Abstract
Neurotrophic tyrosine receptor kinase (NTRK)-rearranged spindle cell neoplasms (NTRK-RSCNs) constitute a rare, heterogeneous subset of soft tissue tumors defined by oncogenic fusions involving NTRK1, NTRK2, or NTRK3 genes. Despite the remarkable efficacy of TRK inhibitor therapy in fusion-positive tumors, the histomorphologic variability of NTRK-RSCNs poses significant diagnostic challenges, and data on malignant transformation remain limited. Herein, we report a unique case of LMNA::NTRK1-rearranged spindle cell neoplasm in a 23-year-old woman, characterized by previously undescribed pigmentation, multiple local recurrences, and fibrosarcoma-like malignant transformation—features that have not been documented in prior literature. Through integrated histopathological, immunohistochemical, and molecular analyses, we characterize the diagnostic nuances, biological behavior, and potential drivers of progression in this entity. Our findings expand the morphological and clinical spectrum of LMNA::NTRK1-rearranged tumors and highlight the need for close follow-up and consideration of adjuvant targeted therapy in high-risk cases.
1 Introduction
Neurotrophic tyrosine receptor kinase (NTRK)-rearranged spindle cell neoplasms (NTRK-RSCNs) are a recently recognized category of mesenchymal tumors, formally classified in the 2020 WHO Classification of Soft Tissue and Bone Tumors as distinct entities based on their molecular signature and therapeutic relevance (1). These tumors are driven by oncogenic fusions involving NTRK genes (NTRK1, NTRK2, NTRK3), which encode tropomyosin receptor kinase (TRK) proteins—transmembrane receptors critical for cell proliferation, differentiation, and survival (2). Among NTRK-RSCN subtypes, LMNA::NTRK1 fusion represents an emerging variant, with only 39 cases reported in the literature to date (2016–2025) (3–27).
LMNA::NTRK1-rearranged tumors exhibit a broad age distribution (0.2–57 years; median 19 years), with slightly more cases occurring in female individuals (52.5%), and a predilection for the limbs/trunk (62.5%), followed by the head/neck (17.5%) and gastrointestinal tract (15%) (3–27). Histopathologically, they typically present as hypocellular spindle cell proliferations in a collagenous stroma (fibromatosis-like), with fascicular growth or lymphoplasmacytic infiltration (inflammatory myofibroblastic tumor-like) and consistent immunophenotypic features including CD34, S100, and Pan-TRK positivity (3, 7, 12). Clinically, the majority of cases follow an indolent course, with an 8.8% local recurrence rate and a 2.9% distant metastasis rate after surgical resection (3–27). However, rare morphological variants (e.g., pigmented lesions) and malignant transformation have not been previously documented, creating gaps in our understanding of the biological spectrum and prognostic factors of these tumors.
Accurate diagnosis of LMNA::NTRK1-rearranged neoplasms is clinically critical due to the availability of TRK inhibitors, which achieve response rates exceeding 75% in NTRK fusion-positive solid tumors (2, 28). Nevertheless, diagnostic challenges persist due to histomorphologic overlap with mimickers such as desmoid-type fibromatosis, solitary fibrous tumor (SFT), and gastrointestinal stromal tumor (GIST) (3, 15). Furthermore, the factors driving recurrence and malignant transformation in these tumors remain unclear, with no prior reports linking p53 mutation to disease progression.
To address these knowledge gaps, we present the first case of LMNA::NTRK1-rearranged spindle cell neoplasm with pigmentation, multiple local recurrences, and fibrosarcoma-like malignant transformation. We discuss the diagnostic utility of integrated histopathological and molecular testing, the potential role of p53 mutation in tumor progression, and the clinical implications for adjuvant therapy.
2 Case presentation
2.1 Clinical information
A 23-year-old Asian woman presented to our institution with a painless scalp nodule that had been present for a duration of 10 years. Physical examination revealed a slightly elevated lesion measuring 1.0 cm × 0.8 cm on the right parietal scalp, with normal overlying skin and no tenderness or lymphadenopathy. The lesion had remained stable in size until 21 months prior to presentation, when the patient noted gradual enlargement. No relevant personal or family history of tumors was identified.
2.2 Pathological findings
2.2.1 Gross pathology
The resected specimen was a 2.0 cm × 1.8 cm × 1.5 cm firm nodule with a grayish-white cut surface, well-circumscribed margins, and no evidence of necrosis. The overlying skin contained intact hair follicles.
2.2.2 Histopathology
Tumor cells were spindle-shaped with uniform morphology, arranged in a matted pattern—defined as irregularly clustered, merging nodules with ill-defined inter-nodular boundaries (distinct from fascicular or storiform architectures) (Figure 1A). Prominent pigment deposition, spindle cell proliferation, and lymphocytic infiltration were observed (Figure 1B). Scattered dendritic pigment-containing cells were identified in the stroma (Figure 1C). Tumor cells exhibited mild nuclear polymorphism with rare mitotic figures (<1/10 high-power fields [HPF]). Lymphocytes, some forming germinal centers, were distributed between spindle cells and adipocytes.
Figure 1

For the excised specimen from the first surgery, fusiform cells of uniform size were arranged, with fat visible in the interstitium (A). The surrounding area showed lymphocyte aggregation (B) and pigment cell deposition (C). Nuclear atypia and pathological mitoses were rare. Immunohistochemically, the tumor cells were focally positive for S100 (D), while diffusely positive for CD34 (E) and P-TRK (F). SMA (G) was completely negative. P53 expression was diffusely positive, consistent with overexpression related to mutation (H). Ki-67 proliferation index was not more than 2% (I).
2.2.3 Immunohistochemistry (IHC)
Tumor cells were positive for β-catenin (diffuse), vimentin (diffuse), S100 (90% of cells; Figure 1D), CD34 (80% of cells; Figure 1E), Bcl-2 (focal), Pan-TRK (diffuse; Figure 1F), and CD99 (focal). They were negative for SMA (Figure 1G), TLE-1, Melan A, EMA, STAT6, Sox10, and Desmin. Diffuse nuclear positivity for p53 was detected (consistent with pathogenic mutation; Figure 1H). The Ki-67 proliferation index was <2% (Figure 1I). Surgical margins were negative for tumor (Figure 2A).
