Abstract
Introduction:
Secukinumab has demonstrated favorable efficacy and safety in the management of plaque psoriasis; however, its use in generalized pustular psoriasis (GPP) remains limited, particularly in children under 6 years of age, with only a few clinical reports available.
Case description:
A 4-month-old infant was admitted with a 2-month history of GPP, which had worsened over the previous week with extensive erythematous rashes and pruritus. More than 70% of the body surface area was affected, with progression toward erythroderma. The Japanese Dermatological Association Severity Index score was 7, indicating moderate severity. Treatment with secukinumab 50 mg weekly, combined with daily emollients, was initiated. Complete clearance of skin lesions was achieved after four injections. One year after discontinuation of secukinumab, recurrence occurred; a single re-administration of secukinumab resulted in near-complete resolution.
Conclusion:
Secukinumab may provide potential clinical benefits with an acceptable safety profile in infants with GPP. Further research is required to establish the optimal treatment duration, dosing intervals, and long-term safety in this population.
Introduction
Generalized pustular psoriasis (GPP) is a rare and severe psoriasis subtype characterized by widespread sterile pustules, often accompanied by systemic manifestations such as fever, arthralgia, and leukocytosis (1). Recurrent and cyclic flares are common, and in severe cases, delayed treatment may lead to secondary infection or even organ failure, highlighting the need for timely medical intervention (1). Secukinumab, a fully human monoclonal antibody targeting interleukin-17A, has demonstrated favorable efficacy and safety in plaque psoriasis (2). However, its use in GPP remains uncommon, particularly in pediatric patients under 6 years of age, with only limited clinical evidence available. The present case describes a 4-month-old infant with severe GPP who achieved complete resolution following treatment with secukinumab.
Case description
A 4-month-old infant was admitted to Zhongshan People's Hospital for a 2-month history of GPP that had aggravated with rashes and itching in recent 1 week. Two months ago, the patient developed generalized erythema without significant desquamation, vesicles, or pustules. She was initially diagnosed with eczema at a local hospital. She was treated with Lianbo Qushi Zhiyang lotion (a traditional Chinese medicine preparation), desonide cream for topical use, and loratadine syrup orally. However, her symptoms did not improve. One week before admission, multiple millet-sized pustules appeared on the background of existing erythema.
