- The Affiliated Huaian No.1 People's Hospital of Nanjing Medical University, Huai'an, China
Primary hepatic malignant mesothelioma (PHMM) is an uncommon and aggressive neoplasm with vague clinical and radiological features, posing challenges for preoperative diagnosis. In our case, a lobulated hepatic mass demonstrated a serpiginous peripheral enhancement pattern on contrast-enhanced CT and MRI. This uncommon imaging manifestation has been sporadically documented in previous reports. By consolidating these findings, our report emphasizes serpiginous peripheral enhancement as a potential diagnostic clue for PHMM. Recognition of this pattern may aid earlier detection, improve differential diagnosis, and guide timely surgical decision-making in affected patients.
Introduction
Malignant mesothelioma is an uncommon yet highly aggressive neoplasm arising from mesothelial cells. It most frequently affects the pleura (approximately 70% of cases) and the peritoneum (about 20%), whereas occurrences in the pericardium and tunica vaginalis are considerably rarer (1). PHMM is extremely rare, with fewer than 20 cases reported globally. Due to its rarity, PHMM lacks established clinical characteristics. Most patients present with nonspecific abdominal discomfort, and laboratory and imaging examinations also lack specificity. As such, preoperative diagnosis is difficult, and final confirmation depends on pathological and immunohistochemical analysis.
Here, we describe a case of PHMM treated at our institution and provide a brief literature review to highlight its diagnostic challenges and therapeutic strategies.
Case data
A 66-year-old male farmer was admitted to our hospital with a recurrence of right upper abdominal discomfort over the past week. He had no history of alcohol abuse, chronic hepatitis, or cirrhosis, denied any asbestos exposure, and reported no other comorbidities. On physical examination, mild right upper quadrant tenderness was noted without hepatomegaly, splenomegaly, or ascites. The patient initially visited an external hospital, where he was misdiagnosed with a liver abscess and underwent a needle biopsy. Despite the poor drainage and the biopsy finding of only liver fibrosis, the patient was advised to continue observation at home while completing a course of oral antibiotics. Three months later, he revisited our hospital for re-evaluation, during which a CT showed that the hepatic mass had not decreased or resolved.
Treatment and diagnosis
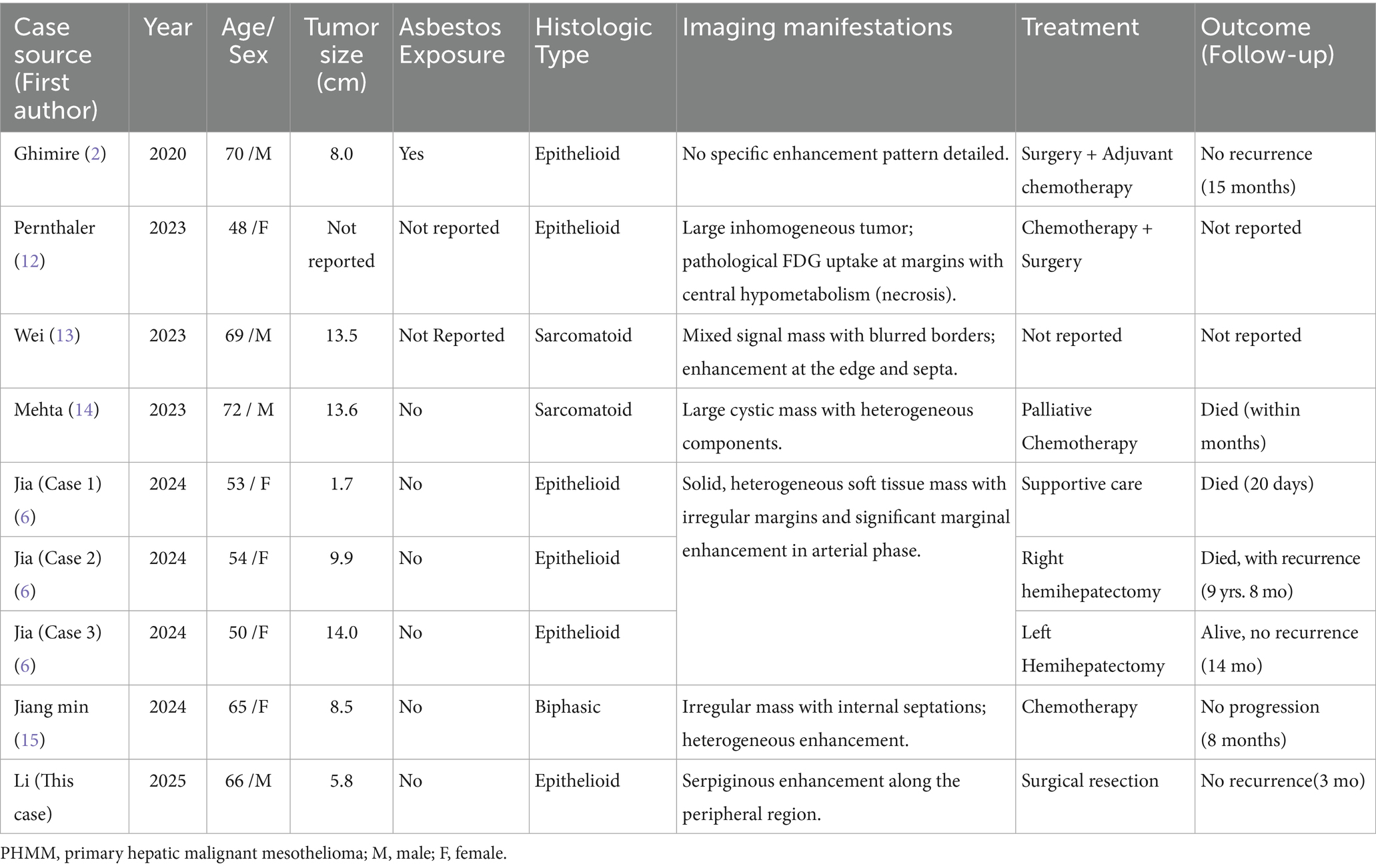
