Abstract
A 69-year-old Chinese male farmer suddenly developed severe lower back pain accompanied by persistent high fever during his recovery from carbon monoxide (CO) poisoning. Clinical examination revealed limited spinal mobility and significant tenderness in the L2–L4 vertebrae and bilateral paravertebral regions, with no palpable masses. Magnetic resonance imaging (MRI) showed fluid accumulation around the bilateral psoas muscles, confirming the presence of bilateral abscesses. We performed ultrasound-guided puncture and drainage of the affected area, and the culture of the drainage fluid identified Carbapenem-Resistant Enterobacteriaceae (CRE). The final diagnosis was bilateral primary psoas abscess (PPA), and targeted antimicrobial therapy for CRE infection was initiated.
1 Introduction
With the advancement of imaging diagnostic technologies, the number of reported cases of psoas abscess (PA) has significantly increased worldwide. This progress has greatly enhanced physicians’ ability to identify these conditions early and manage them clinically, thereby improving patient outcomes. Primary PA is relatively rare in clinical practice and is typically associated with infections caused by pathogens such as Mycobacterium tuberculosis, Brucella, and Staphylococcus aureus, with a higher prevalence among immunocompromised hosts (1–3). Of particular emerging concern are infections caused by Carbapenem-Resistant Enterobacteriaceae (CRE), a critical antimicrobial resistance threat identified by the WHO. CRE resistance primarily arises through three mechanisms: production of carbapenemases (particularly KPC, NDM, and OXA-48 enzymes), alterations in outer membrane porins, and hyperexpression of efflux pumps (4). Globally, KPC variants dominate in the Americas and Europe, while NDM enzymes prevail in Asia and the Middle East (5). Notably, Escherichia coli carrying blaNDM-5 has recently become a major epidemic clone in China’s CRE landscape, demonstrating enhanced virulence and multi-drug resistance profiles (6). This report describes a challenging case of bilateral PA caused by CRE (specifically NDM-producing E. coli) in a patient with a history of carbon monoxide (CO) poisoning, highlighting the complex interplay between antimicrobial resistance and compromised host immunity.
2 Case report
We report the case of a 69-year-old Chinese male farmer who presented to our hospital’s respiratory department with intermittent fever and fatigue lasting over a month, along with dysarthria for two months. He had a history of cerebral infarction and grade 3 hypertension. The patient had no history of diabetes mellitus or inflammatory bowel disease. Two months prior, he had experienced a cerebral infarction and was treated with thrombolytic therapy at a local hospital, after which his condition improved, and he was discharged. Approximately one month ago, he suffered from CO poisoning and received hyperbaric oxygen therapy at a local hospital. CO poisoning is increasingly recognized as a risk factor for immunocompromised states, potentially predisposing patients to opportunistic infections (7). During this period, he suddenly developed persistent high fever and intermittent lower back pain. The local hospital suspected a pulmonary infection and administered intravenous ceftriaxone 2.0 g twice daily and intravenous levofloxacin 0.5 g once daily. Notably, CREC strains frequently exhibit resistance to third-generation cephalosporins (e.g., ceftriaxone) and fluoroquinolones (e.g., levofloxacin) due to extended-spectrum β-lactamase (ESBL) production and plasmid-mediated quinolone resistance genes (8). Due to the lack of improvement, the treatment was switched to intravenous imipenem-cilastatin 1.0 g twice daily, along with other symptomatic treatments. However, carbapenem monotherapy often fails in CRE infections, particularly when carbapenemase production (e.g., NDM enzymes) is present (9). Despite these interventions, the patient continued to experience high fever and chills and was subsequently transferred to our hospital.
