Abstract
Background:
Graft-versus-host disease (GVHD) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation that impairs clinical outcomes. Existing classification systems for GVHD biomarkers remain fragmented, which limits cross-study data integration and clinical translation, creating an urgent need for a systematic classification framework.
Materials and methods:
In this review, a predefined search strategy was used to systematically evaluate the classification systems of GVHD biomarkers. For the search, a systematic literature retrieval was conducted in the PubMed and Web of Science databases, covering the time range from 2012 to 2025, with keywords including “GVHD,” “biomarkers,” and “classification and summarization.” The inclusion criteria for studies were as follows, focusing on the classification or clinical application of GVHD biomarkers: peer-reviewed original articles, reviews, or multicenter trials, and human subjects or well-validated mouse models. After screening, a total of 139 articles were included in this review.
Conclusion:
This review integrates GVHD biomarkers into a three-dimensional system, including pathophysiological mechanisms, clinical application scenarios, and molecular characteristics. It identifies key challenges in biomarker research and application, and proposes feasible integration pathways. This work provides a foundational framework for precision medicine in GVHD management.
1 Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) usually serves as a primary therapeutic modality for hematological diseases; however, graft-versus-host disease (GVHD) remains a major barrier to improving treatment outcomes (1). Biomarkers, which function as indicators of normal physiological processes, pathological conditions, or responses to interventions such as exposure, are widely applied in disease diagnosis, monitoring, and the development of therapeutic approaches (2). They have become critical tools for guiding GVHD diagnosis, prognostic stratification, and treatment response monitoring, yet inconsistencies in their classification severely compromise clinical utility (3). For instance, interleukin-6 (IL-6) is defined as an inflammation-driven marker in preclinical mechanistic studies of GVHD; in clinical cohort studies, however, it is classified as a diagnostic marker for ocular graft-versus-host disease (oGVHD)—such classification discrepancies impede the integration of cross-study data.
Although numerous reviews on GVHD biomarkers have been published, most focus on individual molecules or single application scenarios and fail to address the core issue of “fragmented classification” by proposing targeted solutions. This research gap limits the translation of biomarker research into standardized clinical practice, as clinicians lack a unified framework for interpreting and applying these biomarkers (4). Therefore, by systematically synthesizing existing evidence, the present review aims to describe a coherent, multi-dimensional classification system for GVHD biomarkers, with the goal of resolving classification inconsistencies and facilitating their reliable application in clinical decision-making.
2 An overview of the pathogenesis of GVHD
2.1 Acute GVHD (aGVHD): innate immunity-driven inflammatory cascade
The pathogenic process of aGVHD is centered on a three-stage “initiation-activation-effector” cascade, which proceeds as follows (5) (Figure 1):
-
Initiation stage: pretransplant conditioning induces tissue damage in recipients, triggering the release of damage-associated molecular patterns (DAMPs) (6) that subsequently activate antigen-presenting cells (APCs).
-
Activation stage: activated APCs present recipient alloantigens, which drive the differentiation of donor naive T cells into effector T cell subsets, such as T-helper 1 (Th1) and T-helper 17 (Th17) cells. Notably, interleukin-12 (IL-12) secreted by APCs during this stage acts as a biomarker for early inflammatory activation in aGVHD.
-
Effector stage: effector T cells, along with cytokines interferon-γ (IFN-γ) (7), tumor necrosis factor-α (TNF-α), secrete and infiltrate target organs, including the intestine, skin, and liver, and mediate tissue damage. Among these, regenerating islet-derived protein 3α (REG3α)—which is elevated upon intestinal epithelial injury—and Elafin—associated with skin injury—serve as specific tissue damage biomarkers for intestinal and cutaneous involvement in aGVHD, respectively.
Figure 1
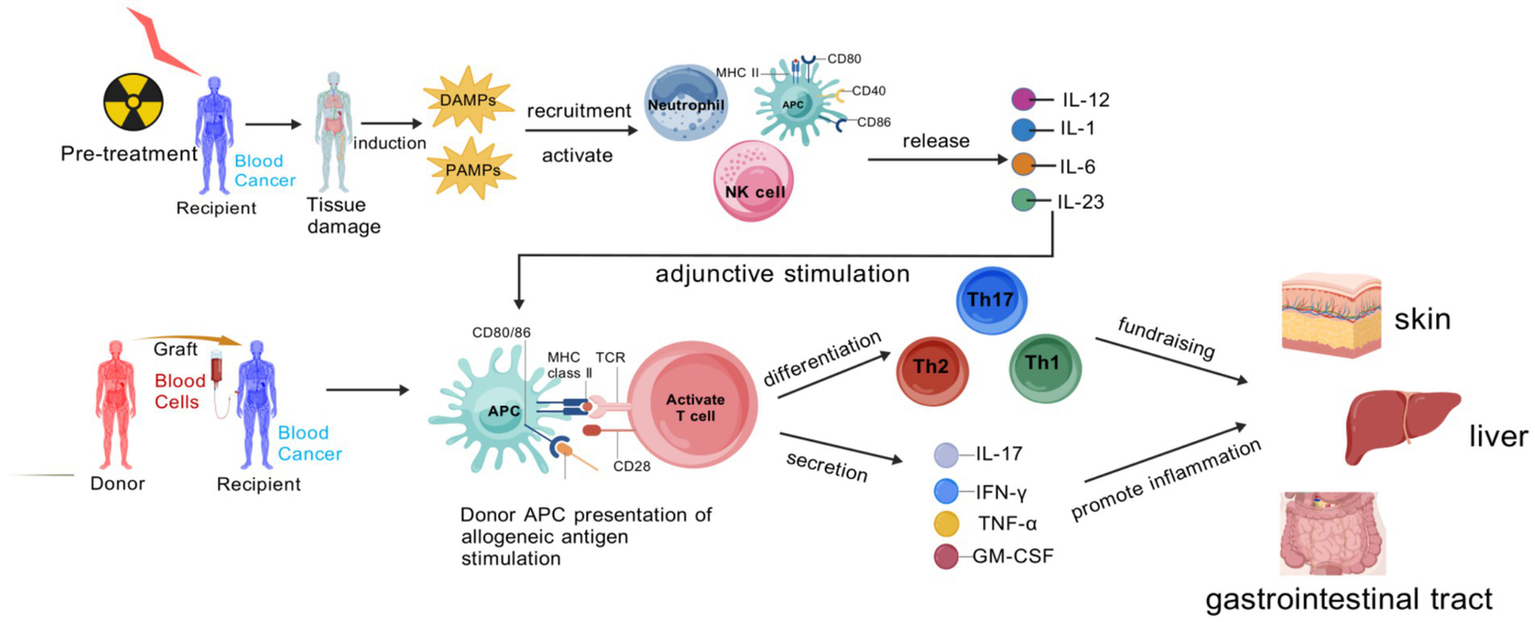
Pathogenic cascade of aGVHD.
The stage-specific immune drivers of aGVHD primarily revolve around the TNF-α/IL-1/IL-6 axis. During the initiation phase, TNF-α and IL-1 are produced by activated innate immune cells, which not only induce local inflammatory responses but also promote immune cell recruitment by upregulating the expression of vascular endothelial cell adhesion molecules (8). IL-6 exhibits pleiotropic effects: it not only promotes T cell proliferation and differentiation but also participates in the synthesis of hepatic acute-phase proteins, exacerbating systemic inflammatory responses. In the activation and effector phases, sustained high expression of these cytokines maintains a “cytokine storm,” further activating effector T cells and enhancing their cytotoxic activity, while exacerbating damage to vascular endothelial cells and target organ cells, leading to tissue necrosis (9).
Pre-transplant conditioning in recipients eradicates malignant cells but also inflicts damage on normal tissues, triggering the release of DAMPs and PAMPs. These molecular patterns mobilize and activate neutrophils, natural killer (NK) cells, and APCs. Once activated, APCs enhance the expression of MHC class II and co-stimulatory molecules, and secrete pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and IL-23, which serve as essential adjuvant signals for T cell activation. Subsequently, donor-derived APCs recognize recipient alloantigens, and through the interaction between MHC class II and T cell receptors (TCRs) during antigen presentation, naive T cells are activated. These activated T cells then differentiate into Th1, Th2, and Th17 subsets, releasing cytokines like IFN-γ, TNF-α, IL-17, and GM-CSF. These cytokines orchestrate inflammatory reactions that ultimately cause tissue injury in target organs. Created with BioGDP.com (10).
2.2 cGVHD: adaptive immune dysregulation and fibrotic remodeling
Chronic graft-versus-host disease (cGVHD) is characterized by immune homeostasis dysregulation and tissue fibrotic remodeling as key features. Its disease course progresses in three phases (11) (Figure 2).
