Abstract
Introduction:
This study aimed to analyze risk factors for mortality in hospitalized patients with bloodstream infections caused by multidrug-resistant Gram-negative bacilli in a retrospective cohort (January–December 2022).
Methods:
Hospitalized patients with positive monomicrobial blood cultures for GNB (from central venous catheters and peripheral venipuncture) were included. Medical records and blood culture isolates were reviewed. The primary endpoint was all-cause mortality at ≤ 30 days. Risk factor analysis was performed using univariate models, survival curves (Cox regression), and an adjusted Cox proportional hazards model.
Results:
A total of 126 patients with Gram-negative bacillus bloodstream infection were included, 36 of them died within ≤ 30 days, representing a mortality rate of 28.6%. Of these deaths, 32/36 (88.9%) were due to carbapenem-resistant bacilli. The most frequently isolated gram-negative bacilli were: Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. According to the univariate analysis, mortality was 13.2 times higher (95% CI 4.3–40.5; p = 0.000) in patients with carbapenem-resistant bacilli and 4.2 times higher (95% CI 1.8–9.6; p = 0.001) in those with carbapenem-resistant A. baumannii. The main factors associated with all-cause mortality within ≤ 30 days were: age ≥46 years, infection with carbapenem-resistant bacilli, ineffective empirical treatment, and septic shock.
Discussion:
Having received ineffective empirical treatment was an independent predictor of mortality, with a hazard ratio (HR) of 10.2 (95% CI: 2.6–39.9; p = 0.001). Mortality due to bloodstream infection was related with a high frequency of patients with isolated infection by carbapenem-resistant gram-negative bacilli, mainly A. baumannii (CRAB).
Introduction
WHO estimates that 4.95 million deaths worldwide in 2019 could be associated with patient infections caused by pathogens resistant to antibacterial drugs, affecting mainly low- and middle-income countries (1). Among nosocomial infections, bloodstream infections (BSIs) caused by multidrug-resistant Gram-negative bacilli (MDR-GNB) have become a significant focus of study, given their high morbidity and mortality and economic burden, which directly affect the provision of health services (2–7).
BSI is a pathological entity caused by the dissemination of bacteria into the bloodstream, normally sterile, characterized by fever or hypothermia, hypotension, tachycardia, altered perfusion, tachypnea, and altered mental status, which can potentially cause septic shock. BSIs can be acquired in the community or in the hospital setting (infections acquired at least 48 h after hospitalization and at least 48 h after device insertion). In the hospital setting, they have been classified as primary BSIs associated with central venous catheters (CVCs) and secondary BSIs related to other sources of nosocomial infection (5, 8).
Patients hospitalized with BSI are exposed to a wide variety of heterogeneous and changing pathogens (mainly MDR) and generally have a wide range of severe clinical syndromes. Knowledge of this variety of pathogens is of great importance for its usefulness in hospital epidemiological research and surveillance in the process of improving medical care and optimizing treatments. In highly developed countries, the most frequently reported pathogens are S. aureus ( ≤ 30-day case-fatality rate: 21%), E. coli, Klebsiella spp., P. aeruginosa, Enterococcus, Streptococcus, and coagulase-negative Staphylococcus. In Latin America, GNB pathogens are more frequent, with reports of up to 70.6% for the A. baumannii-calcoaceticus complex, 28.1% for carbapenem-resistant Enterobacterales (CRE), and 26.3% for P. aeruginosa (9–11).
Abdel Hadi et al. reported, in a retrospective cohort study, a prevalence of 13% for MDR-GNB bacilli, with higher isolation rates of E. coli (62.7%), K. pneumoniae (20.4%), Salmonella species (6.6%), and P. aeruginosa (5.3%). Likewise, they report as risk factors having prolonged hospital stays, presenting multiple comorbidities, having received previous antibiotic treatment, and requiring admission to intensive care units (4).
A mortality of 20%−39% has been reported in BSI due to A. baumannii with an antimicrobial resistance of 86%. The survival of multi-resistant strains of A. baumannii, K. pneumoniae, and E. coli is due to a capsule that protects them from opsonization (5).
At the local and global levels, knowledge and monitoring of microbiological resistance patterns, particularly among patients with BSI due to MDR-GNB, have a direct impact on health services. Therefore, it is necessary to conduct analytical studies that correlate microbiological isolation and bacterial resistance findings with patient demographics, comorbidities, conditions upon admission, conditions during hospitalization, and other risk factors associated with poor patient outcomes (12).
A previous retrospective cohort study in our hospital observed a mortality rate of 78.2% in patients with ventriculitis due to MDR-GNB associated with an external ventricular drain. A. baumannii, K. pneumoniae, and P. aeruginosa were the most frequently isolated bacilli. High resistance to carbapenems was observed in A. baumannii (91.3%) and P. aeruginosa (80.0%) (13). A. baumannii, P. aeruginosa, and K. pneumoniae were also the most frequently isolated bacilli in the present study, so we consider them potentially present in our hospital healthcare settings.
Thus, the objective of this study was to analyze the risk factors associated with mortality in patients hospitalized for BSI due to MDR-GNB in a retrospective cohort selected from January–December 2022.
Methodology
Study design and participants
A retrospective cohort study was conducted among adult patients hospitalized at the National Medical Center of the West, Instituto Mexicano del Seguro Social (January–December 2022), with BSI due to MDR-GNB.
All physical and electronic records of consecutive eligible hospitalized patients with suspected bacteremia in 2022 were reviewed, as were the total blood cultures performed, regardless of results or isolation type. Microbiological results of the isolates were correlated with data from patients suspected of having BSI.
Suspected cases of BSI were defined as those presenting at least two of the following signs and symptoms: fever (above 38.0 °C) or hypothermia (below 36.0 °C), chills, and hypotension (5). The following variables were included in the description and analysis of the selected cohort: age and sex; conditions and comorbidities at admission; conditions during hospitalization (hospitalization service: surgical, medical, or intensive care unit); laboratory and microbiological findings; treatment; and outcomes (mortality). Data were collected from patient admission until discharge due to improvements or death.
Multidrug resistance includes strains resistant to 3 or more classes of antibiotics, particularly carbapenems (14–16).
Outcomes
The primary outcome of the analysis was BSI all-cause mortality at ≤ 30 days. All-cause mortality at ≤ 30 days was estimated, with the first day defined as the date of collection of the positive blood culture. The primary secondary outcomes included the administration of an ineffective empirical treatment (empirical treatment failure), development of resistance, and length of hospital stay.
Empirical treatment consists of administering an antibiotic before the blood culture results and susceptibility pattern are known, given the patient's severe clinical condition.
To classify a patient as having received ineffective empirical treatment (treatment failure), at least one of the following criteria was considered:
(a) Susceptibility Pattern
The isolated bacterium was resistant to the empirically administered antibiotic.
(b) Antibiotic administration
The antibiotic was not administered within the first 24 h of suspected bacterial infection (BSI) (immediately after the blood culture was taken).
(c) Treatment duration (number of consecutive days of therapy)
Although the isolated bacteria were susceptible to the prescribed antibiotic, the patient did not receive treatment for seven consecutive days.
(d) Infection source
The catheter or source of infection should have been removed.
Inclusion criteria: Patients with clinically and microbiologically confirmed BSI, isolated from a GNB. With positive monomicrobial blood cultures, one was obtained from the CVC and another from peripheral venipuncture. With age 18 years or older, regardless of sex, with or without comorbidities, with one or more hospitalization conditions upon admission, with a hospital stay in any medical or surgical department, including the hospital's five intensive care units (general ICU, burn ICU, transplant ICU, coronary ICU, and post-surgical heart ICU), as well as with special conditions during hospitalization, such as the use of invasive mechanical ventilation or parenteral nutrition.
Exclusion criteria: Patients with clinically and microbiologically ruled-out BSI; patients with central venous catheter colonization, with blood cultures showing polymicrobial isolation, or with isolation of a microorganism other than GNB. The patient was on antibiotic treatment (48 h before sample collection)—patients with incomplete data or patients who have requested voluntary discharge within the established retrospective follow-up period.
Blood culture
The peripheral venipuncture site and CVC are disinfected with chlorhexidine or 2% iodine tincture. A 20 to 30 ml blood sample is drawn, which is required for a set of two vials per collection (CVC and peripheral venipuncture): one for aerobic microorganisms and one for anaerobic microorganisms. Blood culture kits are transported to the laboratory at room temperature as soon as possible. Microbiological isolation and antimicrobial susceptibility were obtained by the hospital laboratory using the microdilution method (Vitek 2, BioMérieux). To classify antibiotic susceptibility/resistance, the 30th edition (2020) of the Clinical and Laboratory Standards Institute was used (14–16).
For a BSI infection to be considered associated with a CVC, the blood culture from the CVC must be positive at least 120 min before the blood culture from the peripheral venipuncture, provided that the bacteria are the same. If the bacteria grow earlier during peripheral venipuncture, it is considered a secondary BSI (due to another nosocomial infection with the same bacteria isolated) (3, 8, 17).
An adaptation of the classification proposed by Falcone et al. was made for GNB in the following categories: (1) Carbapenem-susceptible Gram-negative bacilli (CS-GNB); (2) Carbapenem-resistant P. aeruginosa (CRPA) (due to carbapenemases and not porins, according to the results of the antibiogram); (3) Carbapenem-resistant A. baumannii (CRAB); (4) Carbapenem-resistant Enterobacterales (CRE); and (5) Other Fermenting GNB (Stenotrophomonas maltophilia and Alcaligenes faecalis) (18).
Carbapenem- resistance cut-off values by group: CRPA, carbapenems (imipenem and meropenem) ≥8 μg/ml Minimum Inhibitory Concentration (MIC); CRE, carbapenems (imipenem and meropenem) ≥4 μg/ml, and for ertapenem ≥2 μg/ml MIC; and CRAB, carbapenems (imipenem and meropenem) ≥8 μg/ml MIC. Resistance cut-off values for polymyxins (MIC), ≥4 μg/ml for the most critical members of Enterobacterales and Acinetobacter spp., and for P. aeruginosa, were considered (15).
Regarding P. aeruginosa, porin-mediated carbapenem resistance was inferred phenotypically based on the antibiogram pattern, with susceptibility to ceftazidime, cefepime, or piperacillin-tazobactam (19).