Upon admission, the patient’s body temperature was 40.3 °C, accompanied by chills, fatigue, shortness of breath, and tachycardia, but his blood pressure was normal (126/88 mmHg). Laboratory tests showed a white blood cell count of 8.8 × 109/L (normal range: 3.5–9.5 × 109/L), neutrophil count of 83.10 × 109/L (normal range: 1.8–6.3 × 109/L), lymphocyte count of 11.40 × 109/L (normal range: 1.1–3.2 × 109/L), procalcitonin level of 1.175 ng/mL (normal range: <0.05 ng/mL), interleukin-6 level of 84.46 pg/mL (normal range: <7 pg/mL), erythrocyte sedimentation rate (ESR) of 58 mm/h (normal range: 0–15 mm/h), negative tuberculosis PCR, a negative interferon-gamma release assay (IGRA), negative tuberculosis PCR, negative Brucella agglutination test, negative TORCH test results, and multiple negative blood and sputum cultures. Persistently negative conventional cultures in CREC infections are not uncommon, as these pathogens often require specialized media or prolonged incubation for detection (10). CT scans revealed irregular bone structures in the L2–L5 intervertebral discs, with fluid-filled dark areas around the discs and bilateral psoas muscles, suggesting tuberculous abscess changes. Lumbar MRI showed tuberculosis in the L2–L5 vertebrae, with surrounding soft tissue abscess formation, spinal stenosis at the corresponding segments, and compression of the conus medullaris and cauda equina (Figures 1, 2).
Figure 1
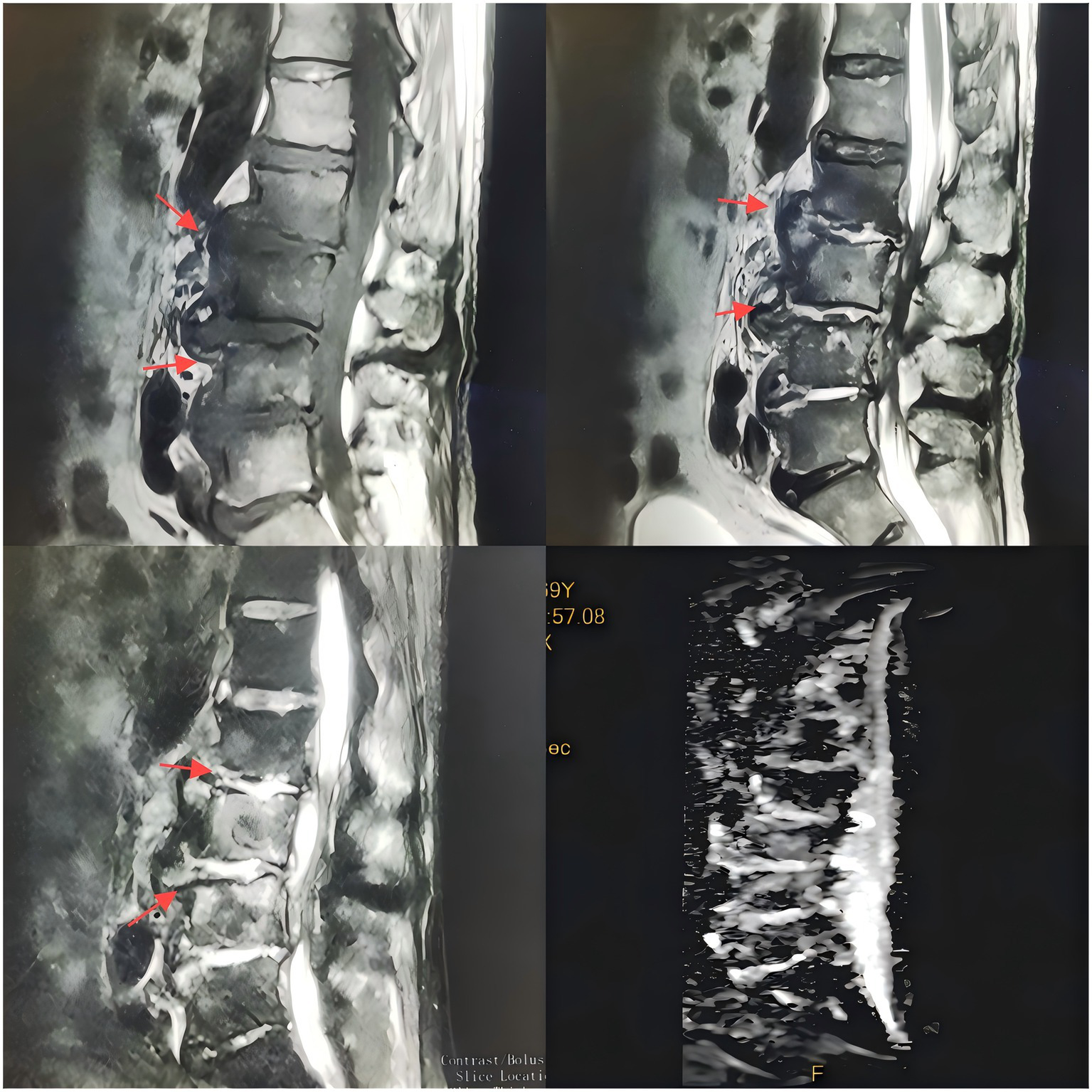
T1-weighted MR images of the intervertebral discs acquired with varying flip angles.
