Abstract
Background:
Patients who undergo radical gastrectomy for gastric cancer often experience multiple complications that affect the recovery process and reduce quality of life. The hemoglobin-to-albumin ratio (HAR) reflects nutritional status and is widely used to assess various diseases. However, its association with complications after radical gastrectomy in gastric cancer patients has not been fully explored.
Methods:
This study retrospectively analyzed clinical and pathological data from 352 gastric cancer patients who underwent radical gastrectomy at the Second Hospital of Lanzhou University between January 2023 and December 2024. The severity of postoperative complications was graded using the Clavien-Dindo classification system, resulting in two groups: non-complication group (n = 254) and Clavien-Dindo ≥II group (n = 98). Multivariate logistic regression models were employed to assess the correlation between HAR and Clavien-Dindo ≥II grade complications. Subsequently, sensitivity analysis, subgroup analysis, restricted cubic spline (RCS) modeling, and threshold effect evaluation were conducted.
Results:
Multivariate logistic regression analysis showed that for every 1-unit increase in HAR, the risk of Clavien-Dindo ≥II grade complications decreased by 62.0% (95% CI: 0.188–0.749; p < 0.05). Sensitivity analysis confirmed this conclusion, demonstrating that patients with higher HAR levels exhibited a 64.4% lower risk of complications compared to patients with lower HAR levels (95% CI: 0.170–0.726; p < 0.05). Restricted cubic spline (RCS) analysis demonstrated a negative correlation between HAR and complications, and the threshold effect analysis determined the critical point of HAR to be 2.87. Subgroup analysis showed that most subgroups did not exhibit significant differences in interaction p values. Interestingly, a significant interaction was observed between HAR and platelet (interaction p = 0.011).
Conclusion:
This study indicates that higher HAR is significantly and negatively correlated with the risk of complications. Higher levels of HAR effectively prevent Clavien-Dindo ≥ II-grade complications. Consequently, HAR can serve as a clinically useful indicator for predicting complications following radical gastrectomy for gastric cancer.
1 Introduction
Gastric cancer constitutes a substantial component of the global cancer burden, with age-standardized incidence and mortality rates ranking fifth and fourth, respectively, among all malignant tumors (1). Radical surgery is the standard treatment for resectable gastric cancer (2). Despite its notable efficacy in enhancing the five-year survival rate of patients (3), postoperative complications persist as a pivotal factor influencing patient recovery and quality of life. According to the Clavien-Dindo postoperative complication grading system (4, 5), the incidence of grade II or higher complications requiring drug treatment or invasive intervention in patients undergoing radical gastrectomy for gastric cancer can reach 25% (6). These complications are significantly associated with prolonged hospital stays, increased medical costs, and reduced three-year survival rates (7).
Current research indicates that preoperative nutritional support can optimize surgical outcomes through multiple mechanisms (8). First, it can regulate excessive inflammatory responses and reduce levels of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Second, studies have confirmed that it can enhance cellular immune function, manifested by increased lymphocyte activity and antibody production capacity (9). The dual effect has been demonstrated to have the capacity to markedly diminish the likelihood of postoperative infectious complications and systemic complications. Patients with malnutrition may experience increased resting energy expenditure, anorexia, loss of adipose tissue, muscle wasting, and reduced protein synthesis, which can lead to severe postoperative complications classified as Clavien-Dindo grade III or higher (10). In the context of clinical nutritional assessment, hemoglobin and albumin serve as pivotal biomarkers, reflecting the body’s oxygen transport capacity and protein reserves. Preoperative hypoalbuminemia has been demonstrated to exhibit an independent association with an augmented risk of postoperative pulmonary infection (11). Concurrent testing of these biomarkers is of significant clinical value. Extensive evidence-based medical evidence indicates that preoperative hypoalbuminemia and low hemoglobin levels are significantly associated with shorter postoperative survival and increased complication rates in patients with malignant tumors (12).
In recent years, the hemoglobin-to-albumin ratio (HAR) has garnered significant attention as a novel composite indicator that integrates nutritional status with inflammatory conditions (13). Hemoglobin, the oxygen-carrying protein, directly reflects tissue oxygen supply and the severity of anemia (14). Albumin plays a central role in maintaining plasma colloid osmotic pressure and acts as a negative acute-phase protein involved in systemic inflammatory regulation (15). The HAR has the capacity to simultaneously capture dual information regarding oxygen-carrying capacity and inflammatory/nutritional status, thus offering a more comprehensive assessment value compared to single indicators. Unlike traditional nutritional indices such as the prognostic nutritional index (PNI), platelet-to-albumin ratio (PAR), and controlling nutritional status (CONUT) score, which primarily focus on nutritional or inflammatory components alone, HAR provides a more integrative perspective that better reflects the complex physiological interplay in surgical patients. Clinical studies has demonstrated that HAR is associated with the prognosis of various diseases, including colorectal cancer and nasopharyngeal cancer (16, 17). However, there is a paucity of research investigating the correlation between HAR and postoperative complications in patients undergoing radical gastrectomy for gastric cancer.
The study aimed to assess the predictive value of HAR in postoperative complications by exploring the correlation between postoperative complications and HAR following radical gastrectomy. The goal was to provide new reference indicators for the perioperative management of gastric cancer patients in clinical practice, enabling the early identification and timely intervention for high-risk patients, reducing the risk of postoperative complications, and improving patient outcomes and quality of life.
2 Materials and methods
2.1 Data and participants
This retrospective study examined 397 gastric cancer patients who underwent radical gastrectomy at the Second Hospital of Lanzhou University between January 2023 and December 2024. These patients underwent radical proximal, distal, or total gastrectomy based on tumor location, size, and stage. Complete tumor resection was confirmed through intraoperative macroscopic observation and postoperative pathology, which demonstrated the absence of residual tumor.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of the Second Hospital of Lanzhou University (Project Number:2025 A-652). As this study is a retrospective and the privacy and personal identity information of the patients were protected, the need for informed consent was waived by the Medical Ethics Committee of the Second Hospital of Lanzhou University.
2.2 Inclusion and exclusion criteria
Inclusion criteria are as follows: (1) Pathologically confirmed gastric cancer post-surgery, patients underwent radical resection of the proximal, distal, or entire stomach; (2) No evidence of tumor invasion into adjacent organs or distant metastasis; (3) Data required for outcome measures are complete and comprehensive. Exclusion criteria include: (1) Patients with gastroesophageal junction cancer who underwent open thoracotomy; (2) Patients who underwent gastric stump surgery, recurrent cancer surgery, or combined surgery with other organ surgeries; (3) Patients with special gastric tumors, including lymphoma, hepatoma, neuroendocrine tumors, or gastrointestinal stromal tumors (GISTs). The final sample included 352 patients (Figure 1).
