- Respiratory Pathophysiology Unit, Department of Cardiac-Thoracic-Vascular Sciences and Public Health, University of Padova, Padova, Italy
Background: Severe asthma remains a major problem despite pharmacological advances. Pulmonary rehabilitation (PR) is established in chronic respiratory disease but its role in severe asthma is unclear.
Objectives: Summarise evidence on PR in severe and uncontrolled asthma, describe PR-modalities, and outline implementation and research priorities.
Methods: Narrative review of systematic reviews and clinical studies of multidimensional PR programmes and isolated components [aerobic training, inspiratory muscle training (IMT), breathing retraining, neuromuscular electrical stimulation (NMES), telerehabilitation]. Outcomes included asthma control, HRQoL, exercise capacity and healthcare utilisation.
Results: Multicomponent PR improves exercise capacity and multiple QoL domains; pooled data show substantial increases in six-minute walk distance. Combined exercise, education and self-management produced clinically meaningful improvements in asthma control and symptoms, notably patients with uncontrolled disease and functional impairment. IMT, NMES and breathing retraining improved inspiratory strength, peripheral muscle function and hyperventilation symptoms. Telerehabilitation expands access but requires attention to digital literacy and adherence. Heterogeneity, small samples and attrition limit generalisability.
Conclusion: PR is a promising personalised, multidisciplinary adjunct for severe asthma. Larger phenotype-stratified trials, harmonised outcome sets and implementation research are needed to define candidate selection, optimal dose and cost-effectiveness; embedding PR within severe asthma centres may optimise outcomes and reduce healthcare use.
1 Introduction
Asthma is a chronic inflammatory disease of the airways, characterized by variable airflow obstruction, bronchial hyperresponsiveness and recurrent respiratory symptoms (1). It affects an estimated 339 million people worldwide and imposes a substantial public-health and socio-economic burden (2). Despite therapeutic advances, including inhaled corticosteroids, long-acting bronchodilators and targeted biologic agents, a proportion of patients continue to experience severe or uncontrolled asthma according to ATS/ERS definitions (3). Difficult-to-treat asthma refers to asthma that remains uncontrolled despite medium- or high-dose inhaled corticosteroids with a second controller, or requires oral corticosteroids to achieve control (1, 3). It is distinguished from severe asthma, a subset in which disease remains uncontrolled despite optimized therapy and management of comorbidities, or worsens when treatment is stepped down. In clinical practice, poor adherence to therapy, incorrect inhaler technique and underuse of maintenance inhaled corticosteroids contribute to disease instability and recurrent exacerbation cycles. Multimorbidity is common in severe asthma; rhinosinusitis, obesity, gastroesophageal reflux, anxiety, depression and dysfunctional behaviours amplify clinical complexity, worsen control and increase healthcare utilisation (4, 5). A comprehensive, multimodal care strategy is therefore required to optimise clinical trajectories for these patients (1). Current guidelines recommend personalised care plans negotiated between patients, caregivers and the multidisciplinary clinical team, within a continuous cycle of assessment, adjustment and review (1). Biologic therapies targeting specific inflammatory pathways have improved outcomes in selected phenotypes, but residual symptom burden and functional impairment persist in many patients, motivating investigation of non-pharmacological adjuncts. In other chronic respiratory diseases, notably COPD, pulmonary rehabilitation (PR) is an evidence-based cornerstone that improves dyspnoea, fatigue, emotional function, quality of life and exercise capacity; telemedicine delivery is an emerging method to increase access (6, 7). This review evaluates PR in severe and uncontrolled asthma, analyses available evidence, describes modalities and practical aspects and identifies research priorities.
2 Pulmonary rehabilitation
Pulmonary rehabilitation (PR) is defined by ATS/ERS as a comprehensive intervention based on detailed patient assessment, followed by individually tailored therapies including exercise training, education and behaviour change to improve physical and psychological condition and to promote long-term adherence to health-enhancing behaviours (8). Despite early consensus statements and efficacy data, access to PR remains limited by insufficient funding, restricted dedicated resources, variable awareness among clinicians and patients, and inconsistent training opportunities for providers; its availability differs widely across countries (9).
GINA recommendations recognises PR as complementary to pharmacological management (level A evidence for improvements in functional exercise capacity and health-related quality of life), although direct evidence for symptom control and long-term durability in asthma is less conclusive (1). PR programmes are multimodal by design and should be adapted to individual needs and comorbidities (9).
