- Department of Intensive Care Unit, PLA 983rd Hospital, Tianjin, China
Background: Although low tidal volume ventilation has been shown to reduce mortality in patients with acute respiratory distress syndrome (ARDS), overall mortality remains high (30%−40%). Ultra-protective ventilation (≤4 mL/kg predicted body weight) has the potential to further decrease ventilator-induced lung injury but may result in severe hypercapnia. Extracorporeal carbon dioxide removal (ECCO2R) could facilitate ultra-protective ventilation by alleviating carbon dioxide retention; however, supporting evidence remains limited.
Objective: To evaluate the efficacy of ECCO2R in enabling ultra-protective ventilation strategies in patients with ARDS.
Methods: A systematic search was conducted in PubMed, Embase, Web of Science, and the Cochrane Library for studies published up to June 2025 that met predefined inclusion criteria. Primary outcomes included changes in gas exchange and ventilator settings 24 h after initiating ECCO2R. All analyses were performed using a random-effects model. Sensitivity and subgroup analyses were conducted to further explore the findings.
Results: Fourteen studies involving 593 ARDS patients were included. ECCO2R significantly reduced driving pressure (weighted mean difference [WMD]: −3.70 cmH2O; 95% CI: −4.05 to −3.34; P < 0.001), plateau pressure (WMD: −3.26 cmH2O; 95% CI: −3.70 to −2.82; P < 0.001), and tidal volume (WMD: −1.68 mL/kg; 95% CI: −1.81 to −1.55; P < 0.001) at 24 h, while it increased positive end-expiratory pressure (WMD: 0.64 cmH2O; 95% CI: 0.44 to 0.85; P < 0.001). No significant changes were observed in PaO2/FiO2 ratio, pCO2, or pH (P > 0.05). The pooled 28-day mortality rate was 29% (95% CI: 19%−38%). Notable complications included bleeding (15%; 95% CI: 8%−21%), circuit clotting (19%; 95% CI: 13%−26%), and hemolysis (15%; 95% CI: 5%−25%).
Conclusion: ECCO2R facilitates the implementation of ultra-protective ventilation by significantly improving respiratory mechanics and mitigating the hypercapnia that would otherwise result from ultra-low tidal volumes. However, its use is associated with a notable risk of device-related complications, necessitating careful patient selection and expert management.
Systematic review registration: INPLASY platform (registration number: INPLASY202570067.
Introduction
Acute respiratory distress syndrome (ARDS) is a critical condition commonly encountered in intensive care units. It is pathologically characterized by diffuse damage to the alveolar–capillary membrane, resulting in refractory hypoxemia and decreased lung compliance (1). Although low tidal volume ventilation has been shown to significantly reduce 28-day mortality, the overall mortality rate remains high, ranging from 30% to 40%, underscoring the need for more effective ventilation strategies (2). While standard low tidal volume ventilation mitigates ventilator-induced lung injury, it does not entirely eliminate the shear stress caused by repetitive alveolar opening and collapse (3). Pathophysiological studies have identified the “baby lung” phenomenon in ARDS, wherein conventional tidal volumes may lead to regional overdistension of the remaining functional lung units (4).
Recently, ultra-protective ventilation—defined as tidal volume ≤ 4 mL/kg predicted body weight—has attracted interest for its potential to further reduce lung injury (5). However, its application is constrained by the risk of severe hypercapnia and acid–base disturbances (6). Animal studies indicate that reducing tidal volumes below 4 mL/kg helps attenuate injury to the alveolar–epithelial–endothelial barrier; nevertheless, consequent carbon dioxide (CO2) retention may lead to pulmonary hypertension, increased cerebral blood flow, and myocardial suppression (7). Therefore, striking a balance between lung protection and CO2 clearance remains a major challenge in optimizing ventilation strategies for ARDS.
Extracorporeal CO2 removal (ECCO2R) utilizes hollow-fiber membrane gas exchange, either through extracorporeal membrane oxygenation (ECMO) or dedicated low-flow devices, to selectively remove CO2 from the blood (8). Recent clinical studies suggest that ECCO2R-assisted ventilation can reduce plateau pressure to below 25 cmH2O while maintaining arterial pH above 7.25 in patients with ARDS (9). However, most available studies are limited by small sample sizes, methodological heterogeneity, and a lack of control groups. Moreover, several key clinical uncertainties remain, including the optimal timing of intervention, standardization of technical parameters, and the effect on definitive patient outcomes such as mortality. In light of these gaps, this systematic review and meta-analysis aims to evaluate the impact of ECCO2R-facilitated ultra-protective ventilation on respiratory mechanics, ventilation parameters, and complications in patients with ARDS.
Methods
Data sources, search strategy, and selection criteria
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10). The study protocol was registered on the INPLASY platform (registration number: INPLASY202570067). A comprehensive literature search was performed in PubMed, Embase, Web of Science, and the Cochrane Library for studies published from database inception through June 2025. Search strategies incorporated both Medical Subject Headings (MeSH) and free-text terms, including: “extracorporeal carbon dioxide removal,” “ECCO2R,” “acute respiratory distress syndrome,” “ARDS,” “ultra-protective ventilation,” and “low tidal volume ventilation.” The complete search strategies for each database are detailed in Supplementary File 1. Manual searches of conference abstracts, gray literature, and backward citation tracking were also conducted to minimize the risk of study omission.
Two reviewers independently screened the identified literature based on a predefined protocol. Any discrepancies were resolved through team consensus. Studies were included if they met the following criteria: (1) population: patients diagnosed with ARDS according to the Berlin Definition; (2) intervention: ECCO2R-assisted ultra-protective ventilation, defined as tidal volume ≤ 4 mL/kg predicted body weight (PBW); (3) study designs: randomized controlled trials (RCTs), quasi-experimental studies, cohort studies, and case-control studies; and (4) outcomes: measures related to respiratory mechanics, ventilation parameters, patient-centered outcomes, and complication rates. Exclusion criteria included: (1) animal studies, reviews, or case reports; (2) studies with critical missing data that were unavailable from authors; and (3) non-Chinese/English publications.
Data collection and quality assessment
Two investigators independently extracted data using predefined electronic forms. Information collected included: first author's surname, year of publication, geographical setting, sample size, patient baseline characteristics (e.g., age, sex, ARDS etiology, Berlin stage), ECCO2R technical parameters (e.g., blood flow rate, membrane surface area, anticoagulation protocol), ventilation details, and outcome measures. Study quality was assessed using appropriate tools: the Cochrane Risk of Bias Tool was used for RCTs to evaluate randomization, allocation concealment, blinding, handling of missing data, and outcome reporting bias (11); the Newcastle-Ottawa Scale (NOS) was applied to observational studies to assess selection, comparability, and outcome/exposure domains (12).
Statistical analysis
Weighted mean difference (WMD) with 95% confidence intervals (CI) was computed for continuous outcomes. Proportions and corresponding 95% CIs were calculated for dichotomous outcomes. Due to anticipated clinical and methodological heterogeneity across studies, a random-effects model was employed for all meta-analyses (13). The certainty of the evidence for primary outcomes was evaluated using the GRADE framework (Grading of Recommendations, Assessment, Development, and Evaluations). Two review authors independently rated the evidence for each outcome based on risk of bias, inconsistency, indirectness, imprecision, and publication bias (14). Heterogeneity was evaluated using the I2 statistic and Cochran's Q test, with I2 ≥ 50% or a Q-test P-value < 0.10 indicating substantial heterogeneity (15, 16). Sensitivity analyses using the leave-one-out method were performed to assess the robustness of the results and the influence of individual studies on heterogeneity (17). To further investigate potential sources of heterogeneity, we performed univariate random-effects meta-regression for the primary outcomes of respiratory mechanics according to study design, mean age, and male proportion (18). Prespecified subgroup analyses were conducted based on study design, patient age, and sex. Publication bias was evaluated through visual inspection of funnel plot symmetry and quantified using Egger's and Begg's tests (19, 20). All statistical analyses were performed using STATA version 12.0 (StataCorp LP, College Station, TX, USA), with a two-sided P-value < 0.05 considered statistically significant.
