- 1Department of Pulmonary Medicine, Xiangyang Hospital Affiliated with Hubei University of Traditional Chinese Medicine, Xiangyang, Hubei, China
- 2Department of Geriatric Medicine, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 3Department of Respiratory and Critical Care Medicine, Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Background: Secreted Phosphoprotein 1 (SPP1), which encodes Osteopontin, a key member of the SIBLING family, is a multifunctional ECM glycoprotein and cytokine. Its interaction with collagen and other ECM components drives pathological remodeling across multiple diseases, yet a unified mechanistic framework remains elusive.
Objective: This review synthesizes current evidence on SPP1-mediated ECM dysregulation, focusing on collagen deposition, epithelial-mesenchymal transition (EMT), and fibrosis, with the goal of elucidating its role as a central pathological hub.
Methods: We synthesize the findings from multi-omics analyses (single-cell RNA sequencing, spatial transcriptomics), machine learning, and in vivo/in vitro experimental studies, aiming to elucidate the role of SPP1 (Osteopontin) in the dysregulation of the extracellular matrix (ECM) across various diseases via a systematic literature review (1990–2025).
Conclusion: SPP1 is a master regulator of pathological ECM dynamics, driven by conserved SPP1+ macrophage-ECM interactions. Targeting the SPP1-collagen axis may offer unified strategies for fibrosis and metastasis suppression. Future work should prioritize in vivo validation in osteoarthritis and clinical translation of SPP1-directed therapies.
1 Introduction
Secreted Phosphoprotein 1 (SPP1), which encodes Osteopontin, is a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of secreted phosphoproteins that participate in bone mineralization (1). The SIBLING family consists of five secreted phosphoglycoproteins: secreted phosphoprotein 1 (SPP1), bone sialoprotein (BSP), dentin matrix protein-1 (DMP1), dentin sialophosphoprotein (DSPP), and matrix extracellular phosphoglycoprotein (MEPE) (2).
SPP1 was first cloned and sequenced by Kiefer et al. (3), revealing a conserved Arg-Gly-Asp (RGD) cell adhesion motif (4). Its mRNA is mainly expressed in osteocytes and the decidua during early stages pregnancy. Kohri et al. (5, 6) demonstrated that Osteopontin, which constitutes the urinary stone matrix, is upregulated in the renal distal tubules and actively participates in calcium oxalate stone formation. Subsequent research confirmed SPP1 expression in normal adult human and monkey kidneys, specifically localized to the distal convoluted and straight tubules in both the cortex and medulla (7). The authors further demonstrated the colocalization of SPP1 with MMPs in human eccrine sweat gland cells, mostly perinuclearly, a pattern consistent with SIBLING family members and their metalloproteinase counterparts; neither SPP1 nor MMPs was identified in the sweat gland stroma or monkey lacrimal gland structures. Shinohara et al. (8) discovered two translational initiation sites in mouse SPP1 mRNA, producing a full-length isoform (including a signal peptide) and a truncated isoform (devoid of signal peptide). These correspond to 75 kDa and 70 kDa proteins in dendritic cells, exhibiting different subcellular localizations: the full-length isoform is transported to secretory vesicles and the Golgi apparatus, while the short isoform is predominantly localizes in the cytoplasm (8).
The Osteopontin encoded by SPP1 promotes the adherence of osteoclasts to the calcified bone matrix, which is intricately associated with the dynamics of the extracellular matrix (ECM) (9). Osteopontin exhibits a strong affinity for the binding with hydroxyapatite. Additionally, the osteoclast vitronectin receptor is situated in the cell membrane. It may engage in binding with the SPP1 protein, which acts as a cytokine that stimulates the synthesis of IFN-γ and IL-12, which promotes EMT and ECM deposition. In particular, SPP1-positive macrophages (SPP1+ macrophages) refer to a subset of macrophages characterized by increased expression of SPP1, identified through immunohistochemistry, flow cytometry, or single-cell RNA sequencing (10). SPP1+ macrophages are common in pathological microenvironments, including fibrosis and malignancies, and play a significant role in matrix remodeling (11). These macrophages produce large amounts of Osteopontin, which acts as matrix-associated glycoprotein, involved in the dynamic regulation of the ECM by facilitating fibrotic deposition, and managing cell-matrix adhesion. In recent years, SPP1 and SPP1+ macrophages have garnered increasing attention in various disease settings. SPP1+ macrophages not only play a crucial role as tumor-associated macrophages (TAMs) within the tumor microenvironment (12–15) but also participate in physiological processes, such as aging (16), and in a wide range of non-cancerous diseases, including rheumatoid arthritis (17), neurodegeneration (18, 19), and fibrosis (20, 21). This perspective provides a theoretical foundation for a deeper understanding of the mechanisms by which SPP1 regulates ECM dynamics and its role in disease (22).
SPP1 and the Osteopontin it encodes are crucial factors influencing ECM dynamics and play a significant role in tumor diseases, cardiovascular diseases, pulmonary diseases, chronic kidney disease, and osteoarthritis (23). It will be introduced in further detail below. The research process is shown in Figure 1.
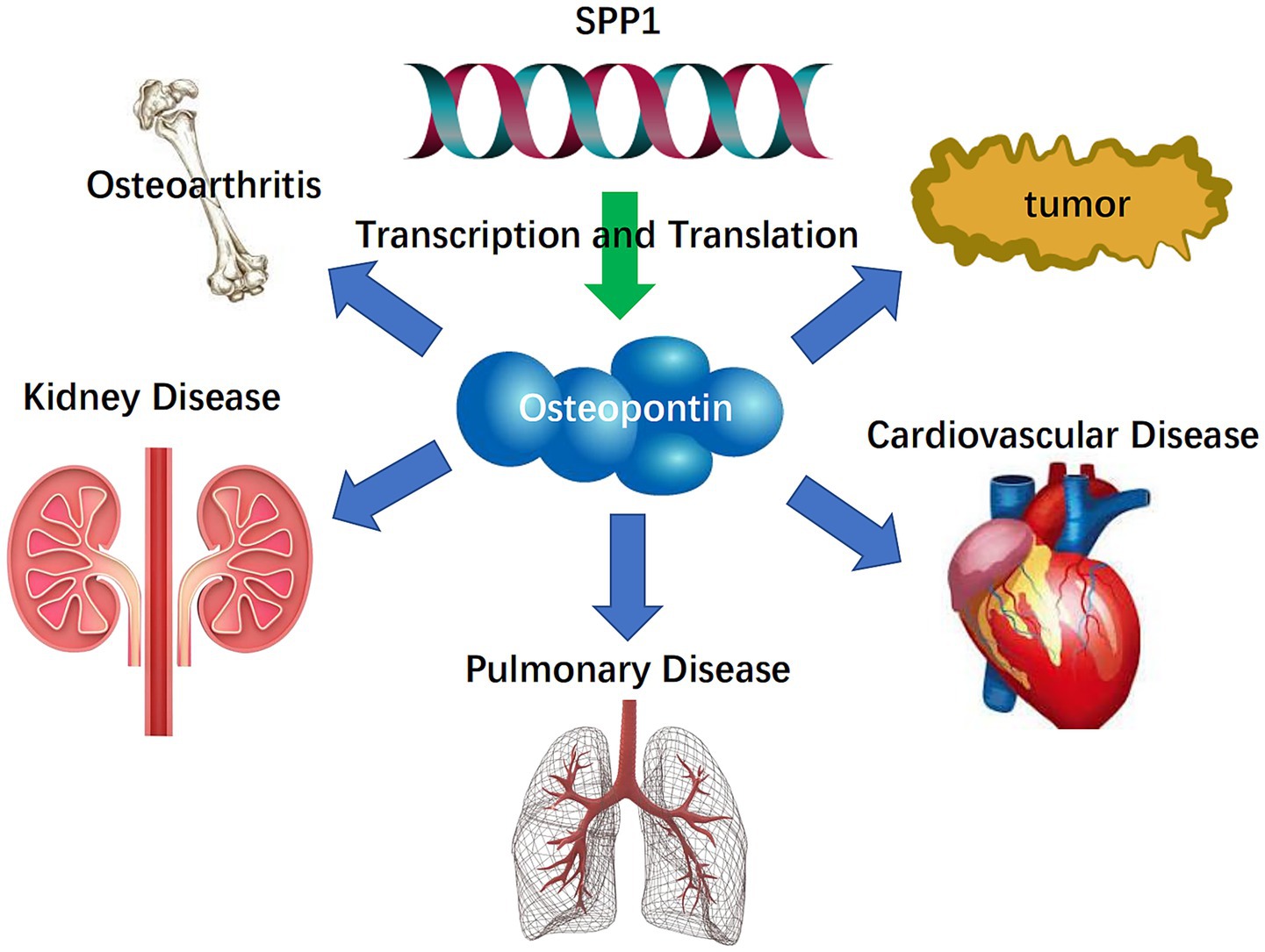
Figure 1. Flowchart of the systematic review process on the relationship between SPP1 (Osteopontin) and ECM.
2 Molecular architecture, function, and signaling of SPP1 (Osteopontin)
The SIBLING protein family is a class of evolutionarily conserved, structurally related ECM glycoproteins. SPP1 is one of the main members of this protein family and is involved in the deposition of the ECM and EMT mechanisms. Each SIBLING protein has a minimum of one “acidic, serine- and aspartic acid-rich motif” (ASARM) and many Ser-x-Glu/pSer sequences that, upon phosphorylation, facilitate biomineralization and ECM deposition. Osteopontin functions by regulating the nucleation and development of hydroxyapatite crystals. The biological activities of Osteopontin are facilitated through interactions with cell surface integrins and the activation of certain MMPs, therefore coordinating a complicated relationship between cellular signaling and ECM remodeling in mineralized tissues (24).
The SPP1 is located in the 4q21-q25 region of chromosome 4, with genomic coordinates (GRCh38): 4:87,975,714–87,983,411 (25), as shown in Figure 2A. According to the Genecard database, SPP1 is a protein-coding gene. SPP1 is associated with various cancers, cardiovascular diseases, respiratory diseases, and kidney diseases. Related pathways include the integrin pathway and ERK signaling pathway. Gene Ontology (GO) annotations associated with this gene cover cytokine activity and ECM binding (26). SPP1 demonstrates significant evolutionary conservation among several species. The SPP1 which encoding Osteopontin consists of seven exons featuring canonical splice sites. While genetic linkage studies initially linked the SPP1 locus to dentinogenesis imperfecta type II, further investigation ruled out coding sequence mutations within its exons as the direct cause of the disease (27). Research shows that SPP1 serves as a specific marker for a profibrotic macrophage subpopulation that expands dramatically in idiopathic pulmonary fibrosis (IPF). SPP1+ macrophages, characterized by co-expression of MERTK, promote fibrosis through Osteopontin deposition and aberrant repair processes, positioning SPP1 as a central mediator and potential therapeutic target in IPF (28).
