- 1All India Institute of Ayurveda (AIIA), Government of India, New Delhi, India
- 2Institute of pharmaceuticals science, Kurukshetra University, Kurukshetra, Haryana, India
- 3Department of Pharmacy, Indira Gandhi National Tribal University, Amarkantak, Madhya Pradesh, India
Background: Common malignancies such as breast, lung, colorectal, prostate, and stomach cancers significantly contribute to cancer related death worldwide. Although conventional therapies including chemotherapy, radiotherapy, immunotherapy, and targeted agents have substantially improved cancer outcomes, their effectiveness is often constrained by off-target toxicities, therapeutic resistance, and limited efficacy in advanced-stage disease.
Objective: This review explores the potential of Ayurvedic formulations and bioactive phytochemicals alongside modern cancer treatments to enhance the safety and efficacy of cancer therapies.
Methods: The review highlights recent advancements in both conventional and alternative cancer treatments, focusing on the pharmacological properties of Ayurvedic botanicals and their integration with modern oncology through translational research and precision medicine.
Results: Ayurvedic plants like Phyllanthus emblica (Amalaki), Piper nigrum (Piperine), Withania somnifera (Ashwagandha) and Curcuma longa (Haridra) contain bioactive phytochemicals that exhibit anti-proliferative, pro-apoptotic, and anti-metastatic effects, effectively targeting key cancer hallmarks. These phytochemicals are integrated into evidence-based oncology through reverse pharmacology. Modern oncology complements this approach with immunotherapies like Pembrolizumab and CAR-T cell therapies, targeted therapies such as Bevacizumab and Dabrafenib, and precision medicines like Imatinib, Trastuzumab, and Osimertinib. Additionally, hormonal therapies, along with innovations like radiopharmaceuticals and PARP inhibitors, expand the range of therapeutic options.
Conclusion: Integrating Ayurvedic phytochemicals with modern oncology provides a comprehensive framework for overcoming the limits of existing cancer treatments. This integrative approach enhances the safety, efficacy, and personalization of cancer medicines by combining conventional knowledge with new advances to generate creative cancer care.
1 Introduction
Cancer continues to pose a significant global health challenge, with its impact steadily increasing and placing immense strain on healthcare systems worldwide (Pesec and Sherertz, 2015). High-incidence malignancies such as breast, lung, colorectal, prostate, and stomach cancers collectively account for a substantial share of cancer-related morbidity and mortality (Torre et al., 2016; Jemal et al., 2010). While the last few decades have witnessed remarkable progress in oncology including advances in chemotherapy, radiotherapy, targeted therapies, immunotherapies, and precision medicine conventional treatments are frequently limited by systemic toxicity (Crawford, 2013), drug resistance, and variable patient responses (Weeks et al., 1998), especially in advanced or metastatic stages.
In response to these limitations, there is a growing interest in integrative oncology, wherein traditional medicinal systems such as Ayurveda are explored as complementary approaches to modern cancer therapeutics (Kaur et al., 2023). Ayurveda, an ancient Indian system of medicine, offers a holistic framework based on personalized diagnosis and natural remedies. Numerous classical Ayurvedic formulations and botanicals have demonstrated therapeutic potential in the context of cancer, exhibiting anti-proliferative, pro-apoptotic, anti-inflammatory, and anti-metastatic activities. They are rich in bioactive phytochemicals (Mondal et al., 2024; Banerjee et al., 2023) capable of modulating various molecular targets associated with cancer pathogenesis, such as NF-κB, MAPK/ERK, PI3K/Akt/mTOR, p53/Bax/Bcl-2, and VEGF pathways.
Modern oncological research has begun to validate these traditional remedies through preclinical studies, clinical trials, and reverse pharmacology approaches. This convergence of Ayurveda with contemporary biomedical science is paving the way for novel integrative strategies aimed at enhancing the efficacy and safety of existing cancer therapies. For instance, combining Ayurvedic phytoconstituents with chemotherapeutic agents has shown potential in overcoming chemoresistance, sensitizing resistant cancer cells, mitigating treatment-induced toxicity, and improving overall therapeutic outcomes. Furthermore, the integration of Ayurvedic diagnostics—such as prakriti-based classification, dosha imbalances, and metabolic profiling with modern tools like genomics and imaging may support more personalized and predictive cancer care.
This review discusses the pharmacological potential of Ayurvedic formulations against high-incidence cancers and explores their synergistic integration with modern oncology. By bridging traditional wisdom with evidence-based medicine, this approach holds promise for advancing early detection, therapeutic precision, and patient-centered treatment strategies in the evolving landscape of cancer care.
2 Methods
This review study utilized a systematic literature review (SLR) approach to thoroughly explore current evidence on the integration of Ayurvedic medicine with conventional cancer treatments, covering publications from 2000 to 2024. A comprehensive search was carried out using several major databases and platforms, including PubMed, Google Scholar, ScienceDirect, Scopus, SpringerLink, Web of Science, the Google search engine (for grey literature), and the AYUSH Research Portal. The search strategy involved targeted use of keywords and Boolean operators such as “Integrative Oncology” AND “Ayurveda”, “Ayurvedic medicine” AND “Cancer therapy”, and “Cancer” AND “Rasayana therapy.” To refine the research question and guide the development of inclusion and exclusion criteria, the PICOC (Population, Intervention, Comparison, Outcome, Context) framework was utilized. The population considered included patients diagnosed with any type of cancer. The intervention focused on Ayurvedic medicine, including Rasayana therapy and other Ayurvedic formulations or practices used in conjunction with conventional cancer treatments. The comparison involved conventional cancer treatments administered alone or in combination with other complementary or integrative therapies. The outcomes of interest included tumor response, survival rates, symptom management, immune modulation, side effects, and quality of life indicators, including patient-reported outcomes. The context encompassed studies conducted in integrative oncology settings and published in English between the years 2000 and 2024. The search was limited to English-language publications within the specified timeframe. The review process followed PRISMA guidelines, and the quality of studies was evaluated using CONSORT standards.
3 Foundations of ayurvedic medicine in cancer care
Ayurveda, conceptualizes health as a dynamic balance of the Tridoshas—Vata, Pitta, and Kapha alongside the proper functioning of Agni (digestive fire), Dhatus (tissues), Malas (waste), and Ojas (vital essence) (Kizhakkeveettil et al., 2024). Disease, including cancer, is viewed as a consequence of doshic imbalance and disrupted tissue homeostasis, often influenced by an individual’s Prakriti, or constitutional makeup (Sharma and Chandola, 2011; Patwardhan et al., 2015) (Figure 1).
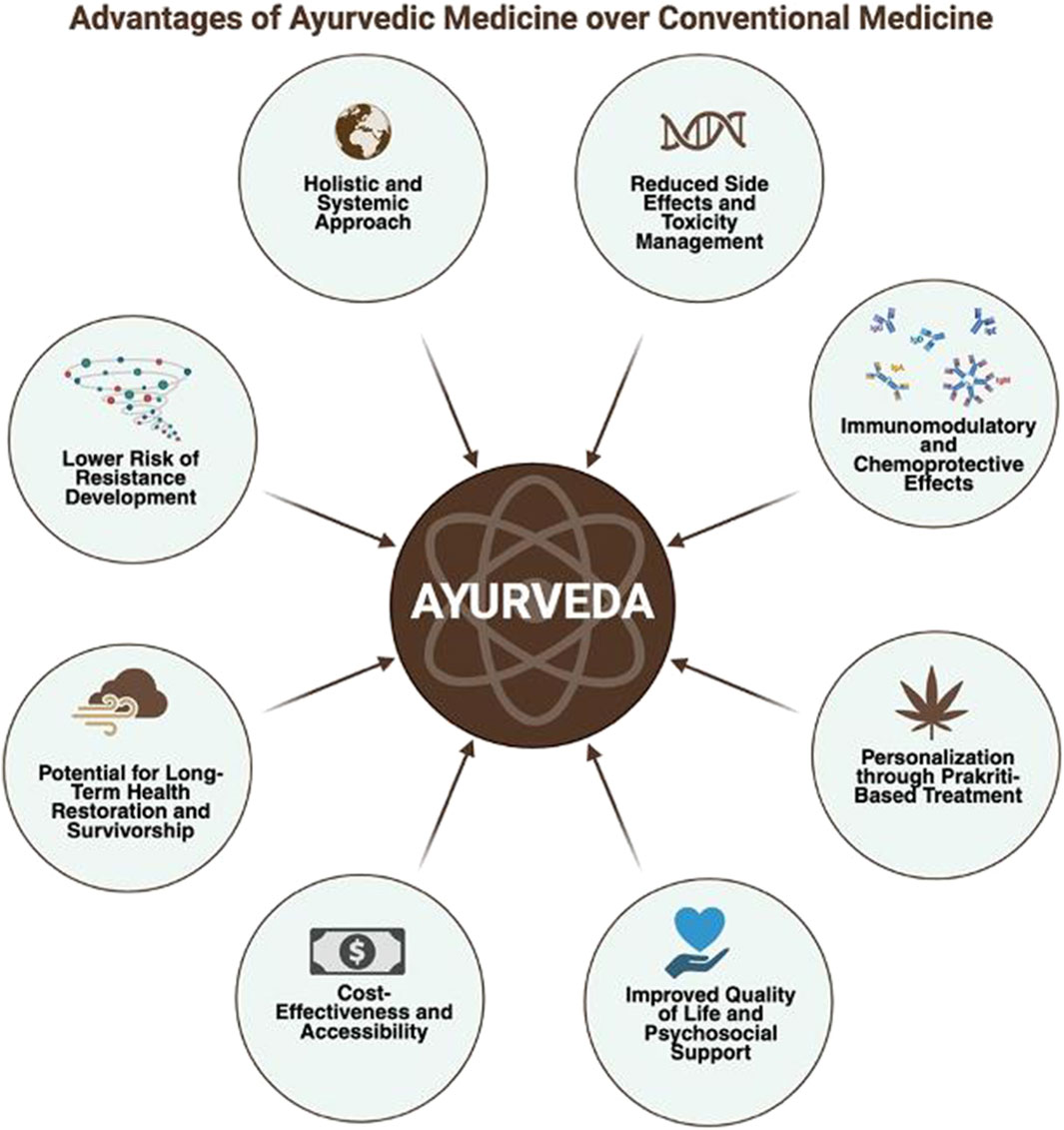
Figure 1. Advantages of ayurvedic medicine over conventional approaches: This figure illustrates the key benefits of Ayurvedic medicine compared to conventional therapies. Ayurveda offers a holistic and systemic approach with reduced side effects, lower risk of resistance development, and immunomodulatory and chemoprotective effects. Additional advantages include personalized treatment based on individual constitution (Prakriti), cost- effectiveness and accessibility, improved quality of life and psychosocial support, and a focus on long-term health restoration and survivorship. These attributes underscore Ayurveda’s potential role in integrative and preventive healthcare models.
Though classical Ayurvedic texts do not explicitly mention “cancer,” they describe pathological entities analogous to tumorigenesis, such as Arbuda (Manohar, 2015; Kalachaveedu et al., 2023)—characterized by large, immobile masses caused by vitiated Doshas infiltrating muscles, blood, and fat tissues—and Granthi, smaller glandular swellings that may represent benign or precursor lesions. Malignant features, including ulceration and rapid tissue destruction, align with conditions described under Dushi Visha (latent toxins) and Sannipataja Vikara (complex systemic disorders) (Sundaramourthy et al., 2024). This systemic perspective highlights Ayurveda’s emphasis on underlying physiological imbalance rather than isolated tumor pathology.
The concept of Prakriti is pivotal in Ayurveda’s personalized approach, influencing disease susceptibility, progression, and therapeutic response (Chatterjee and Pancholi, 2011). Recent Ayurgenomic studies have begun to validate molecular correlates of Prakriti types, linking constitutional phenotypes to variations in immune response and metabolism that may affect cancer risk and treatment efficacy (Sharma and Prajapati, 2020; Prasher et al., 2016; Huang et al., 2022). This supports integrative oncology’s movement toward precision medicine.
Rasayana therapy, a cornerstone of Ayurvedic cancer care, focuses on rejuvenation and immune modulation to enhance Ojas, promote tissue regeneration, and mitigate therapy-related toxicity (Sagar and Sabharwal, 2024). Key Rasayana herbs like Withania somnifera (Ashwagandha), Tinospora cordifolia (Guduchi), Emblica officinalis (Amalaki), and components of Triphala demonstrate immunomodulatory, antioxidant, and cytoprotective effects relevant to cancer prevention and adjunctive treatment.
Furthermore, Ayurveda’s use of polyherbal formulations exemplifies a multi-target, synergistic strategy well-suited to the complex pathophysiology of cancer. Classical preparations such as Kanchnar guggulu, Triphala, and Arogyavardhini have shown promising anti-inflammatory, pro-apoptotic, and detoxifying properties in preliminary studies (Nair and Ashwini, 2019). However, challenges remain in pharmacokinetic standardization, mechanistic elucidation, and rigorous clinical validation to bridge traditional knowledge with modern oncology.
This integrative foundation underscores Ayurveda’s potential to complement contemporary cancer therapeutics by offering personalized, systemic interventions aimed at restoring physiological balance and enhancing patient resilience.
4 Role of medicinal herbs in cancer
Medicinal herbs have long been recognized for their therapeutic potential across a wide range of diseases, including cancer (Huang et al., 2009). In recent decades, there has been a growing scientific interest in exploring the anticancer properties of plant-derived compounds as complementary or alternative approaches to modern oncology. Unlike conventional chemotherapeutic agents that often target single pathways and carry significant toxicity, many herbal formulations exhibit multi-targeted mechanisms of action with comparatively lower side-effect profiles (Ravikumar and Aittokallio, 2018) (Figure 2).
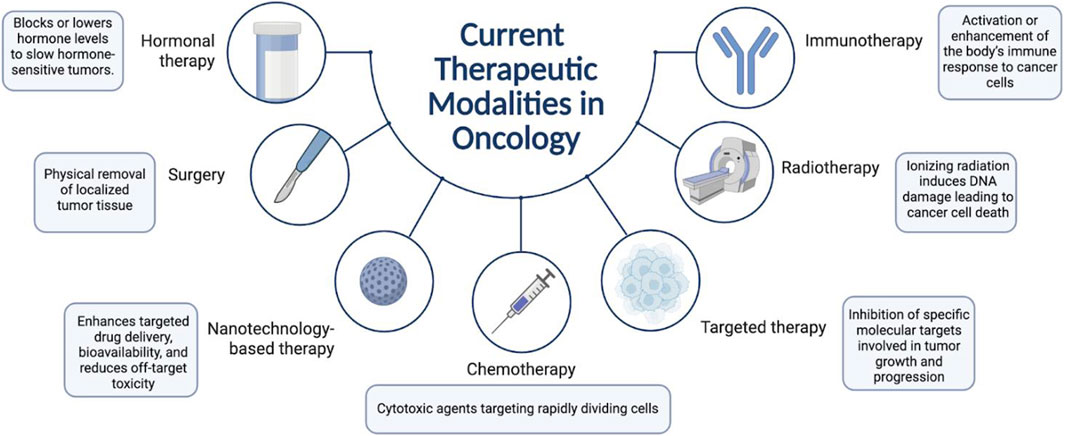
Figure 2. Current therapeutic modalities in oncology: This figure outlines the principal cancer treatment strategies, including surgery, chemotherapy, radiotherapy, hormonal therapy, targeted therapy, immunotherapy, and nanotechnology-based approaches. Each modality operates through distinct mechanisms such as physical tumor removal, induction of DNA damage, hormonal regulation, immune activation, molecular target inhibition, and enhanced drug delivery.
