Abstract
A sustained imbalance between excitatory and inhibitory mechanisms within the glutamatergic and GABAergic systems of the cerebral cortex, induced by noxious stimuli, is a fundamental characteristic in the development and maintenance of chronic pain. This review provides a comprehensive summary of the roles and interaction of glutamatergic and GABAergic systems in the processing of chronic pain signals. Specifically, we present a systematic summary of the processing patterns of the cerebral cortex in the cross-modular integration and output of chronic pain information, according to four aspects, molecular, cellular, neural network and behavioral cognition. These patterns consist of neuronal responses in individual cortical regions, neuron-astrocyte interactions, sharing and cascading of inter-cortical signals, and downward cortical modulation. Furthermore, a number of potential therapeutic approaches to the chronic pain are discussed from the pain management perspective.
1 Introduction
The cerebral cortex, the highest level of central nervous system (CNS), governs sensory-motor functions and emotional responses. Its neural networks excitability is regulated by two key systems: the glutamatergic system and the gamma-aminobutyric acid (GABA) system (Mazo et al., 2022; Wu et al., 2023; Ganguly et al., 2001; Kami et al., 2022; Petroff, 2002; Bell et al., 2021). The glutamatergic system directly encode sensory information and reshapes cortical nerve fiber projections (Tan et al., 2020; Waqas et al., 2018; Cui et al., 2022), whereas the GABAergic system modulates excitatory inputs and optimizes the representation and processing of supra-modal information (Ferguson and Gao, 2018; Tremblay et al., 2016). While the GABAergic system modulates excitatory input and refines the processing of external stimuli, thereby maintaining accurate sensory perception (Tan et al., 2020). The dynamic fluctuations of these systems can, to some extent, reflect the current excitability state of the brain.
Continuous exposure to noxious stimuli can lead to a dysregulation of the balance between excitatory glutamatergic and inhibitory GABAergic systems in the cerebral cortex, which is progressively exacerbated. This impairs normal cortical excitability and sensory output of the noxious sensory network, resulting in neurological network instability, aberrant information transmission (Chen et al., 2022; Marcello et al., 2013; Eto et al., 2012; Chang et al., 2014; Benson et al., 2015) and abnormal nociception (Pigott et al., 2023). Meanwhile, cortical circuits undergo functional modifications and a reduction in their capacity for corrective action (Cheriyan and Sheets, 2020), which ultimately results in an enhanced processing of chronic pain (Pigott et al., 2023; Watson, 2016; Mecca et al., 2021; Zhang et al., 2015; Peek et al., 2020; Niddam et al., 2021; Kang et al., 2021). This process also evoke negative emotions, which interactively exacerbate chronic pain (Song et al., 2024; Li et al., 2023). A cerebral excitation-inhibition (E/I) imbalance is considered a significant factor in the development of pain (Eto et al., 2012; Watson, 2016; Cao et al., 2019), as well as a typical feature of the transition from acute to chronic pain (Bell et al., 2021; Pigott et al., 2023; Peek et al., 2020; Wang et al., 2022; Zunhammer et al., 2016) and the long-term maintenance of chronic pain (Zunhammer et al., 2016; Thiaucourt et al., 2018). This imbalance is also a critical factor in the development and maintenance of central sensitization of the brain (Watson, 2016; Raja et al., 2020; Lyu et al., 2021; Bhatt et al., 2023; Li et al., 2019). Furthermore, there is a positive correlation between the ratio of glutamate to GABA and pain sensitization (Zunhammer et al., 2016; Thiaucourt et al., 2018).
The extant review literature focuses on individual systems, drawing systematic conclusions about for dysregulation of the glutamatergic system (Temmermand et al., 2022; Zanetti et al., 2021; Yang and Chang, 2019; Ozawa et al., 1998) or the GABAergic system (Li et al., 2019; Qian et al., 2023; Fischer, 2017; Comitato and Bardoni, 2021) in chronic pain. Furthermore, it addresses distinctive pain treatment modalities that target the systems and mechanisms that may be associated with the onset or maintenance of chronic pain. Moreover, some literatures have reported the progressive functional and structural changes in the brain that occur in individuals with chronic pain (Yang and Chang, 2019) and changes involve different neuronal types and complex neural networks that process pain information (Kuner and Kuner, 2021; Tan and Kuner, 2021; Mercer Lindsay et al., 2021). However, there is a lack of systematic knowledge regarding temporal dynamics in the E/I balance and the dynamic processing of pain information in the cerebral cortex at the level of the glutamatergic and GABAergic dual systems.
Therefore, this review considers the glutamatergic and GABAergic systems, which are vital for sustaining the typical E/I equilibrium of the brain, as a foundational focus. It exhibits a detailed analysis of the chronological changes in interaction between glutamatergic and GABAergic systems in pain and the processing patterns of pain information for the cerebral cortex, involving various dimensions of chronic pain. The objective is to achieve a systematic understanding of the role of the cerebral cortex in the cross-modular integration and output of chronic pain information at four levels, namely molecular, cellular, neural network and behavioral cognition. Moreover, therapeutic strategies targeting various elements of the glutamatergic and GABAergic systems are classified to facilitate new perspectives into pain treatment.
2 Cortical processing of pain-related information
Pain is a multidimensional sensory experience involving sensory, cognitive, and affective components (Raja et al., 2020). Research focusing on the spinal cord, as the site for receiving, integrating, and gatekeeping primary pain signals, is insufficient to fully elucidate the mechanisms underlying the development and persistence of chronic pain, or to account for its cognitive and affective components (Comitato and Bardoni, 2021; Rankin et al., 2024; Kiani et al., 2024; Zheng et al., 2020). The cortex integrates incoming nociceptive stimuli, extracts key pain features and re-encodes this information (Ziegler et al., 2023; Ishikawa et al., 2023; Guo et al., 2023; Xiao et al., 2023; Gan et al., 2022; Li et al., 2022; Hu et al., 2019; Shao et al., 2023). It then mobilizes hierarchically organized neuronal projections to diverse brain regions (Li et al., 2023; Guo et al., 2023; Li et al., 2022; Chen et al., 2024; Hu et al., 2021; Smith et al., 2021; Cardoso-Cruz et al., 2019; Garcia-Larrea and Peyron, 2013), engaging in bidirectional interactions with glial cells (Kim et al., 2016; Zhou et al., 2025). This dynamic process establishes a cortex-centric pain modulation network. This network governs the propagation and feedback of pain-related information across the brain, orchestrates responses to the multifaceted components of pain, and ultimately drives adaptive changes in behavior, cognition, and emotional state (Gan et al., 2022; Li et al., 2022; Martins et al., 2015; Falconi-Sobrinho et al., 2017; Singh et al., 2020; Buetfering et al., 2022).
Furthermore, sustained nociceptive input progressively worsens the E/I imbalance between glutamatergic and GABAergic systems within the cerebral cortex, leading to functional reorganization (Sanganahalli et al., 2021; Baliki et al., 2012; Obermann et al., 2013). This reorganization is characterized by a spatiotemporal shift in brain activity, transitioning from sensory structures toward anterior emotional and limbic regions (Baliki et al., 2012; Park et al., 2022; Li et al., 2025). Substantial evidence indicates this renders neural networks more susceptible to persistent activation across all pain dimensions (Sanganahalli et al., 2021). Consequently, this elevated and prolonged network activity underlies the prolongation of pain duration, maintenance of the pain state, and the development of associated emotional and cognitive impairments and motor dysfunction (Weizman et al., 2018), thereby promoting pain catastrophizing (Gnall et al., 2025; Korkut, 2025; Jee et al., 2023). Critically, the degree of E/I imbalance and its associated shift in the cortex may reflect the brain’s integrated representation of the perceived intensity of the ongoing chronic pain state, concurrent emotional alterations, prognostic predictions, and the processing mechanisms underlying chronic pain itself (Baliki et al., 2012).
3 Brain regions exhibiting time-varying E/I balance and divergent neural response
Persistent nociceptive input directly targets cortical regions responsible for pain perception and emotional processing. This induces a sustained E/I imbalance, triggering dysregulated of neuronal gene expression, aberrant receptor signaling, and disruption of downstream transduction pathways. Consequently, maladaptive synaptic plasticity develops, which disrupts normal sensory encoding and leads to pathological processing of nociceptive signals. These alterations result in impaired discrimination of noxious stimuli and distorted pain perception, while simultaneously generating negative affective states (as illustrated in Figure 1).
Figure 1

The dynamic changes of glutamatergic and GABAergic system. In physiological state, the two systems work together in response to external stimuli, in part as a function of the current state of arousal. In chronic pain state, prolonged exposure to noxious stimuli can induce sustained excitation of the glutamatergic system in the cerebral cortex. The excitability of the GABAergic system changes from increased to decreased to compensate for the abnormal change in excitability of the CNS. However, this is not sufficient to restore normal homeostasis.
The glutamatergic system plays a dominant role on the initial processing of pain. Noxious stimuli enhance glutamatergic transmission (Cseh et al., 2020), leading to a reflexive increase in glutamate levels (Watson, 2016; Wu et al., 2008; Guida et al., 2015). Concurrently, changes occur in the structure and function of glutamate receptors, such as N-methyl-D-aspartate receptors (NMDARs) (Mao et al., 2022; Zugaib et al., 2014; Fan et al., 2018; Sun et al., 2016; Hogrefe et al., 2022; Ren et al., 2022; López-Avila et al., 2004), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (Sun et al., 2016; Ren et al., 2022; Chen et al., 2014; Liu et al., 2015) and metabotropic glutamate receptors (mGluRs) (Cao et al., 2019; Kim et al., 2016; Ji and Neugebauer, 2011; Notartomaso et al., 2024), inducing synaptic plasticity. This plasticity manifests as alterations in the induction of long-term potentiation (LTP) (Tullis et al., 2023) or the suppression of long-term depression (LTD) (Liu W. et al., 2024). Collectively, these changes result in functional hyperactivity of cortical glutamatergic neurons and widespread neuronal excitation. Evidence shows that following nerve ligation or complete Freund’s adjuvant (CFA) injection, glutamatergic neurons in the secondary somatosensory cortex (S2) (Guo et al., 2023), anterior cingulate cortex (ACC) (Zhang et al., 2017), insular cortex (IC) (Zhang et al., 2023), and prelimbic cortex (PL) (Ma L. et al., 2024) exhibit hyperactivity. This hyperactivity contributes to an imbalance in E/I dysfunction, an increased sensitivity to pain (Wu et al., 2008; Chen et al., 2014; Tullis et al., 2023) and an enhanced aversion behavior (Zhang et al., 2017). Furthermore, excessive excitation of glutamatergic neurons in these pain-processing regions can inhibit glutamatergic neurons in other brain areas (Thompson and Neugebauer, 2019; Ji et al., 2010), thereby prioritizing the perception and transmission of nociceptive signals within the brain.
The modulation of GABAergic system dynamically shifts during chronic pain progression, evolving from an adaptive inhibitory state to a maladaptive disruption of e E/I balance. The GABAergic system acts to counteract neuronal excitation. Co-activated alongside the glutamatergic system, GABAergic activity serves as a marker for persistent changes in pain perception. Painful stimuli have been observed to stimulate GABA release (Jasmin et al., 2003; Peek et al., 2021), enhance GABAA receptor activation via mGluR1 (Ji and Neugebauer, 2011) and promote GluD1 binding—an iGluR family member—with GABA (Piot et al., 2023). Consequently, GABAergic neurons are activated, partially correcting the E/I imbalance (Eto et al., 2012; Zhang et al., 2023). However, the effect of enhanced inhibition is proves insufficient to fully counterbalance the increased glutamatergic excitation and alleviate chronic pain symptoms (Eto et al., 2012; Cao et al., 2019; Zhang et al., 2023). Paradoxically, pain itself can directly hyperactivate the GABAergic system, triggering feedforward inhibition that reduces output neuronal activity, thereby promoting pain persistence (Zhang et al., 2015; Kim et al., 2024). Conversely, when pain becomes sustained or intensifies, the GABAergic system becomes dysregulated. This leads to a further breakdown of E/I balance, facilitating the development of persistent pain sensitization and the emergence of diverse complex pain-related behavioral comorbidities.
Furthermore, a disrupted collapse of the GABAergic system occurs in the absence of the removal of exogenous or endogenous stimuli, resulting in a malignant E/I imbalance, persistent pain sensitization and the emergence of a variety of complex pain-related behaviors. It has identified that inhibitory synaptic deficits in hippocampus (HPC) induces by the increase of α5-containing GABAA receptors (Cai et al., 2022) or GABA release (Saffarpour et al., 2017) mediate cognitive impairment associated with pain. A reduction of the inhibitory synaptic transmission resulting from the decrease in GABA release and expression of vesicular GABA transporter, although the GABA transporter-1 increasing in neuropathic pain (Masocha, 2015), can contribute to the development of pain and anxiety in the ACC in inflammatory pain (Shao et al., 2021; Koga et al., 2018). Interestingly, the addition of exogenous GABA can reverse abnormal excitability caused by paclitaxel-induced neuropathic pain in the ACC (Nashawi et al., 2016). Conversely, the over-release of GABA, induced by the loss function of CB1R in GABAergic neurons of the PL, can lead to depression (Mecca et al., 2021; Li et al., 2022).
