Abstract
Sleep disorders, particularly obstructive sleep apnea (OSA), circadian disruption, and insomnia, are increasingly recognized as contributors to the onset and progression of gynecologic cancers. This review explores the bidirectional interactions between sleep dysfunction and malignancies such as ovarian, endometrial, and cervical cancers. Mechanistically, intermittent hypoxia (IH) from OSA promotes tumor aggressiveness through hypoxia-inducible factor-1 alpha (HIF-1α) stabilization, M2 macrophage polarization, and impaired DNA repair, while circadian disruption alters endocrine signaling and immune regulation. Disrupted sleep also perturbs the gut and vaginal microbiota, promoting systemic inflammation and tumor-supportive environments. Conversely, cancer therapies such as chemotherapy and radiotherapy exacerbate sleep dysfunction via neurotoxicity and fibrotic airway damage, especially in estrogen-deprived states. These interconnected mechanisms not only worsen clinical outcomes but also underscore sleep as a modifiable and actionable therapeutic target. Emerging integrative strategies—such as hypoxia-targeted nanomedicine, circadian-based chronotherapy, and microbiota modulation—offer promising avenues to enhance treatment efficacy and quality of life. Progress in this field hinges on interdisciplinary collaboration and the development of personalized care models that embed sleep health as a core component of gynecologic cancer management.
1 Introduction
Sleep is fundamental physiological process essential for maintaining systemic homeostasis. It regulates immune function, supports cardiovascular stability, and maintains metabolic balance, while also playing a pivotal role in cognitive performance and emotional regulation (1). Disruptions in sleep architecture—such as insomnia, circadian rhythm sleep–wake disorders, sleep-related movement disorders, sleep disordered breathing—compromise these functions, leading to poorer health outcomes and reduced quality of life, particularly during hormonal dynamic periods such as menstrual cycle, pregnancy, and menopause (2, 3). Obstructive sleep apnea (OSA), characterized by recurrent episodes of partial or complete upper airway collapse during sleep, is a prevalent but historically underdiagnosed condition in women. Importantly, growing epidemiological evidence links OSA in women not only with cardiometabolic and cognitive sequelae but also with an elevated risk for site-specific malignancies, particularly gynecological cancers (4). Despite these associations, research explicitly investigating OSA in gynecologic cancer cohorts remains limited. Most existing studies have focused on breast cancer, which is often included in gynecologic analyses due to overlapping hormonal pathways, such as estrogen receptor signaling (5). This lack of targeted data highlights the need for dedicated studies exploring the prevalence, mechanisms, and clinical implications of sleep disorders in ovarian, cervical, endometrial, and other gynecologic cancer sub-types. Therefore, this review aims to synthesize the existing evidence and propose a conceptual framework specifically focusing on the bidirectional crosstalk between sleep disorders (with an emphasis on OSA) and gynecological cancers, to spur further mechanistic and clinical research in this under-explored area.
1.1 Sleep disorders and obstructive sleep apnea in women
OSA, as a representative of sleep disordered breathing, has garnered increasing attention for its dual role in triggering intermittent hypoxia (IH) and contributing to excessive daytime sleepiness and frequent arousals (6, 7). It is noteworthy that the prevalence of OSA varies significantly across age groups and genders. While the overall prevalence in the general population ranges from 9 to 38%, rates are particularly higher among older adults, with some studies reporting rates as high as 78% in elderly women (4), while traditionally considered a male-dominated disorder, significant prevalence exists in females, particularly during peri-menopause and post-menopause.
OSA prevalence in women varies significantly across the lifespan, influenced by hormonal status. During reproductive years, women exhibit roughly a threefold lower prevalence compared to age-matched men (approximating a 1:3 ratio), and this protection is largely attributed to hormonal influences—specifically, progesterone and estrogen—which enhance upper airway stability and reduce ventilatory instability (8–11). In women with concurrent obesity, obstructive sleep apnea (OSA), and endometrial cancer, adipokines (leptin/adiponectin) and estrogen pathways collectively regulate metabolic dysfunction (12–14). ≥50% of their survivor patients were overweight/obeseImportantly, this hormonal benefit is modulated by factors like obesity and endocrine disorders. At menopause, with declining sex hormones, OSA prevalence in women substantially increases, approaching rates seen in men (15). Circadian rhythm disorder can lead to abnormal hormone secretion, which is associated with an elevated risk of fatal ovarian cancer (RR = 1.27). This life-stage variation strongly implicates the critical role of sex hormones in OSA pathogenesis. The prevalence of OSA during pregnancy is 10.5–33.3%, which is closely related to diaphragm elevation caused by uterine enlargement, congestion and edema of airway mucosa caused by estrogen and progesterone, and weight gain (16–18). The prevalence of OSA increases significantly in perimenopausal and postmenopausal women, primarily due to the decline in sex hormone levels (19, 20). On the other hand, OSA patients are at high risk of circadian disruption (21). Such circadian disruption impairs the regulation of endocrine factors governed by the hypothalamic–pituitary axis, including cortisol, growth hormone (GH), prolactin (PRL), thyroid hormones and sex steroids (22). Receptors for female hormones such as estradiol and progesterone are widely distributed in sleep–wake regulatory brain regions, including the basal forebrain, hypothalamus, dorsal raphe nucleus, and locus coeruleus (23). Although the incidence and underlying causes of OSA vary among women of different age groups, these hormonal influences may help explain why sex hormones are consistent risk factors for OSA across the female lifespan. Clinically, investigating the features of OSA in women may elucidate sex-specific health disparities, thereby improving its diagnosis and management.
1.2 Sleep disorders, OSA and gynecological cancer
Gynecological cancers including cervical, endometrial, ovarian, and vulvovaginal cancer have exhibited a rising global incidence and mortality over the last three decades (24). In parallel, sleep disorders have emerged as increasingly prevalent comorbidity that may influence both the development and progression of these malignancies. Disruptions in sleep, particularly circadian rhythm disorders, insomnia, and OSA, are recognized for their potential role in tumor biology. Short sleep duration has been implicated in the pathogenesis of hormone-dependent malignancies, including breast, endometrial, and ovarian cancers, with meta-analyses indicating a modest association between insufficient sleep and cancer incidence (HR = 1.06) (25, 26). Nevertheless, these findings warrant cautious interpretation due to ongoing inconsistencies and methodological limitations across observational studies (27). Meta-analysis by Lu et al. (28) (10 studies) found no significant association between sleep duration and overall cancer risk, though a marginal trend linked short sleep to ovarian cancer development. Emerging pathophysiological models suggest that psychological stressors and insomnia may synergistically disrupt cervicovaginal microbial homeostasis. This dysbiotic state may promote chronic inflammation and enhance the oncogenic potential of human papillomavirus (HPV), potentially accelerating the progression from viral persistence to cervical carcinogenesis (21).
In addition to disruptions in sleep, OSA has been increasingly recognized as a potential risk factor influencing cancer initiation, progression and prognosis (29). Clinical data robustly associate OSA with cancers like breast and prostate (28). The clinical relevance of OSA in oncology was first highlighted by the Spanish Sleep Network in 2013, which identified nocturnal hypoxemia-quantified by Tsat90% (the percentage of nighttime with oxygen saturation <90%)-as a significant predictor of increased cancer incidence (30). Subsequent large-scale studies have reinforced these findings. In a cohort of 34,848 OSA patients versus 77,380 controls, OSA conferred a 53% elevated overall cancer risk (RR = 1.53), with site-specific risks including a 2.09-fold increase in breast cancer (HR = 2.09) (31–33). Notably, severe OSA (apnea-hypopnea index, AHI ≥30) correlates with a 4.8-fold higher cancer mortality risk (HR = 4.8), a relationship that strengthens when Tsat90% replaces AHI as the severity marker (HR = 8.6) (34). These risks are further amplified in cancer survivors, where sleep disorders like insomnia contribute to treatment delays and immune dysfunction, perpetuating a vicious cycle of disease progression (35).
Although it is evident that sleep disorders, especially OSA, are closely related to gynecological cancers, research on the underlying biological mechanisms remains relatively limited. Hormones have been shown to play contributory roles in the development and progression of hormone-sensitive cancers, including breast cancer, prostate cancer, endometrial cancer, and ovarian cancer (36, 37). Circadian rhythm disorder can lead to abnormal hormone secretion, which is associated with an elevated risk of fatal ovarian cancer (RR = 1.27) (38). Moreover, women using estrogen therapy have been reported to exhibit a 40% lower incidence of ovarian cancer compared to postmenopausal women not using hormone replacement (39). Additionally, urinary melatonin levels may be associated with the risk of developing ovarian cancer, suggesting a potential link between circadian regulation and tumorigenesis (OR = 0.79) (40). Physiological and pathological fluctuations in key hormones—including progesterone, melatonin, and cortisol—are significant drivers of circadian disruption in women (41–43). This disruption manifests as altered expression of circadian rhythm hormones (e.g., melatonin itself), core clock genes (e.g., Clock, Bmal1, Per, Cry), and downstream genes regulating growth control and reproductive pathways (44). Critically, the resulting dysregulation reciprocally exacerbates hormonal imbalance, indicating a bidirectional relationship between circadian dysfunction and the endocrine system. Consequently, this established endocrine-circadian axis provides a fundamental mechanistic link connecting chronic sleep disorders like OSA to cancer development and progression.
On the contrary, sleep disturbances are highly prevalent among gynecologic oncology patients, with reported prevalence rates ranging from 30 to 88%—substantially higher than the 4 to 33% observed in the general population and the 30 to 50% reported among general cancer survivors (45–52). Regardless of the specific type of gynecologic cancer, many women experience sleep disturbances around the time of diagnosis (50). In postmenopausal women with breast or endometrial cancer, the prevalence of OSA is notably high, with 58 and 57% of patients, respectively, exhibiting and AHI greater than 15 events/hour (5). Proposed mechanisms of the development of sleep disturbances include the release of cytokines such as tumor necrosis factor (TNF) or C-reactive protein (CRP), depression and distress, cancer-related fatigue, menopausal hormone therapy (MHT), chemotherapy, radiotherapy, and surgery (50, 53–55).
An in-depth understanding of gynecological tumors and the discussion on the role and mechanism of tumor and their treatments in sleep and sleep respiratory disorders is conducive to the formulation of clinical comprehensive management pathways.
2 Molecular mechanisms of sleep disorder-induced cancer initiation and progression
2.1 Circadian disruption and genomic instability
The pooled prevalence of poor sleep quality in patients with cancer was 57.4% [95% confidence interval (CI): 53.3–61.6%], and higher prevalence rates were reported among patients with gynecological cancer (p < 0.01) (56), which results in the circadian disruption. At the molecular level, circadian regulation is mediated by core circadian genes and their protein products, molecular mechanisms and physiological importance of circadian rhythms. The molecular clock mechanism initiates with BMAL1 (Brain and Muscle ARNT-Like Protein 1) and CLOCK (Circadian Locomotor Output Cycles Kaput) forming heterodimeric complexes that bind E-box motifs within promoters of target genes, including clock-controlled genes (CCGs) and negative regulators period (PER) and Cryptochrome (CRY) (Figure 1A). Accumulation of PER/CRY proteins ultimately suppresses CLOCK-BMAL1 transcriptional activity, establishing an autoregulatory loop fundamental to circadian rhythm generation (57). Nevertheless, the International Agency for Research on Cancer (IARC) in 2019 concluded that night-shift work was possibly carcinogenic (58). Epidemiologic studies have yielded conflicting results as to whether circadian clock disruption by night or shift work is carcinogenic (59, 60). Circadian rhythm disruption has been mentioned to be associated with breast cancer in women. No relevant studies have addressed gynecological cancer, either in cohort studies or basic studies, but this deserves further exploration.
Figure 1

Molecular mechanisms of sleep disorder-induced cancer progression. (A) Disruption of circadian rhythms leads to genomic instability. (B) OSA-associated IH produces distinct HIF isoform regulation, with preferential stabilization of HIF-1α. (C) OSA-related hypoxia induces intestinal hyperpermeability and gut dysbiosis. (D) CIPN constitutes structural and functional damage to peripheral nerves. (E) Hormonal regimens aim to suppress systemic estrogen via aromatase inhibitors. Created with Biorender.com.
2.1.1 Clock gene dysregulation
Circadian oscillators coordinate the temporal regulation of cellular proliferation, DNA repair mechanisms, and redox homeostasis. Emerging evidence links circadian disruption to enhanced tumorigenesis and metastatic dissemination through dysregulation of cancer stem cell maintenance and niche remodeling (61). Notably, BMAL1/CLOCK dimerization and cyclical expression of PER1-3 genes regulate critical oncogenic pathways across multiple malignancies. Experimental models demonstrate BMAL1 depletion accelerates endometrial carcinogenesis through mTOR pathway activation. As a circadian rhythm synchronizer, melatonin has been confirmed to regulate central and peripheral clock genes by up-regulating or down-regulating specific clock genes to control cell cycle, survival, and repair mechanisms (62). Hypoxia exposure causes dysregulation of ovarian circadian clock protein (CLOCK, BMAL1, and E4BP4) expression, which mediates female reproductive dysfunction by impairing LHCGR-dependent signaling events (63). Melatonin exhibits significant antitumor effects by modulating various signaling pathways, promoting apoptosis, and suppressing metastasis in breast cancers and gynecological cancers, including ovarian, endometrial, and cervical cancers (64–66). Ovarian malignancies exhibit prognostic correlations with PER1-3 downregulation, suggesting circadian gene dysregulation may influence therapeutic responses (67). Clinical studies in rectal, pancreatic, and endometrial carcinomas, as well as lymphoblastic leukemia, reveal that chronomodulated chemotherapy regimens optimize drug efficacy while minimizing toxicity (68). Therefore, this circadian rhythm disorder caused by obstructive sleep apnea is believed to create a microenvironment conducive to the development of ovarian cancer by promoting genomic instability, impairing DNA damage repair, and allowing cells to proliferate uncontrollably—all of which are key characteristics of ovarian cancer development.