Figure 1
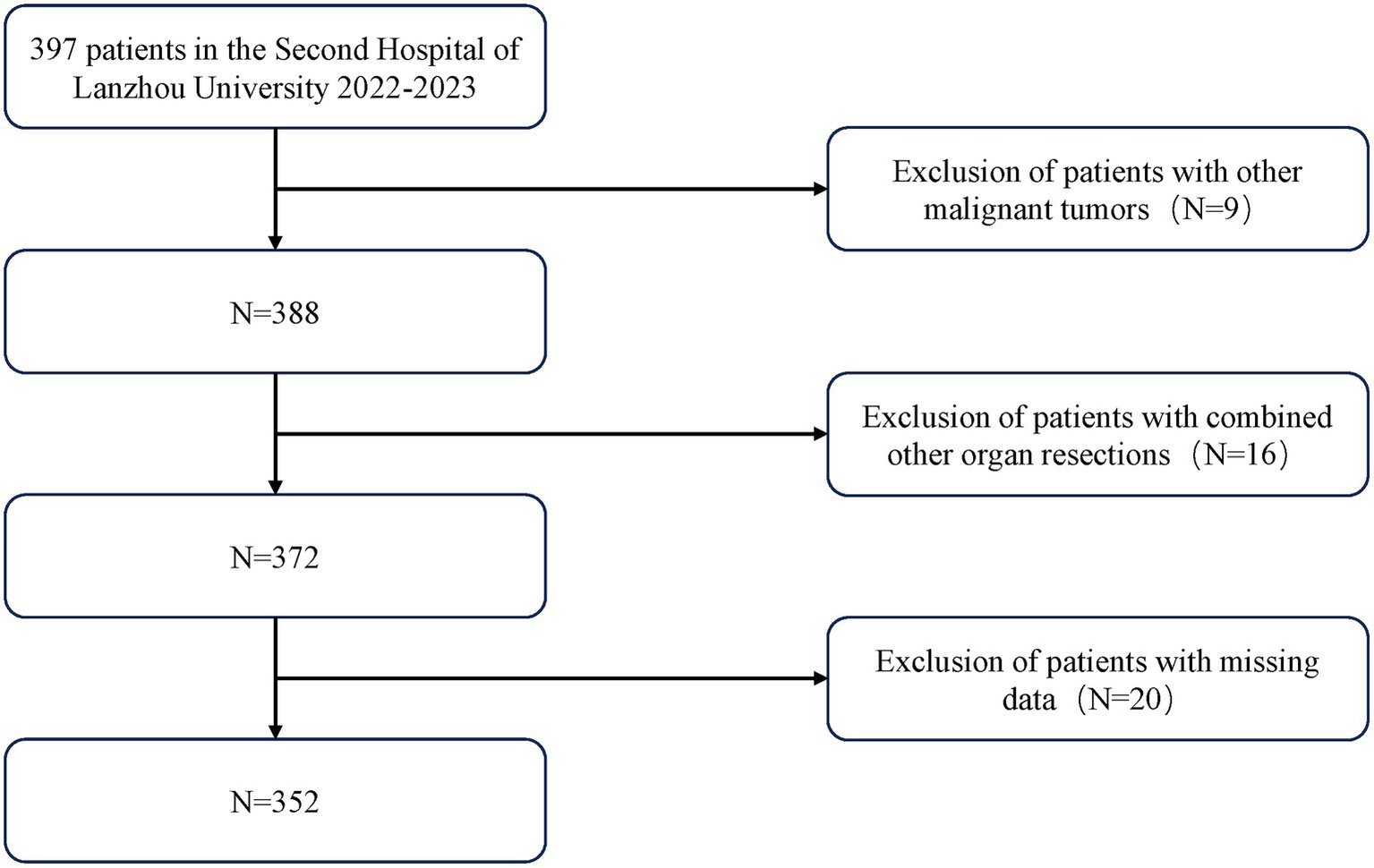
Flow diagram of the selection of eligible patients.
2.3 Assessment of Clavien-Dindo ≥II grade complications
Postoperative complications are issues that arise from surgery within 30 days after the procedure. The diagnosis of these conditions is made through the observation of clinical symptoms, the execution of laboratory tests, and the analysis of imaging examinations. These findings are then meticulously documented in the patient’s medical records. These complications include pulmonary infection, intra-abdominal infection, wound infection, anastomotic obstruction, pleural effusion, intra-abdominal hemorrhage, anastomotic leakage or fistula, acute heart failure, acute respiratory failure, and multiple organ dysfunction, among others (Supplementary Table S1).
The severity of complications is classified according to the Clavien-Dindo classification system, which is structured into five grades. Level one is characterized by mild symptoms that do not pose a threat to life, while level five complications are associated with fatality (Supplementary Table S2). Patients with Clavien-Dindo ≥ II are defined as the complication group, while the remaining patients are defined as the non-complication group.
2.4 Clinical variables
The case data to be observed and collected in this study include: medical history, postoperative recovery status, serological indicators, and risk factors associated with postoperative complications. Medical history includes the presence of hypertension, diabetes, cardiovascular and cerebrovascular diseases, smoking history, alcohol consumption history, neoadjuvant chemotherapy, and history of abdominal surgery. Variables related to postoperative recovery include the status of postoperative recovery, whether complications occurred, and the treatment measures taken for complications. For patients with multiple complications, grading is based on the severity of the most severe complication. Serological tests are conducted for all admitted cases. Relevant serological data were collected, including white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MONO), hemoglobin (HGB), red blood cell distribution width standard deviation (RDWSD), red blood cell distribution width coefficient of variation (RDWCV), platelet count (PLT), albumin (ALB), total cholesterol count (TC), lactate dehydrogenase (LDH), and blood fibrinogen (FIB). Additionally, collect and assess potential risk factors associated with postoperative complications, including sex, age, surgical method, TNM stage, intraoperative blood loss, and operation time. HAR was calculated as the hemoglobin-to-albumin ratio using the most recent routine laboratory results within 24–48 h before anesthesia. To avoid reverse causality, pre-intervention values were used if patients received transfusion, albumin, parenteral nutrition, or large-volume fluids during this period.
2.5 Statistical analysis
This study employed various statistical methods for a systematic analysis of the data. Descriptive statistical analysis was first performed on demographic characteristics. Continuous variables were expressed as mean ± SD and compared between groups using T-tests or Kruskal-Wallis rank sum tests. Categorical variables were presented as percentages and compared using Chi-square tests. For further optimization of variable selection, Lasso regression was employed. Lasso regression, with L1 regularization, shrinks the coefficients of less important variables to zero, achieving dimensionality reduction and enhancing the model’s generalizability, thereby preventing overfitting. To ensure model stability and minimize the risk of multicollinearity, the Variance Inflation Factor (VIF) was used to screen independent variables. VIF quantifies the level of collinearity among variables, and variables with a VIF greater than 5 were excluded to improve model stability and interpretability.
Furthermore, to capture potential non-linear relationships between HAR and Clavien-Dindo ≥ II-grade complications, Restricted Cubic Spline (RCS) analysis was applied. Multivariable logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to assess the association between HAR and Clavien-Dindo ≥ II-grade complications. The optimal HAR cutoff value was determined through threshold effect analysis. Additionally, sensitivity analysis was performed by excluding outliers below the 1st percentile and above the 99th percentile in HAR. Subgroup analysis was also conducted to further explore the relationship between HAR and Clavien-Dindo ≥ II-grade complications.
All statistical analyses were performed using R software (version 4.4.2).
