- Department of Pulmonary and Critical Care Medicine, The Sixth Hospital of Wuhan, Affiliated Hospital of Jianghan University, Wuhan, China
Pulmonary Lymphatic Perfusion Syndrome (PLPS) is a rare condition characterized by abnormal lymphatic drainage into the lungs, leading to symptoms such as chyloptysis and chylous effusions. We report a case of a 54-year-old male with PLPS who presented with persistent cough, milky sputum, and pericardial effusion. Following initial misdiagnosis and ineffective empirical treatment for a presumed pulmonary infection, a definitive diagnosis of PLPS was established. The patient underwent successful percutaneous thoracic duct embolization, resulting in complete resolution of symptoms and imaging findings at follow-up. This case highlights the importance of considering PLPS in patients with unexplained chylous effusions and the efficacy of lymphatic intervention as a treatment option.
Introduction
Pulmonary Lymphatic Perfusion Syndrome (PLPS) is a rare, challenging disorder characterized by abnormal lymphatic flow from the thoracic duct into the lungs and surrounding structures (1). This aberrant perfusion can lead to severe clinical manifestations, including chylothorax, chylopericardium, and plastic bronchitis (1). Although often congenital, PLPS symptoms may be unmasked by secondary triggers like trauma or infection (2). Diagnosing PLPS remains difficult due to its non-specific presentation mimicking other conditions (3). While advanced imaging, particularly dynamic contrast-enhanced magnetic resonance lymphangiography (DCMRL), is crucial for identification (1), effective treatment remains a hurdle. Conservative measures often fail (4), creating a need for more definitive interventions. Percutaneous lymphatic embolization has emerged as a highly effective, minimally invasive treatment to occlude these abnormal pathways. Here, we report a 54-year-old male with PLPS who, after initial misdiagnosis and failed conservative therapy, was successfully treated with percutaneous thoracic duct embolization (5).
Case presentation
A 54-year-old male with a history of chronic bronchitis and hepatitis B presented with an 11-month history of intermittent cough, milky sputum, and fever, with a recent recurrence 20 days prior to admission on August 28, 2023. Initial treatment at another hospital for suspected lung infection and pericardial effusion (with antibiotics and pericardial drainage) provided partial relief of fever and dyspnea but no improvement in cough or sputum. Upon admission, physical examination revealed hypertension (147/108 mmHg) and coarse breath sounds with wet rales in the right lung. Chest CT showed a right lung infectious lesion (Figure 1A) and pericardial effusion (Figure 1B), while bronchoscopy revealed milky white sputum, and pericardial fluid (Figure 1C) analysis confirmed chylous effusion (high triglyceride levels, predominantly lymphocytes).
