Abstract
Introduction:
Astragalus fruticosus Forssk. is a plant species with potential for producing valuable secondary metabolites, such as phloroglucinol, which has pharmaceutical applications. In vitro techniques, including multiple shoot induction and elicitation, offer promising methods to enhance the production of such compounds. This study investigates the use of signal molecules methyl jasmonate (MJ) and salicylic acid (SA) to increase phloroglucinol yield in cultured shoots of A. fruticosus.
Methods:
Stem and leaf explants of A. fruticosus were cultured on Murashige and Skoog (MS) medium supplemented with 1 mg/L 6-benzylaminopurine (BAP) for over 8 weeks to induce multiple shoots. Elicitation was performed by treating the cultured shoots with MJ and SA at concentrations of 50 and 100 μg/mL, individually and in combination (final concentration of 50 μg/mL), over 1–3 weeks. Phloroglucinol content and biomass production were measured to assess the efficacy of the elicitation treatments.
Results:
Elicitation with MJ at 100 μg/mL resulted in a 5.06-fold increase in phloroglucinol content after 3 weeks compared to the unelicited control. Other treatments with MJ, SA, and their combination also enhanced phloroglucinol production, though to a lesser extent. The highest biomass production was observed under the same conditions that maximized phloroglucinol yield.
Discussion:
This study represents the first report of elicitation in A. fruticosus multiple shoot cultures using MJ and SA to enhance phloroglucinol production. The significant increase in phloroglucinol content, particularly with 100 μg/mL MJ, highlights the effectiveness of elicitation as a yield enhancement strategy. These findings suggest potential for optimizing in vitro protocols for large-scale production of phloroglucinol, offering valuable insights for biotechnological applications in pharmaceutical development.
Introduction
Astragalus fruticosus Forssk (family Leguminosae) is a rare perennial herb and is one of the 37 species wild growing in Egypt (Salehi et al., 2021). Astragalus is famed for its medicinal applications, with hepatoprotective, antioxidative, immunostimulant, antiperspirant, diuretic, tonic, and antiviral properties (Li et al., 2014; Pistelli, 2002), which have been attributed to its secondary metabolites including triterpenes, saponins, phenolics, flavonoids, and polysaccharides (Lysiuk and Darmohray, 2016).
Phloroglucinol is a major phenolic compound in A. fruticosus Forssk. It displays many pharmacological activities with anticancer, anti-inflammatory, anti-allergic, anti-microbial, neuro-regenerative, vasodilating, and antioxidant activities besides its use in cosmetics, pesticides, paints, cements, and dyeing. Moreover, phloroglucinol and its derivatives could serve as artificial sweeteners, plasma substitutes, and in vitamin preparations (Singh et al., 2009; Li et al., 2011). Worldwide, there is an increasing demand for plant-based medicines, especially in primary healthcare (Zaheer and Giri, 2015). The field cultivation of A. fruticosus Forssk faces challenges such as long growth time and the uncontrollable production of valuable secondary metabolites. Therefore, an alternative method for the more efficient production of phenolic compounds such as phloroglucinol from A. fruticosus Forssk is urgently required.
Plant cell, tissue, and organ culture has recently been successfully used for the sustainable production of bioactive compounds of commercial interest (Loyola-Vargas and Ochoa-Alejo, 2018). In vitro shoot cultures can provide an alternative source to natural plant populations for the large-scale production of secondary metabolites. However, to the best of our knowledge, there are no reports on the production of phloroglucinol from the shoot cultures of A. fruticosus Forssk. Little is known so far about the strategies for biomass production and secondary metabolites stimulation using elicitors.
Elicitation is a process of creating a scenario of artificial pathogen attack using different means to promote secondary metabolite production (Sidhu, 2011). Elicitors are external stimuli that cause changes in plant cells, leading to a series of reactions that result in the accumulation of secondary metabolites (Largia et al., 2015). Among the commonly used elicitors are methyl jasmonate (MJ) and salicylic acid (SA), which are effective biotechnological tools for the induction of secondary metabolites in plant cultures (Sivanandhan et al., 2013). Methyl jasmonate, a volatile methyl ester of jasmonic acid, is involved in the signal transduction pathway which triggers specific enzymes that catalyze different biochemical reactions in plants to synthesize low-molecular weight defense compounds such as terpenoids, alkaloids, polyphenols, and quinones (Bai et al., 2025). Salicylic acid is a signal molecule that induces plant resistance to stress factors and pathogens through the stimulation of gene expression related to the biosynthesis of secondary metabolites in plants (Gadzovska et al., 2012). Recently, multiple shoot culture systems have been used for elicitation studies and for the production of secondary metabolites.
