- 1Department of Orthopedics, Gusu School, Nanjing Medical University, The First People's Hospital of Kunshan, Suzhou, Jiangsu, China
- 2Kunshan Biomedical Big Data Innovation Application Laboratory, Suzhou, Jiangsu, China
- 3Information Department, Affiliated Kunshan Hospital of Jiangsu University, Suzhou, Jiangsu, China
- 4Kunshan Municipal Health and Family Planning Information Center, Suzhou, Jiangsu, China
- 5Chronic Disease Department, Kunshan Center for Disease Control and Prevention, Suzhou, Jiangsu, China
Background: Osteoporotic fractures (OPF) represent a significant health concern among the elderly population. Frailty, a prevalent condition in this demographic, can be evaluated via the Frailty Index (FI). This study investigated the association between FI and all-cause mortality (ACM) in aged individuals with osteoporosis (OP).
Methods: This retrospective cohort study included 19,332 patients who underwent surgical treatment for fractures at Kunshan First People’s Hospital between January 1, 2017, and August 31, 2023. Among these, 4,782 patients aged ≥ 50 years were diagnosed with OPF. The FI was developed based on 30 health indicators, and it requires the availability of at least 75% of the variables for all patients. Moreover, ACM was monitored from the time of hospitalization until death or the end of the study period. Data on the correlation between FI and ACM were statistically evaluated, including the Cox proportional hazard regression model, interaction test, smooth curve fitting, K-M survival curve, threshold effect, subgroup, and sensitivity analyses.
Results: Among the 3,833 patients, the mean age was 68.77 years, with an average FI of 0.07. A substantial positive correlation was observed between FI and ACM (HR = 1.05, 95% CI: 1.03–1.07, p < 0.01). Importantly, a 0.033 increase in the FI score (equivalent to ~ 1 additional cumulative deficit) was related to a 17% higher risk of ACM (HR: 1.17, 95% CI: 1.10 to 1.24). Subgroup analyses further validated these findings across diverse demographic groups.
Conclusion: This study establishes a significant correlation between the FI and ACM in elderly patients with OPF, underscoring the importance of frailty measurement in clinical management. These findings support the need for targeted interventions to improve outcomes in this high-risk population and emphasize the necessity of further research to develop effective screening and management strategies.
Introduction
As the global population ages, the health challenges among elderly people have become a growing focus (1). Osteoporosis (OP) is a significant public health concern, affecting approximately 39.4% (2) of the elderly population in China. Globally, OPF occurs at an alarming rate of one every 3 s, amounting to approximately 8.9 million cases annually (3). These fractures remarkably impair patients’ quality of life (QoL) and are closely related to elevated mortality rates (4, 5). Studies highlight the high prevalence of OP among older people, particularly in women, with fracture incidence increasing substantially with advancing age (6, 7). The implications of OPF extend beyond reduced physical function to include prolonged hospitalization and long-term care requirements (3, 8). Among older people, OPF is frequently associated with factors such as decreased bone density and a heightened risk of falls (9, 10). Thus, early screening and timely treatment for OP are essential to reduce fracture risk and improve the QoL in this population (11, 12). As the current understanding of OP evolves, future research will focus on developing comprehensive intervention strategies to enhance skeletal health in elderly individuals.
The FI, a comprehensive tool for assessing frailty, incorporates multiple health indicators to evaluate an individual’s physiological reserves and resilience effectively (13–15). Studies have demonstrated a strong correlation between FI and health outcomes in the elderly, particularly in OPF patients (16–20). The presence of frail status may exacerbate post-fracture recovery challenges, increase the risk of complications, and affect overall mortality rates. Furthermore, frailty is not only related to fracture incidence but also closely associated with adverse outcomes such as postoperative recovery, duration of hospitalization, and readmission rates (21–25). Therefore, assessing frailty status in elderly patients is crucial for devising effective clinical interventions to improve their prognosis and QoL (26–30).
Studies have shown a significant correlation between FI and overall mortality rates (31, 32) for the elderly population. However, whether FI is uniformly associated with ACM in OF patients remains unclear. Investigating the role of FI in OPFs is crucial for improving clinical management and prognostic outcomes in elderly patients. This study not only advances the current understanding of the risk factors contributing to OP-related mortality but also serves as a valuable reference for guiding clinical practice. Therefore, this study aimed to investigate the association between the frailty index and all-cause mortality among elderly patients hospitalized with osteoporotic fractures.
Materials and methods
Ethical concern
This study was approved by the Ethics Committee of the Affiliated Kunshan Hospital of Jiangsu University (approval No. 2024-03-053-H00-K01), and conducted per the guidelines outlined in the Declaration of Helsinki. To maintain objectivity and unbiased investigation, all participants provided informed consent (in writing), and their identities were anonymized throughout the investigation.
Study participants
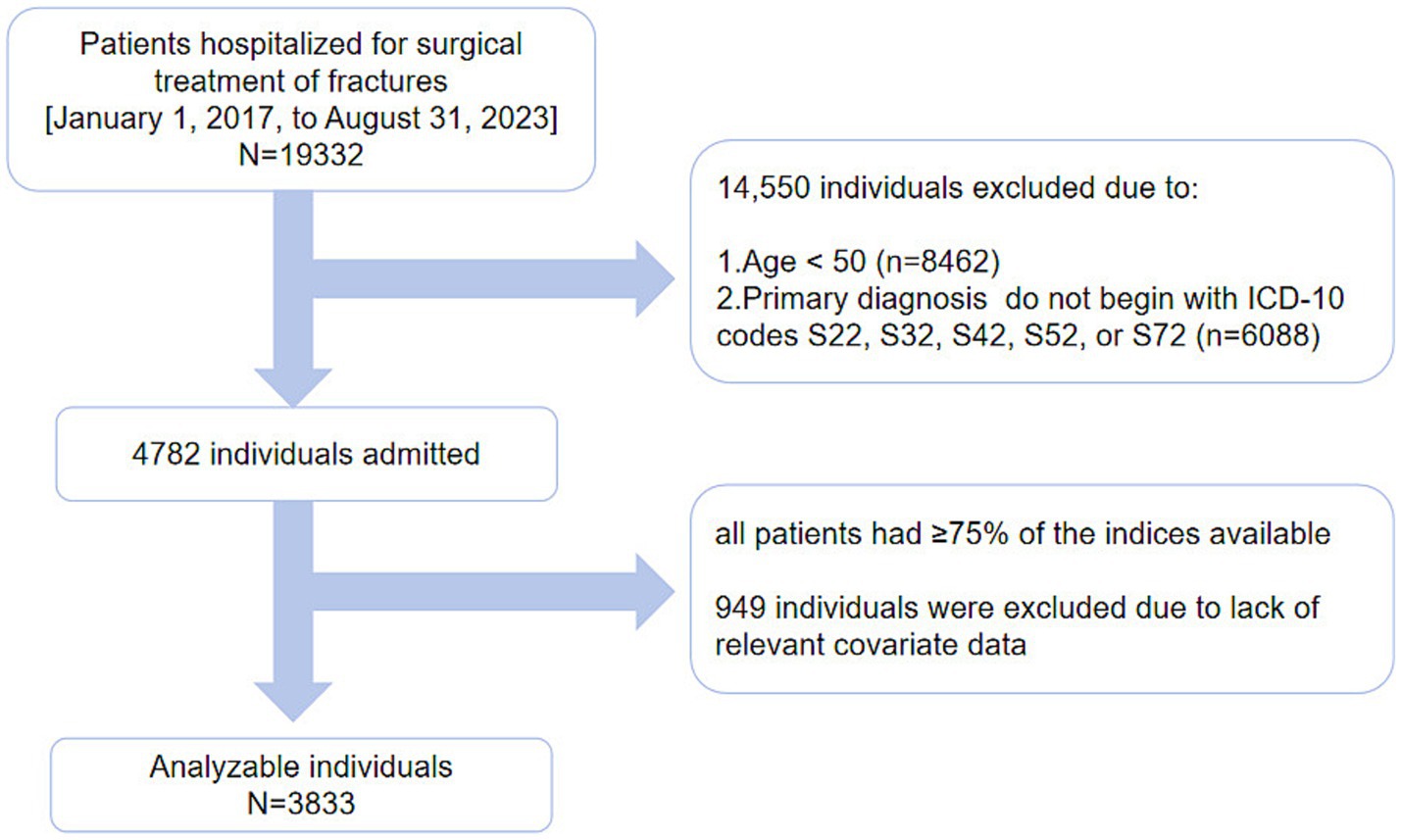
We conducted a cohort study spanning from January 1, 2017, to August 31, 2023. It initially enrolled 19,332 patients who were treated for fractures at the First People’s Hospital of Kunshan. From this group, 8,462 patients under the age of 50 were excluded. A total of 4,782 patients with OPFs who underwent inpatient surgical treatment were included in the study. Patients with diagnoses corresponding to ICD-10 codes S22, S32, S42, S52, or S72, based on the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, were selected. Blood tests were performed for all patients during their hospitalization. Blood samples (in fasting condition) were collected, and all clinical parameters were assessed within 3 days of admission. OP was diagnosed based on fractures and skeletal instability in the absence of bone metabolic disorders or a standard bone mineral density (BMD) T-score of −2.5 or lower, even in the absence of fractures (33). Data for ≥ 75% of the 30 indicators required for FI assessment, equivalent to at least 22 indicators (32), were available for all included patients. A total of 949 patients were excluded due to missing data on other covariates (As shown in Figure 1).