Critically, pain responses exhibit significant heterogeneity even among neurons within a single brain region, underscoring the complexity of cortical pain processing. Distinct subregions and diverse subtypes of glutamatergic and GABAergic neurons within a given area respond differentially to pain signals. In the medial prefrontal cortex (mPFC) of mice, neuropathic injury increases the excitability of parvalbumin-expressing neurons in layer 5 of the PL subdivision (Jones and Sheets, 2020). Conversely, it reduces the excitability of somatostatin-expressing neurons in layers 2/3 (Jones and Sheets, 2020). Painful stimuli attenuate the excitability of glutamatergic neurons in the anterior but not in inferior limbic subregions of the mPFC (Cheriyan and Sheets, 2018). In the midcingulate cortex (MCC), the majority of glutamatergic neurons in zone 2 are inhibited by painful stimuli. Whereas zone 1, they are activated (Hu et al., 2019). Cortical responses to distinct types of pain stimuli also exhibit region-specific heterogeneity. Glutamatergic neurons in the ventral hippocampal CA1 (vCA1) subregion exhibit increased excitability in response to neuropathic pain (Kami et al., 2022), but conversely show reduced excitability following CFA-induced inflammatory pain (Shao et al., 2023). Concurrently, glutamatergic neurons in the dorsal hippocampus also demonstrate decreased excitability during inflammatory pain (Wei et al., 2021). Moreover, distinct subregions of a single brain region can mediate the information transmission of different components. The anterior parts of the IC preferentially mediate somatosensory features of pain, while the posterior parts are more involved in the affective features (Meng et al., 2022). Glutamatergic neurons in layers 5 and 6 of the primary motor cortex (M1) encode downstream projection information for the various components of pain (Gan et al., 2022).
4 Functional homeostatic of neuron-astrocyte interaction
It is clear that the neuron-astrocyte crosstalk is critical for maintaining the normal function of the nervous system. Astrocytes play a critical role in the regulation of synaptic gap homeostasis, which is instrumental in the perception and maintenance of central sensitivity to chronic pain (Ozawa et al., 1998; Kim et al., 2016; Lee et al., 2022). This is achieved by forming the tripartite synapse with neurons (Araque et al., 1999; Perea et al., 2009), involving in the glutamate-glutamine cycle, generating and regulating the release of glutamate (de Ceglia et al., 2023) and GABA (Koh et al., 2023; Vélez-Fort et al., 2012; Cheng et al., 2023) and their own activity in response to stimuli (Cahill et al., 2024). This interaction is disrupted in chronic pain, resulting in an over-excitation of neurons and a diminished inhibitory effect of astrocytes on neurons. This further exacerbates the imbalance between the excitation and inhibition in the brain, leading to disturbances in pain signaling and processing and excitatory neurotoxicity (Ozawa et al., 1998; Lee et al., 2022).
Astrocytes can influence neuronal activity through signaling mechanisms. Sustained nociceptive stimulation induces aberrant glutamate metabolism, which activates astrocytes to drive structural and functional alterations in neuronal synapses via tripartite synaptic connections. These changes provoke localized hyperexcitability within the somatosensory cortex and trigger circuit rewiring, ultimately disrupting pain-processing cortical networks and culminating in chronic pain pathogenesis (Kim et al., 2016; Kim et al., 2017; Danjo et al., 2022). Thus, cortical astrocyte activation both modulates nociceptive input and promotes synaptic plasticity changes, such as dendritic spine formation in the cortex. This process disrupts the maladaptive neural connections formed in the cortex during the transition from acute to chronic pain (Takeda et al., 2022). Notably, a similar phenomenon is observed in the ACC. Sustained noxious stimulation induces hyperactivation of astrocytes. This astrocytic hyperactivation promotes pain persistence and the emergence of anxiety-like behaviors by increasing synapse-related protein expression (Cardoso-Cruz et al., 2019), re-expressed mGluR5 (Shen et al., 2025) or upregulating the signal pathway for S100B (the astrocyte marker S100 calcium binding protein B)-RAGE (The receptor for advanced glycation end-products) (Jiang et al., 2025), thereby altering excitatory neuronal plasticity and enhancing neuronal activity via tripartite synapses. Intriguingly, astrocytes not only directly modulate E/I balance in local brain region (Wei N. et al., 2024), but also influence downstream pain-processing brain regions via neuronal circuit from the ventrolateral orbitofrontal cortex (vlOFC) to the ventrolateral periaqueductal gray (vlPAG) (Islam et al., 2025), thereby contributing to pain persistence.
In addition, astrocytes couple neural activity to energy metabolism. In the ACC, reduced glutamatergic metabolism and impaired glutamate reuptake induce NMDA spike generation in pyramidal neuron dendrites. This stimulates astrocyte activation and proliferation, promoting pain persistence and lowering pain thresholds (Takeda et al., 2022; Romanos et al., 2020). Furthermore, astrocytes can directly modulate pain hypersensitivity (Reid et al., 2025) and pain-related aversive behaviors (Iqbal et al., 2022) through the lactate shuttle. Visceral hypersensitivity can also trigger lactate release from ACC astrocytes, contributing to decision-making deficits comorbid with chronic visceral pain (Wang et al., 2017).
5 Sharing and cascading of inter-cortical signals
Noxious stimulus information is shared across the cerebral cortex and transmitted between sensory and affective centers, as well as between ipsilateral and contralateral brain regions. This intercortical communication mediates synchronized activation of local cortical circuits during pain processing. Importantly, these circuits integrate feedback inhibition and facilitation, undergoing recurrent cycles of signal amplification, cascading recruitment, and dynamic rebalancing. This reverberatory process ultimately promotes the transition to chronic pain (as illustrated in Figure 2). Ipsilateral stimuli can alter the activity of structures involved in pain modulation on the contralateral hemisphere. In individuals with chronic pain, the projection from the ipsilateral ACC (Li et al., 2023; Hu et al., 2021) to the contralateral side of the brain exhibit aberrant activation, which in turn induces primary and secondary nociceptive hypersensitivity (Garcia-Larrea and Peyron, 2013). This plays an important role in the development and maintenance of chronic pain and promotes peripheral and central sensitization, which suggests that the chronic pain state of the organism is more difficult to correct. Additionally, it impairs the feedback loops for the processing of injurious information and disrupts the E/I balance between the cerebral cortex. During chronic pain, the glutamatergic neurons in PL are sustainably activated by the glutamatergic neurons in the primary somatosensory cortex (S1) (Ma L. et al., 2024), the glutamatergic neurons in the ACC are inhibited by decreasing GABAergic interneurons in PL (Li et al., 2022), or the inhibition effect of GABAergic neurons in the Zona incerta (ZI) is reduces by MCC Cg2 glutamatergic neurons (Hu et al., 2019). This leads to loss of cortical–cortical inhibition and an exacerbated and spontaneous pain.
Figure 2
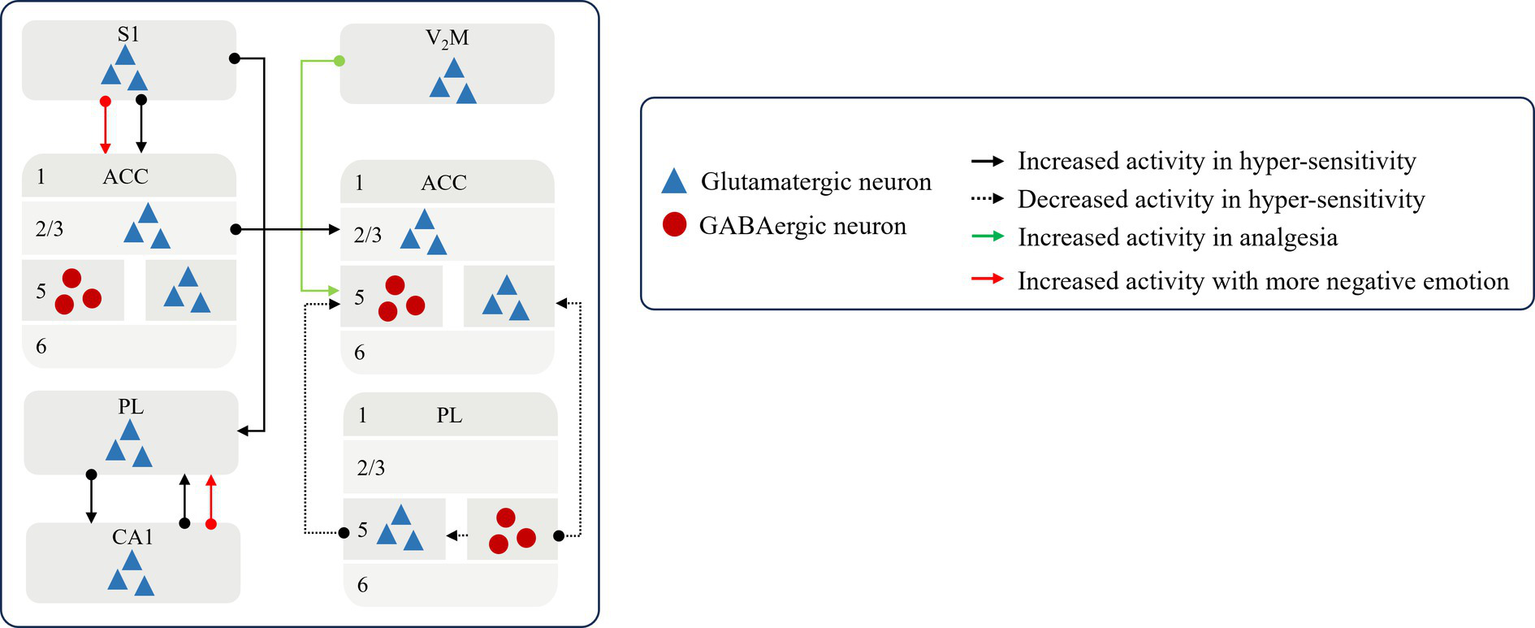
The pattern of sharing and cascading of inter-cortical signals Noxious stimulus signal is transmitted between sensory and affective centers to affect the state of painsensation and emotion. S1, the primary somatosensory cortex; V2M, the secondary visual cortex; ACC, anterior cingulate cortex; PL, prelimbic cortex; CA1.
In contrast, the restoration of a balanced excitation-inhibition equilibrium and information feedback within neural circuits between brain regions has been demonstrated to induce resistance to noxious stimuli and reinstate cortical inhibition. Activation of glutamatergic projections from the medial part of the secondary visual cortex (V2M) to GABAergic neurons in the ACC (Cao et al., 2023) and from the MCC Cg2 to GABAergic neurons in the ZI mitigate pain hypersensitivity, while transient activation of the latter also relieves aversive behaviors associated with spontaneous persistent pain (Hu et al., 2019).
Concurrently, the sensory and affective centers constantly engage in a continuous bidirectional interaction, resulting in the malignant crosstalk in pain sensation with emotion to affect individual’s coping style and emotional state. Enhanced connectivity from the primary somatosensory cortex (S1) to the anterior ACC has been observed, wherein S1 encodes sensory pain signals and the ACC processes the affective consequences of pain, thereby the aversions to nociceptive responses to enrich the negative experience of pain specificity in affective centers (Singh et al., 2020).
6 Downward cortical modulation
Higher central processing in the cerebral cortex converts noxious sensory signals into perceptual signals and input to downstream brain regions (shown in Figure 3). On the one hand, the cerebral cortex controls the flow of afferent sensory stimuli to the downward regions (Ong et al., 2019; Wu et al., 2020; Huo et al., 2005; Yin et al., 2020) by projecting sensory signals downstream to produce downstream elicitation or inhibition. An abnormally large number of perceptual signals are generated and delivered to the downstream analgesic system, whose function and homeostasis are disrupted, eventually resulting in the persistence of pain (Gan et al., 2022; Cheriyan and Sheets, 2018).
