Abstract
The Annonaceae family, a major group of tropical and subtropical flowering plants, is widely recognized for its edible fruits and traditional medicinal uses. While phytochemical research on this family has primarily focused on alkaloids and acetogenins, flavonoids, key polyphenolic compounds with diverse pharmacological properties, have remained comparatively overlooked. This review presents the first comprehensive synthesis of flavonoid chemistry within Annonaceae, analyzing 238 reported structures across multiple genera and regions. Our findings reveal a surprisingly broad structural diversity, with flavonol glycosides and flavanones emerging as the most prevalent classes. Other types, including chalcones, isoflavanones, catechins, and dihydroflavonols, further underscore the family’s underexplored chemical richness. Geographically, Asia dominates the research landscape, particularly Thailand and Vietnam, while Africa and the Americas also contribute notably. Among the most studied genera are Uvaria, Melodorum, Fissistigma, Annona, and Desmos. Leaf extracts represent the primary source of flavonoid isolation, though other plant parts remain underutilized. This review not only consolidates existing data but also highlights critical gaps in taxonomic coverage, biosynthetic understanding, and ecological context. By illuminating the hidden diversity and potential of flavonoids in Annonaceae, this work lays the groundwork for future studies in natural products chemistry, pharmacology, and plant systematics.
1 Introduction
The Annonaceae family, commonly referred to as the custard apple family, is one of the largest and most morphologically diverse families of flowering plants within the order Magnoliales. Comprising over 2,500 species across approximately 108 genera (Nge et al., 2024), Annonaceae is widely distributed in tropical and subtropical regions and holds significant ecological, economic, and ethnobotanical value. Many species are known for producing edible fruits and have been used in traditional medicine systems throughout Africa, Asia, and the Americas (Hernández Fuentes et al., 2021; Al Kazman et al., 2022; Erkens et al., 2023).
Phytochemically, Annonaceae has been extensively studied for its isoquinoline-derived alkaloids, particularly aporphinoids and acetogenins, which are associated with a range of biological activities, including cytotoxic, antimicrobial, antiparasitic, and anti-inflammatory effects (Leboeuf et al., 1980). However, while these chemical classes have garnered considerable scientific interest, other equally important groups of natural products remain comparatively understudied, such as flavonoids (Santos and Salatino, 2000). Flavonoids are polyphenolic compounds with well-established roles in plant physiology and human health (Harborne and Mabry, 2013; Panche et al., 2016). They are known for their antioxidant properties and associated pharmacological activities such as anti-inflammatory, antimicrobial, antidiabetic, and anticancer effects. In many plant families, flavonoids serve as key bioactive principles (Panche et al., 2016; Dias et al., 2021). Surprisingly, despite the biological and ecological relevance of flavonoids, their diversity and distribution within Annonaceae remain poorly understood.
The existing literature suggests that flavonoids, although confirmed in several Annonaceae genera (Santos and Salatino, 2000), have not been systematically investigated across the family. Most reports have focused on isolated findings from individual species, and no comprehensive survey has been conducted to elucidate the structural diversity, biosynthetic patterns, or taxonomic significance of flavonoids within Annonaceae. These findings underscore both the presence and potential significance of flavonoids in Annonaceae while simultaneously highlighting the need for broader and more integrative investigations.
Given the growing interest in plant-derived bioactive compounds and the rich ethnopharmacological background of Annonaceae (Silva et al., 2018a; Silva et al., 2018b; Leite et al., 2020; Mohanty et al., 2023; Mouafon et al., 2025), a systematic review of flavonoids in this family is timely. Such an effort can reveal untapped chemical diversity, inform future pharmacological studies, and contribute to our understanding of the ecological and evolutionary roles of flavonoids in this basal angiosperm lineage. This review aims to fill this knowledge gap by synthesizing the current state of research on flavonoids in Annonaceae. We begin by providing an overview of the family’s classification and global distribution, followed by a discussion of its diagnostic features, economic significance, and general phytochemical profile. We then focus specifically on the occurrence, chemical structure, and biological activities of flavonoids identified in Annonaceae species to date, highlighting gaps in knowledge and proposing future research directions.
1.1 Classification and distribution of Annonaceae family
Annonaceae Juss. is a morphologically diverse family of flowering plants comprising 108 genera and approximately 2,500 species distributed in four subfamilies: Anaxagoreoideae (ca. 30 spp.), Ambavioideae (ca. 60 spp.), Malmeoideae (ca. 800 spp.), and Annonoideae (ca. 1,600 spp.). It is currently the most diverse family in the Magnoliales clade at both the genus and species levels and the most primitive family of angiosperms (Erkens et al., 2023; Nge et al., 2024). Since the formalization of plant family names with the publication of Genera plantarum by de Jussieu in 1789, Annonaceae has been recognized as a distinct and readily identifiable group (Chatrou et al., 2012a). In the past four decades, considerable progress has been made in understanding the systematics and taxonomy of the family, driven by an extensive and collaborative global research effort. As a result, Annonaceae has become one of the most taxonomically well-known tropical plant families (Nge et al., 2024). Undeniably, the understanding of evolutionary relationships within Annonaceae has benefited from advances in molecular phylogenetics (Chatrou et al., 2012b; Guo et al., 2017; Couvreur et al., 2019; Chaowasku, 2020; Dagallier et al., 2023).
A recent phylogenomic study employing a previously developed nuclear bait kit tailored for Annonaceae succeeded in constructing the first comprehensive genus-level phylogeny of the group (Nge et al., 2024). This study incorporated all 108 recognized genera and was based on hundreds of nuclear loci. The resulting tree led to important taxonomic revisions, including the recognition of 25 subtribes, 21 of which are newly proposed. The ability of this phylogenomic framework to clarify intertribal relationships within the subfamily Malmeoideae (Chaowasku, 2020) and to resolve most phylogenetic placements within the tribe Miliuseae (Guo et al., 2017) was a key achievement. Notably, the analysis indicated that Meiocarpidium is sister to Ambavioideae, warranting its elevation to tribal status rather than classification as a distinct subfamily. Moreover, the genus Artabotrys, previously aligned with the tribe Xylopieae based on plastid data, was instead placed within Duguetieae, where it forms a clade with the African genera Letestudoxa and Pseudartabotrys (Nge et al., 2024). In addition to these findings, the study also drew attention to considerable gene tree conflict, particularly near the base of certain clades. This conflict is likely associated with rapid early diversification within the tribe, resulting in extensive incomplete lineage sorting. Such complexities pose challenges for fully resolving the deeper phylogenetic relationships in the family (Nge et al., 2024).
Annonaceae species are widely distributed across tropical and subtropical regions, exhibiting particularly high species richness in tropical lowland rainforests, with their richness and abundance being associated with higher temperatures and increased precipitation (Al Kazman et al., 2022; Erkens et al., 2023; Nge et al., 2024). A recent study based on spatial data combined with published and drafted International Union for Conservation of Nature (IUCN) Red List assessments allowed for an investigation into how Annonaceae are distributed across a human-impacted globe and provided insight into how this taxon is distributed across natural biomes and anthropogenic biomes (anthromes) (Erkens et al., 2023). Based on records of 67,966 specimens, this study revealed that Annonaceae occur in nearly every tropical region and are present in four of the six major terrestrial biome types: the desert biome type, the grassland biome type, the temperate vegetation with trees biome type, and the tropical vegetation with trees biome type (Erkens et al., 2023). At the continental level, Central and South America, in particular, account for more than half (61%) of the specimens and, together with Africa (27%), represent over 85% of the total. Regarding genus distribution, it is noteworthy that only the genus Xylopia is present on all continents. In contrast, 78 out of the 108 genera have their specimens restricted to a single continent. A particularly interesting case is the genus Asimina, which is entirely distributed outside the tropical realm being found exclusively in North America (Erkens et al., 2023).
At the country level, Brazil holds the largest number of Annonaceae specimens, followed by Peru, Gabon, and the United States (Erkens et al., 2023). In Brazil, 33 genera and approximately 400 species are recorded, the vast majority of which occur in the northern region (292 species), especially in the state of Amazonas (214 species). The northeastern (119 species), central-western (87 species), and southeastern (95 species) regions also show significant occurrences, while the southern region has a lower incidence (24 species). Regarding phytogeographic domains, the Amazon rainforest harbors the highest number of species, followed by the Atlantic rainforest and the Central Brazilian savanna. The Caatinga, Pantanal, and Pampa domains host comparatively fewer species (Maas et al., 2015).
1.2 Diagnostic features of Annonaceae family
The Annonaceae family is readily identified by a suite of morphological and anatomical traits that serve as reliable diagnostic features across its taxa. Members of the family are predominantly aromatic trees, shrubs, or, less commonly, lianas, often with lenticellate stems and simple, alternate, distichous leaves (except Tetrameranthus) that lack stipules and exhibit entire margins and brochidodromous or campylodromous venation (Maas et al., 2007; Maas et al., 2015). When cut, many species emit a characteristic odor, and their indumentum may consist of simple, scale-like, or stellate trichomes (Leboeuf et al., 1980; Maas et al., 2007; Maas et al., 2015). Flowers are typically axillary or extra-axillary, rarely terminal, oppositifolious, mostly bracteate, and occur singly or in rhipidiate inflorescences. The pedicels are usually articulated at the base, except in Guatteria. Flowers are bisexual, rarely androdioecious, and actinomorphic, with differentiated perianth parts. Sepals are free or variously connate and arranged in valvate or imbricate aestivation (Leboeuf et al., 1980; Maas et al., 2007; Maas et al., 2015). Petals are usually 6, though sometimes 3, 4, 8, or 12, typically arranged in whorls, subequal to markedly unequal, free or rarely connate, valvate or imbricate, and generally fleshy or thickened. Stamens are few too numerous, spirally arranged, free, and connective, often with a shield-like apical prolongation; anthers are longitudinally dehiscent and may be locellate or not; staminodes are rarely present. Carpels are few too many, free or fused at the base, and rarely completely connate. The ovary is superior, with carpels usually numerous, rarely few, and ovules 1 to many, either basal or parietal (Leboeuf et al., 1980; Maas et al., 2007; Maas et al., 2015). Fruits are mostly apocarpous, formed by free, often stipitate, generally indehiscent monocarps, although syncarpous and dehiscent fruits also occur, particularly in genera such as Annona (Maas et al., 2007). Seeds are often large, sometimes arillate, and characteristically possess a ruminate endosperm with a minute embryo, a feature also shared with the related Myristicaceae (Maas et al., 2007). Additional diagnostic features include the presence of resin canals and septate piths, which are associated with the production of aromatic compounds and ecological interactions (Leboeuf et al., 1980). These combined characteristics aid in taxonomic identification and underscore the family’s phylogenetic position among basal angiosperms.
1.3 Economic importance and traditional uses of Annonaceae family
The economic value of the Annonaceae family is substantial, primarily due to its fruit-bearing species, which hold significant commercial and nutritional importance. Among its genera, only Annona and Asimina stand out for producing economically important edible fruits. Notable species within Annona include A. cherimola Mill. (cherimoya), A. squamosa L. (sugar apple or sweetsop), A. muricata L. (soursop), the hybrid A. cherimola × A. squamosa (atemoya), A. diversifolia Saff. (ilama), A. reticulata L. (Bullock’s heart), A. glabra L. (pond apple), and A. purpurea Moc. and Sessé ex Dunal (soncoya), while A. triloba L. (pawpaw) is the sole economically relevant species in the genus Asimina (Hernández Fuentes et al., 2021; Al Kazman et al., 2022). Regarding production value, it was estimated that in Brazil, only in 2017, the fruits of A. squamosa, A. muricata, and the hybrid A. cherimola × A. squamosa had a combined production value exceeding 65 million reais (IBGE, 2024). Although these fruits are predominantly consumed in their fresh form, they are also subject to industrial processing and commercialization in diverse value-added products, such as alcoholic formulations, desserts, dehydrated flakes, ice creams, jams, jellies, juices, milk-based beverages, nectars, syrups, yogurts, and extracts enriched with bioactive compounds exhibiting medicinal properties. (Hernández Fuentes et al., 2021).
Regarding the medicinal properties of Annonaceae, their value is well documented across tropical and subtropical regions, where Indigenous peoples and local communities have traditionally used these plants for medicinal and cultural purposes (Silva et al., 2018a; Silva et al., 2018b; Leite et al., 2020; Mohanty et al., 2023; Mouafon et al., 2025). For Annona species, the most frequent use is for the treatment of gastrointestinal tract diseases, skin diseases, respiratory system diseases, diabetes, and snakebite treatments, among others. However, it has also been used for the treatment of diseases caused by infectious agents such as malaria and Ebola, as well as for cancer treatment or prevention (Leite et al., 2020; Al Kazman et al., 2022). Similarly, Duguetia species have a long history in traditional medicine and are associated with a wide range of reported biological activities, including anticancer, anti-inflammatory, antimicrobial, antimalarial, antinociceptive, antioxidant, antiparasitic (antiprotozoal, antiplasmodial, leishmanicidal, trypanocidal), antirheumatic, and insecticidal properties (Mouafon et al., 2025). Species of Uvaria, in turn, have been traditionally used to treat diseases such as diabetes, epilepsy, fever, jaundice, malaria, menstrual pain, and minor infections (Mohanty et al., 2023). Similarly, Unonopsis species are widely used in the treatment of conditions such as arthritis, bronchitis, rheumatism, diarrhea, malaria, and age-related cognitive disorders (Silva et al., 2018a; Silva et al., 2018b). In general, preparation methods involving Annonaceae species vary considerably, ranging from decoctions and infusions of leaves, bark, or roots to topical applications, highlighting the importance of traditional knowledge in guiding therapeutic practices (Leite et al., 2020). In this context, ethnobotanical surveys further underscore the global relevance of this traditional knowledge, particularly in India, Africa, South America, and Southeast Asia, where local populations continue to make extensive use of Annonaceae species in folk medicine (Focho et al., 2010; Frausin et al., 2014; Mohanty et al., 2023).
Pharmacological studies have corroborated traditional claims, demonstrating activities such as antidiabetic, anticancer, antipyretic, antimicrobial, antioxidant, and anti-inflammatory effects across various species (Leite et al., 2020; Al Kazman et al., 2022; Mohanty et al., 2023; Christopher, 2022). These pharmacological properties are attributed to the complex chemical profiles of Annonaceae plants, often involving synergistic interactions between multiple secondary metabolites (Leite et al., 2020). However, most pharmacological investigations have focused on limited species and chemical classes, with many species remaining unexplored. This gap highlights the urgent need for integrated approaches combining ethnobotanical surveys, phytochemical analyses, and bioassays to fully exploit the therapeutic potential of Annonaceae biodiversity.
1.4 Phytochemistry of Annonaceae family
The chemical diversity within Annonaceae is remarkable, encompassing a wide array of primary and secondary metabolites. Regarding primary metabolites, carbohydrates, lipids, amino acids, and proteins have been reported (Leboeuf et al., 1980). On the other hand, alkaloids represent the most extensively studied class of secondary metabolites, constituting approximately 76% of identified metabolites in genera such as Duguetia (Leboeuf et al., 1980; Mouafon et al., 2025). Almost all these alkaloids possess an isoquinoline-derived structure and are divided into subclasses such as simple isoquinolines, benzyltetrahydroisoquinolines, bisbenzylisoquinolines, bisbenzyltetrahydroisoquinolines, protoberberines, tetrahydroprotoberberines, and aporphinoids. On the other hand, aporphinoids can be divided into several subclasses, such as aporphines, oxoaporphines, C-7 and/or C-4 substituted aporphines, dehydroaporphines, phenanthrenes, and miscellaneous isoquinoline-type alkaloids. Aporphines, in turn, include noraporphines, aporphines, quaternary aporphines, aporphine N-oxides, and natural N-acylated noraporphines (Leboeuf et al., 1980; Guinaudeau et al., 1994). In addition to alkaloids, Annonaceae species produce terpenoids, flavonoids, acetogenins, and miscellaneous compounds (Leboeuf et al., 1980). Terpenoids include diterpenoids such as xylopic acid isolated from Xylopia aethiopica, which has been the subject of early phytochemical research dating back to the 1950s (Moreira et al., 2013). Acetogenins are another important class known for potent cytotoxic and pesticidal properties, although they have been less explored comparatively (Leboeuf et al., 1980).
Although flavonoids are well-established as biologically active compounds across numerous plant families (Panche et al., 2016; Dias et al., 2021), their presence and diversity within Annonaceae have remained notably underexplored. Despite scattered reports confirming their occurrence in several genera, flavonoids have received far less scientific attention compared to the extensively studied alkaloids and acetogenins traditionally associated with this family. To date, no comprehensive investigation has systematically mapped the structural diversity, distribution, or biosynthetic significance of flavonoids across Annonaceae. A notable exception is the work of Santos and Salatino (2000), which focused on Brazilian species and reported 76 compounds, primarily flavonoid glycosides derived from flavones (e.g., apigenin, luteolin) and flavonols (e.g., kaempferol, quercetin, isorhamnetin), with a clear predominance of flavonol derivatives. While this study underscores the chemical relevance of flavonoids within the family, it also highlights the fragmented nature of current knowledge. The absence of a holistic survey leaves a critical gap in our understanding of how flavonoids contribute to the phytochemical landscape, biological potential, and evolutionary ecology of Annonaceae, a gap this review seeks to address.
2 Flavonoids and their derivatives from Annonaceae
2.1 General comments
The biosynthesis of flavonoids derives from the combination of two main metabolic pathways: the shikimate pathway and the acetate pathway (Figure 1). From the convergence of these routes, the characteristic C6-C3-C6 flavonoid backbone is formed (Chen et al., 2022). The shikimate pathway begins with the condensation of D-erythrose-4-phosphate and phosphoenolpyruvate (PEP), resulting in the formation of a series of intermediates that culminate in the synthesis of shikimic acid. This compound undergoes sequential reactions to be converted into chorismic acid. According to Nabavi et al. (2020), this conversion initially involves an ATP-dependent phosphorylation that yields shikimic acid-3-phosphate. Subsequently, an addition–elimination reaction with a second molecule of PEP occurs, resulting in 5-enolpyruvylshikimate-3-phosphate (EPSP). Finally, the conversion of EPSP into chorismic acid occurs through a 1,4-elimination reaction, which releases a molecule of phosphoric acid. Thus, chorismic acid is the direct precursor of the aromatic amino acids L-phenylalanine and L-tyrosine, which are formed through decarboxylation and aromatization reactions. These aromatic amino acids undergo deamination, leading to the formation of hydroxycinnamic acids, such as p-coumaric acid, which is subsequently activated by a CoA ligase to generate 4-hydroxycinnamoyl-CoA (Cheng et al., 2014).
FIGURE 1
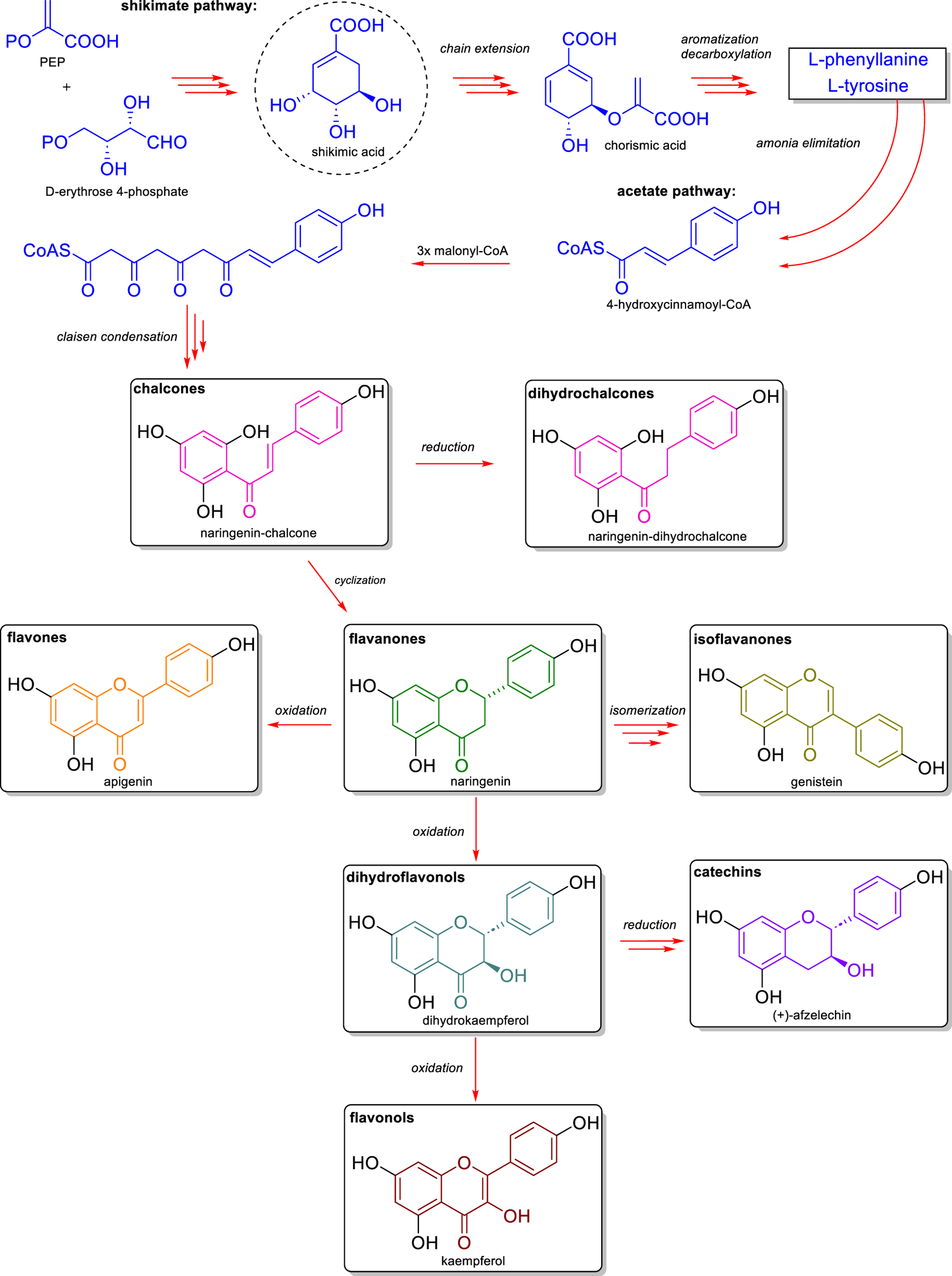
Biosynthetic pathway of flavonoids. The path begins with the shikimate route, which produces the aromatic amino acids L-phenylalanine and L-tyrosine—precursors in the formation of cinnamic acid and its derivatives. The condensation of 4-coumaroyl-CoA with three units of malonyl-CoA gives rise to chalcones, which are central intermediates in flavonoid biosynthesis. From chalcones, various enzymatic modifications—such as reduction, cyclization, isomerization, and oxidation—lead to the formation of the main flavonoid subclasses: flavanones (e.g., naringenin), flavones (e.g., apigenin), isoflavones (e.g., genistein), dihydroflavonols (e.g., dihydrokaempferol), catechins (e.g., (+)-epicatechin), and flavonols (e.g., kaempferol).
