- 1Intensive Care Unit, The Second People’s Hospital of Hefei, Hefei Hospital Affiliated to Anhui Medical University, Hefei, China
- 2The Second Clinical Medical School, Anhui University of Chinese Medicine, Hefei, China
- 3Department of Emergency, Intensive Care Unit, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 4Intensive Care Unit, First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, China
- 5Institute of Artificial Intelligence, Hefei Comprehensive National Science Center, Hefei, China
- 6School of Medicine, Xian Jiaotong University, Xi’an, China
- 7Department of Colorectum, First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, China
Background: The objective of our retrospective multicenter analysis was to identify risk factors and construct a statistical model for predicting acute kidney injury (AKI) among elderly patients with severe pneumonia requiring mechanical ventilation (SPRMV) in elderly patients in different intensive care units (ICU).
Methods: We aimed to utilize a multi-center retrospective analysis, including 353 cases of SPRMV patients diagnosed and treated in the ICU of the Hefei Second People’s Hospital between May 2018 and February 2025 as a training dataset, and 151 participants were admitted to the ICU of the First Affiliated Hospital of Anhui Medical University between June 2020 and March 2025, considered as a validation dataset. Both univariate and multivariate logistic regression analyses were utilized to investigate the risk factors of SPRMV with AKI. After that, our predictive model was evaluated using a nomogram, a receiver operating characteristic (ROC) curve for discrimination, calibration curves, and decision curve analysis (DCA) curves for clinical validity.
Results: Our multivariate logistic regression analysis indicated that CREA, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, and MAP are independent risk factors of SPRMV in the elderly patients with AKI. A nomogram of SPRMV in elderly patients with AKI was constructed. The ROC curve revealed that our predictive model showed good predictive efficacy with an area under curve (AUC) of 0.920 (95% confidence intervals (CI) = 0.892–0.948) with a specificity of 0.993 and sensitivity of 0.763 in the training dataset and an AUC value of 0.938 (95%CI = 0.899–0.977) with a specificity of 0.952 and sensitivity of 0.854 in the validation dataset. Moreover, calibration and DCA curves demonstrated that our predictive model had a good fit, better net benefit, and higher predictive efficiency for SPRMV in the elderly patients with AKI.
Conclusion: Our predictive model demonstrated that CREA, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, and MAP were the independent risk factors of AKI in SPRMV in the elderly patients with high accuracy and good calibration.
Introduction
Severe pneumonia is a serious infectious disease that is common among the elderly and has a high mortality rate (1–7). Mechanical ventilation, as a key life-supporting therapy, is widely used in the treatment of patients with severe pneumonia (8). However, the incidence of acute kidney injury (AKI) in mechanically ventilated patients remains relatively high, which has become an important determinant affecting the prognosis and quality of life of patients (9). AKI not only complicates the treatment process, significantly prolongs hospitalization time, and increases medical costs but also significantly increases the risk of death for patients (10). The risk of AKI during mechanical ventilation is further increased in elderly patients due to weakened immune function, diminished organ reserves, and various underlying diseases (11). At present, there is a validated lack of validated risk-prediction tools for AKI in elderly patients with severe pneumonia undergoing mechanical ventilation, and there is no systematic validation based on large amounts of clinical data from multiple centers, which to some extent limits the accurate identification and early intervention of high-risk patients in clinical practice.
The pathogenesis of AKI is extremely complex in patients with severe pneumonia undergoing mechanical ventilation. Infection and systemic inflammatory response trigger the release of a large amount of inflammatory mediators (12), causing direct damage to renal tubular epithelial cells, inducing microcirculation disorders and cell apoptosis (13, 14). During mechanical ventilation, increased positive chest pressure leads to a decrease in venous return and a decrease in renal blood flow perfusion, which exacerbates renal ischemia and hypoxia (15). Furthermore, common factors such as acid–base imbalance, electrolyte imbalance, and metabolic disorders in severe conditions also significantly increase the burden on the kidneys, promoting the occurrence and development of AKI (16). These pathological processes interact with each other to form a vicious cycle, resulting in a poor prognosis for elderly critically ill patients, which is extremely poor once AKI occurs (17, 18). Specifically, we note that the majority of previous AKI prediction models were constructed for broad-based intensive care units (ICU) populations and did not specifically target elderly patients with severe pneumonia requiring mechanical ventilation (SPRMV). Furthermore, most modeling studies failed to perform any external validation and, in addition, did not consider either ventilation-related or inflammatory parameters relevant to this population. To fill these specific gaps, our study combines clinical, inflammatory, and mechanical ventilation variables from multiple centers to establish and validate a model predictive of AKI that is more directly targeted and better generalizable.
In view of this, this study retrospectively analyzed clinical data from elderly patients with severe pneumonia undergoing mechanical ventilation admitted to multiple centers by comprehensively evaluating infection- and inflammation-related markers, ventilation parameters, underlying diseases, and medication use. A rigorous multivariate statistical method was used to establish and validate an AKI risk prediction model. This model aims to provide a reliable and clinically applicable tool for clinical practice, enabling early prediction and precise management of high-risk patients, promoting the development of individualized treatment plans, and ultimately improving the treatment effectiveness and quality of life of patients.
Materials and methods
Study population and analysis design
We retrospectively collected individuals diagnosed with SPRMV from two multicenter ICU, with a total of 504 SPRMV individuals, of which a total of 353 participants were admitted to the ICU of the Hefei Second People’s Hospital between May 2018 and February 2025 and considered as a training dataset. A total of 151 participants were admitted to the ICU of the First Affiliated Hospital of Anhui Medical University between June 2020 and March 2025 and considered as a validation dataset. Our analysis was approved by the Ethics Committee of both the Hefei Second People’s Hospital and the First Affiliated Hospital of Anhui Medical University, and the study followed the Declaration of Helsinki.
In our study, AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, which include an increase in serum creatinine by ≥ 0.3 mg/dL within 48 h, or an increase to ≥ 1.5 times the baseline within the prior 7 days, or a urine output of < 0.5 mL/kg/h for 6 h. The inclusive and exclusive criteria for our SPRMV patients’ clinical data collection are as follows: Inclusive criteria: (a) elderly patients aged ≥ 65 years old; (b) diagnosed with SPRMV during hospitalization in two ICUs; (c) all patients diagnosed with ARI undergo renal ultrasound examination to rule out posterior renal factors such as ureteral obstruction. The exclusive criteria are as follows: (a) age less than 65 years old; (b) chronic renal disease stage 4–5 (eGFR (glomerular filtration rate) < 30 mL/min/1.73 m2) or previous ≥ 4 weeks of blood/peritoneal dialysis; (c) those who die within 48 h of admission, voluntarily give up treatment, have family members request discharge, or have advanced malignant tumors and receive palliative treatment, with an expected survival period of less than 3 months; (d) individuals with incomplete medical history data.
Data definition
Social-demographic characteristics
We recorded case history information on four social-demographic characteristics, including gender, age, education level, and marital status. Gender was categorized as male or female. The education level was defined as less than primary school, middle school, or upper college. Marital status was defined as married, unmarried, or divorced.
Clinicopathologic characteristics
The following clinicopathologic characteristics were included in our present analysis: drinking consumption (yes, no), smoking consumption (yes, no), daily diet habit (regular, irregular), depression (yes, no), cardiovascular disease (CVD) (yes, no), hypertension (yes, no), diabetes (yes, no), coronary heart disease (CHD) (yes, no), hemoglobin (HGB), red blood cell (RBC) (1,012/L), white blood cell (WBC) (109/L), creatinine (CREA) (μmol/L), lactic acid (LAC) (mmol/L), albumin (ALB) (g/L), total bilirubin (TBIL) (μmol/L), alanine aminotransferase (ALT) (U/L), aspartate aminotransferase (AST) (U/L), procalcitonin (PCT) (ng/L), mechanical ventilation time (h), duration of antibiotic use (h), length of stay in ICU (day), driving pressure (mmHg), mechanical kinetic energy (J), CRP/ALB and mean arterial pressure (MAP) (mmHg), sequential organ failure assessment (SOFA) (19) and acute physiology and chronic health evaluation II (APACHE II) (20), of which mechanical kinetic energy, MAP, SOFA and APACHE II are the worst value within 24 h of admission. To ensure data accuracy and completeness, we used double-checking procedures.
In this study, a dual confirmation process was used for the diagnosis of depressive disorders. First, use the Zung Self-Rating Depression Scale (SDS) for screening (a standard score of ≥ 50 indicates a positive screening result) (21). Subsequently, all screened positive individuals were independently clinically evaluated by specialized physicians in the hospital’s psychological outpatient department. The final diagnosis is made only when both the screening results of the equivalent scale and the clinical judgment of the specialist support a depressive disorder. The basis for determining type 2 diabetes in this study is meeting the following two criteria. First, the patient must have an admission glycated hemoglobin (HbA1c) ≥ 6.5%. Second, a type 2 diabetes diagnosis clearly recorded in the admission history: (a) Must have a clear diagnosis made by a specialist endocrinologist from a second- or higher-level hospital in the past; (b) The medical history should include specific diagnosis dates, treatment plans (such as records of oral hypoglycemic drugs/insulin use), or previous blood glucose monitoring results.
The mechanical kinetic energy (MKE) was the ventilator-derived energy delivered to the respiratory system each minute, with conceptual grounding in the mechanical power theory, as described in (22). It was therefore possible to introduce a valid, though simplified, approximation, enhancing its clinical feasibility (23):
Where RR is respiratory rate (unit: breaths/min); VT is tidal volume (unit: L); Ppeak is peak inspiratory pressure (unit: cmH₂O); Pplat is plateau pressure (unit: cmH₂O); and PEEP is positive end-expiratory pressure (unit: cmH₂O). The value 0.098 is a conversion constant from cmH₂O·L/min to joules per minute (J/min). This approximation has been shown to correlate well with the complete mechanical power model and represents the total mechanical energy load applied to the lung.