Figure 2

In the first (A) and third surgeries (C), no tumor cells were detected at the incision margin on pathological examination, whereas tumor cells were identified at the incision margin in the second surgery (B).
2.2.4 Molecular testing
Targeted RNA sequencing identified an in-frame LMNA::NTRK1 fusion, with breakpoints at LMNA exon 6 and NTRK1 exon 11 (Figures 3A,B). No other pathogenic mutations or fusions were detected.
Figure 3

FISH analysis demonstrated the fusion of the LMNA-NTRK1 gene using an NTRK1 break-apart probe (A). RNA-based sequencing identified an LMNA-NTRK1 fusion (B).
2.3 First recurrence (21 months after initial resection)
The patient presented with a 2.5-cm firm nodule at the same scalp site. Gross pathology: A 2.8 cm × 2.5 cm × 2.0 cm nodule with a tan-yellow cut surface and irregular margins.
2.3.1 Histopathology
Spindle cell proliferation was retained, but with increased cellular atypia, hyperchromatic nuclei, and fibrosarcoma-like malignant transformation (fascicular arrangement of pleomorphic spindle cells; Figures 4A–C). Mitotic activity was increased (3/10 HPF).
Figure 4
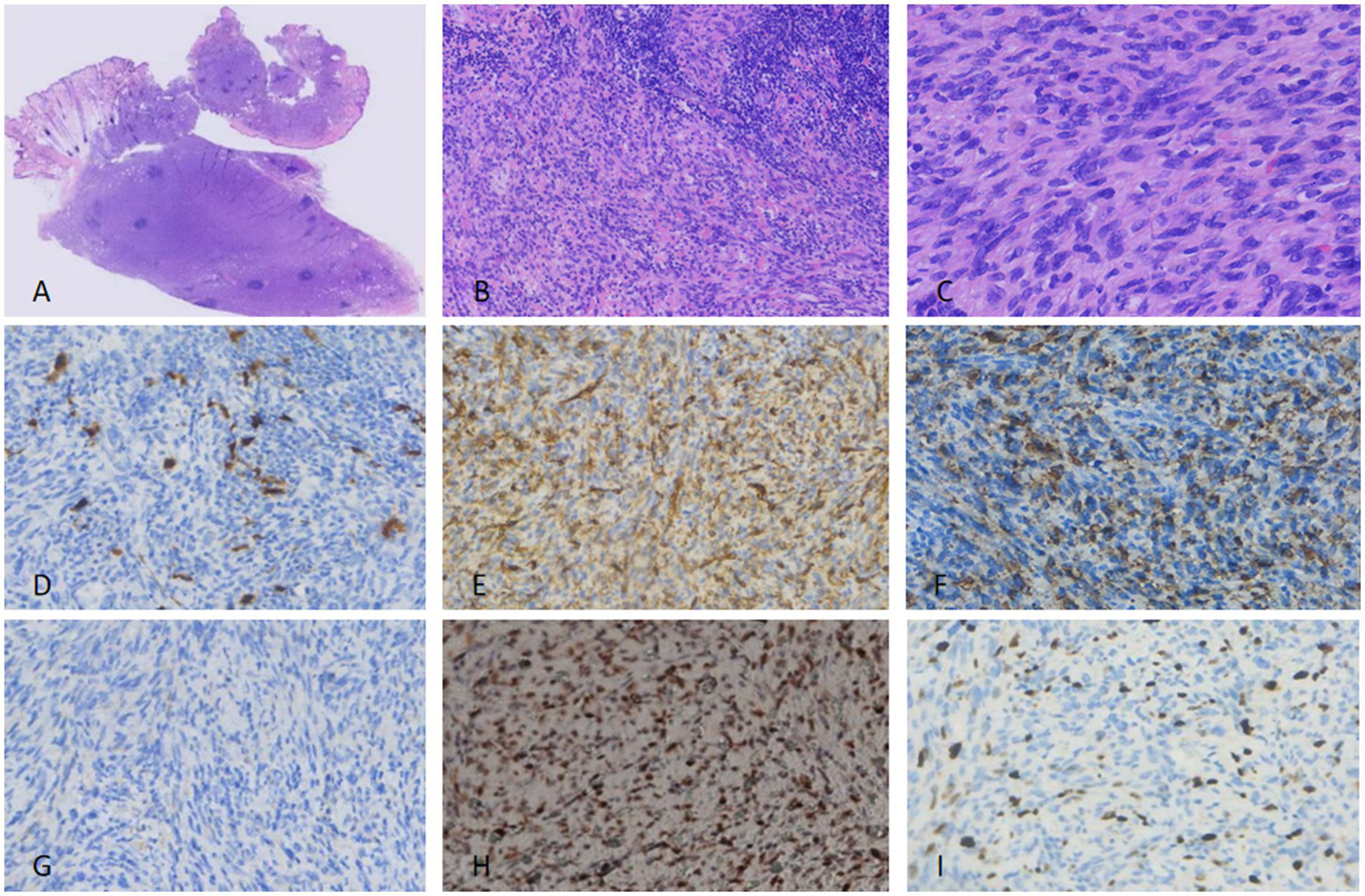
For the excised specimen of the first recurrence, tightly packed spindle-shaped cells were arranged in fascicular, whorled, and storiform patterns with lymphocyte aggregation present. Pigment cells were no longer visible. Moderate nuclear atypia and pathological mitoses were observed (A–C). Immunohistochemically, the tumor cells showed hardly any positivity for S100 (D)—a finding distinct from the primary specimen. CD34 (E) and P-TRK (F) were focally positive, while SMA remained negative (G). P53 xpression was still diffusely positive. (H) Ki-67 proliferation index was nearly 20% (I).
2.3.2 IHC
Results were consistent with the primary tumor, except for the loss of S100 expression (Figure 4D) and an elevated Ki-67 index (20%; Figure 4I). Pan-TRK (Figure 4F), CD34 (Figure 4E), and p53 (Figure 4H) remained positive; SMA was focally positive (Figure 4G). Tumor cells were identified at the surgical margins (Figure 2B).
2.4 Second recurrence (6 months after first re-excision)
The patient developed a third nodule at the same site, requiring re-resection. Gross pathology: A 3.0 cm × 2.8 cm × 2.2 cm firm nodule with a grayish-tan cut surface and infiltrative margins.