Simultaneously, the acetate pathway produces malonyl-CoA units from acetyl-CoA through the action of acetyl-CoA carboxylase. Three malonyl-CoA units condense with one 4-hydroxycinnamoyl-CoA unit in a reaction catalyzed by chalcone synthase, forming a polyketide intermediate. Through a Claisen-type condensation reaction, the compound naringenin chalcone forms the first intermediate with the basic structure of a flavonoid (Nabavi et al., 2020).
Naringenin chalcone can follow two distinct pathways. In one of them, a H2 addition occurs at carbons 7 and 8, forming a dihydrochalcone known as naringenin dihydrochalcone. Dihydrochalcones comprise a flavonoid subclass characterized by the absence of a cyclized C-ring, which confers greater structural flexibility. In the other pathway, a cyclization catalyzed by the enzyme chalcone isomerase occurs via a Michael-type nucleophilic attack of the hydroxyl group on the α,β-unsaturated ketone, resulting in the formation of the flavanone naringenin (Lin et al., 2021). Flavanones have a saturated C-ring and represent key intermediates in the biosynthesis of three flavonoid subclasses.
Through oxidation catalyzed by the enzyme flavone synthase, naringenin is converted into apigenin, a flavone characterized by a double bond between carbons 2 and 3 of the central (C) ring (Liu et al., 2021). Through a radical oxidation, followed by a 1,2-aryl migration and dehydration—reactions catalyzed by 2-hydroxyisoflavanone synthase and 2-hydroxyisoflavanone dehydratase—naringenin is also converted into the isoflavone genistein (Bosse et al., 2021). Isoflavones are distinguished by the B-ring being relocated from position 2 to position 3 of the C-ring. Naringenin may also undergo hydroxylation at position C-3 by the enzyme flavanone 3-hydroxylase, forming dihydrokaempferol, a member of the dihydroflavonol class (Liu et al., 2021). Dihydroflavonols are important biosynthetic intermediates, with a saturated C-ring and a hydroxyl group at position 3.
Dihydrokaempferol can also follow two distinct biosynthetic routes. In one pathway, a dehydrogenation reaction at carbons 2 and 3, catalyzed by the enzyme flavonol synthase, leads to the formation of kaempferol, a flavonol (Meng et al., 2019). Flavonols are characterized by the presence of a C2–C3 double bond and a hydroxyl group at C3, and they are among the most abundant flavonoids in nature, exhibiting diverse biological activities. In the alternative pathway, the reduction of dihydrokaempferol, catalyzed by dihydroflavonol 4-reductase and leucoanthocyanidin reductase, results in the formation of catechins, such as (+)-afzelechin (Raza et al., 2024). Catechins belong to the flavan-3-ol class, compounds that lack a double bond in the C-ring but possess a hydroxyl group at the C-3 position.
The diversity of flavonoids identified in the Annonaceae family can be seen in Figure 2, which shows the proportional distribution of the subclasses that will be described below. Flavonols and flavanones are the most abundant subclasses, followed by chalcones and dihydrochalcones. Flavones, catechins, dihydroflavonols, and miscellaneuos appear in smaller proportions.
FIGURE 2
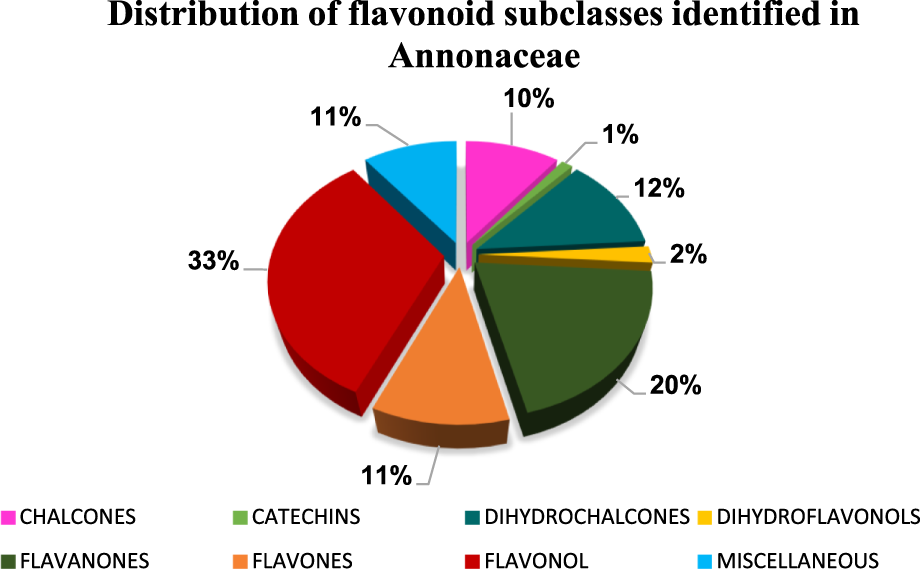
The graph shows the relative proportion of flavonoid subclasses reported in species of the Annonaceae family, including chalcones, dihydrochalcones, flavanones, flavonols, flavones, catechins, dihydroflavonols, and other miscellaneous compounds.
Therefore, Figure 3 shows the geographical distribution of flavonoids isolated from species of the Annonaceae family, as well as the genera associated with each country. There is a clear predominance of studies conducted in Asia, especially in Thailand and Vietnam, which account for the largest number of described compounds and encompass a wide diversity of genera. To a lesser extent, African countries such as Nigeria, Cameroon, and Tanzania also stand out for their contribution of species with phytochemical relevance. The Americas, in turn, show more sporadic records, mainly in Brazil and Mexico. This distribution highlights a strong geographical bias in the investigations, reflecting both the floristic richness of tropical regions and the concentration of active research groups in these areas.
FIGURE 3
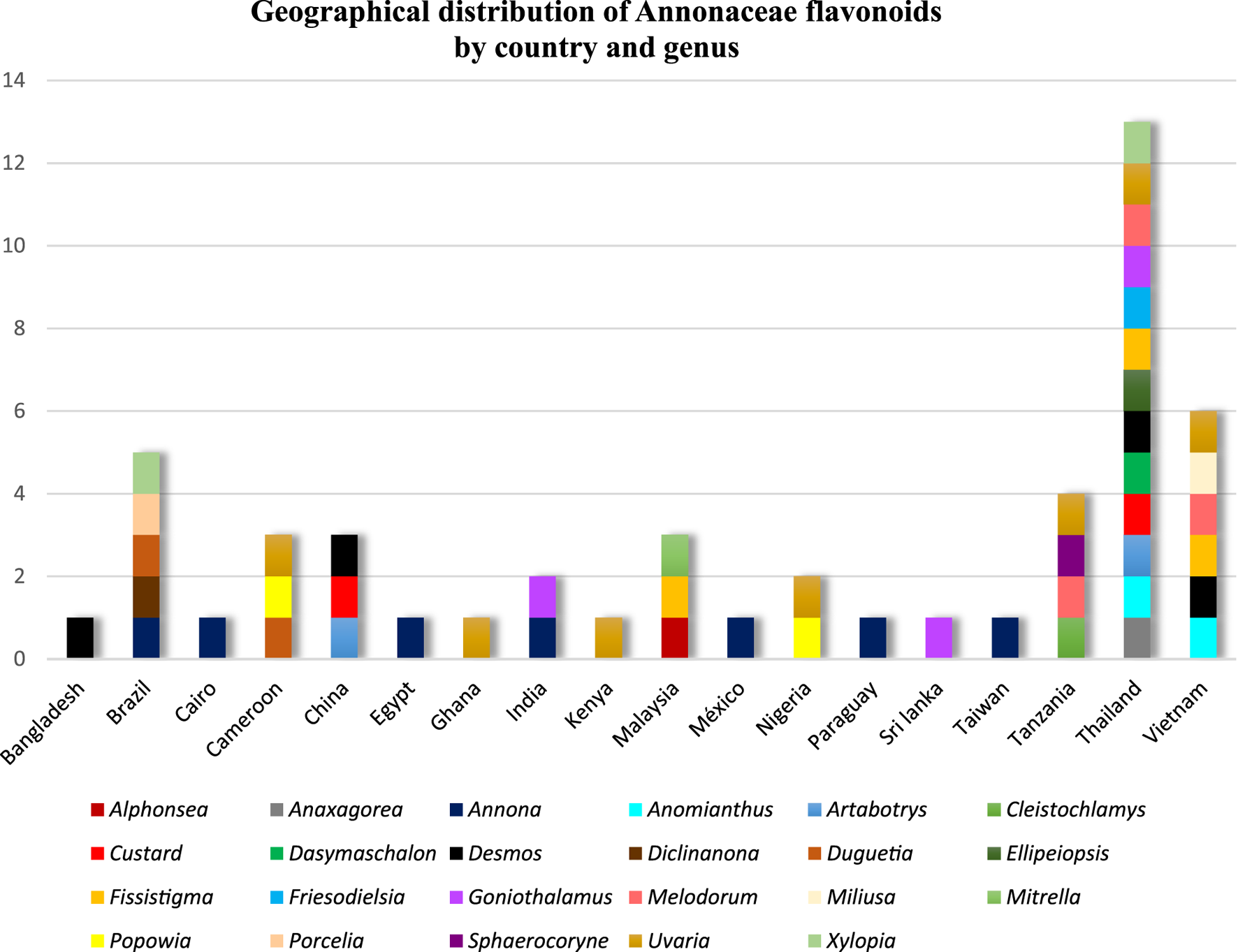
Distribution of flavonoids isolated from species of the Annonaceae family in different countries, also indicating the most representative genera in each region.
2.2 Chalcones
For chalcones, 25 structures (Figure 4) were isolated from various plant species, predominantly from the genera Melodorum, Friesodielsia, Fissistigma, Uvaria, and Desmos, mainly collected in Southeast Asia and Africa, including countries such as Thailand, Vietnam, Sri Lanka, and Cameroon. These chalcones were obtained from different plant parts, including leaves, fruits, roots, stems, and aerial parts, highlighting their wide distribution within plant tissues. The isolated compounds exhibit significant structural diversity, particularly in the substitution patterns of hydroxy, methoxy, and benzyl groups. Biologically, many of these chalcones demonstrated noteworthy anti-inflammatory, antioxidant, antimicrobial, antiplasmodial, and cytotoxic activities, underlining their potential as promising pharmacological agents. The following sections detail the chemical features and biological activities of each identified compound.
FIGURE 4
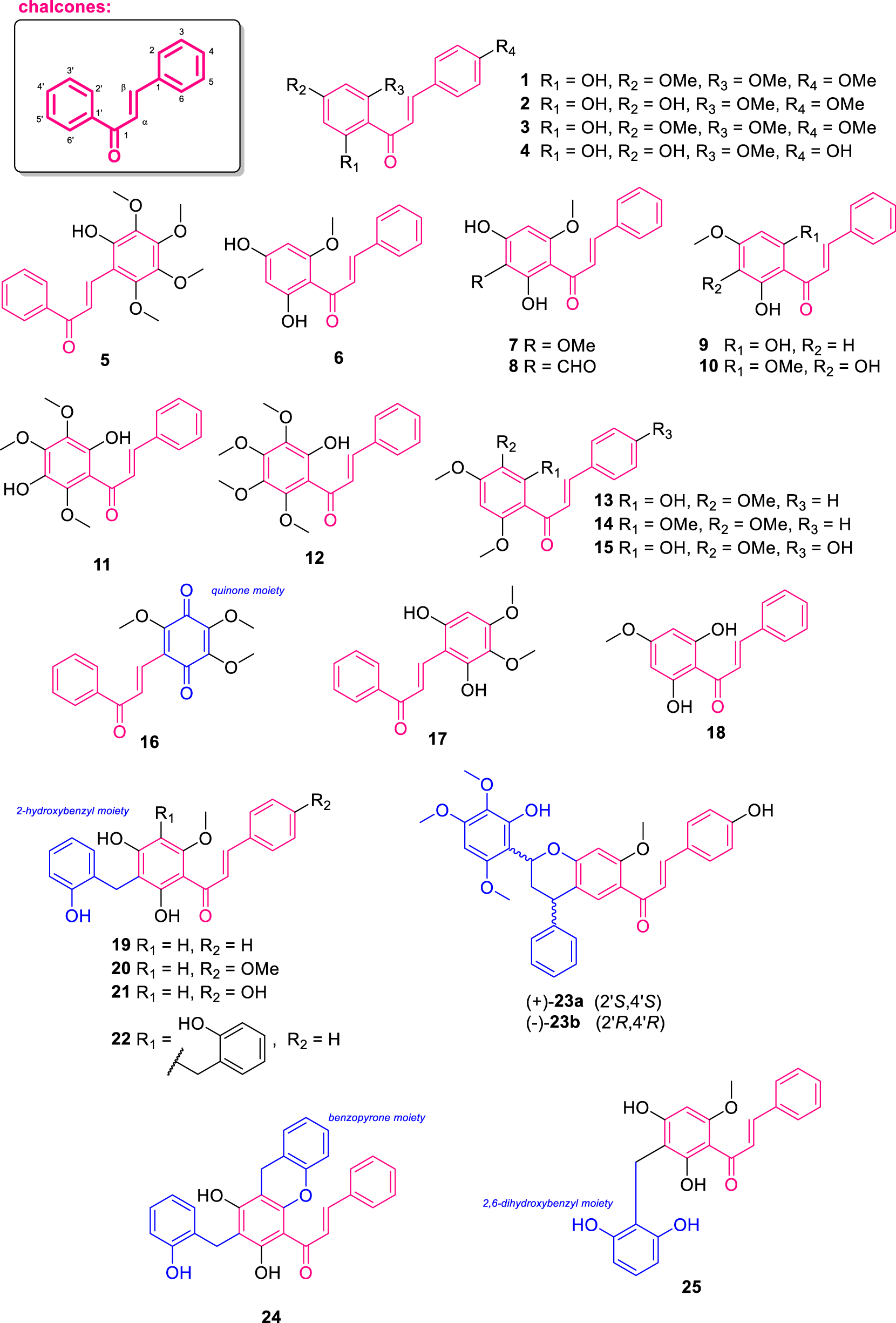
Chemical structures of chalcones isolated from Annonaceae (1–25).
Flavokawain-A (1) and 2′,4′-dihydroxy-4,6′-dimethoxychalcone (2) were isolated from the aerial parts of Goniothalamus gardneri (collected in Sri Lanka) (Seidel et al., 2000). Later, Chan et al. (2013) investigated the anti-inflammatory activity of 1 and observed its ability to inhibit superoxide anions, with a half maximal inhibitory concentration (IC50) of 8.65 μM, as well as modest elastase inhibitory activity. Furthermore, compound 2 exhibited inhibition of Interleukin-8 (IL-8) (a chemokine involved in the inflammatory response) production with an IC50 of 8.6 μM and inhibited tumor necrosis factor alpha (TNF-α) production in lipopolysaccharide (LPS) stimulated human neutrophils with an IC50 of 13.3 μM (Engels et al., 2018). Additional chalcones with a similar substitution pattern, specifically 4,4′-dihydroxy-2′,6′-dimethoxychalcone (3) and helichrysetin (4), were isolated from the fruits of Melodorum siamensis collected in Thailand (Jaidee et al., 2019). Besides, Ngoc et al. (2019) isolated the compound 2-hydroxy-3,4,5,6-tetramethoxychalcone (5) from the stem of Fissistigma polyanthoides from Vietnam. This compound features a B-ring fully substituted with four methoxy groups and one hydroxy group. Additionally, compound 5 demonstrated antioxidant activity by inhibiting the formation of intracellular reactive oxygen species (ROS) in human bronchial epithelial cell line (BEAS-2B) cells treated with 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH), with an IC50 value of 59.9 μM.
The compound cardamonin (6), isolated from the leaves of Desmos cochinchinensis (from Thailand), displayed no substitution at ring B and exhibited potent antioxidant activity and moderate cytotoxicity (Bajgai et al., 2011). Additionally, a study conducted by Meesakul et al. (2017) showed that this compound exhibited an inhibitory effect on nitric oxide (NO) production with an IC50 of 28.14 μM. The compound 2′,4′-dihydroxy-3′,6′-dimethoxychalcone (7) was isolated from the leaves of Friesodielsia desmoides and demonstrated an inhibitory effect on NO production with an IC50 = 37.21 μM. Furthermore, the aldehyde derivative 3′-formyl-2′,4′-dihydroxy-6′-methoxychalcone (8) was isolated from the leaves of Friesodielsia discolor, collected in Thailand (Prawat et al., 2012). Interestingly, compound 8 exhibited antiplasmodial activity against Plasmodium falciparum (IC50 = 2.75 μg/mL), antimicrobial activity against Mycobacterium tuberculosis was observed, with a minimum inhibitory concentration (MIC) of 6.25 μg/mL and cytotoxic activity against the epidermoid carcinoma in the mouth (KB) with an IC50 of 6.50 μg/mL and the human breast adenocarcinoma (MCF-7), with an IC50 value of 4.13 μg/mL. The compound 2′,6′-dihydroxy-4′-methoxychalcone (9), isolated from the leaves of Melodorum fruticosum (from Vietnam), showed inhibition of IL-8 production with an IC50 = 11.6 μM and inhibition of TNF-α production in LPS stimulated human neutrophils with an IC50 = 6.2 μM (Engels et al., 2018). Additionaly, the compound 2′,3′-dihydroxy-4′,6′-dimethoxychalcone (10) was isolated from the leaves of Anomianthus dulcis collected in Thailand and no bioactivity was observed (Sinz et al., 1999).
The compound pedicin (11) was isolated from the leaves of Fissistigma lanuginosum (from Malaysia) (Alias et al., 1995), displaying the ring A fully substituted with three methoxy and two hydroxy groups. Besides, kanakugiol (12), isolated from the fruits of Popowia cauliflora, (from Cameroon) differs from 11 by containing four methoxy groups and one hydroxy group at the ring A (Waterman and Pootakahm, 1979). From the same plant, the additional chalcones 2′-hydroxy-3′,4′,6′-trimethoxychalcone (13), 2′,3′,4′,6′-tetramethoxychalcone (14), and 2′,4-dihydroxy-3′,4′,6′-trimethoxychalcone (15) were isolated (Waterman and Pootakahm, 1979). On the other hand, melosiamensone E (16), obtained from the leaves of Melodorum siamensis collected in Thailand, features a quinone core bearing three methoxy groups in ring B (Jaidee et al., 2019). The compound 2-hydroxy-3,4,6-trimethoxychalcone (17), isolated from the roots of Uvaria dependens (Tanzania) (Nkunya et al., 1993b), exhibited cytotoxic activity against human promyelocytic leukemia cells (HL-60) with an IC50 of 22.9 μM (Moriyasu et al. (2011). From leaves of Melodorum fruticosum (from Vietnam), discovered the compound 2′,6′-dihydroxy-4′-methoxychalcone (18), which showed potential anti-inflammatory activity by inhibiting superoxide anions, with an IC50 value of 8.40 μM (Chan et al., 2013).