2.1 Components and duration of PR programme
Most high-quality data relative to PR programme derive from COPD populations, but practical principles could be at least partially translated to asthma. Meta-analyses and consensus statements recommend a minimum programme duration of approximately eight weeks to achieve clinically meaningful gains, with outpatient models typically delivering two to three supervised sessions per week and inpatient models offering more frequent supervised training (6, 9). A combination of supervised sessions and prescribed home exercise, supported by follow-up and booster cycles, promotes maintenance of benefits; repeated programme cycles produce effects comparable to initial one (9). For asthma patients, no consensus yet exists regarding optimal duration, total session number, intensity schema, or staffing composition—which may include physicians, healthcare professionals, psychologists, nutritionists, and social workers—as heterogeneity in study designs limits generalisability (8). High-quality programmes should include structured supervised exercise, education and behavioural support, comprehensive assessment and personalised outcome measurement, and home-exercise prescription with encouragement to perform daily physical activity (9). Exercise sessions typically last 30–90 min, two to three times per week, and may combine aerobic training (e.g., cycling or treadmill) with strength and flexibility exercises, either with equipment or bodyweight (10, 11). Educational components typically cover safety, treatment use, exacerbation management, and lifestyle aspects such as physical activity, nutrition, emotional well-being, and social interaction (12).
3 Current evidence on pulmonary rehabilitation in severe asthma
Two recent systematic reviews provide complementary perspectives. A Cochrane review of comprehensive PR programmes reported significant improvement in functional capacity, with a pooled mean increase of 79.8 meters in the six-minute walk test (6MWT), while improvements in maximal exercise capacity (VO₂max, VO₂peak) were modest and uncertain due to heterogeneity and limited long-term data (13). Zampogna et al. (14) considered isolated rehabilitation components (aerobic training, inspiratory muscle training, breathing exercises, yoga, education) and found uncertain or limited effects on health-related quality of life (Asthma Quality of Life Questionnaire AQLQ) and asthma control, reflecting methodological diversity across studies.
Studies targeting severe or uncontrolled asthma show promising but heterogeneous results. Majd et al. reported that a 12-week tailored rehabilitation programme yielded improvements in physical performance, health-related quality of life and asthma control in patients with severe disease (15). Schultz et al. evaluated a 3-week inpatient PR programme in patients with Asthma Control Test (ACT) scores <20 and documented clinically meaningful ACT improvements and secondary benefits in AQLQ, SGRQ (St. George’s Respiratory Questionnaire), core respiratory symptoms, self-management skills, illness perception, anxiety and depression; many improvements persisted up to 12 months (16). Ricketts et al. (17) studied an 8-week programme for patients with uncontrolled asthma and BMI ≥ 25 kg·m−2 (Body mass index) combining one supervised weekly session with two home sessions. The intervention improved asthma control, symptoms and exercise tolerance, particularly in activity and symptom domains of the AQLQ, but did not increase accelerometer-measured physical activity, suggesting limited behavioural change. A 12-month follow-up of the cohort demonstrated sustained improvements among completers but highlighted high attrition and barriers including the COVID-19 pandemic, work and personal constraints, and perceived exercise difficulty (18). A retrospective home-based PR analysis showed improved 6MST (six-minute stepper test) step counts and long-term quality of life in severe asthma patients, though anxiety and depression changes were less evident compared with COPD cohorts (19).
In summary these data indicate that PR confers functional, symptomatic and quality-of-life benefits in selected asthma populations, especially those with uncontrolled symptoms or deconditioning; however, small sample sizes, heterogeneous interventions and retention challenges limit definitive conclusions.
4 Strategic approaches and modalities in PR in severe asthma
The most relevant methods utilized for pulmonary rehabilitation in asthma have been summarized and listed in Table 1 and illustrated in the paragraphs below.
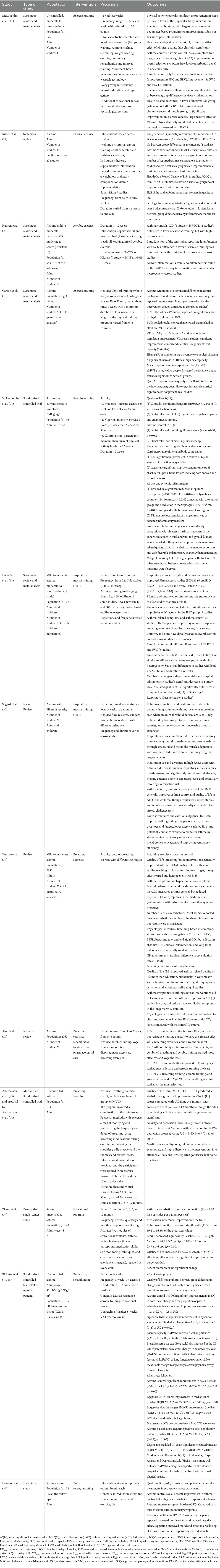
Table 1. Evidence of major PR methods in asthma, with particular attention to the type of interventions, programs and outcomes.
