- 1Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland
- 2Department of Neurology and Research Institute, Medical University Plovdiv, Plovdiv, Bulgaria
- 3Clinica Luganese, Mancucco, Lugano, Switzerland
- 4Department of Neurology, University Hospital Queen Giovanna, Sofia, Bulgaria
- 5Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University Hospital and University of Bern, Bern, Switzerland
- 6Department of Neurosurgery, Inselspital, University Hospital and University of Bern, Bern, Switzerland
- 7Division of Paediatric Neurology, Department of Paediatrics, Children's Hospital Lucerne, Lucerne, Switzerland
- 8Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland
- 9Division of Neuropaediatrics, Istituto Pediatrico della Svizzera Italiana IPSI EOC, Ospedale Regionale di Bellinzona e Valli, Bellinzona, Switzerland
- 10Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland
- 11Netzwerk Radiologie, Kantonsspital St. Gallen, St. Gallen, Switzerland
Aim: The aim of this study was to investigate baseline characteristics and outcome of patients after endovascular therapy (EVT) for acute large vessel occlusion (LVO) in relation to their history of symptomatic vascular disease and sex.
Methods: Consecutive EVT-eligible patients with LVO in the anterior circulation admitted to our stroke center between 04/2015 and 04/2020 were included in this observational cohort study. All patients were treated according to a standardized acute ischaemic stroke (AIS) protocol. Baseline characteristics and successful reperfusion, recurrent/progressive in-hospital ischaemic stroke, symptomatic in-hospital intracranial hemorrhage, death at discharge and at 3 months, and functional outcome at 3 months were analyzed according to previous symptomatic vascular disease and sex.
Results: 995 patients with LVO in the anterior circulation (49.4% women, median age 76 years, median admission NIHSS score 14) were included. Patients with multiple vs. no previous vascular events showed higher mortality at discharge (20% vs. 9.3%, age/sex − adjustedOR = 1.43, p = 0.030) and less independency at 3 months (28.8% vs. 48.8%, age/sex − adjustedOR = 0.72, p = 0.020). All patients and men alone with one or multiple vs. patients and men with no previous vascular events showed more recurrent/progressive in-hospital ischaemic strokes (19.9% vs. 6.4% in all patients, age/sex − adjustedOR = 1.76, p = 0.028) (16.7% vs. 5.8% in men, age-adjustedOR = 2.20, p = 0.035). Men vs. women showed more in-hospital symptomatic intracranial hemorrhage among patients with one or multiple vs. no previous vascular events (23.7% vs. 6.6% in men and 15.4% vs. 5.5% in women, OR = 2.32, p = 0.035/age − adjustedOR = 2.36, p = 0.035).
Conclusions: Previous vascular events increased the risk of in-hospital complications and poorer outcome in the analyzed patients with EVT-eligible LVO-AIS. Our findings may support risk assessment in these stroke patients and could contribute to the design of future studies.
Introduction
Multiple vascular events are predominantly driven by atherosclerosis, especially when cardiac and peripheral locations are affected (1–3).
Patients who suffer an acute ischaemic stroke (AIS) and have a history of multiple vascular events have certain characteristics: they tend to be older, have multiple vascular risk factors and often have more than one possible cause for their stroke (3). Despite the implementation of more aggressive preventive measures, these patients remain more likely to have complications and are at higher risk of recurrent vascular events and vascular-related mortality (3). Notably, previous studies looking at pre-existing vascular disease focused primarily on patients with transient ischaemic attack (TIA) and/or mild to moderate AIS (3–6).
Past studies have uncovered significant sex differences in vascular risk factor profiles. These studies have also identified the impact of specific vascular risk factors such as atrial fibrillation, diabetes mellitus, and arterial hypertension in women (7, 8). Women have been reported to develop AIS at an older age compared to men (7, 8). Furthermore, they are less likely to receive intravenous thrombolysis (IVT) (9), and tend to experience more severe AIS with worse outcomes and higher mortality rates (8, 10, 11). However, data on sex differences in patients eligible for endovascular therapy (EVT) with large vessel occlusion (LVO)-AIS remain limited (12).
The aim of this study was to investigate baseline characteristics and outcome after EVT of patients with LVO-AIS according to history of symptomatic vascular disease and sex.
Methods
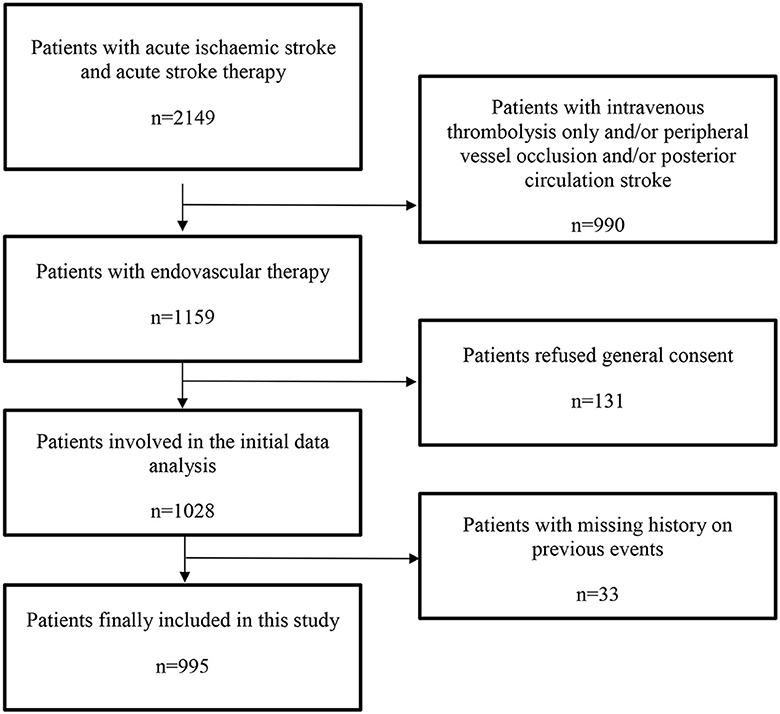
Data were extracted from AIS patients treated between April 2015 and April 2020 from the prospective Bern Stroke Center Registry and retrospectively analyzed. We included all patients with AIS and acute large vessel occlusion (LVO) in the anterior circulation who were treated with EVT. AIS was defined according to the American Stroke Association/American Health Association (ASA/AHA) criteria (13). LVO was defined as acute vessel occlusion of the internal carotid artery (ICA), the carotid terminus (ICA-T), the proximal middle cerebral artery (M1- or M2-segment; MCA) or tandem occlusion (ICA and M1- or M2-segment of the ipsilateral MCA). Patients who were treated with intravenous thrombolysis only and/or had suffered peripheral vascular occlusion and/or posterior circulation stroke were excluded from the study. A flow chart showing the inclusion and exclusion criteria of the study is shown in Figure 1.