Figure 2
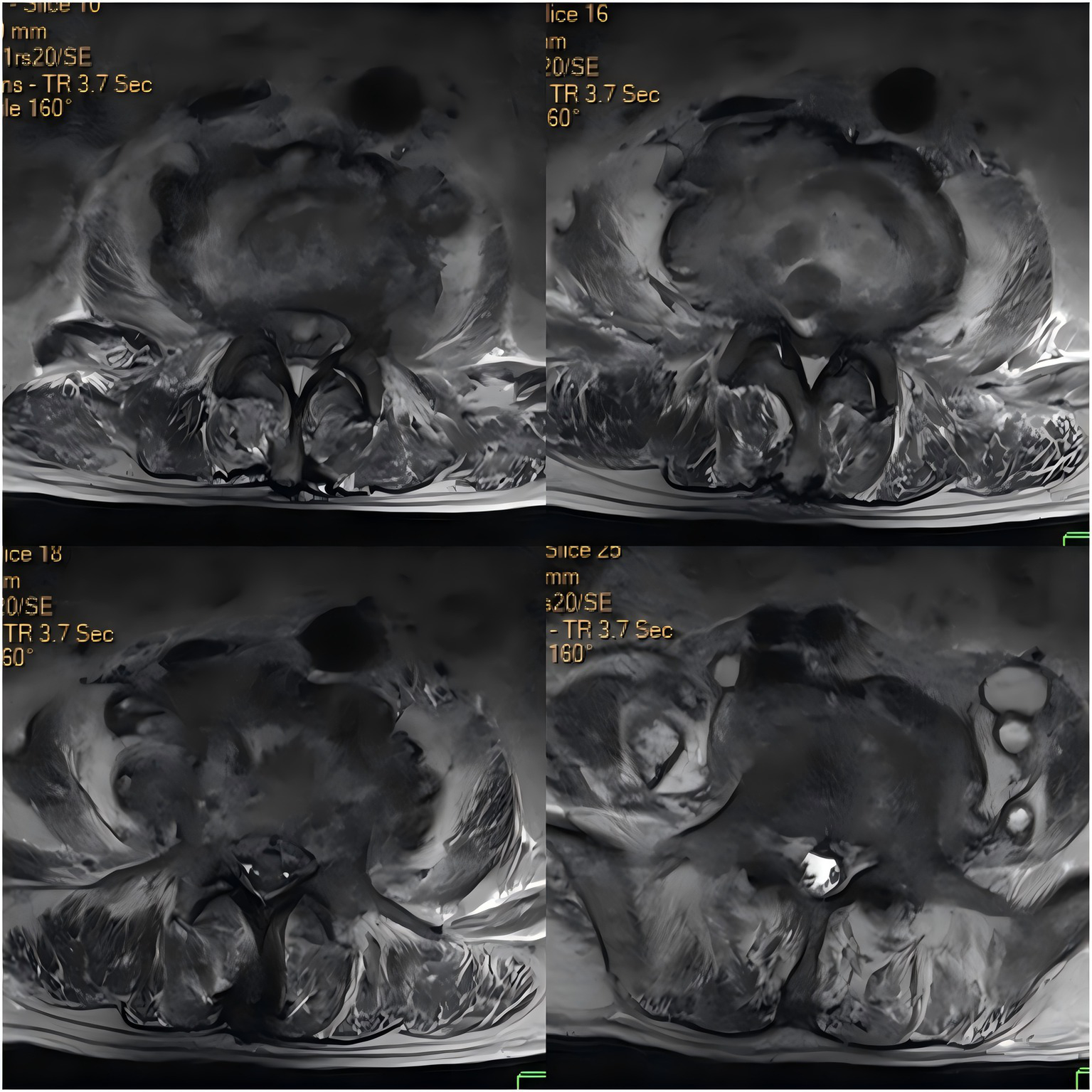
Axial contrast-enhanced CT image of the intervertebral discs.