4.1 Exercise training
Structured exercise training is the principal active component of PR. Aerobic training is safe in adults with asthma and is associated with improved symptom control, HRQoL (health related quality of life), maximal exercise capacity (VO₂max and HRmax) and functional capacity; benefits are more evident when exercise is combined with dietary, educational or behavioural interventions and performed at moderate intensity or above (10, 11, 20–22). Poor evidence is reported on a role played by exercise on inflammation in severe asthma: moderate-intense activity conducted for 12 weeks appears to reduce airway inflammation, but not at a systemic level (22). However, in patients with early-stage asthma, including mild intermittent asthma, there is promising evidence that moderate-intensity supervised aerobic training (SAT), performed three times per week, decreases pulmonary and systemic profibrotic biomarkers while increasing antifibrotic markers, and its anti-inflammatory effect is sensitively detected by FeNO. It also reduces airway eosinophil and macrophage infiltration, as well as peripheral blood eosinophil and lymphocyte counts (23). Quality of life (AQLQ) and asthma control questionnaire (ACQ) showed improvement as a result of moderate- and vigorous-intensity training (24). Resistance training mitigates muscle mass and strength loss, reduces fall risk and exertional dyspnoea compared to sustained high-intensity continuous exercise, improving tolerance and enabling progressive load increases (8). Interval training alternating high-intensity bouts with recovery periods is suitable where dyspnoea or fatigue limit longer continuous efforts, supporting higher training stimulus with lower perceived symptoms (8). Walking aids including adjunctive oxygen support when necessary may assist severely impaired patients by improving respiratory mechanics (25). Use of fitness trackers is recommended for objective monitoring and behaviour support (26), while family involvement, and patient network engagement enhance adherence (27, 28).
4.2 Neuromuscular electrical stimulation (NMES)
NMES is an adjunctive option to strengthen peripheral muscle without active exertion and is particularly useful in highly debilitated patient (4). Recent trials in older adults with mild persistent asthma reported that adding NMES to conventional rehabilitation improved quadriceps strength, endurance, functional capacity, dyspnoea and HRQoL within four weeks, with a favourable safety profile (29).
4.3 Inspiratory muscle training (IMT)
Airflow obstruction and dynamic hyperinflation increase inspiratory workload and promote diaphragm flattening, reducing mechanical efficiency. IMT, applied using threshold or tapered resistive devices, increases maximal inspiratory pressures (PImax), endurance and reduces dyspnoea when training intensity reaches or exceeds approximately 30% of PImax. IMT was found to be most beneficial in patients with documented inspiratory muscle weakness (PImax or PEmax below ~65–80% predicted); in these cases, incremental benefits can be gained by whole-body training are most evident in these cases (29–31).
4.4 Breathing retraining and adjunctive interventions
Breathing retraining methods (Buteyko, Papworth, yoga-based techniques) target dysfunctional breathing patterns, improving symptom perception, HRQoL and psychological outcomes although effects on lung function and exacerbation frequency are limited (32–34). Adjunctive supports such as bronchodilator therapy optimisation, oxygen supplementation, anabolic support, heliox or non-invasive ventilation may enhance exercise tolerance for selected patients but require individualised risk–benefit assessment (8).
4.5 Education and self-management
Education is a core component of PR and is recommended within GINA algorithms for difficult-to-treat asthma (1). Contemporary educational strategies emphasize shared decision-making, problem-solving and self-management skills, addressing medication safety (including biologic therapy and corticosteroid use), exacerbation action plans, inhaler technique and lifestyle counselling (8, 12, 28). Integrated educational modules within PR improve self-efficacy and may enhance adherence, though evaluating the independent effect of education is methodologically challenging (17, 35–37).
4.6 Telerehabilitation
Telerehabilitation offers flexible, incremental delivery—ranging from synchronous supervised sessions to asynchronous educational modules, exercise videos and symptom tracking—and can broaden access for patients with mobility constraints or geographic barriers (38). Hybrid models including initial in-person assessment followed by remote supervision may optimise safety, engagement and cost-efficiency. Challenges include variable digital literacy, technology access and the need to ensure intervention fidelity.
