Abstract
A 32-year-old female patient visited our hospital due to a solitary firm nodule in the right preauricular area for 4 years. Histopathological manifestations of the skin were nested tumor tissue in the dermis, with polygonal tumor cells, which had abundant cytoplasm filled with eosinophilic granules, and were stained pale pink. Immunohistochemical markers like S-100, vimentin and CD68 were positive. She was diagnosed with cutaneous granular cell tumor and treated by complete resection.
1 Introduction
Granular cell tumor is named for its morphological manifestation of substantial eosinophilic granules contained in the cytoplasm. This rare tumor primarily affects adult women (60%), with a mean age at diagnosis of 45. 8 years (1), typically presenting as solitary, reddish-brown or skin-colored subcutaneous nodules. Although usually asymptomatic and benign, it can cause pain, has a tendency for local recurrence, and may rarely undergo malignant transformation with multiple tumors or distant metastasis. This report of a 32-year-old female with a solitary facial nodule highlights the diagnostic challenge of such lesions and informs the differential diagnosis.
2 Case report
A 32-year-old female patient visited our hospital due to a preauricular nodule on the right face for 4 years and pruritus for 1 week. Four years ago, she developed a rice grain-sized dark red nodule on the right side of her face without obvious cause, which gradually increased to the size of a soybean and turned brown. Feeling no subjective symptoms, she did not seek treatment. One week ago, pruritus appeared, so the patient visited our hospital for treatment. She was previously in good health and had no similar family history. The patient had received no prior treatment. Smoking status, family history, and relevant exposures are unknown; Dermoscopy was not performed. The clinical manifestations, pathological features, and immunological test results of the patient are demonstrated in Figures 1–7.
FIGURE 1

Clinical presentation of a brown, solitary nodule in the right preauricular area.
FIGURE 2
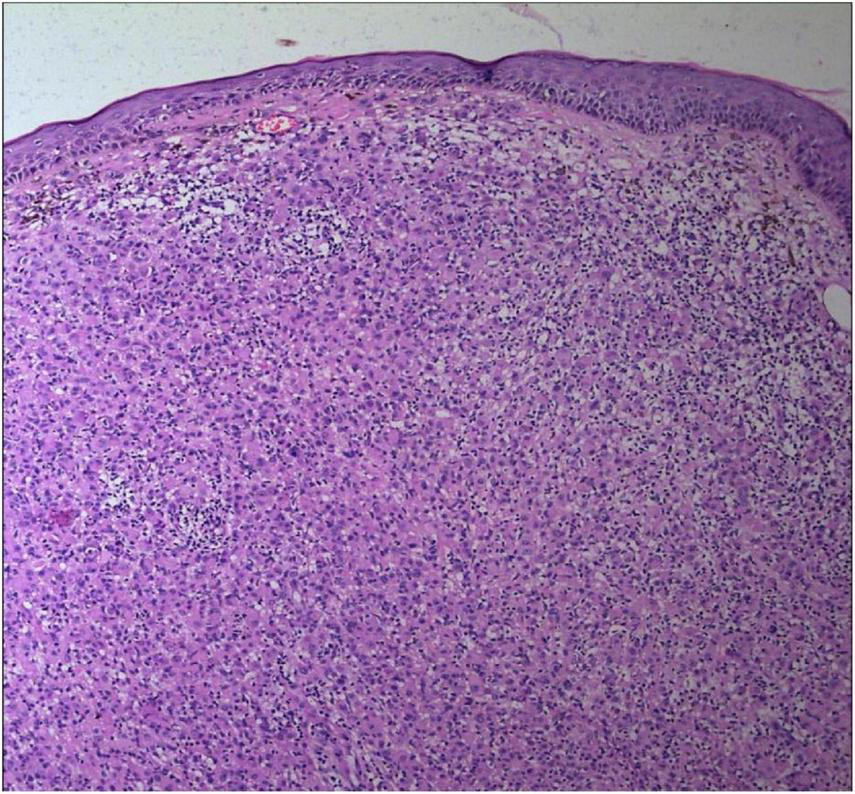
The epidermal processes disappeared, and the dermis was diffusely infiltrated by substantial tumor cells that were in the nested or clumpy form (Hematoxylin and Eosin Stain, × 100).
FIGURE 3

The tumor cells were polygonal, either round or oval, which had abundant cytoplasm filled with eosinophilic granules, and were stained pale pink. The nuclei were small, hyperchromatic and centered, with few mitotic figures, and multiple nuclei were observed in some tumor cells (Hematoxylin and Eosin Stain, × 200).