Patients were assessed on admission to the emergency department using a standardized AIS protocol that included history taking and clinical examination by a board-certified neurologist, laboratory blood tests, electrocardiography and cranial imaging with CT and/or MR arteriography.
The decision for or against EVT was made individually and in consultation with experienced neurologists and neuroradiologists according to international as well as our institutional guidelines (14–16). EVT was performed as early as possible after diagnosis and after consideration of indications and contraindications. All patients underwent diagnostic digital subtraction angiography. Two independent neuroradiologists evaluated the radiological data. After EVT, all patients were hospitalized in the stroke unit, intermediate or intensive care unit at the Bernese stroke center.
A follow-up CT and/or MR arteriography was performed 24 h after EVT or at an earlier time point if secondary neurological deterioration occurred.
A 3-month follow-up was performed clinically by a board-certified neurologist or by telephone by a trained study nurse.
Patients were a priori classified according to history of symptomatic vascular disease in different vascular beds: peripheral, coronary or cerebrovascular, if leading to past hospitalization. Patients were attributed to previous coronary artery event(s) if there was previous myocardial infarction, unstable angina, angina and previous percutaneous coronary intervention or coronary bypass surgery. History was positive for peripheral vascular event(s) with previous aortic aneurysm rupture, aortic dissection, acute limb ischaemia, critical limb ischaemia, acute visceral ischaemia, intermittent claudication, and previous angioplasty or stenting and peripheral arterial bypass grafting or amputation. History was positive for cerebrovascular event(s) in case of a previous transient ischaemic attack, AIS or symptomatic intracranial hemorrhage.
