- 1Department of Biology, University of Ottawa, Ottawa, ON, Canada
- 2Department of Physics, University of Ottawa, Ottawa, ON, Canada
- 3Krembil Brain Institute, University Health Network, Toronto, ON, Canada
- 4Department of Mathematics, University of Toronto, Toronto, ON, Canada
- 5Institute of Mental Health Research at The Royal, Ottawa, ON, Canada
Introduction: Periodic brain stimulation (PBS) techniques, either intracranial or non-invasive, electrical or magnetic, represent promising neuromodulatory tools for the treatment of neurological and neuropsychiatric disorders. Through the modulation of endogenous oscillations, PBS may engage synaptic plasticity, hopefully leading to persistent lasting effects. However, stabilizing such effects represents an important challenge: the interaction between induced electromagnetic fields and neural circuits may yield highly variable responses due to heterogeneous neuronal and synaptic biophysical properties, limiting PBS clinical potential.
Methods: In this study, we explored the conditions on which transcranial alternating current stimulation (tACS) as a common type of non-invasive PBS leads to amplified post-stimulation oscillatory power, persisting once stimulation has been turned off. We specifically examined the effects of heterogeneity in neuron time scales on post-stimulation dynamics in a population of balanced Leaky-Integrate and Fire (LIF) neurons that exhibit synchronous-irregular spiking activity.
Results: Our analysis reveals that such heterogeneity enables tACS to engage synaptic plasticity, amplifying post-stimulation power. Our results show that such post-stimulation aftereffects result from selective frequency- and cell-type-specific synaptic modifications. We evaluated the relative importance of stimulation-induced plasticity amongst and between excitatory and inhibitory populations.
Discussion: Our results indicate that heterogeneity in neurons’ time scales and synaptic plasticity are both essential for stimulation to support post-stimulation aftereffects, notably to amplify the power of endogenous rhythms.
Introduction
Brain stimulation has attracted significant interest in the last decades (Takeuchi and Berényi, 2020; Gschwind and Seeck, 2016; Bronstein et al., 2011). Various simulation techniques have shown promising results, and more are coming. Researchers, experimentally and theoretically, have addressed numerous challenges related to the effects of these interventions on behaviour (Miniussi and Vallar, 2011; Bestmann et al., 2015), brain function (Polanía et al., 2018), as well as pathologies such as epilepsy (Takeuchi and Berényi, 2020; San-Juan et al., 2022), Parkinson’s (Benninger et al., 2010; Madadi Asl et al., 2023), major depressive disorder (MDD) (Riddle et al., 2020; Haller et al., 2020) and stroke (Schlaug et al., 2008; Monti et al., 2013). Despite these promising results, it is still unclear how brain stimulation interventions shape endogenous brain dynamics (Ali et al., 2013; Helfrich et al., 2014; Alagapan et al., 2016; Reato et al., 2010) and the neural circuits that support them (Zaehle et al., 2010; Pariz et al., 2023). Indeed, brain stimulation outcomes remain variable: induced changes in neuron excitability vary remarkably between stimulation sites, repeated trials, and subjects, oftentimes vanishing after stimulation offset (Vogeti et al., 2022; Maeda et al., 2000; Eldaief et al., 2011; López-Alonso et al., 2014; Temperli et al., 2003). Uncovering the source of this variability can help to optimize existing brain stimulation paradigms and stabilize their effect on brain dynamics and plasticity.
Periodic brain stimulation (PBS) techniques, such as transcranial alternating current stimulation (tACS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS) have repeatedly been shown to be capable of altering neurons’ dynamics to interfere with cortical rhythms (Helfrich et al., 2014; Alagapan et al., 2016; Kasten et al., 2022; Kasten et al., 2016; Vossen et al., 2015; Negahbani et al., 2018; Herrmann et al., 2016; Krause et al., 2019; Nowotny et al., 2003; Lubenov and Siapas, 2008), thereby engaging synaptic plasticity by altering the neurons’ dynamics, firing rates and spike-timing (Sjöström et al., 2001) by modulating phase- and/or mode-locking beahviour of neurons (Pariz et al., 2023; Farokhniaee and Large, 2017) to alter network connectivity (Madadi Asl et al., 2023; Kromer and Tass, 2022; Kromer and Tass, 2024). However, the effects of these various types of stimulation may generate widely variable responses, notably due to physiological differences among neurons, while engaging different forms of brain plasticity (Shen et al., 2003). In fact, neural plasticity has been shown to depend on the stimulation frequency (Lea-Carnall et al., 2017; Yamawaki et al., 2012), highlighting the importance of tuning stimulation parameters to elevate its effects.
tACS is thought to work by engaging endogenous oscillations through time-varying electromagnetic waveforms and altering mode-locking behavior via continuous currents (Farokhniaee and Large, 2017; Elyamany et al., 2021), thereby inducing structural and functional changes in targeted regions (Madadi Asl et al., 2023; Herrmann et al., 2016; Hutt et al., 2018) potentially through diverse plasticity mechanisms (Shen et al., 2003; Pfister and Gerstner, 2006). This stimulation paradigm can entrain oscillations and elicit persistent after-effects lasting beyond the stimulation duration (Alagapan et al., 2016; Reato et al., 2010; Krause et al., 2022). Additionally, the efficacy of the entrainment and subsequent post-stimulation effects are state-dependent (Alagapan et al., 2016; Lefebvre et al., 2017), notably because of the competing influences of endogenous oscillations and tACS-induced forcing (Krause et al., 2022; Lefebvre et al., 2017). Multiple hypotheses for such persistent effects have been proposed, ranging from feedback reverberation (Alagapan et al., 2016; Park et al., 2018) to synaptic plasticity (Madadi Asl et al., 2023; Vogeti et al., 2022; Kromer and Tass, 2022; Schwab et al., 2021; Pfister and Tass, 2010). Yet, mechanisms remain poorly understood and outcomes are highly variable (Huang et al., 2017; Goldsworthy et al., 2016; Ridding and Ziemann, 2010).