Statistical analysis
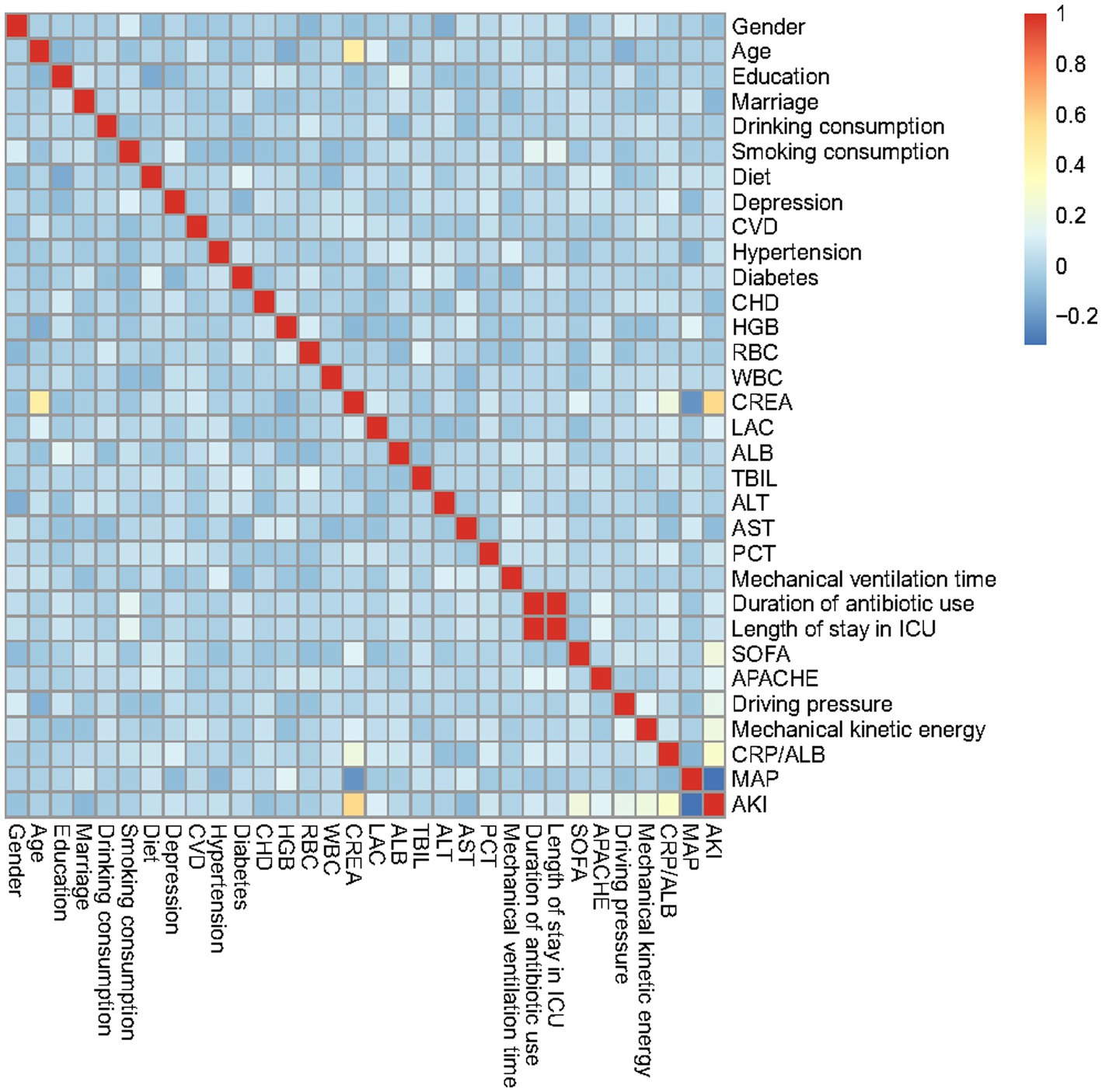
The association between the incidence rate of SPRMV and 32 potential risk factors or related diseases was examined using R software. For continuous clinical variables, baseline is expressed as the mean (standard deviation), and the p-value is calculated using a t-test. Furthermore, categorical variables are displayed as numbers (N) or proportions (%). For large samples (N ≥ 40), the chi-square test is chosen as the testing method; otherwise, Fisher’s exact test is used. Afterwards, univariate logistic regression analysis was used to analyze the recorded clinical data to control for confounding factors. We further utilize multiple logistic regression analysis to explore the association between the obtained independent risk factors and SPRMV. Besides, we conducted a correlation analysis of independent features using the function “cor()” and obtained a heatmap of the correlation matrix, which displays a graphical representation of the strength and direction of relationships between features in a dataset using color coding. According to the Pearson method, the correlation coefficient displayed in each cell of the matrix ranges from −1 to 1 (24). Furthermore, an advanced nomogram was developed to illustrate the risk of AKI in SPRMV elderly patients.
The discrimination of our predictive model is evaluated using receiver operating characteristic (ROC) curve analysis; the larger the area under the ROC (AUC), the better the discrimination ability. To evaluate the accuracy and clinical effectiveness of our regression model, we utilized a calibration curve and decision curve analysis (DCA).
All the establishment of clinical predictive model and analysis were used with R version 4.4.2 (http://www.r-project.org, R Foundation for Statistical Computing). For baseline characteristics, we utilized the “tableone” package and obtained the table based on both the “flextable” and “officer” packages. To establish the linear regression model, both the “rms” and “regplot” packages were used to plot the advanced nomogram for subsequent analysis. Furthermore, we plotted ROC and DCA curves based on the “pROC” and “rmda” packages, respectively. A two-bilateral p value less than 0.05 was considered statistically significant.
Results
Participant baseline characteristics
In the current study, detailed baseline information of the socio-demographic and clinicopathologic characteristics of both the training and validation datasets is presented in Table 1. We recorded 504 SPRMV patients across two hospitals, including 300 AKI and 204 non-AKI patients, as shown in Supplementary Table S1. For the training dataset, there were a total of 353 SPRMV patients with non-AKI (N = 142, 40.2%) and AKI (N = 211, 59.8%), consisting of 223 male patients (63.2%) and 130 female patients (36.8%), and the mean (SD) age was 80.92 ± 7.54 in AKI patients and 81.23 ± 7.69 in non-AKI patients. For the validation dataset, with a total of 151 SPRMV patients with non-AKI (N = 62, 41.1%) and AKI (N = 89, 58.9%), with 90 male patients (59.6%) and 61 female patients (40.4%), the mean (SD) age was 80.33 ± 7.80 in AKI patients and 81.60 ± 7.99 in non-AKI patients, as shown in Table 1 and Supplementary Table S2.
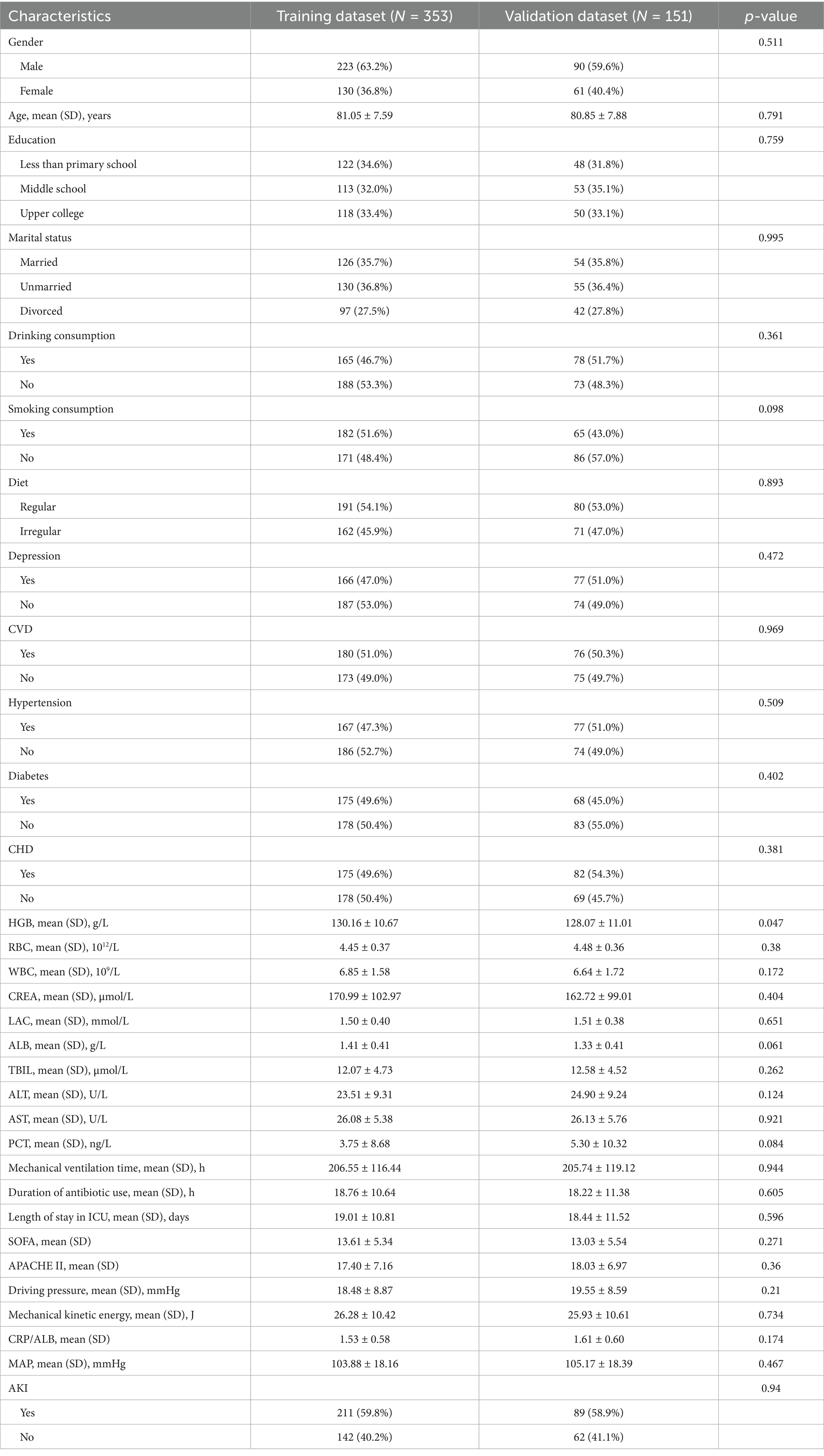
Table 1. Baseline characteristics of SPRMV elderly patients based on training dataset and validation dataset.