Numerous herbs used in traditional systems of medicine, particularly Ayurveda, Traditional Chinese Medicine (TCM), and folk medicine, contain bioactive phytochemicals that exhibit anti-proliferative, pro-apoptotic, anti-inflammatory, anti-metastatic, and immunomodulatory activities. These compounds can modulate key molecular targets such as tumour suppressor genes, oncogenes, angiogenic factors, transcription factors, and immune checkpoints. Moreover, some herbal constituents also help enhance the efficacy of conventional therapies by increasing drug sensitivity, modulating drug resistance proteins or reducing chemotherapy- and radiotherapy-induced toxicity.
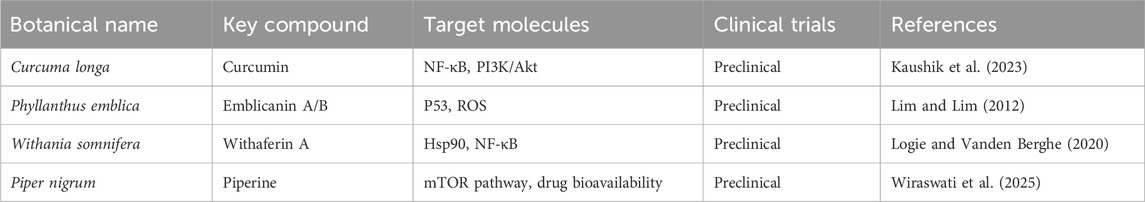
Integrating these herbs into cancer therapy not only offers pharmacological benefits but also aligns with the concept of holistic, patient-centred care, improving quality of life, minimizing treatment-related complications, and potentially reducing recurrence. On-going preclinical, translational and clinical research is crucial to validate these agents, understand their mechanisms, optimize formulations, and ensure their standardized, safe integration into evidence based oncology protocols. Several medicinal herbs have demonstrated significant anticancer properties, offering potential complementary approaches to conventional cancer therapies. We will explore some of the most extensively studied anticancer herbs, highlighting their mechanisms of action, therapeutic advantages, and potential limitation and safety considerations (Table 1).
4.1 Curcuma longa
Curcuma longa, commonly known as turmeric, is a staple spice in Indian and Southeast Asian cuisine, is one of the most studied medicinal herbs for its anticancer properties (Verma et al., 2018). Its primary bioactive constituent, curcumin, has garnered substantial attention for its broad-spectrum antineoplastic effects across various cancer types. Curcumin exerts its anticancer activity through modulation of multiple molecular targets and signaling pathways (Kunnumakkara et al., 2017a). It inhibits cell proliferation by downregulating cyclins and cyclin-dependent kinases, induces apoptosis via activation of caspases (Srivastava et al., 2007) and mitochondrial pathways (Das and Vinayak, 2014), suppresses angiogenesis (Saberi-Karimian et al., 2019) by downregulating vascular endothelial growth factor (VEGF) expression, and interferes with metastatic progression by modulating matrix metalloproteinases (MMPs). Additionally, it also modulates key signaling pathways implicated in cancer pathogenesis, including NF-κB, STAT3, and PI3K/Akt, thereby disrupting the cancer cell’s survival and proliferation, and inflammatory signals (Shishodia et al., 2005).
One of the advantages of curcumin is its low toxicity profile, even at relatively high doses, making it a safe candidate for long-term adjunctive therapy. Curcumin has demonstrated preclinical and clinical efficacy against a variety of malignancies, including breast, colon, prostate, lung, and pancreatic cancers (Aggarwal et al., 2003). Moreover, it enhances the efficacy of conventional chemotherapy and radiotherapy while alleviating associated side effects, such as mucositis, oxidative stress, and systemic inflammation.
However, despite these promising therapeutic attributes, cur cumin’s clinical application is limited by its poor bioavailability, attributed to low absorption, rapid hepatic metabolism, and systemic elimination (Anand et al., 2007). To overcome these limitations, several strategies have been developed, including nanoparticle encapsulation, liposomal formulations, structural analogs and co-administration with other herbs such as piperine (Ma et al., 2019).
4.2 Withania somnifera
Withania somnifera, commonly known as Ashwagandha, widely recognized for its rejuvenating, adaptogenic and immunomodulatory properties. Its principal bioactive constituents, Withanolides and Withaferin A, have demonstrated notable anticancer effects in various preclinical studies (Rai et al., 2016). Withaferin A exerts its anticancer activity primarily by inducing apoptosis in malignant cells. It modulates several signaling pathways, including inhibition of NF-κB, activation of tumor suppressor p53, and interference with the cytoskeletal architecture, which disrupts cancer cell integrity and function (Dutta et al., 2019).
Ashwagandha has exhibited cytotoxic effects against a broad range of cancer cell lines, including breast, colon, lung, and prostate cancers (Singh et al., 2021). Furthermore, studies suggest that Withaferin A may enhance the efficacy of standard chemotherapeutic agents by sensitizing tumor cells to apoptosis, thereby offering potential for synergistic cancer therapy and its adaptogenic nature also supports cancer patients by alleviating fatigue, anxiety, and stress, contributing to improved quality of life.
Despite its promising pharmacological profile, clinical data in humans remain limited. Only a few randomized controlled trials have been conducted, making it difficult to conclusively establish safety and optimal dosing for cancer patients. Side effects by its use include gastrointestinal disturbances, drowsiness, and rare allergic reactions (Tandon and Yadav, 2020). Additionally, Ashwagandha may interact with immunosuppressive agents and thyroid hormone medications, warranting caution in patients on such therapies (Bhaskar et al., 2022).
4.3 Phyllanthus emblica
Phyllanthus emblica, commonly known as Indian Gooseberry or Amla, is a cornerstone of Ayurvedic medicine recognized for its potent antioxidant and rejuvenating properties. Its anticancer potential is primarily attributed to its diverse bioactive constituents, including gallic acid, ellagic acid, and emblicanin A and B. These phytochemicals exert anti carcinogenic effects via multiple mechanisms as they induce apoptosis, inhibit tumor cell proliferation, and modulate oxidative stress (Zhao et al., 2015), thereby disrupting the cellular environment that favors malignant transformation. Phyllanthus emblica modulates transcription factors such as NF-κB and AP-1, which play key roles in inflammation-mediated cancer progression (Naseer et al., 2022).
Amla’s greatest strengths lie in its rich antioxidant profile, which not only combats oxidative damage (Gul et al., 2022), a known contributor to carcinogenesis, but also supports general cellular health. Preclinical studies have demonstrated its ability to inhibit the proliferation of cancer cells from breast, cervical, and ovarian malignancies, and it has also shown promise in enhancing immune surveillance by stimulating cytotoxic T cells and natural killer cell activity.
However, despite promising results from in vitro and in vivo studies, clinical validation in human cancer patients is still limited. Most evidence remains preclinical, underscoring the need for well-designed human studies to confirm therapeutic efficacy and establish standardized dosages. In higher doses, Amla may lead to gastrointestinal disturbances such as bloating or diarrhea. It may also interact with anticoagulant medications due to its blood-thinning potential, thus warranting caution in individuals on such therapies (Abebe, 2019).
4.4 Piper nigrum
Piper nigrum, commonly known as Black Pepper, is a culinary spice that also holds significant therapeutic potential due to its bioactive alkaloid, piperine (Srinivasan, 2009). This has been extensively studied for its pharmacological benefits, and has demonstrated notable anticancer activity through multiple molecular mechanisms. It induces apoptosis and inhibits tumor cell proliferation by modulating key molecular targets involved in oncogenesis, including NF-κB, PI3K/Akt, and MAPK signaling pathways. Piperine plays a critical role in enhancing the bioavailability of other anticancer compounds such as curcumin, by inhibiting hepatic and intestinal glucuronidation processes (Ashokkumar et al., 2021). This bioenhancement capacity makes it an attractive candidate for combination therapy in integrative oncology.
One of the most compelling advantages of piperine is its capacity to potentiate the efficacy of co-administered chemopreventive agents. Studies have shown that it exhibits cytotoxicity against a range of cancer cell lines, including breast, lung, colon, and prostate cancers. Piperine also has the potential to overcome multidrug resistance (MDR) in cancer cells by downregulating P-glycoprotein and other drug-efflux mechanisms, which are major limitations in conventional chemotherapy (Li et al., 2011). However, the use of piperine at high doses may lead to gastrointestinal irritation and discomfort. Moreover, due to its influence on hepatic enzymes like CYP3A4 and CYP2D6, piperine may interact with drugs metabolized in the liver, potentially altering their pharmacokinetics and increasing the risk of toxicity (Mehmood and Gilani, 2010; Haq et al., 2021).
5 Mechanistic and molecular cellular signalling of ayurvedic herbs
5.1 Curcumin
Curcumin also demonstrates significant action via growth factors like fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor-b1 (TGF-b1) and vascular endothelial growth factor (VEGF) (Kunnumakkara et al., 2017b; Sahebkar et al., 2016; Jordan et al., 2016) Curcumin treatment is reported to increase HDAC1, HDAC4 and HDAC8, apoptosis, production of ROS and Nrf2 expression, while decrease VEGF, HIF1-a, GSK-3b, Akt, prostate-specific antigen (PSA) level, PSA mRNA expression, HAT activity and cellular proliferation in prostate and bladder cancer (Taverna et al., 2015). Curcumin treatment also downregulated the expression of VEGF and decreased the phosphorylation of AKT, in leukemia and cervical cancer (Mimeault and Batra, 2011).
Curcumin modulates intracellular signal transduction elements such as p21, p27, inhibitor of growth family member 4 (ING4), cyclin D1, cMyc, VEGF, ICAM-1, MMPS, uPA, COX-2, CXCR-4, Bax, Bad, Bak, Noxa, p53, modulator of apoptosis, caspases, etc. resulting in reversal of cancer incidence, progression and relapse (Kasi et al., 2016).
In cellline studies, Curcumin treatment upregulated E-cadherin while downregulated vimentin and MMPs expressions along with reduced metastasis, cell spreading and cell migration in human papillary thyroid carcinoma cells (Zhang L. et al., 2016). Curcumin treatment induced the arrest of G0/G1 cell cycle phase alongside inhibited the regulatory proteins cyclin D1 and CDK-2 (Sha et al., 2016). Besides, it upregulated the expression of p21, p27 and p53 while downregulated Bcl-2 expression. Further, Curcumin treatment is known to activate caspase (Jemal et al., 2010; Banerjee et al., 2023; Kizhakkeveettil et al., 2024) while decreased Akt, MMP2 and MMP9, Bcl2, Bcl-XL and tumor volume in prostate cancer (Mimeault and Batra, 2011).
Curcumin is known to modulate various cellular signaling cascade like the phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR), ERK5/AP-1, TGF-b signaling, Wnt/b-catenin, PAK1/Ras-related C3 botulinum toxin substrate 1, TLR-4/MyD-88, signal transducers and activators of transcription (STAT) 3 pathway, PPARc-C/EBPa pathway, nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome, p38MAPK, etc. (Shanmugam et al., 2015).
Curcumin treatment downregulated chemokine receptor 4 expression and supressed Wnt signaling. In addition, curcumin treatment downregulated vimentin and upregulated E-cadherin expression, which leads to inhibition of proliferation and epithelial mesenchymal transition in SW620 human colon cancer cells (Zhang Z. et al., 2016).
Curcumin mediated downregulation of viral oncogenes is attributed to its ability to modulate apoptosis and prevent NFkB and AP1 translocation thereby suppressing the transcription of HPVs (Divya and Pillai, 2006; Prusty and Das, 2005).
5.2 Piperine
Piperine exhibits a unique duality in its abilities, functioning as both a blocking and a suppressing agent in cancer prevention and therapy. The main chemopreventive mechanism of action of piperine include activation of apoptotic signalling cascades, inhibition of cell proliferation, cell cycle arrest, alterations in redox homeostatis, modulation of ER stress and autophagy, inhibition of angiogenesis, induction of detoxification enzymes, and sensitization of tumors to radiotherapy and chemotherapy (Manayi et al., 2018). Piperine is a pro-oxidant agent and can stimulate the formation of reactive oxygen species (ROS) in many types of cancer cells. ROS triggers the depolarization of mitochondrial membrane potential (MMP), leading to release of cytochrome c, activation of caspases (Lai et al., 2012), and induction of apoptosis.
Piperine attenuates the UV-R induced damage in human HaCaT keratinocytes through its influence on the NF-κB and Bax/Bcl2 pathways. It is believed that piperine binds to active site of NF-κB and blocks its nuclear localization. Altered NF-κB activity in turs triggers suppression of apoptotic marker genes (Verma et al., 2017). Its effects have been reported for different cancer types. In lung cancer, piperine induces apoptosis via p53 signaling. In breast cancer, this substance suppresses proliferation and metastasis both in vitro and in vivo3. In melanoma cells, piperine inhibitis NF-κβ, c-Fos, ATF-2 and CREB (Lin et al., 2014). Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Piperine inhibits proliferation, causes cell cycle arrest and induces autophagy in prostate cancer cell lines (Lai et al., 2012). In rectal cancer, piperine increases reactive oxygen species leading to apoptosis increase (Pradeep and Kuttan, 2004). Additionally, some effects of piperine on colorectal cancer were reported, such as inhibition of cell proliferation and activation of apoptotic program by endoplasmic reticulum stress (Ouyang et al., 2013). However, piperine molecular mechanisms of action on colorectal cancer have not been elucidated, as well as its relation to the Wnt signaling pathway.
Piperine also blocked the phosphorylation of Ser 473 and Thr 308 residues of Akt involved in regulating endothelial cells and, therefore, angiogenesis (Yaffe et al., 2013). Piperine downregulated MMP-9 and VEGF mRNA expressions in a dose-dependent manner. In addition, piperine treatment increased the expression levels of E-cadherin, a cell adhesion molecule required to maintain extracellular matrix integrity and cell-to-cell contact, thereby supporting the anti-metastatic potential of this alkaloid (Yaffe et al., 2015). Piperine induces various cell death types, including apoptosis, autophagy, ferroptosis, and anoikis, which play significant roles in cancer prevention and therapy (Jafri et al., 2019; Qi et al., 2023; Fofaria and Srivastava, 2015). Piperine can also inhibit the cell cycle at the G2/M phase in cancer cells via the downregulation of G2-associated (cyclin B, CDK1, Cdc25C) proteins, the induction of p21, and the enhancement of the phosphorylation of both CDK1 and checkpoint kinase 2 (Chk2) (Zhang et al., 2015; Greenshields et al., 2015; Pressete et al., 2023). Piperine enhanced the bioavailability of various well-known chemopreventive natural agents (e.g., resveratrol, curcumin), by inhibiting glucuronidation, significantly increasing the plasma concentration of resveratrol when given with resveratrol.
