- 1Department of Pharmacy, Faculty of Pharmacy and Medical Sciences, Hebron University, Hebron, Palestine
- 2Department of Medical Laboratory Science, Hebron University, Hebron, Palestine
- 3College Of Graduate Studies, Hebron University, Hebron, Palestine
Vitiligo (skin depigmentation) affects approximately 2% of the global population. It is characterized by a decrease in the number of pigment-forming cells known as melanocytes in the skin. Vitiligo is classified according to its extent and distribution into segmental (affecting one side of the body) and non-segmental (affecting both sides of the body). Vitiligo is an autoimmune disease characterized by complex and multifactorial genetic and non-genetic factors. The genetic factors are related to gene defects on HLA regions that code the histocompatibility complex, creating autoimmune response. Non-genetic factors include the exposure of melanocytes to oxidative stress and environmental factors. Conventional therapies include corticosteroids, immunomodulators, vitamins, phototherapy, surgery, and laser therapy. The use of complementary medicines such as plants, their extracts, and natural compounds in managing vitiligo has consistently been shown in many studies to be effective in the management and treatment of vitiligo. This review summarizes the most recent advances in understanding the pathogenesis of vitiligo, with an emphasis on the role of the Nrf2 pathway machinery and the effectiveness of herbal medicines and their extracts in vitiligo management and treatment. Plants such as Ginkgo biloba, Olea europaea, Cucumis melo, Camellia sinensis, and Allium sativum exhibit activity against vitiligo. The possible mechanisms by which these plants act are summarized in this review.
1 Introduction
Vitiligo is an acquired asymptomatic autoimmune disease that affects skin pigment (melanin)-forming cells (melanocytes), resulting in the appearance of white (depigmented) patches on the skin, hair, eyes, inner ear, and mucous membranes of the mouth, nose, and eyes (Ortel et al., 1988; Picardo et al., 2015). In its early stages, vitiligo starts as a patch that is paler than the skin and gradually becomes completely white (Ezzedine et al., 2012; Picardo et al., 2015). Vitiligo can develop at any age and affects both sexes equally, with women complaining of it earlier and more frequently (Alikhan et al., 2011; Rodrigues et al., 2017).
Vitiligo prevalence among the world’s population ranges from 0.5% to 2% (Alikhan et al., 2011; Kruger and Schallreuter, 2012; Zhang et al., 2016) with some areas in India and Africa having a higher incidence, reaching up to 9% (Shah et al., 2008; Zhang et al., 2016). In a recent report by Valle (2019), the total number of people with vitiligo worldwide was estimated to range from 65 to 95 million (Valle, 2019). Although the incidence of vitiligo is relatively low, it often has devastating effects on patients’ psychology and quality of life (Alikhan et al., 2011).
The etiology of vitiligo remains unknown (Manga et al., 2016). Many theories have explained the destruction of melanocytes in vitiligo. These theories include metabolic abnormalities, oxidative stress, endoplasmic reticulum stress, environmental factors, and autoimmune processes (Iannella et al., 2016; Picardo et al., 2015). Vitiligo is classified into two types: segmental and non-segmental. Segmental vitiligo affects one side of the body and is less common than non-segmental vitiligo, which affects both body sides (Ezzedine et al., 2012).
Vitiligo is not a life-threatening disease; however, it can alter life because it affects patients’ lives both biologically and psychologically. Hearing loss and ocular problems are the main physical impairments associated with vitiligo. Hearing loss is detected in up to 20% of patients with vitiligo because of the loss of functional melanocytes of striavascularis (Alikhan et al., 2011). Ocular abnormalities such as iritis (inflammation of the iris) and uveitis (inflammation of the middle layer of the eye) are reported in almost 40% of patients with vitiligo. Psychologically, many patients experience serious depression, low self-esteem, anxiety, and problems with confidence; they often feel embarrassment and shame and have to deal with social stigma due to misunderstandings of vitiligo (Sawant et al., 2019). A questionnaire-based study involving 73 British adults with vitiligo reported an increase in bereavement, sleep disturbance, and changes in eating habits compared with other dermatological diseases (73 control patients) (Papadopoulos et al., 1998). Another study conducted in 2003 involving 31 adults with vitiligo reported a decrease in social support and acceptance and an increase in anxiety compared to 116 other patients who experienced other dermatologic diseases (Picardi et al., 2003).
Several studies suggest oxidative stress as a hallmark of vitiligo pathogenesis with particular interest of the elevated level of the reactive oxygen species (ROS) in melanocytes and its role in triggering inflammation, autoimmunity and susceptibility to apoptosis (Chang and Ko, 2023; Colucci et al., 2015). An increase of H2O2 is detected in epidermis from vitiligo patients (Schallreuter et al., 1999) while marked decrease of the antioxidant enzymes have been reported (Zedan et al., 2015). The nuclear factor erythroid 2-related factor 2 (Nrf2) is a master cellular sensor of oxidative stress and a key regulator of the cellular redox state. Once activated, Nrf2 enhance the expression of endogenously antioxidant, detoxification and cytoprotective enzymes that provide cell protection and survival (Jian et al., 2014). The dysfunction of Nrf2 was reported in many studies amplifying melanocyte destruction (Jian et al., 2014). On the contrary, Nrf2 activation by several inducers such as DMF, Sulforaphane and plant extracts present a promising strategy in vitiligo management (Li et al., 2020; Lin et al., 2020; Liu et al., 2007). Recently, there has been an increasing interest in understanding the pathophysiology of vitiligo and the use of complementary herbal treatments. This review aims to summarize the current advances in vitiligo pathophysiology, with an emphasis on oxidative stress affecting melanocytes, and identify the underlying role of Nrf2 pathway as a possible target for herbal plants and natural products involved in vitiligo management.
2 Classification of vitiligo
Vitiligo is classified into two major categories according to its extent and distribution: segmental vitiligo (SV) and non-segmental vitiligo (NSV) (Table 1). Segmental vitiligo accounts for (5%–27.9%) of all vitiligo cases and characterized by its unilateral distribution, stable size, low association with autoimmune diseases, and a tendency to affect younger patients (87% of cases are <30 years old). In some cases, two segments may appear on opposite sides of the body, and vitiligo is called disegmental; if these segments appear on the same side, it is called multisegmental (Picardo et al., 2015; van Geel and Speeckaert, 2017).
Non-segmental vitiligo is the most common type, characterized by symmetrical bilateral depigmented macules, an unpredictable course and activity that increases in size over time, and is associated with autoimmune diseases. It includes all forms of vitiligo that are not classified as segmental (Ezzedine et al., 2012) such as acrofacial, universal (generalized), mixed, and rare cases. In acrofacial cases, lesions primarily affect the distal fingers, facial orifices, face, hands, feet, and head. It can later progress to a universal form, involving lesions in multiple parts of the body in a symmetrical pattern on 80%–90% of the body surface (Alikhan et al., 2011; Ezzedine et al., 2012).
Mucosal vitiligo involves the oral or genital mucosae, or both. When it is associated with other sites of skin depigmentation, it is classified as NSV. However, it can involve only mucosal site for a long time of diagnosis–at least 2 years–and it classified under the undetermined category (Table 1).
The mixed type of vitiligo is characterized by the presence of segmental and non-segmental types; it most often starts with segmental vitiligo and then develops into non-segmental vitiligo, so it is considered the NSV subtype.
2.1 Risk factors and disease association
Individuals at risk of acquiring vitiligo include those with a family history of certain autoimmune diseases, such as type 1 diabetes, alopecia areata, pernicious anemia, rheumatoid arthritis, and autoimmune thyroid diseases (Alikhan et al., 2011; Alkhateeb et al., 2003; Nejad et al., 2013), suggesting the existence of shared common molecular pathways in the development of various autoimmune disorders.
Thyroid diseases are the most prevalent autoimmune diseases associated with vitiligo and are reported in 20% of vitiligo cases. Their prevalence is at least three times higher than that in the normal population (Nejad et al., 2013). In one study, 34.9% (538 1541) participants with vitiligo had comorbid autoimmune disorders (Gill et al., 2016). Similarly, in another study, 34% of patients with vitiligo had a history of thyroid disease (Yaghoobi et al., 2011). These findings are similar to those obtained by Baldini et al. (2017), who reported a higher incidence of thyroid disorders and thyroid hormone antibodies in vitiligo patients and their family members (Baldini et al., 2017).
Several physical and psychological factors are associated with vitiligo pathogenesis. These factors include exposure to sunburns, cuts, radiation, chemicals, and trauma (Rodrigues et al., 2017). Psychological factors, including a high frequency of stressful life events and preceding vitiligo onset, are associated with the development of vitiligo. A questionnaire-based study included 1541 vitiligo patients reported that 56.6% of participants experienced at least one stressor within 2 years prior to vitiligo onset; the stressor was mainly death of a loved one, and other stressors included financial problems, family problems, or end of long-term relationships.
3 Vitiligo pathogenesis
The pathogenesis of vitiligo is poorly understood. However, they are believed to be genetic (Nath et al., 1994; Spritz and Andersen, 2017) and non-genetic factors (Wang et al., 2014; Wang et al., 2016).
3.1 Genetic factors in the pathogenesis of vitiligo
The earliest evidence implicating genetic factors in the pathogenesis of vitiligo was reported by Nath et al., in 1994 (Spritz and Andersen, 2017). Vitiligo affects 1% of the general population (Abreu et al., 2015), but the risk of patients’ siblings (immediate relatives of patients with vitiligo) developing the disease is 12%, approximately 7% for parents (Majumder et al., 1993), and 23% among monozygotic twins (Nath et al., 1994).
Vitiligo is a complex disease that cannot be explained by Mendelian patterns. It is characterized by multi-factorial polygenic inheritance, incomplete penetrance, and multiple susceptibility loci (Shen et al., 2016). Mutations and single nucleotide polymorphisms in genes involved in both innate and adaptive immunity and non-immune genes increase the risk of vitiligo.
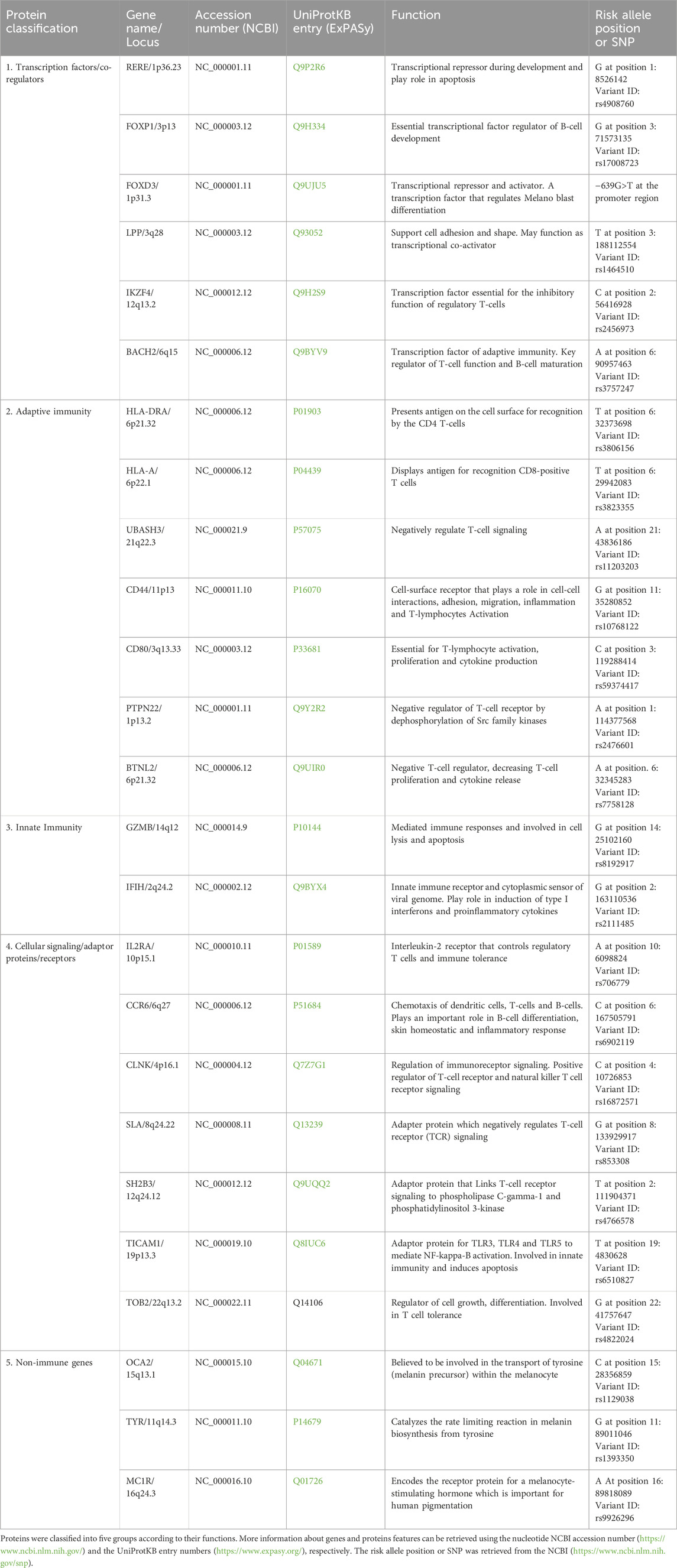
Genome-wide association studies (GWAS) are the gold standard for identifying genetic variations in complex diseases. Using this approach, a set of SNP across the whole genome is examined to identify the most relevant variants associated with the disease (Spritz, 2012). Richard Spritz et al. conducted the largest GWAS on Caucasian populations and identified several genetic locations linked to vitiligo. Genes linked to vitiligo pathogenesis are categorized into five groups: transcription factors/co-regulators, adaptive immunity, innate immunity, cellular signaling/adaptor proteins/receptors, and nonimmune genes (Alkhateeb et al., 2005; Jin et al., 2016; Jin et al., 2012; Jin et al., 2010a; Jin et al., 2010b; Spritz, 2012). Other GWAS carried out in the Han Chinese identified candidate genes, including (CXCR5/DDX6 at 11q23.3, PMEL/IKZF4 at 12q13.2, and SLC29A3/CDH23 at 10q22.1) (Tang et al., 2013). In East Asia, the identified genes included (HLA-B and HLA-C at 6p21.33, RNASET2/FGFR1OP/CCR6 at 6q27, and ZMIZ1 at 10q22.3) (Quan et al., 2010). These differences were probably due to heterogeneity among the populations. The gene names, loci, functions, and risk allele positions are listed in (Table 2).
Genome-wide linkage studies are another unbiased approach used to identify genetic loci associated with complex human diseases. In this approach, a gene with multiple features is detected by scanning the entire genome (Dawn Teare and Barrett, 2005). Linkage studies of families from North America and the United Kingdom with multiple vitiligo have identified susceptible locus at chromosome 1p31 for the gene FOXD3 (Fain et al., 2003). Genetic variations associated with vitiligo, including those in HLA (Yang et al., 2005; Zamani et al., 2001; Zhang et al., 2004), PTPN22 (Cantón et al., 2005; Elmongy and Abu Khalil, 2013; LaBerge et al., 2008; Laddha et al., 2008; Rajendiran et al., 2018), NALP1 or NRLP1 (Jin et al., 2007a; Jin et al., 2007b), and XBP1 (Birlea et al., 2011; Ren et al., 2009).