Understanding the mechanisms underlying post-stimulation effects–critical for the clinical efficacy of tACS–remains challenging due to cellular heterogeneity. Numerous seminal studies show that neural responses to tACS, are influenced by biophysical properties like the membrane time constant (MTC) (Pariz et al., 2023), which shapes neuronal frequency selectivity and varies across cortical regions (Cheng and Lu, 2021; Moradi Chameh et al., 2021; Institute A. Dataset: Allen Institute for Brain Science, 2015). The MTC is a quantity that reflects the agility of neurons in response to time-varying stimuli (Cheng and Lu, 2021), and dictates their varied frequency selectivity (Pariz et al., 2023). The MTC varies significantly across cortical layers, and brain areas, ranging from a few to tens of milliseconds (Moradi Chameh et al., 2021; Institute A. Dataset: Allen Institute for Brain Science, 2015). Such variability has been shown to mediate selective, direction-specific synaptic plasticity under tACS (Pariz et al., 2023) and hence represents a promising candidate in supporting persistent post-stimulation effects. Indeed, stimulation-induced and MTC-dependent changes in neuronal spike timing, further modulated by endogenous oscillations, may solicit Hebbian spike timing dependent plasticity (STDP) to support changes in synaptic weights (Zaehle et al., 2010; Pariz et al., 2023). In this study, we investigated how low-amplitude sinusoidal stimulation (tACS) affects synaptic plasticity across neurons with heterogeneous MTCs and induce transient post-stimulation aftereffects. We explored two network states: a weak-coupling regime dominated by stimulation and a strong-coupling regime dominated by recurrent activity. Please note that in the Results section, we mainly focused on the weak coupling regime, and the strong coupling regime is presented and discussed in the Supplementary Material in details. We found that plasticity outcomes–and resulting changes in oscillatory power–were specific to stimulation amplitude and frequency, with excitatory–excitatory and inhibitory–excitatory connections playing key roles in generating persistent effects (Zaehle et al., 2010; Pariz et al., 2023; Kasten et al., 2016; Vossen et al., 2015). These findings emphasize the importance of accounting for biophysical diversity when designing stimulation protocols (Madadi Asl et al., 2023; Kromer and Tass, 2022; Kromer and Tass, 2024; Pfister and Tass, 2010).
Results
Besides entrainment, which naturally occurs through the oscillatory modulation of targeted regions (Herrmann et al., 2016), one purpose of tACS is to yield persistent effects that outlast stimulation duration. Intuitively, this objective can not be fulfilled unless tACS changes some physiological characteristics of the area under intervention. While sufficiently large amplitude stimulation is capable of altering neuronal spiking activity (Pariz et al., 2023), the nature of the responses will also depend on the neurons’ heterogeneous biophysical attributes. Such a key attribute is the membrane time constant (MTC). The membrane time constant is a key parameter representing the agility of neurons in response to time-varying stimuli (Pariz et al., 2023; Cheng and Lu, 2021; Brette, 2015). Such wide heterogeneity in time scales translates into significant variability in neurons’ response to periodic stimulation: neuron spiking phase (in respect to the stimulation phase in which the neuron spikes) depends on the interplay between stimulation frequency and the neurons’ MTCs (Pariz et al., 2023). For instance, in the Leaky-Integrate and Fire (LIF) neuron model used in this study (see Materials and methods), differences in the spiking phase (i.e,
Network properties and dynamic influenced by tACS