2.4.1 Histopathology
Marked nuclear atypia, increased mitotic activity (5/10 HPF), and prominent fibrosarcoma-like morphology were observed (Figures 5A–C). No necrosis was identified.
Figure 5
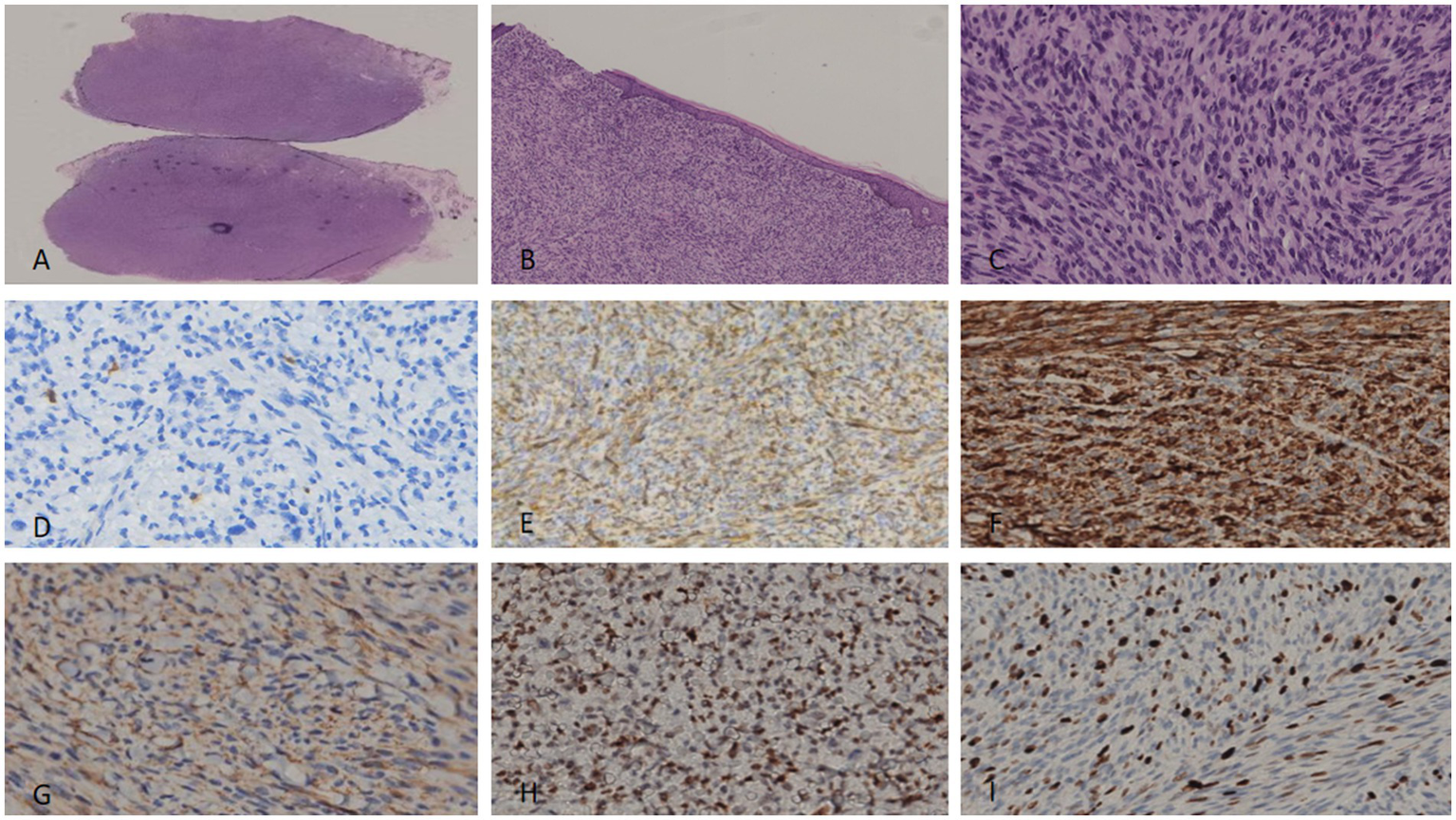
For the excised specimen of the second recurrence, tightly packed spindle-shaped cells were arranged in a storiform pattern, with obvious nuclear atypia and pathological mitoses. Pigment cells had completely disappeared (A–C). Immunohistochemically, the tumor cells were negative for S100 (D), while CD34 (E), P-TRK (F), SMA (G), and P53 (H) showed diffuse positivity. The Ki-67 proliferation index was even higher than before, reaching more than 40% (I).
2.4.2 IHC
S100 remained negative (Figure 5D), while CD34 (Figure 5E), Pan-TRK (Figure 5F), SMA (Figure 5G), and p53 (Figure 5H) were diffusely positive. The Ki-67 index was 40% (Figure 5I). Surgical margins were negative for tumors (Figure 2C).
2.5 Treatment and follow-up
The primary tumor was treated with a wide local excision (margin ≥1 cm). After the first recurrence, the patient underwent re-excision with margin control. Following the second recurrence, radical resection was performed. TRK inhibitor therapy (larotrectinib) was recommended postoperatively, but the patient declined. At 12-month follow-up after the third resection, no evidence of further recurrence or distant metastasis was detected. Clinical and pathological features are summarized in Figure 6.
