Abstract
Introduction:
There is a critical need for innovative therapies beyond the current standard of care for meningiomas and gliomas. Radioligand therapy (RLT), with its theranostic approach, holds significant promise in this regard. Although several reviews on this topic have been published, none yet have combined the utilization of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology with the Critical Appraisal Skills Programme (CASP) analysis, along with a dedicated subsection specifically addressing ongoing and completed clinical trials. This review aims to fill this gap in the literature by providing a comprehensive assessment of the current evidence on RLT in these tumors.
Materials and methods:
Published studies were searched through PubMed, Scopus, and Web of Science up to 30 April 2025. Only original articles and clinical studies were included. Following a structured selection process, data extraction was performed. Study quality was critically appraised using CASP analyses. For clinical trials, an additional search was conducted on ClinicalTrials.gov beginning on 12 May 2025.
Results:
A total of 30 studies were included in the review: 22 on meningiomas (290 patients) and 8 on gliomas (259 patients). For each study, first author, journal, year of publication, somatostatin receptor imaging, study design, radiopharmaceutical used, main topics, response criteria, toxicity assessment, post-therapy scintigraphy, number of patients, WHO grade, demographics, findings and median follow-up were considered. Among clinical trials, 22 were analyzed, including study site, year of first submission, proposed radiopharmaceutical, study type, primary endpoints and status. Efficacy and toxicity data were the primary focus, and the findings were generally encouraging. Studies on RLT in meningiomas was more robust, while in gliomas remained largely experimental. Nevertheless, the authors’ critical appraisal was generally positive. Clinical trials confirmed the more “traditional” nature of research in meningiomas compared to gliomas.
Conclusion:
Despite the heterogeneity of the studies, RLT emerges as a promising therapeutic strategy in neuro-oncology. Its theranostic paradigm offers a distinctive advantage, enabling patient selection, treatment personalization, and response monitoring. The development of potentially novel radiopharmaceuticals and the conduct of well-designed multicenter trials with standardized response criteria are needed to further increase the impact and clinical translation of RLT in neuro-oncology.
Introduction
Meningiomas and gliomas are classified as primary brain tumors, with meningiomas accounting for approximately 30% of cases. According to the World Health Organization (WHO) classification, meningiomas are categorized as typical (grade I, with less aggressive behavior, less risks of recurrence and better prognosis), atypical (grade II), or anaplastic (grade III) (1–3).
Following the latest European Association of Neuro-Oncology (EANO) guidelines, incidental asymptomatic meningiomas should be monitored over time, while for growing or symptomatic ones neurosurgery remains the first-line treatment, when feasible (2). External bean radiation therapy (EBRT) may serve as a complementary treatment or even alternative option to surgery in certain cases (3). Medical treatments— such as bevacizumab, sunitinib, everolimus, temozolomide, irinotecan, imatinib—can be used in cases of recurrent or progressive disease not suitable for surgery or radiotherapy (2).
Gliomas are considered the most common malignant brain tumors. They are classified according to the WHO document by their tumor grade (4) and include different histological subtypes such as astrocytomas, oligodendrogliomas, glioblastomas and ependymomas. These tumors are usually managed through surgical resection, EBRT and chemotherapy, often combined (5), but unfortunately also show a high level of treatment resistance, immune escape and spatiotemporal heterogeneity (6). Multiple factors can explain treatment resistance: first, certain lesions are difficult to reach or resect completely, secondly, and equally important, the presence of the blood–brain barrier (BBB) hinders or postpones the delivery of medications to the tumor (7). Despite efforts to discover new treatments, unfortunately, the prognosis has not much improved and the overall survival (OS) of patients with glioma is still low.
Both meningiomas and gliomas often express somatostatin receptors (SSRs) type 2 (8). Initially, [111In]Pentetreotide scintigraphy (Octreoscan®) was used for imaging these tumors. More recently, hybrid imaging techniques—such as [68Ga]Ga DOTA PET/CT or PET/MRI (DOTA PET)—have been applied in various clinical settings.
Thus, a theranostic approach, based on the use of the same target for both diagnostics and therapy, through peptide receptor radionuclide therapy (PRRT) was proposed for the treatment of meningiomas and gliomas. PRRT is most commonly administered using radiopharmaceuticals such as [177 Lu]Lu-DOTA-TOC and [177 Lu]Lu-DOTA-TATE (LuPRRT) or [90Y]Y-DOTA PRRT (YPRRT). Despite some well-documented differences among the radionuclides employed (9) in terms of type of emission, physical half-life, maximum, and mean β-particle energies and penetration depths in soft tissue, LuPRRT and YPRRT similarly combine a molecular vector targeting the SSRs with a beta-emitting isotope. They can be can be delivered systemically by intravenous injection (more frequently), through intratumoral injection, through injection into the tumor resection cavity (10, 11) and finally through intraarterial administration.
The therapeutic landscape has recently expanded from PRRT to the broader concept of radioligand therapy (RLT), with several novel radiopharmaceuticals currently under investigation, such as [177Lu]Lu-prostate specific membrane antigen (PSMA) radioligand therapy (LuPSMA) and the alpha emitters [213Bi] and 225[Ac] to name but a few. The use of alpha particles, on the other hand, should be considered more suitable for smaller lesions, having a shorter travel distance in the substance and higher linear energy transfer (LET) than beta particles.
It is important to note that, at present, meningiomas and gliomas can be treated with RLT only within investigational trials and only in cases of advanced, progressive, or refractory tumors. Findings from these trials have been reported in multiple scientific publications. Consequently, various reviews have been already published on this argument. However, to the best of our knowledge, there are no reviews specifically including at the same time:
-
the utilization of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) methodology (12).
-
the Critical Appraisal Skills Programme (CASP) analysis.
-
the clinical trials currently available or recently completed.
The aim of this publication is to address the current gap in the literature and provide a comprehensive, up-to-date overview of the use of RLT in patients with meningiomas and gliomas, based on available data and clinical trials, and following PRISMA and CASP methodologies.
Materials and methods
Information sources and search strategy
Published studies
A literature search up to 30 April 2025 was conducted using the PubMed, Scopus, and Web of Science databases. Four authors (M. S., I. G., S. N., and e I. M.) suggested the terms for the research. The following terms were used: meningioma peptide receptor radionuclide therapy/PRRT, meningioma radioligand therapy/meningioma RLT, meningioma 90Y/90Y DOTA, meningioma 177Lu/177Lu DOTA, glioma peptide receptor radionuclide therapy, glioma 90Y/90Y DOTA, and glioma 177Lu/177Lu DOTA. Furthermore, a chronological filter—from 2006 to the present day—was applied to make the research more consistent and aligned with advances in imaging. The search was carried out with the addition of filters, such as English language only and humans subjects only, when possible, that is to say for Pubmed and Scopus. Additionally, filters regarding the type of articles were considered and they were: “Article” for Scopus, while for Pubmed selecting “article” was not possible, so this filter was not applied and the articles were selected later. One reviewer (I. G.) conducted the literature search. Critical appraisal was performed by two reviewers (I. G. and M. S.). and discrepancies, if any, were solved by discussion with the other authors.
Considering the heterogeneity of the studies, a meta-analysis was not performed.
Clinical trials
A search was conducted on clinical trials.gov until May 2025. The terms for the research were suggested by four authors (M. S., I. G., S. N., and e I. M.) and included: peptide receptor radionuclide therapy/PRRT, radioligand therapy/RLT, 90Y/90Y DOTA, 177Lu/177Lu DOTA, and meningioma and glioma regarding “Condition/disease.” No additional filters were used. One author (I. G.) conducted the research.
Selection process
Published studies
The inclusion criteria were established by three authors (I. G., M. S., and I. M.) and included original articles and clinical studies, and the studies primarily focussed on therapeutic interventions.
Exclusion criteria were reported in Figure 1.
Figure 1

Flowchart illustrating the exclusion criteria applied in the study selection process. Criteria include the type of article, language, type of research and subject relevance. This schematic provides a clear overview of the factors leading to study exclusion.
One reviewer (I. G.) screened each record and each report retrieved, without the use of automation tools in the process.
Clinical trials
Two authors (I. G. and M. S.) carried out the selection process. Trials addressing neoplasms other than meningiomas and gliomas, evaluating agents other than radiopharmaceuticals, or investigating radiopharmaceuticals intended solely for diagnostic purposes were excluded as not relevant to the scope of this review.
Data extraction
Published studies
One reviewer (I. G.) handled the extraction of the data. For any article, the following parameters were taken into account: editorial information (first author, journal, and year of publication), material and methods (study design, radiopharmaceutical used, and treatment schedules), main topics, main findings (efficacy and its endpoints, toxicity and its endpoints, the availability of a post-therapy whole-body scintigraphy WBS, single or serial studies), the number of patients, the WHO tumor grade, the presence or absence of demographic data and the follow-up times.
The studies were analyzed according to the Critical Appraisal Skills Programme (CASP) (https://casp-uk.net/casp-tools-checklists) for qualitative studies, based on ten items and questions. Two reviewers (I. G. and F. M.) filled out the form separately and, in case of discrepancy, a discussion among the authorial group was done.
Clinical trials
One reviewer (M. S.) handled the extraction of the data. For any trial, the following parameters were taken into consideration: title, study site(s), year of first submission, investigating radiopharmaceutical, type of study, main endpoints, and current status.
A formal quality assessment was not performed, as results are not yet available.
Results
Published studies
Search strategy
The results of PRISMA search strategy is reported in Figure 2. From the systematic literature search, 30 papers were finally selected, including 22 papers dealing with meningiomas and eight with gliomas, for the sum of 290 patients with meningioma and of 259 patients with glioma.
Figure 2
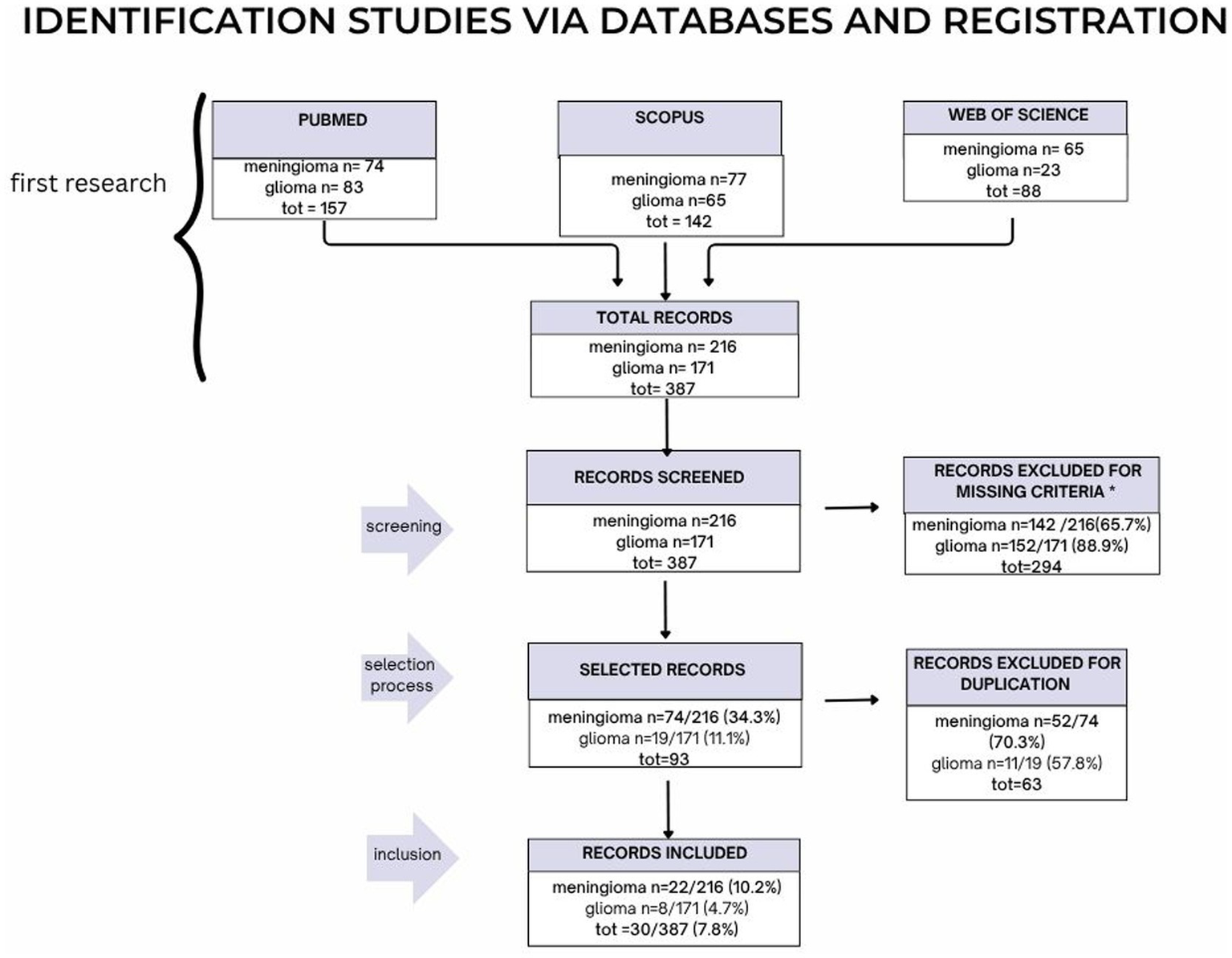
The PRISMA strategy is illustrated here in its parts: screening, selection process and finally inclusion.
Study characteristics
Tables 1, 2 summarize the main findings of the selected studies regarding meningiomas and Tables 3, 4 summarize all the studies regarding gliomas. In Table 5, a comparison is provided between meningioma studies and glioma studies in terms of study design, radiopharmaceuticals, and main findings.