To present the model development steps clearly, we calculated all characteristics and their variance inflation factors (VIFs) to address multicollinearity, as shown in Supplementary Tables S3, S4. Since the VIF values for the characteristics are all less than 10, except for duration of antibiotic use and length of stay in ICU. As shown in the following text, these two characteristics did not participate in the construction of the clinical model in this article. Hence, there is no multicollinearity problem in the subsequent model construction.
Correlation heatmap of predictive characteristics in the training dataset
Correlation analysis was conducted on the predicted features in the training dataset based on the Pearson method, including gender, age, education, marital status, drinking consumption, and smoking.
consumption, diet, depression, CVD, hypertension, diabetes, CHD, HGB, RBC, WBC, CREA, LAC, ALB, TBIL, ALT, AST, PCT, mechanical ventilation time, duration of antibiotic use, length of stay in ICU, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, MAP, and AKI, as depicted in Figure 1. In the relevant heatmap, blue and red boxes represent negative and positive correlations, respectively. For example, the duration of antibiotic use is significantly positively correlated with ICU stay, while CREA is negatively correlated with MAP.
Univariate and multivariate logistic analysis of SPRMV risk factors
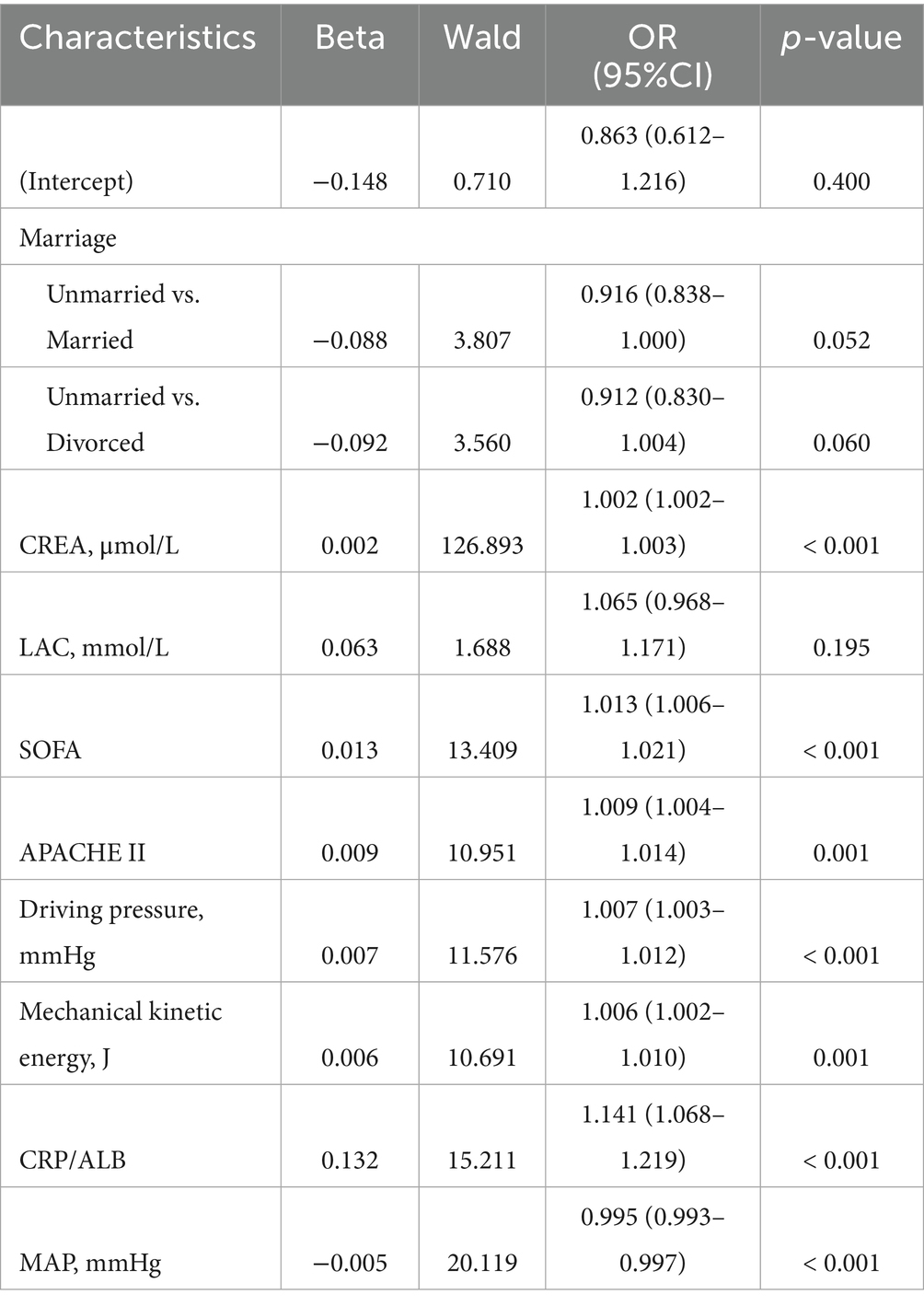
In our univariate logistic regression analysis, a total of 9 potential risk factors of SPRMV in the training dataset showed statistically significant results: marriage (unmarried vs. divorced), CREA, LAC, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, and MAP (p < 0.05), as shown in Table 2. Variables analyzed by the univariate logistic regression method were entered into the multivariate logistic regression model based on clinical expertise combined with statistical significance.
Multivariate logistic regression analysis further revealed that CREA (OR = 1.002, 95%CI = 1.002–1.003), SOFA (OR = 1.013, 95%CI = 1.006–1.021), APACHE II (OR = 1.009, 95%CI = 1.004–1.014), driving pressure (OR = 1.007, 95%CI = 1.003–1.012), mechanical kinetic energy (OR = 1.006, 95%CI = 1.002–1.010), CRP/ALB (OR = 1.141, 95%CI = 1.068–1.219) and MAP (OR = 0.995, 95%CI = 0.993–0.997) are independent risk factors of SPRMV (p < 0.05) in the elderly patients with AKI, as presented in Table 3. According to the results of multivariate logistic regression analysis, the Odds Ratios (ORs) for continuous variables like CREA (OR = 1.002) and MAP (OR = 0.995) are very close to 1. While statistically significant, their clinical interpretation is challenging. Hence, we presented the OR for a clinically relevant increment (e.g., OR for a 50-unit increase in CREA by dividing the original data by 50, or a 10-unit decrease in MAP by multiplying the original data by 10) to make the results more interpretable for clinicians, as shown in Supplementary Table S5. As depicted in Supplementary Table S5, CREA for a 50-unit increase (OR = 1.117, 95%CI = 1.095–1.138) and MAP for a 10-unit decrease (OR = 1, 95%CI = 0.999–1) are also independent risk factors of SPRMV (p < 0.05) in the elderly patients with AKI. However, the regression coefficient for MAP with a 10-unit decrease is zero, and it has no impact on the modified statistical logistic model.