Figure 3
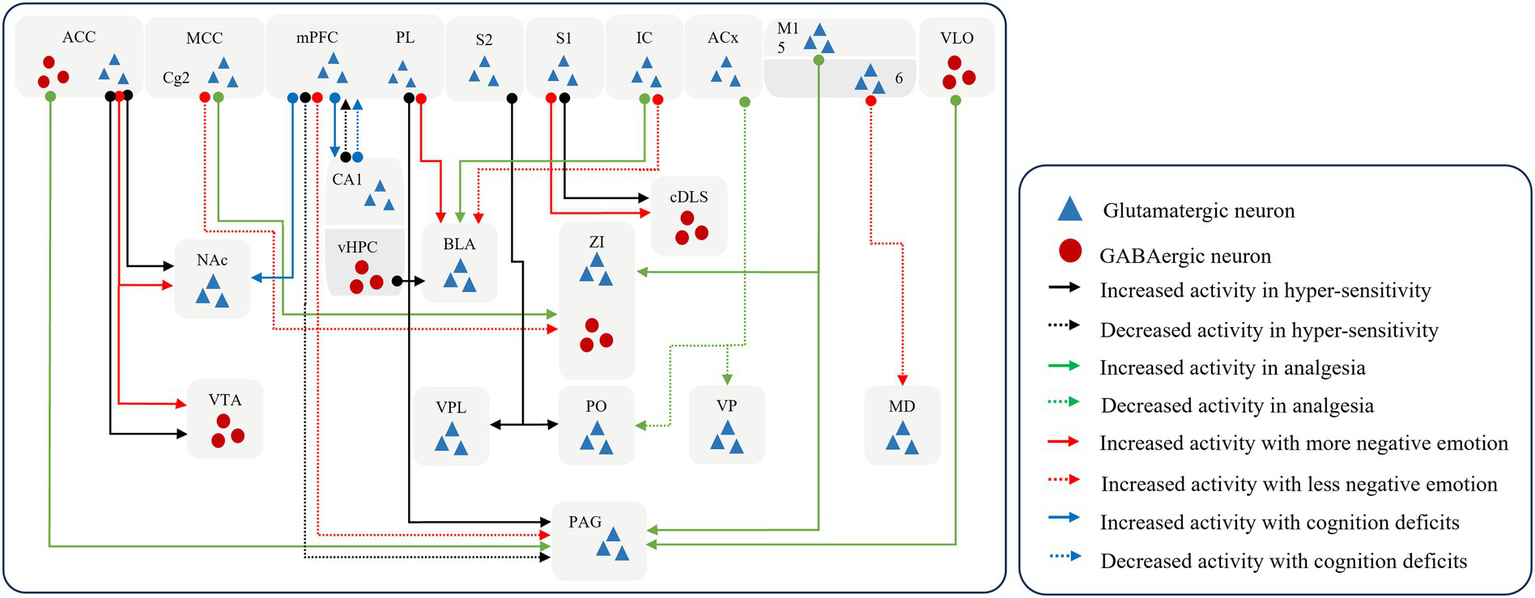
The pattern of downward cortical modulation. The cerebral cortex controls signal propagation by recognizing sensory signals and outputting perceptual signals. And prolonged injurious stimulation can remodel neural networks, leading the cortex to assign additional negative coding to stimulus signals, which in turn leads to the emergence of complex behaviors. S1, the primary somatosensory cortex; S2, the secondary somatosensory cortex; M1, the primary motor cortex; PL, prelimbic cortex; dmPFC, the dorsal medial prefrontal cortex; ACC, anterior cingulate cortex; MCC, the midcingulate cortex; IC, the insular cortex; ACx, the auditory cortex; VLO, the ventrolateral orbital cortex; vHPC, the ventral hippocampus; NAc, the nucleus accumbent; ZI, the zona incerta; VPL, the ventral posterolateral thalamic nucleus; PO, the posterior thalamic nucleus; VP, the ventral posterior nucleus; MD, the mid-dorsal thalamus; VTA, the ventral tegmental area; BLA, the basolateral amygdala; cDLS, the caudal dorsolateral striatum; PAG, the periaqueductal gray.
It has been shown that the disruption of connections of glutamatergic neuronal connections, from the mPFC to the periaqueductal grey (PAG) (Cheriyan and Sheets, 2018), from layer 5 in the M1 to the ZI and PAG (Gan et al., 2022), and from the S2 to the ventral posterolateral thalamic nucleus (VPL) and posterior thalamic nucleus (PO) (Guo et al., 2023) induce sensory hypersensitivity. In contrast, restoration of normal regulation of cortex over downstream brain regions can disrupt the chronic pain cycle, which can block ascending nociceptive input and induces analgesia (Yin et al., 2020; Lucas et al., 2011). The voluntary exercise can activate the GABAergic projections from the ventral HPC to the basolateral amygdala to produce the effect of hypoalgesia and alleviate negative emotions (Minami et al., 2023). Excitation of GABAergic connections from the M1 (de Andrade et al., 2019), ventrolateral orbital cortex (VLO) (Wu et al., 2020; Huo et al., 2005) to the vlPAG may facilitate the proper functioning of the downstream inhibitory system and block further upload of nociceptive information to higher centers. This processing induces analgesia at the spinal cord level. Similarly, the activation of the glutamatergic projection from the dorsal medial prefrontal cortex (dmPFC) to the vlPAG (Yin et al., 2020) and the inhibition of the inputs from the auditory cortex (ACx) to the PO and the ventral posterior nucleus (VP) (Zhou et al., 2022) and from PL (Gao et al., 2023) to vlPAG has also found to relieve nociceptive hypersensitivity.
On the other hand, the cerebral cortex induces a protective response from the organism either by directly producing pain or by generating negative emotions to facilitate the organism’s rapid departure from the environment in which the source of the nociceptive stimulus is located (Chen et al., 2024; Botvinik-Nezer et al., 2024; Kragel et al., 2018). When removed from the worse environment, the organism reverts to a state of normalcy. Hence, the production of negative emotions can be considered a predictive behavior of pain perception. Prolonged hyperexcitability of cerebral cortex results in a pain-specific negative experience. It amplifies the perception of pain and affects the pathways of pain processing in the brain, while causing abnormalities in the nociceptive modulation system and promoting the formation of pain memories (Song et al., 2024; Gan et al., 2022; Meng et al., 2022; Yin et al., 2020; Gao et al., 2023; Zeng et al., 2021).
Glutamatergic projections from the S1 to the caudal dorsolateral striatum (cDLS) (Jin et al., 2020), the M1 to the mid-dorsal thalamus (MD) (Gan et al., 2022), the dmPFC to the vlPAG (Yin et al., 2020), the IC to the basolateral amygdala (BLA) (Meng et al., 2022), the PL to the BLA (Gao et al., 2023) or to the nucleus accumbent (NAc) (Zeng et al., 2021), and the ACC to the ventral tegmental area (VTA) (Song et al., 2024) confer negative emotional messages and promote pain persistence and chronicity. Furthermore, the glutamatergic connections from the IC to the BLA is involved in the formation and consolidation of empathic pain (Zhang et al., 2022). And glutamatergic projection from the ACC to the nucleus accumbens (NAc) is intimately associated with the genesis and induction of empathic pain, in addition to the social transfer of pain and fear (Smith et al., 2021).
As established above, chronic pain involves a shift in brain activity from sensory regions to limbic regions. Pain chronicity leads to persistent activation of the limbic system. Following intense nociceptive stimulation, the limbic cortex becomes unstable and undergoes reorganization, exhibiting structural plasticity during the sustained phase of nociception (Kim et al., 2011). The brain comprehensively encodes and integrates pain perception, pain-related affect, and cognition (Talbot et al., 1991). Chronic pain imbues sensory signals with affective valence via cortico-limbic pathways, facilitating a maladaptive sensory-affective interplay that promotes long-term pain persistence. Furthermore, prolonged affective disturbances disrupt processing of cognitive information within the limbic system—including core networks for emotion and memory processing (mPFC, ACC, hippocampus, amygdala)—culminating in progressive and complex cognitive-behavioral abnormalities (Cai et al., 2022; Shackman et al., 2011). These cognitive impairments, in turn, further exacerbate emotional deterioration.
PL and the CA1 in the HPC are the primary regions for the brain associated with the formation of pain memory and cognitive process (Stegemann et al., 2023). Sciatic nerve injury can inactivate the mPFC via glutamatergic synaptic inhibition in the BLA-mPFC pathway, leading to decision-making deficits (Ji et al., 2010). Conversely, the mPFC itself can induce deficits in pain-related working memory—without affecting pain perception—through hyperactivation of the glutamatergic mPFC-dCA1 circuit (Cardoso-Cruz et al., 2019; Cardoso-Cruz et al., 2013a). Furthermore, the prelimbic mPFC can drive this dysfunction through its glutamatergic projections to the NAc (Cardoso-Cruz et al., 2022), or, in the context of chronic inflammatory pain, via glutamatergic circuitry projecting to the mediodorsal thalamus (Cardoso-Cruz et al., 2013b). Similarly, chronic pain induced by sciatic nerve injury also can impair spatial learning and memory by increasing GABA release and decreasing glutamate levels in the HPC (Saffarpour et al., 2017). Furthermore, it mediates cognitive deficits through the induction of inhibitory synaptic dysfunction in hippocampus (Cai et al., 2022). Inflammatory pain significantly weakens connectivity between the vCA1 and infralimbic cortex (IL) paralleling the onset of cognitive impairments (Shao et al., 2023). Furthermore, CA1 can mediate the comorbidity of neuropathic pain and memory deficits through glutamatergic projections to the mPFC (Han et al., 2023). Other critical limbic components, such as the ACC, are closely associated with processing pain information and decision-making behavior (Davis et al., 1997; Cao et al., 2016). However, the intricate mechanisms linking the amygdala to pain-related cognitive deficits require further investigation.
Even after the noxious stimulus has disappeared, the pain perception persists or intensifies when then pain-inducing external stimulus acts on the body again (Hu et al., 2021; Stegemann et al., 2023). This phenomenon is referred to as pain memory. The formation of memory has been found to impair cognitive function and leads to negative behaviors (Cai et al., 2022; Shackman et al., 2011). In particular, the formation of pain memory can be prevented by restoring E/I balance. This can be achieved by reducing the activity of GABAergic neurons in the MCC (Li et al., 2023) and increasing the activity of the inhibitory neurons in the IC (Xiao et al., 2023).
7 Clinical relevance and therapeutic innovations
Chronic pain progression drives functional and structural reorganization within the brain. Gray matter volume decreases occur across distinct cortical regions (Baliki et al., 2012; Obermann et al., 2013), with the magnitude and distribution of atrophy varying according to the intensity and phenotype of persistent pain (Obermann et al., 2013; Wu et al., 2022; Amirmohseni et al., 2016; Alshelh et al., 2018). Significantly increased c-Fos expression is observed in the mPFC of migraine patients (Wu et al., 2022). Patients with chronic trigeminal neuropathic pain exhibit reduced blood oxygenation level dependent (BOLD) signal intensity detected by functional magnetic resonance imaging (fMRI) in the S1 (Obermann et al., 2013), while those with clinical chronic facial pain show decreased T2 signal intensity in the S1 (Alshelh et al., 2018). As pain constitutes a dynamic network imbalance spanning sensory, affective, and cognitive circuits, the disruption of E/I balance maintained by glutamatergic and GABAergic systems serves as the primary mechanism underlying this reorganization (Eto et al., 2012; Watson, 2016; Cao et al., 2019). Research demonstrates that dynamic alterations in glutamate-driven excitatory and GABA-mediated inhibitory neurotransmission are critical determinants of the transition from acute to chronic pain (Bell et al., 2021; Pigott et al., 2023), individual variability in pain sensitivity (Zunhammer et al., 2016; Thiaucourt et al., 2018; Onderwater et al., 2021; Stærmose et al., 2019) and the long-term maintenance of chronic pain states (Zunhammer et al., 2016; Thiaucourt et al., 2018). Notably, distinct patterns of glutamate and GABA changes emerge in the brain, reflecting pathophysiological heterogeneity across pain subtypes (Peek et al., 2020).
Two 7 Tesla magnetic resonance spectroscopy (MRS) studies reveal elevated GABA concentrations in the visual cortex of adult migraineurs during interictal periods (Onderwater et al., 2021)and stable glutamate and glutamine (Glx) levels in the visual cortex across migraine states (Zielman et al., 2017). Additional MRS investigations demonstrate the decreased level of glutamate in sensorimotor and occipital cortex in pediatric patients within 24 h preictally (Cho et al., 2024), a significantly reduced concentration of glutamate in the visual cortex during migraine attacks (Bell et al., 2021) and further declines of glutamate in sensorimotor and occipital cortex after headache attacking postictally (Cho et al., 2024). Notably, higher sensorimotor GABA/Glx ratios correlate with longer disease duration and lower sensorimotor GABA levels associate with shorter time to next migraine attack (Bell et al., 2021). Another separate MRS study indicates negative correlations between Glx levels in the right dorsolateral prefrontal cortex (dlPFC) and migraine severity in episodic migraine without aura patients (Wang et al., 2024).