4.7 Maintenance strategies and outcomes
Effective maintenance strategies have demonstrated benefit include periodic supervised booster sessions, telephone or text-message support combined with structured home exercise, diaries and activity monitors; these approaches can sustain walking distance and activity, though evidence for preserved HRQoL is not consistent (39). Optimal contact frequency is uncertain; follow-up intervals vary among programmes, with some adopting quarterly reviews and others semi-annual monitoring after the first year (18, 40). Economic analyses and cost-effectiveness data remain scant, with variability across health systems. Core outcome domains include HRQoL, asthma control (ACQ/ACT), exercise capacity (6MWT: Six-minute Walk test, 6MST), objectively measured physical activity, anxiety and depression, respiratory function, inflammatory biomarkers and medication adherence. Long-term follow-up is desirable because PR benefits may reduce within 6–12 months without maintenance (8, 9); however standardized timing criteria remain undefined.
4.8 Key performance indicators
Standardized process and performance indicators facilitate benchmarking and quality assurance. Suggested metrics include clinical outcomes (control scores, exacerbation and hospitalization rates), functional measures (6MWT, exercise tolerance), psychological outcomes, healthcare utilization and cost-effectiveness indicators to support service evaluation and commissioning (9, 13, 41, 42) (Table 2).
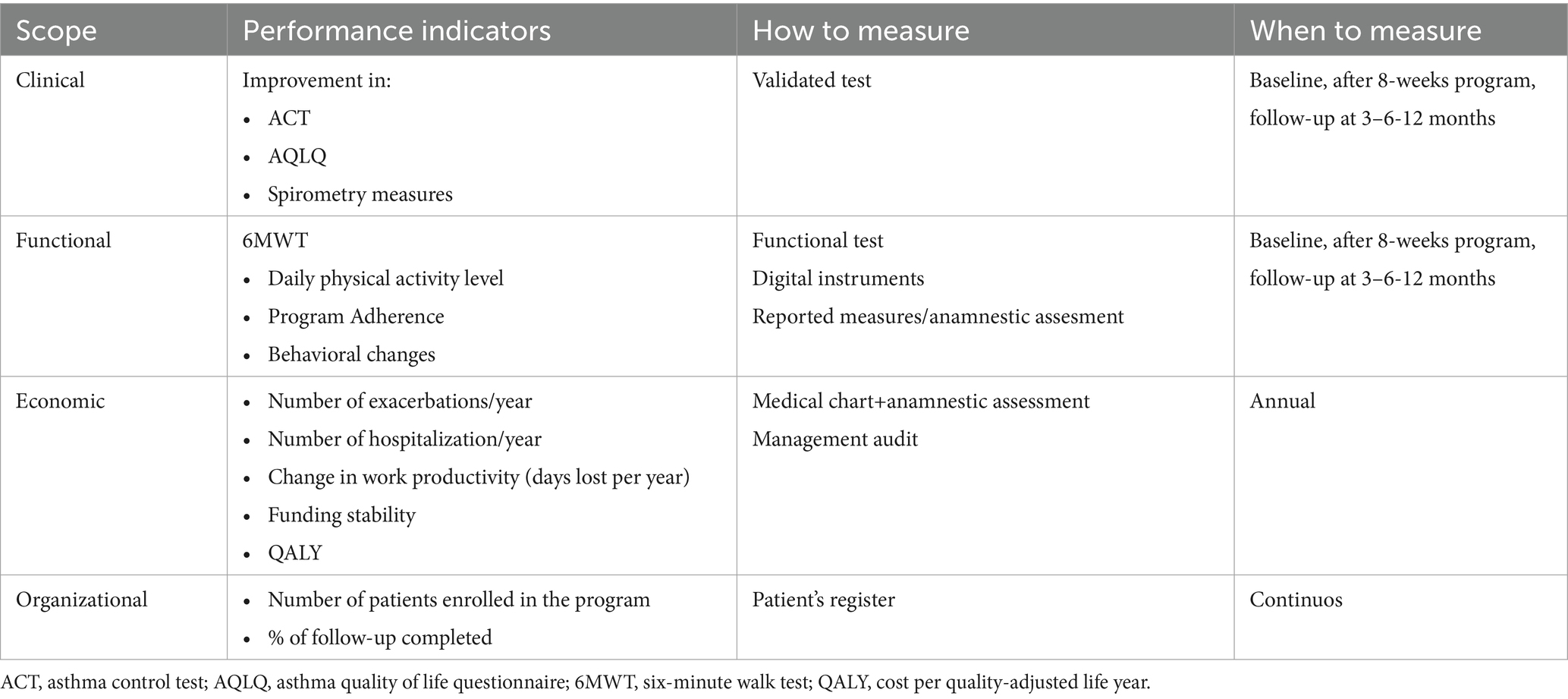
Table 2. Performance indicators base on clinical, functional, economic and organizational scope and timing to measure them (9, 10, 39, 40).