Under ultrasound guidance, we performed puncture and drainage of the abscess and sent the aspirate for culture, drug sensitivity testing, and next-generation sequencing (NGS). Recent studies highlight the utility of NGS in rapidly identifying polymicrobial infections and resistance genes, particularly in culture-negative cases (11). Approximately 50 mL of bloody fluid was drained, followed by thorough irrigation of the abscess cavity with saline, and drainage tubes were inserted bilaterally. While awaiting the test results, the patient continued to experience high fever and severe lower back pain. Given the high radiological suspicion of spinal tuberculosis, empirical anti-tuberculosis therapy was initiated. Empirically, we initiated anti-infective therapy (etimicin sulfate 300 mg intravenously once daily; linezolid 0.6 g intravenously every 12 h), antiviral therapy (peramivir 0.3 g intravenously once daily; ganciclovir 250 mg intravenously once daily), ganciclovir 250 mg intravenously once daily was administered preemptively due to the detection of low-sequence HHV-5 by NGS in this immunocompromised host, anticoagulation, anti-tuberculosis, and pain management.
Subsequently, NGS results showed 89,390 sequences belonging to Escherichia coli (E. coli) and 9 sequences belonging to human herpesvirus 5 (HHV-5). The detection of HHV-5 in this immunocompromised host aligns with reports of viral reactivation following CO poisoning, though its clinical significance in this context remains debated (12). Pus culture and drug sensitivity testing identified CREC, which was sensitive to meropenem and amikacin. Intriguingly, some NDM-producing E. coli retain susceptibility to carbapenems at high inoculum concentrations, as observed in recent pharmacokinetic/pharmacodynamic models (13). Therefore, we selected meropenem, a β-lactam antibiotic effective against Gram-negative bacilli, administered intravenously at 1 g every 8 h. Following this treatment, the patient’s condition gradually improved, and the puncture site healed well. Combined surgical drainage and carbapenem therapy have demonstrated synergistic efficacy in deep-seated CREC infections, achieving cure rates of 65–80% in recent cohort studies (14). He was discharged and continued rehabilitation at a local hospital, with ongoing improvement. At our most recent follow-up (October 2023), the patient had no fever or back pain for two years and was able to walk independently.
3 Discussion
Bilateral PPA caused by CRE infection is a relatively rare condition. In this case, the patient was 69 years old with no history of tuberculosis infection. Although lumbar CT suggested tuberculous abscess changes in the psoas muscles, multiple tuberculosis-related tests were negative, including IGRA, ruling out secondary tuberculous PA. The Brucella serum agglutination test was also negative, and the patient had no history of contact with livestock, thus excluding brucellosis as a cause of secondary PA. This case represents primary bilateral PA in an immunocompromised individual, early surgical drainage and antimicrobial therapy led to a favorable outcome. E. coli typically colonizes the gastrointestinal tract, reproductive tract, and other areas and can become pathogenic in immunocompromised states, leading to bacteremia, pneumonia, urinary tract infections, and device-related sepsis (1). The widespread use of antibiotics has led to an increase in CRE infections. CRE resistance mechanisms include the production of carbapenemases, overexpression of efflux pumps, plasmid-mediated resistance genes, and mutations affecting lipopolysaccharide core synthesis (15). CRE infections are associated with higher mortality rates and are an independent risk factor for patient death, posing significant challenges in clinical practice (16).