Figure 2
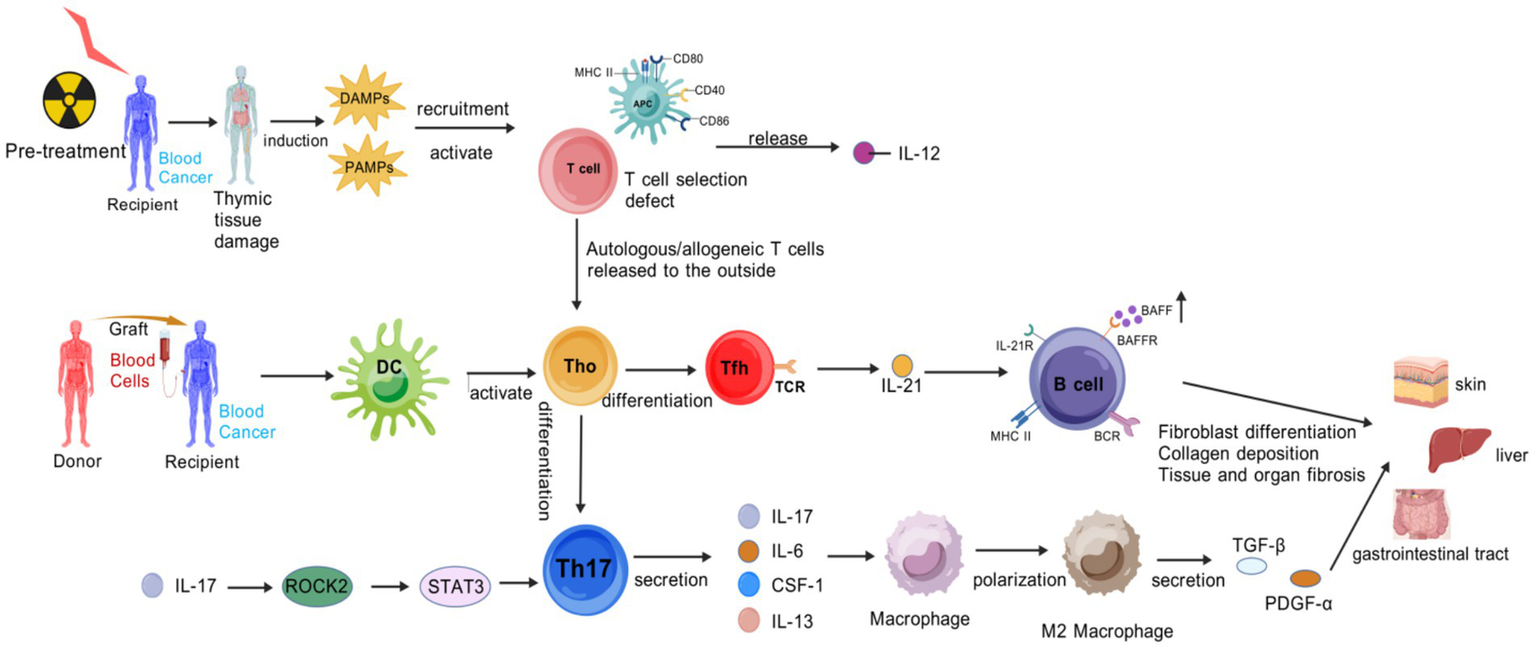
Pathogenic cascade of cGVHD. Pre-transplant conditioning damages the thymus, impairing negative selection of T cells. Autoreactive T cells evade deletion and enter the periphery. Concurrent tissue injury releases DAMPs, and gut microbial translocation releases PAMPs, APCs that secrete IL-12. Donor-derived dendritic cells (DCs) present auto−/alloantigens to naive T cells (Th0), driving their differentiation into two pathogenic subsets: Th17 cells. An autocrine loop via IL-17 → ROCK2 → STAT3 signaling amplifies Th17 differentiation. Th17 secretes cytokines (IL-17, IL-6, CSF-1, IL-13) to recruit and polarize macrophages toward a profibrotic M2 phenotype. Follicular helper T (Tfh) cells: Express TCR and secrete IL-21, which activates B cells through IL-21R, BAFF/BAFFR, and MHC-II/BCR interactions. M2 macrophages secrete TGF-β and PDGF-α, inducing fibroblast differentiation and collagen deposition, leading to fibrosis in target organs. Activated B cells produce autoantibodies and perpetuate T cell activation via antigen presentation, forming a pathogenic feedback loop. Created with BioGDP.com (10).
Early Inflammation and Tissue Injury: Tissue damage induced by transplantation preconditioning releases DAMP/PAMP, which activates APCs to upregulate MHC class II molecules and costimulatory molecules, and secrete IL-12. Donor T cells differentiate into Th1/Th17 subsets under the action of IL-6 and TGF-β, and secrete IL-17, IFN-γ, and IL-17 exacerbate epithelial/endothelial damage, thereby forming a proinflammatory microenvironment. IL-12, IL-6, and other factors are not only regulatory factors, but also inflammation-related biomarkers (12).
Chronic Inflammation and Immunodysregulation: it is centered on abnormal B-cell activation and impaired regulatory T-cell (Treg) function—B cells overproduce autoantibodies and rely on B-cell activating factor (BAFF) for survival, and elevated BAFF levels are closely associated with cGVHD activity (13).
Fibrosis and Tissue Remodeling (14): Myofibroblasts highly express α-smooth muscle actin (α-SMA) and connective tissue growth factor (CTGF), leading to collagen deposition. ROCK2 activation upregulates Th17 transcription via STAT3, suppresses Regulatory T cells, and regulates actin polymerization to reinforce fibrosis. Persistent cytokine secretion by Th17 maintains a vicious cycle of inflammation and fibrosis (14).
The stage-specific immune drivers of cGVHD include B-cell hyperactivity, Tfh cell expansion, and fibrosis-related factors. B-cell hyperactivity manifests as clonal proliferation and autoantibody production, with these autoantibodies targeting multiple tissue antigens to induce immune-mediated damage in target organs (15). Tfh cell expansion serves as a key driver of B cell activation: Tfh cells promote B cell differentiation and antibody production through direct cell–cell interactions and cytokine secretion (16). In terms of fibrosis, cytokines such as TGF-β and PDGF not only drive fibroblast activation and proliferation but also suppress immune cell functions, creating an immunosuppressive microenvironment that impedes normal immune regulation and further accelerates fibrotic progression (17).
3 Current status of multiple classification criteria for biomarkers in GVHD
Currently, the classification of GVHD biomarkers is based on multiple perspectives; however, there exists a certain degree of overlap and exclusivity among these classification methods. This article provides a review and integrative discussion of the current GVHD biomarker classification systems from the perspectives of pathophysiology, clinical application scenarios, and molecular characteristics.
3.1 Pathophysiological perspective
Synthesizing previous studies, from the pathophysiological perspective, biomarkers can be subdivided into three major categories: inflammation-driven, tissue damage-related, and immunoregulatory.
3.1.1 Inflammation-driven biomarkers
The core function of this category of biomarkers is to mediate the inflammatory cascade in GVHD. Studies have shown that the gene expression levels of cytokines in peripheral blood mononuclear cells (PBMCs) of GVHD patients are upregulated, including IFN-γ, TNF, and interleukins (18). IL-6 and IFN-γ regulate immune cell function by activating the Janus kinase 1 (JAK1) signaling pathway (19). When the selective JAK1 inhibitor itacitinib is used in haploidentical hematopoietic stem cell transplantation (HSCT), it reduces the incidence of acute and chronic GVHD without causing severe complications—this finding has been validated in multicenter clinical trials (20). TNF-α, a key mediator of the inflammatory response, exhibits elevated serum levels in patients with aGVHD, a characteristic observed in single-center cohort studies (21). As a member of the interleukin-1 receptor family, growth-stimulated expressed gene 2 protein (ST2) plays a crucial role in inflammatory signaling pathways (22). The signaling axis formed by ST2 and interleukin-33 (IL-33) is closely associated with treatment-refractory aGVHD and non-relapse mortality (NRM) (23). Serum ST2 levels in patients with aGVHD are higher than those in control populations, and this difference has been confirmed by single-center controlled studies (24).
3.1.2 Tissue damage-related biomarkers
This category of biomarkers directly reflects damage to GVHD target organs. REG3α, secreted by intestinal Paneth cells, is a specific biomarker for gastrointestinal GVHD (25). REG3α concentrations were 3-fold higher at the time of GVHD diagnosis in patients who had no response to therapy at 4 weeks than in patients who experienced a complete or partial response; patients responding to therapy still exhibited REG3α concentrations more than 3 times that of non-GVHD controls. And it can predict NRM at 4 weeks and 1 year post-transplantation—this clinical value has been confirmed in multicenter cohort studies (26). Hartwell et al. developed an innovative biomarker analysis algorithm based on single-center cohort data to evaluate blood samples collected on day 7 post-transplantation; this algorithm employs a dual-biomarker model consisting of ST2 and REG3α (27, 28). Galectin-3 (Gal-3) is a fundamental component of the galectin family (29). It induces T cell exhaustion by activating the nuclear factor of activated T cells (NFAT) signaling pathway (30), thereby alleviating tissue damage in aGVHD. The expression intensity of Gal-3 in CD4⁺ T cells is negatively correlated with intestinal pathological scores, and this association was derived from single-center clinical biopsy analyses (31). On day 15 post-haplocytotoxic HSCT, plasma Elafin levels are elevated in patients with severe cutaneous aGVHD. However, this result exhibits heterogeneity due to differences in donor characteristics and conditioning regimens, and it is only based on a single-center “discovery cohort-validation cohort” design—relevant data were obtained from single-center dual-cohort studies (32).
3.1.3 Immunoregulatory biomarkers
This category of biomarkers reflects dynamic changes in the GVHD immunoregulatory system. During aGVHD onset, there is an increase in the number of effector memory T cells (TEM), a decrease in naive T cells, and enhanced proliferative activity of Treg with abnormal expression of functional markers. These dynamic changes were observed in a single-center longitudinal monitoring study at 3 months post-transplantation (33), providing evidence for immune dysregulation in aGVHD. In cGVHD, the number of follicular Tfh in peripheral blood decreases, while the number of peripheral helper T cells (Tph) increases; additionally, tissue-resident helper T cells (Trh) undergo clonal expansion in target organs. This conclusion was first mechanistically confirmed in animal models and subsequently preliminarily validated in single-center samples from patients with moderate-to-severe cGVHD, representing an integrated study combining animal models and single-center clinical correlation (34). The dynamic changes in these T cell subsets during GVHD pathogenesis profoundly reflect the disruption of the body’s immunoregulatory network, are closely associated with prognosis, and provide important evidence for GVHD treatment based on immunoregulatory mechanisms.
3.2 Clinical application scenario perspective
Based on the National Institutes of Health (NIH) Biomarkers, Endpoints, and Surrogate Targets (BEST) Resource, GVHD biomarkers can be further subdivided into diagnostic, predictive, response, prognostic, and risk biomarkers to meet the needs of precision medicine in different clinical scenarios (35).
3.2.1 Diagnostic biomarkers
This category of biomarkers is used to confirm the presence of GVHD and involvement of target organs (3). Regulatory B cells (Bregs) exhibit significant potential in GVHD diagnosis due to their ability to maintain Treg homeostasis, promote Treg proliferation, and inhibit proinflammatory cytokine secretion (36). CD1c⁺ Bregs are induced via the PKA-CREB signaling axis. Post-HSCT, a decrease in the number of CD1c⁺ Bregs is accompanied by enhanced effector T cell activity and reduced immunosuppression, which can assist in GVHD diagnosis. Currently, evidence supporting this diagnostic potential is derived from single-center cellular function exploration studies (37). oGVHD severely impairs patients’ quality of life and visual function (38). Combined detection of IL-6, IL-10, and TNF-α improves diagnostic accuracy for oGVHD. Among these, the diagnostic value of IL-6 for oGVHD-associated dry eye has been confirmed by single-center receiver operating characteristic (ROC) curve analysis (39); further validation via a single-center small-sample correlation study of ocular surface indices demonstrated that combined detection of the three biomarkers enhances diagnostic efficacy (40). The CSF-1R inhibitor pexidartinib reduces T cell infiltration into the skin and alleviates cognitive impairment in a cGVHD mouse model, with relevant mechanisms validated in preclinical animal experiments (41). Chemokine biomarkers are a group of small-molecule cytokines or signaling proteins secreted by cells (42), and they are particularly important in GVHD diagnosis (43). Plasma levels of chemokine ligand 15 (CCL15) are elevated in cGVHD patients and correlate with NRM. This association was cross-validated using animal models and single-center human plasma samples (44), providing a new direction for cGVHD diagnosis.