The HLA region is a crosslinker between the genome and autoimmune diseases. This region encodes the histocompatibility complex (responsible for antigen presentation of antigens) (Matzaraki et al., 2017). Several genes associated with vitiligo are located in the HLA region, which induces an autoimmune response owing to self-antigens that activate T cells. Variations in this region indicate that patients with vitiligo are at a higher risk of developing other autoimmune diseases (Bowcock and Fernandez-Vina, 2012; Hayashi et al., 2016; Jin et al., 2019). PTPN22 increases the risk of vitiligo owing to its effect on protein tyrosine phosphatase, which plays a key role in signal transduction and controls the activity of the T-cell immune system. Affected PTPN22 proteins cause a loss of control of the immune system, which attacks melanocytes in the skin and contributes to vitiligo (Cantón et al., 2005). NALP1 is highly expressed in immune cells, especially in T cells and Langerhans cells. NALP1 associates with other proteins (ASC, caspase 1 and caspase 5) to form NALP1 inflammasome. This complex activating the pro-inflammatory cytokine IL-1β (Kummer et al., 2007), which is clearly elevated in vitiligo patients (Tu et al., 2003). XBP1 plays a central role in unfolded protein response (UPR) signaling. Upon the accumulation of misfolded proteins in the ER, the XBP1 mRNA undergoes specific splicing, generating the spliced XBP1 transcription factor (sXBP1), which subsequently translocates to the nucleus and drives the expression of stress-induced inflammatory genes.
3.2 Non-genetic factors in the pathogenesis of vitiligo
Nongenetic factors include environmental factors, alterations in autoimmune responses, and exposure of melanocytes to OS. Autoimmune responses can be divided into two categories: adaptive and innate (Das et al., 2019). In adaptive immunity the CD8 T cells, activated by binding of INF-γ to cytokine receptors, are consider as the most important cytotoxic T-cell inducing melanocyte injury (Wang et al., 2016).
The mechanism by which T-cells inducing melanocyte damage is mediated by binding of INF-γ to cytokine receptors causing JAK (intracellular Janus kinases) activation (Abdou et al., 2018), which will undergo autophosphorylation due to adenosine triphosphate (ATP) help. This cascade causes a conformational change in signal transducers and activators of transcription (STAT) to activate and bind to each other. This activation helps STAT enter the nucleus and induce the transcription of many mediators, which then activate cytokines, such as CXCL9 and CXCL10 (Ala et al., 2015), which are considered the most potent chemokines that activate other cytotoxic T-cells (Wang et al., 2016). The ratio of CD8\CD4 increases in blood samples obtained from vitiligo patients (Kaur et al., 2017), which is directly correlated with the severity and activity of the disease (Rashighi et al., 2014), and is considered a marker of poor treatment response.
3.2.1 Environment
Vitiligo is triggered by a combination of environmental and physical factors that disrupt melanocyte function, leading to skin depigmentation. Chemical compounds, such as phenols, commonly found in gloves, hair dyes, adhesives, and leather products, act as tyrosine analogs (Toosi et al., 2012). These chemicals interfere with melanin production by disrupting the activity of tyrosinase, a key enzyme involved in melanogenesis (Toosi et al., 2012). This disruption generates harmful free radicals known as semi-quinone radicals that induce cellular stress and trigger the UPR (Toosi et al., 2012). Additionally, physical trauma to the skin such as cuts, abrasions, or friction from clothing, sports equipment, or accessories can initiate or exacerbate vitiligo through the Koebner phenomenon (Sagi and Trau, 2011). This process is linked to oxidative or mechanical stress, which impairs the expression of E-cadherin, a calcium-dependent adhesion molecule critical for maintaining the connection between melanocytes and keratinocytes. When E-cadherin function is compromised, melanocytes detach from the epidermis and are lost in a process known as melanocytorrhagy. Together, these factors, including OS, immune responses, and disrupted melanocyte-keratinocyte adhesion, interact to drive vitiligo development and progression (Al-Shobaili and Rasheed, 2015).
3.2.2 Innate immunity
Melanocyte stress dysregulates the innate immune system, which comprises macrophages, natural killer cells (NK cells and white blood cells), and inflammatory dendritic cells (DC). Similar to the adaptive immune system, innate immunity rapidly initiates responses without specific antigen recognition as in the adaptive immune system (Richmond et al., 2013). Through the activation of pattern recognition receptors (PRRs), such as NOD-like receptors (NLR), which comprise large multiprotein complexes known as inflammasomes. Inflammasomes are responsible for increasing the production and release of pro-inflammatory proteins and cytokines, including heat shock protein (HSP) (Abdou et al., 2013), IL6, and IL8, due to high levels of heat shock protein −70, which can induce inflammatory dendritic cell DC (carry melanocyte-specific antigen to T-cells in the lymph tissue to damage it) (Wang et al., 2014), melanocytes will be damaged, which may causes vitiligo. The destruction of melanocytes through T-cells is considered a cross-link between innate and adaptive immunity.
3.2.3 Oxidative stress
Melanocyte cells synthesize proteins according to genetic information from nucleus DNA, this protein (melanin-synthesis related proteins) become functional due to an appropriate oxidative folding process, in which oxidation of sulfhydryl groups to produce disulfide bridges in proteins is the main step to get a mature folded protein. The endoplasmic reticulum catalyzes disulfide bond formation in proteins through a family of chaperones that are responsible for disulfide bond formation (protein disulfide isomerase (PDI)), which uses a molecule of oxygen in oxidative folding; hydrogen peroxide (Alikhan et al.) is produced as a byproduct, and catalase and glutathione peroxidase eliminate it; in this way, ER hemostasis is maintained (Eletto et al., 2014).
However, high level of ROS (Alikhan et al., 2011) and free radicals, which are mainly produced in the mitochondria (which communicated with ER by mitochondrial associated membrane (MAM)), due to some conditions such as, exposure to UV radiation (290–320 mm) and chemicals that have phenolic groups (as monobenzyl ether of hydroquinone) (Canizares et al., 1958). They create OS (high level of hydrogen peroxide, and decrease the level of catalase enzyme and glutathione peroxidase enzyme) (Agrawal et al., 2004), which leads to ER imbalance (Guan et al., 2015), caused by damage to poly unsaturated fatty acids of the cell membrane and break the disulfide bonds in proteins, that cause an accumulation of unfolded proteins in the ER.
The accumulation of misfolded proteins, will exert mitochondrial stress (Dell’Anna et al., 2001), in which nucleus receives a signal and ultimately activates UPR (Harris, 2016), that help reestablish the ER homeostasis by increasing the protein folding capacity of the ER, and decrease the ER protein folding loads. However, prolonged activity of the UPR (indicating that ER stress cannot be managed) leads to cell apoptosis (melanocyte apoptosis in the case of vitiligo) (Malhotra and Kaufman, 2007). Moreover, stressed melanocytes undergo apoptosis and secrete melanocyte-specific antigens and immunostimulatory signals such as cytokines (IL-6 and IL8) (Bettigole and Glimcher, 2015), which attract autoimmune reactions toward melanocytes and cause disease progression (Colucci et al., 2015).
Some vitamins also play a major role in maintaining cellular hemostasis, such as lipophilic antioxidants, tocopherol (vitamin E), vitamin A, vitamin D, and COQ10. Moreover, hydrophilic antioxidants such as ascorbic acid (vitamin C), which stabilizes free radicals due to its delocalization around the chemical rings of vitamins (Jalel et al., 2009).
4 Oxidative stress and ER stress: central pathways in vitiligo pathogenesis
Vitiligo is associated with metabolic alterations, increased generation of ROS and increased susceptibility to mild exogenous stimuli in the epidermis. OS is a pathogenic event that affects melanocyte degeneration. OS triggers tissue damage and apoptosis, resulting in the production of small immunogenic molecules and an increased risk of autoimmune disorders in susceptible individuals. Endoplasmic reticulum is responsible for protein folding and is highly sensitive to changes in the cellular redox state. OS, ER stress, and the two events are linked (Figure 1) (Chaudhari et al., 2014; Malhotra and Kaufman, 2007).
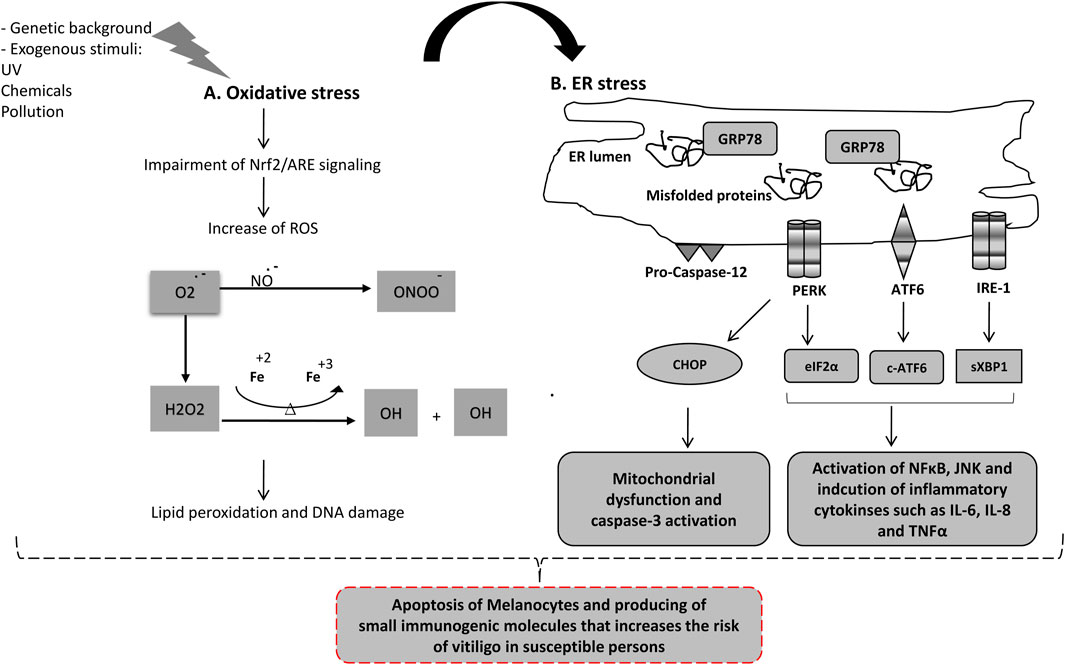
Figure 1. Oxidative stress and ER-stress molecular network in Vitiligo pathogenesis. (A) High level of reactive oxygen species (ROS) is generated due to exposure to radiation, pollution, phenolic chemicals and exogenous xenobiotic compounds or impairment in Nrf2 antioxidant sensor. The high level of ROS including Superoxide, Hydroperoxyl, Hydrogen peroxide, peroxynitrite and organic peroxides leads to damage of plasma membrane, organelles and DNA, which subsequently trigger apoptosis of melanocytes. (B) Oxidative stress and high level of ROS causes accumulation of misfolded proteins in ER inducing ER-stress response. GRP78 protein is released from the three ER-stress sensors enhancing their activation. (1) Activated PERK induces cell cycle arrest and activation of CHOP. (2) Activated ATF6 is cleaved in Golgi generating c-ATF6 (a transcription factor involved in inflammation and cytokines release). (3) Activated IRE-1 generates specific splicing on XBP1 converting it to sXBP1 (a potent transcription factor that induces NFκB and inflammatory response). Prolonged ER-stress induces apoptosis in melanocytes, mainly through CHOP (pro-apoptotic protein), caspase-12, and inflammatory response pathway.
ER stress occurs when the ER accumulates unfolded proteins, and cells initiate the UPR pathway. The UPR consists of three main signaling pathways, PERK, IRE-1, and ATF6., that normally bound by a chaperone called Grp78 and are inactive. ER stress results in the recruitment of GRP78 away from the UPR sensors and activates them. Activated PERK, phosphorylates eukaryotic initiation factor 2 (elF2α) leading to cell cycle arrest and upregulates important transcription factors (ATF4, CHOP and Nrf2). Activated IRE-1 resulted in the activation of the XBP1 transcription factor. ATF6 is processed in the Golgi to active the cleaved-ATF6 transcription factor. Prolonged ER stress leads to the activation of pro-apoptotic signaling (CHOP is a key player) (Almanza et al., 2019; Hetz, 2012). Chronic ER stress also activates the inflammatory response pathways. All UPR sensors participate in NF-Kappa B activation, increasing cytokine productions (IL-1, IL-6, IL-8, TNF-α and MCP1), activates JNK and induces inflammasome formation (Chaudhari et al., 2014; Garg et al., 2012; Hotamisligil, 2010; Mohan et al., 2019). Notably, UPR sensors are involved in regulating the development, differentiation, activation, cytokine production, and apoptosis of multiple immune cell types, including T cells, B cells, dendritic cells (DCs), macrophages, and myeloid-derived suppressor cells (MDSCs) (Cai et al., 2019; So, 2018).
Several studies have highlighted the important role of the UPR in vitiligo pathogenesis (Passeron and Ortonne, 2012; Rashighi and Harris, 2017; Toosi et al., 2012) and it could be a major link between OS and vitiligo autoimmunity (Manga et al., 2016; Park et al., 2019). Furthermore, single-nucleotide polymorphisms XBP1 in patients with vitiligo illuminate the importance of this pathway in the pathogenesis of vitiligo (Birlea et al., 2011).
5 Nrf2 – Antioxidant response pathway
Oxidative stress is considered as a major event in vitiligo pathogenesis (Chang and Ko, 2023). Nuclear factor erythroid-2 related factor 2 (Nrf2) is an important transcription factor and sensor of the cellular redox state. It belongs to the cap ‘n’ collar (CNC) family and has basic leucine zipper (bZip) domain that facilitates DNA binding (Li and Kong, 2009a). In the absence of stimuli, Nrf2 is tethered to the cytosol by its inhibitory partner, Keap-1 that regulates its binding and degradation through Cul3-ubiquitine ligase complex (Figure 2A). The Keap-1 contains three important domains in which BTB domain is require for homodimerization of Keap-1 and mediates the Cul3-ligase binding. IVR domain contains critical cysteine residues and regulate Nrf2/Keap-1 binding. The Kelch/DGR domain essential for Nrf2 binding (Canning et al., 2015). The classical model of Nrf2 activation involved the direct OS-induced Keap-1 modification (Itoh et al., 2004). Keap-1 contains several cysteine residues, among them, three were extensively studied and confirmed as redox sensitizing-cysteine (Cys151 in BTB domain, Cys273 and Cys288 in IVR domain) (Yamamoto et al., 2008). Under normal state, the thiol group of the cysteine residues are present in their reduced state (-SH), allowing complex with Cul3 and Nrf2, targeting the later for ubiquitination and proteosomal degradation. Oxidative and electrophilic stress basically modify the cysteine residues state of Keap-1 causes a conformational change and Nrf2 release. The most common modifications occur on Cys151, Cys273 and Cys288 include: (1) S-oxidation generating sulfenic acid (-SOH) and sulfinic acid (–SO2H) by oxidants (e.g., H2O2, sulforaphane and quinone) (2) S-nitrosylation (e.g., nitric oxide), and (3) S-alkylation induced by electrophiles (e.g., 4-octylitaconate and DMF). Furthermore, other post-translation modifications of keap-1 (glycosylation, phosphorylation, glutathionylation and SUMOylation) is also reported indicating that the type of modifier is critical to determine how Keap-1 is chemically modified and Nrf2 is released (Song et al., 2023). Another model for Nrf2/Keap-1 activation is the indirect Nrf2 phosphorylation by OS-response kinases such as: PKC (Bloom and Jaiswal, 2003), ERK (Buckley et al., 2003), JNK MAP kinase (Keum et al., 2003), PI3K (Kang et al., 2002) and PERK (Cullinan et al., 2003). Upon activation, Nrf2 translocates to the nucleus, binds specifically to the Antioxidant Response Element (ARE) and induces transcription of downstream genes (Baird and Dinkova-Kostova, 2011) (Figure 2B).