2.1.2 DNA repair impairment
The tumor microenvironment (TME) exhibits circadian-modulated immune dynamics, where PER1 expression inversely correlates with infiltrating B lymphocytes, macrophages, and neutrophils in ovarian cancer (69). PER2 and BMAL1 deficiency in ovarian tumors impairs homologous recombination repair (HRR) capacity. PER2 deficiency, in particular, regulates key downstream genes involved in cell cycle control (cyclin A, cyclin B1, cyclin D1, cyclin E) and DNA damage response (p53, c-myc); the dysregulation of these genes disrupts genomic maintenance mediated by SIRT1, BRCA1, BRCA2, and TP53 (70–72).
2.2 Hypoxia-driven tumor plasticity
OSA is closely linked to gynecological cancers due to its hallmark pathophysiological feature—IH. Hypoxia plays a critical role in the progression of gynecologic oncology, such as cervical cancer, by activating adaptive cellular pathways that promote tumor survival, invasion, and resistance to therapy. Here are key mechanistic insights:
2.2.1 HIF-1α/ERα synergy
Hypoxia (tissue pO2 < 10 mmHg), prevalent in 90% of solid tumors (73, 74), activates HIF-1-mediated transcriptional programs governing angiogenesis, glycolytic metabolism, and invasion (75). OSA-associated IH produces distinct HIF isoform regulation, with preferential stabilization of HIF-1α over HIF-2α (Figure 1B) (76, 77). Mechanistically, hypoxia impairs prolyl hydroxylase-mediated HIF-1α degradation, enabling nuclear translocation and heterodimerization with HIF-1β. Subsequent recruitment of CBP/p300 coactivators to hypoxia-response elements (HREs) drives the expression of >100 target genes critical for hypoxic adaptation (78–81).
Experimentally, IH demonstrates accelerated HIF-1α activation compared to sustained hypoxia (82, 83). In gynecologic malignancies, ERα stabilizes HIF-1α to potentiate epithelial-mesenchymal transition (EMT) (84). HIF-mediated VEGF induction promotes tumor neovascularization, though resultant vessels exhibit structural abnormalities that perpetuate hypoxia (85). The hypoxic TME fosters metabolic adaptation via Warburg-effect dominance, generating lactate-rich microenvironments that facilitate immune evasion (86). Concurrent HIF-1α-mediated upregulation of anti-apoptotic Bcl-2/Survivin and suppression of Bax expression enhances tumor cell survival (87). Hypoxia also induces TGF-β-driven EMT programs critical for metastatic dissemination (88), while promoting M2 macrophage polarization and PD-L1-mediated T cell exhaustion (89, 90). Clinical evidence indicates that intratumoral hypoxia is a significant predictor of poor disease-free survival in cervical cancer patients (91, 92). IH mimicking sleep apnoea increases spontaneous tumorigenesis and cancer progression in mice (93, 94). Therefore, there is a link between hypoxia and gynecological tumors, however, how sex-specific pathways, such as estrogen-mediated regulation of hypoxia-inducible factors (e.g., HIF-1α), might contribute to gynecologic tumorigenesis in OSA patients deserves to be further explored.
2.2.2 Metabolic reprogramming
HIF-1α orchestrates metabolic switching through PDK1 upregulation, which phosphorylates and inactivates pyruvate dehydrogenase (PDH). This redirects pyruvate flux toward lactate production via HIF-1α: CEBP-β axis activation under IH conditions (95–97). Increased expression of ABCB1/ABCC1 transporter, along with downregulation of ABCA1, has been observed in tumors exposed to IH, correlating strongly with enhanced chemoresistance (98). Autophagy-related gene BECN1 exhibits a positive correlation with HIF1α expression, indicating potential synergistic adaptation mechanisms that support cell survival within hypoxic tumor microenvironments. In mice, IH-induced neuroinflammation and mitochondrial ROS damage can be ameliorated through activation of the PINK1-Parkin mitophagy pathway (99).
2.3 Microbiota-immune axis dysfunction
2.3.1 Gut barrier breakdown
OSA-related hypoxia has been shown to induce intestinal hyperpermeability, as indicated by increased circulating levels of lipopolysaccharide (LPS) and LBP levels in pediatric cohorts (Figure 1C) (100). Gut dysbiosis in sleep-disordered breathing is characterized by an elevated Firmicutes/Bacteroidetes ratios and the predominance of endotoxin-producing genera (Klebsiella, Prevotella), which promote systemic inflammation through activation of the TLR4/NF-κB signaling pathway (101, 102). The female reproductive tract (FRT) microbiota interacts with the gut and with the urinary tract, defining a vagina–gut axis and a vagina–bladder axis, respectively (103, 104). The gut and vaginal microbiota secret metabolites like endotoxins, bile acids, lipopolysaccharides, genotoxins and conjugated estrogen, which can induce DNA damage and increase genomic instability—factors implicated in carcinogenesis and disease progression (105). Therefore, OSA-altered microbial metabolites (e.g., LPS) and genotoxins can promote tumorigenesis by inducing systemic inflammation (NF-κB activation), ROS-mediated DNA damage, and impaired epithelial barrier function—all established drivers in endometrial and ovarian carcinogenesis. This dysregulation may form a self-sustaining loop: dysbiosis amplifies systemic inflammation and genotoxicity, driving tumor initiation/progression, while cancer-associated inflammation and therapy further disrupt microbial homeostasis. However, these viewpoints still require further research to clarify their potential mechanisms and clinical relevance.
2.3.2 Tumor-associated macrophage polarization
The exposure of IH facilitates remodeling of TEM by inducing M2 macrophage polarization of adipose tissue macrophages (ATMs) and tumor-associated macrophages (TAMs), promoting regulatory T cell (Treg) expansion, and facilitating the adipocyte stem cell (ASC) accumulation (106). Intermittent hypoxia activates the NF-κB/HIF-1α pathway, increasing pro-inflammatory mediators like COX-2, CCL2, CXCL1, PGE2, and CSF1, these mediators recruit monocytes and neutrophils to the tumor microenvironment (TME), differentiating into TAMs and Tumor-Associated Neutrophils (TAN) (107–110). TAM and TAN can both lead to increased secretion of VEGF-A, thereby promoting cancer development (107). Sleep fragmentation-induced IL-10 secretion further reinforces an immunosuppressive TME, potentially facilitating tumor progression by inhibiting effective anti-tumor immune responses. In an animal model of IH, M2-like TAMs are activated in cervical cancer, contributing to a tumor-promoting microenvironment. IH caused by OSA can also cause the polarization of tumor-associated macrophages in mice (111). OSA appears to be closely linked to tumor initiation and progression; however, the mechanisms underlying its comorbidity with gynecological tumors—particularly the role of tumor-associated macrophages (TAMs)—remain underexplored.
3 Cancer therapy-exacerbated sleep disorders
3.1 Chemotherapy neurotoxicity
Chemotherapy-induced oxidative stress and autonomic dysfunction may serve as a critical bridge linking cancer treatment to the worsening of sleep disturbances, particularly OSA exacerbation. Chemotherapy-induced peripheral neurotoxicity (CIPN) constitutes a major dose-limiting complication in oncology practice, characterized by structural and functional damage to peripheral nerves (Figure 1D) (112).
3.1.1 Paclitaxel-induced phrenic neuropathy
Chemotherapeutic agents directly exacerbate sleep-disordered breathing through neuro-autonomic pathways. Central to this phenomenon is paclitaxel-induced diaphragmatic dysfunction, occurring in 4–8% of gynecologic oncology patients due to microtubule stabilization disrupting phrenic nerve conduction (113–115). Approximately 60–70% of patients develop peripheral neuropathy following paclitaxel therapy, with severe manifestations (≥ grade 2) affecting 36% of elderly ovarian cancer patients and 20% of younger cohorts (112, 116).
3.1.2 Chemotherapeutic resistance and oxidative stress crosstalk
Emerging evidence suggests a bidirectional relationship between chemotherapy-related oxidative stress and comorbid OSA, which may reciprocally exacerbate clinical outcomes. The emergence of multidrug resistance (MDR) during chemotherapy remains a major obstacle in cancer therapeutics, significantly compromising treatment efficacy, patient survival, and quality of life. This resistance phenomenon also impacts the clinical utility of reactive oxygen species (ROS) modulators, whether administered as monotherapy or chemo-sensitizing adjuvants (117). Notably, chemotherapeutic agents like paclitaxel and cisplatin—cornerstones of first-line therapy for ovarian carcinomas and other malignancies (118–120)—paradoxically induce therapeutic complications through ROS generation, causing collateral damage to normal tissues while modulating tumor redox dynamics. Doxorubicin exemplifies this pleiotropic interplay, exerting anticancer effects via DNA damage induction alongside complex cell death modulation through apoptosis, senescence, autophagy, ferroptosis, and pyroptosis pathways (121).
Emerging evidence suggests a bidirectional relationship between chemotherapy-related oxidative stress and comorbid OSA, which may reciprocally exacerbate clinical outcomes. Chemotherapeutic activation of hypoxia-inducible factor (HIF) signaling can augment carotid body chemosensitivity, a peripheral oxygen-sensing system critical for ventilatory and sympathetic regulation. Pathological hypersensitization of this system in OSA patients drives sympathetic overactivation and metabolic dysfunction, manifesting as refractory hypertension and insulin resistance (122). These autonomic perturbations are further aggravated by ROS-mediated chemoreflex hyperactivity and baroreflex suppression, creating a vicious cycle of hypertension induction (122–124).
Intriguingly, tumors may co-opt oxygen-chemo-sensing mechanisms analogous to carotid body physiology to facilitate survival and growth within hypoxic tumor microenvironments. This adaptation is mediated by HIF-2α—a transcription factor essential for carotid body development and hypoxic chemotransduction (125)—which also promotes chemoresistance in cancers such as high-grade serous ovarian carcinoma (HGSOC). HIF-2α exerts its protective role through TGFBI-dependent PI3K/Akt pathway activation, which suppresses apoptosis and enhances DNA repair, while its stabilization via the USP9X-HIF-2α proteostatic axis sustains cancer stem cell populations to drive tumor recurrence (126, 127).
Clinically, the IH characteristic of OSA creates a feedforward loop that amplifies both chemoresistance and autonomic dysfunction. IH disrupts HIF homeostasis by stabilizing HIF-1α while destabilizing HIF-2α, skewing redox balance toward ROS overproduction via pro-oxidant enzyme induction and antioxidant suppression (125). The resultant oxidative stress not only accelerates tumor adaptation to chemotherapy but also heightens carotid body chemosensitivity. This dual effect may synergize with chemotherapy-induced HIF activation to exacerbate hypertension and sympathetic overdrive during cancer treatment. These pathophysiological intersections underscore the need for combinatorial therapeutic strategies targeting HIF-ROS signaling networks to simultaneously mitigate chemoresistance and OSA-associated complications.
Collectively, IH-induced HIF-1α stabilization, ROS accumulation, and β-adrenergic signaling foster DNA repair upregulation, drug efflux amplification, and tumor cell survival—directly compromising chemotherapy efficacy (249).
3.2 Hormone deprivation effects on sleep
3.2.1 Estrogen blockade and withdrawal
Evidence suggests that menopausal hormone therapy (MHT) may elevate the risk of gynecological malignancies such as endometrial cancer and ovarian cancer (128, 129). Systemic estrogen suppression via aromatase inhibitors (AIs) in ovarian and endometrial cancers accelerates sarcopenia progression, with 38.8% overall prevalence peaking in endometrial (43.6%) and ovarian (42.5%) malignancies (130–132). This muscle-wasting condition independently predicts poor treatment responsiveness—a critical determinant of survival in patients receiving hormone therapy or chemotherapy for advanced/recurrent disease (133). Interestingly, estrogen depletion may exacerbate sarcopenia progression, given estrogen’s pleiotropic roles in muscle homeostasis: preserving satellite cell function, enhancing membrane stability, and mitigating oxidative stress during mitochondrial dysfunction (134). This pro-sarcopenic effect is clinically consequential, as patients with dual diagnoses of gynecologic malignancies and sarcopenia exhibit significantly worse overall survival compared to those without muscle loss (135–138).
Notably, estrogen-deficient states—whether iatrogenic (AI-induced) or physiological (postmenopausal)—amplify vulnerability to upper airway myopathy under intermittent hypoxic stress, a hallmark of OSA. Ovariectomized models demonstrate sex-specific respiratory muscle deterioration under chronic intermittent hypoxia (CIH), partially reversed by estrogen replacement (139). These findings align with clinical observations of estrogen’s protective role in maintaining upper airway muscle tonicity and ventilatory drive (140), mediated through estrogen receptor α (ERα)-dependent regulation of mitochondrial bioenergetics (141). Consequently, AI-mediated estrogen suppression may inadvertently promote OSA by dual mechanisms: diminishing upper airway dilator muscle function (as evidenced by reduced genioglossus EMG activity post-therapy) (140) and exacerbating sarcopenia-related respiratory weakness. Therefore, when both gynecological cancer and OSA are present, the use of estrogen inhibitors may require careful consideration from multiple aspects.