5.3 Withaferin A
Withania somnifera (Ashwagandha), particularly Withaferin A, exhibits extensive anticancer properties via mitochondrial disruption, reactive oxygen species (ROS) generation, and interference with mitotic spindles, resulting in apoptosis (Widodo et al., 2010; Carmi et al., 2012). It inhibits essential oncogenic pathways, including NF-κB, Sp1, Akt, mTOR, STAT3, and Notch-1, while downregulating angiogenesis and epithelial-mesenchymal transition markers such as VEGF, vimentin, Snail, and Slug (Song et al., 2013). In vivo studies demonstrate a reduction in microvessel formation and tumor regression across various cancers, with liposomal and nanosponge formulations markedly improving efficacy (Garvey et al., 2021; Jendrossek, 2013). Docking studies indicate that garcinol exhibits synergistic effects in inhibiting BCL-2/AKT, demonstrating greater efficacy compared to conventional agents such as venetoclax or melatonin (Olarewaju et al., 2022; Huang et al., 2023). Challenges in solubility and bioavailability remain, highlighting the necessity for additional nanotechnological advancements and clinical validation (Balkrishna et al., 2024a).
WA arrested the cell cycle at the G2/M phase and is correlated with decreasing the levels of cyclin-dependent kinase 1 (Cdk1) and cell division cycle 25C (Cdc25C) protein levels, leading to inhibition of the Cdck1/CyclinB1 kinase complex (Stan et al., 2008). Another mechanism by which WA ceases the cell cycle is by inhibiting the spindle assembly checkpoint (SAC). SAC is a mitotic checkpoint complex consisting of Mad1 and Mad2 components, which are essential for the initiation of anaphase (Das et al., 2014). In the MDA-MB-231 xenograft model, WA showed an anti-metastatic effect through inhibition of vimentin expression (Lee et al., 2015). WA inhibited vimentin assembly by directly binding to cysteine 328 residues through covalent modification and also inhibited proteasomal activity in a vimentin-dependent manner in the case of breast cancer (Thaiparambil et al., 2011). The matrix metalloproteinase inhibition is observed to be mediated through suppression of the Akt pathway (Lee et al., 2013).
5.4 Emblicanin
Phyllanthus emblica (amla), which contains high levels of Emblicanin A/B (ellagitannins), demonstrates significant antioxidant and anti-inflammatory properties by inhibiting reactive oxygen species (ROS), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), cyclooxygenase (COX), and inducible nitric oxide synthase (iNOS) (Im et al., 2018; Yang et al., 2018). Recent findings indicate tumor-suppressive potential in hepatotoxic and nephrotoxic models, as well as anti-fibrotic activity through caspase-3-mediated apoptosis (Kim et al., 2024; Hussein et al., 2020).
Flavonoids in E. officinalis such as apigenin and luteolin can inhibit HCC cell proliferation, migration, and invasion by inducing apoptosis via inhibiting the AKT/osteopontin and PI3K/Akt/mTOR pathways, respectively (Im et al., 2018; Yang et al., 2018). Phenolic compounds such as ferulic acid and vanillic acid have antioxidant properties and can target PTGS2 implicated in inflammation and tumor progression thereby inhibiting NF-κB signaling pathway (Kim et al., 2024). Caffeic acid, chlorogenic acid, o-coumaric acid have been reported to exhibit anti-inflammatory and antioxidant properties that contribute to hepatoprotective effects by targeting tyrosine receptor kinases, EGFR and VEGFR, by modulating MAPK and PI3K/AKT pathways, respectively (Hussein et al., 2020; Yang et al., 2021) Likewise, pyrogallol inhibits PI3K/AKT/Skp2/cMYC signaling pathways in HCC (Ahn et al., 2019).
Caspases serve as a primary mediator of apoptosis located in the cytosolic space (van Loo et al., 2002). The treatment of emblicanin-A to PC-3 cells increased the caspase-3 and-9 mRNA expression compared to untreated cells which might the mechanism that emblicanin-A lead to the release of cytochrome c from mitochondrial space which would have been combined with an adaptor molecule apoptosis protease activating factor 1 and also with an inactive initiator caspase, pro-caspase-9 within a multiprotein complex called the apoptosome, causing a series of caspase and that ultimately would have caused apoptosis (Ghobrial et al., 2005). Hence, it clearly demonstrates that emblicanin-A activates apoptosis by the intrinsic pathway. In support of the present investigation, we found that E. officinalis fruit extract treated rats significantly increased the caspase-3 protein expression (Malik et al., 2016).
5.5 Ayurvedic compound synergy: evidence-based insights
The insight mechanism combining two bioactive ayurvedic compounds is also well explored especially the curcumin and piperine bioactives. Curcumin and piperine together have positive effect on serum IgE levels with no significant changes on serum IL-10 and IL-12 in healthy young women (Bahrami et al., 2022). Administration of oral curcumin with piperine as an adjuvant symptomatic therapy in COVID-19 treatment substantially reduce morbidity and mortality, and ease the logistical and supply-related burdens on the healthcare system (Pawar et al., 2021). Piperine also potentiates curcumin’s inhibitory effect on tumor progression via enhancing its delivery and therapeutic activity (Yüksel et al., 2023). Curcumin-C3complex®/Bioperine® treatment strongly reduces in vitro tumorigenic properties of mesothelioma cells by impairing cellular self-renewal ability, proliferative cell rate and cell migration and delays tumor growth in xenograft mouse model by reducing angiogenesis and increasing apoptosis (Di Meo et al., 2019). Kaur et al. (2018) concluded that curcumin and piperine, either alone or in combination, have the potential to downregulate mTORC1 signaling in the intestinal epithelium with implications for tumorigenesis and inflammation.
5.6 Unified in silico investigation of cancer-targeting phytochemicals from major medicinal herbs
In silico assessments of bioactive compounds derived from Curcuma longa, Phyllanthus emblica, Piper nigrum, and Withania somnifera reveal their significant therapeutic potential across multiple cancer types. Curcumin from Curcuma longa displayed high binding affinity with EGFR (−9.1 kcal/mol) and inhibited PI3K/Akt and NF-κB pathways (Kurmi et al., 2025), while a combination of curcumin and demethoxycurcumin showed multi-targeted interactions with VEGFA, TNF, PTGS2, and AKT1, indicating anti-inflammatory and pro-apoptotic activity. Emblicanin B, gallic acid, quercetin, and ellagic acid from Phyllanthus emblica demonstrated effective docking with CASP3 (−8.7 kcal/mol), TP53 (−8.3 kcal/mol), and MMP9, implicating apoptotic induction and detoxification. From Piper nigrum, piperine exhibited stable binding with BCL2 (−8.5 kcal/mol), AKT1, and STAT3, with supportive QSAR analysis indicating pro-apoptotic effects. Network pharmacology also linked piperine and related alkaloids to MAPK8, RELA, and ESR1, suggesting influence over NF-κB and estrogen pathways. Withania somnifera compounds showed strong anti-cancer interactions as well, with Withaferin A binding hnRNP-K (−9.0 kcal/mol) and demonstrating antiangiogenic potential, while Withanolide D showed robust docking with mTOR (−9.2 kcal/mol) and STAT3 (−8.8 kcal/mol), contributing to cell cycle arrest and suppression of invasion. Collectively, these findings support the multitarget capabilities of plant-derived phytochemicals and reinforce their potential as promising candidates in anticancer drug discovery through molecular docking, dynamics simulations, and network-based modeling (Bhati et al., 2025; Table 2).
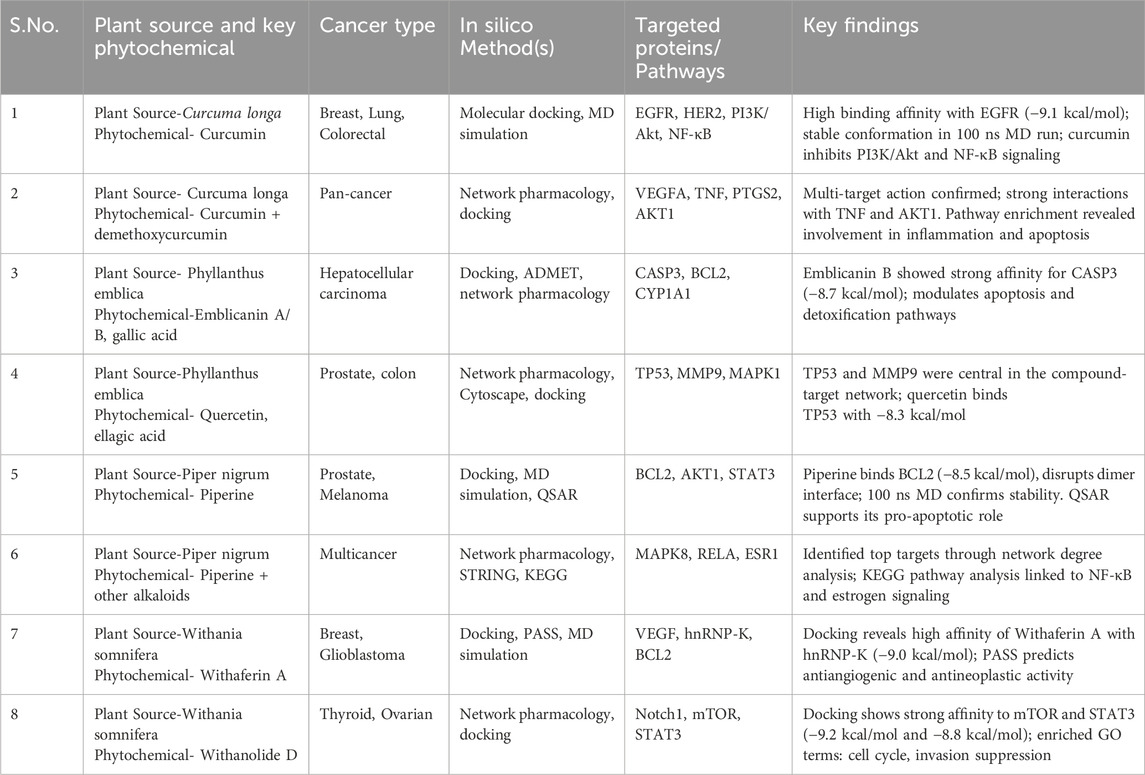
Table 2. In Silico and mechanistic insights into the anticancer mechanisms of phytochemicals derived from Curcuma longa, Phyllanthus emblica, Piper nigrum, and Withania somnifera.
6 Pharmacological analysis of plant derived chemotherapeutic agents
6.1 Taxanes: paclitaxel and docetaxel
Taxanes, particulrly paclitaxel and docetaxel, represent a pivotal class of plant-derived chemotherapeutic agents originally isolated from the bark of Taxus brevifolia and later from Taxus baccata (105) Paclitaxel was the first taxane to be identified and has since been extensively utilized in the treatment of a variety of malignancies. Mechanistically, taxanes exert their antitumor effects by stabilizing microtubules, the structural components essential for cell division (Sati et al., 2024). Unlike other conventional antimitotic drugs that disrupt microtubule formation, paclitaxel and docetaxel promote tubulin polymerization and prevent depolymerization, effectively “freezing” the mitotic spindle in a polymerized state (Abal et al., 2003). This results in mitotic arrest, inhibition of cell division, and eventual induction of apoptosis in rapidly dividing cancer cells.
Clinically, paclitaxel and docetaxel have demonstrated broad-spectrum antineoplastic activity and are widely used in the management of breast cancer, ovarian cancer, non-small cell lung cancer (NSCLC), prostate cancer, and head and neck cancers (Jordan, 2002; Ahmed Khalil et al., 2022). Their efficacy in both adjuvant and metastatic settings has made them a standard part of many chemotherapy regimens. Despite their robust efficacy, these agents are associated with several limitations, including hypersensitivity reactions (due to the solvent Cremophor EL in paclitaxel), neuropathy, myelosuppression, and development of multidrug resistance via P-glycoprotein efflux (Herbst and Khuri, 2003). Thus, taxanes exemplify how natural products can be successfully translated into potent anticancer agents, highlighting the value of phytochemicals in modern oncology.
6.2 Vinca alkaloids: vincristine and vinblastine
Vinca alkaloids, primarily vincristine and vinblastine, are among the earliest plant-derived anticancer agents extracted from Catharanthus roseus (Nobili et al., 2012), a plant traditionally used in various folk medicine systems but not explicitly referenced in classical Ayurvedic literature. These alkaloids have significantly advanced cancer chemotherapy due to their unique mechanism of action and potent efficacy against various malignancies. The primary mechanism of action of Vinca alkaloids is their ability to disrupt microtubule dynamics, which are essential for mitosis. Specifically, vincristine and vinblastine bind to tubulin monomers and inhibit their polymerization into microtubules. This inhibition halts mitotic spindle formation, thereby arresting cells in metaphase and leading to apoptosis (Dhyani et al., 2022). Vinca alkaloids selectively target rapidly dividing cells, renders them particularly effective against hematological malignancies.
Vincristine and Vinblastine are integral to many chemotherapy regimens. Vincristine is commonly used in pediatric oncology, and is a key agent in the treatment of acute lymphoblastic leukemia (ALL), Hodgkin’s and non-Hodgkin’s lymphomas, and neuroblastoma (Jordan, 2002; Škubník et al., 2021) In contrast vinblastine is widely used in the management of testicular cancer, Hodgkin’s lymphoma, breast cancer, and Kaposi’s sarcoma (Lopez-Lopez et al., 2016). Their synergistic use with other chemotherapeutics enhances efficacy and minimize the development of drug resistance.
Despite their therapeutic potential, vinca alkaloids present notable challenges. Neurotoxicity, especially peripheral neuropathy, is a dose-limiting side effect of vincristine, while vinblastine is more often associated with myelosuppression (Samuels and Howe, 1970). The potential integration of Ayurvedic approaches with vinca alkaloid-based chemotherapy lies in mitigating side effects and improving patient quality of life.
6.3 Podophyllotoxins: etoposide and teniposide–natural inhibitors of DNA replication
Podophyllotoxins, particularly etoposide and teniposide, are semi-synthetic derivatives of the natural compound extracted from Podophyllum peltatum (Verstappen et al., 2003; Guerram et al., 2012). These agents belong to the class of epipodophyllotoxins and were developed after identifying the cytotoxic effects of the parent compound podophyllotoxin. The primary mechanism of action of etoposide and teniposide involves inhibition of the enzyme topoisomerase II. This enzyme is essential for DNA replication and cell division as it relieves torsional strain in DNA and stabilize the transient DNA-topoisomerase II complex, preventing the re-ligation of DNA strands (Sharma et al., 2016). This results in persistent DNA breaks, ultimately triggering apoptosis in rapidly dividing cancer cells. The action is cell cycle-specific, most active in the S and G2 phases, making these drugs effective in tumors with high proliferative indices.
Clinically, etoposide is a cornerstone in the treatment of testicular cancer, particularly when combined with cisplatin and bleomycin (Hande, 1998). It is also widely used for small cell lung cancer, lymphomas, and certain leukemias. Teniposide, a closely related compound with a slightly different solubility profile, is predominantly used in pediatric oncology, including acute lymphoblastic leukemia (ALL) and neuroblastoma (Dearnaley et al., 1991). Their efficacy, however, is often tempered by significant toxicities.
6.4 Camptothecins: Irinotecan and topotecan–topoisomerase I inhibitors from nature
Camptothecins, notably irinotecan and topotecan, are potent anticancer agents originally derived from Camptotheca acuminate. These alkaloids were developed following the discovery of camptothecin, a naturally occurring compound that exhibited significant anticancer activity through a novel mechanism of action (Sinkule et al., 1984). Irinotecan and topotecan are semi-synthetic analogs designed to improve solubility and therapeutic efficacy while minimizing toxicity (Gallo et al., 1971).
