- Department of Obstetrics and Reproductive Medicine, Chinese People’s Liberation Army Air Force Specialty Medical Center, Beijing, China
Objective: To establish a predictive model for pregnancy outcomes in patients undergoing assisted reproductive technology (ART) treatment using human chorionic gonadotropin (HCG) day endometrial thickness (EMT), follicle output rate (FORT), serum anti-Müllerian hormone (AMH), and clinical characteristics.
Methods: A retrospective study was conducted on 200 patients who underwent ART treatment at our hospital from August 2019 to August 2024. All patient data were obtained from the electronic medical record system, and patients were divided into a clinical pregnancy group and a non-pregnancy group based on whether they achieved pregnancy. Clinical-related data were collected and compared. Two machine learning methods, Lasso regression and Extreme Gradient Boosting (XGBoost), were used for “overlap coverage” screening of risk factors. The XGBoost model was implemented using the “xgboost” package in R, with the following hyperparameters: max_depth = 6, learning_rate = 0.3, n_estimators = 100, objective = “binary:logistic.” These parameters were selected based on common practices for binary classification tasks and were not extensively tuned due to the sample size limitations. Multivariate logistic analysis was performed to identify risk factors for pregnancy outcomes in ART patients. A regression model was established using R software for prediction and validation.
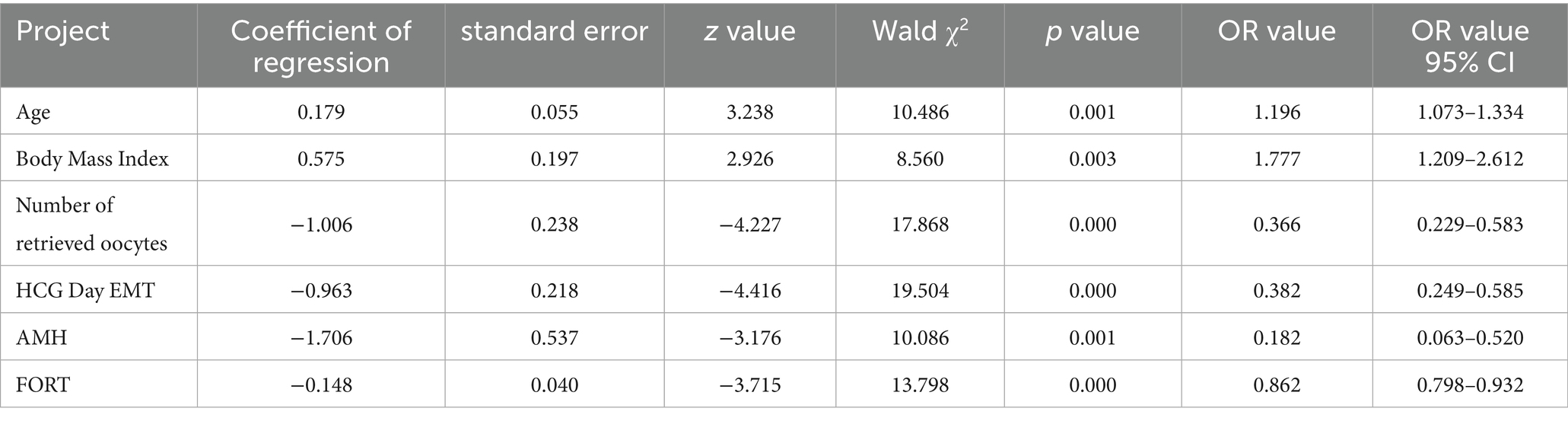
Results: A total of 200 ART patients were included in this study. According to medical record system records, 109 patients did not achieve pregnancy, accounting for 54.50%, and were included in the non-pregnancy group. 91 patients achieved clinical pregnancy, accounting for 45.50%, and were included in the clinical pregnancy group. There were significant statistical differences between the non-pregnant group and the clinically pregnant group in terms of age, body mass index, number of oocytes retrieved, HCG day EMT, AMH, and FORT (p < 0.05); Using LASSO regression and XGBoost machine learning, different risk factors were identified. Through the “overlap coverage” method, six common risk factors for pregnancy outcomes in ART patients were identified and included in the logistic regression model. The results indicated that age and body mass index were both risk factors influencing pregnancy outcomes in ART patients (OR = 1.196, 1.777, p < 0.05); the number of retrieved oocytes, HCG day EMT, AMH, and FORT were protective factors influencing pregnancy outcomes in ART patients (OR = 0.366, 0.382, 0.182, 0.862, p < 0.05); Based on the results of the logistic regression analysis, a risk prediction model for pregnancy outcomes in ART patients was constructed using a nomogram. The ROC curve showed an AUC value of 0.911, with a 95% confidence interval (CI) of 0.871 to 0.951. The model was validated using the Bootstrap method with 1,000 repeated samples, yielding a Cox-Snell R2 of 0.629, Nagelkerke R2 was 0.471, Brier Score was 0.117, model fit p-value was 0.240, and the statistic was 10.369. This indicates that the model’s predictive results align well with the actual occurrence of pregnancy outcomes in patients, with the clinical decision curve generally exceeding the two extreme curves. This suggests that the factors included in the nomogram provide a high net benefit for predicting pregnancy outcomes in ART-treated patients.
Conclusion: There are numerous factors influencing pregnancy outcomes in ART patients, primarily including age, body mass index, number of oocytes retrieved, HCG day EMT, AMH, and FORT. The risk prediction nomogram model constructed based on these factors has certain predictive value for pregnancy outcomes in ART patients. Clinicians should identify high-risk populations early and optimize treatment plans accordingly.
1 Introduction
Assisted reproductive technology (ART) has undergone more than 40 years of development, with over 2 million cycles performed globally each year. However, the clinical pregnancy rate ranges from 40 to 50%, and the live birth rate is even lower, highlighting the urgent need to optimize predictive models for pregnancy outcomes (1, 2). Bianchi C et al. (3) noted that the current bottlenecks affecting ART success rates primarily stem from two core factors: embryo quality and endometrial receptivity. The former is closely related to ovarian responsiveness and oocyte developmental potential, while the latter involves endometrial receptivity. Together, these two factors form the “dual-factor balance” model of embryo implantation. With the rapid advancement of embryo culture and genetic screening technologies, embryo selection has achieved precision, but endometrial receptivity assessment remains heavily reliant on subjective indicators such as ultrasound morphology, lacking standardized biological markers. Furthermore, Gao R et al. (4) noted that there is a dynamic interaction between ovarian reserve function and endometrial receptivity. This bidirectional regulatory mechanism limits the predictive value of single indicators, necessitating the establishment of a multidimensional predictive system that integrates endometrial status and ovarian function.
Previous studies have indicated that endometrial receptivity refers to the specialized functional state of the endometrium that allows embryo adhesion, invasion, and induces endometrial stromal decidualization. Its assessment has historically been a challenge in the field of assisted reproductive technology (ART). Human chorionic gonadotropin (HCG)-day endometrial thickness (EMT) has become the most widely used clinical evaluation indicator due to its non-invasive and quantifiable nature (5, 6). Related clinical studies have pointed out that an EMT of 7–12 mm at the time of ovulation or embryo transfer is ideal, as the endometrium is rich in glands and blood flow at this time, providing a suitable microenvironment for embryo implantation (7, 8). Grynberg M et al. (9) noted that ovarian function indicators such as serum anti-Müllerian hormone (AMH) and follicle output rate (FORT) can effectively quantify ovarian reserve, reflecting the ovarian response to exogenous stimulation and the quality of the follicular microenvironment. AMH is secreted by granulosa cells in preantral and small antral follicles, and its serum concentration directly reflects the size of the primordial follicle pool, making it the most sensitive indicator of ovarian reserve function. The core value of FORT lies in quantifying the sensitivity of the ovaries to gonadotropin stimulation. Unlike AMH, which only reflects static reserve, FORT dynamically captures the conversion efficiency of follicles from recruitment to maturation (10, 11). These indicators cover key stages from ovarian stimulation to embryo implantation in ART cycles. Their combined assessment may overcome the limitations of traditional single-indicator predictions, offering new perspectives for personalized treatment. It is worth noting that with the significant increase in the proportion of frozen–thawed embryo transfer (FET) cycles, non-physiological endometrial preparation protocols further alter the endometrial-embryo synchronization mechanism, making predictive models more complex. Existing models are often limited to a single dimension.
