Abstract
The gut-brain axis is emerging as a key player in Parkinson's disease (PD), with growing attention on how the gut microbiome (GM) shapes microglial activity, a central driver of neuroinflammation and dopaminergic loss. GM dysbiosis, characterized by reduced beneficial microbes and increased proinflammatory taxa, can compromise intestinal barrier integrity, activate systemic immunity, and prime microglia toward a proinflammatory state, potentially facilitating α-synuclein misfolding and propagation from gut to brain. Preclinical studies reveal that probiotics can rebalance microbial communities, enhance short-chain fatty acid production, reinforce intestinal barrier integrity, and modulate immune responses, effects collectively linked to reduced microglial reactivity, lower α-synuclein aggregation, and improved motor outcomes in PD models. Human trials of probiotic supplementation in PD, primarily investigating gastrointestinal and non-motor symptoms, suggest potential benefits for systemic inflammation and neuroimmune signaling, though direct evidence of central microglial modulation is limited. By synthesizing animal and clinical data, this review underscores both the therapeutic promise of probiotics and identifies current gaps in leveraging microbiota-based interventions as non-invasive, disease-modifying strategies for PD.
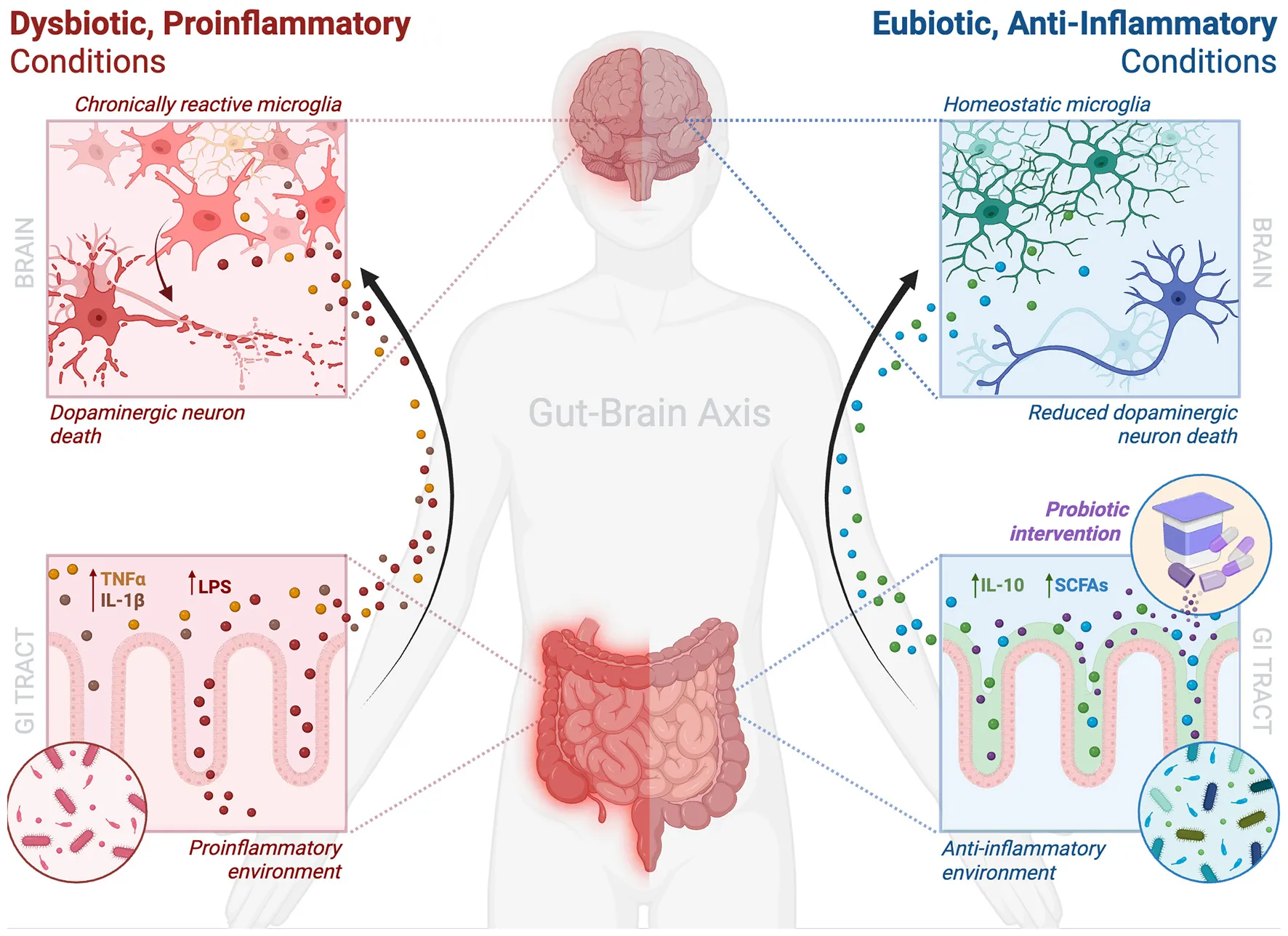
1 Introduction
The human microbiome is a dynamic ecosystem composed of tens of trillions of microorganisms, the majority of which reside in the gastrointestinal (GI) tract, collectively forming the gut microbiome (GM) (Bidell et al., 2022; Sender et al., 2016). Initially recognized for metabolic and digestive roles, the GM influences additional physiological processes such as immune system development and brain function, impacting cognition and neural activity through the gut-brain axis (GBA) (Sanz et al., 2025; Zhang et al., 2025).
Of the GBA's various communication pathways, one connection of increasing interest is the influence of the GM on microglia, the brain's resident immune cells. Microglia are critical regulators of brain health. They sculpt neural circuitry and prune synapses especially during development, and throughout life they continuously survey the central nervous system (CNS) for signs of injury or infection (Augusto-Oliveira et al., 2019; Prinz et al., 2019; Thion et al., 2018a). Importantly, microglia regulate neuroinflammation—a CNS immune response involving glial cells, the release of inflammatory mediators, and the synthesis of reactive oxygen species (ROS) and nitric oxide (NO) in response to trauma, infection, or neurodegenerative disease (Adamu et al., 2024). Research has revealed that microglial development, maturation, and reactivity (marked by upregulation of inflammatory markers and morphological changes such as thicker processes and amoeboid-shaped soma) are governed not solely by local CNS signals but also by cues originating in the gut such as short-chain fatty acids (SCFAs) which are vital microbial metabolites produced by gut bacteria through the fermentation of dietary fibers (Abdel-Haq et al., 2019; Cao et al., 2025; Jurga et al., 2020; Mallick et al., 2024; Silva et al., 2020).
Given their central role in neural health, microglia are increasingly implicated in neurodegenerative diseases such as Parkinson's disease (PD) (Bloem et al., 2021; Gao et al., 2023). PD, a progressive disorder projected to more than double by 2050, is influenced by increasing age and sex, with men consistently being at higher risk (Ben-Shlomo et al., 2024; Ou et al., 2021). Characterized by both motor symptoms (bradykinesia, resting tremor, rigidity, postural instability) and prodromal non-motor symptoms (constipation, cognitive impairment, depression), PD results from the pathogenic loss of dopaminergic neurons in the midbrain (substantia nigra pars compacta) and the toxic accumulation of misfolded alpha-synuclein (αSyn) in Lewy bodies (Bloem et al., 2021; Brás and Outeiro, 2021). Neuroinflammation, particularly from chronically dysregulated microglia, plays a central role in PD progression and genome-wide association studies further implicate microglia by identifying PD risk variants enriched in microglial genes, such as LRRK2, HLA-DRB5, and CD33 (Gelders et al., 2018; Guillot-Sestier and Town, 2018; Masuda et al., 2020; Tansey and Romero-Ramos, 2019).