From the leaves of Ellipeiopsis cherrevensis collected in Thailand, the molecule 2′,4′-dihydroxy-3′-(2-hydroxybenzyl)-6′-methoxychalcone (19) was isolated (Wirasathien et al., 2006). Compound 19 showed cytotoxic activity against human tumor cell lines human small cell lung carcinoma (NCI-H187), KB, and breast cancer (BC), with IC50 values of 1.40, 5.31, and 13.92 μg/mL, respectively (Wirasathien et al., 2006). Also, 19 exhibited antimalarial activity against Pasmodium falciparum, with an IC50 = 7.1 μg/mL and antimicrobial activity against Mycobacterium tuberculosis (MIC = 25 μg/mL) (Wirasathien et al., 2006). In another study, 19 compound also exhibited cytotoxic activity against KB, MCF-7, and NCI-H187 cell lines, with IC50 values of 1.26, 2.08, and 3.98 μg/mL, respectively (Lekphrom et al., 2018). The compound 2′,4′-dihydroxy-3′-(2-hydroxybenzyl)-4,6′-dimethoxychalcone (20) was isolated from the leaves of Melodorum fruticosum (Engels et al., 2018). This compound is structurally similar to 19, as both feature a hydroxybenzyl group on the A-ring but differ by the presence of a methoxy group on the B-ring in 20.
The compounds cherrevenones B (21) and C (22) were isolated from the fruits of Uvaria cherrevensis, from Thailand (Auranwiwat et al., 2018). Compound 21 is the first example of this review that displays the presence of a hydroxybenzyl connected to ring A. Alternatively, 22 contains two hydroxybenzyl groups attached at C-3′ and C-5’. Compound 21 exhibited cytotoxic activity against KB and African green monkey kidney epithelial (Vero) cell lines, with IC50 values of 2.76 and 3.34 μM, respectively, while 22 displayed moderate inhibitory activity against the K1CB1 strain of Plasmodium falciparum (a chloroquine- and pyrimethamine-resistant strain) (IC50 = 43.5 μM) (Auranwiwat et al., 2018). The molecules (−)-melosiamensone A (23a) and (+)-melosiamensone A (23b) were isolated as an enantiomeric mixture from the fruits of Melodorum fruticosum (Jaidee et al., 2019). These compounds display an uncommon chalcone-phenylpropanoid hybrid structure that the authors speculate to be formed via a Michael addiction-like reaction. Their absolute configurations were determined as (2′S,4′S) and (2′R,4′R), respectively, based on chiral reverse-phase liquid chromatography purification and Electronic Circular Dichroism (ECD) analysis with aid of theoretical calculations. On the other hand, cherrevenone A (24), obtained from fruits of Uvaria cherrevensis exhibited a benzopyrone moiety fused with the A-ring at positions C-2′ and C-3’ (Auranwiwat et al., 2018). Moreover, 24 displayed an IC50 of 21.0 μM against the Tm4/8.2 (a chloroquine-sensitive strain) and K1CB1 strains of P. falciparum, as well as cytotoxic activity against KB and Vero cells, with IC50 values of 0.60 and 0.61 μM, respectively (Auranwiwat et al., 2018). Additionally, the compound 2′,4′-dihydroxy-3′-(2,6-dihydroxybenzyl)-6′-methoxychalcone (25) was isolated from the leaves of Desmos chinensis collected in Bangladesh (Rahman et al., 2003). Interestingly, 25 features a 2,6-dihydroxybenzyl substituent attached to the B-ring of the chalcone skeleton.
2.3 Dihydrochalcones
About dihydrochalcones, 29 structures (Figure 5) were isolated from various plant species, with notable representation from the genera Uvaria, Melodorum, Fissistigma, and Miliusa, predominantly collected in Southeast Asia and parts of Africa. These compounds were extracted from diverse plant parts, including roots, leaves, stems, twigs, and aerial portions. The isolated dihydrochalcones display a wide range of substitution patterns, including fully substituted aromatic rings, β-substituted variants (such as β-methoxy and β-ethoxy derivatives), and rare benzopyrone-fused skeletons. Several of these compounds exhibit significant biological activities, including cytotoxicity, antimicrobial effects, and inhibitory effects on the nuclear factor of activated T-cells (NFAT), as well as selective activity against human tumor cell lines and Mycobacterium species. In addition, several dihydrochalcones bearing hydroxybenzyl or 2-(4-hydroxyphenethyl)phenol moieties further enrich the chemical diversity observed within this class. The following section provides a detailed overview of their structures, sources, and reported bioactivities.
FIGURE 5
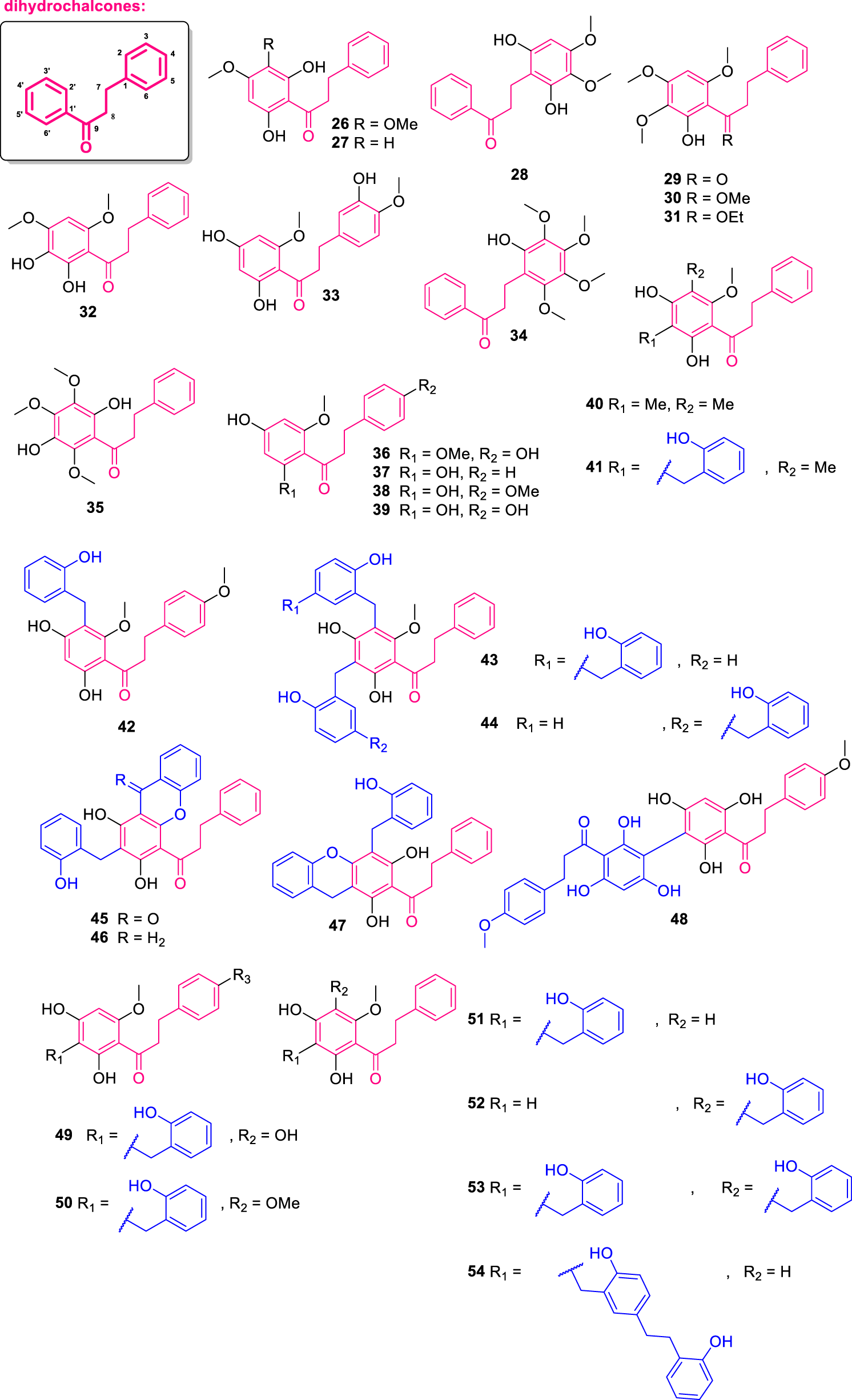
Chemical structures of dihydrochalcones isolated from Annonaceae (26–54).
The compounds dihydropashanone (26) and 2′,6′-dihydroxy-4′-methoxydihydrochalcone (27) were isolated from the twigs and leaves of Miliusa balansae, collected in Vietnam (Kamperdick et al., 2002). To add to that, compounds 2-hydroxy-3′,4′,6′-trimethoxydihydrochalcone (28), 2′-hydroxy-3′,4′,6′-trimethoxydihydrochalcone (29), 2′-hydroxy-3′,4′,6′-trimethoxy-β′-methoxychalcane (30) and 2′-hydroxy-3′,4′,6′-trimethoxy-β′-ethoxychalcone (31) were isolated from the leaves of Fissistigma bracteolatum, also from Vietnam (Lien et al., 2000). Compounds 30 and 31 do not display the common β-keto group from other dihydrochalcones, instead this function was replaced by a methoxy and an ethoxy group, respectively. From the leaves of Anomianthus dulcis (collected in Thailand), the compound 2′,3′-dihydroxy-4′,6′-dimethoxydihydrochalcone (32) was isolated (Sinz et al., 1999). Further investigation displayed an inhibitory activity of 32 against the NFAT transcription factor, with an IC50 of 9.77 μM (Kiem et al., 2005). Additional dihydrochalcones were isolated from the leaves of Melodorum siamensis and named melosiamensone F (33) and 4′,4-dihydroxy-2′,6′-dimethoxydihydrochalcone (36) (Jaidee et al., 2019).
The compounds 34 (2-hydroxy-3,4,5,6-tetramethoxydihydrochalcone) (Ngoc et al., 2019) and 35 (2′,5′-dihydroxy-3′,4′,6′-trimethoxydihydrochalcone) (Alias et al., 1995) were isolated by the species Fissistigma polyanthoides and Fissistigma lanuginosum, respectively. Compound 34 display the ring B fully substituted, while 35 present a fully substituted ring A. Moreover, uvangoletin (37) was obtained from the roots of Uvaria angolensis, collected in Nigeria (Hufford and Oguntimein, 1982). From the aerial parts of Goniothalamus gardneri (from Sri Lanka), the compounds 2′,4′-dihydroxy-4,6′-dimethoxydihydrochalcone (38) and 2′,4,4′-trihydroxy-6′-methoxydihydrochalcone (39) were isolated. These compounds exhibited cytotoxic activity against KB, MCF-7, and NCI-H187 cell lines, with IC50 values of 9.09, 16.72, and 14.26 μM for compound 38, and 5.18, 10.92, and 8.82 μM for compound 39, respectively (Prawat et al., 2013).
From the roots of a Nigerian specimen of Uvaria angolensis, angoletin (40) and anguvetin (41) were isolated (Hufford and Oguntimein, 1982). Both exhibited antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, and Mycobacterium smegmatis with IC50 values of 12.5, 0.8, and 6.3 μM, respectively for 40 and 1.5, 0.2, and 1.5 μM, respectively for 41. Additionally, the compound melosiamensone D (42) was isolated from the leaves of Melodorum siamensis (Jaidee et al., 2019). By studying the roots of Uvaria leptocladon, collected in Thailand, Nkunya et al. (1993a) isolated two C-benzylated dihydrochalcones: triuvaretin (43) and isotriuvaretin (44). These two compounds possess a distinct substitution pattern at ring B, in which a 2-(4-hydroxyphenethyl)phenol-like group is the substituent at C-3′ and C-5′ for 43 and 44, respectively. Beyond, from the roots of Uvaria acuminata, collected in Kenya, the compounds isochamuvaretin (45) and acumitin (46), in both compounds a fused benzopyrone moiety as observed for 24 (Ichimaru et al., 2004). Both 45 and 46 exhibited cytotoxic activity against HL-60 human promyelocytic leukemia cells, with IC50 values of 8.2 and 4.1 μM, respectively.
The compound chamuvaretin (47) was isolated from the roots of Uvaria chamae, which displayed an interesting benzopyrone moiety fused to ring B at C-4′ and C-5’ (Okorie, 1977). From the leaves of Melodorum siamensis, collected in Thailand, the chalcone dimer 3′,3″-bis-[2′,4′,6′-trihydroxy-4-methoxydihydrochalcone] (48) was isolated (Prawat et al., 2013). Additional dihydrochalcones named 2′,4,4′-trihydroxy-6′-methoxy-3′-(2″-hydroxybenzyl)dihydrochalcone (49) and 2′,4′-dihydroxy-4,6′-dimethoxy-3′-(2″-hydroxybenzyl)dihydrochalcone (50) were obtained from the leaves of Melodorum siamensis (Jaidee et al., 2019). Compound 49 exhibited IC50 values of 7.16, 14.86, and 3.66 μM against the KB, MCF-7, and NCI-H187 cell lines, respectively, while compound 50 showed IC50 values of 2.02, 20.03, and 2.73 μM against the same cell lines (Jaidee et al., 2019).
The compound uvaretin (51) was isolated from the roots of Uvaria acuminata by Cole et al. (1976). Then, Ichimaru et al. (2004) evaluated the cytotoxic potential of 51, reporting an IC50 of 9.3 μM against HL-60 cells. Additionally, from the stem bark of Uvaria chamae, the compound isouvaretin (52) (also known as chamuvaretin) was isolated (Hufford and Lasswell, 1976). Compound 52 exhibited cytotoxic activity against HL-60 cells, with an IC50 value of 24.7 μM (Ichimaru et al., 2004). In the study conducted on the roots of Uvaria angolensis, Hufford and Oguntimein (1982) isolated the compound diuvaretin (53), which was further assayed and found to possess cytotoxic activity of this compound against HL-60 cells with an IC50 value of 6.1 μM (Ichimaru et al., 2004). Nkunya et al. (1993a), in a study with the roots of Uvaria leptocladon, (from Tanzania), isolated the compound angoluvarin (54). Compounds 51–53 bear variable patterns of substitution by 2-hydroxybenzil motifs, while 54 display an uncommon 2-(4-hydroxyphenethyl)phenol-like group attached to C-5’.
2.4 Flavanones
Concerning flavanones, 48 structures (Figures 6, 7) were isolated from a wide range of plant species belonging predominantly to the Annonaceae family, including genera such as Melodorum, Uvaria, Desmos, Fissistigma, and Friesodielsia. These compounds were obtained from various plant parts, including leaves, roots, stems, bark, twigs, and heartwood, mainly collected across Southeast Asia and West Africa. The isolated flavanones exhibit significant structural diversity, including diverse methoxy and hydroxy substitution patterns, formyl groups, and rare prenylated and C-benzylated derivatives. Several of these flavanones have been reported to exhibit potent biological activities, including antioxidant, cytotoxic, antiplasmodial, estrogenic, aromatase inhibitory, and α-glucosidase inhibitory properties. In particular, compounds featuring 2-hydroxybenzyl moieties, O-prenylation, and unusual dimeric or polybenzylated architectures stand out for their unique structures and promising bioactivity profiles. The following sections present these compounds in detail, highlighting their natural sources, structural characteristics, and biological evaluations.
FIGURE 6
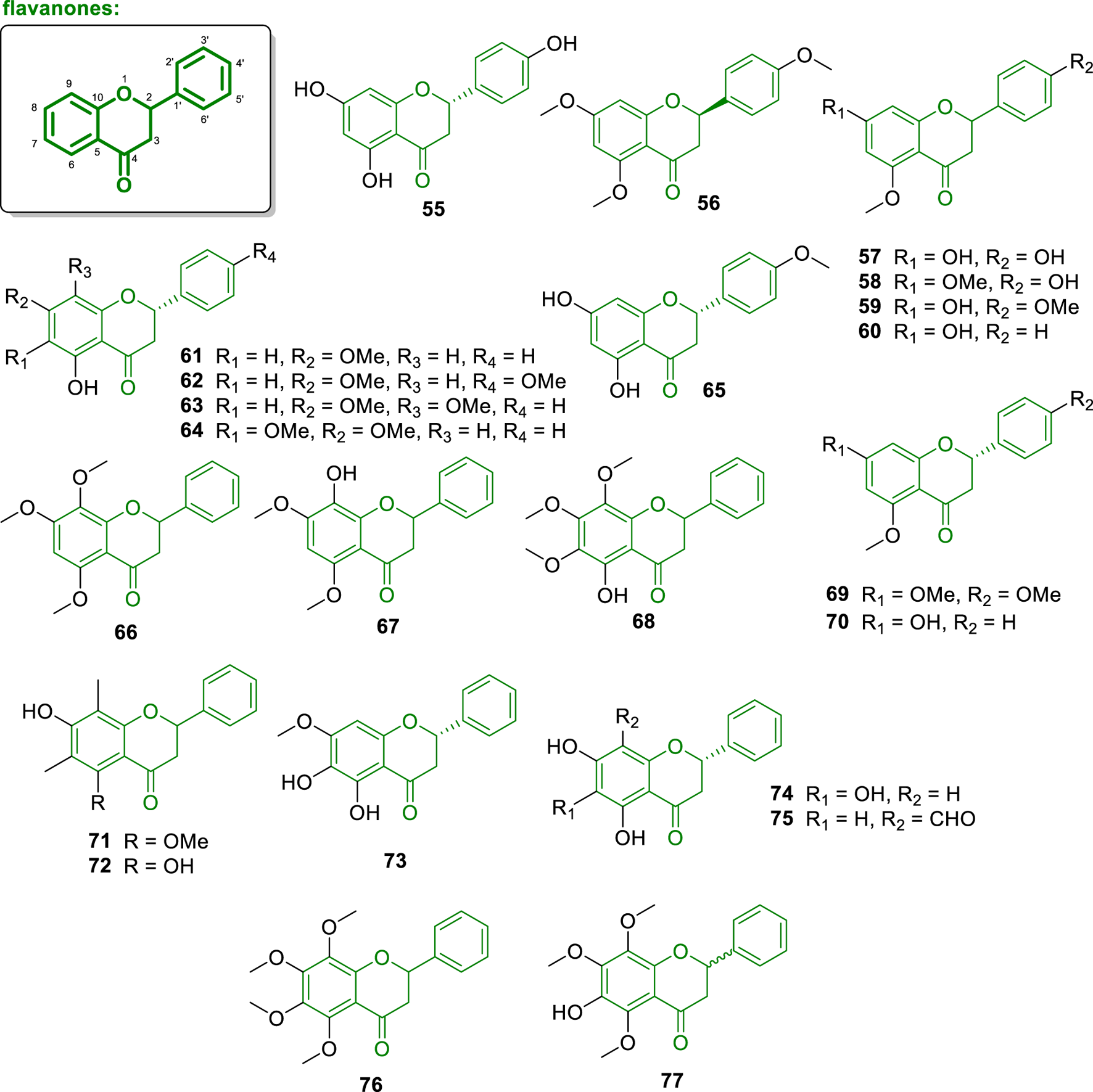
Chemical structures of flavanones isolated from Annonaceae (55–77).
FIGURE 7
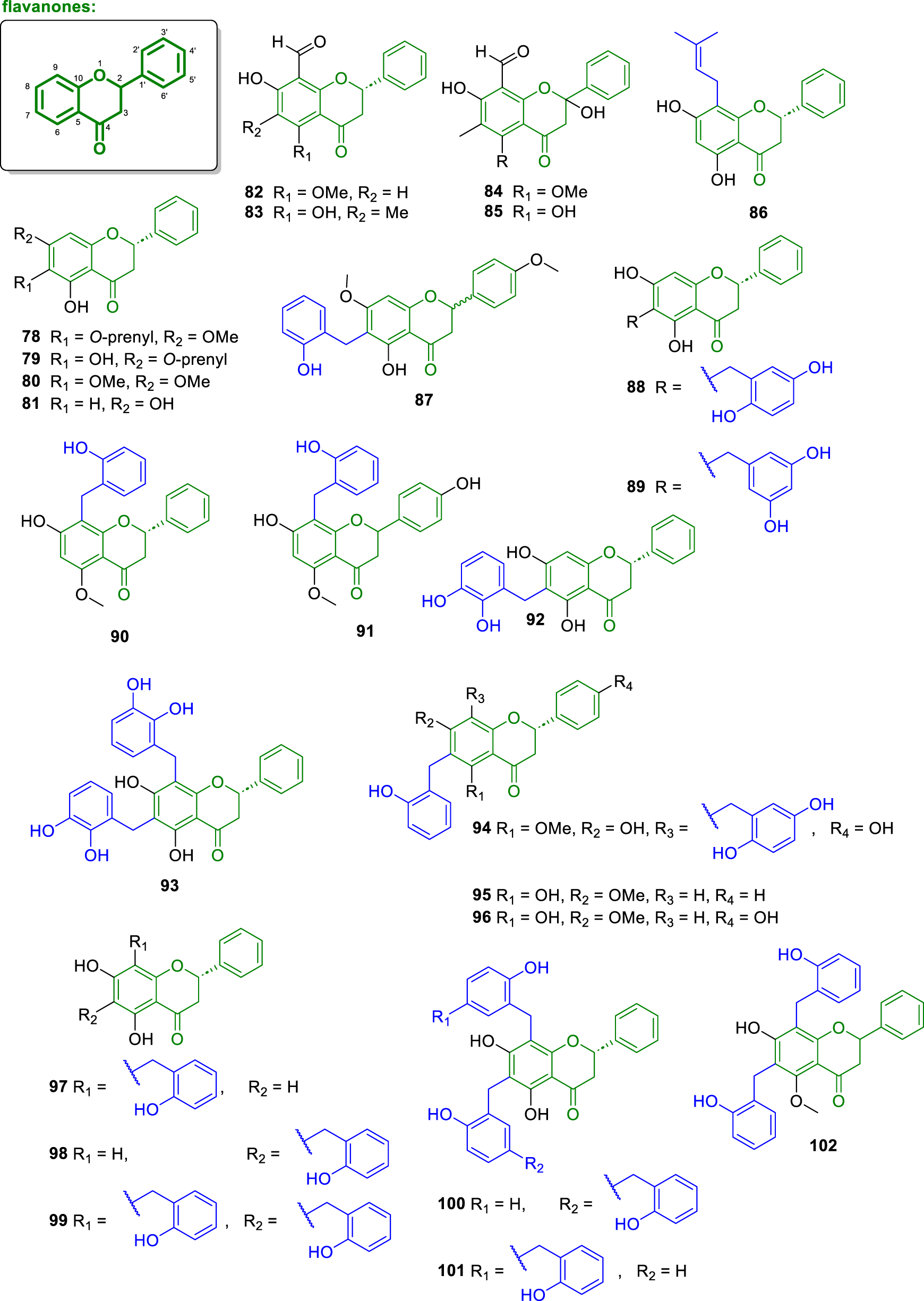
Chemical structures of flavanones isolated from Annonaceae (82–102).