In the current study, we implemented some elicitation strategies to increase the amount of phloroglucinol in vitro. We likewise studied the different factors such as the elicitor concentration, exposure time, and harvest time in MS medium supplemented with a specific plant growth regulator for optimized biomass production and the enhanced accumulation of phloroglucinol using the two elicitors, MJ and SA, either individually or in combination.
Materials and methods
Induction and maintenance of multiple shoots
Multiple shoot cultures of A. fruticosus were induced using explants from the stem and leaf of 28-day-old seedlings that were obtained as we have previously described (Zayed et al., 2022). Full strength MS medium supplemented with 30 g/L sucrose and amended with various concentrations of plant growth regulators were used: 0.5 mg/L of 6-benzylaminopurine (BAP), named “Medium I”; 1.0 mg/L of 6-benzylaminopurine (BAP) named “Medium II:; 0.5 mg/L of thidiazuron (TDZ) named “Medium III”; 1.0 mg/L of kinetin (kn) named “Medium IV: a combination of 1 mg/L of naphthalene acetic acid (NAA) and 0.1 mg/L of BAP named “Medium V”. The pH of the medium was adjusted to 5.6–5.8 using 1N NaOH or 1N HCl, then 0.6% agar was added. The media were autoclaved at 121 °C for 15 min, and the culture jars were incubated at 25 °C under a white fluorescent lamp over a 16-/8-h light/dark period. All cultures were sub-cultured to fresh media after 28 days of culture initiation. The most appropriate medium for shoot induction was selected by determining the number of explants that produced shoots and the number of produced shoots per explant after the third subculture. The experiments were repeated three times and data of respective experiments were recorded.
Elicitation of induced shoots by signal compounds
One-month-old healthy shoots (3–4 cm long) grown on MS medium supplemented with 1 mg/L BAP (Medium II) were cut and individually transferred into jars containing sterilized semi-solid MS media consisting of 4.4 g/l MS containing 30 g/L sucrose, 4 g/L agar, and 1 mg/L BAP. The pH of the medium was adjusted to 5.6–5.8. Methyl jasmonate (MJ) and salicylic acid (SA) elicitors were used to evaluate their influence on phloroglucinol accumulation in multiple shoot cultures of A. fruticosus. MJ and SA (Sigma- Aldrich, United States of America) stock solutions were prepared in 99.9% ethanol and filter-sterilized using a 0.22-µm bacterial filtration unit (Millipore, Ireland). The multiple shoot cluster was allowed to grow in the presence of various concentrations (50 and 100 μg/mL) of MJ, SA, and a combination of both (50 μg/mL) at different durations (1–3 weeks). A small hole (1 cm in diameter) was made in the sterilized semi-solid MS media around the cultured shoots, where different concentrations (50 and 100 μg/mL) of MJ and SA were aseptically added either separately or combined at concentration of 50 μg/mL of both elicitors on the first day of subculture. The cultures were incubated at 25 °C under a white fluorescent lamp with a 16-/8-h light/dark period then harvested at intervals of 1, 2, and 3 weeks to study growth (number of produced shoots and their dry weight) and phloroglucinol production. All treatments were performed in triplicate. Control experiments were prepared by substituting the elicitor with distilled water.
Growth study for multiple shoot cultures
The growth of the control (untreated) and treated shoot cultures was assessed by recording the mean number of shoots branched after 1, 2, and 3 weeks. Shoot biomass was dried at room temperature and dry weight was recorded.