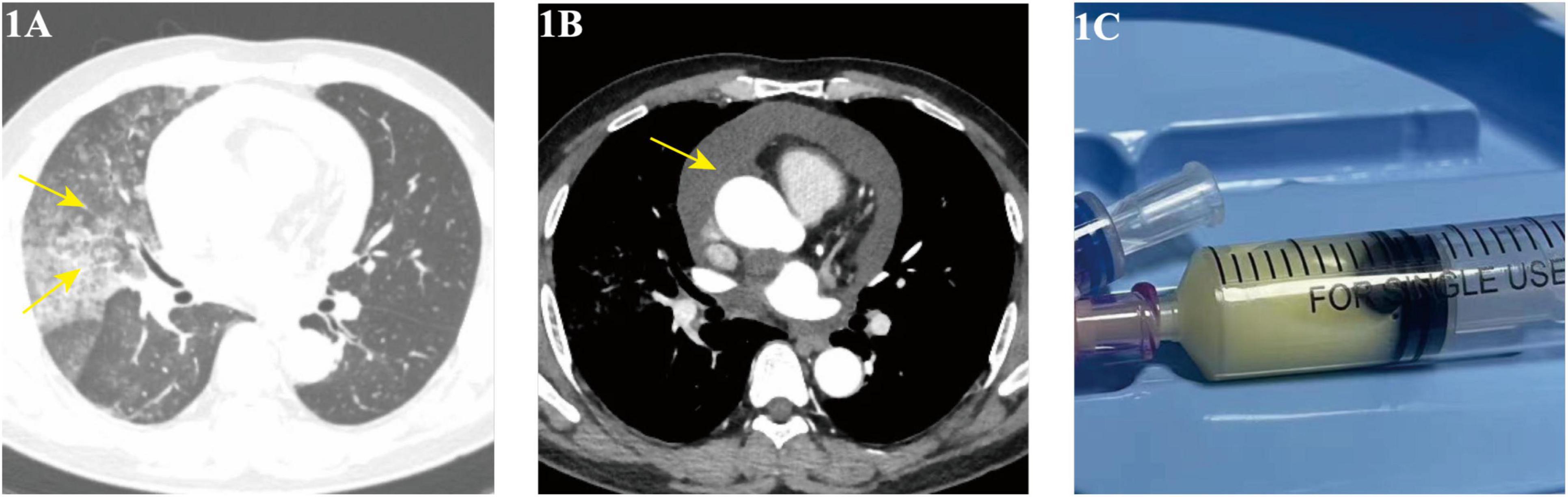
Figure 1. (A,B) Chest CT (August 29, 2023) showing right lung infiltrates and pericardial effusion; (C) pericardiocentesis yielding chylous fluid.
On September 2, 2023, the patient underwent a multi-step lymphatic interventional procedure. Initially, ultrasound-guided intranodal lymphangiography was performed via puncture of an inguinal lymph node, followed by the slow injection of iodized oil (Lipiodol). Imaging revealed lymphatic drainage into the left venous angle and its confluence with the subclavian vein. Concurrently, contrast extravasation was observed from the cisternal segment of the thoracic duct, with leakage extending toward the right lung and pericardium, confirming the diagnosis of PLPS (Figure 2A, Supplementary Video 1). Subsequently, the femoral vein was punctured, and a 5F vascular sheath was inserted. A 5F VERT catheter, advanced over a 0.035-inch guidewire, was navigated to the left subclavian vein to locate the lymphatic orifice. Upon identification of the orifice, a 1.98F microcatheter, supported by 0.014-inch and 0.018-inch microwires, was successfully advanced into the thoracic duct. Embolization of the distal thoracic duct was achieved using 3 × 3 microcoils (Supplementary Video 2), followed by the rapid injection of surgical glue (n-BCA) into the duct (Supplementary Video 3). This combined technique uses the coils to create a physical scaffold, slowing chyle flow and preventing glue migration, while the glue permeates the coil mesh to create a durable, impermeable seal. The microcatheter was promptly withdrawn post-injection. Final Digital Subtraction Angiography (DSA) confirmed satisfactory deposition of the surgical glue within the thoracic duct and demonstrated complete occlusion of the fistula, with no residual contrast extravasation (Figure 2B and Supplementary Video 4). Post-procedure, the patient was placed on a low-fat diet.

Figure 2. Lymphangiography and thoracic duct embolization. (A) Pre-embolization lymphangiography via a microcatheter (white arrow) in the thoracic duct shows the main duct trunk (black arrows) and an active fistula with contrast extravasation into the right lung parenchyma and pericardial cavity (asterisks). (B) Post-embolization DSA confirms successful occlusion of the thoracic duct, with the coil and glue cast visible (white arrow) and no further contrast leakage.
Follow-up imaging on September 4 and 8, 2023, revealed reduced lung infection and minimal pericardial effusion (Figures 3A, B), with complete resolution of both pericardial effusion and lung lesions observed by October 25, 2023 (Figures 3C, D), and no recurrence of symptoms. As of the latest follow-up, one year later, the patient reports no discomfort.