Routine laboratory tests, including complete blood count, liver function, and renal function, were within normal limits. Serum tumor markers including CA19-9, CEA, and AFP were negative. Contrast-enhanced CT: A hypodense lesion in segments VI-VII of the liver measuring approximately 5.8 cm. The lesion showed mild heterogeneous enhancement in the arterial phase and partial washout in the portal venous phase, with a serpiginous distribution of enhancement along the peripheral region (Figure 1A). MRI: A 5.6-cm lobulated lesion in the liver, T1 hypointense and T2 hyperintense, with heterogeneous enhancement after gadolinium injection. Diffusion-weighted imaging showed restricted diffusion. A serpiginous distribution of enhancement was observed along the peripheral region (Figure 1B). Due to the patient’s prior needle biopsy and drainage procedure three months ago, which showed poor drainage and liver fibrosis without resolution of the mass, and the fact that the lesion had not decreased in size after three months of follow-up, the lesion remained indeterminate. Given the indeterminate nature of the lesion and the inability to exclude malignancy based on preoperative imaging, surgical resection was performed to ensure oncological safety. This decision was also in line with the patient’s request for definitive treatment. A liver resection was performed with a margin of at least 1 cm from the tumor to ensure the complete removal of potentially involved tissue while preserving sufficient residual hepatic tissue to maintain adequate liver function and achieve clear surgical margins. During surgery, the tumor, located on the liver surface near the liver-renal interface, posed a significant surgical challenge. Its delicate surrounding tissues made dissection difficult and increased the risk of bleeding. Nevertheless, the team successfully achieved complete tumor resection with negative margins. Intraoperative frozen section analysis confirmed a malignant hepatic tumor with cellular atypia. The patient’s postoperative recovery was uneventful: they began oral fluids on postoperative day (POD) 1, mobilized by POD 3, and was discharged in stable condition on POD 13 following an excellent recovery (Figure 2). This recovery trajectory is consistent with the patient’s good general health and the absence of major postoperative complications. Taking into account the rarity of the tumor, this approach was chosen to ensure oncological safety while also minimizing risks to liver function.