Exposure and outcome variables
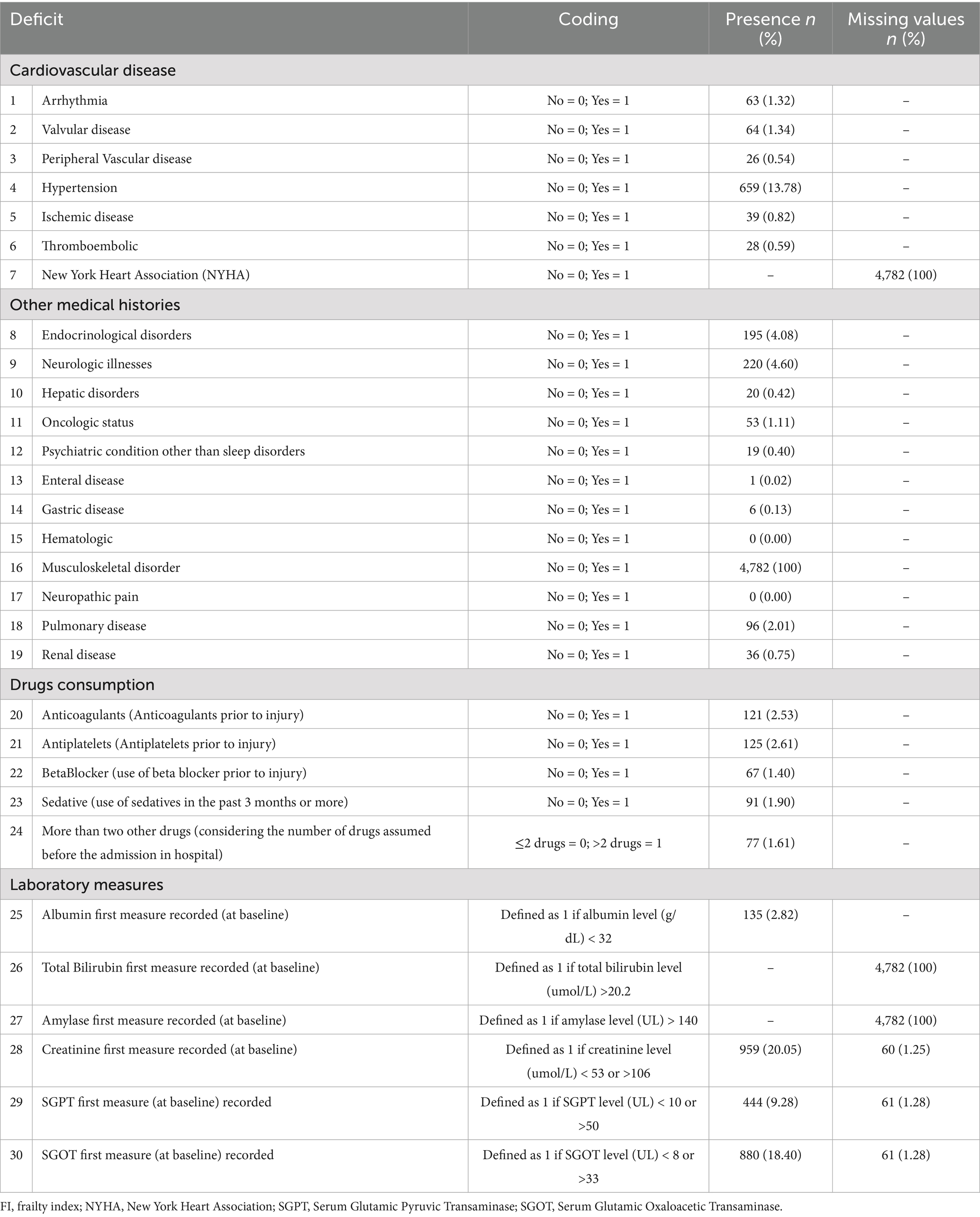
The construction of the FI was based on the methodology established by Galimberti et al. (32). It incorporated 30 variables that reflected the extent of pre-fracture comorbidities, pre-fracture medication use, and post-admission laboratory results. The final FI required the availability of at least 75% of these variables for each individual to maintain its comprehensive assessment. For each item, a score of “0” was assigned if deficits were absent, and a score of “1” indicated the presence of deficits. Abnormal laboratory values were determined based on reported laboratory reference ranges. The cumulative deficit count divided by the non-missing item numbers yielded a standardized score (ranging from 0 to 1, where 0 signifies no frailty and 1 represents complete frailty) (32).
Besides, ACM was defined as the time interval between the admission date for fracture-related hospitalization and the occurrence of death, transfer out of the area, or the study’s end date (April 24, 2024). We could not collect any relevant information about the cases transferred out of this region. All patients who were still alive before the end date of the study were included in the study. Mortality-related data for this study were obtained from the Jiangsu Provincial Death Registration System (PDRS).
Covariate variables
Covariate variables, including age, body mass index (BMI), gender, smoking status, alcohol consumption, American Society of Anesthesiologists (ASA) score, and fracture type, were assessed. BMI was calculated by dividing weight (kg) by the square of height (m). Smoking was classified as current or former smoking within the preceding 12 months. Alcohol consumption was defined as drinking at least once per week over the past 12 months. The ASA score was employed to evaluate overall physical status. Fractures were categorized into the following types: thoracic vertebrae, lumbar vertebrae, wrist, proximal humerus, and femur (34). Some of these patients may have multiple fractures.
Statistical analyses
Continuous data regarding patient demographics and clinical characteristics are depicted as mean ± standard deviation (SD) or median [1st quartile (Q1) to 3rd quartile (Q3)]. Normally distributed data were compared via independent two-tailed t-tests, while non-normally distributed data were analyzed using the Mann–Whitney U test. Categorical data are expressed as frequency (%), with variances evaluated via chi-squared tests. When the assumptions for the chi-squared test were not satisfied, the Fisher exact test was applied as an alternative. Cox proportional hazard regression models were used to assess the association between FI and ACM. The initial multivariate analysis comprised patient age, gender, BMI, alcohol consumption, smoking status, ASA score, and fracture type. A smooth curve fit was applied to generate a plot illustrating the correlation between FI and ACM. Moreover, Kaplan–Meier (K-M) survival curves were constructed to represent patient outcomes. Furthermore, subgroup analyses were conducted based on patient gender, age percentiles, BMI, smoking and drinking habits, ASA score (1, 2, ≥3), and fracture categories. The study also used likelihood ratio tests (LRT) to compare subgroup modifications and correlations. In the sensitivity analysis, follow-up times of 1, 2, 3, and 5 years were used, and K-M curves were generated for each time point to evaluate whether variations in follow-up duration influenced the relationship between FI and ACM in patients.