The primary mechanism of action of camptothecins involves inhibition of DNA topoisomerase I, by inducing single-stranded breaks (Gokduman, 2016). Irinotecan and topotecan stabilize the transient enzyme-DNA complex, preventing re-ligation of the DNA strand ultimately leading to replication fork collapse, DNA damage, and apoptosis. This makes them especially effective against rapidly dividing tumor cells.
Irinotecan is a key component of combination regimens such as FOLFIRI (with 5-fluorouracil and leucovorin) in the treatment of metastatic colorectal cancer (Pommier, 2006). Topotecan is used in the management of ovarian cancer, small cell lung cancer, and as a second-line treatment in cervical cancer (Folprecht et al., 2006). Despite their clinical effectiveness, the use of camptothecins is often limited by significant adverse effects, particularly gastrointestinal toxicity (severe diarrhea) and hematological toxicity (neutropenia).
Other than the above mentioned there are several other exemplary natural compounds which have the anticancer effect. Honokiol, a biphenolic compound extracted from the bark of Magnolia officinalis, modulate multiple molecular targets, such as anti-angiogenic, anti-inflammatory, and pro-apoptotic signalling pathways and inhibits cancer cell proliferation and metastasis (Petrelli et al., 2021). Its lipophilic nature allows it to cross the blood-brain barrier, making it particularly promising in gliomas and brain metastases. Polysaccharide-K (PSK), a protein-bound polysaccharide derived from the medicinal mushroom Trametes versicolor, is another naturally sourced agent approved as an adjuvant therapy for gastric and colorectal cancers (Ong et al., 2019). PSK enhances the immune response by activating dendritic cells, macrophages, and T lymphocytes contributing to improved tumor surveillance. Genistein, a soy-derived isoflavone, represents another compelling natural compound for its anticancer effects, particularly in hormone-sensitive cancers such as breast, prostate, and colon cancers (Habtemariam, 2020). Genistein acts primarily as a tyrosine kinase inhibitor and exerts phytoestrogenic effects by binding to estrogen receptors, particularly ER-β, thereby modulating estrogen signaling. It also interferes with cancer cell growth and metastasis by affecting the PI3K/Akt, Wnt, and MAPK pathways. Furthermore, genistein exhibits antioxidant and anti-inflammatory activities, contributing to its chemopreventive potential (Table 3).
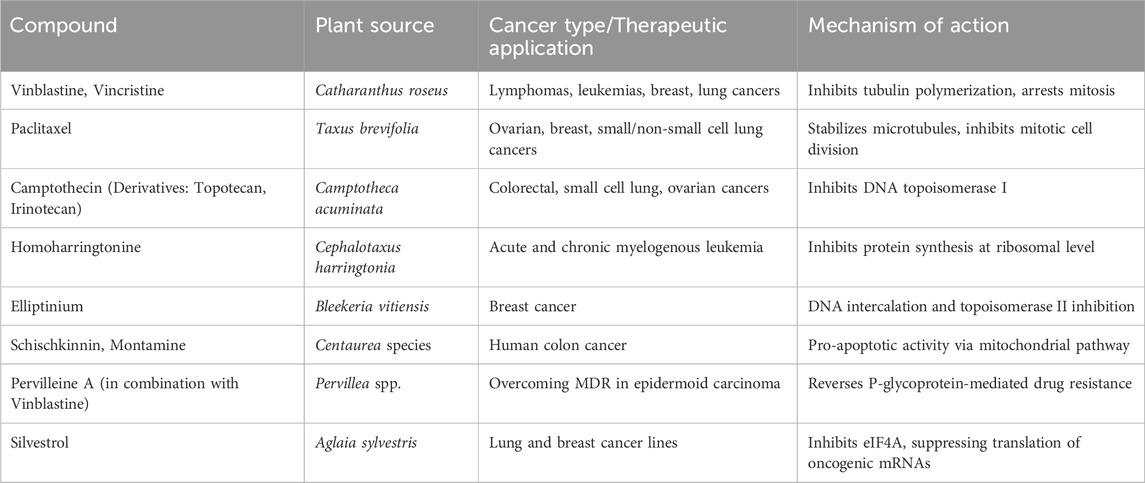
Table 3. Plant-derived anticancer drugs: therapeutic applications and mechanistic insights with their target molecules.
7 Overview of modern oncology: current concepts and challenges
Modern oncology has witnessed remarkable progress over the past few decades, driven by advances in molecular biology, genomics, immunology, and personalized medicine (Liliana et al., 2017). The current oncological paradigm is rooted in the understanding of cancer as a multifactorial and heterogeneous disease characterized by hallmarks such as sustained proliferative signalling, evasion of apoptosis, angiogenesis, metastasis, and immune escape. These insights have guided the development of targeted therapies and immunotherapies that aim to disrupt specific molecular drivers of tumor progression.
Standard oncologic modalities include surgery, chemotherapy, radiotherapy, targeted therapy, hormone therapy, and immunotherapy (Figure 3). Among these, immunotherapy—especially immune checkpoint inhibitors and CAR-T cell therapy—has significantly transformed the treatment landscape in several malignancies, including melanoma, non-small cell lung cancer, and certain hematologic cancers (Kalia, 2015; Sharma and Allison, 2015; June et al., 2018) Molecular diagnostics and next-generation sequencing have enabled stratification of tumors based on genetic mutations and expression profiles, paving the way for precision oncology (Dienstmann et al., 2017) (Table 4).
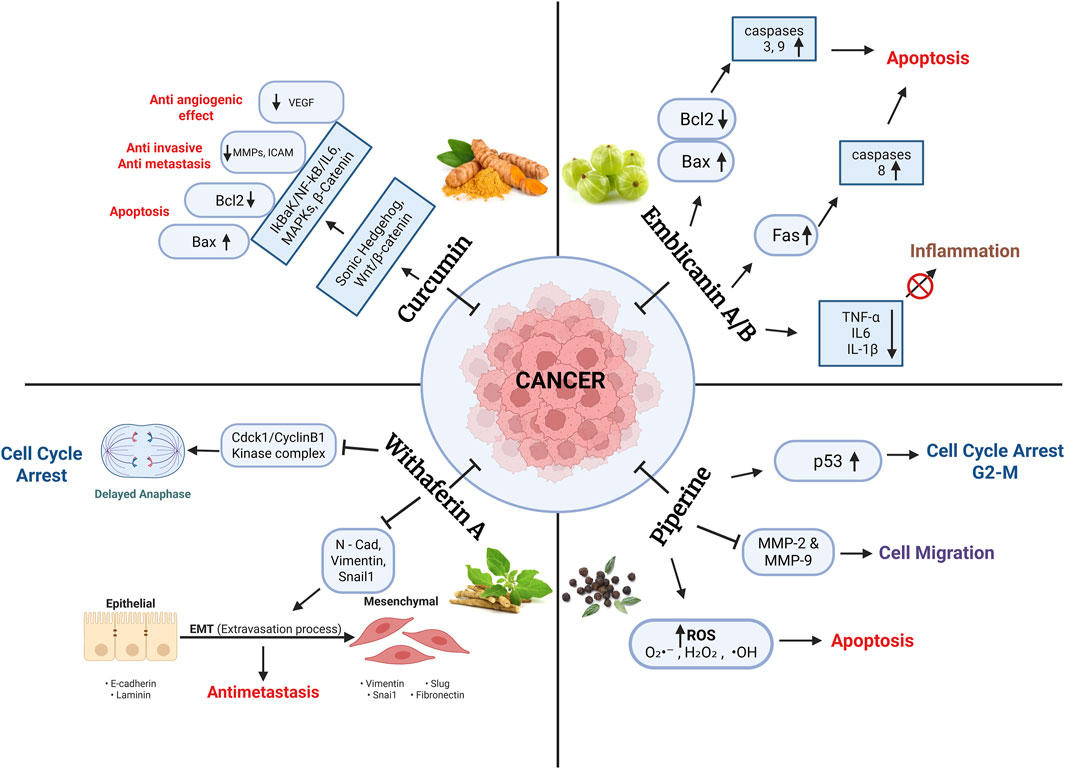
Figure 3. The key bioactive compounds curcumin (from Curcuma longa), Withaferin A (from Withania somnifera), Emblicanin A/B (from Phyllanthus emblica), and Piperine (from Piper nigrum) and their modulation of major oncogenic signaling pathways. These include the regulation of apoptosis (via Bcl-2/Bax and caspase cascades), inhibition of metastasis (via MMPs and EMT markers), suppression of inflammation (via NF-KB, IL-6, TNF-α), induction of oxidative stress (via ROS), and enforcement of cell cycle arrest (via p53 and Cdc2/Cyclin B1). The schematic highlights both shared and distinct targets, demonstrating how Ayurvedic compounds exert multi-targeted actions against various hallmarks of cancer.
However, several challenges continue to impede optimal cancer management. Many cancers remain refractory to existing therapies, and drug resistance both intrinsic and acquired remains a formidable barrier (Holohan et al., 2013). Additionally, conventional treatments often come with significant side effects, including immunosuppression, mucositis, fatigue, and cognitive impairment, which severely impact quality of life (Bower, 2014). Financial toxicity, limited access to care, and disparities in treatment outcomes further complicate cancer care globally.
Another critical gap lies in the integration of holistic, supportive approaches that address the psychosocial, nutritional, and immunological needs of patients. As such, there is a growing interest in complementary and integrative oncology, including non-Western medical systems like Ayurveda, which may offer novel bioactive compounds and person-centred strategies to enhance therapeutic outcomes and patient wellbeing (Deng and Cassileth, 2005; Greenlee et al., 2017).
8 Current research landscape and gaps in evidence
The integration of Ayurvedic medicine into oncology remains an emergent area of scientific inquiry. While preclinical studies have provided compelling evidence for the anticancer potential of various Ayurvedic herbs the majority of these findings are limited to in vitro and animal models (Dar et al., 2015). These studies demonstrate the ability of Ayurvedic botanicals to modulate oncogenic pathways, enhance immunity, and mitigate chemoradiotherapy-induced toxicities. However, translation into clinical practice remains constrained by the lack of rigorous human trials.
Systematic reviews indicate a scarcity of high-quality randomized controlled trials evaluating Ayurvedic formulations in cancer patients (Chattopadhyay et al., 2022). Existing studies often suffer from methodological limitations such as small sample sizes, lack of blinding, heterogeneous interventions, and inconsistent outcome measures. Furthermore, there is minimal standardization in herbal preparations and dosages, complicating reproducibility and cross-comparisons (Lamichhane et al., 2023).
Another key gap lies in the absence of robust pharmacokinetic and pharmacodynamic data for Ayurvedic phytochemicals in human subjects. Bioavailability issues, herb-drug interactions, and safety profiles remain inadequately characterized (Kunnumakkara et al., 2017a). These challenges are further compounded by regulatory ambiguities and the limited inclusion of traditional systems in mainstream research funding and clinical guidelines.
Despite these gaps, interest in integrative oncology is growing. Collaborative research frameworks such as Ayurgenomics and systems biology offer promising avenues for mechanistic exploration and personalized medicine (Patwardhan et al., 2015). The incorporation of advanced omics technologies, artificial intelligence, and standardized protocols in clinical studies could significantly advance the evidence base and bridge the gap between traditional knowledge and modern oncology.
9 Rationale for integrating ayurvedic medicine with contemporary cancer therapeutics
Cancer continues to be one of the most formidable global health challenges, accounting for nearly 10 million deaths in 2022 and with global incidence rates projected to reach 35 million cases annually by 2050 (Chen et al., 2024). While modern cancer treatments including chemotherapy, targeted therapy, immunotherapy, and radiation have led to remarkable advances in survival and tumor control (Vanneman and Dranoff, 2012), they are frequently associated with severe side effects, drug resistance, and high costs (Nikolaou et al., 2018). Moreover, drug resistance and cancer recurrence remain critical hurdles. Many tumors eventually develop resistance to chemotherapy or targeted agents through mutations, altered drug metabolism, or activation of compensatory pathways, leading to treatment failure and poor prognosis which is one of the most pressing challenges in contemporary cancer care. These limitations underscore the necessity for more holistic and personalized therapeutic strategies (Kashid, 2017).
In this context, integrating Ayurveda with modern oncology offers a holistic, multi-dimensional, and patient-centered approach that can fill critical gaps in current cancer care and offers a complementary and integrative approach (Hoenders et al., 2024; Orticelli, 2022; Urban, 2019) aimed at not only supporting the physical body but also balancing mental and emotional health. Ayurvedic interventions majorly include Rasayana therapy, Herbal formulations, Diet and lifestyle modifications, and Mind-body therapies. Rasayana therapy is specialized branch of Ayurveda focused on rejuvenation, immune enhancement, and longevity. Herbs like Withania somnifera (Ashwagandha), Tinospora cordifolia (Guduchi), and Phyllanthus emblica (Amalaki) are known to enhance vitality and resistance to disease. Many Ayurvedic herbs possess anti-inflammatory, antioxidant, hepatoprotective, and adaptogenic properties (Rege et al., 1999; Govindarajan et al., 2005; Lam et al., 2016), which can help mitigate treatment-induced toxicities and improve resilience. Personalized dietary regimens based on Prakriti (constitution) and detoxifying practices (Panchakarma) may support digestion (Agni), reduce oxidative stress, and promote tissue repair. Practices such as Yoga, meditation, and Pranayama have been shown to reduce anxiety, depression, and fatigue, and improve sleep and overall wellbeing in cancer patients.
10 Integrating ayurvedic medicine and modern cancer therapeutics: a multidimensional strategy
The integration of Ayurvedic medicine with conventional oncological therapies represents a paradigm shift in cancer care, offering the potential for synergistic treatment approaches that address not only tumor eradication but also systemic resilience, patient quality of life, and long-term survivorship. As cancer treatment evolves beyond cytotoxicity to include targeted therapy, immunomodulation, and precision medicine, the inclusion of Ayurveda with its emphasis on systemic homeostasis, immune fortification, and individualized care presents a compelling adjunctive framework. This section outlines a multidimensional framework to effectively harmonize Ayurvedic principles with contemporary biomedical oncology, based on emerging clinical evidence, mechanistic understanding, and integrative care models.
10.1 Complementary therapeutic goals: bridging mechanistic and systemic frameworks
Modern oncology primarily targets cellular and molecular pathways associated with carcinogenesis, employing chemotherapy, radiation, immunotherapy, and targeted agents to inhibit tumor proliferation and metastasis. In contrast, Ayurveda aims to restore dosha balance, strengthen agni (digestive/metabolic fire), and enhance ojas (vitality), all of which contribute to systemic immunity and resilience. () The integration of these paradigms allows for the coupling of tumor-targeted cytotoxicity with systemic support, thereby enhancing treatment tolerance and optimizing patient outcomes. For instance, interventions that support gut health, immune competence, and metabolic detoxification can reduce collateral damage from chemotherapeutic agents, improve treatment adherence, and mitigate long-term side effects.