3 Results
3.1 Baseline characteristics of participants
The present study comprised 352 patients diagnosed with gastric cancer who underwent radical gastrectomy. The subjects were divided into two groups: a complication group and a non-complication group. This division was based on the presence or absence of Clavien-Dindo ≥ II-grade complications postoperatively. As demonstrated in Table 1, the preliminary demographic characteristics analysis indicated that the mean age of the complication group (65.2 ± 8.5 years) was considerably higher than that of the non-complication group (57.7 ± 9.4, p < 0.001). With respect to hemoglobin (HGB) levels, the complication group (119.5 ± 27.3) exhibited significantly lower levels in comparison to the non-complication group (139.0 ± 26.6, p < 0.001). The operation time in the complication group (3.8 ± 0.9 h) was significantly longer than that in the non-complication group (4.5 ± 1.0, p < 0.001). Furthermore, the complication group exhibited significantly elevated levels of white blood cell count (WBC), neutrophil count (NEU), monocyte count (MONO), and platelet count (PLT) (p < 0.001). There were no significant differences in gender distribution between the two groups; however, the proportion of elderly patients in the complication group (75.5%) was significantly higher than that in the non-complication group (39.0%, p < 0.001). The prevalence of diabetes mellitus in the complication group (17.3%) was significantly higher than that in the non-complication group (5.1%, p < 0.001). However, there were no significant differences in the prevalence of cardiovascular disease between the two groups. Furthermore, the HAR index in the complication group was 3.5, which is significantly higher than the 3.2 in the non-complication group.
Table 1
| Characteristic | Non-complication | Complication | P-value |
|---|---|---|---|
| N | 254 | 98 | |
| Age (year) | 57.7 ± 9.4 | 65.2 ± 8.5 | <0.001 |
| WBC (103/μL) | 5.4 ± 1.6 | 6.7 ± 2.1 | <0.001 |
| NEU (103/μL) | 3.3 ± 1.3 | 4.6 ± 1.9 | <0.001 |
| LYM (103/μL) | 1.6 ± 0.6 | 1.4 ± 0.6 | 0.005 |
| MONO (103/μL) | 0.4 ± 0.1 | 0.5 ± 0.2 | <0.001 |
| HGB (g/L) | 139.0 ± 26.6 | 119.5 ± 27.3 | <0.001 |
| RDWSD (fL) | 47.8 ± 7.8 | 50.8 ± 10.0 | 0.005 |
| RDWCV (%) | 14.2 ± 2.8 | 15.9 ± 3.9 | <0.001 |
| PLT (103/μL) | 215.4 ± 73.5 | 264.7 ± 96.3 | <0.001 |
| ALB (g/L) | 39.2 ± 4.0 | 37.4 ± 4.3 | <0.001 |
| TC (mmol/L) | 4.0 ± 0.9 | 3.7 ± 0.9 | <0.001 |
| LDH (U/L) | 184.4 ± 58.7 | 199.3 ± 68.9 | 0.011 |
| FIB (g/L) | 2.9 ± 0.6 | 3.5 ± 0.8 | <0.001 |
| CEA (μg/L) | 6.7 ± 21.7 | 8.1 ± 23.1 | 0.117 |
| HAR | 3.5 ± 0.6 | 3.2 ± 0.6 | <0.001 |
| Diabetes, n (%) | <0.001 | ||
| No | 241 (94.9%) | 81 (82.7%) | |
| Yes | 13 (5.1%) | 17 (17.3%) | |
| Hypertension, n (%) | 0.048 | ||
| No | 219 (86.2%) | 76 (77.6%) | |
| Yes | 35 (13.8%) | 22 (22.4%) | |
| Heartdisease, n (%) | 0.3622 | ||
| No | 246 (96.9%) | 93 (94.9%) | |
| Yes | 8 (3.1%) | 5 (5.1%) | |
| Smoking, n (%) | 0.991 | ||
| No | 215 (84.6%) | 83 (84.7%) | |
| Yes | 39 (15.4%) | 15 (15.3%) | |
| Drinking, n (%) | 0.112 | ||
| No | 246 (96.9%) | 98 (100.0%) | |
| Yes | 8 (3.1%) | 0 (0.0%) | |
| Abdominal surgery, n (%) | 0.26 | ||
| No | 224 (88.2%) | 82 (83.7%) | |
| Yes | 30 (11.8%) | 16 (16.3%) | |
| Sex, n (%) | 0.984 | ||
| Male | 205 (80.7%) | 79 (80.6%) | |
| Female | 49 (19.3%) | 19 (19.4%) | |
| Surgical method, n (%) | 0.128 | ||
| Proximal | 17 (6.7%) | 12 (12.2%) | |
| Distal | 127 (50.0%) | 40 (40.8%) | |
| Total | 110 (43.3%) | 46 (46.9%) | |
| T | 0.221 | ||
| 1 | 51 (20.1%) | 13 (13.3%) | |
| 2 | 42 (16.5%) | 13 (13.3%) | |
| 3 | 85 (33.5%) | 33 (33.7%) | |
| 4 | 76 (29.9%) | 39 (39.8%) | |
| N | 0.379 | ||
| 0 | 101 (39.8%) | 34 (34.7%) | |
| 1 | 48 (18.9%) | 14 (14.3%) | |
| 2 | 42 (16.5%) | 18 (18.4%) | |
| 3 | 63 (24.8%) | 32 (32.7%) | |
| TNM | 0.028 | ||
| 1 | 76 (29.9%) | 16 (16.3%) | |
| 2 | 72 (28.3%) | 30 (30.6%) | |
| 3 | 106 (41.7%) | 52 (53.1%) | |
| Intraoperative blood loss (mL) | 89.6 ± 76.1 | 86.8 ± 87.1 | 0.699 |
| Operation time (h) | 3.8 ± 0.9 | 4.5 ± 1.0 | <0.001 |
Population characteristics analysis of patients from 2023 to 2024.
WBC, white blood cell count; NEU, neutrophil count; LYM,: lymphocyte count; MONO, monocyte count; HGB, hemoglobin.
RDWSD, red blood cell distribution width standard deviation; RDWCV, red blood cell distribution width coefficient of variation; PLT, platelet count; ALB, albumin; TC, total cholesterol count; LDH, lactate dehydrogenase; FIB, plasma fibrinogen; CEA, carcinoembryonic antigen; HAR, hemoglobin-to-albumin ratio.
3.2 Lasso regression and variance inflation factor analysis
To further optimize variable selection, we selected 18 significant variables from Table 1 for Lasso regression analysis, including age, white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MONO), hemoglobin (HGB), red blood cell distribution width standard deviation (RDWSD), red blood cell distribution width coefficient of variation (RDWCV), platelet count (PLT), albumin (ALB), total cholesterol count (TC), lactate dehydrogenase (LDH), plasma fibrinogen (FIB), HAR index, diabetes status (Diabetes), hypertension status (Hypertension), TNM stage, and Operation time. We set alpha = 1 for standard L1 regularization. Lasso regression achieves feature selection and dimension reduction by penalizing model coefficients, causing the coefficients of unimportant variables to approach zero. This method helps reduce model complexity and prevent overfitting. During model optimization, the optimal λ value (λ min = 0.008985436) was determined using 5-fold cross-validation, and the model was retrained using this λ value, ultimately incorporating 13 variables (as shown in Figure2).