Reperfusion was assessed using the modified Thrombolysis in Cerebral Infarction Score (mTICI) (17). Successful reperfusion (SR) was defined as mTICI 2b/3. Recurrent/progressive in-hospital ischaemic stroke was defined according to the ASA/AHA criteria and if NIHSS score increased by at least 4 points. In-hospital symptomatic intracranial hemorrhage (sICH) was defined according to the ECASS II criteria (18). Death was classified as vascular if the patient died within 2 weeks of a vascular event. Functional outcome was assessed according to the modified Rankin Scale (mRS). mRS 0–2 was defined as functional independence (FI) and mRS 0–1 as excellent outcome (EO) (19).
Statistical analysis
Statistical analysis was performed using SPSS 25.0 (SPSS Inc., Chicago, Illinois, USA). In univariable analysis, the χ2-test was applied for categorical variables and the ANOVA-test for ordinal and continuous variables to compare baseline characteristics between patients with one or multiple vs. no vascular events and between patients with multiple vs. no vascular events. Sensitivity analyses were performed for patients according to sex and according to cardioembolism only.
A 2-tailed p < 0.05 was considered significant.
Binary logistic regression and ordinal or linear regression analysis were performed for outcome analysis where appropriate. Regression analysis was adjusted for age and sex where appropriate.
Standard protocol approvals, registrations, patient consent and reporting
The Bernese stroke registry was approved by the local ethics committee (KEK Bern 2016–01905) for quality control and research. Informed consent was waived by the ethics committee. Patients were informed about the registry and the potential use of their data for research. In accordance with Swiss law, patients who refused the use of their data for research were excluded from this analysis. This study complies with the Declaration of Helsinki. Data analyses followed strengthening the reporting of observational studies in epidemiology (STROBE) reporting guidelines.
Results
Baseline characteristics of patients according to previous vascular events
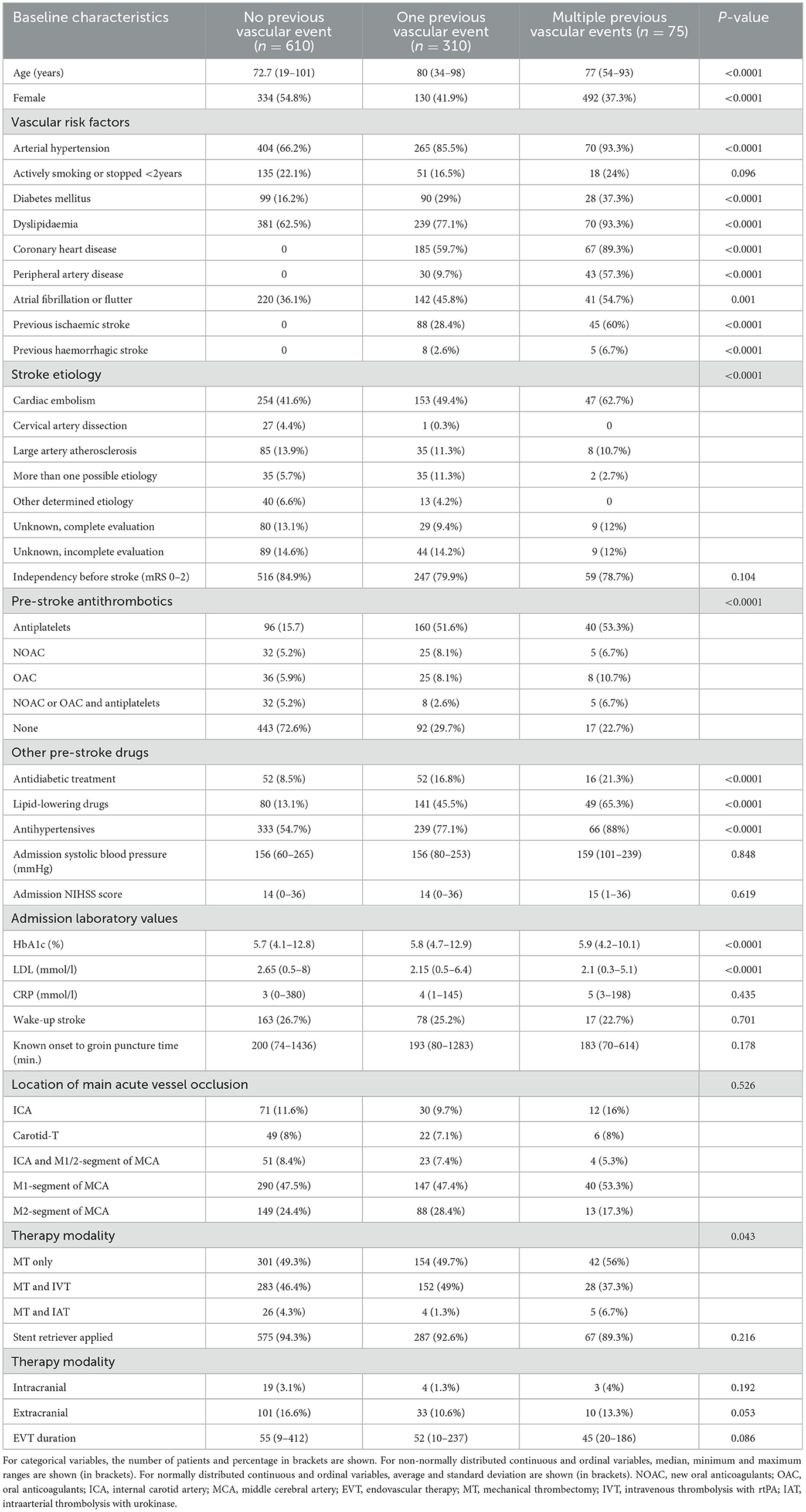