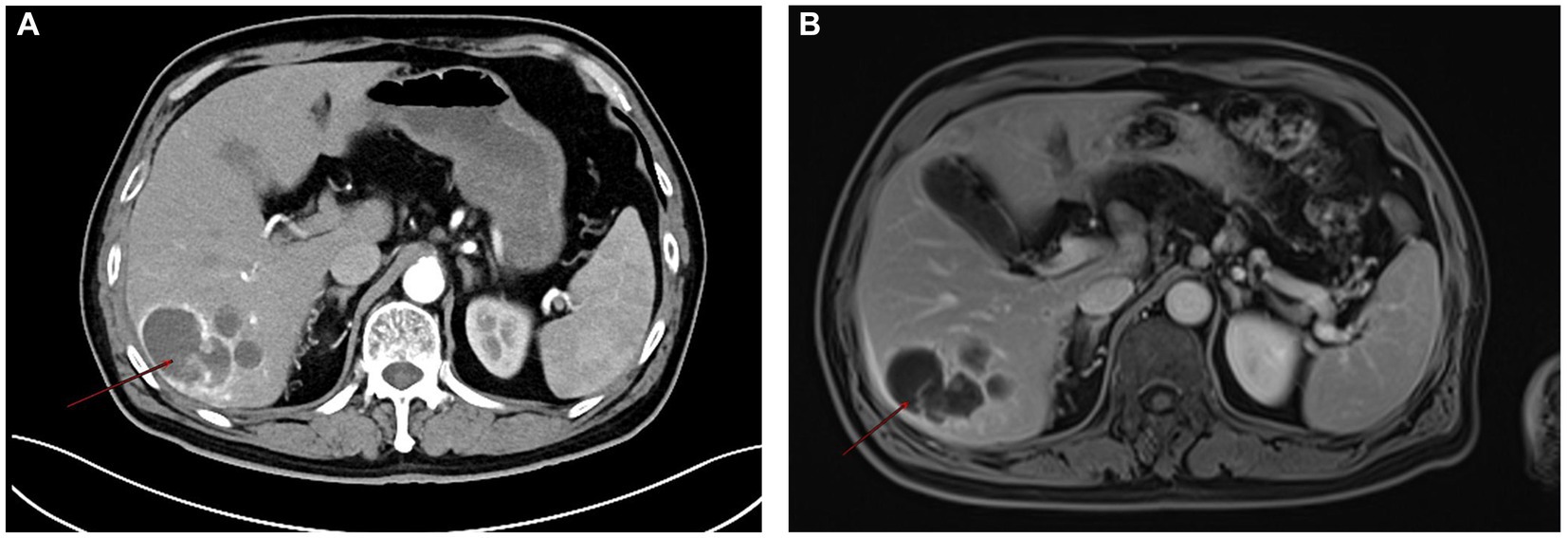
Figure 1. Contrast-enhanced CT (A) showing a hypodense lesion in segments VI-VII of the liver, measuring approximately 5.8 cm. The lesion demonstrates mild heterogeneous enhancement in the arterial phase with a serpiginous, serpentine distribution of enhancement along the peripheral region. MRI (B) also exhibits heterogeneous enhancement after gadolinium injection, with a serpiginous, serpentine distribution of enhancement observed along the peripheral region.
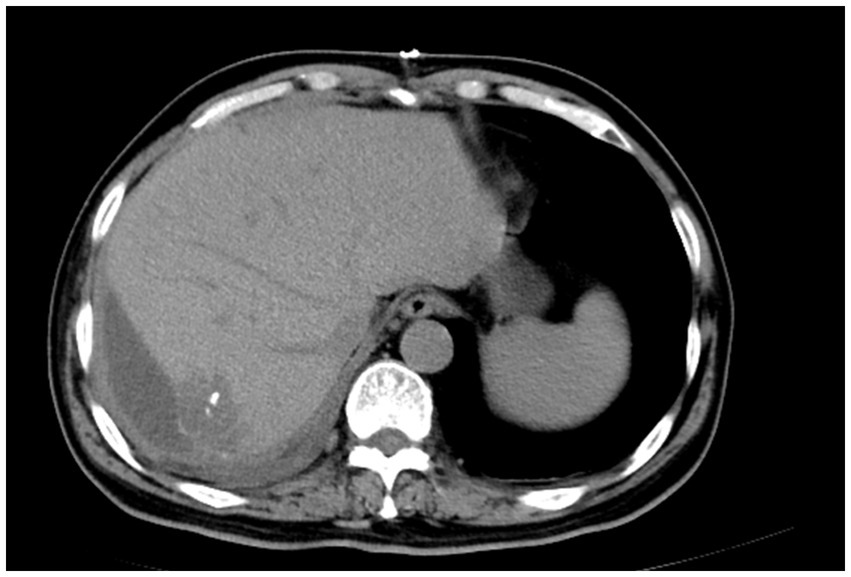
Figure 2. Postoperative CT scan showing complete tumor resection with minimal perihepatic fluid accumulation.