Recent studies have increasingly utilized machine learning (ML) for predicting ART outcomes. For instance, Liu et al. developed an XGBoost model incorporating age, BMI, and ovarian reserve markers to predict live birth, achieving an AUC of 0.89 (12). Similarly, Zhang et al. used a random forest classifier with endometrial and embryological features, reporting improved prediction accuracy over traditional logistic regression (13). Chen et al. applied a deep neural network to predict implantation success, highlighting the value of integrating multi-omics data (14). Wang et al. compared several ML algorithms and found ensemble methods like XGBoost to be superior in handling nonlinear relationships in ART data (15). Finally, Li et al. demonstrated the utility of LASSO for feature selection in a large multicenter ART cohort, reinforcing the importance of variable reduction in high-dimensional clinical settings (16). These studies underscore the potential of ML in enhancing ART outcome prediction. However, many existing models focus on a limited set of predictors or single dimensions (e.g., embryological or endometrial factors alone). In contrast, our study integrates both ovarian function indicators (AMH, FORT) and endometrial receptivity marker (HCG day EMT) alongside key clinical characteristics (age, BMI, number of oocytes retrieved) using a hybrid machine learning approach (LASSO and XGBoost) for feature selection. This comprehensive variable selection strategy, followed by logistic regression modeling, allowed us to develop a nomogram with an AUC of 0.911, which compares favorably to the previously reported models (e.g., Liu et al., AUC = 0.89; Zhang et al., improved accuracy over logistic regression). Our model’s performance, particularly its high discriminative ability and net benefit as shown by the decision curve analysis, suggests that the integration of multifaceted factors may offer superior predictive value for ART pregnancy outcomes compared to models relying on narrower sets of predictors. Based on this, this study selected patients undergoing ART treatment at our hospital for analysis, systematically examining the predictive value of endometrial thickness and ovarian function indicators on pregnancy outcomes on the day of hCG administration. By developing an individualized treatment decision support system using machine learning algorithms, this research aims to advance ART treatment toward precision medicine, ultimately improving pregnancy outcomes.
2 Materials and methods
2.1 Declaration of ethical standards
This study was approved by the review committee and ethics committee of this institution. Given that this study is a retrospective study and only de-identified patient data was used, informed consent was not required as there was no risk or adverse effect to patients. This exemption complies with regulations and ethical guidelines related to retrospective studies.
2.2 Study design
This retrospective study included 200 patients who received ART treatment at our hospital between August 2019 and August 2024. All patient data were obtained from the electronic medical record system and divided into clinical pregnancy and non-pregnancy groups based on pregnancy status.
2.3 Inclusion criteria
Inclusion Criteria: (1) Age > 22 years; (2) Complete clinical records; (3) All meet the clinical indications specified in the “Guidelines for Human Assisted Reproductive Technology” (17); (4) No concomitant severe neurological disorders or cognitive impairments.
Exclusion Criteria: (1) Presence of uterine malformations; (2) History of ovarian surgery; (3) Presence of organ dysfunction (e.g., cardiac, hepatic, renal); (4) Presence of infectious diseases.
2.4 Treatment methods
All patients first undergo ovulation induction therapy, which includes the following protocols: long-acting gonadotropin-releasing hormone (GnRH) protocol, modified long-acting GnRH protocol, GnRH antagonist protocol, and other superovulation protocols. During the superovulation period, clinicians assess follicle development based on vaginal ultrasound and serum hormone levels. When the dominant follicle diameter reaches the standard (typically ≥3 follicles ≥18 mm in diameter), human chorionic gonadotropin (HCG) is administered to induce final follicle maturation. Oocyte retrieval is performed approximately 36 h after HCG injection under vaginal ultrasound guidance. Within 4–6 h after egg retrieval, conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is selected based on patient indications (e.g., semen parameters). Fertilization is observed 16–20 h post-fertilization. After 72 h, embryo quality is assessed, and 1–2 embryos are selected for transfer. Embryo transfer: ① Fresh embryo transfer: For patients who did not experience moderate to severe ovarian hyperstimulation syndrome (OHSS), significant ascites, or other conditions unsuitable for fresh cycle transfer (such as premature progesterone elevation) during the ovulation induction and egg retrieval stages, fresh transfer of cleavage-stage embryos is performed on day 3 post-retrieval. Starting from the day of egg retrieval or embryo transfer, all patients undergoing embryo transfer receive luteal support (intramuscular progesterone or vaginal progesterone preparations) until serum β-HCG levels are measured 2 weeks after embryo transfer. ② Frozen–thawed embryo transfer: For patients who develop moderate to severe OHSS risk or symptoms, significant ascites, premature elevation of progesterone, or other clinical conditions deemed unsuitable for fresh transfer during the ovulation induction or egg retrieval phase, all embryos are cryopreserved via vitrification. After the patient’s condition improves, endometrial preparation is conducted via either natural cycle ovulation monitoring or hormone replacement therapy based on the patient’s condition. When the endometrial thickness reaches the ideal state, the cryopreserved embryos are thawed and transferred. In a natural cycle, luteal support is initiated post-ovulation or on the day of embryo transfer, following the same protocol as in a fresh cycle. In a hormone replacement cycle, estradiol is administered from the start of estrogen supplementation to establish the endometrium. Once the endometrium meets the criteria, progesterone is added to facilitate endometrial transformation, and embryo transfer is performed on the scheduled transfer day. Luteal support continues according to the same protocol as in a fresh cycle.
2.5 Grouping method
Serum β-HCG levels are measured 14 days after embryo transfer to determine biochemical pregnancy (serum β-HCG ≥ 10 mIU/mL is considered positive). A transvaginal ultrasound is performed 28 days after embryo transfer to detect an intrauterine gestational sac and primitive heartbeat to confirm clinical pregnancy. Otherwise, it is considered a non-pregnancy.
2.6 General data collection
General patient data were retrospectively collected through the electronic medical record system, primarily including age, body mass index, family history (yes/no), smoking history (yes/no, defined as daily smoking volume > 1 cigarette, with a continuous duration > 1 year or cessation period < 1 year), alcohol consumption history (defined as daily alcohol intake > 1 drink unit, duration > 1 year, or abstinence < 1 year, where 1 drink unit is equivalent to 45 mL of spirits/360 mL of beer/120 mL of wine) (yes/no), Hypertension [meeting the diagnostic criteria in The Japanese Society of Hypertension Guidelines for Self-monitoring of Blood Pressure at Home (Second Edition) (18)] (Yes/No), Diabetes [meeting the diagnostic criteria in Application of the Chinese Expert Consensus on Diabetes Classification in clinical practice (19)] (Yes/No), Hyperlipidemia [meeting the diagnostic criteria in the “Report of the Japan Atherosclerosis Society (JAS) Guideline for Diagnosis and Treatment of Hyperlipidemia in Japanese Adults” (20)] (Yes/No), duration of infertility, total number of Gn days, initial Gn dose, total Gn dose, number of oocytes retrieved, number of fertilized oocytes, type of transferred embryos, number of cleaved embryos, number of transferred embryos.
2.7 HCG day EMT measurement
All patients were treated with individualized ovulation induction protocols. When three or more follicles reached a diameter of ≥18 mm, HCG was administered to promote final oocyte maturation. On the morning of the HCG injection day, EMT was measured using transvaginal ultrasound.
2.8 AMH and FORT testing
Five milliliters of fasting venous blood was drawn from the patient in the morning during the menstrual cycle 1 month prior to egg retrieval. A centrifuge (manufacturer: Beijing Shidai Beili Centrifuge Co., Ltd.) was used at 3000 r/min for 10 min to separate the patient’s serum. AMH was then tested using chemiluminescence and (Yahuilong Company).