Data was statistically measured using R packages from The R Foundation1 and Empower Stats from X&Y Solutions.2 The significance threshold level was set at p < 0.05 for all two-tailed tests.
Results
Participant features
The current comprehensive data comprising ≥ 75% of the parameters (with at least 22 indicators/individual) were obtained for all patients. After excluding subjects with missing data (n = 949), approximately 3,833 patients were included in the final analysis (Figure 1).
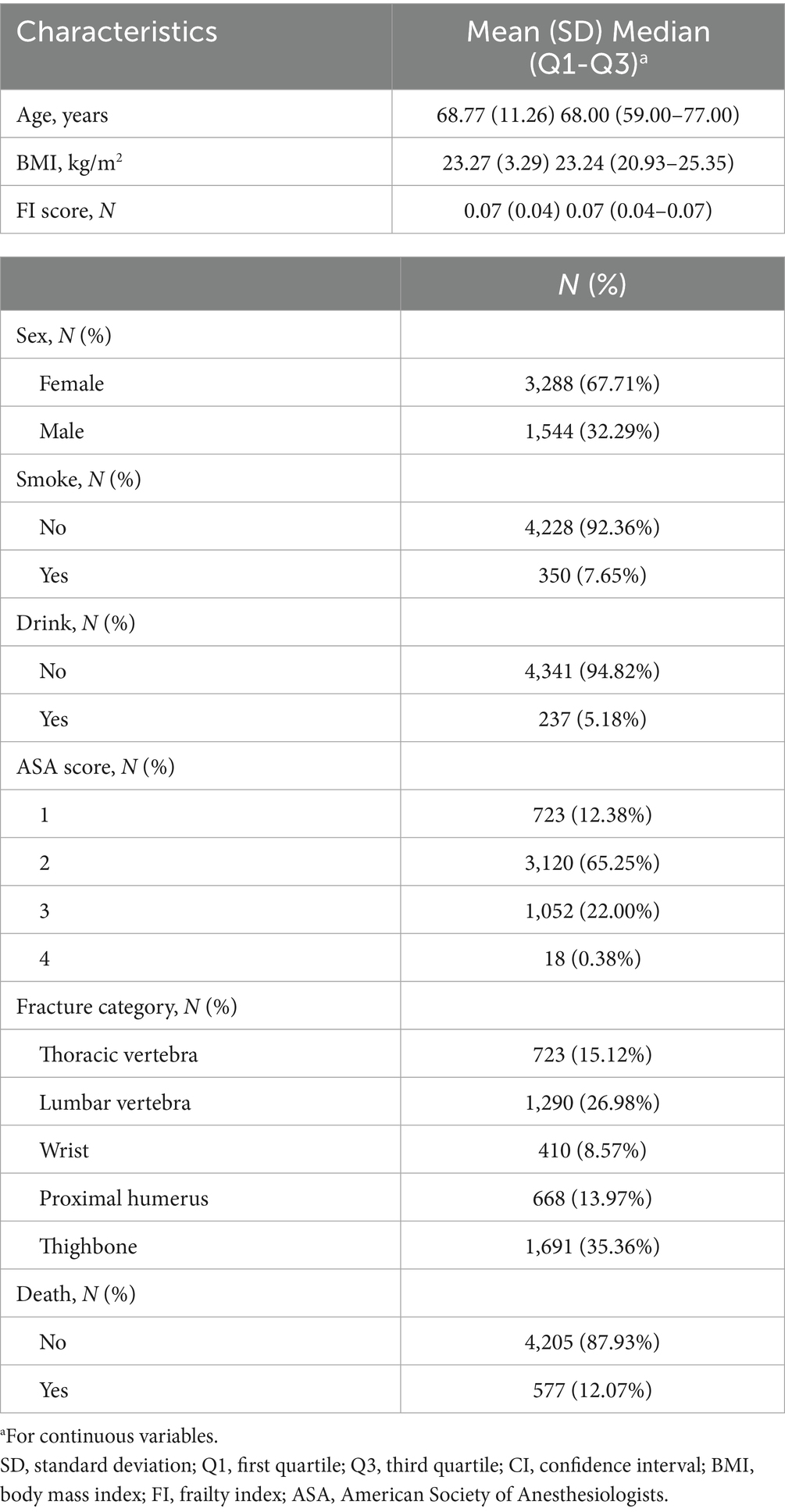
Table 1 summarizes the process of constructing the FI. Table 2 presents the overall characteristics of the study cohort. The mean age of patients was 68.77 ± 11.26 years (interquartile range: 59.00–77.00 years). The participants were mostly females (n = 3,288, 67.71%), while males accounted for 32.29% (n = 1,544) of the sample. The mean BMI was 23.27 ± 3.29 kg/m2 (interquartile range: 20.93–25.35 kg/m2).
The sample contained 350 smokers (7.65%) and 237 drinkers (5.18%). An ASA anesthesia score of 2 was the most prevalent category, with 3,120 individuals (65.25%). For ASA scores of 1, 3, and 4, the distribution was as follows: 723 individuals (12.38%), 1,052 individuals (22.00%), and 18 individuals (0.38%), respectively. In the thoracic vertebrae, lumbar vertebrae, wrist, proximal humerus, and thighbone, the corresponding numbers of fractures were 723 (15.12%), 1,290 (26.98%), 410 (8.57%), 668 (13.97%), and 1,691 (35.36%), respectively.
Figure 2 illustrates the distribution of FI values, with most values clustered < 0.10. The mean FI for all participants was 0.07 ± 0.04. The FI levels of male and female patients were similar, indicating a weak correlation between FI and age and an approximated overall mean level. A total of 577 patients (12.07%) had passed away by the end of the follow-up period.
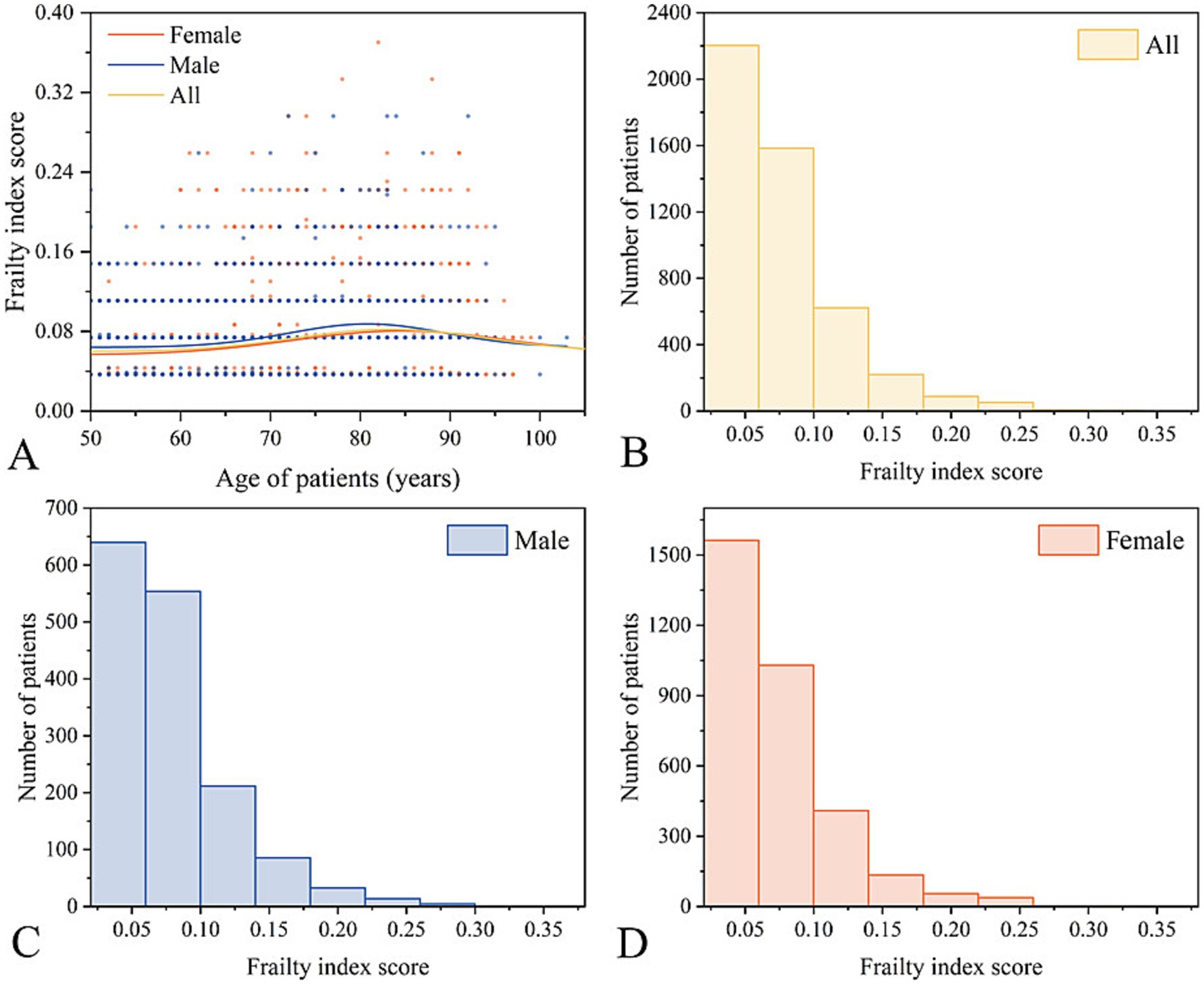
Figure 2. Overview of the frailty index. (A,B) General distribution of FI scores. Distribution of FI scores for males (C) and females (D).