Figure 2
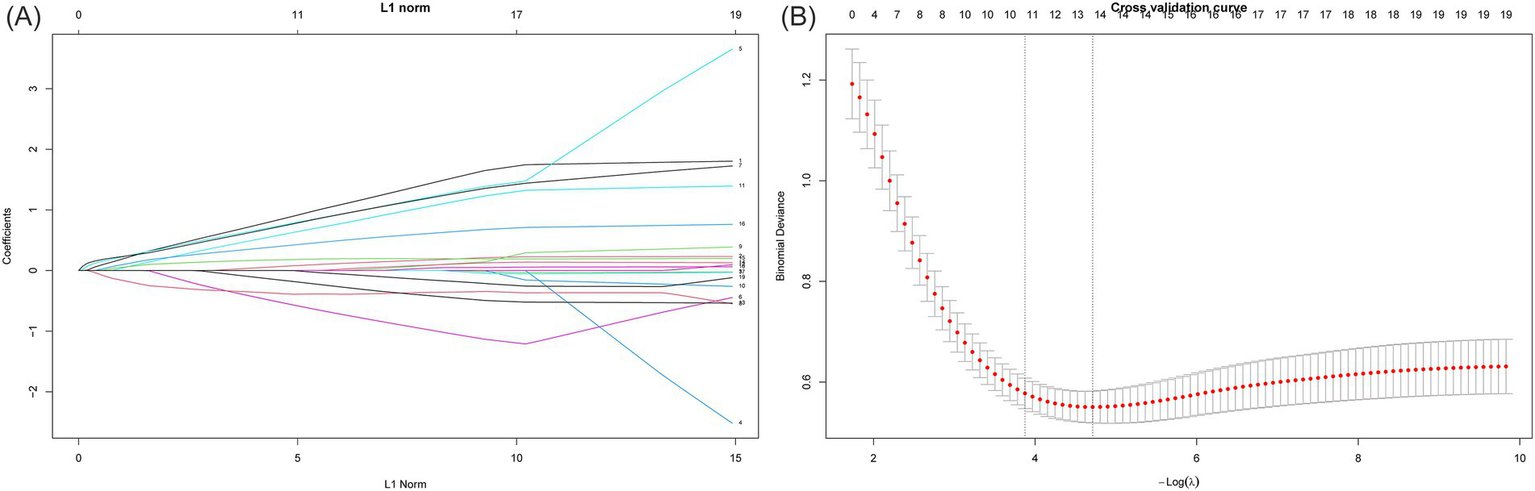
Screening of variables based on Lasso regression. This figure illustrates the process of optimizing variable selection through Lasso regression. In 5-fold cross validation, alpha = 1 is used for L1 regularization, and the optimal regularization parameter λ = 0.008985436 is determined by minimizing the cross validation error. This parameter optimized the performance of the model, and through the λ value, Lasso regression screened out 13 significant variables. (A) The characteristics of the regression coefficients of each variable as a function of L1 regularization intensity. (B) The process of selecting the optimal λ value through cross validation in the Lasso regression model.
Subsequently, we used VIF analysis to address potential multicollinearity issues among variables. The results of the variance inflation factor analysis (Table 2) showed that after excluding hemoglobin level (HGB) and red blood cell distribution width standard deviation (RDWSD), the variance inflation factor values of all remaining variables were less than 5, indicating that there were no multicollinearity issues.
Table 2
| Variables | VIF1 | VIF2 |
|---|---|---|
| Age | 1.702 | 1.689 |
| Diabetes | 1.088 | 1.083 |
| NEU | 1.630 | 1.580 |
| LYM | 1.832 | 1.811 |
| MONO | 1.933 | 1.926 |
| HGB | 5.537 | NA |
| RDWSD | 1.712 | 1.497 |
| PLT | 2.029 | 2.012 |
| TC | 1.251 | 1.192 |
| LDH | 1.203 | 1.155 |
| FIB | 1.157 | 1.142 |
| Operation time | 1.218 | 1.213 |
| HAR | 4.481 | 1.543 |
Variance inflation factor.
NEU, neutrophil count; LYM, lymphocyte count; MOMO, monocyte count; HGB, hemoglobin; RDWSD, red blood cell distribution width standard deviation; PLT, platelet count; TC, total cholesterol count; LDH, lactate dehydrogenase; FIB, plasma fibrinogen; HAR, hemoglobin-to-albumin ratio.
3.3 Association between HAR and Clavien-Dindo ≥II grade complications
Following a two-way stepwise regression analysis, the final model was determined, encompassing seven significant factors: age, neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MONO), platelet count (PLT), total cholesterol count (TC), operation time. To further investigate the relationship between HAR and Clavien-Dindo ≥II grade complications, three multivariable regression models were assessed (Table 3). In Model 1, which did not adjust for covariates, each 1-unit increase in HAR was associated with a 61.6% lower risk of complications (OR = 0.384, 95% CI: 0.255–0.568, p < 0.001). In Model 2, which adjusted for covariates including age, neutrophil count (NEU), lymphocyte count (LYM), the odds ratio (OR) for HAR and complications was 0.274 (95% CI: 0.158–0.459, p < 0.001). In Model 3, which included all covariates, the OR for HAR was further adjusted to 0.380 (95% CI: 0.188–0.749, p = 0.006). Furthermore, with increasing HAR, the risk of complications continued to decrease significantly (P for trend <0.005).
Table 3
| Variables | Crude Model1a | Model2b | Model3c | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| HAR | 0.384(0.255–0.568) | <0.001 | 0.274(0.158–0.459) | <0.001 | 0.380(0.188–0.749) | 0.006 |
| Categories | ||||||
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.556 (0.298–1.028) | 0.063 | 0.278 (0.120–0.618) | 0.002 | 0.646 (0.208–1.965) | 0.443 |
| Q3 | 0.346(0.176–0.662) | 0.002 | 0.193(0.079–0.448) | <0.001 | 0.445(0.148–1.310) | 0.144 |
| Q4 | 0.218(0.102–0.439) | <0.001 | 0.114(0.043–0.283) | <0.001 | 0.172(0.047–0.579) | 0.006 |
| P for trend | <0.001 | <0.001 | 0.006 | |||
Multivariable logistic regression models for the association between HAR and Clavien-Dindo ≥II grade complications.
HAR, hemoglobin-to-albumin ratio.
aWithout adjustment.
bAdjusted for age, NEU, LYM.
cAdjusted for all variables.
d P for trend is calculated by converting the quartiles of HAR into level variables, assigning values of 0, 1, 2, and 3, and then inputting the level variables into the regression model.
Based on Model 3, we performed restricted cubic spline analysis to further explore the relationship between HAR and Clavien-Dindo ≥II complications (Figure 3). The results showed that there was no nonlinear relationship between HAR and complications (nonlinear test p value = 0.4962). To further elucidate the nature of this relationship, we subsequently conducted a threshold effect analysis (Table 4). Threshold effect analysis showed that no significant association was observed when the HAR value was below 2.87; however, when the HAR value was equal to or above 2.87, there was a significant negative correlation with event risk (OR, 0.21; 95% CI: 0.06–0.76; p = 0.018).