The patient's mother has a history of psoriasis with mild skin involvement and has not received regular treatment. At physical examination, the patient showed widespread erythema of varying sizes over the entire body. On the trunk and limbs, the erythematous lesions had largely coalesced into confluent plaques. Numerous pinhead-sized pustules were densely distributed along the margins of the erythema throughout the body. On the trunk, these pustules had merged to form lakes of pus. Some of the erythematous areas showed mild edema. The affected skin area exceeded 70% of the total body surface area, showing a tendency toward erythroderma (Figures 1A–D). No erosions or ulcers were observed in the oral cavity or genital area. Hair, fingernails, and toenails appeared normal.
Figure 1
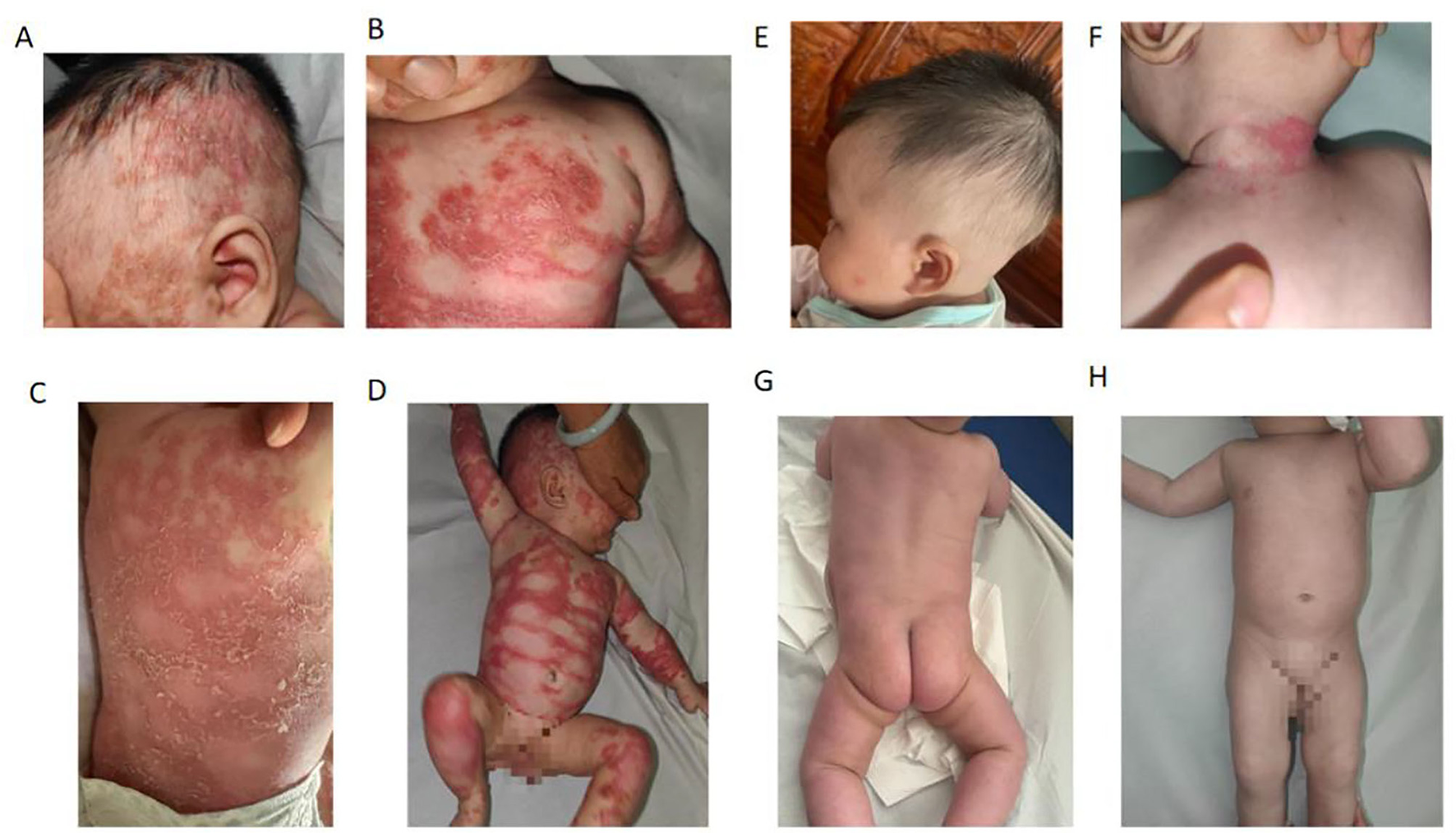
Generalized pustular psoriasis (GPP) in a 4-month-old female before (A–D) and after (E–H) treatment. (A–D) A diffuse erythema covered with confluent pustules all over the trunk and limbs, leading to the formation of a pustular lake with superficial scaling at a later stage. (E–H) The patient was treated with a 50-mg dose of secukinumab once a week and daily application of emollients. The rashes resolved rapidly within 4 weeks.
The pustular lesions were assessed using the Japanese Dermatological Association Severity Index (JDA-SI). The JDA-SI includes the evaluation of cutaneous symptoms (erythema, pustules, and edema; each scored from 0 to 3, total of 0–9 points) and systemic inflammation (fever, white blood cell count, C-reactive protein, and albumin; each scored from 0 to 2, total of 0–8 points). The combined score ranges from mild (0–6) to moderate (7–10) and severe (11–17). This infant was assigned a JDA-SI score of 7 points based on erythema, pustules, and edema, consistent with moderate disease severity.
A skin biopsy was performed in the outpatient clinic, and confirmed the diagnosis of GPP (Figure 2). The epidermal hyperplasia was accompanied by keratosis and hypogranulosis. The granular layer was reduced, and the basal layer was thickened. Additionally, a significant presence of neutrophils was observed in the superficial keratosis and above the spinous layer. Genetic testing revealed a positive mutation in the CARD14 gene in the patient. A specific missense mutation, c.2458C>T, was identified, leading to a substitution of arginine for tryptophan at codon 820 (p.R820W). The report also noted other silent mutations in the CARD14 gene, including a synonymous mutation (c.633G>A, p.E211=) and several intronic and non-coding exonic variants, which are not expected to affect protein function (Supplementary Appendix 1). The parents declined genetic testing for themselves for economic reasons (no medical insurance).