Correlation between FI and ACM
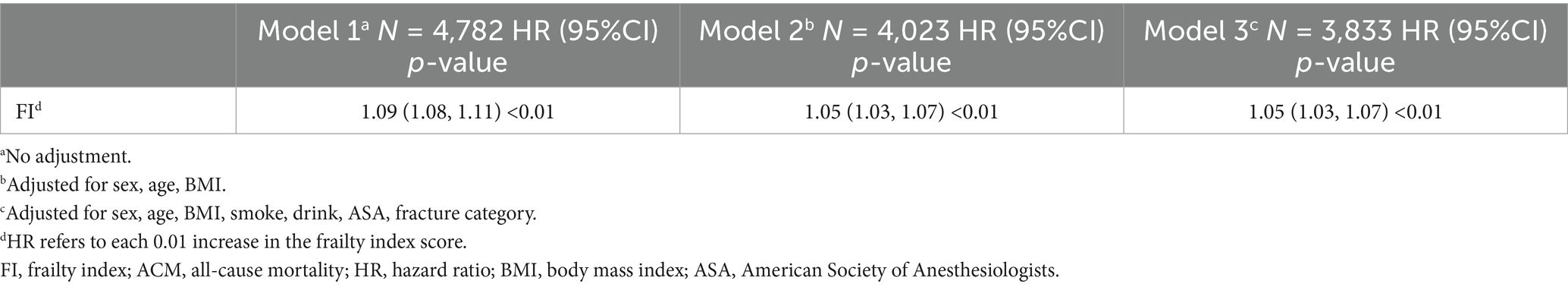
A total of 3 models were employed to examine the relationship between FI and ACM in OPFs (Table 3). In the unadjusted Model 1, a remarkable correlation between FI and ACM was observed (HR = 1.09, 95% CI: 1.08–1.11, p < 0.01). Model 2, which adjusted for age, gender, and BMI, demonstrated consistent findings, with FI remaining considerably correlated with ACM (HR = 1.05, 95% CI: 1.03–1.07, p < 0.01). In Model 3, after adjustments for smoking, alcohol consumption, ASA score, and fracture type, a persistent, substantial positive association between FI and ACM was found (HR = 1.05, 95% CI: 1.03–1.07, p < 0.01). (HR corresponds to the effect of each 0.01 increase in the FI score).
Overall, each 0.033-point increase in the FI score (approximately equivalent to one additional deficit) was related to a 17% higher risk of ACM (HR: 1.17, 95% CI: 1.10–1.24). This indicates that higher FI scores are associated with poorer prognoses for OPFs.
Spline smoothing plot and threshold analysis
The correlation between FI and ACM was assessed via graphical methods to examine its linearity (Figure 3). Generalized Additive Model (GAM) estimates demonstrated a distinct linear association between FI and ACM in the OPF population after adjusting for covariates. No inflection point was observed in the threshold effect analysis.
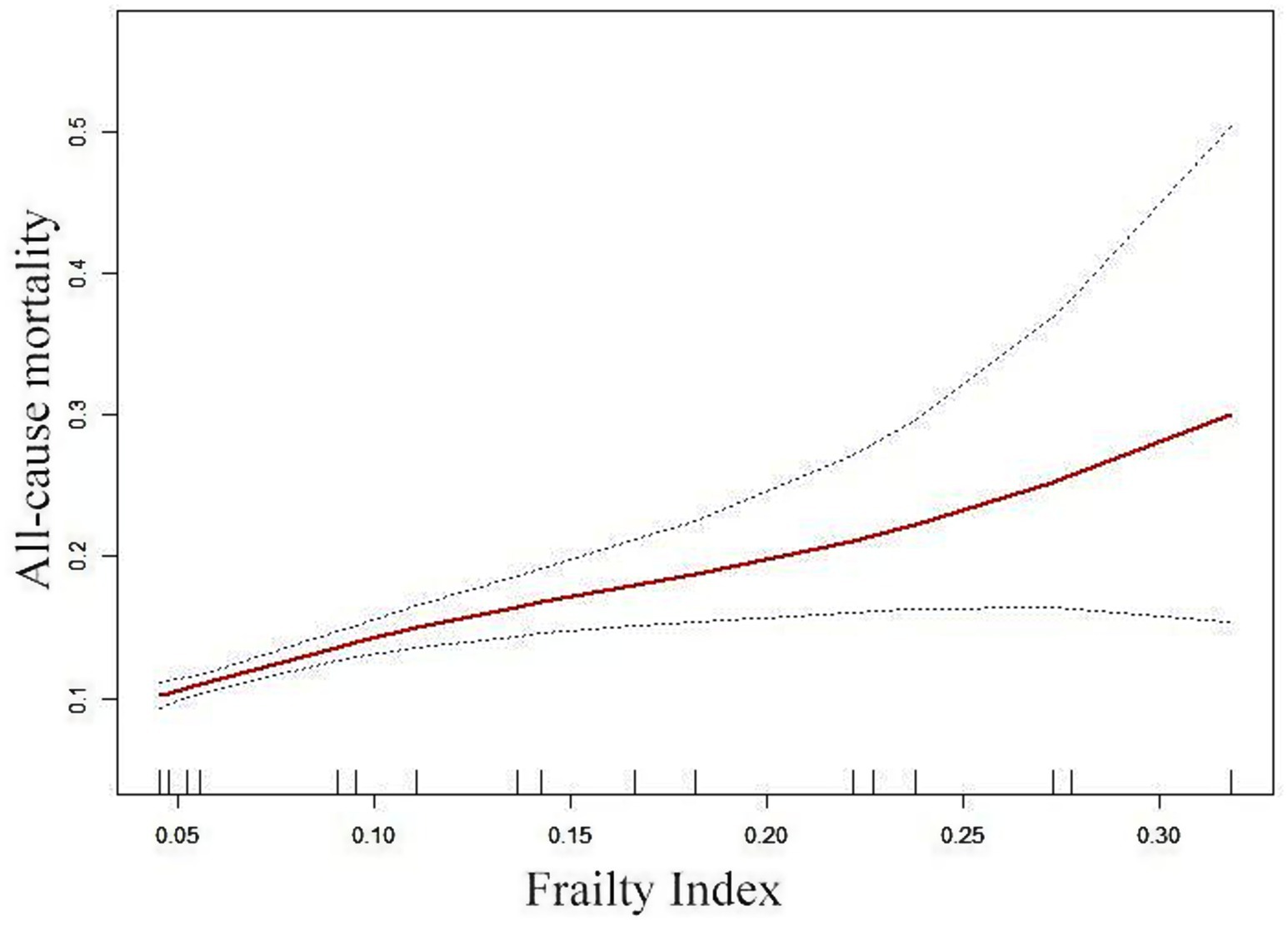
Figure 3. Adjusted smoothed curve analysis illustrates the FI and ACM correlation. The solid red line represents the fitted smooth curve, while the blue bands denote the 95% CI of the fit. The analysis was adjusted for gender, age, BMI, smoking status, alcohol consumption, ASA score, and fracture types.