10.2 Evidence-based ayurvedic botanicals: adjuvant potential in cancer therapy
Several Ayurvedic botanicals have demonstrated significant anti-cancer, immunomodulatory, and chemoprotective activities in both preclinical and clinical settings. Curcuma longa (curcumin), a polyphenolic compound, has shown potent anti-inflammatory (Patel, 2010), antioxidant, and radiosensitizing properties (Saggam et al., 2020) through modulation of oncogenic signaling pathways such as NF-κB, STAT3, and COX-2 (Mishra, 2003; Jurenka, 2009; Sak, 2020). Clinical trials have also indicated that curcumin, when used as an adjuvant with chemotherapeutic agents like paclitaxel and cisplatin (Mohankumar et al., 2021), improves chemosensitivity and mitigates systemic side effects. Similarly, Withania somnifera (ashwagandha), known for its adaptogenic and immunomodulatory properties, has shown efficacy in alleviating fatigue and improving the quality of life (Joshi et al., 2021) in cancer patients undergoing chemotherapy (Lee et al., 2005), with one randomized controlled trial reporting significant improvement among breast cancer patients receiving standardized extracts. Additionally, botanicals such as Tinospora cordifolia and Phyllanthus emblica have exhibited hepatoprotective and antioxidant effects, protecting against hepatic damage and oxidative stress associated with chemotherapy. Piper longum, rich in the alkaloid piperine, has demonstrated the capacity to enhance the oral bioavailability of curcumin and other drugs, serving as a bioenhancer (Hussain et al., 2021). However, despite these promising results, the integration of these botanicals into mainstream oncology is hindered by methodological limitations in existing trials, including small sample sizes, lack of placebo controls, insufficient blinding, and inconsistencies in formulations and dosing protocols. Furthermore, inadequate standardization of phytochemical content and quality control impairs reproducibility across studies. These challenges are compounded by the lack of harmonized regulatory frameworks and tailored clinical guidelines for Ayurvedic interventions.
10.3 Management of treatment-induced toxicity: a therapeutic niche for ayurveda
Conventional cancer treatments (Biswal et al., 2013; Saggam et al., 2020) are frequently associated with adverse effects such as mucositis, gastrointestinal toxicity, fatigue, immunosuppression, and cognitive dysfunction, which can compromise therapy adherence and patient wellbeing (Miller et al., 2008; Cavalcanti et al., 2021). Ayurvedic formulations like Triphala, Shatavari (Asparagus racemosus), and classical Rasayana formulations have been studied for their potential to mitigate such toxicities (dos Santos Guimarães et al., 2013; Andreyev et al., 2012). Triphala (Balasubramani et al., 2011) has demonstrated gastroprotective and antioxidant effects in rodent models of chemotherapy-induced mucositis (Akhtar et al., 2024) while Shatavari has shown mucosal healing and immunoregulatory activity (Peterson et al., 2017). Rasayanas, traditionally used for rejuvenation (Baliga, 2010) and convalescence, may support hematopoietic recovery and improve systemic resilience during and after cytotoxic therapy (Ramnath, 2016; Singh et al., 2018). These interventions should be integrated within a protocolized framework, aligned with treatment timelines and toxicity profiles.
10.4 Mind-body integration: enhancing psychoneuroimmunological resilience
Ayurveda’s incorporation of mind-body practices, including yoga, pranayama, meditation, and mantra therapy (Singh and Rastogi, 2011) aligns with modern psychoneuroimmunology (Gaddipati et al., 2004) models of cancer care. Clinical studies have shown that yoga and meditation reduce cortisol levels, enhance natural killer (NK) cell activity, and improve emotional wellbeing among cancer patients (Vyas et al., 2010; Santra, 2022). The integration of these modalities into oncology rehabilitation programs has been associated with improved treatment tolerance, reduced fatigue, and enhanced quality of life. Ayurvedic mind-body interventions can thus be strategically deployed to buffer the psychological stress and immune suppression (Bauer, 1994) commonly observed during cancer therapy, thereby supporting overall treatment outcomes.
10.5 Personalized and precision oncology: aligning prakriti with molecular profiles
One of Ayurveda’s core principles Prakriti analysis, or assessment of individual constitutional types (Fang et al., 2010) offers a promising parallel to modern precision oncology. Recent developments in Ayurgenomics (Giridharan and Bhana, 2024) have revealed correlations between Prakriti types and genetic, transcriptomic, and metabolic profiles, suggesting a potential framework for personalized integrative care (Huang et al., 2022). Pitta types may exhibit inflammatory tendencies and faster metabolism, possibly influencing drug metabolism and toxicity risk. Combining Prakriti assessment with genomic and biomarker-driven stratification may enhance patient selection for both Ayurvedic and biomedical interventions, allowing for more nuanced, responsive, and individualized cancer care strategies (Luhaste, 2023).
10.6 Scientific validation and protocol development: building the evidence base
Despite compelling theoretical and preclinical support, the integration of Ayurveda into mainstream oncology requires rigorous scientific validation (Soni, 2025; Madgulwar and Shewalkar, 2024). This includes standardized preclinical models to elucidate pharmacodynamics and synergistic mechanisms, well-designed randomized controlled trials to evaluate clinical efficacy, and comprehensive pharmacovigilance to assess herb-drug interactions (Mukerji and Prasher, 2011). Institutions such as the All India Institute of Medical Sciences (AIIMS), Tata Memorial Centre, and international collaborators have initiated clinical trials exploring Ayurvedic interventions in breast, colorectal, and head and neck cancers. However, broader institutional adoption requires the development of standardized protocols, validated herbal formulations (Sharma et al., 2024) and consensus guidelines that can be integrated into existing oncological pathways (Balkrishna et al., 2024b).
10.7 Cross-disciplinary collaboration: Building a translational ecosystem
Effective integration hinges on sustained collaboration between Ayurvedic practitioners, oncologists, pharmacologists, and molecular scientists. Such interdisciplinary efforts are essential for designing translational research, co-developing clinical protocols, and training clinicians in integrative care delivery (Mao et al., 2022; Arnold, 2023). Collaborative platforms should also engage regulatory agencies, industry stakeholders, and patient advocacy groups to ensure that integrative oncology evolves as a scientifically robust, ethically governed, and patient-aligned discipline (Mukherjee et al., 2022). Ultimately, this collaborative model will be critical in translating Ayurvedic insights into clinically actionable, evidence-based interventions.
10.8 Synergistic and holistic integration of Ayurvedic Medicine with modern cancer therapeutics
The therapeutic potential of combining Ayurvedic medicine with conventional cancer treatments lies in two interrelated paradigms: synergy and holism. While modern oncologic agents such as taxanes, vinca alkaloids, and platinum compounds focus on targeted cytotoxicity, Ayurvedic interventions emphasize systemic equilibrium, immune modulation, and long-term resilience. Integrating these approaches offers a synergistic effect, wherein Ayurvedic phytochemicals sensitize tumor cells to chemotherapy, mitigate drug resistance, and reduce treatment-related toxicity.
In a phase II randomized, double-blind clinical trial (n = 150), intravenous curcumin combined with paclitaxel significantly improved the objective response rate (ORR: 51% vs. 33%) and reduced fatigue (3 vs. 10 patients; OR 3.7, p = 0.05) compared to paclitaxel alone, without compromising quality of life (Gupta, 2024). Preclinical studies further elucidate that curcumin enhances paclitaxel-induced apoptosis via suppression of NF-κB and activation of caspases, along with modulation of resistance pathways such as PI3K/Akt and STAT3 (Masucci et al., 2025). For instance, curcumin from Curcuma longa has demonstrated synergistic cytotoxicity when combined with paclitaxel and cisplatin, enhancing therapeutic efficacy while reducing systemic inflammation.
Similarly, in an open-label comparative clinical study (n = 100), administration of Withania somnifera (Ashwagandha) extract (2 g every 8 h) during chemotherapy resulted in significantly lower fatigue scores (p < 0.001) and improved functioning across seven domains of the EORTC QLQ-C30 (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30) (Saghatelyan et al., 2020) Although the observed increase in two-year survival (72% vs. 56%) did not reach statistical significance, the trend supports Ashwagandha’s value in enhancing patient resilience and quality of life during cancer treatment. Withaferin A, a key bioactive constituent, not only potentiates apoptosis in malignant cells but also protects normal tissues from oxidative damage. These effects are mediated through modulation of molecular targets such as NF-κB, PI3K/Akt, and p53, demonstrating the potential of multi-targeted co-therapy in integrative oncology.
Beyond pharmacological synergy, the holistic framework of Ayurveda addresses psycho-oncological dimensions often overlooked in conventional regimens. Rasayana therapies, yoga, pranayama, and personalized dietary interventions based on Prakriti contribute to enhanced mental clarity, immune homeostasis, and overall wellbeing. Clinical studies have reported significant reductions in anxiety, improvements in sleep quality, and enhanced psychological resilience among cancer patients participating in Ayurvedic mind-body interventions (Mustian et al., 2013; Calaf et al., 2018).
The outcomes of such integrative approaches include:
• Enhanced treatment tolerance and reduced drop-out rates during chemotherapy,
• Improved functional status and immune competence,
• Reduced tumor recurrence through sustained metabolic and inflammatory regulation,
• Increased patient empowerment and satisfaction through personalized, constitution-based care.
These preliminary observations provide a compelling rationale for the development of future clinical trials exploring standardized botanical extracts as adjuvants to chemotherapy and immunotherapy. Systems biology and network pharmacology approaches can facilitate the identification of synergistic interactions, while Ayurgenomic profiling may enable patient stratification for personalized integrative care. Collectively, these efforts can pave the way toward the establishment of evidence-based integrative oncology protocols, forming a scientifically validated alliance between Ayurveda and modern cancer therapeutics.
11 Discussion
The convergence of Ayurvedic medicine and modern oncology represents a transformative approach to cancer care one that emphasizes not only tumor eradication but also patient-centered healing, resilience, and quality of life. This review underscores the complementary strengths of these two paradigms: modern oncology offers precision, rapid disease control, and evidence-based pharmacology, while Ayurveda contributes holistic insights into disease causation, constitutional variability, detoxification, and immune fortification (Figure 4).
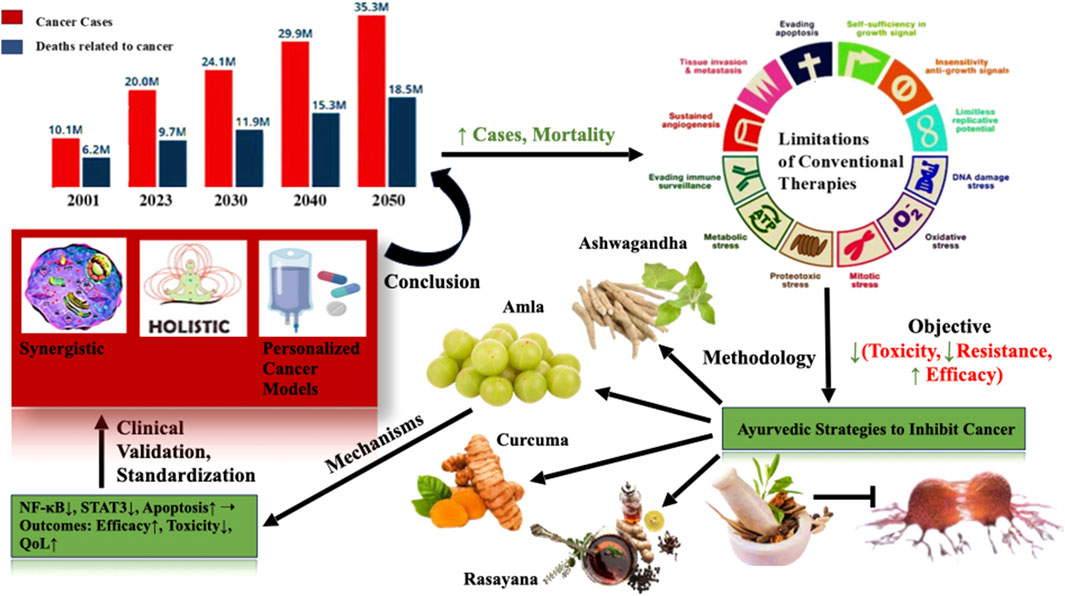
Figure 4. Integrative ayurvedic strategies to inhibit cancer progression: A holistic and mechanistic overview. The figure summarizes rising global cancer incidence and the limitations of conventional therapies, It highlights Ayurvedic interventions such as Curcuma longa, Withania somnifera, Phyllanthus emblica, and Rasayana formulations and their potential to modulate key cancer pathways (e.g., NF-κB, STAT3, apoptosis). Through systems biology and reverse pharmacology, these botanicals offer promising avenues for synergistic, personalized, and low-toxicity cancer management.
Numerous phytochemicals and classical Ayurvedic formulations have shown preclinical and limited clinical promise in reducing tumor burden, enhancing immune function, mitigating therapy-induced toxicity, and improving psychosocial wellbeing. Techniques such as Rasayana therapy, polyherbal synergy, and personalized Prakriti-based interventions are especially valuable for long-term survivorship and palliative care.
However, significant limitations remain. The lack of large-scale, randomized clinical trials, insufficient standardization of herbal products, poor bioavailability of key phytochemicals, and limited mechanistic validation of traditional formulations hinder mainstream integration. Furthermore, interdisciplinary collaboration between oncologists, Ayurveda practitioners, and biomedical researchers is still nascent and requires institutional support.
Future directions must prioritize robust clinical trial designs that evaluate integrative regimens alongside standard therapies. Systems biology and network pharmacology approaches should be advanced to decode multi-target effects. Recent Nano-delivery systems and bio enhancers can be optimize the pharmacokinetics of herbal drugs. Development of regulatory and educational frameworks that uphold safety, efficacy, and patient autonomy should take authority for the same for smooth conduct. Ultimately, an integrative oncology model rooted in scientific rigor and traditional wisdom can improve patient outcomes not merely in terms of survival, but also in enhancing quality of life, reducing therapy-related distress, and empowering individuals through personalized, holistic care.
Author contributions
SJ: Methodology, Writing – review and editing, Writing – original draft, Conceptualization. NS: Methodology, Writing – review and editing, Writing – original draft. OS: Writing – review and editing, Methodology, Writing – original draft. IA: Writing – review and editing. JB: Writing – review and editing. VH: Conceptualization, Funding acquisition, Writing – review and editing, Supervision. RT: Conceptualization, Writing – review and editing, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by All India institute of Ayurveda (AIIA), New Delhi Ministry of Ayush.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AJ declared a shared affiliation with the authors at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abal, M. A. J. M., Andreu, J. M., and Barasoain, I. (2003). Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr. cancer drug targets 3 (3), 193–203. doi:10.2174/1568009033481967
Abebe, W. (2019). Review of herbal medications with the potential to cause bleeding: dental implications, and risk prediction and prevention avenues. EPMA J. 10, 51–64. doi:10.1007/s13167-018-0158-2
Aggarwal, B. B., Kumar, A., and Bharti, A. C. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23 (1/A), 363–398.