A description and analysis of two BSI outbreaks due to the isolation of an opportunistic GNB, which were observed during the cohort follow-up, were included.
Statistical analysis
Qualitative variables were analyzed using the χ2 test or Fisher's exact test. Quantitative variables with normal distributions were summarized using the arithmetic mean and standard deviation and compared using the Student's t-test. Quantitative variables with non-normal distributions were summarized using medians and interquartile ranges, and comparisons between groups were performed using the Mann-Whitney U test. Exact 95% confidence intervals (CIs) were used to compare groups. A bilateral p-value < 0.05 was considered statistically significant. To establish cut-off points for converting quantitative variables to qualitative variables, ROC (receiver operating characteristic) curves were used.
The following statistical analyses were performed using univariate models:
(1) Analysis of the risk factors associated with mortality among survivors and non-survivors due to BSI who died within ≤ 30 days, with the first day defined as the date of collection of the positive blood culture. (2) Analysis of the risk factors for presenting BSI due to CR-GNB vs. CS-GNB (the isolates of S. maltophilia were excluded because it has intrinsic resistance to carbapenems; A. faecalis was also excluded). (3) Analysis of the poor prognostic factors in patients with BSI due to MDR-GNB, stratified according to the bacilli isolated. (4) And, an additional analysis to review the conditions of patients at the Transplant Unit with BSI due to Achromobacter xylosoxidans.
Statistically significant risk factors associated with mortality in univariate analysis (among survivors and non-survivors) were analyzed using survival curves (Cox regression) and the adjusted Cox Proportional Hazard Model (SPSS 25, IBM, Armonk, NY, USA).
Results
The flowchart (Figure 1) presents the results of the review of the physical and electronic records of the included patients, along with the microbiological findings. A total of 942 hospitalized patients with suspected bacteremia were identified from 1884 cultures performed during the study period (942 blood cultures). Two hundred ten patients had positive monomicrobial blood cultures, meeting the clinical and laboratory criteria for bacteremia. Fifty-six patients with Gram-positive cocci, 7 with Gram-positive bacilli, and 21 with fungi were excluded. The remaining 126 patients with BSI and GNB isolation were included.
Figure 1
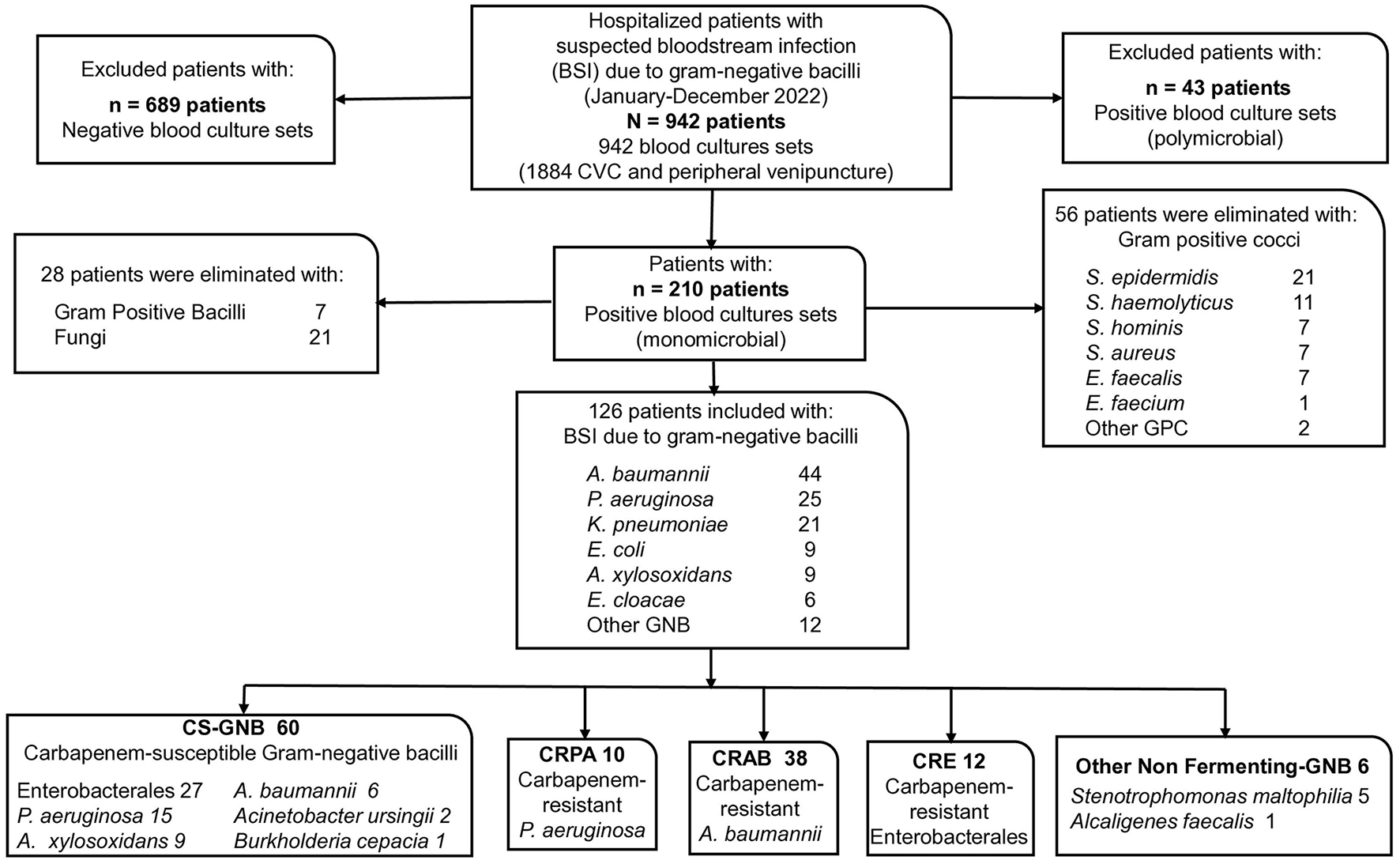
Participant flowchart. CVC, Central venous catheter.
In the 126 blood cultures included, the Gram-negative bacteria (GNB) found, ordered by frequency, were 44 isolates of A. baumannii (34.9%), 25 of P. aeruginosa (19.8%), 21 of K. pneumoniae (16.7%), 9 of E.coli (7.1%), 9 of A. xylosoxidans (7.1%), 6 of Enterobacter cloacae (4.8%), and 12 of other GNB (9.5%).
According to classification, 60 isolates (47.6%) were classified as CS-GNB; 27 belonged to the order Enterobacterales: 15 of P. aeruginosa, 9 of A. xylosoxidans, 6 of A. baumannii, 2 of A. ursingii, and 1 of B. cepacia. And 66 isolates (52.4%) were found to be CR-GNB: 10 CRPA isolates, 38 CRAB isolates, 12 CRE isolates, and six other non-fermenting GNB isolates (5 S. maltophilia and 1 A. faecalis) (Figure 1).
Table 1 presents the demographic characteristics, comorbidities, complications, laboratory findings, and outcomes at ≤ 30 days of follow-up in patients with BSI, along with blood culture isolates and antimicrobial resistance. Of the patients included, 67 (53.1%) were male and 59 (46.9%) female, with an age range of 18 to 82 years and a mean age of 46.5 ± 15.7 years.