3.2.2 Predictive biomarkers
As measurable indicators reflecting the underlying pathophysiological processes of diseases, predictive biomarkers hold critical value in predicting disease progression and assessing dynamic evolution (45). C-X-C motif chemokine ligand 9 (CXCL9) and C-X-C motif chemokine ligand 10 (CXCL10) regulate immunopathological processes via the C-X-C chemokine receptor 3 (CXCR3). Early post-transplant serum CXCL9 levels are positively correlated with cGVHD severity, and this correlation is modulated by single-nucleotide polymorphisms (SNPs) in CXCR3 ligand genes—this finding was confirmed by multicenter cohort analysis (46). The predictive value of soluble ST2 is time-dependent: on post-transplant day 7, it can predict severe aGVHD, with an area under the curve (AUC) of 0.8125, an optimal cut-off value of 2,363 pg/mL, a sensitivity of 83.3%, and a specificity of 75.0%; on day 14, its predictive efficacy for gastrointestinal aGVHD reaches a peak, showing an AUC of 0.8007, a cut-off value of 3,419 pg/mL, a sensitivity of 81.8%, and a specificity of 82.1%; and on day 21, its predictive accuracy for overall aGVHD improves, with an AUC of 0.7092, a cut-off value of 3,464 pg/mL, a sensitivity of 65.0%, and a specificity of 80.0%. These time-dependent characteristics were derived from single-center time-series measurements. These time-dependent characteristics were derived from single-center time-series measurements (47), suggesting that dynamic monitoring is required in clinical practice to enhance predictive accuracy. Combined detection of effector CD4⁺ conventional T cells (Tconv) and CXCL9 on post-transplant day 28 can predict aGVHD, and this prediction model has been jointly validated using multicenter samples (48), providing a feasible tool for early risk stratification of aGVHD.
3.2.3 Prognostic biomarkers
This category of biomarkers is used to estimate the expected disease course in patients with clinically significant conditions (49). Tumor necrosis factor receptor 1 (TNFR1), a member of the TNF receptor superfamily, is widely expressed on cell surfaces and plays important roles in anti-tumor activity and apoptosis regulation (50). Its plasma levels are elevated in aGVHD patients, and the AUC of TNFR1 at aGVHD onset is 0.71—relevant data were obtained from single-center multi-time-point detection analyses (51).
3.2.4 Response biomarkers
This category of biomarkers is used to assess the efficacy of GVHD treatment. BAFF levels increase after cGVHD onset, and it enhances B-cell receptor (BCR) reactivity by upregulating NOTCH2 expression. Changes in BAFF levels can reflect treatment response in cGVHD: the mechanistic component was elucidated using animal models, and clinical relevance was established based on correlation analysis between BAFF levels and disease manifestations in single-center cGVHD patients (52). Syk inhibitors can inhibit B-cell proliferation in cGVHD patients, and BAFF levels are positively correlated with BCR signaling pathway activity, suggesting that BAFF may serve as a potential response biomarker for cGVHD treatment. This hypothesis is currently supported by single-center mechanistic exploration studies (53).
3.2.5 Risk biomarkers
This category of biomarkers is used to predict the risk of GVHD development. Osteopontin (OPN) exacerbates tissue fibrosis by promoting epithelial-mesenchymal transition (EMT). Its plasma levels are upregulated in cGVHD patients, and the biomarker panel consisting of OPN, ST2, CXCL9, and matrix metalloproteinase 3 (MMP3) achieves an AUC of 0.89 for distinguishing cGVHD in the validation cohort. Its value in risk stratification has been validated in multicenter cohorts (54) (see Table 1).
Table 1
| Type of biomarker | Organ | Acute graft-versus-host disease | Chronic graft-versus-host disease | ||
|---|---|---|---|---|---|
| Type of biomarker | Clinical significance | Type of biomarker | Clinical significance | ||
| Diagnostic biomarker | Skins | Elafin (93) | Produced by skin keratinocytes elevate at the onset of cutaneous aGVHD | CXCL9 (94) CCL17 (95) |
Exacerbates local immune responses in the skin by chemotaxis of immune cells, leading to skin tissue damage. |
| Gastrointestinal tract | Reg3a (96) TIM3 (97) |
Elevated concentrations in patients with intestinal aGVHD | CD34 (98) | Concentration correlates with gastrointestinal cGVHD, aids in diagnosis | |
| Whole body/plasma | IL2R (99) HGF (100) IL-8 (64) TNFR-1 (65) Tregs (101) Th17 (13) |
Closely related to immune activation and inflammatory response. | sBAFF (102) ST2 (103) CXCL9 (54) OPN (103) CXCL10 (103) CCL19 (44) MMP 3(103) |
All of these levels are influenced by a variety of factors and the combined application of aids in the diagnosis of cGVHD. | |
| Pulmonary | MMP3 (104) | Not yet widely used and needs to be diagnosed in combination with other markers. | / | / | |
| Predictive biomarker | Whole body/plasma | ST2 (105) Reg3a (105) |
ST2 and Reg3a levels are usually elevated during exacerbations | ST2 (103) CXCL9 (103) |
Predicting treatment resistance or disease progression. |
| Reactive biomarker | Gastrointestinal tract | REG3α (106) | Concentration changes reflect steroid resistance | sBAFF (107) IL-10 (76) |
Treatment response assessment |
| Whole body/plasma | TIM3 (108) ST2 (109) TNFR1 (110) IL-2R (111) Tregs (112) Th17 (112) |
Dynamic changes in their levels reflect whether T cell activation is effectively regulated or not. | TNF-α (113) ST2 (114) |
Reflecting the immune inflammatory state in patients with chronic GVHD | |
| Prognostic biomarker | Gastrointestinal tract | REG3α (115) | High concentration associated with 1-year non-recurrent mortality rate. | MMP9 (116) Reg3a (117) |
Predicting disease progression. |
| Whole body/plasma | ST2 (115) | 14-day post-transplantation level predicts 6-month mortality. | CD163 (118) ST2 (119) CXCL9 (120) |
Associated with moderate/severe cGVHD progression. | |
| Risk biomarker | Whole body/plasma | ST2 (115) REG3α (115) |
Early post-transplant elevations suggest high risk and high concentrations suggest the risk of treatment failure | Reg3α (117) CXCL10 (121) ST2 (103) MMP3 (121) CXCL9 (120) OPN (103) |
Potential value in predicting the risk of developing chronic GVHD. |
| Novel biomarker | Whole body/plasma | MiRNA (122) Extracellular vesicles (EVs) (123) | Assessing severity and trends in aGVHD | IgG glycosylation (124) miRNA (125) EVs (126) |
The different levels of immune regulation, gene expression regulation, and intercellular communication (EVs), respectively, provide new perspectives for understanding the pathological process of cGVHD. |
| Gastrointestinal tract | Gut microflora (127) | Flora imbalance can further increase the risk of infection. | Gut microflora (128) | Based on the results of gut microflora testing, targeted treatments are possible. | |
Classification table of current mainstream GVHD biomarkers.
3.3 Molecular characteristic perspective
3.3.1 Protein biomarkers
Protein biomarkers play an indispensable role in disease diagnosis and assessment of disease severity (55), and they can serve as potential targets for drug development (56) (Figure 3). Interleukin-2 receptor (IL-2R) is upregulated due to donor T cell activation in GVHD, and monitoring its expression levels can assist in the early diagnosis of aGVHD (57). Takehitolmado et al. conducted preclinical studies in a GVHD mouse model and found that hepatocyte growth factor (HGF) gene transfection improves mouse survival and alleviates intestinal and thymic epithelial cell damage—this protective effect is hypothesized to be associated with the anti-apoptotic biological properties of HGF (58). HGF alleviates intestinal epithelial damage via anti-apoptotic effects, and its serum levels are elevated in aGVHD patients—based on single-center small-sample serum level detection (59). Extracellular vesicles (EVs) are secreted by various cell types and play a critical role in the secretion of soluble factors such as cytokines, growth factors, chemokines, and hormones (60). They have emerged as potential novel biomarkers for multiple diseases, including aGVHD (61). Human mesenchymal stem cell-derived exosomes alleviate aGVHD by regulating the miR-16-5p/activating transcription factor 6 (ATF6)/C/EBP homologous protein (CHOP) axis—this mechanism has been confirmed by in vitro cellular experiments and animal models (62).
Figure 3
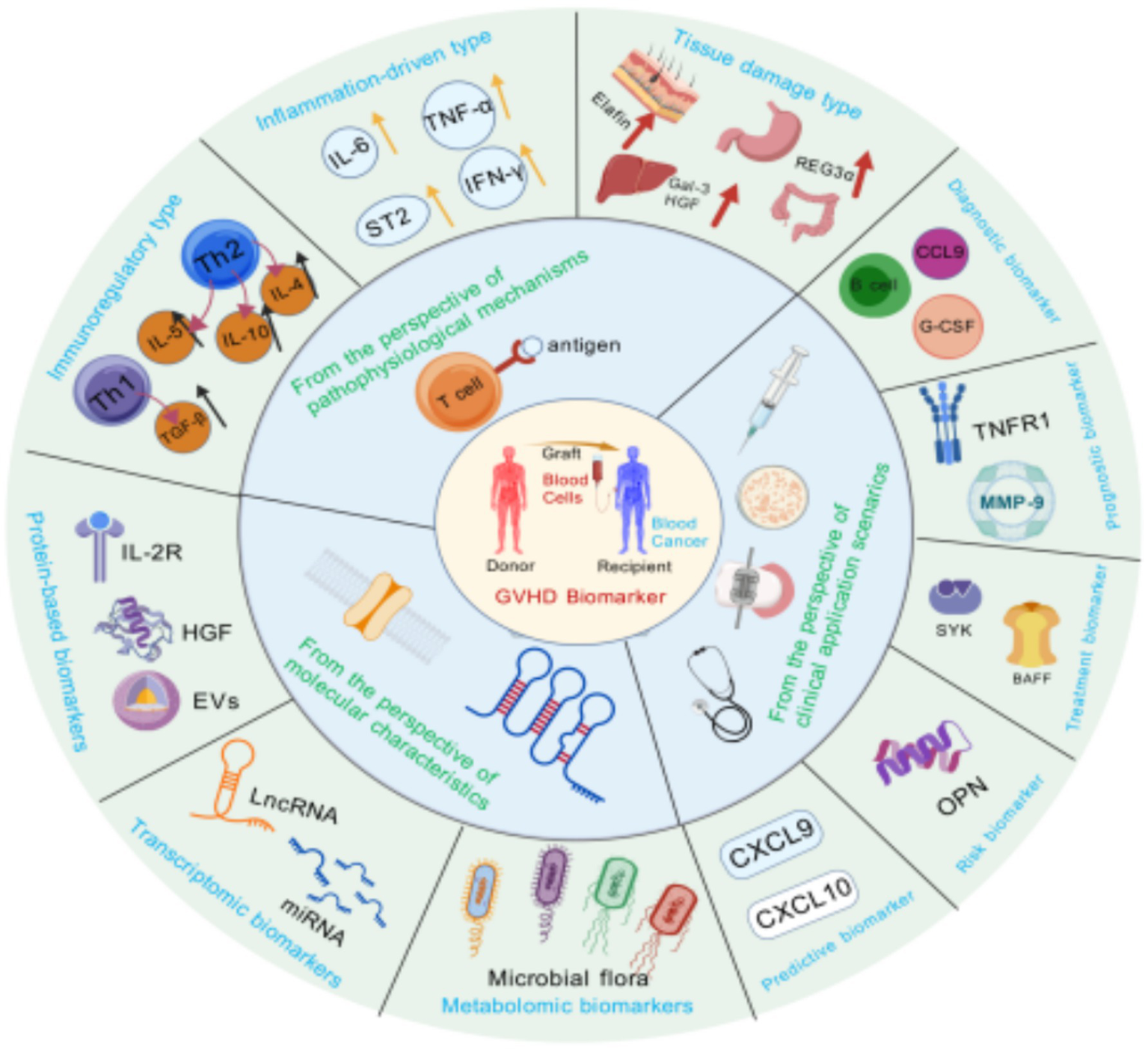
Multi-view classification of GVHD biomarkers. This figure integrates the classification of GVHD biomarkers from three perspectives: pathophysiological mechanisms, clinical scenarios, and molecular characterization. The pathophysiological mechanism perspective covers immunomodulatory, inflammation-driven, and tissue damage biomarkers; the clinical application scenario perspective is categorized into diagnostic, prognostic, response, risk, and predictive biomarkers; and the molecular characterization perspective includes metabolomics, transcriptomics, and protein biomarkers. The classification of different perspectives intersects with each other, which comprehensively demonstrates the diversity of GVHD biomarkers and helps to understand their different roles in the diagnosis and treatment of the disease. Created with BioGDP.com (10).