Probiotics, live microorganisms that can confer health benefits, have gained interest as GBA modulators (Bi et al., 2023; Rosas-Sánchez et al., 2025). By alleviating microbiota dysbiosis (reduced microbial diversity and/or overgrowth of harmful bacteria) and increasing beneficial microbial metabolites, such as SCFAs like butyrate, probiotics may influence microglia by modulating peripheral immune signals, blood–brain barrier (BBB) integrity, and inflammatory microglial gene expression (Alagiakrishnan et al., 2024; Bi et al., 2023). In animal models, specific probiotic strains may even reduce microglial reactivity and proinflammatory cytokines in the brain, highlighting their potential to mitigate neuroinflammatory pathways in neurological diseases (Parra et al., 2023; Sun et al., 2021; Tsao et al., 2024).
PD represents a compelling case to explore the probiotic-microglia influence given its rising global incidence, prevalent prodromal gut-related symptoms, and largely idiopathic nature. Thus, this mini review synthesizes current evidence linking probiotic interventions to microglial modulation in PD, highlighting emerging therapeutic possibilities within the GBA. Given limited human studies in this area, findings from mammalian models are also considered to provide a comprehensive overview and guide future preclinical and clinical research directions.
2 Gut-brain mechanisms of communication
The human gut harbors an incredibly diverse community of bacteria, fungi, parasites, viruses and protozoa that form the GM and contribute to the synthesis of bioactive compounds such as SCFAs, neurotransmitters, tryptophan metabolites, and select vitamins, all essential to host physiology (Pedroza Matute and Iyavoo, 2023; Thursby and Juge, 2017). Microbiota homeostasis is critical for both GM and host health (Khalil et al., 2024). While considerable inter-individual variation exists, research has attempted to identify common microbial patterns associated with health and disease (Pedroza Matute and Iyavoo, 2023). For instance, physiological stress has been correlated with decreased Lactobacilli spp., and reduced Faecalibacterium prausnitzii has been found in patients with Crohn's disease (Bhattarai et al., 2017; Sokol et al., 2008). Unfortunately, no universally consistent microbial signature has been established as a reliable biomarker for disease. This is likely due to the GM's dynamic, complex nature which is shaped by many factors such as diet, environment, genetics, and lifestyle, ultimately complicating causal inferences (Duvallet et al., 2017; Lloyd-Price et al., 2016).
The GBA is a widely studied model for understanding how microbiota shifts influence CNS health. Communication along this axis occurs in a multifaceted manner with three key mechanisms mediating the GM's impact on the brain: immune modulation by microbial products such as SCFAs and lipopolysaccharides (LPS), neural signaling via the vagus nerve, and direct action of microbial metabolites (Fung et al., 2017; Sharon et al., 2016). SCFAs, butyrate being the most well studied, modulate microglial activity and promote anti-inflammatory states, while microbial tryptophan metabolites influence serotonergic signaling and CNS immune tone (Abdel-Haq et al., 2019; O'Mahony et al., 2015). LPS, an outer membrane component of Gram-negative bacteria, can enter systemic circulation during dysbiosis, activating peripheral immunity and promoting neuroinflammation and microglial priming (Banks, 2015; Zhao et al., 2017). Finally, the vagus nerve provides a direct gut-brain conduit, with subdiaphragmatic vagotomy eliminating behavioral and neurochemical effects of certain probiotics in mice, underscoring its role in transmitting microbial signals (Bravo et al., 2011; Carabotti et al., 2015; Zhang et al., 2020). Notably, this communication is bidirectional: the GM influences brain function, while neurological and psychological states, via stress hormones and autonomic signaling, shape the gut environment (Shekarabi et al., 2024).
Among the many CNS components influenced by gut-derived signals, microglia act as a key interface between the gut and the brain. Beyond synaptic remodeling, immune surveillance, and neuroinflammatory responses, evidence from emerging mammalian and human studies suggest that GM-derived signals shape microglial maturation and function, in health and disease (Abdel-Haq et al., 2019; Thion et al., 2018b). In germ-free (GF) mice—raised without a gut microbiota—microglia develop abnormally, with altered morphology, gene expression, and impaired immune responses. Interestingly, these deficits can be partially reversed by colonization with a conventional microbiota or supplementation with SCFAs, suggesting that gut-derived signals are not merely permissive but actively instructive for proper microglial function (Abdel-Haq et al., 2019; Erny et al., 2015).
3 The role of microglia across development and adulthood
Microglia are highly specialized immune cells that enter the CNS early in embryonic development and persist as resident cells throughout life (Prinz et al., 2019). Originating from yolk sac progenitors, they populate the brain prior to the formation of the BBB and continuously survey the CNS microenvironment (Prinz et al., 2019; Tay et al., 2017). In addition to maintaining homeostasis, supporting neuronal development and synaptic pruning, microglia are dynamic integrators of peripheral signals, including those from the GM (Wang et al., 2018). Their capacity to respond adaptively to both endogenous and exogenous stimuli allows microglia to maintain CNS health under physiological conditions and mount appropriate responses to injury or disease (D'Alessandro et al., 2022; Salter and Stevens, 2017).
Neuroinflammation is a common feature across many neurodegenerative disorders, including PD. In this context, microglia are considered as key orchestrators. When exposed to stimuli such as misfolded proteins like αSyn, environmental toxicants, systemic inflammation, or foreign microbial metabolites, microglia can shift into a proinflammatory phenotype (Gao et al., 2023; Perry and Teeling, 2013). This is characterized by increased expression of surface receptors (e.g., Toll-like receptor 4 (TLR4)), production of proinflammatory cytokines (IL-1β, TNF-α, IL-6), and generation of ROS and NO, contributing to oxidative stress and neuronal injury (Franklin et al., 2021; Gao et al., 2023). Although acute inflammation aids in repair and pathogen clearance, chronic or inappropriate microglial reactivity can exacerbate neuronal damage (Gao et al., 2023; Ransohoff, 2016).
Importantly, microglia do not operate in isolation. Their inflammatory responses and physiological activities can be shaped by systemic factors, including signals from the GM. As shown in GF mice, microglia exhibit altered morphology and impaired immune responses, effects that can be partially rescued by microbial colonization or SCFA supplementation, supporting the critical role of the GM in neuroimmune homeostasis (Caetano-Silva et al., 2023; Dalile et al., 2019; Erny et al., 2015). Human and translational studies further link gut microbial alterations with changes in neuroinflammatory tone and microglial reactivity across neurological disorders (Morais et al., 2021).
The responsiveness of microglia to microbial signals positions them as a cellular interface of the GBA, allowing gut-derived factors to influence CNS inflammation (Diaz Heijtz, 2016; Sampson and Mazmanian, 2015). In diseases like PD, where chronic microglial reactivity and neuroinflammation are prominent, understanding upstream modulators of microglial function, including the GM, may offer new therapeutic avenues (Wang et al., 2018). As such, microglia represent not only a pathological hallmark but also a potentially modifiable target within the GBA, especially through interventions like probiotics that aim to correct gut dysbiosis and reduce neuroinflammatory signaling (Zhu et al., 2024).