Table 1
| First author | Journal | Year | SSRs imaging | Study design | RP | Treatment schedules | Main topics | Response criteria | Toxicity measure | WBS | Number of pts | Who grade | Demographics | Main findings | Median FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minczeles | European Journal of Nuclear Medicine and Molecular Imaging (EJNMMI) | 2022 | Octreoscan® and DOTAPET | Retrospective | LuDOTA | Planned TCA of 29.6 GBq, 4 cycles | Efficacy (PFS)and safety | RANO Working Group | CTCAE 4.03 | Y (MIRD-based dosimetry) |
15 | G1, G2, G3, ki 67 10% (4–20). | YES | Efficacy: mOS 13.6 mo TGR declined to 3.1% in surface (p = 0.016) and 5.0% in volume. Toxicity: HT: G3 8 pts., G4 in 1 |
3 mo |
| Gerster-Gilliéron. | The Journal Of Nuclear Medicine (JNM) |
2015 | Octreoscan® | Prospective phase 2 | YDOTA | 7,400 MBq/m2 in 2 fractions. | Efficacy, safety | RECIST | (NCI-CTC V4.0) | Y | 15 | 1 to 3 | yes. | Efficacy: mPSF at least 24 mo. Toxicity: HT > G2 in 5 pts. (33.3%), transient G3 lymphocytopenia in 4 pts., transient NT in 2 pts |
6 to 12 mo |
| Minutoli | Cancer biotherapy and radiopharmaceuticals | 2014 | Octreoscan® | retrospective | [111In]In Pentetreotide | 2–4 cycles, activity per cycle 1.1–7.4 GBq + YPRRT in 1 pt. and LuPRRT in 1 pt. | Safety, efficacy | SWOG | unknown | N | 8 | unknown | yes | Toxicity: no G3-G4. Efficacy (DCR): PR in 2 pts., SD in 5 patients, PD in 1 PT |
Median of 12 mo |
| Bartolomei | EJNMMI | 2009 | Octreoscan® | Prospective | YDOTA | 2.5 GBq/cycle every 2/3 months up to a TCA of 15 GBq | Safety and efficacy | SWOG | WHO criteria | Y | 29 | 1 to 3 | yes | Efficacy: SD in 19 and PD in10, median OS 40 months. Median TTP: 61 months in grade 1 and 13 in grade 2 and 3. No NT, no G3 or G4 other toxicity. | Last update oct 2007 |
| Kreissl | Radioation oncology | 2012 | DOTAPET | Pilot trial | LuDOTA | 7.4 ± 0.3 GBq | Safeyt and efficacy of PRRT + EBRT | ce MRI (volume changes of 25% to define progression or response and DOTAPET) | CTCAE 4.0 | Y | 10 | 1 and 2 | yes | Efficacy: SD in 8/10 pts. 1/10 CR No chronic effects >G 1 |
Median of 13.4 mo |
| Hanscheid | JNM | 2017 | Octreoscan® | retrospective | LuDOTA | 22; 5.3–8.1 GBq | dosimetry | NA | NA | Y OLINDA /EXM based dosimetry |
29, 8 with meningioma | – | no | A single post-therapeutic measurement (4 d after injection) by SPECT/CT could be used be used to estimate the absorbed with minor additional resources and effort. |
– |
| Vonken | JNM | 2021 | DOTAPET | retrospective | LuDOTA (high affinity) |
7.400 MBq/cycle for 4 cycles,the first one intravenous, the subsequent ones intraarterially. |
intrapatient comparison betwwen intravenous and intraarterial treatment (safety and efficacy) |
RANO Working Group 2019 | CTCAE 5.0 | Y | 4 | 2 and 3 | yes | Toxicity: 1 pt. with G3 HT (Leukopenia). Efficacy: 3 pts. completed treatment: 1 was PR, 2 SD. They all Improved clinical conditions |
Median 1.7 y |
| Amerein | JNM | 2024 | DOTAPET | retrospective | LuDOTA (high affinity) | 1–4 cycles with mean activity/cycle of 7.428 MBq |
Safety and efficacy of intraarterial PRRT | RANO Working Group | CTCAE 5.0 | Y | 13 | 1–3 | yes | HT: 3 pts. with G3 thrombocypenia, 5 with G3 lymphocytopenia and 1 with G4 lymphocytopenia. 1 pt. had transient G3-G4, RN (probably not reated to PRRT), 1 pt. with local necrosis (probably related to angiography). Efficacy. 1/10 CR, 1/10 PR, 8/10 SD, 9/13 CI m PFS of 18 mo |
Up to 43 mo |
| Hartrampf | Clinical and Translational Radiation Oncology | 2020 | DOTAPET | retrospective | LuDOTA | 7–7.9 GBq/cycle | Efficacy and safety of PRRT+EBRT | RANO Working Group | CTCAE 5.0 | Y | 10 | 2 and 3 | yes | Toxicity: no severe acute or chronic toxicity. Efficacy: SD in 7/10; mPFS ranging from 13.8 to 107.7 months, OS from 38.2 to 111.4 |
Median of 105.0 mo |
| Kertels | Clinical nuclear medicine | 2021 | DOTAPET | retrospective | LuDOTA | Median of 4 cycles. TCA 9.6–39.5 GBq | PRRT in neurofibromatosis and meningioma, efficacy and safety | RANO Working Group | CTCAE 5.0 | Y | 11 | 1–3 | yes | HT: 5 pts. anemia>G3, 7 pts. trombocytopenia >G3, 9 pts. leukopemia >G3. Efficacy: mPSF 12 mo, OS 37 mo. SD in 6 pts |
Median 27 mo |
| Kletting | JNM | 2016 | – | retrospective | YDOTA | unnkown | Dosimetry Optimizing YPRRT | NA | NA | Y OLINDA /EXM based dosimetry |
4 | unknown | +/− | Optimal activity of 4.2 ± 1.8 GBq for meningioma | – |
| van Essen | JNM | 2006 | Octreoscan® | Prospective? | LuDOTA | 7.4 Gbq/cycle, TCA of 22.2–29.6 | Safety and efficacy of PRRT in different tumors | recist | WHO | Y | 22, 5 with meningioma | unknown | yes | Efficacy: 4 PD, 1 SD. Toxicity for pts. with meningioma unclear |
unclear |
| Graillon | Journal of Neuro-Oncology | 2013 | DOTAPET | retrospective | LuDOTA | Lutathera schedule | Impact of PRRT on 3DVGR | RANO Working Group | no | N | 8 | 1 and 2 | yes | 3DVGR significantly decreased at 3, 6, and 12. | 18 mo |
| Hänscheid | EJNMMI | 2012 | DOTAPET | Pilot trial | LuDOTA | 7.4 GBq | Correlation between tumour uptake of PRRT and PET pre-PRRT | NA | no | Y | 11 | ≥2 | yes | Strong correlation between SUV and TR |
3 mo after PRRT |
| Eigler | JNM | 2024 | DOTAPET | Prospective phase 0 | LuDOTA | 6.9–7.3 GBq of [177 Lu]Lu-DOTATOC followed by 3.3–4.9 GBq of [177 Lu]Lu-DOTA-JR11 at an interval of 10 ± 1wk |
Comparison between [177Lu]Lu-DOTA-JR11 And LuPRRT in terms of therapeutic index (tumor–to– bone marrow and tumor-to-kidney absorbed-dose ratios) |
progressive disease was defined as at least a 40% increase of meningioma volume or new lesions; and stable disease was definedaslessthana 40% increase in volume |
CTCAE 5.0 | Y OLINDA /EXM based dosimetry |
6 | 1–3 | yes | Median absorbed dose of 1 cycle: 3.4 Gy for LuPRRT and 11.5 Gy for DOTA-JR11. Toxicity: 2 pts. with G3 lymphopenia and 1 pt. with G3 lymphopenia and neutropenia after DOTA-JR11. Efficacy DRC: 83% |
18 mo |
| Puranik | Neurology India | 2024 | DOTAPET | Retrospective | LuDOTA | First cycle intravenous, 4 pts. continued with intraarterial 7.4 GBq/cycle every 8–12-weeks |
Efficacy and safety of intavenous and subsequent intrarterial administration of PRRT | RANO Working Group | Unknown | Y OLINDA /EXM based dosimetry |
8 | 1–3 | yes | Efficacy: median TTP of 8.9 months, PD in 2 pts., PR in 2 pts., 4 pts. in SD. Toxicity: unclear |
12 mo |
| Severi | JNM | 2024 | Octreoscan® and DOTAPET | Prospective | LuDOTA and YDOTA | TCA of 11.1 for 90Y and of 22 GBq for Lu, divided into 4 to 5 cycles every 5–8 wks TCA for rechallenge:13 GBq of 177 Lu DOTATATE |
Efficacy and safety of PRRT, rechallenge | RANO Working Group | CTCAE v 4.0 and 5 | NA | 42 | 1–3 | yes | Efficacy: DCR of 57%, mPFS of 16 mo, mOS 36 mo. Toxicity: G3 platelets toxicity in 1 pt. No symptomatic worsening of conditions. Efficacy: For rechallenge: mPFS of 6.5 mo and mOS of 17 mo |
63 mo 75.8 for rechallenge |
| Reuvers | Cancers | 2024 | – | unknown | LuDOTA | NA | In vitro model for PRRT (spheroids) | NA | NA | NA | 16 | 1 and 2 | yes | PRRT induced DNA damage, correlated with SSTR2-expression. |
NA |
| Dubois | Cancer biotherapy and radiopharmaceuticals | 2024 | DOTAPET | restrospective | LuDOTA | Luthathera schedule | Factors associated with safety | NA | CTCEA 5.0 | N | 46 but 40 evaluable, 5 with meningioma | unknown | no | Toxicity: 14 pts. with G3 or higher HT with a single parameter; 3 pts. with 2 parameters; 4 pts. with 3 parameters; and 2 ptswith 4 parameters. 1 pt. had G3 hepatic cytolysis. Risk factors not considered separately for meningioma |
for 6 mo after the last injection. |
| Salgues | Current oncology | 2022 | DOTAPET | restrospective | LuDOTA | 3,200–7,400 MBq for 4 cycles every 8/9 wks | Safety and efficacy | RANO Working Group | CTCAE v6.0 | Y | 8 | 2 | yes | Toxicity: frequent SE transient G1 HT. 3 pts. G3 lymphocytopenia. Efficacy: 5/6 pts. with SD at 12 months. PFS at 6mo was 85.7% and PFS at 12mo was 66.7% |
unknown |
| Seystahal. | Neuro-Oncology | 2016 | DOTAPET | retrospective | LuDOTAand YDOTA | median of 3 cycles, dose/cycle 3,400–7,400 MBq | Safety and efficacy | RANO Working Group | CTCAE v 4.0 | N | 20 | 1–3 | yes | Efficacy: SD in 10/20, mPFS and PFS at 6 mo stratified according to grade, as such as mOS. Ga DOTA uptake was linked to MRI imaging | 20 mo |
| Marincek | JNM | 2015 | Octreoscan® | Prospective phase 2 | LuDOTA and YDOT. | Cycles repeated until tumor progression or permanent toxicity occurred |
Safety and efficacy | RECIST 1.1 | CTCAE v 3.0 | Y | 74 treatments, 34 pts. evaluable | unknown | yes | Efficacy: SD in 23 pts. Relevant HT in 3 pts. and severe RT in 1 pt. MS of 8.6 y from recruitment. SD and and high tumor uptake associated with longer survival. |
21.8 mo |
Overview of published studies on RLT in meningioma (word version).
This table provides a comprehensive summary of clinical studies, including in particular imaging modalities, study design, primary objectives, post therapy WBS and/or dosimetric evaluations, patient and tumor characteristics and outcomes in terms of efficacy and safety. Both retrospective and prospective experiences are included, highlighting heterogeneous protocols and patient populations while illustrating feasibility, efficacy and safety of RLT in progressive or pretreated meningiomas.
RP: radiopharmaceutical; FU: follow-up; Mo: months; Octreoscan®: [111In]In-DTPA-octreotide scintigraphy; DOTA PET: [68 Ga]Ga DOTA PET; LuDOTA: [177Lu]Lu DOTA-TATE or [177Lu]Lu-DOTA-TOC; YDOTA: [90 Y]Y DOTA-TOC; HT: hematological toxicity; NT: neurological toxicity; RN: renal toxicity; DCR: disease control rate; PR: partial responders; SD: stable disease; PD: progression disease; CR: complete responders; TTP: time to progression; CI: clinical improvement; 3DVGR: MRI three-dimensional volume growth rate; TR: PRRT radionuclide tumour retention; [177Lu]Lu-DOTA-JR11: LuJR11; SE: side effect; MS: Mean survival; Y: yes; N: no; WBS: Post-therapy whole-body scintigraphy (single or serial studies).