Extraction and quantitative analysis of phloroglucinol
All elicited and non-elicited shoots were removed from the culture media after 1, 2, and 3 weeks and then washed thoroughly, allowed to dry, and powdered separately. Each dry powder was macerated overnight with the same volume (10 mL) of high-performance liquid chromatography (HPLC)-grade methanol and filtered using Whatman filter paper No. 41. The resulting extracts were concentrated using a rotary evaporator under reduced pressure. The recovered solutions were filtered through a 0.45-µM membrane (MillexHV, Millipore, Ireland) then injected into a HPLC system (Agilent Technology, G1315D) equipped with an autosampler and an Eclipse Plus-C18 column (150 mm × 4.60 mm, 3.5 μm particle size). Isocratic elution was employed with methanol:acetonitrile:water (25: 35: 40, v/v/v) as a mobile phase at 1.0 mL/min flow rate with 20 µL injection volume. The column was maintained at 25 °C. The elution of phloroglucinol was monitored at 256 nm using a photodiode array detector (PDA) at a retention time of 0.9 min. Commercially available authentic standard phlorogluinol (98% purity) was procured from Sigma-Aldrich (United States of America) for analysis. All results were averaged over two consecutive experiments. Phlorogluinol content was expressed as % DW of control and elicited multiple shoot culture samples. The relative concentrations of phloroglucinol in different samples were calculated by comparing their peak areas with standard curve generated using different concentrations (1, 2, 3, 4, 5, 6, 7, 8, and 9 μg/mL) of phloroglucinol standard. Phloroglucinol data were expressed as mg/g dry weight. Each sample was run in triplicate.
Results and discussion
Effect of phytohormones on induction of multiple shoot cultures
Cultivation of stem and leaf explants of 28-day-old sterile seedlings on media I-V resulted in the swelling of the explants as an initial response within 8–10 days of incubation. The green protuberances differentiated into direct shoots within 3 weeks. The highest percentage of explants giving shoot (75.8%) was observed in Medium II, with approximately 10–25 shoot/explants with an average length range of 3–8 cm after the third subculture (Table 1; Figure 1). Hasancebi et al. (2011) reported that BAP was the most commonly used cytokinin for the in vitro culture of different Astragalus species. According to Hill et al. (2015), explant tissues produce a greater amount of undifferentiated mass of cells, which results in viable embryos having the ability to differentiate into healthy shoots in the presence of higher concentrations of cytokinins. Culturing the stem and leaf explants of sterile seedlings on a hormonal-free MS medium resulted in the induction of shoot buds from one cut ending after 2 weeks that gave multiple shoots 3–5 cm long within the 6th week. The other cut ending showed an induction of fine and very tiny white rootlets with average length of 0.1–0.2 cm which showed no further growth (Figure 1F).