K-M survival curve
The K-M survival curve (Figure 4) demonstrates that patients in the high FI group had a higher ACM rate and lower survival probability than those throughout the observation period. Further, the survival difference between both groups progressively widened over time, suggesting that FI may substantially influence patient survival.
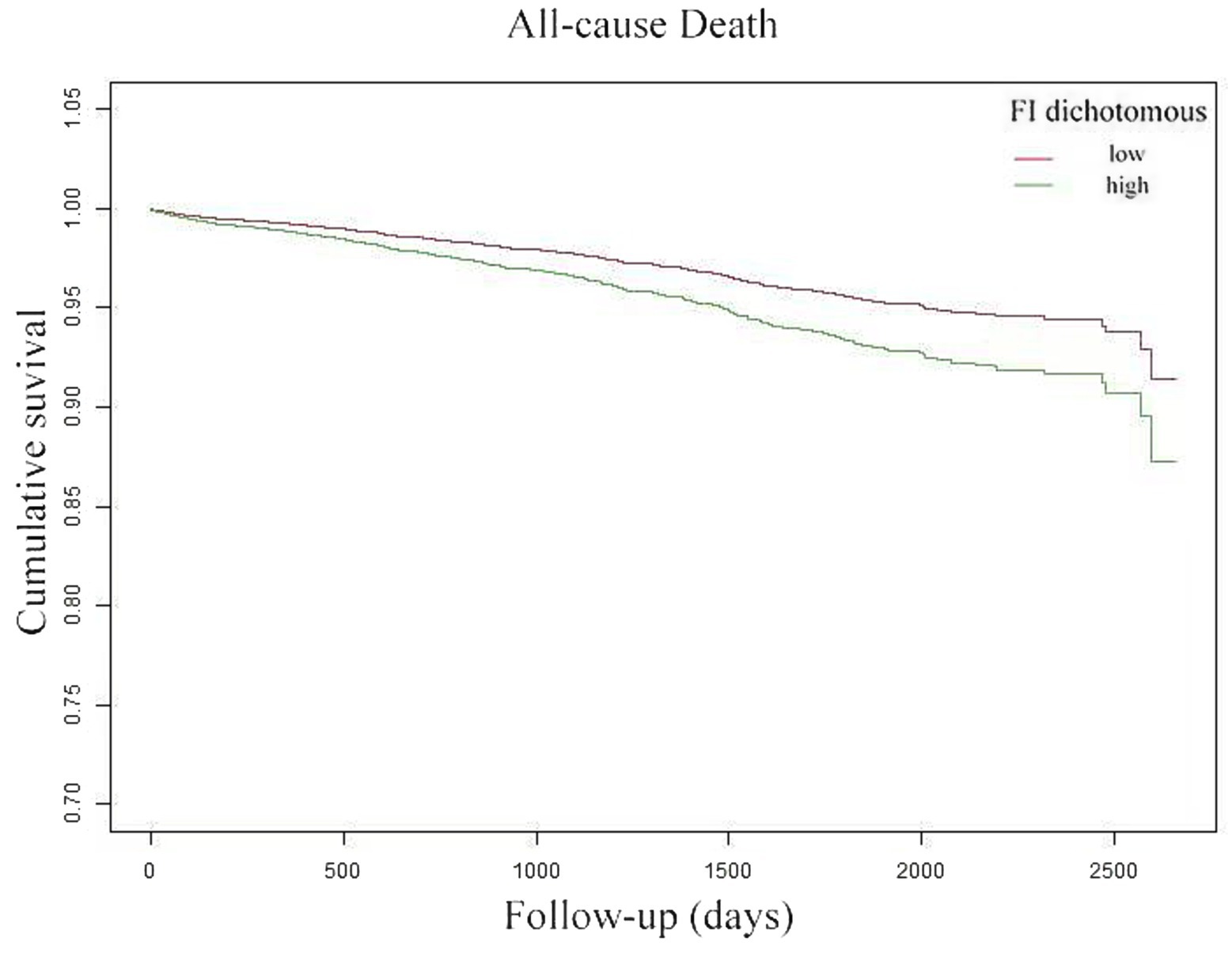
Figure 4. Kaplan–Meier curves reflected the cumulative survival of patients with high FI (green line) and patients with low FI (red line).
Subgroup analysis
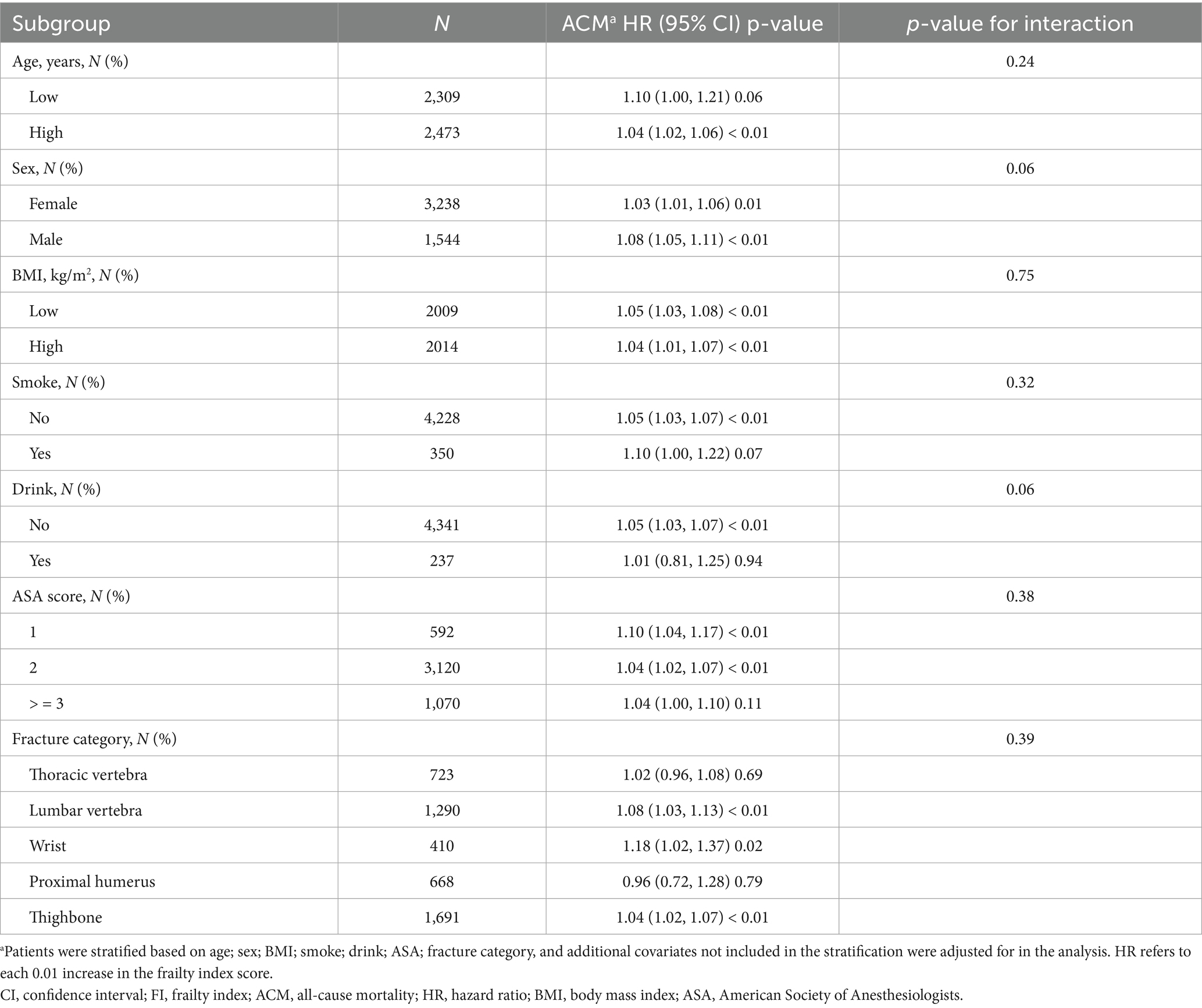
Subgroup analyses were conducted based on patients’ age, gender, BMI, smoking status, alcohol consumption, ASA score, and fracture types to identify possible confounders in a fully adjusted multivariable Cox regression model. All variables were adjusted in addition to the subgroup variables. Table 4 illustrates that there were no significant stratified interactions (all interaction p-values > 0.05), which suggests that the patterns were highly consistent across subgroups.