Table 1
| Variables | Total N = 126 (%) | Survivors N = 90 (%) | Non-survivors N = 36 (28.6) | OR (95% CI) | p |
|---|---|---|---|---|---|
| Man | 67 (53.2) | 48 (53.3) | 19 (52.8) | 0.555 | |
| Age (years), mean ± SD | 46.5 ± 15.7 | 44.4 ± 16.0 | 51.8 ± 13.8 | 0.012a | |
| Age ≥46 years | 61 (48.4) | 35 (38.9) | 26 (72.2) | 4.1 (1.8–9.5) | 0.001 |
| Comorbidities and conditions at admission | |||||
| Immunocompromise | 86 (68.3) | 62 (68.9) | 24 (66.7) | 0.483 | |
| Arterial hypertension | 59 (46.8) | 43 (47.8) | 16 (44.4) | 0.445 | |
| Diabetes mellitus | 35 (27.8) | 18 (20.0) | 17 (47.2) | 3.6 (1.6–8.2) | 0.003 |
| Vascular venous exhaustion | 31 (24.6) | 22 (24.4) | 9 (25.0) | 0.558 | |
| Chronic kidney disease | 28 (22.2) | 24 (26.7) | 4 (11.1) | 0.062 | |
| Hemodialysis | 27 (21.4) | 21 (23.3) | 6 (16.7) | 0.285 | |
| SAH/TBI/brain hemorrhage | 23 (18.3) | 16 (17.8) | 7 (19.4) | 0.505 | |
| Patients admitted for burns | 21 (16.7) | 16 (17.8) | 5 (13.9) | 0.792 | |
| Kidney transplant | 18 (14.3) | 18 (20.0) | 0 | NS | |
| Neoplasia/leukemia | 9 (7.1) | 6 (6.7) | 3 (8.3) | 0.714 | |
| Short bowel syndrome | 9 (7.1) | 8 (8.9) | 1 (2.8) | 0.444 | |
| Ischemic heart disease | 8 (6.3) | 4 (4.4) | 4 (11.1) | 0.224 | |
| Conditions during hospitalization | |||||
| Surgery hospitalization ward | 87 (69.0) | 60 (66.7) | 27 (75.0) | 0.244 | |
| Septic shock | 86 (68.3) | 51 (56.7) | 35 (97.2) | 26.8 (3.5–204) | 0.000 |
| Invasive mechanical ventilation | 76 (60.3) | 46 (51.1) | 30 (83.3) | 4.8 (1.8–12.6) | 0.001 |
| Medical hospitalization ward | 38 (30.2) | 29 (32.2) | 9 (25.0) | 0.283 | |
| Intensive care unit | 56 (44.4) | 37 (41.1) | 19 (52.8) | 0.161 | |
| Parenteral nutrition | 18 (14.3) | 9 (10.0) | 9 (25.0) | 3.0 (1.1–8.3) | 0.033 |
| Post-cardiac arrest syndrome | 9 (7.1) | 4 (4.4) | 5 (13.9) | 0.117 | |
| Central venous catheter (d), median (IQR) | 20 (12–34) | 19 (13–34) | 23 (11–35) | 0.846 | |
| Hospitalization (d), median (IQR) | 31 (20–51) | 36 (23–53) | 25 (14–44) | 0.055b | |
| Hospital admission to BSI (d), median (IQR) | 13 (7–28) | 13 (6–25) | 15 (9–34) | 0.197b | |
| BSI to mortality ≤ 30-d, median (IQR) | 14 (7–23) | 16 (10–30) | 6 (2–18) | 0.000b | |
| Laboratory findings | |||||
| Blood leukocytes (cells/μl), mean ± SD | 15.9 ± 7.6 | 16.2 ± 7.4 | 15.2 ± 8.2 | 0.595a | |
| Procalcitonin (ng/ml), median (IQR) | 5.7 (1.9–18.5) | 4.2 (1.7–18.7) | 7.3 (3.3–19.3) | 0.371b | |
| Resistance by type of bacteria | |||||
| A. baumannii (CRAB and non-CRAB) | 44 (34.9) | 25 (27.8) | 19 (52.8) | 2.9 (1.3–6.5) | 0.008 |
| P. aeruginosa (CRPA and non-CRPA) | 25 (19.8) | 22 (24.4) | 3 (8.3) | NS | |
| Enterobacterales (CRE and non-CRE) | 39 (31.0) | 24 (26.7) | 15 (41.7) | 0.077 | |
| Carbapenem-resistant P. aeruginosa (CRPA) | 10 (7.9) | 8 (8.9) | 2 (5.6) | 0.723 | |
| Carbapenem-resistant A. baumannii (CRAB) | 38 (30.2) | 19 (21.1) | 19 (52.8) | 4.2 (1.8–9.6) | 0.001 |
| Carbapenem-resistant Enterobacterales (CRE) | 12 (9.5) | 5 (5.6) | 7 (19.4) | 4.1 (1.2–14.0) | 0.038 |
| Other NGF-GNB | 6 (4.8) | 2 (2.2) | 4 (11.1) | 0.055 | |
| CR-GNB | 66 (52.4) | 34 (37.8) | 32 (88.9) | 13.2 (4.3–40.5) | 0.000 |
| Carbapenem-resistant CRAB and CRE | 50 (39.7) | 24 (26.7) | 26 (72.2) | 7.2 (3.0–17.0) | 0.000 |
| Antibiotic resistance | |||||
| Meropenem resistance | 67/121 (55.4) | 38/89 (42.7) | 29/32 (90.6) | 13.0 (3.7–45.8) | 0.000 |
| Piperacillin/tazobactam resistance | 65/113 (57.5) | 36/82 (43.9) | 29/31 (93.5) | 18.5 (4.1–82.9) | 0.000 |
| Resistance to cefepime | 76/119 (63.9) | 46/87 (52.9) | 30/32 (93.8) | 13.4 (3.0–59.4) | 0.000 |
| Resistance to cefoxitin | 45/76 (59.2) | 29/57 (50.9) | 16/19 (84.2) | 5.1 (1.4–19.6) | 0.014 |
| Resistance to 3rd-generation cephalosporins | 98/118 (83.1) | 66/86 (76.7) | 32/32 (100.0) | NS | |
| Resistance to quinolones | 83/123 (67.5) | 53/87 (60.9) | 30/36 (83.3) | 3.2 (1.2–8.5) | 0.012 |
| Resistance to aminoglycosides | 59/118 (50.0) | 38/86 (44.2) | 21/32 (65.6) | 2.4 (1.0–5.6) | 0.031 |
| Tigecycline resistance | 29/87 (33.3) | 23/61 (37.7) | 6/26 (23.1) | 0.140 | |
| Colistin resistance | 3/89 (3.4) | 2/62 (3.2) | 1/27 (3.7) | 1.000 | |
| Outcomes | |||||
| Ineffective empirical treatment | 48/126 (38.1) | 15/90 (16.7) | 33/36 (91.7) | 55.0 (15.0–203) | 0.000 |
| From bloodstream infection to mortality ≤ 30-d | 36/126 (28.6) | 0 | 36/36 (100.0) | NA | |
| Overall mortality of patients with BSI | 46/126 (36.5) | 10/90 (11.1) | 36/36 (100.0) | NA | |
Demographic characteristics, comorbidities, complications, laboratory findings, and mortality at ≤ 30 days of follow-up for patients with bloodstream infection; blood culture isolates and antimicrobial resistance.
SAH, Subarachnoid hemorrhage; TBI, Traumatic brain injury; BSI, Bloodstream infections; CVC, Central venous catheter; d, days; CS-GNB, Carbapenem-susceptible Gram-negative bacilli; CR-GNB, Carbapenem-resistant Gram-negative bacilli; Other NGF-GNB, Other non-fermenting Gram-negative bacilli; ng/ml, nanograms/milliliter; OR, Odds ratio; CI, Confidence interval; SD, Standard deviation; IQR, Interquartile range; NS, Not significant; NA, Not applicable.
aStudent's t statistical test.
bMann–Whitney U test.
The most common comorbidities listed among the patients in the cohort, in descending order, were 86 patients (68.3%) who were immunocompromised, 59 (46.8%) with arterial hypertension, 35 (27.8%) with diabetes mellitus, and 31 (24.6%) with vascular access exhaustion. Eighty-seven patients (69.0%) came from surgical hospitalization areas, and 38 patients (30.2%) came from medical hospitalization areas; 86 (68.3%) had septic shock, 76 (60.3%) required invasive mechanical ventilation support, 56 (44.4%) were admitted to an intensive care unit, 18 (14.3%) received parenteral nutrition, and 9 (7.1%) had a post-cardiac arrest syndrome.
The median hospitalization for the selected patients was 31 days (IQR 20–51). No deaths were observed among patients with BSI admitted to the Renal Transplant Intensive Care Unit. A median of 6 days from BSI to mortality ≤ 30 days was estimated (IQR 2–18 d, p = 0.000) (Mann–Whitney U test). A mortality rate of 28.6% (36/126) from all causes was found at ≤ 30 days; of these deaths, 32/36 (88.9%) were CR-GNB (Table 1).
In the univariate analysis between patients with bacteremia who did not survive and those who did survive, it was found that age ≥46 years (OR 4.1 CI 1.8–9.5, p = 0.001), diabetes mellitus (OR 3.6 CI 1.6–8.2, p = 0.003), septic shock (OR 26.8 CI 3.5–204, p = 0.000), use of invasive mechanical ventilation (OR 4.8 CI 1.8–12.6, p = 0.001), need for parenteral nutrition (OR 3.0 CI 1.1–8.3, p = 0.033), presence of CR-GNB (OR 13.2 CI 4.3–40.5, p = 0.000), and having received ineffective empirical treatment (OR 55.0 CI 15.0–203, p = 0.000) were statistically significant factors for mortality risk. Among patients who did not survive ≤ 30 days, 19/36 (52.8%) had CRAB (OR 4.2, CI 1.8–9.6, p = 0.001) (Table 1).
Figure 2 shows a timeline of the study, including hospital admission, follow-up, and patient outcomes. According to the proposed classification for the groups of isolated bacilli, the following mortality outcomes were observed: In the CS-GNB group 4/60 patients (6.7%), in the CRPA group 2/10 patients (20.0%), in the CRAB group 19/38 patients (50.0%), in the CRE group 7/12 (58.3%), and in the other NF-GNB group 4/6 (66.7%) who died within ≤ 30 days.
Figure 2
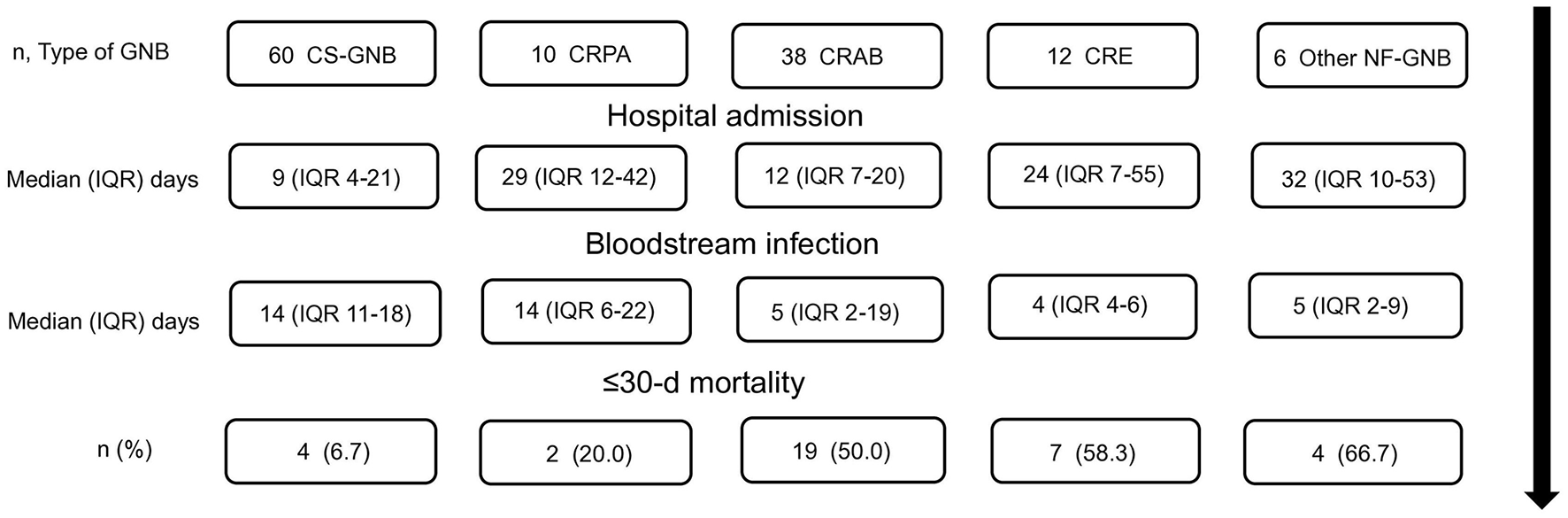
Timeline of the study, patient's hospital admission, follow-up, and outcomes. CS-GNB, Carbapenem-susceptible Gram-negative bacilli; CRPA, carbapenem-resistant P. aeruginosa; CRAB, Carbapenem-resistant A. baumannii; CRE, carbapenem-resistant Enterobacterales; Other NF-GNB, Other Non-fermenting Gram-negative bacilli; IQR, Interquartile range; d, days.