Ahmed Khalil, A., Rauf, A., Alhumaydhi, F. A., Aljohani, A. S., Javed, M. S., Khan, M. A., et al. (2022). Recent developments and anticancer therapeutics of paclitaxel: an update. Curr. Pharm. Des. 28 (41), 3363–3373. doi:10.2174/1381612829666221102155212
Ahn, H., Im, E., Lee, D. Y., Lee, H. J., Jung, J.-H., and Kim, S. H. (2019). Antitumor effect of pyrogallol via miR-134 mediated S phase arrest and inhibition of PI3K/AKT/Skp2/cMyc signaling in hepatocellular carcinoma. Int. J. Mol. Sci. 20, 3985. doi:10.3390/ijms20163985
Akhtar, S., Gupta, A. K., Naik, B., Kumar, V., Ranjan, R., Jha, A. K., et al. (2024). Exploring pharmacological properties and food applications of Asparagus racemosus (shatavari). Food Chem. Adv. 4, 100689. doi:10.1016/j.focha.2024.100689
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm. 4 (6), 807–818. doi:10.1021/mp700113r
Andreyev, H. J. N., Davidson, S. E., Gillespie, C., Allum, W. H., and Swarbrick, E. (2012). Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 61 (2), 179–192. doi:10.1136/gutjnl-2011-300563
Arnold, J. T. (2023). Integrating ayurvedic medicine into cancer research programs part 1: ayurveda background and applications. J. Ayurveda Integr. Med. 14 (2), 100676. doi:10.1016/j.jaim.2022.100676
Ashokkumar, K., Murugan, M., Dhanya, M. K., Pandian, A., and Warkentin, T. D. (2021). Phytochemistry and therapeutic potential of black pepper [piper nigrum (L.)] essential oil and piperine: a review. Clin. Phytoscience 7 (1), 52. doi:10.1186/s40816-021-00292-2
Bahrami, A., Mohammadifard, M., Rajabi, Z., Motahari-Nasab, M., and Ferns, G. A. (2022). Effects of curcumin-piperine supplementation on systemic immunity in young women with premenstrual syndrome and dysmenorrhea: a randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 278, 131–136. doi:10.1016/j.ejogrb.2022.09.021
Balasubramani, S. P., Venkatasubramanian, P., Kukkupuni, S. K., and Patwardhan, B. (2011). Plant-based rasayana drugs from ayurveda. Chin. J. Integr. Med. 17 (2), 88–94. doi:10.1007/s11655-011-0659-5
Baliga, M. S. (2010). Triphala, ayurvedic formulation for treating and preventing cancer: a review. J. Altern. Complementary Med. 16 (12), 1301–1308. doi:10.1089/acm.2009.0633
Balkrishna, A., Kumud, S. K., Goswami, P., and Arya, V. (2024a). An in-depth exploration of ethnomedicinal applications, phytochemical profiles and pharmacological potentials: unveiling Withania somnifera. HORIZON 2 (4), 10–17. doi:10.14719/tcb.4431
Balkrishna, A., Sharma, N., Srivastava, D., Kukreti, A., Srivastava, S., and Arya, V. (2024b). Exploring the safety, efficacy, and bioactivity of herbal medicines: bridging traditional wisdom and modern science in healthcare. Future Integr. Med. 3 (1), 35–49. doi:10.14218/fim.2023.00086
Banerjee, J., Samanta, S., Ahmed, R., and Dash, S. K. (2023). Bioactive pentacyclic triterpenes trigger multiple signalling pathways for selective apoptosis leading to anticancer efficacy: recent updates and future perspectives. Curr. Protein Peptide Sci. 24 (10), 820–842. doi:10.2174/1389203724666230418123409
Bauer, S. M. (1994). Psychoneuroimmunology and cancer: an integrated review. J. Adv. Nurs. 19 (6), 1114–1120. doi:10.1111/j.1365-2648.1994.tb01195.x
Bhaskar, M., Telessy, I. G., and Buttar, H. S. (2022). The importance of drug dose adjustment in elderly patients with special considerations for patients on diverse co-medications and antidepressants. Biomed. Transl. Res. Drug Des. Discov., 231–272. doi:10.1007/978-981-16-9232-1_15
Bhati, R., Zadeng, H., Singh, E., Kumar, A., Jain, M., Senthil Kumaran, J., et al. (2025). Molecular dynamics simulations assisted investigation of phytochemicals as potential lead candidates against anti-apoptotic Bcl-B protein. J. Biomol. Struct. Dyn. 43 (6), 3049–3063. doi:10.1080/07391102.2023.2295385
Biswal, B. M., Sulaiman, S. A., Ismail, H. C., Zakaria, H., and Musa, K. I. (2013). Effect of Withania somnifera (ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr. cancer Ther. 12 (4), 312–322. doi:10.1177/1534735412464551
Bower, J. E. (2014). Cancer-related Fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 11 (10), 597–609. doi:10.1038/nrclinonc.2014.127
Calaf, G. M., Ponce-Cusi, R., and Carrión, F. (2018). Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 40 (4), 2381–2388. doi:10.3892/or.2018.6603
Carmi, C., Mor, M., Petronini, P. G., and Alfieri, R. R. (2012). Clinical perspectives for irreversible tyrosine kinase inhibitors in cancer. Biochem. Pharmacol. 84 (11), 1388–1399. doi:10.1016/j.bcp.2012.07.031
Cavalcanti, I. D. L., Soares, J. C. S., Cavalcanti, I. D. L., and Soares, J. C. S. (2021). “Conventional cancer treatment,” in Advances in cancer treatment: from systemic chemotherapy to targeted therapy, 29–56.
Chatterjee, B., and Pancholi, J. (2011). Prakriti-based medicine: a step towards personalized medicine. AYU (An International Quarterly Journal of Research in Ayurveda) 32 (2), 141–146. doi:10.4103/0974-8520.92539
Chattopadhyay, K., Wang, H., Kaur, J., Nalbant, G., Almaqhawi, A., Kundakci, B., et al. (2022). Effectiveness and safety of Ayurvedic medicines in type 2 diabetes mellitus management: a systematic review and meta-analysis. Frontiers in pharmacology 13, 821810. doi:10.3389/fphar.2022.821810
Chen, J., Cui, Y., Deng, Y., Xiang, Y., Chen, J., Wang, Y., et al. (2024). Global, regional, and national burden of cancers attributable to particulate matter pollution from 1990 to 2019 and projection to 2050: worsening or improving? Journal of hazardous materials 477, 135319. doi:10.1016/j.jhazmat.2024.135319
Crawford, S. (2013). Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Frontiers in pharmacology 4, 68. doi:10.3389/fphar.2013.00068
Dar, N. J., Hamid, A., and Ahmad, M. (2015). Pharmacologic overview of Withania somnifera, the Indian ginseng. Cellular and molecular life sciences 72, 4445–4460. doi:10.1007/s00018-015-2012-1
Das, L., and Vinayak, M. (2014). Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PloS one 9 (6), e99583. doi:10.1371/journal.pone.0099583
Das, T., Roy, K. S., Chakrabarti, T., Mukhopadhyay, S., and Roychoudhury, S. (2014). Withaferin A modulates the spindle assembly checkpoint by degradation of Mad2–Cdc20 complex in colorectal cancer cell lines. Biochem. Pharmacol 91, 31–39. doi:10.1016/j.bcp.2014.06.022
Dearnaley, D. P., Horwich, A., A'Hern, R., Nicholls, J., Jay, G., Hendry, W. F., et al. (1991). Combination chemotherapy with bleomycin, etoposide and cisplatin (BEP) for metastatic testicular teratoma: long-term follow-up. European Journal of Cancer and Clinical Oncology 27 (6), 684–691. doi:10.1016/0277-5379(91)90166-b
Deng, G., and Cassileth, B. R. (2005). Integrative oncology: complementary therapies for pain, anxiety, and mood disturbance. CA a cancer journal for clinicians 55 (2), 109–116. doi:10.3322/canjclin.55.2.109
Dhyani, P., Quispe, C., Sharma, E., Bahukhandi, A., Sati, P., Attri, D. C., et al. (2022). Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer cell international 22 (1), 206. doi:10.1186/s12935-022-02624-9
Dienstmann, R., Vermeulen, L., Guinney, J., Kopetz, S., Tejpar, S., and Tabernero, J. (2017). Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nature reviews cancer 17 (2), 79–92. doi:10.1038/nrc.2016.126
Di Meo, F., Filosa, S., Madonna, M., Giello, G., Di Pardo, A., Maglione, V., et al. (2019). Curcumin C3 complex®/Bioperine® has antineoplastic activity in mesothelioma: an in vitro and in vivo analysis. J Exp Clin Cancer Res 38 (1), 360. doi:10.1186/s13046-019-1368-8
Divya, C. S., and Pillai, M. R. (2006). Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Molecular Carcinogenesis 45 (5), 320–332. doi:10.1002/mc.20170
dos Santos Guimarães, I., Daltoé, R. D., Herlinger, A. L., Madeira, K. P., Ladislau, T., Valadão, I. C., et al. (2013). “Conventional cancer treatment,” in Cancer treatment-conventional and innovative approaches (Brazil: IntechOpen).
Dutta, R., Khalil, R., Green, R., Mohapatra, S. S., and Mohapatra, S. (2019). Withania somnifera (ashwagandha) and withaferin A: potential in integrative oncology. International journal of molecular sciences 20 (21), 5310. doi:10.3390/ijms20215310
Fang, C. Y., Reibel, D. K., Longacre, M. L., Rosenzweig, S., Campbell, D. E., and Douglas, S. D. (2010). Enhanced psychosocial well-being following participation in a mindfulness-based stress reduction program is associated with increased natural killer cell activity. The Journal of Alternative and Complementary Medicine 16 (5), 531–538. doi:10.1089/acm.2009.0018
Fofaria, N. M., and Srivastava, S. K. (2015). STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis 36, 142–150. doi:10.1093/carcin/bgu233
Folprecht, G., Lutz, M. P., Schöffski, P., Seufferlein, T., Nolting, A., Pollert, P., et al. (2006). Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Annals of Oncology 17 (3), 450–456. doi:10.1093/annonc/mdj084
Gaddipati, J. P., Rajeshkumar, N. V., Thangapazham, R. L., Sharma, A., Warren, J., Mog, S. R., et al. (2004). Protective effect of a polyherbal preparation, brahma rasayana against tumor growth and lung metastasis in rat prostate model system. Journal of experimental therapeutics and oncology 4 (3).
Gallo, R. C., Whang-Peng, J., and Adamson, R. H. (1971). Studies on the antitumor activity, mechanism of action, and cell cycle effects of camptothecin. Journal of the National Cancer Institute 46 (4), 789–795.
Garvey, D. R., Chhabra, G., Ndiaye, M. A., and Ahmad, N. (2021). Role of polo-like kinase 4 (PLK4) in epithelial cancers and recent progress in its small molecule targeting for cancer management. Molecular cancer therapeutics 20 (4), 632–640. doi:10.1158/1535-7163.mct-20-0741
Ghobrial, I. M., Witzig, T. E., and Adjei, A. A. (2005). Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 55 (3), 178–194. doi:10.3322/canjclin.55.3.178
Giridharan, S., and Bhana, R. (2024). The impact of yoga on the immune system of cancer patients: a scoping review of current evidence. Int Res J Ayurveda Yoga 7 (2), 33–40. doi:10.48165/irjay.2024.70207
Gokduman, K. (2016). Strategies targeting DNA topoisomerase I in cancer chemotherapy: camptothecins, nanocarriers for camptothecins, organic non-camptothecin compounds and metal complexes. Current drug targets 17 (16), 1928–1939. doi:10.2174/1389450117666160502151707
Govindarajan, R., Vijayakumar, M., and Pushpangadan, P. (2005). Antioxidant approach to disease management and the role of ‘rasayana’herbs of ayurveda. Journal of ethnopharmacology 99 (2), 165–178. doi:10.1016/j.jep.2005.02.035
Greenlee, H., DuPont-Reyes, M. J., Balneaves, L. G., Carlson, L. E., Cohen, M. R., Deng, G., et al. (2017). Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA a cancer journal for clinicians 67 (3), 194–232. doi:10.3322/caac.21397
Greenshields, A. L., Doucette, C. D., Sutton, K. M., Madera, L., Annan, H., Yaffe, P. B., et al. (2015). Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett 357, 129–140. doi:10.1016/j.canlet.2014.11.017
Guerram, M., Jiang, Z. Z., and Zhang, L. Y. (2012). Podophyllotoxin, a medicinal agent of plant origin: past, present and future. Chinese Journal of Natural Medicines 10 (3), 161–169. doi:10.3724/sp.j.1009.2012.00161
Gul, M., Liu, Z. W., Rabail, R., Faheem, F., Walayat, N., Nawaz, A., et al. (2022). Functional and nutraceutical significance of amla (Phyllanthus emblica L.): a review. Antioxidants 11 (5), 816. doi:10.3390/antiox11050816
Gupta, R. (2024). Integration of alternative and complementary medicine with modern Western medicine for enhanced patient care. International Journal of Clinical Case Reports and Reviews 20 (2), 01–09. doi:10.31579/2690-4861/582
Habtemariam, S. (2020). Trametes versicolor (synn. Coriolus versicolor) polysaccharides in cancer therapy: targets and efficacy. Biomedicines 8 (5), 135. doi:10.3390/biomedicines8050135
Hande, K. R. (1998). Etoposide: four decades of development of a topoisomerase II inhibitor. European journal of cancer 34 (10), 1514–1521. doi:10.1016/s0959-8049(98)00228-7
Haq, I. U., Imran, M., Nadeem, M., Tufail, T., Gondal, T. A., and Mubarak, M. S. (2021). Piperine: a review of its biological effects. Phytotherapy research 35 (2), 680–700. doi:10.1002/ptr.6855
Herbst, R. S., and Khuri, F. R. (2003). Mode of action of docetaxel–a basis for combination with novel anticancer agents. Cancer treatment reviews 29 (5), 407–415. doi:10.1016/s0305-7372(03)00097-5
Hoenders, R., Ghelman, R., Portella, C., Simmons, S., Locke, A., Cramer, H., et al. (2024). A review of the WHO strategy on traditional, complementary, and integrative medicine from the perspective of academic consortia for integrative medicine and health. Frontiers in medicine 11, 1395698. doi:10.3389/fmed.2024.1395698
Holohan, C., Van Schaeybroeck, S., Longley, D. B., and Johnston, P. G. (2013). Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer 13 (10), 714–726. doi:10.1038/nrc3599
Huang, F., Ke, P., and Huang, M. (2023). Directed acyclic transformer pre-training for high-quality non-autoregressive text generation. Transactions of the Association for Computational Linguistics 11, 941–959. doi:10.1162/tacl_a_00582
Huang, W. Y., Cai, Y. Z., and Zhang, Y. (2009). Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutrition and cancer 62 (1), 1–20. doi:10.1080/01635580903191585
Huang, Z., Chavda, V. P., Bezbaruah, R., Uversky, V. N., P, S., Patel, A. B., et al. (2022). An ayurgenomics approach: prakriti-based drug discovery and development for personalized care. Frontiers in Pharmacology 13, 866827. doi:10.3389/fphar.2022.866827
Hussain, Y., Islam, L., Khan, H., Filosa, R., Aschner, M., and Javed, S. (2021). Curcumin–cisplatin chemotherapy: a novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytotherapy Research 35 (12), 6514–6529. doi:10.1002/ptr.7225
Hussein, S., Elmosallamy, A., Abdel-Hamid, N., and Srour, L. (2020). Identification of polyphenolic compounds and hepatoprotective activity of artichoke (Cynara scolymus L.) edible part extracts in rats. Egypt J Chem 63, 6–9. doi:10.21608/ejchem.2020.22707.2348
Im, E., Yeo, C., and Lee, E.-O. (2018). Luteolin induces caspase-dependent apoptosis via inhibiting the AKT/osteopontin pathway in human hepatocellular carcinoma SK-Hep-1 cells. Life Sci 209, 259–266. doi:10.1016/j.lfs.2018.08.025
Jafri, A., Siddiqui, S., Rais, J., Ahmad, M. S., Kumar, S., Jafar, T., et al. (2019). Induction of apoptosis by piperine in human cervical adenocarcinoma via ROS mediated mitochondrial pathway and caspase-3 activation. EXCLI J 18, 154–164. doi:10.17179/excli2018-1928
Jemal, A., Center, M. M., DeSantis, C., and Ward, E. M. (2010). Global patterns of cancer incidence and mortality rates and trends. Cancer epidemiology, biomarkers and prevention 19 (8), 1893–1907. doi:10.1158/1055-9965.epi-10-0437
Jendrossek, V. (2013). Targeting apoptosis pathways by celecoxib in cancer. Cancer letters 332 (2), 313–324. doi:10.1016/j.canlet.2011.01.012
Jordan, B. C., Mock, C. D., Thilagavathi, R., and Selvam, C. (2016). Molecular mechanisms of curcumin and its semisynthetic analogues in prostate cancer prevention and treatment. Life Sciences 152, 135–144. doi:10.1016/j.lfs.2016.03.036
Jordan, M. A. (2002). Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Current Medicinal Chemistry-Anti-Cancer Agents 2 (1), 1–17. doi:10.2174/1568011023354290
Joshi, P., Joshi, S., Semwal, D., Bisht, A., Paliwal, S., Dwivedi, J., et al. (2021). Curcumin: an insight into molecular pathways involved in anticancer activity. Mini reviews in medicinal chemistry 21 (17), 2420–2457. doi:10.2174/1389557521666210122153823
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S., and Milone, M. C. (2018). CAR T cell immunotherapy for human cancer. Science 359 (6382), 1361–1365. doi:10.1126/science.aar6711
Jurenka, J. S. (2009). Anti-inflammatory properties of curcumin, a major constituent of curcuma longa: a review of preclinical and clinical research. Alternative medicine review 14 (2).