Other MRS studies across diverse chronic pain conditions reveal converging alterations in glutamate and GABA. Patients with chronic pain syndromes exhibited an increased Glu/GABA ratio in the insula cortex (Thiaucourt et al., 2018). Adolescents with chronic pain shown significantly decreased GABA levels in the left posterior insula, while the Glx level remain unchanged (Pigott et al., 2023). Additionally, elevated levels of circulating soluble interleukin-2 receptor (sCD25) significantly correlate with the Glx/GABA ratio in the ACC, which interplay between peripheral inflammation and central E/I imbalance promotes pain catastrophizing (Huo et al., 2005). Both patients with chronic musculoskeletal pain (Ma J. et al., 2024) and those with primary dysmenorrhea (Chen et al., 2023) demonstrate an increased Glx/GABA ratio in the ACC, whose ratio correlates with comorbid anxiety and depression in chronic pain (Ma J. et al., 2024).
Collectively, these clinical studies demonstrate significant associations between dynamic alterations in glutamate and GABA levels across distinct brain regions and key pain features, including susceptibility to pain attacks, inter-individual variability, and pain persistence (Onderwater et al., 2021; Stærmose et al., 2019). Emerging therapeutic strategies, aimed at restoring cortical E/I balance and directly correcting maladaptive neural circuitry in chronic pain, show promising efficacy. These encompass approaches of traditional Chinese medicine (TCM) and novel non-invasive brain stimulation (NIBS) techniques within chronic pain management frameworks (Pigott et al., 2023; Galafassi et al., 2021; Mawla et al., 2021; Zhang et al., 2025).
Electroacupuncture (EA), a well-established TCM modality for pain relief, demonstrates mechanistic specificity (Mawla et al., 2021; Wei X. Y. et al., 2024; Li R. et al., 2024). Combined resting-state functional MRI (rs-fMRI) and proton magnetic resonance spectroscopy (1H-MRS) evidence reveals that EA ameliorates fibromyalgia pain by indirectly elevating GABA concentrations in the bilateral anterior insula, thereby enhancing functional connectivity between the S1 and bilateral anterior insula regions (Mawla et al., 2021). NIBS techniques, including repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial focused ultrasound (tFUS), transcranial random noise stimulation (tRNS), and temporal interference stimulation (TIS), represent critical alternatives for pharmacotherapy-refractory pain (Jayathilake et al., 2025; Ahn et al., 2019; Liu Y. et al., 2024). They operate by precisely modulating neuroplasticity and restoring E/I balance, while concomitantly exhibiting potential to improve mood and cognition. Recent mechanistic insights show that preemptive tDCS over the M1 attenuates pain-related anxiety via modulation of microstate D-to-E transitions. Preemptive tDCS over dlPFC reduces pain intensity by decreasing microstate D-to-C shifts (Zhang et al., 2025). These innovational treatments improve the precision of pain discrimination and the ability to respond effectively to harmful stimuli, thereby improving pain sensitivity. Concurrently, it can participate in the coding and regulation of pain information, obstruct the downward transmission of sensory signals and induces analgesia, thus rectifying the E/I imbalance and maintaining normal sensory information processing in the cerebral cortex.
8 Discussion
The glutamatergic and GABAergic systems play a critical role in maintaining the equilibrium of E/I balance and in the processing of pain within the CNS. The interaction between these two systems determines the encoding and transmission of pain signals. An imbalance in this system can lead to the emergence of a persistent E/I imbalance, enhance nociceptive perception and induce pain-related negative behaviors by affecting the plasticity and stability of neural networks. Current chronic pain treatments often target a single system, aiming to achieve analgesic effects. The direct inhibition of glutamatergic systems has been found to reduce hyperexcitability, including the modulation of glutamate transporter function (Palmer et al., 1994; Nicholls and Attwell, 1990; Gegelashvili and Bjerrum, 2019), glutamate receptor activity (Li et al., 2020) glutamatergic neurons (Kami et al., 2022; Notartomaso et al., 2024) and output neuronal circuits (Hu et al., 2019; Takeda et al., 2022; Cao et al., 2023; Yin et al., 2020; Zhou et al., 2022). Alternatively, the activation of the GABAergic system produces inhibitory effects through the specific activation of GABAergic receptors (Chaparro et al., 2012), neurons (Xiao et al., 2023; Ren et al., 2022; Li et al., 2023) and their output projections (Cao et al., 2023; Wu et al., 2020; Huo et al., 2005; de Andrade et al., 2019; Gao et al., 2023). In particular, the role of the interaction between the glutamatergic and GABAergic systems in pain modulation warrants careful consideration. The specific mechanism underlying this interaction and the potential for exploiting this property in the treatment of chronic pain (Piot et al., 2023; Wen et al., 2022) remain poorly understood. These two systems form a specific neuronal network for pain modulation. The excitatory neurons in the cerebral cortex are regulated artificially to generate inhibitory action through the projection to GABAergic neurons in downward regions, and vice versa. This represents a straightforward unidirectional output mechanism that can be adjusted by excitatory or inhibitory synapses. However, this pathway lacks physiological feedback from the downstream to the upstream. And this can easily result in further degradation and loss of normal function of the downstream normal regions. Once external interventions are removed, a more pronounced disturbance of the E/I balance and associated side effects may occur. Therefore, the simultaneous reconditioning of the two system that have been overly disrupted systems can prevent impairment of physiological function and normalize the perception and E/I balance.
In addition, this review exclusively discusses the output work pattern dominated by the systems for pain processing, without considering the role of the cerebral cortex in the pattern of receiving inputs of pain information. Further research is required to elucidate the manner in which the cerebral cortex detects, classifies and recodes the various components of sensory stimuli. The brainstem, serving as the central hub for descending modulation and endogenous analgesia, plays a critical role in autonomic and affective responses (Gan et al., 2022; Cheriyan and Sheets, 2018; Wu et al., 2020; Huo et al., 2005; de Andrade et al., 2019). It relays preliminarily processed signals to the cerebral cortex, which sorts, integrates, and transmits pain signals back to the brainstem. The brainstem executes pain signaling, initiates endogenous analgesic mechanisms, and further feeds back arousal and affective signals to the cortex, modifying cortical signal encoding processes. Prior research demonstrates that prolonged noxious sensory input induces hyperexcitability of glutamatergic neurons in the ACC. This enhances GABAergic transmission onto dopaminergic (DA) neurons in the VTA, inhibiting DA neurons and producing antidepressant-like behavioral effects. Consequently, this abolishes the “braking” effect of DA transmission within the ACC, leading to sustained hyperactivation of glutamatergic neurons, thereby promoting persistent pain and affective comorbidities. This ACC-VTA-ACC circuit drives a maladaptive sensory-affective interplay and a vicious cycle, perpetuating chronic pain (Song et al., 2024). Furthermore, two neuroimaging studies reveal that during acute pain states, increased PFC-NAc functional connectivity coupled with decreased NAc-mPFC connectivity is predictive of the transition to chronic pain. Conversely, during the emergence of affective pain, enhanced feedback connectivity from the NAc to the mPFC further facilitates pain persistence (Baliki et al., 2012; Park et al., 2022). The specific manifestations of this cortico-brainstem “decision-execution-feedback” signaling circuit under physiological and pathological conditions require further experimental elucidation.
Meanwhile, the circuitries within the CNS may be subject to the influence of a multitude of factors, including inflammation, injury and psychological processes. This can result in complications with regard to the coordination of circuits and presents a challenge in the context of pain medication. Moreover, this review specifically centers on the glutamatergic and GABAergic systems constituting the E/I balance. Other neural types also play significant roles in pain, including monoaminergic neurons (serotonin, norepinephrine, dopamine), peptide-gic neurons (substance P, calcitonin gene-related peptide, opioid peptides), and cholinergic neurons. Further comprehensive reviews are warranted to elucidate the integration and encoding mechanisms of pain signals by these neuronal populations, the dynamic modulation of regional brain function via these neural circuit mechanisms and the therapeutic targeting strategies for developing novel interventions. Similarly, this review primarily addresses interactions between neurons and astrocytes in relation to pain. Other glial cells, notably oligodendrocytes (Kim and Angulo, 2025; Li D. et al., 2024; Iwata et al., 2024) and microglia (Kohno and Tsuda, 2025; Malcangio and Sideris-Lampretsas, 2025), also engage in crucial bidirectional interactions with neurons. These interactions which are also the key determinants of neuronal excitability, are intricately linked to neuronal development, myelination, and synaptic plasticity, and thus warrant focused investigation in future pain research.
Pain represents a dynamic network imbalance involving sensory, affective, and cognitive circuits. Current mechanistic investigations remain largely confined to rodent-based animal studies. Research in rodent models offers unique advantages for dissecting molecular and cellular mechanisms, permitting invasive genetic manipulations. Through optogenetic and chemogenetic modulation of specific neurons or neural circuits can be manipulated to verify the micro-scale mechanisms and facilitate high-throughput drug screening. However, rodent studies cannot fully recapitulate the affective-cognitive dimensions of human pain, particularly in modeling affective-cognitive comorbidities. The cross-species validation integrating rodents, non-human primates and human models is essential to delineate evolutionary conservation and species-specific divergence in nociceptive pathways. This approach comprehensively elucidates the multidimensional characteristics of pain—spanning sensory, affective, and cognitive domains. The study on non-human primate can bridge this translational gap through their high similarity to humans in neuroanatomical connectivity and advanced behavioral repertoires. NHPs effectively model affective integration of pain and reveal its impact on cognition, directly predicting clinical drug efficacy and validating neuromodulatory mechanisms (Shackman et al., 2011). Human researches constitute the cornerstone for clinical phenotyping and individualized mechanistic profiling. Direct assessment of diverse pain phenotypes in humans enables precision-targeted interventions, guiding tailored therapeutic strategies.
Our analysis highlights a significant positive correlation between elevated glutamate/GABA ratios and pain sensitization. Contemporary studies employ diverse innovative methodologies, such as high-performance liquid chromatography (HPLC) for ex vivo quantification (Cseh et al., 2020), photosensitive photopharmacology for spatiotemporal receptor manipulation (Notartomaso et al., 2024), whole-cell patch-clamp electrophysiology for single-neuron excitability profiling (Tullis et al., 2023; Liu W. et al., 2024), two-photon imaging combined with calcium indicators for circuit-level activity mapping (Guo et al., 2023; Tullis et al., 2023; Liu W. et al., 2024; Zhang et al., 2023), MRS for in vivo neurochemical measurement (Onderwater et al., 2021; Zielman et al., 2017) and fMRI for network connectivity analysis (Mawla et al., 2021). These creative methods are adopted to assess functional alterations in glutamatergic and GABAergic systems, including neurotransmitter concentration shifts, receptor functional dynamics, neuronal excitability changes, and migration of activity in brain regions. Despite substantial advances in mechanistic understanding, key limitations remain. Chronic pain progression involves dynamic co-evolution of glutamatergic and GABAergic systems across extended timescales. Current technologies cannot longitudinally capture multi-transmitter interactions with simultaneous cellular resolution, hindering elucidation compensatory plasticity between excitatory and inhibitory circuits, temporal coordination of neuromodulator release events, and system-wide adaptation thresholds driving pain chronification. Meanwhile, it is also difficult to present time points at which the multi-dimensional pain experiences occur and how these multi-dimensional experiences influence each other. Notably, species differences between rodents and humans result in disparities in the practicality and applicability of technical approaches, posing a significant barrier to the clinical translation of current research findings.
Concurrently, the inherent constraints of single-modality detection techniques hinder the capture of multifaceted characteristics of chronic pain. Multimodal neuroimaging studies enable a comprehensive assessment of cortical excitability changes and facilitates precise targeting of therapeutic interventions (Widman et al., 2025; Quidé et al., 2024; Zhang et al., 2024; Namgung et al., 2025). Consequently, future research must prioritize the integration of cross-species validation with multimodal imaging technologies to analyze critical transition points in the brain from acute to chronic pain (Baliki et al., 2012; Park et al., 2022), to decipher dynamic multidimensional shifts spanning sensory perception, affect, and cognition in the pain experience, and to investigate individual differences in pain sensitivity and underlying genetic susceptibility. Furthermore, leveraging artificial intelligence to co-develop diagnostic and progression models for pain is crucial. These models should guide the timing of therapeutic interventions and optimize neuromodulation parameters for non-invasive neurostimulation therapies, marking a critical direction for future advancements in the field.
9 Conclusion
The cerebral cortex plays a complex role in the processing of pain information, involving a number of different processes, including neuronal responses in individual cortical regions, neuron-astrocytes interaction, the sharing and cascading of inter-cortical signal and downward cortical modulation. Collectively, these processes form a complex pain modulation network that controls the propagation and return of pain information in the brain. However, the modulation network is disrupted in individual with chronic pain, and this disruption is strongly associated with the progression of chronic pain. The onset and persistence of chronic pain is closely linked to an E/I imbalance between glutamatergic and GABAergic systems within the cerebral cortex. This imbalance leads to destabilization of neural networks, the biased output of information and the manifestation of abnormal pain perception. The interaction between neurons and astrocytes is disrupted, which can exacerbate the E/I disequilibrium in the brain, further interfering with the processing of pain signals and potentially leading to excitatory neurotoxicity. The process in sharing and cascading of inter-cortical signal influences how individuals cope with pain and their emotional state and contributes to the pain chronicity. The cerebral cortex converts sensory signals to perceptual signals and causes changes in plasticity functional and structural plasticity through modulation of downstream brain regions. Meanwhile, future studies need to further explore the mechanisms of interaction between these systems and develop more effective treatments to restore the excitatory-inhibitory balance in the cerebral cortex and improve the symptoms of chronic pain patients. This is due to the importance of interactions between glutamatergic and GABAergic systems.