TABLE 1
| Medium | % Of explants giving shoots | Number of shoots /Explant |
|---|---|---|
| Medium I | 63.4% | 8–15 |
| Medium II | 75.8% | 10–25 |
| Medium III | 58.9% | 5–8 |
| Medium IV | 44.7% | 3–7 |
| Medium V | 37.6% | 4–9 |
Shoot induction percentage and the number of produced shoots per explant after the third subculture in different media.
FIGURE 1
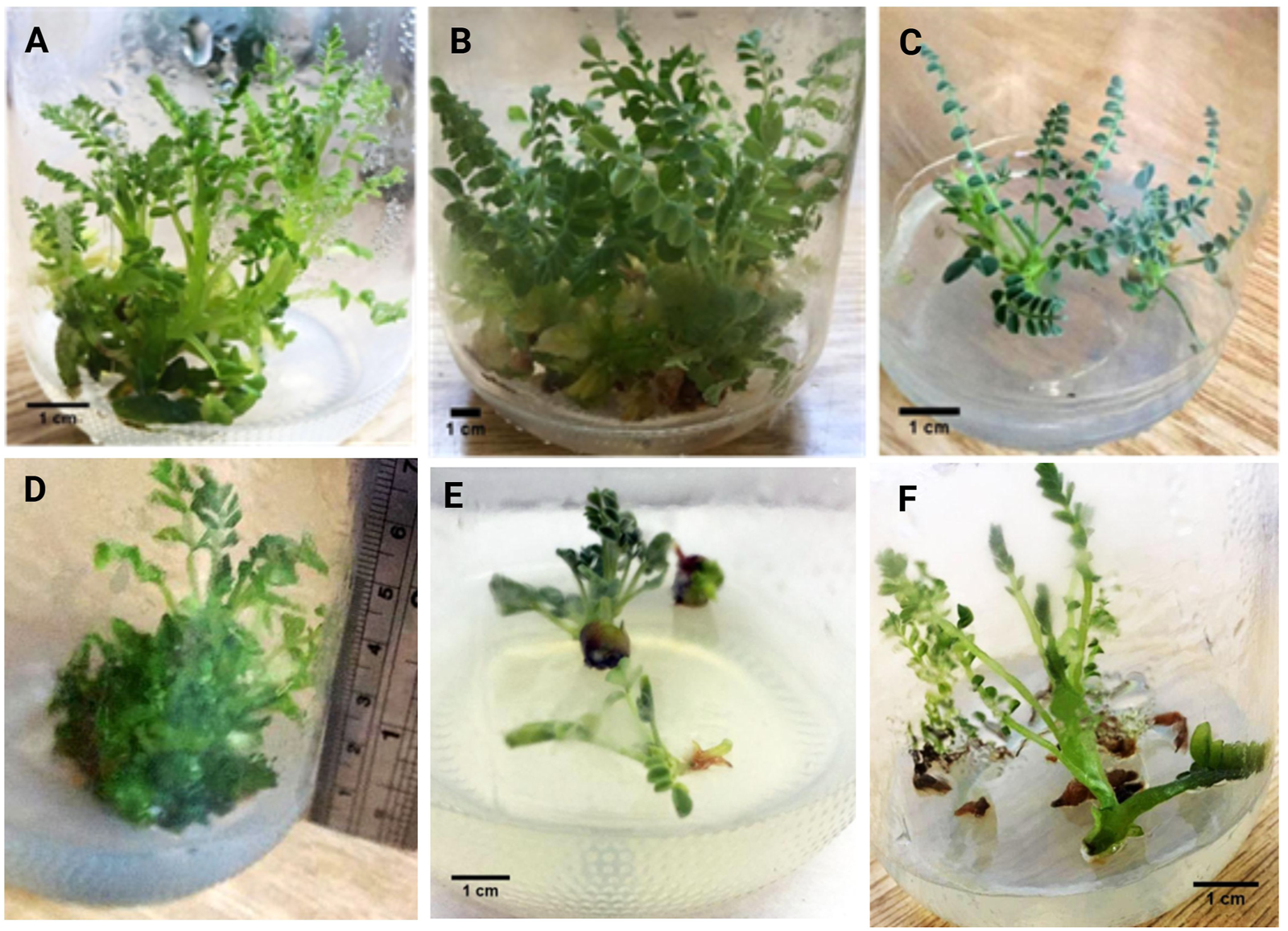
Shoot induction in different media: (A) 3-month-old shoots in Medium I; (B) 3-month-old shoots in Medium II; (C) 3-month-old shoots in Medium III; (D) 3-month-old shoots in Medium IV; (E) 3-week-old shoots in Medium V; (F) 6-week-old plantlet in hormonal-free medium.
Influence of signal compounds (MJ and SA) on growth and phloroglucinol production
Effect of elicitors on shoot culture and biomass accumulation
The effect of MJ and SA, alone or in combination, on A. fruticosus shoot growth rates was monitored by determining the mean number of shoots produced and the resultant dry weight. The applied elicitors showed different influences on the biomass accumulation of shoots in culture in addition to the mean number of produced shoots. MJ and SA were added on the first day of the first subculture at different concentrations (50–100 μg/mL) on different exposure times (1–3 weeks).
Table 2 shows the mean number of shoots produced in samples treated with MJ and SA compared to the non-treated ones at different time intervals. The growth of A. fruticosus shoots was highly affected by the addition of elicitors. The applied concentrations (50–100 μg/mL) of both elicitors and their combination at a concentration of 50 μg/mL showed various inhibitory actions on shoot growth compared to control. Higher concentrations of SA and MJ displayed more toxic effects on the plant and limited the growth of its shoot. Therefore, the production of shoots was inversely proportional to the elicitor concentration. Additionally, it was noted that SA is less toxic than MJ, which is in accordance with Largia et al. (2015), who used SA and MJ at concentrations similar to those described in our study; they similarly reported that SA was less toxic to the plant than MJ. High MJ concentrations showed inhibitory action on cell growth due to an inhibition of the biosynthesis of photosynthetic pigments resulting in the suppression of the photosynthesis process (Ji et al., 2019).