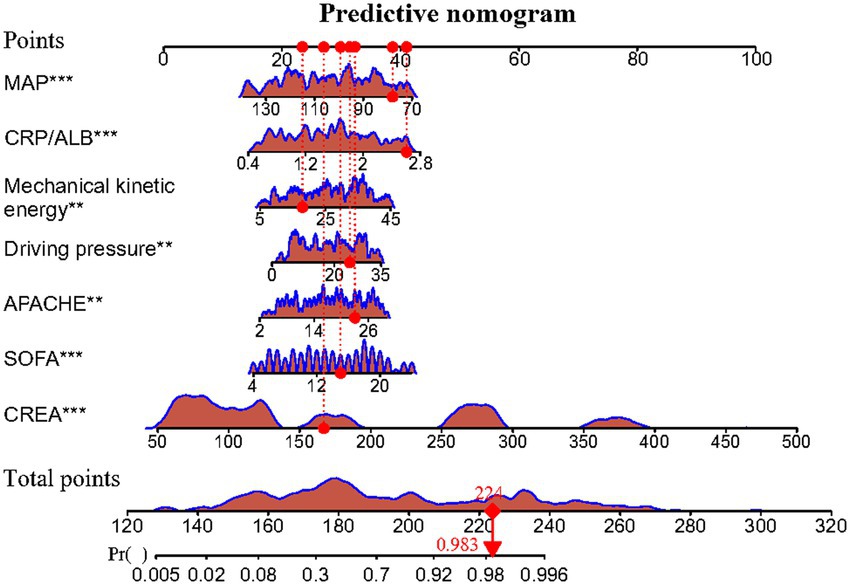
Meanwhile, the predictive model was presented as an advanced nomogram, which constructed CREA, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, and MAP based on the aforementioned relevant indicators, as shown in Figure 2. Our advanced nomogram provides a visual representation of the impact of each factor, helping doctors conduct individualized risk evaluations in clinical practice. In addition to the explanation methods in the advanced nomogram in Figure 2, we also gave another example. For example, if an SPRMV patient has CREA (200 μmol/L), SOFA (14), APACHE II (30), driving pressure (20 mmHg), mechanical kinetic energy (25 J), CRP/ALB (2.4), and MAP (140 mmHg), then the SPRMV patient’s corresponding scores would be about 34, 12, 19, 10, 10, 22, and 0, respectively, with a total score of 107. These results would indicate that the estimated probability of SPRMV in the elderly patients with AKI is approximately 91%. Meanwhile, we offered a step-by-step guide in our advanced Figure 2 nomogram. An SPRMV patient with CREA of 170 μmol/L, SOFA of 15, APACHE II of 23, driving pressure of 26 mmHg, mechanical kinetic energy of 18, CRP/ALB of 2.5, and MAP of 81 mmHg, respectively, with a total score of 224. This result would indicate that the estimated probability of SPRMV in the elderly patients with AKI is approximately 98.3%.
Validation and calibration of predictive model
As shown in Figures 3A,B, the predictive model generated an AUC of 0.920 (95%: 0.892–0.948) with a specificity of 0.993 and sensitivity of 0.763, and an AUC value of 0.938 (95%CI = 0.899–0.977) with a specificity of 0.952 and sensitivity of 0.854 in the validation dataset. The AUC in validation (0.938) has a higher score than in training (0.920), indicating that our predictive model has a good effect. Calibration curves for the advanced nomogram, based on our multivariate logistic regression analysis across the training and validation datasets, are shown in Figures 4A,B, revealing good agreement between predicted and observed outcomes. In clinical practice, calibration curves are commonly used to evaluate and optimize predictive models, such as the probability of postoperative complications in SPRMV in elderly patients with AKI. By comparing the predicted probability with the actual occurrence probability, doctors can develop more accurate postoperative monitoring and intervention plans. Furthermore, DCA across the training and validation datasets indicates that our predictive model consistently outperforms the two extreme strategies (all treatment and no treatment) in terms of net benefits over a wide range of threshold probabilities, representing its potential clinical capabilities, as shown in Figures 5A,B. According to DCA, doctors can choose the most appropriate intervention threshold based on changes in net income. This helps to avoid excessive or inappropriate interventions and improve the quality of medical decision-making for SPRMV in elderly patients with AKI. The good calibration curve and DCA indicate that our predictive model has good calibration, clinical application, and generalizability.
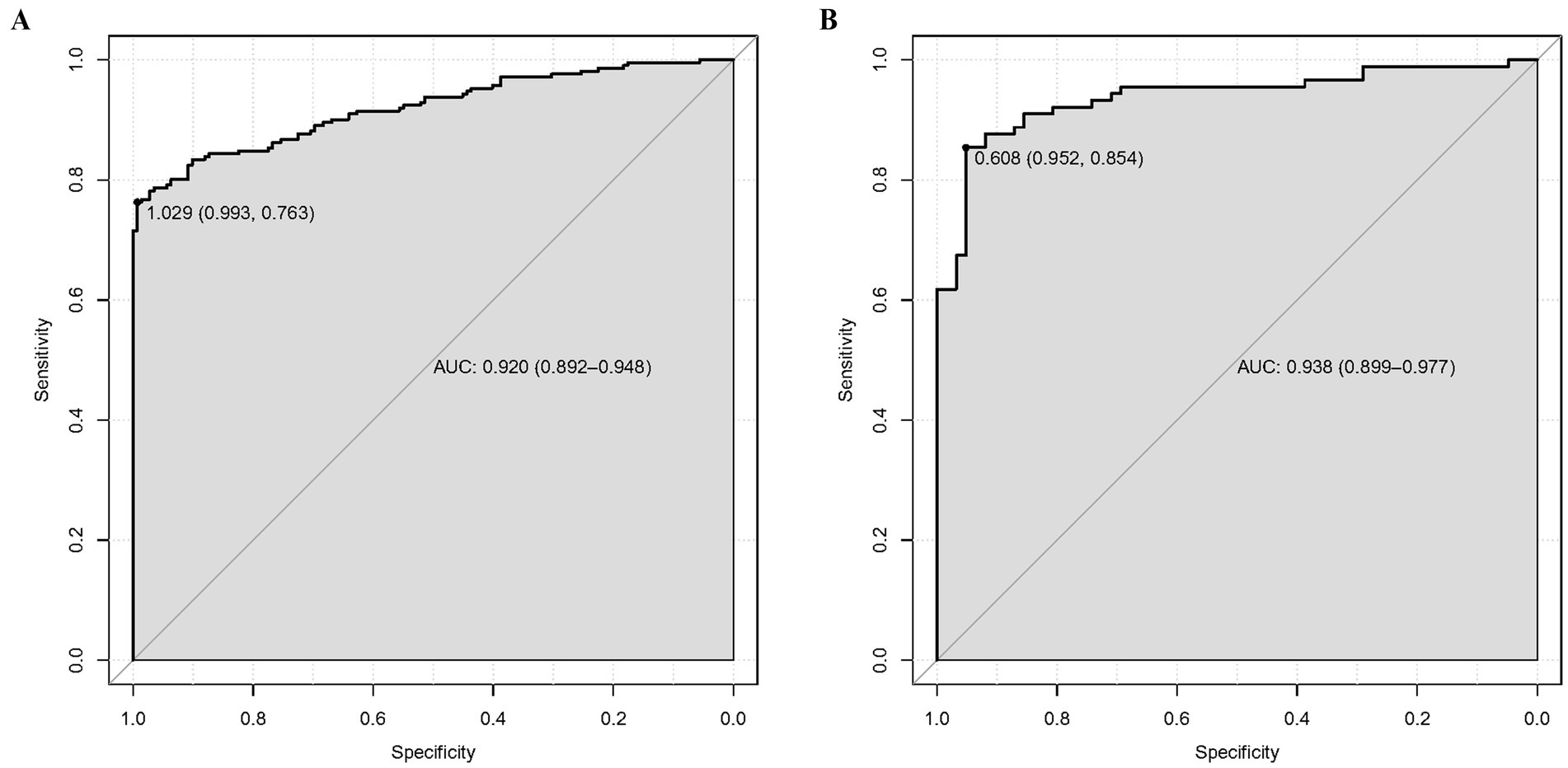
Figure 3. Receiver operating characteristic curve (ROC) of our predictive model. (A) Training dataset. (B) Validation dataset.

Figure 4. Calibrate the curve of our predictive model. (A) Training dataset. (B) Validation dataset.
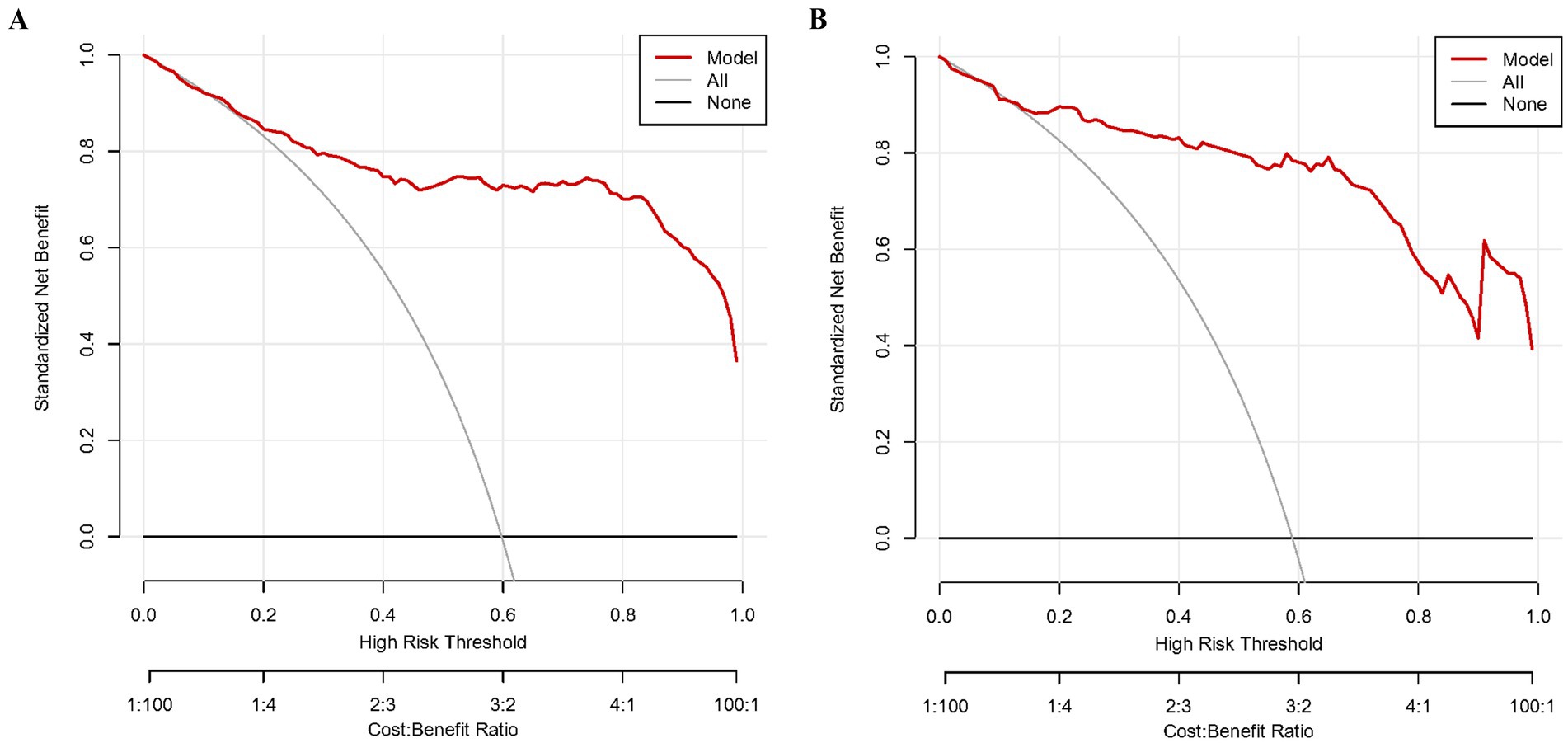
Figure 5. Decision curve analysis (DCA) of our predictive model. (A) Training dataset. (B) Validation dataset.