Statements
Author contributions
DH: Conceptualization, Visualization, Writing – original draft. Y-TD: Writing – original draft. L-XH: Investigation, Visualization, Writing – original draft. R-ZZ: Investigation, Writing – original draft. J-XZ: Investigation, Writing – original draft. SY: Funding acquisition, Supervision, Writing – review & editing. S-GY: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Projects National Natural Science Foundation of China (no. 82230127), National Natural Science Foundation of China (nos. 82374604 and 82004487), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (no. ZYYCXTDD-202003), and Key Project of Natural Science Foundation of Sichuan Provincial Department of Science and Technology (no. 2023NSFSC0696).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AhnS.PrimJ. H.AlexanderM. L.McCullochK. L.FröhlichF. (2019). Identifying and engaging neuronal oscillations by transcranial alternating current stimulation in patients with chronic low back pain: a randomized, crossover, double-blind, sham-controlled pilot study. J. Pain20:277. doi: 10.1016/j.jpain.2018.09.004
2
AlshelhZ.Di PietroF.MillsE. P.VickersE. R.PeckC. C.MurrayG. M.et al. (2018). Altered regional brain T2 relaxation times in individuals with chronic orofacial neuropathic pain. Neuroimage Clin.19, 167–173. doi: 10.1016/j.nicl.2018.04.015
3
AmirmohseniS.SegelckeD.ReichlS.WachsmuthL.GörlichD.FaberC.et al. (2016). Characterization of incisional and inflammatory pain in rats using functional tools of MRI. NeuroImage127, 110–122. doi: 10.1016/j.neuroimage.2015.11.052
4
AraqueA.ParpuraV.SanzgiriR. P.HaydonP. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci.22, 208–215. doi: 10.1016/S0166-2236(98)01349-6
5
BalikiM. N.PetreB.TorbeyS.HerrmannK. M.HuangL.SchnitzerT. J.et al. (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci.15, 1117–1119. doi: 10.1038/nn.3153
6
BellT.StokoeM.KhairaA.WebbM.NoelM.AmoozegarF.et al. (2021). GABA and glutamate in pediatric migraine. Pain162, 300–308. doi: 10.1097/j.pain.0000000000002022
7
BensonC.MifflinK.KerrB.JesudasanS. J.DursunS.BakerG. (2015). Biogenic amines and the amino acids GABA and glutamate: relationships with pain and depression. Mod. Trends Pharmacopsychiatry30, 67–79. doi: 10.1159/000435933
8
BhattR. R.HaddadE.ZhuA. H.ThompsonP. M.GuptaA.MayerE. A.et al. (2023). Mapping brain structure variability in chronic pain: the role of widespreadness and pain type and its mediating relationship with suicide attempt. Biol. Psychiatry95, 473–481. doi: 10.1016/j.biopsych.2023.07.016
9
Botvinik-NezerR.PetreB.CekoM.LindquistM. A.FriedmanN. P.WagerT. D. (2024). Placebo treatment affects brain systems related to affective and cognitive processes, but not nociceptive pain. Nat. Commun.15:6017. doi: 10.1038/s41467-024-50103-8
10
BuetferingC.ZhangZ.PitsianiM.SmallridgeJ.BovenE.McElligottS.et al. (2022). Behaviorally relevant decision coding in primary somatosensory cortex neurons. Nat. Neurosci.25, 1225–1236. doi: 10.1038/s41593-022-01151-0
11
CahillM. K.CollardM.TseV.ReitmanM. E.EtcheniqueR.KirstC.et al. (2024). Network-level encoding of local neurotransmitters in cortical astrocytes. Nature629, 146–153. doi: 10.1038/s41586-024-07311-5
12
CaiX.QiuL.WangC.YangH.ZhouZ.MaoM.et al. (2022). Hippocampal inhibitory synapsis deficits induced by α5-containing GABA(a) receptors mediate chronic neuropathic pain-related cognitive impairment. Mol. Neurobiol.59, 6049–6061. doi: 10.1007/s12035-022-02955-8
13
CaoB.WangJ.MuL.PoonD. C.LiY. (2016). Impairment of decision making associated with disruption of phase-locking in the anterior cingulate cortex in viscerally hypersensitive rats. Exp. Neurol.286, 21–31. doi: 10.1016/j.expneurol.2016.09.010
14
CaoF. L.XuM.GongK.WangY.WangR.ChenX.et al. (2019). Imbalance between excitatory and inhibitory synaptic transmission in the primary somatosensory cortex caused by persistent nociception in rats. J. Pain20, 917–931. doi: 10.1016/j.jpain.2018.11.014
15
CaoP.ZhangM.NiZ.SongX. J.YangC. L.MaoY.et al. (2023). Green light induces antinociception via visual-somatosensory circuits. Cell Rep.42:112290. doi: 10.1016/j.celrep.2023.112290
16
Cardoso-CruzH.LaranjeiraI.MonteiroC.GalhardoV. (2022). Altered prefrontal-striatal theta-band oscillatory dynamics underlie working memory deficits in neuropathic pain rats. Eur. J. Pain26, 1546–1568. doi: 10.1002/ejp.1982
17
Cardoso-CruzH.LimaD.GalhardoV. (2013a). Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J. Neurosci.33, 2465–2480. doi: 10.1523/JNEUROSCI.5197-12.2013
18
Cardoso-CruzH.PaivaP.MonteiroC.GalhardoV. (2019). Bidirectional optogenetic modulation of prefrontal-hippocampal connectivity in pain-related working memory deficits. Sci. Rep.9:10980. doi: 10.1038/s41598-019-47555-0
19
Cardoso-CruzH.SousaM.VieiraJ. B.LimaD.GalhardoV. (2013b). Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain154, 2397–2406. doi: 10.1016/j.pain.2013.07.020
20
ChangC. L.TrimbuchT.ChaoH. T.JordanJ. C.HermanM. A.RosenmundC. (2014). Investigation of synapse formation and function in a glutamatergic-GABAergic two-neuron microcircuit. J. Neurosci.34, 855–868. doi: 10.1523/JNEUROSCI.0229-13.2014
21
ChaparroL. E.WiffenP. J.MooreR. A.GilronI. (2012). Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst. Rev.2020, 230–251. doi: 10.1002/14651858.CD008943.pub2
22
ChenX.HuangZ.WuX.HanS.WuP.LiY. (2023). Assessment of neurotransmitter imbalances within the anterior cingulate cortex in women with primary dysmenorrhea: An initial proton magnetic resonance spectroscopy study. Eur. J. Radiol.167:111079. doi: 10.1016/j.ejrad.2023.111079
23
ChenL.LiX.TjiaM.ThapliyalS. (2022). Homeostatic plasticity and excitation-inhibition balance: the good, the bad, and the ugly. Curr. Opin. Neurobiol.75:102553. doi: 10.1016/j.conb.2022.102553
24
ChenC.NiehausJ. K.DincF.HuangK. L.BarnetteA. L.TassouA.et al. (2024). Neural circuit basis of placebo pain relief. Nature632, 1092–1100. doi: 10.1038/s41586-024-07816-z
25
ChenT.WangW.DongY. L.ZhangM. M.WangJ.KogaK.et al. (2014). Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol. Brain7:76. doi: 10.1186/s13041-014-0076-8
26
ChengY. T.Luna-FigueroaE.WooJ.ChenH. C.LeeZ. F.HarmanciA. S.et al. (2023). Inhibitory input directs astrocyte morphogenesis through glial GABA(B)R. Nature617, 369–376. doi: 10.1038/s41586-023-06010-x
27
CheriyanJ.SheetsP. L. (2018). Altered excitability and local connectivity of mPFC-PAG neurons in a mouse model of neuropathic pain. J. Neurosci.38, 4829–4839. doi: 10.1523/JNEUROSCI.2731-17.2018
28
CheriyanJ.SheetsP. L. (2020). Peripheral nerve injury reduces the excitation-inhibition balance of basolateral amygdala inputs to prelimbic pyramidal neurons projecting to the periaqueductal gray. Mol. Brain13:100. doi: 10.1186/s13041-020-00638-w
29
ChoL. Y.BellT. K.CraddockL.GodfreyK. J.HersheyA. D.KuziekJ.et al. (2024). Region-specific changes in brain glutamate and gamma-aminobutyric acid across the migraine attack in children and adolescents. Pain165, 2749–2761. doi: 10.1097/j.pain.0000000000003289
30
ComitatoA.BardoniR. (2021). Presynaptic inhibition of pain and touch in the spinal cord: from receptors to circuits. Int. J. Mol. Sci.22:414. doi: 10.3390/ijms22010414
31
CsehE. K.VeresG.KörtésiT.PolyákH.NánásiN.TajtiJ.et al. (2020). Neurotransmitter and tryptophan metabolite concentration changes in the complete Freund's adjuvant model of orofacial pain. J. Headache Pain21:35. doi: 10.1186/s10194-020-01105-6
32
CuiL.GuoJ.CranfillS. L.GautamM.BhattaraiJ.OlsonW.et al. (2022). Glutamate in primary afferents is required for itch transmission. Neuron110, 809–823. doi: 10.1016/j.neuron.2021.12.007
33
DanjoY.ShigetomiE.HirayamaY. J.KobayashiK.IshikawaT.FukazawaY.et al. (2022). Transient astrocytic mGluR5 expression drives synaptic plasticity and subsequent chronic pain in mice. J. Exp. Med.219:989. doi: 10.1084/jem.20210989
34
DavisK. D.TaylorS. J.CrawleyA. P.WoodM. L.MikulisD. J. (1997). Functional MRI of pain- and attention-related activations in the human cingulate cortex. J. Neurophysiol.77, 3370–3380. doi: 10.1152/jn.1997.77.6.3370
35
de AndradeE. M.MartinezR. C. R.PaganoR. L.LopesP. S. S.AuadaA. V. V.GouveiaF. V.et al. (2019). Neurochemical effects of motor cortex stimulation in the periaqueductal gray during neuropathic pain. J. Neurosurg.132, 239–251. doi: 10.3171/2018.7.JNS173239
36
de CegliaR.LedonneA.LitvinD. G.LindB. L.CarrieroG.LatagliataE. C.et al. (2023). Specialized astrocytes mediate glutamatergic gliotransmission in the CNS. Nature622, 120–129. doi: 10.1038/s41586-023-06502-w
37
EtoK.IshibashiH.YoshimuraT.WatanabeM.MiyamotoA.IkenakaK.et al. (2012). Enhanced GABAergic activity in the mouse primary somatosensory cortex is insufficient to alleviate chronic pain behavior with reduced expression of neuronal potassium-chloride cotransporter. J. Neurosci.32, 16552–16559. doi: 10.1523/JNEUROSCI.2104-12.2012
38
Falconi-SobrinhoL. L.Dos Anjos-GarciaT.Elias-FilhoD. H.CoimbraN. C. (2017). Unravelling cortico-hypothalamic pathways regulating unconditioned fear-induced antinociception and defensive behaviours. Neuropharmacology113, 367–385. doi: 10.1016/j.neuropharm.2016.10.001
39
FanY. F.GuanS. Y.LuoL.LiY. J.YangL.ZhouX. X.et al. (2018). Tetrahydroxystilbene glucoside relieves the chronic inflammatory pain by inhibiting neuronal apoptosis, microglia activation, and GluN2B overexpression in anterior cingulate cortex. Mol. Pain14:1744806918814367. doi: 10.1177/1744806918814367
40
FergusonB. R.GaoW. J. (2018). PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits.12:37. doi: 10.3389/fncir.2018.00037
41
FischerB. D. (2017). GABA(a) receptors as targets for the Management of Pain-related Disorders: historical perspective and update. CNS Neurol. Disord. Drug Targets16, 658–663. doi: 10.2174/1871527316666170207155149
42
GalafassiG. Z.PiresS.de AguiarP. H.SimmR. F.FranceschiniP. R.FilhoM. P.et al. (2021). Neuromodulation for medically refractory neuropathic pain: spinal cord stimulation, deep brain stimulation, motor cortex stimulation, and posterior insula stimulation. World Neurosurg.146, 246–260. doi: 10.1016/j.wneu.2020.11.048
43
GanZ.GangadharanV.LiuS.KörberC.TanL. L.LiH.et al. (2022). Layer-specific pain relief pathways originating from primary motor cortex. Science378, 1336–1343. doi: 10.1126/science.add4391
44
GangulyK.SchinderA. F.WongS. T.PooM. (2001). GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell105, 521–532. doi: 10.1016/S0092-8674(01)00341-5