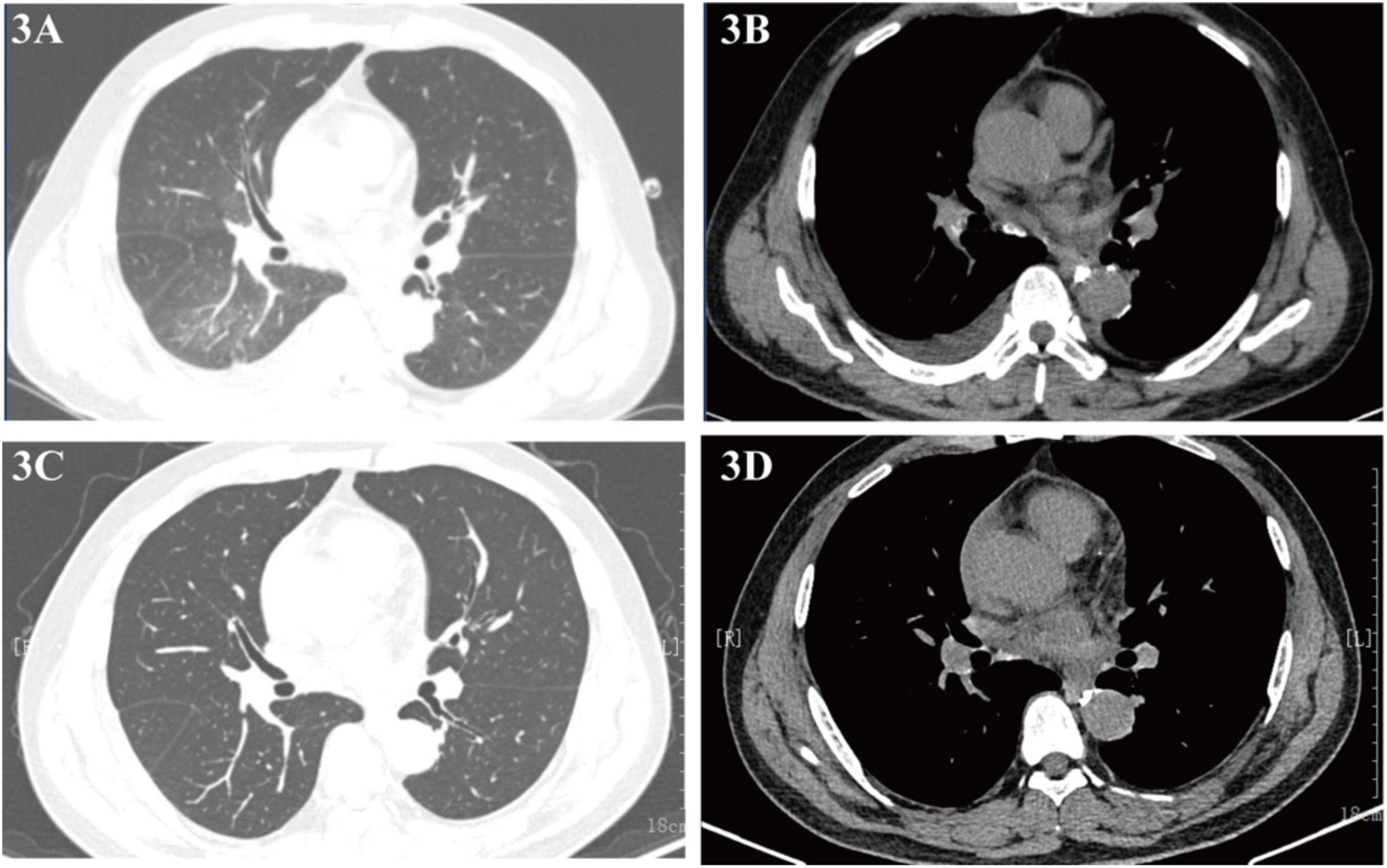
Figure 3. (A,B) Chest CT (September 4, 2023) showing reduced infiltrates and effusion; (C,D) follow-up CT (October 25, 2023) confirming resolution.
Discussion
Pulmonary Lymphatic Perfusion Syndrome (PLPS) is a rare and complex group of lymphatic disorders characterized by the abnormal flow of chyle from the thoracic duct (TD) or other central lymphatic channels into the pulmonary parenchyma, airways, mediastinum, pericardium, or pleural space (6). This case report describes the successful management of a 54-year-old male presenting with the exceedingly rare combination of chyloptysis and chylous pericardial effusion secondary to PLPS, effectively treated with percutaneous thoracic duct embolization (TDE). The initial misdiagnosis as a pulmonary infection with reactive pericardial effusion underscores the diagnostic challenges posed by PLPS, where non-specific symptoms often mimic more common cardiorespiratory conditions (7).