Table 2
| First author | Journal | Year | SSRs imaging | Study design | RP | Treatment schedules | Main topics | Response criteria | Toxicity | WBS | Number of pts | Who grade | Demographics | Main findings | Median FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minczeles | EJNMMI | 2022 | Octreoscan® and DOTAPET | Retrospective | LuDOTA | Planned TCA of 29.6 GBq, 4 cycles | Efficacy (PFS)and safety | RANO Working Group | CTCAE 4.03 | Y | 15 | G1, G2, G3, ki 67 10% (4–20). | YES | Efficacy: mOS 13.6 mo | 3 mo |
| Gerster-Gilliéron. | The Journal Of Nuclear Medicine | 2015 | Octreoscan® | Prospective phase 2 | YDOTA | 7,400 MBq/m2 in 2 fractions. | Efficacy, safety | RECIST | (NCI-CTC V4.0) | Y | 15 | 1 to 3 | Yes. | Efficacy: mPSF at least 24 mo. | 6 to 12 mo |
| Minutoli | Cancer biotherapy and radiopharmaceuticals | 2014 | Octreoscan® | Retrospective | [111In]In Pentetreotide | 2–4 cycles, activity per cycle 1.1–7.4 GBq + YPRRT in 1 pt. and LuPRRT in 1 pt | Safety, efficacy | SWOG | unknown | N | 8 | unknown | yes | Toxicity: no G3-G4. Efficacy (DCR): PR in 2 pts., SD in 5 patients, PD in 1 PT | Median of 12 mo |
| Bartolomei | EJNMMI | 2009 | Octreoscan® | Prospectic | YDOTA | 2.5 GBq/cycle every 2/3 months up to a TCA of 15 GBq | Safety and efficacy | SWOG | WHO criteria | Y | 29 | 1 to 3 | yes | Efficacy: SD in 19 and PD in10, median OS 40 months. Median TTP: 61 months in grade 1 and 13 in grade 2 and 3. No NT, no G3 or G4 other toxicity | Last update oct 2007 |
| Kreissl | Radioation oncology | 2012 | DOTAPET | Pilot trial | LuDOTA | 7.4 ± 0.3 GBq | Safeyt and efficacy of PRRT + EBRT | ce MRI (volume changes of 25% to define progression or response and DOTAPET) | CTCAE 4.0 | Y | 10 | 1 and 2 | yes | Efficacy: SD in 8/10 | Median of 13.4 mo |
| Hanscheid | JNM | 2017 | Octreoscan® | retrospective | LuDOTA | 22; 5.3–8.1 GBq | dosimetry | NA | NA | Y OLINDA/EXM based dosimetry | 29, 8 with meningioma | – | no | A single post-therapeutic measurement could estimate the absorbed dose | – |
| Vonken | JNM | 2021 | DOTAPET | retrospective | LuDOTA (high affinity) | 7.400 MBq/cycle for 4 cycles,the first one intravenous, the subsequent ones intraarterially. | intrapatient comparison betwwen intravenous and intraarterial treatment (safety and efficacy) | RANO Working Group 2019 | CTCAE 5.0 | Y | 4 | 2 and 3 | yes | Toxicity:1 pt. with G3 HT (Leukopenia). Efficacy: 3 pts. completed treatment: 1 was PR, 2 SD. They all Improved clinical conditions | Median 1.7 y |
| Amerein | JNM | 2024 | DOTAPET | retrospective | LuDOTA (high affinity) | 1–4 cycles with mean activity/cycle of 7.428 MBq | Safety and efficacy of intraarterial PRRT | RANO Working Group | CTCAE 5.0 | Y | 13 | 45717.00 | yes | HT: 3 pts. with G3 thrombocypenia, 5 with G3 lymphocytopenia and 1 with G4 lymphocytopenia. RN: 1 pt. with G3-G4, 1 pt. with local necrosis. Efficacy: 1/10 CR, 1/10 PR, 8/10 SD, 9/13 CI m PFS of 18 mo | Up to 43 mo |
| Hartrampf | Clinical and Translational Radiation Oncology | 2020 | DOTAPET | retrospective | LuDOTA | 7–7.9 GBq/cycle | Efficacy and safety of PRRT+EBRT | RANO Working Group | CTCAE 5.0 | Y | 10 | 2 and 3 | yes | no toxicities; mPFS 13.8–107.7 mo, OS 38.2 to 111.4 | Median of 105.0 mo |
| Kertels | Clinical nuclear medicine | 2021 | DOTAPET | retrospective | LuDOTA | Median of 4 cycles. TCA 9.6–39.5 GBq | PRRT in neurofibromatosis and meningioma, efficacy and safety | RANO Working Group | CTCAE 5.0 | Y | 11 | 45717.00 | yes | HT: 5 pts. anemia>G3, 7 pts. trombocytopenia >G3, 9 pts. leukopemia >G3. Efficacy: mPSF 12 mo, OS 37 mo. SD in 6 pts. | Median 27 mo |
| Kletting | JNM | 2016 | – | retrospective | YDOTA | unnkown | Dosimetry Optimizing YPRRT | NA | NA | Y | 4 | unknown | +/− | Optimal activity of 4.2 ± 1.8 GBq for meningioma | – |
| van Essen | JNM | 2006 | Octreoscan® | Prospective? | LuDOTA | 7.4 Gbq/cycle, TCA of 22.2–29.6 | Safety and efficacy of PRRT in different tumors | recist | WHO | Y | 22, 5 with meningioma | unknown | yes | Efficacy: 4 PD, 1 SD. Toxicity uncleae | unclear |
| Graillon | Journal of Neuro-Oncology | 2013 | DOTAPET | retrospective | LuDOTA | Lutathera schedule | Impact of PRRT on 3DVGR | RANO Working Group | no | N | 8 | 1 and 2 | yes | 3DVGR significantly decreased at 3, 6, and 12. | 18 mo |
| Hänscheid | EJNMMI | 2012 | DOTAPET | Pilot trial | LuDOTA | 7.4 GBq | Correlation between tumour uptake of PRRT and PET pre-PRRT | NA | no | Y | 11 | ≥2 | yes | Strong correlation between TR ansd SUV | 3 mo after PRRT |
| Eigler | JNM | 2024 | DOTAPET | prospective phase 0 | LuDOTA | 6.9–7.3 GBq Lu-DOTATOC followed by 3.3–4.9 GBq of [177 Lu]Lu-DOTA-JR11 | comparison between LuDOTA JR11 and LuPRRT | PD atleast a 40% increase volume or new lesions; SD less than 40% increase i | CTCAE 5.0 | Y OLINDA/EXM based dosimetry | 6 | from 1 to 3 | yes | Toxicity: 2 pts. with G3 lymphopenia and 1 pt. with G3 lymphopenia and neutropenia after DOTA-JR11. Efficacy DRC: 83% | 18 mo |
| Puranik. | Neurology India | 2024 | DOTAPET | Retrospective | LuDOTA | First cycle intravenous, 4 pts. continued with intraarterial 7.4 GBq/cycle every 8–12 weeks | Efficacy and safety of intavenous and subsequent intrarterial administration of PRRT | RANO Working Group | Unknown | Y OLINDA/EXM based dosimetry | 8 | from 1 to 3 | yes | Efficacy: median TTP of 8.9 months, PD in 2 pts., PR in 2 pts., 4 pts. in SD Toxicity: unclear | 12 mo |
| Severi | JNM | 2024 | Octreoscan® and DOTAPET | Prospective | LuDOTA and YDOTA | TCA of 11.1 for 90Y and of 22 GBq for Lu, divided into 4 to 5 cycles every 5–8 wks | Efficacy and safety of PRRT, rechallenge | RANO Working Group | CTCAE 4.0 and 5 | NA | 42 | from 1 to 3 | yes | Efficacy: DCR of 57%, mPFS of 16 mo, mOS 36 mo. Toxicity: G3 platelets toxicity in 1 pt. No symptomatic worsening of conditions. Efficacy: For rechallenge: mPFS of 6.5 mo and mOS of 17 mo | 63 mo 75.8 for rechallenge |
| Reuvers | Cancers | 2024 | - | unknown | LuDOTA | NA | In vitro model for PRRT (spheroids) | NA | NA | NA | 16 | 1 and 2 | yes | PRRT induced DNA damage, correlated with SSR expression | NA |
| Dubois | Cancer biotherapy and radiopharmaceuticals | 2024 | DOTAPET | restrospective | LuDOTA | Luthathera schedule | Factors associated with safety | NA | CTCEA 5.0 | N | 46 but 40 evaluable, 5 with meningioma | unknown | no | Toxicity: 14 pts. with G3 or higher HT with a single parameter;3 pts. with 2 parameters; 4 pts. with 3 parameters; and 2 pts. with 4 parameters. 1 pt. had G3 hepatic cytolysis. Risk factors not considered separately for meningioma | for 6 mo after the last injection. |
| Salgues | Current oncology | 2022 | DOTAPET | restrospective | LuDOTA | 3,200–7,400 MBq for 4 cycles every 8/9 wks | Safety and efficacy | RANO Working Group | CTCAE v6.0 | Y | 8 | 2.00 | yes | Toxicity: frequent SE transient G1 HT. 3 pts. G3 lymphocytopenia. Efficacy: 5/6 pts. with SD at 12 months. PFS at 6mo was 85.7% and PFS at 12mo was 66,7% | unknown |
| Seystahal | Neuro-Oncology | 2016 | DOTAPET | retrospective | LuDOTAand YDOTA | median of 3 cycles, dose/cycle 3,400–7,400 MBq | Safety and efficacy | RANO Working Group | CTCAE v 4.0 | N | 20 | from 1 to 3 | yes | Efficacy: SD in 10/20, mPFS and PFS at 6 mo stratified according to grade, as such as mOS. Ga DOTA uptake was linked to MRI | 20 mo |
| Marincek | JNM | 2015 | Octreoscan® | Prospective phase 2 | LuDOTA and YDOT. | Cycles repeated until tumor progressionor permanent toxicity occurred | Safety and efficacy | RECIST 1.1 | CTCAE v 3.0 | Y | 74 treatments, 34 pts. evaluable | unknown | yes | Efficacy: SD in 23 pts. Relevant HT in 3 pts. and severe RT in 1 pt. MS of 8.6 y from recruitment. SD and high tumor uptake associated with longer survival. | 21.8 mo |
Overview of published studies on RLT in meningioma (excel version).
This table provides a comprehensive summary of clinical studies, including in particular imaging modalities, study design, primary objectives, post therapy WBS and/or dosimetric evaluations, patient and tumor characteristics and outcomes in terms of efficacy and safety. Both retrospective and prospective experiences are included, highlighting heterogeneous protocols and patient populations while illustrating feasibility, efficacy and safety of RLT in progressive or pretreated meningiomas.
RP: radiopharmaceutical; FU: follow-up; Mo: months; Octreoscan®: [111In]In-DTPA-octreotide scintigraphy; DOTA PET: [68 Ga]Ga DOTA PET; LuDOTA: [177Lu]Lu DOTA-TATE or [177Lu]Lu-DOTA-TOC; YDOTA: [90 Y]Y DOTA-TOC; HT: hematological toxicity; NT: neurological toxicity; RN: renal toxicity; DCR: disease control rate; PR: partial responders; SD: stable disease; PD: progression disease; CR: complete responders; TTP: time to progression; CI: clinical improvement; 3DVGR: MRI three-dimensional volume growth rate; TR: PRRT radionuclide tumour retention; [177Lu]Lu-DOTA-JR11: LuJR11; SE: side effect; MS: Mean survival; Y: yes; N: no; WBS: Post-therapy whole-body scintigraphy (single or serial studies).
Table 3
| First author | journal | Year | Study design | SSRimaging | RP | Treatment schedules | Main topics | Response criteria | Toxicity measure | WBS | Number of pts | WHO grade | Demographics | Main findings | Median FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | Journal of neurosurgery Online | 2010 | Prospective phase 2 | NA | 125I-mAb425 | 1.8 GBq over a course of 3 weekly administration | RIT:efficacy and safety | Kaplan–Meier curves | CTCAE v.2010 | no | 192 treated with RIT, 60 also with temozolomide (RIT + TMZ) | Grade 4 astrocytoma | yes | mOS 15.7 mo, 1 y survival 62.5%, 2 y survival 25.5%, better in RIT. 7 (3.6%) pts. with acute SE mostly G1-G2 (flushing, nausea, hypotension, skin irritation at the injection site). 4 patients became HAMA positive No Grade 3 or 4 toxicities. TMZ + RIT groups no major toxicities |
unclear |
| Keinfel | EJNMMI | 2007 | prospective | – | [⁹⁰Y]Y-DOTA GAsubstance P |
370–3.330 MBq | dosimetry (using 2 MBq of [111 In]In-substance P) |
NA | NA | Yes | 12 | 4 four glioblastoma(grade 4), 2 anaplastic gliomas (grade 3) and 6 low-grade astrocytomas (grade 2I). |
Yes (not detailed) | Very good agreement between pre- and post-therapeutic dosimetry Good correspondence between the pretherapeutic test injection and the dose deposition |
NA |
| Krolicki | International journal of molecular science | 2023 | Pivotal study | − | [213Bi] Bi/225[Ac] Ac DOTA-substance P | 2–2.5 GBq of [213Bi] Bi DOTA-substance P or 17–35 MBq 225[Ac] Ac DOTA-substance P, intratumoral injection | TAT: efficacy and safety | NA | unknown | no | 11 | oligodendroglioma grade 2 and astrocytoma grade 2 | yes | RFS of 2–16 ys in astrocytoma (8 pts) and of 4–24 ys in oligodendroglioma (3pts) Low neurotoxicity |
1–24 years (median 10) |
| Cordier-Forrer Kneifel | Journal of Neurooncol | 2009 | Prospective phase 1 | – | [⁹⁰Y]Y-DOTA DOTAGA–substance P | Intratumoral injection of [90Y]Y-DOTAGA–substance P at weekly intervals. TCA: 120–345 mCi with dose escalation |
Feasibility and safety | NA | CTCAE v 2.0 | Yes + Test injection before surgerywith [111In]In-DOTAGA-SP |
17 | Unknown | yes | No relevant SE. No increased intracranial pressure. During surgery better resectability and reduced intraoperative bleeding. 1 pt. with epidural and subdural abscess, 1 pt. with local fistula, 1 pt. with acute intracerebral hemorrhage for tumor regrowth. |
unclear |
| Truckenmueller | Frontiers in oncology | 2022 | retrospective cohort study, | PSMA PET |
LuPSMA | 6.03 (5.74–6.10) GBq/cycle for 2 cycle every 9–11 wks | correlation between [68 Ga]Ga-PSMA uptake and histological PSMA expression, TBR and rLuPSMA |
NA | CTCAE v 5.0 | yes | 3 | 1 glioblastoma and 2 astrocytoma | yes | 20 pts. included mSUVmax 4.5 (3.7–6.2) High TBR correlated with increased endothelial PSMA expression. Only 3 pts. had TBRmax>1.0 and qualified for LuPSMA RLT. No SE.observed No efficay data are given |
maximum 15 weeks |
| Heute | JNM | 2010 | prospective | DOTA PET |
YDOTA | TCA 1.7–2.2 GBq in 3 or 4 cycles locally injected into a previously implanted catheter system every 3 mo |
Efficacy and safety | FUI: ceMRI DOTA PET, FDG PET, FET PET | unknown | yes | 3 | Grade 4 glioblastoma | yes | Treatment successful in all 3 patients, with only minor SE, such as epileptic seizure, transient, mild headache 1 pt. CR, 2 pts. PR clinical improvement and improved QOL |
Unclear, probably 4 years |
| Cordier Forrer Bruchertseifer | EJNMMI | 2009 | Pilot trial | − |
213Bi-DOTA -substance P: |
Intratumoral injection, 3–5 injections over 2 days. 4 pts. with TCA 1.07–2.00 GBq, 1 pt. TCA 7.36 GBq divided into 4 cycles |
Feasibility and safety | MRI | CTCAE v2.0 | Yes (with blood sampling) | 5 | glioma (grade 2–4) | yes | feasible and tolerated without additional neurological deficit. No relevant AE. Possible radiation induced necrosis |
unclear |
| Nemati | CNM | 2021 | prospective | Octreoscan® and DOTA PET |
LuDOTA | 1–4 cycles every 1–2 mo, TCA 3.7–26.9 GBq | Efficacy, safety, qol | RANO, Karnoski performance status | CTCAE 4.03 | yes | 16 | 3 and 4 | yes | CR in 2 pts., PR in 5 pts., SD in 3 pts., PD in 6 pts. No relevant SE. No significant improvement of qol | 1–26 months |
Overview of published studies on RLT in gliomas (word version).
This table provides a comprehensive summary of clinical studies, including in particular imaging modalities, study design, primary objectives, post therapy WBS and/or dosimetric evaluations, patient and tumor characteristics and outcomes in terms of efficacy and safety. Both prospective and retrospective studies, as well as pilot and pivotal trials, are included. The table highlights feasibility, tolerability and potential clinical benefit of RLT in malignant gliomas, with generally low rates of severe adverse events.
RP: radiopharmaceutical; FU: follow-up; Mo: months; Octreoscan®: [111In]In-DTPA-octreotide scintigraphy; DOTA PET: [68 Ga]Ga DOTA PET; LuDOTA: [177Lu]Lu DOTA-TATE or [177Lu]Lu-DOTA-TOC; YDOTA: [90 Y]Y DOTA-TOC; HT: hematological toxicity; NT: neurological toxicity; RN: renal toxicity; DCR: disease control rate; PR: partial responders; SD: stable disease; PD: progression disease; CR: complete responders; TTP: time to progression; CI: clinical improvement; 3DVGR: MRI three-dimensional volume growth rate; TR: PRRT radionuclide tumour retention; [177Lu]Lu-DOTA-JR11: LuJR11; SE: side effect; MS: Mean survival; Y: yes; N: no; WBS: Post-therapy whole-body scintigraphy (single or serial studies).