3.2.2 Fatigue, circadian dysregulation, and sleep architecture disruption
Cancer-related fatigue (CRF) in ovarian cancer manifests as a persistent, activity-limiting burden distinct from normal fatigue, intricately linked to sleep disturbances and neuroendocrine dysregulation. Longitudinal studies reveal sustained sleep impairment in ≥60% of epithelial ovarian cancer survivors (EOCS) at one-year follow-up, independent of depression yet correlated with diminished quality of life (142). General analyses based on weighting according to sample size showed a significantly positive correlation between fatigue and circulating levels of inflammatory markers, including significantly positive correlations between fatigue and elevated interleukin-6 (IL-6) (143). IL-6 levels consistently predict both poor sleep quality and fatigue severity, while aberrant cortisol rhythms—characterized by flattened diurnal variability and nocturnal hypercortisolemia—are mechanistically implicated in CRF pathogenesis (144–146).
Pelvic irradiation induces irreversible sleep architecture damage in gynecologic oncology patients, with 88% of irradiated cohorts developing OSA versus 67% in non-irradiated controls (147, 148). This increased risk of radiation-associated OSA likely results from fibrotic damage to upper airway tissues, compounded by preexisting sarcopenia. Crucially, pharmacological sleep aids have limited efficacy in improving long-term sleep quality in epithelial ovarian cancer survivors (EOCS), highlighting the importance of developing and implementing non-pharmacological management strategies (142). These intersecting pathways—cytokine-mediated inflammation, HPA axis dysfunction, and treatment-related anatomical changes—establish a self-perpetuating cycle wherein sleep disruption exacerbates fatigue, subsequently hindering physiological recovery and overall well-being.
4 Clinical challenges and biomarker detection
4.1 Diagnostic dilemmas
4.1.1 Gender-specific OSA phenotypes: female-predominant hypopnea-dominant vs. male-predominant apnea-dominant OSA
Identifying sex- and gender-related differences in sleep research holds the potential to advance personalized care by improving diagnosis, treatment, and prevention of sleep disorders and related comorbidities. Besides, hormonal changes post-puberty has been proposed as a key factor contributing to adult sleep disparities (149, 150). Female sex hormones, such as estradiol and progesterone, are associated with increased sleep fragmentation, including frequent awakenings and prolonged wakefulness (150). The landmark Wisconsin Sleep Cohort Study reported OSA prevalence (AHI ≥5) in 24% of men and 9% of women (151). By 2013, meta-analyses revealed a mean OSA prevalence of 22% (range: 9–37%) in men and 17% (range: 4–50%) in women (151). Research indicates gender-specific phenotypes in OSA, with women more commonly exhibiting hypopnea-dominant OSA, while men tend to present with apnea-dominant forms (152). Potential reasons for these gender differences include anatomical and physiological variations: women have structurally more stable upper airways, men exhibit greater chemoreceptor sensitivity to hypoxic/hypercapnic stimuli, men display higher abdominal and neck fat deposition linked to OSA risk, and women more frequently experience partial upper airway obstruction (152). Hormonal influences may also play a critical role, as postmenopausal women show higher OSA prevalence than premenopausal women (151).
Clinically, male-to-female OSA referral ratios exceed those observed in population studies, suggesting underdiagnosis in women. Contributing factors include social stigma around snoring symptoms leading to fewer women seeking care (153, 154), though women diagnosed with OSA exhibit better treatment adherence. Gender differences in symptom presentation also exist at comparable AHI levels, women report less daytime sleepiness, habitual snoring, and witnessed apneas compared to men (153, 154). Polysomnographic differences further reveal that women exhibit similar OSA severity to men during REM sleep but milder events during NREM sleep (153), resulting in a higher proportion of REM-related respiratory events. Women also demonstrate more respiratory effort-related arousals (RERAs) and upper airway resistance syndrome (UARS) (155), which may be underrepresented by current scoring criteria, increasing the risk of underdiagnosis (156).
Collectively, anatomical, hormonal, behavioral, and diagnostic disparities likely amplify gender-specific OSA prevalence and clinical manifestations, necessitating further research to refine gender-sensitive diagnostic and therapeutic strategies.
4.1.2 Fatigue overlap: distinguishing cancer-related fatigue from OSA symptoms
The overlap of fatigue-related symptoms presents a diagnostic challenge in CRF from OSA. CRF is persistent, intense, longer in duration, and not alleviated by rest as compared to more traditional fatigue (157, 158). Tumor- and/or treatment-associated cytokines have a proposed role in cancer-related fatigue via effects on central nervous system pathways that elicit vegetative behaviors (159–162). Supporting such hypotheses are findings that fatigued breast cancer survivors demonstrated significantly higher elevations of cytokines including IL-1ra, TNF-α, sTNF-RII, IL-6, and neopterin than non-fatigued survivors, and circulating levels of IL-6, IL-1ra, and neopterin have been associated with fatigue in a quantitative review of cancer patients (159–162). Given the strong relationship between excessive daytime sleepiness and complaints of fatigue, depression, and/or insomnia, these symptoms are also more commonly reported by women. Complaints of fatigue, tiredness, or lack of energy may be as important as that of sleepiness to OSAS patients, among whom women appear to have all such complaints more frequently than men (163). The diagnosis of OSA should not be excluded based only on a person’s tendency to emphasize fatigue, tiredness, or lack of energy more than sleepiness (163). Research has shown that 93% of patients with head and neck tumors experience daytime fatigue. Among these patients, 79% had undergone radiotherapy prior to the sleep study, and of this subgroup, 88% were diagnosed with OSA. In contrast, among patients who had not received prior radiotherapy, 67% were found to have OSA (148). These findings suggest a potential link between tumor treatment-radiotherapy and increased OSA prevalence, underscoring the need for routine sleep assessments in this patient population.
4.2 Prognostic markers
4.2.1 Circulating exosomal miRNAs
Exosomal miR-210 is an important regulator of cell function via its downregulation of mitochondrial biogenesis, which attenuates oxidative phosphorylation demand (164, 165). The serum concentration of miR-210 was higher in individuals with OSA, and studies have demonstrated a potentially major role of miR-210 in mediating OSA-induced vascular risk (166). The mechanisms underlying OSA risk may be modulated by SREBP2—miR-210—induced mitochondrial dysfunction in endothelial cells (ECs), providing compelling evidence that miR-210 may be a suitable candidate as an OSA biomarker and a therapeutic target for interventional studies (166). miR-210 expression is upregulated in response to hypoxia in epithelial ovarian cancer specimens and cell lines, with an association to HIF-1α overexpression (167). Furthermore, upregulated miR-210 promoted tumor growth in vitro via targeting PTPN1 and inhibiting apoptosis (167). Sevoflurane and desflurane both promoted SKOV3 cancer cells (an ovarian cancer type) and malignancy via miR-210 (167), highlighting its potential role as a hypoxia-associated predictor of platinum resistance. Therefore, perhaps miR-210 may act as a diagnostic marker for gynecological cancer comorbid with OSA.
4.2.2 Sleep EEG signatures
Slow-wave sleep (SWS) loss correlates with elevated IL-6 and shorter progression-free survival in cancer patients (168). Postoperative sleep disorders (PSD) are characterized by post-surgical alterations in sleep quality (169). Patients who undergo major surgeries experience lower sleep efficiency, disrupted sleep, decreased rapid eye movement (REM) sleep, and, in some cases, absence of the N3 sleep stage (170). OSA patients may have decreased SWS (171). There are also therapeutic methods targeting slow-wave sleep in female patients. SWS and REM sleep can be stimulated by prolactin (PRL) (172), which shares cellular effects exerted through the JAK/STAT signaling cascade, including survival, cell cycle progression, proliferation, migration, high metabolic rates, angiogenesis, and anti-apoptosis (173–176). Notably, renal sympathetic denervation has been shown to ameliorate inflammatory responses in a chronic obstructive sleep apnea (OSA) model via JAK/STAT pathway modulation, demonstrating conserved mechanistic cross-talk between neurohumoral regulators and inflammatory pathophysiology in sleep disorders (177). IL-6 has been shown to enhance non-rapid eye movement (NonREM) sleep in rats and slow wave activity during SWS in humans (166, 178, 179). S-ketamine can improve the prognosis of patients undergoing gynecological laparotomy by improving SWS (180).
The co-occurrence of slow-wave sleep (SWS) deficits and IL-6 elevation manifests as a shared phenotypic feature in both populations with obstructive sleep apnea (OSA) and post-gynecologic oncology patients, suggesting the potential utility of SWS/IL-6 profiling as discriminative biomarkers for OSA screening within gynecologic cancer cohorts.
5 Emerging therapeutic strategies
5.1 Microenvironment-targeted therapies
5.1.1 Hypoxia-targeted interventions and nanoparticles
The hypoxic tumor microenvironment drives malignant progression through HIF-1α-mediated upregulation of vascular endothelial growth factor (VEGF) and glycolytic enzymes (181–183). Emerging evidence indicates that IH induced by OSA exacerbates tumor hypoxia, potentially accelerating oncogenesis and chemoresistance. To counter these challenges, nanoparticle-based delivery systems have been engineered as multifunctional platforms for precision targeting of therapeutic agents, including small interfering RNA (siRNA), hypoxia-responsive nanotherapeutics, and conventional chemotherapeutic drugs (184). While current clinically approved nanomedicines primarily utilize passive tumor targeting via liposomal or polymeric carriers leveraging the enhanced permeability and retention (EPR) effect (185). Hypoxia-activated prodrugs such as mitomycin C remain limited by suboptimal clinical efficacy (186), necessitating innovative strategies for tumor reoxygenation and hypoxia modulation. Recent advancements in hypoxia-targeted nanotherapeutics focus on three synergistic approaches: tumor oxygenation via oxygen-generating biomaterials (e.g., alginate depots encapsulating catalase and calcium peroxide) (187, 188), hypoxia-responsive nanocarriers for spatiotemporally controlled drug release (e.g., doxorubicin-loaded hypoxia-responsive nanoparticles) (Figure 2A), and HIF-pathway inhibition using nanotechnology-enabled gene silencing (Figure 2B) (189). And the exploration of nanosensors which are capable of accurate diagnosis of hypoxic level is in urgent demand to estimate the malignant degree of cancer for subsequent effective and personalized cancer treatments (190). Concurrently, the development of hypoxia nanosensors has enabled dynamic monitoring of tumor microenvironment dynamics. For instance, Liu et al. (191) designed near-infrared (NIR)-activated upconversion nanoparticles (UCNPs) coupled with ruthenium-based complexes, achieving real-time in vivo hypoxia imaging through light conversion mechanisms (Figure 2C). Integration of nanotechnology with multimodal therapies demonstrates enhanced efficacy against resistant malignancies. Notable examples include reactive oxygen species (ROS)-responsive nanoplatforms co-delivering apatinib and doxorubicin for chemo-photodynamic synergy (192), alginate-optimized niosomes co-encapsulating doxorubicin and cisplatin to overcome ovarian cancer resistance (193) and albumin nanoparticles combining HIF-1α siRNA with methylene blue-mediated photodynamic ablation (194, 195). Parallel developments in OSA management reveal therapeutic interactions, where short-term continuous positive airway pressure (CPAP) withdrawal may enhance radiosensitivity through IH preconditioning, while prolonged CPAP application suppresses oncogenic pathways (e.g., JUN/MYC/SMAD3) in specific patient subsets (196). Notably, nanotechnology now enables both therapeutic intervention and physiological monitoring in OSA-related comorbidities (197). Key advances include CNS-targeted hesperidin delivery for leptin sensitization in obesity-associated sleep-disordered breathing, and ROS-responsive nanotherapeutics attenuating intermittent hypoxia-induced cognitive impairment through NRF2/KEAP1/HO-1 pathway modulation (198, 199). hypoxic tumor (200–202). This disparity underscores the necessity for personalized nanomedicine strategies that simultaneously address tumor hypoxia heterogeneity (quantified via nanosensors) and patient-specific physiological barriers.