Discussion
Our multi-center retrospective analysis developed a clinical predictive model for AKI in elderly patients with SPRMV using a multivariate logistic regression algorithm with clinical data from two ICUs (Hefei Second People’s Hospital and the First Affiliated Hospital of Anhui Medical University), and our predictive model is the first research on AKI in elderly patients with SPRMV. For baseline results, only the baseline HGB differed significantly between the two centers (p = 0.047), suggesting good inter-hospital consistency and generalizability of the model. Univariate and multivariate logistic regression analyses identified CREA, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, and MAP as independent factors associated with AKI in SPRMV elderly patients (p 0.05). AKI remains a frequent and severe complication in this population, largely reflecting the combined impact of systemic inflammation, sepsis, hemodynamic instability, and the physiological burden of mechanical ventilation. These interacting factors jointly impair renal perfusion and function in elderly patients with reduced cardiovascular reserve and comorbidities (25–28).
Among these variables, serum creatinine (CREA) plays a central role as a sensitive marker of renal dysfunction. Elevated CREA reflects compromised glomerular filtration and is strongly associated with AKI incidence and poor outcomes, including prolonged ventilation, extended ICU stay, and increased mortality (29–35). Therefore, continuous monitoring of CREA remains crucial for early AKI detection and for guiding fluid management, nephrotoxin avoidance, and renal-protective interventions in this vulnerable group.
The SOFA score is, therefore, an important prognostic and monitoring tool in elderly patients with SPRMV. It reflects the degree of multi-organ dysfunction in these patients and has very strong predictive value for outcome (36). In these vulnerable patients, severe pneumonia is generally associated with systemic inflammation and sepsis, which may cause failure of organs other than the lungs (37). The SOFA score evaluates organ dysfunction in six systems (respiratory, cardiovascular, liver, coagulation, kidney, and neurological), which is crucial for diagnostic work (38). Therefore, currently, it precisely reflects the overall physiological damage. Elderly people are very sensitive to such damage due to reduced physiological reserves, comorbidities, and immune failure. In these patients, pneumonia often rapidly leads to multiple organ failure, resulting in high SOFA scores (39). A very high SOFA score (≥ 11) upon admission or an increase within 48–72 h in the ICU is a clear independent predictor of extremely high mortality, prolonged ventilation dependence, prolonged ICU stay, and high resource utilization, while a decrease in SOFA score indicates good treatment response (40). The renal dysfunction component in the SOFA score is directly associated with the occurrence of AKI (41). Our study shows that an increase in SOFA score is positively correlated with the incidence of AKI. Therefore, monitoring changes in the SOFA score can not only assess patients’ multiple organ dysfunction, but also provide valuable information for early identification of AKI (42). The application of this indicator can help clinical doctors adjust treatment plans in a timely manner, thereby reducing the risk of further deterioration in kidney function.
The APACHE II score is highly correlated with severe SPRMV in elderly patients. It is a critical illness score based on acute physiological changes, age, and chronic health status upon ICU admission (43). In this high-risk group of elderly patients requiring mechanical ventilation, an increase in APACHE II score is significantly correlated with the hospital’s expected mortality rate (44). Therefore, the APACHE II score is of great significance in evaluating initial severity and prognosis, and in discussing treatment intensity and nursing goals with family members, helping to benchmark ICU performance and stratify clinical research interventions for severe pneumonia (45). Although not the best single predictor, this synthesized APACHE II score provides critical prognostic information: the greater the physiological damage in this group of patients, the more susceptible they are to impact and the lower the expected outcome (46). Meanwhile, some parameters included in APACHE II, such as age, cardiovascular function, and respiratory function, are also closely related to renal function (47). Therefore, the increase in APACHE II score not only reflects the aggravation of multi-organ dysfunction, but may also indicate the risk of AKI occurrence (48).
Driving pressure is the difference between platform pressure and positive end-expiratory pressure (PEEP), a key determinant of ventilation management for SPRMV patients, especially elderly patients (49). Considering the impact of age and disease on lung physiology, this value is the most vulnerable threshold for mechanical ventilation-induced lung injury. Increased driving pressure directly indicates changes in global lung stress and strain during tidal ventilation (50). High driving pressure can cause significant regional stress in rigid lung lesions in elderly patients without requiring excessive ventilation (51). This significantly enhances the mechanism of mechanical ventilation-induced lung injury, including barotrauma, volumetric injury, and atelectasis. Therefore, driving pressure has become a top priority for monitoring and minimizing (52). Our research suggests that driving pressure is closely related to renal dysfunction. The reasons may be related to the following factors: (a) High driving pressure and renal hypoxia: High driving pressure may lead to an exacerbation of systemic inflammatory response, affect renal blood flow perfusion, cause renal hypoxia, and ultimately trigger or worsen AKI (53). Especially in elderly patients, their physiological reserves decrease, and the kidney’s tolerance to hypoxia diminishes (54). (b) Impact of driving pressure on renal fluid balance. High driving pressure may increase the fluid load, thereby increasing the burden on the kidneys (55). Fluid overload can reduce renal blood flow, impair renal excretory function, and exacerbate AKI (56). (c) The necessity of adjusting PEEP: Optimizing PEEP to control driving pressure can not only improve lung ventilation, but also help protect kidney function. Reasonable adjustment of PEEP can reduce lung injury caused by high driving pressure and indirectly lower the risk of AKI (57).
The concept of mechanical kinetic energy, called the energy of moving air, lies at the center of elderly patients with SPRMV. The pneumonia process makes breathing much more inefficient because of reduced lung compliance (making the lungs stiffer) and increased airway resistance due to inflammation and secretions (58). Elderly patients with low muscle strength and reduced physiological reserve are particularly sensitive to this increased energy consumption demand, which can easily lead to respiratory failure (59). Changes in mechanical kinetic energy are also related to acute kidney injury. The possible reasons are as follows: (a) Mechanical kinetic energy and risk of respiratory failure: elderly patients need to spend more mechanical kinetic energy to maintain respiratory function in the process of disease deterioration, which will increase the risk of respiratory failure. Respiratory failure may lead to systemic hypoxia, aggravate kidney injury, and then increase the chance of AKI (60). (b) Impact of gas exchange efficiency: a lack or imbalance of mechanical kinetic energy leads to a decrease in gas exchange efficiency and an increase in complications such as carbon dioxide retention and respiratory acidosis. These metabolic alterations can further compromise renal function, increasing the burden on the kidneys to maintain acid–base homeostasis (61). (c) Ventilator settings and kidney protection: while meeting the demand for mechanical kinetic energy, ventilator parameters should be carefully set to avoid double damage to the lung and kidney (62). Appropriate ventilation mode and setting can help maintain renal function and reduce the incidence of AKI (63). By adjusting the parameters such as reasonable waveform, tidal volume, and respiratory rate, the systemic inflammatory response and potential negative effects on the kidney can be reduced (64). In conclusion, for the ventilation management of elderly patients with SPRMV, monitoring and optimizing driving pressure and mechanical kinetic energy are not only crucial for lung function but also essential for protecting kidney function and reducing the risk of AKI.
CRP/ALB is a very important prognostic biomarker in elderly patients with SPRMV. The ratio reflects two important pathophysiological processes: the level of CRP indicates the degree of systemic inflammatory response syndrome due to infection (65), while the level of ALB reflects nutritional status, hepatic synthetic function, and the degree of an underlying catabolic condition (66). Therefore, high CRP/ALB values reflect a high inflammatory burden associated with physiological deterioration, usually seen in critically ill states (67). Accordingly, increased CRP/ALB at the time of admission or its sustained elevation during an ICU stay is related to increased severity, complications such as acute respiratory distress syndrome, sepsis, and multiple organ dysfunction syndrome, prolonged mechanical ventilation, and longer durations in the ICU and hospitals, with mortality exceedingly high in suspected pneumonia cases among the elderly patients with SPRMV (68). This associate measure, from a clinically available perspective, and its composite marker could stratify risk, predict adverse outcomes, and very likely modulate monitoring and treatment approaches among these clinically fragile and high-risk populations. Increased levels indicate that poorly controlled inflammation and malnutrition/cachexia interact to create a bad prognosis (69). First, the relationship between inflammation and renal function: a high CRP/ALB value in elderly patients with SPRMV not only reflects a high inflammatory burden, but also indicates a potential risk of AKI (67). Chronic low-grade inflammation can induce renal tubular injury, increase the incidence of AKI, and worsen renal function. Therefore, monitoring changes in the CRP/ALB ratio can help clinicians early identify the risk of kidney injury, and then carry out corresponding intervention (70). Second, the impact of nutritional status: a reduction in ALB levels is usually associated with malnutrition, which is an important promoting factor of renal dysfunction. The decrease in ALB levels in elderly patients indicates that albumin synthesis in vivo is inhibited, a finding that is significantly correlated with the decline in renal regeneration ability. Therefore, paying attention to the CRP/ALB ratio not only helps monitor the inflammatory state but also reflects the nutritional status of patients, which is related to the risk of AKI (71). Third, the necessity of a comprehensive intervention strategy: combined with the monitoring of the CRP/ALB ratio, a comprehensive intervention strategy should be adopted for elderly patients with SPRMV to reduce the risk of AKI. This includes improving nutritional status, controlling the inflammatory response, and implementing appropriate fluid management to ultimately improve the prognosis of patients (11).