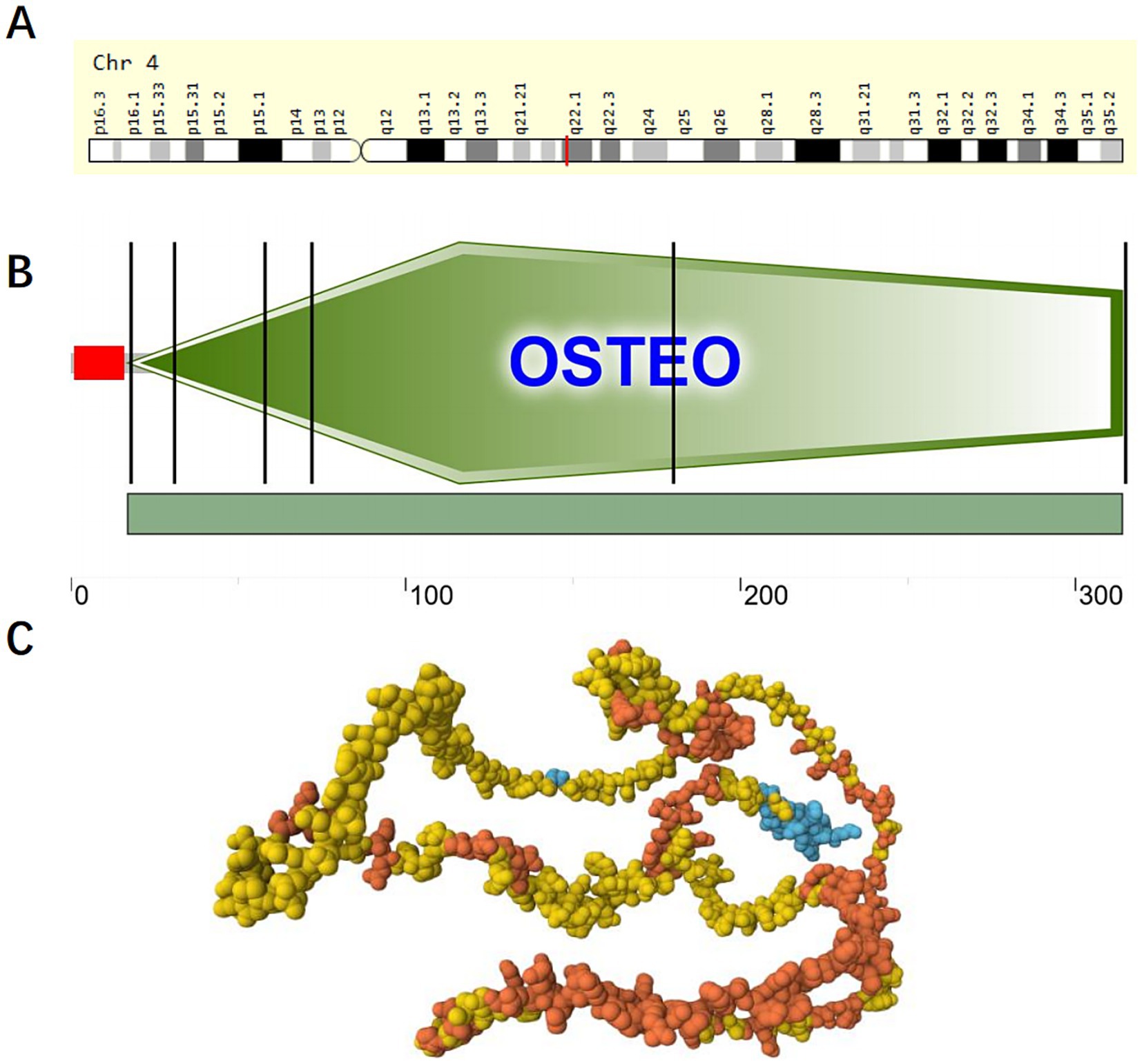
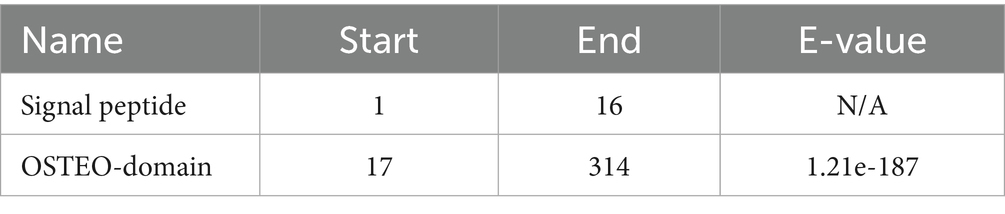
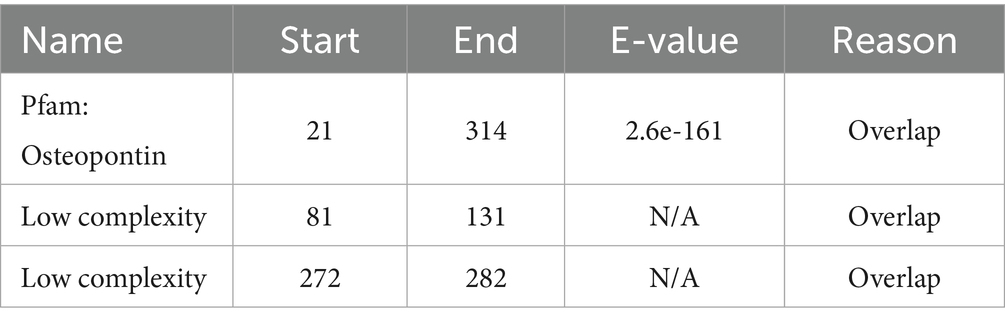
Figure 2. Domains within Homo sapiens protein SPP1 (OSTP_HUMAN, P10451). (A) Location of SPP1 on chromosome 4; (B) Schematic representation of SPP1; The red portion represents the signal peptide. The green portion represents the OSTEO domain. (C) AlphaFold Model of SPP1 (AF-P10451-F1), model confidence: dark blue: very high (pLDDT >90); light blue: confident (90 > pLDDT >70); yellow: low (70 > pLDDT >50); orange: very low (pLDDT <50), AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Some regions below 50 pLDDT may be unstructured in isolation.
Osteopontin, a multifunctional ECM protein, serves dual roles in bone remodeling by facilitating cell-matrix adhesion and in preventing pathological calcification. Baccarani believe that Osteopontin functions as a potent inhibitor of pathological calcification by localizing within elastic fibers of the aorta and skin, where it helps prevent mineral precipitation (29). Osteopontin expression is strongly and specifically induced by elevated extracellular phosphate levels, a product of alkaline phosphatase activity. Beck (30) found that Osteopontin expression is strongly and specifically induced by elevated extracellular phosphate levels, a product of alkaline phosphatase activity, elucidating the relationship between Osteopontin and phosphate. The molecular architecture of Osteopontin remains incompletely elucidated; however, its fundamental composition includes a signal peptide and an OSTEO domain., as shown in Figures 2B,C and Tables 1, 2.
SPP1 (Osteopontin), an essential stromal cell factor, facilitates EMT mechanism and ECM remodeling via many molecular signaling pathways. Osteopontin is predominantly secreted by a distinct SPP1+ macrophage subset located inside the disease microenvironment, and its mode of action commences post-secretion. Secreted Osteopontin then undergoes ligand-receptor interactions with integrins (such as ITGAV/ITGB1) and CD44 on the surfaces of target cells, such as fibroblasts, epithelial cells, and vascular smooth muscle cells. This binding triggers signaling by the ERM protein family or phosphorylated FAK-Src signaling. Downstream signaling converges on pro-fibrotic and pro-inflammatory pathways, including the TGF-β1/Smads, PI3K/AKT, and NF-κB axes. The TGF-β1/Smads pathway is a major driver of the fibrotic response and influences ECM dynamics, while the PI3K/AKT pathway promotes cellular activity, metabolic reprogramming, and the synthesis of specific proteins. These downstream pathways are extensively interconnected, forming a highly integrated and collaborative network. For example, the synergistic relationship between NF-κB and the TGF-β1/Smads pathways exists. Proinflammatory NF-κB signaling can enhance the TGF-β1-driven fibrotic response, while TGF-β1 itself can activate the NF-κB pathway in a non-canonical manner. The integration of these signals ultimately leads to the nuclear translocation of key transcription factors (such as NF-κB, the Smads complex, and β-catenin), which synergistically bind to the promoter regions of SPP1 and ECM-related genes. This transcriptional reprogramming induces physiological changes such as EMT mechanism, ECM remodeling, and myofibroblast activation, establishing a pathological positive feedback loop. The signaling mechanism of SPP1 (Osteopontin) is shown in Figure 3.