2.9 Statistical analysis
This study utilized SPSS 26.0 and R 4.3.2 software for data statistical analysis and visualization. For metric data following a normal distribution, independent samples t-tests were performed, with results presented as (x̅ ± s). For non-normally distributed data, Mann–Whitney U tests were conducted, with results presented as [M (P25, P75)]. For comparisons of categorical variables, chi-square (χ2) tests were used, with results expressed as frequencies. The significance level was set at p < 0.05. The study employed two machine learning methods—LASSO regression and XGBoost—for variable selection. ASSO regression minimizes the following objective function: minβ{1/2 N∑Ni = 1(yi − β0 − ∑pj = 1xijβj)2 + λ∑pj = 1∣βj∣}, where yi is the outcome, xij are the predictors, βj are the coefficients, and λ is the penalty parameter. XGBoost builds an ensemble of decision trees by iteratively adding trees that predict the residuals of previous trees. The objective function for step t is: L(t) = ∑ni = 1l(yi,y^i(t − 1) + ft(xi)) + Ω(ft) where Ω(ft) = γT + 1/2λ∑Tj = 1w2j, with T being the number of leaves, wj the leaf weights, and γ, λ regularization parameters. The overlapping and overlapping important risk factors selected by the two machine learning methods were analyzed, and the selected variables were included in logistic regression analysis. A prediction model based on the rms package was developed, and internal validation was conducted using Bootstrap resampling (1,000 times). ROC curve analysis was used to calculate the AUC value to evaluate discriminative ability, calibration curves were plotted to assess predictive accuracy, and decision curves were applied to evaluate clinical utility.
3 Results
3.1 Comparison of clinical data between the non-pregnant group and the clinically pregnant group
A total of 200 patients undergoing ART treatment were included in this study. According to medical record system records, 109 patients did not become pregnant, accounting for 54.50%, and were included in the non-pregnant group. Ninety-one patients achieved clinical pregnancy, accounting for 45.50%, and were included in the clinically pregnant group. There were significant statistical differences between the non-pregnant group and the clinically pregnant group in terms of age, body mass index, number of oocytes retrieved, HCG day EMT, AMH, and FORT (p < 0.05). However, there were no significant statistical differences in terms of family history and other variables (p > 0.05), as shown in Table 1.
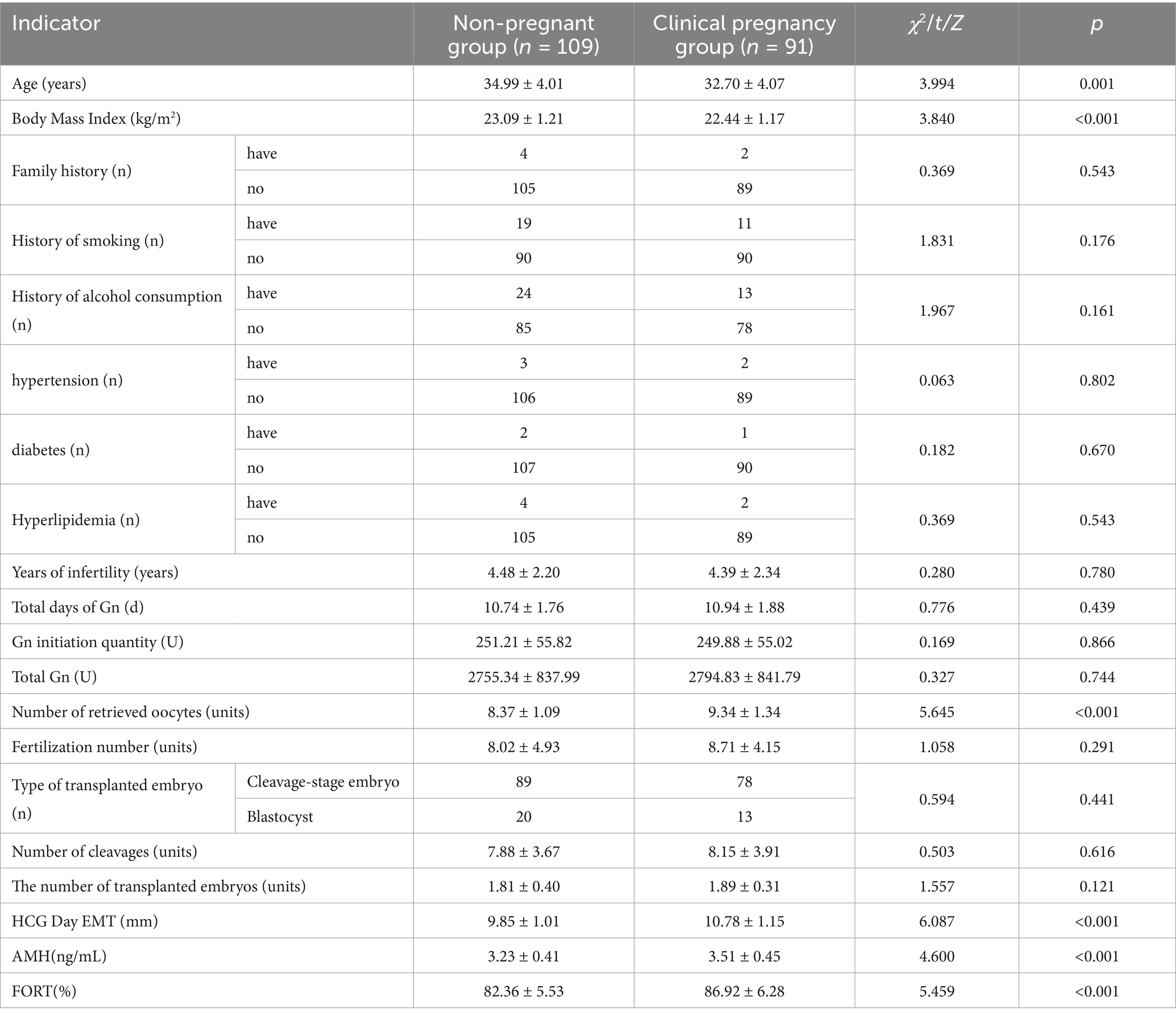
Table 1. Comparison of clinical data between the non-pregnant group and the clinically pregnant group.
3.2 Analysis of important variables affecting pregnancy outcomes in ART patients
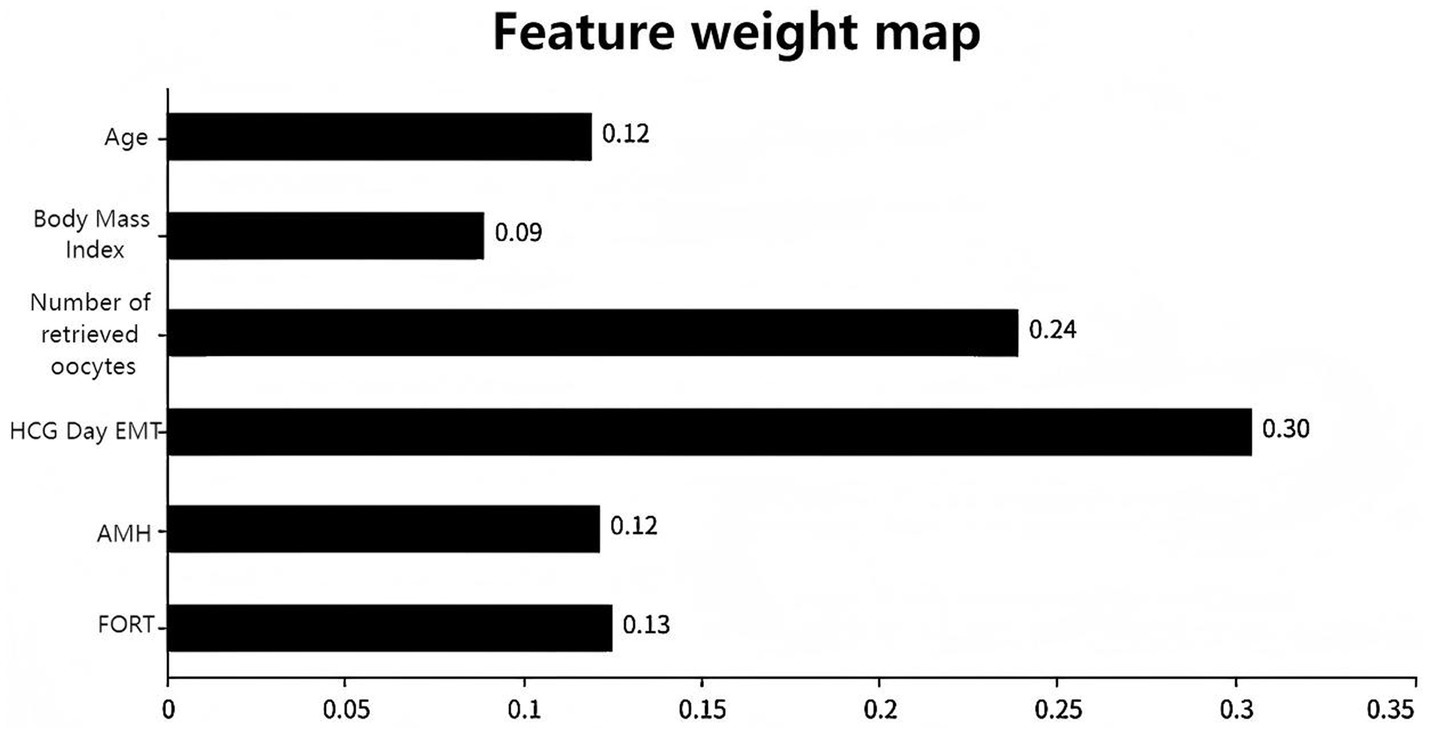
To optimize model performance and address multicollinearity issues, the study used LASSO regression to perform feature selection on the initially screened differential variables (Figure 1A), compressing the regression coefficients of redundant variables to 0. To validate the reliability of the LASSOlogit results, cvlassologit cross-validation was used to determine the optimal λ (Figure 1B). Ultimately, the best fitting results were achieved when λ was 0.006 for minimum mean square error and 0.042 for minimum distance standard error, with six variables included: age, body mass index, number of oocytes retrieved, HCG day EMT, AMH, and FORT. According to XGBoost analysis, the proportions of age, body mass index, number of oocytes retrieved, HCG day EMT, AMH, and FORT were 12, 9, 24, 30, 12, and 13%, respectively, demonstrating clinical interpretability. By applying an “overlap coverage” analysis to the two machine learning models’ parallel evaluations of important variables, six meaningful factors were jointly identified: age, body mass index, number of oocytes retrieved, HCG day EMT, AMH, and FORT.