We built a network of 10,000 leaky integrate-and-fire (LIF) neurons, consisting of 8,000 excitatory (E) and 2,000 inhibitory (I) units, with a
To promote entrainment and improve the signal-to-noise ratio (i.e., contrast between endogenous oscillations and tACS), we set the system in a weak-coupling regime. In this configuration, the ratio of synaptic input to stimulation amplitude remains comparable, especially during the early stages of the simulation, before plasticity significantly alters connectivity. Although individual synaptic weights are small in this regime (see Table 1), the net synaptic current amplitude is comparable to stimulation-induced fluctuations: the average maximum synaptic current during population synchronous spiking is approximately
We subjected this network to periodic stimulation of various amplitudes
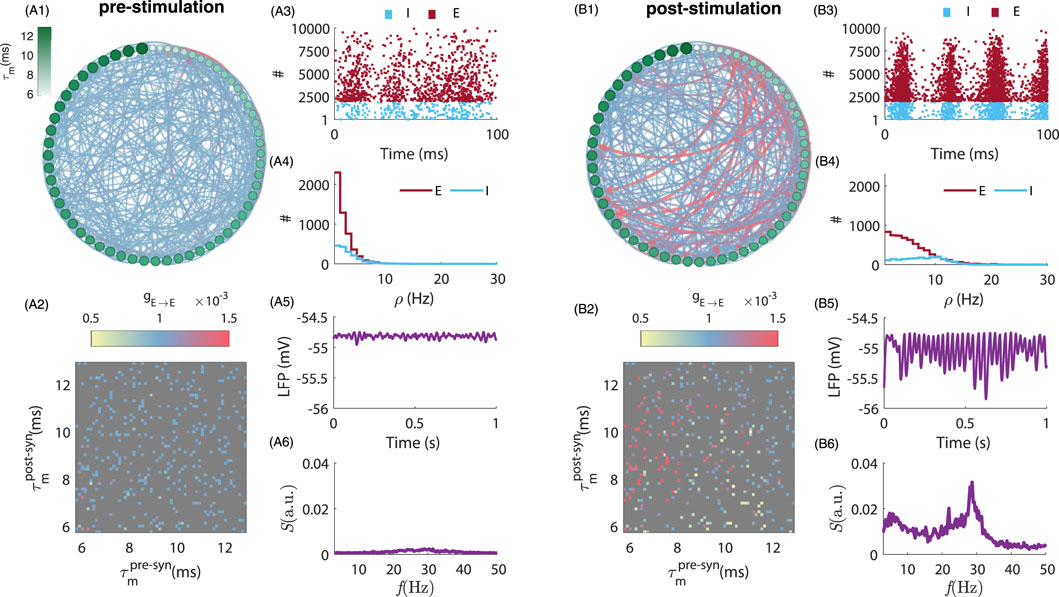
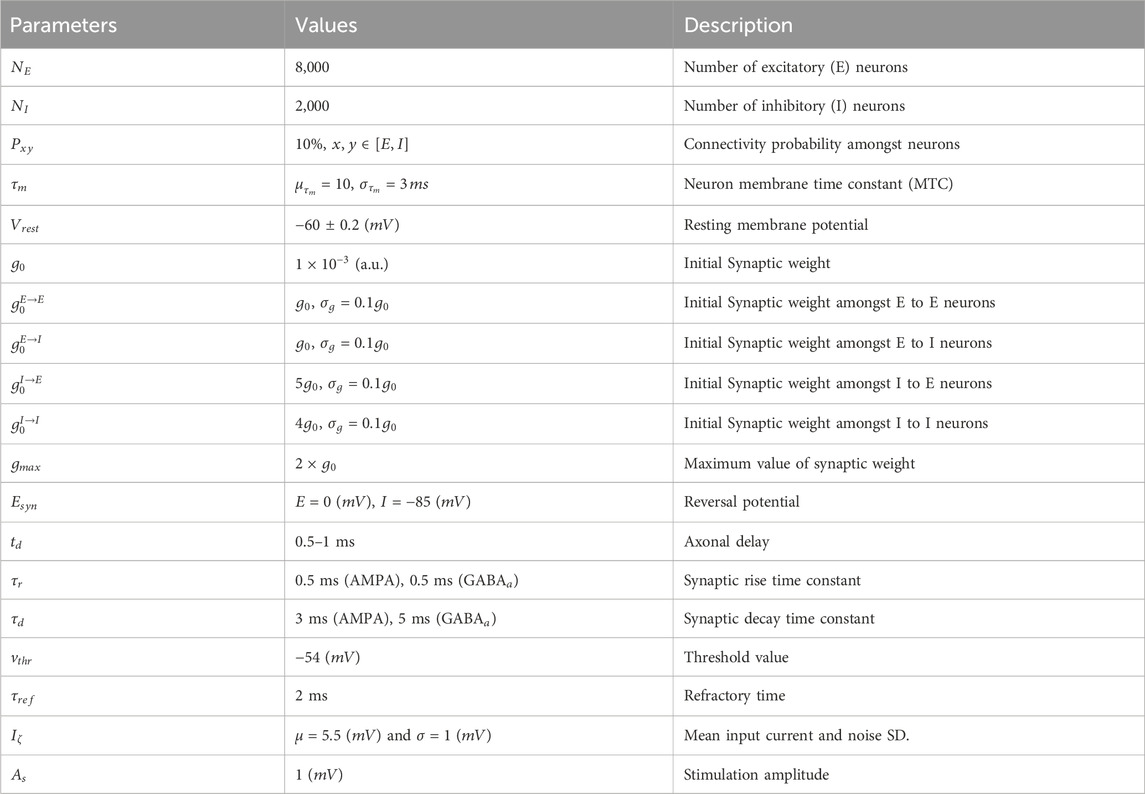
Figure 1. Comparison of neuronal network connectivity and dynamics before and after stimulation. (A1,B1) depict the pre- and post-stimulation population connectivity diagram, highlighting the changes in synaptic weights resulting from tACS. Here we plotted the connectivity amongst 60 randomly selected excitatory neurons during pre-
Post-stimulation aftereffects depend on stimulation parameters
Having identified post-stimulation amplification in endogenous oscillations, we next evaluated how this phenomenon depends on stimulation parameters. In Figures 2A,B, we plot the peak LFP power spectrum for various stimulation frequencies, both during and after stimulation offset. Stimulating at frequencies ranging from
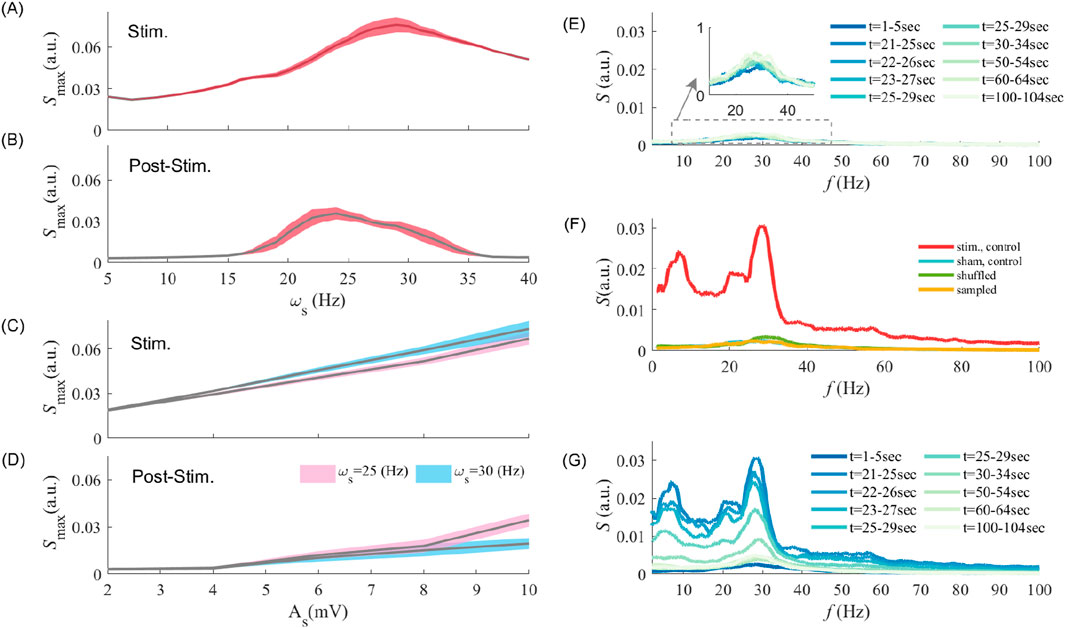
Figure 2. Interaction between stimulation frequency and amplitude in driving synaptic plasticity and post-stimulation aftereffects (A,B) show the maximum value of the LFP power spectrum at different stimulation frequencies during entrainment and post-stimulation epochs, respectively. Note that the maximum peak power may occur at different frequency other than the endogenous frequency, but fluctuates around the endogenous frequency
Stimulation amplitude is also crucial to elicit - and possibly maintain - persistent entrainment and associated changes in synaptic coupling. We plotted in Figures 2C,D the peak LFP power as a function of stimulation amplitude (i.e.,
We further investigated whether and how MTC heterogeneity is involved in generating those results. Is the LFP power amplification observed post-stimulation due to a global, non-specific increase in synaptic coupling, or is it instead due to selective, MTC-mediated synaptic plasticity? To answer this question, we first explored the effects of STDP on post-stimulation power amplification. As shown in Figure 2E, in the absence of stimulation (i.e., sham;
Stimulation-induced amplification in post-stimulation power was found to rely heavily on selective synaptic modifications, i.e., synapse-specific directional changes resulting from periodic entrainment of neurons possessing distinct MTCs (Pariz et al., 2023). To expose the role of such selectivity, we randomly shuffled synaptic weights amongst neurons of the same cell-type while preserving their overall statistics (see Materials and methods). Figure 2F compares the spectral power obtained without stimulation (sham control;
Our results indicate that despite the significance of oscillatory amplification and its manifest reliance on MTC heterogeneity, all reported post-stimulation after-effects were found to be transient, as reported in several studies (Zaehle et al., 2010; Kasten et al., 2016; Vossen et al., 2015) and dissipate over time after stimulation is turned off. Upon stimulation offset, prevailing endogenous synchronous irregular activity engages STDP to bring synaptic connectivity back to baseline (see Figure 2G).