Table 4
| First author | Journal | Year | Study design | SSRimaging | RP | Treatment schedules | Main topics | Response criteria | Toxicity measure | WBS | Number of pts | WHO grade | Demographics | Main findings | Median FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | Journal of neurosurgery Online | 2010 | Prospective phase 2 | NA | 125I-mAb425 | 1.8 GBq over a course of 3 weekly administration | RIT:efficacy and safety | Kaplan–Meier curves | CTCAE v.2010 | no | 192 treated with RIT, 60 also with temozolomide (RIT + TMZ) | Grade 4 astrocytoma | yes | mOS 15.7 mo, 1 y survival 62.5%, 2 y survival 25.5%, better in RIT.7 (3.6%) pts. with acute SE mostly G1-G2 (flushing, nausea, hypotension, skin irritation at the injection site). 4 patients became HAMA positive No Grade 3 or 4 toxicities. TMZ + RIT groups no major toxicities | unclear |
| Keinfel | EJNMMI | 2007 | prospective | – | [⁹⁰Y]Y-DOTA GAsubstance P | 370–3.330 MBq | dosimetry (using 2 MBq of [111 In]In-substance P) | NA | NA | Yes | 12 | 4 four glioblastoma (grade 4), 2 anaplastic gliomas (grade 3) and 6 low-grade astrocytomas (grade 2I). | Yes (not detailed) | Very good agreement between pre- and post-therapeutic dosimetry Good correspondence between the pretherapeutic test injection and the dose deposition | NA |
| Krolicki | International journal of molecular science | 2023 | Pivotal study | – | [213Bi] Bi/225[Ac] Ac DOTA-substance P | 2–2.5 GBq of [213Bi] Bi DOTA-substance P or 17–35 MBq 225[Ac] Ac DOTA-substance P, intratumoral injection | TAT: efficacy and safety | NA | unknown | no | 11 | oligodendroglioma grade 2 and astrocytoma grade 2 | yes | RFS of 2–16 ys in astrocytoma (8 pts) and of 4–24 ys in oligodendroglioma (3pts) Low neurotoxicity | 1–24 years (median 10) |
| Cordier-Forrer Kneifel | Journal of Neurooncol | 2009 | Prospective phase 1 | – | [⁹⁰Y]Y-DOTA DOTAGA–substance P | Intratumoral injection of [90Y]Y-DOTAGA–substance P atweekly intervals. TCA: 120–345 mCi with dose escalation | Feasibility and safety | NA | CTCAE v 2.0 | Yes | 17 | Unknown | yes | No relevant SE. No increased intracranial pressure. During surgery better resectability and reduced intraoperative bleeding. 1 pt. with epidural and subdural local fistula, 1 pt. with acute intracerebral hemorrhage for tumor regrowth. | unclear |
| Truckenmueller | Frontiers in oncology | 2022 | retrospective cohort study | PSMA PET | LuPSMA | 6.03 (5.74–6.10) GBq/cycle for 2 cycle every 9–11 wks | correlation between [68 Ga]Ga-PSMA uptake and histological PSMA expression, TBR and rLuPSMA | NA | CTCAE v 5.0 | yes | 3 | 1 glioblastoma and 2 astrocytoma | yes | 20 pts. included mSUVmax 4.5 (3.7–6.2)High TBR correlated with increased endothelial PSMA expression. Only 3 pts. had TBRmax>1.0 and qualified for LuPSMA RLT. No SE.observed. No efficacy data | maximum 15 weeks |
| Heute | JNM | 2010 | prospective | DOTA PET | YDOTA | TCA 1.7–2.2 GBq in 3 or 4 cycles locally injected into a previously implanted catheter system every 3 mo | Efficacy and safety | FUI: ceMRI DOTA PET, FDG PET, FET PET | unknown | yes | 3 | Grade 4 glioblastoma | yes | Treatment successful in all 3 patients, with only minor SE, such as epileptic seizure, transient, mild headache 1 pt. CR, 2 pts. PR clinical improvement and improved QOL | Unclear, probably 4 years |
| Cordier Forrer Bruchertseifer | EJNMMI | 2009 | Pilot trial | − | 213Bi-DOTA -substance P | Intratumoral injection, 3–5 injections over 2 days. 4 pts. with TCA 1.07–2.00 GBq, 1 pt. TCA 7.36 GBq divided into 4 cycles | Feasibility and safety | MRI | CTCAE v2.0 | Yes (with blood sampling) | 5 | glioma (grade 2–4) | yes | feasible and tolerated without additional neurological deficit. No relevant AE. Possible radiation induced necrosis | unclear |
| Nemati | CNM | 2021 | prospective | Octreoscan® and DOTA PET | LuDOTA | 1–4 cycles every 1–2 mo, TCA 3.7–26.9 GBq | Efficacy, safety, qol | RANO, Karnoski performance status | CTCAE 4.03 | yes | 16 | 3 and 4 | yes | CR in 2 pts., PR in 5 pts., SD in 3 pts., PD in 6 pts. No relevant SE. No significant improvement of qol | 1–26 months |
Overview of published studies on RLT in gliomas (excel version).
This table provides a comprehensive summary of clinical studies, including in particular imaging modalities, study design, primary objectives, post therapy WBS and/or dosimetric evaluations, patient and tumor characteristics and outcomes in terms of efficacy and safety. Both prospective and retrospective studies, as well as pilot and pivotal trials, are included. The table highlights feasibility, tolerability and potential clinical benefit of RLT in malignant gliomas, with generally low rates of severe adverse events.
RP: radiopharmaceutical; FU: follow-up; Mo: months; Octreoscan®: [111In]In-DTPA-octreotide scintigraphy; DOTA PET: [68 Ga]Ga DOTA PET; LuDOTA: [177Lu]Lu DOTA-TATE or [177Lu]Lu-DOTA-TOC; YDOTA: [90 Y]Y DOTA-TOC; HT: hematological toxicity; NT: neurological toxicity; RN: renal toxicity; DCR: disease control rate; PR: partial responders; SD: stable disease; PD: progression disease; CR: complete responders; TTP: time to progression; CI: clinical improvement; 3DVGR: MRI three-dimensional volume growth rate; TR: PRRT radionuclide tumour retention; [177Lu]Lu-DOTA-JR11: LuJR11; SE: side effect; MS: Mean survival; Y: yes; N: no; WBS: Post-therapy whole-body scintigraphy (single or serial studies).
Table 5
| First author | Tumor | Study design | RF | Main findings |
|---|---|---|---|---|
| Minczeles | meningioma | Retrospective | LuDOTA | Efficacy: mOS 13.6 mo |
| Gerster-Gilliéron. | meningioma | Prospective phase 2 | YDOTA | Efficacy: mPSF at least 24 mo. |
| Minutoli | meningioma | Retrospective | [111In]In Pentetreotide | Toxicity: no G3-G4. Efficacy (DCR): PR in 2 pts., SD in 5 patients, PD in 1 PT |
| Bartolomei | meningioma | Prospectic | YDOTA | Efficacy: SD in 19 and PD in10, median OS 40 months. Median TTP: 61 months in grade 1 and 13 in grade 2 and 3. No NT, no G3 or G4 other toxicity |
| Kreissl | meningioma | Pilot trial | LuDOTA | Efficacy: SD in 8/10 |
| Hanscheid | meningioma | retrospective | LuDOTA | A single post-therapeutic measurement could estimate the absorbed dose |
| Vonken | meningioma | retrospective | LuDOTA (high affinity) | Toxicity:1 pt. with G3 HT (Leukopenia). Efficacy: 3 pts. completed treatment: 1 was PR, 2 SD. They all Improved clinical conditions |
| Amerein | meningioma | retrospective | LuDOTA (high affinity) | HT: 3 pts. with G3 thrombocypenia, 5 with G3 lymphocytopenia and 1 with G4 lymphocytopenia. RN: 1 pt. with G3-G4, 1 pt. with local necrosis. Efficacy: 1/10 CR, 1/10 PR, 8/10 SD, 9/13 CI m PFS of 18 mo |
| Hartrampf | meningioma | retrospective | LuDOTA | no toxicities; mPFS 13.8–107.7 mo, OS 38.2 to 111.4 |
| Kertels | meningioma | retrospective | LuDOTA | HT: 5 pts. anemia>G3, 7 pts. trombocytopenia >G3, 9 pts. leukopemia >G3. Efficacy: mPSF 12 mo, OS 37 mo. SD in 6 pts. |
| Kletting | meningioma | retrospective | YDOTA | Optimal activity of 4.2 ± 1.8 GBq for meningioma |
| van Essen | meningioma | Prospective? | LuDOTA | Efficacy: 4 PD, 1 SD. Toxicity uncleae |
| Graillon | meningioma | retrospective | LuDOTA | 3DVGR significantly decreased at 3, 6, and 12. |
| Hänscheid | meningioma | Pilot trial | LuDOTA | Strong correlation between TR ansd SUV |
| Eigler | meningioma | prospective phase 0 | LuDOTA | Toxicity: 2 pts. with G3 lymphopenia and 1 pt. with G3 lymphopenia and neutropenia after DOTA-JR11. Efficacy DRC: 83% |
| Puranik. | meningioma | Retrospective | LuDOTA | Efficacy: median TTP of 8.9 months, PD in 2 pts., PR in 2 pts., 4 pts. in SD Toxicity: unclear |
| Severi | meningioma | Prospective | LuDOTA and YDOTA | Efficacy: DCR of 57%, mPFS of 16 mo, mOS 36 mo. Toxicity: G3 platelets toxicity in 1 pt. No symptomatic worsening of conditions. Efficacy: For rechallenge: mPFS of 6.5 mo and mOS of 17 mo |
| Reuvers | meningioma | unknown | LuDOTA | PRRT induced DNA damage, correlated with SSR expression |
| Dubois | meningioma | restrospective | LuDOTA | Toxicity: 14 pts. with G3 or higher HT with a single parameter;3 pts. with 2 parameters; 4 pts. with 3 parameters; and 2 pts. with 4 parameters. 1 pt. had G3 hepatic cytolysis. Risk factors not considered separately for meningioma |
| Salgues | meningioma | restrospective | LuDOTA | Toxicity: frequent SE transient G1 HT. 3 pts. G3 lymphocytopenia. Efficacy: 5/6 pts. with SD at 12 months. PFS at 6mo was 85.7% and PFS at 12mo was 66,7% |
| Seystahal | meningioma | retrospective | LuDOTAand YDOTA | Efficacy: SD in 10/20, mPFS and PFS at 6 mo stratified according to grade, as such as mOS. Ga DOTA uptake was linked to MRI |
| Marincek | meningioma | Prospective phase 2 | LuDOTA and YDOT. | Efficacy: SD in 23 pts. Relevant HT in 3 pts. and severe RT in 1 pt. MS of 8.6 y from recruitment. SD and high tumor uptake associated with longer survival. |
| Li | glioma | Prospective phase 2 | 125I-mAb425 | mOS 15.7 mo, 1 y survival 62.5%, 2 y survival 25.5%, better in RIT.7 (3.6%) pts. with acute SE mostly G1-G2 (flushing, nausea, hypotension, skin irritation at the injection site). 4 patients became HAMA positive No Grade 3 or 4 toxicities. TMZ + RIT groups no major toxicities |
| Keinfel | glioma | prospective | [⁹⁰Y]Y-DOTA GAsubstance P | Very good agreement between pre- and post-therapeutic dosimetry Good correspondence between the pretherapeutic test injection and the dose deposition |
| Krolicki | glioma | Pivotal study | [213Bi] Bi/225[Ac] Ac DOTA-substance P | RFS of 2–16 ys in astrocytoma (8 pts) and of 4–24 ys in oligodendroglioma (3pts) Low neurotoxicity |
| Cordier-Forrer Kneifel | glioma | Prospective phase 1 | [⁹⁰Y]Y-DOTA DOTAGA–substance P | No relevant SE. No increased intracranial pressure. During surgery better resectability and reduced intraoperative bleeding. 1 pt. with epidural and subdural local fistula, 1 pt. with acute intracerebral hemorrhage for tumor regrowth. |
| Truckenmueller | glioma | retrospective cohort study | LuPSMA | 20 pts. included mSUVmax 4.5 (3.7–6.2)High TBR correlated with increased endothelial PSMA expression. Only 3 pts. had TBRmax>1.0 and qualified for LuPSMA RLT. No SE.observed. No efficacy data |
| Heute | glioma | prospective | YDOTA | Treatment successful in all 3 patients, with only minor SE, such as epileptic seizure, transient, mild headache 1 pt. CR, 2 pts. PR clinical improvement and improved QOL |
| Cordier Forrer Bruchertseifer | glioma | Pilot trial | 213Bi-DOTA -substance P | feasible and tolerated without additional neurological deficit. No relevant AE. Possible radiation induced necrosis |
| Nemati | glioma | prospective | LuDOTA | CR in 2 pts., PR in 5 pts., SD in 3 pts., PD in 6 pts. No relevant SE. No significant improvement of qol |
Published clinical experiences with RLT in meningiomas and gliomas.
This table summarizes key results of the comparison between published studies on meningiomas and gliomas. Study design, radiopharmaceuticals and main findings were considered. Overall, these studies demonstrate feasibility, tolerability and potential clinical benefit of RLTs in both tumor types. Notably, meningioma studies predominantly used 177Lu- and 90Y-labeled DOTA-peptides, whereas glioma studies included RIT and alpha targeted therapies. Glioma studies were generally smaller and more heterogeneous.
RP: radiopharmaceutical; FU: follow-up; Mo: months; Octreoscan®: [111In]In-DTPA-octreotide scintigraphy; DOTA PET: [68 Ga]Ga DOTA PET; LuDOTA: [177Lu]Lu DOTA-TATE or [177Lu]Lu-DOTA-TOC; YDOTA: [90 Y]Y DOTA-TOC; HT: hematological toxicity; NT: neurological toxicity; RN: renal toxicity; DCR: disease control rate; PR: partial responders; SD: stable disease; PD: progression disease; CR: complete responders; TTP: time to progression; CI: clinical improvement; 3DVGR: MRI three-dimensional volume growth rate; TR: PRRT radionuclide tumour retention; [177Lu]Lu-DOTA-JR11: LuJR11; SE: side effect; MS: Mean survival; Y: yes; N: no; WBS: Post-therapy whole-body scintigraphy (single or serial studies).
Studies about meningiomas
Patients selection according to SSRs
In 12 studies patients were selected according to DOTA PET; in six studies, selection was based on Octreoscan®; in two studies, both modalities were used; and in two studies the selection method was not reported. In particular, Octreoscan® alone was used in studies published between 2006 and 2017, whereas DOTA PET alone was used in studies published between 2012 and 2024.
Study design
13 Studies were retrospective (59.1%), six were prospective (27.3%), two (9.1%) were pilot trials, in one case (4.5%) the study could not be classified in this sense.
Radiopharmaceutical and treatment schedule
In 15 studies (68.3%), patients were treated with LuPRRT; in three studies (13.6%), with YPRRT; in another three studies (13.6%), with a combination of Lu- and Y-based PRRT; and in one study (4.5%), with [111 In]In-Pentetreotide, administered in two patients together with a beta-emitting radiolabelled peptide (13). In the case of LuPRRT, total cumulative activity (TCA) amounted between 7.4 (14) and 39.5 GBq (15). With the exception of two studies, where intra-arterial usage was required, the administration method was intravenous. In the case of intra-arterial administration, [177Lu]Lu- high-affinity (HA)-DOTATATE was used: It exhibits some structural and functional differences when compared to the DOTATATE compound currently employed (16, 17). In the phase-0 PROMENADE study, the use of LuDOTA was combined with the use of [177Lu]Lu-DOTA-JR11 (18). This latter agent is a radiolabelled SSRs antagonist designed for PRRT. Unlike somatostatin receptor agonists, which internalize upon binding to the receptor, JR11 binds to a broader range of SSR subtype 2 sites. This includes both internalizing and non-internalizing sites, leading to higher tumor uptake and prolonged tumor retention.