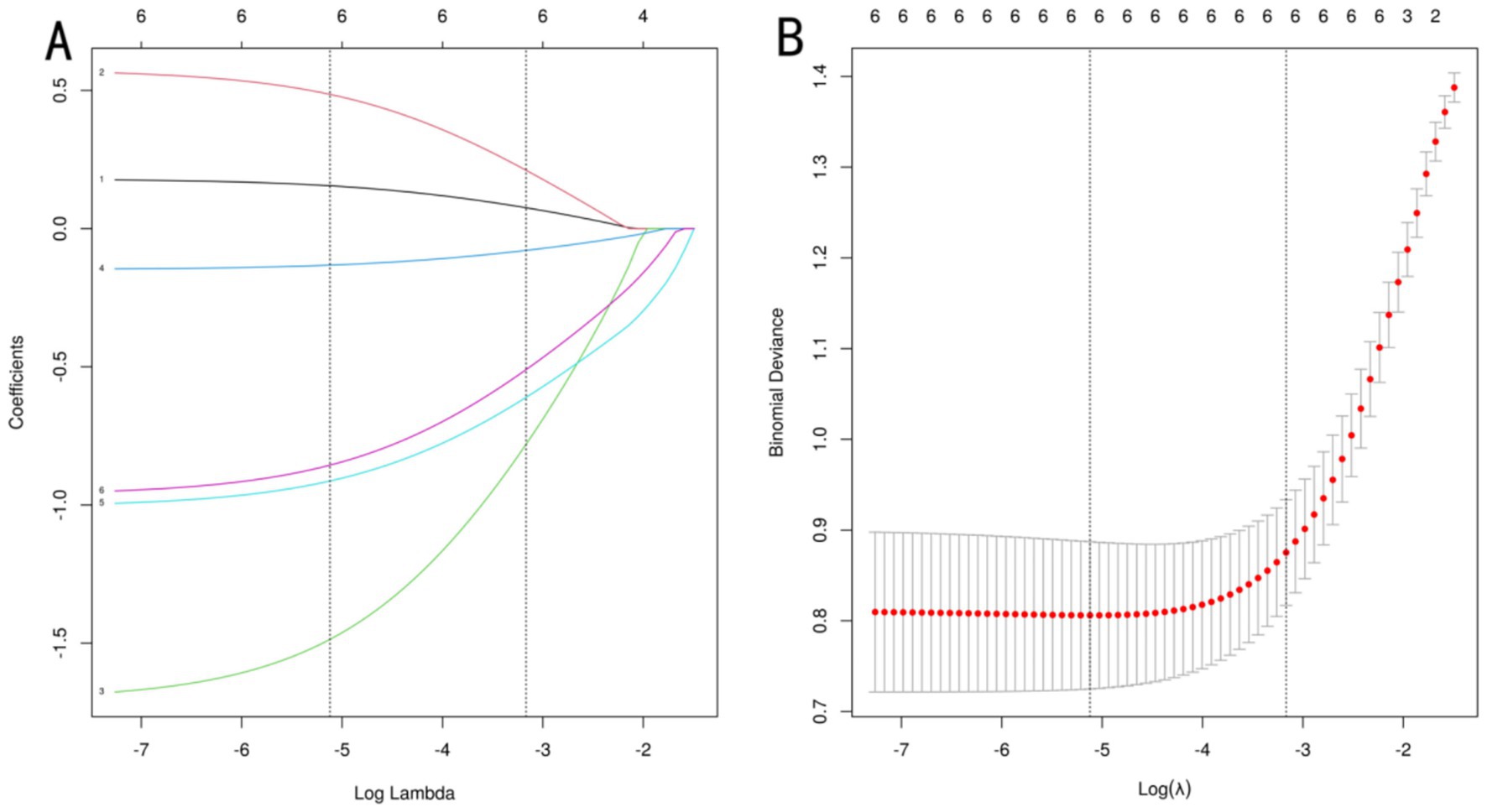
Figure 1. LASSO regression analysis. (A) uses a dual horizontal axis design, with the lower axis displaying the logarithmic value of log(λ) and the upper axis displaying the number of retained variables. The vertical axis presents the standardized coefficients of each feature, with their change trajectories intuitively displayed through colored curves. (B) shows the mean squared error on the vertical axis. The red vertical line indicates the optimal variable subset corresponding to the minimum error value λ.min, which represents the number of independent variables in the model when the mean squared error is minimized. The black vertical line λ.lse represents the simplified model selection when the error increases by one standard error.
Figure 1A uses a dual horizontal axis design, with the lower axis displaying the logarithmic value of log(λ) and the upper axis displaying the number of retained variables. The vertical axis presents the standardized coefficients of each feature, with their change trajectories intuitively displayed through colored curves. Figure 1B shows the mean squared error on the vertical axis. The red vertical line indicates the optimal variable subset corresponding to the minimum error value λ.min, which represents the number of independent variables in the model when the mean squared error is minimized. The black vertical line λ.lse represents the simplified model selection when the error increases by one standard error. Figure 2 shows the importance scores of the variables identified by the XGBoost model, with AMH having the highest score and age having the lowest.
3.3 Logistic regression analysis of factors influencing pregnancy outcomes in patients undergoing ART treatment
Variables selected using LASSO regression and XGBoost algorithms were included in the logistic analysis. The results indicated that age and body mass index were both risk factors influencing pregnancy outcomes in patients undergoing ART treatment (OR = 1.196, 1.777, p < 0.05), while the number of oocytes retrieved, HCG day EMT, AMH, and FORT were protective factors influencing pregnancy outcomes in patients undergoing ART treatment (OR = 0.366, 0.382, 0.182, 0.862, p < 0.05), as shown in Table 2.
3.4 Development of a risk-stratification model for predicting pregnancy outcomes in patients undergoing ART treatment
Based on the results of the statistical analysis, the following regression model was established: Logit(P) = −18.290 + 0.179 × age + 0.575 × body mass index—1.006 × number of oocytes retrieved—0.963 × HCG day EMT—1.706 × AMH - 0.148 × FORT. Based on the six independent predictive factors identified through screening, a visual scoring system was developed. Each risk factor corresponds to an independent scale axis, with the scale length intuitively reflecting the weight of that factor’s contribution to the prognosis. The total score is obtained by summing the scores corresponding to each variable, and the individualized risk prediction value is mapped onto the probability scale, enabling a quantitative assessment of pregnancy outcomes in ART patients, as shown in Figure 3.