5 Discussion
Severe and uncontrolled asthma presents ongoing challenges despite pharmacological advances. PR offers a multidimensional strategy addressing deconditioning, dysfunctional breathing, multimorbidity and self-management deficits. Evidence indicates that structured exercise, particularly when combined with education and behavioural support, improves exercise capacity, dyspnoea and HRQoL and may reduce exacerbation burden in selected cohorts (18–22). IMT delivers important gains in inspiratory strength and symptom relief when respiratory muscle weakness is present (30, 31). Breathing retraining is effective on symptom perception and psychological measures but does not improve pulmonary function test spirometry nor reduce exacerbation risk substantially (32–34, 43, 44). Biking is safe, adaptable and appropriate for patients with osteopenia or osteoporosis, which are common comorbidities in severe asthma (45).
Referral criteria should consider persistent symptoms, functional limitation, frequent exacerbations and healthcare utilization as well as abnormal pulmonary function test; contraindications are uncommon and most barriers can be addressed by program adaptation (8).
Program heterogeneity and small study sample sizes limited the strength of recommendations and generalisability. High dropout indicates to the need for flexible, patient-centred delivery models that may combine employment commitments and individual preferences; hybrid programmes, evening or weekend sessions and telerehabilitation options may improve access and retention (13, 17, 22). Adherence is supported by engaging, varied exercise prescriptions, fitness trackers, family involvement and educational initiatives that increase perceived benefits and self-efficacy (26–28). Safety considerations include cardiovascular and musculoskeletal screening, attention to corticosteroid-related risks and clear procedures for managing exacerbations during rehabilitation programmes (2, 8). In particular it is known that Exercise-induced bronchoconstriction (EIB) may occur in otherwise well-controlled asthma, typically 5–10 min post-exercise, with symptoms such as dyspnea, wheeze, chest tightness, or cough. GINA guidelines recommend pre-medicate with a rapid-acting inhaled b2-agonist prior to exercise, with leukotriene receptor antagonists or cromones as alternatives, and include a gradual warm-up (1, 46).
From an economic standpoint, PR may be cost-effective by preventing escalation of pharmacotherapy, reducing unplanned visits and hospital admissions and improving productivity through reduced absenteeism and presenteeism. PR shows a higher QALY (Cost per quality-adjusted life year) gain compared to other treatments (9, 47). Rigorous health-economic analyses are needed to guide policymakers.
Additional considerations include the high prevalence of comorbid conditions—chronic rhinosinusitis, obesity, gastroesophageal reflux and psychiatric disorders—which may influence rehabilitation needs and outcomes and should be proactively managed within multidisciplinary programmes (5). Multidisciplinary team commonly includes respiratory physicians, physiotherapists/exercise physiologists, specialist nurses, psychologists and dietitians; competency frameworks and training are required to ensure safe, standardised delivery (9).
Scientific evidence highlights the importance of fidelity and adherence metrics; trials should prespecify adherence thresholds and report intention-to-treat effects. Standardised reporting of intervention dose, intensity and adherence will enable meta-analysis and possibility to identify active strategies.
Technological supports including wearables, smartphone apps and secure telehealth platforms may enable objective monitoring, remote supervision and patients’education, yet digital inequities and data governance require attention (22, 38, 39). In order to disseminate rehabilitation in severe asthma, efforts should be done to address equity, ensuring educational materials and community delivery options to underserved populations who bear disproportionate asthma morbidity.
6 Conclusion
Pulmonary rehabilitation represents a promising adjunctive intervention for patients with severe or uncontrolled asthma. Multidimensional programmes that combine exercise training, education and behavioural support produce clinically relevant improvements in asthma control, dyspnoea, exercise capacity and quality of life in selected populations. However, the evidence remains heterogeneous and limited in severe asthma patients. Future priorities include large, phenotype-stratified randomized trials, harmonised core outcome sets including patient-reported and economic endpoints, standardisation of programme and implementation of research to determine optimal delivery models and cost-effectiveness. Recommendations and future directions include adopting a personalised, phenotype-informed approach to rehabilitation referral and design, with early identification of patients who are deconditioned, physically inactive, or experiencing frequent exacerbations despite optimal pharmacotherapy. Multidisciplinary teams should tailor interventions to address comorbid contributors such as obesity, psychological distress and sinonasal disease, and should incorporate objective activity monitoring, stratified goal setting, and family or community support to promote long-term behaviour change. Programmes should also consider occupational and social determinants of health and offer flexible scheduling and hybrid delivery models. Safety screening, inhaler technique optimisation, action plans and integration with severe asthma Clinics are essential components. Shared core outcome sets and registries will facilitate pooled analyses and benchmarking. Education plans should be standardised across programmes while allowing local adaptation. Funding should prioritise pragmatic trials and implementation research, and healthcare systems should consider reimbursement mechanisms to support multidisciplinary rehabilitation teams. Ultimately, embedding PR within strategic approach of severe asthma Centers may optimise patient-centred outcomes and reduce healthcare utilisation.