Survival curves for all-cause mortality at ≤ 30 days among patients with BSI, stratified by GNB group, are presented in Figure 3.
Figure 3
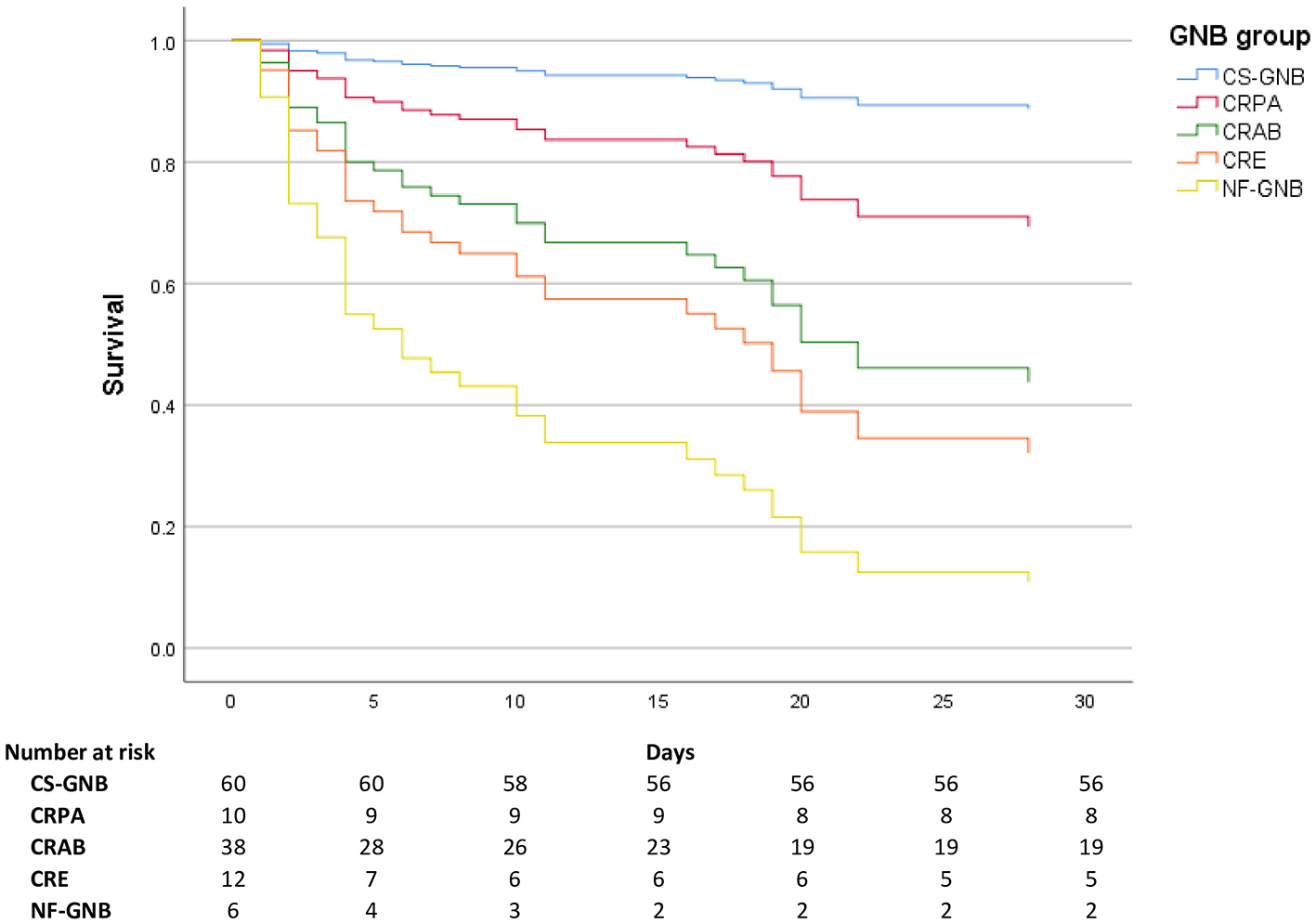
Survival curves by bacterial group. CS-GNB, Carbapenem-susceptible Gram-negative bacilli; CRPA, carbapenem-resistant P. aeruginosa; CRAB, Carbapenem-resistant A. baumannii; CRE, carbapenem-resistant Enterobacterales; Other NF-GNB, Other Non-fermenting Gram-negative bacilli.
Figure 4 displays the survival curves (Cox regression) for the principal risk factors associated with all-cause mortality at ≤ 30 days in patients with BSI due to MDR-GNB: age ≥46 years, CR-GNB infection, ineffective empirical treatment, and septic shock. Where having received an ineffective empirical treatment was statistically significant (p = 0.001).
Figure 4
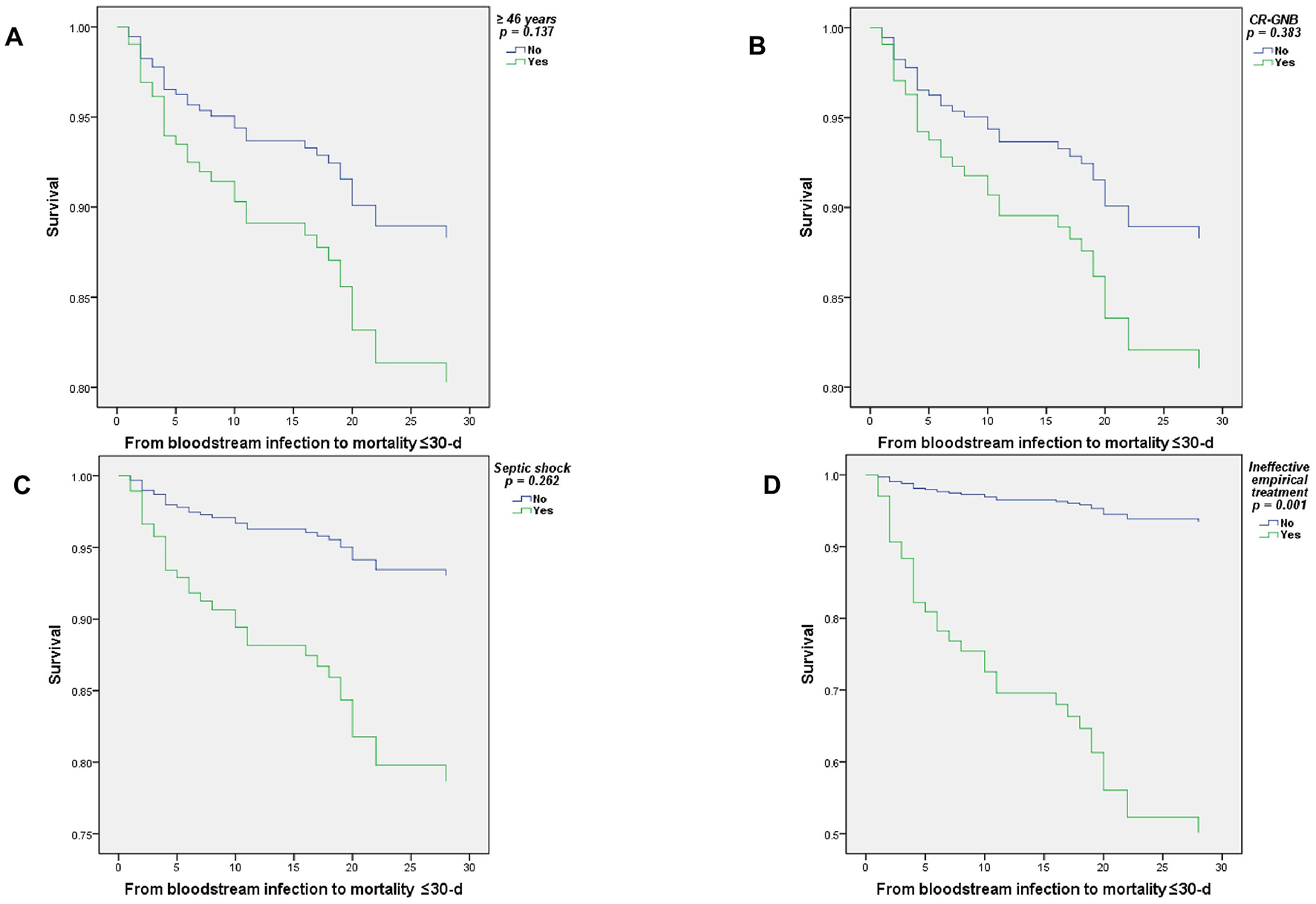
Cox regression survival curves of patients with BSI due to multidrug-resistant Gram-negative bacilli, ≤ 30-d mortality. (A) ≥46 years, (B) CR-GNB, (C) Septic shock, and (D) Ineffective empirical treatment.
Table 2 shows the characteristics of patients with BSI due to CS-GNB vs. CR-GNB isolates.