Figure 2
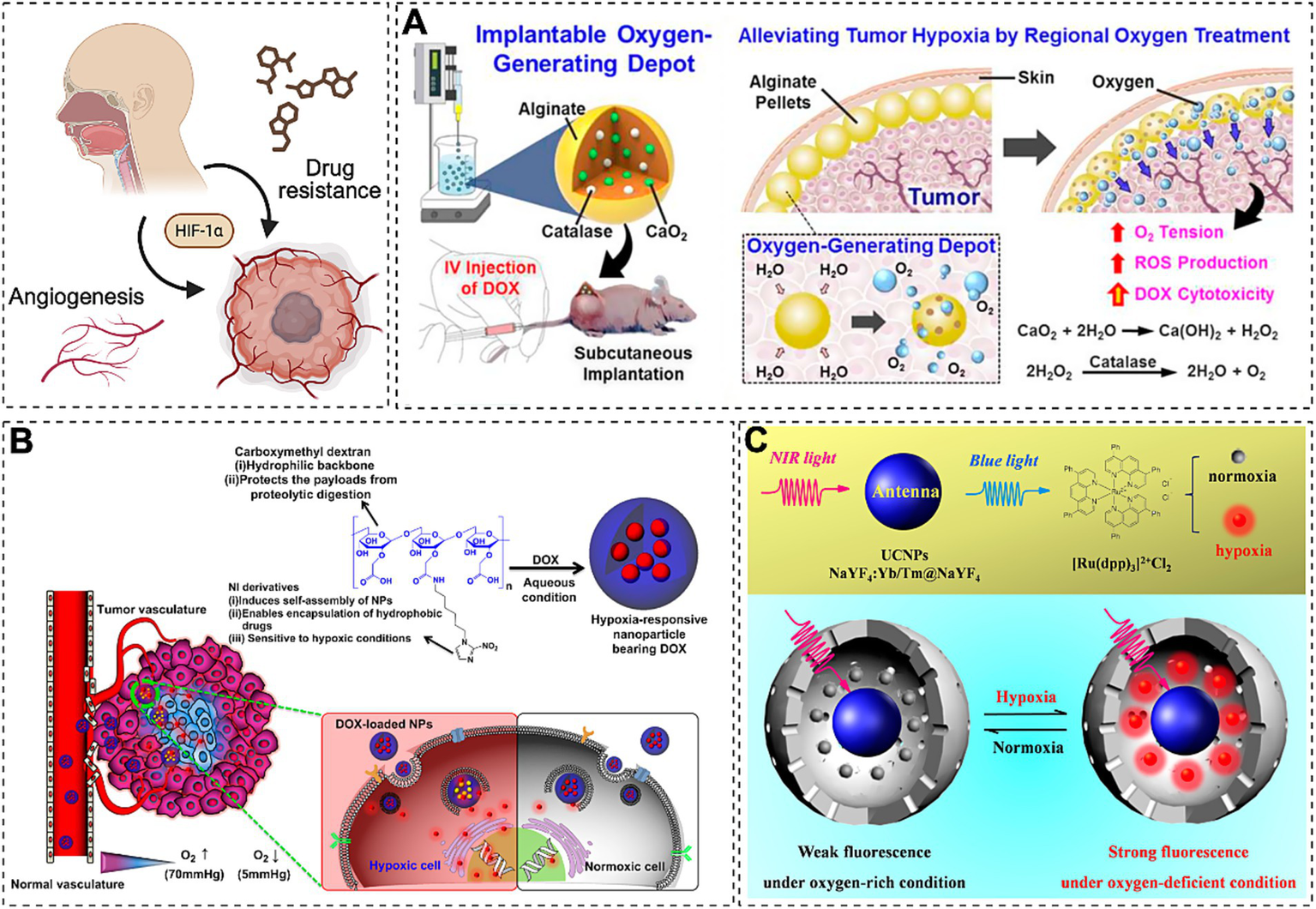
Emerging therapies target HIF-1α-driven VEGF and glycolytic enzyme upregulation to combat drug resistance. (A) Oxygen-delivering alginate pellet structure and DOX cytotoxicity enhancement in tumors (Reprinted with permission (126), licensed under CC BY). (B) EPR-mediated hypoxia-responsive drug release in tumors (Reprinted with permission (189), Copyright © 2013 Elsevier Ltd.). (C) NIR-activated UCNP-Ru hybrids enable real-time in vivo hypoxia imaging via photon upconversion (Reprinted with permission) (191).
5.1.2 Fecal microbiota transplantation
The impact of gut microbiota on the body’s immune system and hormonal balance is significant, dysbiosis of gut microbiota has been associated with the promotion of common gynecological diseases such as PCOS, endometriosis, and malignant tumors (203). Recent studies have shown that sleep insufficiency/deprivation alters the composition of gut microbiota in both humans and rodents (204, 205). Fecal microbiota transplantation (FMT) is being evaluated for its potential to enhance immune checkpoint blockade therapy in clinical studies (primarily for metastatic melanoma) (206). HPV is linked to cervical cancer (207). In a study by Xu et al. (208), ovarian cancer cells transplanted into mice with gut microbiota dysbiosis exhibited increased xenograft tumor sizes; this dysbiosis stimulated macrophages, elevating circulating interleukin (IL)-6 and tumor necrosis factor-α levels, thereby inducing ovarian cancer epithelial-mesenchymal transition. Lactobacillus plantarum HL2 mitigated PCOS-like pathological changes in ovaries (209). Melatonin ameliorates cadmium-induced intestinal mucosal damage by scavenging ROS and increasing goblet cell numbers, while Akkermansia muciniphila regulates melatonin production via increased enterochromaffin cells (210). Melatonin acts as a safe nutraceutical to limit skeletal muscle frailty, prolong physical performance, and target mitochondrial function by reducing oxidative damage (211–213). At week 12, FMT recipients showed higher insomnia remission rates versus controls (37.9% vs. 10.0%; p = 0.018), with significant reductions in ISI, PSQI, GAD-7, ESS scores, and cortisol (p < 0.05), while controls displayed no changes (214). Targeting microbiota may alleviate sleep deprivation effects (215). OSA alters gut microbiome diversity, and IH-induced GM changes can mediate sleep disturbances independently (215). Melatonin supplementation in NLRP3 KO mice delayed sarcopenia onset in aged animals, suggesting gut microbiota regulation (including FMT) as a potential sleep disorder therapy. The balance of gut microbiota benefits both gynecological tumors and OSA (210). Thus, exploring mechanistic connections and therapeutic synergies between these conditions is warranted.
5.2 Circadian optimization
Many cellular functions including the cell cycle and cell division are, at least in part, controlled by the molecular clock components (CLOCK, BMAL1, CRYs, PERs), it has also been expected that appropriate timing of chemotherapy may increase the efficacy of chemotherapeutic drugs and ameliorate their side effect (216, 217). Chronomodulated chemotherapy, such as timing oxaliplatin to PER3 expression peaks, improves endometrial cancer response and reduces treatment toxicity (218). OSA-related circadian rhythm disorders are linked to hypoxia-induced HIF1α augmentation, with HIF1α mRNA levels positively correlating with Bmal1, Cry1, and CK1δ expression (219). The combined expression of CRY1 and PER3 enhances prediction of severe OSA (220). In ovarian cancer, time-regulated 5-fluorouracil/leucovorin combined with radiotherapy is well-tolerated (221). Light therapy using blue wavelengths resets circadian phase, reducing cancer-related insomnia (222). While artificial light at night (ALAN) increases cancer risk and metabolic/mood disorders (222), blue light exposure disrupts cervical cell metabolism and reduces melatonin secretion via “darkness deficiency” (223, 224). Appropriately timed bright light exposure synchronizes biological rhythms and improves sleep–wake cycles, offering a nonpharmacological option for cancer patients (225).
5.3 Probiotic cocktails
Growing evidence has revealed the intimate relationship between the gut microbiota and anticancer treatments, including chemotherapy (226), radiotherapy (227), targeted therapy (228), and immunotherapy (229). Probiotics help maintain the integrity of the intestinal barrier and reduce chemotherapy drug-induced damage to the intestinal tract, thereby lowering the incidence of gastrointestinal side effects (230). Probiotics can improve patients’ quality of life by regulating the intestinal microbiota and reducing toxic reactions caused by chemotherapy drugs such as cisplatin. The early cytotoxicity of cisplatin depends on reactive oxygen species (ROS) generation, and probiotics enhance cisplatin efficacy by modulating the oxidative stress response (231). When probiotics (e.g., Lactobacillus) are combined with cisplatin, they induce tumor cell apoptosis, enhancing cisplatin’s anticancer effect (232). OSA-associated IH alters the gut microbiome, contributing to cardiovascular dysfunction; probiotics and prebiotics mitigate these effects by increasing cecal acetate concentrations (232, 233). Interestingly, the benefits of gut microbiota seem to manifest simultaneously in both tumor occurrence and development, as well as in the severity and complications of OSA.
6 Future directions
6.1 Mechanistic insights
6.1.1 Single-cell multiomics
In cancer research, hypoxic tumor niches represent a critical focus. These oxygen-deprived regions are linked to tumor aggressiveness, therapy resistance, and immune evasion. Single-cell multiomics enables precise mapping of these niches and their interactions with neighboring cells. Sleep disorders impair immune function, potentially altering immune cell states within the TME. For instance, sleep deprivation may reduce T cell and natural killer (NK) cell efficacy, compromising immune surveillance and tumor clearance.
Single-cell multiomics simultaneously analyzes multiple molecular profiles (genome, transcriptome, epigenome, proteome) at the single-cell level, offering unprecedented resolution for studying complex systems like TME (Figure 3) (234). Utilizing this approach, Yao et al. (235) identified a tumor immune barrier (TIB) composed of SPP1+ macrophages and cancer-associated fibroblasts (CAFs) near tumor boundaries, influencing immune checkpoint blockade efficacy. Hypoxia-driven SPP1 expression promotes macrophage-CAF interactions, stimulating extracellular matrix remodeling and TIB formation, limiting immune infiltration in tumor cores. This technology elucidates hypoxic niche–sleep-disrupted immune cell interplay, identifying therapeutic targets to enhance immunotherapy.
Figure 3

Future exploration of sleep disorders-gynecological cancer links. Step 1: Single-cell multiomics unravels dynamic tumor ecosystem complexities. Step 2: AI assists PSG scoring. Step 3: Wearable technologies monitor real-time SpO2 and activity data. Step 4: Repurposing melatonin agonists may offer safer circadian restoration and chemo-sensitization. Created with Biorender.com.
6.1.2 Artificial intelligence
Artificial intelligence (AI) enables “big data” analysis combining clinical, environmental, and laboratory metrics to advance sleep disorder understanding. Its medical applications include clinician-assisted image interpretation, health system workflow optimization, and patient self-monitoring (236). In sleep centers, AI assists polysomnography (PSG) scoring (Figure 3) (237). Tumor genomics identifies mutations and expression patterns for personalized treatment, while AI predicts therapeutic responses and drug targets. Esophageal pressure (Pes) monitoring, the gold standard for assessing respiratory effort during apneas/hypopneas, is invasive and rarely used. AI models trained on PSG-derived features simulated Pes in 1,119 individuals (237), highlighting AI’s potential to integrate PSG, cytokine, and genomic data for multimodal predictive modeling.
6.2 Translational opportunities
6.2.1 Wearable technologies
Smartwatch SpO2 monitoring via photoplethysmography is critical for tracking respiratory health in cancer patients, particularly those with chemotherapy/radiotherapy-related respiratory dysfunction or OSA (237). Circadian rest-activity metrics show moderate-to-strong associations with cancer patient overall survival (238). Leveraging wearable devices (e.g., Apple Watch, BioStamp, Dreem, Oura) and machine learning techniques, perioperative cancer monitoring and mortality risk prediction in advanced cancer patients can be achieved through continuous measurement of core physiological indicators including body temperature, average heart rate, step count, and blood oxygen saturation (239–241). Meanwhile, real-time SpO₂ and activity data concurrently facilitate early detection of respiratory compromise and sleep disturbances, enhancing therapeutic optimization (Figure 3) (242). Future advancements may integrate biomarkers such as heart rate variability and dermal thermoregulation patterns for holistic assessment. Ultimately, wearable data streams are projected to catalyze comprehensive diagnostic and therapeutic paradigm shifts for sleep disorders and gynecological malignancies.
6.2.2 Drug repurposing
Traditional insomnia treatments (benzodiazepines/nonbenzodiazepines) carry risks of cognitive impairment, falls, and dependence (243). Safe alternatives include slow-release melatonin (Circadian) and synthetic agonists (ramelteon, tasimelteon, agomelatine) (Figure 3) (244). Agomelatine, a MT1/MT2 agonist and 5-HT2C antagonist, improves depression-related sleep without cognitive side effects (245). Melatonin enhances tamoxifen efficacy by up-regulating estrogen receptors and activating PKC/PKA pathways via MT1-mediated p27Kip1 induction (246–248). Repurposing melatonin agonists may offer safer circadian restoration and chemo-sensitization, though clinical validation remains essential.
7 Conclusion
These intertwined mechanisms highlight sleep as a modifiable therapeutic target in gynecologic cancer. Integrative strategies—like hypoxia-targeted nanomedicine (e.g., hypoxia-targeted nanoparticles), chronotherapy (e.g., circadian-optimized immunotherapy timing), and microbiota modulation (probiotics)—offer promising paths to improve outcomes through personalized, interdisciplinary care.
Statements
Author contributions
HM: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Investigation, Methodology. CZ: Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. HJ: Methodology, Software, Writing – review & editing. WQ: Formal analysis, Resources, Writing – original draft, Data curation. XL: Investigation, Methodology, Writing – original draft. YX: Formal analysis, Methodology, Writing – review & editing. WW: Project administration, Validation, Visualization, Funding acquisition, Writing – original draft, Writing – review & editing. YS: Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. W-YL: Funding acquisition, Resources, Software, Writing – original draft, Writing – review & editing, Formal analysis, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82370093 and 82270107) and Shenyang Medical and Industrial Special Project (No. 240906).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Meyer N Harvey AG Lockley SW Dijk D-J . Circadian rhythms and disorders of the timing of sleep. Lancet. (2022) 400:1061–78. doi: 10.1016/S0140-6736(22)00877-7
2.
Kloss JD Perlis ML Zamzow JA Culnan EJ Gracia CR . Sleep, sleep disturbance, and fertility in women. Sleep Med Rev. (2015) 22:78–87. doi: 10.1016/j.smrv.2014.10.005
3.
Sateia MJ . International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
4.
Senaratna CV Perret JL Lodge CJ Lowe AJ Campbell BE Matheson MC et al . Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. (2017) 34:70–81. doi: 10.1016/j.smrv.2016.07.002
5.
Madut A Fuchsova V Man H Askar S Trivedi R Elder E et al . Increased prevalence of obstructive sleep apnea in women diagnosed with endometrial or breast cancer. PLoS One. (2021) 16:e0249099. doi: 10.1371/journal.pone.0249099
6.
Zinchuk AV Gentry MJ Concato J Yaggi HK . Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev. (2017) 35:113–23. doi: 10.1016/j.smrv.2016.10.002
7.
Jordan AS McSharry DG Malhotra A . Adult obstructive sleep apnoea. Lancet. (2014) 383:736–47. doi: 10.1016/S0140-6736(13)60734-5
8.