3.3.2 Transcriptomic biomarkers
The core of this category of biomarkers is microRNAs (miRNAs)—a class of non-coding single-stranded RNA molecules encoded by endogenous genes (63). miRNA-155 is upregulated in effector T cells of aGVHD animal models (64), and inhibiting its expression reduces aGVHD severity. Relevant mechanistic exploration was conducted using preclinical animal models (65). miRNAs activate Toll-like receptors 7/8 (TLR7/8) in target cells via endocytosis, thereby inducing dendritic cell maturation and donor T cell proliferation. The conclusion that this pathway is associated with target organ damage in aGVHD was derived from pathway exploration in animal models (6).
3.3.3 Metabolomic biomarkers
Metabolomic biomarkers are small-molecule metabolites produced by bodily metabolic activities, including amino acids, sugars, lipids, nucleotides, and their derivatives (66). The serum stearic acid/palmitic acid (SA/PA) ratio decreases on post-transplant day 7, which can diagnose grade II-IV aGVHD. Its efficacy in predicting aGVHD prognosis has been validated via multicenter metabolomic analysis (67), demonstrating potential for clinical application. The gut microbiota is a complex and important microecosystem in the human body, and it has been confirmed to participate in immune system development and influence host susceptibility to aGVHD (68, 69). Butyrate, a metabolite of the gut microbiota, exerts a protective effect on GVHD target organs—this protective effect was confirmed in animal models (70). In human samples, only an association between gut microbiota composition and MHC-II expression in intestinal epithelial cells has been observed, with relevant analyses derived from an integrated study combining animal models and single-center microbiota research (71).
4 Challenges to basic research and clinical application of GVHD biomarkers
4.1 Specificity and sensitivity of biomarkers
Despite the identification of numerous potential biomarkers for GVHD, their specificity and sensitivity remain inadequate for clinical application (72) (Figure 4). Although TNF-α is elevated in the serum of patients with aGVHD, it is also highly expressed in other inflammatory conditions, such as post-transplant sepsis, and thus cannot be used alone to distinguish aGVHD (73). As a candidate biomarker for cutaneous aGVHD, elastase exhibits variations in plasma level cutoffs and diagnostic efficacy across different studies due to differences in donor characteristics and conditioning regimens; furthermore, the small sample sizes in these studies result in insufficient stability of the findings. Consequently, further validation and optimization of biomarker combinations are necessary to enhance the accuracy of diagnostic and prognostic evaluations.
Figure 4
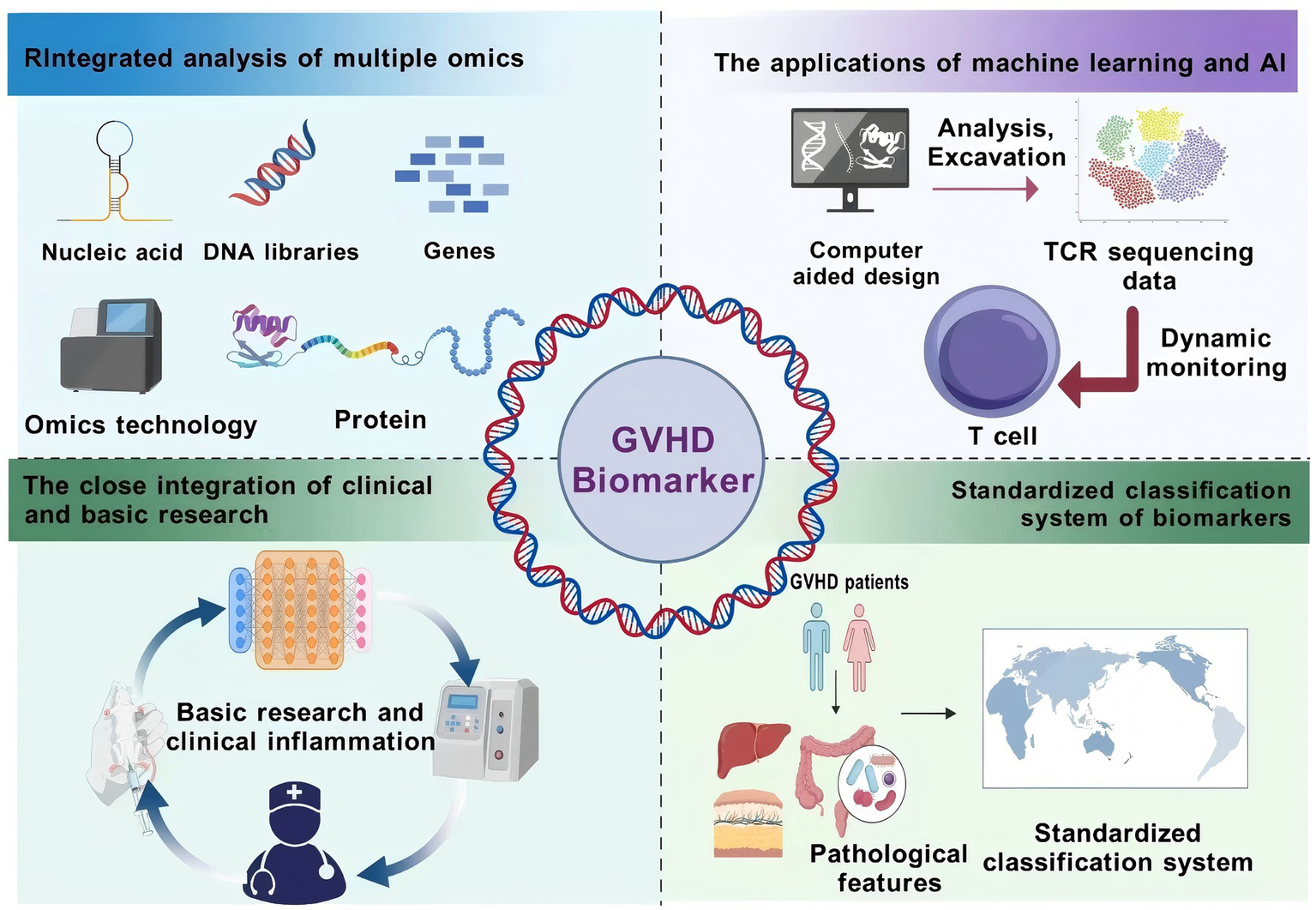
GVHD Biomarker Integration Pathway Advocacy. This figure presents the integration path of GVHD biomarkers. Multi-omics joint analysis integrates genomics, transcriptomics, and other multi-omics data to comprehensively analyze the pathogenesis; machine learning and artificial intelligence are used to analyze the biomarker data, mine potential markers, and monitor their dynamic changes; clinical and basic research are closely integrated to validate the validity and feasibility of the biomarkers and to promote the translation of the results; and a standardized classification system is established to unify the testing methods and standards and to improve the clinical application value. These paths provide a direction for solving the current challenges of biomarker research. Created with BioGDP.com (10).
4.2 Dynamics and time dependence of biomarkers
GVHD is characterized by a dynamic pathological process, where biomarker expression levels fluctuate over time.
Consequently, a single biomarker assessment may not adequately capture disease progression and treatment response, as it reflects only a specific temporal snapshot and fails to encompass the disease’s dynamic nature. The predictive value of soluble ST2 is time-dependent; relying solely on detection at a single time point easily leads to missed identification of specific organ involvement or misjudgment of disease severity. REG3α is elevated during the onset of gastrointestinal aGVHD and decreases following effective treatment. The lack of dynamic monitoring for REG3α may result in missed early intervention windows or incorrect assessment of treatment response. To more accurately assess the status of GVHD, it is imperative to establish a dynamic monitoring system capable of real-time tracking of biomarker fluctuations and generating continuous data. Integrating these data with clinical symptoms and treatment protocols for comprehensive evaluation can enhance physicians’ understanding of disease evolution (74), facilitate timely adjustments to therapeutic strategies, and ultimately improve treatment efficacy and patient prognosis.
4.3 Individual differences and heterogeneity of biomarkers
Significant individual differences and heterogeneity among patients with GVHD present challenges for biomarker research and its applications. Variations in immune response, genetic background, and treatment experience both prior to and following transplantation may influence biomarker expression levels (75). The degree of donor-recipient human leukocyte antigen (HLA) matching influences the expression of C-X-C motif CXCL9. Variations in conditioning regimens alter the serum levels of HGF: patients who receive total body irradiation (TBI) have higher HGF levels than those undergoing chemotherapy-based conditioning. Notably, HGF levels show no direct correlation with the incidence of aGVHD, which can easily interfere with risk assessment. Additionally, patients’ underlying diseases can increase OPN levels, weakening the correlation between OPN and the degree of fibrosis in cGVHD. Therefore, when developing personalized biomarker testing protocols, these individual differences must be fully considered.
4.4 Clinical validation and standardization of biomarkers
Numerous potential biomarkers for GVHD remain in the research phase and have not yet undergone extensive clinical validation (76). Although exosomes have emerged as novel biomarkers for aGVHD, their miRNA expression profiles associated with the disease have only been validated in single-center small-sample studies, and no multicenter validation has been conducted. Protein biomarkers are commonly detected using enzyme-linked immunosorbent assay (ELISA), while metabolomic biomarkers rely on mass spectrometry (MS) for detection. Differences in these technical platforms result in variations in clinical accessibility of these biomarkers. It is imperative to establish standardized testing protocols and validation procedures to enhance the clinical applicability of these biomarkers (77).