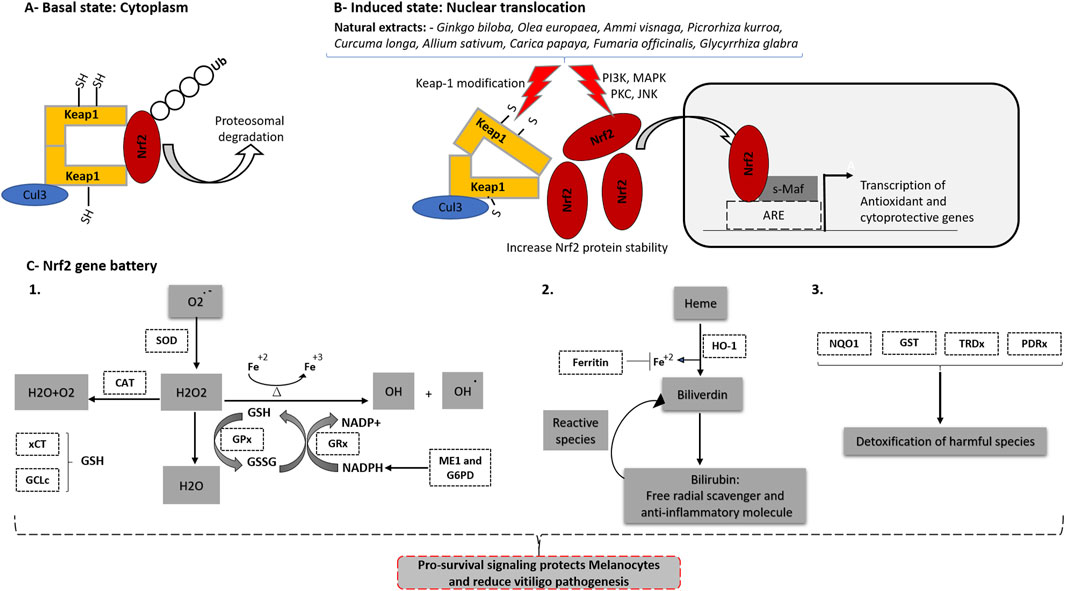
Figure 2. Potential role of Herbal Medicine in activating Nrf2 and its promising target to manage Vitiligo. (A) At the basal state, Nrf2 binds to keap1 protein that mediates its proteosomal degradation. (B) Nrf2 is activated by changes of cellular redox state or chemicals/drugs that affect Nrf2 binding to keap1. Natural extracts used to treat vitiligo such as (Ginkgo biloba, Olea europaea, Ammi visnaga, Picrorhiza kurroa, Curcuma longa, Allium sativum, Carica papaya, Fumaria officinalis, and Glycyrrhiza glabra) enhance Nrf2 translocation from cytoplasm to nucleus. Activated Nrf2 binds to s-Maf proteins and then to Antioxidant Response Element (ARE) which is present in the promoter sequence of large number of antioxidant and detoxification genes. (C) Nrf2 induces the transcription of “Nrf2 gene battery“ including: 1) Genes involved in antioxidant (SOD and CAT) and glutathione homeostasis (xCT, GCLc, GPx, ME1 and G6PD). 2) Genes involved in biliverdin-bilirubin redox cycle (HO-1) and (Ferritin) that blocks Fenton reaction. 3) Large number of detoxification/cytoprotective genes (NQO1, GST, GRDx and PDRx).
Nrf2 regulates plethora of genes called “Nrf2 gene battery” that function together to restore redox homeostasis (Figure 2C). Several high-throughput microarray and CHIP/sequencing profiling approaches have been proposed, and several Nrf2 target gene candidates have been identified. The first group function in glutathione homeostasis including amino acid uptake transporters (SLC7A11 and SLC6A9), GSH synthesizing enzymes (γ-GCLc and γ-GCLm), GSH metabolizing enzymes that eliminate ROS through GSSG/GSH oxidation-reduction cycle (GPx and GRx) and GSH complementary enzymes such as glutathione transferases (GST). The second group comprises the antioxidant/detoxification enzymes. Heme oxygenase-1 (HO-1) is the rate-limiting enzyme in heme catabolism and is considered the main target of Nrf2 (Qaisiya et al., 2014). Cytoprotection by HO-1 against OS results from the generation of three products: carbon monoxide (CO), biliverdin-bilirubin, and ferrous iron (Fe+2). These have important physiological roles against OS, inflammation, and apoptosis (Pae et al., 2008). The ferritin heavy chain (FTH) effectively sequesters pro-oxidative Fe+2 that would otherwise participate in the Fenton reaction (Vile and Tyrrell, 1993). The NADPH quinoneoxidoreductase-1 (NQO1) is a multifunctional flavoprotein capable of reducing or metabolizing a broad range of substrates in a single two-electron step, avoiding the generation of semi-reactive species (Dinkova-Kostova and Talalay, 2010). Superoxide dismutase (SOD) and catalase are essential for ROS elimination. The third group is involved in NADPH production and includes enzymes, such as ME1 and glucose-6-phosphate dehydrogenase. NADPH is an abundant antioxidant that acts as an electron donor for several oxidoreductase cycles, such as the GSSG-GSH cycle, biliverdin-bilirubin cycle, and the NQO1 detoxification process (Malhotra et al., 2010; Tonelli et al., 2018). Beside its classical role in regulation of the cellular antioxidant and detoxification processes, Nrf2 cross-talk with NF-κB which is major regulator of inflammation and immune response (Barnabei et al., 2021). Nrf2 knockout animal models showed an increase in NF-κB activity and upregulation of its downstream target genes (such as TNF-α, IL-1β and IL-6 production) confirming the negative interplay between Nrf2 and NF-κB (Gao et al., 2021).
5.1 Nrf2 signaling is impaired in vitiligo
The dysfunction of Nrf2 signaling is significant to vitiligo pathogenesis. Jian et al., shows that melanocytes from vitiligo patients have impaired Nrf2-ARE signaling, reduced Nrf2 translocation to nucleus, decreased induction of HO-1 antioxidant genes and lower activity of SOD and GPx (Jian et al., 2014). Knockdown of Nrf2 reduces melanocyte viability but not the viability of keratinocytes and fibroblasts, indicating that melanocytes are preferentially dependent on Nrf2 (Arowojolu et al., 2017). HO-1 suppresses T cell proliferation by inhibiting IL-2 production (Pae et al., 2004). The lower levels of HO-1 in patients with vitiligo are correlated with higher serum levels of IL-2, suggesting that serum HO-1 can be used indirectly to assess the antioxidant capacity of patients with vitiligo (Jian et al., 2014). Furthermore, serum bilirubin levels (the final product of HO-1) are significantly lower in vitiligo patients (Turkmen, 2020). Interestingly, genetic studies on the Han Chinese population have shown that variations in Nrf2 are associated with susceptibility to vitiligo (Guan et al., 2008; Song et al., 2016). In contrast, several studies demonstrated that the Nrf2/HO-1 axis could be exploited as a therapeutic strategy to counter oxidative damage in melanocytes and treatment of vitiligo (Gęgotek and Skrzydlewska, 2015). Keap-1 siRNA and Nrf2 activation protects melanocyte cell lines from oxidative damage (Jian et al., 2011). On the contrary, Nrf2 downregulation increases H2O2-induced melanocytes toxicity (He et al., 2017). Induction of HO-1 in cultured melanocytes from vitiligo patient was able to reduce cell death (Elassiuty et al., 2011). Melanocytes treated with curcumin (an Nrf2 inducer) showed a reduced apoptosis and high level of NQO-1 Nrf2-target genes (Elassiuty et al., 2011; Fang et al., 2020; Gęgotek and Skrzydlewska, 2015; He et al., 2017; Jian et al., 2011; Natarajan et al., 2010). Vitiligo melanocytes treated with H2 induces Nrf2 and revers H2O2-induced cell apoptosis (Fang et al., 2020; Fang et al., 2020). All these data and many others confirmed the crucial role of Nrf2 activation in protecting melanocytes form oxidative damage.
6 Management of vitiligo
No treatment ensures a complete cure for vitiligo owing to its complex and multifactorial pathogenesis (Cai et al., 2019). However, different methods of vitiligo management exist for stabilizing and repigmenting lesions. These include medical drugs (topical or systemic corticosteroids, topical immunomodulators, and vitamins), phototherapy, surgical techniques, lasers (Bleuel and Eberlein, 2018), and medicinal plants owing to their immunomodulatory and antioxidant properties. However, combination therapy may be more successful than monotherapy.
6.1 Therapeutic options in vitiligo management
Topical corticosteroids, immunomodulators, and phototherapy are effective in stable and localized cases. However, in cases of generalized rapid progression, oral corticosteroids are required to decrease disease progression.
Topical corticosteroids seem to be the first option for the treatment of vitiligo owing to their anti-inflammatory and immunosuppressive effects, which decrease the spread and progression of the disease, and have clear repigmentation effects on the face and neck, especially in recent lesions. However, the course of treatment should be at least 6 months to obtain good results, which may have side effects, including skin atrophy, telangiectasia (spider vein, dilation of blood vessels), and acneiform eruptions (in this case, it is advisable to switch to topical immunomodulators).
Tacrolimus is considered a prototype immunomodulatory drug because it inhibits calcineurin synthesis, decreases T-cell activity, and induces the production of proinflammatory cytokines. However, it also increases melanocyte migration and pigmentation. As mentioned for topical corticosteroids, topical tacrolimus also has an evident result, especially on the face, and it could be an alternative to topical corticosteroids in some cases, such as acneiform eruption, or when topical corticosteroids show no sign of recovery on the face (Choi et al., 2008).
Phototherapy, which includes ultraviolet A and B, is an efficient way to induce repigmentation in vitiligo owing to its ability to produce melanin and induce the differentiation of melanocytes (Farag et al., 2019). Patients should be aware of the treatment duration, expected results, and side effects of phototherapy (Esmat et al., 2016). This treatment results in partial repigmentation in the majority of cases, instead of complete repigmentation, which appears in only one or two cases, and is treated for 12 months by narrow-band UVB (NA-UVB), which is the best choice for phototherapy (Bordere et al., 2009). Nonetheless, the risk of vitiligo in repigmented skin after the discontinuation of therapy (relapse) has also been found; therefore, topical tacrolimus should be used after stopping phototherapy to decrease the risk of recurrent lesions (Bleuel and Eberlein, 2018).
Dexamethasone (2.5–10) mg is an oral corticosteroid used in vitiligo treatment as an immunosuppressant and anti-inflammatory agent, and when administered twice weekly, it can prevent the progression and spread of disease (Dellatorre et al., 2020). However, common side effects of corticosteroids, such as weight gain, hypertrichosis (abnormal hair growth over the body), acne, and menstrual disturbances, occur during the treatment period, which limit the use of these drugs.
The combination of UVB and topical therapies, including dexamethasone or immunomodulators, is more effective at reducing disease progression and achieving rapid repigmentation (Dellatorre et al., 2020), than monotherapy alone. However, it is necessary to be careful about the long-term side effects of UVB in combination with systemic immunosuppressants or steroids.
6.2 Herbal medicine and natural product in management of vitiligo
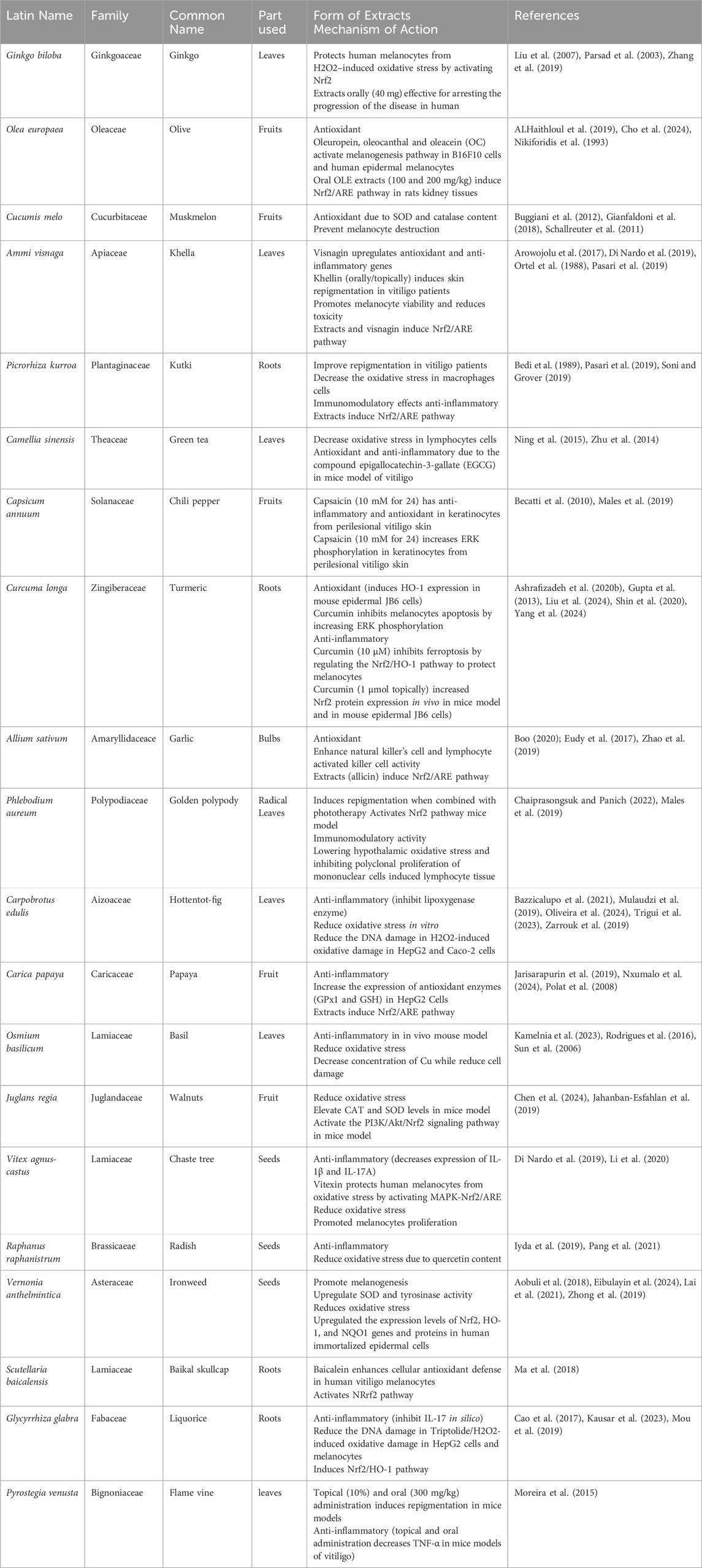
The involvement of traditional medicine in vitiligo management has received considerable attention over the past 2 decades. This is largely due to the fact that vitiligo - until now - has no cure and herbal medicine or their purified natural compounds could provide safe alternative/supportive therapy for vitiligo in conjunction with existing pharmacological drugs (Table 3). Among the several herbal drugs implicated in the treatment of vitiligo are Gingko biloba and Vernohia anthelmintica–Chinese herb– (Pang et al., 2021; Zhang et al., 2019). In cell culture and animal models, both plants consistently exhibited melanogenesis and antioxidant capacity comparable to those of the positive controls (Huo et al., 2017; Lai et al., 2021; Zhang et al., 2019). In a recent study by Li et al., 2009 the ethanolic extracts of V. anthelmintica seeds (0.5 mg/mL) significantly promoted melanogenesis in B16 murine melanoma cells (Lai et al., 2021). However, in hydroquinone-induced vitiligo in mice, Butin (3′,4′,7′-Trihydroxyflavan-4-one) – major compound in the seed of V. anthelmintica–administration (4.25 and 45.5 mg/kg) significantly increases the basal layer melanocytes and melanin-containing epidermal cells (Huo et al., 2017). Lai et al. (2021) further confirmed previous findings on butin. In their study, butin (40 μmol/L) is reported to induce melanin production in B16 murine melanoma cells and in H2O2–induced zebrafish model at a concentration of 10, 40, 80 and 100 μmol/L (Lai et al., 2021). Another compound extracted from V. anthelmintica, cynarine (1,5-Dicaffeoylquinic acid) induced increased melanogenesis in B16 murine melanoma cells (Mamat et al., 2018). Although several studies using in vitro and in vivo models have implicated the role of V. anthelmintica and its derived metabolites in anti-vitiligo, no clinical studies have been conducted to confirm its effects on humans.