Table 2
| Variables | Total n = 120 (%) | CS-GNB n = 60 (50) | CR-GNB n = 60 (50) | OR (95% CI) | p |
|---|---|---|---|---|---|
| Man | 65 (54.2) | 35 (58.3) | 30 (50.0) | 0.232 | |
| Age (years), mean ± SD | 47 ± 16 | 46 ± 17 | 48 ± 15 | 0.483a | |
| Age ≥46 years | 59 (49.2) | 27 (45.0) | 32 (53.3) | 0.233 | |
| Comorbidities and conditions at admission | |||||
| Immunocompromise | 81(67.5) | 42 (70.0) | 39 (65.0) | 0.348 | |
| Arterial hypertension | 58 (48.3) | 33 (55.0) | 25 (41.7) | 0.100 | |
| Diabetes mellitus | 32 (26.7) | 12 (20.0) | 20 (33.3) | 0.074 | |
| Vascular access exhaustion | 29 (24.2) | 13 (21.7) | 16 (26.7) | 0.335 | |
| Chronic kidney disease | 27 (22.5) | 19 (31.7) | 8 (13.3) | NS | |
| Hemodialysis | 26 (21.7) | 19 (31.7) | 7 (11.7) | NS | |
| SAH/TBI/brain hemorrhage | 22 (18.3) | 6 (10.0) | 16 (26.7) | 3.3 (1.2–9.1) | 0.016 |
| Patients admitted for burns | 20 (16.7) | 9 (15.0) | 11 (18.3) | 0.404 | |
| Kidney transplant | 18 (15.0) | 16 (26.7) | 2 (3.3) | NS | |
| Neoplasia/leukemia | 9 (7.5) | 3 (5.0) | 6 (10.0) | 0.491 | |
| Short bowel syndrome | 9 (7.5) | 5 (8.3) | 4 (6.7) | 1.000 | |
| Ischemic heart disease | 8 (6.7) | 3 (5.0) | 5 (8.3) | 0.717 | |
| Conditions during hospitalization | |||||
| Central venous catheter (d), median (IQR) | 19 (12–35) | 20 (12–32) | 19 (11–38) | 0.973b | |
| Hospitalization in the surgical area | 82 (68.3) | 34 (56.7) | 48 (80.0) | 3.1 (1.4–6.9) | 0.005 |
| Septic shock | 82 (68.3) | 32 (53.3) | 50 (83.3) | 4.4 (1.9–10.2) | 0.000 |
| Invasive mechanical ventilation | 73 (60.8) | 24 (40.0) | 49 (81.7) | 6.7 (3.0–15.4) | 0.000 |
| Hospitalization in the medical area | 37 (30.8) | 25 (41.7) | 12 (20.0) | NS | |
| Intensive care unit | 52 (43.3) | 18 (30.0) | 34 (56.7) | 3.1 (1.4–6.5) | 0.003 |
| Parenteral nutrition | 17 (14.2) | 7 (11.7) | 10 (16.7) | 0.301 | |
| Ventilator-associated pneumonia | 35 (29.2) | 12 (20.0) | 23 (38.3) | 2.5 (1.1–5.6) | 0.022 |
| Skin and soft tissue infection | 18 (15.0) | 8 (13.3) | 10 (16.7) | 0.399 | |
| Post-cardiac arrest syndrome | 9 (7.5) | 4 (6.7) | 5 (8.3) | 1.000 | |
| Hospitalization (d), mean ± SD | 37 ± 22 | 33 ± 19 | 40 ± 24 | 0.095a | |
| Hospital admission to BSI (d), median (IQR) | 13 (6–25) | 9 (4–21) | 14 (8–29) | 0.048b | |
| BSI to mortality ≤ 30–d, median (IQR) | 7 (14–25) | 14 (10–23) | 15 (4–30) | 0.987b | |
| Antibiotic resistance | |||||
| Meropenem resistance | 66/118 (55.9) | 6/58 (10.3) | 60 (100.0) | NS | |
| Resistance to 3rd generation cephalosporins | 97/117 (82.9) | 38/57 (66.7) | 59 (98.3) | 29.5 (3.8–229) | 0.000 |
| Resistance to quinolones | 82/117 (70.1) | 25/58 (43.1) | 57/59 (96.6) | 37.6 (8.4–169) | 0.000 |
| Resistance to aminoglycosides | 58/117 (49.6) | 20/58 (34.5) | 38/59 (64.4) | 3.4 (1.6–7.4) | 0.001 |
| Tigecycline resistance | 26/86 (33.7) | 10/41 (24.4) | 19/45 (42.2) | 0.064 | |
| Colistin resistance | 3/89 (3.4) | 1/38 (2.6) | 2/51 (3.9) | 1.000 | |
| Laboratory findings | |||||
| Blood leukocytes × 103 cells/μl, mean ± SD | 15.8 ± 7.5 | 16.8 ± 7.9 | 14.9 ± 7.0 | 0.155a | |
| Procalcitonin (ng/ml), median (IQR) | 5.5 (1.9–17.7) | 3.4 (1.5–18.0) | 6.6 (2.2–17.7) | 0.452b | |
| Ineffective empirical treatment | 45/120 (37.5) | 4/60 (6.7) | 41/60 (68.3) | 30.2 (9.6–92.5) | 0.000 |
| From bloodstream infection to mortality ≤ 30-d | 32 (26.7) | 4 (6.7) | 28 (46.7) | 12.3 (3.9–38.1) | 0.000 |
| Overall mortality of patients with BSI | 42 (35.0) | 8 (13.3) | 34 (56.7) | 8.5 (3.4–21.0) | 0.000 |
Demographic characteristics, comorbidities, complications, and laboratory findings of patients with bloodstream infections due to carbapenem-susceptible Gram-negative bacilli vs. carbapenem-resistant Gram-negative bacilli.
SAH, Subarachnoid hemorrhage; TBI, Traumatic brain injury; BSI, bloodstream infections; CVC, Central venous catheter; d, days; CS-GNB, Carbapenem-susceptible Gram-negative bacilli; CR-GNB, Carbapenem-resistant Gram-negative bacilli; ng/ml, nanograms/milliliter; OR, Odds ratio; CI, Confidence interval; SD, Standard deviation; IQR, Interquartile range; NS, Not significant.
aStudent's t statistical test.
bMann–Whitney U test.
The following risk factors (according to univariate analysis) were identified as statistically significant for patients with CR-GNB: subarachnoid hemorrhage (SAH), head trauma (TBI), cerebral hemorrhage (OR 3.3; CI 1.2–9.1; p = 0.016), conditions during hospitalization, particularly in surgical areas (OR 3.1; CI 1.4–6.9; p = 0.005), septic shock (OR 4.4; CI 1.9–10.2; p = 0.000), invasive mechanical ventilation (IMV) (OR 6.7; CI 3.0–15.4; p = 0.000) and intensive care unit (general and burn) (OR 3.1; CI 1.4–6.5; p = 0.003). Patients with ventilator-associated pneumonia (OR 2.5; CI 1.1–5.6; p = 0.022). The number of days from admission to the day of a positive blood culture was also statistically significant (p = 0.048). We found that having received ineffective empirical treatment was statistically significant (OR 30.2, CI 9.6–92.5, p = 0.000), as well as mortality ≤ 30 days by BSI (OR 12.3, CI 3.9–38.1, p = 0.000) and overall mortality (OR 8.5, CI 3.4–21.0, p = 0.000).
Table 3 shows the poor prognostic factors for death in patients with BSI according to the four main categories specified. For patients who had a CRAB isolation, 33/38 (86.8%) had septic shock, and 13/38 (34.2%) had SAH/TBI/brain hemorrhage. Statistical significance was observed in those who presented SAH/TBI/brain hemorrhage (OR 4.1, IC 1.6–10.4, p = 0.003), IMV (OR 6.9, IC 2.5–19.3, p = 0.000), septic shock (OR 4.4, IC 1.6–12.2, p = 0.003), and intensive care unit (OR 2.6, IC 1.2–5.6, p = 0.014). One hundred percent of CRAB were resistant to piperacillin/tazobactam, cefepime, third-generation cephalosporins, and quinolones; 65.8% were resistant to aminoglycosides, and 44.7% were resistant to tigecycline, with statistical significance for resistance to aminoglycosides (OR 2.6, CI 1.2–5.8, p = 0.015), having received an ineffective empirical treatment (OR 5.4, CI 2.4–12.4, p = 0.000), and not surviving the first 30 days of BSI (OR 4.2, CI 1.8–9.6, p = 0.001).
Table 3
| Variables | Carbapenem-resistant A. baumannii (CRAB) | Carbapenem-resistant Enterobacterales (CRE) | ||||
|---|---|---|---|---|---|---|
| n = 38 (%) | OR (95% CI) | p | n = 12 (%) | OR (95% CI) | p | |
| Diabetes mellitus | 11 (28.9) | 0.504 | 6 (50.0) | 0.075 | ||
| SAH/TBI/brain hemorrhage | 13 (34.2) | 4.1 (1.6–10.4) | 0.003 | 3 (25.0) | 0.458 | |
| Chronic kidney disease | 4 (10.5) | 0.060 | 3 (25.0) | 0.728 | ||
| Hemodialysis | 4 (10.5) | 0.060 | 2 (16.7) | 1.000 | ||
| Kidney transplant | 1 (2.6) | NS | 0 | NS | ||
| Invasive mechanical ventilation | 33 (86.8) | 6.9 (2.5–19.3) | 0.000 | 8 (66.7) | 0.762 | |
| Septic shock | 33 (86.8) | 4.4 (1.6–12.2) | 0.003 | 9 (75.0) | 0.751 | |
| Intensive care unit | 23 (60.5) | 2.6 (1.2–5.6) | 0.014 | 7 (58.3) | 0.368 | |
| Piperacillin/tazobactam resistance | 38 (100.0) | NS | 11/11 (100.0) | NS | ||
| Resistance to cefepime | 38 (100.0) | NS | 11 (91.7) | 0.054 | ||
| Resistance to 3rd-generation cephalosporins | 38 (100.0) | NS | 12 (100.0) | NS | ||
| Resistance to quinolones | 38 (100.0) | NS | 11/11 (100.0) | NS | ||
| Resistance to aminoglycosides | 25 (65.8) | 2.6 (1.2–5.8) | 0.015 | 5/11 (45.5) | 1.000 | |
| Tigecycline resistance | 17 (44.7) | 0.040 | 2/7 (28.6) | 1.000 | ||
| Colistin resistance | 0 | NS | 1/9 (11.1) | 0.277 | ||
| Ineffective empirical treatment | 25 (65.8) | 5.4 (2.4–12.4) | 0.000 | 9 (75.0) | 5.8 (1.5–22.5) | 0.010 |
| From bloodstream infection to mortality ≤ 30-d | 19 (50.0) | 4.2 (1.8–9.6) | 0.001 | 7 (58.3) | 4.1 (1.2–14.0) | 0.038 |
| Variable | Carbapenem-susceptible Gram-negative bacilli (CS-GNB) | Carbapenem-resistant P. aeruginosa (CRPA) | ||||
| n = 60 (%) | OR (95% CI) | p | n = 10 (%) | OR (95% CI) | p | |
| Diabetes mellitus | 12 (20.0) | NS | 3 (30.0) | 1.000 | ||
| SAH/TBI/brain hemorrhage | 6 (10.0) | NS | 0 | NS | ||
| Chronic kidney disease | 19 (31.7) | NS | 1 (10.0) | 0.456 | ||
| Hemodialysis | 19 (31.7) | NS | 1 (10.0) | 0.688 | ||
| Kidney transplant | 16 (26.7) | NS | 1 (10.0) | 1.000 | ||
| Invasive mechanical ventilation | 24 (40.0) | NS | 8 (80.0) | 0.313 | ||
| Septic shock | 32 (53.3) | NS | 8 (80.0) | 0.501 | ||
| Intensive care unit | 18 (30.0) | NS | 4 (40.0) | 1.000 | ||
| Piperacillin/tazobactam resistance | 9/56 (16.1) | NS | 6/7 (85.7) | 0.235 | ||
| Resistance to cefepime | 17/58 (29.3) | NS | 9 (90.0) | 0.092 | ||
| Resistance to 3rd-generation cephalosporins | 38/57 (66.7) | NS | 9 (90.0) | 1.000 | ||
| Resistance to quinolones | 25/58 (43.1) | NS | 8 (80.0) | 0.721 | ||
| Resistance to aminoglycosides | 20/58 (34.5) | NS | 8 (80.0) | 0.094 | ||
| Tigecycline resistance | 10/41 (24.4) | 0.074 | NA | NA | ||
| Colistin resistance | 1/38 (2.6) | 1.000 | 1/8 (12.5) | 0.249 | ||
| Ineffective empirical treatment | 4 (6.7) | NS | 7 (70.0) | NS | ||
| From bloodstream infection to mortality ≤ 30-d | 4 (6.7) | NS | 2 (20.0) | 0.723 | ||
Poor prognostic factors in patients with bloodstream Infection due to multidrug-resistant Gram-negative bacilli.