45
GaoF.HuangJ.HuangG. B.YouQ. L.YaoS.ZhaoS. T.et al. (2023). Elevated prelimbic cortex-to-basolateral amygdala circuit activity mediates comorbid anxiety-like behaviors associated with chronic pain. J. Clin. Invest.133:356. doi: 10.1172/JCI166356
46
Garcia-LarreaL.PeyronR. (2013). Pain matrices and neuropathic pain matrices: a review. Pain154, S29–S43. doi: 10.1016/j.pain.2013.09.001
47
GegelashviliG.BjerrumO. J. (2019). Glutamate transport system as a key constituent of glutamosome: molecular pathology and pharmacological modulation in chronic pain. Neuropharmacology161:107623. doi: 10.1016/j.neuropharm.2019.04.029
48
GnallK. E.JochimsenK. N.BrewerJ. R.BakhshaieJ.VranceanuA. M. (2025). Pain catastrophizing and pain anxiety mediate changes in physical function in a mind-body intervention for adults with traumatic orthopedic injuries. Pain166, 1418–1424. doi: 10.1097/j.pain.0000000000003477
49
GuidaF.LuongoL.MarmoF.RomanoR.IannottaM.NapolitanoF.et al. (2015). Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain8:47. doi: 10.1186/s13041-015-0139-5
50
GuoF.LinS. D.DuY.HuT. T.WangY.ChenZ.et al. (2023). Secondary somatosensory cortex glutamatergic innervation of the thalamus facilitates pain. Pain165, 1142–1153. doi: 10.1097/j.pain.0000000000003117
51
HanS.RenJ.LiZ.WenJ.JiangB.WeiX. (2023). Deactivation of dorsal CA1 pyramidal neurons projecting to medial prefrontal cortex contributes to neuropathic pain and short-term memory impairment. Pain165:3100. doi: 10.1097/j.pain.0000000000003100
52
HogrefeN.BlomS. M.ValentinovaK.NtamatiN. R.JonkerL. J. E.NevianN. E.et al. (2022). Long-lasting, pathway-specific impairment of a novel form of spike-timing-dependent Long-term depression by neuropathic pain in the anterior cingulate cortex. J. Neurosci.42, 2166–2179. doi: 10.1523/JNEUROSCI.0326-21.2022
53
HuT. T.WangR. R.DuY.GuoF.WuY. X.WangY.et al. (2019). Activation of the intrinsic pain inhibitory circuit from the Midcingulate Cg2 to zona Incerta alleviates neuropathic pain. J. Neurosci.39, 9130–9144. doi: 10.1523/JNEUROSCI.1683-19.2019
54
HuS. W.ZhangQ.XiaS. H.ZhaoW. N.LiQ. Z.YangJ. X.et al. (2021). Contralateral projection of anterior cingulate cortex contributes to Mirror-image pain. J. Neurosci.41, 9988–10003. doi: 10.1523/JNEUROSCI.0881-21.2021
55
HuoF. Q.WangJ.LiY. Q.ChenT.HanF.TangJ. S. (2005). Gabaergic neurons express mu-opioid receptors in the ventrolateral orbital cortex of the rat. Neurosci. Lett.382, 265–268. doi: 10.1016/j.neulet.2005.03.070
56
IqbalZ.LiuS.LeiZ.RamkrishnanA. S.AkterM.LiY. (2022). Astrocyte l-lactate signaling in the ACC regulates visceral pain aversive memory in rats. Cells12:26. doi: 10.3390/cells12010026
57
IshikawaT.MurataK.OkudaH.PotapenkoI.HoriK.FuruyamaT.et al. (2023). Pain-related neuronal ensembles in the primary somatosensory cortex contribute to hyperalgesia and anxiety. iScience.26:106332. doi: 10.1016/j.isci.2023.106332
58
IslamJ.RahmanM. T.AliM.KimH. K.KcE.ParkY. S. (2025). Optogenetic inhibition of ventrolateral orbitofrontal cortex astrocytes facilitates ventrolateral periaqueductal gray glutamatergic activity to reduce hypersensitivity in infraorbital nerve injury rat model. J. Headache Pain26:41. doi: 10.1186/s10194-025-01977-6
59
IwataK.HayashiY.HitomiS.TsuboiY.ShinodaM. (2024). Non-neuronal cells act as crucial players in neuropathic orofacial pain. J. Oral Biosci.66, 491–495. doi: 10.1016/j.job.2024.07.005
60
JasminL.RabkinS. D.GranatoA.BoudahA.OharaP. T. (2003). Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature424, 316–320. doi: 10.1038/nature01808
61
JayathilakeN. J.PhanT. T.KimJ.LeeK. P.ParkJ. M. (2025). Modulating neuroplasticity for chronic pain relief: noninvasive neuromodulation as a promising approach. Exp. Mol. Med.57, 501–514. doi: 10.1038/s12276-025-01409-0
62
JeeH. J.ZhuE.SunM.LiuW.ZhangQ.WangJ. (2023). Anterior cingulate cortex regulates pain catastrophizing-like behaviors in rats. Mol. Brain16:71. doi: 10.1186/s13041-023-01060-8
63
JiG.NeugebauerV. (2011). Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(a) receptors. J. Neurophysiol.106, 2642–2652. doi: 10.1152/jn.00461.2011
64
JiG.SunH.FuY.LiZ.Pais-VieiraM.GalhardoV.et al. (2010). Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci.30, 5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010
65
JiangW.GongM.ShenL.YuC.RuanH.ChenP.et al. (2025). The receptor for advanced glycation end-products in the mouse anterior cingulate cortex is involved in neuron–astrocyte coupling in chronic inflammatory pain and anxiety comorbidity. Mol. Neurobiol.62, 7183–7204. doi: 10.1007/s12035-025-04713-y
66
JinY.MengQ.MeiL.ZhouW.ZhuX.MaoY.et al. (2020). A somatosensory cortex input to the caudal dorsolateral striatum controls comorbid anxiety in persistent pain. Pain161, 416–428. doi: 10.1097/j.pain.0000000000001724
67
JonesA. F.SheetsP. L. (2020). Sex-specific disruption of distinct mPFC inhibitory neurons in spared-nerve injury model of neuropathic pain. Cell Rep.31:107729. doi: 10.1016/j.celrep.2020.107729
68
KamiK.TajimaF.SenbaE. (2022). Brain mechanisms of exercise-induced Hypoalgesia: to find a way out from "fear-avoidance belief". Int. J. Mol. Sci.23:886. doi: 10.3390/ijms23052886
69
KangD.Hesam-ShariatiN.McAuleyJ. H.AlamM.TrostZ.RaeC. D.et al. (2021). Disruption to normal excitatory and inhibitory function within the medial prefrontal cortex in people with chronic pain. Eur. J. Pain25, 2242–2256. doi: 10.1002/ejp.1838
70
KianiF. A.LiH.NanS.LiQ.LeiQ.YinR.et al. (2024). Electroacupuncture relieves neuropathic pain via adenosine 3 receptor activation in the spinal cord dorsal horn of mice. Int. J. Mol. Sci.25:242. doi: 10.3390/ijms251910242
71
KimW.AnguloM. C. (2025). Unraveling the role of oligodendrocytes and myelin in pain. J. Neurochem.169:e16206. doi: 10.1111/jnc.16206
72
KimS. K.HayashiH.IshikawaT.ShibataK.ShigetomiE.ShinozakiY.et al. (2016). Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J. Clin. Invest.126, 1983–1997. doi: 10.1172/JCI82859
73
KimS. K.KatoG.IshikawaT.NabekuraJ. (2011). Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain. Mol. Pain7:87. doi: 10.1186/1744-8069-7-87
74
KimW.KimS. K.NabekuraJ. (2017). Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J. Neurochem.141, 499–506. doi: 10.1111/jnc.14012
75
KimH. R.LongM.SekerkováG.MaesA.KennedyA.MartinaM. (2024). Hypernegative GABA(a) reversal potential in pyramidal cells contributes to medial prefrontal cortex deactivation in a mouse model of neuropathic pain. J. Pain25, 522–532. doi: 10.1016/j.jpain.2023.09.021
76
KogaK.ShimoyamaS.YamadaA.FurukawaT.NikaidoY.FurueH.et al. (2018). Chronic inflammatory pain induced GABAergic synaptic plasticity in the adult mouse anterior cingulate cortex. Mol. Pain14:1744806918783478. doi: 10.1177/1744806918783478
77
KohW.KwakH.CheongE.LeeC. J. (2023). GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci.24, 523–539. doi: 10.1038/s41583-023-00724-7
78
KohnoK.TsudaM. (2025). Neuron-microglia interactions modulating neuropathic pain. Int. Immunol.2:dxaf022. doi: 10.1093/intimm/dxaf022
79
KorkutS. (2025). Comparison of the predictive role of spiritual well-being and pain intensity on pain catastrophizing in acute and chronic pain. Pain Manag. Nurs.26, e254–e260. doi: 10.1016/j.pmn.2024.12.024
80
KragelP. A.KanoM.Van OudenhoveL.LyH. G.DupontP.RubioA.et al. (2018). Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nat. Neurosci.21, 283–289. doi: 10.1038/s41593-017-0051-7
81
KunerR.KunerT. (2021). Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev.101, 213–258. doi: 10.1152/physrev.00040.2019
82
LeeH. G.WheelerM. A.QuintanaF. J. (2022). Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov.21, 339–358. doi: 10.1038/s41573-022-00390-x
83
LiC.LeiY.TianY.XuS.ShenX.WuH.et al. (2019). The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol. Pain15:1744806919847366. doi: 10.1177/1744806919847366
84
LiX. H.ShiW.ChenQ. Y.HaoS.MiaoH. H.MiaoZ.et al. (2023). Activation of the glutamatergic cingulate cortical-cortical connection facilitates pain in adult mice. Commun. Biol.6:1247. doi: 10.1038/s42003-023-05589-1
85
LiR.SunJ.LuoK.LuoN.SunR.GaoF.et al. (2024). Electroacupuncture and carbamazepine for patients with trigeminal neuralgia: a randomized, controlled, 2 × 2 factorial trial. J. Neurol.271, 5122–5136. doi: 10.1007/s00415-024-12433-x
86
LiD.YangK.LiJ.XuX.GongL.YueS.et al. (2024). Single-cell sequencing reveals glial cell involvement in development of neuropathic pain via myelin sheath lesion formation in the spinal cord. J. Neuroinflammation21:213. doi: 10.1186/s12974-024-03207-3
87
LiH.ZhanX.ZhaoX.ZhouJ.ChenK.ChenY.et al. (2025). Exploring the differences in resting state functional magnetic resonance imaging brain activity in patients with chronic low back pain based on ALE meta-analysis. J. Pain32:105442. doi: 10.1016/j.jpain.2025.105442
88
LiJ.ZhangL.XuC.LinY. H.ZhangY.WuH. Y.et al. (2020). Prolonged use of NMDAR antagonist develops analgesic tolerance in neuropathic pain via nitric oxide reduction-induced GABAergic disinhibition. Neurotherapeutics17, 1016–1030. doi: 10.1007/s13311-020-00883-w
89
LiM.ZhouH.TengS.YangG. (2022). Activation of VIP interneurons in the prefrontal cortex ameliorates neuropathic pain aversiveness. Cell Rep.40:111333. doi: 10.1016/j.celrep.2022.111333
90
LiX.ZhuY.SunH.ShenZ.SunJ.XiaoS.et al. (2023). Electroacupuncture inhibits pain memory and related anxiety-like behaviors by blockading the GABA(B) receptor function in the Midcingulate cortex. Mol. Neurobiol.60, 6613–6626. doi: 10.1007/s12035-023-03467-9
91
LiuW.ChenQ. Y.LiX. H.ZhouZ.ZhuoM. (2024). Cortical tagged synaptic Long-term depression in the anterior cingulate cortex of adult mice. J. Neurosci.44:e0028242024. doi: 10.1523/JNEUROSCI.0028-24.2024
92
LiuY.SunJ.WuC.RenJ.HeY.SunN.et al. (2024). Characterizing the opioidergic mechanisms of repetitive transcranial magnetic stimulation-induced analgesia: a randomized controlled trial. Pain165, 2035–2043. doi: 10.1097/j.pain.0000000000003220
93
LiuS. B.ZhangM. M.ChengL. F.ShiJ.LuJ. S.ZhuoM. (2015). Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain. Mol. Brain8:76. doi: 10.1186/s13041-015-0169-z
94
López-AvilaA.CoffeenU.Ortega-LegaspiJ. M.del AngelR.PellicerF. (2004). Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain111, 136–143. doi: 10.1016/j.pain.2004.06.010
95