TABLE 2
| Elicitor conc (µg/mL) | Mean number of produced shoots | ||
|---|---|---|---|
| After 1 week | After 2 weeks | After 3 weeks | |
| Control | 10.67 | 17.33 | 24.67 |
| SA 50 | 8.67 | 15.33 | 22.67 |
| SA 100 | 4.33 | 10.67 | 13.33 |
| MJ 50 | 5.33 | 9.67 | 12.67 |
| MJ 100 | 3.67 | 7.67 | 9.33 |
| MJ 50 + SA 50 | 2.67 | 6.33 | 8.33 |
Effect of different concentrations of elicitors and their combination on the mean number of produced shoots in shoot cultures of A. fruticosus.
Elicitors affected the dry weight of the shoots in a manner similar to the mean number of growing shoots, where the elicitors’ inhibitory effect was concentration-dependent. Table 3 presents the biomass production after shoot treatment with SA and/or MJ. The biomass of MJ-elicited shoots was dramatically reduced after 3 weeks of treatment at a concentration of 100 μg/mL (2.26 g) compared to control (4.34 g). Biomass reduction was minimal in SA-treated cultures after treatment with a concentration of 50 μg/mL (3.72 g) relative to control (4.34 g). A combination of both elicitors at a concentration of 50 μg/mL showed a synergistic action that caused the highest biomass reduction (2.03 g) relative to control (4.34 g). Similar findings on Withania somnifera were likewise reported where elicitation of multiple shoot culture using SA and/or MJ resulted in various effects on biomass reduction. Similarly, SA-treated cultures did not show much variation in biomass accumulation compared with control (Sivanandhan et al., 2013). Previous studies on the effect of jasmonic acid and SA elicitors on biomass production in shoot cultures of Hypericum hirsutum and Hypericum maculatum showed effects similar to those observed in the present study (Coste et al., 2011). A high concentration of SA has been reported to inhibit the multiple shoot culture of Andrographis paniculata (Zaheer and Giri, 2015).
TABLE 3
| Elicitor conc (µg/mL) | Dry weight (g) | ||
|---|---|---|---|
| After 1 week | After 2 weeks | After 3 weeks | |
| Control | 1.63 | 2.43 | 4.34 |
| SA 50 | 1.28 | 2.05 | 3.72 |
| SA 100 | 0.82 | 1.74 | 2.84 |
| MJ 50 | 0.98 | 1.85 | 3.19 |
| MJ 100 | 0.61 | 1.46 | 2.26 |
| MJ 50 + SA 50 | 0.58 | 1.31 | 2.03 |
Effect of different concentrations of elicitors and their combination on biomass accumulation in shoot cultures of A. fruticosus.
Effect of elicitors on phloroglucinol content