Results
Literature search
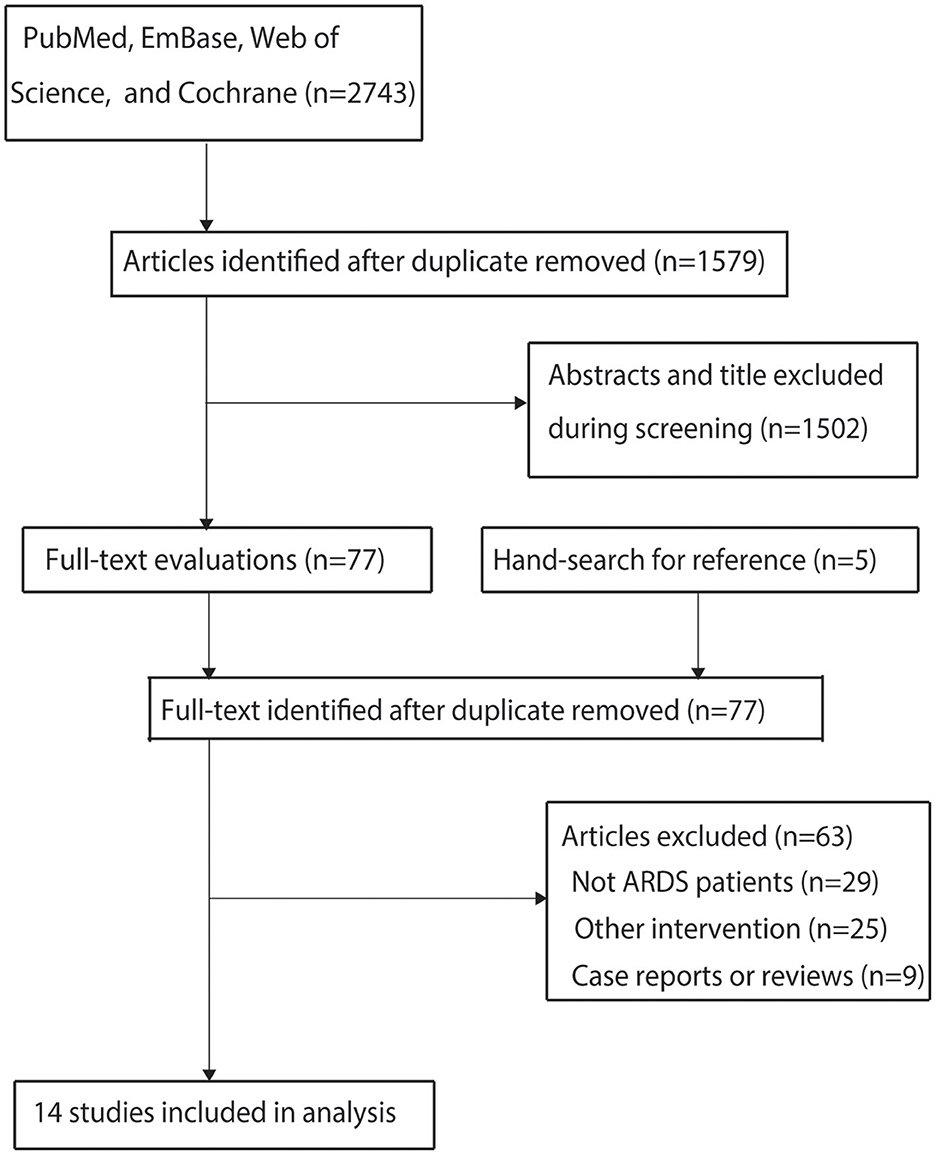
The initial electronic database search identified 2,743 articles. After removing duplicates, 1,579 studies remained. Screening of titles and abstracts excluded 1,502 studies as irrelevant to the research topic. The remaining 77 studies underwent full-text review, of which 63 were excluded for the following reasons: inclusion of non-ARDS patients (n = 29), absence of an ultra-protective ventilation strategy (n = 25), or study design as case reports or reviews (n = 9). A manual search of reference lists from relevant articles did not yield additional eligible studies. Ultimately, 14 studies met the inclusion criteria and were included in the meta-analysis (21–34). The literature search and study selection process are illustrated in Figure 1.
Study characteristics
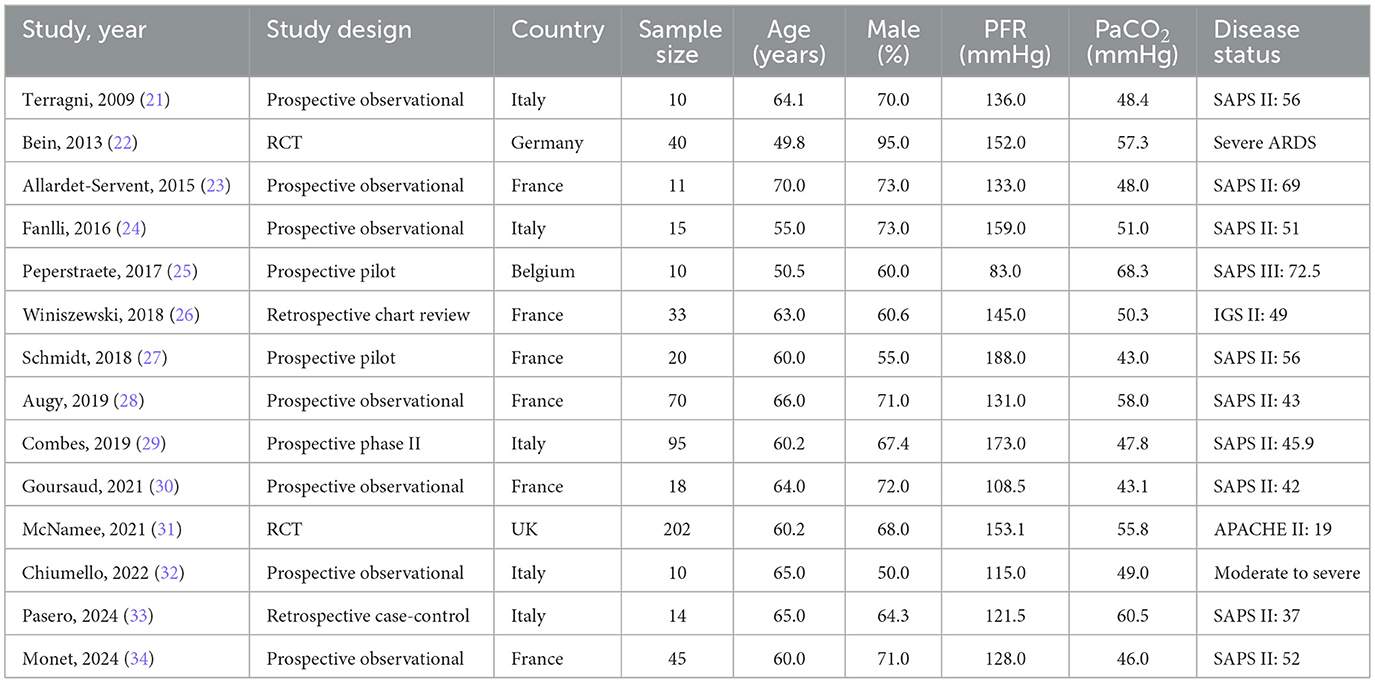
Table 1 summarizes the baseline characteristics of the included studies and patients. All studies were conducted in Europe and involved a total of 593 ARDS patients. Among them, two were RCTs, 10 were prospective observational studies, and two were retrospective observational studies. In recognition of the differing validity of evidence derived from various study designs, and in line with the reviewer's comment, we have stratified our primary analysis and interpretation based on this hierarchy. The inclusion of observational studies, including retrospective series, allows for the exploration of “real-world” feasibility and the spectrum of complications, but the estimates of physiological efficacy from these studies must be interpreted with caution due to inherent risks of confounding and selection bias. The results from the two RCTs are therefore emphasized as providing the most robust evidence within this dataset. The mean age of participants ranged from 49.8 to 70.0 years, and the proportion of male patients varied between 50.0% and 95.0%. Quality assessments of the included studies are provided in Supplementary File 2. The detailed risk of bias assessment is provided in Supplementary File 2. Of the two RCTs, one was judged to have a “low” risk of bias, while the other raised “some concerns” primarily due to unclear information regarding allocation concealment and blinding of participants and personnel. Observational studies were evaluated using the NOS, with three studies scoring 7 points, four scoring 6 points, and five scoring 5 points. The observational studies, as expected, were susceptible to selection bias and confounding due to their non-randomized design. This high risk of bias across the majority of the body of evidence is a key consideration when interpreting the pooled effect estimates, as it likely contributes to an overestimation of the treatment effects observed in the single-arm cohorts.