4 Consequences of gut dysbiosis in Parkinson's disease
4.1 Gut dysbiosis, barrier dysfunction, and microglial reactivity in Parkinson's disease
GI dysfunction, particularly constipation, is common in the prodromal phase of PD, suggesting a role for the gut in disease onset. In PD, proinflammatory microbial imbalances are thought to compromise gut barrier integrity, alter metabolite production, and disrupt immune signaling, with downstream consequences for CNS function (Cryan et al., 2019; Nie and Ge, 2023). Evidence from animal models supports this link: transplants from PD patients into GF, αSyn-overexpressing (ASO) mice worsen motor deficits and drive microglia toward a proinflammatory state (Cryan et al., 2019; Sampson et al., 2016). In humans, sequencing and metagenomic studies in patients with PD revealed decreased abundance of SCFA-producing genera and increases in proinflammatory taxa (Boktor et al., 2023; Pavan et al., 2023). Across geographically diverse cohorts, these shifts point toward a reproducible dysbiotic signature that may actively contribute to PD pathogenesis, potentially through microglial priming and promoting neuroinflammation.
A key consequence of gut dysbiosis is impaired intestinal barrier integrity. Loss of SCFA-producing microbes, particularly butyrate-producers, can weaken the mucus layer via decreased mucin production—glycoproteins that form the mucus layer to protect intestinal epithelium—and impair tight junction assembly via downregulation of key proteins such as zonula occludens-1 (ZO-1), occludin, and claudin-1 (Di Vincenzo et al., 2024; Kang et al., 2022; Peng et al., 2009; Pérez-Reytor et al., 2021; Wang et al., 2012). This allows for the translocation of microbial metabolites and pathogen-associated molecular patterns (PAMPs) such as LPS into the lamina propria and systemic circulation. There, these molecules engage pattern recognition receptors (PRRs) like TLR4, driving release of proinflammatory cytokines (TNF-α, IL-1β, IL-6) (Di Vincenzo et al., 2024). Resulting peripheral immune activation can prime microglia, lowering their ‘activation' threshold and increasing susceptibility to neuroinflammatory cascades in PD (Chen et al., 2021; Dogra et al., 2021). Supporting this, patients with PD exhibited increased intestinal permeability, reduced colonic ZO-1 expression, elevated mucosal TLR4+ cells, and decreased butyrate-producing bacteria (Perez-Pardo et al., 2019). Further, in a rotenone-induced PD mouse model, TLR4-knockout mice were protected against phagocytic, proinflammatory microglial activity, dopaminergic neuronal loss, and motor impairments, reinforcing the link between gut barrier dysfunction, microglial activity, and neuroinflammation in PD (Perez-Pardo et al., 2019).
Peripheral inflammation affects the brain both directly and indirectly. Circulating cytokines and LPS can cross and potentially disrupt the BBB, altering endothelial and astrocytic modulation and increasing cerebral prostaglandin E2 (Gryka-Marton et al., 2025; Varatharaj and Galea, 2017). Similarly, peripheral cytokines enhance BBB permeability and promote endothelial activation, establishing a neuroinflammatory milieu (Varatharaj and Galea, 2017). Microglia are exquisitely sensitive to such inflammatory cues, particularly LPS and TNF-α, which can act on microglia via TLR4, leading to the production of IL-1β, IL-6, TNF-α, and ROS (Walker et al., 2014; Woodburn et al., 2021). This chronically reactive state enhances phagocytosis, antigen presentation, and upregulates CD86 (T-cell co-stimulation) and CD68 (phagolysosomal activity), ultimately promoting dopaminergic neurotoxicity and impaired homeostatic surveillance (Fornari Laurindo et al., 2023). Preclinical studies have demonstrated that peripheral LPS induces microglial reactivity and accelerates dopaminergic cell loss across various PD models while TLR4 inhibition reduces microglial proinflammatory activity and neurodegeneration (García-Domínguez et al., 2018; Perez-Pardo et al., 2019; Xie et al., 2023; Zhang et al., 2022). Further, GF mice, which display immature microglia, regain normal inflammatory responsiveness after colonization with a conventional microbiota or SCFA supplementation, reinforcing the importance of microbial signals in microglial homeostasis (Erny et al., 2015).
4.2 α-Synuclein pathology and gut-brain propagation
Alongside neuroinflammation, PD is defined by the aggregation of αSyn into Lewy bodies and neurites (Tansey and Romero-Ramos, 2019; Xiang, 2025). Growing evidence suggests that αSyn misfolding may begin in the gut, having been detected in GI tract and brain of patients with PD prior to onset of motor symptoms (Brás and Outeiro, 2021; Chen and Lin, 2022; de Lataillade et al., 2020; Nie and Ge, 2023). It is hypothesized the GM dysbiosis may drive this process: the loss of anti-inflammatory and SCFA-producing bacteria stresses enteric neurons, inducing αSyn expression, misfolding, and aggregation (Chen and Lin, 2022; Claudino dos Santos et al., 2023). Supporting this, in a rotenone PD model, bacterial endotoxins activated signaling pathways that promoted αSyn aggregation, an effect reversed by antibiotics or fecal transplants from healthy donors (Fang et al., 2024). Notably, LPS may further accelerate this process by binding directly to αSyn and facilitating amyloid fibril formation (Bhattacharyya et al., 2019).
Preclinical and clinical studies provide further support for gut-to-brain spread. Following duodenal human αSyn injections, αSyn fibrils were detected in regions of the vagus nerve connected to the gut in transgenic rats, a process prevented by vagotomy (Kim et al., 2019; Van Den Berge et al., 2019). In human patients, two independent studies of vagotomised patients suggested a reduced PD risk, though long-term follow-up indicated this protection may not be absolute (Liu et al., 2017; Svensson et al., 2015; Tysnes et al., 2015). Together, this research suggests that GM imbalances not only foster αSyn misfolding locally but set the stage for its propagation to the brain.
4.3 Clinical implications and therapeutic potential
Recognition of the GBA in PD opens new avenues for clinical intervention, shifting attention toward earlier diagnosis, disease modification, and even, potential prevention. GI αSyn pathology, barrier dysfunction, and gut dysbiosis offer a window into the prodromal phase of PD, sparking interest in identifying early biomarkers of GBA dysfunction, such as altered SCFA levels, increased fecal zonulin (intestinal permeability marker), or loss of butyrate-producing bacteria, that could stratify PD risk or monitor therapeutic response. However, biomarker standardization is lacking and their predictive power for PD progression remains uncertain (Aho et al., 2021; Nie et al., 2022).
Therapeutically, direct manipulation of the GM is under investigation. Beyond current dopaminergic symptom management, mounting evidence suggests that gut-level interventions may reduce systemic inflammation, microglial phenotypic transformation, and αSyn pathology (Alam et al., 2024; Bloem et al., 2021). Probiotics thus offer an attractive approach due to their non-invasive, relatively low-risk, modifiable nature, and potential to reshape microbial ecology and host immune responses. Preclinical and small human studies suggest they may:
-
Restore gut barrier function by upregulating expression of tight junction proteins like occludin and ZO-1;
-
Reduce systemic and neuroinflammation by modulating cytokine production and microglial reactivity;
-
Suppress αSyn misfolding and propagation via SCFA-mediated effects on protein homeostasis and enteric neuronal stress (Ahn et al., 2022; Lorente-picón and Laguna, 2021; Zhu et al., 2022).