In elderly patients with SPRMV, adequate MAP must be maintained at all times. These patients have a series of high-risk factors for hemodynamic instability: pre-existing cardiovascular disease, low vascular compliance, and systemic inflammatory response caused by severe pneumonia (72). In this context, mechanical ventilation, especially positive-pressure mechanical ventilation, may reduce venous return and cardiac output, thereby exerting complex effects on mechanisms of blood pressure regulation (73). In general, any MAP level above a certain threshold (at least 65 mmHg) will ensure that the perfusion pressure to important organs, especially the kidney and brain, reaches the minimum standard (74). Insufficient MAP will weaken the tissue oxygen and nutrient supply due to infection, further worsen organ dysfunction, and increase mortality. First, the effect of MAP on renal perfusion: the kidney is highly sensitive to blood flow, and a lack of MAP directly affects the renal perfusion pressure, leading to renal hypoxia and AKI. Therefore, maintaining appropriate MAP is an important measure to protect renal function and reduce the risk of AKI (31). Second, the key to fluid management: in the context of mechanical ventilation, a precise fluid resuscitation strategy is needed to ensure effective hemodynamic support and maintain MAP level, thereby maintaining renal perfusion and function. This implies that during fluid resuscitation, hemodynamics needs to be continuously monitored to ensure patients receive the best support (75). Third, monitoring and intervention for critically ill patients: given the urgent need for appropriate MAP, continuous MAP monitoring and necessary interventions are essential. Through standardized monitoring and targeted intervention, it can maintain organ perfusion and improve prognosis in the setting of severe infection and mechanical ventilation, especially by actively protecting renal function (76). In conclusion, the CRP/ALB ratio and MAP not only play an important role in evaluating the severity and prognosis of elderly patients with SPRMV but are also associated with the occurrence of AKI. Effective monitoring and management of these indicators can guide clinical decision-making and improve patient outcomes and quality of life.
Moreover, our multivariate logistic regression model revealed that CREA, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB ratio, and MAP will be independent risk factors for AKI among SPRMV patients. Even after analysis removes the influence of confounding factors through intervention, it becomes relatively straightforward to view an important determinant of AKI as a complex, multivariate phenomenon in this critically ill population. One variable might act through one or more important interactions with others, thus modifying the net impact on renal outcomes that any of the measured variables could independently be expected to have. For the interaction of hemodynamics and inflammation, a usually low MAP may interact unfavorably with a high CRP/ALB ratio, an inflammatory marker and indicator of systemic oxidative stress (77). Inflammation could interfere with renal autoregulation, thus increasing the kidney’s vulnerability to even brief episodes of relative hypotension. The nephrotoxic effects of inflammation might be lessened by strict hemodynamic management (78). And when it comes to ventilatory-induced kidney injury and severity of illness, the association between the determinants of ventilator-induced lung injury and AKI may be modified by a patient’s pre-existing physiologic reserve. High driving pressure or mechanical kinetic energy deteriorates renal venous congestion and cardiac output; therefore, these parameters would be magnified in those patients with high APACHE II or SOFA scores with greater baseline organ dysfunction and hemodynamic instability (79, 80). Besides, our predictive model can be integrated into clinical practice. Based on our multivariate logistic regression, we outline a possible path for clinical integration, such as embedding the model in the electronic health record (EHR) as a decision-support tool to flag high-risk patients for physician review. Furthermore, we note that effective facilitators include an interface that is easy to use and fits with current clinical practices, whereas significant barriers include concerns over data privacy, regulatory approval (e.g., FDA clearance), and the need for clinical validation. We believe that this addition greatly enhances the practical relevance of our study.
Furthermore, there are certain limitations in our multi-center retrospective analysis. First of all, as a multi-center study, the cohort may not represent broader demographic or geographic populations, and the exclusion of patients with chronic renal disease stage 4–5 will affect pre-existing renal dysfunction, limiting model generalizability. Meanwhile, unmeasured confounding factors may also affect the predictive model, and further research will be carried out in the future. Furthermore, our model does not explicitly mention whether a power calculation was conducted to determine the appropriate sample size. The Events per Variable (EPV) metric is intended to estimate the sample size of a predictive model in the future (26, 81). Second, univariate and multivariate logistic regression are prevailing methods used in our analysis. Furthermore, machine learning algorithms can be used to establish new predictive models with better performance (82). Third, there may be biases in the recording of more effective features of our predictive model, including short follow-up time, serological and histological variability, and histopathological grading, which are influenced by inter-laboratory variability and inter-observer differences. In the future, the standardization and linkage of electronic health records will promote the standardization and interoperability of EHR data across different medical institutions and form a regional or national health information database. This will greatly expand the sample size, improve representativeness, and reduce selection bias. And integrating multi-source data will combine traditional medical data with medical insurance settlement data, death registration data, environmental monitoring data, and even health data generated by wearable devices. This data fusion can more comprehensively describe the individual’s exposure history and health status and provide a new research perspective.
Conclusion
In our multi-center retrospective analysis, we found a relationship between several independent factors (CREA, SOFA, APACHE II, driving pressure, mechanical kinetic energy, CRP/ALB, and MAP) and AKI in SPRMV patients and developed a predictive model to evaluate the clinical diagnosis of SPRMV.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of both the Hefei Second People’s Hospital and the First Affiliated Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. WD: Methodology, Validation, Writing – review & editing. JZ: Conceptualization, Methodology, Project administration, Visualization, Writing – original draft. XF: Conceptualization, Data curation, Visualization, Writing – original draft. KN: Data curation, Writing – review & editing. DM: Visualization, Writing – review & editing. TC: Methodology, Project administration, Writing – review & editing. JL: Methodology, Writing – review & editing. YF: Conceptualization, Methodology, Writing – review & editing. YZ: Conceptualization, Validation, Writing – review & editing. GL: Conceptualization, Writing – review & editing. WW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Anhui Province Traditional Chinese Medicine Inheritance and Innovation Research Project (project no. 2024CCCX073): Clinical Evaluation of the Effect of Sizi Wenfei Decoction on Airway Mucus Hypersecretion and the Expression of IL-33/ST2 Pathway in AECPOD Mechanical Ventilation Patients.