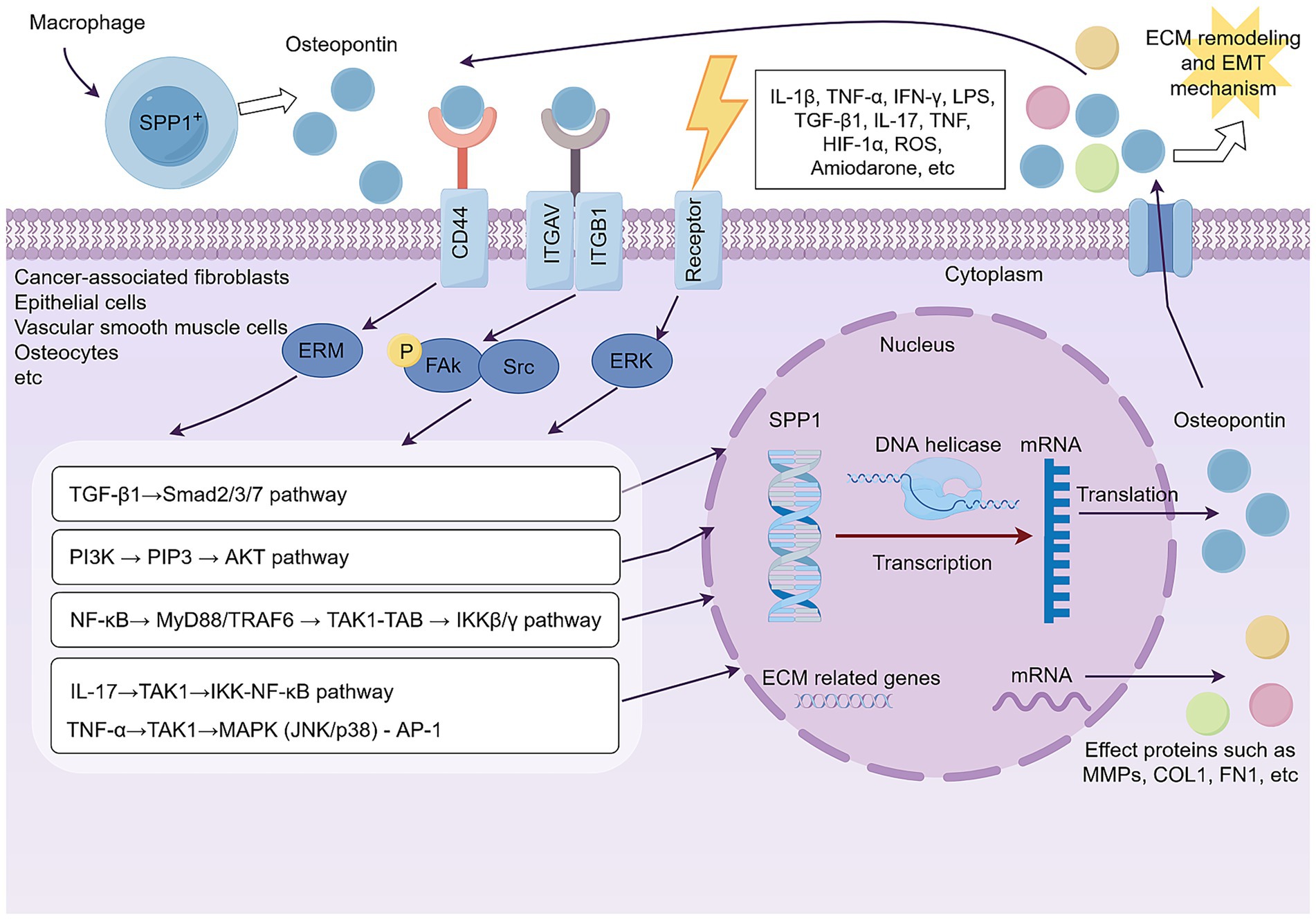
Figure 3. SPP1 (Osteopontin) secreted by SPP1+ macrophages orchestrate a downstream signaling network that converges to drive pathological ECM remodeling and EMT mechanism in target cells. By Figdraw.
3 Relationship between SPP1 (Osteopontin) and ECM dynamics in tumor growth and metastasis
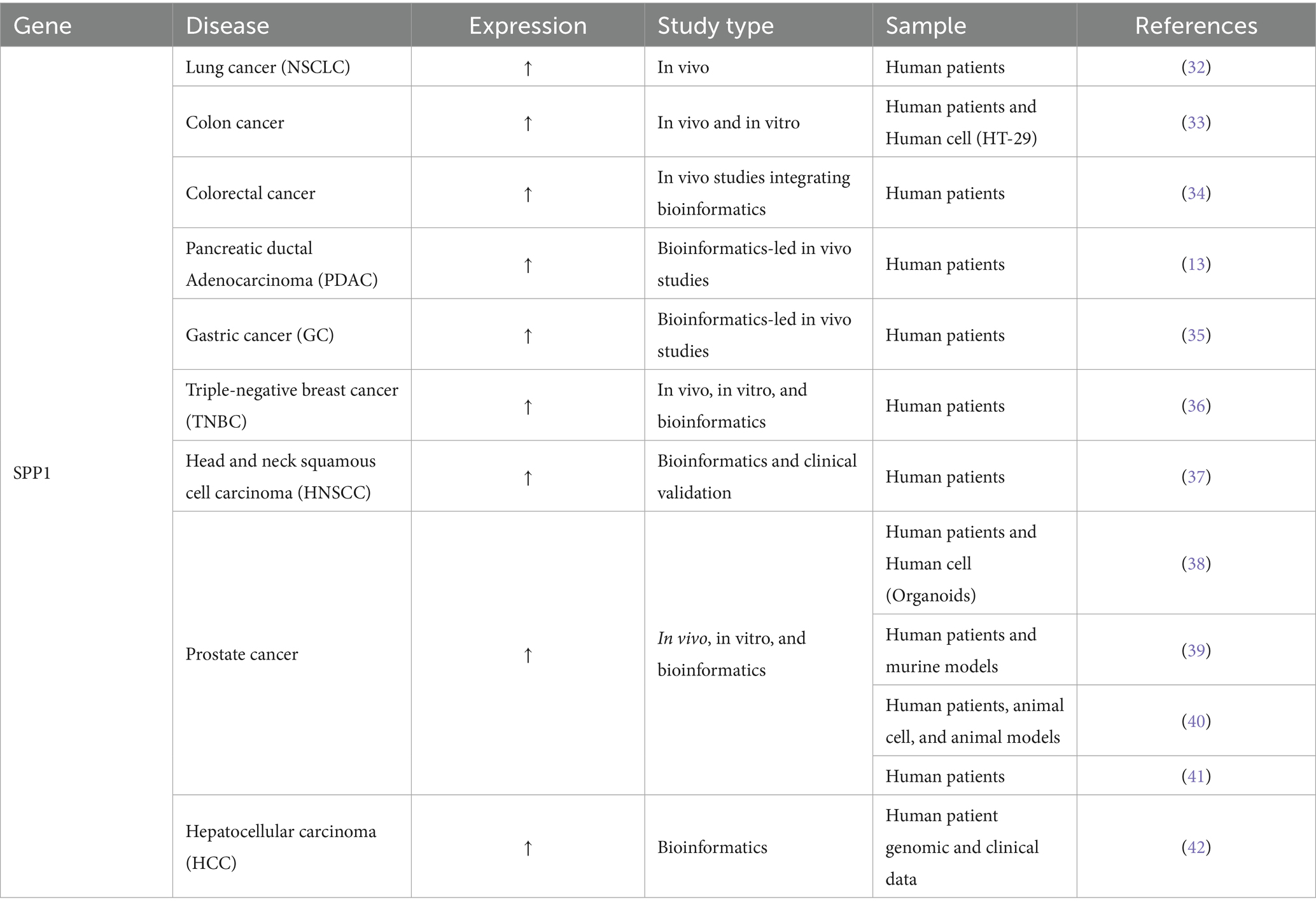
According to the 2025 Cancer Statistics report published by the American Cancer Society, data from the US national cancer registry and demographic analyses indicate a continuing rise in the overall cancer burden, with an estimated 2,041,910 new cancer cases projected in 2025 (31). Notably, the following cancer types exhibit significantly high incidence rates: lung cancer, colorectal cancer, pancreatic ductal adenocarcinoma, gastric cancer, breast cancer, head and neck squamous cell carcinoma, prostate cancer, and hepatocellular carcinoma. Extensive independent studies have demonstrated that SPP1, as an oncogene, promotes the progression of these malignancies, particularly through its role in mediating pathological remodeling of the ECM across these cancer types. Table 3 illustrates the function of SPP1 in tumor growth and metastasis.
Rouanne et al. (32) demonstrated through clinical cohort analysis that elevated serum levels of SPP1, an ECM protein, significantly correlate with tumor progression in non-small cell lung cancer (NSCLC). Their data revealed that each 50 ng/mL increase in serum SPP1 was associated with a 69% higher risk of metastasis (HR 1.69, 95% CI 1.12–2.56, p = 0.01) and 95% increased mortality risk (HR 1.95, 95% CI 1.15–3.32, p = 0.01), suggesting SPP1-mediated ECM dysregulation may drive malignant progression. Gómez de Segura et al. (33) demonstrated through integrated clinical and in vitro analyses that decreased expression of microfibril-associated glycoprotein-1 (MAGP-1, encoded by MFAP2 gene) in obesity-associated colon cancer leads to dysregulated TGF-β1 signaling and subsequent upregulation of SPP1. Their work revealed that SPP1-mediated ECM remodeling promotes collagen VI (COL6A3) and decorin (DCN) deposition, creating a fibrotic tumor microenvironment that physically excludes cytotoxic T lymphocytes (CTLs) and contributes to immune evasion. This study provides mechanistic insight into how metabolic dysregulation in obesity drives tumor progression through the SPP1-ECM axis (33). Through scRNA-seq analysis of liver tissues from Microsatellite stable metastatic-type metastatic colorectal cancer (MSS-mCRC), normal liver tissues, and PBMCs, Sathe et al. (34) revealed that SPP1+ macrophages in the metastatic tumor microenvironment (TME) secrete SPP1, which binds to integrin receptors (ITGAV/ITGB1) on cancer-associated fibroblasts (CAFs). This interaction activates CAFs to produce excessive collagen, ECM glycoproteins (e.g., FN1), and remodeling enzymes (e.g., MMPs, LOXL2), driving aberrant ECM deposition. The resulting dense ECM promotes MSS-mCRC progression and therapy resistance by impairing CD8+ T cell infiltration, increasing matrix stiffness to facilitate invasive metastasis, and inducing angiogenesis (34). Evan et al. demonstrated that SPP1 primarily promotes ECM deposition and EMT by orchestrating the crosstalk between TAMs and myofibroblastic cancer-associated fibroblasts (myCAFs). This interaction fosters an immunosuppressive tumor microenvironment (TME), ultimately driving the progression of pancreatic ductal adenocarcinoma (PDAC) (13). Thus, SPP1 emerges as a central regulator of tumor malignancy by orchestrating pathogenic ECM remodeling. Su et al. uncovered the heterogeneity of the tumor immune microenvironment (TiME) in gastric cancer (GC) patients with different mismatch repair (MMR) statuses. Their study highlights that the proficient MMR (pMMR) TiME is characterized by hypoxia, pro-angiogenic signaling, and ECM remodeling, driven by the presence of GC2 cells, SPP1+ macrophages, FAP + fibroblasts, and E2 endothelial cells. These findings are critical for developing targeted immunotherapies tailored to pMMR GC patients (35). By integrating single-cell transcriptomics, spatial multi-omics, and functional validation, Lu et al. elucidated a hypoxia-mediated immunosuppressive axis in triple-negative breast cancer (TNBC), wherein SPP1+ macrophages orchestrate tumor progression through dual secretion of SPP1 and TGF-β1. These cytokines directly program stromal fibroblasts to differentiate into ECM-producing CAFs (ecmCAFs), which in turn drive pathological ECM remodeling featuring excessive collagen deposition and stromal fibrosis (36). Through integrative bioinformatics analysis of multiple datasets (GSE6791, GSE29330, GSE58911), Cheon et al. identified SPP1 as a hub gene significantly upregulated in head and neck squamous cell carcinoma (HNSCC). Their study demonstrated that SPP1 expression is directly associated with functional enrichment of ECM organization and degradation processes. Clinically, elevated SPP1 expression correlated with advanced tumor grade, progressive clinical stage, and poor prognosis in HNSCC patients (37). Pang et al. established SPP1 as a pivotal molecular nexus linking ECM remodeling to metastatic castration-resistant progression in prostate cancer (mCRPC). Their integrative multi-omics and organoid approach revealed that SPP1 orchestrates a feedforward loop: it not only activates Androgen receptor (AR) signaling to promote therapy resistance but also directly mediates ECM reorganization through collagen crosslinking and fibronectin assembly. This dual functionality creates a permissive microenvironment for metastatic dissemination, positioning SPP1-ECM crosstalk as a promising therapeutic target in mCRPC (38). Similarly, the role of SPP1 in prostate cancer has been confirmed in several other studies (39–41). Based on molecular subtyping of the ECM in the TCGA-LIHC cohort, SPP1 was identified as a core gene for constructing an ECM-related prognostic model in hepatocellular carcinoma (HCC). High SPP1 expression marked pathological ECM remodeling features and was significantly associated with advanced histological grade, resistance to immunotherapy, and poor prognosis. These findings provide a mechanistic rationale for targeting the SPP1-ECM axis to reprogram the immunosuppressive tumor microenvironment in HCC (42). In summary, SPP1 acts as a pivotal oncogenic driver by orchestrating pathological remodeling of the ECM during tumorigenesis and progression across multiple malignancies. Cross-cancer analyses demonstrate that SPP1-mediated collagen deposition, stromal fibrosis, and integrin-dependent ECM receptor signaling collectively fuel metastatic dissemination and therapy resistance.