Figure 6
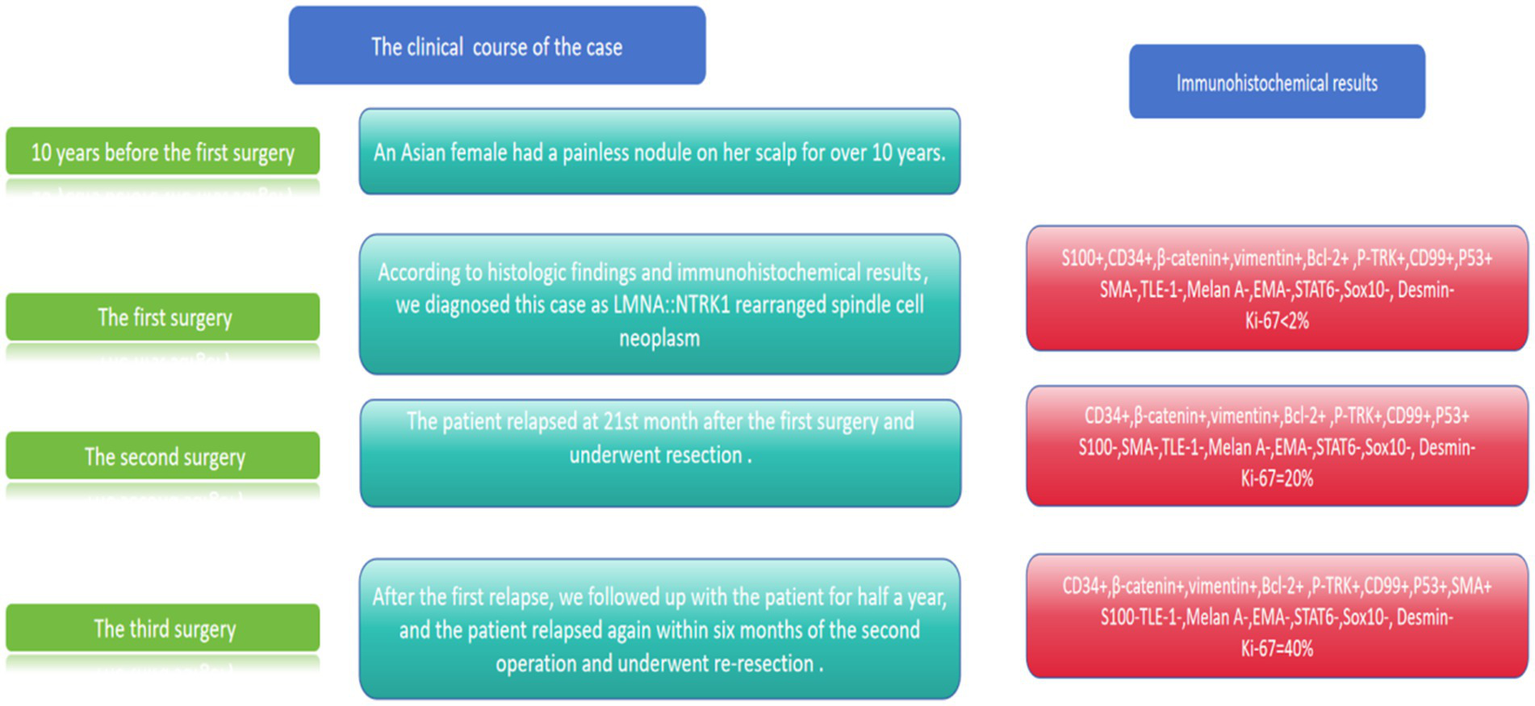
Clinical course and immunohistochemical results of our case.
3 Literature review
3.1 Search strategy
A comprehensive literature search was conducted in PubMed, Embase, and Web of Science databases using the terms (“LMNA::NTRK1” OR “LMNA-NTRK1 fusion”) AND (“spindle cell neoplasm” OR “soft tissue tumor” OR “sarcoma”) from January 2016 to March 2025. Studies were included if they reported clinical, pathological, or molecular data on LMNA::NTRK1-rearranged spindle cell neoplasms. Duplicates, review articles, and non-English studies were excluded. Two independent researchers screened titles, abstracts, and full texts, with discrepancies resolved by consensus.
3.2 Summary of published cases
Including our case, 40 patients with LMNA::NTRK1-rearranged spindle cell neoplasms have been reported (Table 1). Demographic features: Male-to-female ratio 19:21 (47.5%:52.5%), age range 0.2–57 years (mean ± SD 16.5 ± 17.5 years; median 19 years). Tumor locations: Limbs/trunk (25 cases, 62.5%), gastrointestinal tract (6 cases, 15%), head/neck (7 cases, 17.5%), and lungs (2 cases, 5%).
Table 1
| Case | Author | Age/Gender | Site | Gene fusion type | Positive immunohistochemistry | Negative immunohistochemistry | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Haller et al. (4) | 2 years/Female | Paravertebral lumbar | LMNA::NTRK1 | α-SMA | Desmin, h-caldesmon, CD34, STAT6, EMA, CK | Excision, radiotherapy | No evidence of disease |
| 2 | Agaram et al. (5) | 4 years/Female | Thigh | LMNA:NTRK1 | S100, CD34, NTRK1 | Sox10, HMB45, Melan A, Desmin, GFAP, STAT6 | Tumor resection | No evidence of disease |
| 3 | Agaram et al. (5) | 28 years/Female | Flank | LMNA::NTRK1 | S100 | NTRK1, Sox10, HMB45, Melan A, Desmin, GFAP, STAT6 | Tumor resection | No evidence of disease |
| 4 | Agaram et al. (5) | 15 years/Male | Forearm | LMNA::NTRK1 | S100, NTRK1 | Sox10, HMB45, Melan A, Desmin, GFAP, STAT6 | NA | NA |
| 5 | Agaram et al. (5) | 12 years/Male | Arm | LMNA::NTRK1 | S100, CD34, NTRK1 | Sox10, HMB45, Melan A, Desmin, GFAP, STAT6 | Tumor resection | No evidence of disease |
| 6 | Davis et al. (6) | 2 months/Female | Back | LMNA::NTRK1 | S100, CD34, NTRK1, CD30 | SMA | Tumor resection | No evidence of disease |
| 7 | Kohsaka et al. (7) | 6 years/Female | Right elbow | LMNA::NTRK1 | CD34, S100, EMA, CK | myogenic marker | Tumor resection | No evidence of disease |
| 8 | Warren et al. (8) | 3 years/Female | Back | LMNA::NTRK1 | S100, CD34, NTRK1 | Myogenin, Desmin, CD117, DOG1, Bcl2, CD68, factor XIIIA | Tumor resection | No evidence of disease |
| 9 | Malik et al. (9) | 3 years/Female | Right buttock | LMNA::NTRK1 | S100, CD34, SMA, Pan-TRK | Desmin, CD117, ERG | Tumor resection | NA |
| 10 | Dupuis et al. (10) | 21 years/Male | Lumber | LMNA::NTRK1 | CD34,pan-TRK, S100, SMA, SATB2, H3K27me3 | STAT6, DOG1, Desmin, TLE-1, Melan-A, Sox10 | Targeted therapy, surgery | No evidence of disease |
| 11 | Yin et al. (11) | 3 months/Female | Knee | LMNA::NTRK1 | Vimentin, CD34, MSA, SMA, Pan-TRK | Alk | Chemotherapy | No recurrence |
| 12 | Yin et al. (11) | 3 years/Male | Thigh | LMNA::NTRK1 | Vimentin, CD34, MSA, SMA, Desmin, Pan-TRK | Alk | Chemotherapy, targeted therapy | NA |
| 13 | Kang et al. (12) | 3 years/Male | Forehead | LMNA::NTRK1 | Trk, S100, CD34, nestin, vimentin, CD3 | CD56, SMA, Desmin, myogenin, STAT6, EMA, CK, CD1a, CD21, CD35, CD43, WT-1, MelanA, HMB45, BRAF, ALK | Tumor resection | No evidence of disease |
| 14 | So et al. (13) | 40 years/Female | Calf | LMNA::NTRK1 | S100, CD34, SMA, Rb, INI-1, Pan-TRK | SoxX10, STAT6, GFAP, calponin, Desmin, MUC4, CD56, CDK4, CD31, CAM5.2, MNF116, CK, EMA, p63 | Tumor resection, chemotherapy | Lung metastasis |
| 15 | Atiq et al. (14) | 4 years/Female | Stomach | LMNA::NTRK1 | Trk, S100, CD34 | Sox10, EMA Kit, DOG1, Desmin, STAT6, ALK, CK | Tumor resection | NA |