Postoperative histopathological analysis revealed the tumor was composed of epithelioid cells arranged in nests and sheets, with nuclear pleomorphism, frequent mitotic figures, and focal necrosis (Figure 3A). Immunohistochemistry showed tumor cells were positive for CK (3+), WT-1 (1+), CK5/6 (1+), D2-40 (2+), CR (3+) and negative for Hepatocyte, CD31, CD34, SMA, HMB45 (Figure 3B). These findings were consistent with PHMM.
Figure 3. Histopathological (A) image of primary hepatic malignant mesothelioma (PHMM) stained with hematoxylin and eosin (HE). The tumor exhibits epithelioid cells arranged in sheets and nests, with nuclear pleomorphism, frequent mitotic figures, and invasive growth into the surrounding liver tissue, characteristic of malignant mesothelioma. Immunohistochemistry (B) showing positive staining for Calretinin (CR) and WT-1, indicative of mesothelial origin. The tumor cells also show strong D2-40 (Podoplanin) positivity, supporting the diagnosis of PHMM.
The patient was scheduled for the first follow-up visit three months after discharge. During this follow-up, contrast-enhanced CT imaging was performed, which showed no signs of tumor recurrence (Figure 4). Given the rarity of PHMM and the lack of well-established treatment guidelines, it was decided to adopt a more conservative approach in terms of follow-up. As such, the patient will continue with a surveillance plan similar to that of other malignant liver tumors, with CT imaging scheduled every three months for the first two years post-surgery. The clinical timeline figure following the CARE guidelines has been added as Figure 5.
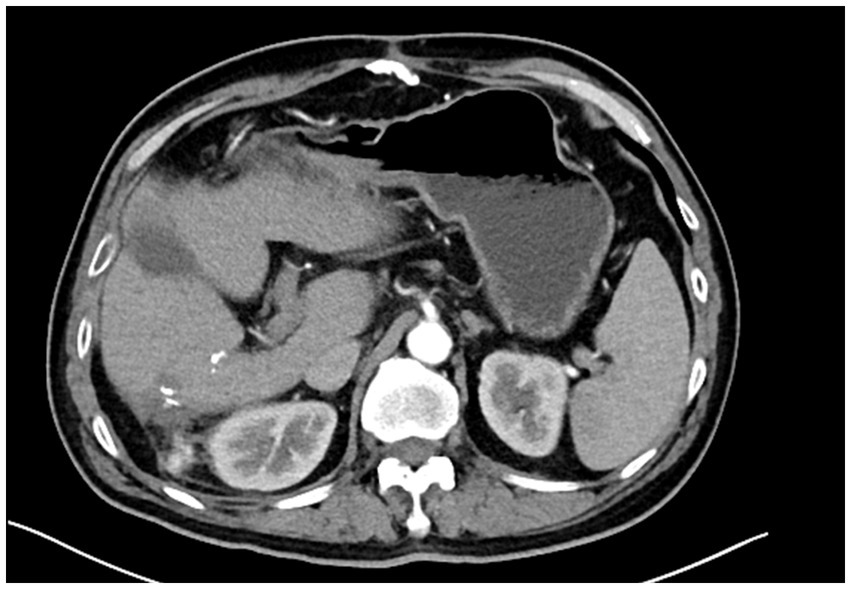
Figure 4. Postoperative follow-up CT scan after three months showing no signs of recurrence, with stable liver and surrounding structures.