Acknowledgments
We would like to thank the support from the Hefei Second People’s Hospital and First Affiliated Hospital of Anhui Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1685110/full#supplementary-material
References
1. Lanspa, MJ, Jones, BE, Brown, SM, and Dean, NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. (2013) 8:83–90. doi: 10.1002/jhm.1996
2. Jiang, JP, Wang, LL, Hu, YY, Chen, X, Li, P, Zhang, JF, et al. Global emergence of Carbapenem-resistant Hypervirulent Klebsiella pneumoniae driven by an IncFIIK34 KPC-2 plasmid. EBioMedicine. (2025) 113:105627. doi: 10.1016/j.ebiom.2025.105627
3. Zhou, H, Zhu, SP, Li, YJ, Ren, XD, Zhu, YC, Chen, XY, et al. Pre-admission fine particulate matter exposure is associated with invasive pulmonary aspergillosis in patients with severe pneumonia, results from two multicenter cohort studies. EBioMedicine. (2025) 121:105971. doi: 10.1016/j.ebiom.2025.105971
4. Hertoghs, N, Roels, S, Brückner, M, Sadoff, J, Banbury, BL, Akers, NK, et al. Vaccine-induced T cell responses correlate with reduced risk of severe COVID-19 in a placebo-controlled efficacy trial. EBioMedicine. (2025) 117:105809. doi: 10.1016/j.ebiom.2025.105809
5. Wang, Y, Xu, BC, Zhang, P, Li, SY, and Xie, Y. Efficacy and safety of reduning injection for severe pneumonia: a systematic review and meta-analysis. Front Pharmacol. (2025) 16:1591136. doi: 10.3389/fphar.2025.1591136
6. Tie, YF, Liu, H, Zhang, T, Meng, TW, and Liang, Q. Efficacy and safety of modified Xuanbai Chengqi decoction as an adjunctive treatment for severe pneumonia: a systematic review and meta-analysis. Front Pharmacol. (2025) 16:1573025. doi: 10.3389/fphar.2025.1573025
7. Xu, J, Han, MF, Ma, KS, Zhang, T, Wang, RR, Zhang, JJ, et al. Longitudinal relationships between serum ferritin and prognosis among severe community-acquired pneumonia patients. Front Pharmacol. (2025) 16:1556185. doi: 10.3389/fphar.2025.1556185
8. Ramirez, P, Lopez-Ferraz, C, Gordon, M, Gimeno, A, Villarreal, E, Ruiz, J, et al. From starting mechanical ventilation to ventilator-associated pneumonia, choosing the right moment to start antibiotic treatment. Crit Care. (2016) 20:169. doi: 10.1186/s13054-016-1342-1
9. Soliman, IW, Frencken, JF, Peelen, LM, Slooter, AJ, Cremer, OL, Delden, JJ, et al. The predictive value of early acute kidney injury for long-term survival and quality of life of critically ill patients. Crit Care. (2016) 20:242. doi: 10.1186/s13054-016-1416-0
10. Chertow, GM, Burdick, E, Honour, M, Bonventre, JV, and Bates, DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. (2005) 16:3365–70. doi: 10.1681/ASN.2004090740
11. Faubel, S, and Edelstein, CL. Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol. (2016) 12:48–60. doi: 10.1038/nrneph.2015.158
12. Murashima, M, Nishimoto, M, Kokubu, M, Hamano, T, Matsui, M, Eriguchi, M, et al. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci Rep. (2019) 9:20260. doi: 10.1038/s41598-019-56615-4
13. Liu, AB, Tan, B, Yang, P, Tian, N, Li, JK, Wang, SC, et al. The role of inflammatory response and metabolic reprogramming in sepsis-associated acute kidney injury: mechanistic insights and therapeutic potential. Front Immunol. (2024) 15:1487576. doi: 10.3389/fimmu.2024.1487576
14. Li, SL, Wang, F, and Sun, D. The renal microcirculation in chronic kidney disease: novel diagnostic methods and therapeutic perspectives. Cell Biosci. (2021) 11:90. doi: 10.1186/s13578-021-00606-4
15. Seubert, ME, and Goeijenbier, M. Controlled mechanical ventilation in critically ill patients and the potential role of venous bagging in acute kidney injury. J Clin Med. (2024) 13:1504. doi: 10.3390/jcm13051504
16. Pochineni, V, and Rondon-Berrios, H. Electrolyte and acid-base disorders in the renal transplant recipient. Front Med. (2018) 5:261. doi: 10.3389/fmed.2018.00261
17. Kane-Gill, SL, Sileanu, FE, Murugan, R, Trietley, GS, Handler, SM, and Kellum, JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis. (2015) 65:860–9. doi: 10.1053/j.ajkd.2014.10.018
18. Albaschmidt, E, Rosa, S, Muller, J, Husing, P, Daniels, R, Theile, P, et al. Acute kidney injury predicts mortality in very elderly critically-ill patients. Eur J Intern Med. (2024) 127:119–25. doi: 10.1016/j.ejim.2024.05.007
19. Vincent, JL, Moreno, R, Takala, J, Willatts, S, Mendon¸ca, A, Bruining, H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure: on behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine (see contributors to the project in the appendix). Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
20. Gall, JR, Loirat, P, and Alp’erovitch, A. Apache II-A severity of disease classification system. Crit Care Med. (1986) 14:754–5. doi: 10.1097/00003246-198608000-00027
21. Romera, I, Delgado-Cohen, H, Perez, T, Caballero, L, and Gilaberte, I. Factor analysis of the Zung self-rating depression scale in a large sample of patients with major depressive disorder in primary care. BMC Psychiatry. (2008) 8:4. doi: 10.1186/1471-244X-8-4
22. Gattinoni, L, Tonetti, T, Cressoni, M, Cadringher, P, Herrmann, P, Moerer, O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. (2016) 42:1567–75. doi: 10.1007/s00134-016-4505-2
23. Giosa, L, Busana, M, Pasticci, I, Bonifazi, M, Macri, MM, Romitti, F, et al. Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp. (2019) 7:61. doi: 10.1186/s40635-019-0276-8
24. Tiessen, A, Cubedo-Ruiz, EA, and Winkler, R. Improved representation of biological information by using correlation as distance function for heatmap cluster analysis. Am J Plant Sci. (2017) 8:3. doi: 10.4236/ajps.2017.83035
25. Chang, YM, Chou, YT, Kan, WC, and Shiao, CC. Sepsis and acute kidney injury: a review focusing on the bidirectional interplay. Int J Mol Sci. (2022) 23:9159. doi: 10.3390/ijms23169159
26. Geri, G, Ferrer, L, Tran, N, Celi, LA, Jamme, M, Lee, J, et al. Cardio-pulmonary-renal interactions in ICU patients. Role of mechanical ventilation, venous congestion and perfusion deficit on worsening of renal function: insights from the MIMIC-III database. J Crit Care. (2021) 64:100–7. doi: 10.1016/j.jcrc.2021.03.013
27. Lameire, N, and Kellum, JAKDIGO AKI Guideline Work Group. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (part 2). Crit Care. (2013) 17:205. doi: 10.1186/cc11455
28. Acharya, M, Berger, R, and Popov, AF. The role of the ADVanced organ support (ADVOS) system in critically ill patients with multiple organ failure. Artif Organs. (2022) 46:735–46. doi: 10.1111/aor.14188
29. Chen, DW, Yuan, HB, Cao, CC, Liu, ZH, Jiang, LL, Tan, Y, et al. Impact of acute kidney injury on in-hospital outcomes in Chinese patients with community acquired pneumonia. BMC Pulm Med. (2021) 21:143. doi: 10.1186/s12890-021-01511-9
30. Blot, S, Koulenti, D, Dimopoulos, G, Martin, C, Komnos, A, Krueger, WA, et al. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit Care Med. (2014) 42:601–9. doi: 10.1097/01.ccm.0000435665.07446.50
31. Ko, CH, Lan, YW, Chen, YC, Cheng, TT, Yu, SF, Cidem, A, et al. Effects of mean artery pressure and blood pH on survival rate of patients with acute kidney injury combined with acute hypoxic respiratory failure: a retrospective study. Medicina. (2021) 57:1243. doi: 10.3390/medicina57111243
32. Chen, XK, Wang, XT, Honore, PM, Spapen, HD, and Liu, DW. Renal failure in critically ill patients, beware of applying (central venous) pressure on the kidney. Ann Intensive Care. (2018) 8:91. doi: 10.1186/s13613-018-0439-x
33. Elyasi, S, Khalili, H, Dashti-Khavidaki, S, and Mohammadpour, A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. (2012) 68:1243–55. doi: 10.1007/s00228-012-1259-9
34. Pan, SW, Kao, HK, Lien, TC, Chen, YW, Kou, YR, and Wang, JH. Acute kidney injury on ventilator initiation day independently predicts prolonged mechanical ventilation in intensive care unit patients. J Crit Care. (2011) 26:586–92. doi: 10.1016/j.jcrc.2011.04.009
35. Phua, J, Dean, NC, Guo, Q, Kuan, WS, Lim, HF, and Lim, TK. Severe community-acquired pneumonia: timely management measures in the first 24 hours. Crit Care. (2016) 20:237. doi: 10.1186/s13054-016-1414-2
36. Bingold, TM, Lefering, R, Zacharowski, K, Meybohm, P, Waydhas, C, Rosenberger, P, et al. Individual organ failure and concomitant risk of mortality differs according to the type of admission to ICU—a retrospective study of SOFA score of 23,795 patients. PLoS One. (2015) 10:e0134329. doi: 10.1371/journal.pone.0134329
37. Kumar, V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. (2020) 11:1722. doi: 10.3389/fimmu.2020.01722
38. Polkki, A, Pekkarinen, PT, Takala, J, Selander, T, and Reinikainen, M. Association of Sequential Organ Failure Assessment (SOFA) components with mortality. Acta Anaesthesiol Scand. (2022) 66:731–41. doi: 10.1111/aas.14067
39. Ahnert, P, Creutz, P, Horn, K, Schwarzenberger, F, Kiehntopf, M, Hossain, H, et al. Sequential organ failure assessment score is an excellent operationalization of disease severity of adult patients with hospitalized community acquired pneumonia-results from the prospective observational PROGRESS study. Crit Care. (2019) 23:110. doi: 10.1186/s13054-019-2316-x
40. Minne, L, Abu-Hanna, A, and Jonge, E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care. (2008) 12:R161. doi: 10.1186/cc7160
41. Mendonca, A, Vincent, JL, Suter, PM, Moreno, R, Dearden, NM, Antonelli, M, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. (2000) 26:915–21. doi: 10.1007/s001340051281