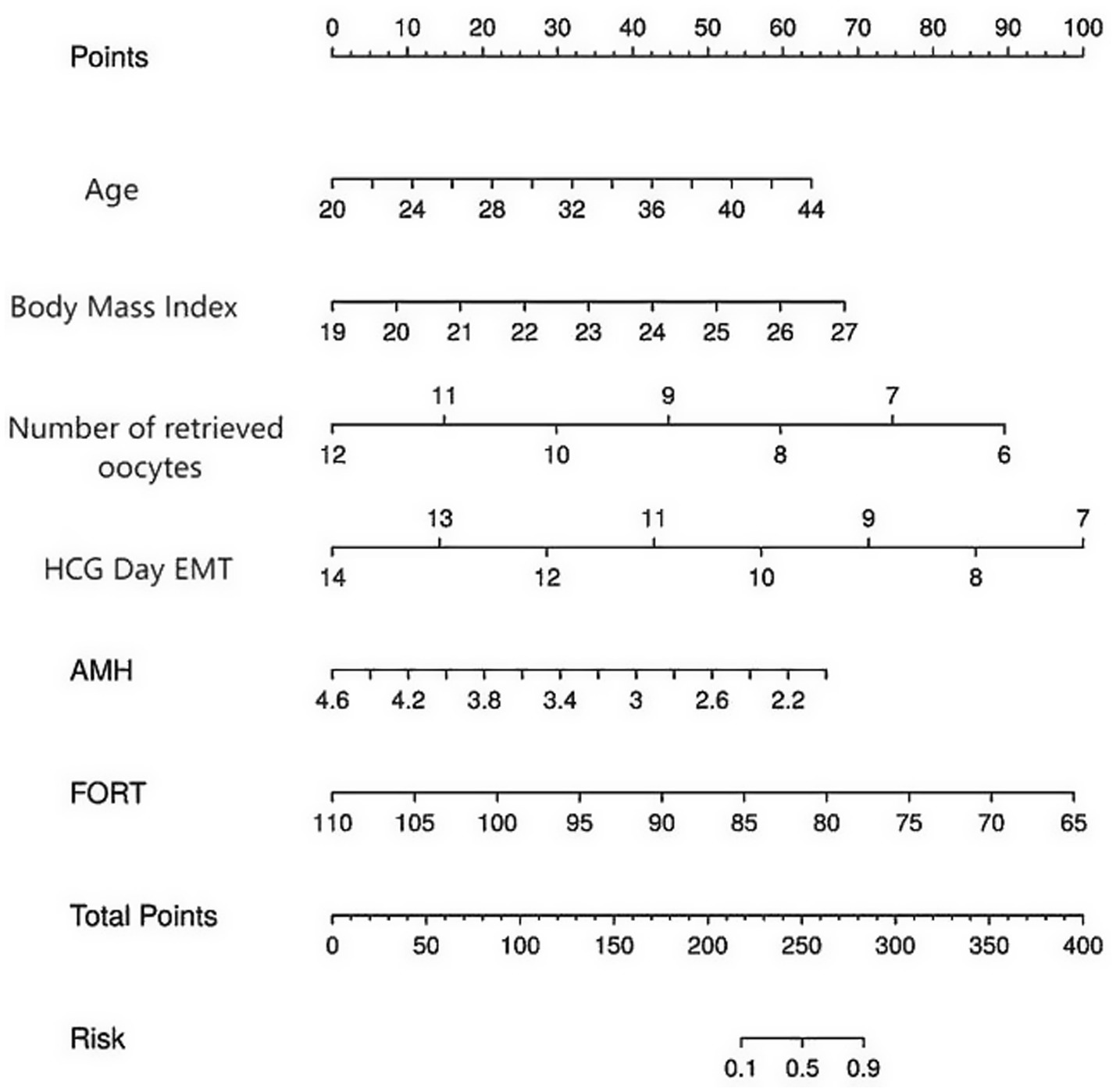
Figure 3. A risk prediction nomogram for pregnancy outcomes in patients treated with ART. The nomogram is used by summing the points assigned to each variable (Age, BMI, Number of Retrieved Oocytes, HCG Day EMT, AMH, FORT) and projecting the total points to the “Total Points” axis. The corresponding value on the “Risk of Non-pregnancy” axis indicates the predicted probability of non-pregnancy.
3.5 Model performance evaluation
3.5.1 ROC curve
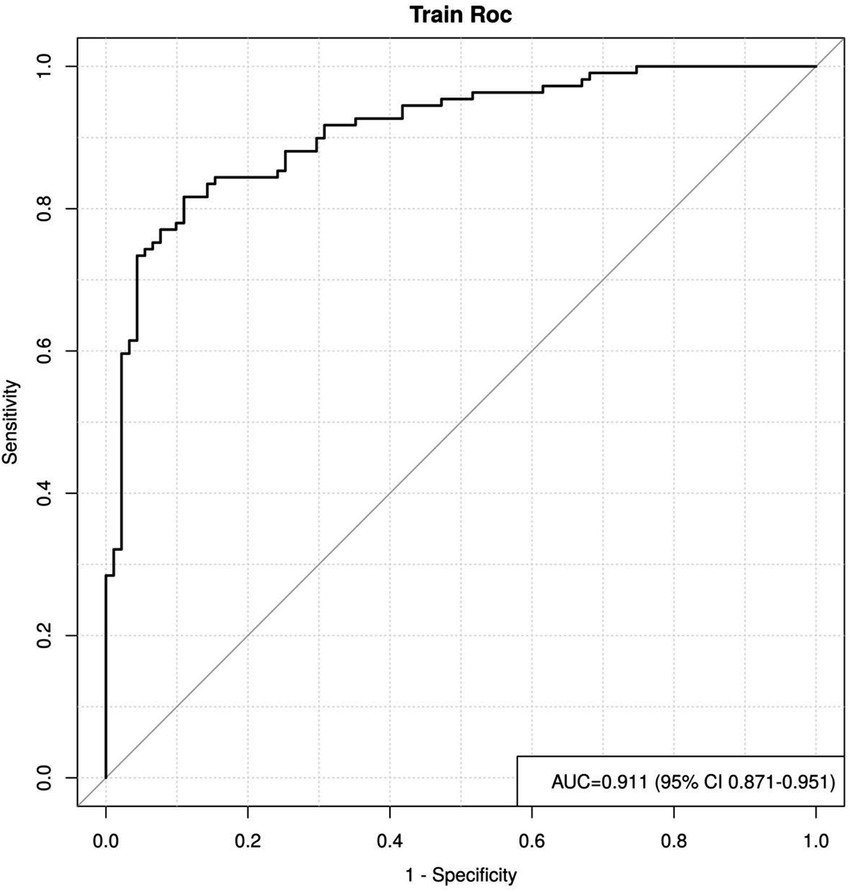
By plotting the ROC curve, we can see that the AUC value is 0.911, with a 95% CI of 0.871 to 0.951, indicating that the reclassified curve model has good discriminatory power, as shown in Figure 4.
3.5.2 Calibration curve and decision curve
The model was validated using the Bootstrap method with 1,000 repeated samples. The Cox-Snell R2 was 0.629, the Nagelkerke R2 was 0.471, the Brier Score was 0.117, and the model fit p-value was 0.240. the statistic is 10.369, indicating that the column plot model exhibits good calibration, as shown in Figure 5A; the DCA curve is higher than the two extreme curves, indicating that the net predictive gain of the relevant factors in the column plot is high, as shown in Figure 5B.