The compound naringenin (55) was isolated from the heartwood of Anaxagorea luzonensis, collected in Thailand (Sabphon et al., 2015), while the compound (2S)-4′,5,7-trimethoxyflavanone (56) was isolated from the leaves of Melodorum fruticosum, collected in Vietnam (Engels et al., 2018). Moreover, the investigation of leaves of Melodorum fruticosum afforded the compound 4′,7-dihydroxy-5-methoxyflavanone (57). Compound 57 exhibited a superoxide anion inhibition activity with IC50 = 2.20 μM. Additionally, 57 also showed cytotoxic activity against KB and NCI-H187 cell lines, with IC50 values of 17.45 and 16.97 μg/mL, respectively (Chan et al., 2013).
The compound 4′-hydroxy-5,7-dimethoxyflavanone (58) was isolated from the leaves of Melodorum fruticosum (Engels et al., 2018). Tsugafolin (59) was isolated from the aerial parts of Goniothalamus gardneri, collected in India (Seidel et al., 2000). According to Chan et al. (2013), 58 exhibited superoxide anion inhibition with an IC50 = 2.50 μM. On the other hand, Prawat et al. (2013) demonstrated that this compound possesses cytotoxic activity against KB and NCI-H187 cell lines with IC50 values of 20.29 and 17.74 μg/mL, respectively. Furthermore, the compound 7-hydroxy-5-methoxyflavanone (60) was described from the leaves of Melodorum fruticosum (Engels et al., 2018) and pinostrobin (61) the stems of Uvaria chamae (Lasswell and Hufford, 1977). From the leaves and twigs of Miliusa balansae (collected in Vietnam), the compounds 5-hydroxy-4′,7-dimethoxyflavanone (62), 5-hydroxy-7,8-dimethoxyflavanone (63), and 5-hydroxy-6,7-dimethoxyflavanone (64) were isolated (Kamperdick et al., 2002). Besides, in the study conducted by Chan et al. (2013), the compound ponciretin (65) was isolated from the leaves of Melodorum fruticosum and showed a superoxide anion inhibition with an IC50 = 7.69 μM.
Additional flavanone analogues were isolated from Annonaceae plants, such as 5,7,8-trimethoxyflavanone (66) from the stems of Popowia cauliflora (from Nigeria) (Panichpol and Waterman, 1978), 8-hydroxy-5,7-dimethoxyflavanone (67) was isolated from the leaves of Anomianthus dulcis (from Thailand) (Sinz et al., 1999), 5-hydroxy-6,7,8-trimethoxyflavanone (68) from Fissistigma polyanthoides (from Vietnam) (Ngoc et al., 2019), naringenin trimethyl ether (69) from the aerial parts of Goniothalamus gardneri (from India) (Seidel et al., 2000), alpinetin (70) and 5,6,7-trihydroxyflavanone (74) from the leaves of Friesodielsia desmoides (from Thailand) (Meesakul et al., 2017), (+)-6,8-C-dimethylpinocembrin 5-methyl ether (71) and demethoxymatteucinol (72) from roots of Uvaria angolensis (from Nigeria) (Hufford and Oguntimein, 1982) and 5,6-dihydroxy-7-methoxyflavanone (73) from leaves of Desmos chinensis (from Vietnam) (Kiem et al., 2005).
The compound containing a formyl group at the C-8 position of the A-ring named 8-formyl-5,7-dihydroxyflavanone (75) was isolated from the leaves of Friesodielsia discolor (Prawat et al., 2012). An error in the carbon numbering of the structure is present in the original article, which refers to positions 4a and 8a instead of the correct ones, 5 and 10. Morevoer, the compound kanakugin (76) was isolated from the fruits of Popowia cauliflora, collected in Cameroon. Interestingly, this compound features an A-ring fully substituted with four methoxy groups (Waterman and Pootakahm, 1979). Furthermore, from the trunk barks of Fissistigma oldhamii, collected in Taiwan, was isolated the compound isopedicin (77) (Hwang et al., 2009). This molecule potently and concentration-dependently inhibited superoxide anion production in human neutrophils activated by formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP), with an IC50 value of 0.34 μM. In Vietnam, the stem barks of Melodorum fruticosum were collected and phytochemically studied to afford the molecules melodorone B (78), melodorone C (79), and onysilin (80) (Do and Sichaem, 2022). Compounds 78 and 79 are distinguished by the presence of O-prenyl groups at C-8 and C-7, respectively. Flavanones 78–80 were evaluated for their α-glucosidase inhibitory activity, displaying IC50 values of 3.33 μM (78), 4.00 μM (79), and 192 μM (80). Additionally, compounds 78 and 79 demonstrated cytotoxicity against KB (IC50 = 62.1 and 59.0 μM, respectively), human liver hepatocellular carcinoma (HepG2) (IC50 = 44.8 and 80.0 μM, respectively), and MCF-7 (IC50 = 73.7 μM, only for 78) cell lines (Do and Sichaem, 2022).
Pinocembrin (81) was isolated from the stem of Uvaria chamae (from Ghana) (Lasswell and Hufford, 1977). Beyond, Bajgai et al. (2011), assayed this compound towards inhibition of the enzyme aromatase obtaining an IC50 value of 0.9 μM. In another study conducted by Meesakul et al. (2019), pinocembrin also inhibited the α-glucosidase enzyme with an IC50 value of 4.3 μM. The compounds 8-formyl-7-hydroxy-5-methoxyflavanone (82) and lawinal (83) were isolated from the leaves of Friesodielsia discolor (Prawat et al., 2012). Compound 82 exhibited antiplasmodial activity against Plasmodium falciparum (IC50 = 2.78 μg/mL), antimicrobial activity against Mycobacterium tuberculosis (MIC = 25.00 μg/mL), and cytotoxic activity against KB (IC50 = 12.51 μg/mL) and MCF-7 (IC50 = 10.27 μg/mL) cell lines. Desmosflavanone II (84) was isolated from the roots of Desmos cochinchinensis (from China) (Wu et al., 1997). Compound 84 displayed ring A is fully substituted with formyl, hydroxyl, methoxyl, and methyl groups. From the leaves and stems of Desmos chinensis, collected in Japan was isolated the flavanone desmal (85), which inhibited the growth of human epidermoid carcinoma cells (A431), retrovirus-transformed cell line (ER12), and rous sarcoma virus-transformed normal rat kidney cells (RSV'-NRK) (Kakeya et al., 1993).
The compound (2S)-8-isoperitenylnaringenin (86) was isolated from the heartwood of Anaxagorea luzonensis (from Thailand), exhibited strong estrogenic activity with an IC50 value of 140 nM (Kitaoka et al., 1998). The compound 5-hydroxy-6-(2-hydroxybenzyl)-4′,7-dimethoxyflavanone (87) was isolated from the leaves of Melodorum fruticosum (Engels et al., 2018). This compound is notable for containing a 2-hydroxybenzyl group at ring A, as also observed for chalcones and dihydrochalcones. Two compounds, (−)-(2S)-desmoscochinflavanones A (88) and B (89) were isolated from the twigs and leaves of Desmos cochinchinensis, (Meesakul et al., 2019). From the roots of Uvaria angolensis, was isolated the compound (±)-chamanetin 5-methyl ether (90) (Hufford and Oguntimein, 1982). Later, Lekphrom et al. (2018) described the cytotoxic activity against KB, MCF-7, and NCI-H187 cell lines of 90 with IC50 values of 42.37, 27.82, and 8.17 μg/mL, respectively.
Melosiamensone B (91), a flavone containing a 2-hydroxybenzyl at ring A, was isolated from the leaves of Melodorum siamensis (Jaidee et al., 2019). In the study by Maeda et al. (2020), the compounds 3″-hydroxyisochamanetin (92) and 3″-hydroxygracinol (93) were isolated from the leaves of Sphaerocoryne gracilis, collected in Tanzania. Both compounds exhibit (S)-configuration at C-2 of the C-ring. The compounds cherrevenone D (94), isochamanetin 7-methyl ether (95), and epi-methylphelligrin (96) were isolated from the fruits of Uvaria cherrevensis in Thailand, as described by Auranwiwat et al. (2018). All three compounds exhibit relative α-oriented ring C and a 2-hydroxybenzyl group at ring A. The compounds chamanetin (97), isochamanetin (98), and dichamanetin (99) were isolated from the stem of Uvaria chamae (Lasswell and Hufford, 1977). Compounds 97–99 exhibited cytotoxic activity against KB, human cervical cancer cells (HeLa), MCF-7, and HepG-2 cell lines (Hongnak et al., 2015). Compounds 98 and 99 also exhibited cytotoxic activity against NCI-H187 and Vero cell lines, with IC50 values of 40.28 and 19.31 μM for compound 98, and 17.26 and 22.29 μM for compound 99 (Chokchaisiri et al., 2017). In the study by Costa et al. (2021), compound 98 exhibited cytotoxic activity against HepG2 and human lung fibroblast cell line (MRC-5) cell lines (IC50 = 19.79 and 24.69 μg/mL, respectively). In contrast, compound 99 was cytotoxic against HL-60, MCF-7, and human colorectal carcinoma cell line (HCT116) cell lines, with IC50 values of 15.78, 23.59, and 18.99 μg/mL, respectively.
In the work of Hufford et al. (1979), the compound uvarinol (100) was isolated from the stem of Uvaria chamae. This compound features two 2-hydroxybenzyl groups on the A-ring, one of which (at C-6) is further substituted with another 2-hydroxybenzyl group. The compound isouvarinol (101) was isolated from two Uvaria species, U. lucida and U. doeringii (Achenbach et al., 1997). This compound also possesses two 2-hydroxybenzyl groups on the A-ring, with the group at C-8 being substituted with an additional 2-hydroxybenzyl group. From the stem and root of Uvaria cherrevensis, (±)-dichamanetin 5-methyl ether (102) was isolated, which exhibited cytotoxic activity against the Vero cell line, with an IC50 = 38.6 μM (Auranwiwat et al., 2017). Moreover, 102 also showed cytotoxicity against KB, MCF-7, and NCI-H187 cell lines, with IC50 values of 11.36, 6.45, and 12.81 μg/mL, respectively (Lekphrom et al., 2018).
2.5 Flavone aglycones and isoflavones
To flavone aglycones and isoflavones, 25 structures (Figure 8) were isolated from various Annonaceae species, notably from the genera Anomianthus, Desmos, Fissistigma, Friesodielsia, Melodorum, Popowia, Uvaria, and Cleistochlamys. These compounds were primarily obtained from leaves, stems, trunks, and roots of plants collected across Southeast Asia and Africa. The identified flavones and isoflavones display considerable structural variation, including O-methylation, O-prenylation, and substitution with hydroxybenzyl groups, as well as the presence of formyl and methoxy functionalities. Several of these compounds have been reported to exhibit notable biological properties, including cytotoxic, antiplasmodial, anti-inflammatory, antioxidant, and α-glucosidase inhibitory activities. The presence of dihydroxybenzyl-substituted flavones and diverse isoflavone profiles further highlights the chemodiversity and pharmacological potential of flavonoids within this plant family.
FIGURE 8
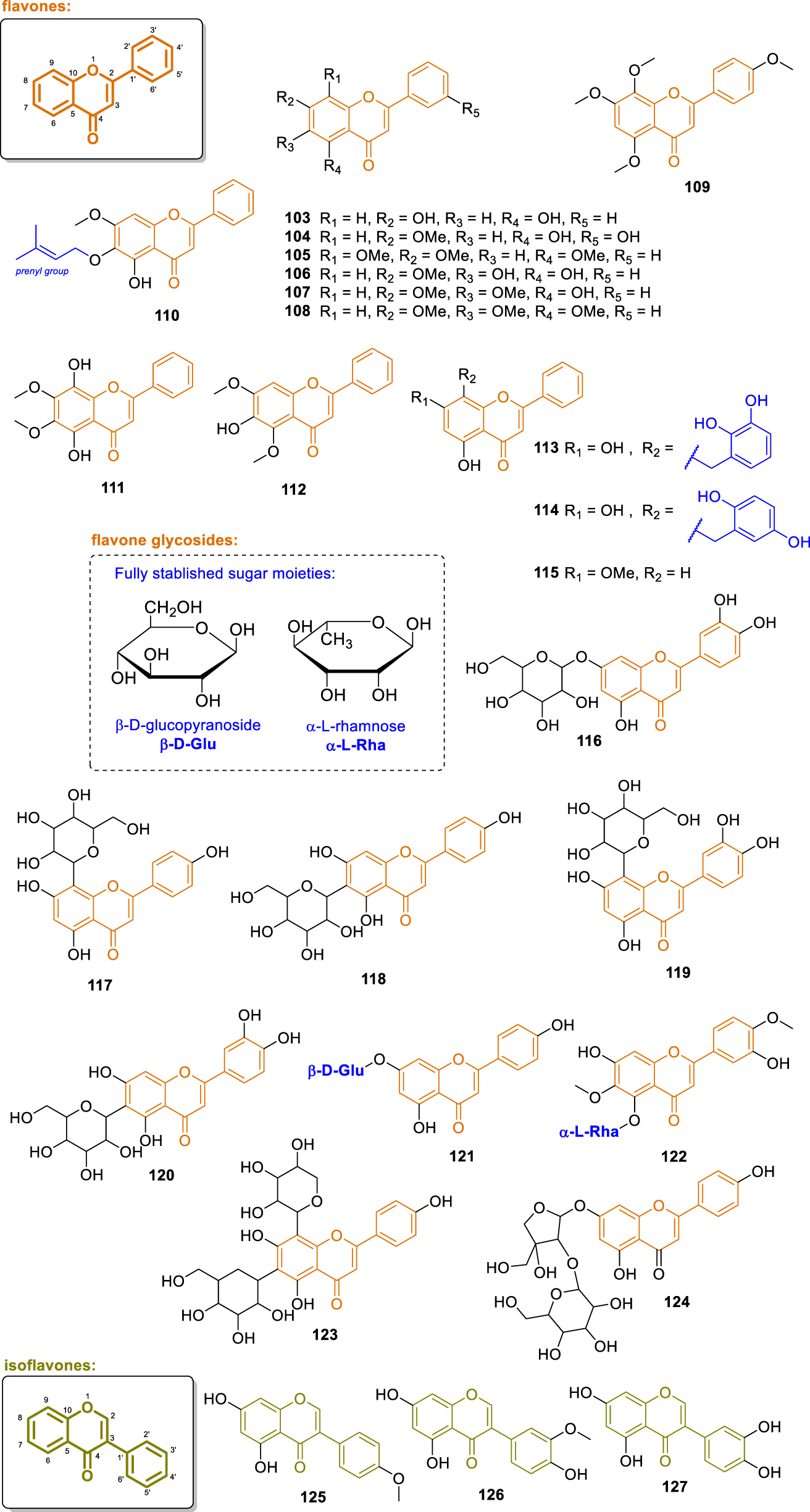
Chemical structures of flavones and isoflavones isolated from Annonaceae (103–127).
The chemical investigation of the leaves of Anomianthus dulcis yielded the isolation of chrysin (103) (Sinz et al., 1999). Compound 103, when isolated from other Annonaceae sources, was assayed in different models. When evaluated for its cytotoxicity, it exhibited activity against HepG2 and human acute lymphoblastic leukemia T (MOLT-3) cell lines, with IC50 values of 9.7 and 7.2 μg/mL, respectively (Bajgai et al., 2011). In the study by Saadawi et al. (2012), 103 showed strong dose-dependent inhibitory activity on prostaglandin E2 (PGE2) production, with an IC50 value of 25.5 μM, and also demonstrated inhibitory effects on thromboxane B2 (TXB2) production, with an IC50 = 39.3 μM. In the study by Hongnak et al. (2015), compound 103 exhibited cytotoxic activity against KB, HeLa, MCF-7, and HepG-2 cell lines, with IC50 values (μM) of 26.9, 33.9, 32.5, and 28.9, respectively. The study by Hsu et al. (2016) showed inhibitory effects of 103 on superoxide anion generation and elastase release, with IC50 values of 2.25 and 2.44 μM, respectively. The study by Meesakul et al. (2017) exhibited an inhibitory effect of 103 on NO production, with an IC50 = 7.56 μM. In other studies, α-glucosidase inhibitory activity was demonstrated for 103 (Meesakul et al., 2019; Do and Sichaem, 2022).
The compound 5,3′-dihydroxy-7-methoxyflavone (104) was isolated from the leaves of Friesodielsia discolor (Prawat et al., 2012). Compound 104 exhibited cytotoxic activity against the MCF-7 cell line, with an IC50 value of 3.45 μg/mL. In the study by Moriyasu et al. (2011), 5,7,8-trimethoxyflavone (105) was isolated from the roots of Uvaria welwitschia (from Kenya). From the leaves of Desmos chinensis collected in Vietnam, negletein (106) was isolated and showed inhibitory activity against NFAT transcription, with an IC50 = 3.89 μM (Kiem et al., 2005). Panichpol and Waterman (1978) isolated the compounds 5-hydroxy-6,7-dimethoxyflavone (107) and baicalein trimethyl ether (108) from the stems of Popowia cauliflora (from Cameroon). Compound 107, also known as isoflavone (Clement et al., 2017), exhibited inhibitory activity on superoxide anion generation with an IC50 value of 1.19 μM (Hsu et al., 2016). Moreover, the molecule tetramethylscutellarein (109) was isolated from the leaves of Cleistochlamys kirkii (from Tanzania) was reported (Nyandoro et al., 2017).
The chemical investigation of the stem bark of Melodorum fruticosum afforded the O-prenylated molecule melodorone A (110), which showed α-glucosidase inhibitory activity with an IC50 of 2.59 μM (Do and Sichaem, 2022). The compound 5,8-dihydroxy-6,7-dimethoxyflavone (111) was isolated from the leaves of Fissistigma lanuginosum (from Malaysia), displaying a fully substituted ring A (Alias et al., 1995). From the leaves and trunks of Uvaria flexuosa the compound 6-hydroxy-5,7-dimethoxyflavone (112) was isolated (Hsu et al., 2016). Furthermore, two compounds, named desmoscochinflavones A (113) and B (114), were isolated from the leaves of Desmos cochinchinensis (Meesakul et al., 2019). Compounds 113 and 114 contain a dihydroxybenzyl group on the A-ring, differing only in the position of the hydroxyl groups within this substituent. Moreover, they demonstrated α-glucosidase inhibitory activity, each with an IC50 value of 0.9 μM. In the study by Maeda et al. (2020), these compounds were also isolated but referred to with different names. Additionally, tectochrysin (115) was isolated from the leaves of Friesodielsia discolor (Prawat et al., 2012). Compound 115 exhibited antiplasmodial activity against Plasmodium falciparum (IC50 = 2.08 μg/mL) and cytotoxic activity against KB (IC50 = 14.82 μg/mL) and MCF-7 (IC50 = 4.49 μg/mL) cell lines.
From the heartwood of Anaxagorea luzonensis, collected in Thailand, Gonda et al. (2000) isolated three isoflavones: biochanin A (125), 3′-methylorobol (126), and orobol (127). In the B-ring, compound 125 contains a methoxy group; compound 126 contains both a methoxy and a hydroxyl group; and compound 127 contains two hydroxyl groups.
2.6 Flavone glycosides
About flavone glycosides, nine structures (Figure 8) were isolated from members of the Annonaceae family, notably from the genera Artabotrys, Alphonsea, and Annona. These compounds were primarily obtained from leaf extracts of species collected in Asia, including China, Thailand, India, and Malaysia. Structurally, these flavonoids include both C-glycosylated and O-glycosylated derivatives of common aglycones such as apigenin and luteolin. These glycosides demonstrated notable anti-inflammatory activity, as evidenced by their inhibitory effects on the production of PGE2, cyclooxygenase-2 (COX-2), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) in vitro. The occurrence of rare glycosylation patterns, including rhamnosides and apiosyl-glucosides, highlights the structural diversity and potential therapeutic relevance of flavone glycosides in Annonaceae species.