Synaptic weights evolution depends on stimulation parameters and neurons’ properties
We examined the evolution of synaptic weights between all types of synapses in Figure 3 with respect to differences in MTCs, i.e.,
Figure 3. Frequency- and cell-type–specific effects of MTC heterogeneity on synaptic and spectral dynamics. Figure groups A (i.e., A1-A4), B (i.e., B1-B4), and C (i.e, C1-C4) are related to the stimulation frequencies
Influence of cell-type heterogeneity and synaptic plasticity on post-stimulation effects
Heterogeneity amongst and between different cell types, either excitatory or inhibitory, has different consequences on the post-stimulation power. To quantify this, we explored in Figure 4 the effects of cell-type MTC heterogeneity on post-stimulation LFP power. As shown in Figure 4A, MTC heterogeneity among excitatory neurons (i.e.,
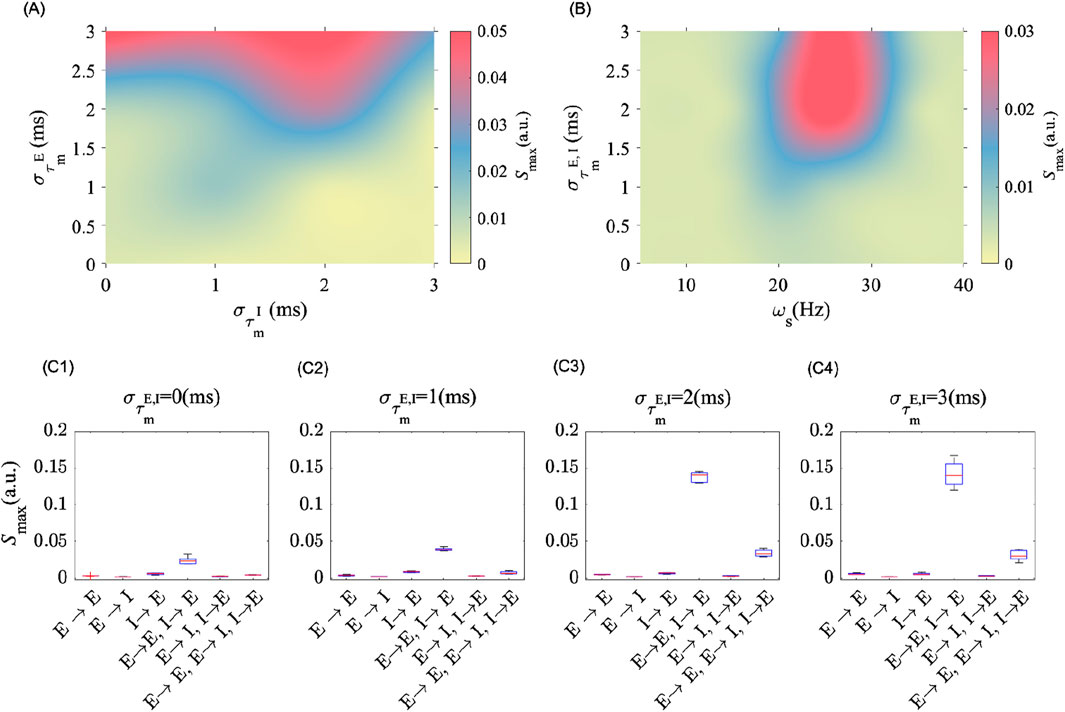
Figure 4. MTC heterogeneity amongst cell types modulates post-stimulation oscillation power. (A) Shows the peak spectral power in the post-stimulation epoch as the level of MTC heterogeneity of E (i.e.,
These results highlight the importance of considering plasticity among and between neuron subtypes. To investigate which synapses are more significantly involved in mediating the post-stimulation aftereffects, we applied periodic electrical stimulation on the same population at different degrees of MTC heterogeneity while selectively turning ON and OFF STDP amongst different cell types. This enabled the identification of synapses whose plasticity is more significantly solicited during stimulation. In Figures 4C1–C4, we show that plasticity between excitatory to excitatory neurons (that is,
Materials and methods
Spiking neuron model
We modelled a population of excitatory and inhibitory Leaky-Integrate and Fire (LIF) neurons (Brette, 2015; Tuckwell, 2006). The differential equation for the evolution of the subthreshold membrane potential of each neuron is
where
where
where
Individual synaptic weights are randomly chosen from a normal distribution with mean and standard deviation as given in Table 1 (sham control). In shuffled (see Figure 2F), first we let the simulation run for
Spike timing dependent plasticity (STDP)
Plasticity in our population amongst connected neurons is modelled using Hebbian pair-based spike-timing dependent plasticity (Choe et al., 2013; Gütig et al., 2003; Sjöström et al., 2010). To avoid biased synaptic changes (i.e., preferential LTP/LTD.) we chose a symmetric STDP Hebbian learning rule (Bi and Mm, 2001; Gütig et al., 2003; Sjöström et al., 2010). The synaptic weight dynamics in our model follows the below equations:
The
Baseline synaptic coupling and threshold were selected to set the network in a weak-coupling regime, sub-threshold regime, in which an isolated presynaptic spike does not guarantee postsynaptic firing. This regime achieved by choosing the synaptic weight from a narrow distribution (see Table. 1) and at the early stage of simulation. Despite weak synaptic coupling, the afferent synchronous synaptic input each neuron receives from the rest of the network is comparable to the stimulation amplitude, i.e., the average of maximum synaptic input (at the onset of every synchronous spiking activity) and its standard deviation is
Network model
We modelled a randomly connected sparse network of 10,000, LIF neurons (see Equation 1) with a 4:1 ratio of E (8000) and I (2000) neurons with a fixed connection probability of 0.1 (Vogels and Abbott, 2005; Bryson et al., 2021; Campagnola et al., 2022). To balance physiological relevance and computational tractability for the network sizes we used the LIF neurons model (Burkitt, 2006). The synaptic weights and other neurons’ parameters have been selected within the reported physiological range (Campagnola et al., 2022) to be in line with previous studies on LIF cortical network models (see (Burkitt, 2006; Kobay and ashi, 2009; Brunel and Wang, 2003) and references therein), and are further summarized in Table 1. To study the effect of MTC heterogeneity, we randomly sampled neuronal MTCs
Power spectral analysis
To perform spectral analysis of the network’s mean activity, we first calculated the local field potential (LFP),
where the relative proportion of excitatory (0.8) versus inhibitory interneurons (0.2) cells is taken into consideration. The power spectral density of
Discussion
To better understand the mechanism underlying post-stimulation amplification in oscillatory activity observed in experiments (Alagapan et al., 2016; Clancy et al., 2022), we extended the framework of selective STDP (Pariz et al., 2023) in a synchronous, sparsely connected neuronal network of heterogeneous spiking neurons. We computationally showed that in the presence of endogenous synchronous activity, near-resonant periodic stimulation may amplify post-stimulation power through selective synaptic changes, whose magnitude and direction rely on intrinsic differences in MTC. Stimulation at the near-resonant frequency was found to engage STDP so that the population expresses higher endogenous oscillatory power (see Figure 2B), resulting in transient yet prolonged overlasting effects. We confirmed that selective, directional changes in synaptic coupling - both within and between cell types - are responsible for such amplification, while any shuffled, randomly assigned synaptic weights, or intrinsic synaptic weight changes in the absence of stimulation, are insufficient for generating aftereffects on their own (see Figures 2E–G). The level of heterogeneity in neuronal MTC was found to determine the efficacy of stimulation on post-stimulation power magnitude and duration (see Figure 4A). Indeed, in a homogeneous network (i.e., where the