A total of 995 patients (49.4% women, median age 76 years, median admission NIHSS score 14) were included in the current study. A total of 610 (61.3%) patients had no previous vascular event, 310 (31.2%) one and 75 (7.5%) multiple (Table 1). Patients with one or multiple previous vascular events were older (median age 80 and 79 years respectively vs. 72.7 in patients with no events, p < 0.0001) and less frequently female (41.9% and 37.3% respectively vs. 54.8% in patients with no events, p < 0.0001). They more frequently had a history of vascular risk factors, were more likely to be treated with antithrombotics (70.3% and 77.3% vs. 27.4% in patients with no events, p < 0.0001) and other (antidiabetic, lipid-lowering and antihypertensive) drugs (p < 0.0001) pre-stroke. They more often had higher admission HbA1c values (5.8% and 5.9% vs. 5.7% in patients with no events, p < 0.0001), lower LDL values (2.15 mmol/l and 2.1 mmol/l vs. 2.65 mmol/l in patients with no events, p < 0.0001) and a different pattern of stroke etiology (p < 0.0001) as well as a different applied therapy strategy during emergency care (p = 0.043). The three groups were similar with respect to other baseline characteristics such as admission NIHSS, location of main acute vessel occlusion, extra/intracranial therapy modality and EVT duration. All baseline data are shown in Table 1.
Outcome characteristics of patients according to previous vascular events
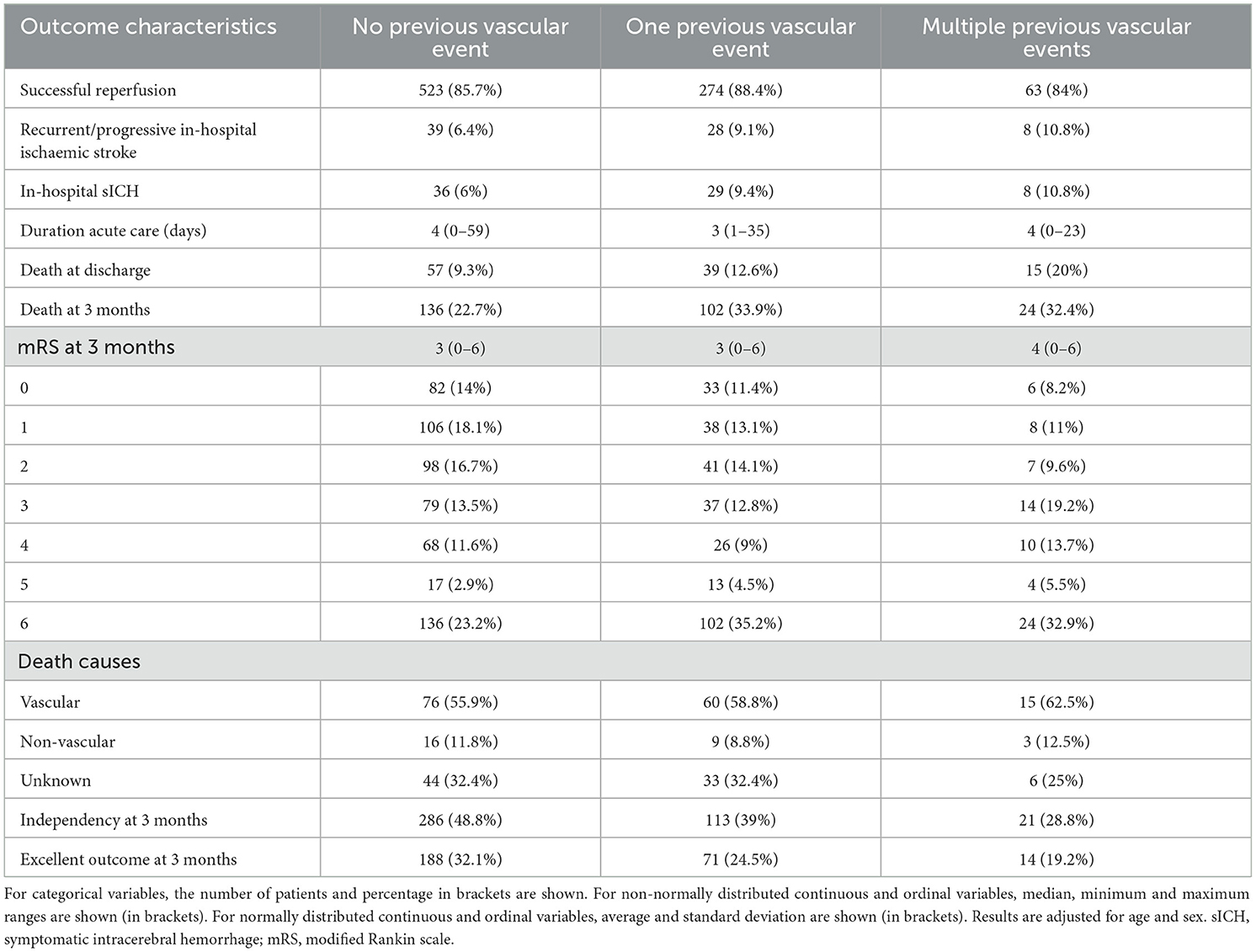
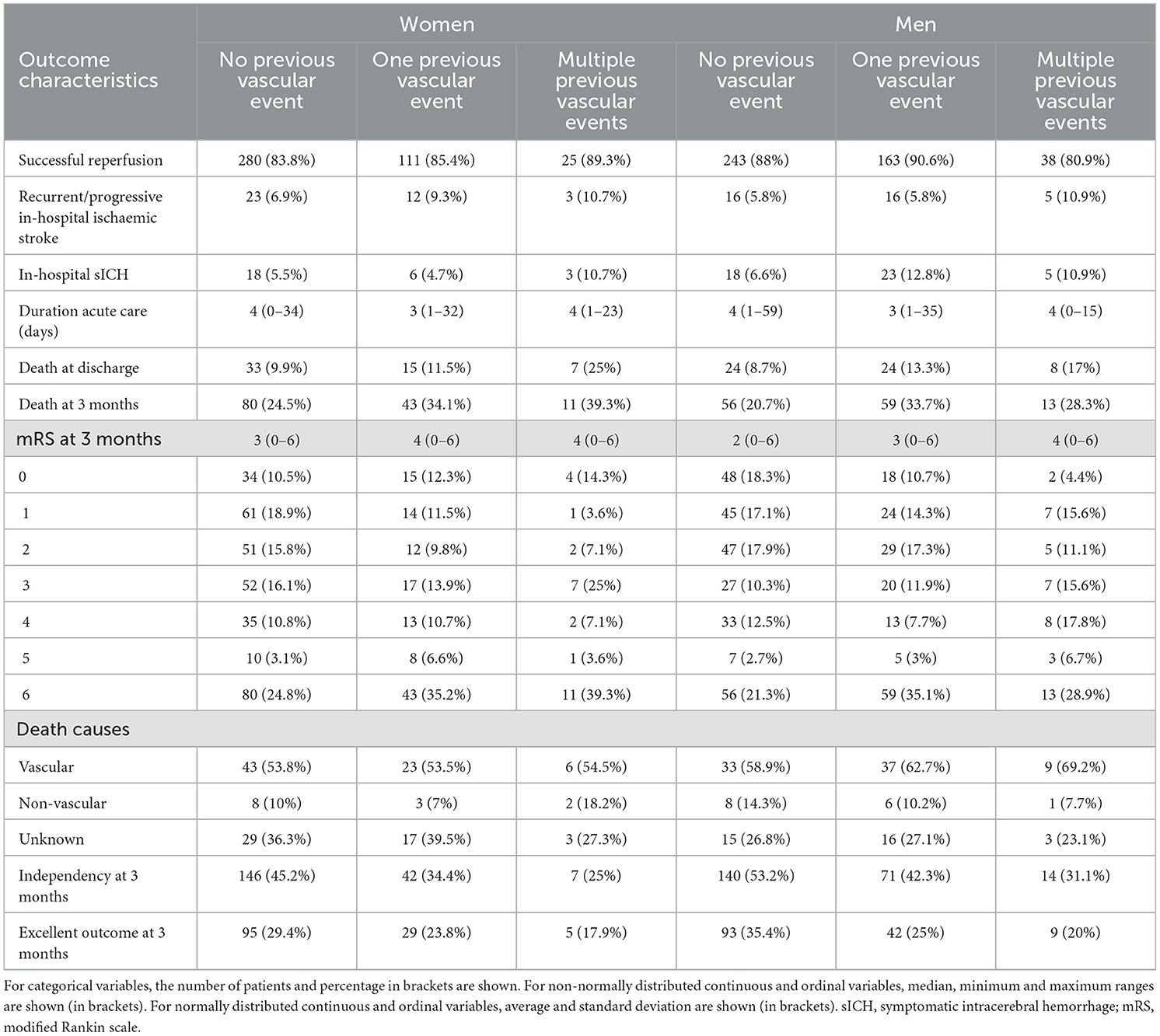
In univariable analysis (Table 2, Figure 2A), patients with one or multiple vs. no previous vascular events showed more in hospital sICH (20.2% vs. 6%, OR = 1.69, p = 0.031), death at discharge (22.6% vs. 9.3%, OR = 1.58, p = 0.023) and at 3 months (69.3% vs. 22.7%, OR = 1.72, p < 0.0001), less independency (67.8% vs. 48.8%, OR = 0.61, p < 0.0001) and less excellent outcome at 3 months (43.7% vs. 32.1%, OR = 0.65, p = 0.004) and a worse mRS shift at 3 months (p < 0.0001). Patients with multiple vs. no previous vascular events (Table 2, Figure 2B) showed higher mortality at discharge (20% vs 9.3%, OR = 1.56, p = 0.006) and less independency (28.8% vs. 48.8%, OR = 0.65, p = 0.002) and less excellent outcome at 3 months (19.2% vs. 32.1%, OR = 0.71, p = 0.026) and a worse mRS shift at 3 months (p = 0.005).