Driving pressure, plateau pressure, and tidal volume
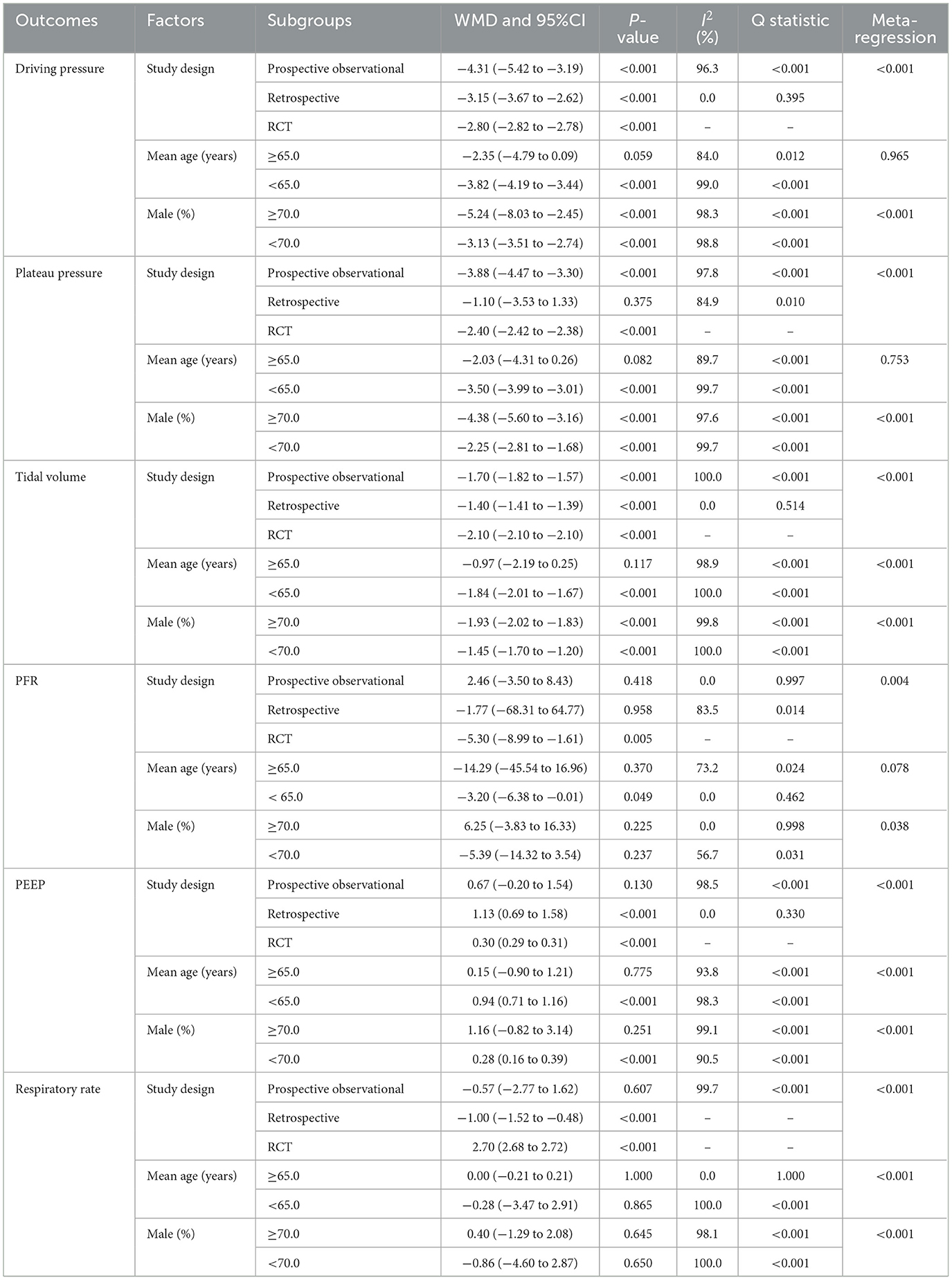
Changes in driving pressure, plateau pressure, and tidal volume after 24 h of ECCO2R-facilitated ultra-protective ventilation were reported in 10, 12, and 12 studies, respectively. Significant reductions were observed in driving pressure (WMD: −3.70 cm H2O; 95% CI: −4.05 to −3.34; P < 0.001), plateau pressure (WMD: −3.26 cm H2O; 95% CI: −3.70 to −2.82; P < 0.001), and tidal volume (WMD: −1.68 mL/kg; 95% CI: −1.81 to −1.55; P < 0.001; Figure 2). Considerable heterogeneity was detected for driving pressure (I 2 = 98.7%; P < 0.001), plateau pressure (I2 = 99.6%; P < 0.001), and tidal volume (I2 = 100.0%; P < 0.001). The consistently extreme heterogeneity (I2 > 95%) indicates that the included studies do not estimate a single common effect size, but rather a distribution of true effects. Therefore, the pooled WMD from the random-effects model should not be interpreted as a precise estimate of efficacy, but rather as a descriptive summary of the average reported effect across highly variable clinical settings, patient populations, and ECCO2R management protocols. The primary value of this analysis lies in confirming the direction of this effect and in exploring the potential sources of its substantial variability. Sensitivity analyses confirmed that the pooled estimates remained robust after sequential exclusion of individual studies (Supplementary File 3). Study design and male proportion were significant predictor affect the treatment effect of ECCO2R-facilitated ultra-protective ventilation on driving pressure, plateau pressure, and tidal volume, while mean age of patients was a significant predictor for tidal volume (Table 2). Subgroup analyses indicated significant reductions in driving pressure and tidal volume across all subgroups except among patients aged ≥65.0 years. Plateau pressure was significantly reduced in all subgroups except in retrospective studies and patients aged ≥65.0 years (Table 2). No significant publication bias was detected for driving pressure (Egger's P = 0.231; Begg's P = 0.858), plateau pressure (Egger's P = 0.422; Begg's P = 0.837), or tidal volume (Egger's P = 0.372; Begg's P = 0.945) at 24 h post-ECCO2R initiation (Supplementary File 4).