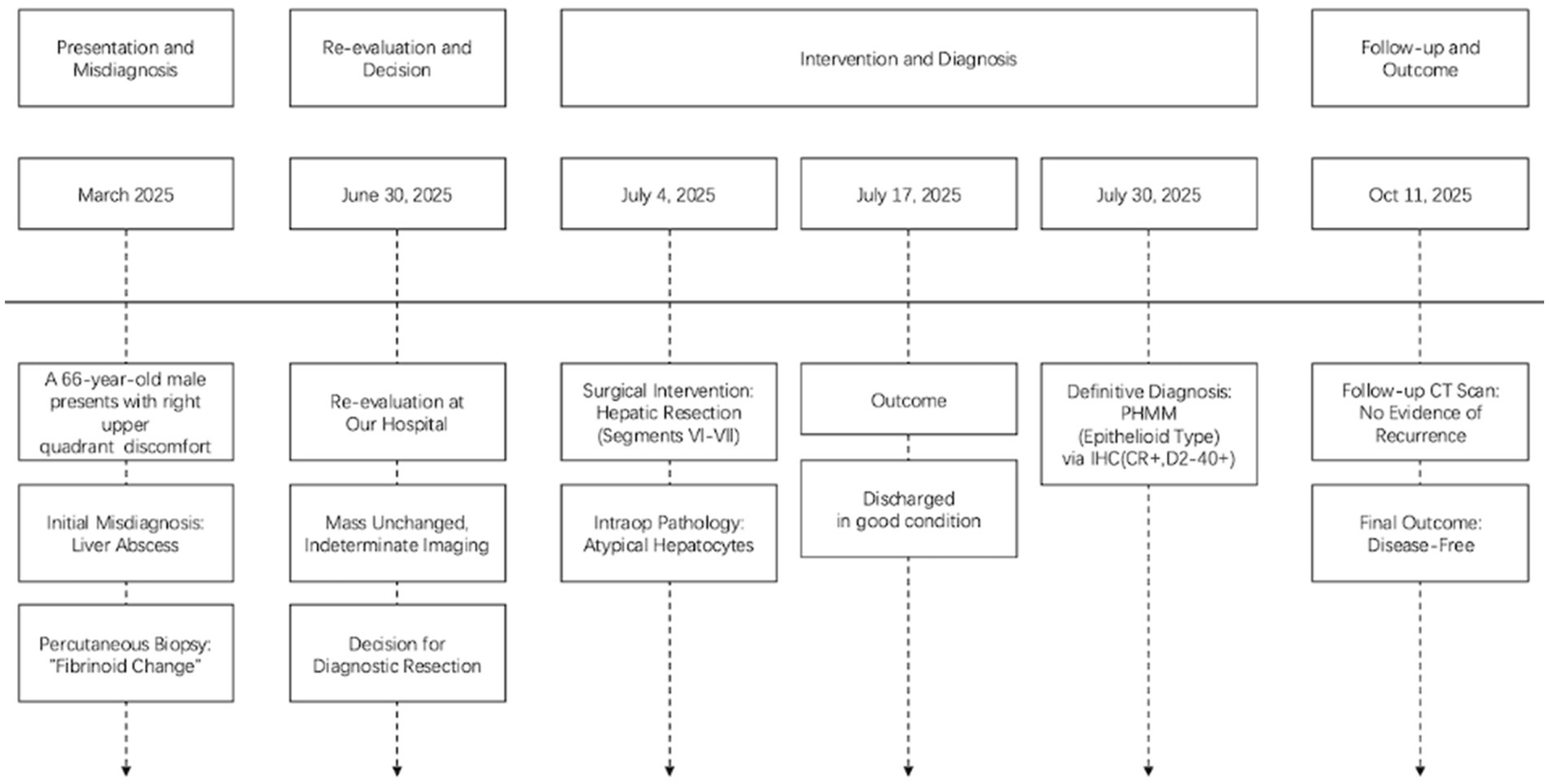
Figure 5. Clinical timeline summarizing key events of presentation, diagnosis, treatment, and follow-up, aligned with the CARE guidelines.