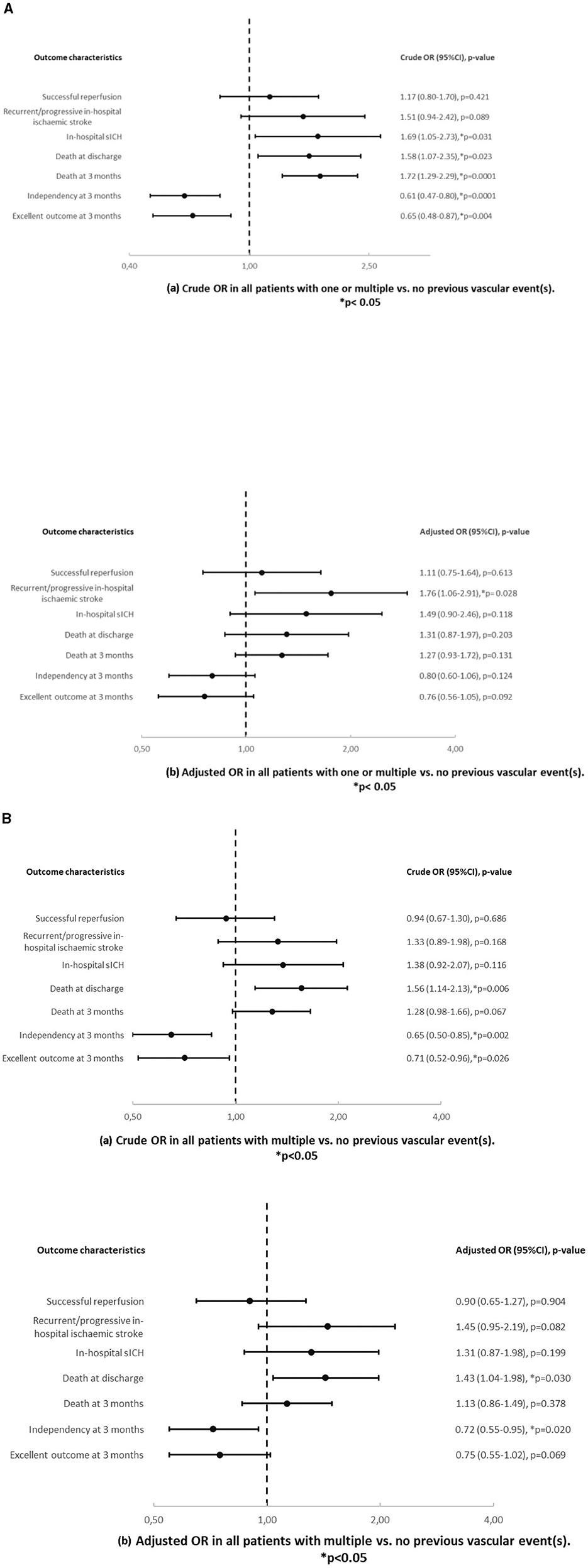
Figure 2. (A) Crude (a) and adjusted (b) odds ratios and p-values in all patients with one or multiple vs. no previous vascular event(s). Results are adjusted for age and sex. (B) Crude (a) and adjusted (b) odds ratios and p-values in all patients with multiple vs. no previous vascular event(s). Results are adjusted for age and sex.
In multivariable analysis (Table 2, Figures 2A, B), patients with one or multiple vs. no previous vascular events showed more recurrent/progressive in-hospital ischaemic strokes (19.9% vs. 6.4%, age/sex − adjustedOR = 1.76, p = 0.028) but otherwise similar outcomes. Patients with multiple vs. no previous vascular events showed higher mortality at discharge (20% vs. 9.3%, age/sex − adjusted OR = 1.43, p = 0.030) and less independency at 3 months (28.8% vs. 48.8%, age/sex − adjustedOR = 0.72, p = 0.020) but otherwise similar outcomes.
Baseline characteristics of patients according to sex
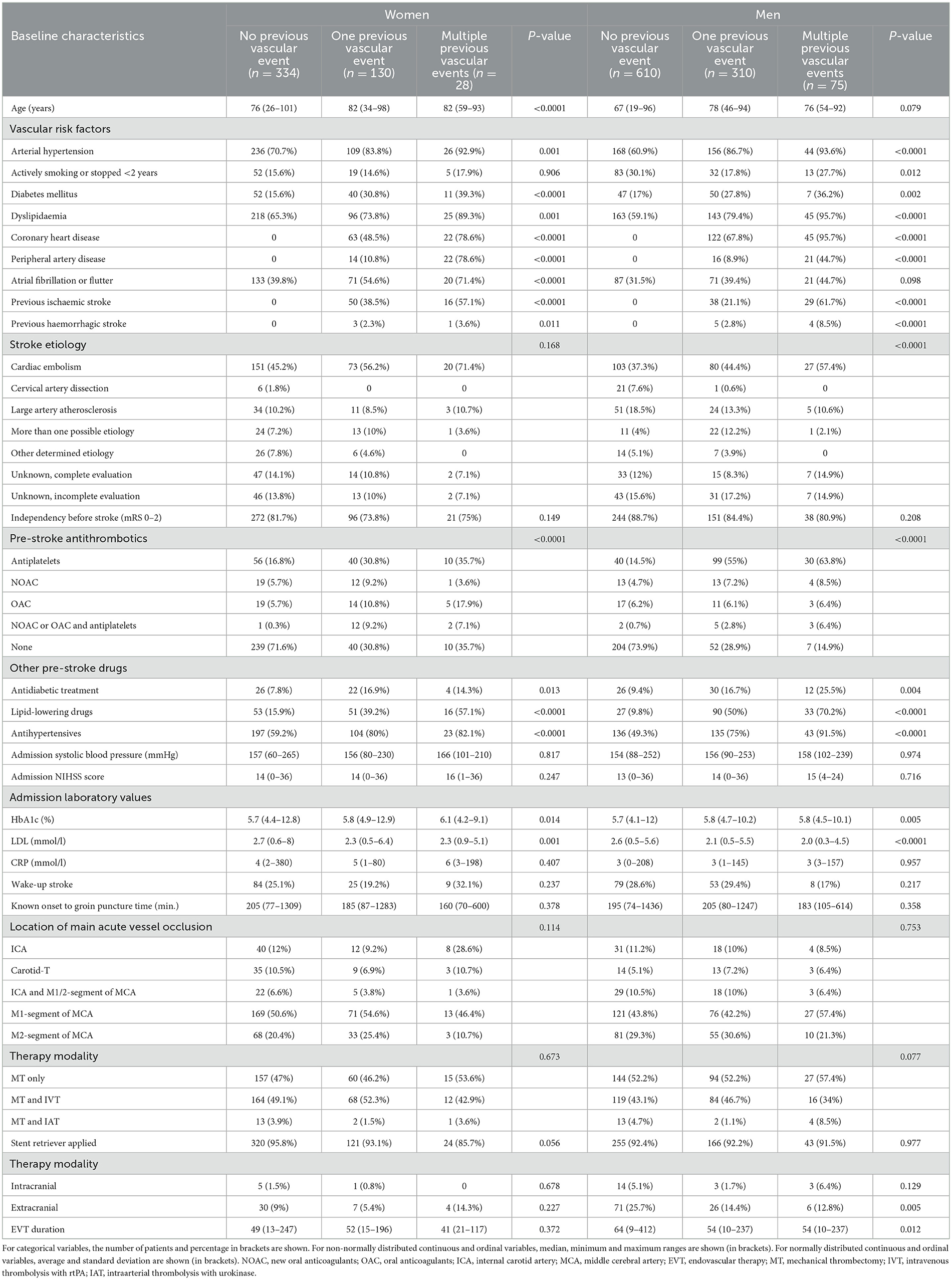
Women with one or multiple previous vascular events were older (median age 82 and 82 years respectively vs. 76 years in patients with no events, p < 0.001), more frequently had a history of vascular risk factors, of pre-stroke antithrombotics (69.2% and 64.3% respectively vs. 28.4% in patients with no events, p < 0.0001) and other drugs (p ≤ 0.013), of higher admission HbA1c values (5.8% and 6.1% vs. 5.7% in patients with no events, p = 0.014) and lower LDL values (2.3 mmol/l and 2.3 mmol/l vs. 2.7 mmol/l, p = 0.001). All other baseline characteristics did not differ between women in this group comparison (Table 3).