| 16 | Brčić et al. (15) | 50 years/Male | Neck | LMNA::NTRK1 | pan-TRK, CD34, S100 | Sox10, H3K27me3 | Excised with clear margins | No evidence of disease |
| 17 | Panse et al. (3) | 31 years/Female | Scalp | LMNA: NTRK1 | CD34, S100, pan-TRK | Sox10, CK, EMA, Desmin, CD21, CD23, ALK | Excised with clear margins | No recurrence |
| 18 | Yin et al. (26) | 9 months/Male | Back | LMNA::NTRK1 | S100, CD34, H3K27Me3, TRK-A, Pan-TRK | Sox-10 | Tumor resection | NA |
| 19 | Yin et al. (26) | 3 years/Female | Abdominal wall | LMNA::NTRK1 | S100, CD34, H3K27Me3, TRK-A, Pan-TRK | Sox-10 | Extended tumor resection | Local recurrence |
| 20 | Yin et al. (26) | 4 years/Female | Right upper arm | LMNA::NTRK1 | S100, CD34, H3K27Me3, TRK-A, Pan-TRK | Sox-10 | Extended tumor resection | Local recurrence |
| 21 | Yin et al. (26) | 22 years/Male | Right buttock | LMNA::NTRK1 | S100, CD34, H3K27Me3, TRK-A, Pan-TRK | Sox-10 | Tumor resection | No evidence of disease |
| 22 | Yin et al. (26) | 23 years/Male | Rectum | LMNA::NTRK1 | S100, CD34, H3K27Me3, TRK-A, Pan-TRK | Sox-10 | Tumor resection | NA |
| 23 | Zhu et al. (23) | 31 years/Male | Lung(Right upper lobe) | LMNA::NTRK1 | S100, CD34, H3K27Me3, TRK-A, Pan-TRK | Sox-10, α-SMA, Desmin, ALK, STAT6, calponin | Tumor resection | No evidence of disease |
| 24 | Tsai et al. (20) | 34 years/Male | Right lung | LMNA::NTRK1 | CD34, H3K27Me3, P53, Pan-TRK | S100, P16 | No resection | Alive with disease |
| 25 | Rahim et al. (18) | 20 years/Female | Ileum | LMNA::NTRK1 | Pan-TRK, S100, CD34, H3K27Me3 | CD117, DOG1, STAT6, Sox-10, CK, EMA, Desmin, SMA, HMB-45, Melan-A, ALK1,β-calponin, SYN. CgA | Tumor resection | No recurrence |
| 26 | Tauziede-Espariat et al. (22) | 21 years/Female | Thorax | LMNA::NTRK1 | CD34, S100 | Sox-10 | NA | NA |
| 27 | Kobayashi et al. (21) | 23 years/Female | Lower leg | LMNA::NTRK1 | CD34, S100 | STAT6 | Tumor resection | Complete disease-free |
| 28 | Kobayashi et al. (21) | 35 years/Male | Perineal | LMNA::NTRK1 | CD34, S100 | STAT6 | Tumor resection | Complete disease-free |
| 29 | Czaja et al. (17) | 11 years/Male | Back | LMNA::NTRK1 | Pan-TRK, S100, CD34, CD30 | Sox-10 | Excised with clear margins | Free of disease |
| 30 | Wei et al. (16) | 57 years/Male | Right buttock | LMNA::NTRK1 | Pan-TRK, S100, CD34, Caldesmin | Sox-10, SMA, Desmin, EMA, ALK, STAT6, CD31, ALK | Tumor resection | No recurrence |
| 31 | Jian et al. (25) | 47 years/Male | Ascending colon | LMNA::NTRK1 | S100, CD34, Pan-TRK | Sox-10, CK, EMA, SMA, CD117, Desmin, DOG1, ALK | Tumor resection | No recurrence |
| 32 | Gao et al. (24) | 7 years/Male | Descending colon | LMNA::NTRK1 | S100, CD34, H3K27Me3, Pan-TRK | Sox-10, AE1/AE3, SMA, CD117, Desmin, DOG1, ALK, STAT6 | Tumor resection | No evidence of disease |
| 33 | Gao et al. (24) | 45 years/Female | Transverse colon | LMNA::NTRK1 | CD34, H3K27Me3, TRK-A, Pan-TRK | S100, Sox-10, AE1/AE3, SMA, CD117, Desmin, DOG1, ALK, STAT6 | Tumor resection | No evidence of disease |
| 34 | Gao et al. (24) | 34 years/Female | Ascending colon | LMNA::NTRK1 | CD34, H3K27Me3 | Sox-10, AE1/AE3, SMA, CD117, Desmin, DOG1, ALK, STAT6 | Tumor resection | No evidence of disease |
| 35 | Suurmeijer et al. (19) | 4 years/Male | Mandible | LMNA::NTRK1 | S100, CD34 | NA | Tumor resection | No recurrence |
| 36 | Suurmeijer et al. (19) | 13 years/Male | Maxilla | LMNA::NTRK1 | S100, CD34 | Sox-10 | Tumor resection | No recurrence |
| 37 | Klubíčková N et al. (27) | 3 years/Female | Lower eyelid | LMNA::NTRK1 | S100, CD34, Pan-TRK | NA | Surgery, targeted therapy | Local recurrence |
| 38 | Klubíčková N et al. (27) | 37 years/Female | Instep | LMNA::NTRK1 | S100, CD34, Pan-TRK | NA | Tumor resection | No evidence of disease |
| 39 | Klubíčková N et al. (27) | 43 years/Male | Hip | LMNA::NTRK1 | S100, CD34, Pan-TRK | NA | Tumor resection | No evidence of disease |
Clinicopathological features of LMNA::NTRK1 spindle cell neoplasm in the literature.