However, large-scale human trials are needed to determine whether probiotic use can meaningfully alter the trajectory of PD beyond merely GI symptom relief (Leta et al., 2021).
5 Probiotics as modulators of microglia in Parkinson's disease
A healthy GM is essential for numerous biological processes, including proper microglial development and function (Erny et al., 2015). In PD, where microglial dysfunction contributes to dopaminergic neurodegeneration, targeting microglia via the GM has gained increasing interest (Alam et al., 2024; Salim et al., 2023). Probiotics offer a promising, non-invasive approach to restoring microbial balance, enhancing gut barrier integrity, and modulating immune and microglial responses, mechanisms thought to affect both the prodromal and symptomatic stages of PD (Leta et al., 2021). Although several clinical trials have evaluated probiotics in PD, most have not directly assessed microglial activity, limiting insight into their central immunomodulatory effects (Magistrelli et al., 2024; Yang et al., 2023; Zali et al., 2024). Consequently, much of our understanding arises from preclinical research. This section highlights key evidence from human and animal studies on probiotics in PD, focusing on how restoring a balanced GM may ameliorate disease symptoms by promoting microglial homeostasis and dampening neuroinflammation.
Animal studies provide compelling evidence that probiotics modulate microglial function and attenuate neuroinflammation in PD models. In ASO mice, prebiotics (non-digestible fibers promoting beneficial bacterial growth) and probiotics reduced microglial reactivity, normalized neuroimmune signaling, and improved motor outcomes (Abdel-Haq et al., 2022; Parra et al., 2023). Abdel-Haq et al. demonstrated that a prebiotic-enriched diet restored microglial homeostasis, reduced αSyn aggregation in the substantia nigra, and improved motor function, effects further negated by microglial depletion, confirming their central role in neuroprotection (Abdel-Haq et al., 2022). In an inflammatory PD rat model (intra-striatal LPS injection), Parra et al. reported that Microbiot® (Lactobacillus rhamnosus GG and Bifidobacterium animalis lactis) reduced proinflammatory microglial responses, although dopaminergic neuron degeneration persisted (Parra et al., 2023). Similarly, Symprove™, a commercial probiotic formulation, decreased proinflammatory microglial markers, circulating proinflammatory cytokines and LPS, and dopaminergic neuron loss in an early-stage neurotoxin-induced rat model (Sancandi et al., 2023). Sun et al. demonstrated that Clostridium butyricum supplementation in MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine)-induced PD mice decreased motor deficits and reversed proinflammatory microglial phenotypes and neuroinflammation through GM modulation and enhancing glucagon-like peptide-1 (GLP-1) signaling (Sun et al., 2021). Collectively, these studies indicate that probiotics act via the GBA to shape microglial activity and confer neuroprotection in PD.
Translating preclinical findings to humans remains a challenge, but emerging trials suggest clinical relevance with several reporting improvements in motor symptoms, inflammatory markers, and gut barrier integrity. A randomized controlled trial (RCT) of 128 patients with PD found that Lacticaseibacillus paracasei strain Shirota significantly improved constipation and non-motor symptoms without causing major shifts in GM composition (Yang et al., 2023). Notably, fecal L-tyrosine decreased while plasma L-tyrosine increased, suggesting enhanced absorption and potential support for neurotransmitter synthesis (Yang et al., 2023). Another RCT of 40 patients reported that a Bifidobacterium cocktail improved motor and non-motor symptoms and reduced plasma proinflammatory cytokines (Magistrelli et al., 2024). Further, a cohort study of 46 patients with PD also found that probiotic supplementation with vitamin D lowered levels of proinflammatory cytokines while improving GI and cognitive symptoms, suggesting a systemic, anti-inflammatory effect of probiotics via the GM (Zali et al., 2024).
Together, these clinical and preclinical findings summarized in Table 1 support the hypothesis that probiotics influence central immune responses through peripheral microbial modulation. While further studies, especially in human populations, are needed, current evidence suggests that targeting microglial dysregulation through the GBA may represent a novel therapeutic strategy to slow neuroinflammation and modify disease progression in PD.
Table 1
| Model | Intervention | Key outcomes | Microglia outcome | GBA relevance | Ref. |
|---|---|---|---|---|---|
| Preclinical studies | |||||
| αSyn-overexpressing (ASO) male mice | High-fiber prebiotic-enriched diet | Improved motor function; ↓αSyn aggregation in SN | ↓ Microglial proinflammatory activity; benefits abolished with microglial depletion | Restored microglial homeostasis via GM; increased major SCFAs | Abdel-Haq et al., 2022 |
| LPS-induced PD male Wistar rat model | Microbiot® (L. rhamnosus GG + B. animalis ssp. lactis) | ↓ Striatal microgliosis; no prevention of DA neuron loss | ↑ Proportion of homeostatic microglia | Suggests anti-inflammatory effects via GM modulation | Parra et al., 2023 |
| Early-stage 6-OHDA PD male Wistar rat model | Symprove™ (multi-strain probiotic) | ↓ Motor deficits; ↓ systemic LPS; ↓ dopaminergic neuron loss | ↓ Reactive microglial morphology; ↓ proinflammatory plasma cytokines | Enhanced gut barrier and systemic inflammation control; prevented SCFA decrease | Sancandi et al., 2023 |
| MPTP-induced PD male C57BL/6 mouse model | Clostridium butyricum | Improved motor function; ↓ dopaminergic neuron loss; ↑ GLP-1 signaling | ↓ Microglial proinflammatory responses; ↓ neuroinflammation | Demonstrates GM-GLP-1 pathway in CNS immune regulation | Sun et al., 2021 |
| Clinical studies | |||||
| 128 PD patient RCT | Lacticaseibacillus paracasei strain Shirota fermented milk | Improved constipation and non-motor symptoms | Not investigated | Potential support for neurotransmitter synthesis via GM | Yang et al., 2023 |
| 40 PD patient RCT | Bifidobacterium probiotic cocktail | ↓ Motor and non-motor symptoms; ↓ proinflammatory serum cytokines (IFNγ, IL-6) | Not investigated | Potential influence on neuroimmune interactions via GBA | Magistrelli et al., 2024 |
| 46 PD patients (Iranian cohort) | Probiotic cocktail + vitamin D | ↓ disease severity, non-motor symptoms; ↓ proinflammatory serum cytokines (IFNγ, IL-6, IL-1β); ↑ anti-inflammatory serum cytokine (IL-10) | Not investigated | Modulates gut–immune signaling; reduces systemic inflammation and enhances gut-mediated immune responses via GBA | Zali et al., 2024 |
Overview of preclinical and clinical studies examining probiotics supplementation in Parkinson's disease, with a focus on gut–microglia interactions and neuroinflammatory outcomes.
6 Discussion
Growing evidence supports the existence of a bidirectional GBA in PD, with gut dysbiosis contributing to or potentially even driving microglial dysfunction and neurodegeneration (Pfaffinger et al., 2025; Xiang, 2025). Preclinical studies demonstrate that probiotics have potential to restore GI eubiosis (a balanced, healthy gut microbiota) and dampen microglial-mediated neuroinflammation, improving motor outcomes and reduced α-synuclein aggregation, a hallmark of PD. However, translating these findings to the clinic remains complex.