In the case of YPRRT, the TCA ranged between 7.4 GBq (19) and 15 GBq (20). In one prospective phase 2 study, which combined the use of LuPRRT and YPRRT, the cycles were repeated until progression or until toxicity manifests (21).
Main topic and evaluation criteria
Safety was the main topic in 17 studies (77.3%) and the registration of the adverse events (AEs) was its endpoint (20, 22).
Efficacy was evaluated in 16 studies (72.7%), with the endpoints of progression-free survival (PFS) (2, 15, 17, 19, 23, 24), disease control rate (DCR) (2, 13, 20, 25), OS (15, 23), and time to progression (TPP) (20, 25). Response criteria were variable: In eight studies, they were based on Response Assessment in Neuro-Oncology Working Group (RANO) criteria (26); in three on Southwest Oncology Group standard response criteria (SWOG) criteria (27); and in two on Response Evaluation Criteria in Solid Tumors (RECIST) criteria (28).
WBS
WBS was performed in 16 studies (72.7%), mostly using LuPRRT. In four cases, OLINDA/EXM software was used. Dosimetry was the main topic in two studies (18, 25, 29, 30). Only two of these studies reported the use of YPRRT. In particular, one study aimed exclusively to optimize the peptide amount and activity for YPRRT, while in the other, a sub-cohort of 14 patients received co-infusion of [111In]In DOTA−TOC to assess YPRRT biodistribution (20).
Main findings
With regard to safety, the authors primarily focused on major toxicities, of hematological or renal nature, as shown in the synoptic table. In a study by Severi, patients were treated with YPRRT (five) or LuPRRT (37) and with different TCA, based on the presence of risk factors for toxicity. According to this study, the treatment was well tolerated, and only one patient had G3 toxicity on platelets. No deterioration of clinical conditions occurred in any case. Some authors reported no major (G3 or G4) toxicities with YPRRT (20), LuPRRT (23) or with [111In]In Pentetreotide (13).
In other studies, toxicities were more significant. Amerein reported retrospective data on patients treated with intra-arterial HA-LuPRRT. Three patients experienced G3 thrombocytopenia, five had G3 lymphocytopenia, and one developed G4 lymphocytopenia. In addition, one patient showed transient G3/G4 renal toxicity, probably unrelated to PRRT, and another developed local necrosis likely associated with angiography. Kertels also reported higher toxicity rates in patients with meningioma and neurofibromatosis treated with LuPRRT, describing severe anemia in five cases, severe thrombocytopenia in seven and severe leukopenia in nine.
Eigler and the PROMENADE group conducted a phase-0 study on patients treated with one cycle of LuPRRT followed by two or three cycles of [177Lu]Lu-DOTA-JR11 PRRT. They reported G3 lymphopenia in two patients, and combined G3 lymphopenia and neutropenia in one patient.
Finally, in a study by Dubois, short-term toxicity prediction was retrospectively investigated in patients with SSRs tumors treated with LuPRRT. Only five of them were affected by meningioma. The authors reported considerations about the increased risk of toxicity generally, without separating between the different primary tumours, and found that risk factors were gastrointestinal primary tumor diagnosis, bone metastases, peritoneal metastases, pancreatic metastases or pulmonary metastases, and high tumor grade (31).
With regard to efficacy, DCR was investigated by Severi and it was reported of 57%. Other studies on LuPRRT reported stable disease (SD), respectively, in 8/10 patients (14, 17), in 4/5 patients (22), and in 5/6 patients (32). In patients treated with [111In]In Pentetreotide SD was found in 5/8 patients[13]. When associated with antagonist, according to the study on [177Lu]Lu-DOTA-JR11 PRRT reported above, the DCR could reach 83% (18). Together with DCR, also PFS and OS were considered. In the study by Hartrampf, patients were treated with the combination of LuPRRT and EBRT with a long-term follow-up (median of 105 months). Median PFS (mPFS) was reported ranging from 13.8 to 107.7 months, median OS (mOS) from 38.2 to 111.4 months.
To provide an alternative definition of treatment response, some researchers looked for a volumetric parameter. A study by Graillon evaluated the impact of PRRT with Lutathera in nonanaplastic meningiomas, according to the three-dimensional volume growth rate (3DVGR) measured with MRI (33). 3DVGR significantly decreased at 3, 6, and 12 months after treatment initiation, analysing each lesion separately. In particular, at 3, 6, and 12 months after treatment initiation, 4/8, 6/7, and 5/6 patients were class 2 (stabilization or severe 3DVGR slowdown), respectively. Moreover, its antitumor activity persisted for 12–18 months following treatment initiation (34).
Other studies focused on dosimetry. Hänscheid retrospectively assessed activity kinetics from planar images in patients with various tumors, including meningioma, treated with LuPRRT. Mono- or biexponential functions were applied to data from the kidneys, liver, spleen, and neuroendocrine tumor (NET) lesions. Tissue-specific deviations of the approximation from the time integral were calculated at 24, 48, 72, 96, 120, and 144 h. The authors concluded that a single quantitative measurement of abdominal activity concentration by SPECT/CT, performed four days after PRRT, could provide a three-dimensional dose map and estimate the actual absorbed doses.
In a study by Kletting, a whole-body physiologically based pharmacokinetic (PBPK) model was developed to determine in YPRRT biologically effective doses (BEDs) to the tumor, to the liver, to the spleen, and to the red marrow for a maximal kidney BED (20 Gy 2.5) for different peptide amounts and activities. The authors found that these two parameters depended on tumor perfusion and receptor density. For meningioma and for YPRRT, the optimal amount and pertaining activity was found to be 76 ± 46 nmol (118 ± 71 μg) and 4.2 ± 1.8 GBq.
Finally, to assess the short-term response to radiation therapies such as PRRT and EBRT, Reuvers et al. developed a 3D spheroidal culture model for meningiomas. The authors noticed that in general meningioma spheroids retained characteristics of the parental tumor during the initial days of culturing, but a subset of tumors veered toward a more aggressive phenotype. PRRT induced DNA damage which was detectable for an extended timeframe as compared to EBRT. Furthermore, levels of DNA damage in spheroids after PRRT correlated with SSR2-expression levels of parental tumors (35).
Follow-up times
Follow-up times were extremely variable, ranging from 3 months (36, 37) to a median of 105.0 months (23).
Studies about gliomas
Patients’ selection according to SSRs
Given the heterogeneity of the radiopharmaceuticals used, only in two cases patients were selected according to SSRs imaging and in particular with DOTA PET (38) and both (DOTAPET and Octreoscan®) (39). In one study, patients were selected through [68Ga]Ga-PSMA (PSMA PET) (40).
Study design
Three of the included studies were prospective (39, 41, 42); one was a retrospective cohort study (40); one was a pilot trial (43); and for the other three studies, a similar classification was not possible (38, 44, 45).
Radiopharmaceutical and treatment schedule
YPRRT was proposed in the study by Heute on three patients. A TCA of 1.7–2.2 GBq was delivered in three or four cycles and locally injected into a previously implanted catheter system every 3 months (38).
LuPRRT was proposed in a study by Nemati, who treated 16 patients for 1–4 cycles every 1–2 months and with a TCA of 3.7–26.9 GBq.
Other radiopharmaceutical used were:
-
[125I]I-labeled anti-epidermal growth factor receptor 425 murine monoclonal antibody (125I-mAb425) is a murine monoclonal antibody directed against the epidermal growth factor receptor (EGFR) and conjugated with 125I. This antibody binds to a part of the extracellular domain of human EGFR. The used schedule was of 1.8 GBq over a course of three weekly administration and for a TCA of 5.4 GBq, approximately 4–6 weeks after surgery and EBRT. A total of 192 patients were treated over a course of 20 years, starting from 1987 (41).
-
[111 In]In/[90Y]Y –DOTAGA substance P, used in two studies before surgery and through intratumoral injections. Substance P is the main ligand of neurokinin type 1 (NK-1) receptors, commonly overexpressed in gliomas and tumor neovasculature. One of the two studies was addressed to dosimetry and, in particular, to establish a protocol for intratumoral radiopeptide using 2 MBq of [111 In]In-substance P and 370–3.330 MBq of /[90Y]Y-substance P (44). The other study was addressed to evaluate feasibility of intratumoral injection of [90Y]Y-DOTAGA–substance P at weekly intervals, with a TCA of 120–345 mCi with dose escalation and before surgery (42).
-
[213Bi] Bi/ [225Ac] Ac DOTA-substance P, considered in two studied and based on the combination of targeted alpha therapy (TAT) with a linear, small-peptide vector. [213Bi] Bi emits high LET alpha particles (~5–9 MeV, ~100 keV/μm) with a short tissue range (< 0.1 mm), 225Ac has longer half-life (~9.9 days), decays via a cascade yielding ^{213}Bi among daughters, delivering up to five alpha particles per decay. The alpha-particle radiopharmaceuticals are considered to delivery radiation to malignant sites while sparing healthy tissue. In one study, addressed to efficacy and toxicity in 11 patients, 2–2.5 GBq of [213Bi]Bi DOTA-substance P or 17–35 MBq [225Ac]Ac DOTA-substance P were locally injected directly into the tumor via a stereotactic insertion of a capsule-catheter system (45). In another study, the radiopharmaceutical was used in five patients through intratumoral injection, three to five times and over 2 days, with a TCA of 1.07–2.00 GBq in 4 cases and of 7.36 GBq divided into 4 cycles in one case (43).
LuPSMA was considered in one study (40). Several previous studies had shown that PSMA is overexpressed in the tumor-associated neovasculature of high-grade gliomas (46–48), so building on this premise, 20 patients were firstly evaluated with [68Ga]Ga-PSMA PET/MRI (PSMA PET). Three of them likely to benefit from LuPSMA according to the maximum tumor-to-background ratio (TBRmax) of tumor and liver, which had been chosen by the authors as a criterion for recommending LuPSMA, with a predefined cut-off set at 1. In 11 samples, immunohistochemical PSMA expression was determined using the H-score (49) and correlated with uptake on PSMA PET. The H-score in the three patients eligible for LuPSMA was higher than the H-score in patients who were not (respectively 65 versus 30, p = 0.08). While this review primarily focuses on primary brain tumors, it is essential to recognize that LuPSMA may play a role also in brain metastases, according to recent evidence from the literature (50).
Main topics and evaluation criteria
In seven studies, safety was the main topic and the registration of the adverse events (AEs) its endpoint (38, 41–43, 45). Four studies regarded efficacy, in terms of recurrence-free survival and DCR (38, 39, 45).
One study regarded dosimetry (44). It was conducted through injections of [111 In] In-substance P and [90 Y] Y-substance P in 12 patients with malignant gliomas. Over a period of 24 h, serial SPECT scans were performed on a dual-head SPECT camera. Quantitative voxelwise dose distribution maps (in Gy/ GBq) were computed from these data and pre- and post-therapeutic values were compared.
Post-therapy whole-body scintigraphy (WBS, single, or serial studies)
WBS was performed in all studies, except for two (41, 45).
Main findings
With regard to toxicity, in the case of radioimmunotherapy (RIT), the combination with temozolomide (TMZ) was not associated with an increase in toxicity. Adverse events occurred in seven patients (3.6%), and were generally mild, including flushing, nausea, hypotension, and local skin irritation at the injection site. Only four patients developed human anti-mouse antibodies (HAMA). Regarding the studies on TAT, neurotoxicity appeared to be minimal. In the case of [111 In]In/[90Y]Y –DOTAGA substance P, no short-term side effects were observed, too; moreover, intraoperative bleeding appeared to be reduced. For YPRRT, side effects were mild and an overall improvement in quality of life was reported (38, 41, 42).
In terms of efficacy, the combination of TAT and substance P provided a recurrence-free survival time ranging from 2 to 16 years. This was observed in eight patients with astrocytoma, who were treated with TAT following a biopsy or tumor debulking. Regarding oligodendrogliomas, the recurrence-free survival time was 24 years in the first case treated and 4 and 5 years in the two second-line cases, with low toxicities (45). Furthermore, the use of YPRRT in three patients showed the following results: One patient had a complete response (CR) and two patients a partial response (PR) at follow-up imaging with ceMRI, DOTA PET, [18F]F FDG PET, and [18F]F Fuoroethyltyrosine PET. Clinical improvements and a global improvement in quality of life (qol) was noticed, too (38). Finally, in the study by Nemati on LuPSMA, the radiological response according to RANO criteria was considered: Two patients showed a CR, five patients a PR, three patients a SD, and six patients a progression disease (PD). No significant improvement of qol was noticed.
With regard to the dosimetric study, it was found a very good agreement between pre- and post-therapeutic dosimetry and a good correspondence between the pretherapeutic test injection and the dose deposition (44).
Follow-up times
In most cases, the follow-up period was not clearly defined. In one study, it ranged from 1 to 24 years (median 10 years) (45); in another, patients were monitored for up to 15 weeks (40); and in a third study, the follow-up period ranged from 1 to 26 months (39).
CASP analysis of the selected studies
The results of CASP analysis is reported in detail in Table 6 and Figure 3 for meningiomas and in Table 7 and Figure 4 for gliomas. Below, the most noteworthy data are summarized.
Table 6
| First author | (1) Was there a clear statement of the aims of the research? | (2) Is a qualitative methodology appropriate? | (3) Was the research design appropriate to address the aims of the research? | (4) Was the recruitment strategy appropriate to the aims of the research? | (5) Was the data collected in a way that addressed the research issue? | (6) Has the relationship between researcher and participants been adequately considered? | (7) Have ethical issues been taken into consideration? | (8) Was the data analysis sufficiently rigorous? | (9) Is there a clear statement of findings? | (10) How valuable is the research? |
|---|---|---|---|---|---|---|---|---|---|---|
| Minczeles | yes | yes | yes | yes | yes | Cannot Tell | yes | Yes | Yes | Low(number of pts) |
| Gilliéron | yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Cannot tell (pochi pz) | yes | Moderate |
| Minutoli | no | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell | yes | Cannot tell | yes | Moderate |
| Bartolomei | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | high |
| Kreissl | yes | yes | yes | yes | yes | no | Yes | Cannot tell (pochi pz) | no | low |
| Hanscheid | Yes | Yes | Yes | Cannot tell | Yes | no | Cannot tell | Yes | Yes | moderate |
| Vonken | Yes | Cannot tell | Cannot tell | Cannot tell | Cannot tell | no | yes | Cannot tell | yes | Low (very small number of pts) |
| Amerein | yes | yes | yes | yes | yes | yes | yes | yes | yes | Moderate |
| Hartrampf | Yes | yes | Yes | Yes | Yes | Yes | yes | Yes | Yes | Moderate |
| Kertels | Yes | yes | Yes | Yes | Yes | Yes | yes | Yes | Yes | High |
| Kletting | Yes | Yes | Yes | Yes | Yes | Cannot tell | Cannot tell | Yes | Yes | Low (very small number of pts) |
| van Essen | Yes | Cannot tell | Cannot tell | Cannot tell | Yes | Yes | Yes | Cannot tell | Yes | Low (very small number of pts) |
| Graillon | Yes | yes | Yes | Yes | Yes | Yes | yes | Yes | Yes, but complexly (in modo complesso) | high |
| Hänscheid | Yes | Cannot tell | Cannot tell | Cannot tell | yes | no | yes | Cannot tell | yes | moderate |
| Eigler | Yes | yes | yes | yes | yes | Cannot tell | Yes | Yes | yes | moderate (very small number of pts) |
| Puranik | Yes | Cannot tell | Cannot tell | Yes | Yes | No | yes | Cannot tell | No | low |
| Severi | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | high |
| Reuvers | yes | Yes | Yes | Yes | Yes | Cannot tell | Cannot tell | Yes | Yes | Moderate/high |
| Dubois | Yes | Cannot tell | Yes | Yes | Cannot tell | no | Yes | Cannot tell | Yes | moderate |
| Salgues | Yes | Yes | Yes | Yes | Yes | no | Yes | Cannot tell | Yes | moderate |
| Seystahal | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | moderate |
| Marincek | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | high |
CASP analysis of published RLT studies on meningiomas.