5 Suggested integration pathways for GVHD biomarkers
In basic research and practical clinical applications, it is not difficult to find that the three classification methods of GVHD biomarkers based on different perspectives have various limitations:
The classification of GVHD biomarkers according to pathophysiological mechanisms is grounded in scientific and theoretical principles; however, it presents certain limitations. A single marker, such as ST2, may be involved in multiple pathways, resulting in ambiguous classification (78). Various biomarker types interact and exert cross-influences (79), such as inflammatory factors impacting tissue damage and immune regulation. Focusing on a single marker while neglecting its synergistic effects and dynamic changes within the pathological process fails to fully capture the complex pathological nature of GVHD.
Biomarkers for GVHD, when considered from the perspective of clinical application scenarios, hold significant potential in the realms of diagnosis, prediction, prognosis, and the assessment of response and risk. However, they encounter challenges in practical implementation. The functionality of these biomarkers may vary throughout the disease course; initially, they may enhance immune response and disease progression, thereby aiding in diagnosis. In later stages, they may contribute to immune homeostasis or facilitate tissue repair, thus aiding in the prediction of patient survival (80), demonstrating their dual efficacy as biomarkers. For instance, the levels of BAFF may be influenced by the stage of the disease, therapeutic interventions, and other immunological factors, with its predictive value and risk assessment potentially evolving over time.
There are differences in the technology platforms required for the detection of GVHD biomarkers based on molecular characterization. For example, protein-based markers are commonly detected using ELISA (81), whereas metabolomic markers mostly rely on mass spectrometry (82). This dependence on technological platforms leads to differences in the clinical accessibility of different markers and affects their widespread use.
5.1 Joint multi-omics analysis
Joint multi-omics analysis involves the integration and examination of genomics, transcriptomics, proteomics, metabolomics, and other multi-omics data (83). An allo-HSCT cohort encompassing subgroups of aGVHD, cGVHD, and non-GVHD was selected, and genomic data, transcriptomic data, proteomic data, and metabolomic data were collected simultaneously from this cohort (84). After processing the data using batch correction methods, core variables were screened via LASSO regression, and a correlation network was constructed using weighted gene co-expression network analysis (WGCNA). Finally, the efficacy of the integrated panel was validated in independent multicenter cohorts to ensure its ability to distinguish between GVHD and non-GVHD, as well as to identify different target organ involvement scenarios. This work aims to further advance the clinical pilot application of the panel. This approach enables a comprehensive understanding of the pathogenesis of GVHD at various levels and facilitates the identification of additional potential biomarkers (85). Furthermore, combined multi-omics analysis can uncover the interrelationships and synergistic effects among different markers, thereby providing a theoretical foundation for the development of a standardized biomarker classification system (85).
5.2 Applications of machine learning and artificial intelligence
Machine learning and artificial intelligence technologies are increasingly employed in biomarker research. Through the application of machine learning algorithms, extensive biomarker datasets can be analyzed and mined to identify potential biomarkers (86). For instance, a study utilized machine learning algorithms to analyze TCR sequencing data, thereby revealing the dynamic changes in T cell clones of varying grades in GVHD patients (48). With biomarker data and clinical indicators from multicenter cohorts used as input variables, a logistic regression algorithm was employed to construct the model, which outputs GVHD development risk scores and treatment response probabilities. Following this, the model was validated in multiple independent centers, and risk score thresholds were set to guide the formulation of clear decisions during the clinical translation phase. It realizes the transformation from biomarkers to individualized treatment decisions through machine learning, providing a new paradigm for precision management of aGVHD (87).
5.3 Close integration of clinical and basic research
The integration of clinical and basic research is crucial for enhancing the clinical applicability of biomarkers (88). Clinical research serves to verify the validity and feasibility of biomarkers in practical settings, while basic research offers theoretical support for their discovery and application. Mechanisms of biomarkers were validated using cGVHD mouse models: BAFF regulates B cell activation, Gal-3 affects T cell cytotoxicity via the NFAT signaling pathway (88). Meanwhile, the levels of corresponding biomarkers were measured in clinical cohorts to confirm their correlation coefficients with disease activity. Furthermore, close integration of clinical and basic research facilitates the translational application of these biomarkers and accelerates the clinical translation of research findings.
5.4 Establishment of a standardized biomarker classification system
Establishing a standardized biomarker classification system is essential for enhancing the clinical utility of biomarkers (89). The MAGIC consortium has developed a biomarker-based classification system for evaluating the severity of aGVHD (90).
Inclusion thresholds for various types of biomarkers should be defined: for example, inflammation-driven biomarkers need to be demonstrated to have a correlation coefficient of no less than 0.5 with GVHD inflammatory indicators in at least two multicenter cohorts, while diagnostic biomarkers should have an AUC of no less than 0.75 when detected alone. Unified technical protocols for detection should be established: ELISA should be adopted for protein biomarkers, and standardized mass spectrometry parameters should be used for metabolomic biomarkers. Application guidelines should be developed based on clinical scenarios; for instance, the diagnosis of gastrointestinal aGVHD requires combined detection of REG3αand ST2. Furthermore, a standardized biomarker classification system should consider the interrelationships and synergistic effects between different biomarkers to enhance the clinical utility of these biomarkers.
6 Discussion on current standard protocols for the clinical application of biomarkers
Currently, despite significant progress in the discovery and validation of biomarkers, there remains a lack of widely recognized and uniformly applied standardized clinical practice guidelines for the use of biomarkers in the management of GVHD following allo-HSCT. A variety of promising biomarkers (91)—such as ST2 and REG3α for GVHD, and CXCL9 and soluble BAFF for cGVHD—have been validated in at least two independent cohorts via large-scale proteomics and reproducible detection methods. However, consensus has not been reached on key clinical parameters, including optimal detection time points, unified cut-off values, and standards for combining biomarkers with clinical indicators. This has hindered their integration into routine standardized clinical practice (92).
Currently, in clinical practice, the application of biomarkers remains primarily in the exploratory and experimental phase rather than in standardized use. For instance, patients with standard-risk aGVHD are stratified to receive treatment with sirolimus or prednisone; some centers continuously monitor ST2 to assess treatment response in steroid-refractory aGVHD. Nevertheless, such practices are limited to specific clinical trials or single-center protocols, lacking multi-institutional validation and regulatory approval (76).
Establishing standardized clinical practice guidelines for biomarker use requires prioritizing prospective, multicenter studies to address existing gaps. These studies should focus on validating well-established biomarkers to develop standardized protocols—including optimal sampling timings days 7–14 post-allo-HSCT for aGVHD risk stratification and day 100 post-allo-HSCT for cGVHD screening, clinically meaningful cut-off values, and algorithms that combine biomarkers with clinical assessments. Maintaining consistent consensus standards for biomarker identification is crucial for translating promising biomarkers into reproducible, widely applicable clinical standards. This will facilitate improved risk stratification, treatment decision-making, and post-transplant outcomes.
7 Conclusion
This review systematically examines the current state of research on GVHD biomarkers, including their classification systems and associated challenges. The analysis encompasses multiple dimensions, such as pathophysiological mechanisms, clinical application scenarios, and molecular properties, highlighting the fragmentation within the existing biomarker classification system and underscoring the urgent need for its integration. Furthermore, the paper identifies challenges related to specificity, sensitivity, dynamic changes, individual differences, and clinical validation in current research. It proposes feasible approaches, including multi-omics joint analysis, the application of machine learning and artificial intelligence, the integration of clinical and basic research, and the establishment of a standardized classification system.
Despite notable advancements in the investigation of GVHD biomarkers, their clinical implementation continues to encounter several obstacles. Future research should focus on the integration and analysis of multi-omics data in conjunction with machine learning and artificial intelligence technologies to enhance the specificity and sensitivity of these biomarkers. Furthermore, large-scale clinical validation and the development of standardized assays are essential for advancing the clinical application of biomarkers. A limitation of this review is that, although an integrated classification framework was proposed, specific implementation and validation data were not provided. Future research should build upon this foundation, further refine the classification criteria, and validate their efficacy through multicenter clinical studies to offer a more reliable basis for the precise diagnosis and treatment of GVHD.
Statements
Author contributions
XY: Conceptualization, Investigation, Methodology, Writing – original draft. SH: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. XL: Writing – original draft. YiZ: Writing – original draft. XJ: Writing – original draft. MF: Writing – original draft. YuZ: Writing – original draft. LM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the School of Clinical Medicine, Shandong Second Medical University in Weifang City, Shandong Province, and Yuhuangding Hospital in Yantai City for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Penack O Marchetti M Aljurf M Arat M Bonifazi F Duarte RF et al . Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. (2024) 11:e147–59. doi: 10.1016/S2352-3026(23)00342-3
2.
Biyun L Yahui H Yuanfang L Xifeng G Dao W . Risk factors for invasive fungal infections after haematopoietic stem cell transplantation: a systematic review and meta-analysis. Clin Microbiol Infect. (2024) 30:601–10. doi: 10.1016/j.cmi.2024.01.005
3.
Califf RM . Biomarker definitions and their applications. Exp Biol Med. (2018) 243:213–21. doi: 10.1177/1535370217750088
4.
Akahoshi Y Spyrou N Weber D Aguayo-Hiraldo P Ayuk F Chanswangphuwana C et al . Novel MAGIC composite scores using both clinical symptoms and biomarkers best predict treatment outcomes of acute GVHD. Blood. (2024) 144:1010–21. doi: 10.1182/blood.2024025106
5.
Hill GR Koyama M . Cytokines and costimulation in acute graft-versus-host disease. Blood. (2020) 136:418–28. doi: 10.1182/blood.2019000952
6.
Zitzer NC Garzon R Ranganathan P . Toll-like receptor stimulation by MicroRNAs in acute graft-vs.-host disease. Front Immunol. (2018) 9:2561. doi: 10.3389/fimmu.2018.02561
7.
Li A Abraham C Wang Y Zhang Y . New insights into the basic biology of acute graft-versus-host-disease. Haematologica. (2020) 105:2540–9. doi: 10.3324/haematol.2019.240291
8.
Pierini A Strober W Moffett C Baker J Nishikii H Alvarez M et al . TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. (2016) 128:866–71. doi: 10.1182/blood-2016-04-711275
9.
Holtan SG Pasquini M Weisdorf DJ . Acute graft-versus-host disease: a bench-to-bedside update. Blood. (2014) 124:363–73. doi: 10.1182/blood-2014-01-514786
10.