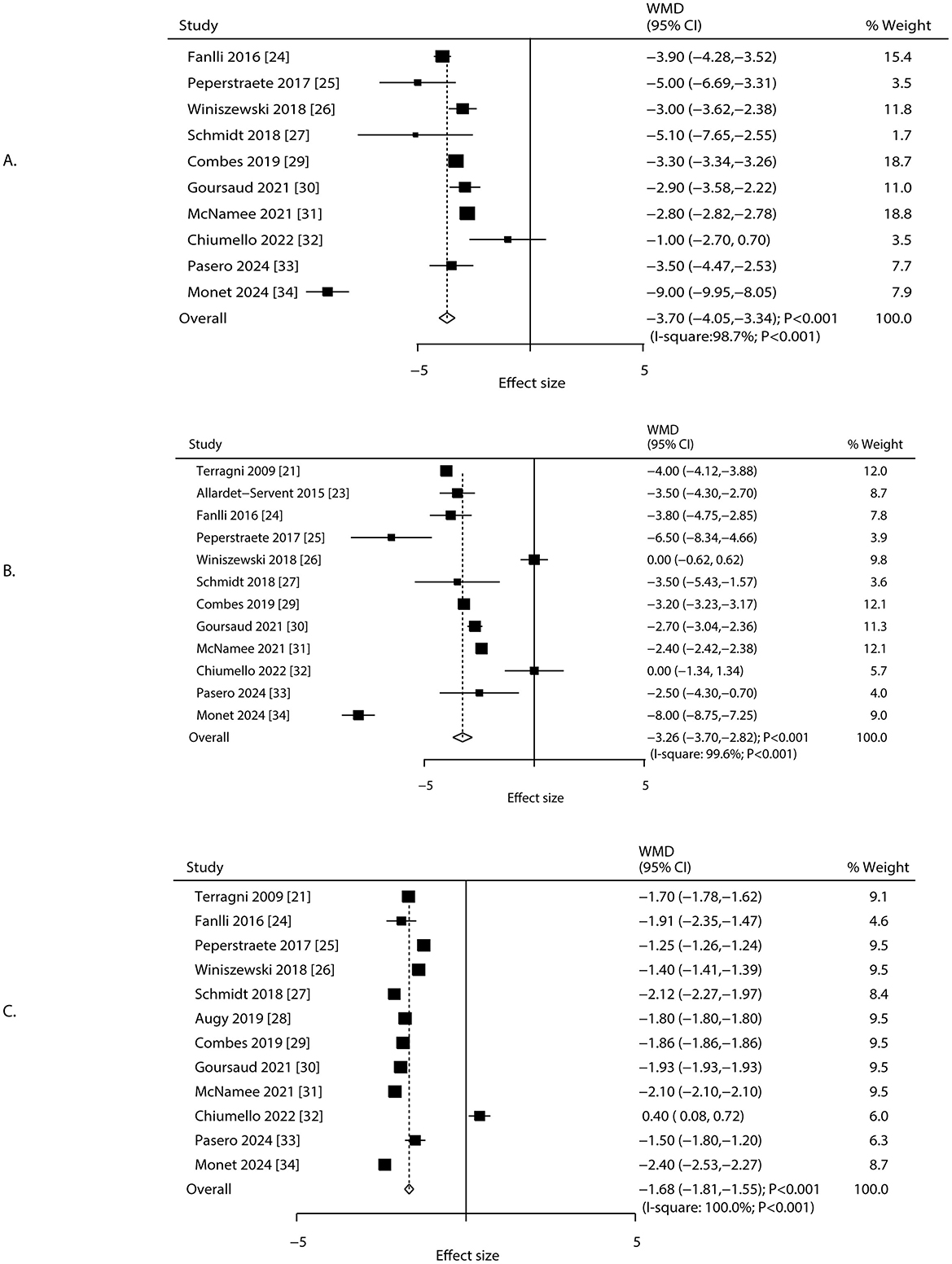
Figure 2. Changes in driving pressure, plateau pressure, and tidal volume after 24 h of ECCO2R, facilitating the application of ultraprotective ventilation. (A) Driving pressure, (B) plateau pressure, (C) tidal volume.
PaO2/FiO2, positive end-expiratory pressure, and respiratory rate
Changes in PaO2/FiO2, positive end-expiratory pressure (PEEP), and respiratory rate after 24 h of ECCO2R were reported in 12, 12, and 10 studies, respectively (Figure 3). No statistically significant changes were observed in PaO2/FiO2 (WMD: −2.63; 95% CI: −9.49 to 4.23; P = 0.452) or respiratory rate (WMD: −0.22 breaths/min; 95% CI: −2.95 to 2.50; P = 0.872). In contrast, PEEP increased significantly (WMD: 0.64 cm H2O; 95% CI: 0.44 to 0.85; P < 0.001). Heterogeneity was significant for PaO2/FiO2 (I2 = 39.8%; P = 0.075), PEEP (I2 = 98.1%; P < 0.001), and respiratory rate (I2 = 100.0%; P < 0.001). Sensitivity analyses supported the stability of these pooled estimates (Supplementary File 3). The treatment effect of ECCO2R-facilitated ultra-protective ventilation on PaO2/FiO2 could affected by study design and male proportion, while study design, mean age, and male proportion were significant predictors for PEEP, and respiratory rate (Table 2). Subgroup analyses indicated that PaO2/FiO2 decreased significantly in RCTs and among patients aged < 65.0 years. PEEP increased significantly in most subgroups except in retrospective studies, patients aged ≥65.0 years, and studies with ≥70.0% male participants. Respiratory rate decreased significantly in retrospective studies but increased in RCTs (Table 2). No significant publication bias was identified for PaO2/FiO2 (Egger's P = 0.483; Begg's P = 0.631), PEEP (Egger's P = 0.539; Begg's P = 0.945), or respiratory rate (Egger's P = 0.310; Begg's P = 0.210) at 24 h after ECCO2R initiation (Supplementary File 4).