FIGURE 4

S-100 (+) (Immunohistochemical staining, × 200).
FIGURE 5

Vimentin (+) (Immunohistochemical staining, × 200).
FIGURE 6
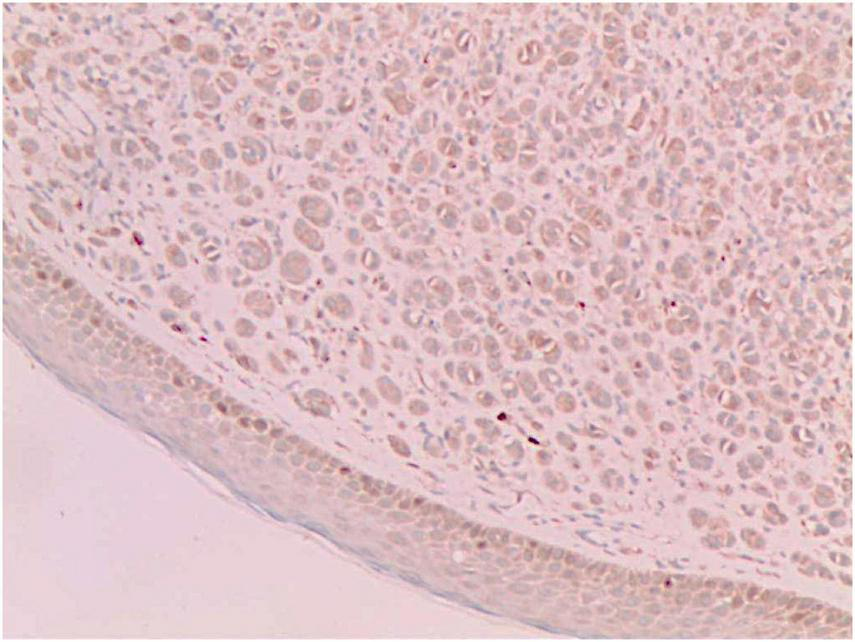
CD68 (+) (Immunohistochemical staining, × 200).
FIGURE 7
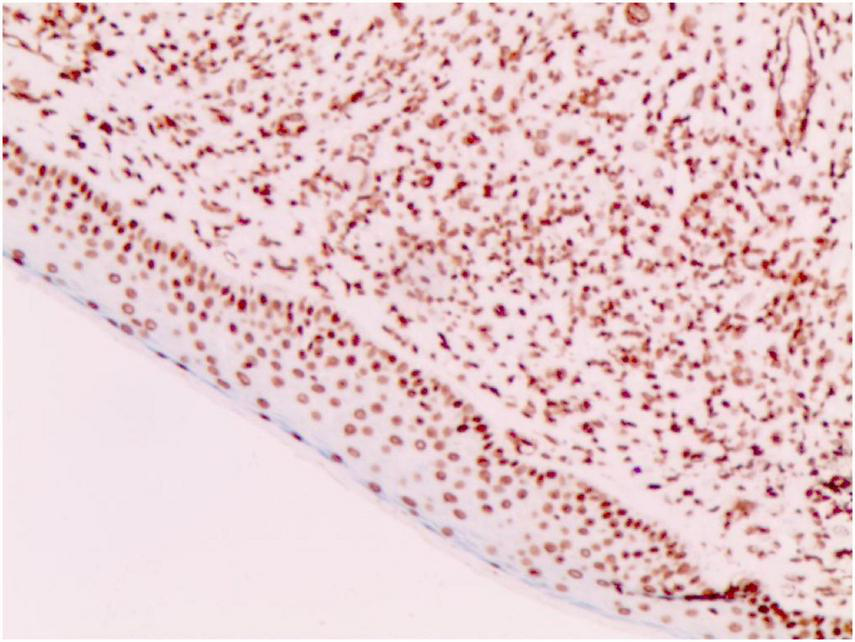
SAM (+ –) (Weakly positive, Immunohistochemical staining, × 200).
2.1 Physical examination
No abnormalities were detected upon systemic examination. Dermatological findings: A soybean-sized brown nodule was visible in the right preauricular area, which had a clear boundary and bulged on the skin surface. The nodule surface was smooth without ulceration, and telangiectasia was seen. The texture was moderate and there was no tenderness.