A lack of standardization in probiotics research regarding strain specificity, dosage, treatment duration, and outcome measures limits reproducibility, cross-study comparisons and strain-specific effects (Rosas-Sánchez et al., 2025; Smolinska et al., 2025). More studies comparing different types of probiotics are needed to determine which strains ameliorate PD symptoms and to clarify their mechanisms of action on the GBA. Additionally, most clinical trials focus on symptomatic improvements and peripheral immune markers, with few directly assessing microglial activity or central inflammation. Techniques such as positron emission tomography (PET) using TSPO-targeting radioligands (translocator protein notably upregulated in reactive microglia) and emerging radioligands, or magnetic resonance imaging (MRI) sensitive to microglial morphology and neuroinflammatory signatures, could assess microglia-specific changes in vivo (Garcia-Hernandez et al., 2022; Guilarte, 2019; Lavisse et al., 2021). Currently, this gap hinders the mechanistic validation of probiotics' neuroimmune benefits in humans, even as peripheral changes suggest indirect support for such effects.
While targeting microglia with probiotics is promising, further high-quality, mechanistically informed trials are needed. Future research should identify reliable, non-invasive biomarkers such as SCFA levels, fecal calprotectin (a marker of intestinal inflammation), or gut permeability markers to detect early GBA dysfunction and monitor therapeutic response (Chai et al., 2025). Emerging neuroimaging techniques and cerebrospinal fluid-based assays may allow researchers to assess microglial activity more directly in vivo. Integrative approaches combining probiotics with prebiotics, dietary modulation, or agents like GLP-1 agonists, which enhance gut barrier function and reduce neuroinflammation, may yield synergistic benefits for both gut and brain health (Loh et al., 2024; Menozzi et al., 2025). With appropriate clinical tools and biomarkers, probiotics may one day complement conventional PD therapies by targeting both motor and non-motor symptoms at their neuroimmune root.
Statements
Author contributions
IK: Writing – original draft, Writing – review & editing. M-ÈT: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. M-ÈT is a Tier 2 Canada Research Chair in Neurobiology of Aging and Cognition.
Acknowledgments
We respectfully acknowledge that the University of British Columbia's Vancouver campus is located on the traditional, ancestral, and unceded territory of the xwmɘθkwɘýɘm (Musqueam) People. We also acknowledge with gratitude the Lɘkwɘηɘn (Songhees and Xwsepsɘm/Esquimalt) Peoples on whose territory the University of Victoria stands, and the Lɘkwɘηɘn and WSÁNEC Peoples whose historical relationships with the land continue to this day.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
αSyn, alpha-synuclein; ASO, alpha-synuclein overexpressing; SN, substantia nigra; GM, gut microbiome; SCFA, short-chain fatty acid; LPS, lipopolysaccharide; DA, dopamine; OHDA, 6-hydroxydopamine; GLP-1, glucagon-like peptide-1; CNS, central nervous system; RCT, randomized controlled trial; IFNγ, interferon gamma; IL, interleukin.
References
1
Abdel-Haq R. Schlachetzki J.C.M. Glass C.K. Mazmanian S.K. (2019). Microbiome–microglia connections via the gut-brain axis. J. Exp. Med. 216, 41–59. 10.1084/jem.20180794
2
Abdel-Haq R. Schlachetzki J. C. M. Boktor J. C. Cantu-Jungles T. M. Thron T. Zhang M. et al . (2022). A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. Elife11:e81453. 10.7554/eLife.81453.sa2
3
Adamu A. Li S. Gao F. Xue G. (2024). The role of neuroinflammation in neurodegenerative diseases: current understanding and future therapeutic targets. Front. Aging Neurosci. 16:1347987. 10.3389/fnagi.2024.1347987
4
Ahn S. I. Cho S. Jeon E. Park M. Chae B. Ditengou I. C. P. et al . (2022). The effect of probiotics on intestinal tight junction protein expression in animal models: a meta-analysis. Appl. Sci. Switz. 12:4680. 10.3390/app12094680
5
Aho V. T. E. Houser M. C. Pereira P. A. B. Chang J. Rudi K. Paulin L. et al . (2021). Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol. Neurodegener. 16:6. 10.1186/s13024-021-00427-6
6
Alagiakrishnan K. Morgadinho J. Halverson T. (2024). Approach to the diagnosis and management of dysbiosis. Front. Nutr. 11:1330903. 10.3389/fnut.2024.1330903
7
Alam M. Abbas K. Mustafa M. Usmani N. Habib S. (2024). Microbiome-based therapies for Parkinson's disease. Front. Nutr. 11:1496616. 10.3389/fnut.2024.1496616
8
Augusto-Oliveira M. Arrifano G. P. Lopes-Araújo A. Santos-Sacramento L. Takeda P. Y. Anthony D. C. et al . (2019). What do microglia really do in healthy adult brain?Cells8:1293. 10.3390/cells8101293
9
Banks W. A. (2015). The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain. Behav. Immun. 44, 1–8. 10.1016/j.bbi.2014.08.007
10
Ben-Shlomo Y. Darweesh S. Llibre-Guerra J. Marras C. San Luciano M. Tanner C. et al . (2024). The epidemiology of Parkinson's disease. Lancet403, 283–292. 10.1016/S0140-6736(23)01419-8
11
Bhattacharyya D. Mohite G. M. Krishnamoorthy J. Gayen N. Mehra S. Navalkar A. et al . (2019). Lipopolysaccharide from gut microbiota modulates α-synuclein aggregation and alters its biological function. ACS Chem. Neurosci. 10, 2229–2236. 10.1021/acschemneuro.8b00733
12
Bhattarai Y. Pedrogo D. A. M. Kashyap P. C. (2017). Irritable bowel syndrome: a gut microbiota-related disorder?Am. J. Physiol. Gastrointest. Liver Physiol.312, 52–62. 10.1152/ajpgi.00338.2016
13
Bi M. Liu C. Wang Y. Liu S. J. (2023). Therapeutic prospect of new probiotics in neurodegenerative diseases. Microorganisms11:1527. 10.3390/microorganisms11061527
14
Bidell M. R. Hobbs A. L. V. Lodise T. P. (2022). Gut microbiome health and dysbiosis: a clinical primer. Pharmacotherapy42, 849–857. 10.1002/phar.2731
15
Bloem B. R. Okun M. S. Klein C. (2021). Parkinson's disease. Lancet397, 2284–2303. 10.1016/S0140-6736(21)00218-X
16
Boktor J. C. Sharon G. Verhagen Metman L. A. Hall D. A. Engen P. A. Zreloff Z. et al . (2023). Integrated multi-cohort analysis of the Parkinson's disease gut metagenome. Mov. Disord. 38, 399–409. 10.1002/mds.29300
17
Brás I. C. Outeiro T. F. (2021). Alpha-synuclein: mechanisms of release and pathology progression in synucleinopathies. Cells10, 1–19. 10.3390/cells10020375
18
Bravo J. A. Forsythe P. Chew M. V. Escaravage E. Savignac H. M. Dinan T. G. et al . (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 108, 16050–16055. 10.1073/pnas.1102999108
19
Caetano-Silva M. E. Rund L. Hutchinson N. T. Woods J. A. Steelman A. J. Johnson R. W. et al . (2023). Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 13:1053. 10.1038/s41598-022-27086-x
20
Cao Q. Shen M. Li R. Liu Y. Zeng Z. Zhou J. et al . (2025). Elucidating the specific mechanisms of the gut-brain axis: the short-chain fatty acids-microglia pathway. J. Neuroinflamm.22:133. 10.1186/s12974-025-03454-y
21
Carabotti M. Scirocco A. Antonietta Maselli M. Severi C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol.28, 203–209.