Gingko biloba is one of the few plants marketed in some countries owing to its ability to slow disease progression in humans with no side effects (Parsad et al., 2003; Szczurko et al., 2011). Two independent studies have consistently implicated G. biloba extract in slowing the progression of vitiligo in humans (Parsad et al., 2003; Szczurko et al., 2011). In Canada, in patients with vitiligo (n = 11), ingestion of 60 mg of G. biloba extract twice daily for a period of 12 weeks has been associated with a significant improvement, as assessed by measuring vitiligo area scoring index (VASI) and vitiligo European task force (VETF) spread (Szczurko et al., 2011). However, in another clinical study involving patients with gradually progressive (slow-spreading) vitiligo (focal, vulgaris, and acrofacial) divided into two groups, group A (receiving G. bilopa extract 40 mg three times daily) and group B (receiving placebo). The progression of vitiligo in patients receiving G. bilopa extracts (n = 27) was arrested in twenty patients and skin repigmentation was observed in ten patients. However, compared with placebo treated patients (n = 22) the progression of vitiligo was observed on only five patients with only two patients showed skin repigmentation (Parsad et al., 2003).
Consistent with the protective evidence implicating G. biloba extract (EGB761, standardized extract of dried leaves of G. biloba containing 24% ginkgo-flavonol glycosides, 6% terpene lactones such as ginkgolides A, B, C, J and bilobalides) as anti-vitiligo, applying EGB761 extracts (100 μg/mL) have also protected human melanocyte cells pretreated with H2O2-induced OS and apoptosis. This protective effect has been associated with the activation of the Nrf2 pathway (Zhang et al., 2019). This effect has also been reported for baicalein (a flavonoid extracted from Scutellaria baicalensis), bilobides (terpenoid constituents of Ginkgo biloba extract), and methylcobalmine (activated forms of vitamin B12) (An et al., 2021; Lu et al., 2016; Ma et al., 2018).
Ammi species positively reduce vitiligo symptoms by inducing skin repigmentation in humans (Sidi and Bourgeois-Gavardin, 1952). The use of Ammi majus by older Egyptians for the treatment of skin conditions was supported by Sidi et al., in 1952. In a previous study, A. majus oral (tablet–containing Ammoidin 10 mg and Ammidin 5 mg–main active constituent in A. majus) and topical (Ammoidin and Ammidin, 7 and 2 mg/mL, respectively) administration followed by sun exposure or a combination of oral and topical administration caused remarkable skin repigmentation in all six patients tested, irrespective of the method of administration (Sidi and Bourgeois-Gavardin, 1952) although some reversible side effects such as skin burning were observed (Sidi and Bourgeois-Gavardin, 1952). These findings have triggered further research to identify safer options for Ammi species. Ammi visnaga, commonly known as Khella, was tested for its activity in the treatment of vitiligo in vivo by Egyptian scholars in 1982 (Abdel-Fattah et al., 1982). Khellin, the main active constituent of A. visnaga, administered orally (100 mg/day) for 4 months followed by exposure to sunlight for 15 min (45 min after khellin administration) effectively and variably induced stable skin repigmentation in 23 individuals out of 30 (Abdel-Fattah et al., 1982). In later clinical studies, administration of khellin, either orally or topically, followed by ultraviolet A or sunlight exposure, consistently achieved more than 70% skin repigmentation with minimal and often no side effects typically associated with other furanocoumarins present in A. visnaga (Orecchia and Perfetti, 1992; Ortel et al., 1988). Visnagin (an analog of khellin) has been reported to upregulate the Nrf2 pathway in rat models, attenuating OS (Abukhalil et al., 2021). Moreover, in in vitro model involving acinar cells, visnagin enhances expression Nrf2 leading to reduced inflammation in acute pancreatitis (Pasari et al., 2019). Although A visnaga seems to have A positive impact on vitiligo, the underlying molecular mechanisms, targets, and markers, unlike G. biloba, are yet to be identified and require further investigation despite the involvement of the Nrf2 pathway.
Picrorhiza kurroa rhizomes (Scrophulariaceae) promote skin repigmentation in humans, likely due to their immunomodulatory and anti-inflammatory activities (Bedi et al., 1989). Administration of 200 mg P. kurroa rhizome capsules (picrolex) twice daily as a supplement with methoxsalen (a furanocoumarin drug), followed by exposure to light, significantly eliminated the vitiligo patches in 27 of the 30 patients involved in the study (Bedi et al., 1989). Although the underlying molecular pathways remain unclear, recent studies have implicated picrosides (iridoid glycosides in P kurroa extracts) in activating Nrf2 pathways, leading to the increased expression of detoxifying enzymes (Soni and Grover, 2019).
Curcuma longa (turmeric) has been the subject of many dermatological studies because of its overwhelmingly positive impact on skin health and vitiligo (Guarneri et al., 2021; Jalalmanesh et al., 2022; Schallreuter and Rokos, 2006; Vaughn et al., 2016). In a recent clinical trial involving 30 patients with mild to moderate vitiligo, turmeric cream application twice daily for 4 months reduced lesion size and skin pigmentation compared to the placebo group (Jalalmanesh et al., 2022). Several topical turmeric-based formulations have been developed and marketed in various countries (Narayanaswamy and Ismail, 2018). Turmeric exerts antivitiligo effects through several mechanisms. Turmeric is demonstrated to inhibit the production of several key inflammatory cytokines such as IL-1, TNF-α promoting melanocytes death (Singh et al., 2021). Turmeric also reduced the OS associated with vitiligo pathogenesis. Curcumin, the main active constituent of turmeric, scavenges free radicals, increases the expression of antioxidant enzymes, and modulates Nrf2 expression by regulating antioxidant responses.
6.2.1 Plants contribute to reduced oxidative stress in vitiligo management
Antioxidants are substances that can prevent or inhibit damage to cells caused by free radicals that the body produces in response to environmental stress or other pressures (Basilicata et al., 2019). Antioxidants can be extracted from many herbs and plants, affect OS in vitiligo, and have been used either alone or in combination with sunlight/ultraviolet light (UV), which removes free radicals by donating an electron that protects the cells and organ systems of the body from OS by against free radical (Zhong et al., 2019). Cells naturally produce antioxidants such as glutathione peroxidase and catalase, which protect mitochondria from stress (Hosseinzadeh et al., 2019). Sometimes, the body cannot create antioxidants on its own, which can come from several different sources, such as plants in dietary supplements, and some antioxidants do not remove free radicals but enhance endogenous activity that may be classified as antioxidants (Mothana et al., 2019).
The most efficient enzymatic antioxidants are catalase, glutathione peroxidase (GPx) and superoxide dismutase (SOD). Non-enzymatic antioxidants include vitamins E and C, thiol antioxidants (glutathione, thioredoxin, and lipoic acid), melatonin, carotenoids, and natural flavonoids (Nandi et al., 2019). Plants used in vitiligo management, such as Ginkgo biloba, Oleaeuropaea, Cucumis melo, Camellia sinensis, Allium sativum, Carpobrotus edulis (Di Nardo et al., 2019), exhibit different mechanisms of direct and indirect antioxidant activity (Table 3; Figure 2).
6.2.2 Plants contribute to immune modulation in vitiligo management
Anti-inflammation refers to the paraphernalia of substances that reduce swelling or inflammation (Khan et al., 2019). Many bioactive compounds extracted from plants and herbs exhibit anti-inflammatory activities and are used to manage vitiligo (Mihaila et al., 2019). Recently, many herbal remedies have been prescribed for vitiligo patients (Kim et al., 2019), for example, Ammi visnaga, Picrorhiza kurroa, Capsicum annuum, Curcuma longa, Phlebodium aureum.
6.2.3 Plants induces Nrf2 pathways: a promising therapeutic agents to treat vitiligo
At molecular level, Nrf2 causes wide spectrum of responses that inhibits the progression of vitiligo pathogenesis including: (1) The coordinated upregulation of antioxidant and detoxification genes. (2) It inhibits the pro-inflammatory cells (T helper 1 and T helper 17) and activates immunosuppressive regulatory cells (T reg and T helper). (3) It plays a crucial role in counteracting NF-κB-inducing inflammatory response. (4) It reduces ER-stress (Cuadrado et al., 2018; Sarcinelli et al., 2020; Surh and Na, 2008). Furthermore, the availability of numerous natural and synthetic Nrf2 inducers isothiocynate (sulforaphan), polyphenols (curcumin and resveratrol), flavonoids (Quercetin and kaempferol), and terpenoids (Carnosic acid) is one of the major advantage for targeting this pathway (Wang et al., 2024).
Interestingly, many herbal medicinal plants used to treat vitiligo have been implicated in inducing the Nrf2 pathway and related genes. This mechanism may partially explain their use in the management of vitiligo through the reduction of oxidative damage. Plants consistently demonstrate activation of the Nrf2 pathway, including Ginkgo biloba, Olea europaea, Ammi visnaga, Picrorhiza kurroa, Curcuma longa, Allium sativum, Carica papaya, Fumaria officinalis, Paeonia lactiflora and Glycyrrhiza glabra (Table 3). Ginko biloba extract induces Nrf2 mainly thorough the disruption of Keap1-Nrf2 interaction decreasing the content of Keap1 and releasing more Nrf2 to nucleus (Liu et al., 2007). It’s worth noting that Ginko biloba extract contains bioactive flavonoids and terpenoids that act as mild electrophiles that disrupt keap1-Nrf2 interaction (Noor et al., 2022).
Olea europaea is rich with polyphenolic compounds such as oleacin, oleuropein and hydroxytyrosol that mediate keap-1 degradation and Nrf2 release (Zhou et al., 2019). Hydroxytyrosl is also reported to induce Nrf2 in PI3K dependent manner (Martín et al., 2010). Benolea® is a standardized dry olive leaf extract that is suggested as a pro-pigmenting agent for vitiligo treatment in range of concentration between 10 and 200 μg/ml (Goenka and Simon, 2021). Ammi visnaga extract contains bioactive compounds such as khellin and visnagin that are able to induce Nrf2 and downregulate keap-1 (Table 3) (Abukhalil et al., 2021). The extract is also rich with phenolic compounds and flavonoids that may affect keap-1-Nrf2 association (Khalil et al., 2020). Activation of Nrf2 pathway by Picrorhiza kurroa is reported to be mediated through signal transduction kinases (MAPK, PKC and PI3K) (Soni and Grover, 2019). Different mechanisms were reported for Nrf2 activation by Curcuma longa extract including the improvement of Nrf2 translocation, keap-1 inhibition and affecting upstream signaling modulators of Nrf2 (Ashrafizadeh et al., 2020a). Although, Allum sativa extract is rich with direct antioxidants, studies demonstrated its potent ability to induce the endogenous antioxidant machinery of Nrf2 by enhancing its phosphorylation (by PKC and p38 MAPK) and decreasing its ubiquitination (Capasso, 2013). Allium sativa extracts contain allicin, diallyl disulfied and diallyl trisulfide that are able to modify directly the Cys288 of keap-1 (Kim et al., 2014). Carica papaya extract stimulates Nrf2 in a mechanism that depends on the interaction of the Carica papaya flavonoids with the Nrf2 binding pocket of keap-1, leading to release of Nrf2 and its nuclear accumulation (Kumar et al., 2024). Fumaria officinale contains bioactive fumaric acid derivative, isoquinoline alkaloids and high level of polyphenols (Ahmoda et al., 2025). Fumaric acid derivatives activate Nrf2 via S-alkylation of keap-1 and nuclear exit of the Nrf2 repressor (Bach1) (Ahuja et al., 2016). Paeonia lactiflora extract (mainly Paeoniflorin (PF)) induces Nrf2/HO-1 signaling pathway by JNK phosphorylation and may serve as a potential therapy for vitiligo. Pf pretreatment of melanocytes with 50 μMPF significantly reduced cell death and suppressed oxidative damage (Lu et al., 2024; Yuan et al., 2020).
Glycyrrhizin (GR) is a triterpenoid extracted from Glycyrrhiza glabra. GR (at 1 mM) protects melanocytes from high dose of 0.5 mM H2O2 by induces Nrf2/HO-1. GR generates a mild electrophilic stress that leads to modifying cysteine residues of keap-1 (Mou et al., 2019). Other studies showed that GR activates Nrf2 by stress response kinases (PI3K/AKT) (Aqeel et al., 2025). It is worth noting that the type of cells and the model used is important to determine the appropriate concentration of the extract and its outcome on the Nrf2/keap-1 signaling.
The Nrf2 inducers have been a subject of many clinical trials, dimethyl fumarate (DMF) is now in clinical use to treat the skin disorder “psoriasis” (Cuadrado et al., 2018; Robledinos-Antón et al., 2019). The DMF protects healthy and vitiligo melanocytes against monobenzone-induced toxicity via Nrf2 activation, demonstrating its therapeutic potential in vitiligo treatment (Arowojolu et al., 2017). Future clinical studies is needed to investigate the use of the herbal medicine extracts in the treatment of vitiligo, focusing on the potential role of Nrf2.
7 Conclusion
Complementary herbal therapies have gained attention as an adjunct approach in treatment of vitilio. Herbal treatments offer potential synergistic effects when combined with standard treatments, thereby enhancing outcomes while minimizing adverse reactions.