Figure 2
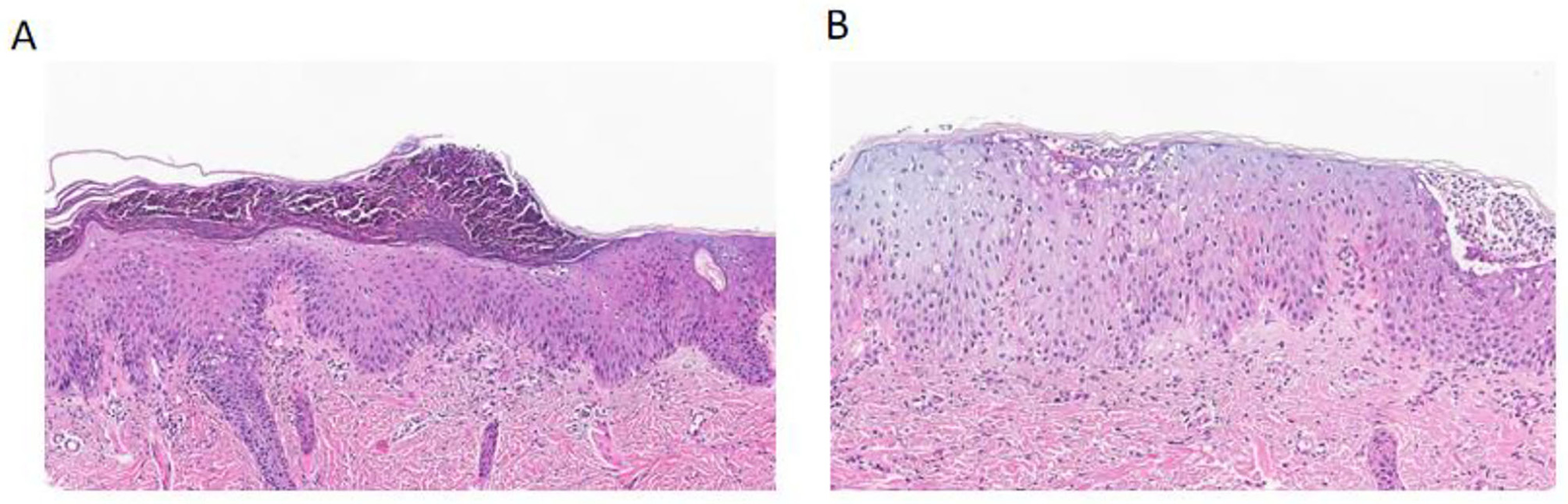
Histopathological findings of generalized pustular psoriasis compared with normal skin. (A) Hematoxylin and eosin (H&E) staining of the patient's lesional skin shows marked epidermal hyperplasia with keratosis and hypogranulosis, thinning of the granular layer, and thickening of the basal layer. Numerous neutrophils are observed within the stratum corneum and above the spinous layer, forming Munro's microabscesses, consistent with generalized pustular psoriasis. (B) H&E staining of normal skin demonstrates a well-preserved epidermal architecture without keratosis, hypogranulosis, or neutrophilic infiltration.
Pesolizumab was first considered for treatment, but its cost in mainland China is about 34 times higher than secukinumab, and the patient had no commercial medical insurance. Therefore, the patient was treated with a 50-mg dose of secukinumab once a week and daily application of emollients. The recommended dosing regimen for secukinumab is 75 mg for patients weighing < 25 or 25–50 kg, 150 mg for patients weighing 25–50 or ≥50 kg, and 300 mg for patients weighing ≥50 kg. All doses are administered subcutaneously at weeks 0, 1, 2, 3, and 4, followed by administration every 4 weeks thereafter. Given that the patient was only 4 months old and the treatment constituted an off-label use, she was administered secukinumab 50 mg subcutaneously once weekly (qw). After four injections, the patient's skin lesions had completely resolved (Figures 1E–H).
The patient experienced a recurrence of skin lesions 1 year after discontinuation of secukinumab (Figure 3). In April of this year, she was retreated with secukinumab 75 mg subcutaneously once weekly (qw). On follow-up visit on May 13, the skin lesions were observed to have fully disappeared (Figure 4).