22
Chai Z. Ouyang Y. Debebe A. Picker M. Lee W-. J. Fenton S. et al . (2025). Intestinal biomarkers, microbiota composition, and genetic predisposition to inflammatory bowel disease as predictors of Parkinson's disease manifestation. J. Park. Dis. 15, 766–779. 10.1177/1877718X251328567
23
Chen S. J. Lin C. H. (2022). Gut microenvironmental changes as a potential trigger in Parkinson's disease through the gut-brain axis. J. Biomed. Sci. 29:43. 10.1186/s12929-022-00839-6
24
Chen X. Feng W. Ou R. Liu J. Yang J. Fu J. et al . (2021). Evidence for peripheral immune activation in Parkinson's disease. Front. Aging Neurosci. 13, 617370. 10.3389/fnagi.2021.617370
25
Claudino dos Santos J. C. Lima M. P. P. Brito G. A. de C. Viana G.S. de B. (2023). Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 84:101812. 10.1016/j.arr.2022.101812
26
Cryan J. F. O, K.J. M Cowan C.S. Sandhu K.V. S Bastiaanssen T.F. Boehme M. et al . (2019). The microbiota-gut-brain axis. Physiol. Rev.99, 1877–2013. 10.1152/physrev.00018.2018
27
D'Alessandro G. Marrocco F. Limatola C. (2022). Microglial cells: sensors for neuronal activity and microbiota-derived molecules. Front. Immunol. 13:1011129. 10.3389/fimmu.2022.1011129
28
Dalile B. Van Oudenhove L. Vervliet B. Verbeke K. (2019). The role of short-chain fatty acids in microbiota–gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. 10.1038/s41575-019-0157-3
29
de Lataillade A de G. Lebouvier T. Noble W. Leclair-Visonneau L. Derkinderen P. (2020). Enteric synucleinopathy: from trendy concept to real entity. Free Neuropathol. 1, 1–26. 10.17879/freeneuropathology-2020-2920
30
Di Vincenzo F. Del Gaudio A. Petito V. Lopetuso L. R. Scaldaferri F. (2024). Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern. Emerg. Med. 19, 275–293. 10.1007/s11739-023-03374-w
31
Diaz Heijtz R. (2016). Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin. Fetal. Neonatal Med. 21, 410–417. 10.1016/j.siny.2016.04.012
32
Dogra N. Mani R. J. Katare D. P. (2021). The gut-brain axis: two ways signaling in Parkinson's disease. Cell. Mol. Neurobiol. 42, 315–332. 10.1007/s10571-021-01066-7
33
Duvallet C. Gibbons S. M. Gurry T. Irizarry R. A. Alm E. J. (2017). Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8:1784. 10.1038/s41467-017-01973-8
34
Erny D. De Angelis A. L. H. Jaitin D. Wieghofer P. Staszewski O. David E. et al . (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. 10.1038/nn.4030
35
Fang X. Liu S. Muhammad B. Zheng M. Ge X. Xu Y. et al . (2024). Gut microbiota dysbiosis contributes to α-synuclein-related pathology associated with C/EBPβ/AEP signaling activation in a mouse model of Parkinson's disease. Neural Regen. Res. 19, 2081–2088. 10.4103/1673-5374.391191
36
Fornari Laurindo L. Aparecido Dias J. Cressoni Araújo A. Torres Pomini K. Machado Galhardi C. Rucco Penteado Detregiachi C. et al . (2023). Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 14:1305933. 10.3389/fimmu.2023.1305933
37
Franklin H. Clarke B. E. Patani R. (2021). Astrocytes and microglia in neurodegenerative diseases: lessons from human in vitro models. Prog. Neurobiol. 200:101973. 10.1016/j.pneurobio.2020.101973
38
Fung T. C. Olson C. A. Hsiao E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. 10.1038/nn.4476
39
Gao C. Jiang J. Tan Y. Chen S. (2023). Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 8:220. 10.1038/s41392-023-01588-0
40
García-Domínguez I. Veselá K. García-Revilla J. Carrillo-Jiménez A. Roca-Ceballos M. A. Santiago M. et al . (2018). Peripheral inflammation enhances microglia response and nigral dopaminergic cell death in an in vivo MPTP model of Parkinson's disease. Front. Cell. Neurosci. 12:398. 10.3389/fncel.2018.00398
41
Garcia-Hernandez R. Cerdán Cerdá A. Trouve Carpena A. Drakesmith M. Koller K. Jones D. K. et al . (2022). Mapping microglia and astrocyte activation in vivo using diffusion MRI. Sci. Adv. 8:eabq2923. 10.1126/sciadv.abq2923
42
Gelders G. Baekelandt V. Van der Perren A. (2018). Linking neuroinflammation and neurodegeneration in parkinson's disease. J. Immunol. Res. 2018:4784268. 10.1155/2018/4784268
43
Gryka-Marton M. Grabowska A. D. Szukiewicz D. (2025). Breaking the barrier: the role of proinflammatory cytokines in BBB dysfunction. Int. J. Mol. Sci. 26:3532. 10.3390/ijms26083532
44
Guilarte T. R. (2019). TSPO in diverse CNS pathologies and psychiatric disease: a critical review and a way forward. Pharmacol. Ther. 194, 44–58. 10.1016/j.pharmthera.2018.09.003
45
Guillot-Sestier M. V. Town T. (2018). Let's make microglia great again in neurodegenerative disorders. J. Neural Transm. 125, 751–770. 10.1007/s00702-017-1792-x
46
Jurga A. M. Paleczna M. Kuter K. Z. (2020). Overview of general and discriminating markers of differential microglia phenotypes. Front. Cell. Neurosci. 14:198. 10.3389/fncel.2020.00198
47
Kang Y. Park H. Choe B-. H. Kang B. (2022). The role and function of mucins and its relationship to inflammatory bowel disease. Front. Med. 9:848344. 10.3389/fmed.2022.848344
48
Khalil M. Di Ciaula A. Mahdi L. Jaber N. Di Palo D. M. Graziani A. et al . (2024). Unraveling the role of the human gut microbiome in health and diseases. Microorganisms12:2333. 10.3390/microorganisms12112333
49
Kim S. Kwon S. H. Kam T. I. Panicker N. Karuppagounder S. S. Lee S. et al . (2019). Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron103, 627–641.e7. 10.1016/j.neuron.2019.05.035
50
Lavisse S. Goutal S. Wimberley C. Tonietto M. Bottlaender M. Gervais P. et al . (2021). Increased microglial activation in patients with Parkinson disease using [18F]-DPA714 TSPO PET imaging. Parkinsonism Relat. Disord. 82, 29–36. 10.1016/j.parkreldis.2020.11.011
51
Leta V. Ray Chaudhuri K. Milner O. Chung-Faye G. Metta V. Pariante C. M. et al . (2021). Neurogenic and anti-inflammatory effects of probiotics in Parkinson's disease: a systematic review of preclinical and clinical evidence. Brain. Behav. Immun. 98, 59–73. 10.1016/j.bbi.2021.07.026
52
Liu B. Pedersen N. L. Tillander A. Ludvigsson J. F. Ekbom A. Svenningsson P. et al . (2017). Vagotomy and Parkinson disease A Swedish register-based matched-cohort study, Neurology. 88, 1996–2002. 10.1212/WNL.0000000000003961