Discussion
PHMM is a rare mesothelial malignancy. Reports of PHMM in the past 5 years are shown in Table 1. Unlike pleural mesothelioma, asbestos exposure is not clearly associated with hepatic mesothelioma (2). Its pathogenesis remains uncertain, with hypotheses including mesothelial cell rests in Glisson’s capsule undergoing malignant transformation (3). Owing to its nonspecific radiological manifestations, the preoperative diagnosis of PHMM remains challenging. In the present case, contrast-enhanced CT revealed a serpiginous peripheral enhancement pattern, an imaging feature that is unusual in common hepatic tumors but has been sporadically described in previously reported PHMM cases. Serter et al. (4), Leonardou et al. (5), and Jia et al. (6) have clearly revealed a serpiginous peripheral enhancement pattern in their studies, which aligns with the imaging findings observed in our case (4–6). We also observed a serpiginous peripheral structure on the enhanced CT images provided in the study by Dong et al. (7). However, this feature was not explicitly emphasized by the authors. It is important to note, however, that serpiginous peripheral enhancement is not unique to PHMM. Similar findings may be encountered in other hepatic lesions: hepatic hemangiomas typically show peripheral nodular enhancement with centripetal fill-in, a dynamic pattern not observed in PHMM (8). On MRI, hemangiomas often demonstrate the characteristic “light-bulb sign,” appearing markedly hyperintense on T2-weighted images, which further distinguishes them from PHMM. Focal nodular hyperplasia (FNH) is often associated with a central scar that enhances in a radiating fashion on delayed phases (9). Intrahepatic cholangiocarcinoma (ICC) usually demonstrates progressive delayed enhancement and is frequently accompanied by bile duct dilatation and elevated tumor markers such as CA19-9 or CEA (10), whereas in our case, all tumor markers were within normal limits.
Epithelioid, sarcomatoid, and biphasic are the three types of malignant mesothelioma, with epithelioid being the most common (2). Our case also presents as the epithelioid type. The positive results for immunohistochemical markers Calretinin (CR), WT-1, and D2-40 (Podoplanin), along with a negative result for CD34, support the diagnosis of primary hepatic mesothelioma (11). Due to its rarity, there is no standardized treatment for this condition. In previous studies, most early-stage patients underwent surgical treatment and achieved favorable prognoses. Therefore, surgical resection was also chosen for this patient, with the hope of a positive outcome.
The distinctive contribution of this report lies in consolidating the serpiginous peripheral enhancement pattern as a recurrent imaging feature of PHMM. While individual cases have sporadically described this manifestation, our report not only documents the finding in detail but also contextualizes it within the existing literature. By emphasizing this uncommon yet reproducible feature, the present case adds to the limited pool of evidence that may assist radiologists and clinicians in suspecting PHMM earlier and differentiating it from other hepatic tumors.
Conclusion
While the imaging characteristics of PHMM are not yet fully defined, the presence of serpiginous peripheral enhancement warrants attention. With the accumulation of more case reports, this finding may contribute to earlier recognition and more accurate differential diagnosis of PHMM.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Huai’an First People’s Hospital, Affiliated to Nanjing Medical University, Huai’an, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GL: Writing – original draft, Resources, Methodology, Conceptualization. SH: Conceptualization, Writing – review & editing. TH: Investigation, Writing – review & editing. JX: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC) [Grant No. 82203722].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PHMM, Primary Hepatic Malignant Mesothelioma; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; HE, Hematoxylin and Eosin; CR, Calretinin; WT-1, Wilms’ Tumor 1; D2-40, Podoplanin; AFP, Alpha-Fetoprotein; CEA, Carcinoembryonic Antigen; CA19-9, Carbohydrate Antigen 19–9; CK, Cytokeratin; SMA, Smooth Muscle Actin; CD31, Cluster of Differentiation 31; CD34, Cluster of Differentiation 34; POD, Postoperative Day.