Figure 3
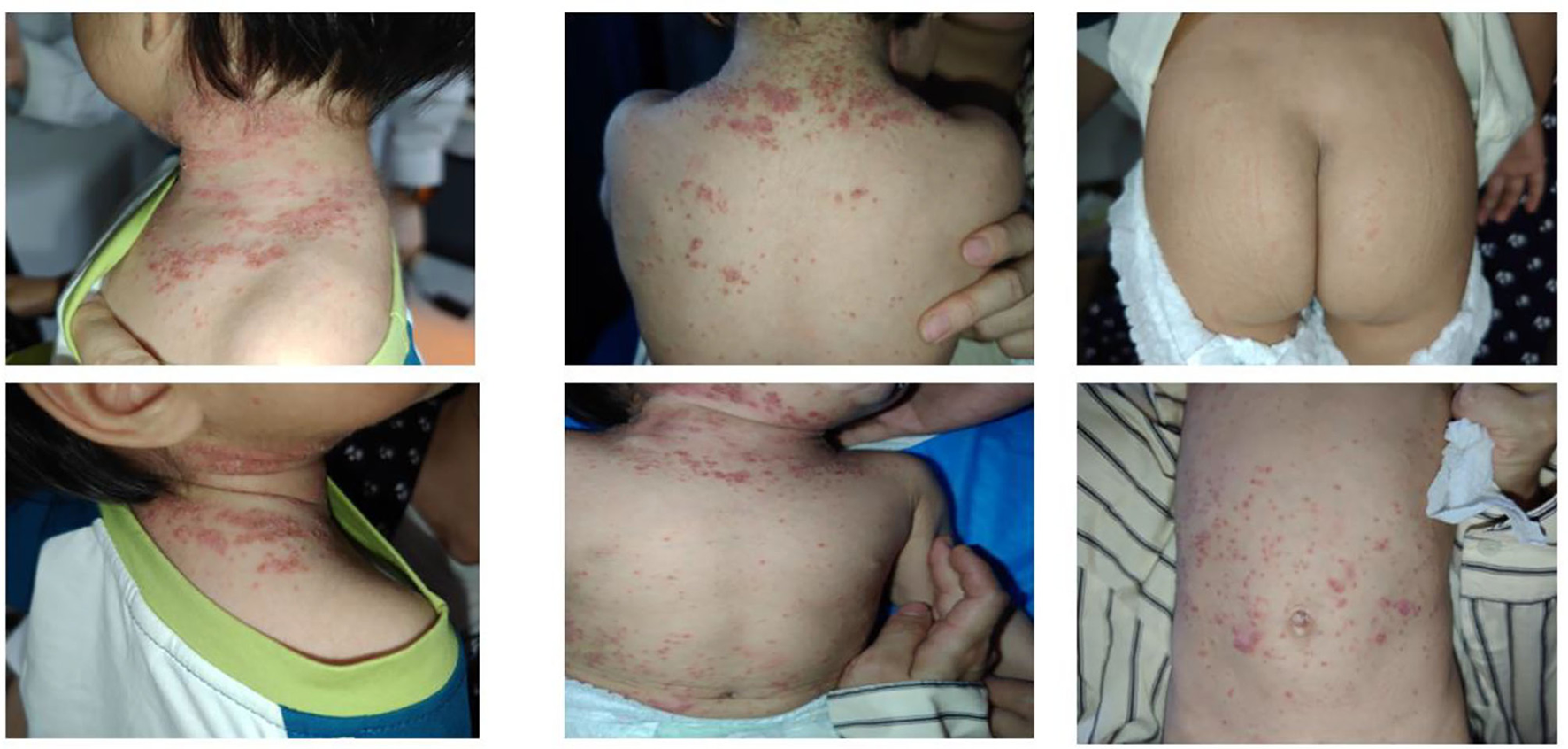
Recurrence observed at follow-up on April 22, 2025.