53
Lloyd-Price J. Abu-Ali G. Huttenhower C. (2016). The healthy human microbiome. Genome Med. 8:51. 10.1186/s13073-016-0307-y
54
Loh J. S. Mak W. Q. Tan L. K. S. Ng C. X. Chan H. H. Yeow S. H. et al . (2024). Microbiota–gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 9:37. 10.1038/s41392-024-01743-1
55
Lorente-picón M. Laguna A. (2021). New avenues for parkinson's disease therapeutics: disease-modifying strategies based on the gut microbiota. Biomolecules11, 1–39. 10.3390/biom11030433
56
Magistrelli L. Contaldi E. Visciglia A. Deusebio G. Pane M. Amoruso A. et al . (2024). The impact of probiotics on clinical symptoms and peripheral cytokines levels in Parkinson's disease: preliminary in vivo data. Brain Sci. 14:1147. 10.3390/brainsci14111147
57
Mallick R. Basak S. Das R. K. Banerjee A. Paul S. Pathak S. et al . (2024). Roles of the gut microbiota in human neurodevelopment and adult brain disorders. Front. Neurosci. 18:1446700. 10.3389/fnins.2024.1446700
58
Masuda T. Sankowski R. Staszewski O. Prinz M. (2020). Microglia heterogeneity in the single-cell era. Cell Rep. 30, 1271–1281. 10.1016/j.celrep.2020.01.010
59
Menozzi E. Schapira A. H. V. Borghammer P. (2025). The gut-brain axis in Parkinson disease: emerging concepts and therapeutic implications. Mov. Disord. Clin. Pract. 12, 904–916. 10.1002/mdc3.70029
60
Morais L. H. Schreiber H. L. Mazmanian S. K. (2021). The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. 10.1038/s41579-020-00460-0
61
Nie S. Ge Y. (2023). The link between the gut microbiome, inflammation, and Parkinson's disease. Appl. Microbiol. Biotechnol. 107, 6737–6749. 10.1007/s00253-023-12789-6
62
Nie S. Wang J. Deng Y. Ye Z. Ge Y. (2022). Inflammatory microbes and genes as potential biomarkers of Parkinson's disease. Npj Biofilms Microbiomes8:50. 10.1038/s41522-022-00367-z
63
O'Mahony S. M. Clarke G. Borre Y. E. Dinan T. G. Cryan J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. 10.1016/j.bbr.2014.07.027
64
Ou Z. Pan J. Tang S. Duan D. Yu D. Nong H. et al . (2021). Global trends in the incidence, prevalence, and years lived with disability of Parkinson's disease in 204 countries/territories from 1990 to 2019. Front. Public Health9:776847. 10.3389/fpubh.2021.776847
65
Parra I. Martínez I. Vásquez-Celaya L. Gongora-Alfaro J. L. Tizabi Y. Mendieta L. et al . (2023). Neuroprotective and immunomodulatory effects of probiotics in a rat model of Parkinson's disease. Neurotox. Res. 41, 187–200. 10.1007/s12640-022-00627-y
66
Pavan S. Gorthi S. P. Prabhu A. N. Das B. Mutreja A. Vasudevan K. et al . (2023). Dysbiosis of the Beneficial gut bacteria in patients with Parkinson's disease from india. Ann. Indian Acad. Neurol. 26, 908–916. 10.4103/aian.aian_460_23
67
Pedroza Matute S. Iyavoo S. (2023). Exploring the gut microbiota: lifestyle choices, disease associations, and personal genomics. Front. Nutr. 10. 10.3389/fnut.2023.1225120
68
Peng L. Li Z. R. Green R. S. Holzman I. R. Lin J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625. 10.3945/jn.109.104638
69
Perez-Pardo P. Dodiya H. B. Engen P. A. Forsyth C. B. Huschens A. M. Shaikh M. et al . (2019). Role of TLR4 in the gut-brain axis in Parkinson's disease: a translational study from men to mice. Gut68, 829–843. 10.1136/gutjnl-2018-316844
70
Pérez-Reytor D. Puebla C. Karahanian E. García K. (2021). Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front. Physiol. 12:650313. 10.3389/fphys.2021.650313
71
Perry V. H. Teeling J. (2013). Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 35, 601–612. 10.1007/s00281-013-0382-8
72
Pfaffinger J. M. Hays K. E. Seeley J. Ramesh Babu P. Ryznar R. (2025). Gut dysbiosis as a potential driver of Parkinson's and Alzheimer's disease pathogenesis. Front. Neurosci. 19:1600148. 10.3389/fnins.2025.1600148
73
Prinz M. Jung S. Priller J. (2019). Microglia biology: one century of evolving concepts. Cell179, 292–311. 10.1016/j.cell.2019.08.053
74
Ransohoff R. M. (2016). How neuroinflammation contributes to neurodegeneration. Science353, 772–777. 10.1126/science.aag2590
75
Rosas-Sánchez G. U. Germán-Ponciano L. J. Puga-Olguín A. Soto M. E. F. Medina A. Y. N. Muñoz-Carillo J. L. et al . (2025). Gut-brain axis in mood disorders: a narrative review of neurobiological insights and probiotic interventions. Biomedicines13:1831. 10.3390/biomedicines13081831
76
Salim S. Ahmad F. Banu A. Mohammad F. (2023). Gut microbiome and Parkinson's disease: perspective on pathogenesis and treatment. J. Adv. Res. 50, 83–105. 10.1016/j.jare.2022.10.013
77
Salter M. W. Stevens B. (2017). Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027. 10.1038/nm.4397
78
Sampson T. R. Debelius J. W. Thron T. Janssen S. Shastri G. G. Ilhan Z. E. et al . (2016). Gut Microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's Disease. Cell167, 1469–1480.e12. 10.1016/j.cell.2016.11.018
79
Sampson T. R. Mazmanian S. K. (2015). Control of brain development, function, and behavior by the microbiome. Cell Host Microbe17, 565–576. 10.1016/j.chom.2015.04.011
80
Sancandi M. De Caro C. Cypaite N. Marascio N. Avagliano C. De Marco C. et al . (2023). Effects of a probiotic suspension SymproveTM on a rat early-stage Parkinson's disease model. Front. Aging Neurosci. 14:986127. 10.3389/fnagi.2022.986127
81
Sanz Y. Cryan J. F. Deschasaux-Tanguy M. Elinav E. Lambrecht R. Veiga P. et al . (2025). The gut microbiome connects nutrition and human health. Nat. Rev. Gastroenterol. Hepatol. 22, 534–555. 10.1038/s41575-025-01077-5
82
Sender R. Fuchs S. Milo R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14:e1002533. 10.1371/journal.pbio.1002533
83
Sharon G. Sampson T. R. Geschwind D. H. Mazmanian S. K. (2016). The central nervous system and the gut microbiome. Cell167, 915–932. 10.1016/j.cell.2016.10.027
84
Shekarabi A. Qureishy I. Puglisi C. H. Dalseth M. Vuong H. E. (2024). Host–microbe interactions: communication in the microbiota–gut-brain axis. Curr. Opin. Microbiol. 80:102494. 10.1016/j.mib.2024.102494
85