Sensitivity analysis
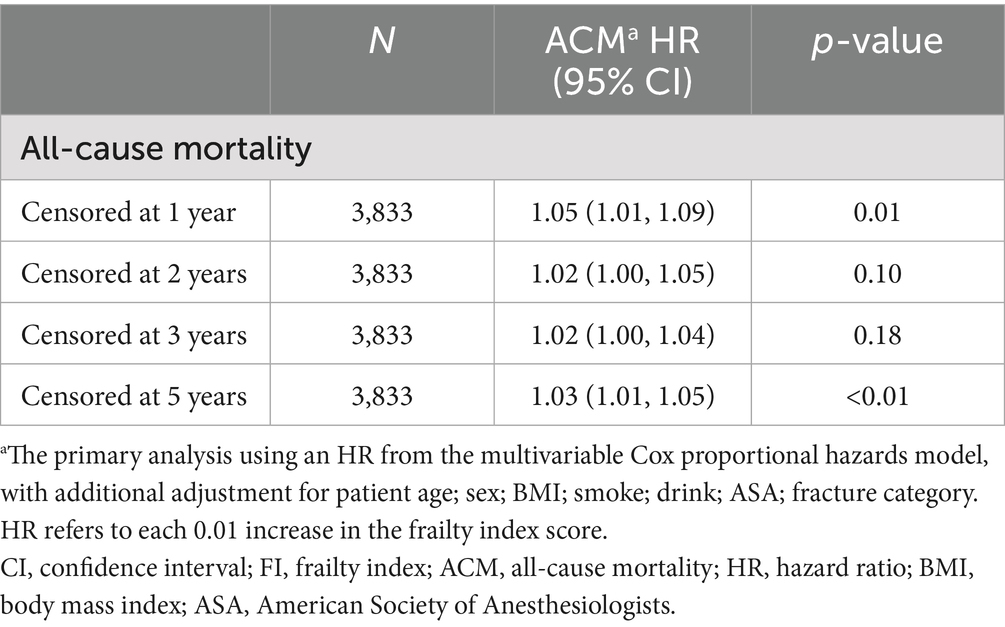
Comprehensive sensitivity analyses were conducted by examining data with varying truncation times. Follow-up data at one, two, three, and 5 years were extracted for regression and survival analyses. Detailed results, presented in Figure 5 and Table 5, confirm the consistency of these findings with the primary study outcomes.
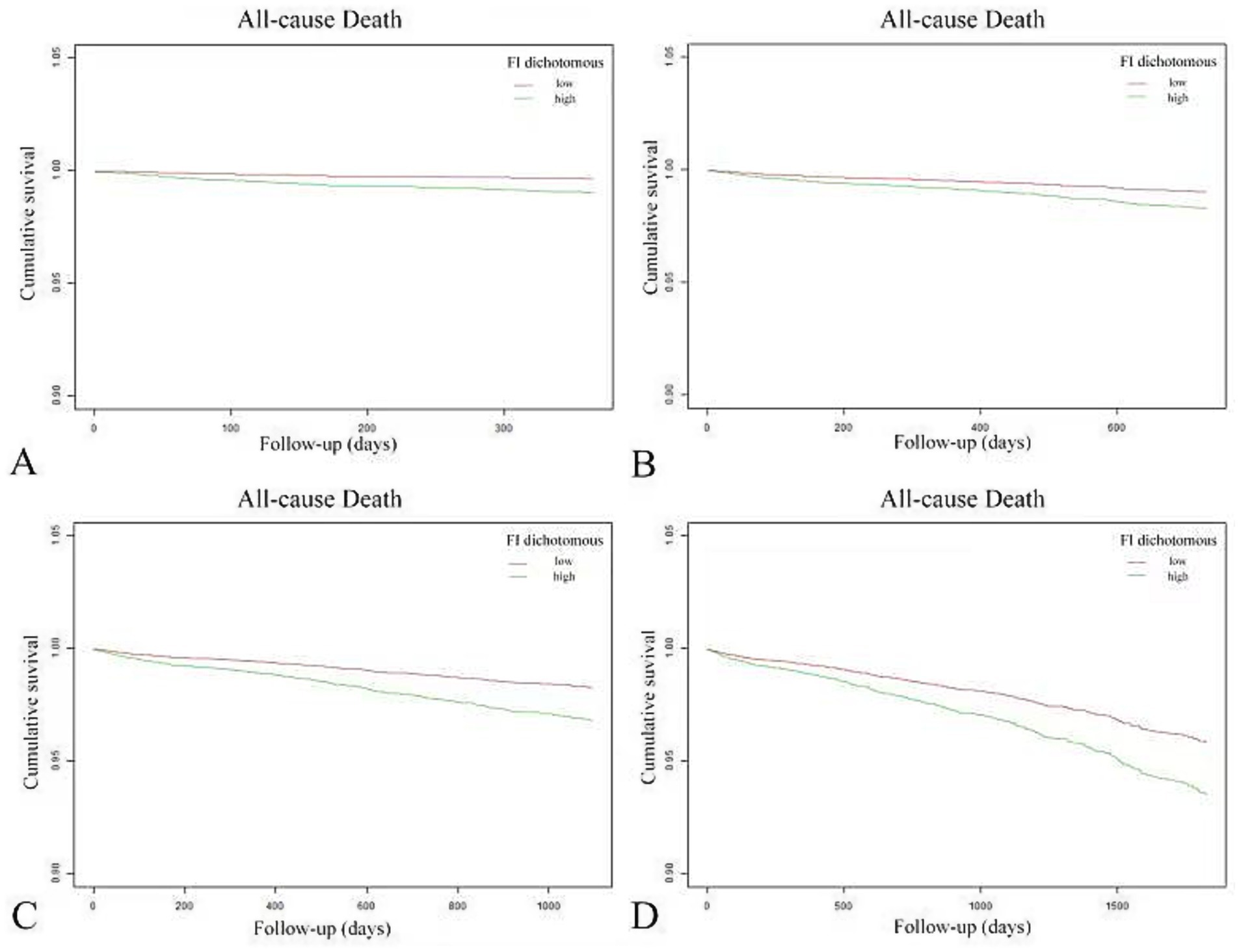
Figure 5. Variable sensitivity analysis of Kaplan–Meier curves for cumulative survival in patients with high FI (green line) and low FI (red line). Panels (A–D) correspond to follow-up periods of 1, 2, 3, and 5 years, respectively.
Discussion
This study examined the correlation between the FI and ACM in OPFs. The current findings demonstrated a substantial positive correlation between FI and ACM (HR = 1.05, 95% CI: 1.03–1.07, p < 0.01), indicating a 5% increase in mortality risk for every 0.01 rise in FI score. Specifically, each 0.033-point increase in FI score, which approximates 1 additional deficit, was related to a 17% elevation in the risk of ACM (HR = 1.17, 95% CI: 1.10–1.24). These results suggest that higher levels of frailty are related to poorer prognoses in patients with OPFs.