Both elicitors (at the studied concentrations) exhibited changeable responses on phloroglucinol content in multiple shoot cultures of A. fruticosus. The phloroglucinol content of the untreated shoots showed limited increase over time. Meanwhile, there were different degrees of phloroglucinol enhancement when both elicitors and their combination were applied (Figure 2).
FIGURE 2
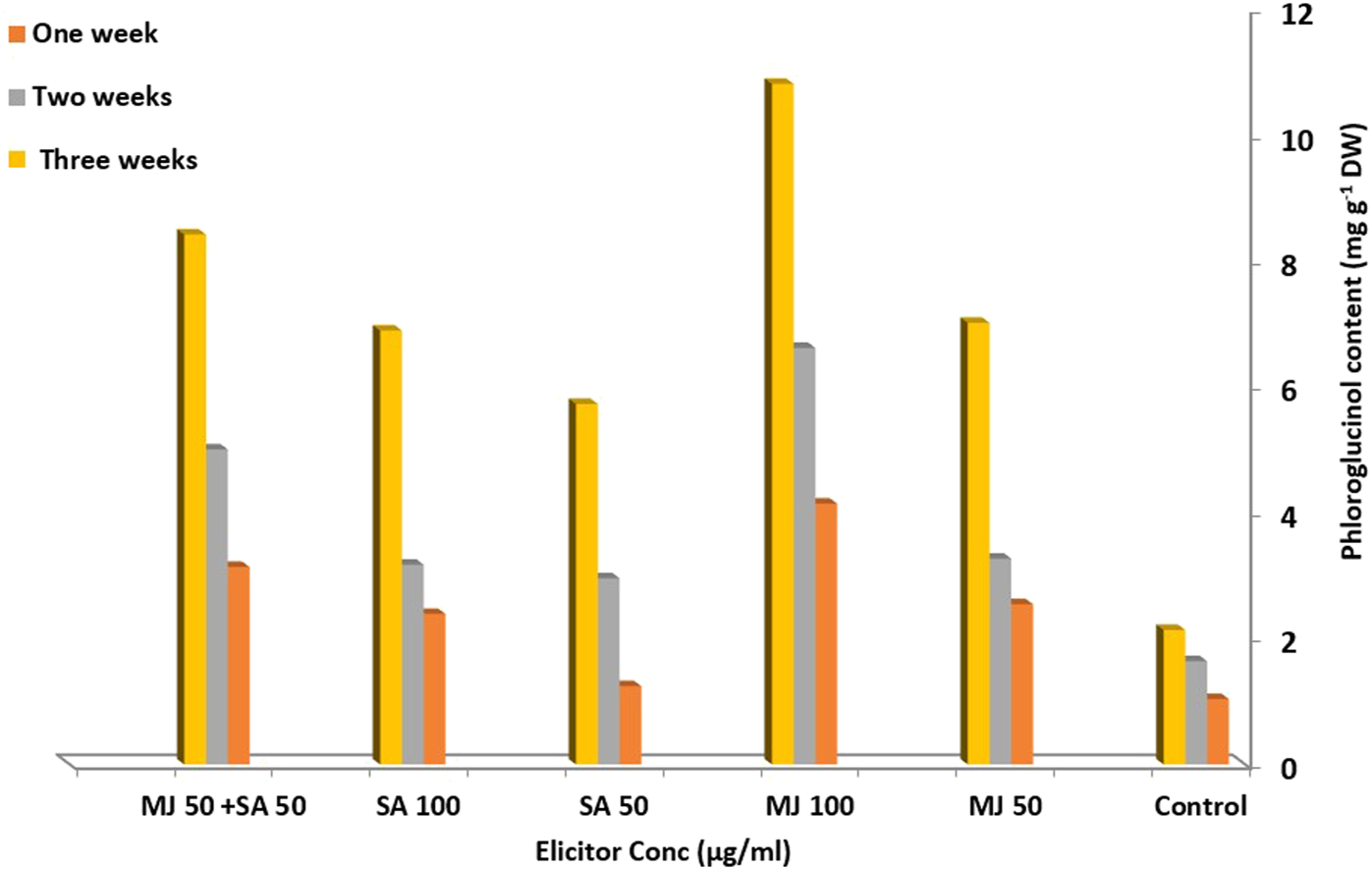
Effect of different concentrations of elicitors and their combination on phloroglucinol content in A. fruticosus shoot cultures.
Elicitation by SA (50 μg/mL) showed the least phloroglucinol production enhancement, around 2.7-fold of the control level (2.13 mg/g DW) after 3 weeks. After increasing the SA concentration to 100 μg/mL, phloroglucinol production raised to 3.2-fold compared to control level after 3 weeks. This was in accordance with Beygi et al. (2021) who showed that trigonelline biosynthesis was enhanced in fenugreek callus culture after treatment with 100 µM SA. In contrast, another study revealed that the addition of 50 μg/mL SA to shoot cultures of Hypericum hirsutum and H. maculatum induced the significant accumulation of hypericin, while the addition of SA at a concentration of 100 μg/mL led to decrease in hypericin production (Coste et al., 2011). MJ highly enhanced phloroglucinol production, and the response depended on the concentration of MJ where 50 μg/mL MJ exhibited a 3.29-fold increase in phloroglucinol content after 3 weeks compared to control. Increasing the concentration of MJ to 100 μg/mL increased the phloroglucinol content to 5.06-fold compared to control. This result was in accordance with previous studies which reported that MJ at concentration of 100 μmol/L showed significant enhancement in the accumulation of polysaccharides in the multiple shoot culture of Codonopsis pilosula (Ji et al., 2019). The optimum concentration for the stimulation of secondary metabolites from in vitro cultured plant species was 100 µM (Shabani et al., 2009; Zaheer and Giri, 2015). In Peganum harmala root cultures, MJ enhanced the production of carboline and quinoline alkaloids 5-fold and 7-8-fold in root and hairy root cultures, respectively, compared to the untreated control (Zayed and Wink, 2005). Additionally, MJ promoted the production of bacoside A, a triterpenoid saponin, from the in vitro shoot cultures of Bacopa monnieri by 1.8-fold (compared to control) after 1 week (Sharma et al., 2013).