This study suggests that many plants play promising roles in the management of vitiligo. These plants function through two primary mechanisms: immunomodulation and antioxidation. Immunomodulatory plants, such as Ginkgo biloba, Ammi visnaga, Capsicum annuum, Curcuma longa, Carica papaya, and Vitex agnus-castus, support immune regulation, potentially reducing the autoimmune response that contributes to vitiligo. In contrast, plants with antioxidant properties, including Olea europaea, Cucumis melo, Ocimum basilicum, Juglans regia, Camellia sinensis, Raphanus raphanistrum, Phlebodium aureum, and Carpobrotus edulis help neutralize OS, which is known to play a role in pigment cell damage. Moreover, many herbal plants influence OS response pathways, including the Nrf2 pathway, which protects melanocytes against oxidative damage. The Nrf2 pathway regulates the expression of various antioxidant genes, helping restore cellular balance by enhancing antioxidant defenses and reducing OS. This pathway is particularly relevant to vitiligo, as oxidative damage to melanocytes and pigment-producing cells contributes to depigmentation. Nrf2 controls the expression of some cytoprotective genes, including those encoding antioxidant enzymes such as HO-1, GST, and NQO1. Several Mediterranean herbs contain bioactive compounds that naturally activate Nrf2 and can be effective in managing vitiligo (Lu et al., 2022). For example, rosmarinic acid from Rosmarinus officinalis (rosemary) and carnosic acid from Salvia officinalis (sage) have been shown to activate Nrf2 and upregulate downstream antioxidant genes (Hu et al., 2023; Lu et al., 2022). Similarly, apigenin from Petroselinum crispum (parsley) and thymol from Thymus vulgaris (thyme) also modulate oxidative pathways via Nrf2 activation (Ren et al., 2024; Zhang et al., 2020). By targeting Nrf2, these phytochemicals enhance the skin’s endogenous defense mechanisms, reduce oxidative damage, and create a protective environment for melanocyte survival. Therefore, local Mediterranean herbs represent a promising natural source of Nrf2 activators in the development of adjunct therapies for vitiligo and may explain why incidence of vitiligo in the Mediterranean region remains less than other parts of the world.
Author contributions
AQ: Writing – review and editing, Investigation, Writing – original draft, Supervision, Data curation, Conceptualization, Project administration. MQ: Data curation, Validation, Investigation, Writing – review and editing, Writing – original draft. AI: Conceptualization, Writing – original draft, Investigation, Data curation. DK: Data curation, Conceptualization, Writing – original draft, Investigation. SS: Data curation, Investigation, Writing – original draft, Conceptualization. SF: Writing – review and editing. DH: Writing – review and editing. HS: Writing – review and editing. AK: Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Authors acknowledge Rezq Basheer-Salimia and Mohannad Jazzar for their insightful advises.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Fattah, A., Aboul-Enein, M. N., Wassel, G. M., and El-Menshawi, B. S. (1982). An approach to the treatment of vitiligo by khellin. Dermatologica 165 (2), 136–140. doi:10.1159/000249932
Abdou, A. G., Maraee, A., Yassien, H., and Sarhan, M. (2018). Immunohistochemistry of Janus kinase 1 (JAK1) expression in vitiligo. J. Pathol. Transl. Med. 52 (6), 363–368. doi:10.4132/jptm.2018.09.18
Abdou, A. G., Maraee, A. H., and Reyad, W. (2013). Immunohistochemical expression of heat shock protein 70 in vitiligo. Ann. Diagn Pathol. 17 (3), 245–249. doi:10.1016/j.anndiagpath.2012.11.005
Abreu, A. C., Duarte, G. G., Miranda, J. Y., Ramos, D. G., Ramos, C. G., and Ramos, M. G. (2015). Immunological parameters associated with vitiligo treatments: a literature review based on clinical studies. Autoimmune Dis. 2015, 1–5. doi:10.1155/2015/196537
Abukhalil, M. H., Hussein, O. E., Aladaileh, S. H., Althunibat, O. Y., Al-Amarat, W., Saghir, S. A., et al. (2021). Visnagin prevents isoproterenol-induced myocardial injury by attenuating oxidative stress and inflammation and upregulating Nrf2 signaling in rats. J. Biochem. Mol. Toxicol. 35 (11), e22906. doi:10.1002/jbt.22906
Agrawal, D., Shajil, E. M., Marfatia, Y. S., and Begum, R. (2004). Study on the antioxidant status of vitiligo patients of different age groups in Baroda. Pigment. Cell Res. 17 (3), 289–294. doi:10.1111/j.1600-0749.2004.00149.x
Ahmoda, R. A., Pirković, A., Milutinović, V., Milošević, M., Marinković, A., and Jovanović, A. A. (2025). Fumaria officinalis dust as a source of bioactives for potential dermal application: optimization of extraction procedures, phytochemical profiling, and effects related to skin health benefits. Plants 14 (3), 352. doi:10.3390/plants14030352
Ahuja, M., Ammal Kaidery, N., Yang, L., Calingasan, N., Smirnova, N., Gaisin, A., et al. (2016). Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson's-like disease. J. Neurosci. 36 (23), 6332–6351. doi:10.1523/jneurosci.0426-16.2016
Ala, Y., Pasha, M. K., Rao, R. N., Komaravalli, P. L., and Jahan, P. (2015). Association of IFN-gamma: IL-10 cytokine ratio with nonsegmental vitiligo pathogenesis. Autoimmune Dis. 2015, 1–8. doi:10.1155/2015/423490
Alhaithloul, H. A. S., Alotaibi, M. F., Bin-Jumah, M., Elgebaly, H., and Mahmoud, A. M. (2019). Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 111, 676–685. doi:10.1016/j.biopha.2018.12.112
Alikhan, A., Felsten, L. M., Daly, M., and Petronic-Rosic, V. (2011). Vitiligo: a comprehensive overview. J. Am. Acad. Dermatol 65 (3), 473–491. doi:10.1016/j.jaad.2010.11.061
Alkhateeb, A., Fain, P. R., and Spritz, R. A. (2005). Candidate functional promoter variant in the FOXD3 melanoblast developmental regulator gene in autosomal dominant vitiligo. J. Invest. Dermatol 125 (2), 388–391. doi:10.1111/j.0022-202X.2005.23822.x
Alkhateeb, A., Fain, P. R., Thody, A., Bennett, D. C., and Spritz, R. A. (2003). Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment. Cell Res. 16 (3), 208–214. doi:10.1034/j.1600-0749.2003.00032.x
Almanza, A., Carlesso, A., Chintha, C., Creedican, S., Doultsinos, D., Leuzzi, B., et al. (2019). Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. Febs J. 286 (2), 241–278. doi:10.1111/febs.14608
Al-Shobaili, H. A., and Rasheed, Z. (2015). Oxidized tyrosinase: a possible antigenic stimulus for non-segmental vitiligo autoantibodies. J. Dermatol Sci. 79 (3), 203–213. doi:10.1016/j.jdermsci.2015.06.009
An, R., Li, D., Dong, Y., She, Q., Zhou, T., Nie, X., et al. (2021). Methylcobalamin protects melanocytes from H(2)O(2)-induced oxidative stress by activating the Nrf2/HO-1 pathway. Drug Des. Devel Ther. 15, 4837–4848. doi:10.2147/dddt.s336066
Aobuli, A., Maitusong, J., Bakri, M., Lu, X., Maiwulanjiang, M., and Aisa, H. A. (2018). The Effect of volatile oil from Vernonia anthelmintica seeds on melanin synthesis in B16 Cells and its chemical analysis by GC-QTOF-MS. Evid. Based Complement. Altern. Med. 2018, 6291281. doi:10.1155/2018/6291281
Aqeel, M., Upadhayay, S., Devi, R., Jangid, K., Kumar, V., and Kumar, P. (2025). Glycyrrhizic acid mitigates haloperidol-induced neurotoxicity in SHSY-5Y cells and rats via activation of PI3k/Akt/Nrf2 Pathways. Neurochem. Res. 50 (1), 75. doi:10.1007/s11064-024-04319-1
Arowojolu, O. A., Orlow, S. J., Elbuluk, N., and Manga, P. (2017). The nuclear factor (erythroid-derived 2)-like 2 (NRF2) antioxidant response promotes melanocyte viability and reduces toxicity of the vitiligo-inducing phenol monobenzone. Exp. Dermatol 26 (7), 637–644. doi:10.1111/exd.13350
Ashrafizadeh, M., Ahmadi, Z., Mohammadinejad, R., Farkhondeh, T., and Samarghandian, S. (2020a). Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr. Mol. Med. 20 (2), 116–133. doi:10.2174/1566524019666191016150757
Ashrafizadeh, M., Zarrabi, A., Hashemi, F., Moghadam, E. R., Hashemi, F., Entezari, M., et al. (2020b). Curcumin in cancer therapy: a novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 256, 117984. doi:10.1016/j.lfs.2020.117984
Baird, L., and Dinkova-Kostova, A. T. (2011). The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 85 (4), 241–72. doi:10.1007/s00204-011-0674-5
Baldini, E., Odorisio, T., Sorrenti, S., Catania, A., Tartaglia, F., Carbotta, G., et al. (2017). Vitiligo and autoimmune thyroid disorders. Front. Endocrinol. (Lausanne) 8, 290. doi:10.3389/fendo.2017.00290
Barnabei, L., Laplantine, E., Mbongo, W., Rieux-Laucat, F., and Weil, R. (2021). NF-κB: at the borders of autoimmunity and inflammation. Front. Immunol. 12, 716469. doi:10.3389/fimmu.2021.716469
Basilicata, M. G., Pepe, G., Rapa, S. F., Merciai, F., Ostacolo, C., Manfra, M., et al. (2019). Anti-inflammatory and antioxidant properties of dehydrated potato-derived bioactive compounds in intestinal cells. Int. J. Mol. Sci. 20 (23), 6087. doi:10.3390/ijms20236087
Bazzicalupo, M., Cornara, L., Burlando, B., Cascini, A., Denaro, M., Smeriglio, A., et al. (2021). Carpobrotus edulis (L.) NE Br. extract as a skin preserving agent: from traditional medicine to scientific validation. J. Integr. Med. 19 (6), 526–536. doi:10.1016/j.joim.2021.09.002
Becatti, M., Prignano, F., Fiorillo, C., Pescitelli, L., Nassi, P., Lotti, T., et al. (2010). The involvement of Smac/DIABLO, p53, NF-kB, and MAPK pathways in apoptosis of keratinocytes from perilesional vitiligo skin: protective effects of curcumin and capsaicin. Antioxid. Redox Signal 13 (9), 1309–1321. doi:10.1089/ars.2009.2779
Bedi, K. L., Zutshi, U., Chopra, C. L., and Amla, V. (1989). Picrorhiza kurroa, an ayurvedic herb, may potentiate photochemotherapy in vitiligo. J. Ethnopharmacol. 27 (3), 347–352. doi:10.1016/0378-8741(89)90009-3
Bettigole, S. E., and Glimcher, L. H. (2015). Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138. doi:10.1146/annurev-immunol-032414-112116
Birlea, S. A., Jin, Y., Bennett, D. C., Herbstman, D. M., Wallace, M. R., McCormack, W. T., et al. (2011). Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J. Invest. Dermatol 131 (2), 371–381. doi:10.1038/jid.2010.337
Bleuel, R., and Eberlein, B. (2018). Therapeutic management of vitiligo. J. Dtsch. Dermatol Ges. 16 (11), 1309–1313. doi:10.1111/ddg.13680
Bloom, D. A., and Jaiswal, A. K. (2003). Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 278 (45), 44675–44682. doi:10.1074/jbc.M307633200
Boo, Y. C. (2020). Natural Nrf2 modulators for skin protection. Antioxidants 9 (9), 812. doi:10.3390/antiox9090812
Bordere, A. C., Lambert, J., and van Geel, N. (2009). Current and emerging therapy for the management of vitiligo. Clin. Cosmet. Investig. Dermatol 2, 15–25. doi:10.2147/ccid.s3627
Bowcock, A. M., and Fernandez-Vina, M. (2012). Targeting skin: vitiligo and autoimmunity. J. Invest. Dermatol 132 (1), 13–15. doi:10.1038/jid.2011.353
Buckley, B. J., Marshall, Z. M., and Whorton, A. R. (2003). Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem. Biophysical Res. Commun. 307 (4), 973–979. doi:10.1016/S0006-291X(03)01308-1
Buggiani, G., Tsampau, D., Hercogovà, J., Rossi, R., Brazzini, B., and Lotti, T. (2012). Clinical efficacy of a novel topical formulation for vitiligo: compared evaluation of different treatment modalities in 149 patients. Dermatol Ther. 25 (5), 472–476. doi:10.1111/j.1529-8019.2012.01484.x
Cai, M., Zhou, L., Liao, J., Huang, Q., Xia, Z., and Shang, J. (2019). IFN-gamma inhibits 5-HT-induced melanin biosynthesis via downregulation of 5-HT receptors in vivo/in vitro. J. Pharmacol. Sci. 141 (1), 1–8. doi:10.1016/j.jphs.2019.05.005
Canizares, O., Uribe Jaramillo, F., and Kerdel Vegas, F. (1958). Leukomelanoderma subsequent to the application of monobenzyl ether of hydroquinone; a vitiligoid reaction observed in Colombia and Venezuela. AMA Arch. Derm. 77 (2), 220–223. doi:10.1001/archderm.1958.01560020074009
Canning, P., Sorrell, F. J., and Bullock, A. N. (2015). Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 88 (Pt B), 101–107. doi:10.1016/j.freeradbiomed.2015.05.034
Cantón, I., Akhtar, S., Gavalas, N. G., Gawkrodger, D. J., Blomhoff, A., Watson, P. F., et al. (2005). A single-nucleotide polymorphism in the gene encoding lymphoid protein tyrosine phosphatase (PTPN22) confers susceptibility to generalised vitiligo. Genes Immun. 6 (7), 584–587. doi:10.1038/sj.gene.6364243
Cao, L.-J., Hou, Z.-Y., Li, H.-D., Zhang, B.-K., Fang, P.-F., Xiang, D.-X., et al. (2017). The ethanol extract of licorice (Glycyrrhiza uralensis) protects against triptolide-induced oxidative stress through activation of Nrf2. Evidence-Based Complementary Altern. Med. 2017 (1), 2752389. doi:10.1155/2017/2752389
Capasso, A. (2013). Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules 18 (1), 690–700. doi:10.3390/molecules18010690
Chaiprasongsuk, A., and Panich, U. (2022). Role of phytochemicals in skin photoprotection via regulation of Nrf2. Front. Pharmacol. 13, 823881. doi:10.3389/fphar.2022.823881
