When analyzing the dominant genera with statistical differences between the two groups, we found that both Escherichia-Shigella and Megamonas were present in the MHT- group simultaneously. Coincidentally, both are closely related to inflammatory responses. Escherichia-Shigella is the most common pathogen causing bacillary dysentery in humans (26); Megamonas is also closely associated with local intestinal inflammatory diseases, colorectal cancer, systemic obesity, ankylosing spondylitis, and autism spectrum disorders (27). In polycystic ovary syndrome, a female endocrine and metabolic disorder, the abundance of Escherichia-Shigella also increased, suggesting a possible link with hormonal imbalance (28). Correlation studies have confirmed that this bacterium is negatively correlated with estrogen levels and bone density, while positively correlated with increased bone metabolism indicators (P < 0.05).
The abundance of Clostridia also varies among different bone mass groups, being higher in the normal bone density group. This finding is consistent with previous reports (29), indicating significant differences in component abundance. LDA analysis suggests that Clostridia abundance is higher in the MHT+ group and positively correlated with bone mass, serum estradiol, and vitamin D levels, while negatively correlated with bone metabolism indicators such as P1NP and CTX moderately (P < 0.05). Other genera with similar correlations include Coprococcus, Eubacterium, Colidextribacter, and Lachnospiraceae_NC2004_group. The protective effect of MHT on bone mass is likely the result of the combined action of multiple microbial groups. These genera may play important bone-protective roles under the regulation of estrogen in MHT and all belong to the Clostridia class of the Firmicutes phylum. Coprococcus is an important genus in the human gut, capable of actively fermenting carbohydrates and is a significant producer of butyric acid among intestinal microorganisms (30). Bacteria of the Coprococcus genus help suppress immune responses in the body and have been found to be associated with constipation, language development in children, depression, and chronic fatigue (31, 32). In terms of bone metabolic disease, Coprococcus and Blautia potentially perturb glucose homeostasis via the generation of SCFAs, thereby influencing osteoclastic metabolism and contributing to the pathogenesis of tibial dyschondroplasia (33).
We also conducted SCFAs analysis and correlation analysis with the intestinal microbiota. The results indicated that many genera positively correlated with bone protection are also associated with the production of certain SCFAs. Adlercreutzia belongs to the Firmicutes phylum and Bifidobacteriaceae family. They can metabolize and ferment in the human gut, producing xylose, arabinose, and SCFAs such as acetic acid, propionic acid, and butyric acid (34). It plays an important role in regulating the host's intestinal environment, immune status, promoting nutrient absorption, and maintaining intestinal health. In this study, its abundance in the MHT+ group was nearly five times that in the MHT– group, suggesting it may also be a factor in the protective effect of MHT on menopausal women. Although the abundance of many beneficial bacteria related to butyric acid production significantly increased in the MHT+ group, the butyric acid production in the MHT+ group was not higher than that in the MHT- group. This may be related to the relatively low proportion of other major butyric acid-producing bacteria such as Butyricicoccus in the MHT+ group.
This study explored the impact of MHT on the gut microbiota in postmenopausal women and its relationship with bone metabolism, laying a foundation for exploring the role of the gut microbiota in bone mass protection. However, it still has certain limitations. Firstly, the number of subjects enrolled in the two groups was relatively small, which might compromised the precision and statistical power of the results. Secondly, although this study included different MHT options, the effects of different MHT options on the gut microbiota may also vary, which requires further investigation in subsequent studies. Thirdly, this study only conducted a correlation study between bacterial genera and bone metabolism indicators, without further verification and exploration of the underlying specific mechanisms, which awaits validation through animal experiments.
This study contributes to a better understanding and promotion of MHT and is of great significance in exploring new approaches to prevention and treatment of osteoporosis.