4 Relationship between SPP1 (Osteopontin) and ECM dynamics in cardiovascular disease
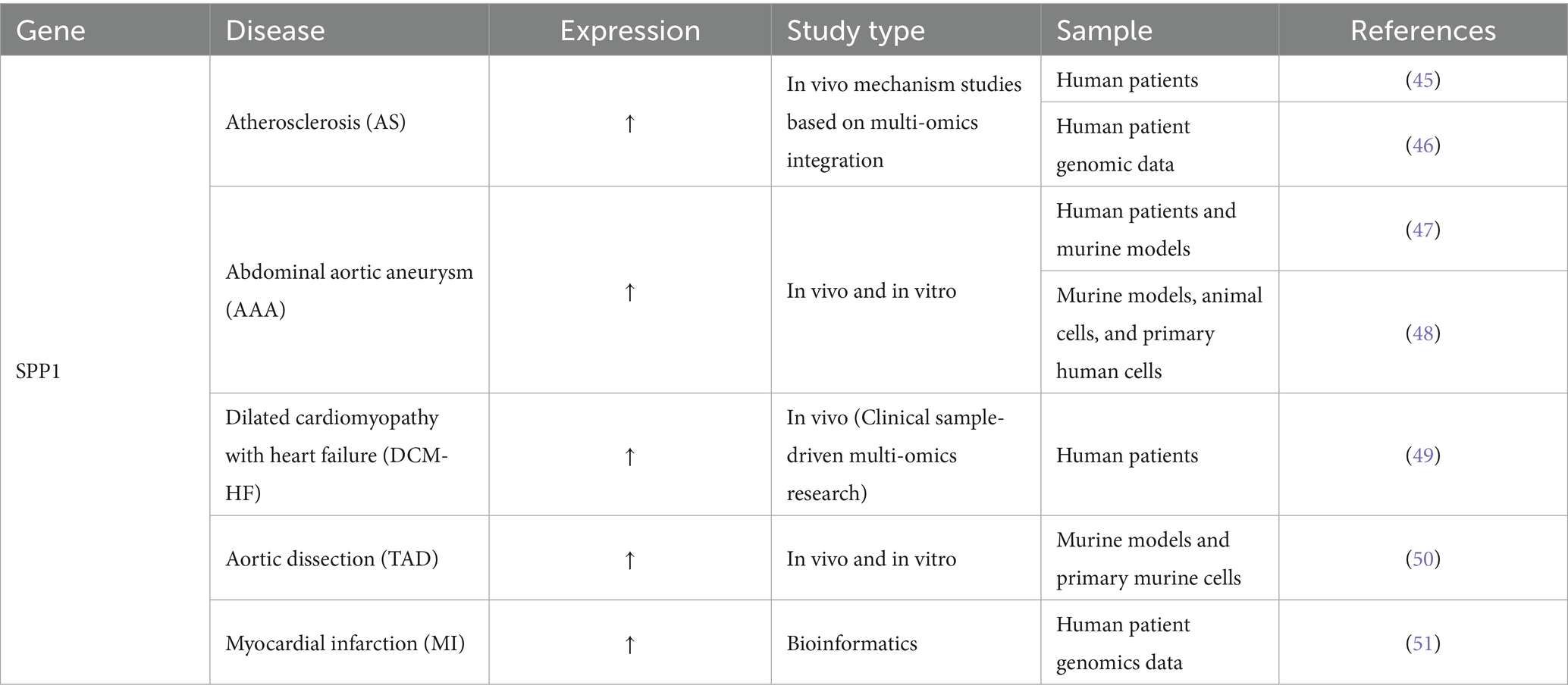
Cardiovascular disease (CVD) is the leading cause of death worldwide, accounting for approximately 18.5 million deaths (9.6 million men and 8.9 million women), or about one-third of all deaths globally (43). CVD is also the leading cause of death in China, accounting for nearly 4 million deaths, highlighting its enormous disease burden and risk of premature death, which is on the rise year by year (44). Numerous studies suggest that SPP1 may contribute to the development and progression of cardiovascular diseases, including atherosclerosis, abdominal aortic aneurysm, dilated cardiomyopathy with heart failure, thoracic aortic dissection, and myocardial infarction, via the EMT pathway. Table 4 illustrates the function of SPP1 in Cardiovascular Disease.
Single-cell and spatial analyses revealed a significant interaction between SPP1+ macrophages and ITLN1+ foam cells, mediated by the SPP1-CD44 ligand-receptor axes, which accelerates arterial lipid accumulation and EMT transformation, which is necessary to develop effective immunotherapeutic strategies against atherosclerosis (AS) (45). LASSO regression and SVM-REF also verified the pivotal role of SPP1 in atherosclerosis (46). Markus et al. found that platelets play a key role in promoting the formation of abdominal aortic aneurysm (AAA) by regulating inflammation and degrading the ECM. Platelets are responsible for upregulating the expression of the SPP1 gene in macrophages and aortic tissue, which triggers inflammation and remodeling, while promoting platelet adhesion and migration to the abdominal aortic wall and intraluminal thrombus (ILT) (47). It was found that SPP1 is upregulated in vascular smooth muscle cells (VSMCs) induced by Di-(2-ethylhexyl) phthalate (DEHP), and its phenotypic switch is significantly accelerated, indicating that M1 macrophage polarization and VSMC phenotypic switch can exacerbate the progression of AAA, which also involves the EMT process (48). Furthermore, A study analyzed the expression levels of cardiovascular-related proteins in patients with dilated cardiomyopathy with heart failure (DCM-HF) (n = 20) and healthy controls (Normal) (n = 18). Using Olink proteomics analysis, five key proteins, including SPP1, were identified and validated in human serum samples via ELISA, indicating that SPP1 is equally important in DCM-HF and may be involved in the EMT mechanism of the disease (49). Similarly, Suwei et al. established a thoracic aortic dissection (TAD) mouse model by perfusing angiotensin (Ang) II into mice administered β-aminopropionitrile. Through mouse experiments, they confirmed that upregulation of SPP1 is a key sign of the pathological phenotype transition of aortic smooth muscle cells from a contractile state to a synthetic state, and IGFBP3 can directly inhibit this process, thereby maintaining vascular homeostasis (50). Finally, GEO database analysis showed that SPP1, as a biomarker for myocardial infarction (MI), participated in fibroblast proliferation and myocardial remodeling, and was also involved in the EMT mechanism (51). In summary, SPP1 coordinates immune cell interactions, drives cell phenotypic transformation, and promotes pathological tissue remodeling, thereby affecting ECM dynamics and becoming a common pathogenic factor in the progression of multiple cardiovascular diseases.