The application of both elicitors in combination at a concentration of 50 μg/mL showed an enhancement in phloroglucinol production after 3 weeks of exposure, with the highest induction effect at the third week (four-fold increase compared to control) as shown in Figure 2. It was reported that a combination of MJ and SA at various concentrations exhibited synergistic action for the intensified production of saponins from Bacopa monnieri (Largia et al., 2015). In the callus cultures of Rosa hybrida, the combination of both elicitors resulted in higher production of anthocyanins than in control (Ram et al., 2013). Overall, the results showed that the enhancement of phloroglucinol accumulation by elicitor treatment is dose-dependent and is accompanied by a notable suppression of biomass production. To the best of our knowledge, this is the first report on the enhanced production of phloroglucinol in multiple shoots of A. fruticosus by the influence of elicitors (MJ and SA).
Conclusion
The accumulation of phloroglucinol in multiple shoot culture of Astragalus fruticosus was influenced by the concentration of the elicitors (methyl jasmonate and salicylic acid) bringing about enhancement and variation. Our results showed that the application of signal molecules allowed the optimal production of phloroglucinol in multiple shoot cultures of A. fruticosus. Synthesis of secondary metabolites requires the plant tissue to perceive and react to various environmental signals in an interactive manner. The outcome of the present study may be exploited for further enhancement of phloroglucinol production through biotechnological interventions using molecular approaches.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Author contributions
RZ: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. AE-S: Writing – original draft, Writing – review and editing. AE: Writing – original draft, Writing – review and editing. WI: Writing – original draft, Writing – review and editing. ME-S: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. SF: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Bai X. Lee H.-S. Han J.-E. Murthy H. N. Park S.-Y. (2025). Enhancement of phenolic and polyacetylene accumulation in Lobelia chinensis (Chinese lobelia) plantlet cultures through yeast extract and salicylic acid elicitation. Horticulturae11, 612. 10.3390/horticulturae11060612
2
Beygi Z. Nezamzadeh Z. Rabiei M. Mirakhorli N. (2021). Enhanced accumulation of trigonelline by elicitation and osmotic stresses in fenugreek callus culture. Plant Cell, Tissue Organ Cult. (PCTOC)147, 169–174. 10.1007/s11240-021-02055-w
3
Coste A. Vlase L. Halmagyi A. Deliu C. Coldea G. (2011). Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell, Tissue Organ Cult. (PCTOC)106, 279–288. 10.1007/s11240-011-9919-5
4
Gadzovska S. Maury S. Delaunay A. Spasenoski M. Hagège D. Courtois D. et al (2012). The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell, Tissue Organ Cult. (PCTOC)113, 25–39. 10.1007/s11240-012-0248-0
5
Hasancebi S. Kara N. T. Çakir Ö. Ari Ş. (2011). Micropropagation and root culture of Turkish endemic Astragalus chrysochlorus (Leguminosae). Turkish J. Bot.35, 203–210. 10.3906/bot-1007-48
6
Hill P. Gutierrez B. Carmack L. Kopp O. R. (2015). Micropropagation of Astragalus holmgreniorum (Holmgren milkvetch), an endemic and endangered species. Plant Cell, Tissue Organ Cult. (PCTOC)121, 381–387. 10.1007/s11240-015-0708-4
7
Ji J.-J. Feng Q. Sun H.-F. Zhang X.-J. Li X.-X. Li J.-K. et al (2019). Response of bioactive metabolite and biosynthesis related genes to methyl jasmonate elicitation in Codonopsis pilosula. Molecules24, 533. 10.3390/molecules24030533
8
Largia M. J. V. Pothiraj G. Shilpha J. Ramesh M. (2015). Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.). Plant Cell, Tissue Organ Cult. (PCTOC)122, 9–20. 10.1007/s11240-015-0745-z
9
Li T. T. Zhang Y. S. He L. Li N. S. Peng J. Li Y. J. (2011). Protective effect of phloroglucinol against myocardial ischaemia–reperfusion injury is related to inhibition of myeloperoxidase activity and inflammatory cell infiltration. Clin. Exp. Pharmacol. Physiology38, 27–33. 10.1111/j.1440-1681.2010.05457.x
10
Li X. Qu L. Dong Y. Han L. Liu E. Fang S. et al (2014). A review of recent research progress on the astragalus genus. Molecules19, 18850–18880. 10.3390/molecules191118850
11
Loyola-Vargas V. M. Ochoa-Alejo N. (2018). “An introduction to plant tissue culture: advances and perspectives,” in Plant cell culture protocols. Editors Loyola-VargasV. M.Ochoa-AlejoN. (New York, NY: Springer New York).