42. He, Y, Luo, Q, Wang, H, Zheng, Z, Luo, H, and Ooi, OC. Real-time estimated sequential organ failure assessment (SOFA) score with intervals: improved risk monitoring with estimated uncertainty in health condition for patients in intensive care units. Health Inf Sci Syst. (2024) 13:12. doi: 10.1007/s13755-024-00331-5
43. Ho, KM, Lee, KY, Williams, T, Finn, J, Knuiman, M, and Webb, SA. Comparison of acute physiology and chronic health evaluation (APACHE) II score with organ failure scores to predict hospital mortality. Anaesthesia. (2007) 62:466–73. doi: 10.1111/j.1365-2044.2007.04999.x
44. Zhou, XY, Ben, SQ, Chen, HL, and Ni, SS. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int J Infect Dis. (2015) 30:144–7. doi: 10.1016/j.ijid.2014.11.005
45. Pereira, JM, Paiva, JA, and Rello, J. Assessing severity of patients with community-acquired pneumonia. Semin Respir Crit Care Med. (2012) 33:272–83. doi: 10.1055/s-0032-1315639
46. Nielsen, AB, Thorsen-Meyer, HC, Belling, K, Nielsen, AP, Thomas, CE, Chmura, PJ, et al. Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: a retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit Health. (2019) 1:e78–89. doi: 10.1016/S2589-7500(19)30024-X
47. Chen, YC, Hsu, HH, Kao, KC, Fang, JT, and Huang, CC. Outcomes and APACHE II predictions for critically ill patients with acute renal failure requiring dialysis. Ren Fail. (2001) 23:61–70. doi: 10.1081/JDI-100001284
48. Gao, Z, Mu, DW, Guo, L, Li, XM, and Lun, LD. Etiological factors, prognostic assessment, and outcomes of patients with acute kidney injury and multiple organ dysfunction syndrome. Genet Mol Res. (2014) 13:8378–84. doi: 10.4238/2014.October.20.13
49. Liang, GP, and Zhang, ZW. Positive end expiratory pressure titration guided by plateau pressure in chronic obstructive pulmonary disease patients. Clin Respir J. (2018) 12:674–80. doi: 10.1111/crj.12578
50. Tawfik, P, Syed, MKH, Elmufdi, FS, Evans, MD, Dries, DJ, and Marini, JJ. Static and dynamic measurements of compliance and driving pressure: a pilot study. Front Physiol. (2022) 13:773010. doi: 10.3389/fphys.2022.773010
51. Dickson, RP, Erb-Downward, JR, and Huffnagle, GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med. (2014) 2:238–46. doi: 10.1016/S2213-2600(14)70028-1
52. Cruz, FF, Ball, L, Rocco, PRM, and Pelosi, P. Ventilator-induced lung injury during controlled ventilation in patients with acute respiratory distress syndrome: less is probably better. Expert Rev Respir Med. (2018) 12:403–14. doi: 10.1080/17476348.2018.1457954
53. Scholz, H, Boivin, FJ, Schmidt-Ott, KM, Bachmann, S, Eckardt, KU, Scholl, UI, et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat Rev Nephrol. (2021) 17:335–49. doi: 10.1038/s41581-021-00394-7
54. Colloca, G, Santoro, M, and Gambassi, G. Age-related physiologic changes and perioperative management of elderly patients. Surg Oncol. (2010) 19:124–30. doi: 10.1016/j.suronc.2009.11.011
55. Prowle, JR, Echeverri, JE, Ligabo, EV, Ronco, C, and Bellomo, R. Fluid balance and acute kidney injury. Nat Rev Nephrol. (2010) 6:107–15. doi: 10.1038/nrneph.2009.213
56. Prowle, JR, Kirwan, CJ, and Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. (2014) 10:37–47. doi: 10.1038/nrneph.2013.232
57. Bugedo, G, Retamal, J, and Bruhn, A. Driving pressure: a marker of severity, a safety limit, or a goal for mechanical ventilation? Crit Care. (2017) 21:199. doi: 10.1186/s13054-017-1779-x
58. Roquilly, A, Torres, A, Villadangos, JA, Netea, MG, Dickson, R, Becher, B, et al. Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir Med. (2019) 7:710–20. doi: 10.1016/S2213-2600(19)30140-7
59. Desler, C, Hansen, TL, Frederiksen, JB, Marcker, ML, Singh, KK, and Juel Rasmussen, L. Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res. (2012) 2012:192503. doi: 10.1155/2012/192503
60. Bellelli, G, Bruni, A, Malerba, M, Mazzone, A, Aliberti, S, Pesci, A, et al. Geriatric multidimensional assessment for elderly patients with acute respiratory diseases. Eur J Intern Med. (2014) 25:304–11. doi: 10.1016/j.ejim.2014.03.002
61. Panza, L, Piamonti, D, and Palange, P. Pulmonary gas exchange and ventilatory efficiency during exercise in health and diseases. Expert Rev Respir Med. (2024) 18:355–67. doi: 10.1080/17476348.2024.2370447
62. Pannu, N, and Mehta, RL. Effect of mechanical ventilation on the kidney. Best Pract Res Clin Anaesthesiol. (2004) 18:189–203. doi: 10.1016/j.bpa.2003.08.002
63. Kuiper, JW, Groeneveld, AB, Slutsky, AS, and Plotz, FB. Mechanical ventilation and acute renal failure. Crit Care Med. (2005) 33:1408–15. doi: 10.1097/01.ccm.0000165808.30416.ef
64. Gyuraszova, M, Gurecka, R, Babickova, J, and Tothova, L. Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxidative Med Cell Longev. (2020) 2020:1–11. doi: 10.1155/2020/5478708
65. Lindvig, KP, Henriksen, DP, Nielsen, SL, Jensen, TG, Kolmos, HJ, Pedersen, C, et al. How do bacteraemic patients present to the emergency department and what is the diagnostic validity of the clinical parameters; temperature, C-reactive protein and systemic inflammatory response syndrome? Scand J Trauma Resusc Emerg Med. (2014) 22:39. doi: 10.1186/1757-7241-22-39
66. Spinella, R, Sawhney, R, and Jalan, R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. (2016) 10:124–32. doi: 10.1007/s12072-015-9665-6
67. Wang, JJ, Zhao, K, Mao, XH, Zhang, YB, Shao, J, Fan, WH, et al. Relationship between CRP albumin ratio and the mortality in critically ill patients with AKI: a retrospective observational study. Biomed Res Int. (2021) 2021:9957563. doi: 10.1155/2021/9957563
68. Yu, YH, Wu, WW, Dong, YY, and Li, JL. C-reactive protein-to-albumin ratio predicts sepsis and prognosis in patients with severe burn injury. Mediat Inflamm. (2021) 2021:6621101. doi: 10.1155/2021/6621101
69. Huang, XY, Peng, G, Kong, YQ, Cao, XJ, and Zhou, X. The prognostic value of CRP/Alb ratio in predicting overall survival for hepatocellular carcinoma treated with transcatheter intra-arterial therapy combined with molecular-targeted agents and PD-1/PD-L1 inhibitors. J Inflamm Res. (2025) 18:203–17. doi: 10.2147/JIR.S483208
70. Giordano, M, Ciarambino, T, Castellino, P, Cataliotti, A, Malatino, L, Ferrara, N, et al. Long-term effects of moderate protein diet on renal function and low-grade inflammation in older adults with type 2 diabetes and chronic kidney disease. Nutrition. (2014) 30:1045–9. doi: 10.1016/j.nut.2014.03.007
71. Levitt, DG, and Levitt, MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. (2016) 9:229–55. doi: 10.2147/IJGM.S102819
72. Durante, A, Mazzapicchi, A, and Baiardo, RM. Systemic and cardiac microvascular dysfunction in hypertension. Int J Mol Sci. (2024) 25:13294. doi: 10.3390/ijms252413294
73. Kreit, J. Respiratory-cardiovascular interactions during mechanical ventilation: physiology and clinical implications. Compr Physiol. (2022) 12:3425–48. doi: 10.1002/j.2040-4603.2022.tb00219.x
74. Correa, TD, Vuda, M, Takala, J, Djafarzadeh, S, Silva, E, and Jakob, SM. Increasing mean arterial blood pressure in sepsis: effects on fluid balance, vasopressor load and renal function. Crit Care. (2013) 17:R21. doi: 10.1186/cc12495
75. Messina, A, Calatroni, M, Castellani, G, De Rosa, S, Ostermann, M, and Cecconi, M. Understanding fluid dynamics and renal perfusion in acute kidney injury management. J Clin Monit Comput. (2025) 39:73–83. doi: 10.1007/s10877-024-01209-3
76. Pinsky, MR, Cecconi, M, Chew, MS, De Backer, D, Douglas, I, Edwards, M, et al. Effective hemodynamic monitoring. Crit Care. (2022) 26:294. doi: 10.1186/s13054-022-04173-z
77. Cho, AR, Lee, SB, Hong, KW, and Jung, DH. C-reactive protein-to-albumin ratio and 8-year incidence of type 2 diabetes: the Korean genome and epidemiology study. Acta Diabetol. (2021) 58:1525–32. doi: 10.1007/s00592-021-01755-1
78. Post, EH, and Vincent, JL. Renal autoregulation and blood pressure management in circulatory shock. Crit Care. (2018) 22:81. doi: 10.1186/s13054-018-1962-8
79. Smeden, M, Moons, KG, de Groot, JA, Collins, GS, Altman, DG, Eijkemans, MJ, et al. Sample size for binary logistic prediction models: beyond events per variable criteria. Stat Methods Med Res. (2019) 28:2455–74. doi: 10.1177/0962280218784726
80. Vittinghoff, E, and McCulloch, CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. (2007) 165:710–8. doi: 10.1093/aje/kwk052
81. Chapalain, X, Vermeersch, V, Egreteau, PY, Prat, G, Alavi, Z, Vicaut, E, et al. Association between fluid overload and SOFA score kinetics in septic shock patients: a retrospective multicenter study. J Intensive Care. (2019) 7:42. doi: 10.1186/s40560-019-0394-0
Keywords: severe pneumonia requiring mechanical ventilation, acute kidney injury, predictive model, risk factors, nomogram
Citation: Yao L, Ding W, Zhao J, Fang X, Niu K, Ma D, Chen T, Li J, Fu Y, Zhan Y, Ling G and Wang W (2025) Efficacy and validation of a clinical model to predict acute kidney injury in severe pneumonia requiring mechanical ventilation in elderly patients: a multicenter retrospective observational analysis. Front. Med. 12:1685110. doi: 10.3389/fmed.2025.1685110
Edited by:
Pierluigi Marzuillo, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Zhen Wang, Huazhong University of Science and Technology, ChinaHongsheng Wu, Southern Medical University, China
Copyright © 2025 Yao, Ding, Zhao, Fang, Niu, Ma, Chen, Li, Fu, Zhan, Ling and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yao, eWxpcm4xODlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Li Yao1*†
Li Yao1*† Wenjing Ding
Wenjing Ding Xiang Fang
Xiang Fang