5 Relationship between SPP1 (Osteopontin) and ECM dynamics in pulmonary disease
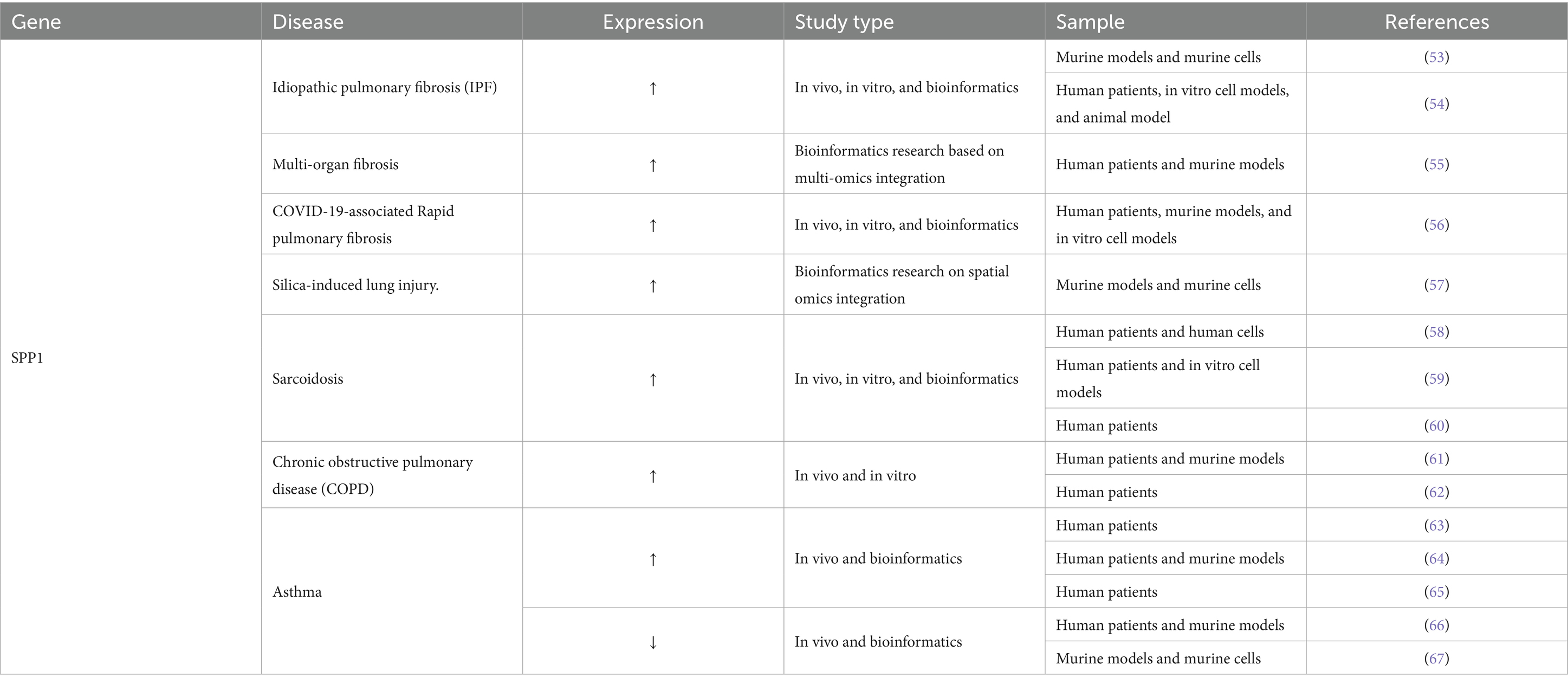
Data from the Worldwide Burden of Disease Study indicate that chronic respiratory disorders (CRDs) pose a significant global health challenge. Annually, they result in the deaths of 4.4 million individuals and impact approximately 468 million people. From 1990 to 2021, the age-standardized prevalence rate decreased by 1.01%, whereas it increased by 0.20% during the pandemic from 2019 to 2021, exhibiting significant regional disparities in risk factors (52). Numerous studies indicate that SPP1-mediated ECM remodeling and EMT mechanisms are the primary determinants in the onset of several chronic lung diseases, including pulmonary fibrosis, silicosis, sarcoidosis, and chronic airway diseases. Table 5 illustrates the function of SPP1 in Pulmonary Disease.
Single-cell analysis verified that monocyte-derived interstitial macrophages (Mo-IMs) exhibit a pro-fibrotic phenotype in the initial stages of pulmonary fibrosis and engage with fibroblasts via the SPP1 signaling pathway, facilitating EMT and ECM deposition, thereby advancing disease progression (53). Conversely, knockdown of SPP1 expression inhibits macrophage-induced EMT in epithelial cells and fibroblasts. In vivo treatment with an SPP1 inhibitor enhances lung function and ameliorates idiopathic pulmonary fibrosis (IPF). Inhibiting SPP1 expression in vivo effectively mitigates the progression of IPF, suggesting that SPP1 in macrophages may be a potential therapeutic target for IPF (54). Furthermore, SPP1+ macrophages demonstrate a conserved matrisome-associated macrophage (MAM) polarization in multi-organ fibrosis, particularly in pulmonary and liver fibrosis, directly facilitating fibrosis via ECM remodeling and metabolic reprogramming, thereby uncovering novel targets for cross-tissue intervention (55). Integrated multi-omics analyses of COVID-19-associated Rapid pulmonary fibrosis (RPF) patients and murine models reveal that CD163+ macrophages drive rapid pulmonary fibrosis progression via SPP1 secretion, identifying the CD163+-SPP1 axis as a potential therapeutic target (56). Tao et al. (57) identified a unique stress-induced epithelial subset (C0) that enhances immunological crosstalk, activates EMT pathways, increases ECM deposition, and facilitates tissue remodeling in silica-induced lung damage via the SPP1-CD44 signaling pathway. Therefore, SPP1, as a common molecular hub connecting immune cells, epithelial cells and ECM remodeling, plays a core role in the process of pulmonary fibrosis driven by different causes. SPP1 is raised in the blood and granulomatous tissue of individuals with sarcoidosis, elucidating the EMT pathway in disease development (58, 59). Research indicates that single-nucleotide polymorphisms (SNPs) in the SPP1 gene are strongly associated with susceptibility to pulmonary lesions in sarcoidosis, which also encompasses the EMT pathway (60). In chronic obstructive pulmonary disease (COPD), SPP1 expression is markedly increased, particularly in airway cells and antigen-presenting cells of patients with emphysema. This elevation facilitates Th1/Th17-mediated inflammation and Th2-mediated inflammatory responses, promotes neutrophil recruitment, and leads to MMP9-dependent tissue destruction (61, 62). SPP1 expression is increased in serum, sputum, and bronchial tissue in asthma, correlating with disease severity, late-onset asthma, and airway remodeling (63). It can promote Th2 inflammation, smooth muscle proliferation, and collagen deposition through pathways such as PI3K/AKT, thus exacerbating the disease (64, 65), while also exhibiting anti-inflammatory properties at specific stages. In a house dust mite (HDM) induced allergic asthma mouse model, SPP1/Osteopontin was shown to significantly enhance the host’s ability to defend against Streptococcus pneumoniae infection by inhibiting airway inflammatory cell infiltration, alleviating tissue damage, and reducing proinflammatory cytokine levels, as manifested by a significant reduction in bacterial load in alveolar lavage fluid and lung tissue (66). Furthermore, Samitas (67) found that SPP1/Osteopontin plays a critical role in the modulation of allergic asthma by preserving the homeostasis of the gut-lung axis. Its deficiency leads to increased airway inflammation, which is associated with gut barrier dysfunction, microbiota dysbiosis, and the PD-1/PD-L1-mediated disruption of the Treg/Th17 balance, as evidenced by fecal microbiota transplantation and pathway analysis. Therefore, SPP1 plays a dual role in enabling ECM remodeling and EMT processes in asthma and COPD. In summary, SPP1 constitutes a common core pathogenic mechanism of various chronic lung diseases by driving ECM remodeling, EMT and immune inflammation regulation.
6 Relationship between SPP1 (Osteopontin) and ECM dynamics in kidney disease
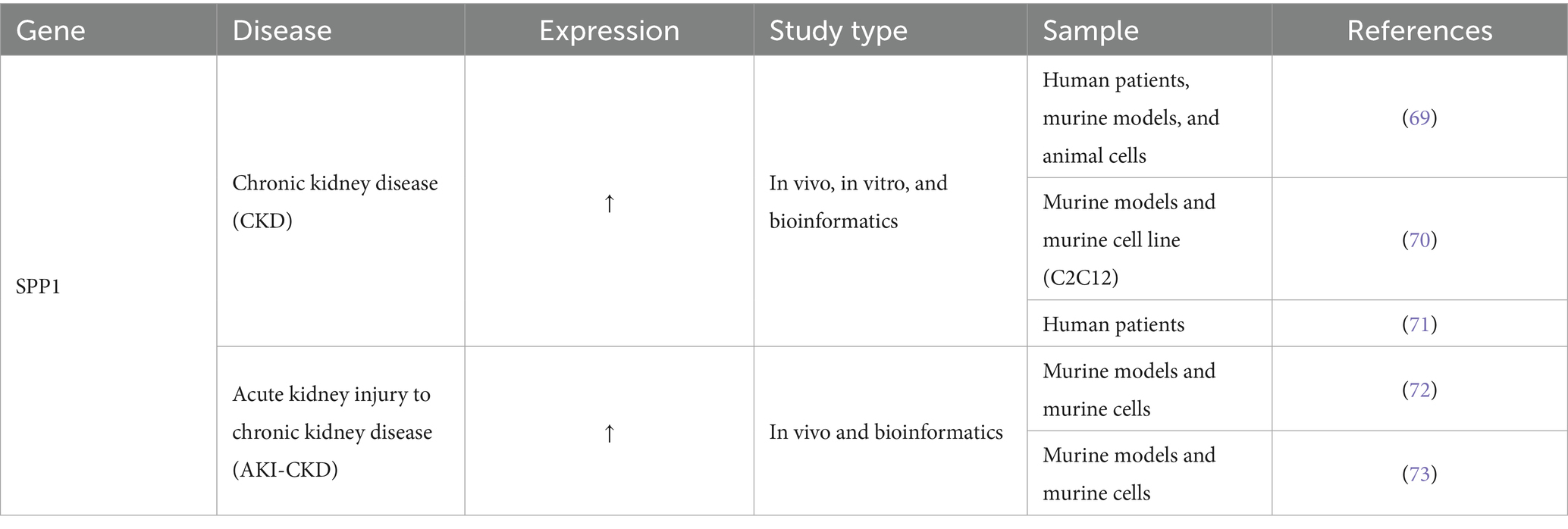
The Lancet Global Burden of Disease Study indicates that chronic kidney disease (CKD) impacts over 700 million individuals globally (prevalence rate 9.1%), ranks as the 12th greatest cause of mortality (constituting 4.6% of global deaths), and represents a significant threat to human health (68). SPP1 affects the occurrence, development, and prognosis of CKD by affecting ECM dynamics, and is also important in the process of acute kidney injury to chronic kidney disease (AKI-CKD) transformation. Table 6 illustrates the function of SPP1 in Kidney Disease. Table 6 illustrates the function of SPP1 in Kidney Disease.