Author contributions
GG: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. MP: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AV: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open Access funding provided by Universitã degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GINA Scientific Commitee. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma. (2025). Available online at: https://ginasthma.org/2025-gina-strategy-report/ (Accessed May, 2024).
2. Busse, WW, and Kraft, M. Current unmet needs and potential solutions to uncontrolled asthma. Eur Respir Rev. (2022) 31:210176. doi: 10.1183/16000617.0176-2021
3. Chung, KF, Wenzel, SE, Brozek, JL, Bush, A, Castro, M, Sterk, PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73. doi: 10.1183/09031936.00202013
4. Scortichini, M, Mennini, FS, Marcellusi, A, Paoletti, M, Tomino, C, and Sciattella, P. The economic burden of asthma in Italy: evaluating the potential impact of different treatments in adult patients with severe eosinophilic asthma. Eur J Health Econ. (2024) 26:869–76. doi: 10.1007/s10198-024-01736-5
5. Chen, W, Safari, A, FitzGerald, JM, Sin, DD, Tavakoli, H, and Sadatsafavi, M. Economic burden of multimorbidity in patients with severe asthma: a 20-year population-based study. Thorax dicembre. (2019) 74:1113–9. doi: 10.1136/thoraxjnl-2019-213223
6. McCarthy, B, Casey, D, Devane, D, Murphy, K, Murphy, E, and Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Airways Group, curatore. Cochrane Database Syst Rev. (2015) 2015. doi: 10.1002/14651858.CD003793.pub3
7. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention od Chronic Obstructive Lung Disease (GOLD 2025 Report). Global Initiative for Chronic Obstructive Lung Disease (GOLD). (2025) 63–67. Avaliable online at: https://goldcopd.org/2025-gold-report/
8. Spruit, MA, Singh, SJ, Garvey, C, ZuWallack, R, Nici, L, Rochester, C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. (2013) 188:e13–64. doi: 10.1164/rccm.201309-1634ST
9. Rochester, CL, Vogiatzis, I, Holland, AE, Lareau, SC, Marciniuk, DD, Puhan, MA, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. (2015) 192:1373–86. doi: 10.1164/rccm.201510-1966ST
10. Carson, KV, Chandratilleke, MG, Picot, J, Brinn, MP, Esterman, AJ, and Smith, BJ. Physical training for asthma. Cochrane airways group, curatore. Cochrane Database Syst Rev. (2013) 2013. doi: 10.1002/14651858.CD001116.pub4
11. McLoughlin, RF, Clark, VL, Urroz, PD, Gibson, PG, and McDonald, VM. Increasing physical activity in severe asthma: a systematic review and meta-analysis. Eur Respir J. (2022) 60:2200546. doi: 10.1183/13993003.00546-2022
12. Laurence, R, Ancel, J, Devilliers, MA, Carre, S, Dury, S, Dormoy, V, et al. Patient education needs in severe asthma, a pilot study. BMC Pulm Med. (2024) 24:134. doi: 10.1186/s12890-024-02960-8
13. Osadnik, CR, Gleeson, C, McDonald, VM, and Holland, AE. Pulmonary rehabilitation versus usual care for adults with asthma. Cochrane Database Syst Rev. (2022) 2022. doi: 10.1002/14651858.CD013485.pub2
14. Zampogna, E, Paneroni, M, Cherubino, F, Pignatti, P, Rudi, M, Casu, G, et al. Effectiveness of a pulmonary rehabilitation program on persistent asthma stratified for severity. Respir Care. (2019) 64:1523–30. doi: 10.4187/respcare.06761
15. Majd, S, Apps, L, Chantrell, S, Hudson, N, Eglington, E, Hargadon, B, et al. A feasibility study of a randomized controlled trial of asthma-tailored pulmonary rehabilitation compared with usual Care in Adults with severe asthma. J Allergy Clin Immunol Pract. (2020) 8:3418–27. doi: 10.1016/j.jaip.2020.05.052