12
Lysiuk R. Darmohray R. (2016). Pharmacology and ethnomedicine of the genus Astragalus. Int. J. Pharmacol. Phytochemistry Ethnomedicine3, 46–53. 10.18052/www.scipress.com/ijppe.3.46
13
Pistelli L. (2002). “Secondary metabolites of genus Astragalus: structure and biological activity,” in Studies in natural products chemistry. Elsevier.
14
Ram M. Prasad K. Singh S. Hada B. Kumar S. (2013). Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell, Tissue Organ Cult. (PCTOC)113, 459–467. 10.1007/s11240-013-0287-1
15
Salehi B. Carneiro J. N. P. Rocha J. E. Coutinho H. D. M. Morais Braga M. F. B. Sharifi-Rad J. et al (2021). Astragalus species: insights on its chemical composition toward pharmacological applications. Phytotherapy Res.35, 2445–2476. 10.1002/ptr.6974
16
Shabani L. Ehsanpour A. Asghari G. Emami J. (2009). Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ. J. Plant Physiology56, 621–626. 10.1134/s1021443709050069
17
Sharma P. Yadav S. Srivastava A. Shrivastava N. (2013). Methyl jasmonate mediates upregulation of bacoside A production in shoot cultures of Bacopa monnieri. Biotechnol. Lett.35, 1121–1125. 10.1007/s10529-013-1178-6
18
Sidhu Y. (2011). In vitro micropropagation of medicinal plants by tissue culture.
19
Singh I. P. Sidana J. Bansal P. Foley W. J. (2009). Phloroglucinol compounds of therapeutic interest: global patent and technology status. Expert Opin. Ther. Pat.19, 847–866. 10.1517/13543770902916614
20
Sivanandhan G. Rajesh M. Arun M. Jeyaraj M. Dev G. K. Arjunan A. et al (2013). Effect of culture conditions, cytokinins, methyl jasmonate and salicylic acid on the biomass accumulation and production of withanolides in multiple shoot culture of Withania somnifera (L.) Dunal using liquid culture. Acta physiol. plant.35, 715–728. 10.1007/s11738-012-1112-x
21
Zaheer M. Giri C. C. (2015). Multiple shoot induction and jasmonic versus salicylic acid driven elicitation for enhanced andrographolide production in Andrographis paniculata. Plant Cell, Tissue Organ Cult. (PCTOC)122, 553–563. 10.1007/s11240-015-0787-2
22
Zayed R. Wink M. (2005). β-Carboline and quinoline alkaloids in root cultures and intact plants of Peganum harmala. Z. für Naturforsch. C60, 451–458. 10.1515/znc-2005-5-614
23
Zayed R. El-Sayed A. S. Ismaeil W. M. (2022). In vitro micropropagation, biological activities and phenolic profile of Astragalus fruticosus Forssk. Asian J. Plant Sci.21, 192–202. 10.3923/ajps.2022.192.202
Summary
Keywords
methyl jasmonate, phloroglucinol, salicylic acid, Astragalus fruticosus , biomass
Citation
Zayed R, El-Sayed A, Elissawy AM, Ismaeil W, El-Shazly M and Fayez S (2025) Methyl jasmonate and salicylic acid synergism enhances phloroglucinol content in shoot cultures of Astragalus fruticosus Forssk. Front. Nat. Prod. 4:1618336. doi: 10.3389/fntpr.2025.1618336
Received
25 April 2025
Accepted
31 July 2025
Published
29 August 2025
Volume
4 - 2025
Edited by
Benedetta Chiancone, University of Parma, Italy
Reviewed by
Rafia Rehman, National University of Medical Sciences (NUMS), Pakistan
Ramazan Erenler, Gaziosmanpaşa University, Türkiye
Updates
Copyright
© 2025 Zayed, El-Sayed, Elissawy, Ismaeil, El-Shazly and Fayez.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rawia Zayed, rawiazayed@hotmail.com; Mohamed El-Shazly, mohamed.elshazly@pharma.asu.edu.e
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.