References
1. Jain, M, Crites, MK, Rich, P, and Bajantri, B. Malignant pleural mesothelioma: a comprehensive review. JCM. (2024) 13:5837. doi: 10.3390/jcm13195837
2. Ghimire, S, Regmi, N, Yang, T, Shah, H, Srivatana, U, Sarker, A, et al. Primary malignant mesothelioma of the liver: case report and review of the literature. Eur J Case Reports Internal Medicine. (2020) 7:002128. doi: 10.12890/2020_002128
3. Ismael, H, and Cox, S. Primary intrahepatic mesotheliomas: a case presentation and literature review. Int J Surg Case Rep. (2018) 47:1–6. doi: 10.1016/j.ijscr.2018.04.004
4. Serter, A, Buyukpinarbasili, N, Karatepe, O, and Kocakoc, E. An unusual liver mass: primary malignant mesothelioma of the liver: CT and MRI findings and literature review. Jpn J Radiol. (2015) 33:102–6. doi: 10.1007/s11604-014-0379-9
5. Leonardou, P, Semelka, RC, Kanematsu, M, Braga, L, and Woosley, JT. Primary malignant mesothelioma of the liver: MR imaging findings. Magn Reson Imaging. (2003) 21:1091–3. doi: 10.1016/S0730-725X(03)00197-8
6. Jia, J, Tan, X, Gao, F, Shao, Z, and Zhang, M. Primary intrahepatic mesothelioma: case series and systematic review of literature. J Gastrointest Canc. (2024) 55:1520–9. doi: 10.1007/s12029-024-01075-x
7. Dong, A, Dong, H, and Zuo, C. Multiple primary hepatic malignant mesotheliomas mimicking cystadenocarcinomas on enhanced CT and FDG PET/CT. Clin Nucl Med. (2014) 39:619–22. doi: 10.1097/RLU.0b013e31828da61d
8. Kacała, A, Dorochowicz, M, Matus, I, Puła, M, Korbecki, A, Sobański, M, et al. Hepatic hemangioma: review of imaging and therapeutic strategies. Medicina. (2024) 60:449. doi: 10.3390/medicina60030449
9. Yao, ZHT, Gao, XY, Xu, QS, Chen, YT, and Song, YP. Evaluation of the characteristics of hepatic focal nodular hyperplasia: correlation between dynamic contrast-enhanced multislice computed tomography and pathological findings. OTT. (2016) 9:5217–24. doi: 10.2147/OTT.S103647
10. Fábrega-Foster, K, Ghasabeh, MA, Pawlik, TM, and Kamel, IR. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. (2017) 6:67–78. doi: 10.21037/hbsn.2016.12.10
11. Zhou, D, Quan, Z, and Wang, J. Current status of malignant mesothelioma with liver involvement in China: a brief report and review of the literature. IRDR. (2018) 7:112–9. doi: 10.5582/irdr.2018.01052
12. Pernthaler, B, Brcic, L, Aigner, RM, Fuchsjäger, M, and Talakic, E. 18F-FDG PET/CT in primary mesothelioma of the liver. Clin Nucl Med. (2023) 48:49–51. doi: 10.1097/RLU.0000000000004445
13. Wei, C, Sun, W, Cui, M, and Wang, J. Primary sarcomatoid malignant mesothelioma of the liver: a case report. Asian J Surg. (2023) 46:4058–9. doi: 10.1016/j.asjsur.2023.04.052
14. Mehta, K, Mehta, S, Joshi, M, Bharadwaj, HR, Ardeshana, G, and Tenkorang, PO. Challenging diagnosis of sarcomatoid hepatic mesothelioma: a case report with review of literature. Annals Medicine Surgery. (2023) 85:5123–6. doi: 10.1097/MS9.0000000000001148
Keywords: primary hepatic malignant mesothelioma, serpiginous peripheral enhancement, contrast-enhanced CT/MRI, immunohistochemistry, hepatic resection
Citation: Li G, Hong S, He T and Xu J (2025) Case Report: Whispers of the serpent: exploring uncommon imaging features in primary hepatic malignant mesothelioma. Front. Med. 12:1713971. doi: 10.3389/fmed.2025.1713971
Edited by:
Yan Huang, Anhui Medical University, ChinaCopyright © 2025 Li, Hong, He and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbo Xu, eHUyNDAxODk5MzFAMTYzLmNvbQ==
 Guoan Li
Guoan Li Jianbo Xu
Jianbo Xu