Chang, W. L., and Ko, C. H. (2023). The role of oxidative stress in vitiligo: an update on its pathogenesis and therapeutic implications. Cells 12 (6), 936. doi:10.3390/cells12060936
Chaudhari, N., Talwar, P., Parimisetty, A., Lefebvre d'Hellencourt, C., and Ravanan, P. (2014). A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell Neurosci. 8, 213. doi:10.3389/fncel.2014.00213
Chen, L., Chen, S., Li, P., Zhao, X., Sun, P., Liu, X., et al. (2024). Exploration of the mechanism of Qinglongyi-Buguzhi drug pair in treating vitiligo based on network pharmacology, molecular docking and experimental verification. J. Ethnopharmacol. 334, 118595. doi:10.1016/j.jep.2024.118595
Cho, J., Bejaoui, M., Tominaga, K., and Isoda, H. (2024). Comparative analysis of olive-derived phenolic compounds’ pro-melanogenesis effects on B16F10 cells and epidermal human melanocytes. Int. J. Mol. Sci. 25 (8), 4479. doi:10.3390/ijms25084479
Choi, C. W., Chang, S. E., Bak, H., Choi, J. H., Park, H. S., Huh, C. H., et al. (2008). Topical immunomodulators are effective for treatment of vitiligo. J. Dermatol 35 (8), 503–507. doi:10.1111/j.1346-8138.2008.00511.x
Colucci, R., Dragoni, F., and Moretti, S. (2015). Oxidative stress and immune system in vitiligo and thyroid diseases. Oxid. Med. Cell Longev. 2015, 1–7. doi:10.1155/2015/631927
Cuadrado, A., Manda, G., Hassan, A., Alcaraz, M. J., Barbas, C., Daiber, A., et al. (2018). Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol. Rev. 70 (2), 348–383. doi:10.1124/pr.117.014753
Cullinan, S. B., Zhang, D., Hannink, M., Arvisais, E., Kaufman, R. J., and Diehl, J. A. (2003). Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 23 (20), 7198–7209. doi:10.1128/mcb.23.20.7198-7209.2003
Das, D., Akhtar, S., Kurra, S., Gupta, S., and Sharma, A. (2019). Emerging role of immune cell network in autoimmune skin disorders: an update on pemphigus, vitiligo and psoriasis. Cytokine Growth Factor Rev. 45, 35–44. doi:10.1016/j.cytogfr.2019.01.001
Dawn Teare, M., and Barrett, J. H. (2005). Genetic linkage studies. Lancet 366 (9490), 1036–1044. doi:10.1016/s0140-6736(05)67382-5
Dell’Anna, M. L., Maresca, V., Briganti, S., Camera, E., Falchi, M., and Picardo, M. (2001). Mitochondrial impairment in peripheral blood mononuclear cells during the active phase of vitiligo. J. Invest. Dermatol 117 (4), 908–913. doi:10.1046/j.0022-202x.2001.01459.x
Dellatorre, G., Antelo, D. A. P., Bedrikow, R. B., Cestari, T. F., Follador, I., Ramos, D. G., et al. (2020). Consensus on the treatment of vitiligo - Brazilian Society of Dermatology. An Bras Dermatol. 95 (Suppl 1), 70–82. doi:10.1016/j.abd.2020.05.007
Di Nardo, V., Barygina, V., Franca, K., Tirant, M., Valle, Y., and Lotti, T. (2019). Functional nutrition as integrated approach in vitiligo management. Dermatol Ther. 32 (4), e12625. doi:10.1111/dth.12625
Dinkova-Kostova, A. T., and Talalay, P. (2010). NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 501 (1), 116–123. doi:10.1016/j.abb.2010.03.019
Eibulayin, G., Rahman, N., Fu, J. H., and Turxuntayi, A. (2024). Study on the protective effect of flavonoids in Vernonia anthelmintica (L.) Willd on oxidative stress damage of HaCaT cells. Nat. Product. Commun. 19 (4), 1934578X241245866. doi:10.1177/1934578x241245866
Elassiuty, Y. E., Klarquist, J., Speiser, J., Yousef, R. M., El Refaee, A. A., Hunter, N. S., et al. (2011). Heme oxygenase-1 expression protects melanocytes from stress-induced cell death: implications for vitiligo. Exp. Dermatol 20 (6), 496–501. doi:10.1111/j.1600-0625.2010.01232.x
Eletto, D., Chevet, E., Argon, Y., and Appenzeller-Herzog, C. (2014). Redox controls UPR to control redox. J. Cell Sci. 127 (Pt 17), 3649–3658. doi:10.1242/jcs.153643
Elmongy, N. N., and Abu Khalil, R. E. (2013). PTPN22 gene polymorphism in Egyptian females with non-segmental vitiligo. Comp. Clin. Pathol. 22 (5), 961–964. doi:10.1007/s00580-012-1508-4
Esmat, S., Mostafa, W., Hegazy, R. A., Shalaby, S., Sheth, V., Youssef, R., et al. (2016). Phototherapy: the vitiligo management pillar. Clin. Dermatol 34 (5), 594–602. doi:10.1016/j.clindermatol.2016.05.009
Eudy, B. J., Carter, R. E., Abed, S. A., Svoboda, S. A., and Percival, S. S. (2017). Activation of Nrf2 target genes by aged garlic extract in THP-1 macrophages: regulation by glutathione depletion. FASEB J. 31 (S1), 974. doi:10.1096/fasebj.31.1_supplement.974.24
Ezzedine, K., Lim, H. W., Suzuki, T., Katayama, I., Hamzavi, I., Lan, C. C. E., et al. (2012). Revised classification/nomenclature of vitiligo and related issues: the vitiligo global issues consensus conference. Pigment Cell and melanoma Res. 25 (3), E1–E13. doi:10.1111/j.1755-148X.2012.00997.x
Fain, P. R., Gowan, K., LaBerge, G. S., Alkhateeb, A., Stetler, G. L., Talbert, J., et al. (2003). A genomewide screen for generalized vitiligo: confirmation of AIS1 on chromosome 1p31 and evidence for additional susceptibility loci. Am. J. Hum. Genet. 72 (6), 1560–1564. doi:10.1086/375451
Fang, W., Tang, L., Wang, G., Lin, J., Liao, W., Pan, W., et al. (2020). Molecular hydrogen protects human melanocytes from oxidative stress by activating Nrf2 signaling. J. Invest. Dermatol 140 (11), 2230–2241.e9. doi:10.1016/j.jid.2019.03.1165
Farag, A. G. A., Hammam, M. A., Al-Sharaky, D. R., and El-Boghdady, G. M. (2019). Leucine-rich glioma inactivated 3: a novel keratinocyte-derived melanogenic cytokine in vitiligo patients. Bras Dermatol 94 (4), 434–441. doi:10.1590/abd1806-4841.20198250
Gao, W., Guo, L., Yang, Y., Wang, Y., Xia, S., Gong, H., et al. (2021). Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front. Cell Dev. Biol. 9, 809952. doi:10.3389/fcell.2021.809952
Garg, A. D., Kaczmarek, A., Krysko, O., Vandenabeele, P., Krysko, D. V., and Agostinis, P. (2012). ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol. Med. 18 (10), 589–598. doi:10.1016/j.molmed.2012.06.010
Gęgotek, A., and Skrzydlewska, E. (2015). The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol Res. 307 (5), 385–396. doi:10.1007/s00403-015-1554-2
Gianfaldoni, S., Wollina, U., Tirant, M., Tchernev, G., Lotti, J., Satolli, F., et al. (2018). Herbal compounds for the treatment of vitiligo: a review. Open Access Maced. J. Med. Sci. 6 (1), 203–207. doi:10.3889/oamjms.2018.048
Gill, L., Zarbo, A., Isedeh, P., Jacobsen, G., Lim, H. W., and Hamzavi, I. (2016). Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J. Am. Acad. Dermatol 74 (2), 295–302. doi:10.1016/j.jaad.2015.08.063
Goenka, S., and Simon, S. R. (2021). A novel pro-melanogenic effect of standardized dry olive leaf extract on primary human melanocytes from lightly pigmented and moderately pigmented skin. Pharm. (Basel) 14 (3), 252. doi:10.3390/ph14030252
Guan, C., Xu, W., Hong, W., Zhou, M., Lin, F., Fu, L., et al. (2015). Quercetin attenuates the effects of H2O2 on endoplasmic reticulum morphology and tyrosinase export from the endoplasmic reticulum in melanocytes. Mol. Med. Rep. 11 (6), 4285–4290. doi:10.3892/mmr.2015.3242
Guan, C. P., Zhou, M. N., Xu, A. E., Kang, K. F., Liu, J. F., Wei, X. D., et al. (2008). The susceptibility to vitiligo is associated with NF-E2-related factor2 (Nrf2) gene polymorphisms: a study on Chinese Han population. Exp. Dermatol 17 (12), 1059–1062. doi:10.1111/j.1600-0625.2008.00752.x
Guarneri, F., Bertino, L., Pioggia, G., Casciaro, M., and Gangemi, S. (2021). Therapies with antioxidant potential in psoriasis, vitiligo, and lichen planus. Antioxidants 10 (7), 1087. doi:10.3390/antiox10071087
Gupta, S. C., Patchva, S., and Aggarwal, B. B. (2013). Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 15 (1), 195–218. doi:10.1208/s12248-012-9432-8
Harris, J. E. (2016). Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol. Rev. 269 (1), 11–25. doi:10.1111/imr.12369
Hayashi, M., Jin, Y., Yorgov, D., Santorico, S. A., Hagman, J., Ferrara, T. M., et al. (2016). Autoimmune vitiligo is associated with gain-of-function by a transcriptional regulator that elevates expression of HLA-A*02:01 in vivo. Proc. Natl. Acad. Sci. U. S. A. 113 (5), 1357–1362. doi:10.1073/pnas.1525001113
He, Y., Li, S., Zhang, W., Dai, W., Cui, T., Wang, G., et al. (2017). Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci. Rep. 7 (1), 42394. doi:10.1038/srep42394
Hetz, C. (2012). The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13 (2), 89–102. doi:10.1038/nrm3270
Hosseinzadeh, A., Houshmand, G., Goudarzi, M., Sezavar, S. H., Mehrzadi, S., Mansouri, E., et al. (2019). Ameliorative effect of gallic acid on sodium arsenite-induced spleno-cardio- and hemato-toxicity in rats. Life Sci. 217, 91–100. doi:10.1016/j.lfs.2018.11.050
Hotamisligil, G. S. (2010). Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140 (6), 900–917. doi:10.1016/j.cell.2010.02.034
Hu, S., Liu, B., Yang, M., Mao, S., Ju, H., Liu, Z., et al. (2023). Carnosic acid protects against doxorubicin-induced cardiotoxicity through enhancing the Nrf2/HO-1 pathway. Food and Funct. 14 (8), 3849–3862. doi:10.1039/d2fo03904d
Huo, S. X., Wang, Q., Liu, X. M., Ge, C. H., Gao, L., Peng, X. M., et al. (2017). The effect of butin on the vitiligo mouse model induced by hydroquinone. Phytother. Res. 31 (5), 740–746. doi:10.1002/ptr.5794
Iannella, G., Greco, A., Didona, D., Didona, B., Granata, G., Manno, A., et al. (2016). Vitiligo: pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 15 (4), 335–343. doi:10.1016/j.autrev.2015.12.006
Itoh, K., Tong, K. I., and Yamamoto, M. (2004). Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 36 (10), 1208–1213. doi:10.1016/j.freeradbiomed.2004.02.075
Iyda, J. H., Fernandes, A., Ferreira, F. D., Alves, M. J., Pires, T., Barros, L., et al. (2019). Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 121, 714–722. doi:10.1016/j.foodres.2018.12.046
Jahanban-Esfahlan, A., Ostadrahimi, A., Tabibiazar, M., and Amarowicz, R. (2019). A comprehensive review on the chemical constituents and functional uses of Walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 20 (16), 3920. doi:10.3390/ijms20163920
Jalalmanesh, S., Mansouri, P., Rajabi, M., and Monji, F. (2022). Therapeutic effects of turmeric topical cream in vitiligo: a randomized, double-blind, placebo-controlled pilot study. J. Cosmet. dermatology 21 (10), 4454–4461. doi:10.1111/jocd.14814
Jalel, A., Soumaya, G. S., and Hamdaoui, M. H. (2009). Vitiligo treatment with vitamins, minerals and polyphenol supplementation. Indian J. Dermatol 54 (4), 357–360. doi:10.4103/0019-5154.57613
Jarisarapurin, W., Sanrattana, W., Chularojmontri, L., Kunchana, K., and Wattanapitayakul, S. K. (2019). Antioxidant properties of unripe Carica papaya fruit extract and its protective effects against endothelial oxidative stress. Evid. Based Complement. Altern. Med. 2019, 4912631–15. doi:10.1155/2019/4912631
Jian, Z., Li, K., Liu, L., Zhang, Y., Zhou, Z., Li, C., et al. (2011). Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J. Invest. Dermatol 131 (7), 1420–1427. doi:10.1038/jid.2011.56
Jian, Z., Li, K., Song, P., Zhu, G., Zhu, L., Cui, T., et al. (2014). Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. J. Invest. Dermatol 134 (8), 2221–2230. doi:10.1038/jid.2014.152
Jin, Y., Andersen, G., Yorgov, D., Ferrara, T. M., Ben, S., Brownson, K. M., et al. (2016). Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat. Genet. 48 (11), 1418–1424. doi:10.1038/ng.3680
Jin, Y., Birlea, S. A., Fain, P. R., Ferrara, T. M., Ben, S., Riccardi, S. L., et al. (2012). Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat. Genet. 44 (6), 676–680. doi:10.1038/ng.2272
Jin, Y., Birlea, S. A., Fain, P. R., Gowan, K., Riccardi, S. L., Holland, P. J., et al. (2010a). Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N. Engl. J. Med. 362 (18), 1686–1697. doi:10.1056/NEJMoa0908547
Jin, Y., Birlea, S. A., Fain, P. R., Mailloux, C. M., Riccardi, S. L., Gowan, K., et al. (2010b). Common variants in FOXP1 are associated with generalized vitiligo. Nat. Genet. 42 (7), 576–578. doi:10.1038/ng.602
Jin, Y., Birlea, S. A., Fain, P. R., and Spritz, R. A. (2007a). Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J. Invest. Dermatol 127 (11), 2558–2562. doi:10.1038/sj.jid.5700953
Jin, Y., Mailloux, C. M., Gowan, K., Riccardi, S. L., LaBerge, G., Bennett, D. C., et al. (2007b). NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 356 (12), 1216–1225. doi:10.1056/NEJMoa061592
Jin, Y., Roberts, G. H. L., Ferrara, T. M., Ben, S., van Geel, N., Wolkerstorfer, A., et al. (2019). Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat. Commun. 10 (1), 391. doi:10.1038/s41467-019-08337-4
Kamelnia, E., Mohebbati, R., Kamelnia, R., El-Seedi, H. R., and Boskabady, M. H. (2023). Anti-inflammatory, immunomodulatory and anti-oxidant effects of Ocimum basilicum L. and its main constituents: a review. Iran. J. Basic Med. Sci. 26 (6), 617–627. doi:10.22038/ijbms.2023.67466.14783
Kang, K. W., Lee, S. J., Park, J. W., and Kim, S. G. (2002). Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol. Pharmacol. 62 (5), 1001–1010. doi:10.1124/mol.62.5.1001