In retrospect, the initial empirical antibiotic regimen (etimicin and linezolid) lacked adequate coverage against Gram-negative bacteria, particularly CRE. This highlights a critical learning point. In a patient with extensive prior antibiotic exposure and poor response to initial broad-spectrum therapy (including carbapenems), the possibility of infection with multidrug-resistant Gram-negative organisms should be considered earlier, even when imaging findings suggest alternative diagnoses. The definitive diagnosis in this case underscores the indispensable value of obtaining pus samples for microbiological culture and susceptibility testing to guide appropriate therapy.
PA can be classified as primary or secondary based on etiology. Primary PA is usually caused by hematogenous or lymphatic spread from a distant (often occult) focus and is relatively rare in clinical practice (17). Risk factors include diabetes, intravenous drug use, HIV infection, renal failure, and other immunocompromised states, which are more common in elderly patients (18, 19). Secondary PA results from direct spread of infection from adjacent structures, such as spinal infections, gastrointestinal infections, cervical cancer metastasis, or retroperitoneal germ cell tumor metastasis (20–22). Additionally, PA can occur postpartum, after miscarriage, or following intrauterine device insertion (22). Pulmonary infections, including the spread of Mycobacterium tuberculosis to the vertebrae and involvement of paravertebral soft tissues, can also lead to PA (23). Typically, the source of infection is unclear, and such cases are more common in younger patients.
Following CO poisoning, the patient’s immune system may have been compromised through multiple synergistic mechanisms. First, CO-induced oxidative stress (24) disrupts neutrophil chemotaxis and phagocytosis by impairing NADPH oxidase activity and cytoskeletal dynamics (25). This innate immune dysfunction likely reduced bacterial clearance, facilitating CRE invasion. Second, mitochondrial dysfunction caused by CO binding to cytochrome c oxidase exacerbates ATP depletion (26, 27), which suppresses lymphocyte proliferation and cytokine production (e.g., IL-6, TNF-α) (28), further weakening adaptive immunity. Third, CO poisoning may have synergized with prior antibiotic use to disrupt the gut microbiome. Animal studies demonstrate that CO exposure alters microbiota diversity and increases intestinal permeability (29), potentially enabling CRE translocation from the gut to systemic circulation. This dual insult—immune suppression and gut barrier compromise—provides a plausible pathway for CREC hematogenous seeding of the psoas muscles.
Comparisons with similar cases reinforce this hypothesis. A 2018 case series documented two patients developing multidrug-resistant Pseudomonas aeruginosa bacteremia within 14 days of CO poisoning (30), while a 2020 cohort study found prolonged lymphopenia and monocyte dysfunction in CO-poisoned patients (31). Although no identical cases of CRE-associated PA post-CO poisoning exist in the literature, these reports suggest a broader pattern of CO-mediated susceptibility to opportunistic pathogens. The temporal sequence in our patient—CO exposure followed by CRE bacteremia and PA—aligns with the known timeline of CO-induced immunometabolic disturbances, which may persist for weeks post-exposure.
The clinical presentation of PA is characterized by various nonspecific symptoms. Paresthesia, anorexia, and weight loss are common and may lead to delayed diagnosis, increasing morbidity and mortality (3, 32). The classic triad includes fever, back pain, and lower limb weakness (33). Since the psoas muscle is innervated by the L2, L3, and L4 nerve roots, pain may radiate anteriorly to the hip and thigh. Extension of the affected hip joint exacerbates the pain, known as the “psoas sign” (34). Therefore, most patients adopt a supine position with the knees moderately flexed and the hips slightly externally rotated (35). For clinicians, the combination of the “psoas sign” and relief of hip pain upon flexion is an important diagnostic indicator.
Saylam et al. (36) reported a case of PA in which the patient presented with fever, swelling of the right thigh, and cramping pain radiating from the left hip down the entire left leg. In this case, the patient presented with persistent high fever, back pain, limited spinal mobility, and tenderness in the L2–L4 region. The diagnosis of PA relies on a comprehensive assessment of symptoms, physical examination, laboratory tests, imaging studies, and microbiological analysis. Imaging is considered the gold standard for diagnosing PA, and pus culture can identify the causative pathogen (37). For abscesses larger than 3 cm, CT-guided percutaneous drainage combined with antibiotic therapy is recommended, while smaller abscesses may be effectively managed with antibiotics alone (2).