Peppard PE Young T Barnet JH Palta M Hagen EW Hla KM . Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. (2013) 177:1006–14. doi: 10.1093/aje/kws342
9.
Kirkness JP Schwartz AR Schneider H Punjabi NM Maly JJ Laffan AM et al . Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. (2008) 104:1618–24. doi: 10.1152/japplphysiol.00045.2008
10.
Kahal H Kyrou I Uthman OA Brown A Johnson S Wall PDH et al . The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep Breath. (2020) 24:339–50. doi: 10.1007/s11325-019-01835-1
11.
Pancholi C Chaudhary SC Gupta KK Sawlani KK Verma SK Singh A et al . Obstructive sleep apnea in hypothyroidism. Ann Afr Med. (2022) 21:403–9. doi: 10.4103/aam.aam_134_21
12.
Ray I Meira LB Michael A Ellis PE . Adipocytokines and disease progression in endometrial cancer: a systematic review. Cancer Metastasis Rev. (2021) 41:211–42. doi: 10.1007/s10555-021-10002-6
13.
Zeng F Wang X Hu W Wang L . Association of adiponectin level and obstructive sleep apnea prevalence in obese subjects. Medicine. (2017) 96:e7784. doi: 10.1097/MD.0000000000007784
14.
Brown KA Scherer PE . Update on adipose tissue and cancer. Endocr Rev. (2023) 44:961–74. doi: 10.1210/endrev/bnad015
15.
Vgontzas AN Bixler EO Chrousos GP . Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. (2003) 254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x
16.
Pien GW Pack AI Jackson N Maislin G Macones GA Schwab RJ . Risk factors for sleep-disordered breathing in pregnancy. Thorax. (2014) 69:371–7. doi: 10.1136/thoraxjnl-2012-202718
17.
Tantrakul V Sirijanchune P Panburana P Pengjam J Suwansathit W Boonsarngsuk V et al . Screening of obstructive sleep apnea during pregnancy: differences in predictive values of questionnaires across trimesters. J Clin Sleep Med. (2015) 11:157–63. doi: 10.5664/jcsm.4464
18.
Cai X-H Xie Y-P Li X-C Qu WL Li T Wang HX et al . The prevalence and associated risk factors of sleep disorder-related symptoms in pregnant women in China. Sleep Breath. (2013) 17:951–6. doi: 10.1007/s11325-012-0783-2
19.
Jordan AS McEvoy RD . Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. (2003) 7:377–89. doi: 10.1053/smrv.2002.0260
20.
Resta O Caratozzolo G Pannacciulli N Stefàno A Giliberti T Carpagnano GE et al . Gender, age and menopause effects on the prevalence and the characteristics of obstructive sleep apnea in obesity. Eur J Clin Investig. (2003) 33:1084–9. doi: 10.1111/j.1365-2362.2003.01278.x
21.
Gabryelska A Turkiewicz S Karuga FF Sochal M Strzelecki D Białasiewicz P . Disruption of circadian rhythm genes in obstructive sleep apnea patients-possible mechanisms involved and clinical implication. Int J Mol Sci. (2022) 23:709. doi: 10.3390/ijms23020709
22.
Gamble KL Berry R Frank SJ Young ME . Circadian clock control of endocrine factors. Nat Rev Endocrinol. (2014) 10:466–75. doi: 10.1038/nrendo.2014.78
23.
Mong JA Baker FC Mahoney MM Paul KN Schwartz MD Semba K et al . Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. (2011) 31:16107–16. doi: 10.1523/JNEUROSCI.4175-11.2011
24.
Tran C Diaz-Ayllon H Abulez D Chinta S Williams-Brown MY Desravines N . Gynecologic cancer screening and prevention: state of the science and practice. Curr Treat Options in Oncol. (2025) 26:167–78. doi: 10.1007/s11864-025-01301-z
25.
Hurley S Goldberg D Bernstein L Reynolds P . Sleep duration and cancer risk in women. Cancer Causes Control. (2015) 26:1037–45. doi: 10.1007/s10552-015-0579-3
26.
Rai R Nahar M Jat D Gupta N Mishra SK . A systematic assessment of stress insomnia as the high-risk factor for cervical cancer and interplay of cervicovaginal microbiome. Front Cell Infect Microbiol. (2022) 12:1042663. doi: 10.3389/fcimb.2022.1042663
27.
Savard J Miller SM Mills M O'Leary A Harding H Douglas SD et al . Association between subjective sleep quality and depression on immunocompetence in low-income women at risk for cervical cancer. Psychosom Med. (1999) 61:496–507. doi: 10.1097/00006842-199907000-00014
28.
Lu Y Tian N Yin J Shi Y Huang Z . Association between sleep duration and cancer risk: a meta-analysis of prospective cohort studies. PLoS One. (2013) 8:e74723. doi: 10.1371/journal.pone.0074723
29.
Brenner R Kivity S Peker M Reinhorn D Keinan-Boker L Silverman B et al . Increased risk for cancer in young patients with severe obstructive sleep apnea. Respiration. (2019) 97:15–23. doi: 10.1159/000486577
30.
Campos-Rodriguez F Martinez-Garcia MA Martinez M Duran-Cantolla J Peña M Masdeu MJ et al . Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. (2013) 187:99–105. doi: 10.1164/rccm.201209-1671OC
31.
Chang W-P Liu M-E Chang W-C Yang AC Ku YC Pai JT et al . Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med. (2014) 15:1016–20. doi: 10.1016/j.sleep.2014.05.026
32.
Palamaner Subash Shantha G Kumar AA Cheskin LJ Pancholy SB . Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. (2015) 16:1289–94. doi: 10.1016/j.sleep.2015.04.014
33.
Fang H-F Miao N-F Chen C-D Sithole T Chung M-H . Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J Cancer. (2015) 6:1140–7. doi: 10.7150/jca.12490
34.
Nieto FJ Peppard PE Young T Finn L Hla KM Farré R . Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. (2012) 186:190–4. doi: 10.1164/rccm.201201-0130OC
35.
Huang H-Y Lin S-W Chuang L-P Wang CL Sun MH Li HY et al . Severe OSA associated with higher risk of mortality in stage III and IV lung cancer. J Clin Sleep Med. (2020) 16:1091–8. doi: 10.5664/jcsm.8432
36.
Jeon S-Y Hwang K-A Choi K-C . Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol. (2016) 158:1–8. doi: 10.1016/j.jsbmb.2016.02.005
37.
Folkerd EJ Dowsett M . Influence of sex hormones on cancer progression. J Clin Oncol. (2010) 28:4038–44. doi: 10.1200/JCO.2009.27.4290
38.
Carter BD Diver WR Hildebrand JS Patel AV Gapstur SM . Circadian disruption and fatal ovarian cancer. Am J Prev Med. (2014) 46:S34–41. doi: 10.1016/j.amepre.2013.10.032
39.
Hartge P Hoover R McGowan L Lesher L Norris HJ . Menopause and ovarian cancer. Am J Epidemiol. (1988) 127:990–8. doi: 10.1093/oxfordjournals.aje.a114902
40.
Poole EM Schernhammer E Mills L Hankinson SE Tworoger SS . Urinary melatonin and risk of ovarian cancer. Cancer Causes Control. (2015) 26:1501–6. doi: 10.1007/s10552-015-0640-2
41.
Moeller JS Bever SR Finn SL Phumsatitpong C Browne MF Kriegsfeld LJ . Circadian regulation of hormonal timing and the pathophysiology of circadian dysregulation. Compr Physiol. (2022) 12:4185–214. doi: 10.1002/j.2040-4603.2022.tb00246.x
42.
Pines A . Circadian rhythm and menopause. Climacteric. (2016) 19:551–2. doi: 10.1080/13697137.2016.1226608
43.
Baker FC Driver HS . Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. (2007) 8:613–22. doi: 10.1016/j.sleep.2006.09.011
44.
Sephton S Spiegel D . Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease?Brain Behav Immun. (2003) 17:321–8. doi: 10.1016/S0889-1591(03)00078-3
45.
Savard J Morin CM . Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. (2001) 19:895–908. doi: 10.1200/JCO.2001.19.3.895
46.
Baker F Denniston M Smith T West MM . Adult cancer survivors: how are they faring?Cancer. (2005) 104:2565–76. doi: 10.1002/cncr.21488
47.
Westin SN Sun CC Tung CS Lacour RA Meyer LA Urbauer DL et al . Survivors of gynecologic malignancies: impact of treatment on health and well-being. J Cancer Surviv. (2016) 10:261–70. doi: 10.1007/s11764-015-0472-9
48.
Christman NJ Oakley MG Cronin SN . Developing and using preparatory information for women undergoing radiation therapy for cervical or uterine cancer. Oncol Nurs Forum. (2001) 28:93–8. Available at: https://pubmed.ncbi.nlm.nih.gov/11198902 PMID:
49.
Tian J Chen GL Zhang HR . Sleep status of cervical cancer patients and predictors of poor sleep quality during adjuvant therapy. Support Care Cancer. (2015) 23:1401–8. doi: 10.1007/s00520-014-2493-8
50.
Zhao C Grubbs A Barber EL . Sleep and gynecological cancer outcomes: opportunities to improve quality of life and survival. Int J Gynecol Cancer. (2022) 32:669–75. doi: 10.1136/ijgc-2022-003404
51.
Al Maqbali M Al Sinani M Alsayed A Gleason AM . Prevalence of sleep disturbance in patients with cancer: a systematic review and meta-analysis. Clin Nurs Res. (2022) 31:1107–23. doi: 10.1177/10547738221092146
52.
Fox RS Ancoli-Israel S Roesch SC Merz EL Mills SD Wells KJ et al . Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Support Care Cancer. (2020) 28:845–55. doi: 10.1007/s00520-019-04834-w
53.
Tuyan İlhan T Uçar MG Gül A Saymaz İlhan T Yavaş G Çelik Ç . Sleep quality of endometrial cancer survivors and the effect of treatments. Turk J Obstet Gynecol. (2017) 14:243–8. doi: 10.4274/tjod.59265
54.
Pozzar RA Hammer MJ Paul SM Cooper BA Kober KM Conley YP et al . Distinct sleep disturbance profiles among patients with gynecologic cancer receiving chemotherapy. Gynecol Oncol. (2021) 163:419–26. doi: 10.1016/j.ygyno.2021.09.002
55.
Bonsignore MR Saaresranta T Riha RL . Sex differences in obstructive sleep apnoea. Eur Respir Rev. (2019) 28:190030. doi: 10.1183/16000617.0030-2019
56.
Chen M-Y Zheng W-Y Liu Y-F Li XH Lam MI Su Z et al . Global prevalence of poor sleep quality in cancer patients: a systematic review and meta-analysis. Gen Hosp Psychiatry. (2024) 87:92–102. doi: 10.1016/j.genhosppsych.2023.12.004
57.
Patke A Young MW Axelrod S . Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. (2020) 21:67–84. doi: 10.1038/s41580-019-0179-2
58.
Sancar A Van Gelder RN . Clocks, cancer, and chronochemotherapy. Science. (2021) 371:eabb0738. doi: 10.1126/science.abb0738
59.
Dun A Zhao X Jin X Wei T Gao X Wang Y et al . Association between night-shift work and cancer risk: updated systematic review and meta-analysis. Front Oncol. (2020) 10:1006. doi: 10.3389/fonc.2020.01006
60.
Yuan X Zhu C Wang M Mo F Du W Ma X . Night shift work increases the risks of multiple primary cancers in women: a systematic review and meta-analysis of 61 articles. Cancer Epidemiol Biomarkers Prev. (2018) 27:25–40. doi: 10.1158/1055-9965.EPI-17-0221
61.
Wang Y Narasimamurthy R Qu M Shi N Guo H Xue Y et al . Circadian regulation of cancer stem cells and the tumor microenvironment during metastasis. Nat Cancer. (2024) 5:546–56. doi: 10.1038/s43018-024-00759-4
62.
Jung-Hynes B Reiter RJ Ahmad N . Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res. (2010) 48:9–19. doi: 10.1111/j.1600-079X.2009.00729.x
63.
Ding M Lu Y Huang X Xing C Hou S Wang D et al . Acute hypoxia induced dysregulation of clock-controlled ovary functions. Front Physiol. (2022) 13:1024038. doi: 10.3389/fphys.2022.1024038
64.
Hosseinzadeh A Alinaghian N Sheibani M Seirafianpour F Naeini AJ Mehrzadi S . Melatonin: current evidence on protective and therapeutic roles in gynecological diseases. Life Sci. (2024) 344:122557. doi: 10.1016/j.lfs.2024.122557
65.
Dana PM Sadoughi F Mobini M Shafabakhsh R Chaichian S Moazzami B et al . Molecular and biological functions of melatonin in endometrial cancer. Curr Drug Targets. (2020) 21:519–26. doi: 10.2174/1389450120666190927123746
66.
Mafi A Rezaee M Hedayati N Hogan SD Reiter RJ Aarabi MH et al . Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Commun Signal. (2023) 21:33. doi: 10.1186/s12964-023-01047-x
67.
Angelousi A Kassi E Ansari-Nasiri N Randeva H Kaltsas G Chrousos G . Clock genes and cancer development in particular in endocrine tissues. Endocr Relat Cancer. (2019) 26:R305–17. doi: 10.1530/ERC-19-0094
68.
Barrett RJ Blessing JA Homesley HD Twiggs L Webster KD . Circadian-timed combination doxorubicin-cisplatin chemotherapy for advanced endometrial carcinoma. A phase II study of the Gynecologic Oncology Group. Am J Clin Oncol. (1993) 16:494–6. doi: 10.1097/00000421-199312000-00007
69.