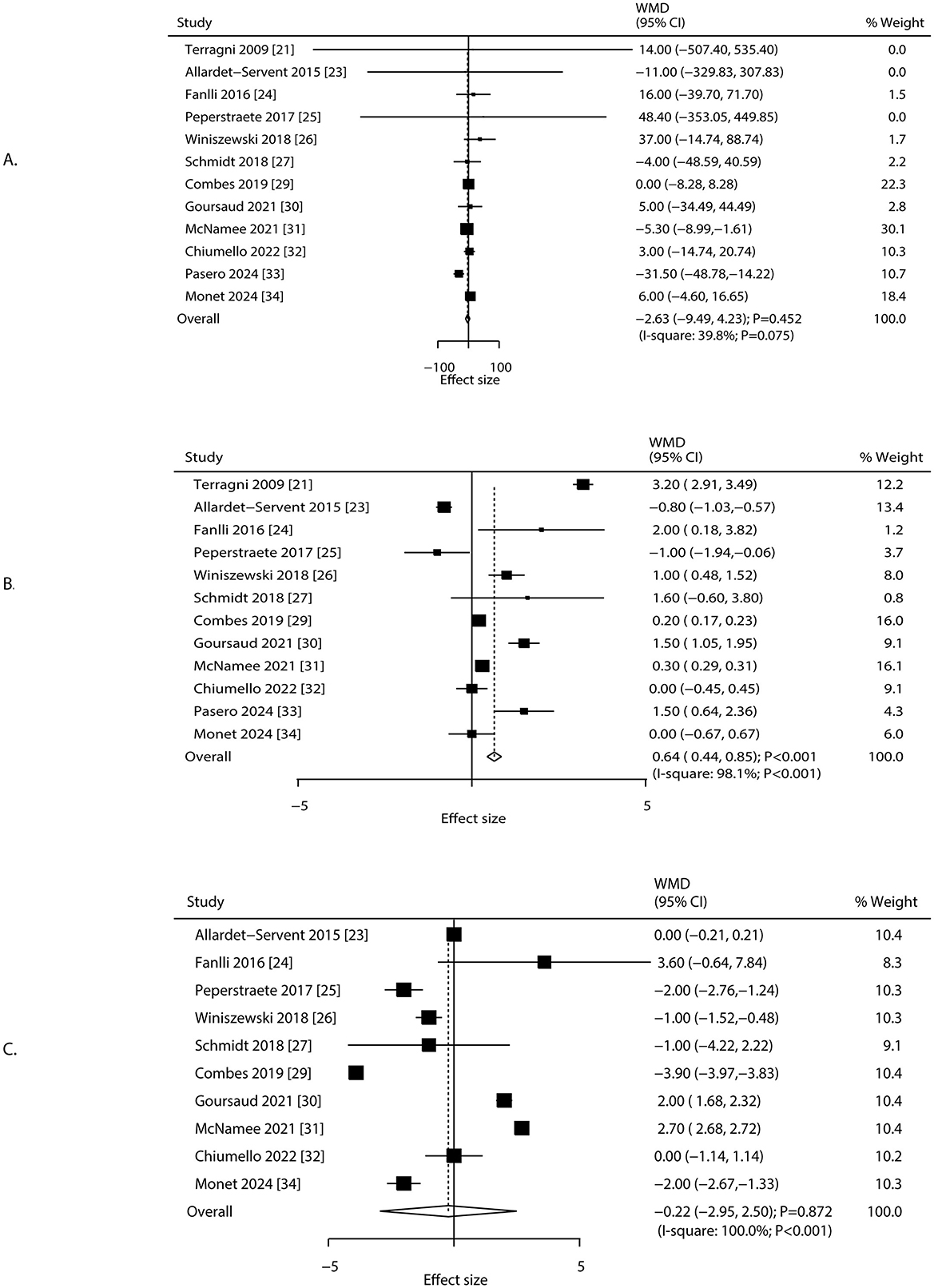
Figure 3. Changes in PaO2/FiO2, PEEP, and respiratory rate after 24 h of ECCO2R, facilitating the application of ultra-protective ventilation. (A) PFR, (B) PEEP, (C) respiratory rate.
pCO2 and pH
Changes in pCO2 and pH after 24 h of ECCO2R were reported in 13 and 11 studies, respectively (Figure 4). The implementation of ECCO2R-assisted ultra-protective ventilation resulted in no significant net change in pCO2 (WMD: −0.11 mmHg; 95% CI: −2.92 to 2.70; P = 0.938) or pH (WMD: 0.02; 95% CI: −0.01 to 0.05; P = 0.205) at 24 h. Substantial heterogeneity was observed for both pCO2 (I2 = 99.6%; P < 0.001) and pH (I2 = 99.9%; P < 0.001). Sensitivity analyses confirmed that the results were not driven by any single study (Supplementary File 3). The treatment effect of ECCO2R-facilitated ultra-protective ventilation on pCO2 and pH could affected by study design, mean age, and male proportion (Table 2). Subgroup analyses showed that pCO2 decreased significantly in retrospective studies but increased in RCTs. pH increased significantly in retrospective studies and among patients aged ≥65.0 years, but decreased in RCTs (Table 2). No significant publication bias was detected for pCO2 (P-value for Egger: 0.399; P-value for Begg: 0.360), or pH (P-value for Egger: 0.687; P-value for Begg: 0.638) at 24 h after initiating ECCO2R (Supplementary File 4).
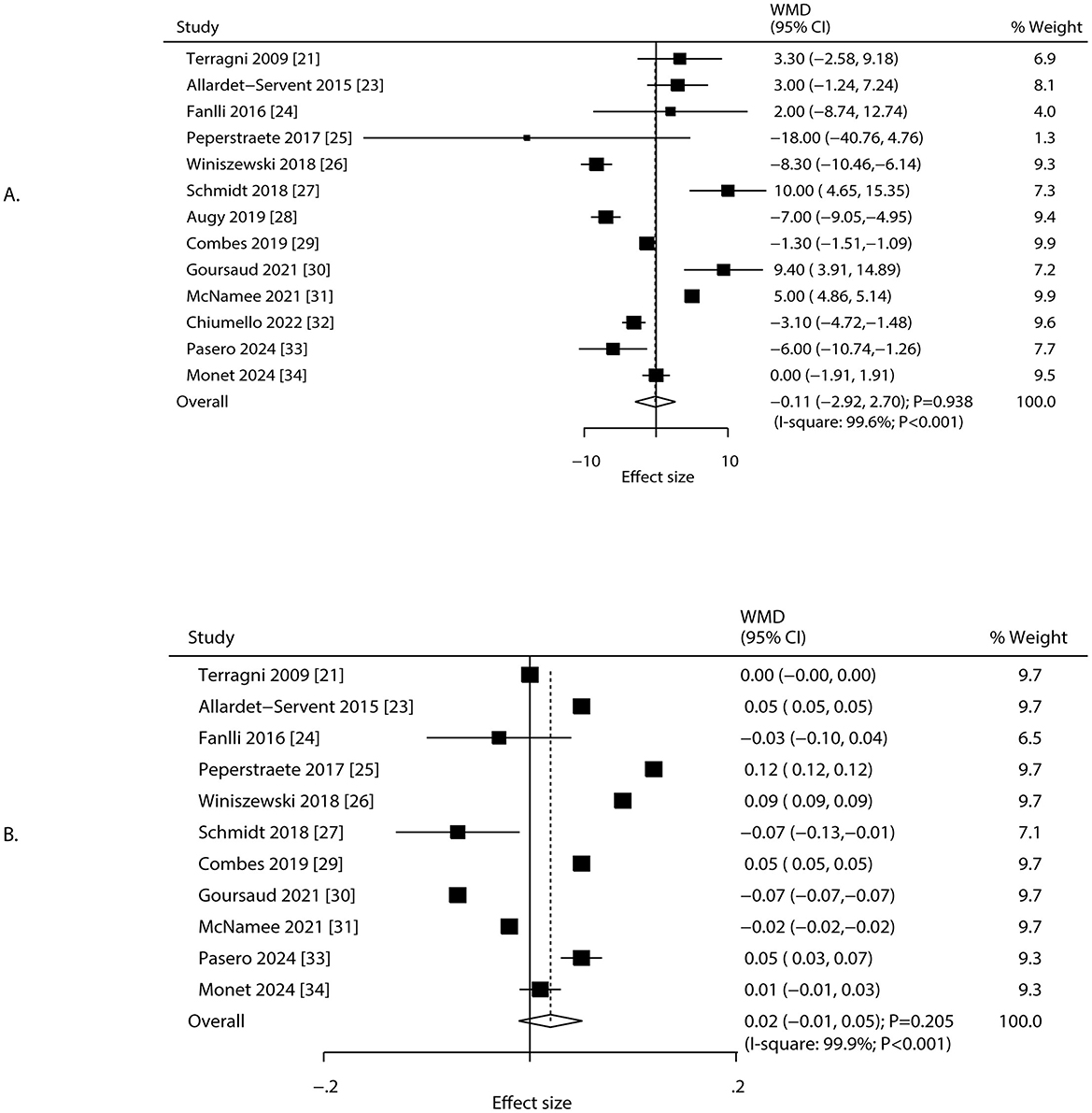
Figure 4. Changes in pCO2 and pH after 24 h of ECCO2R, facilitating the application of ultra-protective ventilation. (A) pCO2, (B) pH.