The simultaneous occurrence of chyloptysis (indicative of lymphatic leakage into the bronchial tree or lung parenchyma) and chylopericardium (chyle accumulation in the pericardial sac) is an exceptionally uncommon clinical scenario. While primary or idiopathic chylopericardium is itself a rarity, with historical estimates of around 100 reported cases over decades (8–10), and chyloptysis is considered even rarer, with fewer than 20 cases documented by a 2018 review (2, 11), their co-existence strongly implicates a singular, central lymphatic pathology affecting major lymphatic pathways. PLPS provides a coherent pathophysiological explanation for such multi-compartmental chylous leakage (8). In this patient, lymphangiography confirmed active leakage from the thoracic duct into both the right lung and the pericardium (Figure 2A), directly supporting PLPS as the unifying cause of his symptoms. This abnormal perfusion is thought to arise from congenital anatomical lymphatic variants (1), where triggers such as infection or increased central venous pressure can unmask the underlying defect by increasing lymphatic flow or pressure (4).
The diagnostic pathway in this case highlights critical aspects of evaluating suspected lymphatic disorders. Confirmation of the chylous nature of the expectorated sputum and pericardial fluid was paramount, achieved through bronchoscopy revealing milky sputum and pericardial fluid analysis showing high triglyceride levels and a predominance of lymphocytes (8, 12). Although dynamic contrast-enhanced magnetic resonance lymphangiography (DCMRL) is the preferred non-invasive examination for evaluating PLPS, its diagnostic utility can be limited by a variety of technical and patient-specific factors. These include motion artifact, concomitant venous enhancement that may obscure lymphatic channels, and insufficient signal-to-noise ratio, particularly when the central lymphatic ducts are not pathologically dilated (13, 14), In our patient, the attempted DCMRL was non-diagnostic due to poor signal quality, a recognized challenge of the technique. We therefore proceeded with conventional catheter lymphangiography, which remains an essential tool for definitive diagnosis and interventional planning when advanced imaging is inconclusive or unavailable (15). The ability of lymphangiography to pinpoint the direct source of bilateral leakage (8) was crucial for both confirming PLPS and planning the subsequent interventional procedure (16).
Management of PLPS and associated chylous effusions has evolved significantly. Conservative measures, including a low-fat diet (8) (often supplemented with medium-chain triglycerides) and somatostatin analogs (17) (e.g., octreotide), aim to reduce chyle production and flow (2). However, these approaches often have limited success, particularly in cases of high-output or persistent leaks (18), or when complex anatomical abnormalities like PLPS are present, as was the experience with this patient prior to definitive intervention. The failure rates for conservative management, especially in non-traumatic contexts, can exceed 50–60% (17).
In contrast, percutaneous lymphatic interventions, particularly TDE or selective lymphatic embolization (SLE), have emerged as highly effective and minimally invasive treatments (16, 19, 20). TDE aims to occlude the thoracic duct at or near the point of leakage, thereby preventing further chylous extravasation. In this patient, embolization of the thoracic duct fistula using microcoils and surgical glue resulted in the rapid and complete resolution of both chyloptysis and pericardial effusion, with sustained clinical improvement at one-year follow-up. The combined embolization technique using microcoils and n-BCA glue is an established strategy to ensure durable fistula occlusion. The coils provide a stable 3D scaffold that slows flow and prevents distal migration of the liquid embolic, while the n-BCA glue permeates this framework to create a completely impermeable seal, thereby minimizing the risk of recanalization. This outcome is consistent with a growing body of evidence supporting TDE as a first-line invasive treatment for non-traumatic chylous effusions and specific manifestations of PLPS (16), such as plastic bronchitis (21) and chylothorax. Systematic reviews and institutional series report technical success rates for TDE in traumatic chylothorax as high as 70–90% (22), and with the advent of advanced imaging-guided algorithms, success in complex non-traumatic chylothorax, including PLPS, can reach over 90% in specialized centers (6). Compared to traditional thoracic duct ligation (TDL), TDE offers significant advantages in terms of lower procedural morbidity, shorter recovery times, and reduced invasiveness. While TDL remains a highly effective treatment, it typically requires open thoracic surgery and is associated with the risks of a major operation. The current treatment paradigm for refractory chyle leaks therefore positions TDE as the first-line invasive intervention, with TDL reserved for cases where percutaneous access is unsuccessful or the intervention fails. Table 1 summarizes a comparison of the two approaches. However, potential complications of TDE, though infrequent, must be acknowledged, including infection, non-target embolization, glue migration, and, rarely, long-term sequelae of TD occlusion such as peripheral edema, chronic diarrhea, or chylous ascites (22).