MTCs are identical), neurons respond similarly to a given stimulus. Because of the symmetric nature of our STDP rule, such homogeneity might prevent stimulation-induced synaptic plasticity, even in the presence of noise. This means that a minimum level of heterogeneity is essential for pushing STDP in one direction or another, especially while interacting with time-varying inputs. Taken together, these results echo previous studies (Madadi Asl et al., 2023; Kromer and Tass, 2022; Pfister and Tass, 2010) by revealing one potential mechanism behind the effectiveness of tACS for therapeutic purposes, specifically the stabilization of stimulation effects on neural dynamics and connectivity. We argue that heterogeneity in neuronal time scales represents a dominant contributor mediating tACS efficacy, affirming the neurophysiological bases of persistent entrainment towards the development and/or optimization of clinical interventions. The results were qualitatively similar in both early stage of simulation and in the late stage where the synaptic weights modification, in the absence of stimulation, reaches a steady state. Further results for the latter case can be found in the Supplementary Material section. In short, we showed that even in the steady state, where the synaptic input currents are larger with respect to stimulation amplitude (up to three times the stimulation amplitude; i.e., strong-coupling regime), the post-stimulation aftereffects still depend on stimulation frequency and amplitude, as well as the MTC heterogeneity level (See. S6 and S9).
It should be noted that the results we report here extend to a broad range of endogenous frequencies. For instance, networks expressing oscillations within the alpha range may need different stimulation frequencies to solicit selectivity in synaptic plasticity (Lefebvre et al., 2017). This has important implications given the broad variety of frequencies characterizing oscillopathies (Takeuchi and Berényi, 2020; Hammond et al., 2007; Uhlhaas and Singer, 2013), where tACS hold promise to perturb pathological rhythms to unveil the mechanisms and potentially treat neurological and/or neuropsychiatric disorders. Interestingly, while stimulating at resonant/endogenous frequency expectedly yields higher entrainment (Lefebvre et al., 2017) (see Figure 2A), this does not always accompany significant post-stimulation aftereffects (see Figure 2B). We point out that our simulations also support a state-dependent dependence on stimulation efficacy. Indeed, weak background synaptic activity resulted in a high signal-to-noise ratio i.e., stimulation-induced modulation in neuronal membrane potential was significant enough to trigger depolarization and hence recruit STDP. In the presence of strong synaptic activity, however, the effects may fade away (Krause et al., 2022; Lefebvre et al., 2017). We also emphasize that to engage populations expressing a wide range of MTC, stimulation amplitude must scale accordingly, potentially influencing neuronal firing rates (Pariz et al., 2023). The precise relationship between stimulation frequency, synaptic plasticity, and persistent entrainment remains to be fully explored.
Nonetheless, our model suffers from limitations. First, we considered a neuronal network with random local (i.e., close spatial proximity where axonal conduction delays are considered small) connectivity, among cell types (
Synaptic plasticity selectivity is not limited to heterogeneity in MTC: other sources of heterogeneity, such as the resting membrane potential, rheobase, and/or spiking threshold, may promote cell-to-cell differences in spike timing. Lastly, we have mapped neurons’ MTC using a normal distribution, whose variance
Another limitation arises from our choice of using the same MTC distribution for both excitatory and inhibitory neurons. This choice was motivated by the need to balance physiological relevance and computational tractability - as well as limiting the dimensionality of the analysis. While the introduction of cell-type specific MTC distributions would certainly influence our results, we note that by construction, excitatory and inhibitory cells in our network already display differences in firing rates (e.g., see Figure 1). Further investigations are warranted to thoroughly examine such additional sources of heterogeneity. We however, hypothesize that as long as the overall activity of the neuronal population remains within an oscillatory synchronous irregular state, characterized by a low level of coherency, similar results would be observed.
Conclusion
Brain stimulation techniques offer invasive and non-invasive treatments for brain-related disorders. The promising results in the application of these techniques attracted a wide range of interdisciplinary researchers to investigate the response of brain cells to these interventions and devise more effective and reliable methods. Towards this goal, our study expanded the knowledge of how periodic stimulation may enhance and stabilize post-stimulation effects. Our results emphasize the importance of neural timescale variability in the interaction between synaptic plasticity and tACS. Overall, our results elucidate one potential mechanism by which tACS affects neural population connectivity, and conditions under which such intervention can lead to amplified, overlasting effects.
Data availability statement
The datasets presented in this article are not readily available because our study is a modeling study in which we simulated neuronal activity and analyzed the results. The codes we used are publicly available on GitHub https://github.com/arefpz/neuronal_population. Requests to access the datasets should be directed to cGFyaXouYXJlZkBnbWFpbC5jb20=.