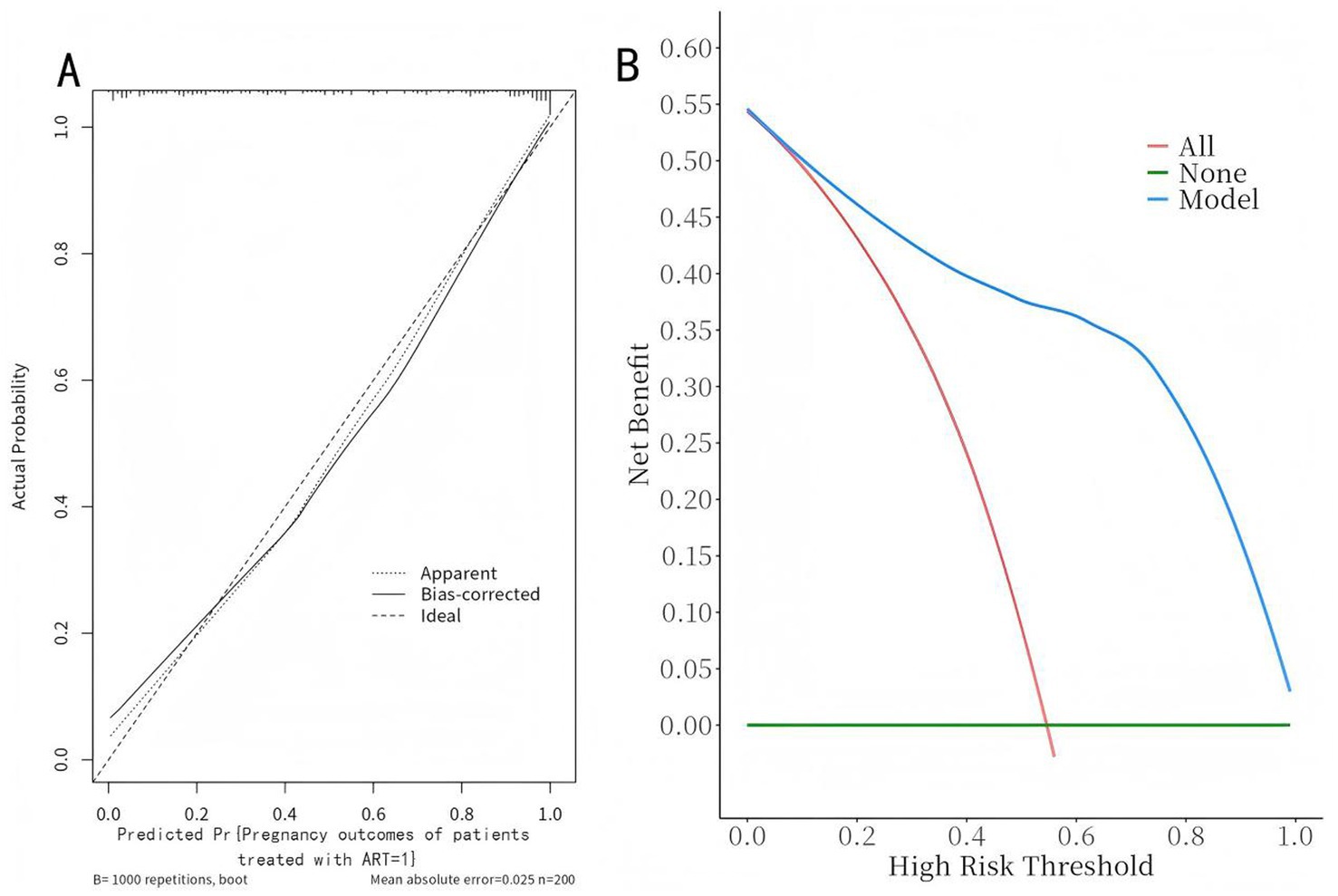
Figure 5. Calibration curves and decision curves of the nomogram model. (A) Calibration curve: The x-axis represents the predicted probability of non-pregnancy, and the y-axis represents the actual observed frequency. The dashed line is the reference line of perfect prediction. The solid line indicates the performance of the nomogram. Pregnancy Outcomes in ART-Treated Patients (=1). (B) Decision curve: The x-axis represents the threshold probability, and the y-axis represents the net benefit. The solid black line represents the nomogram, the gray line “None” assumes no patients experience the event, and the black line “All” assumes all patients experience the event.
4 Discussion
ART is currently an important method for treating infertility. During treatment, patients generally use the same ovulation-inducing drugs, and techniques such as gamete handling and embryo culture are relatively mature (21, 22). Graham ME et al. (23) noted that ART is a medical technique that assists infertile couples in achieving pregnancy, encompassing two main categories: artificial insemination and in vitro fertilization-embryo transfer (IVF-ET) and its derivative techniques. On one hand, exogenous gonadotropins are used to induce synchronized development of multiple follicles, combined with GnRH analogs to precisely trigger ovulation at the optimal time, thereby obtaining an adequate number of high-quality oocytes; on the other hand, after oocytes are retrieved, fertilization is completed in vitro, directly addressing severe male infertility issues. Embryos are cultured to the cleavage stage or blastocyst stage, with precise regulation of gamete development, fertilization environment, embryo selection, and endometrial status, forming a multi-step synergistic effect. This ultimately overcomes the physiological limitations of natural conception, reduces miscarriage rates, and improves live birth rates (24). However, some patients still experience poor pregnancy outcomes due to the combined effects of multiple factors, which severely impact both physical and mental health. Therefore, actively analyzing risk factors influencing pregnancy outcomes in ART patients and constructing predictive models is of significant clinical importance for implementing corresponding preventive and therapeutic measures.
HCG day EMT refers to the endometrial thickness measured on the day of HCG injection in assisted reproductive technology, which is crucial for assessing endometrial receptivity, embryo implantation success rates, and pregnancy outcomes; FORT refers to the ratio of the number of mature follicles with a diameter ≥14 mm in both ovaries on the day of HCG injection to the number of antral follicles with a diameter of 2–8 mm before ovulation induction, which is an important indicator for assessing ovarian responsiveness; AMH is secreted by preantral follicles and small antral follicles in the ovaries, and its levels are directly related to the number of remaining follicles in the ovaries. It is used to assess ovarian reserve function, reflecting the quantity and quality of remaining oocytes in the ovaries, and is closely associated with the efficacy of reproductive treatment (25–27). Based on the data from this study, significant differences were observed between the non-pregnant group and the clinically pregnant group in terms of EMT, AMH, and FORT on the day of HCG administration (p < 0.05). Logistic regression analysis indicated that AMH, EMT on the day of HCG administration, and FORT are all risk factors influencing pregnancy outcomes in patients undergoing ART (p < 0.05). Previous studies have indicated that synchronized endometrial development is essential for embryo implantation. When EMT > 10 mm, estrogenization is optimal, facilitating embryo implantation; when EMT is between 7 mm and 10 mm, the pregnancy rate increases with increasing EMT (28). Martins RS et al. (29) reported that insufficient EMT on the day of HCG administration is an important risk factor for pregnancy failure in ART cycles, primarily involving impaired endometrial receptivity, impaired blood flow perfusion, and abnormal molecular microenvironment. On one hand, a decrease in EMT on the HCG day is often accompanied by increased uterine spiral artery blood flow resistance, delayed glandular epithelial development, and reduced vascular endothelial growth factor expression, leading to inadequate endometrial blood supply, reduced oxygen and nutrient delivery, and impaired normal endometrial proliferation and decidualization. When the endometrium is too thin, receptor density is insufficient, weakening the role of HCG in promoting endometrial thickening and establishing receptivity; On the other hand, decreased EMT on the HCG day indicates endometrial thinning, and inadequate decidualization may fail to support early placental formation, leading to endometrial receptivity defects, ectopic implantation of the embryo, and an increased risk of clinical non-pregnancy. Under normal physiological conditions, AMH has a stimulatory effect on follicle development and can also prevent excessive follicle depletion to some extent, thereby enhancing ovarian reserve function. Referring to the study by Peigné M et al. (27), estrogen and progesterone imbalance is an important cause of irregular changes in the endometrium. AMH is a glycoprotein differentiation factor primarily produced by granulosa cells of primordial follicles, which can enter the systemic circulation via follicular fluid. Serum AMH levels fundamentally reflect ovarian antral follicle reserve. Abnormal serum AMH levels may impair oocyte maturation, thereby reducing egg and embryo quality, leading to poor embryo quality and adverse pregnancy outcomes. Previous studies have indicated that FORT is a novel assessment indicator applied in clinical reproductive systems in recent years, first proposed by Genro et al. in 2011. It objectively reflects the developmental potential of follicles within the ovarian follicle, independent of the original number of follicles in the ovarian follicle, and is instead correlated with the overall health status of follicles (30). FORT assessment is an important indicator for predicting the extent of follicle response to medication. Its expression is not influenced by factors such as age, exhibiting uniqueness and high stability. It quantitatively assesses the follicular potential of the ovaries in patients with relatively regular menstrual cycles. As FORT values increase, the number of retrieved oocytes and high-quality embryos also increases, playing a positive role in achieving favorable assisted reproductive outcomes.