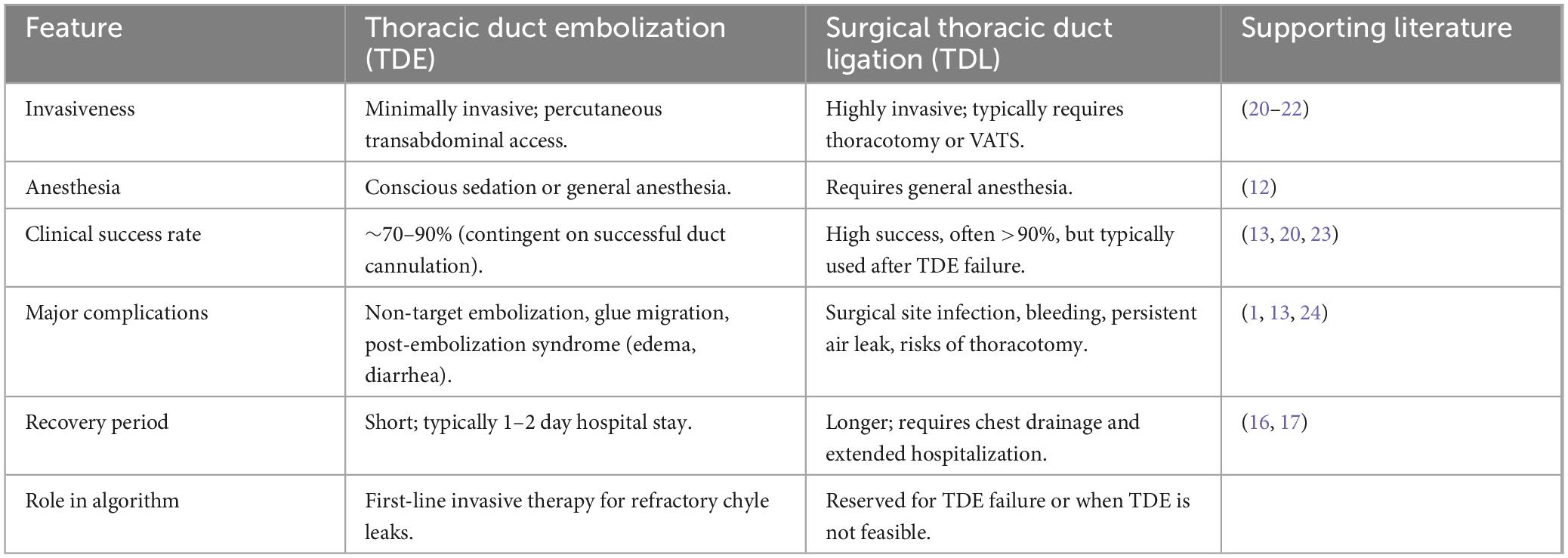
Table 1. Comparative analysis of thoracic duct embolization (TDE) vs. surgical thoracic duct ligation (TDL).