Jiang S Li H Zhang L Mu W Zhang Y Chen T et al . Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. (2025) 53:D1670–6. doi: 10.1093/nar/gkae973
11.
Cooke KR Luznik L Sarantopoulos S Hakim FT Jagasia M Fowler DH et al . The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2017) 23:211–34. doi: 10.1016/j.bbmt.2016.09.023
12.
MacDonald KP Blazar BR Hill GR . Cytokine mediators of chronic graft-versus-host disease. J Clin Invest. (2017) 127:2452–63. doi: 10.1172/JCI90593
13.
Jiang H Fu D Bidgoli A Paczesny S . T cell subsets in graft versus host disease and graft versus tumor. Front Immunol. (2021) 12:761448. doi: 10.3389/fimmu.2021.761448
14.
Vadakkel G Eng S Proli A Ponce DM . Updates in chronic graft-versus-host disease: novel treatments and best practices in the current era. Bone Marrow Transplant. (2024) 59:1360–8. doi: 10.1038/s41409-024-02370-8
15.
Bracken SJ Suthers AN DiCioccio RA Su H Anand S Poe JC et al . Heightened TLR7 signaling primes BCR-activated B cells in chronic graft-versus-host disease for effector functions. Blood Adv. (2024) 8:667–80. doi: 10.1182/bloodadvances.2023010362
16.
Forcade E Kim HT Cutler C Wang K Alho AC Nikiforow S et al . Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. (2016) 127:2489–97. doi: 10.1182/blood-2015-12-688895
17.
Belle L Fransolet G Somja J Binsfeld M Delvenne P Drion P et al . Limited impact of Imatinib in a murine model of Sclerodermatous chronic graft-versus-host disease. PLoS One. (2016) 11:e0167997. doi: 10.1371/journal.pone.0167997
18.
Pinto GR Sarmento VA Carvalho-Filho PC Fortuna VA Costa R Conceição RR et al . Gene expression profile of chronic oral graft-versus-host disease. PLoS One. (2022) 17:e0267325. doi: 10.1371/journal.pone.0267325
19.
Mannina D Kröger N . Janus kinase inhibition for graft-versus-host disease: current status and future prospects. Drugs. (2019) 79:1499–509. doi: 10.1007/s40265-019-01174-1
20.
Abboud R Schroeder MA Rettig MP Gao F Ritchey JK Abboud CN et al . Itacitinib for prevention of graft-versus-host disease and cytokine release syndrome with T-cell replete peripheral blood haploidentical transplantation. Blood. (2023) 142:651. doi: 10.1182/blood-2023-180289
21.
Maximova N Nisticò D Riccio G Maestro A Barbi E Faganel Kotnik B et al . Advantage of first-line therapeutic drug monitoring-driven use of infliximab for treating acute intestinal and liver GVHD in children: a prospective, single-center study. Cancers. (2023) 15:3605. doi: 10.3390/cancers15143605
22.
Reichenbach DK Schwarze V Matta BM Tkachev V Lieberknecht E Liu Q et al . The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. (2015) 125:3183–92. doi: 10.1182/blood-2016-07-728048
23.
Paczesny S . Post-haematopoietic cell transplantation outcomes: why ST2 became a “golden nugget” biomarker. Br J Haematol. (2021) 192:951–67. doi: 10.1111/bjh.16497
24.
Aladağ Karakulak E Demİroğlu H Malkan UY Akman U Göker H Büyükaşik Y . Assessment of ST2 and Reg3a levels in patients with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Turk J Med Sci. (2021) 51:355–8. doi: 10.3906/sag-2007-17
25.
Zhao D Kim Y-H Jeong S Greenson JK Chaudhry MS Hoepting M et al . Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest. (2018) 128:4970–9. doi: 10.1172/JCI99261
26.
Harris AC Levine JE Ferrara JLM . Have we made progress in the treatment of GVHD?Best Pract Res Clin Haematol. (2012) 25:473–8. doi: 10.1016/j.beha.2012.10.010
27.
Hartwell MJ Özbek U Holler E Renteria AS Major-Monfried H Reddy P et al . An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. (2017) 2:e89798. doi: 10.1172/jci.insight.89798
28.
Smullin CP Venick RS Marcus EA Naini BV Farmer DG . REG3α is a novel biomarker that potentially correlates with acute allograft rejection after intestinal transplantation. Clin Transpl. (2021) 35:e14378. doi: 10.1111/ctr.14378
29.
Meng Y Zhao Q Sang Y Liao J Ye F Qu S et al . GPNMB+Gal-3+ hepatic parenchymal cells promote immunosuppression and hepatocellular carcinogenesis. EMBO J. (2023) 42:e114060. doi: 10.15252/embj.2023114060
30.
Mohammadpour H Tsuji T MacDonald CR Sarow JL Rosenheck H Daneshmandi S et al . Galectin-3 expression in donor T cells reduces GvHD severity and lethality after allogeneic hematopoietic cell transplantation. Cell Rep. (2023) 42:112250. doi: 10.1016/j.celrep.2023.112250
31.
Mohammadpour H Tsuji T MacDonald CR Sarow JL Qiu J Matsuzaki J et al . Galectin-3 signaling in donor T cells regulates acute graft versus host disease (aGvHD) after allogenic transplantation. Blood. (2021) 138:2765. doi: 10.1182/blood-2021-150777
32.
Solán L Carbonell D Muñiz P Dorado N Landete E Chicano-Lavilla M et al . Elafin as a predictive biomarker of acute skin graft-versus-host disease after Haploidentical stem cell transplantation using post-transplant high-dose cyclophosphamide. Front Immunol. (2021) 12:516078. doi: 10.3389/fimmu.2021.516078
33.
Latis E, Michonneau D, Leloup C, Varet H, Peffault de Latour R, CRYOSTEM Consortium, Bianchi E, Socié G, Rogge L . Cellular and molecular profiling of T-cell subsets at the onset of human acute GVHD. Blood Adv. (2020) 4:3927–42. doi: 10.1182/bloodadvances.2019001032
34.
Kong X Wu X Wang B Zeng D Cassady K Nasri U et al . Trafficking between clonally related peripheral T-helper cells and tissue-resident T-helper cells in chronic GVHD. Blood. (2022) 140:2740–53. doi: 10.1182/blood.2022016581
35.
Alexoudi V-A Gavriilaki E Cheva A Sakellari I Papadopoulou S Paraskevopoulos K et al . Graft-versus-host disease: can biomarkers assist in differential diagnosis, prognosis, and therapeutic strategy?Pharmaceuticals (Basel). (2024) 17:298. doi: 10.3390/ph17030298
36.
Hu Y He G-L Zhao X-Y Zhao X-S Wang Y Xu L-P et al . Regulatory B cells promote graft-versus-host disease prevention and maintain graft-versus-leukemia activity following allogeneic bone marrow transplantation. Onco Targets Ther. (2017) 6:e1284721. doi: 10.1080/2162402X.2017.1284721
37.
Bao Y Liu J Li Z Sun Y Chen J Ma Y et al . Ex vivo-generated human CD1c+ regulatory B cells by a chemically defined system suppress immune responses and alleviate graft-versus-host disease. Mol Ther. (2024) 32:4372–82. doi: 10.1016/j.ymthe.2024.10.026
38.
Soleimani M Mahdavi Sharif P Cheraqpour K Koganti R Masoumi A Baharnoori SM et al . Ocular graft-versus-host disease (oGVHD): from a to Z. Surv Ophthalmol. (2023) 68:697–712. doi: 10.1016/j.survophthal.2023.02.006
39.
Hu B Qiu Y Hong J . Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease(GVHD). Ocul Surf. (2020) 18:298–304. doi: 10.1016/j.jtos.2019.12.005
40.
Chen Y Zhuang X Wang L Xu Y Sun Z Ren Y et al . The role of IL-6, IL-10, and TNF-α in ocular GVHD following allogeneic transplantation. Ocul Immunol Inflamm. (2024) 32:1788–95. doi: 10.1080/09273948.2024.2302445
41.
Shaikh SN Willis EF Dierich M Xu Y Stuart SJS Gobe GC et al . CSF-1R inhibitor PLX3397 attenuates peripheral and brain chronic GVHD and improves functional outcomes in mice. J Neuroinflammation. (2023) 20:300. doi: 10.1186/s12974-023-02984-7
42.
Guerrero S Sánchez-Tirado E Agüí L González-Cortés A Yáñez-Sedeño P Pingarrón JM . Simultaneous determination of CXCL7 chemokine and MMP3 metalloproteinase as biomarkers for rheumatoid arthritis. Talanta. (2021) 234:122705. doi: 10.1016/j.talanta.2021.122705
43.
Ahmed SS Wang XN Norden J Pearce K El-Gezawy E Atarod S et al . Identification and validation of biomarkers associated with acute and chronic graft versus host disease. Bone Marrow Transplant. (2015) 50:1563–71. doi: 10.1038/bmt.2015.191
44.
Du J Flynn R Paz K Ren H-G Ogata Y Zhang Q et al . Murine chronic graft-versus-host disease proteome profiling discovers CCL15 as a novel biomarker in patients. Blood. (2018) 131:1743–54. doi: 10.1182/blood-2017-08-800623
45.
El Nahhas OSM Loeffler CML Carrero ZI van Treeck M Kolbinger FR Hewitt KJ et al . Regression-based deep-learning predicts molecular biomarkers from pathology slides. Nat Commun. (2024) 15:1253. doi: 10.1038/s41467-024-45589-1
46.
Shino MY Todd JL Neely ML Kirchner J Frankel CW Snyder LD et al . Plasma CXCL9 and CXCL10 at allograft injury predict chronic lung allograft dysfunction. Am J Transplant. (2022) 22:2169–79. doi: 10.1111/ajt.17108
47.
Huang K Yang M Huang J Cao Y Zhou Y Pang G et al . Soluble ST2 as a predictive biomarker for acute graft-versus-host disease post -allogeneic stem cell transplantation. Clin Transpl. (2025) 39:e70108. doi: 10.1111/ctr.70108
48.
McCurdy SR Radojcic V Tsai H-L Vulic A Thompson E Ivcevic S et al . Signatures of GVHD and relapse after posttransplant cyclophosphamide revealed by immune profiling and machine learning. Blood. (2022) 139:608–23. doi: 10.1182/blood.2021013054
49.
FDA-NIH Biomarker Working Group . BEST (biomarkers, EndpointS, and other tools) resource. Silver Spring (MD): Food and Drug Administration (US) (2016).
50.
Alam MS Gaida MM Witzel HR Otsuka S Abbasi A Guerin T et al . TNFR1 signaling promotes pancreatic tumor growth by limiting dendritic cell number and function. Cell Rep Med. (2024) 5:101696. doi: 10.1016/j.xcrm.2024.101696
51.
Mir E Palomo M Rovira M Pereira A Escolar G Penack O et al . Endothelial damage is aggravated in acute GvHD and could predict its development. Bone Marrow Transplant. (2017) 52:1317–25. doi: 10.1038/bmt.2017.121
52.