In this table a methodological quality assessment was performed. Following the CASP critical appraisal guidelines, key aspects included: clarity of research aims, appropriateness of study design, recruitment strategy, data collection and analysis, consideration of researcher-participant relationships, ethical compliance and clarity of findings. Studies on meningiomas generally demonstrated consistent methodological approaches.
Figure 3

In this figure CASP analysis on meningiomas studies is reported sinoptically.
Table 7
| First author | (1) Was there a clear statement of the aims of the research? | (2) Is a qualitative methodology appropriate? | (3) Was the research design appropriate to address the aims of the research? | (4) Was the recruitment strategy appropriate to the aims of the research? | (5) Was the data collected in a way that addressed the research issue? | (6) Has the relationship between researcher and participants been adequately considered? | (7) Have ethical issues been taken into consideration? | (8) Was the data analysis sufficiently rigorous? | (9) Is there a clear statement of findings? | (10) How valuable is the research? |
|---|---|---|---|---|---|---|---|---|---|---|
| Li | Yes | Yes | Yes | Cannot tell | Yes | Yes | Cannot tell | Yes | Yes | High |
| Keinfel | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | High |
| Krolicki | no | Cannot tell | Cannot tell | Cannot tell | Cannot tell | no | Cannot tell | Cannot tell | Cannot tell | low |
| Cordier- Forrer-Kneifel | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | high |
| Truckenmueller | Yes | Yes | yes | Cannot tell | yes | Cannot tell | yes | yes | yes | low |
| Heute | yes | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell | yes | moderate |
| Cordier Forrer Bruchertseifer. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | high |
| Nemati | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | moderate |
CASP analysis of published RLT studies on gliomas.
In this table, a methodological quality assessment was conducted. As for published studies on meningiomas, the CASP critical appraisal guideline were applied. Key aspects regarded mostly: study rigor, data collection and analysis and ethical compliance. Overall, most studies demonstrated rigorous design and reporting. Some studies showed limitations in qualitative methodology or reporting completeness.
Figure 4

In this figure CASP analysis on gliomas studies is reported sinoptically.
Regarding meningiomas, with the exception of one paper, all of the works had clear aims. The statement of findings was evidently reported in 20/22 (91%) of the papers, too. The general quality of the researches and the quality of the methodology, of the study design, of the recruiting strategy, and of the data analysis were variable. In more detail, the quality of the methodology was considered appropriate in 16/22 (72.7%) of the studies, and the study design was considered adequate in 17/22 (77.3%), as was the recruiting strategy. The overall value of the research was found high in 5/22 (22.7%) of the papers, moderate in 11/22 (50%) of them, and low in 6/22 (27.3%).
Regarding gliomas, all of the works had clear aims but one, as well as regards the clear statements of findings.
The following parameters, however, showed variability: The ethical issues, which have been taken into consideration in five out of eight studies, the quality of the methodology, which was considered as appropriate in six, the quality of the research design, considered as appropriate in six, the quality of the recruiting strategy, considered as adequate in four.
The general quality of the research resulted high in four studies, moderate in two, and low in two.
Clinical trials
Search strategy
The results of the search strategy is reported in Figure 5.
Figure 5
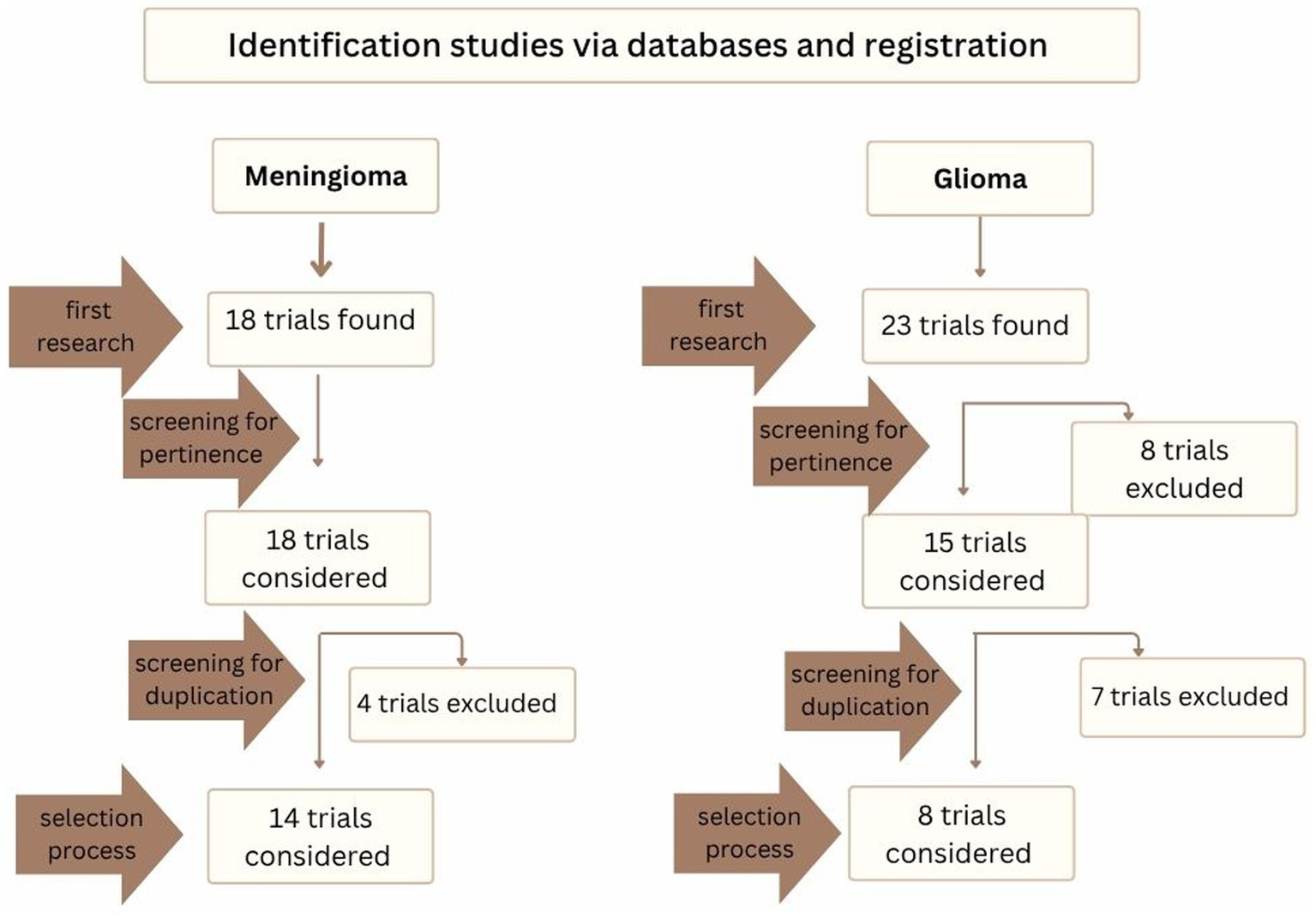
In this figure the selection process for clinical trials is reported, from the first research to selection process.
Fourteen studies on meningiomas and eight on gliomas were included in the final analysis. The characteristics of the trials on meningiomas and gliomas are presented in detail in Tables 8, 9, respectively, so what follows is strictly an overview of the trials.
Table 8
| Title | Study site (s) | First submitted (year) | Radiopharmaceutical | Study type | Main endpoints | Status |
|---|---|---|---|---|---|---|
| (1) Dosimetry Guided PRRT with 90Y-DOTATOC | University of Iowa | 2017 | Y DOTA | Phase 2 | safety and efficacy of 90Y-DOTATOC, the role of 68Ga-DOTATOC | completed |
| (2) Treatment of Recurrent or Progressive Meningiomas With the Radiolabelled Somatostatin Antagonist 177Lu-satoreotide | University Hospital, Basel, Switzerland | 2021 | [177Lu]Lu Satoreotide | phase 0 study followed by Phase I/II study. | Comparison of the therapeutic index of 177Lu-DOTA-JR11 and 177Lu-DOTATOC, dosimetry (tumour absorbed dose), early onset toxicity, QOL | recruting |
| (3) Peptide Receptor Radionuclide Therapy Administered to Participants With Meningioma With 67Cu-SARTATE | Royal North Shore Hospital Sydney | 2019 | [67 Cu]Cu-SARTATE™ | Phase 1/2 | Safety and tolerability of multiple doses of Cu-67 SARTATE, dosimtery | completed |
| (4) Semiautomated Segmentation Methods of SSTR PET for Dosimetry Prediction |
Central Hospital, Nancy, France | 2022 | LuDOTA | Observational retrospective | Correlation between SUVmean and SUVmax for tumor absorbed dose, a new semi-automated segmentation for determining pretherapy metabolic tumor volume | completed |
| (5) Combination of Everolimus and 177Lu-DOTATATE in the Treatment of Grades 2 and 3 Refractory Meningioma: a Phase IIb Clinical Trial | Central Hospital, Nancy, France | 2023 | LuDOTA and Everolimus | Phase 2 | PFS, OS, QOL, toxicity | recruting |
| (6) Radiolabeled Octreotide in Treating Children With Advanced or Refractory Solid Tumors | University of Iowa | 2002 | YDOTA | Phase 1 | safety and effectiveness of radiolabeled octreotide in treating children | completed |
| (7) Lutathera for the Treatment of Inoperable, Progressive Meningioma After External Beam Radiation Therapy | Mayo Clinic | 2020 | LuDOTA | Phase 2 | Efficacy, safety, PFS, OS, QOL, dosimetry | recruting |
| (8) 177Lu-DOTATATE for Recurrent Meningioma (LUMEN-1) | France, Norway, Austria | 2024 | Local standard of Care vs. LuDOTA | Phase 2, Randomized | PFS, OS, Radiological response rate, QOL | recruiting |
| (9) Semi-automatic Segmentation Method for Determining 177Lu-DOTATATE Tumor Dosimetry completato |
|
2024 | LuDOTA | Observational Cohort Time Perspective Retrospective |
Dice index between semi-automatic segmentation and reference segmentation | completed |
| (10) Phase II Study of 177Lu-DOTATATE Radionuclide in Adults With Progressive or High-risk Meningioma | NYU Langone Health | 2019 | LuDOTA | Phase 2 | PFS, ORR, OS | active not rectruinting |
| (11) MOMENTUM-1: A Multicenter, Randomized, Open-Label, Phase II Study of [177 LU]LU-DOTATATE in Adults With Progressive Intracranial Grade 1–3 Meningioma | RTOG Foundation, Inc | 2025 | LuDOTA | Phase 2, randomized | Comparing [177Lu]Lu-DOTATATE to Standard of Care in tems of PFS, OS, DCR, toxicity | not yet recritug |
| (12) Tumor Absorbed Dose–Response Relationship in Patients Treated With 177Lu-DOTATATE for Meningioma (DATUM) | Central Hospital, Nancy, France | 2024 | LuDOTA | Observational Cohort Retrospective |
diagnostic accuracy for PFS | completed |
| (13) Lutathera for Treatment of Recurrent or Progressive High-Grade CNS Tumors | Nationwide Children’s Hospital | LuDOTA | Phase 1/2 | MTD, recommended Phase II dose (RP2D) toxicity, PFS | recruting | |
| (14) Study in Children and Adolescents of 177Lu-DOTATATE (Lutathera®) Combined with the PARP Inhibitor Olaparib for the Treatment of Recurrent or Relapsed Solid Tumours Expressing Somatostatin Receptor (SSTR) (LuPARPed). (LUPARPED) | Fundación de investigación HM |
|
Phase 2 | ORR | recruting |
Overview of clinical trials of RLT in meningiomas.
This table summarizes study design, the proposed radiopharmaceuticals, primary endpoints and study status of the trials. Early-phase and randomized trials are reported. Regarding radiopharmaceuticals, they are also combined with targeted agents such as everolimus or olaparib. Most studies focus on safety, dosimetry, efficacy and quality of life. Overall, these trials demonstrate the feasibility and translational potential of RLT in meningiomas.
LuDOTA: [177Lu]Lu DOTA-TATE or [177Lu]Lu-DOTA-TOC or LUTATHERA ®; YDOTA: [90 Y]Y DOTA-TOC; QOL: Quality Of Life; ORR: overall response rate (ORR); MTD: maximally tolerated dose.
Table 9
| Title | Study site (s) | First submitted (year) | Radiopharmaceutical | Study type | Main endpoints | status |
|---|---|---|---|---|---|---|
| 1. Yttrium Y 90 SMT 487 in Treating Patients With Refractory or Recurrent Cancer | ? in USA | 2000 | [90Y]Y-SMT 487 | Phase 1 | MTD and safety | completed |
| 2. A Feasibility Study to Evaluate the Safety of the TheraSphere Glioblastoma (GBM) Device in Patients With Recurrent GBM | California, Florida, Illinois, Maryland USA | 2022 | Y-90 Glass Microsphere System (TheraSphere GBM) | Phase 1 | PFS, OS, ORR, toxicity | recruting |
| 3. 68Ga/177Lu-PSMA Theranostics in Recurrent Grade 3 and Grade 4 Glioma | St. Olavs Hospital Norway | 2022 | [177Lu]Lu-PSMA I&T | pilot-study | Efficacy, toxicity, dosimetry, QOL; role of [68 Ga]Ga-PSMA uptake | recruting |
| 4. 177Lu-J591 Antibody in Patients With Nonprostate Metastatic Solid Tumors | Weill Medical College of Cornell University, New Yors, USA | 2005 | [177Lu]Lu Radiolabeled Monoclonal Antibody HuJ591 | Phase 1 | DCR, safety | completed |
| 5. Dose Finding Study of [177Lu]Lu-NeoB in Newly Diagnosed Glioblastoma and in Recurrent Glioblastoma | Novartis Pharmaceuticals | 2023 | [177Lu]Lu -NeoB in combination with RT and TMZ | Phase 1 | DLTs toxicity, PFS, OS, | recruiting |
| 6. Radioimmunotherapy with Lu-177 Labeled 6A10 Fab-fragments in Patients with Glioblastoma After Standard Treatmen | University Hospital Muenster, Germany | 2022 | -[177Lu]Lu - Labeled 6A10 Fab-fragments | Phase 1 | Pharmacokinetics, absorbed dose, PFS and OS | recruiting |
| 7. A Dose Finding Study of [177Lu]Lu-DOTA-TATE in Newly Diagnosed Glioblastoma in Combination With Standard of Care and in Recurrent Glioblastoma as a Single Agent. | Novartis Pharmaceuticals | 2021 | [177Lu]Lu-DOTA-TATE | Phase Ib | DLTs, PFS, OS, ORR, dosimetry | recruiting |
| 8. A Phase I Study of [177Lu]Lu-FF58 in Patients With Advanced Solid Tumors. | Novartis Pharmaceuticals | 2023 | [177Lu]Lu-FF58 | Phase 1 | Dose escalation and dose expansion | Terminated |
Overview of clinical trials of RLT in gliomas.