Author contributions
JL: Funding acquisition, Investigation, Validation, Writing – review and editing. AP: Conceptualization, Formal Analysis, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We thank the National Research Council of Canada (NSERC GRANT RGPIN-2017-06662) as well as the Canadian Institute for Health Research (CIHR GRANT NO PJT-156164) for funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnetp.2025.1621283/full#supplementary-material
References
Abbott, L. F., and Nelson, S. B. (2000). Synaptic plasticity: taming the beast. Nat. Neurosci. 3 (11), 1178–1183. doi:10.1038/81453
Alagapan, S., Schmidt, S. L., Lefebvre, J., Hadar, E., Shin, H. W., and Frohlich, F. (2016). Modulation of cortical oscillations by low-frequency direct cortical stimulation is state-dependent. PLOS Biol. 14 (3), e1002424. doi:10.1371/journal.pbio.1002424
Ali, M. M., Sellers, K. K., and Fröhlich, F. (2013). Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 33 (27), 11262–11275. doi:10.1523/jneurosci.5867-12.2013
Bastian, M., Heymann, S., and Jacomy, M. (2009). Gephi: an open source software for exploring and manipulating networks. Proc. Int. AAAI Conf. Web Soc. Media 3, 361–362. doi:10.1609/icwsm.v3i1.13937
Bazhenov, M., Stopfer, M., Rabinovich, M., Huerta, R., Abarbanel, H. D., Sejnowski, T. J., et al. (2001). Model of transient oscillatory synchronization in the locust antennal lobe. Neuron 30 (2), 553–567. doi:10.1016/s0896-6273(01)00284-7
Benninger, D. H., Lomarev, M., Lopez, G., Wassermann, E. M., Li, X., Considine, E., et al. (2010). Transcranial direct current stimulation for the treatment of Parkinson’s disease. J. Neurology, Neurosurg. and Psychiatry 81 (10), 1105–1111. doi:10.1136/jnnp.2009.202556
Bestmann, S., de Berker, A. O., and Bonaiuto, J. (2015). Understanding the behavioural consequences of noninvasive brain stimulation. Trends cognitive Sci. 19 (1), 13–20. doi:10.1016/j.tics.2014.10.003
Bi, G., and Poo, M. (2001). Synaptic modification BY correlated. Annu. Rev. Neurosci. 24, 139–166. doi:10.1146/annurev.neuro.24.1.139
Brette, R. (2015). What is the most realistic single-compartment model of spike initiation? PLoS Comput. Biol. 11 (4), e1004114. doi:10.1371/journal.pcbi.1004114
Bronstein, J. M., Tagliati, M., Alterman, R. L., Lozano, A. M., Volkmann, J., Stefani, A., et al. (2011). Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Archives neurology 68 (2), 165. doi:10.1001/archneurol.2010.260
Brunel, N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 8, 183–208. doi:10.1023/a:1008925309027
Brunel, N., and Wang, X. J. (2003). What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J. neurophysiology 90 (1), 415–430. doi:10.1152/jn.01095.2002
Bryson, A., Berkovic, S. F., Petrou, S., and Grayden, D. B. (2021). State transitions through inhibitory interneurons in a cortical network model. PLOS Comput. Biol. 17 (10), e1009521. doi:10.1371/journal.pcbi.1009521
Burkitt, A. N. (2006). A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biol. Cybern. 95, 1–19. doi:10.1007/s00422-006-0068-6
Buzsáki, G., and Mizuseki, K. (2014). The log-dynamic brain: how skewed distributions affect network operations. Nat. Rev. Neurosci. 15 (4), 264–278. doi:10.1038/nrn3687
Campagnola, L., Seeman, S. C., Chartrand, T., Kim, L., Hoggarth, A., Gamlin, C., et al. (2022). Local connectivity and synaptic dynamics in mouse and human neocortex. Science 375 (6585), eabj5861. doi:10.1126/science.abj5861
Caporale, N., and Dan, Y. (2008). Spike timing–dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46. doi:10.1146/annurev.neuro.31.060407.125639
Cheng, C. Y., and Lu, C. C. (2021). The agility of a neuron: phase shift between sinusoidal current input and firing rate curve. J. Comput. Biol. 28 (2), 220–234. doi:10.1089/cmb.2020.0224
Choe, Y., Jaeger, D., and Jung, R. (2013). Anti-hebbian learning (New York, NY: Springer New York), 1–4. doi:10.1007/978-1-4614-7320-6_675-1
Clancy, K. J., Andrzejewski, J. A., You, Y., Rosenberg, J. T., Ding, M., and Li, W. (2022). Transcranial stimulation of alpha oscillations up-regulates the default mode network. Proc. Natl. Acad. Sci. 119 (1), e2110868119. doi:10.1073/pnas.2110868119
D’amour, J. A., and Froemke, R. C. (2015). Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 86 (2), 514–528. doi:10.1016/j.neuron.2015.03.014
Dan, Y., and Poo, Mm (2004). Spike timing-dependent plasticity of neural circuits. Neuron 44 (1), 23–30. doi:10.1016/j.neuron.2004.09.007
Eldaief, M. C., Halko, M. A., Buckner, R. L., and Pascual-Leone, A. (2011). Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci. 108 (52), 21229–21234. doi:10.1073/pnas.1113103109
Elyamany, O., Leicht, G., Herrmann, C. S., and Mulert, C. (2021). Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur. Archives Psychiatry Clin. Neurosci. 271 (1), 135–156. doi:10.1007/s00406-020-01209-9
Farokhniaee, A., and Large, E. W. (2017). Mode-locking behavior of Izhikevich neurons under periodic external forcing. Phys. Rev. E 95 (6), 062414. doi:10.1103/PhysRevE.95.062414
Gerstner, W., Kistler, W. M., Naud, R., and Paninski, L. (2014). Neuronal dynamics: from single neurons to networks and models of cognition. Cambridge University Press.
Goldsworthy, M. R., Vallence, A. M., Yang, R., Pitcher, J. B., and Ridding, M. C. (2016). Combined transcranial alternating current stimulation and continuous theta burst stimulation: a novel approach for neuroplasticity induction. Eur. J. Neurosci. 43 (4), 572–579. doi:10.1111/ejn.13142
Gschwind, M., and Seeck, M. (2016). Transcranial direct-current stimulation as treatment in epilepsy. Expert Rev. Neurother. 16 (12), 1427–1441. doi:10.1080/14737175.2016.1209410
Gütig, R., Aharonov, R., Rotter, S., and Sompolinsky, H. (2003). Learning input correlations through nonlinear temporally asymmetric Hebbian plasticity. J. Neurosci. 23 (9), 3697–3714. doi:10.1523/jneurosci.23-09-03697.2003
Haller, N., Senner, F., Brunoni, A. R., Padberg, F., and Palm, U. (2020). Gamma transcranial alternating current stimulation improves mood and cognition in patients with major depression. J. psychiatric Res. 130, 31–34. doi:10.1016/j.jpsychires.2020.07.009
Hammond, C., Bergman, H., and Brown, P. (2007). Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 30 (7), 357–364. doi:10.1016/j.tins.2007.05.004
Helfrich, R. F., Schneider, T. R., Rach, S., Trautmann-Lengsfeld, S. A., Engel, A. K., and Herrmann, C. S. (2014). Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 24 (3), 333–339. doi:10.1016/j.cub.2013.12.041
Herrmann, C. S., Murray, M. M., Ionta, S., Hutt, A., and Lefebvre, J. (2016). Shaping intrinsic neural oscillations with periodic stimulation. J. Neurosci. 36 (19), 5328–5337. doi:10.1523/JNEUROSCI.0236-16.2016
Huang, Y. Z., Lu, M. K., Antal, A., Classen, J., Nitsche, M., Ziemann, U., et al. (2017). Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin. Neurophysiol. 128 (11), 2318–2329. doi:10.1016/j.clinph.2017.09.007
Hutt, A., Griffiths, J. D., Herrmann, C. S., and Lefebvre, J. (2018). Effect of stimulation waveform on the non-linear entrainment of cortical alpha oscillations. Front. Neurosci. 12, 376. doi:10.3389/fnins.2018.00376
Institute A. Dataset: Allen Institute for Brain Science (2015). Allen cell types database – human morphology-electrophysiology.