Figure 3
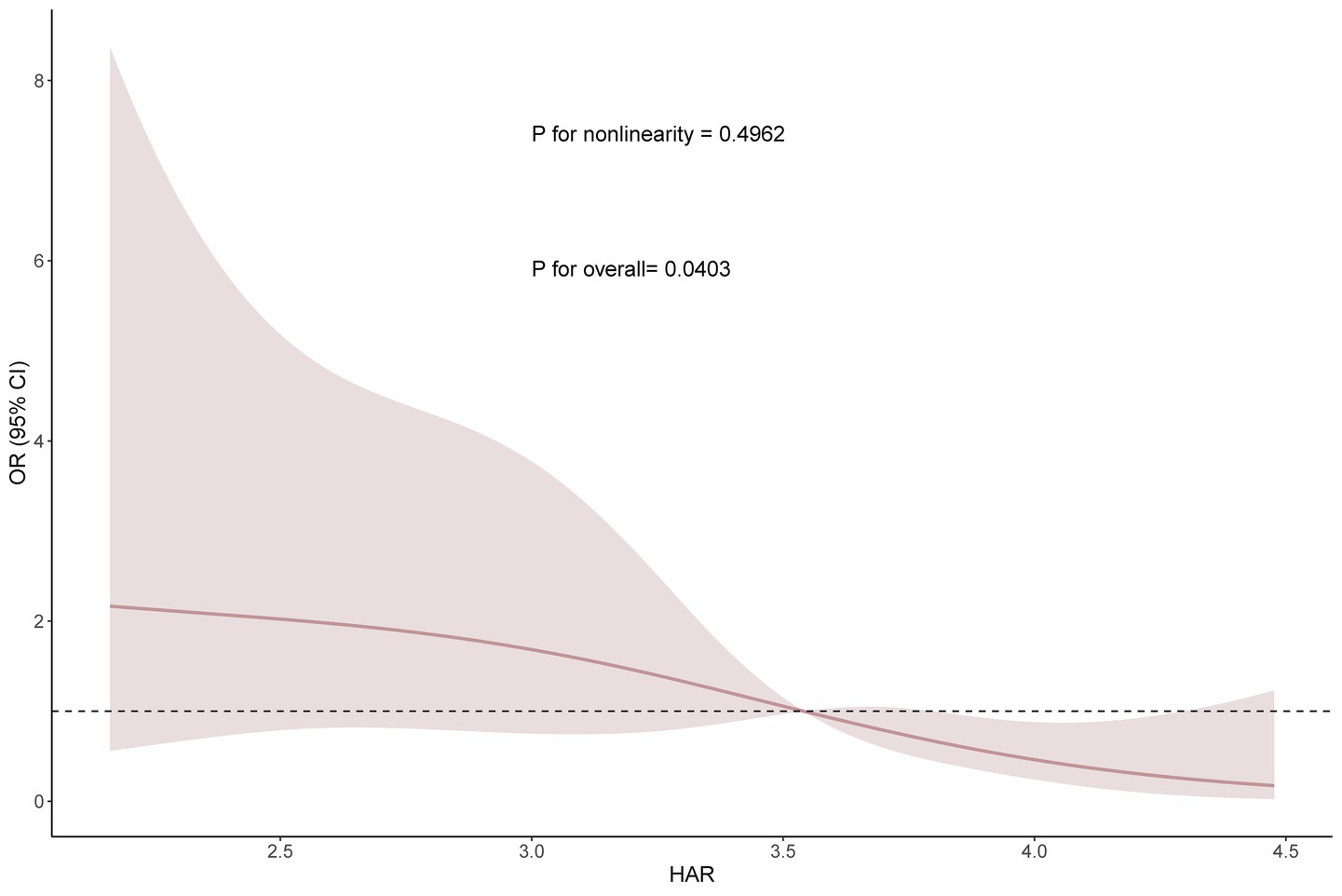
Smooth curve fitting (RCS analysis) between HAR and complications based on Model 3.
Table 4
| Threshold effect analysis | OR (95%CI) | P |
|---|---|---|
| Inflection point | 2.87 | |
| <2.87 | 1.32 (0.05–37.08) | 0.871 |
| ≥2.87 | 0.21 (0.06–0.76) | 0.018 |
| P for likelihood test | 0.408 |
Threshold effect analysis of HAR and complications using a two-piecewise logistic regression model.
3.4 Sensitivity analysis
Sensitivity analysis showed that after excluding the upper and lower 1% of outliers before and after HAR (Table 5), the association remained statistically significant, with an adjusted odds ratio (OR) of 0.175 (95% confidence interval, 0.048–0.593; p = 0.006), indicating a significant negative correlation between the highest HAR group and event risk. Trend analysis further supported the conclusion that higher HAR values are associated with lower event risk (trend p-value = 0.006). The ROC curve shows that the AUC of HAR is higher than that of PNI, PAR, and CONUT score (Supplementary Figure S1).
Table 5
| Variables | OR | CI_lower | CI_upper | P for trend |
|---|---|---|---|---|
| HAR | 0.356 | 0.170 | 0.726 | 0.005 |
| Categories | ||||
| Q1 | Reference | Reference | Reference | |
| Q2 | 0.613 | 0.196 | 1.870 | 0.392 |
| Q3 | 0.436 | 0.145 | 1.280 | 0.133 |
| Q4 | 0.175 | 0.048 | 0.593 | 0.006 |
| P for trend | 0.006 | |||
Sensitivity analysis.
P for trend is calculated by converting the quartiles of HAR into level variables, assigning values of 0, 1, 2, and 3, and then inputting the level variables into the regression model.
3.5 Subgroup analyses
Further subgroup analyses (Figure 4) showed that HAR was generally negatively associated with the risk of Clavien-Dindo ≥II grade complications. This trend was observed in both the normal lymphocyte (LYM) group (OR, 0.399, 95% CI, 0.170–0.876), the abnormal group (OR, 0.204, 95% CI, 0.054–0.624), monocytes (MONO) normal group (OR, 0.412, 95% CI, 0.185–0.878), and total cholesterol (TC) normal group (OR, 0.368, 95% CI, 0.180–0.732), and also in patients with operation time <4 h(OR, 0.241, 95% CI, 0.075–0.683). Interestingly, HAR had a protective effect against complications in both the normal platelet (PLT) group (OR, 0.384, 95% CI, 0.193–0.735) and the abnormal platelet group (OR, 0.001, 95% CI, 0.001–0.021), and there was a significant interaction between the two groups (P for interaction = 0.011).