4 Conclusion
Bilateral PA caused by CRE in a patient recovering from CO poisoning is relatively rare. This represents PPA in an immunocompromised individual. In this case, early surgical drainage and antimicrobial therapy led to a favorable outcome. Overall, PA is considered a rare but serious infectious disease. Clinically, PA caused by CREC is even rarer and often presents with nonspecific symptoms, making early diagnosis challenging. For patients who do not respond to empirical anti-infective therapy and have an unclear etiology, the possibility of multidrug-resistant bacterial infection should be considered, especially in those with recent immune dysfunction. Early diagnosis, standardized treatment, and improved outcomes can be achieved by comprehensively considering symptoms, signs, and relevant auxiliary test results and completing pathogen testing. Future studies should investigate the precise mechanisms by which CO poisoning increases susceptibility to CRE infections, including in vitro and animal studies examining CO’s effects on immune cell function (e.g., neutrophil activity, macrophage phagocytosis) and bacterial virulence factors. Additionally, enhanced clinical surveillance protocols should be established to monitor CO-poisoned patients—particularly those receiving antibiotics—for atypical infections, which may inform guideline revisions for early detection. Healthcare facilities must prioritize strict infection control measures, including enhanced hand hygiene, environmental disinfection, and antimicrobial stewardship programs, to curb CRE transmission. While the specific pathway linking CO-induced immune dysfunction to multidrug-resistant E. coli invasion of the psoas muscle remains unclear, multicenter collaborations to collect comparable case data and systematic analyses of host-pathogen interactions in similar scenarios may yield clinically actionable insights.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Gansu Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MY: Writing – original draft. WH: Resources, Writing – original draft, Writing – review & editing. SC: Writing – original draft. ZC: Methodology, Resources, Writing – review & editing. MH: Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
All authors would like to thank the patient and his family for allowing this case study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Jang J Hur HG Sadowsky MJ Byappanahalli MN Yan T Ishii S . Environmental Escherichia coli: ecology and public health implications—a review. J Appl Microbiol. (2017) 123:570–81. doi: 10.1111/jam.13468,
2.
Mougui A El Bouchti I . Primary psoas abscess as a complication of postpartum: a case report. SAGE Open Med Case Rep. (2024) 12:2050313X241260184. doi: 10.1177/2050313X241260184
3.
Akhaddar A Hall W Ramraoui M Nabil M Elkhader A . Primary tuberculous psoas abscess as a postpartum complication: case report and literature review. Surg Neurol Int. (2018) 9:239. doi: 10.4103/sni.sni_329_18,
4.
Zeng M Xia J Zong Z Shi Y Ni Y Hu F et al . Guidelines for the diagnosis, treatment, prevention and control of infections caused by carbapenem-resistant gram-negative bacilli. J Microbiol Immunol Infect. (2023) 56:653–71. doi: 10.1016/j.jmii.2023.01.017,
5.
Jean S Harnod D Hsueh P . Global threat of carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol. (2022) 12:823684. doi: 10.3389/fcimb.2022.823684,
6.
Riley LW . Distinguishing pathovars from nonpathovars: Escherichia coli. Microbiol Spectr. (2020) 8:1–23. doi: 10.1128/microbiolspec.AME-0014-2020,
7.
Chenoweth JA Albertson TE Greer MR . Carbon monoxide poisoning. Crit Care Clin. (2021) 37:657–72. doi: 10.1016/j.ccc.2021.03.010,
8.
Huang Y Kuo Y Teng L Huang Y-T Kuo Y-W Teng L-J et al . Comparison of Etest and broth microdilution for evaluating the susceptibility of Staphylococcus aureus and Streptococcus pneumoniae to ceftaroline and of carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa to ceftazidime/avibactam. J Glob Antimicrob Resist. (2021) 26:301–7. doi: 10.1016/j.jgar.2021.06.016,
9.
Paul M Carrara E Retamar P Tängdén T Bitterman R Bonomo RA et al . European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. (2022) 28:521–47. doi: 10.1016/j.cmi.2021.11.025,
10.