Data availability statement
Due to the sensitive nature of the data collected for this study, which includes potentially identifying patient information, the data are not publicly available. However, the data are available from the corresponding author upon reasonable request, subject to the approval of an institutional ethics committee and a data use agreement is acceptable.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Jiangxi Provincial Maternal and Child Health Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JX: Data curation, Conceptualization, Writing – original draft. LL: Project administration, Investigation, Writing – original draft. MA: Validation, Supervision, Writing – review & editing. YT: Data curation, Methodology, Writing – original draft, Investigation. KT: Writing – original draft, Investigation, Methodology, Supervision. LL: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1682925/full#supplementary-material
References
1. Dothard MI, Allard SM, Gilbert JA. The effects of hormone replacement therapy on the microbiomes of postmenopausal women. Climacteric. (2023) 26:182–92. doi: 10.1080/13697137.2023.2173568
2. Subarajan P, Arceo-Mendoza RM, Camacho PM. Postmenopausal osteoporosis: a review of latest guidelines. Endocrinol Metab Clin North Am. (2024) 53:497–512. doi: 10.1016/j.ecl.2024.08.008
3. de Villiers TJ, Hall JE, Pinkerton JV, Pérez SC, Rees M, Yang C, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas. (2016) 91:153–5. doi: 10.1016/j.maturitas.2016.06.001
4. Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, Quintana-Navarro GM, Garcia-Carpintero S, Malagon MM, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. (2018) 116:43–53. doi: 10.1016/j.maturitas.2018.07.008
5. Wang Y, Zhang X, Tang G, Deng P, Qin Y, Han J, et al. The causal relationship between gut microbiota and bone mineral density: a Mendelian randomization study. Front Microbiol. (2023) 14:1268935. doi: 10.3389/fmicb.2023.1268935
6. Yang X, Chang T, Yuan Q, Wei W, Wang P, Song X, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. (2022) 13:930244. doi: 10.3389/fimmu.2022.930244
7. Yan L, Wang X, Yu T, Qi Z, Li H, Nan H, et al. Characteristics of the gut microbiota and serum metabolites in postmenopausal women with reduced bone mineral density. Front Cell Infect Microbiol. (2024) 14:1367325. doi: 10.3389/fcimb.2024.1367325
8. Chen L, Yan S, Yang M, Yu F, Wang J, Wang X, et al. The gut microbiome is associated with bone turnover markers in postmenopausal women. Am J Transl Res. (2021) 13:12601–13.
9. Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. (2016) 126:2049–63. doi: 10.1172/JCI86062
10. Shieh A, Epeldegui M, Karlamangla AS, Greendale GA. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight. (2020) 30:e134092. doi: 10.1172/jci.insight.134092
11. Yang M, Wen S, Zhang J, Peng J, Shen X, Xu L. Systematic review and meta-analysis: changes of gut microbiota before and after menopause. Dis Markers. (2022) 25:3767373. doi: 10.1155/2022/3767373
12. Yasuda H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. (2021) 39:2–11. doi: 10.1007/s00774-020-01175-1
13. Song J, Chang W, Wang Y, Gao P, Zhang J, Xiao Z, et al. Inhibitors of the Wnt pathway in osteoporosis: a review of mechanisms of action and potential as therapeutic targets. Biomol Biomed. (2025) 25:511–24. doi: 10.17305/bb.2024.11200
14. Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. (2015) 2015:897639. doi: 10.1155/2015/897639
15. Wallimann A, Magrath W, Thompson K, Moriarty T, Richards RG, Akdis CA, et al. Gut microbial-derived short-chain fatty acids and bone: a potential role in fracture healing. Eur Cell Mater. (2021) 41:454–70. doi: 10.22203/eCM.v041a29
16. Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. (2021) 8:2004831. doi: 10.1002/advs.202004831
17. Grajeda-Iglesias C, Durand S, Daillère R, Iribarren K, Lemaitre F, Derosa L, et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging. (2021) 13:6375–405. doi: 10.18632/aging.202739
18. Wang Z, Chen K, Wu C, Chen J, Pan H, Liu Y, et al. An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am J Clin Nutr. (2021) 114:1304–13. doi: 10.1093/ajcn/nqab194