Recent studies have shown a remarkable correlation between frailty and ACM in older patients. For instance, frailty evaluated via the Study of OF (SOF) index was substantially associated with postoperative mortality in older gastric cancer patients undergoing gastrectomy, with frail patients demonstrating over threefold increased risk of death (HR > 3) (35). However, cognitive frailty has been identified as an important predictor of ACM and dementia risk in older adults (HR = 1.93 and HR = 3.66, respectively) (36). Galimberti et al. (32) conducted a multicenter cohort study aimed at developing and validating an FI to predict outcomes in traumatic brain injury patients 6 months post-injury. The study revealed that a higher CENTER-TBI frailty index was considerably correlated with an increased risk of negative outcomes, with the association being more pronounced in patients who were managed in non-intensive care units (32). Collectively, these studies underscore that frailty is not only an independent risk factor faced by older individuals but also substantially impacts clinical outcomes, highlighting the importance of addressing frailty in the management of elderly OPFs.
This study aligns with recent literature, reinforcing the substantial correlation between FI and ACM in older individuals, thereby underscoring the importance of frailty assessment in clinical settings. The present study examines frailty in elderly OPF patients using a comprehensive FI based on 30 health indicators. However, other studies focus on different patient populations, such as those with gastric cancer or traumatic brain injury, and employ different frailty assessment tools, such as the SOF index. Despite variations in study populations and assessment methods, a consistent trend emerges: greater frailty severity is related to an increased mortality risk across diverse patient groups. This reinforces the concept of frailty as a universal independent risk factor that substantially impacts the clinical outcomes of older adults. Therefore, it is crucial to implement targeted screening and intervention strategies to address frailty, thereby enhancing the quality of care and outcomes for vulnerable elderly individuals.
The potential mechanisms underlying the positive association between FI in OPFs and ACM are as follows: First, frailty may decrease the body’s physiological reserve, impairing the patient’s ability to recover from surgical trauma. Second, frailty is related to multi-organ dysfunction, which heightens the risk of complications such as pneumonia, deep vein thrombosis, and cardiovascular events. Finally, frailty is often associated with malnutrition and reduced physical activity, which further exacerbates the progression of OPFs and overall health deterioration, thereby impacting survival rates.
The current findings on the FI offer a valuable tool for identifying frail populations in advance, allowing for developing targeted management strategies for elderly patients with frailty to improve their QoL. These results also underscore the need for further research into accelerated aging in younger populations and the development of relevant screening and intervention programs.
This study has several strengths. It specifically focuses on middle-aged and elderly OPF patients who require inpatient orthopedic surgical treatment. By investigating the feasibility of applying the FI within the field of orthopedics, the study aims to effectively identify high-risk populations, thus providing a scientific foundation for clinical decision-making. Further, key strengths of this study include the use of representative individuals and statistical models that account for multiple substantial confounding factors. However, there are some limitations. For example, this study only focused on middle-aged and elderly patients with osteoporosis who require inpatient surgical treatment, which limits its applicability to young people or OF patients who choose conservative treatment, resulting in a certain degree of selection bias. It is impossible to determine whether the diagnosis of osteoporosis in the patient was made before or after admission. There are still many important factors affecting mortality that were not collected in this study, such as the method of surgical anesthesia, whether the patient’s cause of death was fracture or related complications. These need to be further refined in future research.
Conclusion
This study revealed that FI and ACM are positively correlated in OPF patients. The results highlight the essential role of frailty assessment in clinical settings, considering that higher FI scores are associated with a higher mortality risk. These findings were consistent with existing literature, affirming frailty as a strong predictor of adverse outcomes in older adults. Future research should aim to elucidate the mechanisms relating frailty to mortality and to develop targeted interventions to improve outcomes for frail elderly patients with OPFs. Overall, the FI represents a valuable tool for identifying high-risk elderly individuals with OPFs, informing personalized treatments designed to enhance survival and QoL.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The IRB of Affiliated Kunshan Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PZ: Data curation, Writing – review & editing, Software, Writing – original draft. KL: Supervision, Writing – review & editing, Writing – original draft. Y-qG: Writing – review & editing, Writing – original draft, Software. JJ: Writing – review & editing, Writing – original draft, Supervision. W-bH: Writing – original draft, Writing – review & editing, Supervision. CL: Writing – original draft, Supervision, Writing – review & editing. YY: Writing – original draft, Writing – review & editing, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Suzhou City Major Disease Multicenter Clinical Research Project (CN) (DZXYJ202312), Special Funding for Jiangsu Province Science and Technology Plan (Key Research and Development Program for Social Development) (CN) (BE2023738), Medical Education Collaborative Innovation Fund of Jiangsu University (JDY2022013), Key Laboratory Project in Suzhou City (SZS2024018) and Gusu Health Talent Plan Scientific Research Project (CN) (GSWS2022105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1697650/full#supplementary-material
Footnotes
References
1. Luo, L. Analysis of coupling coordination degree between big health industry and pension service. J Healthc Eng. (2022) 2022:1–6. doi: 10.1155/2022/6427024
2. Salari, N, Darvishi, N, Bartina, Y, Larti, M, Kiaei, A, Hemmati, M, et al. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg. (2021) 16:669. doi: 10.1186/s13018-021-02821-8
3. Lin, S, Wu, J, Chen, B, Li, S, and Huang, H. Identification of a potential MiRNA–mRNA regulatory network for osteoporosis by using bioinformatics methods: a retrospective study based on the gene expression omnibus database. Front Endocrinol. (2022) 13:844218. doi: 10.3389/fendo.2022.844218
4. Wu, B, Zhu, X-F, Yang, X-Q, Wang, W-Y, and Lu, J-H. Effects of osthole on osteoporotic rats: a systematic review and meta-analysis. Pharm Biol. (2022) 60:1625–34. doi: 10.1080/13880209.2022.2110267
5. Oo, WM, Naganathan, V, Bo, MT, and Hunter, DJ. Clinical utilities of quantitative ultrasound in osteoporosis associated with inflammatory rheumatic diseases. Quant Imaging Med Surg. (2018) 8:100–13. doi: 10.21037/qims.2018.02.02
6. Jedynasty, K, Zięba, M, Adamski, J, Czech, M, Głuszko, P, Gozdowski, D, et al. Seasonally dependent change of the number of fractures after 50 years of age in Poland—analysis of combined health care and climate datasets. Int J Environ Res Public Health. (2022) 19:9467. doi: 10.3390/ijerph19159467
7. Shao, B-Y, Wang, L, Yu, Y, Chen, L, Gan, N, and Huang, W-M. Effects of CD4+ T lymphocytes from ovariectomized mice on bone marrow mesenchymal stem cell proliferation and osteogenic differentiation. Exp Ther Med. (2020) 20:84. doi: 10.3892/etm.2020.9212