Histopathology: The majority of cases exhibited fibromatosis-like (hypocellular spindle cells in collagenous stroma) or inflammatory myofibroblastic tumor-like (fascicular growth with lymphoplasmacytic infiltration) morphology. Hemangiopericytoma-like vessels and CD34 positivity were common, mimicking SFT. Myxoid degeneration was rare. Typically, tumors showed low mitotic activity (<2/10 HPF) and minimal pleomorphism. Pigmentation and fibrosarcoma-like transformation were not reported in any prior case.
3.2.1 Immunophenotype
Consistent findings included CD34 positivity (36/37 cases, 97.3%), S100 positivity (33/35 cases, 94.3%), and Pan-TRK positivity (23/23 cases, 100%). All cases were negative for Sox10 (22/22), STAT6 (17/17), DOG1 (8/8), and CD117 (7/7). SMA was positive in 60% (9/15) of cases, while Desmin was uniformly negative (19/19).
3.2.2 Clinical outcomes
Follow-up data were available for 34 patients. The majority of patients (28 cases, 82.4%) remained disease-free after surgical resection. Local recurrence could occur in 3 cases (8.8%), and lung metastasis in 1 case (2.9%). Five patients (14.7%) received chemotherapy or targeted therapy. No prior cases of multiple recurrences or malignant transformations were documented.
4 Discussion
To our knowledge, this is the first reported case of LMNA::NTRK1-rearranged spindle cell neoplasm with three key novel features: (1) pigmentation (dendritic pigment-containing stromal cells), (2) multiple local recurrences (two episodes within 27 months), and (3) fibrosarcoma-like malignant transformation. These findings expand the morphological and clinical spectrum of LMNA::NTRK1-rearranged tumors, challenging the notion that these tumors uniformly follow an indolent course.
4.1 Diagnostic considerations
LMNA::NTRK1-rearranged neoplasms must be distinguished from several mimickers, particularly given their overlapping histomorphology and immunophenotype.
Pigmented dermatofibrosarcoma protuberans (DFSP): DFSP typically shows storiform spindle cell proliferation and is characterized by COL1A1: PDGFB fusion. Unlike our case, DFSP is S100-negative and STAT6-negative (29).
Melanoma often exhibits marked nuclear pleomorphism and is positive for Melan A, HMB45, and Sox10—all negative in our case (30).
ALK-rearranged melanocytic myxoid spindle cell tumor (MMySTAR): MMySTAR is characterized by myxoid stroma, melanocytic differentiation (Melan A+/HMB45+/Sox10+), and ALK fusion—all of which are features absent in our case (31).
FMR1-ALK-rearranged cutaneous myxoid spindle cell neoplasm: This entity shows myxoid stroma and whole spindle cell arrangement but lacks pigmentation and NTRK fusion (32).
PRRX1-NCOA1-rearranged fibroblastic tumor: These tumors exhibit pigmentation and S100 positivity but are Sox10-positive and harbor PRRX1-NCOA1 fusion (33, 34).
The key diagnostic triad for LMNA::NTRK1-rearranged neoplasms—CD34+, S100+, Pan-TRK + —was present in our case, confirming the diagnosis. Loss of S100 expression in recurrent lesions may represent a marker of malignant transformation. With the increasing application of bioinformatics analyses, additional biomarkers are being identified in these tumors (35, 36).
4.2 Mechanisms of malignant transformation
The molecular drivers of recurrence and malignant transformation in our case remain to be fully elucidated, but several factors may contribute:
-
p53 mutation: Diffuse p53 positivity in all tumor specimens suggests a pathogenic TP53 mutation. TP53 is a critical tumor suppressor gene; mutations are associated with increased genomic instability, malignant progression, and poor prognosis in soft tissue sarcomas (37, 38). Our findings suggest that p53 mutation may cooperate with LMNA::NTRK1 fusion to drive tumor progression, representing a potential prognostic biomarker.
-
Surgical margin status: Tumor cells at the margin of the first recurrence specimen may have contributed to subsequent progression, emphasizing the importance of wide local excision.
-
Absence of adjuvant therapy: The patient declined TRK inhibitor therapy, which may have prevented a recurrence. TRK inhibitors have been shown to induce durable responses in NTRK fusion-positive tumors, even at advanced stages (2, 28).
4.3 Clinical implications
Our case highlights three critical clinical implications: (1) LMNA::NTRK1-rearranged tumors may exhibit malignant transformation, requiring long-term follow-up (39), (2) p53 positivity may serve as a prognostic marker for high-risk disease, and (3) adjuvant TRK inhibitor therapy should be considered in cases with adverse features (e.g., positive margins, pleomorphism, and p53 mutation) (40). Further studies are needed to validate these findings and establish optimal treatment algorithms.
4.4 Limitations
This study has several limitations: (1) it is a single-case report, limiting generalizability, (2) TP53 sequencing was not performed to confirm the mutation, and (3) long-term follow-up is ongoing to assess for distant metastasis.
5 Conclusion
We report the first case of LMNA::NTRK1-rearranged spindle cell neoplasm with pigmentation, multiple recurrences, and fibrosarcoma-like malignant transformation. Our findings expand the morphological and clinical spectrum of this rare entity and suggest that p53 mutation may contribute to disease progression. Accurate diagnosis via integrated histopathological and molecular testing is critical, and adjuvant TRK inhibitor therapy should be considered in high-risk cases. Further collection of cases is needed to better understand the biological behavior and optimal management of LMNA::NTRK1-rearranged neoplasms.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YZ: Writing – original draft, Validation, Visualization, Conceptualization, Writing – review & editing. ZW: Writing – original draft, Visualization, Writing – review & editing. HX: Writing – original draft, Writing – review & editing, Validation. WL: Writing – original draft, Writing – review & editing, Validation. MS: Supervision, Writing – original draft, Writing – review & editing. XL: Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1691619/full#supplementary-material
References
1.
de Alves Castro JV D'Almeida Costa F Torrezan GT Carraro DM Nicolau UR do Nascimento AG . NTRK-rearranged mesenchymal tumour with epithelioid features: expanding the morphological spectrum of NTRK-fused neoplasms. Histopathology. (2022) 80:736–9. doi: 10.1111/his.14546
2.