Kasten, F. H., Dowsett, J., and Herrmann, C. S. (2016). Sustained aftereffect of α-tACS lasts up to 70 min after stimulation. Front. Hum. Neurosci. 10, 245. doi:10.3389/fnhum.2016.00245
Kasten, F. H., and Herrmann, C. S. (2022). The hidden brain-state dynamics of tACS aftereffects. NeuroImage. 264, 119713. doi:10.1016/j.neuroimage.2022.119713
Kobayashi, R. (2009). The influence of firing mechanisms on gain modulation. J. Stat. Mech. Theory Exp. 2009 (01), P01017. doi:10.1088/1742-5468/2009/01/p01017
Krause, M. R., Vieira, P. G., Csorba, B. A., Pilly, P. K., and Pack, C. C. (2019). Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc. Natl. Acad. Sci. 116 (12), 5747–5755. doi:10.1073/pnas.1815958116
Krause, M. R., Vieira, P. G., Thivierge, J. P., and Pack, C. C. (2022). Brain stimulation competes with ongoing oscillations for control of spike timing in the primate brain. PLOS Biol. 20 (5), e3001650. doi:10.1371/journal.pbio.3001650
Kromer, J. A., and Tass, P. A. (2022). Synaptic reshaping of plastic neuronal networks by periodic multichannel stimulation with single-pulse and burst stimuli. PLOS Comput. Biol. 18 (11), e1010568. doi:10.1371/journal.pcbi.1010568
Kromer, J. A., and Tass, P. A. (2024). Coordinated reset stimulation of plastic neural networks with spatially dependent synaptic connections. Front. Netw. Physiology 4, 1351815. doi:10.3389/fnetp.2024.1351815
Lea-Carnall, C. A., Trujillo-Barreto, N. J., Montemurro, M. A., El-Deredy, W., and Parkes, L. M. (2017). Evidence for frequency-dependent cortical plasticity in the human brain. Proc. Natl. Acad. Sci. 114 (33), 8871–8876. doi:10.1073/pnas.1620988114
Lefebvre, J., Hutt, A., and Frohlich, F. (2017). Stochastic resonance mediates the state-dependent effect of periodic stimulation on cortical alpha oscillations. Elife 6, e32054. doi:10.7554/eLife.32054
Limpert, E., Stahel, W. A., and Abbt, M. (2001). Log-normal distributions across the Sciences: keys and clues. BioScience 51 (5), 341–352. doi:10.1641/0006-3568(2001)051[0341:lndats]2.0.co;2
López-Alonso, V., Cheeran, B., Río-Rodríguez, D., and Fernández-del Olmo, M. (2014). Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 7 (3), 372–380. doi:10.1016/j.brs.2014.02.004
Lubenov, E. V., and Siapas, A. G. (2008). Decoupling through synchrony in neuronal circuits with propagation delays. Neuron 58 (1), 118–131. doi:10.1016/j.neuron.2008.01.036
Madadi Asl, M., Valizadeh, A., and Tass, P. A. (2018). Dendritic and axonal propagation delays may shape neuronal networks with plastic synapses. Front. physiology 9, 1849. doi:10.3389/fphys.2018.01849
Madadi Asl, M., Valizadeh, A., and Tass, P. A. (2023). Decoupling of interacting neuronal populations by time-shifted stimulation through spike-timing-dependent plasticity. PLOS Comput. Biol. 19 (2), e1010853. doi:10.1371/journal.pcbi.1010853
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., and Pascual-Leone, A. (2000). Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. brain Res. 133 (4), 425–430. doi:10.1007/s002210000432
Mazzoni, A., Lindén, H., Cuntz, H., Lansner, A., Panzeri, S., and Einevoll, G. T. (2015). Computing the local field potential (LFP) from integrate-and-fire network models. PLOS Comput. Biol. 11 (12), e1004584. doi:10.1371/journal.pcbi.1004584
Miniussi, C., and Vallar, G. (2011). Brain stimulation and behavioural cognitive rehabilitation: a new tool for neurorehabilitation? Neuropsychol. Rehabil. 21 (5), 553–559. doi:10.1080/09602011.2011.622435
Monti, A., Ferrucci, R., Fumagalli, M., Mameli, F., Cogiamanian, F., Ardolino, G., et al. (2013). Transcranial direct current stimulation (tDCS) and language. J. Neurology, Neurosurg. and Psychiatry 84 (8), 832–842. doi:10.1136/jnnp-2012-302825
Moradi Chameh, H., Rich, S., Wang, L., Chen, F. D., Zhang, L., Carlen, P. L., et al. (2021). Diversity amongst human cortical pyramidal neurons revealed via their sag currents and frequency preferences. Nat. Commun. 12 (1), 2497–15. doi:10.1038/s41467-021-22741-9
Negahbani, E., Kasten, F. H., Herrmann, C. S., and Fröhlich, F. (2018). Targeting alpha-band oscillations in a cortical model with amplitude-modulated high-frequency transcranial electric stimulation. Neuroimage. 173, 3–12. doi:10.1016/j.neuroimage.2018.02.005
Nowotny, T., Zhigulin, V. P., Selverston, A. I., Abarbanel, H. D., and Rabinovich, M. I. (2003). Enhancement of synchronization in a hybrid neural circuit by spike-timing dependent plasticity. J. Neurosci. 23 (30), 9776–9785. doi:10.1523/jneurosci.23-30-09776.2003
Pariz, A., Trotter, D., Hutt, A., and Lefebvre, J. (2023). Selective control of synaptic plasticity in heterogeneous networks through transcranial alternating current stimulation (tACS). PLOS Comput. Biol. 19 (4), e1010736. doi:10.1371/journal.pcbi.1010736
Park, S. H., Griffiths, J. D., Longtin, A., and Lefebvre, J. (2018). Persistent entrainment in non-linear neural networks with memory. Front. Appl. Math. Statistics 4, 31. doi:10.3389/fams.2018.00031
Pfister, J. P., and Gerstner, W. (2006). Triplets of spikes in a model of spike timing-dependent plasticity. J. Neurosci. 26 (38), 9673–9682. doi:10.1523/JNEUROSCI.1425-06.2006