Hansen GT . Continuous evolution: perspective on the epidemiology of Carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect Dis Ther. (2021) 10:75–92. doi: 10.1007/s40121-020-00395-2,
11.
Mandlik JS Patil AS Singh S . Next-generation sequencing (NGS): platforms and applications. J Pharm Bioallied Sci. (2024) 16:S41–5. doi: 10.4103/jpbs.jpbs_838_23,
12.
Huang CC Ho CH Chen YC Hsu CC Lin HJ Wang JJ et al . Autoimmune connective tissue disease following carbon monoxide poisoning: a nationwide population-based cohort study. Clin Epidemiol. (2020) 12:1287–98. doi: 10.2147/CLEP.S266396
13.
El-Gamal MI Brahim I Hisham N Aladdin R Mohammed H Bahaaeldin A . Recent updates of carbapenem antibiotics. Eur J Med Chem. (2017) 131:185–95. doi: 10.1016/j.ejmech.2017.03.022,
14.
Shamsuzzaman M Kim S Kim J . Therapeutic potential of novel phages with antibiotic combinations against ESBL-producing and carbapenem-resistant Escherichia coli. J Glob Antimicrob Resist. (2025) 43:86–97. doi: 10.1016/j.jgar.2025.04.005,
15.
Ma J Song X Li M Yu Z Cheng W Yu Z et al . Global spread of Carbapenem-Resistant Enterobacteriaceae: epidemiological features, resistance mechanisms, detection and therapy. Microbiol Res. (2023) 266:127249. doi: 10.1016/j.micres.2022.127249,
16.
Gao Y Chen M Cai M Liu K Wang Y Zhou C et al . An analysis of risk factors for Carbapenem-Resistant Enterobacteriaceae infection. J Glob Antimicrob Resist. (2022) 30:191–8. doi: 10.1016/j.jgar.2022.04.005,
17.
Büyükbebeci O Seçkiner I Karslı B Karakurum G Başkonuş I Bilge O et al . Retroperitoneoscopic drainage of complicated psoas abscesses in patients with tuberculous lumbar spondylitis. Eur Spine J. (2012) 21:470–3. doi: 10.1007/s00586-011-2049-2,
18.
Spiegel ST Pachtman SL Rochelson B . Bacterial spinal epidural and psoas abscess in pregnancy associated with intravenous drug use. Case Rep Obstet Gynecol. (2018) 2018:1797421. doi: 10.1155/2018/1797421
19.
Xu C Zhou Z Wang S Ren W Yang X Chen H et al . Psoas abscess: an uncommon disorder. Postgrad Med J. (2024) 100:482–7. doi: 10.1093/postmj/qgad110,
20.
Mehdorn M Petersen TO Bartels M Jansen-Winkeln B Kassahun WT . Psoas abscess secondary to retroperitoneal distant metastases from squamous cell carcinoma of the cervix with thrombosis of the inferior vena cava and duodenal infiltration treated by Whipple procedure: a case report and review of the literature. BMC Surg. (2016) 16:55. doi: 10.1186/s12893-016-0169-7,
21.
Verlage KR Underwood L de Riese WT . Retroperitoneal metastatic germ cell tumor presenting as a psoas abscess: a rare clinical occurrence and review of the literature. Int Urol Nephrol. (2016) 48:1839–40. doi: 10.1007/s11255-016-1385-x
22.
Schröder C Calite E Böckenhoff P Büttner T Stein J Gembruch U et al . Psoas abscess in pregnancy: a review of the literature and suggestion of minimally invasive treatment options. Arch Gynecol Obstet. (2024) 309:987–92. doi: 10.1007/s00404-023-06970-5,
23.
Miloudi M Belabbes S Sbaai M Kamouni YE Arsalane L Zouhair S . Psoas abscess due to Candida tropicalis: a case report. Ann Biol Clin. (2018) 76:571–3. doi: 10.1684/abc.2018.1365
24.
Wang T Zhang Y . Mechanisms and therapeutic targets of carbon monoxide poisoning: a focus on reactive oxygen species. Chem Biol Interact. (2024) 403:111223. doi: 10.1016/j.cbi.2024.111223,
25.