Men with one or multiple previous vascular events, more frequently had a history of vascular risk factors, of pre-stroke antithrombotics (71.1% and 85.1% respectively vs. 26.1% in patients with no events, p < 0.0001) and other drugs (p ≤ 0.004), they showed higher admission HbA1c values (5.8% and 5.8% respectively vs. 5.7% in patients with no events, p = 0.005) and lower LDL values (2.1 mmol/l and 2.2mmol/l respectively vs. 2.6 mmol/l in patients with no events, p < 0.0001). They presented a different pattern of stroke etiology (p < 0.0001), more permanent extracranial stent placements (p = 0.005) and a shorter EVT duration (54 vs. 64 min in patients with no events, p = 0.012). All other baseline characteristics did not differ between men in this group comparison (Table 3).
Outcome characteristics of patients according to sex
In univariable analysis (Table 4 and Figure 3A), women with one or multiple vs. no previous vascular events showed higher mortality at 3 months (73.4% vs. 24.5%, OR = 1.67, p = 0.016), less independency at 3 months (59.4% vs. 45.2%, OR = 0.59, p = 0.010) and a worse mRS shift at 3 months (OR = 0.007). However, in multivariable analysis, there was no difference of outcome in this group comparison.
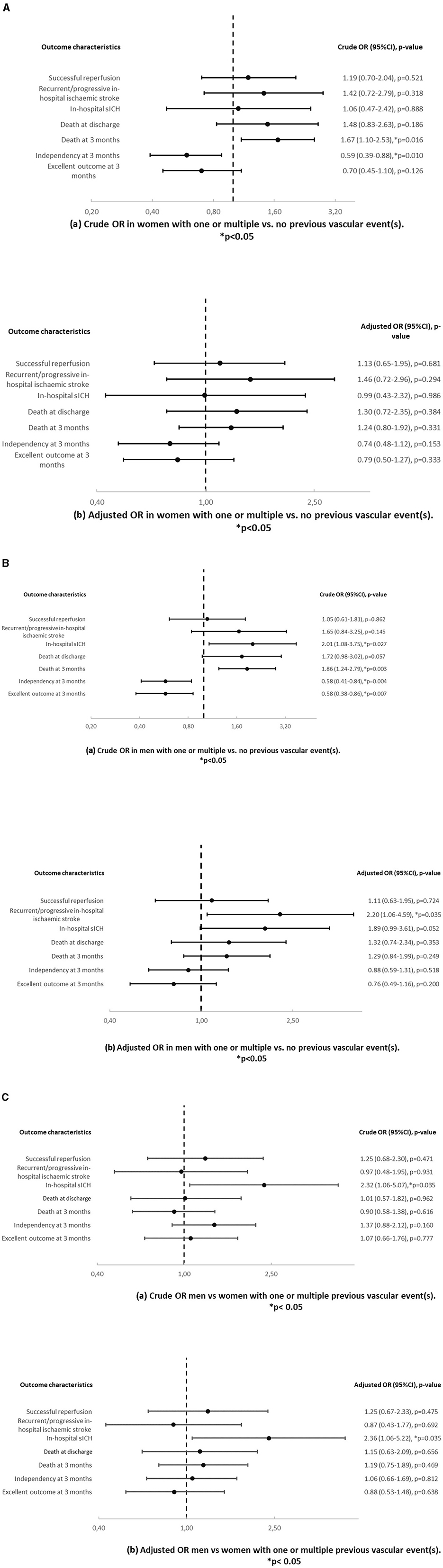
Figure 3. (A) Crude (a) and adjusted (b) odds ratios and p-values in women with one or multiple vs. no previous vascular event(s). Results are adjusted for age. (B) Crude (a) and adjusted (b) odds ratios and p-values in men with one or multiple vs. no previous vascular event(s). Results are adjusted for age. (C) Crude (a) and adjusted (b) odds ratios and p-values in men vs. women with one or multiple previous vascular event(s). Results are adjusted for age.
In univariable analysis (Table 4 and Figure 3B), men with one or multiple vs. no previous vascular events showed more in hospital sICH (23.7% vs. 6.6%, OR = 2.01, p = 0.027), death at 3 months (62.0% vs. 20.7%, OR = 1.86, p = 0.003), less independency (73.4% vs. 53.2%, OR = 0.58, p = 0.004) and excellent outcome (45% vs. 35.4%, OR = 0.58, p = 0.007) at 3 months and a worse mRS shift (p < 0.0001) at 3 months. In multivariable analysis, men with one or multiple vs. no previous vascular events showed more recurrent/progressive in-hospital ischaemic strokes (16.7% vs. 5.8%, age − adjustedOR = 2.20, p = 0.035).
In univariable and multivariable analysis (Table 4 and Figure 3C), men vs. women showed more in-hospital sICH among patients with one or multiple vs. no previous vascular events (23.7% vs. 6.6% in men and 15.4% vs. 5.5% in women, OR = 2.32, p = 0.035/age − adjustedOR = 2.36, p = 0.035).