Kaur, M., Bagga, P. K., Kaur, T., and Kataria, A. S. (2017). Evaluation of histologically and histochemically proven cases of vitiligo and its correlation with CD4+ and CD8+ lymphocyte counts using flow cytometry. J. Clin. Diagn Res. 11 (5), EC09–EC12. doi:10.7860/JCDR/2017/25665.9821
Kausar, M., Pandey, P., Bisht, D., Sengupta, S., Kumar, D., and Arya, R. K. K. (2023). In-silico anti-vitiligo activity of glycyrrhizin as potential IL-17 inhibitor and in-vitro antioxidant potential of isolated glycyrrhizin from G. glabra. South Afr. J. Bot. 162, 381–390. doi:10.1016/j.sajb.2023.09.027
Keum, Y.-S., Owuor, E. D., Kim, B.-R., Hu, R., and Kong, A. N. T. (2003). Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC). Pharm. Res. 20 (9), 1351–1356. doi:10.1023/A:1025737622815
Khalil, N., Bishr, M., Desouky, S., and Salama, O. (2020). Ammi visnaga L., a potential medicinal plant: a review. Molecules 25 (2), 301. doi:10.3390/molecules25020301
Khan, H., Sureda, A., Belwal, T., Cetinkaya, S., Suntar, I., Tejada, S., et al. (2019). Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 18 (7), 647–657. doi:10.1016/j.autrev.2019.05.001
Kim, H., Park, S. Y., and Lee, G. (2019). Potential therapeutic applications of bee venom on skin disease and its mechanisms: a literature review. Toxins (Basel) 11 (7), 374. doi:10.3390/toxins11070374
Kim, S., Lee, H. G., Park, S. A., Kundu, J. K., Keum, Y. S., Cha, Y. N., et al. (2014). Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One 9 (1), e85984. doi:10.1371/journal.pone.0085984
Kruger, C., and Schallreuter, K. U. (2012). A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int. J. Dermatol 51 (10), 1206–1212. doi:10.1111/j.1365-4632.2011.05377.x
Kumar, S. S., K, K., Maria, E., and John, M. (2024). Exploring the cytoprotective potential of flavonoid fraction from Carica papaya L. Cultivar ‘Red Lady' Leaf: insights into Nrf2 activation via in-vitro and in-silico approaches. South Afr. J. Bot. 172, 373–387. doi:10.1016/j.sajb.2024.07.048
Kummer, J. A., Broekhuizen, R., Everett, H., Agostini, L., Kuijk, L., Martinon, F., et al. (2007). Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem Cytochem 55 (5), 443–452. doi:10.1369/jhc.6A7101.2006
LaBerge, G. S., Bennett, D. C., Fain, P. R., and Spritz, R. A. (2008). PTPN22 is genetically associated with risk of generalized vitiligo, but CTLA4 is not. J. Invest. Dermatol 128 (7), 1757–1762. doi:10.1038/sj.jid.5701233
Laddha, N. C., Dwivedi, M., Shajil, E. M., Prajapati, H., Marfatia, Y. S., and Begum, R. (2008). Association of PTPN22 1858C/T polymorphism with vitiligo susceptibility in Gujarat population. J. Dermatol Sci. 49 (3), 260–262. doi:10.1016/j.jdermsci.2007.10.002
Lai, Y., Feng, Q., Zhang, R., Shang, J., and Zhong, H. (2021). The great capacity on promoting melanogenesis of three compatible components in Vernonia anthelmintica (L.) Willd. Int. J. Mol. Sci. 22 (8), 4073. doi:10.3390/ijms22084073
Li, X. S., Tang, X. Y., Su, W., and Li, X. (2020). Vitexin protects melanocytes from oxidative stress via activating MAPK-Nrf2/ARE pathway. Immunopharmacol. Immunotoxicol. 42 (6), 594–603. doi:10.1080/08923973.2020.1835952
Li, W., and Kong, A. N. (2009). Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 48 (2), 91–104. doi:10.1002/mc.20465
Lin, X., Meng, X., Song, Z., and Lin, J. (2020). Nuclear factor erythroid 2-related factor 2 (Nrf2) as a potential therapeutic target for vitiligo. Arch. Biochem. Biophys. 696, 108670. doi:10.1016/j.abb.2020.108670
Liu, L. Y., He, S. J., Luo, J., Huang, J. K., Yuan, J. X., Yuan, C. J., et al. (2024). Network pharmacology, molecular docking and experimental study on the mechanism of Curcumin's anti-ferroptosis in melanocytes. Biochem. Biophysical Res. Commun. 736, 150871. doi:10.1016/j.bbrc.2024.150871
Liu, X. P., Goldring, C. E., Copple, I. M., Wang, H.-Y., Wei, W., Kitteringham, N. R., et al. (2007). Extract of Ginkgo biloba induces phase 2 genes through Keap1-Nrf2-ARE signaling pathway. Life Sci. 80 (17), 1586–1591. doi:10.1016/j.lfs.2007.01.034
Lu, L., Wang, S., Fu, L., Liu, D., Zhu, Y., and Xu, A. (2016). Bilobalide protection of normal human melanocytes from hydrogen peroxide-induced oxidative damage via promotion of antioxidase expression and inhibition of endoplasmic reticulum stress. Clin. Exp. Dermatol 41 (1), 64–73. doi:10.1111/ced.12664
Lu, Y., Yin, L., Yang, W., Wu, Z., and Niu, J. (2024). Antioxidant effects of Paeoniflorin and relevant molecular mechanisms as related to a variety of diseases: a review. Biomed. Pharmacother. 176, 116772. doi:10.1016/j.biopha.2024.116772
Lu, Y. H., Hong, Y., Zhang, T. Y., Chen, Y. X., Wei, Z. J., and Gao, C. Y. (2022). Rosmarinic acid exerts anti-inflammatory effect and relieves oxidative stress via Nrf2 activation in carbon tetrachloride-induced liver damage. Food Nutr. Res. 66. doi:10.29219/fnr.v66.8359
Ma, J., Li, S., Zhu, L., Guo, S., Yi, X., Cui, T., et al. (2018). Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic. Biol. Med. 129, 492–503. doi:10.1016/j.freeradbiomed.2018.10.421
Majumder, P. P., Nordlund, J. J., and Nath, S. K. (1993). Pattern of familial aggregation of vitiligo. Arch. Dermatol 129 (8), 994–998. doi:10.1001/archderm.1993.01680290066010
Males, Z., Drvar, D. L., Duka, I., and Zuzul, K. (2019). Application of medicinal plants in several dermatovenerological entities. Acta Pharm. 69 (4), 525–531. doi:10.2478/acph-2019-0045
Malhotra, D., Portales-Casamar, E., Singh, A., Srivastava, S., Arenillas, D., Happel, C., et al. (2010). Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 38 (17), 5718–5734. doi:10.1093/nar/gkq212
Malhotra, J. D., and Kaufman, R. J. (2007). Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal 9 (12), 2277–2294. doi:10.1089/ars.2007.1782
Mamat, N., Lu, X. Y., Kabas, M., and Aisa, H. A. (2018). Potential anti-vitiligo properties of cynarine extracted from Vernonia anthelmintica (L.) Willd. Int. J. Mol. Med. 42 (5), 2665–2675. doi:10.3892/ijmm.2018.3861
Manga, P., Elbuluk, N., and Orlow, S. J. (2016). Recent advances in understanding vitiligo. F1000Research 5, 2234. doi:10.12688/f1000research.8976.1
Martín, M. A., Ramos, S., Granado-Serrano, A. B., Rodríguez-Ramiro, I., Trujillo, M., Bravo, L., et al. (2010). Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 54 (7), 956–966. doi:10.1002/mnfr.200900159
Matzaraki, V., Kumar, V., Wijmenga, C., and Zhernakova, A. (2017). The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol. 18 (1), 76. doi:10.1186/s13059-017-1207-1
Mihaila, B., Dinica, R. M., Tatu, A. L., and Buzia, O. D. (2019). New insights in vitiligo treatments using bioactive compounds from Piper nigrum. Exp. Ther. Med. 17 (2), 1039–1044. doi:10.3892/etm.2018.6977
Mohan, S., R, P. R. M., Brown, L., Ayyappan, P., and G, R. K. (2019). Endoplasmic reticulum stress: a master regulator of metabolic syndrome. Eur. J. Pharmacol. 860, 172553. doi:10.1016/j.ejphar.2019.172553
Moreira, C. G., Carrenho, L. Z. B., Pawloski, P. L., Soley, B. S., Cabrini, D. A., and Otuki, M. F. (2015). Pre-clinical evidences of Pyrostegia venusta in the treatment of vitiligo. J. Ethnopharmacol. 168, 315–325. doi:10.1016/j.jep.2015.03.080
Mothana, R. A., Khaled, J. M., El-Gamal, A. A., Noman, O. M., Kumar, A., Alajmi, M. F., et al. (2019). Comparative evaluation of cytotoxic, antimicrobial and antioxidant activities of the crude extracts of three Plectranthus species grown in Saudi Arabia. Saudi Pharm. J. 27 (2), 162–170. doi:10.1016/j.jsps.2018.09.010
Mou, K., Pan, W., Han, D., Wen, X., Cao, F., Miao, Y., et al. (2019). Glycyrrhizin protects human melanocytes from H2O2-induced oxidative damage via the Nrf2-dependent induction of HO-1. Int. J. Mol. Med. 44 (1), 253–261. doi:10.3892/ijmm.2019.4200
Mulaudzi, R. B., Aremu, A. O., Rengasamy, K. R. R., Adebayo, S. A., McGaw, L. J., Amoo, S. O., et al. (2019). Antidiabetic, anti-inflammatory, anticholinesterase and cytotoxicity determination of two Carpobrotus species. South Afr. J. Bot. 125, 142–148. doi:10.1016/j.sajb.2019.07.007
Nandi, A., Yan, L. J., Jana, C. K., and Das, N. (2019). Role of catalase in oxidative stress -and age-associated degenerative diseases. Oxid. Med. Cell Longev. 2019, 1–19. doi:10.1155/2019/9613090
Narayanaswamy, R., and Ismail, I. S. (2018). Role of herbal medicines in vitiligo treatment-current status and future perspectives. Asian J. Pharm. Clin. Res. 11, 19–23. doi:10.22159/ajpcr.2018.v11i9.26830
Natarajan, V. T., Singh, A., Kumar, A. A., Sharma, P., Kar, H. K., Marrot, L., et al. (2010). Transcriptional upregulation of Nrf2-dependent phase II detoxification genes in the involved epidermis of vitiligo vulgaris. J. Invest. Dermatol 130 (12), 2781–2789. doi:10.1038/jid.2010.201
Nath, S. K., Majumder, P. P., and Nordlund, J. J. (1994). Genetic epidemiology of vitiligo: multilocus recessivity cross-validated. Am. J. Hum. Genet. 55 (5), 981–990.
Nejad, S. B., Qadim, H. H., Nazeman, L., Fadaii, R., and Goldust, M. (2013). Frequency of autoimmune diseases in those suffering from vitiligo in comparison with normal population. Pak J. Biol. Sci. 16 (12), 570–574. doi:10.3923/pjbs.2013.570.574
Nikiforidis, G. C., Tsambaos, D. G., Karamitsos, D. S., Koutsojannis, C. C., and Georgiou, S. V. (1993). Abnormalities of the auditory brainstem response in vitiligo. Scand. Audiol. 22 (2), 97–100. doi:10.3109/01050399309046024
Ning, W., Wang, S., Dong, X., Liu, D., Fu, L., Jin, R., et al. (2015). Epigallocatechin-3-gallate (EGCG) suppresses the trafficking of lymphocytes to epidermal melanocytes via inhibition of JAK2: its implication for vitiligo treatment. Biol. Pharm. Bull. 38 (11), 1700–1706. doi:10.1248/bpb.b15-00331
Noor, E. T., Das, R., Lami, M. S., Chakraborty, A. J., Mitra, S., Tallei, T. E., et al. (2022). Ginkgo biloba: a treasure of functional phytochemicals with multimedicinal applications. Evid. Based Complement. Altern. Med. 2022, 8288818. doi:10.1155/2022/8288818
Nxumalo, M. B., Ntanzi, N., Kumalo, H. M., and Khan, R. B. (2024). Mitigating hyperglycaemic oxidative stress in HepG2 cells: the role of Carica papaya leaf and root extracts in promoting glucose uptake and antioxidant defence. Nutrients 16 (20), 3496. doi:10.3390/nu16203496
Oliveira, D., Hayrapetyan, R., Dias, M. I., Barros, L., Séverin, I., Custódio, L., et al. (2024). Protective properties of the edible halophyte Carpobrotus edulis (L.) N.E.Br. towards neoformed food contaminants-related oxidative stress and genotoxicity. Food Biosci. 61, 104447. doi:10.1016/j.fbio.2024.104447
Orecchia, G., and Perfetti, L. (1992). Photochemotherapy with topical khellin and sunlight in vitiligo. Dermatology 184 (2), 120–123. doi:10.1159/000247517
Ortel, B., Tanew, A., and Hönigsmann, H. (1988). Treatment of vitiligo with khellin and ultraviolet A. J. Am. Acad. Dermatol 18 (4 Pt 1), 693–701. doi:10.1016/s0190-9622(88)70092-4
Pae, H. O., Lee, Y. C., and Chung, H. T. (2008). Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat. Inflamm. Allergy Drug Discov. 2 (3), 159–165. doi:10.2174/187221308786241929
Pae, H. O., Oh, G. S., Choi, B. M., Chae, S. C., Kim, Y. M., Chung, K. R., et al. (2004). Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol. 172 (8), 4744–4751. doi:10.4049/jimmunol.172.8.4744
Pang, Y., Wu, S., He, Y., Nian, Q., Lei, J., Yao, Y., et al. (2021). Plant-derived compounds as promising therapeutics for vitiligo. Front. Pharmacol. 12, 685116. doi:10.3389/fphar.2021.685116
Papadopoulos, L., Bor, R., Legg, C., and Hawk, J. L. (1998). Impact of life events on the onset of vitiligo in adults: preliminary evidence for a psychological dimension in aetiology. Clin. Exp. Dermatol 23 (6), 243–248. doi:10.1046/j.1365-2230.1998.00384.x
Park, K., Lee, S. E., Shin, K. O., and Uchida, Y. (2019). Insights into the role of endoplasmic reticulum stress in skin function and associated diseases. Febs J. 286 (2), 413–425. doi:10.1111/febs.14739
Parsad, D., Pandhi, R., and Juneja, A. (2003). Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin. Exp. Dermatology 28 (3), 285–287. doi:10.1046/j.1365-2230.2003.01207.x
Pasari, L. P., Khurana, A., Anchi, P., Aslam Saifi, M., Annaldas, S., and Godugu, C. (2019). Visnagin attenuates acute pancreatitis via Nrf2/NFκB pathway and abrogates associated multiple organ dysfunction. Biomed. Pharmacother. 112, 108629. doi:10.1016/j.biopha.2019.108629
Passeron, T., and Ortonne, J. P. (2012). Activation of the unfolded protein response in vitiligo: the missing link? J. Invest. Dermatol 132 (11), 2502–2504. doi:10.1038/jid.2012.328
Picardi, A., Pasquini, P., Cattaruzza, M. S., Gaetano, P., Melchi, C. F., Baliva, G., et al. (2003). Stressful life events, social support, attachment security and alexithymia in vitiligo. Psychother. Psychosom. 72 (3), 150–158. doi:10.1159/000069731
Picardo, M., Dell'Anna, M. L., Ezzedine, K., Hamzavi, I., Harris, J. E., Parsad, D., et al. (2015). Vitiligo. Nat. Rev. Dis. Prim. 1, 15011. doi:10.1038/nrdp.2015.11
Polat, M., Oztas, P., Dikilitas, M. C., and Alli, N. (2008). Phytophotodermatitis due to Ficus carica. Dermatol Online J. 14 (12), 9. doi:10.5070/d3046507z8