Figure 4
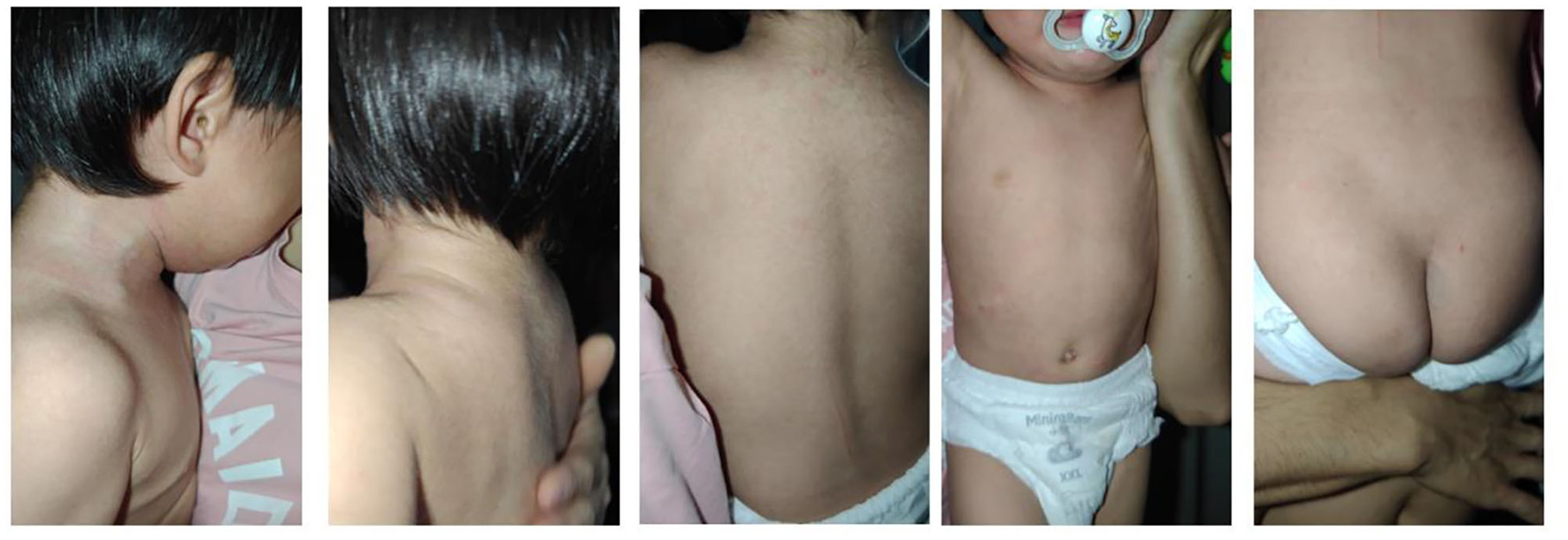
Follow-up visit on May 13, 2025.
Discussion
Secukinumab has demonstrated favorable efficacy and safety in the treatment of plaque psoriasis. Still, it is rarely used for pustular psoriasis, and there are a few clinical reports of secukinumab for psoriasis in children under 6 years old with GPP. The case reported here suggests that secukinumab may have clinical benefits in infants with GPP.
The management of GPP remains inadequate, partly due to the rarity of GPP (3). Avitrin, cyclosporine, and methotrexate are considered the preferred medications for acute GPP. Therefore, long-term treatment is usually necessary to minimize recurrence (1). Due to the limited availability of high-quality data on the efficacy of GPP treatment, the optimal treatment approach remains uncertain (1). In recent years, biologics targeting IL-12/23p40, IL-17A, and IL-36 have been reported for the treatment of GPP. Recent studies suggest that secukinumab can rapidly improve inflammatory markers in patients with GPP (4–8). Still, none of these reports included a patient as young as 4 months. Although no conclusion can be drawn from a single case, the present study suggests that it may be possible to treat infants with secukinumab. Of course, such a use should be reserved for moderate-to-severe cases, such as the one reported here, with lesions covering 70% of the body surface. Treatments should be performed as soon as possible in such young patients to avoid long-term complications. Of course, classical drugs for GPP, such as cyclosporine and methotrexate, are contraindicated in infants. For children younger than 6 years with plaque psoriasis, 4 years with enthesitis-related arthritis, and 2 years with psoriatic arthritis, the safety and efficacy of secukinumab have not been established, and its use is not recommended (9). If a clinical scenario involves a pediatric specialist, they should direct an infant's therapy, which is likely to involve alternative treatments. The use of secukinumab should be discussed in the team and with pharmacovigilance committees to ensure that no other options are available.