16. Schultz, K, Wittmann, M, Wagner, R, Lehbert, N, and Schwarzkopf, L. Szentes, B et al In-patient pulmonary rehabilitation to improve asthma control. Dtsch Arztebl Int. (2021). doi: 10.3238/arztebl.m2021.0003
17. Ricketts, HC, Sharma, V, Steffensen, F, Goodfellow, A, Mackay, E, MacDonald, G, et al. A pragmatic randomised controlled trial of tailored pulmonary rehabilitation in participants with difficult-to-control asthma and elevated body mass index. BMC Pulm Med. (2022) 22:363. doi: 10.1186/s12890-022-02152-2
18. Ricketts, H, Sharma, V, Steffensen, F, Mackay, E, MacDonald, G, Buchan, D, et al. Immediate and one-year outcomes of an asthma-tailored pulmonary rehabilitation programme in overweight and obese people with difficult-to-treat asthma. J Asthma Allergy. (2024) 17:911–28. doi: 10.2147/JAA.S466894
19. Grosbois, JM, Coquart, J, Fry, S, Le Rouzic, O, Grosbois, T, Wallaert, B, et al. Long-term effect of home-based pulmonary rehabilitation in severe asthma. Respir Med ottobre. (2019) 157:36–41. doi: 10.1016/j.rmed.2019.08.015
20. Xing, S, Feng, S, and Zeng, D. Effect of exercise intervention on lung function in asthmatic adults: a network meta-analysis. Ann Med. (2023) 55:2237031. doi: 10.1080/07853890.2023.2237031
21. Kuder, MM, Clark, M, Cooley, C, Prieto-Centurion, V, Danley, A, Riley, I, et al. A systematic review of the effect of physical activity on asthma outcomes. J Allergy Clin Immunol Pract. (2021) 9:3407–3421.e8. doi: 10.1016/j.jaip.2021.04.048
22. Hansen, ESH, Pitzner-Fabricius, A, Toennesen, LL, Rasmusen, HK, Hostrup, M, Hellsten, Y, et al. Effect of aerobic exercise training on asthma in adults: a systematic review and meta-analysis. Eur Respir J. (2020) 56:2000146. doi: 10.1183/13993003.00146-2020
23. Moraes-Ferreira, R, Brandao-Rangel, MAR, Gibson-Alves, TG, Silva-Reis, A, Souza-Palmeira, VH, Aquino-Santos, HC, et al. Physical training reduces chronic airway inflammation and mediators of remodeling in asthma. Oxid Med Cell Longev gennaio. (2022) 2022:5037553. doi: 10.1155/2022/5037553
24. Valkenborghs, SR, Wood, LG, Callister, R, Upham, JW, Grainge, CL, Anderson, S, et al. Effects of moderate- versus vigorous-intensity exercise training on asthma outcomes in adults. J Allergy Clin Immunol Pract ottobre. (2024) 12:2744–2753.e8. doi: 10.1016/j.jaip.2024.06.015
25. Probst, VS, Troosters, T, Coosemans, I, Spruit, MA, Pitta, FDO, Decramer, M, et al. Mechanisms of improvement in exercise capacity using a rollator in patients with COPD. Chest. (2004) 126:1102–7. doi: 10.1378/chest.126.4.1102
26. Ferguson, T, Olds, T, Curtis, R, Blake, H, Crozier, AJ, Dankiw, K, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. (2022) 4:e615–26. doi: 10.1016/S2589-7500(22)00111-X
27. Muijsenberg, AJ, Houben-Wilke, S, Tatousek, J, Lacroix, J, Spruit, MA, and Janssen, DJ. Educational needs of people with COPD or asthma entering pulmonary rehabilitation and their significant others: a cross-sectional study. Chron Respir Dis. (2025) 22:14799731251316891 doi: 10.1177/14799731251316891
28. Muijsenberg, AJL, Haesevoets, S, Houben-Wilke, S, Tatousek, J, Lacroix, J, Spruit, MA, et al. Motivation and preferences for learning of patients with COPD or asthma and their significant others in pulmonary rehabilitation: a qualitative study. ERJ Open Res. (2024) 10:01021–2023. doi: 10.1183/23120541.01021-2023
29. Develi, E, Muammer, R, and Kucukardali, Y. Effects of neuromuscular electrical stimulation on muscle strength, functional capacity, and quality of life among older patients with asthma. Altern Ther Health Med. (2024) 30:6–15.
30. Sogard, AS, and Mickleborough, TD. The therapeutic role of inspiratory muscle training in the management of asthma: a narrative review. Am J Physiol-Regul Integr comp Physiol. (2023) 325:R645–63.