Chen M Zhang L Liu X Ma Z Lv L . PER1 is a prognostic biomarker and correlated with immune infiltrates in ovarian cancer. Front Genet. (2021) 12:697471. doi: 10.3389/fgene.2021.697471
70.
Brown SA Ripperger J Kadener S Fleury-Olela F Vilbois F Rosbash M et al . PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science. (2005) 308:693–6. doi: 10.1126/science.1107373
71.
Hernández-Rosas F Hernández-Oliveras A Flores-Peredo L Rodríguez G Zarain-Herzberg Á Caba M et al . Histone deacetylase inhibitors induce the expression of tumor suppressor genes Per1 and Per2 in human gastric cancer cells. Oncol Lett. (2018) 16:1981–90. doi: 10.3892/ol.2018.8851
72.
Xiang R Cui Y Wang Y Xie T Yang X Wang Z et al . Circadian clock gene Per2 downregulation in non-small cell lung cancer is associated with tumour progression and metastasis. Oncol Rep. (2018) 40:3040–8. doi: 10.3892/or.2018.6704
73.
Bristow RG Hill RP . Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. (2008) 8:180–92. doi: 10.1038/nrc2344
74.
Ye Y Hu Q Chen H Liang K Yuan Y Xiang Y et al . Characterization of hypoxia-associated molecular features to aid hypoxia-targeted therapy. Nat Metab. (2019) 1:431–44. doi: 10.1038/s42255-019-0045-8
75.
Semenza GL . Targeting HIF-1 for cancer therapy. Nat Rev Cancer. (2003) 3:721–32. doi: 10.1038/nrc1187
76.
Martinez C-A Jiramongkol Y Bal N Alwis I Nedoboy PE Farnham MMJ et al . Intermittent hypoxia enhances the expression of hypoxia inducible factor HIF1A through histone demethylation. J Biol Chem. (2022) 298:102536. doi: 10.1016/j.jbc.2022.102536
77.
Prabhakar NR Peng Y-J Nanduri J . Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest. (2020) 130:5042–51. doi: 10.1172/JCI137560
78.
Wu D Zhang R Zhao R Chen G Cai Y Jin J . A novel function of novobiocin: disrupting the interaction of HIF 1α and p300/CBP through direct binding to the HIF1α C-terminal activation domain. PLoS One. (2013) 8:e62014. doi: 10.1371/journal.pone.0062014
79.
Majmundar AJ Wong WJ Simon MC . Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. (2010) 40:294–309. doi: 10.1016/j.molcel.2010.09.022
80.
Yu Z Liu Y Zhu J Han J Tian X Han W et al . Insights from molecular dynamics simulations and steered molecular dynamics simulations to exploit new trends of the interaction between HIF-1α and p300. J Biomol Struct Dyn. (2020) 38:1–12. doi: 10.1080/07391102.2019.1580616
81.
Schumacker PT . Hypoxia-inducible factor-1 (HIF-1). Crit Care Med. (2005) 33:S423–5. doi: 10.1097/01.ccm.0000191716.38566.e0
82.
Yuan G Nanduri J Bhasker CR Semenza GL Prabhakar NR . Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. (2005) 280:4321–8. doi: 10.1074/jbc.M407706200
83.
Iyer NV Kotch LE Agani F Leung SW Laughner E Wenger RH et al . Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. (1998) 12:149–62. doi: 10.1101/gad.12.2.149
84.
Huang X Ruan G Liu G Gao Y Sun P . Immunohistochemical analysis of PGC-1α and ERRα expression reveals their clinical significance in human ovarian cancer. Onco Targets Ther. (2020) 13:13055–62. doi: 10.2147/OTT.S288332
85.
Carmeliet P . VEGF as a key mediator of angiogenesis in cancer. Oncology. (2005) 69:4–10. doi: 10.1159/000088478
86.
Vaupel P Schmidberger H Mayer A . The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. (2019) 95:912–9. doi: 10.1080/09553002.2019.1589653
87.
Jing X Yang F Shao C Wei K Xie M Shen H et al . Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. (2019) 18:157. doi: 10.1186/s12943-019-1089-9
88.
Gao T Li J-Z Lu Y Zhang CY Li Q Mao J et al . The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment. Biomed Pharmacother. (2016) 80:393–405. doi: 10.1016/j.biopha.2016.02.044
89.
Wu Q You L Nepovimova E Heger Z Wu W Kuca K et al . Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. (2022) 15:77. doi: 10.1186/s13045-022-01292-6
90.
Bai R Li Y Jian L Yang Y Zhao L Wei M . The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: mechanisms and clinical treatment strategies. Mol Cancer. (2022) 21:177. doi: 10.1186/s12943-022-01645-2
91.
Höckel M Knoop C Schlenger K Vorndran B Bauβnann E Mitze M et al . Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. (1993) 26:45–50. doi: 10.1016/0167-8140(93)90025-4
92.
Hockel M Schlenger K Aral B Mitze M Schaffer U Vaupel P . Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. (1996) 56:4509–15.
93.
Almendros I Montserrat JM Ramírez J Torres M Duran-Cantolla J Navajas D et al . Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. (2012) 39:215–7. doi: 10.1183/09031936.00185110
94.
Gallego-Martin T Farré R Almendros I Gonzalez-Obeso E Obeso A . Chronic intermittent hypoxia mimicking sleep apnoea increases spontaneous tumorigenesis in mice. Eur Respir J. (2017) 49:1602111. doi: 10.1183/13993003.02111-2016
95.
Semenza GL . Hypoxia-inducible factors in physiology and medicine. Cell. (2012) 148:399–408. doi: 10.1016/j.cell.2012.01.021
96.
Patel MS Korotchkina LG . Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. (2006) 34:217–22. doi: 10.1042/BST20060217
97.
Hsu PP Sabatini DM . Cancer cell metabolism: Warburg and beyond. Cell. (2008) 134:703–7. doi: 10.1016/j.cell.2008.08.021
98.
Salaroglio IC Belisario DC Akman M la Vecchia S Godel M Anobile DP et al . Mitochondrial ROS drive resistance to chemotherapy and immune-killing in hypoxic non-small cell lung cancer. J Exp Clin Cancer Res. (2022) 41:243. doi: 10.1186/s13046-022-02447-6
99.
Wu X Gong L Xie L Gu W Wang X Liu Z et al . NLRP3 deficiency protects against intermittent hypoxia-induced neuroinflammation and mitochondrial ROS by promoting the PINK1-Parkin pathway of mitophagy in a murine model of sleep apnea. Front Immunol. (2021) 12:628168. doi: 10.3389/fimmu.2021.628168
100.
Rajasingham R Govender NP Jordan A Loyse A Shroufi A Denning DW et al . The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. (2022) 22:1748–55. doi: 10.1016/S1473-3099(22)00499-6
101.
Collado MC Katila MK Vuorela NM Saarenpää-Heikkilä O Salminen S Isolauri E . Dysbiosis in snoring children: an interlink to comorbidities?J Pediatr Gastroenterol Nutr. (2019) 68:272–7. doi: 10.1097/MPG.0000000000002161
102.
Mashaqi S Gozal D . Obstructive sleep apnea and systemic hypertension: gut dysbiosis as the mediator?J Clin Sleep Med. (2019) 15:1517–27. doi: 10.5664/jcsm.7990
103.
Thomas-White K Forster SC Kumar N van Kuiken M Putonti C Stares MD et al . Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun. (2018) 9:1557. doi: 10.1038/s41467-018-03968-5
104.
Baker JM Al-Nakkash L Herbst-Kralovetz MM . Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
105.
D'Antonio DL Marchetti S Pignatelli P Piattelli A Curia MC . The oncobiome in gastroenteric and genitourinary cancers. Int J Mol Sci. (2022) 23:9664. doi: 10.3390/ijms23179664
106.
Almendros I Gileles-Hillel A Khalyfa A Wang Y Zhang SX Carreras A et al . Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett. (2015) 361:233–9. doi: 10.1016/j.canlet.2015.03.010
107.
Korbecki J Simińska D Gąssowska-Dobrowolska M Listos J Gutowska I Chlubek D et al . Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. (2021) 22:701. doi: 10.3390/ijms221910701
108.
Korbecki J Kojder K Barczak K Simińska D Gutowska I Chlubek D et al . Hypoxia alters the expression of CC chemokines and CC chemokine receptors in a tumor-a literature review. Int J Mol Sci. (2020) 21:5647. doi: 10.3390/ijms21165647
109.
Kacinski BM . CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. (1995) 27:79–85. doi: 10.3109/07853899509031941
110.
Xu J Ding L Mei J Hu Y Kong X Dai S et al . Dual roles and therapeutic targeting of tumor-associated macrophages in tumor microenvironments. Signal Transduct Target Ther. (2025) 10:268. doi: 10.1038/s41392-025-02325-5
111.
Almendros I Wang Y Becker L Lennon FE Zheng J Coats BR et al . Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. (2014) 189:593–601. doi: 10.1164/rccm.201310-1830OC
112.
Tew WP Muss HB Kimmick GG Von Gruenigen VE Lichtman SM . Breast and ovarian cancer in the older woman. J Clin Oncol. (2014) 32:2553–61. doi: 10.1200/JCO.2014.55.3073
113.
Msaad S Kotti A Zouari Gdoura H Moussa N Feki W Kammoun S . Coexisting central and obstructive sleep apnea and mild diurnal hypoventilation associated with unilateral diaphragmatic dysfunction and brainstem lesion. Neurophysiol Clin. (2020) 50:375–81. doi: 10.1016/j.neucli.2020.06.003
114.
Lu Z Tang X Huang X . Phrenic nerve conduction and diaphragmatic motor evoked potentials: evaluation of respiratory dysfunction. Chin Med J. (1998) 111:496–9.
115.
Rowe LM Connor NP Russell JA . Respiratory-swallow coordination in a rat model of chemoradiation. Head Neck. (2021) 43:2954–66. doi: 10.1002/hed.26782
116.
Mols F Beijers T Vreugdenhil G van de Poll-Franse L . Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. (2014) 22:2261–9. doi: 10.1007/s00520-014-2255-7
117.
Cui Q Wang J-Q Assaraf YG Ren L Gupta P Wei L et al . Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. (2018) 41:1–25. doi: 10.1016/j.drup.2018.11.001
118.
Fidanboylu M Griffiths LA Flatters SJL . Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One. (2011) 6:e25212. doi: 10.1371/journal.pone.0025212
119.
Petrelli F Coinu A Riboldi V Borgonovo K Ghilardi M Cabiddu M et al . Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: a systematic review and meta-analysis of published studies. Oral Oncol. (2014) 50:1041–8. doi: 10.1016/j.oraloncology.2014.08.005
120.
Song M Cui M Liu K . Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur J Med Chem. (2022) 232:114205. doi: 10.1016/j.ejmech.2022.114205
121.
Kciuk M Gielecińska A Mujwar S Kołat D Kałuzińska-Kołat Ż Celik I et al . Doxorubicin-an agent with multiple mechanisms of anticancer activity. Cells. (2023) 12:659. doi: 10.3390/cells12040659
122.
Palma J-A Gileles-Hillel A Norcliffe-Kaufmann L Kaufmann H . Chemoreflex failure and sleep-disordered breathing in familial dysautonomia: implications for sudden death during sleep. Auton Neurosci. (2019) 218:10–5. doi: 10.1016/j.autneu.2019.02.003
123.
Sato F Nishimura M Shinano H Saito H Miyamoto K Kawakami Y . Heart rate during obstructive sleep apnea depends on individual hypoxic chemosensitivity of the carotid body. Circulation. (1997) 96:274–81.
124.
Prabhakar NR . Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp Physiol. (2016) 101:975–85. doi: 10.1113/EP085624
125.
Platero-Luengo A González-Granero S Durán R Díaz-Castro B Piruat JI García-Verdugo JM et al . An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell. (2014) 156:291–303. doi: 10.1016/j.cell.2013.12.013
126.
Ma S Wang J Cui Z Yang X Cui X Li X et al . HIF-2α-dependent TGFBI promotes ovarian cancer chemoresistance by activating PI3K/Akt pathway to inhibit apoptosis and facilitate DNA repair process. Sci Rep. (2024) 14:3870. doi: 10.1038/s41598-024-53854-y
127.
Zhang Z Yu X Wen L Wang J Li Z Zhang Y et al . USP9X integrates TGF-β and hypoxia signalings to promote ovarian cancer chemoresistance via HIF-2α-maintained stemness. Cell Death Dis. (2025) 16:312. doi: 10.1038/s41419-025-07646-5
128.
Beral V Gaitskell K Hermon C Moser K Reeves G Peto R . Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. (2015) 385:1835–42. doi: 10.1016/S0140-6736(14)61687-1
129.
Beral V Bull D Reeves G . Endometrial cancer and hormone-replacement therapy in the million women study. Lancet. (2005) 365:1543–51. doi: 10.1016/S0140-6736(05)66455-0
130.
Zheng H Kavanagh JJ Hu W Liao Q Fu S . Hormonal therapy in ovarian cancer. Int J Gynecol Cancer. (2007) 17:325–38. doi: 10.1111/j.1525-1438.2006.00749.x
131.
Altman AD Nelson GS Chu P Nation J Ghatage P . Uterine sarcoma and aromatase inhibitors: Tom Baker Cancer Centre experience and review of the literature. Int J Gynecol Cancer. (2012) 22:1006–12. doi: 10.1097/IGC.0b013e31825b7de8
132.
Jiang C Chen Q Yu D Zhou Q Tang C Qiao C . Prevalence and prognostic significance of sarcopenia in gynecologic oncology: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2025) 16:e13699. doi: 10.1002/jcsm.13699
133.