SAH, Subarachnoid hemorrhage; TBI, Traumatic brain injury; BSI, bloodstream infections; d, days; OR, Odds ratio; CI, Confidence interval; SD, Standard deviation; IQR, Interquartile range; NS, Not significant; NA, Not applicable.
For patients with CRE isolation, having received an ineffective empirical treatment (OR 5.8, IC 1.5–22.5, p = 0.010) and death within ≤ 30 days of BSI (OR 4.1, IC 1.2–14.0, p = 0.038) were estimated to be statistically significant. No poor prognostic factors were observed in patients with BSI in the CS-GNB or CRPA groups.
To run the adjusted Cox proportional hazards model, the significant variables from the previous univariate models were included: age ≥46 years, diabetes mellitus, septic shock, use of invasive mechanical ventilation, BSI due to CR-GNB, receipt of parenteral nutrition, and prescription of ineffective empirical treatment. The final model included the variables: age ≥46 years, septic shock, BSI due to CR-GNB, and ineffective empirical treatment. Furthermore, for all variables, 95% confidence intervals and p-values ≤ 0.05 were considered statistically significant.
Table 4 summarizes the results of this analysis, finding that having received ineffective empirical treatment was associated with a statistically significant increase in mortality (HR = 10.2, 95% CI = 2.6–39.9, p = 0.001).
Table 4
| Variable | HR (95% CI) | p |
|---|---|---|
| ≥46 years | 1.8 (0.84–3.7) | 0.137 |
| Septic shock | 3.3 (0.41–27.2) | 0.262 |
| CR-GNB | 1.7 (0.52–5.4) | 0.383 |
| Ineffective empirical treatment | 10.2 (2.6–39.9) | 0.001 |
Mortality risks according to the adjusted Cox proportional hazards model.
CI, Confidence interval; CR-GNB, Carbapenem-resistant Gram-negative bacilli; HR, Hazard Ratio, adjusted for age ≥46 years, diabetes mellitus, septic shock, invasive mechanical ventilation; CR-GNB, parenteral nutrition, and ineffective empirical treatment.
Two outbreaks involving a total of 9 patients were detected in the Intensive Care Unit for kidney transplant patients: March 2022 (6 cases) and September 2022 (3 cases), both involving CVC-associated BSI due to A. xylosoxidans. Table 5 shows the demographic data, comorbidities, and conditions of these patients. Statistically significant resistance to tigecycline was found for A. xylosoxidans (OR 8.9, IC 1.7–46.3, p = 0.006). No death events were recorded due to this GNB.
Table 5
| Variable | n = 9 | OR (95 % CI) | p |
|---|---|---|---|
| Age, mean ± SD | 35 ± 8.4 | 0.027a | |
| Diabetes mellitus | 1 (11.1) | 0.443 | |
| SAH/TBI/brain hemorrhage | 0 | NA | |
| Chronic kidney disease | 9 (100.0) | NS | |
| Hemodialysis | 9 (100.0) | NS | |
| Kidney transplant | 9 (100.0) | NS | |
| Conditions during hospitalization | |||
| Invasive mechanical ventilation | 0 | NA | |
| Septic shock | 3 (33.3) | NS | |
| Transplant Intensive Care Unit | 9 (100.0) | NS | |
| BSI associated with the central venous catheter | 9 (100.0) | NS | |
| Endocarditis | 0 | NA | |
| Hospitalization (d), mean ± SD | 15 ± 5 | 0.000a | |
| Hospital admission to BSI (d), median (IQR) | 4 (3–7) | 0.001b | |
| BSI to discharge or mortality ≤ 30-d, mean ± SD | 9 ± 5.4 | 0.071a | |
| Central venous catheter (d), mean, ± SD | 13 ± 3.6 | 0.005a | |
| Laboratory findings | |||
| Blood leukocytes (cells/μl), mean ± SD | 16.6 ± 9.9 | 0.822 | |
| Procalcitonin (ng/ml), median (IQR) | 2.5 (1.1–18.9) | 0.315 | |
| Antibiotic resistance | |||
| Resistance to carbapenems | 0 | NA | |
| Piperacillin/tazobactam resistance | 0 | NA | |
| Resistance to cefoxitin | 2/6 (33.3) | 0.218 | |
| Resistance to cefepime | 1 (11.1) | NS | |
| Resistance to 3rd-generation cephalosporins | 7/8 (87.5) | 1.000 | |
| Resistance to quinolones | 9 (100.0) | NS | |
| Resistance to aminoglycosides | 9 (100.0) | NS | |
| Tigecycline resistance | 7 (77.8) | 8.9 (1.7–46.3) | 0.006 |
| Colistin resistance | 0 | NA | |
| Outcomes | |||
| Ineffective empirical treatment | 0 | NA | |
| From bloodstream infection to mortality ≤ 30-d | 0 | NA | |
Patients with central venous catheter-associated bacteremia due to Achromobacter xylosoxidans.
Outbreaks in March and September 2022.
SAH, Subarachnoid hemorrhage; TBI, Traumatic brain injury; BSI, Bloodstream infections; ng/ml, nanograms/milliliter; SD, Standard deviation; CI, Confidence interval; IQR, Interquartile range; d, days; NS, Not significant; NA, Not applicable; OR, Odds ratio; CI, Confidence interval; SD, Standard deviation; IQR, Interquartile range; NS, Not significant; NA, Not applicable.
aStudent's t statistical test.
bMann–Whitney U test.
Discussion
Of 210 patients with positive monomicrobial blood cultures who met the clinical and microbiological criteria for BSI, 40% (84/210) were excluded, with positive isolates of Gram-positive cocci, Gram-positive bacilli, and fungi. One hundred and twenty-six patients with BSI due to GNB were selected, representing 60% (126/210) of the total.
Our hospital provides tertiary care and serves as a referral center. We routinely receive patients with complex, advanced-stage diseases. We found a mortality of 28.6% in ≤ 30 days due to the GNB BSI. The estimated mortality is higher than that reported in other published studies, which reported 21.6% in ≤ 30 days for GNB-BSI in a multicenter study in Italian hospitals (12) and 17.4% in ≤ 30 days in a cohort study in Spanish hospitals (18).
Mortality due to BSI was related to a high frequency of patients with infection by CR-GNB, mainly CRAB; according to the results of the univariate analysis, mortality was 13.2 (CI 4.3–40.5, p = 0.000) times higher in patients with CR-GNB isolation and 4.2 (CI 1.8–9.6, p = 0.001) times higher in those with CRAB.
Male gender and advanced age have been reported as a frequent demographic profile of patients with BSI. We also found slightly more men but a younger patient group, with statistical significance only in univariate analysis. The younger age found in our patients is notable, which we consider has to do with the severe conditions with which patients are referred to our hospital, since it is documented that older adults have a higher risk of CR-GNB infection, given that they usually have multiple comorbidities, are continuously exposed to antibiotic therapy, and have a higher number of hospitalizations (4, 12, 20).
The most frequent comorbidities found were immunocompromise, arterial hypertension, diabetes mellitus, and vascular access exhaustion. The main factors associated with mortality were being ≥46 years of age, having diabetes mellitus and septic shock, requiring IMV, needing parenteral nutrition, exposure to CR-GNB, presenting BSI due to CRAB, and having received ineffective empirical treatment.
In the multivariate analysis, García-Rodríguez and Mariño-Callejo showed that severe sepsis/septic shock were predictive factors of ≤ 30-day mortality in patients with BSI due to MRD-GNB. And that adequate antimicrobial treatment was a protective factor (12). Also, Abubakar et al. found, in a multivariate regression analysis, that patients presenting with septic shock were a predictor of mortality, and that receiving active (appropriate) antibiotic treatment was protective, according to univariate analysis (21).
Greater vulnerability was found, as expected, by univariate analysis among CR-GNB patients compared with CS-GNB patients across risk factors and outcomes. Ineffective empirical treatment was found to be 30.2 times more frequent in CR-GNB patients, and ≤ 30-day mortality was 12.3 times more frequent in CR-GNB patients.
The main species responsible for GNB BSI were a small subset: E. coli, K. pneumoniae, P. aeruginosa, and A. baumannii (5). Abdel Hadi et al. reported in their cohort study that the predominant MDR-GNB pathogens were E. coli, K. pneumoniae, Salmonella species, and P. aeruginosa (4). Abubakar et al. report that A. baumannii, Enterobacterales, and P. aeruginosa are the most frequent MDR-GNB isolates among hospitalized patients in Malaysia. In our study, the most frequently isolated GNB were A. baumannii, P. aeruginosa, and K. pneumoniae (20). Also, in our study, 47.6% of the isolated GNB were susceptible to carbapenems, and 52.4% were resistant. In a multicenter retrospective study (19 Italian hospitals) that included 1,276 patients with GNB BSI, 56.7% were estimated to be susceptible to carbapenems and 43.3% were estimated to be resistant (18).