Qaisiya, M., Coda Zabetta, C. D., Bellarosa, C., and Tiribelli, C. (2014). Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell Signal 26 (3), 512–520. doi:10.1016/j.cellsig.2013.11.029
Quan, C., Ren, Y. Q., Xiang, L. H., Sun, L. D., Xu, A. E., Gao, X. H., et al. (2010). Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat. Genet. 42 (7), 614–618. doi:10.1038/ng.603
Rajendiran, K. S., Rajappa, M., Chandrashekar, L., and Thappa, D. M. (2018). Association of PTPN22 gene polymorphism with non-segmental vitiligo in South Indian Tamils. Postepy Dermatol Alergol. 35 (3), 280–285. doi:10.5114/ada.2018.76225
Rashighi, M., Agarwal, P., Richmond, J. M., Harris, T. H., Dresser, K., Su, M. W., et al. (2014). CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 6 (223), 223ra23. doi:10.1126/scitranslmed.3007811
Rashighi, M., and Harris, J. E. (2017). Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 35 (2), 257–265. doi:10.1016/j.det.2016.11.014
Ren, Q., Qu, L., Yuan, Y., and Wang, F. (2024). Natural modulators of key signaling pathways in skin Inflammageing. Clin. Cosmet. Investigational Dermatology 17, 2967–2988. doi:10.2147/ccid.s502252
Ren, Y., Yang, S., Xu, S., Gao, M., Huang, W., Gao, T., et al. (2009). Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genet. 5 (6), e1000523. doi:10.1371/journal.pgen.1000523
Richmond, J. M., Frisoli, M. L., and Harris, J. E. (2013). Innate immune mechanisms in vitiligo: danger from within. Curr. Opin. Immunol. 25 (6), 676–682. doi:10.1016/j.coi.2013.10.010
Robledinos-Antón, N., Fernández-Ginés, R., Manda, G., and Cuadrado, A. (2019). Activators and inhibitors of Nrf2: a review of their potential for clinical development. Oxid. Med. Cell Longev. 2019, 9372182. doi:10.1155/2019/9372182
Rodrigues, L. B., Oliveira Brito Pereira Bezerra Martins, A., Cesário, F. R., Ferreira, E. C. F., de Albuquerque, T. R., Martins Fernandes, M. N., et al. (2016). Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: in vivo mouse models. Chem. Biol. Interact. 257, 14–25. doi:10.1016/j.cbi.2016.07.026
Rodrigues, M., Ezzedine, K., Hamzavi, I., Pandya, A. G., and Harris, J. E. (2017). New discoveries in the pathogenesis and classification of vitiligo. J. Am. Acad. Dermatol 77 (1), 1–13. doi:10.1016/j.jaad.2016.10.048
Sagi, L., and Trau, H. (2011). The Koebner phenomenon. Clin. Dermatol 29 (2), 231–236. doi:10.1016/j.clindermatol.2010.09.014
Sarcinelli, C., Dragic, H., Piecyk, M., Barbet, V., Duret, C., Barthelaix, A., et al. (2020). ATF4-dependent NRF2 transcriptional regulation promotes antioxidant protection during endoplasmic reticulum stress. Cancers (Basel) 12 (3), 569. doi:10.3390/cancers12030569
Sawant, N. S., Vanjari, N. A., and Khopkar, U. (2019). Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res. Pract. 2019, 1–10. doi:10.1155/2019/6879412
Schallreuter, K. U., Moore, J., Wood, J. M., Beazley, W. D., Gaze, D. C., Tobin, D. J., et al. (1999). In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Dermatol Symp. Proc. 4 (1), 91–96. doi:10.1038/sj.jidsp.5640189
Schallreuter, K. U., Panske, A., and Chiuchiarelli, G. (2011). Ineffective topical treatment of vitiligo with Cucumis melo extracts. Int. J. Dermatol 50 (3), 374–375. doi:10.1111/j.1365-4632.2009.04415.x
Schallreuter, K. U., and Rokos, H. (2006). Turmeric (curcumin): a widely used curry ingredient, can contribute to oxidative stress in Asian patients with acute vitiligo. Indian J. Dermatol Venereol. Leprol. 72, 57. doi:10.4103/0378-6323.19722
Shah, H., Mehta, A., and Astik, B. (2008). Clinical and sociodemographic study of vitiligo. Indian J. Dermatol Venereol. Leprol. 74 (6), 701. doi:10.4103/0378-6323.45144
Shen, C., Gao, J., Sheng, Y., Dou, J., Zhou, F., Zheng, X., et al. (2016). Genetic susceptibility to vitiligo: GWAS approaches for identifying vitiligo susceptibility genes and loci. Front. Genet. 7, 3. doi:10.3389/fgene.2016.00003
Shin, J. W., Chun, K.-S., Kim, D.-H., Kim, S.-J., Kim, S. H., Cho, N.-C., et al. (2020). Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 173, 113820. doi:10.1016/j.bcp.2020.113820
Sidi, E., and Bourgeois-Gavardin, J. (1952). The treatment of vitiligo with Ammi majus linn: a preliminary note. J. Investigative Dermatology 18 (5), 391–395. doi:10.1038/jid.1952.46
Singh, M., Mansuri, M. S., Kadam, A., Palit, S. P., Dwivedi, M., Laddha, N. C., et al. (2021). Tumor Necrosis Factor-alpha affects melanocyte survival and melanin synthesis via multiple pathways in vitiligo. Cytokine 140, 155432. doi:10.1016/j.cyto.2021.155432
So, J. S. (2018). Roles of endoplasmic reticulum stress in immune responses. Mol. Cells 41 (8), 705–716. doi:10.14348/molcells.2018.0241
Song, P., Li, K., Liu, L., Wang, X., Jian, Z., Zhang, W., et al. (2016). Genetic polymorphism of the Nrf2 promoter region is associated with vitiligo risk in Han Chinese populations. J. Cell Mol. Med. 20 (10), 1840–1850. doi:10.1111/jcmm.12874
Song, Y., Qu, Y., Mao, C., Zhang, R., Jiang, D., and Sun, X. (2023). Post-translational modifications of Keap1: the state of the art. Front. Cell Dev. Biol. 11, 1332049. doi:10.3389/fcell.2023.1332049
Soni, D., and Grover, A. (2019). “Picrosides” from Picrorhiza kurroa as potential anti-carcinogenic agents. Biomed. and Pharmacother. 109, 1680–1687. doi:10.1016/j.biopha.2018.11.048
Spritz, R. A. (2012). Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J. Invest. Dermatol 132 (2), 268–273. doi:10.1038/jid.2011.321
Spritz, R. A., and Andersen, G. H. (2017). Genetics of vitiligo. Dermatol Clin. 35 (2), 245–255. doi:10.1016/j.det.2016.11.013
Sun, X., Xu, A., Wei, X., Ouyang, J., Lu, L., Chen, M., et al. (2006). Genetic epidemiology of vitiligo: a study of 815 probands and their families from south China. Int. J. Dermatol 45 (10), 1176–1181. doi:10.1111/j.1365-4632.2006.02907.x
Surh, Y. J., and Na, H. K. (2008). NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2 (4), 313–317. doi:10.1007/s12263-007-0063-0
Szczurko, O., Shear, N., Taddio, A., and Boon, H. (2011). Ginkgo biloba for the treatment of vitilgo vulgaris: an open label pilot clinical trial. BMC Complement. Altern. Med. 11, 21. doi:10.1186/1472-6882-11-21
Tang, X. F., Zhang, Z., Hu, D. Y., Xu, A. E., Zhou, H. S., Sun, L. D., et al. (2013). Association analyses identify three susceptibility Loci for vitiligo in the Chinese Han population. J. Invest. Dermatol 133 (2), 403–410. doi:10.1038/jid.2012.320
Tonelli, C., Chio, I. I. C., and Tuveson, D. A. (2018). Transcriptional regulation by Nrf2. Antioxid. Redox Signal 29 (17), 1727–1745. doi:10.1089/ars.2017.7342
Toosi, S., Orlow, S. J., and Manga, P. (2012). Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J. Invest. Dermatol 132 (11), 2601–2609. doi:10.1038/jid.2012.181
Trigui, E., Ben Hassen, H., Zaghden, H., Trigui, M., and Achour, S. (2023). A Bioinformatic study on the potential anti-vitiligo activity of a Carpobrotus edulis compound. Molecules 28 (22), 7545. doi:10.3390/molecules28227545
Tu, C. X., Gu, J. S., and Lin, X. R. (2003). Increased interleukin-6 and granulocyte–macrophage clony stimulating factor levels in the sera of patients with non-segmental vitiligo. J. Dermatol Sci. 31 (1), 73–78. doi:10.1016/s0923-1811(02)00151-2
Turkmen, D. (2020). Serum bilirubin and uric acid antioxidant levels in rosacea patients. J. Cosmet. dermatology 19 (10), 2717–2720. doi:10.1111/jocd.13395
Valle, Y. (2019). World Vitiligo Day-A grassroots campaign to improve the quality of life of vitiligo patients. Dermatol Ther. 32 (5), e13050. doi:10.1111/dth.13050
van Geel, N., and Speeckaert, R. (2017). Segmental vitiligo. Dermatol Clin. 35 (2), 145–150. doi:10.1016/j.det.2016.11.005
Vaughn, A. R., Branum, A., and Sivamani, R. K. (2016). Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytotherapy Res. 30 (8), 1243–1264. doi:10.1002/ptr.5640
Vile, G. F., and Tyrrell, R. M. (1993). Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J. Biol. Chem. 268 (20), 14678–14681. doi:10.1016/S0021-9258(18)82386-9
Wang, S., Zhou, M., Lin, F., Liu, D., Hong, W., Lu, L., et al. (2014). Interferon-gamma induces senescence in normal human melanocytes. PLoS One 9 (3), e93232. doi:10.1371/journal.pone.0093232
Wang, T., Liu, M., Li, X., Zhang, S., Gu, H., Wei, X., et al. (2024). Naturally-derived modulators of the Nrf2 pathway and their roles in the intervention of diseases. Free Radic. Biol. Med. 225, 560–580. doi:10.1016/j.freeradbiomed.2024.09.035
Wang, X. X., Wang, Q. Q., Wu, J. Q., Jiang, M., Chen, L., Zhang, C. F., et al. (2016). Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br. J. Dermatol 174 (6), 1318–1326. doi:10.1111/bjd.14416
Yaghoobi, R., Omidian, M., and Bagherani, N. (2011). Vitiligo: a review of the published work. J. Dermatol 38 (5), 419–431. doi:10.1111/j.1346-8138.2010.01139.x
Yamamoto, T., Suzuki, T., Kobayashi, A., Wakabayashi, J., Maher, J., Motohashi, H., et al. (2008). Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell Biol. 28 (8), 2758–2770. doi:10.1128/mcb.01704-07
Yang, S., Wang, J. Y., Gao, M., Liu, H. S., Sun, L. D., He, P. P., et al. (2005). Association of HLA-DQA1 and DQB1 genes with vitiligo in Chinese Hans. Int. J. Dermatol 44 (12), 1022–1027. doi:10.1111/j.1365-4632.2004.02389.x
Yang, Y., Du, Y., and Cui, B. (2024). Polyphenols targeting multiple molecular targets and pathways for the treatment of vitiligo. Front. Immunol. 15, 1387329. doi:10.3389/fimmu.2024.1387329
Yuan, J., Lu, Y., Wang, H., Feng, Y., Jiang, S., Gao, X. H., et al. (2020). Paeoniflorin resists H2O2-induced oxidative stress in melanocytes by JNK/Nrf2/HO-1 pathway. Front. Pharmacol. 11, 536. doi:10.3389/fphar.2020.00536
Zamani, M., Spaepen, M., Sghar, S. S., Huang, C., Westerhof, W., Nieuweboer-Krobotova, L., et al. (2001). Linkage and association of HLA class II genes with vitiligo in a Dutch population. Br. J. Dermatol 145 (1), 90–94. doi:10.1046/j.1365-2133.2001.04288.x
Zarrouk, A., Smach, M. A., Hafsa, J., Sghaier, R., Majdoub, H., Hammami, M., et al. (2019). Effects of Carpobrotus edulis extract on oxidative stress and 158N oligodendrocyte death. Biomed. Environ. Sci. 32 (4), 291–299. doi:10.3967/bes2019.039
Zedan, H., Abdel-Motaleb, A. A., Kassem, N. M., Hafeez, H. A., and Hussein, M. R. (2015). Low glutathione peroxidase activity levels in patients with vitiligo. J. Cutan. Med. Surg. 19 (2), 144–148. doi:10.2310/7750.2014.14076
Zhang, B., Wang, J., Zhao, G., Lin, M., Lang, Y., Zhang, D., et al. (2020). Apigenin protects human melanocytes against oxidative damage by activation of the Nrf2 pathway. Cell Stress Chaperones 25 (2), 277–285. doi:10.1007/s12192-020-01071-7
Zhang, S., Yi, X., Su, X., Jian, Z., Cui, T., Guo, S., et al. (2019). Ginkgo biloba extract protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J. Cell Mol. Med. 23 (8), 5193–5199. doi:10.1111/jcmm.14393
Zhang, X. J., Liu, H. S., Liang, Y. H., Sun, L. D., Wang, J. Y., Yang, S., et al. (2004). Association of HLA class I alleles with vitiligo in Chinese Hans. J. Dermatol Sci. 35 (2), 165–168. doi:10.1016/j.jdermsci.2004.05.003
Zhang, Y., Cai, Y., Shi, M., Jiang, S., Cui, S., Wu, Y., et al. (2016). The prevalence of vitiligo: a meta-analysis. PLoS One 11 (9), e0163806. doi:10.1371/journal.pone.0163806
Zhao, J., Wang, C., Xu, X., Zhang, Y., Ren, H., Ren, Z., et al. (2019). Coexistence of autoimmune encephalitis and other systemic autoimmune diseases. Front. Neurol. 10, 1142. doi:10.3389/fneur.2019.01142
Zhong, H., Zhou, J., An, X. H., Hua, Y. R., Lai, Y. F., Zhang, R., et al. (2019). Natural product-based design, synthesis and biological evaluation of 2',3,4,4'-tetrahydrochalcone analogues as antivitiligo agents. Bioorg Chem. 87, 523–533. doi:10.1016/j.bioorg.2019.03.054
Zhou, Y., Jiang, Z., Lu, H., Xu, Z., Tong, R., Shi, J., et al. (2019). Recent advances of natural polyphenols activators for Keap1-Nrf2 signaling pathway. Chem. and Biodivers. 16 (11), e1900400. doi:10.1002/cbdv.201900400
Keywords: vitiligo, ROS, Nrf2 pathway, er stress, HO-1, HLA, herbal plants, ginkgo
Citation: Qawasmeh A, Qaisiya M, Ishnaiwer A, Khdour D, Shawar S, Fallah S, Hashlamon D, Sinokrot H and Kharaiwesh A (2025) Insights into the role of Nrf2 in vitiligo pathogenesis: a target for herbal medicine. Front. Nat. Prod. 4:1593684. doi: 10.3389/fntpr.2025.1593684
Received: 17 March 2025; Accepted: 07 July 2025;
Published: 23 July 2025.
Edited by:
Pankaj Kumar, Hemwati Nandan Bahuguna Garhwal University, IndiaReviewed by:
Ashwin Kotnis, All India Institute of Medical Sciences, Bhopal, IndiaIsmael Vásquez Moctezuma, National Polytechnic Institute (IPN), Mexico
Hesham Elshamy, Dermatology department, Menoufia University, Egypt
Copyright © 2025 Qawasmeh, Qaisiya, Ishnaiwer, Khdour, Shawar, Fallah, Hashlamon, Sinokrot and Kharaiwesh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdel Qawasmeh, YWJlZHFAaGVicm9uLmVkdQ==
 Abdel Qawasmeh
Abdel Qawasmeh Mohammad Qaisiya2
Mohammad Qaisiya2 Shoroq Shawar
Shoroq Shawar