CKD is defined as a syndrome marked by a sustained reduction in glomerular filtration rate lasting at least three months, accompanied by tubular atrophy, interstitial fibrosis, and advancing systemic complications. The pathological progression is influenced by epigenetic regulation and intercellular vesicle signaling. Research indicates that circulating small extracellular vesicles (sEVs) originating from CKD promote pathological calcification of vascular smooth muscle cells (VSMCs) by depleting protective miRNAs, which in turn releases the inhibition of VEGFA signaling, marked by a significant upregulation of genes such as SPP1 (69). An additional investigation revealed the significant function of SPP1 in modulating ECM dynamics in chronic kidney disease, accompanied by sarcopenia. In animal experiments on CKD, increased secretion of SPP1 by the kidneys, which circulates to skeletal muscle, directly activating the expression of the muscle atrophy marker Murf-1 and promoting smaller myotubes. Pharmacological inhibition of SPP1 in vivo significantly increases the weight of the gastrocnemius and tibialis anterior muscles, improves the atrophy phenotype, and reprograms the muscle transcriptome, thereby confirming SPP1 as the central pathogenic factor and therapeutic target in the CKD-muscle axis (70). Another study involving the European CKD population genome confirmed that genetic variation upstream of the SPP1 influences the progression of CKD by directly regulating Osteopontin expression levels. This mechanism has been validated through rare variant aggregation analysis and multi-level cross-cohort assessments (71). Furthermore, SPP1 is integral to the progression from acute kidney injury to chronic kidney disease (AKI-CKD). Research in single-cell transcriptomics has identified SPP1 as a crucial hub molecule in polyploid cells during the transition from acute kidney injury to chronic kidney disease, involving the EMT mechanism. In vivo gene deletion of SPP1 enhances renal fibrosis via influencing ECM dynamics, substantiating the targeting of SPP1 to impede the advancement of renal fibrosis (72). A related study confirmed that in NF-κB deficient mouse models, the expression of the key inflammatory factor SPP1 in proximal tubule cells (FR-PTCs) was significantly reduced, resulting in decreased pathological damage associated with AKI-CKD (73). This establishes a molecular foundation for the inhibition of SPP1 and other NF-κB effector molecules to impede the advancement of AKI-CKD. In summary, SPP1 establishes its core position in CKD and AKI-CKD transformation by driving ECM remodeling and affecting ECM dynamics, and is a highly potential pleiotropic therapeutic target.
7 Relationship between SPP1 (Osteopontin) and ECM dynamics in osteoarthritis
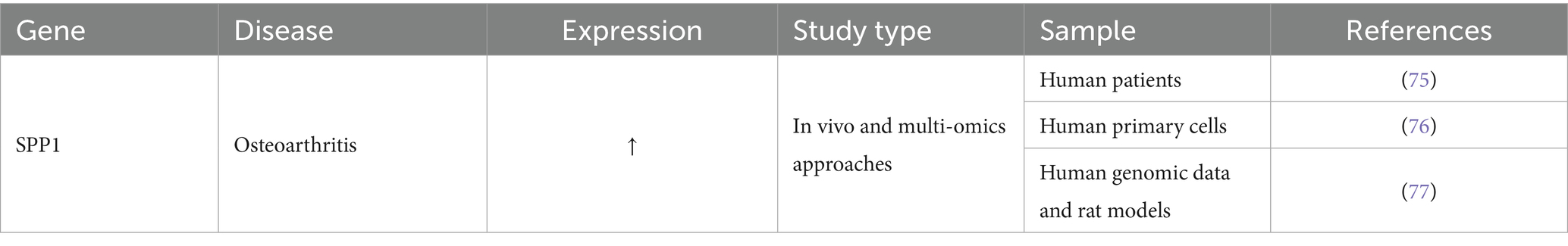
Osteoarthritis (OA) is a very debilitating chronic joint disorder marked by the progressive deterioration of articular cartilage, synovial inflammation, and remodeling of subchondral bone. The pathogenic process is influenced by an imbalance of mechanical stress, immunological metabolic abnormalities, and hereditary variables. The China OA Disease Burden Study (1990–2021) revealed that the age-standardized incidence rate (ASIR), prevalence rate (ASPR), and disability-adjusted life rate (ASDR) showed a continuous upward trend, and are predicted to continue to rise by 2050 (74). Table 7 illustrates the function of SPP1 in OA.
He et al. discovered through multi-omics integrated analysis and machine learning that SPP1 serves as a pivotal immunological metabolic hub gene in OA progression, instigating the aberrant activation of EMT-related pathways. PCR verification indicated considerable overexpression, and the predictive model developed as a key marker may effectively distinguish OA subtypes (75). Another study utilizing a simulated microgravity model of the human meniscus discovered that mechanical unloading of the knee articular cartilage initiates the inflammation-calcification cascade by specifically upregulating SPP1 expression in chondrocytes, highlighting its sex-specific regulatory role as a central factor in the perception of mechanical stress and subsequent pathological transformation (76). Similarly, Yang et al. found that SPP1, as a core molecule of OA, drives EMT by synergizing with the IL-17/TNF inflammatory pathway, providing a new intervention framework for targeting SPP1 to regulate OA immune metabolism (77). Unfortunately, most studies on the role of SPP1 in osteoarthritis are based on bioinformatics analysis, with few in vivo experiments. We look forward to subsequent researchers conducting more in vivo studies to provide more evidence that SPP1 affects osteoarthritis through ECM dynamics. Therefore, SPP1, as a hub molecule connecting mechanical stress, inflammatory signals and ECM remodeling, plays a core role in the progression of osteoarthritis, but its precise mechanism requires additional validation through in vivo research.
8 Conclusion
SPP1 (Osteopontin) serves as a principal regulator of aberrant ECM remodeling and is prevalent in numerous conditions, including cancer, cardiovascular illness, pulmonary disease, chronic renal disease, and osteoarthritis. SPP1+ macrophages are pivotal biological agents of ECM dysregulation, facilitating collagen deposition, fibroblast activation, immunological modulation, and EMT. Targeting SPP1 and its interactions with ECM components presents a possible therapeutic approach to alleviate fibrosis, immunological evasion, and tissue dysfunction. Future research should concentrate on verifying these pathways in vivo, particularly in osteoarthritis, and enhancing the therapeutic use of SPP1-targeted medicines.
Author contributions
YL: Writing – original draft. XY: Writing – review & editing. RF: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported in part by the National Natural Science Foundation of China (81874442); and Special fund for Taishan Scholars’ construction project (ts201712096). Jointly Constructed Project by the Bureau and the Province (GZY-KJS-SD-2023-044).
Acknowledgments
The authors extend profound gratitude to Professor Wei Zhang for his invaluable guidance throughout this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ogbureke, KU, and Fisher, LW. Sibling expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. (2007) 55:17210923:403–9. doi: 10.1369/jhc.6A7075.2007
2. Bellahcène, A, Castronovo, V, Ogbureke, KU, Fisher, LW, and Fedarko, NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. (2008) 8:212–26. doi: 10.1038/nrc2345
3. Kiefer, MC, Bauer, DM, and Barr, PJ. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. (1989) 17:3306. doi: 10.1093/nar/17.8.3306
4. Young, MF, Kerr, JM, Termine, JD, Wewer, UM, Wang, MG, McBride, OW, et al. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. (1990) 7:491–502. doi: 10.1016/0888-7543(90)90191-v
5. Kohri, K, Suzuki, Y, Yoshida, K, Yamamoto, K, Amasaki, N, Yamate, T, et al. Molecular cloning and sequencing of cDNA encoding urinary stone protein, which is identical to osteopontin. Biochem Biophys Res Commun. (1992) 184:859–64. doi: 10.1016/0006-291x(92)90669-c
6. Kohri, K, Nomura, S, Kitamura, Y, Nagata, T, Yoshioka, K, Iguchi, M, et al. Structure and expression of the mRNA encoding urinary stone protein (osteopontin). J Biol Chem. (1993) 268:15180–4.