LucasJ. M.JiY.MasriR. (2011). Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain152, 1398–1407. doi: 10.1016/j.pain.2011.02.025
96
LyuZ.GuoY.GongY.FanW.DouB.LiN.et al. (2021). The role of neuroglial crosstalk and synaptic plasticity-mediated central sensitization in acupuncture analgesia. Neural Plast.2021, 1–18. doi: 10.1155/2021/8881557
97
MaJ.SubramaniamP.YanceyJ. R.FarringtonA. A.McGladeE. C.RenshawP. F.et al. (2024). Elevated circulating soluble interleukin-2 receptor (sCD25) level is associated with prefrontal excitatory-inhibitory imbalance in individuals with chronic pain: a proton MRS study. Brain Behav. Immun.120, 1–9. doi: 10.1016/j.bbi.2024.05.020
98
MaL.YueL.LiuS.XuS.TongJ.SunX.et al. (2024). A distinct neuronal ensemble of prelimbic cortex mediates spontaneous pain in rats with peripheral inflammation. Nat. Commun.15:7922. doi: 10.1038/s41467-024-52243-3
99
MalcangioM.Sideris-LampretsasG. (2025). How microglia contribute to the induction and maintenance of neuropathic pain. Nat. Rev. Neurosci.26, 263–275. doi: 10.1038/s41583-025-00914-5
100
MaoX.CaiD.LouW. (2022). Music alleviates pain perception in depression mouse models by promoting the release of glutamate in the hippocampus of mice to act on GRIK5. Nucleosides Nucleotides Nucleic Acids41, 463–473. doi: 10.1080/15257770.2022.2051048
101
MarcelloL.CavaliereC.ColangeloA. M.BiancoM. R.CirilloG.AlberghinaL.et al. (2013). Remodelling of supraspinal neuroglial network in neuropathic pain is featured by a reactive gliosis of the nociceptive amygdala. Eur. J. Pain17, 799–810. doi: 10.1002/j.1532-2149.2012.00255.x
102
MartinsI.CarvalhoP.de VriesM. G.Teixeira-PintoA.WilsonS. P.WesterinkB. H. C.et al. (2015). GABA acting on GABAB receptors located in a medullary pain facilitatory area enhances nociceptive behaviors evoked by intraplantar formalin injection. Pain156, 1555–1565. doi: 10.1097/j.pain.0000000000000203
103
MasochaW. (2015). Comprehensive analysis of the GABAergic system gene expression profile in the anterior cingulate cortex of mice with paclitaxel-induced neuropathic pain. Gene Expr.16, 145–153. doi: 10.3727/105221615X14181438356337
104
MawlaI.IchescoE.ZöllnerH. J.EddenR. A. E.ChenevertT.BuchtelH.et al. (2021). Greater somatosensory Afference with acupuncture increases primary somatosensory connectivity and alleviates fibromyalgia pain via insular γ-aminobutyric acid: a randomized neuroimaging trial. Arthritis Rheumatol.73, 1318–1328. doi: 10.1002/art.41620
105
MazoC.NissantA.SahaS.PeroniE.LledoP. M.LepousezG. (2022). Long-range GABAergic projections contribute to cortical feedback control of sensory processing. Nat. Commun.13:6879. doi: 10.1038/s41467-022-34513-0
106
MeccaC. M.ChaoD.YuG.FengY.SegelI.ZhangZ.et al. (2021). Dynamic change of endocannabinoid signaling in the medial prefrontal cortex controls the development of depression after neuropathic pain. J. Neurosci.41, 7492–7508. doi: 10.1523/JNEUROSCI.3135-20.2021
107
MengX.YueL.LiuA.TaoW.ShiL.ZhaoW.et al. (2022). Distinct basolateral amygdala excitatory inputs mediate the somatosensory and aversive-affective components of pain. J. Biol. Chem.298:102207. doi: 10.1016/j.jbc.2022.102207
108
Mercer LindsayN.ChenC.GilamG.MackeyS.ScherrerG. (2021). Brain circuits for pain and its treatment. Sci. Transl. Med.13:7360. doi: 10.1126/scitranslmed.abj7360
109
MinamiK.KamiK.NishimuraY.KawanishiM.ImashiroK.KamiT.et al. (2023). Voluntary running-induced activation of ventral hippocampal GABAergic interneurons contributes to exercise-induced hypoalgesia in neuropathic pain model mice. Sci. Rep.13:2645. doi: 10.1038/s41598-023-29849-6
110
NamgungJ. Y.NohE.JangY.LeeM. J.ParkB. Y. (2025). A robust multimodal brain MRI-based diagnostic model for migraine: validation across different migraine phases and longitudinal follow-up data. J. Headache Pain26:5. doi: 10.1186/s10194-024-01946-5
111
NashawiH.MasochaW.EdafioghoI. O.KombianS. B. (2016). Paclitaxel causes electrophysiological changes in the anterior cingulate cortex via modulation of the γ-aminobutyric acid-ergic system. Med. Princ. Pract.25, 423–428. doi: 10.1159/000447775
112
NichollsD.AttwellD. (1990). The release and uptake of excitatory amino acids. Trends Pharmacol. Sci.11, 462–468. doi: 10.1016/0165-6147(90)90129-V
113
NiddamD. M.WangS. J.TsaiS. Y. (2021). Pain sensitivity and the primary sensorimotor cortices: a multimodal neuroimaging study. Pain162, 846–855. doi: 10.1097/j.pain.0000000000002074
114
NotartomasoS.AntenucciN.MazzitelliM.RoviraX.BoccellaS.RicciardiF.et al. (2024). A 'double-edged' role for type-5 metabotropic glutamate receptors in pain disclosed by light-sensitive drugs. eLife13:94931. doi: 10.7554/eLife.94931
115
ObermannM.Rodriguez-RaeckeR.NaegelS.HolleD.MuellerD.YoonM. S.et al. (2013). Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. NeuroImage74, 352–358. doi: 10.1016/j.neuroimage.2013.02.029
116
OnderwaterG. L. J.WijnenJ. P.NajacC.van DongenR. M.RonenI.WebbA.et al. (2021). Cortical glutamate and gamma-aminobutyric acid over the course of a provoked migraine attack, a 7 tesla magnetic resonance spectroscopy study. Neuroimage Clin.32:102889. doi: 10.1016/j.nicl.2021.102889
117
OngW. Y.StohlerC. S.HerrD. R. (2019). Role of the prefrontal cortex in pain processing. Mol. Neurobiol.56, 1137–1166. doi: 10.1007/s12035-018-1130-9
118
OzawaS.KamiyaH.TsuzukiK. (1998). Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol.54, 581–618. doi: 10.1016/S0301-0082(97)00085-3
119
PalmerA. M.RobichaudP. J.ReiterC. T. (1994). The release and uptake of excitatory amino acids in rat brain: effect of aging and oxidative stress. Neurobiol. Aging15, 103–111. doi: 10.1016/0197-4580(94)90150-3
120
ParkS. H.BakerA. K.KrishnaV.MackeyS. C.MartucciK. T. (2022). Altered resting-state functional connectivity within corticostriatal and subcortical-striatal circuits in chronic pain. Sci. Rep.12:12683. doi: 10.1038/s41598-022-16835-7
121
PeekA. L.LeaverA. M.FosterS.PutsN. A.OeltzschnerG.HendersonL.et al. (2021). Increase in ACC GABA+ levels correlate with decrease in migraine frequency, intensity and disability over time. J. Headache Pain22:150. doi: 10.1186/s10194-021-01352-1
122
PeekA. L.RebbeckT.PutsN. A.WatsonJ.AguilaM. R.LeaverA. M. (2020). Brain GABA and glutamate levels across pain conditions: a systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. NeuroImage210:116532. doi: 10.1016/j.neuroimage.2020.116532
123
PereaG.NavarreteM.AraqueA. (2009). Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci.32, 421–431. doi: 10.1016/j.tins.2009.05.001
124
PetroffO. A. (2002). GABA and glutamate in the human brain. Neuroscientist8, 562–573. doi: 10.1177/1073858402238515
125
PigottT.McPeakA.de ChastelainA.DeMayoM. M.RasicN.RaynerL.et al. (2023). Changes in brain GABA and glutamate and improvements in physical functioning following intensive pain rehabilitation in youth with chronic pain. J. Pain24, 1288–1297. doi: 10.1016/j.jpain.2023.02.027
126
PiotL.HerovenC.BossiS.ZamithJ.MalinauskasT.JohnsonC.et al. (2023). GluD1 binds GABA and controls inhibitory plasticity. Science382, 1389–1394. doi: 10.1126/science.adf3406
127
QianX.ZhaoX.YuL.YinY.ZhangX. D.WangL.et al. (2023). Current status of GABA receptor subtypes in analgesia. Biomed. Pharmacother.168:115800. doi: 10.1016/j.biopha.2023.115800
128
QuidéY.JahanshadN.AndohJ.AntoniouG.ApkarianA. V.AsharY. K.et al. (2024). ENIGMA-chronic pain: a worldwide initiative to identify brain correlates of chronic pain. Pain165, 2662–2666. doi: 10.1097/j.pain.0000000000003317
129
RajaS. N.CarrD. B.CohenM.FinnerupN. B.FlorH.GibsonS.et al. (2020). The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain161, 1976–1982. doi: 10.1097/j.pain.0000000000001939
130
RankinG.ChirilaA. M.EmanuelA. J.ZhangZ.WoolfC. J.DrugowitschJ.et al. (2024). Nerve injury disrupts temporal processing in the spinal cord dorsal horn through alterations in PV(+) interneurons. Cell Rep.43:113718. doi: 10.1016/j.celrep.2024.113718
131
ReidP.SchererK.HalaszD.SimalA. L.TangJ.ZaheerF.et al. (2025). Astrocyte neuronal metabolic coupling in the anterior cingulate cortex of mice with inflammatory pain. Brain Behav. Immun.125, 212–225. doi: 10.1016/j.bbi.2024.12.025
132
RenD.LiJ. N.QiuX. T.WanF. P.WuZ. Y.FanB. Y.et al. (2022). Anterior cingulate cortex mediates hyperalgesia and anxiety induced by chronic pancreatitis in rats. Neurosci. Bull.38, 342–358. doi: 10.1007/s12264-021-00800-x
133
RomanosJ.BenkeD.PietrobonD.ZeilhoferH. U.SantelloM. (2020). Astrocyte dysfunction increases cortical dendritic excitability and promotes cranial pain in familial migraine. Sci. Adv.6:1584. doi: 10.1126/sciadv.aaz1584
134
SaffarpourS.ShaabaniM.NaghdiN.FarahmandfarM.JanzadehA.NasirinezhadF. (2017). In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol. Behav.175, 97–103. doi: 10.1016/j.physbeh.2017.03.025
135
SanganahalliB. G.ChitturiJ.HermanP.ElkabesS.HearyR.HyderF.et al. (2021). Supraspinal sensorimotor and pain-related reorganization after a Hemicontusion rat cervical spinal cord injury. J. Neurotrauma38, 3393–3405. doi: 10.1089/neu.2021.0190
136
ShackmanA. J.SalomonsT. V.SlagterH. A.FoxA. S.WinterJ. J.DavidsonR. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci.12, 154–167. doi: 10.1038/nrn2994
137
ShaoF. B.FangJ. F.WangS. S.QiuM. T.XiD. N.JinX. M.et al. (2021). Anxiolytic effect of GABAergic neurons in the anterior cingulate cortex in a rat model of chronic inflammatory pain. Mol. Brain14:139. doi: 10.1186/s13041-021-00849-9
138
ShaoS.ZhengY.FuZ.WangJ.ZhangY.WangC.et al. (2023). Ventral hippocampal CA1 modulates pain behaviors in mice with peripheral inflammation. Cell Rep.42:112017. doi: 10.1016/j.celrep.2023.112017
139
ShenW.ChenF.TangY.ZhaoY.ZhuL.XiangL.et al. (2025). mGluR5-mediated astrocytes hyperactivity in the anterior cingulate cortex contributes to neuropathic pain in male mice. Commun Biol.8:266. doi: 10.1038/s42003-025-07733-5
140
SinghA.PatelD.LiA.HuL.ZhangQ.LiuY.et al. (2020). Mapping cortical integration of sensory and affective pain pathways. Curr. Biol.30, 1703–1715. doi: 10.1016/j.cub.2020.02.091
141
SmithM. L.AsadaN.MalenkaR. C. (2021). Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science371, 153–159. doi: 10.1126/science.abe3040
142
SongQ.WeiA.XuH.GuY.JiangY.DongN.et al. (2024). An ACC-VTA-ACC positive-feedback loop mediates the persistence of neuropathic pain and emotional consequences. Nat. Neurosci.27, 272–285. doi: 10.1038/s41593-023-01519-w