2.2 Laboratory findings
No abnormalities were found upon routine blood and urine tests, coagulation function test and syphilis/HIV screening. Chest X-ray and abdominal B-ultrasound also revealed no abnormalities. Cutaneous histopathology: epidermal processes disappeared, and the dermis was diffusely infiltrated by substantial tumor cells that were in the nested or clumpy form. These polygonal tumor cells were round or oval, which had abundant cytoplasm filled with eosinophilic granules, and were stained pale pink. The cellular nuclei were small, hyperchromatic and centered, with few mitotic figures, and multiple nuclei were observed in some tumor cells. Immunohistochemistry: S-100 (+), CD56 (−), NSE (−), vimentin (+), CD68 (+), Ki-67 (+), cell proportion: 5%, p53 (−), SAM (+−). CK (−), calponin (−), CD163 (−), desmin (−), CD34 (−), CD45 (−), CD30 (−), SMA (1A4) (−), ALK (−), HMB45 (−), melan-A (−).
The mark “(+)” represents a positive immunohistochemistry result. The mark “( + −) represents a focally/weakly positive immunohistochemistry result. The mark “(−)” represents a negative immunohistochemistry result. The diagnostic significance of these negative immunohistochemical findings lies in their ability to differentiate granular cell tumors from potential mimickers, including gastrointestinal stromal tumors, smooth muscle tumors, anaplastic large cell lymphoma, and melanoma.
2.3 Diagnosis
Cutaneous granular cell tumor.
2.3.1 Cutaneous lesion characteristics
A soybean-sized brown nodule was visible in the right preauricular area.
2.3.2 The histopathological features
The dermis was diffusely infiltrated by substantial tumor cells that were in the nested or clumpy form. These polygonal tumor cells were round or oval, which had abundant cytoplasm filled with eosinophilic granules.
2.3.3 The immunohistochemical results
S-100 (+), vimentin (+), CD68 (+), Ki-67 (+), cell proportion: 5%, SAM (+ −). Immunohistochemistry rules out melanoma, tumors of lymphoid origin, smooth muscle tumors, keratoacanthoma, SCC, xanthoma, and dermatofibroma, etc.
2.4 Treatment and prognosis
Complete excision of the mass, undertaken under local infiltration anesthesia, was achieved with a circumferential margin of 0. 5 cm. The long-term prognosis remains to be determined through ongoing follow-upNo recurrence was found during the 1-year follow-up, and the follow-up is ongoing.
This case report adheres to the CARE guidelines.
3 Discussion
As a rare tumor formerly known as granular cell myoblastoma, the granular cell tumor (GCT) was first reported by Abrikossoff in 1926 (2), which is named for the granular shape of tumor cells. Granular cell tumors most frequently affect women between 40 and 60 years of age and are common on the head and limbs, but reports of their occurrence on facial skin are rare. We report a case of a solitary GCT on the face of a 32-year-old female. The nodule was clinically notable for its potential to be misdiagnosed as an epidermal cyst, adnexal neoplasm, melanoma, dermatofibroma, or basal cell carcinoma. This case underscores the importance of considering GCT in the differential diagnosis of solitary facial nodules. GCTs occurring in the skin are mostly painless skin nodules, and the patients are generally asymptomatic.
Granular cell tumors most frequently occur in the mucosa, breast, and skin, with a relatively high incidence in the digestive tract. The esophagus is the most commonly affected site, while in the oral cavity, the tongue high nce on facial skin are rare. We report a case of a solitary GCT on the face of a 32-year-oldlso be found in the floor of the mouth, upper lip, and lower lip. Most tumors present as solitary, painless, yellow or pink nodules on the anterior tongue (3). Unlike granular cell tumors in other sites, S-100-negative granular cell tumors have been identified in the oral cavity, which are considered to be of non-neural origin (4).
The majority of GCTs are benign, while aggressive malignant GCTs account for only 1%–he majority of GCTs are benign (5), which are characterized by nuclear pleomorphism, tumor cell spindling, vesicular nuclei with large nucleoli, increased nuclear-to-cytoplasmic ratio, necrosis, and increased mitotic rate (>2 mitoses/10HPF) (1). Ki-67, a nuclear antigen specifically expressed in proliferating cells, serves as a proliferation index. Its expression level differs significantly between benign and malignant soft tissue tumors (6). A Ki-67 index of <10% indicates that most tumor cells are quiescent with slow proliferation. Some authors have demonstrated that the upregulation of both p53 and Ki-67 is well correlated with an aggressive clinical course and malignant behavior in granular cell tumors. The origin of GCT remains controversial, with most research results implying its derivation from the neural tissue or Schwann cells (7). In this case, the tumor was positive for S-100, indicating a neural origin. Nevertheless, there are also case reports of non-neuronal GCTs of the skin (8, 9). Compared to the traditional GCTs, the non-neuronal type lacks the expression of S-100 and can show stronger nuclear atypia and mitotic activity.