Kalachaveedu, M., Senthil, R., Azhagiyamanavalan, S., Ravi, R., Meenakshisundaram, H., and Dharmarajan, A. (2023). Traditional medicine herbs as natural product matrices in cancer chemoprevention: a trans pharmacological perspective (scoping review). Phytotherapy Research 37 (4), 1539–1573. doi:10.1002/ptr.7747
Kalia, M. (2015). Biomarkers for personalized oncology: recent advances and future challenges. Metabolism 64 (3), S16–S21. doi:10.1016/j.metabol.2014.10.027
Kashid, V. A. (2017). Current management approach of cancer in ayurveda. Journal of Ayurveda and Integrated Medical Sciences 2 (03), 113–120. doi:10.21760/jaims.v2i3.8218
Kasi, P. D., Tamilselvam, R., Skalicka-Wozniak, K., Nabavi, S. F., Daglia, M., Bishayee, A., et al. (2016). Molecular targets of curcumin for cancer therapy: an updated review. Tumor Biology 37 (10), 13017–13028. doi:10.1007/s13277-016-5183-y
Kaur, H., He, B., Zhang, C., Rodriguez, E., Hage, D. S., and Moreau, R. (2018). Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: implications for the inhibition of protein synthesis and TNFα signaling. J Nutr Biochem. 57, 276–286. doi:10.1016/j.jnutbio.2018.04.010
Kaur, R., Bhardwaj, A., and Gupta, S. (2023). Cancer treatment therapies: traditional to modern approaches to combat cancers. Molecular biology reports 50 (11), 9663–9676. doi:10.1007/s11033-023-08809-3
Kaushik, S., Masand, N., Iyer, M. R., and Patil, V. M. (2023). Preclinical to clinical profile of Curcuma longa as antidiabetic therapeutics. Current Topics in Medicinal Chemistry 23 (24), 2267–2276. doi:10.2174/1568026623666230428101440
Kim, W., Ye, Z., Simonenko, V., Shahi, A., Malikzay, A., Long, S. Z., et al. (2024). Codelivery of TGFβ and Cox2 siRNA inhibits HCC by promoting T-cell penetration into the tumor and improves response to immune checkpoint inhibitors. NAR Cancer 6 (1), zcad059. doi:10.1093/narcan/zcad059
Kizhakkeveettil, A., Parla, J., Patwardhan, K., Sharma, A., and Sharma, S. (2024). “History, present and prospect of ayurveda,” in History, present and prospect of world traditional medicine, 1–72.
Kunnumakkara, A. B., Bordoloi, D., Harsha, C., Banik, K., Gupta, S. C., and Aggarwal, B. B. (2017a). Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clinical science 131 (15), 1781–1799. doi:10.1042/cs20160935
Kunnumakkara, A. B., Bordoloi, D., Padmavathi, G., Monisha, J., Roy, N. K., Prasad, S., et al. (2017b). Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. British Journal of Pharmacology 174 (11), 1325–1348. doi:10.1111/bph.13621
Kurmi, S. P. C., Thapa, S., and Karati, D. (2025). Molecular docking and pharmacokinetic evaluations of curcumin-based scaffolds as MDM2-p53 inhibitors. Discov. Chem. 2, 53. doi:10.1007/s44371-025-00128-9
Lai, L. H., Fu, Q. H., Liu, Y., Jiang, K., Guo, Q. m., Chen, Q. y., et al. (2012). Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin 33 (4), 523–530. doi:10.1038/aps.2011.209
Lam, P., Cheung, F., Tan, H. Y., Wang, N., Yuen, M. F., and Feng, Y. (2016). Hepatoprotective effects of Chinese medicinal herbs: a focus on anti-inflammatory and anti-oxidative activities. International Journal of Molecular Sciences 17 (4), 465. doi:10.3390/ijms17040465
Lamichhane, S., Sahariah, B. J., Das, B., Khatiwara, D., Sola, P., Adhikari, R. P., et al. (2023). Herbal drug standardization: a systemic review. Journal of Drug Delivery and Therapeutics 13 (4), 149–153. doi:10.22270/jddt.v13i4.6012
Lee, D. H., Lim, I.-H., Sung, E. –G., Kim, J. –Y., Song, I.-H., Park, Y. K., et al. (2013). Withaferin A inhibits matrix metalloproteinase-9 activity by suppressing the akt signaling pathway. Oncol Rep 30, 933–938. doi:10.3892/or.2013.2487
Lee, J., Hahm, E. R., Marcus, A. I., and Singh, S. V. (2015). Withaferin A inhibits experimental epithelial–mesenchymal transition in MCF-10A cells and suppresses vimentin protein level in vivo in breast tumors. Mol Carcinog 54, 417–429. doi:10.1002/mc.22110
Lee, J., Im, Y. H., Jung, H. H., Kim, J. H., Park, J. O., Kim, K., et al. (2005). Curcumin inhibits interferon-α induced NF-κB and COX-2 in human A549 non-small cell lung cancer cells. Biochemical and biophysical research communications 334 (2), 313–318. doi:10.1016/j.bbrc.2005.06.093
Li, S., Lei, Y., Jia, Y., Li, N., Wink, M., and Ma, Y. (2011). Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 19 (1), 83–87. doi:10.1016/j.phymed.2011.06.031
Liliana, M., Rusu, T., Wang, T. Y., Park, J. B., and Clapa, D. (2017). Soy-derived microbial metabolite equol in cancer prevention. Agric. Sci. Pract, 1–2 (101-102).
Lim, T. K., and Lim, T. K. (2012). Phyllanthus emblica. Edible medicinal and non-medicinal plants. Fruits Vol. 4, 258–296. doi:10.1007/978-94-017-9511-1
Lin, Y., Xu, J., Liao, H., Li, L., and Pan, L. (2014). Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumor Biol 35, 3305–3310. doi:10.1007/s13277-013-1433-4
Logie, E., and Vanden Berghe, W. (2020). Tackling chronic inflammation with withanolide phytochemicals—A withaferin a perspective. Antioxidants 9 (11), 1107. doi:10.3390/antiox9111107
Lopez-Lopez, E., Gutierrez-Camino, A., Astigarraga, I., Navajas, A., Echebarria-Barona, A., Garcia-Miguel, P., et al. (2016). Vincristine pharmacokinetics pathway and neurotoxicity during early phases of treatment in pediatric acute lymphoblastic leukemia. Pharmacogenomics 17 (7), 731–741. doi:10.2217/pgs-2016-0001
Luhaste, V. (2023). Maharishi ayurveda integrated approach to mental health: the effect of maharishi AyurVeda treatments for anxiety and depression symptoms. A mixed methods study within a whole system research project (WSR). Iowa: Maharishi University of Management.
Ma, Z., Wang, N., He, H., and Tang, X. (2019). Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. Journal of Controlled Release 316, 359–380. doi:10.1016/j.jconrel.2019.10.053
Madgulwar, Y. D., and Shewalkar, K. J. (2024). The intersection of ayurveda and genomics: exploring ayurgenomics for personalized health solutions. Journal of Ayurveda and Integrated Medical Sciences 9 (10), 168–177. doi:10.21760/jaims.9.10.28
Malik, S., Suchal, K., Bhatia, J., Khan, S. I., Vasisth, S., Tomar, A., et al. (2016). Therapeutic potential and molecularmechanisms of EmblicaofficinalisGaertn in counteringnephrotoxicity in ratsinduced by the chemotherapeuticagentcisplatin. Front Pharmacol 7, 350. doi:10.3389/fphar.2016.00350
Manayi, A., Nabavi, S. M., Setzer, W. N., and Jafari, S. (2018). Piperine as a potential anti-cancer agent: a review on preclinical studies. Curr Med Chem 25 (37), 4918–4928. doi:10.2174/0929867324666170523120656
Manohar, P. R. (2015). Descriptions and classification of cancer in the classical Ayurvedic texts. Indian Journal of History of Science 50, 187–195. doi:10.16943/ijhs/2015/v50i2/48234
Mao, J. J., Pillai, G. G., Andrade, C. J., Ligibel, J. A., Basu, P., Cohen, L., et al. (2022). Integrative oncology: addressing the global challenges of cancer prevention and treatment. CA a cancer journal for clinicians 72 (2), 144–164. doi:10.3322/caac.21706
Masucci, M., Del Villar Pérez, J., Mazzocato, P., Ernberg, I., and Brommels, M. (2025). Implementing personalized cancer medicine: insights from a qualitative interview study. Journal of Personalized Medicine 15 (4), 150. doi:10.3390/jpm15040150
Mehmood, M. H., and Gilani, A. H. (2010). Pharmacological basis for the medicinal use of Black pepper and piperine in gastrointestinal disorders. Journal of medicinal food 13 (5), 1086–1096. doi:10.1089/jmf.2010.1065
Miller, S., Stagl, J., Wallerstedt, D. B., Ryan, M., and Mansky, P. J. (2008). Botanicals used in complementary and alternative medicine treatment of cancer: clinical science and future perspectives. Expert Opinion on Investigational Drugs 17 (9), 1353–1364. doi:10.1517/13543784.17.9.1353
Mimeault, M., and Batra, S. K. (2011). Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chinese Medicine 6 (1), 31. doi:10.1186/1749-8546-6-31
Mohankumar, K., Francis, A. P., Pajaniradje, S., and Rajagopalan, R. (2021). Synthetic curcumin analog: inhibiting the invasion, angiogenesis, and metastasis in human laryngeal carcinoma cells via NF-kB pathway. Molecular Biology Reports 48 (8), 6065–6074. doi:10.1007/s11033-021-06610-8
Mondal, A., Banerjee, S., Terang, W., Bishayee, A., Zhang, J., Ren, L., et al. (2024). Capsaicin: a chili pepper bioactive phytocompound with a potential role in suppressing cancer development and progression. Phytotherapy Research 38 (3), 1191–1223. doi:10.1002/ptr.8107
Mukerji, M., and Prasher, B. (2011). Ayurgenomics: a new approach in personalized and preventive medicine. Sci Cult 77 (1-2), 10–17.
Mukherjee, P. K., Banerjee, S., Gupta, B. D., and Kar, A. (2022). “Evidence-based validation of herbal medicine: translational approach,” in Evidence-based validation of herbal medicine (Elsevier), 1–41.
Mustian, K. M., Sprod, L. K., Janelsins, M., Peppone, L. J., Palesh, O. G., Chandwani, K., et al. (2013). Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J. Clinical Oncology 31 (26), 3233–3241. doi:10.1200/jco.2012.43.7707
Nair, D. K., and Ashwini, B. N. (2019). AYUSH cancer conclave 2019 accepted posters papers with abstracts. AYU (An International Quarterly Journal of Research in Ayurveda) 40 (Suppl. 1), S35–S64.
Naseer, F., Ahmed, M., Majid, A., Kamal, W., and Phull, A. R. (2022). “Green nanoparticles as multifunctional nanomedicines: insights into anti-inflammatory effects, growth signaling and apoptosis mechanism in cancer,” Seminars in Cancer Biology 86 310–324. doi:10.1016/j.semcancer.2022.06.014
Nikolaou, M., Pavlopoulou, A., Georgakilas, A. G., and Kyrodimos, E. (2018). The challenge of drug resistance in cancer treatment: a current overview. Clinical and Experimental Metastasis 35, 309–318. doi:10.1007/s10585-018-9903-0
Nobili, S., Landini, I., Mazzei, T., and Mini, E. (2012). Overcoming tumor multidrug resistance using drugs able to evade p-glycoprotein or to exploit its expression. Medicinal research reviews 32 (6), 1220–1262. doi:10.1002/med.20239
Olarewaju, O. O., Fajinmi, O. O., Naidoo, K. K., Arthur, G. D., and Coopoosamy, R. M. (2022). A review of the medicinal plants with immune-boosting potential. Journal of Medicinal Plants for Economic Development 6 (1), 158. doi:10.4102/jomped.v6i1.158
Ong, C. P., Lee, W. L., Tang, Y. Q., and Yap, W. H. (2019). Honokiol: a review of its anticancer potential and mechanisms. Cancers 12 (1), 48. doi:10.3390/cancers12010048
Orticelli, B. (2022). Integrating prayer and faith in complementary alternative medicine into treating breast cancer: lived experiences.
Ouyang, D.-Y., Zeng, L. h., Pan, H., Xu, L. h., Wang, Y., Liu, K. p., et al. (2013). Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 60, 424–430. doi:10.1016/j.fct.2013.08.007
Patel, M. R. (2010). Applying the knowledge of ayurveda to appraise the US nutritional paradigm. City, CA: California College of Ayurveda, Nevada.
Patwardhan, B., Mutalik, G., and Tillu, G. (2015). Integrative approaches for health: biomedical research, ayurveda and yoga. Academic Press.