This case contributes significantly to the understanding of PLPS by documenting a rare dual presentation of chyloptysis and chylopericardium successfully managed with TDE. It reinforces the necessity of maintaining a high index of suspicion for underlying lymphatic anomalies in patients presenting with unexplained chylous effusions in multiple thoracic compartments. Furthermore, it illustrates the indispensable role of lymphatic imaging in confirming the diagnosis and guiding targeted, minimally invasive therapy, even when the preferred modality (DCMRL) is not viable. The successful outcome achieved with TDE champions its role in the evolving treatment paradigm for PLPS, shifting from expectant or highly invasive approaches toward precise, image-guided lymphatic interventions.
This report is subject to the inherent limitations of a single case study, and the findings may not be universally generalizable.
Future efforts should focus on increasing awareness of PLPS among clinicians across various specialties to facilitate earlier diagnosis. Collaborative, multicenter studies or registries are warranted to better delineate the full clinical spectrum and natural history of PLPS, standardize diagnostic algorithms incorporating advanced imaging, and further refine interventional treatment protocols. Long-term follow-up of patients undergoing embolization for PLPS is also crucial to understand the durability of the intervention and to monitor for potential late recurrences or the development of other lymphatic pathway abnormalities, given the presumed congenital basis of the underlying lymphatic variant.
Conclusion
This case report demonstrates the successful treatment of PLPS with percutaneous thoracic duct embolization after initial misdiagnosis and ineffective conservative therapy. It highlights the importance of considering PLPS in patients with unexplained chylous effusions and the efficacy of lymphatic intervention as a therapeutic option. Increased awareness and timely diagnosis are essential for optimal management of this rare condition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Jianghan University Affiliated Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
QL: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. JW: Writing – original draft. YC: Conceptualization, Methodology, Writing – review & editing. WQ: Supervision, Writing – review & editing. ZZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to assist in generating keywords from the abstract and to brainstorm potential running titles. All AI-generated content was reviewed, edited, and verified by the authors for accuracy and appropriateness.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1696594/full#supplementary-material
References
1. Itkin M. Interventional treatment of pulmonary lymphatic anomalies. Tech Vasc Interv Radiol. (2016) 19:299–304. doi: 10.1053/j.tvir.2016.10.005
2. Luo X, Zhang Z, Wang S, Gu X, Wang X. Chyloptysis with chylopericardium, a rare case and mini-review. BMC Pulm Med. (2018) 18:21. doi: 10.1186/s12890-018-0583-y
3. Hirano T, Fujino N, Takase K, Ota H, Tanaka R, Saito R, et al. Pulmonary lymphatic perfusion syndrome. Am J Respir Crit Care Med. (2019) 199:529–30. doi: 10.1164/rccm.201806-1125IM
4. Thamkittikun C, Tovichien P. Clinical approach for pulmonary lymphatic disorders. World J Clin Cases. (2024) 12:6020–6. doi: 10.12998/wjcc.v12.i27.6020
5. Itkin M, McCormack F, Dori Y. Diagnosis and treatment of lymphatic plastic bronchitis in adults using advanced lymphatic imaging and percutaneous embolization. Ann Am Thorac Soc. (2016) 13:1689–96. doi: 10.1513/AnnalsATS.201604-292OC
6. Hur S. Novel interventional radiology for the treatment of various lymphatic leakages: Lymphatic intervention and embolization. Vasc Spec Int. (2023) 39:42. doi: 10.5758/vsi.230082
7. Duletzke N, Kiraly L, Martindale R. Chylothorax and chylous ascites: overview, management, and nutrition. Nutr Clin Pract. (2023) 38:557–63. doi: 10.1002/ncp.10973