Mortality and complications
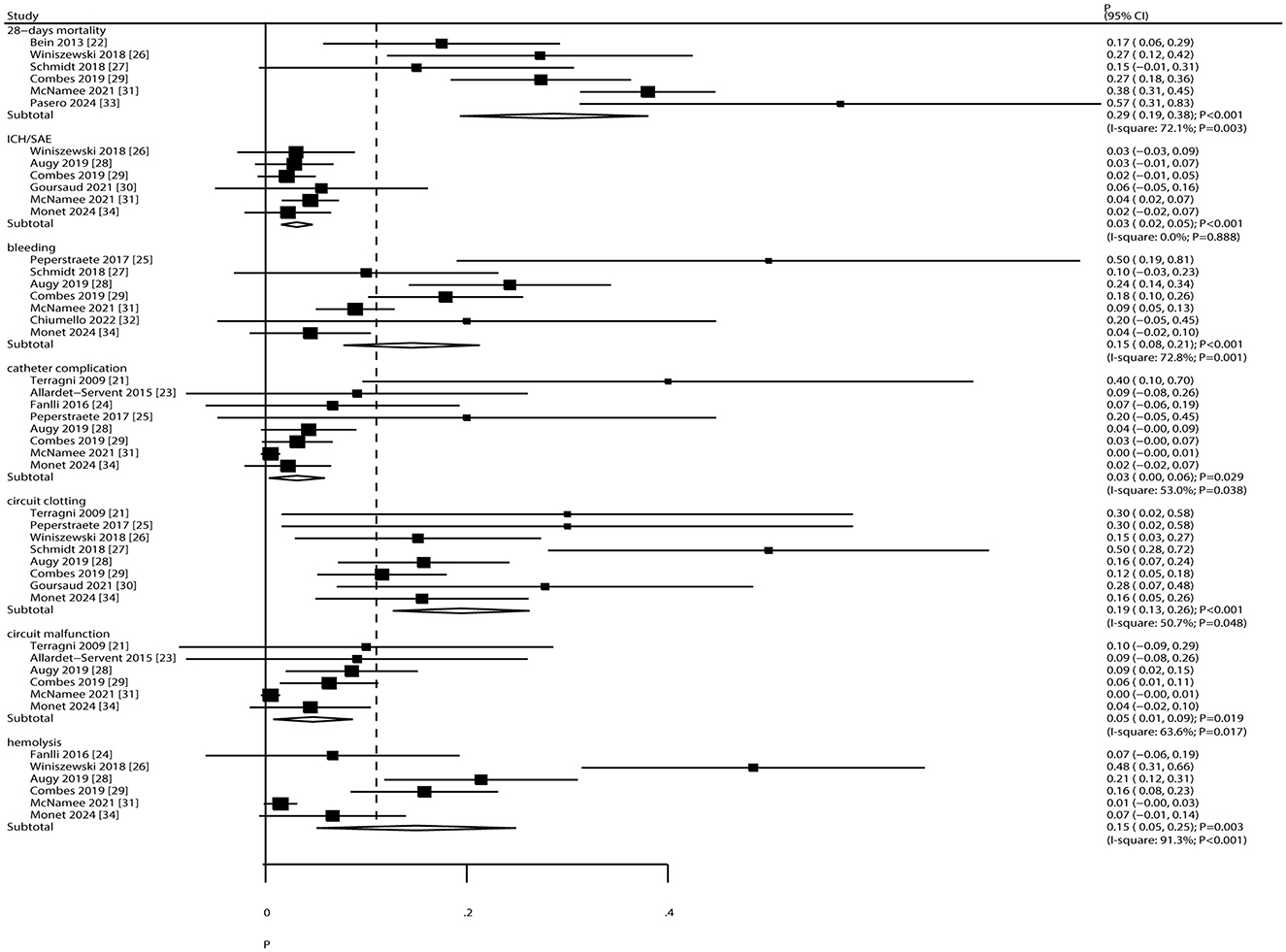
The pooled incidence of 28-day mortality and complications is presented in Figure 5. The overall 28-day mortality rate was 29% (95% CI: 19%−38%). The incidence of specific complications was as follows: intracranial hemorrhage/subarachnoid events (ICH/SAE), 3% (95% CI: 2%−5%); bleeding, 15% (95% CI: 8%−21%); catheter complications, 3% (95% CI: 0%−6%); circuit clotting, 19% (95% CI: 13%−26%); circuit malfunction, 5% (95% CI: 1%−9%); and hemolysis, 15% (95% CI: 5%−25%). Significant heterogeneity was observed for 28-day mortality (I2 = 72.1%; P = 0.003), bleeding (I2 = 72.8%; P = 0.001), catheter complications (I2 = 53.0%; P = 0.038), circuit clotting (I2 = 50.7%; P = 0.048), circuit malfunction (I2 = 63.6%; P = 0.017), and hemolysis (I2 = 91.3%; P < 0.001). No heterogeneity was observed for ICH/SAE (I2 = 0.0%; P = 0.888).
Quality of evidence
The certainty of the evidence for the primary outcomes was formally evaluated using the GRADE framework. As summarized in Supplementary File 5, the overall certainty of evidence was rated as low for all outcomes. The primary reasons for downgrading the evidence were: (1) risk of bias, due to the predominance of non-randomized observational studies which are susceptible to confounding; and (2) inconsistency, due to substantial statistical heterogeneity (I2 > 90%) in the pooled estimates for most respiratory mechanics parameters. Consequently, while the point estimates suggest significant improvements in respiratory mechanics, the true magnitude of these effects is uncertain, and the findings should be interpreted with caution.
Discussion
This meta-analysis demonstrates that ECCO2R-assisted ultra-protective ventilation facilitates clinically meaningful adjustments in ventilation parameters—consistent with lung-protective goals—without directly altering the inherent respiratory mechanics, which remained unchanged. Specifically, ECCO2R enables reductions in driving pressure (−3.70 cmH2O), plateau pressure (−3.26 cmH2O), and tidal volume (−1.68 mL/kg) by mitigating CO2 retention, thereby allowing the safe implementation of ultra-protective ventilation without the risk of severe hypercapnia. These adjustments are intended to reduce ventilator-induced lung injury but do not modify the underlying pathophysiology of ARDS. These findings help reconcile the conflict between lung protection and CO2 management inherent in conventional ventilation strategies. The observed increase in PEEP (0.64 cmH2O) suggests a synergistic effect with ECCO2R in promoting alveolar recruitment, which may further reduce ventilator-induced lung injury. This aligns with preclinical evidence indicating that low tidal volume combined with optimal PEEP decreases alveolar shear stress. However, the certainty of this evidence, as formally assessed by the GRADE framework, is low, primarily due to the inclusion of non-randomized studies with high risk of bias and substantial inconsistency. The effect sizes were consistently larger in observational studies than in the limited RCT data, underscoring the need for cautious interpretation.
Previous meta-analyses have evaluated the efficacy of ECCO2R. One synthesis of 49 studies confirmed that ECCO2R reduces PaCO2 and acidosis, facilitating less invasive ventilation. However, its impact on clinical outcomes remains uncertain, as RCTs showed no mortality benefit and reported more adverse events. That analysis did not specifically focus on ultra-protective strategies or exclude non-ARDS populations (35). Another meta-analysis of 10 studies found that ECCO2R-assisted ultra-protective ventilation reduced driving pressure by 3.56 cmH2O and tidal volume by 1.89 mL/kg from baseline, though no significant changes were observed in oxygenation, respiratory rate, or PEEP. Bleeding and hemolysis were frequently reported complications. However, the study was limited by incomplete literature retrieval and a lack of exploratory analyses (36). Our meta-analysis aimed to address these gaps by comprehensively evaluating the efficacy and safety of ECCO2R-facilitated ultra-protective ventilation specifically in patients with ARDS.