Buxbaum NP . BAD(FF) to the bone: misbehaving B cells in cGVHD. Blood. (2021) 137:2426–7. doi: 10.1182/blood.2021011008
53.
Allen JL Tata PV Fore MS Wooten J Rudra S Deal AM et al . Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood. (2014) 123:2108–15. doi: 10.1182/blood-2013-10-533562
54.
Yu J Storer BE Kushekhar K Abu Zaid M Zhang Q Gafken PR et al . Biomarker panel for chronic graft-versus-host disease. J Clin Oncol. (2016) 34:2583–90. doi: 10.1200/JCO.2015.65.9615
55.
Zainal NHM Abas R Mohamad Asri SF . Childhood allergy disease, early diagnosis, and the potential of salivary protein biomarkers. Mediat Inflamm. (2021) 2021:9198249. doi: 10.1155/2021/9198249
56.
X Q R D . Sex hormone-binding globulin (SHBG) as an early biomarker and therapeutic target in polycystic ovary syndrome. Int J Mol Sci. (2020) 21:191. doi: 10.3390/ijms21218191
57.
Nakamura H Komatsu K Ayaki M Kawamoto S Murakami M Uoshima N et al . Serum levels of soluble IL-2 receptor, IL-12, IL-18, and IFN-gamma in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J Allergy Clin Immunol. (2000) 106:S45–50. doi: 10.1067/mai.2000.106774
58.
Imado T Iwasaki T Kataoka Y Kuroiwa T Hara H Fujimoto J et al . Hepatocyte growth factor preserves graft-versus-leukemia effect and T-cell reconstitution after marrow transplantation. Blood. (2004) 104:1542–9. doi: 10.1182/blood-2003-12-4309
59.
Okamoto T Takatsuka H Fujimori Y Wada H Iwasaki T Kakishita E . Increased hepatocyte growth factor in serum in acute graft-versus-host disease. Bone Marrow Transplant. (2001) 28:197–200. doi: 10.1038/sj.bmt.1703095
60.
Carneiro TX Marrese DG Dos Santos MG Gonçalves MV Novis YAS Rizzatti EG et al . Circulating extracellular vesicles as a predictive biomarker for acute graft-versus-host disease. Exp Hematol. (2023) 117:15–23. doi: 10.1016/j.exphem.2022.11.004
61.
Dixson AC Dawson TR Di Vizio D Weaver AM . Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. (2023) 24:454–76. doi: 10.1038/s41580-023-00576-0
62.
Li W Si Y Wang Y Chen J Huo X Xu P et al . hUCMSC-derived exosomes protect against GVHD-induced endoplasmic reticulum stress in CD4+ T cells by targeting the miR-16-5p/ATF6/CHOP axis. Int Immunopharmacol. (2024) 135:112315. doi: 10.1016/j.intimp.2024.112315
63.
Anelli L Zagaria A Specchia G Musto P Albano F . Dysregulation of miRNA in leukemia: exploiting miRNA expression profiles as biomarkers. Int J Mol Sci. (2021) 22:7156. doi: 10.3390/ijms22137156
64.
Bala S Csak T Saha B Zatsiorsky J Kodys K Catalano D et al . The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. (2016) 64:1378–87. doi: 10.1016/j.jhep.2016.01.035
65.
Pitea M Canale FA Porto G Verduci C Utano G Policastro G et al . The role of MicroRNA in graft-versus-host-disease: a review. Genes (Basel). (2023) 14:1796. doi: 10.3390/genes14091796
66.
Qiu S Cai Y Yao H Lin C Xie Y Tang S et al . Small molecule metabolites: discovery of biomarkers and therapeutic targets. Signal Transduct Target Ther. (2023) 8:132. doi: 10.1038/s41392-023-01399-3
67.
Wu X Xie Y Wang C Han Y Bao X Ma S et al . Prediction of acute GVHD and relapse by metabolic biomarkers after allogeneic hematopoietic stem cell transplantation. JCI Insight. (2018) 3:e99672. doi: 10.1172/jci.insight.99672
68.
Holler E Butzhammer P Schmid K Hundsrucker C Koestler J Peter K et al . Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. (2014) 20:640–5. doi: 10.1016/j.bbmt.2014.01.030
69.
Jenq RR Ubeda C Taur Y Menezes CC Khanin R Dudakov JA et al . Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. (2012) 209:903–11. doi: 10.1084/jem.20112408
70.
Mathewson ND Jenq R Mathew AV Koenigsknecht M Hanash A Toubai T et al . Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. (2016) 17:505–13. doi: 10.1038/ni.3400
71.
Koyama M Hippe DS Srinivasan S Proll SC Miltiadous O Li N et al . Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity. Immunity. (2023) 56:1876–1893.e8. doi: 10.1016/j.immuni.2023.06.024
72.
Akahoshi Y Spyrou N Hogan WJ Ayuk F DeFilipp Z Weber D et al . Incidence, clinical presentation, risk factors, outcomes, and biomarkers in de novo late acute GVHD. Blood Adv. (2023) 7:4479–91. doi: 10.1182/bloodadvances.2023009885
73.
Prado-Acosta M Jeong S Utrero-Rico A Goncharov T Webster JD Holler E et al . Inhibition of RIP1 improves immune reconstitution and reduces GVHD mortality while preserving graft-versus-leukemia effects. Sci Transl Med. (2023) 15:eadf8366. doi: 10.1126/scitranslmed.adf8366
74.
Katsivelos N Spyrou N Weber D Vasova I Ayuk F Choe H et al . Serial clinical and biomarker monitoring during graft-versus-host disease treatment identifies distinct risk strata including an ultra-low risk group. Transplant Cell Ther. (2025) 31:10.e1–9. doi: 10.1016/j.jtct.2024.10.012
75.
Cheung TS Bertolino GM Giacomini C Bornhäuser M Dazzi F Galleu A . Mesenchymal stromal cells for graft versus host disease: mechanism-based biomarkers. Front Immunol. (2020) 11:1338. doi: 10.3389/fimmu.2020.01338
76.
Paczesny S . Biomarkers for posttransplantation outcomes. Blood. (2018) 131:2193–204. doi: 10.1182/blood-2018-02-791509
77.
Zelić Kerep A Olivieri A Schoemans H Lawitschka A Halter J Pulanic D et al . Chronic gvhd dictionary-eurograft cost action initiative consensus report. Bone Marrow Transplant. (2023) 58:68–71. doi: 10.1038/s41409-022-01837-w
78.
Kotsiou OS Gourgoulianis KI Zarogiannis SG . IL-33/ST2 Axis in organ fibrosis. Front Immunol. (2018) 9:2432. doi: 10.3389/fimmu.2018.02432
79.
Zhang D Hu W Xie J Zhang Y Zhou B Liu X et al . TIGIT-fc alleviates acute graft-versus-host disease by suppressing CTL activation via promoting the generation of immunoregulatory dendritic cells. Biochim Biophys Acta Mol basis Dis. (2018) 1864:3085–98. doi: 10.1016/j.bbadis.2018.06.022
80.
Jeon Y Lim J-Y Im K-I Kim N Cho S-G . BAFF blockade attenuates acute graft-versus-host disease directly via the dual regulation of T- and B-cell homeostasis. Front Immunol. (2022) 13:995149. doi: 10.3389/fimmu.2022.995149
81.
Neumair J Kröger M Stütz E Jerin C Chaker AM Schmidt-Weber CB et al . Flow-based CL-SMIA for the quantification of protein biomarkers from nasal secretions in comparison with Sandwich ELISA. Biosensors. (2023) 13:670. doi: 10.3390/bios13070670
82.
Khamis MM Adamko DJ El-Aneed A . Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom Rev. (2017) 36:115–34. doi: 10.1002/mas.21455
83.
Vandereyken K Sifrim A Thienpont B Voet T . Methods and applications for single-cell and spatial multi-omics. Nat Rev Genet. (2023) 24:494–515. doi: 10.1038/s41576-023-00580-2
84.
Liu X Yue Z Cao Y Taylor L Zhang Q Choi SW et al . Graft-versus-host disease-free antitumoral signature after allogeneic donor lymphocyte injection identified by proteomics and systems biology. JCO Precis Oncol. (2019) 3:PO.18.00365. doi: 10.1200/po.18.00365
85.
Huang Q Su J Zhang W Chang S Li S Zhou J et al . Multi-omics analysis of biomarkers and molecular mechanism of rheumatoid arthritis with bone destruction. Joint Bone Spine. (2022) 89:105438. doi: 10.1016/j.jbspin.2022.105438
86.
Mann M Kumar C Zeng W-F Strauss MT . Artificial intelligence for proteomics and biomarker discovery. Cell Syst. (2021) 12:759–70. doi: 10.1016/j.cels.2021.06.006
87.
Levine JE . Prediction and prognostication of acute graft-versus-host disease by MAGIC biomarkers. Am J Hematol. (2025) 100:5–13. doi: 10.1002/ajh.27594
88.
Schrezenmeier EV Barasch J Budde K Westhoff T Schmidt-Ott KM . Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf). (2017) 219:554–72. doi: 10.1111/apha.12764
89.
Murray AD . A new biomarker classification system for AD, independent of cognition: agnosticism is a start. Neurology. (2016) 87:456–7. doi: 10.1212/WNL.0000000000002931
90.
Srinagesh HK Özbek U Kapoor U Ayuk F Aziz M Ben-David K et al . The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv. (2019) 3:4034–42. doi: 10.1182/bloodadvances.2019000791
91.
Wu P Wang Z Sun Y Cheng Z Wang M Wang B . Extracellular vesicles: a new frontier in diagnosing and treating graft-versus-host disease after allogeneic hematopoietic cell transplantation. J Nanobiotechnol. (2025) 23:251. doi: 10.1186/s12951-025-03297-y
92.
Bidgoli A DePriest BP Saatloo MV Jiang H Fu D Paczesny S . Current definitions and clinical implications of biomarkers in graft-versus-host disease. Transplant Cell Ther. (2022) 28:657–66. doi: 10.1016/j.jtct.2022.07.008
93.
Wegner J Weidenthaler-Barth B Engelbert J Knothe M Braun C Helbig D et al . Immunohistochemical markers for histopathological diagnosis and differentiation of acute cutaneous graft-versus-host disease. Exp Dermatol. (2021) 30:1814–9. doi: 10.1111/exd.14416
94.
Dai H Rachakonda SP Penack O Blau IW Blau O Radujkovic A et al . Polymorphisms in CXCR3 ligands predict early CXCL9 recovery and severe chronic GVHD. Blood Cancer J. (2021) 11:42. doi: 10.1038/s41408-021-00434-2
95.