Drilon A . TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. (2019) 30:viii23–30. doi: 10.1093/annonc/mdz282
3.
Panse G Reisenbichler E Snuderl M Wang WL Laskin W Jour G . LMNA-NTRK1 rearranged mesenchymal tumor (lipofibromatosis-like neural tumor) mimicking pigmented dermatofibrosarcoma protuberans. J Cutan Pathol. (2021) 48:290–4. doi: 10.1111/cup.13772
4.
Haller F Knopf J Ackermann A Bieg M Kleinheinz K Schlesner M et al . Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: a subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. J Pathol. (2016) 238:700–10. doi: 10.1002/path.4701
5.
Agaram NP Zhang L Sung YS Chen CL Chung CT Antonescu CR et al . Recurrent NTRK1 gene fusions define a novel subset of locally aggressive Lipofibromatosis-like neural tumors. Am J Surg Pathol. (2016) 40:1407–16. doi: 10.1097/PAS.0000000000000675
6.
Davis JL Lockwood CM Albert CM Tsuchiya K Hawkins DS Rudzinski ER . Infantile NTRK-associated mesenchymal tumors. Pediatr Dev Pathol. (2018) 21:68–78. doi: 10.1177/1093526617712639
7.
Kohsaka S Saito T Akaike K Suehara Y Hayashi T Takagi T et al . Pediatric soft tissue tumor of the upper arm with LMNA-NTRK1 fusion. Hum Pathol. (2018) 72:167–73. doi: 10.1016/j.humpath.2017.08.017
8.
Warren M Anselmo D Takeda M Shillingford N Hiemenz MC Shah R . NTRK-rearranged mesenchymal tumour in a 3-year-old female: a diagnostic quandary. Histopathology. (2019) 75:772–5. doi: 10.1111/his.13920
9.
Malik F Santiago T Newman S McCarville B Pappo AS Clay MR . An addition to the evolving spectrum of lipofibromatosis and lipofibromatosis-like neural tumor: molecular findings in an unusual phenotype aid in accurate classification. Pathol Res Pract. (2020) 216:152942. doi: 10.1016/j.prp.2020.152942
10.
Dupuis M Shen Y Curcio C Meis JM Wang WL Amini B et al . Successful treatment of lipofibromatosis-like neural tumor of the lumbar spine with an NTRK-fusion inhibitor. Clin Sarcoma Res. (2020) 10:14. doi: 10.1186/s13569-020-00136-6
11.
Yin MZ Ma J He Q Shen P Chen JF Jin XT et al . Clinicopathological characteristics of NTRK-rearranged mesenchymal tumors in childhood. Zhonghua Bing Li Xue Za Zhi. (2020) 49:675–80. doi: 10.3760/cma.j.cn112151-20200214-00095
12.
Kang J Park JW Won JK Bae JM Koh J Yim J et al . Clinicopathological findings of pediatric NTRK fusion mesenchymal tumors. Diagn Pathol. (2020) 15:114. doi: 10.1186/s13000-020-01031-w
13.
So YK Chow C To KF Chan JKC Cheuk W . Myxoid spindle cell sarcoma with LMNA-NTRK fusion: expanding the morphologic spectrum of NTRK-rearranged tumors. Int J Surg Pathol. (2020) 28:574–8. doi: 10.1177/1066896920905888
14.
Atiq MA Davis JL Hornick JL Dickson BC Fletcher CDM Fletcher JA et al . Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: a clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod Pathol. (2021) 34:95–103. doi: 10.1038/s41379-020-0623-z
15.
Brčić I Godschachner TM Bergovec M Igrec J Till H Lackner H et al . Broadening the spectrum of NTRK rearranged mesenchymal tumors and usefulness of pan-TRK immunohistochemistry for identification of NTRK fusions. Mod Pathol. (2021) 34:396–407. doi: 10.1038/s41379-020-00657-x
16.
Wei X Wu HY Pu XH Wang XD Li ZW Sun Q . NTRK rearranged spindle cell tumor: a case report. Zhonghua Bing Li Xue Za Zhi. (2023) 52:1278–80.
17.
Czaja R Layng KV Barrett A Hooper P Alomari A Warren S . An NTRK-1-associated soft tissue tumor showing distinctive nodules with peripheral accentuation of cellularity. J Cutan Pathol. (2023) 50:343–8. doi: 10.1111/cup.14355
18.
Rahim S Alkhaldi SS Alasousi K Ali RH . Intestinal LMNA::NTRK1-fused spindle cell neoplasm with S100 and CD34 coexpression: a new case. BMJ Case Rep. (2022) 15:e251270. doi: 10.1136/bcr-2022-251270
19.
Suurmeijer AJH Xu B Torrence D Dickson BC Antonescu CR . Kinase fusion positive intra-osseous spindle cell tumors: a series of eight cases with review of the literature. Genes Chromosomes Cancer. (2024) 63:e23205. doi: 10.1002/gcc.23205
20.
Tsai JW Lee JC Hsieh TH Huang SC Lee PH Liu TT et al . Adult NTRK-rearranged spindle cell neoplasms of the viscera: with an emphasis on rare locations and heterologous elements. Mod Pathol. (2022) 35:911–21. doi: 10.1038/s41379-021-01005-3
21.
Kobayashi H Teramura Y Yamashita K Makise N Ae K Tanaka S . Imaging findings of NTRK-rearranged spindle cell neoplasms: a case series. Mol Clin Oncol. (2023) 18:14. doi: 10.3892/mco.2023.2610
22.
Tauziède-Espariat A Duchesne M Baud J le Quang M Bochaton D Azmani R et al . NTRK-rearranged spindle cell neoplasms are ubiquitous tumours of myofibroblastic lineage with a distinct methylation class. Histopathology. (2023) 82:596–607. doi: 10.1111/his.14842
23.