Our findings are consistent with previous reports indicating that ECCO2R helps maintain plateau pressure below 25 cmH2O. However, the reduction in driving pressure observed here (−3.70 cmH2O) exceeds the −1.2 cmH2O reported in earlier trials (37, 38). This discrepancy may stem from the inclusion of more observational studies, in which selection bias could amplify treatment effects. Although ECCO2R is primarily designed for CO2 removal, its neutral effects on PaO2/FiO2, pCO2, and pH are consistent with device mechanisms operating at low blood flow rates, which clear CO2 without improving oxygenation. The lack of a significant change in pCO2 and pH at 24 h should not be interpreted as ECCO2R having no effect on gas exchange. This finding is physiologically consistent with the intervention's purpose: ECCO2R was used to remove the additional CO2 produced by the deliberate reduction in minute ventilation. A stable pCO2 in the context of a significantly reduced tidal volume demonstrates the efficacy of ECCO2R in preventing severe hypercapnia, thereby “permitting” the use of ultra-protective settings (39). A true “control” group without ECCO2R would have been expected to develop significant respiratory acidosis. We have amended our abstract and conclusion to reflect this more precise interpretation.
The 28-day mortality rate of 29%—lower than that in conventional ARDS cohorts—should be interpreted cautiously due to the inclusion of only two RCTs and considerable heterogeneity (40). While ECCO2R facilitates improvements in respiratory mechanics, this meta-analysis highlights that its use is associated with a substantial and clinically important burden of device-related complications. The pooled incidence of bleeding was 15%, circuit clotting 19%, and hemolysis 15%. These are not trivial events; major bleeding can lead to hemodynamic instability and transfusion requirements, circuit clotting can abruptly terminate therapy and potentially cause thromboembolic events, and significant hemolysis can contribute to organ dysfunction (41). These complication rates critically inform the risk-benefit calculus for ECCO2R. The therapy's potential to reduce ventilator-induced lung injury must be weighed against its inherent risks of causing iatrogenic harm. The high rate of circuit clotting underscores the challenge of managing anticoagulation in a critically ill, often coagulopathic, population. Therefore, the application of ECCO2R should be reserved for settings with extensive expertise in extracorporeal support and vigilant monitoring for these specific complications. The “safety” of this approach is relative and is entirely dependent on a team's ability to prevent and manage these frequent adverse events.
The most significant finding of this meta-analysis may be the profound heterogeneity observed in the reported physiological effects of ECCO2R. Our meta-regression indicates that this variability is not random; it is partially explained by study design, mean age, and male proportion. Substantial heterogeneity (I2 >95%) was observed in driving and plateau pressures, attributable to three main factors: (1) variation in patient age (range: 49.8–70.0 years)—subgroup analysis showed attenuated respiratory improvements in patients aged ≥65 years, possibly due to comorbidities and reduced pulmonary compliance; (2) technical variations, including differences in blood flow rates, membrane surface areas, and anticoagulation protocols affecting CO2 clearance efficiency; and (3) inconsistent intervention strategies, such as strictly ultra-protective vs. transitional ventilation approaches. Prospective studies demonstrated greater reductions in plateau pressure compared to retrospective designs, underscoring the influence of study rigor. Additionally, the lack of a significant PEEP increase in subgroups with ≥70% male participants may suggest sex-related differences in chest wall compliance, warranting further investigation.
Based on these findings, early initiation of ECCO2R should be considered for patients with ARDS who exhibit a driving pressure >15 cmH2O during ventilation with a tidal volume of 6 mL/kg, to enable ultra-protective strategies. ECCO2R may also help stabilize transpulmonary pressure in patients with reduced chest wall compliance, such as those with obesity or thoracic deformities. Future high-quality RCTs should determine the optimal timing for ECCO2R initiation using 90-day mortality and ventilator-free days as primary outcomes. Research should also focus on developing predictive models that incorporate patient-specific factors and ECCO2R parameters to guide therapy titration. Technological advancements should aim to reduce dependence on systemic anticoagulation or implement regional anticoagulation strategies to minimize bleeding risks.
This study has several limitations: (1) inclusion of only two small-sample RCTs and a lack of large multicenter data; (2) absence of long-term outcomes such as ventilator-free days or organ recovery, limiting comprehensive prognostic assessment; (3) no direct parallel-group comparisons—future studies should compare ECCO2R-assisted ultra-protective ventilation directly with standard care in ARDS; (4) persistent heterogeneity despite subgroup and sensitivity analyses; and (5) reliance on published data, which may restrict in-depth exploration and increase the risk of publication bias.
Conclusion
In conclusion, data from primarily observational studies suggest that ECCO2R is a feasible strategy to facilitate ultra-protective ventilation, leading to significant improvements in respiratory mechanics and preventing consequential hypercapnia. The certainty of this evidence is low, and the strategy is associated with a distinct profile of device-related risks. Therefore, it should not be considered a standard of care but rather a reserved intervention within a shared decision-making framework at experienced centers, pending further confirmation from high-quality RCTs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WZ: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. XZ: Data curation, Investigation, Methodology, Writing – review & editing. ZS: Investigation, Methodology, Writing – review & editing. YZ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Tianjin Municipal Health Commission Science and Technology Project (No: TIWJ2023MS068).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1707596/full#supplementary-material
References
1. Xu H, Sheng S, Luo W, Xu X, Zhang Z. Acute respiratory distress syndrome heterogeneity and the septic ARDS subgroup. Front Immunol. (2023) 14:1277161. doi: 10.3389/fimmu.2023.1277161
2. Tanios M, Wu TT, Nguyen HM, Smith L, Mahidhara R, Devlin JW. Comparing the impact of targeting limited driving pressure to low tidal volume ventilation on mortality in mechanically ventilated adults with COVID-19 ARDS: an exploratory target trial emulation. BMJ Open Respir Res. (2024) 11:e002439. doi: 10.1136/bmjresp-2024-002439
3. Isenberg DL, Bloom B, Gentile N, Reimer H, Glaze OD, Palumbo P, et al. Males receive low-tidal volume component of lung protective ventilation more frequently than females in the emergency department west. J Emerg Med. (2020) 21:684–7. doi: 10.5811/westjem.2020.2.45191
4. Nieman G, Kollisch-Singule M, Ramcharran H, Satalin J, Blair S, Gatto LA, et al. Unshrinking the baby lung to calm the VILI vortex. Crit Care. (2022) 26:242. doi: 10.1186/s13054-022-04105-x
5. Graf PT, Boesing C, Brumm I, Biehler J, Müller KW, Thiel M, et al. Ultraprotective versus apneic ventilation in acute respiratory distress syndrome patients with extracorporeal membrane oxygenation: a physiological study. J Intensive Care. (2022) 10:12. doi: 10.1186/s40560-022-00604-9
6. Dell'Amore A, D'Andrea R, Caroli G, Mazzoli CA, Rocca A, Stella F, et al. Intraoperative management of hypercapnia with an extracorporeal carbon dioxide removal device during giant bullectomy. Innovations. (2016) 11:142–5. doi: 10.1097/imi.0000000000000250
7. Güldner A, Kiss T, Bluth T, Uhlig C, Braune A, Carvalho N, et al. Effects of ultraprotective ventilation, extracorporeal carbon dioxide removal, and spontaneous breathing on lung morphofunction and inflammation in experimental severe acute respiratory distress syndrome. Anesthesiology. (2015) 122:631–46. doi: 10.1097/ALN.0000000000000504
8. Wang M, Yao Q, Zhu M. Questioning the classification of “high blood flow” versus “low blood flow” ECCO2R in ultra-low tidal volume ventilation studies: a call for functional classification. Crit Care. (2025) 29:121. doi: 10.1186/s13054-025-05352-4
9. Staudinger T. Is there still a place for ECCO2R? Med Klin Intensivmed Notfmed. (2024) 119:59–64. doi: 10.1007/s00063-024-01197-x