The phytochemical study with the leaves of Artabotrys hexapetalus (from China) yielded the compound glucoluteolin (116) (Li et al., 1997). Moreover, from the leaves of Alphonsea elliptica collected in Malaysia, Attiq et al. (2021) isolated vitexin (117), isovitexin (118), orientin (119), and isoorientin (120). These compounds are notable for the presence of a C-glycosidic group on the ring A of their structures. These substances exhibited inhibitory activity in PGE2 and COX-2 assays, with IC50 values of 20.5 and 16.6 μM, respectively, for compound 117; 17.7 and 12.8 μM for 118; 14.7 and 9.5 μM for 119; and 11.4 and 7.1 μM for 120. Furthermore, these compounds showed inhibitory activity in IL-1β and IL-6 assays, with IC50 values of 14.5 and 14.0 μM, respectively, for 117; 10.8 and 11.5 μM for 118; 6.2 and 5.9 μM for 119; and 4.8 and 4.0 μM for 120. In Thailand, Somanawat et al. (2012) isolated the compound apigenin 7-O-β-D-glucopyranoside (121) from the leaves of Artabotrys hexapetalus.
The molecule 5,7,4′-trihydroxy-6,3′-dimethoxyflavone 5-O-α-L-rhamnopyranoside (122) was isolated from the leaves of Annona squamosa (from India) (Panda and Kar, 2015). Additionally, the compound schaftoside (123) was isolated from the leaves of Alphonsea elliptica (Attiq et al., 2021). Compound 123 exhibited inhibitory activity in the PGE2 assay, with an IC50 value of 77.6 μM. Furthermore, the compound apigenin 7-O-apiosyl (1→2) glucoside (124) was isolated from the leaves of Artabotrys hexapetalus (Li et al., 1997).
2.7 Flavonol aglycones
Regarding flavonol aglycones, 19 distinct structures (Figure 9) have been isolated from various Annonaceae species, particularly from genera such as Anaxagorea, Duguetia, Goniothalamus, Melodorum, Miliusa, Alphonsea, and Friesodielsia. These flavonols were primarily obtained from different plant parts, including leaves, stem bark, heartwood, fruits, and aerial parts, collected across Asia, Africa, and South America. This group encompasses widely known natural flavonols, such as quercetin, kaempferol, and myricetin, as well as a range of polymethoxylated or hydroxylated derivatives. Some of these compounds exhibited notable biological activities, including anti-inflammatory, urease-inhibitory, antioxidant, and antiparasitic effects. The widespread occurrence of this subclass in Annonaceae underscores its chemodiversity and therapeutic potential, particularly given the broad spectrum of bioactivities reported for several of these molecules.
FIGURE 9
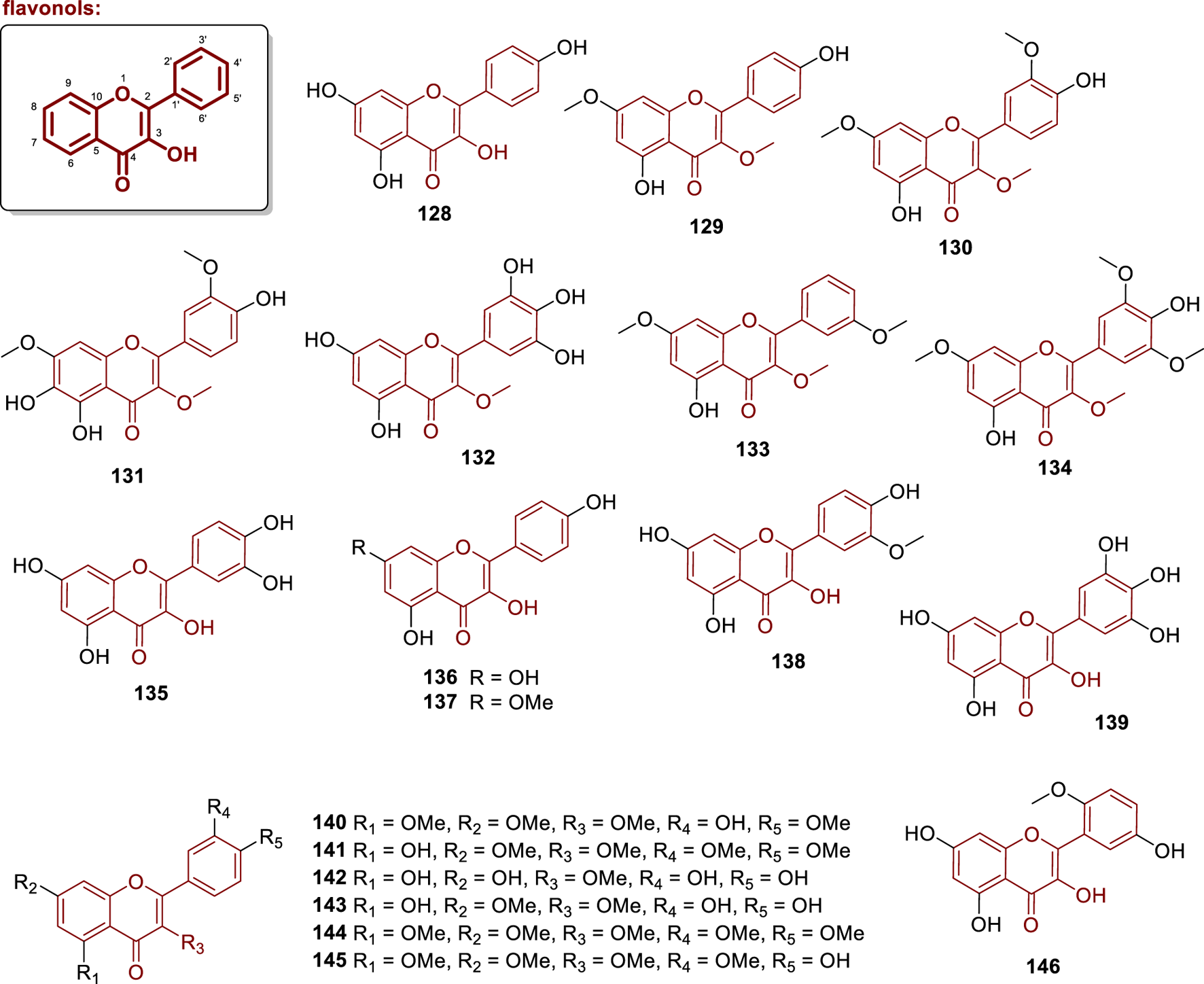
Chemical structures of flavonol aglycones isolated from Annonaceae (128–146).
The compound aromadendrin (128) was isolated from the heartwood of Anaxagorea luzonensis (Sabphon et al., 2015). Ngouonpe et al. (2019) isolated the compounds kumatakenin (129) and pachypodol (130) from the stem barks of Duguetia staudtii (from Cameroon). Compounds 129 and 130 exhibited potent urease inhibitory activity, with IC50 values of 17.5 and 14.5 μg/mL, respectively, as well as strong anti-inflammatory activity by inhibiting both the myeloperoxidase-dependent (luminol/zymosan) and -independent (lucigenin/PMA) oxidative burst, with potencies expressed as IC50 ranging from 4.99 to 14.13 μg/mL (Ngouonpe et al., 2019). Additionally, the compounds 5-hydroxy-3,7-dimethoxy-2-(3-methoxyphenyl)-4H-1-benzopyran-4-one (133) and 5,4′-dihydroxy-3,7,3′,5′-tetramethoxyflavone (134) were also isolated by Ngouonpe et al. (2019). Compound 134 exhibited potent urease inhibitory activity (IC50 = 10.9 μg/mL) and strong anti-inflammatory activity by inhibiting both myeloperoxidase-dependent and -independent oxidative burst, with IC50 values ranging from 3.89 to 8.55 μg/mL. Furthermore, from the leaves of Miliusa balansae, the compound chrysosplenol C (131) was described (Thao et al., 2015), while annulatin (132) was isolated from the aerial parts of Goniothalamus gardneri, (from India) (Seidel et al., 2000).
In the work by Gonda et al. (2000), quercetin (135), kaempferol (136) and 3,5,7,4′-tetrahydroxy-2′-methoxyflavone (146) were isolated from the heartwood of Anaxagorea luzonensis. These molecules were repeatedly isolated from different Annonaceae sources, and their biological activities were reviewed elsewhere (Wang et al., 2022; Periferakis et al., 2022). The compound kaemferide (137) was isolated from the fruits of Melodorum siamensis (Jaidee et al., 2019), while isorhamnetin (138) was obtained from leaves and twigs of Duguetia furfuracea (from Brazil) (Carollo et al., 2006). Compound 138 exhibited antiparasitic activity against Trypanosoma cruzi, with an IC50 = 4.63 μM. Additionally, the compound myricetin (139) was isolated from the leaves of Alphonsea elliptica (Attiq et al., 2021). The molecules 3′-hydroxy-3,5,7,4′-tetramethoxyflavone (140), 5-hydroxy-3,7,3′,4′-tetramethoxyflavone (141), 5,7,3′,4′-tetrahydroxy-3-methoxyflavone (142), 5,3′,4′-trihydroxy-3,7-dimethoxyflavone (143), 3,5,7,3′,4′-pentamethoxyflavone (144), and 4′-hydroxy-3,5,7,3′-tetramethoxyflavone (145) were isolated from the leaves of Goniothalamus tenuifolius (from Thailand) (Likhitwitayawuid et al., 2006). Among these, only compounds 143 and 144 exhibited antioxidant activity, with IC50 values of 6.4 and 5.8 μM, respectively.
2.8 Flavonol glycosides
Flavonol glycosides represent the most diverse flavonoid subclass identified in Annonaceae, comprising at least 59 isolated compounds (Figures 10–13) from a wide range of species, particularly from genera such as Annona, Goniothalamus, Uvaria, Artabotrys, Fissistigma, Ellipeiopsis, Porcelia, Dasymaschalon, and Melodorum. These glycosides are predominantly O-glycosylated at the C-3 position of the aglycone and feature mono-, di-, or trisaccharide moieties composed of glucose, galactose, rhamnose, arabinose, xylose, or apiose, often in branched configurations. Several derivatives also contain acyl substituents, such as p-coumaroyl, feruloyl, or sinapoyl groups. The structural complexity is reflected in a wide array of biological properties, including antioxidant, cytotoxic, anti-inflammatory, and larvicidal activities. This high structural variability and consistent biological potential underscore the phytochemical richness and pharmacological relevance of flavonol glycosides within the family.
FIGURE 10
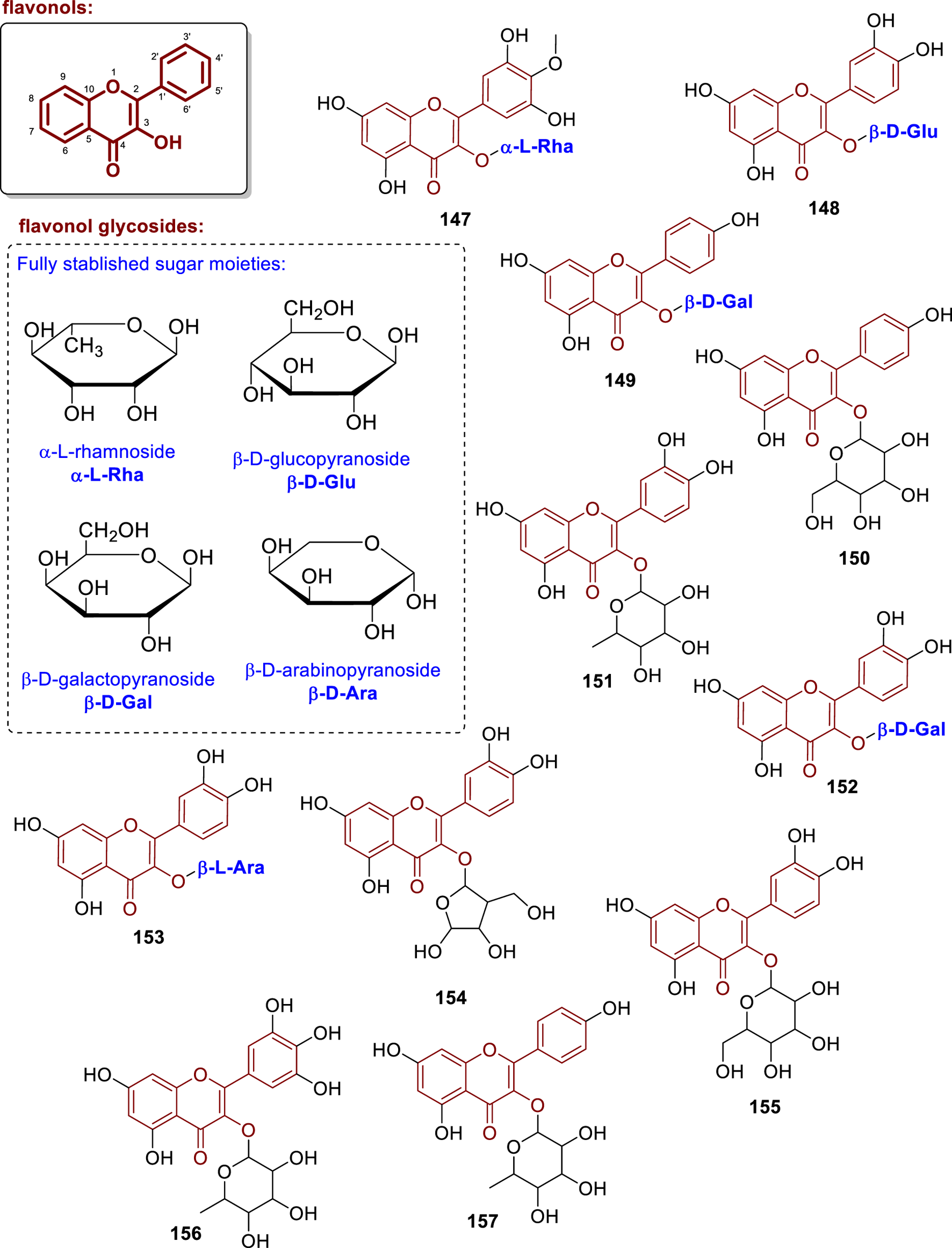
Chemical structures of flavonol glycosides isolated from Annonaceae (147–157).
FIGURE 11
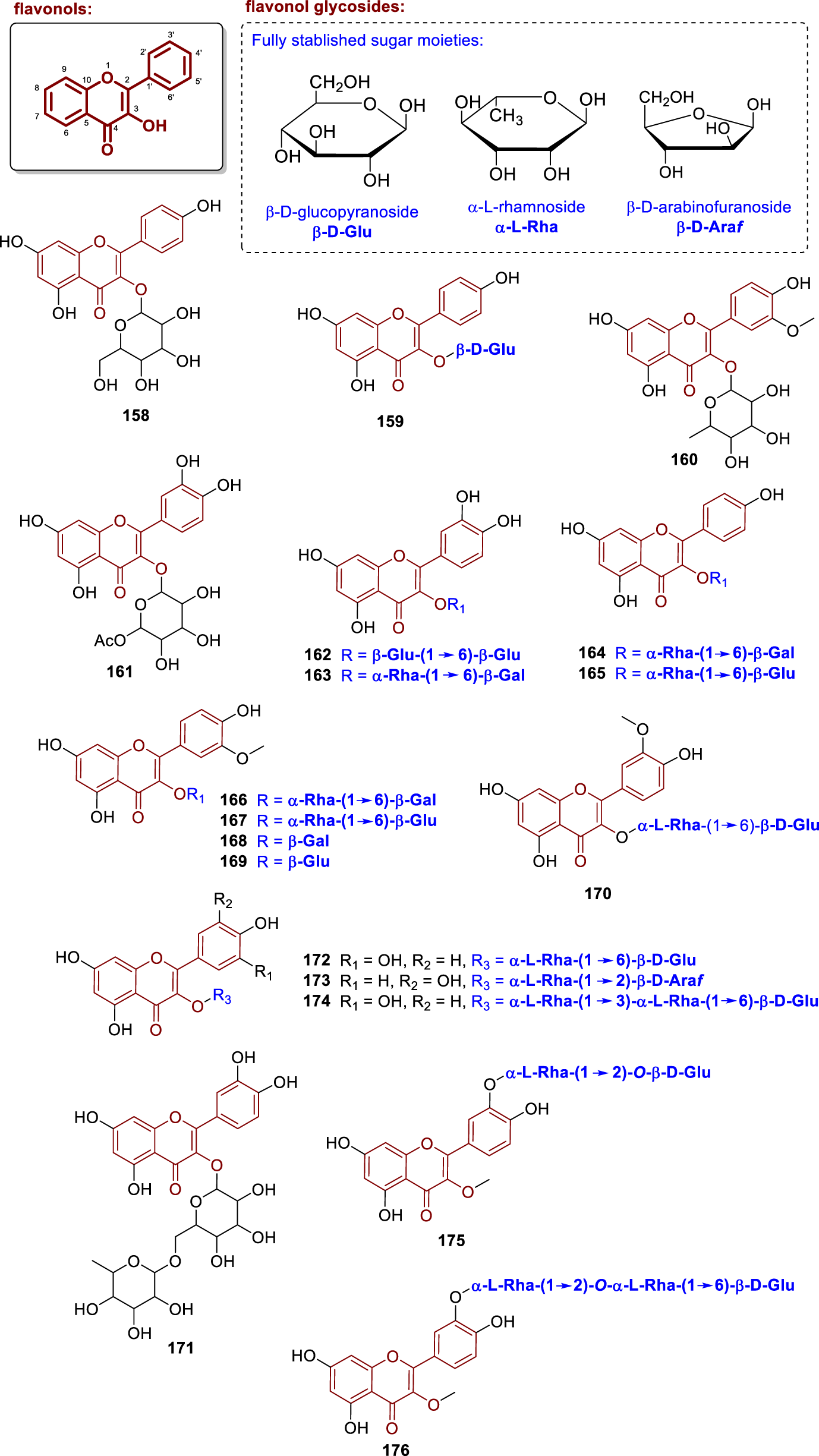
Chemical structures of flavonol glycosides isolated from Annonaceae (158–176).
FIGURE 12
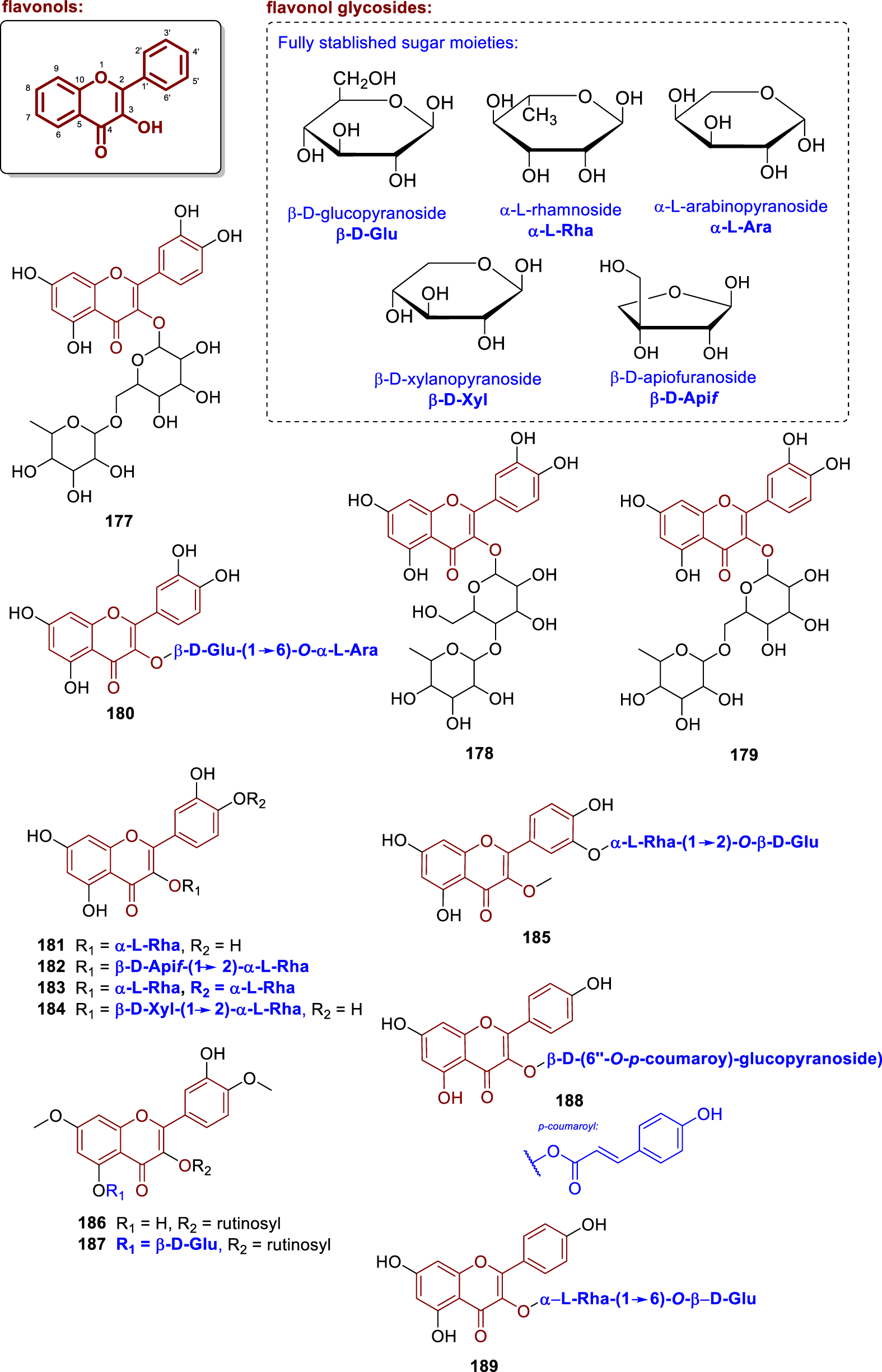
Chemical structures of flavonol glycosides isolated from Annonaceae (177–189).