Pawar, K. S., Mastud, R. N., Pawar, S. K., Bhoite, R. R., Bhoite, R. R., et al. (2021). Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol 12, 669362. doi:10.3389/fphar.2021.669362
Pesec, M., and Sherertz, T. (2015). Global health from a cancer care perspective. Future Oncology 11 (15), 2235–2245. doi:10.2217/fon.15.142
Peterson, C. T., Denniston, K., and Chopra, D. (2017). Therapeutic uses of triphala in ayurvedic medicine. The Journal of Alternative and Complementary Medicine 23 (8), 607–614. doi:10.1089/acm.2017.0083
Petrelli, F., Ghidini, A., and Luciani, A. (2021). Topotecan or other agents as second-line therapy for relapsed small-cell lung cancer: a meta-analysis of randomized studies. Molecular and Clinical Oncology 15 (4), 218. doi:10.3892/mco.2021.2383
Pommier, Y. (2006). Topoisomerase I inhibitors: camptothecins and beyond. Nature Reviews Cancer 6 (10), 789–802. doi:10.1038/nrc1977
Pradeep, C., and Kuttan, G. (2004). Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F–10 melanoma cells. Int. Immunopharmacol. 4, 1795–1803. doi:10.1016/j.intimp.2004.08.005
Prasher, B., Gibson, G., and Mukerji, M. (2016). Genomic insights into ayurvedic and western approaches to personalized medicine. Journal of genetics 95, 209–228. doi:10.1007/s12041-015-0607-9
Pressete, C. G., Viegas, F. P., Campos, T. G., Caixeta, E. S., Hanemann, J. A., Ferreira-Silva, G. Á., et al. (2023). Piperine–chlorogenic acid hybrid inhibits the proliferation of the SK-MEL-147 melanoma cells by modulating mitotic kinases. Pharmaceuticals 16, 145. doi:10.3390/ph16020145
Prusty, B. K., and Das, B. C. (2005). Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. International Journal of Cancer 113 (6), 951–960. doi:10.1002/ijc.20668
Qi, Y., Yao, L., Liu, J., and Wang, W. (2023). Piperine improves the sensitivity of osteosarcoma cells to doxorubicin by inducing apoptosis and inhibiting the PI3K/AKT/GSK-3β pathway. J. Orthop. Surg. Res 18, 180. doi:10.1186/s13018-023-03642-7
Rai, M., Jogee, P. S., Agarkar, G., and Santos, C. A. D. (2016). Anticancer activities of withania somnifera: current research, formulations, and future perspectives. Pharmaceutical biology 54 (2), 189–197. doi:10.3109/13880209.2015.1027778
Ramnath, R. (2016). A comprehensive assessment of mucositis due to radiation for head and neck cancer: pathophysiology, clinical and patient-reported outcomes, and a proposal for a novel treatment based on ayurvedic medicine
Ravikumar, B., and Aittokallio, T. (2018). Improving the efficacy-safety balance of polypharmacology in multi-target drug discovery. Expert opinion on drug discovery 13 (2), 179–192. doi:10.1080/17460441.2018.1413089
Rege, N. N., Thatte, U. M., and Dahanukar, S. A. (1999). Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytotherapy Research 13 (4), 275–291. doi:10.1002/(sici)1099-1573(199906)13:4<275::aid-ptr510>3.3.co;2-j
Saberi-Karimian, M., Katsiki, N., Caraglia, M., Boccellino, M., Majeed, M., and Sahebkar, A. (2019). Vascular endothelial growth factor: an important molecular target of curcumin. Critical reviews in food science and nutrition 59 (2), 299–312. doi:10.1080/10408398.2017.1366892
Sagar, H., and Sabharwal, P. (2024). Amalgamation of ayurveda in cancer treatment in horizon of pranic healing. Journal of Integrated Health Sciences 12 (2), 99–103. doi:10.4103/jihs.jihs_4_23
Saggam, A., Tillu, G., Dixit, S., Chavan-Gautam, P., Borse, S., Joshi, K., et al. (2020). Withania somnifera (L.) dunal: a potential therapeutic adjuvant in cancer. Journal of Ethnopharmacology 255, 112759. doi:10.1016/j.jep.2020.112759
Saghatelyan, T., Tananyan, A., Janoyan, N., Tadevosyan, A., Petrosyan, H., Hovhannisyan, A., et al. (2020). Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: a comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 70, 153218. doi:10.1016/j.phymed.2020.153218
Sahebkar, A., Cicero, A. F., Simental-Mendıa, L. E., Aggarwal, B. B., and Gupta, S. C. (2016). Curcumin downregulates human tumor necrosis factor-a levels: a systematic review and meta-analysis of randomized controlled trials. Pharmacological Research 107, 234–242. doi:10.1016/j.phrs.2016.03.026
Sak, K. (2020). Radiosensitizing potential of curcumin in different cancer models. Nutrition and cancer 72 (8), 1276–1289. doi:10.1080/01635581.2019.1681480
Samuels, M. L., and Howe, C. D. (1970). Vinblastine in the management of testicular cancer. Cancer 25 (5), 1009–1017. doi:10.1002/1097-0142(197005)25:5<1009::aid-cncr2820250504>3.0.co;2-b
Santra, G. (2022). Yoga and the need of its integration in modern medicine. J Assoc Physicians India 70 (12), 80–84. doi:10.5005/japi-11001-0142
Sati, P., Sharma, E., Dhyani, P., Attri, D. C., Rana, R., Kiyekbayeva, L., et al. (2024). Paclitaxel and its semi-synthetic derivatives: comprehensive insights into chemical structure, mechanisms of action, and anticancer properties. European journal of medical research 29 (1), 90. doi:10.1186/s40001-024-01657-2
Sha, J., Li, J., Wang, W., Pan, L., Cheng, J., Li, L., et al. (2016). Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by Down regulating notch signaling. Biomedicine and Pharmacotherapy 84, 177–184. doi:10.1016/j.biopha.2016.09.037
Shanmugam, M. K., Rane, G., Kanchi, M. M., Arfuso, F., Chinnathambi, A., Zayed, M., et al. (2015). The multifaceted role of curcumin in cancer prevention and treatment. Molecules (Basel, Switzerland) 20 (2), 2728–2769. doi:10.3390/molecules20022728
Sharma, H., and Chandola, H. M. (2011). Prameha in ayurveda: correlation with obesity, metabolic syndrome, and diabetes mellitus. Part 2—Management of prameha. The Journal of Alternative and Complementary Medicine 17 (7), 589–599. doi:10.1089/acm.2010.0397
Sharma, K., Ramachandran, A., and Patel, A. (2024). Scope of integrative approach in present era. Journal of Ayurveda and Integrated Medical Sciences 9 (9), 56–67. doi:10.21760/jaims.9.9.8
Sharma, P., and Allison, J. P. (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161 (2), 205–214. doi:10.1016/j.cell.2015.03.030
Sharma, P., Verma, A. K., Chauhan, S., Kharwar, S., and Shrivastava, P. (2016). Review on podophyllotoxin: sources, extraction, applications and current perspectives.
Sharma, R., and Prajapati, P. K. (2020). Predictive, preventive and personalized medicine: leads from ayurvedic concept of prakriti (human constitution). Current Pharmacology Reports 6 (6), 441–450. doi:10.1007/s40495-020-00244-3
Shishodia, S., Amin, H. M., Lai, R., and Aggarwal, B. B. (2005). Curcumin (diferuloylmethane) inhibits constitutive NF-κB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochemical pharmacology 70 (5), 700–713. doi:10.1016/j.bcp.2005.04.043
Singh, A. K., Srivastava, A., Kumar, V., and Singh, K. (2018). Phytochemicals, medicinal and food applications of shatavari (asparagus racemosus): an updated review. The Natural Products Journal 8 (1), 32–44. doi:10.2174/2210315507666170922145258
Singh, N., Yadav, S. S., Rao, A. S., Nandal, A., Kumar, S., Ganaie, S. A., et al. (2021). Review on anticancerous therapeutic potential of Withania somnifera (L.) dunal. Journal of Ethnopharmacology 270, 113704. doi:10.1016/j.jep.2020.113704
Singh, R. H., and Rastogi, S. (2011). “Rasayana therapy and rejuvenation,” in Evidence-based practice in complementary and alternative medicine: perspectives, protocols, problems and potential in ayurveda, 177–189.
Sinkule, J. A., Stewart, C. F., Crom, W. R., Melton, E. T., Dahl, G. V., and Evans, W. E. (1984). Teniposide (VM26) disposition in children with leukemia. Cancer research 44 (3), 1235–1237.
Škubník, J., Pavlíčková, V. S., Ruml, T., and Rimpelová, S. (2021). Vincristine in combination therapy of cancer: emerging trends in clinics. Biology 10 (9), 849. doi:10.3390/biology10090849
Song, H. R., Kim, H. N., Kweon, S. S., Choi, J. S., Shim, H. J., Cho, S. H., et al. (2013). Genetic variations in the PRKAA1 and ZBTB20 genes and gastric cancer susceptibility in a Korean population. Molecular carcinogenesis 52 (S1), 155–160. doi:10.1002/mc.22063
Soni, G. (2025). Prakriti: interdisciplinary perspectives on ayurvedic constitutions. India: Deep Science Publishing. doi:10.70593/978-93-49307-81-0
Srinivasan, K. (2009). “Black pepper (Piper nigrum) and its bioactive compound, piperine,” in Molecular targets and therapeutic uses of spices: modern uses for ancient medicine, 25–64.
Srivastava, R. K., Chen, Q., Siddiqui, I., Sarva, K., and Shankar, S. (2007). Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21/WAF1/CIP1. Cell Cycle 6 (23), 2953–2961. doi:10.4161/cc.6.23.4951
Stan, S. D., Zeng, Y., and Singh, S. V. (2008). Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer 60, 51–60. doi:10.1080/01635580802381477
Sundaramourthy, D., Venkateshperumal, S. K., Mishra, B., and Muzzammel, M. (2024). Cancer through the ayurvedic lens: an agadatantra path to healing. Ayush Journal of Integrative Oncology 1 (3 and 4), 40–45. doi:10.4103/ajio.ajio_12_24
Tandon, N., and Yadav, S. S. (2020). Safety and clinical effectiveness of Withania somnifera (linn.) dunal root in human ailments. Journal of ethnopharmacology 255, 112768. doi:10.1016/j.jep.2020.112768
Taverna, S., Giallombardo, M., Pucci, M., Flugy, A., Manno, M., Raccosta, S., et al. (2015). Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget 6, 21918–21933. doi:10.18632/oncotarget.4204
Thaiparambil, J. T., Bender, L., Ganesh, T., Kline, E., Patel, P., Liu, Y., et al. (2011). Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer 129, 2744–2755. doi:10.1002/ijc.25938
Torre, L. A., Siegel, R. L., Ward, E. M., and Jemal, A. (2016). Global cancer incidence and mortality rates and trends—an update. Cancer epidemiology, biomarkers and prevention 25 (1), 16–27. doi:10.1158/1055-9965.epi-15-0578
Urban, W. J. (2019). The future of medicine and health care: integrative holistic team prevention, diagnosis, and treatment. California: AuthorHouse.
van Loo, G., Saelens, X., Matthijssens, F., Schotte, P., Beyaert, R., Declercq, W., et al. (2002). Caspases arenot localized in mitochondria during life or death. Cell Death Differ. 9 (11), 1207–1211. doi:10.1038/sj.cdd.4401101
Vanneman, M., and Dranoff, G. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nature reviews cancer 12 (4), 237–251. doi:10.1038/nrc3237
Verma, A., Kushwaha, H. N., Srivastava, A. K., Srivastava, S., Jamal, N., Srivastava, K., et al. (2017). Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: an application for photoprotection. J Photochem Photobiol B 172, 139–148. doi:10.1016/j.jphotobiol.2017.05.018
Verma, R. K., Kumari, P., Maurya, R. K., Kumar, V., Verma, R. B., and Singh, R. K. (2018). Medicinal properties of turmeric (Curcuma longa L.): a review. Int. J. Chem. Stud 6 (4), 1354–1357.
Verstappen, C. C., Heimans, J. J., Hoekman, K., and Postma, T. J. (2003). Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 63, 1549–1563. doi:10.2165/00003495-200363150-00003
Vyas, P., Thakar, A. B., Baghel, M. S., Sisodia, A., and Deole, Y. (2010). Efficacy of rasayana avaleha as adjuvant to radiotherapy and chemotherapy in reducing adverse effects. AYU (An International Quarterly Journal of Research in Ayurveda) 31 (4), 417–423. doi:10.4103/0974-8520.82029
Weeks, J. C., Cook, E. F., O'Day, S. J., Peterson, L. M., Wenger, N., Reding, D., et al. (1998). Relationship between cancer patients' predictions of prognosis and their treatment preferences. Jama 279 (21), 1709–1714. doi:10.1001/jama.279.21.1709
Widodo, N., Priyandoko, D., Shah, N., Wadhwa, R., and Kaul, S. C. (2010). Selective killing of cancer cells by ashwagandha leaf extract and its component withanone involves ROS signaling. PloS one 5 (10), e13536. doi:10.1371/journal.pone.0013536
Wiraswati, H. L., Ma'ruf, I. F., Sharifi-Rad, J., and Calina, D. (2025). Piperine: an emerging biofactor with anticancer efficacy and therapeutic potential. BioFactors 51 (1), e2134. doi:10.1002/biof.2134
Yaffe, P. B., Doucette, C. D., Walsh, M., and Hoskin, D. W. (2013). Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 94, 109–114. doi:10.1016/j.yexmp.2012.10.008
Yaffe, P. B., Power Coombs, M. R., Doucette, C. D., Walsh, M., and Hoskin, D. W. (2015). Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 54, 1070–1085. doi:10.1002/mc.22176
Yang, G., Li, X., Liu, J., Huang, S., Weng, Y., Zhu, J., et al. (2021). Hsa_circ_0008537 facilitates liver carcinogenesis by upregulating MCL1 and Snail1 expression via miR 153 3p. Oncol Rep 45, 1072–1082. doi:10.3892/or.2021.7941
Yang, J., Pi, C., and Wang, G. (2018). Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother 103, 699–707. doi:10.1016/j.biopha.2018.04.072
Yüksel, B., Hızlı Deniz, A. A., Şahin, F., Sahin, K., and Türkel, N. (2023). Cannabinoid compounds in combination with curcumin and piperine display an anti-tumorigenic effect against colon cancer cells. Front Pharmacol 14, 1145666. doi:10.3389/fphar.2023.1145666
Zhang, J., Zhu, X., Li, H., Li, B., Sun, L., Xie, T., et al. (2015). Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 24, 50–58. doi:10.1016/j.intimp.2014.11.012
Zhang, L., Cheng, X., Gao, Y., Zhang, C., Bao, J., Guan, H., et al. (2016). Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Experimental Cell Research 341 (2), 157–165. doi:10.1016/j.yexcr.2016.01.006
Zhang, Z., Chen, H., Xu, C., Song, L., Huang, L., Lai, Y., et al. (2016). Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the wnt signaling pathway and upregulating NKD2 expression in Colon cancer cells. Oncology Reports 35 (5), 2615–2623. doi:10.3892/or.2016.4669
Keywords: ayurveda, cancer, integrative approach, phytochemicals, molecular signaling mechanism
Citation: Jha SK, Singh N, Shanker OR, Antil I, Baghel JS, Huddar V and Tripathi R (2025) A review on integrative approaches in oncology: bridging ayurvedic medicine and modern cancer therapeutics. Front. Nat. Prod. 4:1635197. doi: 10.3389/fntpr.2025.1635197
Received: 26 May 2025; Accepted: 23 July 2025;
Published: 22 August 2025.
Edited by:
Hee-Sung Chae, University of Mississippi, United StatesReviewed by:
Ahmad Bikharudin, Okayama University, JapanAishwarya Joglekar, University of Delhi, India
Salma Batool, University of Central Punjab, Pakistan
Jameema Sidhic, St. Joseph’s College (Devagiri), India
Copyright © 2025 Jha, Singh, Shanker, Antil, Baghel, Huddar and Tripathi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richa Tripathi, cmljaGEudHJwdGhzQGdtYWlsLmNvbQ==; Vittal Huddar, ZHIudmdodWRkYXJAYWlpYS5nb3YuaW4=
†These authors have contributed equally to this work and share first authorship
 Sudhanshu Kumar Jha
Sudhanshu Kumar Jha Neha Singh
Neha Singh Ozasvi R. Shanker
Ozasvi R. Shanker Ishika Antil2
Ishika Antil2 Vittal Huddar
Vittal Huddar Richa Tripathi
Richa Tripathi