This table summarizes study design, the proposed radiopharmaceuticals, primary endpoints and study status of the trials. The trials were early-phase and pilot studies and investigate a range of radiopharmaceuticals. Most studies focus on safety, dose-finding, pharmacokinetics, dosimetry, efficacy and quality of life. Overall, these trials highlight the feasibility, tolerability and translational potential of RLT in gliomas.
MTD: maximum tolerated dose; PFS: Progression-Free Survival; OS: Overall Survival; ORR: Objective Response Rate; QOL: Quality Of Life; DCR: Disease Control Rate; Dose Limiting Toxicity (DLTs).
Overview of the selected studies
Trials about meningiomas
A total of 10 trials focus on the use of LuPRRT, two on YPRRT, one on the SSRs antagonist Satoreotide labeled with [177 Lu] one on [67Cu]Cu-SARTATE™. Satoreotide has been tested above all in NETs (51, 52). [67Cu]Cu-SARTATE™ consists of SARTATE, an octreotate-derived ligand, radiolabeled with 67Cu, a beta-emitting isotope that also provides gamma emissions suitable for imaging, thereby enabling theranostic applications. Tumor types targeted by [67Cu]Cu-SARTATE™ potentially include also gastroenteropancreatic GEP-NETs, bronchial NETs and neuroblastomas (53, 54). The association of LuPRRT with other antitumor agents, such as everolimus, an immunosuppressive agent belonging to the class of mechanistic target of rapamycin kinase (mTOR) inhibitors, and olaparib, a poly ADP-ribose polymerase (PARP) inhibitor, was proposed.
Furthermore, eleven trials are prospective and one is retrospective. The studies status is distributed as follows: Six trials are completed, six are recruiting, and two are active but not recruiting.
Trials about gliomas
One trial will investigate the use of LuPRRT, one trial of LuPSMA.
The remaining six trials will test, respectively:
-
[90Y]Y spheres (Glass Microsphere System, TheraSphere GBM), a form of selective internal radiation therapy (SIRT) involving glass microspheres embedded 90Y and originally developed for the treatment of unresectable hepatocellular carcinoma (HCC) via intra-arterial delivery (55). In the context of GBM, TheraSphere are administered via intra-arterial cerebral infusion, enabling localized high-dose beta radiation directly to the tumor bed, while minimizing exposure to surrounding healthy brain tissue. This approach aims to overcome the limitations of EBRT and the blood–brain barrier, offering a promising avenue for treating recurrent or resistant GBM.
-
[90Y]Y-SMT 487, a radiopharmaceutical consisting of the somatostatin analog SMT 487 labeled with 90Y. It primarily targets tumors that overexpress SSRs, especially type 2 and has been evaluated above all in NETs (56).
-
[177Lu]Lu Radiolabeled Monoclonal Antibody HuJ591, a radiolabeled monoclonal antibody that targets PSMA radiolabelled with 177 Lu (57).
-
[177Lu]Lu - Labeled 6A10 Fab-fragments, which consists of Fab fragments derived from the monoclonal antibody 6A10, radiolabeled with 177 Lu. The 6A10 antibody targets carbonic anhydrase XII (CA XII), a membrane-associated enzyme overexpressed in several solid tumors, including renal and lung cancers. It is used for its improved tumor penetration and reduced off-target toxicity due to the smaller size of the Fab fragment (58).
-
NeoB, a gastrin-releasing peptide receptor (GRPR)-binding peptide, also known as bombesin receptor subtype 2. When labeled with 177 Lu, it results in the formation of the radiopharmaceutical [177Lu]Lu-NeoB. As a GRPR antagonist, [177Lu]Lu-NeoB enables selective radiation delivery to GRPR-expressing tumors with enhanced tumor uptake and faster clearance from non-target tissues compared with agonist radioligands. (59, 60).
-
[177Lu]Lu-FF58, a radiolabeled peptide designed to target proteins known as integrins: alpha-v beta-3 integrin (αvβ3) and alpha-v beta-5 integrin (αvβ5).
All the trials were prospective. Five are still recruiting while three have completed the enrolment.
Discussion
There is a critical unmet need for innovative therapeutic strategies to complement the current standard of care in meningiomas and gliomas, particularly in cases where conventional treatments are no longer viable or tolerated.
In this context, RLT holds significant promise due to its theranostic paradigm and personalized approach. More in detail, the theranostic paradigm —“treating what is seen and seeing what is treated”—allows pretherapeutic assessment of receptor expression (e.g., SSRs via DOTA PET or Octreoscan® or PSMA PET tracers) and the personalized approach enables optimized patient selection and dosimetric planning.
Alternative radiopharmaceuticals, including alpha-emitters, may be considered even in patients with low receptor expression, offering reduced risk of edema and collateral damage. RLT can be administered either systemically or locally (e.g., intra-arterial). This approach helps overcome the blood–brain barrier, deliver higher tumor doses, and minimize generalized toxicity. Overall, it is generally well tolerated and has demonstrated potential for disease control in multiple studies.
Following these considerations, which are applicable across studies involving both meningiomas and gliomas, the discussion then diverges to emphasize the contrasting findings reported in the literature concerning the two tumor types.
Published papers on RLT in meningiomas appear more robust and “traditional” and focused predominantly on PRRT, which has demonstrated an acceptable safety profile and encouraging signs of efficacy. Unfortunately, currently approved radiopharmaceuticals are not indicated for the treatment of meningioma, so the available data remain limited, heterogeneous and based on experimental studies. Toxicity is generally minimal and manageable, with hematologic and renal adverse events occasionally reported. It is important to highlight that in the largest study identified (Severi et al.), the treatment course was carefully individualized, particularly with regard to pre-treatment toxicity risk stratification at the time of patient enrollment. Therapy individualization is a key factor also behind the dosimetric studies, whose aim was to optimise PRRT. Toxicity may also be influenced by factors unrelated to the patient, as well as the administration route and the radioligand type. For example, intra-arterial LuPRRT, was associated with high grade hematologic toxicity and one instance of transient high grade renal toxicity, as reported by Amerein. Similarly, Eigler and the PROMENADE group documented relevant hematological toxicities in patients treated with LuPRRT and Lu-DOTA-JR11.
Regarding efficacy, the DCR varied considerably across studies but remained generally encouraging. Evidence also points to potential synergistic benefits when PRRT is combined with EBRT, suggesting opportunities for multimodal strategies, as explored by Hartrampf. In addition to clinical and imaging data, preclinical insights were provided to evaluate meningioma response to PRRT and EBRT, as the 3D spheroid culture model by Reuvers, demonstrating a potential synergy between the two therapies.
CASP analyses confirm methodological rigor. Most reviewed studies clearly defined their research questions, with appropriate study designs and data collection methods.
Clinical trials also tend to focus primarily on already known compounds, with the exception of [177Lu]Lu Satoreotide, of [67 Cu]Cu-SARTATE™. Finally, they evaluate the association of LuPRRT with everolimus and with olaparib, thereby suggesting the potential for combining PRRT with antitumor agents.
In gliomas, the landscape remains largely experimental and heterogeneous. Published papers show a more pioneering character, evident even upon preliminary examination, particularly in the study design. Notably, three studies reported data from early-phase trials, indicating that the clinical translation of preclinical data remains at an early stage. The radiopharmaceuticals used were newly tested in these clinical settings, such as RIT and alpha emitters. In the study focusing on RIT, the lack of consistent toxicity and encouraging data on efficacy were reported, but the most interesting aspects of the research concern the notable number of patients enrolled and the fact that recruitment spanned over 20 years. Regarding alpha emitters, treatments with 213 Bi and 225 Ac were proposed, through intratumoral injections. The advantages of this approach are numerous: First of all, a single injection of this radiopharmaceutical was found to be sufficient to achieve long-lasting tumor control, even if closely spaced injections can be also proposed. Furthermore, the local administration and above all the very favorable dose range of alpha emitters have shown evidence of limiting local neurotoxicity and of minimizing the risk of other side effects. Even with the inherent limitation of a small sample size, the results exposed by Krolicki are striking, showing RFS of up to 16 years in astrocytomas and 24 years in oligodendrogliomas. As the massive pre-treatments (radiotherapy, chemotherapy or PRRT) may increase the risk of secondary toxicity, especially in cases with pre-existing neurological deficits, the authors suggest to anticipate TAT and to use it in neo-adjuvant settings.
Notably, data on toxicity suggest that it is either negligible or limited to low grades, regardless of the radiopharmaceutical used. This holds true despite the limitations related to the small number of patients included in several of the studies reviewed. Similar numerical limitations apply to efficacy, although the data still suggest a potential for disease control.
Within the glioma literature, several aspects—most notably follow-up—remain insufficiently standardized and clearly defined. However, the reported CASP scores reflected an overall reviewers’ positive appraisal of the studies.
The innovative, pioneering character of this approach—predominantly oriented toward RLT rather than PRRT in gliomas—is underscored by the analysis of clinical trials, given that they:
-
are all prospective and phase 1 or pilot study.
-
tend to focus primarily on exploratory radiopharmaceuticals, or.
-
rely on the use of established radiopharmaceuticals which, however, are usually employed in the treatment of other malignancies.
Limitations
This review has some limitations.
First, despite the use of targeted keywords, a number of non-relevant articles still need to be excluded. Major scientific databases remain an essential resource, but they have certain limitations. These include automatic or partial indexing, semantic ambiguity of search terms, the lack of controlled vocabularies (e.g., MeSH, which are not implemented in Scopus and Web of Science), and the retrieval of articles that mention keywords only marginally, without substantively addressing the topic of interest. Notably, a marked difference emerged between studies on meningiomas and those on gliomas: Only 10.2% of the initially assessed meningioma studies met the inclusion criteria, compared with just 4.6% of glioma studies.
Second, remaining within the context of numerical differences, the uneven distribution of the studies—22 on meningiomas versus eight on gliomas—represents a limitation which could have had some impact on the overall balance and completeness of the evidence synthesis.
Finally, the heterogeneity of some parameters, such as study designs, radiopharmaceuticals, endpoints, times of follow-up, and patient populations, which limits the comparability across studies and precludes the possibility of performing a meta-analysis or draw definitive clinical recommendations.
Conclusion
Overall, RLT emerges as a promising therapeutic approach in neuro-oncology. Its theranostic paradigm represents a distinctive advantage, enabling patient selection, treatment personalization and response monitoring. However, it is important to note that the current body of evidence is limited by the heterogeneity and small size of the studies reviewed, which significantly hampers the ability to generalize the findings. Therefore, future research should prioritize well-designed prospective and multicenter trials, standardized response criteria, and the exploration of novel radiopharmaceuticals (e.g., somatostatin receptor antagonists, PSMA ligands, and alpha-emitters). Such efforts may pave the way for future therapeutic applications in these challenging diseases.
Statements
Author contributions
IG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Software. MS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing – review & editing, Visualization. FM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing, Resources, Visualization. IM: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – review & editing. PC: Conceptualization, Data curation, Funding acquisition, Investigation, Validation, Writing – review & editing. MC: Data curation, Formal analysis, Investigation, Software, Validation, Writing – review & editing. LF: Investigation, Validation, Visualization, Writing – review & editing. VR: Investigation, Validation, Visualization, Writing – review & editing. LG: Investigation, Validation, Visualization, Writing – review & editing. NR: Investigation, Validation, Visualization, Writing – review & editing. AR: Investigation, Validation, Visualization, Writing – review & editing. IB: Investigation, Validation, Visualization, Writing – review & editing. VD: Investigation, Validation, Visualization, Writing – review & editing. AS: Investigation, Validation, Visualization, Writing – review & editing. DA: Investigation, Validation, Visualization, Writing – review & editing. SN: Data curation, Investigation, Validation, Visualization, Writing – review & editing, Conceptualization, Methodology.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
The authors would like to thank all the staff of the Nuclear Medicine Department, as well as the colleagues of the Neuro-Oncology multidisciplinary group.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FM declars that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Louis DN Perry A Wesseling P Brat DJ Cree IA Figarella-Branger D et al . The 2021 WHO classification of Tumors of the central nervous system: a summary. Neuro-Oncology. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106,
2.
Severi S Grassi I Bongiovanni A Nicolini S Marini I Arpa D et al . Peptide receptor radionuclide therapy in advanced refractory meningiomas: efficacy and toxicity in a long follow-up. J Nucl Med. (2024) 65:1409–15. doi: 10.2967/jnumed.123.266956,
3.
Goldbrunner R Stavrinou P Jenkinson MD Sahm F Mawrin C Weber DC et al . EANO guideline on the diagnosis and management of meningiomas. Neuro-oncol. (2021) 23:1821–34. doi: 10.1093/neuonc/noab150,
4.
Osborn AG Louis DN Poussaint TY Linscott LL Salzman KL . The 2021 World Health Organization classification of tumors of the central nervous system: what neuroradiologists need to know. AJNR. (2022) 43:928–37. doi: 10.3174/ajnr.A7462,
5.
Perkins A Liu G . Primary brain Tumors in adults: diagnosis and treatment. Am Fam Physician. (2016) 93:211–7.
6.
Tolboom N Verger A Albert NL Fraioli F Guedj E Traub-Weidinger T et al . Theranostics in Neurooncology: heading toward new horizons. J Nucl Med. (2024) 65:167–73. doi: 10.2967/jnumed.123.266205,
7.
Ostrom QT Gittleman H Liao P Vecchione-Koval T Wolinsky Y Kruchko C et al . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology. (2017) 19:v1–v88. doi: 10.1093/neuonc/nox158,
8.
Dutour A Kumar U Panetta R Ouafik L'H Fina F Sasi R et al . Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. (1998) 76:620–7. doi: 10.1002/(SICI)1097-0215(19980529)76:5<620::AID-IJC2>3.0.CO;2-S,
9.
Bodei L Mueller-Brand J Baum RP Pavel ME Hörsch D O'Dorisio MS et al . The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2013) 40:800–16. doi: 10.1007/s00259-012-2330-6. Erratum in: Eur J Nucl Med Mol Imaging. 2014; 41(3):584. O'Dorisiol, T M [corrected to O’Dorisio, T M],
10.
Raghavan R Howell RW Zalutsky MR . A model for optimizing delivery of targeted radionuclide therapies into resection cavity margins for the treatment of primary brain cancers. Biomed Phys Eng Express. (2017) 3:035005. doi: 10.1088/2057-1976/aa6db9,
11.
Reubi JC Mäcke HR Krenning EP . Candidates for peptide receptor radiotherapy today and in the future. J Nucl Med. (2005) 46:67S–75S.