The data from this study revealed significant statistical differences in age, body mass index, and number of oocytes retrieved between the non-pregnant group and the clinically pregnant group (p < 0.05). Logistic regression analysis indicated that age, body mass index, and number of oocytes retrieved are all risk factors influencing pregnancy outcomes in patients undergoing ART treatment (p < 0.05). Previous studies have indicated that overweight and obesity are closely associated with clinical symptoms, hormonal abnormalities, and lipid metabolism abnormalities in infertile patients (31). Sex hormones in the human body are primarily metabolized through adipose tissue. Abnormal increases in adipose tissue can lead to abnormalities in drug metabolism kinetics, resulting in reduced levels of follicle-stimulating hormone (FSH) and subsequently affecting follicle quality (32). Referring to the study by Li YL et al. (33), it is known that in obese women, more leptin in follicles originates from adipose tissue. Therefore, leptin significantly inhibits the stimulatory effect of follicle-stimulating hormone on steroid synthesis in granulosa cells, leading to reduced ovarian responsiveness and increased risk of clinical non-pregnancy. In this study, low oocyte retrieval numbers were identified as a core risk factor for treatment failure in ART. The mechanism may involve a dual decline in both oocyte quantity and quality due to reduced ovarian reserve function and low ovarian responsiveness. Insufficient oocyte retrieval directly limits the acquisition of euploid embryos, while egg quality deteriorates simultaneously, with increased granulosa cell apoptosis and impaired cumulus cell function, affecting oocyte maturation and subsequent embryonic developmental potential. This further reduces the number of available eggs, negatively impacts endometrial receptivity, and impedes embryo implantation, leading to ART treatment failure through a combination of egg quantity deficiency, reduced embryonic developmental potential, and endometrial receptivity imbalance (34). Female fertility begins to decline after the age of 35 and shows a significant decline after the age of 37, with an irreversible decline as age increases. The number of oocytes decreases, and egg aging accelerates, manifested as mitochondrial dysfunction and metabolic disorders, leading to a significant reduction in fertilization rates and embryonic development potential, which is an important factor contributing to poor pregnancy outcomes. Additionally, studies have shown that aging leads to reduced estrogen receptor expression, decreased endometrial blood flow perfusion, and inadequate glandular development in women. Even with exogenous hormone supplementation, adequate proliferation is difficult to achieve, resulting in reduced endometrial thickness, which impedes embryo adhesion and placental formation. Systemic functional decline is exacerbated, and through the synergistic effects of germ cell aging, accumulated genetic abnormalities, and systemic metabolic imbalance, the risk of ART-related pregnancy failure increases (35).
In this study, a nomogram prediction model was constructed by combining various factors, transforming complex multivariate regression equations into graphical representations, thereby making abstract data outcomes visualizable and readable. Different predictive variables were used to assess the approximate probability of adverse pregnancy outcomes in patients. The AUC value of the ROC curve was 0.911, indicating that the model has excellent discriminative ability and can accurately distinguish between clinically pregnant and non-pregnant groups. The 95% CI (0.871–0.951) also indicates that this result is highly reliable. This performance is comparable to or even better than some recent ML-based ART prediction models, such as the XGBoost model by Liu et al. (AUC = 0.89) (12) and the random forest model by Zhang et al. (13), and outperforms traditional logistic regression models commonly used in clinical practice. The calibration curve shows that the model’s predictive results are well aligned with the actual occurrence of adverse pregnancy outcomes. This means that the consistency between the model’s predictive results and the actual observed outcomes is high, further enhancing the model’s credibility. The clinical decision curve is generally higher than the two extreme curves, indicating that the factors included in the nomogram have a high net benefit in predicting adverse pregnancy outcomes, providing stronger support for clinical decision-making. The integration of both ovarian and endometrial factors, along with core clinical characteristics, likely contributes to this robust performance, addressing the limitation of single-dimensional models highlighted in previous studies (3, 4). Limitations of this study: (1) Due to the limitations of single-center data collection, the small sample size reduces statistical power, potentially leading to a lack of representativeness in patient analysis; (2) Although the constructed nomogram model was validated using methods such as ROC curves, calibration curves, and decision curves, the validation was primarily based on internal validation and lacked corresponding external validation; (3) Long-term outcomes were not included, as this study primarily focused on short-term pregnancy outcomes through follow-up studies. Future research can address these limitations to improve and refine the risk prediction model, thereby enhancing its accuracy and practicality.
5 Conclusion
In this retrospective cohort study, age, body mass index, number of oocytes retrieved, EMT on HCG day, AMH, and FORT were identified as risk factors for non-pregnancy in patients undergoing ART treatment. The risk prediction nomogram model constructed based on these factors demonstrated predictive value for the risk of adverse pregnancy outcomes in patients, with an AUC of 0.911, which is competitive with existing models, and offers a clinically useful tool via a visual nomogram. Early clinical screening of high-risk populations using this integrated model holds significant value.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by all research is in strict accordance with and adheres to the Declaration of Helsinki. The study was approved by the Ethics Committee of Chinese People’s Liberation Army Air Force Specialty Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LZ: Writing – review & editing, Methodology, Conceptualization. XL: Data curation, Software, Writing – review & editing. JB: Project administration, Writing – original draft, Investigation. YW: Validation, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zegers-Hochschild, F, Mansour, R, Ishihara, O, Adamson, GD, de Mouzon, J, Nygren, KG, et al. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology. Fertil Steril. (2014) 101:366–78. doi: 10.1016/j.fertnstert.2013.10.005
2. Laube, R, Tran, Y, Paramsothy, S, and Leong, RW. Assisted reproductive technology in Crohn's disease and ulcerative colitis: a systematic review and meta-analysis. Am J Gastroenterol. (2021) 116:2334–44. doi: 10.14309/ajg.0000000000001537
3. Bianchi, C, Brocchi, A, Baronti, W, Nicolì, F, Citro, F, Aragona, M, et al. Assisted reproductive technology,risk of gestational diabetes,and perinatal outcomes in singleton pregnancies. Diabetes Metab Res Rev. (2023) 39:e3625. doi: 10.1002/dmrr.3625
4. Gao, R, Zeng, R, Qing, P, Meng, C, Cheng, K, Zhang, S, et al. Antiphospholipid antibodies and pregnancy outcome of assisted reproductive treatment: a systematic review and meta-analysis. Am J Reprod Immunol. (2021) 86:e13470. doi: 10.1111/aji.13470
5. Dogra, Y, Singh, N, and Vanamail, P. Autologous platelet-rich plasma optimizes endometrial thickness and pregnancy outcomes in women with refractory thin endometrium of varied aetiology during fresh and frozen-thawed embryo transfer cycles. JBRA Assist Reprod. (2022) 26:13–21. doi: 10.5935/1518-0557.20210037