Diagnosis of GCTs is based primarily on the histopathological examination. Regarding the histopathological features of GCTs, the lesions are located mainly in the dermal, subcutaneous or submucosal tissue, and the tumor cells mostly exhibit banded or nested growth. The tumor cells are large, polygonal and filled with fine eosinophilic granules in the cytoplasm. The cellular nuclei are small and centered, which are either round or oval. Common immunohistochemical markers: S-100 protein (+), NSE (+), CD68 (+), CK (−), SMA (−), GFAP (−), etc.
The skin lesion of the patient in this report was a solitary painless firm nodule on the face. Base on the histopathological findings and immunohistochemical results, she was diagnosed with granular cell tumor. Clinically, this disease needs to be differentiated from keratoacanthoma, nodular squamous cell carcinoma, nodular xanthoma, dermatofibroma, nodular basal cell carcinoma, malignant granular cell tumor, rhabdomyomas and leiomyomas. Currently, GCTs are treated chiefly by surgical resection, and the prognosis is good when no residual tumor cells are left at the surgical margin, although close follow-up is still required.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JS: Writing – original draft, Investigation. XH: Resources, Writing – original draft. LZ: Methodology, Writing – review & editing. YC: Methodology, Writing – review & editing. CH: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Berger O Arnon O Lomeli BA Ofek N Talisman R . Granular Cell Tumor Initially Masquerading as Cellulitis.Plast Reconstr Surg Glob Open. (2024) 12:e5992. 10.1097/GOX.0000000000005992
2.
Rehan S Paracha H Masood R Wang R . Granular cell tumor of the abdominal wall, a case report and review of literature.AME Case Rep. (2021) 5:28. 10.21037/acr-20-160
3.
van de Loo S Thunnissen E Postmus P van der Waal I . Granular cell tumor of the oral cavity; a case series including a case of metachronous occurrence in the tongue and the lung.Med Oral Patol Oral Cir Bucal. (2015) 20:e30–3. 10.4317/medoral.19867
4.
Rawal YB Dodson TB . S-100 negative granular cell tumor (so-called primitive polypoid non-neural granular cell tumor) of the oral cavity.Head Neck Pathol. (2017) 11:404–12. 10.1007/s12105-016-0760-3
5.
Gayen T Das A Shome K Bandyopadhyay D Das D Saha A . Granular cell tumor: an uncommon benign neoplasm.Indian J Dermatol. (2015) 60:322. 10.4103/0019-5154.156453
6.
Yang C Zhang J Ding M Xu K Li L Mao L et al Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. (2018) 20:570–5. 10.1007/s12094-017-1774-3
7.
Houcine Y Mlika M Moussa C Rouis H Brahem E Ismail O et al Granular cell tumor of the lung and tracheobronchial tree: two case-presentation with a review of the literature. Rare Tumors. (2023) 15:20363613231187822. 10.1177/20363613231187822
8.
El Ochi MR Essaoudi A Allaoui M Abrid JE Touri S Moussaoui N et al Dermal nonneural granular cell tumor: a case report. J Surg Case Rep. (2022) 2022:rjac317. 10.1093/jscr/rjac317
9.
Kim NY Jang JW Huh YJ Ro YS Paik SS Ko JY . Dermatofibroma-like dermal non-neural granular cell tumor.J Cutan Pathol. (2023) 50:316–20. 10.1111/cup.14336
Summary
Keywords
granular cell tumor, cutaneous, face, preauricular, immunohistochemistry, S-100 protein, CD68, dermatopathology
Citation
Song J, Han X, Zhao L, Cheng Y and Hu C (2025) Cutaneous granular cell tumor presenting as a solitary preauricular nodule: a case report. Front. Med. 12:1689453. doi: 10.3389/fmed.2025.1689453
Received
12 September 2025
Accepted
28 October 2025
Published
17 November 2025
Volume
12 - 2025
Edited by
Sebastian Yu, Kaohsiung Chang Gung Memorial Hospital, Taiwan
Reviewed by
Ori Berger, Barzilai Medical Center, Israel
Ciniraj Raveendran, Government Medical College, India
Updates
Copyright
© 2025 Song, Han, Zhao, Cheng and Hu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caixia Hu, 48201485@hebmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.