Silva Y. P. Bernardi A. Frozza R. L. (2020). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11:25. 10.3389/fendo.2020.00025
86
Smolinska S. Popescu F-. D. Zemelka-Wiacek M. (2025). A review of the influence of prebiotics, probiotics, synbiotics, and postbiotics on the human gut microbiome and intestinal integrity. J. Clin. Med. 14:3673. 10.3390/jcm14113673
87
Sokol H. né dicte Pigneur B. Watterlot L. Lakhdari O. Bermú dez-Humará L.G. Gratadoux J.-J. et al . (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A.105, 16731–16736. 10.1073/pnas.0804812105
88
Sun J. Li H. Jin Y. Yu J. Mao S. Su K. P. et al . (2021). Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson's disease via gut microbiota-GLP-1 pathway. Brain. Behav. Immun. 91, 703–715. 10.1016/j.bbi.2020.10.014
89
Svensson E. Horváth-Puhó E. Thomsen R. W. Djurhuus J. C. Pedersen L. Borghammer P. et al . (2015). Vagotomy and subsequent risk of Parkinson's disease. Ann. Neurol. 78, 522–529. 10.1002/ana.24448
90
Tansey M. G. Romero-Ramos M. (2019). Immune system responses in Parkinson's disease: early and dynamic. Eur. J. Neurosci. 49, 364–383. 10.1111/ejn.14290
91
Tay T. L. Savage J. C. Hui C. W. Bisht K. Tremblay M. È. (2017). Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol. 595, 1929–1945. 10.1113/JP272134
92
Thion M. S. Ginhoux F. Garel S. (2018a). Microglia and early brain development: an intimate journey. Science362, 185–189. 10.1126/science.aat0474
93
Thion M. S. Low D. Silvin A. Chen J. Grisel P. Schulte-Schrepping J. et al . (2018b). Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell172, 500–516.e16. 10.1016/j.cell.2017.11.042
94
Thursby E. Juge N. (2017). Introduction to the human gut microbiota. Biochem. J.474, 1823–1836. 10.1042/BCJ20160510
95
Tsao S. P. Yeh T. H. Lin Y. T. Pan C. H. Lee Y. K. Wu C. H. et al . (2024). Supplementation with Bifidobacterium animalis subsp. lactis MH-022 for remission of motor impairments in a 6-OHDA-induced Parkinson's disease rat model by reducing inflammation, reshaping the gut microbiome, and fostering specific microbial taxa. Food Funct.15, 9368–9389. 10.1039/D4FO02039A
96
Tysnes O. B. Kenborg L. Herlofson K. Steding-Jessen M. Horn A. Olsen J. H. et al . (2015). Does vagotomy reduce the risk of Parkinson's disease?Ann. Neurol. 78, 1011–1012. 10.1002/ana.24531
97
Van Den Berge N. Ferreira N. Gram H. Mikkelsen T. W. Alstrup A. K. O. Casadei N. et al . (2019). Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol.138, 535–550. 10.1007/s00401-019-02040-w
98
Varatharaj A. Galea I. (2017). The blood-brain barrier in systemic inflammation. Brain. Behav. Immun. 60, 1–12. 10.1016/j.bbi.2016.03.010
99
Walker F. R. Beynon S. B. Jones K. A. Zhao Z. Kongsui R. Cairns M. et al . (2014). Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain. Behav. Immun. 37, 1–14. 10.1016/j.bbi.2013.12.010
100
Wang H. B. Wang P. Y. Wang X. Wan Y. L. Liu Y. C. (2012). Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 57, 3126–3135. 10.1007/s10620-012-2259-4
101
Wang Y. Wang Z. Wang Y. Li F. Jia J. Song X. et al . (2018). The gut-microglia connection: implications for central nervous system diseases. Front. Immunol. 9:2325. 10.3389/fimmu.2018.02325
102
Woodburn S. C. Bollinger J. L. Wohleb E. S. (2021). The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J. Neuroinflamm.18:258. 10.1186/s12974-021-02309-6
103
Xiang H. (2025). The interplay between α-synuclein aggregation and necroptosis in Parkinson's disease: a spatiotemporal perspective. Front. Neurosci. 19:1567445. 10.3389/fnins.2025.1567445
104
Xie J. Tuo P. Zhang W. Wang S. (2023). Inhibition of the TLR4/NF-κB pathway promotes the polarization of LPS-induced BV2 microglia toward the M2 phenotype. NeuroReport34, 834–844. 10.1097/WNR.0000000000001961
105
Yang X. He X. Xu S. Zhang Y. Mo C. Lai Y. et al . (2023). Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson's disease. Food Funct. 14, 6828–6839. 10.1039/D3FO00728F
106
Zali A. Hajyani S. Salari M. Tajabadi-Ebrahimi M. Mortazavian A. M. Pakpour B. et al . (2024). Co-administration of probiotics and vitamin D reduced disease severity and complications in patients with Parkinson's disease: a randomized controlled clinical trial. Psychopharmacology241, 1905–1914. 10.1007/s00213-024-06606-9
107
Zhang J. Ma L. Chang L. Pu Y. Qu Y. Hashimoto K. et al . (2020). A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry10:186. 10.1038/s41398-020-00878-3
108
Zhang J. Xue B. Jing B. Tian H. Zhang N. Li M. et al . (2022). LPS activates neuroinflammatory pathways to induce depression in Parkinson's disease-like condition. Front. Pharmacol. 13:961817. 10.3389/fphar.2022.961817
109
Zhang R. Ding N. Feng X. Liao W. (2025). The gut microbiome, immune modulation, and cognitive decline: insights on the gut-brain axis. Front. Immunol. 16:1529958. 10.3389/fimmu.2025.1529958
110
Zhao Y. Jaber V. Lukiw W. J. (2017). Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 7:318. 10.3389/fcimb.2017.00318
111
Zhu F. Yin S. Wang Y. Zhong Y. Ji Q. Wu J. et al . (2024). Effects of probiotics on neurodegenerative disease-related symptoms and systemic inflammation: a systematic review. Int. J. Gen. Med. 17, 5941–5958. 10.2147/IJGM.S499406
112
Zhu M. Liu X. Ye Y. Yan X. Cheng Y. Zhao L. et al . (2022). Gut microbiota: a novel therapeutic target for Parkinson's disease. Front. Immunol. 13:937555. 10.3389/fimmu.2022.937555
Summary
Keywords
Parkinson's disease, gut-brain axis, probiotics, neuroinflammation, microglia
Citation
Kroker Kimber I and Tremblay M-È (2025) Food for thought: probiotic modulation of microglial activity in Parkinson's disease. Front. Mol. Neurosci. 18:1690507. doi: 10.3389/fnmol.2025.1690507
Received
22 August 2025
Accepted
17 September 2025
Published
09 October 2025
Volume
18 - 2025
Edited by
Jochen C. Meier, Technical University of Braunschweig, Germany
Reviewed by
Debosree Ghosh, Government General Degree College, Kharagpur II, India
Updates
Copyright
© 2025 Kroker Kimber and Tremblay.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Ève Tremblay evetremblay@uvic.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.