31. Lista-Paz, A, Bouza Cousillas, L, Jácome, C, Fregonezi, G, Labata-Lezaun, N, Llurda-Almuzara, L, et al. Effect of respiratory muscle training in asthma: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2023) 66:101691. doi: 10.1016/j.rehab.2022.101691
32. Pourdowlat, G, Hejrati, R, and Lookzadeh, S. The effectiveness of relaxation training in the quality of life and anxiety of patients with asthma. Adv Respir Med. (2019) 87:146–51. doi: 10.5603/ARM.2019.0024
33. Bruton, A, Lee, A, Yardley, L, Raftery, J, Arden-Close, E, Kirby, S, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med. (2018) 6:19–28. doi: 10.1016/S2213-2600(17)30474-5
34. Santino, TA, Chaves, GS, Freitas, DA, Fregonezi, GA, and Mendonça, KM. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. (2020):3. doi: 10.1002/14651858.CD001277.pub4
35. Lanario, JW, Davies, D, Cartwright, L, Hyland, ME, and Masoli, M. A lifestyle educational course as an adjunct to biologic administration in patients with severe asthma: a feasibility study. PEC Innov. (2025) 6:100364. doi: 10.1016/j.pecinn.2024.100364
36. Zampogna, E, Zappa, M, Spanevello, A, and Visca, D. Pulmonary rehabilitation and asthma. Front Pharmacol. (2020) 11:542. doi: 10.3389/fphar.2020.00542/full
37. Zhang, X, Lai, Z, Qiu, R, Guo, E, Li, J, Zhang, Q, et al. Positive change in asthma control using therapeutic patient education in severe uncontrolled asthma: a one-year prospective study. Asthma Res Pract. (2021) 7:10. doi: 10.1186/s40733-021-00076-y
38. Amin, R, Suvarna, V, Neelapala, YVR, Parmar, ST, and Vaishali, K. Use of telerehabilitation platforms for delivering patient education among patients with asthma: a scoping review. Curr Med Res Opin. (2024) 40:1421–30. doi: 10.1080/03007995.2024.2380006
39. Malaguti, C, Dal Corso, S, Janjua, S, and Holland, AE. Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2021) 2022. doi: 10.1002/14651858.CD013569.pub2
40. Şahi̇n, ME, Satar, S, and Ergün, P. Long-term efficiency of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease, bronchiectasis, and asthma: does it differ? Turk. J Med Sci. (2023) 53:814–23. doi: 10.55730/1300-0144.5644
41. World Health Organization. ICF Checklist version 2.1a, Clinical form. (2003). Available online at: https://www.who.int/docs/default-source/classification/icf/icfchecklist.pdf by World Health Organization
42. Bolton, CE, Bevan-Smith, EF, Blakey, JD, Crowe, P, Elkin, SL, Garrod, R, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax settembre. (2013) 68:ii1–30. doi: 10.1136/thoraxjnl-2013-203808
43. Andreasson, KH, Skou, ST, Ulrik, CS, Madsen, H, Sidenius, K, Jacobsen, JS, et al. Protocol for a multicentre randomised controlled trial to investigate the effect on asthma-related quality of life from breathing retraining in patients with incomplete asthma control attending specialist care in Denmark. BMJ Open. (2019) 9:e032984. doi: 10.1136/bmjopen-2019-032984
44. Andreasson, KH, Skou, ST, Ulrik, CS, Madsen, H, Sidenius, K, Assing, KD, et al. Breathing exercises for patients with asthma in specialist care: a multicenter randomized clinical trial. Ann Am Thorac Soc. (2022) 19:1498–506. doi: 10.1513/AnnalsATS.202111-1228OC
45. Kumarathas, I, Harsløf, T, Andersen, CU, Langdahl, B, Hilberg, O, Bjermer, L, et al. The risk of osteoporosis in patients with asthma. Eur Clin Respir J. (2020) 7:1763612. doi: 10.1080/20018525.2020.1763612
46. Holland, AE, Wadell, K, and Spruit, MA. How to adapt the pulmonary rehabilitation programme to patients with chronic respiratory disease other than COPD. Eur Respir Rev dicembre. (2013) 22:577–86. doi: 10.1183/09059180.00005613
Keywords: aerobic training, inspiratory muscle training, breathing retraining, telerehabilitation, maintenance strategies
Citation: Guarnieri G, Pozza M and Vianello A (2025) The role of pulmonary rehabilitation in severe asthma: a comprehensive review. Front. Med. 12:1709710. doi: 10.3389/fmed.2025.1709710
Edited by:
Dawei Yang, Fudan University, ChinaReviewed by:
Douglas Cowan, NHS Greater Glasgow and Clyde, United KingdomCopyright © 2025 Guarnieri, Pozza and Vianello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriella Guarnieri, Z2FicmllbGxhLmd1YXJuaWVyaUB1bmlwZC5pdA==
 Gabriella Guarnieri
Gabriella Guarnieri Michela Pozza
Michela Pozza