We observed lower mortality in patients with CS-GNB isolates, estimated at 6.7% (4/60), compared with a multicenter retrospective study reporting a mortality rate of 13.7% (99/723) for this subgroup (18). This is evident in our survival curves, where patients with CS-GNB isolation show a practically flat curve (Figure 3).
During follow-up (as part of the CS-GNB group), two outbreaks of A. xylosoxidans were documented in patients undergoing kidney transplantation, without serious problems. A. xylosoxidans, belonging to the Alcaligenaceae family, is usually considered an opportunistic and emerging pathogen in the hospital environment. It is a GNB, aerobic, and non-lactose fermenting. Outbreaks of nosocomial catheter-associated bacteremia due to A. xylosoxidans have been described, which were caused by contaminated environmental sources or devices, or by water faucets or hemodialysis systems, mainly in immunocompromised patients (21–23).
In patients with P. aeruginosa isolation, we estimated lower mortality in the CRPA group, 20.0% (2/10) vs. 32.8% (20/61) in the comparison group of Falcone et al. (18).
The mortality in the CRAB group was 50.0% (19/38), higher than that of this subgroup in the same comparison study (43.2%; 48/111) (18). In specific research on BSI by A. baumannii, Ngiam et al. reported a mortality of 73.3% for CRAB vs. 16.1% for carbapenem-susceptible A. baumannii (p < 0.001) (24).
The higher number of patients classified as CRAB is consistent with a previous study in our hospital for patients with nosocomial infectious ventriculitis caused by MDR-GNB associated with external ventricular drainage, where a higher percentage of isolation for A. baumannii was observed (40.6% of the total cerebrospinal fluid culture isolates) with 91.3% resistance to carbapenems (13).
In a meta-analysis, Du et al. reported factors associated with mortality in patients with BSI due to CRAB, including, as in the present study, ineffective empirical treatment, septic shock, total parenteral nutrition, and invasive mechanical ventilation (25).
In a retrospective cohort study, Son et al. evaluated risk factors for ≤ 30-day mortality in A. baumannii BSI and found septic shock and inappropriate antimicrobial therapy as independent risk factors for mortality (multivariate analysis). And, as an antibiotic strategy, they discovered that colistin combined with tigecycline or other antibiotics was significantly associated with lower mortality (after adjusting for confounding factors) (26). A. baumannii is responsible for a large number of nosocomial infections, with a significant capacity to survive in the hospital environment and to present antimicrobial resistance. Kumar et al., in a review, summarized the main resistance mechanisms of A. baumannii, including metallo-β-lactamase-type, OXA-type, and KPC-type carbapenemases, as well as efflux pumps responsible for multidrug resistance and aminoglycoside-modifying enzymes (27).
In patients with CRAB, we found 100% resistance to the entire spectrum of available older β-lactam antibiotics and quinolones, high resistance to aminoglycosides (65.8%), and resistance to tigecycline (44.7%).
A retrospective study that included a prognostic-matching analysis of BSI for A. baumannii, Wang et al., explains that providing appropriate empirical therapy to patients with septic shock, mechanical ventilation, and elevated SOFA scores can significantly improve survival (28). They point out that BSI preferentially affects critically ill patients. Of the patients with carbapenem-resistant A. baumannii, more than half presented with this condition; 23/38 (60.5%) were in an ICU, 33/38 (86.8%) required invasive mechanical ventilation, and 33/38 (86.8%) presented with septic shock; half died. Wang et al. also conclude that drug resistance in A. baumannii leads to inadequate empirical antibiotic therapy and is a direct predictor of mortality (28). These are also conclusive findings from our work. However, in clinical practice, we used this treatment due to the patient's condition. Previous studies explain that A. baumannii is very common in developing countries, so if there is no response or clinical improvement, colistin (polymyxin E) should be prescribed. Notably, colistin is also appropriate for definitive treatments (5, 24, 28, 29).
For the CRE group, a high mortality rate was observed, estimated at 58.3% (7/12), presenting one of the steepest survival curves in our work versus Falcone et al. (18) with an estimated mortality (KPC-type and, metallo-β-lactamase-type carbapenemases) of 40.0% (109/272). The high mortality rate found suggests that this group of GN bacilli should not be ignored. In particular, the CRE group stands out in the global panorama, with a significant increase in detection in the Asia Pacific, Europe, and Latin America regions (30).
In the Other Non-Fermenting GNB group, the high mortality rate was influenced by the S. maltophilia burden. This group showed the worst survival curve, but still outperformed the CRAB and CRE groups. S. maltophilia, an environmental aerobic GNB, acting mainly as an opportunistic pathogen, is of special interest. This is considered an essential nosocomial pathogen because it causes potentially fatal invasive infections. In our study, 4/5 patients with a positive culture for this GNB died (immunocompromised patients). According to the meta-analysis by Huang et al., there is insufficient data on an appropriate and specific therapy for S. maltophilia infection. Carbapenems are usually used empirically for any BSI due to GNB, which is inappropriate for S. maltophilia (intrinsically carbapenem-resistant GNB) (31). However, levofloxacin, trimethoprim/sulfamethoxazole, or the combined therapy of levofloxacin and trimethoprim/sulfamethoxazole can be used with favorable outcomes (30–33).
Among the P. aeruginosa isolates, six were classified as CS-GNB despite carbapenem resistance. Conversely, these same strains were found to be susceptible to cephalosporins (cefepime and ceftazidime) and piperacillin/tazobactam. Given this susceptibility, the reported resistance to carbapenems is likely due to porins or efflux pumps rather than carbapenemases. It has been suggested that alterations in the OprD porins responsible for the permeability of molecules such as carbapenems (through a gene produced by P. aeruginosa) and the presence of an active efflux pump are the main mechanisms associated with the resistance to carbapenems of this GNB. Furthermore, patients with these isolates were treated with cephalosporins with favorable outcomes (19, 34–37).
Along with the increase in the incidence of GNB bacteremia, the emergence of antimicrobial resistance in these bacilli has been reported, directly affecting treatment and increasing mortality. Inappropriate use of antibiotics has been reported in Malaysia, where up to 50% of prescriptions among hospitalized patients may not meet the specifications (5, 20). Although CR-GNB infections in the present study were associated with ineffective empirical treatment, the virulence of the isolated bacilli and delays in diagnosis should be considered. Notably, receiving ineffective treatment was an independent factor in mortality, according to the proportional HR model.
By obtaining an early diagnosis, it is easier to provide timely and appropriate treatment, which should be in accordance with the guidelines of the European Society of Clinical Microbiology (ESCMID) and the guidelines of the Infectious Diseases Society of America (IDSA), to improve prevention and stop the effects of drug resistance (3, 19, 27, 34, 38, 39). Furthermore, it is necessary to understand local epidemiological data and strengthen infection control programs.
Limitations
International organizations closely monitor global fluctuations in behavior, as well as variations in the incidence, prevalence, and mortality of antimicrobial resistance, in which resistance to GNB plays a significant role. Despite limitations, primarily stemming from the retrospective nature of this study, the fact that the data correspond to a single hospital with complex characteristics, a lack of resources for performing high-tech microbiological typing (molecular typing and other methods), and potential operational differences with other research that affect the comparability of results, our work allowed us to epidemiologically profile what occurred during the study period. This profile reflects the complexity of managing human and material resources at our hospital, as well as its capacity to address emerging microorganisms.
During the study period, the hospital lacked the necessary technological resources to perform rapid, state-of-the-art tests for carbapenemase detection. This is consistent with the frequency with which patients receive ineffective empirical treatments.
In our hospital setting, therapeutic options for the treatment of BSI were limited during the study period, mainly due to the unavailability of colistin, ceftolozane/tazobactam, and ceftazidime/avibactam.
Conclusions
Mortality in the bacteremia cohort of this study was associated with a high frequency of patients with CR-GNB, primarily due to CRAB, who received ineffective empirical treatment.
The information obtained through the follow-up and analysis of this cohort will undoubtedly improve medical care, accelerating the development of faster diagnostic tools and providing more effective treatments to our patients.
Since the data were collected over an entire year, a compilation of all BSI events, particularly those involving hospitalized patients due to GNB, is also presented, reflecting the complexity of these pathogens in the epidemiological and clinical landscape of our workplace, The Specialty Hospital, with 500 census beds.
Greater efforts are needed to improve epidemiological surveillance systems through Healthcare-Associated Infection and Antimicrobial Stewardship Committees. Furthermore, to adopt and implement universal healthcare measures, including different prevention packages (for each type of healthcare-associated infection) and other general measures such as staff training, water quality, environmental decontamination, antiseptic use, disinfection and sterilization, appropriate antibiotic use, use of isolation systems for proper patient management, and designated areas to improve medication preparation, among others. It is also necessary to promote education, monitoring, and feedback on guidelines for the prevention and control of healthcare-associated infections.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted according to the Good Health Research Practices of the International Conference on Harmonization and the Declaration of Helsinki and was approved by the Hospital Ethics Committee with the R-2023-1301-083 Registry. Only the medical records were reviewed. As it was a retrospective cohort study, it was not necessary to obtain the consent of the included patients. Patients' identities were kept strictly confidential. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AC-N: Data curation, Conceptualization, Project administration, Formal analysis, Writing – review & editing, Investigation, Methodology, Writing – original draft, Supervision. MA-M: Writing – review & editing, Supervision, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. SU-R: Methodology, Supervision, Investigation, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. MV-A: Investigation, Writing – review & editing, Conceptualization, Writing – original draft, Supervision, Validation, Data curation, Methodology. JC-M: Methodology, Writing – original draft, Data curation, Validation, Supervision, Writing – review & editing, Conceptualization. EG-E: Project administration, Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing, Writing – original draft, Formal analysis. JA-S: Data curation, Investigation, Validation, Methodology, Conceptualization, Supervision, Writing – review & editing, Formal analysis, Writing – original draft, Software. AN-Z: Project administration, Writing – review & editing, Formal analysis, Methodology, Writing – original draft, Investigation, Conceptualization. LG-C: Project administration, Methodology, Writing – original draft, Supervision, Conceptualization, Formal analysis, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
World Health Organization . Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. WHO, editor. Geneva: World Health Organization (2024).
2.
Guidelines Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities . Geneva: World Health Organization (2017).
3.
Paul M Carrara E Retamar P Tängdén T Bitterman R Bonomo RA et al . European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. (2022) 28:521–47. doi: 10.1016/j.cmi.2021.11.025
4.