12.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001,
13.
Minutoli F Amato E Sindoni A Cardile D Conti A Herberg A et al . Peptide receptor radionuclide therapy in patients with inoperable meningiomas: our experience and review of the literature. Cancer Biother Radiopharm. (2014) 29:193–9. doi: 10.1089/cbr.2013.1599,
14.
Kreissl MC Hänscheid H Löhr M Verburg FA Schiller M Lassmann M et al . Combination of peptide receptor radionuclide therapy with fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Radiat Oncol. (2012) 7:99. doi: 10.1186/1748-717X-7-99,
15.
Kertels O Breun M Hänscheid H Kircher M Hartrampf PE Schirbel A et al . Peptide receptor radionuclide therapy in patients with Neurofibromatosis type 2: initial experience. Clin Nucl Med. (2021) 46:e312–6. doi: 10.1097/RLU.0000000000003627,
16.
Vonken EPA Bruijnen RCG Snijders TJ Seute T Lam MGEH Keizer B et al . Intraarterial administration boosts 177Lu-HA-DOTATATE accumulation in salvage meningioma patients. J Nucl Med. (2022) 63:406–9. doi: 10.2967/jnumed.121.262491,
17.
Amerein A Maurer C Kircher M Gäble A Krebold A Rinscheid A et al . Intraarterial Administration of Peptide Receptor Radionuclide Therapy in patients with advanced meningioma: initial safety and efficacy. J Nucl Med. (2024) 65:jnumed.124.268217–1916. doi: 10.2967/jnumed.124.268217,
18.
Eigler C McDougall L Bauman A Bernhardt P Hentschel M Blackham KA et al . Radiolabeled somatostatin receptor antagonist versus agonist for peptide receptor radionuclide therapy in patients with therapy-resistant meningioma: PROMENADE phase 0 study. J Nucl Med. (2024) 65:573–9. doi: 10.2967/jnumed.123.266817,
19.
Gerster-Gilliéron K Forrer F Maecke H Mueller-Brand J Merlo A Cordier D . 90Y-DOTATOC as a therapeutic option for complex recurrent or progressive meningiomas. J Nucl Med. (2015) 56:1748–51. doi: 10.2967/jnumed.115.155853,
20.
Bartolomei M Bodei L De Cicco C Grana CM Cremonesi M Botteri E et al . Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging. (2009) 36:1407–16. doi: 10.1007/s00259-009-1115-z,
21.
Marincek N Radojewski P Dumont RA Brunner P Müller-Brand J Maecke HR et al . Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med. (2015) 56:171–6. doi: 10.2967/jnumed.114.147256,
22.
van Essen M Krenning EP Kooij PP Bakker WH Feelders RA de Herder WW et al . Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. (2006) 47:1599–606.
23.
Hartrampf PE Hänscheid H Kertels O Schirbel A Kreissl MC Flentje M et al . Long-term results of multimodal peptide receptor radionuclide therapy and fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Clin Transl Radiat Oncol. (2020) 22:29–32. doi: 10.1016/j.ctro.2020.03.002,
24.
Seystahl K Stoecklein V Schüller U Rushing E Nicolas G Schäfer N et al . Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro-Oncology. (2016) 18:1538–47. doi: 10.1093/neuonc/now060,
25.
Puranik AD Dev ID Rangarajan V Kulkarni S Shetty N Gala K et al . PRRT with Lu-177 DOTATATE in treatment-refractory progressive meningioma: initial experience from a tertiary-care neuro-oncology Center. Neurol India. (2024) 72:278–84. doi: 10.4103/NI.Neurol-India-D-23-00252,
26.
van den Bent MJ Vogelbaum MA Cloughesy T Galldiks N Albert NL Tonn JC et al . Response Assessment in Neuro-Oncology (RANO) 2009-2025: Broad scope and implementation - a Progress report. Neuro Oncol 2025:noaf118. (2025) 27:2209–24. doi: 10.1093/neuonc/noaf118,
27.
Green S Weiss GR . Southwest oncology group standard response criteria, endpoint definitions and toxicity criteria. Investig New Drugs. (1992) 10:239–53. doi: 10.1007/BF00944177,
28.
Eisenhauer EA Therasse P Bogaerts J Schwartz LH Sargent D Ford R et al . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026,
29.
Hänscheid H Lapa C Buck AK Lassmann M Werner RA . Dose mapping after Endoradiotherapy with 177Lu-DOTATATE/DOTATOC by a single measurement after 4 days. J Nucl Med. (2018) 59:75–81. doi: 10.2967/jnumed.117.193706,
30.
Kletting P Kull T Maaß C Malik N Luster M Beer AJ et al . Optimized peptide amount and activity for 90Y-Labeled DOTATATE therapy. J Nucl Med. (2016) 57:503–8. doi: 10.2967/jnumed.115.164699,
31.
Dubois J Tosato G Garrigue P Taieb D Guillet B Nail V . Short-term biological toxicity prediction of [177Lu]lutetium-Oxodotreotide: an original retrospective analysis. Cancer Biother Radiopharm. (2024) 39:381–9. doi: 10.1089/cbr.2023.0195,
32.
Salgues B Graillon T Horowitz T Chinot O Padovani L Taïeb D et al . Somatostatin receptor Theranostics for refractory meningiomas. Curr Oncol. (2022) 29:5550–65. doi: 10.3390/curroncol29080438,
33.
Graillon T Ferrer L Siffre J Sanson M Peyre M Peyrière H et al . Role of 3D volume growth rate for drug activity evaluation in meningioma clinical trials: the example of the CEVOREM study. Neuro-Oncology. (2021) 23:1139–47. doi: 10.1093/neuonc/noab019,
34.
Graillon T Salgues B Horowitz T Padovani L Appay R Tabouret E et al . Peptide radionuclide radiation therapy with Lutathera in multirecurrent nonanaplastic meningiomas: antitumoral activity study by growth rate analysis. J Neuro-Oncol. (2024) 167:427–36. doi: 10.1007/s11060-024-04622-5,
35.
Reuvers TGA Grandia V Brandt RMC Arab M Maas SLN Bos EM et al . Investigating the radiobiological response to peptide receptor radionuclide therapy using patient-derived meningioma spheroids. Cancers (Basel). (2024) 16:2515. doi: 10.3390/cancers16142515,
36.
Minczeles NS Bos EM de Leeuw RC Kros JM Konijnenberg MW Bromberg JEC et al . Efficacy and safety of peptide receptor radionuclide therapy with [177Lu]Lu-DOTA-TATE in 15 patients with progressive treatment-refractory meningioma. Eur J Nucl Med Mol Imaging. (2023) 50:1195–204. doi: 10.1007/s00259-022-06044-9,
37.
Hänscheid H Sweeney RA Flentje M Buck AK Löhr M Samnick S et al . PET SUV correlates with radionuclide uptake in peptide receptor therapy in meningioma. Eur J Nucl Med Mol Imaging. (2012) 39:1284–8. doi: 10.1007/s00259-012-2124-x,
38.
Heute D Kostron H von Guggenberg E Ingorokva S Gabriel M Dobrozemsky G et al . Response of recurrent high-grade glioma to treatment with (90)Y-DOTATOC. J Nucl Med. (2010) 51:397–400. doi: 10.2967/jnumed.109.072819,
39.
Nemati R Shooli H Rekabpour SJ Ahmadzadehfar H Jafari E Ravanbod MR et al . Feasibility and therapeutic potential of peptide receptor radionuclide therapy for high-grade gliomas. Clin Nucl Med. (2021) 46:389–95. doi: 10.1097/RLU.0000000000003599,
40.
Truckenmueller P Graef J Scheel M Vajkoczy P Capper D Kaul D et al . [68Ga]ga-PSMA PET/MRI, histological PSMA expression and preliminary experience with [177Lu]Lu-PSMA therapy in relapsing high-grade glioma. Front Oncol. (2022) 12:980058. doi: 10.3389/fonc.2022.980058
41.
Li L Quang TS Gracely EJ Kim JH Emrich JG Yaeger TE et al . A phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg. (2010) 113:192–8. doi: 10.3171/2010.2.JNS091211,
42.
Cordier D Forrer F Kneifel S Sailer M Mariani L Mäcke H et al . Neoadjuvant targeting of glioblastoma multiforme with radiolabeled DOTAGA-substance P--results from a phase I study. J Neuro-Oncol. (2010) 100:129–36. doi: 10.1007/s11060-010-0153-5
43.
Cordier D Forrer F Bruchertseifer F Morgenstern A Apostolidis C Good S et al . Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8,met(O2)11]-substance P: a pilot trial. Eur J Nucl Med Mol Imaging. (2010) 37:1335–44. doi: 10.1007/s00259-010-1385-5
44.
Kneifel S Bernhardt P Uusijärvi H Good S Plasswilm L Buitrago-Téllez C et al . Individual voxelwise dosimetry of targeted 90Y-labelled substance P radiotherapy for malignant gliomas. Eur J Nucl Med Mol Imaging. (2007) 34:1388–95. doi: 10.1007/s00259-006-0351-8,
45.
Krolicki L Kunikowska J Cordier D Slavova N Koziara H Bruchertseifer F et al . Long-term tumor control following targeted alpha therapy (TAT) of low-grade gliomas (LGGs): a new treatment paradigm?Int J Mol Sci. (2023) 24:15701. doi: 10.3390/ijms242115701,
46.
Holzgreve A Biczok A Ruf VC Liesche-Starnecker F Steiger K Kirchner MA et al . PSMA expression in glioblastoma as a basis for theranostic approaches: a retrospective, correlational panel study including immunohistochemistry, clinical parameters and PET imaging. Front Oncol. (2021) 11:861. doi: 10.3389/fonc.2021.646387
47.
Gao Y Zheng H Li L Feng M Chen X Hao B et al . Prostate-specific membrane antigen (PSMA) promotes angiogenesis of glioblastoma through interacting with ITGB4 and regulating NF-kB signaling pathway. Front Cell Dev Biol. (2021) 9:462. doi: 10.3389/fcell.2021.598377,
48.
Nomura N Pastorino S Jiang P Lambert G Crawford JR Gymnopoulos M et al . Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. (2014) 14:1–9. doi: 10.1186/1475-2867-14-26
49.
Gill S Peston D Vonderhaar B Shousha S . Expression of prolactin receptors in normal, benign, and malignant breast tissue: an immunohistological study. J Clin Pathol. (2001) 54:956–60. doi: 10.1136/jcp.54.12.956,
50.
Urso L Bonatto E Nieri A Castello A Maffione AM Marzola MC et al . The role of molecular imaging in patients with brain metastases: a literature review. Cancers (Basel). (2023) 15:2184. doi: 10.3390/cancers15072184,
51.
Schürrle SB Eberlein U Ansquer C Beauregard JM Durand-Gasselin L Grønbæk H et al . Dosimetry and pharmacokinetics of [177Lu]Lu-satoreotide tetraxetan in patients with progressive neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2024) 51:2428–41. doi: 10.1007/s00259-024-06682-1,
52.
Wild D Grønbæk H Navalkissoor S Haug A Nicolas GP Pais B et al . A phase I/II study of the safety and efficacy of [177Lu]Lu-satoreotide tetraxetan in advanced somatostatin receptor-positive neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2023) 51:183–95. doi: 10.1007/s00259-023-06383-1,
53.
Dearling JLJ van Dam EM Harris MJ Packard AB . Detection and therapy of neuroblastoma minimal residual disease using [64/67Cu]cu-SARTATE in a preclinical model of hepatic metastases. EJNMMI Res. (2021) 11:20. doi: 10.1186/s13550-021-00763-0,
54.
Hicks RJ Jackson P Kong G Ware RE Hofman MS Pattison DA et al . 64Cu-SARTATE PET imaging of patients with neuroendocrine Tumors demonstrates high tumor uptake and retention, potentially allowing prospective dosimetry for peptide receptor radionuclide therapy. J Nucl Med. (2019) 60:777–85. doi: 10.2967/jnumed.118.217745,
55.
Lam M Salem R Toskich B Kappadath SC Chiesa C Fowers K et al . Clinical and dosimetric considerations for yttrium-90 glass microspheres radioembolization of intrahepatic cholangiocarcinoma, metastatic colorectal carcinoma, and metastatic neuroendocrine carcinoma: recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging. (2025) 52:3820–32. doi: 10.1007/s00259-025-07229-8,
56.
Bushnell D O'Dorisio T Menda Y Carlisle T Zehr P Connolly M et al . Evaluating the clinical effectiveness of 90Y-SMT 487 in patients with neuroendocrine tumors. J Nucl Med. (2003) 44:1556–60.
57.
Smith-Jones PM . Radioimmunotherapy of prostate cancer. Q J Nucl Med Mol Imaging. (2004) 48:297–304.
58.
Fiedler L Kellner M Gosewisch A Oos R Böning G Lindner S et al . Evalu- ation of (177)Lu[Lu]-CHX-A″-DTPA-6A10 fab as a radioimmunotherapy agent targeting carbonic anhydrase XII. Nucl Med Biol. (2018) 60:55–62. doi: 10.1016/j.nucmedbio.2018.02.004,
59.
Ruigrok EAM Verhoeven M Konijnenberg MW de Blois E de Ridder CMA Stuurman DC et al . Safety of [177Lu]Lu-NeoB treatment: a preclinical study characterizing absorbed dose and acute, early, and late organ toxicity. Eur J Nucl Med Mol Imaging. (2022) 49:4440–51. doi: 10.1007/s00259-022-05926-2,
60.
Ramos-Álvarez I Moreno P Mantey SA Nakamura T Nuche-Berenguer B Moody TW et al . Insights into bombesin receptors and ligands: highlighting recent advances. Peptides. (2015) 72:128–44. doi: 10.1016/j.peptides.2015.04.026,
Summary
Keywords
meningioma peptide receptor radionuclide therapy/PRRT, meningioma radioligand therapy/meningioma RLT, meningioma 90Y/90Y DOTA, meningioma 177Lu/177Lu DOTA, glioma peptide receptor radionuclide therapy, glioma 90Y/90Y DOTA, glioma 177Lu/177Lu DOTA, peptide receptor radionuclide therapy/PRRT
Citation
Grassi I, Sansovini M, Matteucci F, Marini I, Caroli P, Celli M, Fantini L, Rossetti V, Gurrieri L, Riva N, Rossi A, Bronico I, Iorio VD, Sarnelli A, Arpa D and Nicolini S (2026) Radioligand therapy for primary brain tumors: a PRISMA-based systematic review of meningiomas and gliomas. Front. Med. 12:1728797. doi: 10.3389/fmed.2025.1728797
Received
20 October 2025
Revised
21 November 2025
Accepted
25 November 2025
Published
23 January 2026
Volume
12 - 2025
Edited by
Domenico Albano, University of Brescia, Italy
Reviewed by
Angelo Castello, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
Friedrich Weitzer, Medical University of Graz, Austria
Updates
Copyright
© 2026 Grassi, Sansovini, Matteucci, Marini, Caroli, Celli, Fantini, Rossetti, Gurrieri, Riva, Rossi, Bronico, Iorio, Sarnelli, Arpa and Nicolini.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irene Marini, irene.marini@irst.emr.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.