8. Yu X, Jia N, Ye S, Zhou M, Liu D. Primary chylopericardium: a case report and literature review. Exp Ther Med. (2018) 15:419–25. doi: 10.3892/etm.2017.5383
9. Biko D, Dori Y, Savoca M, Krishnamurthy G, Smith C, Laje P, et al. Pediatric pulmonary lymphatic flow disorders: diagnosis and management. Paediatr Respir Rev. (2020) 36:2–7. doi: 10.1016/j.prrv.2019.11.002
10. Pamarthi V, Stecker M, Schenker M, Baum R, Killoran T, Suzuki Han A, et al. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol JVIR. (2014) 25:1398–404. doi: 10.1016/j.jvir.2014.03.027
11. Han Z, Li S, Jing H, Liu H. Primary idiopathic chylopericardium: a retrospective case series. BMC Surg. (2015) 15:61. doi: 10.1186/s12893-015-0047-8
12. Dib C, Tajik A, Park S, Kheir M, Khandieria B, Mookadam F. Chylopericardium in adults: a literature review over the past decade (1996-2006). J Thorac Cardiovasc Surg. (2008) 136:650–6. doi: 10.1016/j.jtcvs.2008.03.033
13. Researchgate. Nontraumatic chylothorax: Diagnostic Algorithm and Treatment Options. (n.d.). doi: 10.1053/j.tvir.2016.10.008
14. Negm A, Collins J, Bendel E, Takahashi E, Knavel Koepsel E, Gehling K, et al. MR lymphangiography in lymphatic disorders: clinical applications, institutional experience, and practice development. Radiogr Rev Publ Radiol Soc N Am Inc. (2024) 44:e230075. doi: 10.1148/rg.230075
15. Chavhan G, Amaral J, Temple M, Itkin M. MR lymphangiography in children: technique and potential applications. Radiogr Rev Publ Radiol Soc N Am Inc. (2017) 37:1775–90. doi: 10.1148/rg.2017170014
16. Savla J, Itkin M, Rossano J, Dori Y. Post-operative chylothorax in patients with congenital heart disease. Jacc. (2017) 69:2410–22. doi: 10.1016/j.jacc.2017.03.021
17. Verma B, Kumar A, Verma N, Agrawal A, Yesilyaprak A, Furqan M, et al. Clinical characteristics, evaluation and outcomes of chylopericardium: a systematic review. Heart. (2023) 109:1281–5. doi: 10.1136/heartjnl-2022-321798
19. Agrawal A, Verma B, Brooksbank J, Khayata M, Klein A. Symptomatic recurrent chylopericardium: how to manage this rare entity? JACC Case Rep. (2021) 3:1318–21. doi: 10.1016/j.jaccas.2021.06.011
20. Srinivasan A, Smith C, Krishnamurthy G, Escobar F, Da Costa N, Ford B, et al. Selective lymphatic duct embolization for treatment of thoracic lymphatic flow disorders in children: technical aspects and comparison with thoracic duct embolization. J Vasc Interv Radiol JVIR. (2025) 36:88–98.e1. doi: 10.1016/j.jvir.2024.09.004
21. Haddad R, Dautry R, Bonnet D, Malekzadeh-Milani S. Transvenous retrograde thoracic duct embolization for effective treatment of recurrent plastic bronchitis after fontan palliation. Catheter Cardiovasc Interv. (2023) 101:863–9. doi: 10.1002/ccd.30611
22. Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Interv Radiol. (2011) 28:63–74. doi: 10.1055/s-0031-1273941
23. McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. (2010) 104:1–8. doi: 10.1016/j.rmed.2009.08.010
Keywords: pulmonary lymphatic perfusion syndrome (PLPS), chylous pericardial effusion, chyloptysis/milky sputum, thoracic duct embolization, lymphatic intervention
Citation: Liu Q, Wang J, Chen Y, Qin W and Zhu Z (2025) Successful treatment of pulmonary lymphatic perfusion syndrome with lymphatic intervention embolization: a case report. Front. Med. 12:1696594. doi: 10.3389/fmed.2025.1696594
Received: 10 October 2025; Revised: 07 November 2025; Accepted: 12 November 2025;
Published: 02 December 2025.
Edited by:
Raymond N. Haddad, INSERM Biologie cellulaire, développement et évolution, FranceReviewed by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeVadlamudi Nagendra, National Institute of Medical Sciences and Research, India
Copyright © 2025 Liu, Wang, Chen, Qin and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziyang Zhu, NDg1OTQ0MTZAcXEuY29t
†These authors have contributed equally to this work
 Qunxiang Liu
Qunxiang Liu JiaQi Wang†
JiaQi Wang† Wei Qin
Wei Qin