19. He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging. (2020) 12:8583–604. doi: 10.18632/aging.103168
20. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
21. Wang H, Shi F, Zheng L, Zhou W, Mi B, Wu S, et al. Gut microbiota has the potential to improve health of menopausal women by regulating estrogen. Front Endocrinol. (2025) 16:1562332. doi: 10.3389/fendo.2025.1562332
22. So SY, Savidge TC. Sex-bias in irritable bowel syndrome: linking steroids to the gut-brain axis. Front Endocrinol. (2021) 12:684096. doi: 10.3389/fendo.2021.684096
23. Liu Q, Luo Y, Ke X. Interaction between the gut microbiota and intestinal motility. Evid Based Complement Alternat Med. (2022) 2022:3240573. doi: 10.1155/2022/3240573
24. Leite G, Barlow GM, Parodi G, Pimentel ML, Chang C, Hosseini A, et al. Duodenal microbiome changes in postmenopausal women: effects of hormone therapy and implications for cardiovascular risk. Menopause. (2022) 29:264–75. doi: 10.1097/GME.0000000000001917
25. Sun P, Zhang C, Huang Y, Yang J, Zhou F, Zeng J, et al. Jiangu granule ameliorated OVX rats bone loss by modulating gut microbiota-SCFAs-Treg/Th17 axis. Biomed Pharmacother. (2022) 150:112975. doi: 10.1016/j.biopha.2022.112975
26. Chibuye M, Mende DR, Spijker R, Simuyandi M, Luchen CC, Bosomprah S, et al. Systematic review of associations between gut microbiome composition and stunting in under-five children. NPJ Biofilms Microbiomes. (2024) 10:46. doi: 10.1038/s41522-024-00517-5
27. He Q, Li G, Zhao J, Zhu H, Mo H, Xiong Z, et al. The impact of dysbiosis in oropharyngeal and gut microbiota on systemic inflammatory response and short-term prognosis in acute ischemic stroke with preceding infection. Front Microbiol. (2024) 15:1432958. doi: 10.3389/fmicb.2024.1432958
28. Yang Y, Cheng J, Liu C, Zhang X, Ma N, Zhou Z, et al. Gut microbiota in women with polycystic ovary syndrome: an individual based analysis of publicly available data. EClinicalMedicine. (2024) 77:102884. doi: 10.1016/j.eclinm.2024.102884
29. Coskun M, Babayeva A, Barlas T, Muhittin Yalcin M, Akturk M, Balos Toruner F, et al. Relationship between gut microbiome and bone deficits in primary hyperparathyroidism: A proof-of-concept pilot study. J Investig Med. (2024) 72:541–52. doi: 10.1177/10815589241251695
30. Xu TT, Chen P, Zhang CD, Shaukat A, Lin LX, Yue K, et al. Gut microbiome dysregulation drives bone damage in broiler tibial dyschondroplasia by disrupting glucose homeostasis. NPJ Biofilms Microbiomes. (2023) 9:1. doi: 10.1038/s41522-022-00360-6
31. Xu L, Wang S, Wu L, Cao H, Fan Y, Wang X, et al. Coprococcus eutactus screened from healthy adolescent attenuates chronic restraint stress-induced depression-like changes in adolescent mice: potential roles in the microbiome and neurotransmitter modulation. J Affect Disord. (2024) 356:737–52. doi: 10.1016/j.jad.2024.04.050
32. Yang R, Shan S, Shi J, Li H, An N, Li S, et al. Coprococcus eutactus, a potent probiotic, alleviates colitis via acetate-mediated iga response and microbiota restoration. J Agric Food Chem. (2023) 71:3273–84. doi: 10.1021/acs.jafc.2c06697
33. Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K, et al. Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes. (2019) 5:28. doi: 10.1038/s41522-019-0101-x
Keywords: postmenopausal, MHT, bone metabolism, gut microbiota, osteoporosis
Citation: Xiong J, Li L, Ao M, Tu Y, Tu K and Li L (2025) Effects of menopausal hormone therapy on gut microbiota in postmenopausal women and the relationship with bone metabolism. Front. Med. 12:1682925. doi: 10.3389/fmed.2025.1682925
Received: 10 August 2025; Accepted: 29 September 2025;
Published: 27 October 2025.
Edited by:
Teresa Nogueira, National Institute for Agrarian and Veterinary Research (INIAV), PortugalReviewed by:
Jan Josef Stepan, Charles University, CzechiaKirtal Hansdah, National Institutes of Health Clinical Center, United States
Copyright © 2025 Xiong, Li, Ao, Tu, Tu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longyu Li, MjU0OTExNTY4QHFxLmNvbQ==
 Jieqi Xiong
Jieqi Xiong Lijun Li2
Lijun Li2