Figure 4
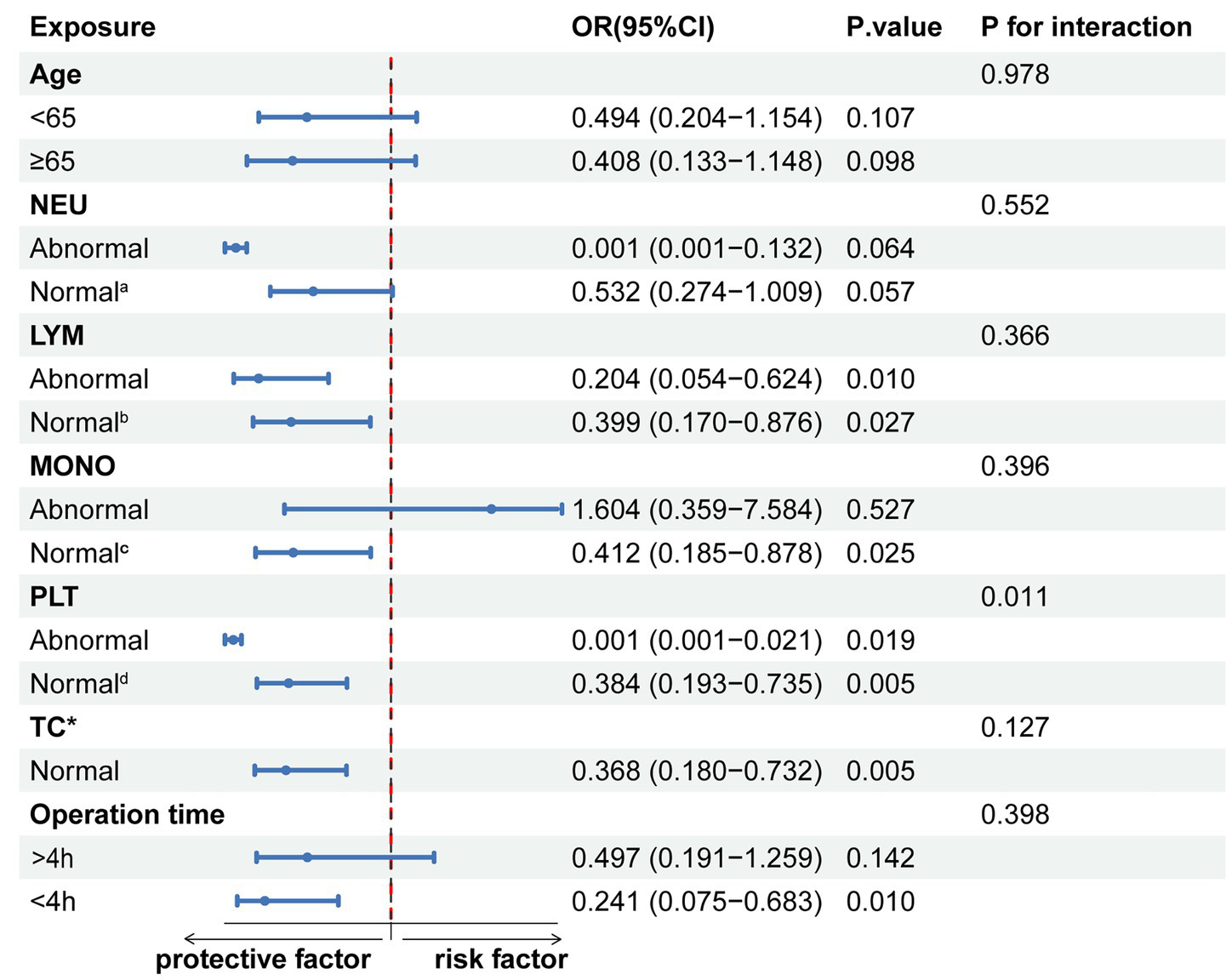
Subgroup stratified analysis of HAR and Clavien-Dindo ≥II grade complications. a: 1.8–6.3 × 109/L; b: 1.1–3.2 × 109/L; c: 0.1–0.6 × 109/L; d: 125–350 × 109/L. *Insufficient data for the abnormal total cholesterol (TC) group.
4 Discussion
This study retrospectively analyzed clinical data from 352 gastric cancer patients who underwent radical gastrectomy at the Second Hospital of Lanzhou University between January 2023 and December 2024. The findings indicated a substantial negative correlation between hemoglobin-to-albumin ratio (HAR) and Clavien-Dindo ≥II grade complications. Specifically, after adjusting for all covariates in Model 3, each 1-unit increase in HAR was associated with a 62.0% reduction in the risk of postoperative complications. Furthermore, the occurrence of complications was found to be significantly lower in the high HAR group compared to the low HAR group, exhibiting a statistically significant overall trend (P for trend = 0.006). RCS analysis further revealed a linear negative correlation between HAR and Clavien-Dindo ≥II grade complications. A subsequent threshold effect analysis identified a significant negative correlation between HAR values of 2.87 or higher and the risk of postoperative complications. Sensitivity analysis further confirmed the stability and consistency of the study results. Also, HAR outperformed established indices such as PNI, PAR, and CONUT in predicting postoperative complications.
HAR is a novel nutritional biomarker calculated based on albumin and hemoglobin. Previous studies have shown that low preoperative HAR is associated with short survival in gastric cancer patients (18). HAR’s clinical significance primarily lies in its integration of hemoglobin and albumin, two fundamental physiological indicators. These indicators influence postoperative complications in gastric cancer patients through a complex network of interactions that regulate oxygen transport, inflammatory balance, and immune homeostasis (19). Compared with established indices such as PNI, PAR, and CONUT, which mainly capture nutritional or inflammatory status alone, HAR provides a more comprehensive reflection of both oxygen-carrying capacity and systemic inflammation, thereby offering superior prognostic value.
Gastric cancer commonly reduces hemoglobin through multiple, reinforcing mechanisms. Friable tumor mucosa and abnormal neovasculature lead to chronic occult or overt bleeding (20). Tumor-associated inflammation elevates hepcidin levels, sequesters iron, shortens red cell lifespan, and diminishes erythropoietin responsiveness, resulting in anemia of inflammation (21). Moreover, reduced dietary intake, gastric outlet obstruction, and mucosal atrophy or malabsorption contribute to deficiencies in iron, folate, and vitamin B12 (10). This sustained, disease-specific downward pressure on hemoglobin decreases oxygen-carrying capacity, lowers tissue oxygen tension, and activates the HIF-1α signaling pathway (22). The ensuing hypoxic response upregulates VEGF expression and promotes the formation of hypoxia-driven but functionally inadequate neovessels, which compromises microvascular perfusion, delays tissue repair, and increases the risk of postoperative complications such as anastomotic leaks (23). In parallel, gastric cancer often lowers albumin through a tumor-driven negative acute-phase response, cancer-related malnutrition, and cachexia (24). Concurrently, low albumin levels decrease plasma colloid osmotic pressure, causing tissue edema and accelerating the spread of inflammatory factors (25). This impairs albumin’s antioxidant function, leading to elevated pro-inflammatory factor levels and triggering an inflammatory cascade reaction. This increases the risk of sepsis (26, 27). Furthermore, reduced hemoglobin and albumin levels can impair immune cell function by causing mitochondrial hypoxia in T cells, decreasing natural killer (NK) cell activity, impairing lymphocyte proliferation, and activating the complement system (28), thereby increasing the incidence of postoperative infection and abdominal abscesses (29, 30). Threshold effect analysis shows that the HAR index has no statistically significant effect on postoperative complications when it is less than 2.87. At this point, patients often have severe anemia and hypoalbuminemia, which trigger complications dominated by confounding factors, such as multiple organ failure and systemic inflammation (31). These factors mask the effect of HAR. However, when the HAR index is ≥2.87, a 1-unit increase in the HAR index reduces the risk of postoperative complications by 82.0%. In summary, the HAR index significantly influences the occurrence of postoperative complications in gastric cancer patients and can serve as an important biomarker for risk assessment.