Watabe Y Giam Chuang VT Sakai H Ito C Enoki Y Kohno M et al . Carbon monoxide alleviates endotoxin-induced acute lung injury via NADPH oxidase inhibition in macrophages and neutrophils. Biochem Pharmacol. (2025) 233:116782. doi: 10.1016/j.bcp.2025.116782
26.
Zazzeron L Franco W Anderson R . Carbon monoxide poisoning and phototherapy. Nitric Oxide. (2024) 146:31–6. doi: 10.1016/j.niox.2024.04.001,
27.
Rose JJ Wang L Xu Q McTiernan CF Shiva S Tejero J et al . Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am J Respir Crit Care Med. (2017) 195:596–606. doi: 10.1164/rccm.201606-1275CI,
28.
Piantadosi CA . Biological chemistry of carbon monoxide. Antioxid Redox Signal. (2002) 4:259–70. doi: 10.1089/152308602753666316
29.
Motterlini R Foresti R Bassi R . Carbon monoxide and the gut microbiome: implications for bacterial translocation. Front Pharmacol. (2021) 12:678912. doi: 10.3389/fphar.2021.678912
30.
Lee JH Park YS Cho YJ . Multidrug-resistant infections after carbon monoxide intoxication: a case series. J Crit Care Med. (2018) 4:145–9. doi: 10.2478/jccm-2018-002
31.
Moon JM Chun BJ Cho YS . The predictive value of scores based on peripheral complete blood cell count for long-term neurological outcome in acute carbon monoxide intoxication. Basic Clin Pharmacol Toxicol. (2019) 124:500–10. doi: 10.1111/bcpt.13157
32.
Dali A Guidara B Hini JD . Iliopsoas muscle abscess after a vaginal delivery. A case report. Gynecol Obstet Fertil Senol. (2024) 52:490–1. doi: 10.1016/j.gofs.2024.01.013,
33.
Garner JP Meiring PD Ravi K Gupta R . Psoas abscess—not as rare as we think?Color Dis. (2007) 9:269–74. doi: 10.1111/j.1463-1318.2006.01135.x,
34.
Xu BY Vasanwala FF Low SG . A case report of an atypical presentation of pyogenic iliopsoas abscess. BMC Infect Dis. (2019) 19:58. doi: 10.1186/s12879-019-3675-2,
35.
Shields D Robinson P Crowley TP . Iliopsoas abscess—a review and update on the literature. Int J Surg. (2012) 10:466–9. doi: 10.1016/j.ijsu.2012.08.016,
36.
Saylam K Anaf V Kirkpatrick C . Successful medical management of multifocal psoas abscess following cesarean section: report of a case and review of the literature. Eur J Obstet Gynecol Reprod Biol. (2002) 102:211–4. doi: 10.1016/S0301-2115(01)00604-2,
37.
Idris I Aburas M Ibarra Martinez F Osei-Kuffuor E Adams K Dizadare T et al . Primary psoas abscess in a pediatric patient: a case report. Cureus. (2022) 14:e26206. doi: 10.7759/cureus.26206
Summary
Keywords
Carbapenem-Resistant Escherichia coli , psoas abscess, CO poison, inflammation, immunology
Citation
Jia Cui Z, Hao W, Chengxin S, Xin Yue M and He Ping M (2025) Case Report: Psoas abscess caused by Carbapenem-Resistant Enterobacteriaceae in a CO poisoning patient. Front. Med. 12:1702821. doi: 10.3389/fmed.2025.1702821
Received
11 September 2025
Revised
10 November 2025
Accepted
19 November 2025
Published
16 December 2025
Volume
12 - 2025
Edited by
Ozgur Karcioglu, University of Health Sciences (Türkiye), Türkiye
Reviewed by
Fatma Tuğba Çetin, Cukurova University, Türkiye
Iveta Golubovska, University of Latvia, Latvia
Updates
Copyright
© 2025 Jia Cui, Hao, Chengxin, Xin Yue and He Ping.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Jia Cui, 1504059310@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.