Lim J-Y Ryu D-B Lee S-E Park G Min C-K . Mesenchymal stem cells (MSCs) attenuate cutaneous Sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model. J Invest Dermatol. (2017) 137:1895–904. doi: 10.1016/j.jid.2017.02.986
96.
Etra A Gergoudis S Morales G Spyrou N Shah J Kowalyk S et al . Assessment of systemic and gastrointestinal tissue damage biomarkers for GVHD risk stratification. Blood Adv. (2022) 6:3707–15. doi: 10.1182/bloodadvances.2022007296
97.
Leotta S Sapienza G Camuglia MG Avola G Marco AD Moschetti G et al . Preliminary results of a combined score based on sIL2-rα and TIM-3 levels assayed early after hematopoietic transplantation. Front Immunol. (2019) 10:3158. doi: 10.3389/fimmu.2019.03158
98.
Barba P Hilden P Devlin SM Maloy M Dierov D Nieves J et al . Ex vivo CD34+-selected T cell-depleted peripheral blood stem cell grafts for allogeneic hematopoietic stem cell transplantation in acute leukemia and myelodysplastic syndrome is associated with low incidence of acute and chronic graft-versus-host disease and high treatment response. Biol Blood Marrow Transplant. (2017) 23:452–8. doi: 10.1016/j.bbmt.2016.12.633
99.
Shen M-Z Li J-X Zhang X-H Xu L-P Wang Y Liu K-Y et al . Meta-analysis of Interleukin-2 receptor antagonists as the treatment for steroid-refractory acute graft-versus-host disease. Front Immunol. (2021) 12:749266. doi: 10.3389/fimmu.2021.749266
100.
Robin M Porcher R Michonneau D Taurines L de Fontbrune FS Xhaard A et al . Prospective external validation of biomarkers to predict acute graft-versus-host disease severity. Blood Adv. (2022) 6:4763–72. doi: 10.1182/bloodadvances.2022007477
101.
Gao Y Li W Bu X Xu Y Cai S Zhong J et al . Human amniotic mesenchymal stem cells inhibit aGVHD by regulating balance of Treg and T effector cells. J Inflamm Res. (2021) 14:3985–99. doi: 10.2147/JIR.S323054
102.
McManigle W Youssef A Sarantopoulos S . B cells in chronic graft-versus-host disease. Hum Immunol. (2019) 80:393–9. doi: 10.1016/j.humimm.2019.03.003
103.
Milosevic E Babic A Iovino L Markovic M Grce M Greinix H . Use of the NIH consensus criteria in cellular and soluble biomarker research in chronic graft-versus-host disease: a systematic review. Front Immunol. (2022) 13:1033263. doi: 10.3389/fimmu.2022.1033263
104.
Inamoto Y Martin PJ Onstad LE Cheng G-S Williams KM Pusic I et al . Relevance of plasma matrix Metalloproteinase-9 for bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplantation. Transplant Cell Ther. (2021) 27:759.e1–8. doi: 10.1016/j.jtct.2021.06.006
105.
Wang B Yin Y Li Y Liang Z Liu W Sun Y et al . Combination of ST2 with organ-specific biomarker is more sensitive and specific for the diagnosis of acute graft-vs-host disease. Transplant Proc. (2023) 55:1706–14. doi: 10.1016/j.transproceed.2023.04.043
106.
Yang J Peng B Wang L Li X Li F Jin X et al . Elevated REG3α predicts refractory aGVHD in patients who received steroids-ruxolitinib as first-line therapy. Ann Hematol. (2022) 101:621–30. doi: 10.1007/s00277-021-04727-1
107.
Jia W Poe JC Su H Anand S Matsushima GK Rathmell JC et al . BAFF promotes heightened BCR responsiveness and manifestations of chronic GVHD after allogeneic stem cell transplantation. Blood. (2021) 137:2544–57. doi: 10.1182/blood.2020008040
108.
Talvard-Balland N Braun LM Dixon KO Zwick M Engel H Hartmann A et al . Oncogene-induced TIM-3 ligand expression dictates susceptibility to anti-TIM-3 therapy in mice. J Clin Invest. (2024) 134:e177460. doi: 10.1172/JCI177460
109.
Griesenauer B Jiang H Yang J Zhang J Ramadan AM Egbosiuba J et al . ST2/MyD88 deficiency protects mice against acute graft-versus-host disease and spares regulatory T cells. J Immunol. (2019) 202:3053–64. doi: 10.4049/jimmunol.1800447
110.
Li X Chen T Gao Q Zhang W Xiao Y Zhu W et al . A panel of 4 biomarkers for the early diagnosis and therapeutic efficacy of aGVHD. JCI Insight. (2019) 4:130413. doi: 10.1172/jci.insight.130413
111.
Huang A Zhao X Li M Tang G Fei Y Wang R et al . Suppression of hematopoietic primitive cells in patients with secondary failure of platelet recovery after acute graft-versus-host disease. Biol Blood Marrow Transplant. (2020) 26:1840–54. doi: 10.1016/j.bbmt.2020.06.003
112.
Luo Y Xu C Wang B Niu Q Su X Bai Y et al . Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human Treg compartment. Nat Commun. (2021) 12:3913. doi: 10.1038/s41467-021-24213-6
113.
Wang L Dai B Gao W Wang J Wan M Wang R et al . Clinical significance of Haplo-fever and cytokine profiling after graft infusion in allogeneic stem cell transplantation from Haplo-identical donors. Front Med. (2022) 9:820591. doi: 10.3389/fmed.2022.820591
114.
Wolff D Greinix H Lee SJ Gooley T Paczesny S Pavletic S et al . Biomarkers in chronic graft-versus-host disease: quo vadis?Bone Marrow Transplant. (2018) 53:832–7. doi: 10.1038/s41409-018-0092-x
115.
Chai Y-J Lu N-D Li P Su S-F Wei H-X Xu Y et al . The value of REG3α, sST2, and TNFR1 in risk stratification and prognostic evaluation of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation in children. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2024) 32:1566–70. doi: 10.19746/j.cnki.issn.1009-2137.2024.05.041
116.
El Jurdi N Hamilton BK Pidala JA Onstad L Mun C Jain S et al . Longitudinal tear cytokine biomarkers: an analysis from the close assessment and testing for chronic graft-versus-host disease (CATCH) protocol. Transplant Cell Ther. (2025) 31:226.e1–9. doi: 10.1016/j.jtct.2025.02.004
117.
DePriest BP Li H Bidgoli A Onstad L Couriel D Lee SJ et al . Regenerating islet-derived protein 3-α is a prognostic biomarker for gastrointestinal chronic graft-versus-host disease. Blood Adv. (2022) 6:2981–6. doi: 10.1182/bloodadvances.2021005420
118.
Motta A Zhan Q Larson A Lerman M Woo S-B Soiffer RJ et al . Immunohistopathological characterization and the impact of topical immunomodulatory therapy in oral chronic graft-versus-host disease: a pilot study. Oral Dis. (2018) 24:580–90. doi: 10.1111/odi.12813
119.
Cuvelier GDE Ng B Abdossamadi S Nemecek ER Melton A Kitko CL et al . A diagnostic classifier for pediatric chronic graft-versus-host disease: results of the ABLE/PBMTC 1202 study. Blood Adv. (2023) 7:3612–23. doi: 10.1182/bloodadvances.2022007715
120.
Giesen N Schwarzbich M-A Dischinger K Becker N Hummel M Benner A et al . CXCL9 predicts severity at the onset of chronic graft-versus-host disease. Transplantation. (2020) 104:2354–9. doi: 10.1097/TP.0000000000003108
121.
Logan BR Fu D Howard A Fei M Kou J Little MR et al . Validated graft-specific biomarkers identify patients at risk for chronic graft-versus-host disease and death. J Clin Invest. (2023) 133:e168575. doi: 10.1172/JCI168575
122.
Crossland RE Norden J Ghimire S Juric MK Pearce KF Lendrem C et al . Profiling tissue and biofluid miR-155-5p, miR-155*, and miR-146a-5p expression in graft vs. host disease. Front Immunol. (2021) 12:639171. doi: 10.3389/fimmu.2021.639171
123.
Lia G Di Vito C Bruno S Tapparo M Brunello L Santoro A et al . Extracellular vesicles as biomarkers of acute graft-vs.-host disease after Haploidentical stem cell transplantation and post-transplant cyclophosphamide. Front Immunol. (2021) 12:816231. doi: 10.3389/fimmu.2021.816231
124.
Crossland RE Perutelli F Bogunia-Kubik K Mooney N Milutin Gašperov N Pučić-Baković M et al . Potential novel biomarkers in chronic graft-versus-host disease. Front Immunol. (2020) 11:602547. doi: 10.3389/fimmu.2020.602547
125.
Wu Y Mealer C Schutt S Wilson CL Bastian D Sofi MH et al . MicroRNA-31 regulates T-cell metabolism via HIF1α and promotes chronic GVHD pathogenesis in mice. Blood Adv. (2022) 6:3036–52. doi: 10.1182/bloodadvances.2021005103
126.
Zavaro M Dangot A Bar-Lev TH Amit O Avivi I Ram R et al . The role of extracellular vesicles (EVs) in chronic graft vs. host disease, and the potential function of placental cell-derived EVs as a therapeutic tool. Int J Mol Sci. (2023) 24:8126. doi: 10.3390/ijms24098126
127.
Li X Lin Y Li X Xu X Zhao Y Xu L et al . Tyrosine supplement ameliorates murine aGVHD by modulation of gut microbiome and metabolome. EBioMedicine. (2020) 61:103048. doi: 10.1016/j.ebiom.2020.103048
128.
Rashidi A Pidala J Hamilton BK Pavletic SZ Kim K Zevin A et al . Oral and gut microbiome alterations in Oral chronic GVHD disease: results from close assessment and testing for chronic GVHD (CATCH study). Clin Cancer Res. (2024) 30:4240–50. doi: 10.1158/1078-0432.CCR-24-0875
Summary
Keywords
graft-versus-host disease, biomarker, classification system, integration pathways, allogeneic hematopoietic stem cell transplantation, precision medicine
Citation
Yu X, Huang S, Li X, Zhao Y, Jin X, Fan M, Zhang Y and Ma L (2025) Current status, challenges, and integration pathways of biomarker classification systems in graft-versus-host disease: a preliminary exploration. Front. Med. 12:1690221. doi: 10.3389/fmed.2025.1690221
Received
21 August 2025
Accepted
16 October 2025
Published
31 October 2025
Volume
12 - 2025
Edited by
Diana Cenariu, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Reviewed by
Delia Dima, Oncology Institute Prof. Dr. Ion Chiricuta, Romania
Andra Daniela Marcu, Carol Davila University of Medicine and Pharmacy, Romania
Updates
Copyright
© 2025 Yu, Huang, Li, Zhao, Jin, Fan, Zhang and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanfeng Zhang, yuanfengzhang126@126.com; Lusheng Ma, mls13854502448@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.