Outcome characteristics of patients according to cardioembolism only
Patients with cardioembolic stroke and one or multiple vs. no previous vascular event(s) showed more death at discharge (OR 2.10, p = 0.018) and at 3 months (OR 1.72, p = 0.011) and less independency (OR 0.52, p = 0.0001) and excellent outcome at 3 months (OR 0.59, p = 0.014) in univariable analysis (Figure 4A). In multivariable analysis, these patients showed more death at discharge (OR 1.88, p = 0.049) and less independency (OR 0.66, p = 0.046) at 3 months (OR 0.66, p = 0.046). Patients with cardioembolic stroke and multiple vs. no previous vascular event(s) (Figure 4B) had less independency at 3 months (OR 0.66, p = 0.016) in univariable analysis. We also observed that these patients experienced more death at discharge in univariable (OR 1.83, p = 0.005) and in multivariable analysis (OR 1.93, p = 0.005). Women with cardioembolic stroke and one or multiple vs. no previous vascular event(s) showed more death at 3 months (OR 1.98, p = 0.020) and less independency (OR 0.47, p = 0.009) in univariable analysis (Figure 4C). Men with cardioembolic stroke and one or multiple vs. no previous vascular event(s) also had less independency (OR 0.53, p = 0.025) and less excellent outcome (OR 0.55, p = 0.046) at 3 months in univariable analysis (Figure 4D). All other analyses were similar (Figures 4A–E).
Figure 4. (A) Crude (a) and adjusted (b) odds ratios and p-values in patients with cardioembolic stroke and one or multiple vs. no previous vascular event(s). Results are adjusted for age and sex. (B) Crude (a) and adjusted (b) odds ratios and p-values in patients with cardioembolic stroke and multiple vs. no previous vascular event(s). Results are adjusted for age and sex. (C) Crude (a) and adjusted (b) odds ratios and p-values in women with cardioembolic stroke and one or multiple vs. no previous vascular event(s). Results are adjusted for age. (D) Crude (a) and adjusted (b) odds ratios and p-values in men with cardioembolic stroke and one or multiple vs. no previous vascular event(s). Results are adjusted for age. (E) Crude (a) and adjusted (b) odds ratios and p-values in men vs. women with cardioembolic stroke and one or multiple previous vascular event(s). Results are adjusted for age.
Discussion
In this monocentric, retrospectively analyzed, observational cohort study, we investigated baseline characteristics and outcome after EVT of patients with LVO-AIS according to history of symptomatic vascular disease and sex.
The main findings of our study are as follows:
Two out of every five patients in our study had one or multiple previous vascular events. Compared to patients without previous vascular events, these patients had a higher burden of vascular risk factors and use of preventive medications prior to stroke, also when women and men were analyzed separately. In the overall study population but specifically in men, patients with one or multiple vs. no vascular event showed a different pattern of stroke etiology and of other EVT strategies. In the adjusted analysis, patients overall and men alone with one or multiple vs. no previous vascular events showed more recurrent/progressive in-hospital ischaemic strokes. Patients with multiple vs. no previous vascular events showed higher mortality at discharge and less independency at 3 months. In patients with one or multiple vs. no previous vascular events, men had more in-hospital sICH than women.
Prevalence of symptomatic vascular disease
Previous studies have examined the prevalence of co-existing symptomatic vascular disease in patients with ischaemic stroke but this was prior to the introduction of EVT. In the OXVASC study (a population-based study which focused on patients with minor strokes and TIAs) (3), 27.9% of patients had a history of previous symptomatic vascular disease. In the REACH registry (a prospective study) (5, 6), 40% of patients with cerebrovascular disease had previous symptomatic vascular disease affecting additional vascular beds. In the CRUSADE registry (a study with focus on coronary artery disease) (20), 12.8% of patients had known disease in at least two vascular territories. Our study revealed, that two out of every five patients had one or multiple previous vascular events. The variations in the reported percentages among these studies may be attributed to differences in settings, patient groups, and definitions of co-existing symptomatic vascular disease. Furthermore, our findings suggest that with increasing life expectancy and disease prevalence, there will be a growing number of patients with multiple vascular diseases. This poses a major challenge for healthcare providers in managing these complex patients.
Association with vascular risk factors
Both, the OXVASC study (3) as well as the CRUSADE registry (20) have demonstrated that the severity of vascular disease is closely associated with an increased burden of vascular risk factors.
In the OXVASC study, hypercholesterolemia (OR 6.80, p < 0.0001) exhibited a particularly strong association with triple-vascular territory disease. Moreover, arterial hypertension (age/sex-adjusted OR 3.43, p < 0.0001), diabetes mellitus (OR 2.89, p < 0.0001), hypercholesterolemia (OR 4.67, p < 0.0001) and current or previous smoking (OR 1.52, p < 0.0001) were more often found in patients with multiple- vs. single-vascular territory disease. In the CRUSADE registry (20), risk factors such as arterial hypertension (p < 0.0001), diabetes mellitus (p < 0.0001), dyslipidaemia (p < 0.0001), renal insufficiency (p < 0.0001), and prior congestive heart failure (p < 0.0001) were closely related to the number of affected vascular territories.
In our study, we also observed that patients who had experienced one or more vascular events had a greater prevalence of vascular risk factors, such as high blood pressure (p < 0.0001), diabetes mellitus (p < 0.0001), and abnormal lipid levels (p < 0.0001), when compared to patients without any previous vascular events. There was also a trend toward a higher burden of atrial fibrillation, particularly among women with one or multiple vascular diseases (p < 0.0001). Atrial fibrillation and multiple vascular diseases often coexist and share common risk factors, including age, high blood pressure, diabetes mellitus, and obesity (21, 22). The presence of multiple vascular diseases can further complicate the management of atrial fibrillation, particularly with regard to antithrombotic therapy. In our study, there is a discrepancy between atrial fibrillation and NOAC/OAC on admission. This difference indicates that in most patients atrial fibrillation was newly discovered on admission.
It is important to note that the studies we and others have conducted primarily focused on classical vascular risk factors, most of which have been found to be more prevalent in men. However, the evaluation of specific vascular risk factors, such as hormone status, factors related to pregnancy, and age at menopause, may introduce new considerations for risk assessment in women (23).
Preventive medication
In addition, the OXVASC trial (3), the REACH registry (5, 6) and the CRUSADE registry (20) have established that patients with advanced vascular disease are more likely to use preventive medications to reduce the risk of vascular events. In the REACH registry (6), it was also noted that the utilization of preventive antiplatelet and lipid-lowering drugs was more prevalent among men compared to women (93.5% vs. 90.1%, p < 0.0001 and 73.6% vs. 71.4%, respectively), and this difference could not be entirely explained by variations in vascular risk factor profiles. Our study aligns with these earlier findings. We observed that patients with one or more previous vascular events were more likely to be on preventive medications prior to stroke, even when we analyzed men and women separately.