Mitra S Lami MS Ghosh A Das R Tallei TE Fatimawali et al . Hormonal therapy for gynecological cancers: how far has science progressed toward clinical applications?Cancers. (2022) 14:759. doi: 10.3390/cancers14030759
134.
Pellegrino A Tiidus PM Vandenboom R . Mechanisms of estrogen influence on skeletal muscle: mass, regeneration, and mitochondrial function. Sports Med. (2022) 52:2853–69. doi: 10.1007/s40279-022-01733-9
135.
Rutten IJG van Dijk DPJ Kruitwagen RFPM Beets-Tan RGH Olde Damink SWM van Gorp T . Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. (2016) 7:458–66. doi: 10.1002/jcsm.12107
136.
Staley SA Tucker K Newton M Ertel M Oldan J Doherty I et al . Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane-based chemotherapy. Gynecol Oncol. (2020) 156:695–700. doi: 10.1016/j.ygyno.2020.01.003
137.
Ubachs J Koole SN Lahaye M Fabris C Bruijs L Schagen van Leeuwen J et al . No influence of sarcopenia on survival of ovarian cancer patients in a prospective validation study. Gynecol Oncol. (2020) 159:706–11. doi: 10.1016/j.ygyno.2020.09.042
138.
Williams GR Dunne RF Giri S Shachar SS Caan BJ . Sarcopenia in the older adult with cancer. J Clin Oncol. (2021) 39:2068–78. doi: 10.1200/JCO.21.00102
139.
O’Halloran KD Lewis P McDonald F . Sex, stress and sleep apnoea: decreased susceptibility to upper airway muscle dysfunction following intermittent hypoxia in females. Respir Physiol Neurobiol. (2017) 245:76–82. doi: 10.1016/j.resp.2016.11.009
140.
Popovic RM White DP . Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. (1998) 84:1055–62. doi: 10.1152/jappl.1998.84.3.1055
141.
Mahboobifard F Pourgholami MH Jorjani M Dargahi L Amiri M Sadeghi S et al . Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother. (2022) 156:113808. doi: 10.1016/j.biopha.2022.113808
142.
Chan Y-N Jheng Y-W Wang Y-J . Chemotherapy-induced peripheral neurotoxicity as a risk factor for poor sleep quality in breast cancer survivors treated with docetaxel. Asia Pac J Oncol Nurs. (2021) 8:68–73. doi: 10.4103/apjon.apjon_51_20
143.
Schubert C Hong S Natarajan L Mills PJ Dimsdale JE . The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. (2007) 21:413–27. doi: 10.1016/j.bbi.2006.11.004
144.
Weinrib AZ Sephton SE DeGeest K Penedo F Bender D Zimmerman B et al . Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. (2010) 116:4410–9. doi: 10.1002/cncr.25299
145.
Schrepf A Clevenger L Christensen D DeGeest K Bender D Ahmed A et al . Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. (2013) 30:S126–34. doi: 10.1016/j.bbi.2012.07.022
146.
Rohleder N Aringer M Boentert M . Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. (2012) 1261:88–96. doi: 10.1111/j.1749-6632.2012.06634.x
147.
Iovoli AJ Smith K Yu H Kluczynski MA Jungquist CR Ray AD et al . Association of insomnia and obstructive sleep apnea with worse oral mucositis and quality of life in head and neck cancer patients undergoing radiation therapy. Cancers. (2024) 16:1335. doi: 10.3390/cancers16071335
148.
Faiz SA Balachandran D Hessel AC Lei X Beadle BM William WN Jr et al . Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. (2014) 19:1200–6. doi: 10.1634/theoncologist.2014-0176
149.
Krishnan V Collop NA . Gender differences in sleep disorders. Curr Opin Pulm Med. (2006) 12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a
150.
Knutson KL . The association between pubertal status and sleep duration and quality among a nationally representative sample of U.S. adolescents. Am J Hum Biol. (2005) 17:418–24. doi: 10.1002/ajhb.20405
151.
Franklin KA Lindberg E . Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. (2015) 7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11
152.
Anttalainen U Tenhunen M Rimpilä V Polo O Rauhala E Himanen SL et al . Prolonged partial upper airway obstruction during sleep—an underdiagnosed phenotype of sleep-disordered breathing. Eur Clin Respir J. (2016) 3:31806. doi: 10.3402/ecrj.v3.31806
153.
Valipour A . Gender-related differences in the obstructive sleep apnea syndrome. Pneumologie. (2012) 66:584–8. doi: 10.1055/s-0032-1325664
154.
Young T Finn L . Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. (1998) 53:S16–9. doi: 10.1136/thx.53.2008.S16
155.
O'Connor C Thornley KS Hanly PJ . Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. (2000) 161:1465–72. doi: 10.1164/ajrccm.161.5.9904121
156.
Theorell-Haglöw J Miller CB Bartlett DJ Yee BJ Openshaw HD Grunstein RR . Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults—What do we know? A clinical update. Sleep Med Rev. (2018) 38:28–38. doi: 10.1016/j.smrv.2017.03.003
157.
Bower JE . Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. (2007) 21:863–71. doi: 10.1016/j.bbi.2007.03.013
158.
Poulson MJ . Not just tired. J Clin Oncol. (2003) 21:112s–3s. doi: 10.1200/JCO.2003.01.191
159.
Meek CL Wallace AM Forrest LM McMillan DC . The relationship between the insulin-like growth factor-1 axis, weight loss, an inflammation-based score and survival in patients with inoperable non-small cell lung cancer. Clin Nutr. (2010) 29:206–9. doi: 10.1016/j.clnu.2009.08.013
160.
Dantzer R . Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. (2001) 933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x
161.
Bower JE Ganz PA Aziz N Fahey JL . Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. (2002) 64:604–11. doi: 10.1097/00006842-200207000-00010
162.
Collado-Hidalgo A Bower JE Ganz PA Cole SW Irwin MR . Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. (2006) 12:2759–66. doi: 10.1158/1078-0432.CCR-05-2398
163.
Chervin RD . Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. (2000) 118:372–9. doi: 10.1378/chest.118.2.372
164.
Chen Z Li Y Zhang H Huang P Luthra R . Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. (2010) 29:4362–8. doi: 10.1038/onc.2010.193
165.
Devlin C Greco S Martelli F Ivan M . miR-210: more than a silent player in hypoxia. IUBMB Life. (2011) 63:94–100. doi: 10.1002/iub.427
166.
Shang F Wang S-C Gongol B Han SY Cho Y Schiavon CR et al . Obstructive sleep apnea-induced endothelial dysfunction is mediated by miR-210. Am J Respir Crit Care Med. (2023) 207:323–35. doi: 10.1164/rccm.202202-0394OC
167.
Li L Huang K You Y Fu X Hu L Song L et al . Hypoxia-induced miR-210 in epithelial ovarian cancer enhances cancer cell viability via promoting proliferation and inhibiting apoptosis. Int J Oncol. (2014) 44:2111–20. doi: 10.3892/ijo.2014.2368
168.
Archer SN Schmidt C Vandewalle G Dijk D-J . Phenotyping of PER3 variants reveals widespread effects on circadian preference, sleep regulation, and health. Sleep Med Rev. (2018) 40:109–26. doi: 10.1016/j.smrv.2017.10.008
169.
Rosenberg J . Sleep disturbances after non-cardiac surgery. Sleep Med Rev. (2001) 5:129–37. doi: 10.1053/smrv.2000.0121
170.
Gögenur I Wildschiøtz G Rosenberg J . Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. (2008) 100:45–9. doi: 10.1093/bja/aem340
171.
Loredo JS Ancoli-Israel S Dimsdale JE . Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens. (2001) 14:887–92. doi: 10.1016/s0895-7061(01)02143-4
172.
Frieboes RM Murck H Stalla GK Antonijevic IA Steiger A . Enhanced slow wave sleep in patients with prolactinoma. J Clin Endocrinol Metab. (1998) 83:2706–10. doi: 10.1210/jcem.83.8.5016
173.
Kelly MP Hickey C Makonnen S Coetzee S Jalal S Wang Y et al . Preclinical activity of the novel anti-prolactin receptor (PRLR) antibody-drug conjugate REGN2878-DM1 in PRLR-positive breast cancers. Mol Cancer Ther. (2017) 16:1299–311. doi: 10.1158/1535-7163.MCT-16-0839
174.
Hakim S Craig JM Koblinski JE Clevenger CV . Inhibition of the activity of cyclophilin A impedes prolactin receptor-mediated signaling, mammary tumorigenesis, and metastases. iScience. (2020) 23:101581. doi: 10.1016/j.isci.2020.101581
175.
Yang X Meyer K Friedl A . STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis. J Biol Chem. (2013) 288:21184–96. doi: 10.1074/jbc.M113.481119
176.
Neradugomma NK Subramaniam D Tawfik OW Goffin V Kumar TR Jensen RA et al . Prolactin signaling enhances colon cancer stemness by modulating notch signaling in a Jak2-STAT3/ERK manner. Carcinogenesis. (2014) 35:795–806. doi: 10.1093/carcin/bgt379
177.
Burgos I Richter L Klein T Fiebich B Feige B Lieb K et al . Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. (2006) 20:246–53. doi: 10.1016/j.bbi.2005.06.007
178.
May U Schiffelholz T Baier PC Krueger JM Rose-John S Scheller J . IL-6-trans-signalling increases rapid-eye-movement sleep in rats. Eur J Pharmacol. (2009) 613:141–5. doi: 10.1016/j.ejphar.2009.04.023
179.
Benedict C Scheller J Rose-John S Born J Marshall L . Enhancing influence of intranasal interleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J. (2009) 23:3629–36. doi: 10.1096/fj.08-122853
180.
Zhang T Song N Li S Yu L Xie Y Yue Z et al . S-ketamine improves slow wave sleep and the associated changes in serum protein among gynecological abdominal surgery patients: a randomized controlled trial. Nat Sci Sleep. (2023) 15:903–13. doi: 10.2147/NSS.S430453
181.
Shannon AM Bouchier-Hayes DJ Condron CM Toomey D . Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. (2003) 29:297–307. doi: 10.1016/S0305-7372(03)00003-3
182.
Chiche J Brahimi-Horn MC Pouysségur J . Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. (2010) 14:771–94. doi: 10.1111/j.1582-4934.2009.00994.x
183.
Ying H Kimmelman AC Lyssiotis CA Hua S Chu GC Fletcher-Sananikone E et al . Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. (2012) 149:656–70. doi: 10.1016/j.cell.2012.01.058
184.
Di Lorenzo G Ricci G Severini GM Romano F Biffi S . Imaging and therapy of ovarian cancer: clinical application of nanoparticles and future perspectives. Theranostics. (2018) 8:4279–94. doi: 10.7150/thno.26345
185.
Maeda H Nakamura H Fang J . The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. (2013) 65:71–9. doi: 10.1016/j.addr.2012.10.002
186.
Iyer VN Szybalski W . Mitomycins and porfiromycin: chemical mechanism of activation and cross-linking of DNA. Science. (1964) 145:55–8. doi: 10.1126/science.145.3627.55
187.
Wang L Huo M Chen Y Shi J . Tumor microenvironment-enabled nanotherapy. Adv Healthc Mater. (2018) 7:e1701156. doi: 10.1002/adhm.201701156
188.
Huang C-C Chia W-T Chung M-F Lin KJ Hsiao CW Jin C et al . An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J Am Chem Soc. (2016) 138:5222–5. doi: 10.1021/jacs.6b01784
189.
Thambi T Deepagan VG Yoon HY Han HS Kim SH Son S et al . Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. (2014) 35:1735–43. doi: 10.1016/j.biomaterials.2013.11.022
190.
Vaupel P Mayer A . Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. (2007) 26:225–39. doi: 10.1007/s10555-007-9055-1
191.
Liu J Liu Y Bu W Bu J Sun Y Du J et al . Ultrasensitive nanosensors based on upconversion nanoparticles for selective hypoxia imaging in vivo upon near-infrared excitation. J Am Chem Soc. (2014) 136:9701–9. doi: 10.1021/ja5042989
192.
Wei X Liu L Guo X Wang Y Zhao J Zhou S . Light-activated ROS-responsive nanoplatform codelivering apatinib and doxorubicin for enhanced chemo-photodynamic therapy of multidrug-resistant tumors. ACS Appl Mater Interfaces. (2018) 10:17672–84. doi: 10.1021/acsami.8b04163
193.
Safari Sharafshadeh M Tafvizi F Khodarahmi P Ehtesham S . Preparation and physicochemical properties of cisplatin and doxorubicin encapsulated by niosome alginate nanocarrier for cancer therapy. Int J Biol Macromol. (2023) 235:123686. doi: 10.1016/j.ijbiomac.2023.123686
194.
Yu J Hsu C-H Huang C-C Chang P-Y . Development of therapeutic au-methylene blue nanoparticles for targeted photodynamic therapy of cervical cancer cells. ACS Appl Mater Interfaces. (2015) 7:432–41. doi: 10.1021/am5064298
195.
Maji SK Kim DH . AgInS2-coated upconversion nanoparticle as a photocatalyst for near-infrared light-activated photodynamic therapy of cancer cells. ACS Appl Bio Mater. (2018) 1:1628–38. doi: 10.1021/acsabm.8b00467
196.
Khalyfa A Masa JF Qiao Z González M Marti S Khalyfa AA et al . Plasma exosomes in obesity hypoventilation syndrome patients drive lung cancer cell malignant properties: effect of long-term adherent CPAP treatment. Biochim Biophys Acta Mol basis Dis. (2022) 1868:166479. doi: 10.1016/j.bbadis.2022.166479
197.