FIGURE 13
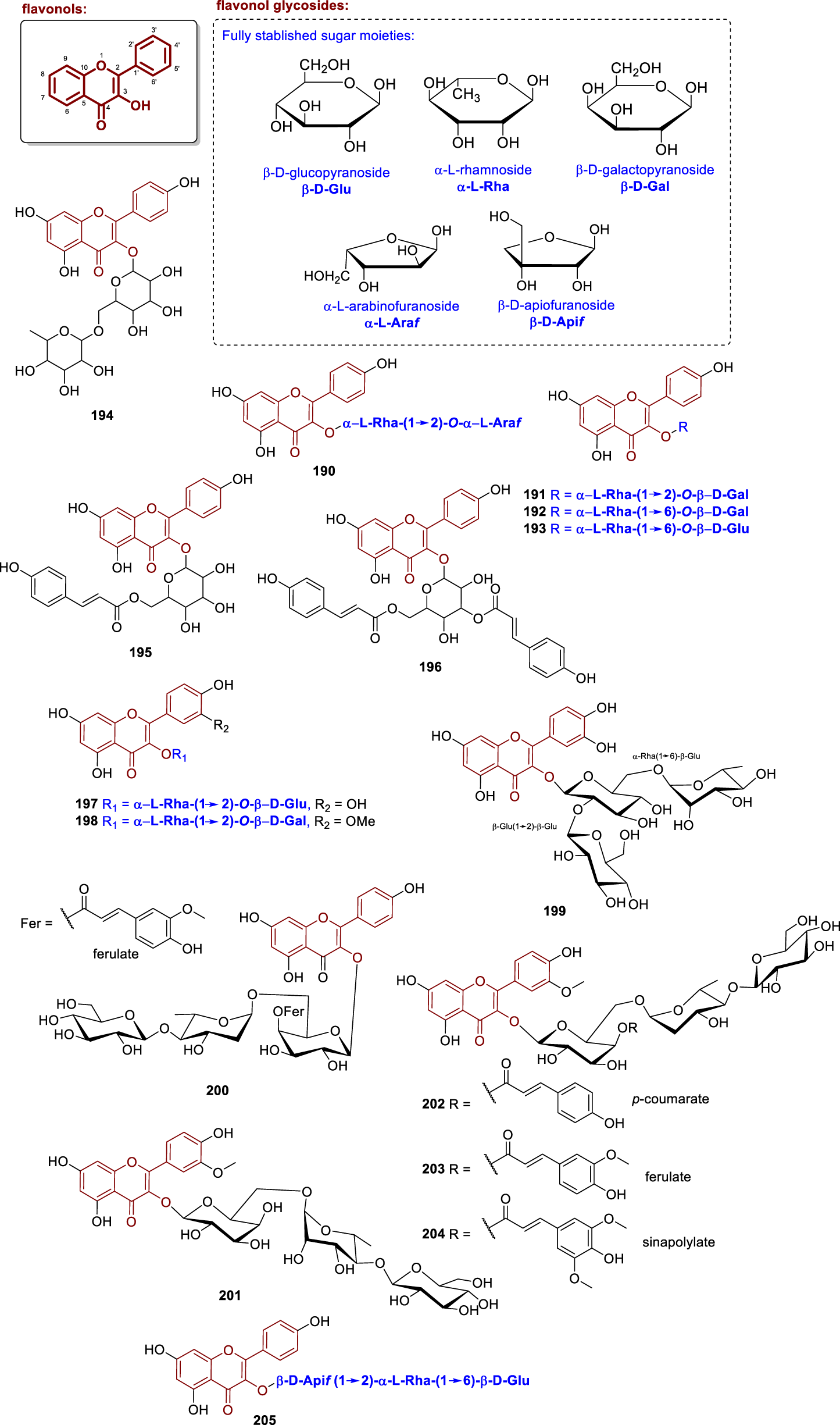
Chemical structures of flavonol glycosides isolated from Annonaceae (190–205).
This group of substances is widespread among Annonaceae with several examples, such as the isolation of mearnsitrin (147), which contains an O-α-L-rhamnopyranose at C-3 from the aerial parts of Goniothalamus gardneri (Seidel et al., 2000). The compound isoquercitrin (148) was first isolated in Annonaceae from the leaves of Uvaria rufa (Deepralard et al., 2009). Later, it was re-isolated and pharmacologically evaluated by Ngoc et al. (2019), showing prevention of intracellular ROS formation in BEAS-2B cells treated with AAPH, with an IC50 = 24.1 μM. Moreover, in the work of Zhu et al. (2020), this compound exhibited antioxidant activity, with an IC50 value of 69.13 μg/mL. Furthermore, the compounds kaempferol 3-O-β-D-galactopyranoside (149) (leaves of Annona dioica, Paraguay) (Vega et al., 2007) and 3-O-β-glucoside (150) (leaves of Annona classiflora, Brazil) (Rocha et al., 2016) were isolated, and solely 149 had the glycosyl moiety fully established. Additionally, quercetin-3-O-rhamnoside (151) was isolated from the leaves of Annona purpurea (From Taiwan) (Chang et al., 1998).
From the leaves of Annona crassiflora (from Brazil), the compounds quercetin-3-O-β-D-galactopyranoside (152) and quercetin-3-O-β-L-arabinopyranoside (153), which exhibited larvicidal activity with LD50 values of 103.74 and 109.52 μg/mL, respectively (Lage et al., 2014). Additionally, quercetin-3-O-arabinofuranoside (154) from the leaves of Xylopia emarginata (from Brazil) (Moreira et al., 2003), quercetin-3-O-β-galactoside (155) and kaempferol-3-O-β-galactoside (158) from the leaves of Annona dioica (from Brazil) (Formagio et al., 2013), tanarixetin-3-O-rhamnoside (156) and kaempferol-3-O-rhamnoside (157) from Annona purpurea (from Taiwan) (Chang et al., 1998) were isolated, but the absolute stereochemistry of the glycosyls not established.
Astragalin (159), displaying a 3-O-β-D-glucopyranoside, was isolated from the leaves of Uvaria rufa (Deepralard et al., 2009). Compound 159, when evaluated by Araujo-Padilla et al. (2022), displayed cytotoxic activity against human immortalized keratinocyte cell line (HaCaT), HeLa, HepG2, and human triple-negative breast cancer cell line (MDA-MB-231) cell lines, with IC50 values of 170.2, 92.85, 81.70, and 84.28 μg/mL, respectively. Besides, the compounds isorhamnetin-3-O-rhamnoside (160) from the leaves of Annona purpurea (Chang et al., 1998), isoquercitrin-6-acetate (161) from the leaves of Uvaria rufa (Deepralard et al., 2009) were isolated.
The compounds quercetin-3-O-gentiobioside (162), quercetin-3-O-robinobioside (163), biorobin (164), nicotiflorin (165), keioside (166), narcissin (167), cacticin (168), and isorhamnetin-3-O-β-D-glucoside (169) were isolated from the leaves of Annona coriacea (from Brazil) (Novaes et al., 2018). Compound 162 features two glucose units connected via a β1→6 glycosidic bond, whereas 163 has a galactose at C-3 linked to a rhamnose at C-6″ (β1→6α). Compounds 164 and 165 follow the same pattern but with kaempferol as the aglycone. Compounds 166 and 167 also share this linkage pattern, with isorhamnetin as the aglycone. Finally, 168 and 169 are monoglycosides containing galactose and glucose, respectively, directly linked at the C-3 position of isorhamnetin (Novaes et al., 2018).
From the leaves of Fissistigma acuminatissima, Van et al. (2007) isolated isorhamnetin-3-O-rutinoside (170), a diglycosylated flavonoid whose structure contains a glucose unit in pyranoside form, with β-anomeric and D-absolute configurations, O-glycosidically linked to the hydroxyl group at the C-3 position of the aglycone. Furthermore, this glucose is branched at position 6″ by a rhamnose unit in pyranoside form through an (α1→6β) linkage. Moreover, quercetin-3-O-β-glucoside-6-O-α-rhamnoside (171) was isolated from the pulp of Annona cacans (from Brazil) (Volobuff et al., 2019).
Somanawat et al. (2012), studied the leaves of Artabotrys hexapetalus (from Thailand) and isolated the compound quercetin 3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (172) and 3-O-α-L-rhamnopyranosyl-(1→3)-O-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside] (174). Compound 174 is a triglycosylated flavonoid with a glucose unit in pyranoside form, bearing β-anomeric and D-absolute configuration, O-glycosidically linked to the hydroxyl group at the C-3 position of quercetin. The glucose is branched at positions 6″ and 3″ by two rhamnose units in pyranoside form, both with α-anomeric and L-absolute configuration, via (α1→6α) and (α1→3β) linkages, respectively. Moreover, the compound arapetaloside A (173) was isolated from the leaves of Artabotrys hexapetalus (from China) by Li et al. (1997). This compound contains an arabinose unit in furanoside form, with α-anomeric and L-absolute configuration, O-glycosidically linked to the C-3 position of quercetin. In addition, this arabinose is branched at position 2″ by a rhamnose unit in pyranoside form, linked via an (α1→2β) bond.
The compounds quercetin-3,7-dimethyl ether 3′-O-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranoside (175) and quercetin-3,7-dimethyl ether 3′-O-α-L-rhamnopyranosyl-(1→2)-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (176) were isolated from the leaves of Dasymaschalon sootepense (from Thailand) (Sinz et al., 1998). These compounds differ by the presence of an additional rhamnose unit at the 6″ position of the glucose moiety in compound 176, whereas compound 175 contains only the rhamnose at position 2″ of the galactose unit.
Furthermore, other analogues were isolated from different Annonaceae sources, such as 3-O-robinoside (177) and quercetin 3-O-neohesperidoside (178) from the leaves of Annona muricata (from Egypt) (Nawwar et al., 2012), quercetin 3-O-rutinoside (rutin, 179) from the leaves of Uvaria rufa (Deepralard et al., 2009), quercetin-3-O-β-D-glucopyranosyl-(1→6)-O-α-L-arabinoside (180) from the leaves of Annona crassiflora (Lage et al., 2014) and quercetin 3-O-α-L-rhamnopyranoside (181) from the leaves of Xylopia emarginata (from Brazil) (Moreira et al., 2003). Compound 181 was re-isolated by Ngoc et al. (2019) and had its potential to prevent intracellular ROS formation in BEAS-2B cells treated with AAPH evaluated, reporting an IC50 of 18.8 μM.
The compounds quercetin 3-O-β-D-apiofuranosyl-(1→2)-α-L-rhamnopyranoside (182), quercetin 3,3′-O-di-α-L-rhamnopyranoside (183), quercetin 3-O-β-D-xylopyranosyl-(1→2)-α-L-rhamnopyranoside (184), and quercetin 3-methoxy-3′-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (185) were isolated from the stems of Fissistigma polyanthoides (from Vietnam) (Ngoc et al., 2019). Compounds 182–184 were evaluated for their antioxidant activity in BEAS-2B cells, with the following IC50 values for inhibition of intracellular ROS formation: 38.5 μM (182), 18.8 μM (183), 35.3 μM (184).
From the branches of Porcelia macrocarpa collected in Brazil, Chaves et al. (2004) isolated the glycosylated flavonoids quercetin-3-O-rutinoside-7,4′-dimethyl ether (186) and quercetin-5-O-glycoside-3-O-rutinoside-7,4′-dimethyl ether (187). Compound 186 contains two sugar units (rutinosides) and two methoxyl groups at positions 7 and 4′ of the flavonoid core, with a free hydroxyl group at C-5. In comparison, compound 187 contains three sugar units (rutinoside and glucoside), however the absolute stereochemistry was not determined. On the other hand, Wirasathien et al. (2006) isolated the compound tiliroside (188) from the aerial parts of Ellipeiopsis cherrevensis (from Thailand). Compound 188 consists of a kaempferol core glycosylated at position C-3 with a β-D-glucopyranose unit, which is acylated at position 6″ with a p-coumaroyl group. Furthermore, the leaves of Annona squamosa, collected in China, were studied by Zhu et al. (2020), from which the compound kaempferol-3-O-robinobioside (189) was isolated. Additionally, antioxidant activity was reported, with an IC50 value of 191.67 μg/mL in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. Compound 189 consists of a kaempferol unit linked at position C-3 to a robinobiose moiety, established as L-Rha(α1→6 β)D-Glu.
From the leaves of Artabotrys hexapetalus, the compound arapetaloside B (190) was isolated and determined to contain two sugar units, forming a diglycoside in which a rhamnose is linked to an arabinose, which is in turn attached to kaempferol at the C-3 position (Li et al., 1997). Moreover, from the leaves of Fissistigma pallens, collected in Vietnam, three glycosylated flavonoids: kaempferol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranoside (191), kaempferol 3-O-α-L-rhamnopyranosyl-(1→6)-β-D-galactopyranoside (192), and kaempferol 3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (193) were isolated (Nhiem et al., 2019). These compounds were evaluated for cytotoxic potential against human colorectal adenocarcinoma (HT-29), human malignant melanoma (A-2058), and human lung adenocarcinoma (A-549) cell lines. Compound 191 showed IC50 values of 141.9 µM, 144.9 µM, and 136.2 µM, respectively. Compound 192, the observed values were 150.5 µM, 143.6 µM, and 149.0 µM. Compound 193 exhibited IC50 values of 147.4 µM, 143.3 µM, and 145.7 µM against the same cell lines, respectively. Additionally, from the aerial parts of Ellipeiopsis cherrevensis, the compound kaempferol 3-O-rutinoside (194) was isolated (Wirasathien et al., 2006).
Vega et al. (2007) isolated the flavonoids 6″-O-p-hydroxycinnamoyl-β-galactopyranosyl-kaempferol (195) and 3-O-[3″,6″-di-O-p-hydroxycinnamoyl]-β-galactopyranosyl-kaempferol (196) from the leaves of Annona dioica. Both compounds were evaluated for cytotoxic activity against Ehrlich carcinoma cells, exhibiting IC50 values of 14.7 µM for compound 195 and 10.9 µM for compound 196. Moreover, the flavonoids rhamnetin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (197) and isorhamnetin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranoside (198) were isolated from the leaves of Fissistigma pallens (Nhiem et al., 2019). Both compounds were evaluated for cytotoxic activity against HT-29, A-2058, and A-549 cell lines, showing IC50 values of 137.9, 156.6, and 144.2 µM for compound 197 and 150.0, 153.8, and 141.3 µM for compound 198, respectively. Moreover, the same study described the flavonoids fissflavoside A (202), fissflavoside B (203), and fissflavoside C (204). These compounds are acylated kaempferol triglycosides, with a trisaccharide linked at position 3 of the aglycone, composed of β-D-galactopyranose (acylated at position 4), L-Rha(α1→6), and D-Glu(β1→4). The difference among the three compounds lies in the acyl group present on the galactose unit: p-coumaroyl in 202, feruloyl in 203 and sinapoyl in 204. All three flavonoids were evaluated for their cytotoxic activity against HT-29, A-2058, and A-549 cell lines, with IC50 values of 150.5, 162.6, and 161.7 µM for 202, 161.6, 149.1, and 160.4 µM for 203 and 158.7, 158.7, and 155.7 µM for 204, respectively (Nhiem et al., 2019).
Furthermore, the compound quercetin 3-O-α-rhamnosyl-(1″→6″)-β-sophoroside (199) was isolated from the leaves of Annona muricata (Nawwar et al., 2012). The chemical investigation of the leaves of Fissistigma maclurei collected in Vietnam, yielded the isolation of the compounds kaempferol 3-O-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→6)-[4-(E)-feruloyl]-β-D-galactopyranoside (200) and fissmacoside A (201) (Ba et al., 2020). Compound 202 is a triglycosylated flavonol consisting of an isorhamnetin moiety linked at position C-3 to a trisaccharide composed of D-Glu(β1→4α)L-Rha(α1→6β)D-Gal. Finally, the leaves of Melodorum fruticosum were studied by Chan et al. (2013), who isolated the flavonoid kaempferol 3-O-β-D-apiofuranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside (205).
2.9 Dihydroflavonols
Dihydroflavonols are less frequently reported in Annonaceae but include notable representatives characterized by 2,3-dihydro-2-phenylchromen-4-one cores with hydroxylation patterns similar to their flavonol counterparts. A total of five compounds (Figure 14) have been identified, primarily featuring glycosylation at the C-3 position, with sugar residues such as glucose, galactose, quinovose, and rhamnose. The absolute stereochemistry, predominantly (2R, 3R), was confirmed for several compounds through circular dichroism analysis or comparison with authentic standards. While structurally simpler than other subclasses, dihydroflavonols have demonstrated antioxidant and moderate enzyme-inhibitory activities, as evidenced by their ability to inhibit ROS. These findings indicate that, despite their lower diversity, dihydroflavonols represent a biologically relevant but underexplored class within the Annonaceae.
FIGURE 14
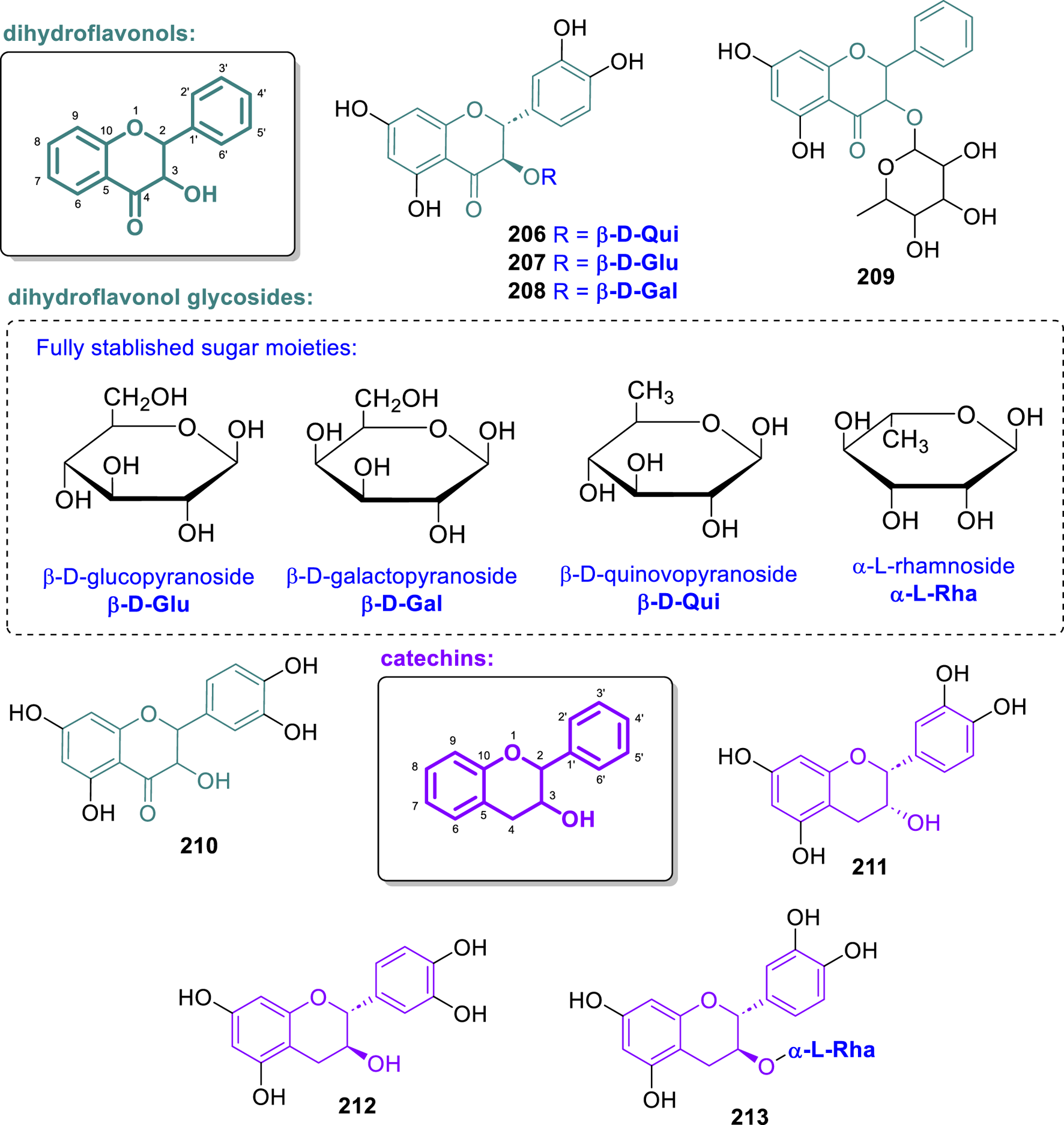
Chemical structures of dihydroflavonols and catechins isolated from Annonaceae (206–213).