7. Ogbureke, KU, and Fisher, LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int. (2005) 68:155–66. doi: 10.1111/j.1523-1755.2005.00389.x
8. Shinohara, ML, Kim, HJ, Kim, JH, Garcia, VA, and Cantor, H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. (2008) 105:7235–9. doi: 10.1073/pnas.0802301105
9. Zhang, X, Yang, F, Han, L, Su, Q, Gao, Y, Wu, R, et al. Identification of pivotal genes and crucial pathways in liver fibrosis through WGCNA analysis. Technol Health Care. (2025) 33:431–48. doi: 10.3233/thc-241142
10. Palma, A. The landscape of SPP1(+) macrophages across tissues and diseases: a comprehensive review. Immunology. (2025) 176:179–96. doi: 10.1111/imm.13952
11. Sica, A, and Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. doi: 10.1172/jci59643
12. Wang, Y, Wang, Q, Tao, S, Li, H, Zhang, X, Xia, Y, et al. Identification of SPP1(+) macrophages in promoting cancer stemness via vitronectin and CCL15 signals crosstalk in liver cancer. Cancer Lett. (2024) 604:217199. doi: 10.1016/j.canlet.2024.217199
13. Li, E, Cheung, HCZ, and Ma, S. CTHRC1(+) fibroblasts and SPP1(+) macrophages synergistically contribute to pro-tumorigenic tumor microenvironment in pancreatic ductal adenocarcinoma. Sci Rep. (2024) 14:17412. doi: 10.1038/s41598-024-68109-z
14. Su, X, Liang, C, Chen, R, and Duan, S. Deciphering tumor microenvironment: CXCL9 and SPP1 as crucial determinants of tumor-associated macrophage polarity and prognostic indicators. Mol Cancer. (2024) 23:13. doi: 10.1186/s12943-023-01931-7
15. van Baarle, L, De Simone, V, Schneider, L, Santhosh, S, Abdurahiman, S, Biscu, F, et al. IL-1R signaling drives enteric glia-macrophage interactions in colorectal cancer. Nat Commun. (2024) 15:6079. doi: 10.1038/s41467-024-50438-2
16. Bi, W, Yang, M, Shi, M, Hou, M, Jiang, C, Fan, G, et al. A comprehensive single-cell RNA transcriptomic analysis identifies a unique SPP1+ macrophages subgroup in aging skeletal muscle. Sci Rep. (2024) 14:18156. doi: 10.1038/s41598-024-69284-9
17. Murthy, S, Karkossa, I, Schmidt, C, Hoffmann, A, Hagemann, T, Rothe, K, et al. Danger signal extracellular calcium initiates differentiation of monocytes into SPP1/osteopontin-producing macrophages. Cell Death Dis. (2022) 13:53. doi: 10.1038/s41419-022-04507-3
18. Dräger, NM, Sattler, SM, Huang, CT, Teter, OM, Leng, K, Hashemi, SH, et al. A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states. Nat Neurosci. (2022) 25:1149–62. doi: 10.1038/s41593-022-01131-4
19. De Schepper, S, Ge, JZ, Crowley, G, Ferreira, LSS, Garceau, D, Toomey, CE, et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer's disease. Nat Neurosci. (2023) 26:406–15. doi: 10.1038/s41593-023-01257-z
20. Wendisch, D, Dietrich, O, Mari, T, von Stillfried, S, Ibarra, IL, Mittermaier, M, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. (2021) 184:6243–6261.e27. doi: 10.1016/j.cell.2021.11.033
21. Uezumi, A, Ito, T, Morikawa, D, Shimizu, N, Yoneda, T, Segawa, M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. (2011) 124:3654–64. doi: 10.1242/jcs.086629
22. Reggio, A, Fuoco, C, Deodati, R, and Palma, A. SPP1 macrophages across diseases: a call for reclassification? FASEB J. (2025) 39:e70448. doi: 10.1096/fj.202403227R
23. Jung, S, Ha, J, Park, JH, and Yoo, KH. Decoding SPP1 regulation: genetic and nongenetic insights into its role in disease progression. Mol Cells. (2025) 48:100215. doi: 10.1016/j.mocell.2025.100215
24. Whyte, MP, Amalnath, SD, McAlister, WH, McKee, MD, Veis, DJ, Huskey, M, et al. Hypophosphatemic osteosclerosis, hyperostosis, and enthesopathy associated with novel homozygous mutations of DMP1 encoding dentin matrix protein 1 and SPP1 encoding osteopontin: the first digenic SIBLING protein osteopathy? Bone. (2020) 132:115190. doi: 10.1016/j.bone.2019.115190
25. Crosby, AH, Lyu, MS, Lin, K, McBride, OW, Kerr, JM, Aplin, HM, et al. Mapping of the human and mouse bone sialoprotein and osteopontin loci. Mamm Genome. (1996) 7:149–51. doi: 10.1007/s003359900037
26. Stelzer, G, Rosen, N, Plaschkes, I, Zimmerman, S, Twik, M, Fishilevich, S, et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. (2016) 54:1.30.1–1.30.33. doi: 10.1002/cpbi.5
27. Crosby, AH, Edwards, SJ, Murray, JC, and Dixon, MJ. Genomic organization of the human osteopontin gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics. (1995) 27:155–60. doi: 10.1006/geno.1995.1018
28. Morse, C, Tabib, T, Sembrat, J, Buschur, KL, Bittar, HT, Valenzi, E, et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J. (2019) 54:1802441. doi: 10.1183/13993003.02441-2018
29. Baccarani-Contri, M, Taparelli, F, and Pasquali-Ronchetti, I. Osteopontin is a constitutive component of normal elastic fibers in human skin and aorta. Matrix Biol. (1995) 14:553–60. doi: 10.1016/s0945-053x(05)80004-6
30. Beck, GR Jr, Zerler, B, and Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. (2000) 97:8352–7. doi: 10.1073/pnas.140021997
31. Siegel, RL, Kratzer, TB, Giaquinto, AN, Sung, H, and Jemal, A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
32. Rouanne, M, Adam, J, Goubar, A, Robin, A, Ohana, C, Louvet, E, et al. Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer. (2016) 16:483. doi: 10.1186/s12885-016-2541-5
33. Gómez de Segura, I, Ahechu, P, Gómez-Ambrosi, J, Rodríguez, A, Ramírez, B, Becerril, S, et al. Decreased Levels of Microfibril-Associated Glycoprotein (MAGP)-1 in Patients with Colon Cancer and obesity are associated with changes in extracellular matrix Remodelling. Int J Mol Sci. (2021) 22:8485. doi: 10.3390/ijms22168485
34. Sathe, A, Mason, K, Grimes, SM, Zhou, Z, Lau, BT, Bai, X, et al. Colorectal Cancer metastases in the liver establish immunosuppressive spatial networking between tumor-associated SPP1+ macrophages and fibroblasts. Clin Cancer Res. (2023) 29:244–60. doi: 10.1158/1078-0432.Ccr-22-2041
35. Su, Z, He, Y, You, L, Chen, J, Zhang, G, and Liu, Z. SPP1+ macrophages and FAP+ fibroblasts promote the progression of pMMR gastric cancer. Sci Rep. (2024) 14:26221. doi: 10.1038/s41598-024-76298-w
36. Lu, X, Gou, Z, Chen, H, Li, L, Chen, F, Bao, C, et al. Extracellular matrix cancer-associated fibroblasts promote stromal fibrosis and immune exclusion in triple-negative breast cancer. J Pathol. (2025) 265:385–99. doi: 10.1002/path.6395
37. Cheon, J, Kim, B, Park, J, Shin, J, and Kim, TH. Unveiling biomarkers in head and neck squamous cell carcinoma through bioinformatics: the role of SPP1 and KRT78. Int J Mol Sci. (2024) 25:12062. doi: 10.3390/ijms252212062
38. Pang, X, Xie, R, Zhang, Z, Liu, Q, Wu, S, and Cui, Y. Identification of SPP1 as an extracellular matrix signature for metastatic castration-resistant prostate Cancer. Front Oncol. (2019) 9:924. doi: 10.3389/fonc.2019.00924
39. Mei, S, Zhang, H, Hirz, T, Jeffries, NE, Xu, Y, Baryawno, N, et al. Single-cell and spatial Transcriptomics reveal a tumor-associated macrophage subpopulation that mediates prostate Cancer progression and metastasis. Mol Cancer Res. (2025) 23:653–65. doi: 10.1158/1541-7786.Mcr-24-0791
40. Sanchis, P, Sabater, A, Lechuga, J, Rada, J, Seniuk, R, Pascual, G, et al. PKA-driven SPP1 activation as a novel mechanism connecting the bone microenvironment to prostate cancer progression. Oncogene. (2025) 44:3568–79. doi: 10.1038/s41388-025-03511-z
41. Wu, T, Li, X, Zheng, F, Liu, H, and Yu, Y. Intercellular communication between FAP+ fibroblasts and SPP1+ macrophages in prostate cancer via multi-omics. Front Immunol. (2025) 16:1560998. doi: 10.3389/fimmu.2025.1560998
42. Tang, H, You, T, Sun, Z, Bai, C, and Wang, Y. Extracellular matrix-based gene expression signature defines two prognostic subtypes of hepatocellular carcinoma with different immune microenvironment characteristics. Front Mol Biosci. (2022) 9:839806. doi: 10.3389/fmolb.2022.839806
43. Roth, GA, Mensah, GA, and Fuster, V. The global burden of cardiovascular diseases and risks: a compass for global action. J Am Coll Cardiol. (2020) 76:2980–1. doi: 10.1016/j.jacc.2020.11.021
44. Liu, S, Li, Y, Zeng, X, Wang, H, Yin, P, Wang, L, et al. Burden of cardiovascular diseases in China, 1990-2016: findings from the 2016 global burden of disease study. JAMA Cardiol. (2019) 4:342–52. doi: 10.1001/jamacardio.2019.0295
45. Li, Y, Wang, S, Zhang, R, Gong, Y, Che, Y, Li, K, et al. Single-cell and spatial analysis reveals the interaction between ITLN1(+) foam cells and SPP1(+) macrophages in atherosclerosis. Front Cardiovasc Med. (2025) 12:1510082. doi: 10.3389/fcvm.2025.1510082
46. Wu, X, Pan, X, Zhou, Y, Pan, J, Kang, J, Yu, JJJ, et al. Identification of key genes for atherosclerosis in different arterial beds. Sci Rep. (2024) 14:6543. doi: 10.1038/s41598-024-55575-8
47. Wagenhäuser, MU, Mulorz, J, Krott, KJ, Bosbach, A, Feige, T, Rhee, YH, et al. Crosstalk of platelets with macrophages and fibroblasts aggravates inflammation, aortic wall stiffening, and osteopontin release in abdominal aortic aneurysm. Cardiovasc Res. (2024) 120:417–32. doi: 10.1093/cvr/cvad168
48. Wang, Y, Qiu, M, Yue, B, Wang, M, Xia, L, Li, F, et al. Mechanistic insight into plasticizer di-(2-ethylhexyl) phthalate as an environmental hazard for abdominal aortic aneurysm: evidence from in vitro and in vivo studies. Ecotoxicol Environ Saf. (2025) 302:118689. doi: 10.1016/j.ecoenv.2025.118689
49. Xu, S, Zhang, G, Tan, X, Zeng, Y, Jiang, H, Jiang, Y, et al. Plasma Olink proteomics reveals novel biomarkers for prediction and diagnosis in dilated cardiomyopathy with heart failure. J Proteome Res. (2024) 23:4139–50. doi: 10.1021/acs.jproteome.4c00522