Abdel Hadi H Dargham SR Eltayeb F Ali MOK Suliman J Ahmed SAM et al . Epidemiology, clinical, and microbiological characteristics of multidrug-resistant gram-negative bacteremia in Qatar. Antibiotics (Basel). (2024) 13:320. doi: 10.3390/antibiotics13040320
5.
Holmes CL Anderson MT Mobley HLT Bachman MA . Pathogenesis of gram-negative bacteremia. Clin Microbiol Rev. (2021) 34:e00234–20. doi: 10.1128/CMR.00234-20
6.
Kumar M Tandel K Shergill SPS Bhalla GS Mahajan P Swarnim V et al . Rapid detection of carbapenem resistance among gram-negative organisms directly from positive blood culture bottles. Med J Armed Forces India. (2023) 79:267–74. doi: 10.1016/j.mjafi.2021.03.026
7.
Luo Q Lu P Chen Y Shen P Zheng B Ji J et al . ESKAPE in China: epidemiology and characteristics of antibiotic resistance. Emerg Microbes Infect. (2024) 13:2317915. doi: 10.1080/22221751.2024.2317915
8.
NHSN National Healthcare Safety Network . CDC/NHSN Surveillance Definitions for Specific Types of Infections. Available online at: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (Accessed January 6, 2025).
9.
Kern WV Rieg S . Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect. (2020) 26:151–7. doi: 10.1016/j.cmi.2019.10.031
10.
Munro C Zilberberg MD Shorr AF . Bloodstream infection in the intensive care unit: evolving epidemiology and microbiology. Antibiotics (Basel Switzerland). (2024) 13:123. doi: 10.3390/antibiotics13020123
11.
Diekema DJ Hsueh PR Mendes RE Pfaller MA Rolston KV Sader HS et al . The microbiology of bloodstream infection: 20-year trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. (2019) 63:e00355–19. doi: 10.1128/AAC.00355-19
12.
García-Rodríguez JF Mariño-Callejo A . The factors associated with the trend in incidence of Bacteremia and associated mortality over 30 years. BMC Infect Dis. (2023) 23:69. doi: 10.1186/s12879-023-08018-0
13.
Corona-Nakamura AL Arias-Merino MJ Ávila-Esparza EI et al . Ventriculitis due to multidrug-resistant gram-negative bacilli associated with external ventricular drain: evolution, treatment, and outcomes. Front Neurol. (2024) 15:1384206. doi: 10.3389/fneur.2024.1384206
14.
Magiorakos AP Srinivasan A Carey RB Carmeli Y Falagas ME Giske CG et al . Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
15.
Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute (2020).
16.
Pintado V Ruiz-Garbajosa P Aguilera-Alonso D Baquero-Artigao F Bou G Cantón R et al . Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) on the diagnosis and antimicrobial treatment of infections due to carbapenem-resistant Gram-negative bacteria. Enferm Infecc Microbiol Clin (Engl Ed). (2023) 41:360–70. doi: 10.1016/j.eimce.2022.06.014
17.
Miller JM Binnicker MJ Campbell S Carroll KC Chapin KC Gonzalez MD et al . Guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2024 update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. (2024) 1–123. doi: 10.1093/cid/ciae104
18.
Falcone M Tiseo G Carbonara S Marino A Di Caprio G Carretta A et al . Mortality Attributable to bloodstream infections caused by different carbapenem-resistant gram-negative bacilli: results from a nationwide study in Italy (ALARICO network). Clin Infect Dis. (2023) 76:2059–69. doi: 10.1093/cid/ciad100
19.
Tamma PD Aitken SL Bonomo RA Mathers AJ van Duin D Clancy CJ . Infectious diseases society of America 2022 Guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. (2022) 75:187–212. doi: 10.1093/cid/ciac268
20.
Abubakar U Zulkarnain AI Rodríguez-Baño J Kamarudin N Elrggal ME Elnaem MH . Krawczyk, J, et al. Treatments and predictors of mortality for carbapenem-resistant gram-negative bacilli infections in Malaysia: a retrospective cohort study. Trop Med Infect Dis. (2022) 7:415. doi: 10.3390/tropicalmed7120415
21.
Houlihan E Lucey M Pandian A Hanahoe B Higgins F DeLappe N et al . Case of recurrent Achromobacter xylosoxidans bacteraemia and PICC (peripherally-inserted central catheter) line infection in an immunocompromised patient. Infect Prev Pract. (2022) 4:100202. doi: 10.1016/j.infpip.2022.100202
22.
Kar M Singh R Tejan N Jamwal A Dubey A Chaudhary et al . One year of experience of Achromobacter bacteremia at a tertiary care hospital in Northern India. Access Microbiol. (2023) 5:000588.v3. doi: 10.1099/acmi.0.000588.v3
23.
Casale R Boattini M Comini S Bastos P Corcione S De Rosa FG et al . Clinical and microbiological features of positive blood culture episodes caused by non-fermenting gram-negative bacilli other than Pseudomonas and Acinetobacter species (2020-2023). Infection. (2025) 53:183–96. doi: 10.1007/s15010-024-02342-6
24.
Ngiam JN Koh MCY Chew KL . Clinical characteristics and treatment outcomes of Acinetobacter baumannii bloodstream infections in a setting with high carbapenem susceptibility among isolates. Microbiol Spectr. (2025) 13:e0160725. doi: 10.1128/spectrum.01607-25
25.
Du X Xu X Yao J Deng K Chen S Shen Z et al . Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Am J Infect Control. (2019) 47:1140–5. doi: 10.1016/j.ajic.2019.03.003
26.
Son H-J Cho EB Bae M Lee SC Sung H Kim MN et al . Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. Open Forum Infect Dis. (2020) 7:ofaa378. doi: 10.1093/ofid/ofaa378
27.
Kumar S Anwer R Azzi A . Virulence potential and treatment options of multidrug-resistant (MDR) Acinetobacter baumannii. Microorganisms. (2021) 9:2104. doi: 10.3390/microorganisms9102104
28.
Wang J Zhang J Wu ZH et al . Clinical characteristics and prognosis analysis of Acinetobacter baumannii bloodstream infection based on propensity matching. Infect Drug Resist. (2022) 15:6963–74. doi: 10.2147/IDR.S387898
29.
Huang C Gao Y Lin H Fan Q Chen L Feng Y et al . Prognostic factors that affect mortality in patients with Acinetobacter baumannii bloodstream infection. Infect Drug Resist. (2024) 17:3825–37. doi: 10.2147/IDR.S475073
30.
Maraolo AE Licciardi F Gentile I Saracino A Belati A Bavaro DF . Stenotrophomonas maltophilia infections: a systematic review and meta-analysis of comparative efficacy of available treatments, with critical assessment of novel therapeutic options. Antibiotics (Basel, Switzerland). (2023) 12:910. doi: 10.3390/antibiotics12050910
31.
Huang C Lin L Kuo S . Risk factors for mortality in Stenotrophomonas maltophilia bacteremia: a meta-analysis. Infect Dis (London, England). (2024) 56:335–47. doi: 10.1080/23744235.2024.2324365
32.
Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 33rd ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute (2023).
33.
Gezer Y Tayşi MR Tarakçi A Gökçe Ö Danaci G Toplu SA et al . Evaluation of clinical outcomes and risk factors associated with mortality in patients with Stenotrophomonas maltophilia bloodstream infection: a multicenter study. BMC Infect Dis. (2024) 24:1396. doi: 10.1186/s12879-024-10348-6
34.
Tamma PD Simner PJ . Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J Clin Microbiol. (2018) 56:e01140–18. doi: 10.1128/JCM.01140-18
35.
Karruli A Catalini C D'Amore C Foglia F Mari F Harxhi A et al . Evidence-based treatment of Pseudomonas aeruginosa infections: a critical reappraisal. Antibiotics (Basel Switzerland). (2023) 12:399. doi: 10.3390/antibiotics12020399
36.
Kao CY Chen SS Hung KH Wu H-M Hsueh P-R Yan JJ et al . Overproduction of active efflux pump and variations of OprD dominate in imipenem-resistant Pseudomonas aeruginosa isolated from patients with bloodstream infections in Taiwan. BMC Microbiol. (2016) 16:107. doi: 10.1186/s12866-016-0719-2
37.
Vila J Marco F . Lectura interpretada del antibiograma de bacilos gramnegativos no fermentadores [Interpretive reading of the non-fermenting gram-negative bacilli antibiogram]. Enferm Infecc Microbiol Clin. (2010) 28:726–36. doi: 10.1016/j.eimc.2010.05.001
38.
Zeng M Xia J Zong Z Shi Y Ni Y Hu F et al . Guidelines for the diagnosis, treatment, prevention and control of infections caused by carbapenem-resistant gram-negative bacilli. J Microbiol Immunol Infect. (2023) 56:653–71. doi: 10.1016/j.jmii.2023.01.017
39.
Kadri SS Adjemian J Lai YL Spaulding AB Ricotta E Prevots DR et al . Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. (2018) 67:1803–14. doi: 10.1093/cid/ciy378
Summary
Keywords
bloodstream infections, multidrug-resistant Gram-negative bacilli, mortality, carbapenem-resistant Acinetobacter baumannii, ineffective empirical treatment
Citation
Corona-Nakamura AL, Arias-Merino MJ, Urbina-Rosas SA, Vázquez-Arias ME, Corona-Macías JF, González-Espinoza E, Andrade-Sierra J, Nava-Zavala AH and Govea-Camacho LH (2026) Mortality risk factors in patients with bloodstream infections due to multidrug-resistant Gram-negative bacilli. Front. Med. 12:1693317. doi: 10.3389/fmed.2025.1693317
Received
26 August 2025
Revised
25 November 2025
Accepted
26 November 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Daniel Diaz, National Autonomous University of Mexico, Mexico
Reviewed by
Valentina Scheggi, Careggi University Hospital, Italy
Sajad A. Dar, Jazan University, Saudi Arabia
Updates
Copyright
© 2026 Corona-Nakamura, Arias-Merino, Urbina-Rosas, Vázquez-Arias, Corona-Macías, González-Espinoza, Andrade-Sierra, Nava-Zavala and Govea-Camacho.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Humberto Govea-Camacho, lhgovea@yahoo.com; Jorge Andrade-Sierra, Jorg_Andrade@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.