The compounds (2R,3R)-taxifolin-3-O-β-D-quinovopyranoside (206), (2R,3R)-taxifolin-3-O-β-D-glucopyranoside (207), and (2R,3R)-taxifolin-3-O-β-D-galactopyranoside (208) were isolated from the stems of Fissistigma polyanthoides (Ngoc et al., 2019). The absolute configuration of the isolated compounds was determined by circular dichroism. Compounds 206 and 207 demonstrated the ability to inhibit the intracellular formation of reactive oxygen species (ROS) in BEAS-2B cells, with IC50 values of 61.4 μM and 63.5 μM, respectively. Moreover, from the leaves of Desmos chinensis, collected in Vietnam, the compound astilbin (209), a dihydroflavonol 3-O-α-L-rhamnoside, was isolated, as reported by Kiem et al. (2005). Its absolute configuration was determined as (2R,3R) by comparison with literature data. In the NFAT-mediated transcription inhibition assay, 209 did not show an IC50 lower than 50 μM. Besides, from the leaves of Artabotrys hexapetalus, collected in China, taxifolin (210) was isolated (Li et al., 1997). Compound 210 exhibited inhibitory activity against phosphodiesterase type 5 (PDE5), with inhibition rates of 14.0% and 7.8% at concentrations of 100 μM and 10 μM, respectively (Sabphon et al., 2015).
2.10 Catechins
A total of three catechin-related compounds (Figure 14) have been isolated primarily from the leaves and stems of species belonging to the genera Annona and Fissistigma. The compound (−)-epicatechin (211) was isolated from the leaves of Annona classiflora, (Lage et al., 2014). Its antimicrobial activity was evaluated, showing a growth inhibition (GI) of 86% against Staphylococcus aureus and 5.5% against Escherichia coli. In the study by Ngoc et al. (2019), this compound exhibited an IC50 of 47.0 µM in the prevention of intracellular reactive oxygen species (ROS) formation in BEAS-2B cells treated with AAPH. Moreover, the chemical study of the leaves of Fissistigma acuminatissima (from Vietnam) was isolated (+)-catechin (212). Ngoc et al. (2019) studied the stems of Fissistigma polyanthoides and isolated catechin-3-O-α-L-rhamnopyranoside (213). This compound showed an IC50 value of 47.9 µM in the prevention of intracellular ROS formation in BEAS-2B cells treated with AAPH.
2.11 Miscellaneous compounds
A diverse array of 25 structurally complex flavonoid-related compounds (Figure 15), classified as miscellaneous derivatives, has been isolated from various Annonaceae species. These compounds include rare biflavonoids, sesquiterpene-flavonoid hybrids, flavan–flavanone dimers, and acylated flavonoids, among others. The majority were obtained from the leaves and branches, with a few isolated from roots and aerial parts. Genera such as Fissistigma, Desmos, Friesodielsia, Goniothalamus, and Uvaria were the primary sources of these unusual metabolites.
FIGURE 15
Chemical structures of miscellaneous compounds isolated from Annonaceae (214–238).
The phytochemical study of the leaves and branches of Fissistigma bracteolatum collected in Vietnam, afforded four compounds: fissistigmatins A (214), B (215), C (216), and D (217) (Porzel et al., 2000). Compounds 214–217 are hybrids of flavonoids with sesquiterpenes. The absolute configuration was determined by X-ray crystallography and molecular modeling studies, with congeners A, C, and D assigned as (4R), and fissistigmatin B as (4S). Furthermore, from the leaves of Desmos cochinchinensis (from Thailand) were isolated the compounds desmosflavan A (218) and B (219), characterized as flavan-chalcones dimers (Bajgai et al., 2011). Their absolute configurations were determined by circular dichroism as (2S,4S) for compound 218 and (2S,4R) for compound 219. In the same study, compound 218 exhibited lipoxygenase inhibitory activity with an IC50 of 4.4 µM, whereas compound 219 was inactive. In cytotoxicity assays, compound 218 showed activity against HuCCA-1 (2.25 μg/mL), HepG2 (3.75 μg/mL), A549 (2.45 μg/mL), and MOLT-3 (0.29 μg/mL), while compound 219 exhibited IC50 values of 22.0 μg/mL, 20.3 μg/mL, 27.0 μg/mL, and 1.71 μg/mL against the same cell lines, respectively (Bajgai et al., 2011).
The compounds friesodielsones A (220), B (221), and C (222), classified as flavan–flavanones dimers, were isolated from the leaves and branches of Friesodielsia desmoides (Meesakul et al., 2017). Their absolute configurations were determined as 2S,4S based on circular dichroism analysis. Compound 220 exhibited an IC50 of 10.21 µM in nitric oxide inhibition assays in J774.A1 macrophages. Moreover, Rittiwong et al. (2011), in their study of the leaves of Desmos chinensis collected in Thailand, isolated saiyutones A (223), B (224), C (225), and D (226), unusual biflavonoids. Compounds 223 and 224 possess a unique biflavonoid skeleton with a 3–6″ linkage through a methylene group, while the formation of a cyclic hemiacetal was proposed as a key step in the biosynthetic pathway of saiyutones 225 and 226.
The flavan derivatives 5,7-dimethoxy-8-formyl-4-hydroxy-6-methylflavan (227), 5-methoxy-8-formyl-4,7-dihydroxy-6-methylflavan (228), 5,7-dimethoxy-4-hydroxy-6,8-dimethylflavan (229), 5-methoxy-8-formyl-4,7-dihydroxyflavan (230) and 4,5-dimethoxy-8-formyl-7-hydroxyflavan (231) were isolated from the roots of Desmos cochinchinensis (Prachyawarakorn et al., 2013). Compounds 227–231exhibited aromatase inhibitory activity with IC50 values of 80.0 nM, 1,010 nM, 10,700 nM, 40.0 nM, and 90.0 nM, respectively. Furthermore, Lan et al. (2012), in their study of Fissistigma latifolium, isolated 2,5,6,7-tetramethoxyflavan (232), which showed superoxide anion inhibition activity in fMLP (N-formylmethionyl-leucyl-phenylalanine)/cytochalasin B-activated human neutrophils, with an IC50 of 6.0 µM.
From the roots of Uvaria welwitschii collected in Kenya were isolated the compounds dependensin (233), welwitschin E (234), welwitschin F (235), welwitschin G (236), and welwitschin H (237) (Moriyasu et al., 2011). Additionally, from the aerial parts of Goniothalamus gardneri was isolated (rel)-1β,2α-di-(2,4-dihydroxy-6-methoxybenzoyl)-3β,4α-di-(4-methoxyphenyl)-cyclobutane (238), a symmetric dimer derived from dihydroxychalcones (Seidel et al., 2000). This compound features a symmetrical chalcone dimer structure, containing a cyclobutane ring substituted with two identical A/A′ rings bearing 2,4-dihydroxy-6-methoxy groups and two identical B/B′ rings with 4-methoxyphenyl groups. The presence of a symmetry plane and the arrangement of the aromatic rings in opposite positions allowed the assignment of the relative stereochemistry as (rel)-1β,2α,3β,4α.
A comprehensive summary of all identified flavonoid compounds and their reported biological activities is presented in Supplementary Table S1 at the end of this article.
3 Concluding remarks
This review highlights the remarkable, yet underexplored, flavonoid diversity within the Annonaceae family. Our analysis confirms that flavonol glycosides are the most frequently reported class of flavonoids, closely followed by flavanones. Other structural types such as chalcones, dehydrochalcones, isoflavanones, flavonol aglycones, dehydroflavonols, catechins, and miscellaneous compounds were also identified, underscoring a broad chemical spectrum within the family. Notably, prior reports have identified glycosides of both flavones (e.g., apigenin, luteolin) and flavonols (e.g., kaempferol, quercetin, isorhamnetin), with a clear predominance of flavonol derivatives across multiple species. Geographically, Asia emerges as the most significant contributor to the current knowledge base, with Thailand and Vietnam standing out due to the high frequency of studies. Additional contributions from Malaysia, India, Bangladesh, Taiwan, and China reinforce Asia’s significant role in the phytochemical investigation of Annonaceae. Africa also plays a prominent part, especially through work conducted in Tanzania, Ghana, Nigeria, Kenya, and Cameroon. In the Americas, Brazil leads the regional representation, followed distantly by Mexico and Paraguay. Notably, no relevant reports were identified from Europe, Oceania, or Antarctica, reflecting potential gaps in geographic sampling or research prioritization. The most cited genus across studies was Uvaria, with numerous species such as U. lucida, U. chamae, U. ferruginea, and U. angolensis frequently appearing in the literature. Melodorum was also highly represented, especially M. fruticosum, followed by genera such as Fissistigma, Annona, Desmos, Friesodielsia, Goniothalamus, Xylopia, and Anaxagorea. These genera demonstrate not only high species richness but also strong research interest due to their phytochemical potential. The analysis of plant material sources revealed a marked preference for leaf extracts, which are by far the most commonly used in flavonoid studies within Annonaceae. This is followed by extracts from roots and stem bark, while branches and fruits have been investigated less frequently. In summary, while significant strides have been made in identifying flavonoids within Annonaceae, this review underscores a critical need for more systematic and integrative research. A clearer understanding of flavonoid distribution, biosynthesis, and biological roles across the family could illuminate new avenues in pharmacology, taxonomy, and plant ecology.
Statements
Author contributions
IG: Writing – review and editing, Writing – original draft, Methodology, Data curation. MB: Writing – review and editing, Data curation, Methodology, Writing – original draft. MP: Writing – review and editing. FD: Data curation, Validation, Software, Project administration, Formal Analysis, Methodology, Visualization, Funding acquisition, Conceptualization, Writing – review and editing, Investigation, Supervision, Resources, Writing – original draft. HK: Project administration, Writing – original draft, Supervision, Data curation, Visualization, Methodology, Formal Analysis, Validation, Conceptualization, Software, Investigation, Funding acquisition, Resources, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) – Finance Code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grants: 315040/2021-1; 302141/2025-1 and 443823/2024-3 - Chamada CNPq/MCTI/FNDCT N° 19/2024 PRO AMAZONIA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fntpr.2025.1657095/full#supplementary-material
References
1
Achenbach H. Höhn M. Waibel R. Nkunya M. H. Jonker S. A. Muhie S. (1997). Oxygenated pyrenes, their potential biosynthetic precursor and benzylated dihydroflavones from two African Uvaria species. Phytochemistry44 (2), 359–364. 10.1016/S0031-9422(96)00448-7
2
Al Kazman B. S. Harnett J. E. Hanrahan J. R. (2022). Traditional uses, phytochemistry and pharmacological activities of Annonacae. Molecules27 (11), 3462. 10.3390/molecules27113462
3
Alias Y. Awang K. Hadi A. H. Thoison O. Sévenet T. Pais M. (1995). An antimitotic and cytotoxic chalcone from Fissistigma lanuginosum. J. Nat. Prod.58 (8), 1160–1166. 10.1021/np50122a002
4
Araujo-Padilla X. Ramón-Gallegos E. Díaz-Cedillo F. Silva-Torres R. (2022). Astragalin identification in graviola pericarp indicates a possible participation in the anticancer activity of pericarp crude extracts: in vitro and in silico approaches. Arab. J. Chem.15 (4), 103720. 10.1016/j.arabjc.2022.103720
5
Attiq A. Jalil J. Husain K. Mohamad H. F. Ahmad A. (2021). Luteolin and apigenin derived glycosides from Alphonsea elliptica abrogate LPS-induced inflammatory responses in human plasma. J. Ethnopharmacol.275, 114120. 10.1016/j.jep.2021.114120
6
Auranwiwat C. Wongsomboon P. Thaima T. Rattanjak R. Kamchonwongpaisan S. Willis A. C. et al (2017). 2-Phenylnaphthalenes and a polyoxygenated cyclohexene from the stem and root extracts of Uvaria cherrevensis (Annonaceae). Fitoterapia120, 103–107. 10.1016/j.fitote.2017.06.002
7
Auranwiwat C. Rattanjak R. Kamchonwongpaisan S. Laphookhieo S. Pyne S. G. Limtharakul T. (2018). Four new C-benzyl flavonoids from the fruit of Uvaria cherrevensis. Fitoterapia130, 198–202. 10.1016/j.fitote.2018.08.020
8
Ba N. V. Men C. V. Khi N. T. Tiet T. V. Khanh P. G. Quang L. B. et al (2020). Flavonol glycosides from Fissistigma maclurei. J. Asian Nat. Prod. Res.22 (11), 1011–1017. 10.1080/10286020.2019.1671374
9
Bajgai S. P. Prachyawarakorn V. Mahidol C. Ruchirawat S. Kittakoop P. (2011). Hybrid flavan-chalcones, aromatase and lipoxygenase inhibitors, from Desmos cochinchinensis. Phytochemistry72, 2062–2067. 10.1016/j.phytochem.2011.07.002
10
Bosse M. A. da Silva M. B. Marques de Oliveira N. G. R. de Araujo M. A. Rodrigues C. de Azevedo J. P. et al (2021). Physiological impact of flavonoids on nodulation and ureide metabolism in legume plants. Plant Physiology Biochem.166, 512–521. 10.1016/j.plaphy.2021.06.007
11
Carollo C. A. Hellmann-Carollo A. R. de Siqueira J. M. Albuquerque S. (2006). Alcaloides e um flavonóide de partes aéreas (folhas e galhos) de Duguetia furfuracea - Annonaceae. J. Chil. Chem. Soc.51 (2), 837–841. 10.4067/S0717-97072006000200001
12
Chan H.-H. Hwang T.-L. Thang T. D. Lee Y.-L. Kuo P.-C. Nguyet B. T. M. et al (2013). Isolation and synthesis of melodamide A, a new anti-inflammatory phenolic amide from the leaves of Melodorum fruticosum. Planta Medica79 (4), 288–294. 10.1055/s-0032-1328131
13
Chang F.-R. Wei J.-L. Teng C.-M. Wu Y.-C. (1998). Antiplatelet aggregation constituents from Annona purpurea. J. Nat. Prod.61 (12), 1457–1461. 10.1021/np9800046
14
Chaowasku T. (2020). Toward a phylogenetic reclassification of the subfamily Ambavioideae (Annonaceae): establishment of a new subfamily and a new tribe. Acta Bot. Bras.34, 522–529. 10.1590/0102-33062020abb0051
15
Chatrou L. W. Erkens R. H. J. Richardson J. E. Saunders R. M. K. Fay M. F. (2012a). The natural history of Annonaceae. Bot. J. Linn. Soc.169, 1–4. 10.1111/j.1095-8339.2012.01242.x
16
Chatrou L. W. Pirie M. D. Erkens R. H. J. Couvreur T. L. P. Neubig K. M. Abbott J. R. et al (2012b). A new subfamilial and tribal classification of the pantropical flowering plant family Annonaceae informed by molecular phylogenetics. Bot. J. Linn. Soc.169, 5–40. 10.1111/j.1095-8339.2012.01235.x
17
Chaves M. H. Roque N. F. Ayres M. C. C. (2004). Steroids and flavonoids of Porcelia macrocarpa. J. Braz. Chem. Soc.15 (4), 608–613. 10.1590/S0103-50532004000400026
18
Chen S. Wang X. Cheng Y. Gao H. Chen X. (2022). A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules27 (5), 4982. 10.3390/molecules28134982
19
Cheng A.-X. Han X.-J. Wu Y.-F. Lou H.-X. (2014). The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci.15 (1), 1080–1095. 10.3390/ijms15011080
20
Chokchaisiri R. Kunkaewom S. Chokchaisiri S. Gauranoo L. Chalermglin R. Suksamrarn A. (2017). Potent cytotoxicity against human small cell lung cancer cells of the heptenes from the stem bark of Xylopia pierrei Hance. Med. Chem. Res.26, 1291–1296. 10.1007/s00044-017-1843-8
21
Christopher R. (2022). Plant species of the genus Uvaria: ethnobotanical uses, biological activities and phytochemistry. Nat. Prod. Res.36 (11), 2946–2961. 10.1080/14786419.2021.1929972
22
Clement J. A. Ondeyka J. G. Goetz M. A. (2017). Benzoate esters and flavonoids from Desmos pedunculosus. Phytochem. Lett.22, 117–121. 10.1016/j.phytol.2017.09.017
23
Cole J. R. Torrance S. J. Wiedhopf R. M. Arora S. K. Bates R. B. (1976). Uvaretin, a new antitumor agent from Uvaria acuminata (Annonaceae). J. Org. Chem.41 (10), 1852–1855. 10.1021/jo00872a037
24
Costa E. V. Soares L. N. da Silva Chaar J. Silva V. R. Santos L. S. Koolen H. H. F. et al (2021). Benzylated dihydroflavones and isoquinoline-derived alkaloids from the bark of Diclinanona calycina (Annonaceae) and their cytotoxicities. Molecules26 (12), 3714. 10.3390/molecules26123714
25
Couvreur T. L. P. Helmstetter A. J. Koenen E. J. M. Bethune K. Brandão R. D. Little S. A. et al (2019). Phylogenomics of the major tropical plant family Annonaceae using targeted enrichment of nuclear genes. Front. Plant Sci.9, 1941. 10.3389/fpls.2018.01941
26
Dagallier L.-P. M. J. Mbago F. M. Couderc M. Gaudeul M. Grall A. Loup C. et al (2023). Phylogenomic inference of the African tribe Monodoreae (Annonaceae) and taxonomic revision of Dennettia, Uvariodendron and Uvariopsis. PhytoKeys233, 1–200. 10.3897/phytokeys.233.103096
27
Deepralard K. Kawanishi K. Moriyasu M. Pengsuparp T. Suttisri R. (2009). Flavonoid glycosides from the leaves of Uvaria rufa with advanced glycation end-products inhibitory activity. Thai J. Pharm. Sci.33 (2-3), 84–90. 10.56808/3027-7922.2187
28
Dias M. C. Pinto D. C. Silva A. M. (2021). Plant flavonoids: chemical characteristics and biological activity. Molecules26 (17), 5377. 10.3390/molecules26175377
29
Do L. T. M. Sichaem J. (2022). New flavonoid derivatives from Melodorum fruticosum and their α-glucosidase inhibitory and cytotoxic activities. Molecules27 (13), 4023. 10.3390/molecules27134023
30
Engels N. S. Waltenberger B. Michalak B. Huynh L. Tran H. Kiss A. K. et al (2018). Inhibition of pro-inflammatory functions of human neutrophils by constituents of Melodorum fruticosum leaves. Chem. and Biodivers.15, e1800269. 10.1002/cbdv.201800269
31
Erkens R. H. Blanpain L. M. Carrascosa Jara I. Runge K. Verspagen N. Cosiaux A. et al (2023). Spatial distribution of Annonaceae across biomes and anthromes: knowledge gaps in spatial and ecological data. Plants, People, Planet5 (4), 520–535. 10.1002/ppp3.10321
32
Focho D. A. Egbe E. A. Chuyong G. B. Fongod A. G. N. Fonge B. A. Ndam W. T. et al (2010). An ethnobotanical investigation of the Annonaceae on Mount Cameroon. J. Med. Plants Res.4 (20), 2148–2158.
33
Formagio A. S. N. Kassuya C. A. L. Neto F. F. Volobuff C. R. F. Iriguchi E. K. K. Vieira M. do C. et al (2013). The flavonoid content and antiproliferative, hypoglycaemic, anti-inflammatory and free radical scavenging activities of Annona dioica St. Hill. BMC Complementary Altern. Med.13, 14. 10.1186/1472-6882-13-14
34
Frausin G. Lima R. B. S. Hidalgo A. D. F. Maas P. Pohlit A. M. (2014). Plants of the Annonaceae traditionally used as antimalarials: a review. Rev. Bras. Frutic.36, 315–337. 10.1590/S0100-29452014000500038
35
Gonda R. Takeda T. Akiyama T. (2000). Studies on the constituents of Anaxagorea luzonensis A. Gray. Chem. and Pharm. Bull.48 (8), 1219–1222. 10.1248/cpb.48.1219
36
Guinaudeau H. Lebœuf M. Cavé A. (1994). Aporphinoid alkaloids, V. J. Nat. Prod.57 (8), 1033–1135. 10.1021/np50110a001
37
Guo X. Tang C. C. Thomas D. C. Couvreur T. L. P. Saunders R. M. K. (2017). A mega-phylogeny of the Annonaceae: taxonomic placement of five enigmatic genera and support for a new tribe, Phoenicantheae. Sci. Rep.7, 7323. 10.1038/s41598-017-07252-2
38
Harborne J. B. Mabry T. J. (2013). The flavonoids: Advances in research. Springer. 10.1007/978-1-4899-2915-0
39
Hernández Fuentes L. M. Montalvo González E. García Magaña M. L. Anaya Esparza L. M. Nolasco González Y. Villagrán Z. et al (2021). Current situation and perspectives of fruit Annonaceae in Mexico: biological and agronomic importance and bioactive properties. Plants11 (1), 7. 10.3390/plants11010007
40
Hongnak S. Jongaramruong J. Khumkratok S. Siriphong P. Tip-pyang S. (2015). Chemical constituents and derivatization of melodorinol from the roots of Melodorum fruticosum. Nat. Product. Commun.10 (4), 1934578X1501000426. 10.1177/1934578X1501000426
41
Hsu Y.-M. Wu T.-Y. Du Y.-C. El-Shazly M. Beerhues L. Thang T. D. et al (2016). 3-Methyl-4,5-dihydro-oxepine, polyoxygenated seco-cyclohexenes and cyclohexenes from Uvaria flexuosa and their anti-inflammatory activity. Phytochemistry122, 184–192. 10.1016/j.phytochem.2015.12.013
42
Hufford C. D. Lasswell W. L. (1976). Uvaretin and isouvaretin. Two novel cytotoxic C-benzylflavanones from Uvaria chamae L. J. Org. Chem.41 (7), 1297–1298. 10.1021/jo00869a062
43
Hufford C. D. Oguntimein B. O. (1982). New dihydrochalcones and flavanones from Uvaria angolensis. J. Nat. Prod.45 (3), 337–342. 10.1021/np50021a016
44
Hufford C. D. Lasswell Jr W. L. Hirotsu K. Clardy J. (1979). Uvarinol: a novel cytotoxic tribenzylated flavanone from Uvaria chamae. J. Org. Chem.44 (25), 4709–4710. 10.1021/jo00393a054
45
Hwang T.-L. Li G.-L. Lan Y.-H. Chia Y.-C. Hsieh P.-W. Wu Y.-H. et al (2009). Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. and Med.46 (4), 520–528. 10.1016/j.freeradbiomed.2008.11.014
46
IBGE (2024). Produção agropecuária. Available online at: https://www.ibge.gov.br/explica/producao-agropecuaria/ (Accessed June 7, 2025).