6. Mahutte, N, Hartman, M, and Meng, L. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. (2022) 117:792–800. doi: 10.1016/j.fertnstert.2021.12.025
7. Al-Lamee, H, Stone, K, Powell, SG, Wyatt, J, Drakeley, AJ, Hapangama, DK, et al. Endometrial compaction to predict pregnancy outcomes in patients undergoing assisted reproductive technologies: a systematic review and meta-analysis. Hum Reprod Open. (2024) 2024:hoae040. doi: 10.1093/hropen/hoae040
8. Ata, B, Liñán, A, Kalafat, E, Ruíz, F, Melado, L, Bayram, A, et al. Effect of the endometrial thickness on the live birth rate: insights from 959 single euploid frozen embryo transfers without a cutoff for thickness. Fertil Steril. (2023) 120:91–8. doi: 10.1016/j.fertnstert.2023.02.035
9. Grynberg, M, Pytel, S, Peigne, M, and Sonigo, C. The follicular output rate in normo-ovulating women undergoing ovarian stimulation is increased after unilateral oophorectomy. Hum Reprod. (2023) 38:1162–7. doi: 10.1093/humrep/dead056
10. Sellami, I, Barbotin, AL, Bernard, V, Robin, G, Catteau-Jonard, S, Sonigo, C, et al. Anti-Mullerian hormone assessment in assisted reproductive technique outcome and natural conception. Semin Reprod Med. (2024) 42:25–33. doi: 10.1055/s-0044-1787273
11. Wang, X, Tian, J, Tian, L, Chen, X, Zhang, Z, Diao, H, et al. The follicular output rate was improved with 3-day letrozole administration compared with 5-day letrozole administration under progestin-primed ovarian stimulation. Drug Des Devel Ther. (2025) 19:4397–406. doi: 10.2147/DDDT.S521554
12. Liu, X, Chen, P, Wang, M, Zhao, W, Jin, L, Shi, J, et al. Association between pre-gravid body mass index and clinical outcomes in in vitro fertilization: a multicentered retrospective cohort study. BMC Pregnancy Childbirth. (2024) 24:469. doi: 10.1186/s12884-024-06661-2
13. Amitai, T, Kan-Tor, Y, Or, Y, Shoham, Z, Shofaro, Y, Richter, D, et al. Embryo classification beyond pregnancy: early prediction of first trimester miscarriage using machine learning. J Assist Reprod Genet. (2023) 40:309–322. doi: 10.1007/s10815-022-02619-5
14. AlSaad, R, Abusarhan, L, Odeh, N, Abd-Alrazaq, A, Choucair, F, Zegour, R, et al. Deep learning applications for human embryo assessment using time-lapse imaging: scoping review. Front Reprod Health. (2025) 7:1549642. doi: 10.3389/frph.2025.1549642
15. Lantzi, MA, Papakonstantinou, E, and Vlachakis, D. Bioinformatic Analysis of Complex In Vitro Fertilization Data and Predictive Model Design Based on Machine Learning: The Age Paradox in Reproductive Health. Biology (Basel). (2025) 14:556. doi: 10.3390/biology14050556
16. Genovese, H, Mayo, CA, Kalafat, E, Fatemi, H, Ata, B, Garcia-Velasco, J, et al. Does endometrial thickness impact live birth rate following a frozen embryo transfer: outcomes of 30 676 euploid single embryo transfers. Hum Reprod. (2025) 40:1919–1927. doi: 10.1093/humrep/deaf129
17. Jiang, L, Chen, Y, Wang, Q, Wang, X, Luo, X, Chen, J, et al. A Chinese practice guideline of the assisted reproductive technology strategies for women with advanced age. J Evid Based Med. (2019) 12:167–84. doi: 10.1111/jebm.12346
18. Imai, Y, Otsuka, K, Kawano, Y, Shimada, K, Hayashi, H, Tochikubo, O, et al. Japanese society of hypertension (JSH) guidelines for self-monitoring of blood pressure at home. Hypertens Res. (2003) 26:771–82. doi: 10.1291/hypres.26.771
19. Yang, ST, Deng, C, He, BB, Chen, X, Li, X, and Zhou, ZG. Application of the Chinese expert consensus on diabetes classification in clinical practice. Zhonghua Nei Ke Za Zhi. (2023) 62:1085–92. doi: 10.3760/cma.j.cn112138-20230131-00043
20. Hata, Y, Mabuchi, H, and Saito, Y. Report of the Japan atherosclerosis society (JAS) guideline for diagnosis and treatment of hyperlipidemia in Japanese adults. J Atheroscler Thromb. (2002) 9:1–27. doi: 10.5551/jat.9.1
21. Chih, HJ, Elias, FTS, and Gaudet, L. Assisted reproductive technology and hypertensive disorders of pregnancy: systematic review and meta-analyses. BMC Pregnancy Childbirth. (2021) 21:449. doi: 10.1186/s12884-021-03938-8
22. Fedele, F, Bulfoni, A, Parazzini, F, Levi-Setti, PE, and Busnelli, A. Assisted reproductive technology outcomes in women with congenital uterine anomalies: a systematic review. Arch Gynecol Obstet. (2024) 310:2315–32. doi: 10.1007/s00404-024-07666-0
23. Graham, ME, Jelin, A, and Hoon, AH Jr. Assisted reproductive technology: short‐ and long‐term outcomes. Dev Med Child Neurol. (2023) 65:38–49. doi: 10.1111/dmcn.15332
24. Lucas, AF, Gemechu, DB, and Du Plessis, SS. Fertility and pregnancy outcome among women undergoing assisted reproductive technology treatment in Windhoek, Namibia. J Assist Reprod Genet. (2021) 38:635–43. doi: 10.1007/s10815-020-02046-4
25. Turkgeldi, E, Yildiz, S, Kalafat, E, Keles, I, Ata, B, and Bozdag, G. Can endometrial compaction predict live birth rates in assisted reproductive technology cycles? A systematic review and meta-analysis. J Assist Reprod Genet. (2023) 40:2513–2522. doi: 10.1007/s10815-023-02942-5
26. Sunkara, SK, Schwarze, JE, Orvieto, R, Fischer, R, Dahan, MH, Esteves, SC, et al. Expert opinion on refined and extended key performance indicators for individualized ovarian stimulation for assisted reproductive technology. Fertil Steril. (2025) 123:653–64. doi: 10.1016/j.fertnstert.2024.10.001
27. Peigné, M, Bernard, V, and Dijols, L. Using serum anti-Müllerian hormone levels to predict the chance of live birth after spontaneous or assisted conception: a systematic review and meta-analysis. Hum Reprod. (2023) 38:1789–806. doi: 10.1093/humrep/dead147
28. Zhao, J, Hao, J, Xu, B, Wang, Y, and Li, Y. Impact of previous caesarean section on reproductive outcomes after assisted reproductive technology:systematic review and meta-analyses. Reprod Biomed Online. (2021) 43:197–204. doi: 10.1016/j.rbmo.2021.04.007
29. Martins, RS, Oliani, AH, Oliani, DV, and de Oliveira, JM. Continuous endometrial volumetric analysis for endometrial receptivity assessment on assisted reproductive technology cycles. BMC Pregnancy Childbirth. (2020) 20:663. doi: 10.1186/s12884-020-03372-2
30. Vanderhoff, AC, Lanes, A, Herz-Roiphe, R, Mecklai, K, Camacho, OL, Srouji, SS, et al. Outcomes for female physicians compared with nonphysicians after assisted reproductive technology. Obstet Gynecol. (2025) 145:e24–30. doi: 10.1097/AOG.0000000000005767
31. Zhang, L, Feng, Y, Sun, X, Yi, S, Xiao, X, and Ma, F. Impact of body mass index on assisted reproductive technology outcomes in patients with polycystic ovary syndrome: a meta-analysis. Reprod Biomed Online. (2024) 48:103849. doi: 10.1016/j.rbmo.2024.103849
32. Kidera, N, Ishikawa, T, Kawamura, T, and Miyasaka, N. Maternal body mass index is not associated with assisted reproductive technology outcomes. Sci Rep. (2023) 13:14817. doi: 10.1038/s41598-023-41780-4
33. Li, YL, Yan, EQ, Zhao, GN, Jin, L, and Ma, B-X. Effect of body mass index on ovarian reserve and ART outcomes in infertile women: a large retrospective study. J Ovarian Res. (2024) 17:195. doi: 10.1186/s13048-024-01521-1
34. Glujovsky, D, Quinteiro Retamar, AM, Alvarez Sedo, CR, Ciapponi, A, Cornelisse, S, and Blake, D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. (2022) 5:CD002118. doi: 10.1002/14651858.CD002118.pub6
Keywords: assisted reproductive technology, pregnancy outcomes, human chorionic gonadotropin, endometrial thickness, serum anti-Müllerian hormone, follicle output rate
Citation: Zhang L, Liu X, Bai J and Wang Y (2025) Building a predictive model for pregnancy outcomes in ART patients based on AMH, FORT, HCG day EMT, and clinical characteristics. Front. Med. 12:1699477. doi: 10.3389/fmed.2025.1699477
Edited by:
Liliana Ibeth Barbosa Santillan, University of Guadalajara, MexicoReviewed by:
Leticia Flores-Pulido, Universidad Autonoma de Tlaxcala, MexicoBertha Patricia Guzman-Velazquez, National Institute of Astrophysics, Optics and Electronics (INAOE), Mexico
Copyright © 2025 Zhang, Liu, Bai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, ZG9uZ3JsMTAyM0AxNjMuY29t
 Lei Zhang
Lei Zhang Xiaofang Liu
Xiaofang Liu