Sanz-Rubio D Khalyfa A Qiao Z Ullate J Marin JM Kheirandish-Gozal L et al . Cell-selective altered cargo properties of extracellular vesicles following in vitro exposures to intermittent hypoxia. Int J Mol Sci. (2021) 22:5604. doi: 10.3390/ijms22115604
198.
Wang Y Zhao Q Zhang Q Wu X Liu X Wu S et al . Targeted delivery of CNS-specific hesperidin as a leptin sensitizer for treating obesity-associated sleep-disordered breathing. Adv Sci. (2025):e06182. doi: 10.1002/advs.202506182
199.
Huang Y Xie H Liu L Zhao H Li B Zhang F . Bioactive ROS-responsive nanotherapeutics attenuate intermittent hypoxia-induced cognitive impairment via NRF2/KEAP1/HO-1 signaling. Neurochem Int. (2025) 188:105997. doi: 10.1016/j.neuint.2025.105997
200.
Patel SR Bakker JP Stitt CJ Aloia MS Nouraie SM . Age and sex disparities in adherence to CPAP. Chest. (2021) 159:382–9. doi: 10.1016/j.chest.2020.07.017
201.
Woehrle H Graml A Weinreich G . Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med. (2011) 12:1034–6. doi: 10.1016/j.sleep.2011.05.008
202.
Edmonds JC Yang H King TS Sawyer DA Rizzo A Sawyer AM . Claustrophobic tendencies and continuous positive airway pressure therapy non-adherence in adults with obstructive sleep apnea. Heart Lung. (2015) 44:100–6. doi: 10.1016/j.hrtlng.2015.01.002
203.
Wang M-Y Sang L-X Sun S-Y . Gut microbiota and female health. World J Gastroenterol. (2024) 30:1655–62. doi: 10.3748/wjg.v30.i12.1655
204.
Wang Z Yuan K Ji Y-B Li SX Shi L Wang Z et al . Alterations of the gut microbiota in response to total sleep deprivation and recovery sleep in rats. Nat Sci Sleep. (2022) 14:121–33. doi: 10.2147/NSS.S334985
205.
Benedict C Vogel H Jonas W Woting A Blaut M Schürmann A et al . Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. (2016) 5:1175–86. doi: 10.1016/j.molmet.2016.10.003
206.
Xu H Cao C Ren Y Weng S Liu L Guo C et al . Antitumor effects of fecal microbiota transplantation: implications for microbiome modulation in cancer treatment. Front Immunol. (2022) 13:949490. doi: 10.3389/fimmu.2022.949490
207.
Crosbie EJ Einstein MH Franceschi S Kitchener HC . Human papillomavirus and cervical cancer. Lancet. (2013) 382:889–99. doi: 10.1016/S0140-6736(13)60022-7
208.
Xu S Liu Z Lv M Chen Y Liu Y . Intestinal dysbiosis promotes epithelial-mesenchymal transition by activating tumor-associated macrophages in ovarian cancer. Pathog Dis. (2019) 77:ftz019. doi: 10.1093/femspd/ftz019
209.
He Y Wang Q Li X Wang G Zhao J Zhang H et al . Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. (2020) 11:5192–204. doi: 10.1039/C9FO02554E
210.
Xie S Zhang R Li Z Liu C Xiang W Lu Q et al . Indispensable role of melatonin, a scavenger of reactive oxygen species (ROS), in the protective effect of Akkermansia muciniphila in cadmium-induced intestinal mucosal damage. Free Radic Biol Med. (2022) 193:447–58. doi: 10.1016/j.freeradbiomed.2022.10.316
211.
Stacchiotti A Favero G Rodella LF . Impact of melatonin on skeletal muscle and exercise. Cells. (2020) 9:288. doi: 10.3390/cells9020288
212.
Rodriguez MI Escames G López LC García JA Ortiz F López A et al . Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J Endocrinol. (2007) 194:637–43. doi: 10.1677/JOE-07-0260
213.
Lee J-Y Kim J-H Lee D-C . Urine melatonin levels are inversely associated with sarcopenia in postmenopausal women. Menopause. (2014) 21:39–44. doi: 10.1097/GME.0b013e318291f6c8
214.
Lau RI Su Q Ching JYL Lui RN Chan TT Wong MTL et al . Fecal microbiota transplantation for sleep disturbance in post-acute COVID-19 syndrome. Clin Gastroenterol Hepatol. (2024) 22:2487–2496.e6. doi: 10.1016/j.cgh.2024.06.004
215.
Chen H Wang C Bai J Song J Bu L Liang M et al . Targeting microbiota to alleviate the harm caused by sleep deprivation. Microbiol Res. (2023) 275:127467. doi: 10.1016/j.micres.2023.127467
216.
Yang Y Lindsey-Boltz LA Vaughn CM Selby CP Cao X Liu Z et al . Circadian clock, carcinogenesis, chronochemotherapy connections. J Biol Chem. (2021) 297:101068. doi: 10.1016/j.jbc.2021.101068
217.
Savvidis C Koutsilieris M . Circadian rhythm disruption in cancer biology. Mol Med. (2012) 18:1249–60. doi: 10.2119/molmed.2012.00077
218.
Printezi MI Kilgallen AB Bond MJG Štibler U Putker M Teske AJ et al . Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol. (2022) 23:e129–43. doi: 10.1016/S1470-2045(21)00639-2
219.
Xie T Guo D Luo J Guo Z Zhang S Wang A et al . The relationship between HIF1α and clock gene expression in patients with obstructive sleep apnea. Nat Sci Sleep. (2022) 14:381–92. doi: 10.2147/NSS.S348580
220.
Yang M-Y Lin P-W Lin H-C Lin PM Chen IY Friedman M et al . Alternations of circadian clock genes expression and oscillation in obstructive sleep apnea. J Clin Med. (2019) 8:1634. doi: 10.3390/jcm8101634
221.
Do VT Thomas GM Bjarnason GA . Postoperative concurrent chronomodulated 5-fluorouracil/leucovorin infusion and pelvic radiotherapy for squamous cell carcinoma of the ovary arising from mature cystic teratoma. Int J Gynecol Cancer. (2001) 11:418–21. doi: 10.1136/ijgc-00009577-200109000-00014
222.
Walker WH Bumgarner JR Walton JC Liu JA Meléndez-Fernández OH Nelson RJ et al . Light pollution and cancer. Int J Mol Sci. (2020) 21:9360. doi: 10.3390/ijms21249360
223.
Gao Q Xu Y Galluzzi M Xing Q Geng J . Enhanced cancer cell specificity through combined blue light therapy and starvation strategies. Adv Biol. (2025) 9:e2400264. doi: 10.1002/adbi.202400264
224.
Minich DM Henning M Darley C Fahoum M Schuler CB Frame J . Is melatonin the “next vitamin D”?: a review of emerging science, clinical uses, safety, and dietary supplements. Nutrients. (2022) 14:3934. doi: 10.3390/nu14193934
225.
Wu H-S Davis JE Chen L . Bright light shows promise in improving sleep, depression, and quality of life in women with breast cancer during chemotherapy: findings of a pilot study. Chronobiol Int. (2021) 38:694–704. doi: 10.1080/07420528.2021.1871914
226.
Alexander JL Wilson ID Teare J Marchesi JR Nicholson JK Kinross JM . Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. (2017) 14:356–65. doi: 10.1038/nrgastro.2017.20
227.
Shiao SL Kershaw KM Limon JJ You S Yoon J Ko EY et al . Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. (2021) 39:1202–1213.e6. doi: 10.1016/j.ccell.2021.07.002
228.
Wong CW Yost SE Lee JS Gillece JD Folkerts M Reining L et al . Analysis of gut microbiome using explainable machine learning predicts risk of diarrhea associated with tyrosine kinase inhibitor neratinib: a pilot study. Front Oncol. (2021) 11:604584. doi: 10.3389/fonc.2021.604584
229.
Sepich-Poore GD Zitvogel L Straussman R Hasty J Wargo JA Knight R . The microbiome and human cancer. Science. (2021) 371:6536. doi: 10.1126/science.abc4552
230.
Legesse Bedada T Feto TK Awoke KS Garedew AD Yifat FT Birri DJ . Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother. (2020) 129:110409. doi: 10.1016/j.biopha.2020.110409
231.
Ting NL-N Lau HC-H Yu J . Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. (2022) 71:1412–25. doi: 10.1136/gutjnl-2021-326264
232.
Lee KA Luong MK Shaw H Nathan P Bataille V Spector TD . The gut microbiome: what the oncologist ought to know. Br J Cancer. (2021) 125:1197–209. doi: 10.1038/s41416-021-01467-x
233.
Ganesh BP Nelson JW Eskew JR Ganesan A Ajami NJ Petrosino JF et al . Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. (2018) 72:1141–50. doi: 10.1161/HYPERTENSIONAHA.118.11695
234.
Henon C Vibert J Eychenne T Gruel N Colmet-Daage L Ngo C et al . Single-cell multiomics profiling reveals heterogeneous transcriptional programs and microenvironment in DSRCTs. Cell Rep Med. (2024) 5:110409. doi: 10.1016/j.xcrm.2024.101582
235.
Liu Y Xun Z Ma K Liang S Li X Zhou S et al . Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. (2023) 78:770–82. doi: 10.1016/j.jhep.2023.01.011
236.
Topol EJ . High-performance medicine: the convergence of human and artificial intelligence. Nat Med. (2019) 25:44–56. doi: 10.1038/s41591-018-0300-7
237.
Goldstein CA Berry RB Kent DT Kristo DA Seixas AA Redline S et al . Artificial intelligence in sleep medicine: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. (2020) 16:605–7. doi: 10.5664/jcsm.8288
238.
Masoumian Hosseini M Masoumian Hosseini ST Qayumi K Hosseinzadeh S Sajadi Tabar SS . Smartwatches in healthcare medicine: assistance and monitoring; a scoping review. BMC Med Inform Decis Mak. (2023) 23:248. doi: 10.1186/s12911-023-02350-w
239.
Liu J-H Shih C-Y Huang H-L Peng JK Cheng SY Tsai JS et al . Evaluating the potential of machine learning and wearable devices in end-of-life care in predicting 7-day death events among patients with terminal cancer: cohort study. J Med Internet Res. (2023) 25:e47366. doi: 10.2196/47366
240.
Wang R Zheng J Guo W Huang H Wang Q Li Y et al . Integrating a multimodal digital device for continuous perioperative monitoring in patients with lung cancer undergoing thoracic surgery: development and usability study. JMIR Mhealth Uhealth. (2025) 13:e69512. doi: 10.2196/69512
241.
Pavic M Klaas V Theile G Kraft J Tröster G Blum D et al . Mobile health technologies for continuous monitoring of cancer patients in palliative care aiming to predict health status deterioration: a feasibility study. J Palliat Med. (2019) 23:678–85. doi: 10.1089/jpm.2019.0342
242.
Kos M Brouwer CG van Laarhoven HWM Hopman MTE van Oijen MGH Buffart LM . The association between wearable device metrics and clinical outcomes in oncology: a systematic review with evidence synthesis and meta-analysis. Crit Rev Oncol Hematol. (2023) 185:103979. doi: 10.1016/j.critrevonc.2023.103979
243.
Laudon M Frydman-Marom A . Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. (2014) 15:15924–50. doi: 10.3390/ijms150915924
244.
Wafford KA Ebert B . Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nat Rev Drug Discov. (2008) 7:530–40. doi: 10.1038/nrd2464
245.
Liu J Clough SJ Hutchinson AJ Adamah-Biassi EB Popovska-Gorevski M Dubocovich ML . MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol. (2016) 56:361–83. doi: 10.1146/annurev-pharmtox-010814-124742
246.
Lissoni P Ardizzoia A Barni S Paolorossi F Tancini G Meregalli S et al . A randomized study of tamoxifen alone versus tamoxifen plus melatonin in estrogen receptor-negative heavily pretreated metastatic breast-cancer patients. Oncol Rep. (1995) 2:871–3. doi: 10.3892/or.2.5.871
247.
Lissoni P Barni S Meregalli S Fossati V Cazzaniga M Esposti D et al . Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br J Cancer. (1995) 71:854–6. doi: 10.1038/bjc.1995.164
248.
Tam CW Mo CW Yao K-M Shiu SYW . Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res. (2007) 42:191–202. doi: 10.1111/j.1600-079X.2006.00406.x
249.
de Zambotti M Goldstein C Cook J Menghini L Altini M Cheng P et al . State of the science and recommendations for using wearable technology in sleep and circadian research. Sleep. (2024) 47:zsad325. doi: 10.1093/sleep/zsad325
Summary
Keywords
sleep disorders, obstructive sleep apnea, gynecological cancers, circadian rhythm, hypoxia-inducible factor, tumor micro-environment
Citation
Mei H, Zhao C, Jin H, Qi W, Lu X, Xin Y, Wang W, Sun Y and Li W-Y (2025) Bidirectional crosstalk between sleep disorders and gynecological cancers: unraveling molecular synergies and precision therapeutics. Front. Med. 12:1717587. doi: 10.3389/fmed.2025.1717587
Received
02 October 2025
Accepted
03 November 2025
Published
21 November 2025
Volume
12 - 2025
Edited by
Ling Zhou, Huazhong University of Science and Technology, China
Reviewed by
Yuenan Ni, Sichuan University, China
Yibing Chen, People’s Liberation Army General Hospital, China
Updates
Copyright
© 2025 Mei, Zhao, Jin, Qi, Lu, Xin, Wang, Sun and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yakai Sun, huangjinjianshi2@163.com; Wen-Yang Li, 20122043@cmu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.