8. Shim, J, Kim, K-T, Kim, KG, Choi, U-Y, Kyung, JW, Sohn, S, et al. Safety and efficacy of Wharton’s jelly-derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: a phase I/IIa study. Stem Cells Transl Med. (2020) 10:554. doi: 10.1002/sctm.20-0308
9. Rahme, M, Sharara, S-L, Baddoura, R, Habib, RH, Halaby, G, Arabi, A, et al. Impact of calcium and two doses of vitamin D on bone metabolism in the elderly: a randomized controlled trial. J Bone Miner Res. (2017) 32:1486–95. doi: 10.1002/jbmr.3122
10. Winzenberg, T, Powell, S, Shaw, KA, and Jones, G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. (2011) 342:c7254. doi: 10.1136/bmj.c7254
11. Gao, C, Song, H, Chen, B, Zhang, Z, and Yue, H. The assessment of the osteoporosis self-assessment tool for Asians and calcaneal quantitative ultrasound in identifying osteoporotic fractures and falls among Chinese people. Front Endocrinol. (2021) 12:684334. doi: 10.3389/fendo.2021.684334
12. Kim, J-W, Ha, Y-C, and Lee, Y-K. Factors affecting bone mineral density measurement after fracture in South Korea. J Bone Metab. (2017) 24:217–22. doi: 10.11005/jbm.2017.24.4.217
13. Uchai, S, Andersen, LF, Hopstock, LA, and Hjartåker, A. Body mass index, waist circumference and pre-frailty/frailty: the Tromsø study 1994−2016. BMJ Open. (2023) 13:e065707. doi: 10.1136/bmjopen-2022-065707
14. Bernabeu-Mora, R, Oliveira-Sousa, SL, Sánchez-Martínez, MP, García-Vidal, JA, Gacto-Sánchez, M, and Medina-Mirapeix, F. Frailty transitions and associated clinical outcomes in patients with stable COPD: a longitudinal study. PLoS One. (2020) 15:e0230116. doi: 10.1371/journal.pone.0230116
15. Huang, W-C, Huang, Y-C, Lee, M-S, Doong, J-Y, Pan, W-H, and Chang, H-Y. The combined effects of dietary diversity and frailty on mortality in older Taiwanese people. Nutrients. (2022) 14:3825. doi: 10.3390/nu14183825
16. Dominguez, LJ, Donat-Vargas, C, Sayon-Orea, C, Barberia-Latasa, M, Veronese, N, Rey-Garcia, J, et al. Rationale of the association between Mediterranean diet and the risk of frailty in older adults and systematic review and meta-analysis. Exp Gerontol. (2023) 177:112180. doi: 10.1016/j.exger.2023.112180
17. Lunt, EK, Gordon, AL, Greenhaff, PL, and Gladman, JFR. The influence of immobility on muscle loss in older people with frailty and fragility fractures. Geroscience. (2024) 46:5473–84. doi: 10.1007/s11357-024-01177-1
18. Sagona, A, Ortega, CA, Wang, L, Brameier, DT, Selzer, F, Zhou, L, et al. Frailty is more predictive of mortality than age in patients with hip fractures. J Orthop Trauma. (2024) 38:e278–87. doi: 10.1097/BOT.0000000000002844
19. Olmos Martínez, JM, Hernández Martínez, P, and González Macías, J. Frailty, sarcopenia and osteoporosis. Med Clin (Barc). (2024) 163:e17–23. doi: 10.1016/j.medcli.2024.03.004
20. Maharani, A, Sinclair, DR, Chandola, T, Bower, P, Clegg, A, Hanratty, B, et al. Household wealth, neighbourhood deprivation and frailty amongst middle-aged and older adults in England: a longitudinal analysis over 15 years (2002-2017). Age Ageing. (2023) 52:afad034. doi: 10.1093/ageing/afad034
21. Tarazona-Santabalbina, FJ, Naval, E, De la Cámara-de las Heras, JM, Cunha-Pérez, C, and Viña, J. Is frailty diagnosis important in patients with COPD? A narrative review of the literature. Int J Environ Res Public Health. (2023) 20:1678. doi: 10.3390/ijerph20031678
22. Sun, Q-Q, Tan, K, Tang, H-Y, Liu, Y-Y, Zhu, H, Qin, H, et al. Incidence and predictive value of social frailty among community-dwelling older adults in Southwest China: a prospective cohort study. Front Public Health. (2023) 11:1103651. doi: 10.3389/fpubh.2023.1103651
23. Díez-Villanueva, P, Jiménez-Méndez, C, Bonanad, C, Ortiz-Cortés, C, Barge-Caballero, E, Goirigolzarri, J, et al. Sex differences in the impact of frailty in elderly outpatients with heart failure. Front Cardiovasc Med. (2022) 9:1000700. doi: 10.3389/fcvm.2022.1000700
24. Kozicka, I, Guligowska, A, Chrobak-Bień, J, Czyżewska, K, Doroba, N, Ignaczak, A, et al. Factors determining the occurrence of frailty syndrome in hospitalized older patients. Int J Environ Res Public Health. (2022) 19:12769. doi: 10.3390/ijerph191912769
25. de Sire, A, Ferrillo, M, Lippi, L, Agostini, F, de Sire, R, Ferrara, PE, et al. Sarcopenic dysphagia, malnutrition, and Oral frailty in elderly: a comprehensive review. Nutrients. (2022) 14:982. doi: 10.3390/nu14050982
26. Chehrehgosha, M, Vatan, RF, Alizadeh Khoei, M, Sharifi, F, Aminalroaya, R, Vahabi, Z, et al. Role of frailty in prediction of hospitalized older adult patient’s outcomes: a prospective study. Turk J Med Sci. (2021) 51:2324–33. doi: 10.3906/sag-2012-332
27. Dibello, V, Lozupone, M, Manfredini, D, Dibello, A, Zupo, R, Sardone, R, et al. Oral frailty and neurodegeneration in Alzheimer’s disease. Neural Regen Res. (2021) 16:2149–53. doi: 10.4103/1673-5374.310672
28. Gandossi, CM, Zambon, A, Oliveri, G, Codognola, M, Szabo, H, Cazzulani, I, et al. Frailty, post-operative delirium and functional status at discharge in patients with hip fracture. Int J Geriatr Psychiatry. (2021) 36:1524–30. doi: 10.1002/gps.5561
29. Hembree, T, Theou, O, Thirlwell, S, Reich, RR, Cao, B, Sehovic, M, et al. A simple test-based frailty index to predict survival among cancer patients with an unplanned hospitalization: an observational cohort study. Cancer Med. (2021) 10:5765–74. doi: 10.1002/cam4.4107
30. Passias, PG, Kummer, N, Williamson, TK, Moattari, K, Lafage, V, Lafage, R, et al. Highest achievable outcomes for patients undergoing cervical deformity corrective surgery by frailty. Neurosurgery. (2022) 91:693–700. doi: 10.1227/neu.0000000000002091
31. Fan, J, Yu, C, Guo, Y, Bian, Z, Sun, Z, Yang, L, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. (2020) 5:e650. doi: 10.1016/S2468-2667(20)30113-4
32. Galimberti, S, Graziano, F, Maas, AIR, Isernia, G, Lecky, F, Jain, S, et al. Effect of frailty on 6-month outcome after traumatic brain injury: a multicentre cohort study with external validation. Lancet Neurol. (2022) 21:153–62. doi: 10.1016/S1474-4422(21)00374-4
33. Aibar-Almazán, A, Voltes-Martínez, A, Castellote-Caballero, Y, Afanador-Restrepo, DF, Carcelén-Fraile, MDC, and López-Ruiz, E. Current status of the diagnosis and management of osteoporosis. Int J Mol Sci. (2022) 23:9465. doi: 10.3390/ijms23169465
34. Lu, K, Wu, Y-M, Shi, Q, Gong, Y-Q, Zhang, T, and Li, C. A novel fracture liaison service using digital health: impact on mortality in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. (2024) 35:53–67. doi: 10.1007/s00198-023-06905-5
35. Jeong, J-R, Choi, J-W, Ryu, S-Y, and Choe, Y-R. Relationship between frailty and mortality after gastrectomy in older patients with gastric cancer. J Geriatr Oncol. (2022) 13:67–73. doi: 10.1016/j.jgo.2021.06.010
Keywords: frailty index, all-cause mortality, osteoporotic fractures, elderly patients, hospitalization
Citation: Zhou P, Lu K, Gong Y-q, Jin J, Hu W-b, Li C and Yin Y (2025) Association between frailty index and all-cause mortality in hospitalized elderly patients with osteoporotic fractures: a retrospective cohort study. Front. Med. 12:1697650. doi: 10.3389/fmed.2025.1697650
Edited by:
Guanwu Li, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Hakan Zora, Kestel State Hospital, TürkiyeYu Cheng Lo, Changhua Christian Hospital, Taiwan
Copyright © 2025 Zhou, Lu, Gong, Jin, Hu, Li and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong Li, bGljaG9uZzE3MDVAMTYzLmNvbQ==; Yi Yin, eXktMTk3MjNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
$ORCID: Peng Zhou, orcid.org/0009-0006-7726-9114
Ke Lu, orcid.org/0000-0002-0029-7874
Ya-qin Gong, orcid.org/0000-0001-8695-4048
Jian Jin, orcid.org/0009-0009-4300-884X
Wen-bin Hu, orcid.org/0000-0002-7278-5084
Chong Li, orcid.org/0000-0002-1526-221X
Yi Yin, orcid.org/0000-0002-8385-4153
 Peng Zhou
Peng Zhou Ke Lu
Ke Lu Ya-qin Gong
Ya-qin Gong Jian Jin
Jian Jin Wen-bin Hu5$
Wen-bin Hu5$ Chong Li
Chong Li Yi Yin
Yi Yin