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
12. Wells G, Shea B, O'Connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Hospital Research Institute (2009).
13. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. (2005) 25:646–54. doi: 10.1177/0272989X05282643
14. Piggott T, Morgan RL, Cuello-Garcia CA, Santesso N, Mustafa RA, Meerpohl JJ, et al. GRADE Working Group. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: extremely serious, GRADE's terminology for rating down by three levels. J Clin Epidemiol. (2020) 120:116–20. doi: 10.1016/j.jclinepi.2019.11.019
15. Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In:Higgins J, Green S, , editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration (2008).
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. (1999) 47:15–7.
18. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. (2003) 326:219. doi: 10.1136/bmj.326.7382.219
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
21. Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. (2009) 111:826–35. doi: 10.1097/ALN.0b013e3181b764d2
22. Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. (2013) 39:847–56. doi: 10.1007/s00134-012-2787-6
23. Allardet-Servent J, Castanier M, Signouret T, Soundaravelou R, Lepidi A, Seghboyan JM. Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury: the pulmonary and renal support in acute respiratory distress syndrome study. Crit Care Med. (2015) 43:2570–81. doi: 10.1097/CCM.0000000000001296
24. Fanelli V, Ranieri MV, Mancebo J, Moerer O, Quintel M, Morley S, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care. (2016) 20:36. doi: 10.1186/s13054-016-1211-y
25. Peperstraete H, Eloot S, Depuydt P, De Somer F, Roosens C, Hoste E. Low flow extracorporeal CO2 removal in ARDS patients: a prospective short-term crossover pilot study. BMC Anesthesiol. (2017) 17:155. doi: 10.1186/s12871-017-0445-9
26. Winiszewski H, Aptel F, Belon F, Belin N, Chaignat C, Patry C, et al. Daily use of extracorporeal CO2 removal in a critical care unit: indications and results. J Intensive Care. (2018) 6:36. doi: 10.1186/s40560-018-0304-x
27. Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. (2018) 22:122. doi: 10.1186/s13054-018-2038-5
28. Augy JL, Aissaoui N, Richard C, Maury E, Fartoukh M, Mekontso-Dessap A, et al. A 2-year multicenter, observational, prospective, cohort study on extracorporeal CO2 removal in a large metropolis area. J Intensive Care. (2019) 7:45. doi: 10.1186/s40560-019-0399-8
29. Combes A, Fanelli V, Pham T, Ranieri VM. European society of intensive care medicine trials group and the “Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) investigators. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. (2019) 45:592–600. doi: 10.1007/s00134-019-05567-4
30. Goursaud S, Valette X, Dupeyrat J, Daubin C, du Cheyron D. Ultraprotective ventilation allowed by extracorporeal CO2 removal improves the right ventricular function in acute respiratory distress syndrome patients: a quasi-experimental pilot study. Ann Intensive Care. (2021) 11:3. doi: 10.1186/s13613-020-00784-3
31. McNamee JJ, Gillies MA, Barrett NA, Perkins GD, Tunnicliffe W, Young D, et al. Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical trial. JAMA. (2021) 326:1013–23. doi: 10.1001/jama.2021.13374
32. Chiumello D, Pozzi T, Mereto E, Fratti I, Chiodaroli E, Gattinoni L, et al. Long term feasibility of ultraprotective lung ventilation with low-flow extracorporeal carbon dioxide removal in ARDS patients. J Crit Care. (2022) 71:154092. doi: 10.1016/j.jcrc.2022.154092
33. Pasero D, Pistidda L, Piredda D, Liperi C, Cossu A, Esposito R, et al. Lung (extracorporeal CO2 removal) and renal (continuous renal replacement therapy) support: the role of ultraprotective strategy in Covid 19 and non-Covid 19 ARDS. A case-control study. J Anesth Analg Crit Care. (2024) 4:27. doi: 10.1186/s44158-024-s00164-4
34. Monet C, Renault T, Aarab Y, Pensier J, Prades A, Lakbar I, et al. Feasibility and safety of ultra-low volume ventilation (≤3 ml/kg) combined with extra corporeal carbon dioxide removal (ECCO2R) in acute respiratory failure patients. Crit Care. (2024) 28:433. doi: 10.1186/s13054-024-05168-8
35. Stommel AM, Herkner H, Kienbacher CL, Wildner B, Hermann A, Staudinger T. Effects of extracorporeal CO2 removal on gas exchange and ventilator settings: a systematic review and meta-analysis. Crit Care. (2024) 28:146. doi: 10.1186/s13054-024-04927-x
36. Worku E, Brodie D, Ling RR, Ramanathan K, Combes A, Shekar K. Venovenous extracorporeal CO2 removal to support ultraprotective ventilation in moderate-severe acute respiratory distress syndrome: a systematic review and meta-analysis of the literature. Perfusion. (2023) 38:1062–79. doi: 10.1177/02676591221096225
37. Ayzac L, Girard R, Baboi L, Beuret P, Rabilloud M, Richard JC, et al. Ventilator-associated pneumonia in ARDS patients: the impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Med. (2016) 42 :871–8. doi: 10.1007/s00134-015-4167-5
38. Loria V, Aparicio A, Hildesheim A, Cortés B, Barrientos G, Retana D, et al. Cohort profile: evaluation of immune response and household transmission of SARS-CoV-2 in Costa Rica: the RESPIRA study. BMJ Open. (2023) 13:e071284. doi: 10.1136/bmjopen-2022-071284
39. Cobeta P, Gomis A, Claver S, Encuentra M, Rubin S, Salvador E, et al. Extracorporeal CO2 removal in severe respiratory acidotic intubated patients: a seven year experience observational study. Respir Med. (2025) 240:108011. doi: 10.1016/j.rmed.2025.108011
40. Duggal A, Conrad SA, Barrett NA, Saad M, Cheema T, Pannu S, et al. Extracorporeal carbon dioxide removal to avoid invasive ventilation during exacerbations of chronic obstructive pulmonary disease: VENT-AVOID Trial-A randomized clinical trial. Am J Respir Crit Care Med. (2024) 209:529–42. doi: 10.1164/rccm.202403-0618LE
Keywords: extracorporeal carbon dioxide removal, ultra-protective ventilation, acute respiratory distress syndrome, systematic review, meta-analysis
Citation: Zhen W, Zhang X, Shi Z and Zhu Y (2025) Effects of extracorporeal carbon dioxide removal in facilitating ultra-protective ventilation strategies for patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Front. Med. 12:1707596. doi: 10.3389/fmed.2025.1707596
Received: 17 September 2025; Accepted: 17 October 2025;
Published: 12 November 2025.
Edited by:
Tommaso Tonetti, University of Bologna, ItalyReviewed by:
Pietro Bertini, Azienda Ospedaliero Universitaria Pisana, ItalyMaria Paparoupa, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2025 Zhen, Zhang, Shi and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhu, emh1eXVsaW85OTlAMTYzLmNvbQ==
 Weifeng Zhen
Weifeng Zhen Yu Zhu
Yu Zhu