GPP is a severe and uncommon variant of psoriasis characterized by widespread pustule formation, fever, and systemic inflammation. Rapid symptom control is crucial, especially in pediatric patients. GPP exhibits high expression of IL-17A, IL-36, TNF-α, and neutrophil-attracting chemokines. IL-17A is particularly potent in driving neutrophil migration to the epidermis, an essential mechanism for pustule development (10, 11). IL-23 is upstream of IL-17A; it promotes the differentiation and survival of Th17 cells, which then produce IL-17A. However, in GPP, increased IL-36 activity uniquely amplifies IL-17A signaling and neutrophil infiltration, making IL-17 a more direct target (11). IL-17 inhibitors (e.g., secukinumab) show more rapid and pronounced clearance of pustules, fever reduction, and systemic symptom relief compared to IL-23 inhibitors (10, 12, 13). Studies demonstrated significantly higher rates of PASI 90/100 responses with IL-17 inhibitors at week 12 (PASI 90: IL-17 54% vs. IL-23 19%; PASI 100: IL-17 40% vs. IL-23 6%). At week 24, more patients on IL-17 inhibition had complete responses (IL-17 74% vs. IL-23 25%) (10). Secukinumab in GPP led to complete pustule clearance within 72 h for most, while IL-23 inhibitors (e.g., ustekinumab) took a week (11, 13). Some mechanistic reasons may support the superiority of IL-17 inhibition over IL-23 inhibition. IL-17A activates keratinocytes to release CXCL1, CXCL8, and other neutrophil chemoattractants more potently than IL-23. This drives the hallmark neutrophilic infiltrate seen in pustular lesions (11). IL-17 inhibitors directly block this signal, preventing neutrophil accumulation and pustule formation, whereas IL-23 inhibitors act upstream and may not fully suppress IL-17A activity (10, 14). IL-36 (a driver in GPP) acts in synergy with IL-17A rather than IL-23 (10). Hence, those reasons may explain the rapid onset and greater efficacy of IL-17 inhibition in pustular cases. Some IL-17 inhibitors are available. Secukinumab targets IL-17A. It demonstrates rapid onset, high PASI response rates, and sustained efficacy in GPP, including in infants and pediatric cases (7, 11, 12). Ixekizumab is also an IL-17A inhibitor; some studies suggest it offers similar or slightly improved long-term efficacy and drug survival compared to secukinumab, though tolerability is slightly lower (14, 15). Brodalumab targets the IL-17 receptor A, thus blocking both IL-17A and IL-17F. It may offer broader blockade and effective skin clearance, but has unique safety considerations (e.g., depression risk) (16). Therefore, IL-17 inhibitors, particularly secukinumab, appear to provide superior and fast control of pustular psoriasis by targeting the final common pathway for neutrophil recruitment and pustule formation. IL-23 inhibitors, acting upstream, do not fully suppress this process and show slower, less complete responses. Nevertheless, treatment affordability (e.g., in the case of pesolizumab) or availability (e.g., brodalumab is not available everywhere in China) by the patients should be considered.
Since the patient was very young, although the literature suggests that a 75-mg dose could be used, the authors had no prior clinical experience with biologic therapy for infantile GPP. Therefore, to ensure safety, a 50-mg dose was administered. The subsequent clinical outcome showed that the lesion clearance rate with the 50-mg dose was highly satisfactory.
This case suggests that secukinumab could be a promising therapeutic option for infants with GPP. However, further research is needed to determine when to discontinue secukinumab and whether infants need to be dosed at intervals during treatment. More clinical data is required to support this conclusion.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by Zhongshan City People's Hospital clinical research and animal experiment Ethic Committee (2025-092). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants‘ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Methodology, Writing – review & editing, Writing – original draft, Software, Conceptualization. YL: Resources, Writing – review & editing, Writing – original draft, Methodology, Conceptualization. CL: Project administration, Supervision, Writing – review & editing, Writing – original draft, Investigation, Funding acquisition. LZh: Data curation, Validation, Formal analysis, Writing – review & editing, Writing – original draft. LZe: Writing – original draft, Visualization, Formal analysis, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1713628/full#supplementary-material
References
1.
Rivera-Díaz R Daudén E Carrascosa JM Cueva P Puig L . Generalized pustular psoriasis: a review on clinical characteristics, diagnosis, and treatment. Dermatol Ther. (2023) 13:673–88. doi: 10.1007/s13555-022-00881-0
2.
Yang EJ Beck KM Liao W . Secukinumab in the treatment of psoriasis: patient selection and perspectives. Psoriasis. (2018) 8:75–82. doi: 10.2147/PTT.S146004
3.
Choon SE Lai NM Mohammad NA Nanu NM Tey KE Chew SF . Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. (2014) 53:676–84. doi: 10.1111/ijd.12070
4.