47
Ichimaru M. Nakatani N. Takahashi T. Nishiyama Y. Moriyasu M. Kato A. et al (2004). Cytotoxic C-benzylated dihydrochalcones from Uvaria acuminata. Chem. Pharm. Bull.52 (1), 138–141. 10.1248/cpb.52.138
48
Jaidee W. Andersen R. J. Chavez M. A. G. Wang Y. A. Patrick B. O. Pyne S. G. et al (2019). Amides and flavonoids from the fruit and leaf extracts of Melodorum siamensis. J. Nat. Prod.82 (2), 283–292. 10.1021/acs.jnatprod.8b00696
49
Kakeya H. Imoto M. Tabata Y. Iwami J. Matsumoto H. Nakamura K. et al (1993). Isolation of a novel substrate-competitive tyrosine kinase inhibitor, desmal, from the plant Desmos chinensis. FEBS Lett.320 (2), 169–172. 10.1016/0014-5793(93)80085-9
50
Kamperdick C. Van N. H. Sung T. V. (2002). Constituents from Miliusa balansae (Annonaceae). Phytochemistry61 (8), 991–994. 10.1016/S0031-9422(02)00374-6
51
Kiem P. V. Minh C. V. Huong H. T. Lee J. J. Lee I. S. Kim Y. H. (2005). Phenolic constituents from Desmos chinensis and their inhibitory effects on NFAT transcription factor. Archives Pharmacal Res.28 (12), 1345–1349. 10.1007/BF02977900
52
Kitaoka M. Kadokawa H. Sugano M. Ichikawa K. Taki M. Takashi S. et al (1998). Prenylflavonoids: a new class of non-steroidal phytoestrogen (part 1). Isolation of 8-isopentenylnaringenin and an initial study on its structure-activity relationship. Planta Medica64 (6), 511–515. 10.1055/s-2006-957504
53
Lage G. A. Medeiros F. S. Furtado V. L. Takahashi J. A. Souza Filho J. D. Pimenta L. P. S. (2014). The first report on flavonoid isolation from Annona crassiflora Mart. Nat. Prod. Res.28 (11), 808–811. 10.1080/14786419.2014.885518
54
Lan Y.-H. Peng Y.-T. Thang T.-D. Hwang T.-L. Dai D.-N. Leu Y.-L. et al (2012). New flavan and benzil isolated from Fissistigma latifolium. Chem. and Pharm. Bull.60 (2), 280–282. 10.1248/cpb.60.280
55
Lasswell W. L. Hufford C. D. (1977). Cytotoxic C-benzylated flavonoids from Uvaria chamae. J. Org. Chem.42 (8), 1295–1302. 10.1021/jo00428a006
56
Leboeuf M. Cavé A. Bhaumik P. K. Mukherjee B. Mukherjee R. (1980). The phytochemistry of the Annonaceae. Phytochemistry21 (12), 2783–2813. 10.1016/0031-9422(80)85046-1
57
Leite D. O. de Fa Nonato C. Camilo C. J. de Carvalho N. K. da Nobrega M. G. Pereira R. C. et al (2020). Annona genus: traditional uses, phytochemistry and biological activities. Curr. Pharm. Des.26 (33), 4056–4091. 10.2174/1381612826666200325094422
58
Lekphrom R. Kanokmedhakul K. Schevenels F. Kanokmedhakul S. (2018). Antimalarial polyoxygenated cyclohexene derivatives from the roots of Uvaria cherrevensis. Fitoterapia127, 420–424. 10.1016/j.fitote.2018.01.018
59
Li T.-M. Li W.-K. Yu J.-G. (1997). Flavonoids from Artabotrys hexapetalus. Phytochemistry45 (4), 831–833. 10.1016/S0031-9422(97)00012-5
60
Lien T. P. Porzel A. Schmidt J. Sung T. V. Adam G. (2000). Chalconoids from Fissistigma bracteolatum. Phytochemistry53 (8), 991–995. 10.1016/S0031-9422(99)00570-1
61
Likhitwitayawuid K. Klongsiriwet C. Jongbunprasert V. Sritularak B. Wongseripipatana S. (2006). Flavones with free radical scavenging activity from Goniothalamus tenuifolius. Archives Pharmacal Res.29 (3), 199–202. 10.1007/BF02969393
62
Lin S. Singh R. K. Navarre D. A. (2021). R2R3–MYB transcription factors, StmiR858 and sucrose mediate potato flavonol biosynthesis. Hortic. Res.8, 25. 10.1038/s41438-021-00463-9
63
Liu W. X. Feng Y. Yu S. Zhengqi F. Li X. Li J. Y. et al (2021). The flavonoid biosynthesis network in plants. Int. J. Mol. Sci.22 (23), 12824. 10.3390/ijms222312824
64
Maas P. J. M. Maas H. Miralha J. M. S. Junikka L. (2007). Flora da Reserva Ducke, Amazonas, Brasil: Annonaceae. Rodriguésia58 (03), 617–662. 10.1590/2175-7860200758307
65
Maas P. Lobão A. Rainer H. (2015). Annonaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Available online at: http://floradobrasil2015.jbrj.gov.br/FB110219 (Accessed June 7, 2025).
66
Maeda G. Munissi J. J. E. Lindblad S. Duffy S. Pelletier J. Avery V. M. et al (2020). A meroisoprenoid, heptenolides, and C-benzylated flavonoids from Sphaerocoryne gracilis ssp. gracilis. J. Nat. Prod.83 (2), 316–322. 10.1021/acs.jnatprod.9b00721
67
Meesakul P. Pudhom K. Pyne S. G. Laphookhieo S. (2017). Hybrid flavan-flavanones from Friesodielsia desmoides and their inhibitory activities against nitric oxide production. RSC Adv.7 (28), 17545–17550. 10.1039/c7ra02528a
68
Meesakul P. Richardson C. Pyne S. G. Laphookhieo S. (2019). α-Glucosidase inhibitory flavonoids and oxepinones from the leaf and twig extracts of Desmos cochinchinensis. J. Nat. Prod.82 (4), 741–747. 10.1021/acs.jnatprod.8b00581
69
Meng X. Li Y. Zhou T. Sun W. Shan X. Gao X. et al (2019). Functional differentiation of duplicated flavonoid 3-O-glycosyltransferases in the flavonol and anthocyanin biosynthesis of Freesia hybrida. Front. Plant Sci.10, 1330. 10.3389/fpls.2019.01330
70
Mohanty S. P. Barik D. P. Moharana A. (2023). A comprehensive review on Uvaria species: conservation status, ethnobotanical uses and pharmacological activities. Drug Pharm. Sci. Archives3 (03), 56–67. 10.47587/DPSA.2023.3301
71
Moreira I. C. Lago J. H. G. Roque N. F. (2003). Alkaloid, flavonoids and terpenoids from leaves and fruits of Xylopia emarginata (Annonaceae). Biochem. Syst. Ecol.31 (5), 535–537. 10.1016/S0305-1978(02)00180-1
72
Moreira I. C. Roque N. F. Vilegas W. Zalewski C. A. Lago J. H. G. Funasaki M. (2013). Genus Xylopia (Annonaceae): chemical and biological aspects. Chem. Biodivers.10, 1921–1943. 10.1002/cbdv.201100308
73
Moriyasu M. Nakatani N. Ichimaru M. Nishiyama Y. Kato A. Mathenge S. G. et al (2011). Chemical studies on the roots of Uvaria welwitschii. J. Nat. Med.65, 313–321. 10.1007/s11418-010-0498-2
74
Mouafon I. L. Mountessou B. Y. G. Ngouonpe A. W. Mbobda A. S. W. Ngadjui B. T. Katerere D. R. et al (2025). Unravelling the chemical and pharmacological contour of plants of the genus Duguetia A. St.-Hil. (Annonaceae): a mini-review. Phytochem. Rev., 1–15. 10.1007/s11101-025-10108-7
75
Nabavi S. M. Šamec D. Tomczyk M. Milella L. Russo D. Habtemariam S. et al (2020). Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol. Adv.38, 107316. 10.1016/j.biotechadv.2018.11.005
76
Nawwar M. Ayoub N. Hussein S. Hashim A. El-Sharawy R. Wende K. et al (2012). A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Archives Pharmacal Res.35 (5), 761–767. 10.1007/s12272-012-0501-4
77
Nge F. J. Chaowasku T. Damthongdee A. Wiya C. Soulé V. R. Rodrigues-Vaz C. et al (2024). Complete genus-level phylogenomics and new subtribal classification of the pantropical plant family Annonaceae. Taxon73 (06), 1341–1369. 10.1002/tax.13260
78
Ngoc H. N. Mair L. Nghiem D. T. Le K. T. Gostner J. M. Stuppner H. et al (2019). Phenolic compounds from the stems of Fissistigma polyanthoides and their anti-oxidant activities. Fitoterapia137, 104252. 10.1016/j.fitote.2019.104252
79
Ngouonpe A. W. Mbobda A. S. W. Happi G. M. Mbiantcha M. Tatuedom O. K. Ali M. S. et al (2019). Natural products from the medicinal plant Duguetia stauditi (Annonaceae). Biochem. Syst. Ecol.83, 22–25. 10.1016/j.bse.2018.12.015
80
Nhiem N. X. Thinh N. S. Thu N. T. B. Anh L. T. Ngoc T. M. Tai B. H. et al (2019). Three new flavonol glycosides from Fissistigma pallens. Biosci. Biotechnol. Biochem.83 (12), 2177–2182. 10.1080/09168451.2019.1654848
81
Nkunya M. H. Weenen H. Renner C. Waibel R. Achenbach H. (1993a). Benzylated dihydrochalcones from Uvaria leptocladon. Phytochemistry32 (5), 1297–1300. 10.1016/S0031-9422(00)95109-4
82
Nkunya M. H. H. Waibel R. Achenbach H. (1993b). Three flavonoids from the stem bark of the antimalarial Uvaria dependens. Phytochemistry34 (3), 853–856. 10.1016/0031-9422(93)85372-X
83
Novaes P. Ferreira M. J. P. dos Santos D. Y. A. C. (2018). Flavonols from Annona coriacea Mart. (Annonaceae). Biochem. Syst. Ecol.78, 77–80. 10.1016/j.bse.2018.04.006
84
Nyandoro S. S. Munissi J. J. E. Gruhonjic A. Duffy S. Pan F. Puttreddy R. et al (2017). Polyoxygenated cyclohexenes and other constituents of Cleistochlamys kirkii leaves. J. Nat. Prod.80 (1), 114–125. 10.1021/acs.jnatprod.6b00759
85
Okorie D. A. (1977). New benzyldihydrochalcones from Uvaria chamae. Phytochemistry16 (10), 1591–1594. 10.1016/0031-9422(77)84030-2
86
Panche A. N. Diwan A. D. Chandra S. R. (2016). Flavonoids: an overview. J. Nutr. Sci.5, e47. 10.1017/jns.2016.41
87
Panda S. Kar A. (2015). Protective effects of 5,7,4'-trihydroxy-6,3'-dimethoxy-flavone 5-O-α-L-rhamnopyranoside, isolated from Annona squamosa leaves in thyrotoxicosis and in hepatic lipid peroxidation in rats. Bioorg. and Med. Chem. Lett.25 (24), 5726–5728. 10.1016/j.bmcl.2015.10.090
88
Panichpol K. Waterman P. G. (1978). Novel flavonoids from the stem of Popowia cauliflora. Phytochemistry17 (8), 1363–1367. 10.1016/S0031-9422(00)94590-4
89
Periferakis A. Periferakis K. Badarau I. A. Petran E. M. Popa D. C. Caruntu A. et al (2022). Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci.23 (23), 15004. 10.3390/ijms232315004
90
Porzel A. Lien T. P. Schmidt J. Drosihn S. Wagner C. Merzweiler K. et al (2000). Fissistigmatins A–D: novel type natural products with flavonoid–sesquiterpene hybrid structure from Fissistigma bracteolatum. Tetrahedron56 (7), 865–872. 10.1016/S0040-4020(99)01049-2
91
Prachyawarakorn V. Sangpetsiripan S. Surawatanawong P. Mahidol C. Ruchirawat S. Kittakoop P. (2013). Flavans from Desmos cochinchinensis as potent aromatase inhibitors. Med. Chem. Comm.4 (12), 1590–1594. 10.1039/c3md00166k
92
Prawat U. Phupornprasert D. Butsuri A. Salae A.-W. Boonsri S. Tuntiwachwuttikul P. (2012). Flavonoids from Friesodielsia discolor. Phytochem. Lett.5 (4), 809–813. 10.1016/j.phytol.2012.09.007
93
Prawat U. Chairerk O. Phupornprasert U. Salae A. W. Tuntiwachwuttikul P. (2013). Two new C-benzylated dihydrochalcone derivatives from the leaves of Melodorum siamensis. Planta Medica79 (01), 83–86. 10.1055/s-0032-1327950
94
Rahman M. M. Qais N. Rashid M. A. (2003). A new C-benzylated chalcone from Desmos chinensis. Fitoterapia74 (5), 511–514. 10.1016/S0367-326X(03)00116-3
95
Raza A. Chen C. Luo L. Asghar M. A. Liu L. Shoaib N. et al (2024). Combined application of organic and chemical fertilizers improved the catechins and flavonoids biosynthesis involved in tea quality. Sci. Hortic.337, 113518. 10.1016/j.scienta.2024.113518
96
Rittiwong T. Mutrapat T. Ponglimanont C. Mahabusarakam W. Chakthong S. (2011). Saiyutones A–D: four new unusual biflavones from Desmos chinensis. Tetrahedron67 (29), 5444–5449. 10.1016/j.tet.2011.05.070
97
Rocha R. S. Kassuya C. A. L. Formagio A. S. N. Mauro M. O. Andrade-Silva M. Monreal A. C. D. et al (2016). Analysis of the anti-inflammatory and chemopreventive potential and description of the antimutagenic mode of action of the Annona crassiflora methanolic extract. Pharm. Biol.54 (1), 35–47. 10.3109/13880209.2015.1014567
98
Saadawi S. Jalil J. Jasamai M. Jantan I. (2012). Inhibitory effects of acetylmelodorinol, chrysin and polycarpol from Mitrella kentii on prostaglandin E2 and thromboxane B2 production and platelet activating factor receptor binding. Molecules17 (5), 4824–4835. 10.3390/molecules17054824
99
Sabphon C. Temkitthavon P. Ingkaninan K. Sawasdee P. (2015). Phosphodiesterase inhibitory activity of the flavonoids and xanthones from Anaxagorea luzonensis. Nat. Product. Commun.10 (2), 301–303. 10.1177/1934578X1501000222
100
Santos D. Y. A. C. D. Salatino M. L. F. (2000). Foliar flavonoids of Annonaceae from Brazil: taxonomic significance. Phytochemistry55 (6), 567–573. 10.1016/S0031-9422(00)00227-2
101
Seidel V. Bailleul F. Waterman P. G. (2000). (Rel)-1β,2α-di-(2,4-dihydroxy-6-methoxybenzoyl)-3β,4α-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii. Phytochemistry55 (5), 439–446. 10.1016/S0031-9422(00)00346-0
102
Silva F. M. A. Silva Filho F. A. Souza C. D. Maciel J. B. Costa E. V. Barison A. et al (2018a). Morphinadienone and other isoquinoline-derived alkaloids from the trunk bark of Unonopsis floribunda Diels (Annonaceae). Biochem. Syst. Ecol. 79, 12–14. 10.1016/j.bse.2018.04.013
103
Silva F. M. A. Silva-Filho F. A. Souza C. A. S. Maciel J. B. Costa E. V. Barison A. et al (2018b). Isoquinoline-derived alkaloids from leaves of Unonopsis stipitata Diels (Annonaceae). Biochem. Syst. Ecol. 79, 69–71. 10.1016/j.bse.2018.05.007
104
Sinz A. Matusch R. Santisuk T. Chaichana S. Reutrakul V. (1998). Flavonol glycosides from Dasymaschalon sootepense. Phytochemistry47 (7), 1393–1396. 10.1016/S0031-9422(97)00719-X
105
Sinz A. Matusch R. van Elsäcker F. Santisuk T. Chaichana S. Reutrakul V. (1999). Phenolic compounds from Anomianthus dulcis. Phytochemistry50 (7), 1069–1072. 10.1016/S0031-9422(98)00646-3
106
Somanawat J. Talangsri N. Deepolngam S. Kaewamatawong R. (2012). Flavonoid and megastigmane glycosides from Artabotrys hexapetalus leaves. Biochem. Syst. Ecol.44, 124–127. 10.1016/j.bse.2012.04.023
107
Thao N. P. Luyen B. T. T. Tai B. H. Cuong N. M. Kim Y. C. Minh C. V. et al (2015). Chemical constituents of Millusa balansae leaves and inhibition of nitric oxide production in lipopolysaccharide-induced RAW 264.7 cells. Bioorg. and Med. Chem. Lett.25 (18), 3887–3892. 10.1016/j.bmcl.2015.07.056
108
Van N. H. Thuy T. T. Sung T. V. (2007). Flavonoids from Fissistigma acuminatissima. J. Chem.45 (5), 648–651. 10.15625/4806
109
Vega M. R. G. Esteves-Souza A. Vieira I. J. C. Mathias L. Braz-Filho R. Echevarria A. (2007). Flavonoids from Annona dioica leaves and their effects in Ehrlich carcinoma cells, DNA-topoisomerase I and II. J. Braz. Chem. Soc.18 (8), 1554–1559. 10.1590/S0103-50532007000800016
110
Volobuff C. R. F. Pederiva M. M. C. Benites R. S. R. Lima C. J. Argandofia E. J. S. Cardoso C. A. L. et al (2019). Bioguided fractionation, and antioxidant, antiproliferative, and anti-inflammatory activity of Annona cacans warm. J. Med. Food22 (10), 1078–1086. 10.1089/jmf.2018.0198
111
Wang G. Wang Y. Yao L. Gu W. Zhao S. Shen Z. et al (2022). Pharmacological activity of quercetin: an updated review. Evidence-Based Complementary Altern. Med.2022, 1–12. 10.1155/2022/3997190
112
Waterman P. G. Pootakahm K. (1979). Chemical studies on the Annonaceae. V. The flavonoids of the fruit of Popowia cauliflora CHIPP. Planta Medica35, 366–369. 10.1055/s-0028-1097231
113
Wirasathien L. Pengsuparp T. Moriyasu M. Kawanishi K. Suttisri R. (2006). Cytotoxic C-benzylated chalcone and other constituents of Ellipeiopsis cherrevensis. Archives Pharmacal Res.29 (6), 497–502. 10.1007/BF02969423
114
Wu J. H. Liao S. X. Mao S. L. Liang H. Q. Wang Y. L. Su Z. W. (1997). Desmosflavanone II: a new flavanone from Desmos cochinchinensis lour. J. Chin. Pharm. Sci.6 (3), 119.
115
Zhu H. Chen L. Yu J. Cui L. Ali I. Song X. et al (2020). Flavonoid epimers from custard apple leaves, a rapid screening and separation by HSCCC and their antioxidant and hypoglycaemic activities evaluation. Sci. Rep.10 (1), 8819. 10.1038/s41598-020-65769-5
Summary
Keywords
flavonols, flavanones, glycosides, Uvaria , Annona
Citation
Graça IRS, Belém MVN, Pinheiro MLB, Da Silva FMA and Koolen HHF (2025) The chemistry of flavonoids from Annonaceae: a comprehensive review. Front. Nat. Prod. 4:1657095. doi: 10.3389/fntpr.2025.1657095
Received
30 June 2025
Accepted
17 September 2025
Published
31 October 2025
Volume
4 - 2025
Edited by
Luciana Scotti, Federal University of Paraíba, Brazil
Reviewed by
Yue Yuan, Chinese Academy of Sciences (CAS), China
Abdullahi Muhammad, Federal University of Health Sciences Azare, Nigeria
Updates
Copyright
© 2025 Graça, Belém, Pinheiro, Da Silva and Koolen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector H. F. Koolen, hkoolen@uea.edu.br; Felipe M. A. Da Silva, felipe.silva@inpa.gov.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.