Pfister, J. P., and Tass, P. A. (2010). STDP in oscillatory recurrent networks: theoretical conditions for desynchronization and applications to deep brain stimulation. Front. Comput. Neurosci. 4, 22. doi:10.3389/fncom.2010.00022
Polanía, R., Nitsche, M. A., and Ruff, C. C. (2018). Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 21 (2), 174–187. doi:10.1038/s41593-017-0054-4
Reato, D., Rahman, A., Bikson, M., and Parra, L. C. (2010). Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci. 30 (45), 15067–15079. doi:10.1523/JNEUROSCI.2059-10.2010
Ridding, M., and Ziemann, U. (2010). Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. physiology 588 (13), 2291–2304. doi:10.1113/jphysiol.2010.190314
Riddle, J., Rubinow, D. R., and Frohlich, F. (2020). A case study of weekly tACS for the treatment of major depressive disorder. Brain Stimul. Basic, Transl. Clin. Res. Neuromodulation 13 (3), 576–577. doi:10.1016/j.brs.2019.12.016
Rubinov, M., Sporns, O., Thivierge, J. P., and Breakspear, M. (2011). Neurobiologically realistic determinants of self-organized criticality in networks of spiking neurons. PLOS Comput. Biol. 7 (6), e1002038. doi:10.1371/journal.pcbi.1002038
San-Juan, D., Espinoza-López, D. A., Vázquez-Gregorio, R., Trenado, C., Aragón, M. F. G., Pérez-Pérez, D., et al. (2022). A pilot randomized controlled clinical trial of Transcranial Alternating Current Stimulation in patients with multifocal pharmaco-resistant epilepsy. Epilepsy and Behav. 130, 108676. doi:10.1016/j.yebeh.2022.108676
Schlaug, G., Renga, V., and Nair, D. (2008). Transcranial direct current stimulation in stroke recovery. Archives neurology 65 (12), 1571–1576. doi:10.1001/archneur.65.12.1571
Schwab, B. C., König, P., and Engel, A. K. (2021). Spike-timing-dependent plasticity can account for connectivity aftereffects of dual-site transcranial alternating current stimulation. NeuroImage. 237, 118179. doi:10.1016/j.neuroimage.2021.118179
Schwab, B. C., Misselhorn, J., and Engel, A. K. (2019). Modulation of large-scale cortical coupling by transcranial alternating current stimulation. Brain Stimul. 12 (5), 1187–1196. doi:10.1016/j.brs.2019.04.013
Shen, K. Z., Zhu, Z. T., Munhall, A., and Johnson, S. W. (2003). Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse 50 (4), 314–319. doi:10.1002/syn.10274
Sjöström, J., and Gerstner, W. (2010). Spike-timing dependent plasticity. Spike-timing Depend. Plast. 35. doi:10.4249/scholarpedia.1362
Sjöström, P. J., Turrigiano, G. G., and Nelson, S. B. (2001). Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32 (6), 1149–1164. doi:10.1016/s0896-6273(01)00542-6
Soltesz, I. (2006). Diversity in the neuronal machine: order and variability in interneuronal microcircuits. Oxford University Press.
Takeuchi, Y., and Berényi, A. (2020). Oscillotherapeutics – time-targeted interventions in epilepsy and beyond. Neurosci. Res. 152, 87–107. doi:10.1016/j.neures.2020.01.002
Temperli, P., Ghika, J., Villemure, J. G., Burkhard, P., Bogousslavsky, J., and Vingerhoets, F. (2003). How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60 (1), 78–81. doi:10.1212/wnl.60.1.78
Tuckwell, H. C. (2006). Cortical network modeling: analytical methods for firing rates and some properties of networks of LIF neurons. J. Physiology-Paris 100 (1-3), 88–99. doi:10.1016/j.jphysparis.2006.09.001
Uhlhaas, P. J., and Singer, W. (2013). High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin. Neurosci. 15 (3), 301–313. doi:10.31887/DCNS.2013.15.3/puhlhaas
Vogels, T. P., and Abbott, L. F. (2005). Signal propagation and logic gating in networks of integrate-and-fire neurons. J. Neurosci. 25 (46), 10786–10795. doi:10.1523/JNEUROSCI.3508-05.2005
Vogeti, S., Boetzel, C., and Herrmann, C. S. (2022). Entrainment and spike-timing dependent plasticity–A review of proposed mechanisms of transcranial alternating current stimulation. Front. Syst. Neurosci. 16. doi:10.3389/fnsys.2022.827353
Vossen, A., Gross, J., and Thut, G. (2015). Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 8 (3), 499–508. doi:10.1016/j.brs.2014.12.004
Yamawaki, N., Magill, P., Woodhall, G., Hall, S., and Stanford, I. (2012). Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus. Neuroscience 203, 1–11. doi:10.1016/j.neuroscience.2011.12.027
Keywords: brain stimulation, post-stimulation after-effects, stimulation-induced, heterogeneity, neurons timescale diversity, network physiology
Citation: Lefebvre J and Pariz A (2025) Amplifying post-stimulation oscillatory dynamics by engaging synaptic plasticity with transcranial alternating current stimulation. Front. Netw. Physiol. 5:1621283. doi: 10.3389/fnetp.2025.1621283
Received: 30 April 2025; Accepted: 30 June 2025;
Published: 18 July 2025.
Edited by:
Mojtaba Madadi Asl, Institute for Research in Fundamental Sciences (IPM), IranReviewed by:
AmirAli Farokhniaee, University College Dublin, IrelandHan Lu, Helmholtz Association of German Research Centers (HZ), Germany
Copyright © 2025 Lefebvre and Pariz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aref Pariz, YXBhcml6QHVvdHRhd2EuY2E=
 Jeremie Lefebvre1,2,3,4
Jeremie Lefebvre1,2,3,4 Aref Pariz
Aref Pariz