Subgroup analysis revealed that the protective effect of HAR was observed across various subgroups, including those defined by age, neutrophil count, lymphocyte count, monocyte count, and total cholesterol level. HAR significantly reduced the risk of Clavien-Dindo ≥II-grade complications through its dual mechanisms of optimizing oxygen transport via hemoglobin and attenuating the inflammatory cascade via albumin. Notably, this protective effect was observed in both the normal and abnormal platelet count groups, although it appeared stronger in patients with abnormal platelet levels. Mechanistically, abnormal platelet counts reflect a state of metabolic and inflammatory stress, which is frequently associated with coagulation disorders, platelet hyperreactivity, and microcirculatory dysfunction (32). These alterations aggravate tissue hypoxia and trigger amplification of inflammatory cascades. Under such conditions, higher hemoglobin levels may compensate by enhancing oxygen-carrying capacity, thereby alleviating hypoxic stress at the tissue level (33). Hemoglobin also promotes VEGF-mediated angiogenesis under hypoxic conditions, which facilitates microvascular repair and oxygen redistribution (34). Albumin, on the other hand, counteracts the deleterious consequences of platelet dysfunction and inflammation. It exerts antioxidant effects by scavenging reactive oxygen species, reduces endothelial injury, and downregulates NF-κB signaling, thereby attenuating inflammation-driven vascular permeability (35, 36). In addition, albumin stabilizes the endothelial glycocalyx, which is otherwise disrupted by platelet-derived inflammatory mediators, and preserves microvascular barrier integrity (37). Together, these mechanisms provide resilience against the detrimental effects of platelet abnormalities, explaining the stronger protective role of HAR in the abnormal platelet group. Even in patients with normal platelet counts, perioperative trauma and surgical stress invariably activate systemic inflammation and coagulation pathways (38). In this context, a higher HAR still offers protection by maintaining physiological balance, reflecting the integrated contribution of oxygen delivery, nutritional reserves, and inflammatory modulation.
4.1 Limitations
This study has several limitations. First, although HAR consistently showed a negative association with postoperative complications across multivariable regression, sensitivity, and RCS analyses, a potential concern is that both hemoglobin and albumin may increase during nutritional recovery, raising the question of whether their ratio could fluctuate unpredictably. However, these biomarkers reflect distinct physiological domains that often change at different rates. Thus, HAR should not be regarded as a pure nutritional marker but rather as a composite index integrating anemia, protein reserves, and systemic inflammation. Nevertheless, in extreme cases of severe anemia combined with hypoalbuminemia, its predictive value may be limited, and future studies should consider combining HAR with established nutritional scoring systems for validation. Second, this was a single-center retrospective study, which may restrict the generalizability of the findings. Third, although we adjusted for multiple established risk factors, some potential confounders were not fully captured, such as detailed perioperative nutritional interventions (e.g., enteral nutrition) and certain comorbidities (e.g., chronic liver disease). Fourth, current evidence supports validation of HAR solely in gastric cancer. Its applicability to other malignancies remains uncertain and should be assessed in prospective, disease-specific multicenter cohorts with external validation before clinical extrapolation. Finally, the retrospective design inherently carries the risk of selection and information bias. Therefore, our findings should be interpreted with caution, and prospective multicenter studies with more comprehensive data collection are warranted to further confirm and extend these results.
5 Conclusion
This study found a significant negative linear correlation between higher HAR levels and the risk of Clavien-Dindo grade II or higher complications in gastric cancer patients. Higher HAR levels were associated with a lower risk of postoperative complications. Specifically, for every one-unit increase in HAR, the risk of complications decreases by 62.0%. This relationship is statistically significant when HAR values are ≥2.87. These results support the potential of HAR as a predictive tool for postoperative Clavien-Dindo ≥II-grade complications in gastric cancer patients, providing an important foundation for early diagnosis and targeted interventions in clinical practice.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Second Hospital of Lanzhou University (Project Number: 2025 A-652). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because As this study is a retrospective and the privacy and personal identity information of the patients were protected, the need for informed consent was waived by the Medical Ethics Committee of the Second Hospital of Lanzhou University.
Author contributions
WL: Project administration, Formal analysis, Writing – original draft, Data curation, Conceptualization, Visualization, Writing – review & editing, Investigation, Methodology, Validation, Software. RL: Methodology, Writing – review & editing, Formal analysis, Writing – original draft, Investigation, Data curation, Conceptualization. CP: Investigation, Writing – review & editing, Writing – original draft, Formal analysis. CL: Project administration, Formal analysis, Writing – original draft, Methodology, Resources, Validation, Data curation, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Gansu Province Traditional Chinese Medicine Research Project (No. GZKG-2024-75); Lanzhou City Chengguan District Science and Technology Program Project (No. 2024SHFZ0018).
Acknowledgments
We are grateful for the financial support provided by the Gansu Provincial Administration of Traditional Chinese Medicine and the Lanzhou Chengguan District Science and Technology Bureau.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1683276/full#supplementary-material
References
1.
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2.
Japanese Gastric Cancer Association . Japanese gastric Cancer treatment guidelines 2021 (6th edition). Gastric Cancer. (2023) 26:1–25. doi: 10.1007/s10120-022-01331-8
3.
Etoh T Ohyama T Sakuramoto S Tsuji T Lee S-W Yoshida K et al . Five-year survival outcomes of laparoscopy-assisted vs open distal gastrectomy for advanced gastric cancer: the JLSSG0901 randomized clinical trial. JAMA Surg. (2023) 158:445–54. doi: 10.1001/jamasurg.2023.0096
4.
Dindo D Demartines N Clavien P-A . Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
5.
Clavien PA Barkun J de Oliveira ML Vauthey JN Dindo D Schulick RD et al . The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187. doi: 10.1097/SLA.0b013e3181b13ca2
6.
Ri M Hayami M Ohashi M Makuuchi R Irino T Sano T et al . Possibly more favorable short-term outcomes with minimally invasive surgery than with open surgery in total gastrectomy for locally advanced gastric cancer: a single high-volume center study. Ann Gastroenterol Surg. (2025) 9:439–47. doi: 10.1002/ags3.12881
7.
Idriss AM Tfeil Y Baba JS Boukhary SM Deddah MA . Applicability of the clavien-dindo classification in the evaluation of postoperative complications at the surgery department of the national hospital center of Nouakchott: observational study of 834 cases. Pan Afr Med J. (2019) 33:254. doi: 10.11604/pamj.2019.33.254.18024
8.
Yamamoto K Nagatsuma Y Fukuda Y Hirao M Nishikawa K Miyamoto A et al . Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer. (2017) 20:913–8. doi: 10.1007/s10120-016-0683-4
9.
Yu J Yuan A Liu Q Wang W Sun Y Li Z et al . Effect of preoperative immunonutrition on postoperative short-term clinical outcomes in patients with gastric cancer cachexia: a prospective randomized controlled trial. World J Surg Oncol. (2024) 22:101. doi: 10.1186/s12957-024-03348-y
10.
Kang MK Lee H-J . Impact of malnutrition and nutritional support after gastrectomy in patients with gastric cancer. Ann Gastroenterol Surg. (2024) 8:534–52. doi: 10.1002/ags3.12788
11.
Chen X Liu X Pan J You P Ren S . Preoperative risk factors for predicting postoperative human serum albumin infusion after hip fracture surgery: development and validation of a nomogram. Balkan Med J. (2023) 40:40–50. doi: 10.4274/balkanmedj.galenos.2022.2022-7-26
12.
Seid A Debebe Z Ayelign A Abeje M Endris BS Assefa M et al . Malnutrition diagnosed by patient-generated subjective global assessment and the risk of all-cause mortality in adults with gastrointestinal cancer: a systematic review and meta-analysis. J Hum Nutr Diet. (2025) 38:e70012. doi: 10.1111/jhn.70012
13.
Zhang L-L Xu F Song D Huang M-Y Huang Y-S Deng Q-L et al . Development of a nomogram model for treatment of nonmetastatic nasopharyngeal carcinoma. JAMA Netw Open. (2020) 3:e2029882. doi: 10.1001/jamanetworkopen.2020.29882
14.
Bellelli A Tame JRH . Hemoglobin allostery and pharmacology. Mol Asp Med. (2022) 84:101037. doi: 10.1016/j.mam.2021.101037
15.