Zhu P Wang J . Primary NTRK-rearranged spindle cell neoplasm of the lung: a Clinicopathologic and molecular analysis of 3 cases. Am J Surg Pathol. (2022) 46:1007–13. doi: 10.1097/PAS.0000000000001880
24.
Gao X Xu S Zhu P Lao IW Yu L Wang J . Primary NTRK-rearranged spindle cell neoplasm of the gastrointestinal tract: a clinicopathological and molecular analysis of 8 cases. Am J Surg Pathol. (2024) 48:623–31. doi: 10.1097/PAS.0000000000002202
25.
Jian XY Gao HQ Zhao ZH Wang F Zhang L Ma YH . Clinicopathological and molecular characteristics of NTRK-rearranged spindle cell neoplasms in the gastrointestinal tract. Zhonghua Bing Li Xue Za Zhi. (2024) 53:598–604. doi: 10.3760/cma.j.cn112151-20231020-00280
26.
Yin L Shi C He X Qiu Y Chen H Chen M et al . NTRK-rearranged spindle cell neoplasms: a clinicopathological and molecular study of 13 cases with peculiar characteristics at one of the largest institutions in China. Pathology. (2023) 55:362–74. doi: 10.1016/j.pathol.2022.10.003
27.
Klubíčková N Dermawan JK Mosaieby E Martínek P Vaněček T Hájková V et al . Comprehensive clinicopathological, molecular, and methylation analysis of mesenchymal tumors with NTRK and other kinase gene aberrations. J Pathol. (2024) 263:61–73. doi: 10.1002/path.6260
28.
Nishikubo H Kawabata K Kanei S Aoyama R Ma D Sano T et al . Multi-Cancer genome profiling for neurotrophic tropomyosin receptor kinase (NTRK) fusion genes: analysis of profiling database of 88,688 tumors. Cancers (Basel). (2025) 17:2250. doi: 10.3390/cancers17132250
29.
Acosta AE Vélez CS . Dermatofibrosarcoma protuberans. Curr Treat Options in Oncol. (2017) 18:56. doi: 10.1007/s11864-017-0498-5
30.
Ahmed M Ardor GD Hanna H Alhaj AM Nassar A . Two unique cases of metastatic malignant melanoma of the gastrointestinal tract. Int J Surg Case Rep. (2023) 103:107907. doi: 10.1016/j.ijscr.2023.107907
31.
Perron E Pissaloux D Charon Barra C Karanian M Lamant L Parfait S et al . Melanocytic Myxoid spindle cell tumor with ALK rearrangement (MMySTAR): report of 4 cases of a nevus variant with potential diagnostic challenge. Am J Surg Pathol. (2018) 42:595–603. doi: 10.1097/PAS.0000000000000973
32.
Kasago I Aypar U Sukhadia P Vanderbilt C Ladanyi M Hurd T et al . A novel case of cutaneous myxoid spindle cell neoplasm with FMR1-ALK gene fusion and CD34/S100 co-expression. J Cutan Pathol. (2023) 50:505–10. doi: 10.1111/cup.14354
33.
Cloutier JM Maloney NS Wang WL Lazar AJ . Pigmented PRRX1::NCOA1-rearranged fibroblastic tumor: a rare morphologic variant of an emerging mesenchymal tumor. J Cutan Pathol. (2022) 49:802–7. doi: 10.1111/cup.14262
34.
Cheng X Wang J Fang R Xu J Wang S Zhao M . PRRX1-fused mesenchymal neoplasm: a novel PRRX1::NCOA1 fusion transcript. J Cutan Pathol. (2024) 51:828–33. doi: 10.1111/cup.14683
35.
Yinan Zhu Xuyong Lin . Identification of key genes for non-alcoholic steatohepatitis and gastric cancer by bioinformatics analysis. Hepat Mon. (2025) 25:e151044. doi: 10.5812/hepatmon-151044
36.
Zhu Y He S Wang Z Xi H Lu W Lin X . Predictive and clinicopathological importance of HMGB2 in various carcinomas: a meta and bioinformatic approach. Sci Rep. (2025) 15:11003. doi: 10.1038/s41598-025-95505-w
37.
Zhu Y Sun M Wang Z Lin X . Undifferentiated high-grade sarcoma with UES-like features in the retroperitoneum: a case report with TP53 p.R306 mutation. Front Oncol. (2025) 15:1672264. doi: 10.3389/fonc.2025.1672264
38.
Ramos-García P González-Moles MÁ Warnakulasuriya S . Significance of p53 overexpression in the prediction of the malignant transformation risk of oral potentially malignant disorders: a systematic review and meta-analysis. Oral Oncol. (2022) 126:105734. doi: 10.1016/j.oraloncology.2022.105734
39.
Nishikawa T Kuwano Y Nakata M Rokutan K Nishida K . Multiple G-quadruplexes in the LMNA promoter regulate LMNA variant 6 transcription and promote colon cancer cell growth. Biochim Biophys Acta Gene Regul Mech. (2021) 1864:194746. doi: 10.1016/j.bbagrm.2021.194746
40.
Doebele RC Drilon A Paz-Ares L Siena S Shaw AT Farago AF et al . Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. (2020) 21:271–82. doi: 10.1016/S1470-2045(19)30691-6
Summary
Keywords
NTRK fusion, spindle cell neoplasm, LMNA::NTRK1, malignant transformation, recurrence, pigmentation
Citation
Zhu Y, Wang Z, Xi H, Lu W, Sun M and Lin X (2025) LMNA-NTRK1-rearranged spindle cell neoplasm with multiple relapses: a case report and literature review. Front. Med. 12:1691619. doi: 10.3389/fmed.2025.1691619
Received
25 August 2025
Revised
10 November 2025
Accepted
14 November 2025
Published
02 December 2025
Volume
12 - 2025
Edited by
Gerardo Cazzato, University of Bari Aldo Moro, Italy
Reviewed by
Zhichang Zhang, Shanghai No.6 People's Hospital, China
Wendong Yu, The University of Texas, United States
Updates
Copyright
© 2025 Zhu, Wang, Xi, Lu, Sun and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingfang Sun, sunmingfang0425@163.com; Xuyong Lin, linxuyong@hotmail.com; cmupatho@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.