López-Sánchez C Falla LM Roé-Crespo E Arostegui JI Mozos A Bernal S et al . Excellent response to secukinumab in an infant with severe generalized pustular psoriasis. J Dermatol. (2021) 48:907–10. doi: 10.1111/1346-8138.15673
5.
Nishida M Takeichi T Kono M Imanishi A Maekawa N Akiyama M et al . Successful secukinumab treatment of recalcitrant juvenile generalized pustular psoriasis. J Dermatol. (2020) 47:e77–8. doi: 10.1111/1346-8138.15228
6.
Polesie S Lidholm AG . Secukinumab in the treatment of generalized pustular psoriasis: a case report. Acta Derm Venereol. (2017) 97:124–5. doi: 10.2340/00015555-2467
7.
Sun ZL Liu ZL Xu YY Zhang XL Zhang CL Guan X . Successful treatment of generalized pustular psoriasis with secukinumab: a report of two cases. Chin Med J. (2020) 133:3015–6. doi: 10.1097/CM9.0000000000001244
8.
Akaji K Nakagawa Y Kakuda K Takafuji M Kiyohara E Murase C et al . Generalized pustular psoriasis associated with systemic lupus erythematosus successfully treated with secukinumab. J Dermatol. (2021) 48:e43–4. doi: 10.1111/1346-8138.15645
9.
Blair HA . Secukinumab: a review in moderate to severe pediatric plaque psoriasis. Paediatr Drugs. (2021) 23:601–8. doi: 10.1007/s40272-021-00476-w
10.
Avallone G Maronese CA Murgia G Carrera CG Mastorino L Roccuzzo G et al . Interleukin-17 vs. interleukin-23 inhibitors in pustular and erythrodermic psoriasis: a retrospective, multicentre cohort study. J Clin Med. (2023) 12:1662. doi: 10.3390/jcm12041662
11.
Ruan SF Su X Xiao Z Zhang Y Lin T Luo R et al . Comparative efficacy and safety of ustekinumab and secukinumab in the treatment of generalized pustular psoriasis: a 48-week retrospective cohort study with genetic background analysis. J Inflamm Res. (2024) 17:6707–21. doi: 10.2147/JIR.S472338
12.
Wei K Li P He X Yang D Lang J Lai L et al . Clinical efficacy and safety of secukinumab in the treatment of generalized pustular psoriasis in the pediatric population: a systematic review of the literature. Front Med. (2024) 11:1377381. doi: 10.3389/fmed.2024.1377381
13.
Fujita H Terui T Hayama K Akiyama M Ikeda S Mabuchi T et al . Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. (2018) 45:1235–70. doi: 10.1111/1346-8138.14523
14.
Cui L Chen R Subedi S Yu Q Gong Y Chen Z et al . Efficacy and safety of biologics targeting IL-17 and IL-23 in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. (2018) 62:46–58. doi: 10.1016/j.intimp.2018.06.020
15.
Lam J Cazzaniga S Seyed Jafari SM Maul JT Feldmeyer L Bossart S et al . Treatment of psoriasis with II-17 inhibitors: comparison of long-term effectiveness and drug survival of secukinumab vs ixekizumab in real-world practice. Psoriasis. (2025) 15:71–84. doi: 10.2147/PTT.S509495
16.
Kearns DG Uppal S Chat VS Wu JJ . Comparison of guidelines for the use of interleukin-17 inhibitors for psoriasis in the United States, Britain, and Europe: a critical appraisal and comprehensive review. J Clin Aesthet Dermatol. (2021) 14:55–9.
Summary
Keywords
generalized pustular psoriasis, infant, secukinumab, efficacy and safety, case report
Citation
Wang L, Li Y, Li C, Zhang L and Zeng L (2025) Successful treatment with secukinumab in a 4-month-old infant with severe generalized pustular psoriasis: a case report. Front. Med. 12:1713628. doi: 10.3389/fmed.2025.1713628
Received
26 September 2025
Accepted
28 October 2025
Published
14 November 2025
Volume
12 - 2025
Edited by
Paulo Filipe, Centro Hospitalar Lisboa Norte (CHLN), Portugal
Reviewed by
Roman J. Nowicki, Medical University of Gdansk, Poland
Gustavo Almeida-Silva, Santa Maria Hospital, Portugal
Updates
Copyright
© 2025 Wang, Li, Li, Zhang and Zeng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Li, 414110094@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.