Xu H Zheng X Ai J Yang L . Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: a systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. (2023) 114:109496. doi: 10.1016/j.intimp.2022.109496
16.
Lv Q Rao S-Q Xiang Z . Preoperative hemoglobin to albumin ratio as a prognostic predictor for patients with colorectal cancer surgery. Updat Surg. (2025) 77:761–9. doi: 10.1007/s13304-025-02061-z
17.
Zhang C Wang S-F Zhang Y-L Teng C-X . Peripheral hemoglobin to albumin ratio predicts prognosis in patients with nasopharyngeal carcinoma underwent concurrent chemoradiotherapy. BMC Cancer. (2024) 24:1012. doi: 10.1186/s12885-024-12763-z
18.
Hu C-G Hu B-E Zhu J-F Zhu Z-M Huang C . Prognostic significance of the preoperative hemoglobin to albumin ratio for the short-term survival of gastric cancer patients. World J Gastrointest Surg. (2022) 14:580–93. doi: 10.4240/wjgs.v14.i6.580
19.
Luo X Chen X-D Chen J-L Wang W-B Madan A Song H-N et al . High albumin-bilirubin grade predicts worse short-term complications in gastric cancer patients with metabolic syndrome: a retrospective study. J Gastrointest Oncol. (2023) 14:2039–47. doi: 10.21037/jgo-23-599
20.
Ramachandran R Grantham T Parvataneni S Budh D Gollapalli S Reddy M et al . Gastric cancer: clinical features, screening, diagnosis, treatment, and prevention. J Community Hosp Intern Med Perspect. (2024) 14:49–57. doi: 10.55729/2000-9666.1304
21.
Weiss G Ganz T Goodnough LT . Anemia of inflammation. Blood. (2019) 133:40–50. doi: 10.1182/blood-2018-06-856500
22.
Retamal MA Salazar-Onfray F González FE Tittarelli A . Tumor hypoxia shapes natural killer cell anticancer activities. J Mol Med. (2025) 103:755–77. doi: 10.1007/s00109-025-02557-6
23.
Qiu T-H Wen H-Y Huang Y-L . Significance of hemoglobin and hematocrit changes in predicting patient survival and efficacy of neoadjuvant chemotherapy for advanced gastric cancer. World J Gastrointest Oncol. (2025) 17:104592. doi: 10.4251/wjgo.v17.i6.104592
24.
Soria Rivas A Escobar Álvarez Y Blasco Cordellat A Majem Tarruella M Molina Mata K Motilla de la Cámara M et al . SEOM clinical guidelines for cancer anorexia-cachexia syndrome (2023). Clin Transl Oncol. (2024) 26:2866–76. doi: 10.1007/s12094-024-03502-8
25.
Takayoshi K Kusaba H Aikawa T Koreishi S Sagara K Nakano M et al . Hypoalbuminemia for the prediction of venous thromboembolism and treatment of direct oral anticoagulants in metastatic gastric cancer patients. Gastric Cancer. (2019) 22:988–98. doi: 10.1007/s10120-019-00930-2
26.
Evans DC Corkins MR Malone A Miller S Mogensen KM Guenter P et al . The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
27.
Kaya Bilir D Çekmen N Tepeoğlu M Özturan Özer HE . Effects of intraperitoneal magnesium sulfate on perioperative inflammatory response in rats with pneumoperitoneum. BMC Anesthesiol. (2025) 25:336. doi: 10.1186/s12871-025-03139-2
28.
Scharping NE Rivadeneira DB Menk AV Vignali PDA Ford BR Rittenhouse NL et al . Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. (2021) 22:205–15. doi: 10.1038/s41590-020-00834-9
29.
Zou Y Li L Jia K Tian L He M Huang D . Effect of preoperative nutritional risk index on 30-day postoperative complications in patients with gastric cancer: a retrospective cohort study. Front Oncol. (2025) 15:1475381. doi: 10.3389/fonc.2025.1475381
30.
Zhang L-K Zheng H-L Zheng X-Y Xu B-B Tang Y-H Zheng Z-W et al . Development and validation of a novel modified cancer cachexia index in patients with locally advanced gastric cancer undergoing neoadjuvant chemotherapy: a multicenter cohort study. Int J Surg (lond Engl). (2025) 111:5868–5881. doi: 10.1097/JS9.0000000000002707
31.
Zhou J Yu P Shi Y Tang B Hao Y Zhao Y et al . Evaluation of clavien-dindo classification in patients undergoing total gastrectomy for gastric cancer. Med Oncol (northwood Lond Engl). (2015) 32:120. doi: 10.1007/s12032-015-0573-3
32.
Giannakeas V Kotsopoulos J Brooks JD Cheung MC Rosella L Lipscombe L et al . Platelet count and survival after cancer. Cancer. (2022) 14:549. doi: 10.3390/cancers14030549
33.
Lee C Kim M-J Kumar A Lee H-W Yang Y Kim Y . Vascular endothelial growth factor signaling in health and disease: from molecular mechanisms to therapeutic perspectives. Signal Transduct Target Ther. (2025) 10:170. doi: 10.1038/s41392-025-02249-0
34.
Cheng Y Zhang M Li C Su L Fu L Wu S et al . Endothelial AGGF1 promotes retinal angiogenesis by coordinating TNFSF12/FN14 signalling. Nat Commun. (2025) 16:1332. doi: 10.1038/s41467-025-55970-3
35.
Heckmann M Karlsberger L Blank-Landeshammer B Klanert G Sadova N Stadlbauer V et al . 3-O-trans-p-coumaroyl esterification enhances the anti-inflammatory effects of tormentic acid by targeting NF-κB signaling. Redox Biol. (2025) 85:103731. doi: 10.1016/j.redox.2025.103731
36.
Imeci O Asci H Asci S Sevuk MA Sarikaya HS Ozmen O . Dapagliflozin mitigates lipopolysaccharide-induced neuroinflammation through potential involvement of the IL-17A/GSK3β signaling pathway and modulation of inflammatory cytokines. Eur J Pharmacol. (2025) 1003:177913. doi: 10.1016/j.ejphar.2025.177913
37.
Shi SM Suh RJ Shon DJ Garcia FJ Buff JK Atkins M et al . Glycocalyx dysregulation impairs blood-brain barrier in ageing and disease. Nature. (2025) 639:985–94. doi: 10.1038/s41586-025-08589-9
38.
Margraf A Ludwig N Zarbock A Rossaint J . Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. (2020) 131:1693–707. doi: 10.1213/ANE.0000000000005175
Summary
Keywords
hemoglobin-to-albumin ratio, radical gastrectomy, gastric cancer, postoperative complications, Clavien-Dindo classification, nutritional biomarker
Citation
Luo W, Li R, Pan C and Luo C (2025) Correlation between hemoglobin-to-albumin ratio and complications after radical gastrectomy in gastric cancer patients. Front. Med. 12:1683276. doi: 10.3389/fmed.2025.1683276
Received
10 August 2025
Accepted
10 October 2025
Published
23 October 2025
Volume
12 - 2025
Edited by
Xinying Wang, Nanjing General Hospital of Nanjing Military Command, China
Reviewed by
Liang Wang, Affiliated Hospital of Qinghai University, China
Fengmin Zhang, Tongji University, China
Updates
Copyright
© 2025 Luo, Li, Pan and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changjiang Luo, luocj@lzu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.