143
StærmoseT. G.KnudsenM. K.KaschH.BlicherJ. U. (2019). Cortical GABA in migraine with aura -an ultrashort echo magnetic resonance spectroscopy study. J. Headache Pain20:110. doi: 10.1186/s10194-019-1059-z
144
StegemannA.LiuS.Retana RomeroO. A.OswaldM. J.HanY.BerettaC. A.et al. (2023). Prefrontal engrams of long-term fear memory perpetuate pain perception. Nat. Neurosci.26, 820–829. doi: 10.1038/s41593-023-01291-x
145
SunT.WangJ.LiX.LiY. J.FengD.ShiW. L.et al. (2016). Gastrodin relieved complete Freund's adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int. Immunopharmacol.41, 66–73. doi: 10.1016/j.intimp.2016.10.020
146
TakedaI.YoshiharaK.CheungD. L.KobayashiT.AgetsumaM.TsudaM.et al. (2022). Controlled activation of cortical astrocytes modulates neuropathic pain-like behaviour. Nat. Commun.13:4100. doi: 10.1038/s41467-022-31773-8
147
TalbotJ. D.MarrettS.EvansA. C.MeyerE.BushnellM. C.DuncanG. H. (1991). Multiple representations of pain in human cerebral cortex. Science251, 1355–1358. doi: 10.1126/science.2003220
148
TanL. L.KunerR. (2021). Neocortical circuits in pain and pain relief. Nat. Rev. Neurosci.22, 458–471. doi: 10.1038/s41583-021-00468-2
149
TanL.TringE.RingachD. L.ZipurskyS. L.TrachtenbergJ. T. (2020). Vision changes the cellular composition of binocular circuitry during the critical period. Neuron108, 735–47.e6. doi: 10.1016/j.neuron.2020.09.022
150
TemmermandR.BarrettJ. E.FontanaA. C. K. (2022). Glutamatergic systems in neuropathic pain and emerging non-opioid therapies. Pharmacol. Res.185:106492. doi: 10.1016/j.phrs.2022.106492
151
ThiaucourtM.ShabesP.SchlossN.SackM.BaumgärtnerU.SchmahlC.et al. (2018). Posterior insular GABA levels inversely correlate with the intensity of experimental mechanical pain in healthy subjects. Neuroscience387, 116–122. doi: 10.1016/j.neuroscience.2017.09.043
152
ThompsonJ. M.NeugebauerV. (2019). Cortico-limbic pain mechanisms. Neurosci. Lett.702, 15–23. doi: 10.1016/j.neulet.2018.11.037
153
TremblayR.LeeS.RudyB. (2016). GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron91, 260–292. doi: 10.1016/j.neuron.2016.06.033
154
TullisJ. E.LarsenM. E.RumianN. L.FreundR. K.BoxerE. E.BrownC. N.et al. (2023). LTP induction by structural rather than enzymatic functions of CaMKII. Nature621, 146–153. doi: 10.1038/s41586-023-06465-y
155
Vélez-FortM.AudinatE.AnguloM. C. (2012). Central role of GABA in neuron-glia interactions. Neuroscientist18, 237–250. doi: 10.1177/1073858411403317
156
WangL.PuH.ZhouJ.LiuW.ZhangS.TanQ.et al. (2024). Abnormal metabolites in the dorsolateral prefrontal cortex of female epilepsy patients with migraine without aura. Neuroreport35, 1155–1162. doi: 10.1097/WNR.0000000000002110
157
WangJ.TuJ.CaoB.MuL.YangX.CongM.et al. (2017). Astrocytic l-lactate signaling facilitates amygdala-anterior cingulate cortex synchrony and decision making in rats. Cell Rep.21, 2407–2418. doi: 10.1016/j.celrep.2017.11.012
158
WangW.ZhangX.BaiX.ZhangY.YuanZ.TangH.et al. (2022). Gamma-aminobutyric acid and glutamate/glutamine levels in the dentate nucleus and periaqueductal gray with episodic and chronic migraine: a proton magnetic resonance spectroscopy study. J. Headache Pain23:83. doi: 10.1186/s10194-022-01452-6
159
WaqasM.GaoS.Iram UsS.AliM. K.MaY.LiW. (2018). Inner ear hair cell protection in mammals against the noise-induced cochlear damage. Neural Plast.2018:3170801. doi: 10.1155/2018/3170801
160
WatsonC. J. (2016). Insular balance of glutamatergic and GABAergic signaling modulates pain processing. Pain157, 2194–2207. doi: 10.1097/j.pain.0000000000000615
161
WeiX.CentenoM. V.RenW.BorrutoA. M.ProcissiD.XuT.et al. (2021). Activation of the dorsal, but not the ventral, hippocampus relieves neuropathic pain in rodents. Pain162, 2865–2880. doi: 10.1097/j.pain.0000000000002279
162
WeiN.GuoZ.QiuM.YeR.ShaoX.LiangY.et al. (2024). Astrocyte activation in the ACC contributes to comorbid anxiety in chronic inflammatory pain and involves in the excitation-inhibition imbalance. Mol. Neurobiol.61, 6934–6949. doi: 10.1007/s12035-024-04027-5
163
WeiX. Y.WangX.ShiG. X.TuJ. F.YangJ. W.RenM. M.et al. (2024). Acupuncture modulation of chronic neuropathic pain and its association with brain functional properties. J. Pain25:104645. doi: 10.1016/j.jpain.2024.104645
164
WeizmanL.DayanL.BrillS.Nahman-AverbuchH.HendlerT.JacobG.et al. (2018). Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology91, e1285–e1294. doi: 10.1212/WNL.0000000000006293
165
WenY.DongZ.LiuJ.Axerio-CiliesP.DuY.LiJ.et al. (2022). Glutamate and GABA(a) receptor crosstalk mediates homeostatic regulation of neuronal excitation in the mammalian brain. Signal Transduct. Target. Ther.7:340. doi: 10.1038/s41392-022-01148-y
166
WidmanA. J.BasharT.BurtonA.ClausenD. M.GuptaP.WolfD. K.et al. (2025). Chronic, battery-free, fully implantable multimodal spinal cord stimulator for pain modulation in small animal models. Adv. Sci.12:e2415963. doi: 10.1002/advs.202415963
167
WuY.FuD.GuQ.LiY.QianZ.HanJ.et al. (2020). Activation of CB1 receptors on GABAergic interneurons in the ventrolateral orbital cortex induces analgesia. Neurosci. Lett.736:135286. doi: 10.1016/j.neulet.2020.135286
168
WuS.RenX.ZhuC.WangW.ZhangK.LiZ.et al. (2022). A c-Fos activation map in nitroglycerin/levcromakalim-induced models of migraine. J. Headache Pain23:128. doi: 10.1186/s10194-022-01496-8
169
WuL. J.SteenlandH. W.KimS. S.IsiegasC.AbelT.KaangB. K.et al. (2008). Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Mol. Pain4:40. doi: 10.1186/1744-8069-4-40
170
WuX. Q.TanB.DuY.YangL.HuT. T.DingY. L.et al. (2023). Glutamatergic and GABAergic neurons in the vLGN mediate the nociceptive effects of green and red light on neuropathic pain. Neurobiol. Dis.183:106164. doi: 10.1016/j.nbd.2023.106164
171
XiaoS.SunH.ZhuY.ShenZ.ZhuX.YaoP. A.et al. (2023). Electroacupuncture alleviates the relapse of pain-related aversive memory by activating KOR and inhibiting GABAergic neurons in the insular cortex. Cereb. Cortex33, 10711–10721. doi: 10.1093/cercor/bhad321
172
YangS.ChangM. C. (2019). Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int. J. Mol. Sci.20:130. doi: 10.3390/ijms20133130
173
YinJ. B.LiangS. H.LiF.ZhaoW. J.BaiY.SunY.et al. (2020). dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. J. Clin. Invest.130, 6555–6570. doi: 10.1172/JCI127607
174
ZanettiL.RegoniM.RattiE.ValtortaF.SassoneJ. (2021). Presynaptic AMPA receptors in health and disease. Cells10:2260. doi: 10.3390/cells10092260
175
ZengF.ZhangQ.LiuY.SunG.LiA.TalayR. S.et al. (2021). AMPAkines potentiate the corticostriatal pathway to reduce acute and chronic pain. Mol. Brain14:45. doi: 10.1186/s13041-021-00757-y
176
ZhangZ.GadottiV. M.ChenL.SouzaI. A.StemkowskiP. L.ZamponiG. W. (2015). Role of Prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep.12, 752–759. doi: 10.1016/j.celrep.2015.07.001
177
ZhangM. M.GengA. Q.ChenK.WangJ.WangP.QiuX. T.et al. (2022). Glutamatergic synapses from the insular cortex to the basolateral amygdala encode observational pain. Neuron110, 1993–2008. doi: 10.1016/j.neuron.2022.03.030
178
ZhangY.LiX.QiuS.JinR.PengW. (2025). Preemptive transcranial direct current stimulation mitigates susceptibility to persistent pain. Commun. Biol.8:865. doi: 10.1038/s42003-025-08304-4
179
ZhangM.LiC.XueQ.LuC. B.ZhaoH.MengF. C.et al. (2023). Activation of cannabinoid receptor 1 in GABAergic neurons in the rostral anterior insular cortex contributes to the analgesia following common peroneal nerve ligation. Neurosci. Bull.39, 1348–1362. doi: 10.1007/s12264-023-01029-6
180
ZhangQ.MandersT.TongA. P.YangR.GargA.MartinezE.et al. (2017). Chronic pain induces generalized enhancement of aversion. eLife6:25302. doi: 10.7554/eLife.25302
181
ZhangH.ZhaoL.LuX.PengW.ZhangL.ZhangZ.et al. (2024). Multimodal covarying brain patterns mediate genetic and psychological contributions to individual differences in pain sensitivity. Pain165, 1074–1085. doi: 10.1097/j.pain.0000000000003103
182
ZhengY.ZhouY.WuQ.YueJ.YingX.LiS.et al. (2020). Effect of electroacupuncture on the expression of P2 × 4, GABAA γ 2 and long-term potentiation in spinal cord of rats with neuropathic pain. Brain Res. Bull.162, 1–10. doi: 10.1016/j.brainresbull.2020.04.020
183
ZhouW.YeC.WangH.MaoY.ZhangW.LiuA.et al. (2022). Sound induces analgesia through corticothalamic circuits. Science377, 198–204. doi: 10.1126/science.abn4663
184
ZhouQ.ZhongQ.LiuZ.ZhaoZ.WangJ.ZhangZ. (2025). Modulating anxiety-like behaviors in neuropathic pain: role of anterior cingulate cortex astrocytes activation. CNS Neurosci. Ther.31:e70227. doi: 10.1111/cns.70227
185
ZieglerK.FolkardR.GonzalezA. J.BurghardtJ.Antharvedi-GodaS.Martin-CorteceroJ.et al. (2023). Primary somatosensory cortex bidirectionally modulates sensory gain and nociceptive behavior in a layer-specific manner. Nat. Commun.14:2999. doi: 10.1038/s41467-023-38798-7
186
ZielmanR.WijnenJ. P.WebbA.OnderwaterG. L. J.RonenI.FerrariM. D.et al. (2017). Cortical glutamate in migraine. Brain140, 1859–1871. doi: 10.1093/brain/awx130
187
ZugaibJ.CoutinhoM. R.FerreiraM. D.Menescal-de-OliveiraL. (2014). Glutamate/GABA balance in ACC modulates the nociceptive responses of vocalization: an expression of affective-motivational component of pain in guinea pigs. Physiol. Behav.126, 8–14. doi: 10.1016/j.physbeh.2013.12.004
188
ZunhammerM.SchweizerL. M.WitteV.HarrisR. E.BingelU.Schmidt-WilckeT. (2016). Combined glutamate and glutamine levels in pain-processing brain regions are associated with individual pain sensitivity. Pain157, 2248–2256. doi: 10.1097/j.pain.0000000000000634
Summary
Keywords
chronic pain, cerebral cortex, glutamatergic system, GABAergic system, neural network
Citation
Huang D, Dong Y-T, He L-X, Zhou R-Z, Zhou J-X, Yang S and Yu S-G (2025) Decoding chronic pain: the glutamate-GABA tug of war in the cerebral cortex. Front. Mol. Neurosci. 18:1572775. doi: 10.3389/fnmol.2025.1572775
Received
07 February 2025
Accepted
01 July 2025
Published
23 July 2025
Volume
18 - 2025
Edited by
Ildikó Rácz, University Hospital Bonn, Germany
Reviewed by
Ulises Coffeen, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), Mexico
Xueyan Shen, Fudan University, China
Updates
Copyright
© 2025 Huang, Dong, He, Zhou, Zhou, Yang and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Yang, yangsha@cdutcm.edu.cn; Shu-Guang Yu, ysg@cdutcm.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.