50. Chen, S, Chen, H, Zhong, Y, Ge, Y, Li, C, Qiao, Z, et al. Insulin-like growth factor-binding protein 3 inhibits angiotensin II-induced aortic smooth muscle cell phenotypic switch and matrix metalloproteinase expression. Exp Physiol. (2020) 105:1827–39. doi: 10.1113/ep088927
51. Wu, ATH, Lawal, B, Tzeng, YM, Shih, CC, and Shih, CM. Identification of a novel Theranostic signature of metabolic and immune-inflammatory dysregulation in myocardial infarction, and the potential therapeutic properties of Ovatodiolide, a Diterpenoid derivative. Int J Mol Sci. (2022) 23:1281. doi: 10.3390/ijms23031281
52. Cao, Z, He, L, Luo, Y, Tong, X, Zhao, J, Huang, K, et al. Burden of chronic respiratory diseases and their attributable risk factors in 204 countries and territories, 1990-2021: results from the global burden of disease study 2021. Chin Med J Pulm Crit Care Med. (2025) 3:100–10. doi: 10.1016/j.pccm.2025.05.005
53. Wang, S, Li, J, Wu, C, Lei, Z, Wang, T, Huang, X, et al. Single-cell RNA sequencing reveals monocyte-derived interstitial macrophages with a pro-fibrotic phenotype in Bleomycin-induced pulmonary fibrosis. Int J Mol Sci. (2024) 25:11669. doi: 10.3390/ijms252111669
54. Yang, X, Liu, Z, Zhou, J, Guo, J, Han, T, Liu, Y, et al. SPP1 promotes the polarization of M2 macrophages through the Jak2/Stat3 signaling pathway and accelerates the progression of idiopathic pulmonary fibrosis. Int J Mol Med. (2024) 54:89. doi: 10.3892/ijmm.2024.5413
55. Ouyang, JF, Mishra, K, Xie, Y, Park, H, Huang, KY, Petretto, E, et al. Systems level identification of a matrisome-associated macrophage polarisation state in multi-organ fibrosis. eLife. (2023) 12:12. doi: 10.7554/eLife.85530
56. Ding, W, Deng, S, Wang, Z, Chen, X, Xu, Z, Zhong, J, et al. CD163(+) macrophages drive rapid pulmonary fibrosis via osteopontin secretion. Int Immunopharmacol. (2025) 161:114976. doi: 10.1016/j.intimp.2025.114976
57. Wang, T, Hao, J, Li, B, Hyraht, A, Wang, J, Xia, H, et al. Single-cell and spatial transcriptomics reveal a stress-induced EMT-like epithelial subset driving immune activation in silica-injured lung. Front Immunol. (2025) 16:1609616. doi: 10.3389/fimmu.2025.1609616
58. Lavi, H, Assayag, M, Schwartz, A, Arish, N, Fridlender, ZG, and Berkman, N. The association between osteopontin gene polymorphisms, osteopontin expression and sarcoidosis. PLoS One. (2017) 12:e0171945. doi: 10.1371/journal.pone.0171945
59. Maeda, K, Takahashi, K, Takahashi, F, Tamura, N, Maeda, M, Kon, S, et al. Distinct roles of osteopontin fragments in the development of the pulmonary involvement in sarcoidosis. Lung. (2001) 179:279–91. doi: 10.1007/s004080000068
60. Maver, A, Medica, I, Salobir, B, Tercelj, M, and Peterlin, B. Genetic variation in osteopontin gene is associated with susceptibility to sarcoidosis in Slovenian population. Dis Markers. (2009) 27:295–302. doi: 10.3233/dma-2009-0675
61. Schneider, DJ, Lindsay, JC, Zhou, Y, Molina, JG, and Blackburn, MR. Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB J. (2010) 24:70–80. doi: 10.1096/fj.09-140772
62. Papaporfyriou, A, Loukides, S, Kostikas, K, Simoes, DCM, Papatheodorou, G, Konstantellou, E, et al. Increased levels of osteopontin in sputum supernatant in patients with COPD. Chest. (2014) 146:951–8. doi: 10.1378/chest.13-2440
63. Xu, H, Lou, W, and Fu, F. Association between osteopontin expression and asthma: a meta-analysis. J Int Med Res. (2019) 47:3513–21. doi: 10.1177/0300060519860684
64. King, EM, Zhao, Y, Moore, CM, Steinhart, B, Anderson, KC, Vestal, B, et al. Gpnmb and Spp1 mark a conserved macrophage injury response masking fibrosis-specific programming in the lung. JCI Insight. (2024) 9:e182700. doi: 10.1172/jci.insight.182700
65. Samitas, K, Zervas, E, Vittorakis, S, Semitekolou, M, Alissafi, T, Bossios, A, et al. Osteopontin expression and relation to disease severity in human asthma. Eur Respir J. (2011) 37:331–41. doi: 10.1183/09031936.00017810
66. Kasetty, G, Bhongir, RKV, Papareddy, P, Tufvesson, E, Stenberg, H, Bjermer, L, et al. Osteopontin protects against pneumococcal infection in a murine model of allergic airway inflammation. Allergy. (2019) 74:663–74. doi: 10.1111/all.13646
67. Huang, J, Qiao, H, Li, Q, Zhang, Y, Zhang, C, Su, H, et al. Osteopontin protects from ovalbumin-induced asthma by preserving the microbiome and the intestinal barrier function. mSystems. (2025) 10:e0038925. doi: 10.1128/msystems.00389-25
68. Cockwell, P, and Fisher, LA. The global burden of chronic kidney disease. Lancet. (2020) 395:662–4. doi: 10.1016/s0140-6736(19)32977-0
69. Koide, T, Mandai, S, Kitaoka, R, Matsuki, H, Chiga, M, Yamamoto, K, et al. Circulating extracellular vesicle-propagated microRNA signature as a vascular calcification factor in chronic kidney disease. Circ Res. (2023) 132:415–31. doi: 10.1161/circresaha.122.321939
70. Wang, M, You, L, He, X, Peng, Y, Wang, R, Zhang, Z, et al. Multiomics analysis reveals therapeutic targets for chronic kidney disease with sarcopenia. J Cachexia Sarcopenia Muscle. (2025) 16:e13696. doi: 10.1002/jcsm.13696
71. Cheng, Y, Li, Y, Scherer, N, Grundner-Culemann, F, Lehtimäki, T, Mishra, BH, et al. Genetics of osteopontin in patients with chronic kidney disease: the German chronic kidney disease study. PLoS Genet. (2022) 18:e1010139. doi: 10.1371/journal.pgen.1010139
72. Li, F, Lan, Q, Wang, Y, Xiong, J, Xiao, T, Gong, S, et al. Single-cell analysis of proximal tubular cells with different DNA content reveals functional heterogeneity in the acute kidney injury to chronic kidney disease transition. Kidney Int. (2025) 108:90–104. doi: 10.1016/j.kint.2025.03.025
73. Cheng, SY, Koppitch, K, Guo, J, Moy, N, Simonian, TL, Wilson, PC, et al. Nfkb1 removal from proximal tubule cells improves renal tubular outcomes following ischemia reperfusion injury. Kidney360. (2025) 6:1292–304. doi: 10.34067/kid.0000000868
74. Cao, X, Zhu, R, Liu, D, Cheng, Y, Sun, Y, and Huang, Z. Epidemiological trends in burden of osteoarthritis in China: an analysis from 1990 to 2021 with forecasts for 2022-2050. Front Public Health. (2025) 13:1612596. doi: 10.3389/fpubh.2025.1612596
75. He, H, Zhao, X, Zhang, B, Zhao, S, and Wu, Y. Integrated multi-omics and machine learning reveals immune-metabolic signatures in osteoarthritis: from bulk RNA-seq to single-cell resolution. Front Immunol. (2025) 16:1599930. doi: 10.3389/fimmu.2025.1599930
76. Ma, Z, Li, DX, Lan, X, Bubelenyi, A, Vyhlidal, M, Kunze, M, et al. Short-term response of primary human meniscus cells to simulated microgravity. Cell Commun Signal. (2024) 22:342. doi: 10.1186/s12964-024-01684-w
77. Yang, L, Yu, X, Liu, M, and Cao, Y. A comprehensive analysis of biomarkers associated with synovitis and chondrocyte apoptosis in osteoarthritis. Front Immunol. (2023) 14:1149686. doi: 10.3389/fimmu.2023.1149686
Glossary
AKI-CKD - Acute Kidney Injury to Chronic Kidney Disease
Ang II - Angiotensin II
AR - Androgen Receptor
AS - Atherosclerosis
ASARM - Acidic, Serine- and Aspartic acid-Rich Motif
AAA - Abdominal Aortic Aneurysm
CAFs - Cancer-Associated Fibroblasts
CKD - Chronic Kidney Disease
COPD - Chronic Obstructive Pulmonary Disease
CRDs - Chronic Respiratory Diseases
CTLs - Cytotoxic T Lymphocytes
CVD - Cardiovascular Disease
DCM-HF - Dilated Cardiomyopathy with Heart Failure
DEHP - Di-(2-ethylhexyl) Phthalate
ECM - Extracellular Matrix
ecmCAFs - ECM-producing Cancer-Associated Fibroblasts
EMT - Epithelial-Mesenchymal Transition
GC - Gastric Cancer
GO - Gene Ontology
HCC - Hepatocellular Carcinoma
HDM - House Dust Mite
HNSCC - Head and Neck Squamous Cell Carcinoma
IFN-γ - Interferon-gamma
IL - Interleukin
ILT - Intraluminal Thrombus
IPF - Idiopathic Pulmonary Fibrosis
MAM - Matrisome-Associated Macrophage
MI - Myocardial Infarction
MMPs - Matrix Metalloproteinases
Mo-IMs - Monocyte-derived Interstitial Macrophages
mCRPC - metastatic Castration-Resistant Prostate Cancer
MSS-mCRC - Microsatellite Stable metastatic-type metastatic Colorectal Cancer
myCAFs - myofibroblastic Cancer-Associated Fibroblasts
NF-κB - Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
NSCLC - Non-Small Cell Lung Cancer
OA - Osteoarthritis
PDAC - Pancreatic Ductal Adenocarcinoma
RPF - Rapid Pulmonary Fibrosis
RGD - Arg-Gly-Asp
scRNA-seq - Single-Cell RNA Sequencing
sEVs - Small Extracellular Vesicles
SIBLING - Small Integrin-Binding Ligand N-linked Glycoprotein
SNPs - Single-Nucleotide Polymorphisms
SPP1 - Secreted Phosphoprotein 1
TAD - Thoracic Aortic Dissection
TAMs - Tumor-Associated Macrophages
TGF-β1 - Transforming Growth Factor Beta 1
Th1 - T-helper 1
Th17 - T-helper 17 cells
TiME - Tumor Immune Microenvironment
TNBC - Triple-Negative Breast Cancer
TME - Tumor Microenvironment
VSMCs - Vascular Smooth Muscle Cells
Keywords: SPP1, Osteopontin, extracellular matrix (ECM), SPP1+ macrophages, epithelial-mesenchymal transition (EMT)
Citation: Liu Y, Yan X and Fan R (2025) Relationship between SPP1 (Osteopontin) and extracellular matrix dynamics: a comprehensive review. Front. Med. 12:1700652. doi: 10.3389/fmed.2025.1700652
Edited by:
Alessandro Palma, Sapienza University of Rome, ItalyReviewed by:
Alessio Reggio, Saint Camillus International University of Health and Medical Sciences, ItalyAlessio Torcinaro, National Research Council (CNR), Italy
Copyright © 2025 Liu, Yan and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Fan, ZmFucnVpYWxiZXJ0QGZveG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Ying Liu
Ying Liu Xiaoyan Yan
Xiaoyan Yan Rui Fan
Rui Fan