Interestingly, we also observed lower LDL values in patients with a higher burden of vascular events, indicating treatment adherence. However, patients still failed to meet secondary prevention LDL treatment goals. This underscores the necessity for not only more intensive and consistently adhered treatment, but also for rigorous control of vascular risk factors, including regular follow-up and personalized lifestyle interventions. Conversely, HbA1c levels were significantly higher in patients with a higher burden of vascular events. Additionally, this effect was more pronounced in women than in men, highlighting the urgent need for improved treatment strategies in women.
Stroke etiology
In the overall study population, and particularly in men, we observed a distinct pattern of stroke etiology among patients with multiple previous vascular events in our study. A similar observation was made in the OXVASC study, which also noted a different stroke etiology pattern in patients with multiple-territory vascular disease compared to those with single-territory disease (4). However, in contrast to the OXVASC study, we identified higher rates of cardioembolic strokes in patients with more extensive vascular disease. This divergence in findings is likely due to variations in study settings and patient populations. Interestingly, when we conducted separate analyses by sex, we found that atrial fibrillation was a significant factor contributing to vascular risk in women, but not in men. This observation is consistent with the existing literature (24, 25).
Therapy modality
In our study, there is a slight difference connected with applied therapy modality as patients with one or multiple vs. no previous vascular events had higher rates of receiving mechanical thrombectomy only as opposed to combined treatment strategies. The selection of treatment approach for patients with multiple vascular diseases has not been thoroughly studied in randomized controlled trials and may depend on both the vascular condition and the potential risks of complications. Looking only at men, those without a history of previous vascular events had longer EVT durations and higher rates of extracranial stent placements. This observation may be attributed to the likelihood that these patients had a higher rate of large artery atherosclerosis as the underlying cause of their stroke. Previous research has indicated that EVT procedures may take longer in patients with large artery atherosclerosis due to the challenges of accessing the affected area, fewer first-past successes, and a higher need for permanent stent placements (26, 27).
Treatment outcomes
The association of vascular risk profile and treatment outcomes after EVT was indirectly assessed in previous studies (28–30). In those studies comorbidities such as cerebrovascular and cardiovascular diseases were assessed as part of Hospital Frailty Risk Scores and were shown to be short and long term outcome predictors after EVT.
In our study, in the adjusted analysis, patients overall and men alone with one or multiple vs. no previous vascular events showed more recurrent/progressive in-hospital ischaemic stroke and patients with multiple vs. no previous vascular events experienced more often death at discharge and less independency at 3 months. Poor outcome, higher in-hospital mortality rates, increased risk of stroke recurrence and vascular death in patients with multiple-territory vascular disease were consistently observed across several studies, including the OXVASC study, the REACH registry, and the CRUSADE registry (3, 6, 20). In all of these studies, including our own study, men were more likely to be affected by multiple-territory vascular diseases.
In our analysis we also found that men with one or multiple vs. no previous vascular events were more affected by in-hospital ICHs. To the best of our knowledge, previous studies (12, 31–33) have not established sex as a predictor of symptomatic intracerebral hemorrhage (sICH) following EVT. Possible explanations for this result are the higher prevalence of prior haemorrhagic strokes, pre-stroke antithrombotic medication use, and smoking in our study (34).
Finally, an interesting finding from our multivariable analysis is that women with one or multiple vs. no previous vascular events did not show any difference in their outcome after EVT. This result may be attributed to various factors, including comorbidities among women. Additionally, the limited sample size of women with a history of previous vascular disease may have resulted in limited statistical power to detect differences.
Outlook
It may be necessary to consider sex-specific calculations for the risk of recurrent vascular events and complications, as well as evaluating the need for different follow-up regimens for women compared to men.
Additionally, it has previously been pointed out that high-risk patients could benefit more from stricter preventive measures. A more intensive lipid-lowering approach (3, 35–37) and the inclusion of novel promising therapies such as PCSK9 inhibitors, interfering RNA (siRNA) agents, and inhibitors of angiopoietin-like protein 3 (ANGPTL3) have the potential to improve outcomes (38–41). Various approaches concerning the choice of antiplatelet therapy in high-risk patients have been analyzed in several randomized controlled trials (RCTs) (42–45). More intensive antithrombotic strategies were successfully investigated in the COMPASS study (46). The authors reported a 50% relative reduction in the risk of ischaemic stroke in patients with carotid artery disease and concomitant coronary or peripheral artery disease when using the combination of rivaroxaban plus aspirin compared to aspirin alone. However, it is important to note that the results were limited by an increased risk of major bleeding, predominantly gastrointestinal bleeding.
Future studies should also consider the selection of the therapy approach, as patients with multiple vascular diseases often receive more aggressive preventive treatments that may be incompatible with certain acute therapies. Furthermore, these patients frequently experience complications and unfavorable outcomes following treatment.
Strengths
The strength of this study is the large cohort of EVT-eligible LVO patients. Furthermore, the study was conducted in an experienced stroke center with an established stroke pathway.
Limitations
The main limitation of this study is its retrospective analysis and monocentric design. In addition, patients were included over a long period of time during which guidelines, treatment strategies and devices have evolved. We must also acknowledge the potential impact of referral bias, as our institution is a tertiary stroke center providing advanced EVT, which may have resulted in more severe or complex stroke patients being referred to our center. Moreover, the sample size was partly rather small, especially for some outcome variables. Furthermore, we did not assess sex-specific vascular risk factors such as hormone status, pregnancy, or age at menopause. It is also important to note that both biological differences and social aspects related to sex/gender may have influenced our results. Therefore, further research focusing on identifying sex/gender-specific factors could improve the accuracy of risk assessment, refine treatment strategies and address inequalities.
Conclusions
Previous vascular events increased the risk of in-hospital complications and poorer outcome in the analyzed patients with EVT-eligible LVO-AIS. Our findings may support risk assessment in stroke patients and could contribute to the design of future studies assessing the potential influence of previous symptomatic vascular disease and sex.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the local Ethics Committee (KEK Bern 2016–01905). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MP: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. GP: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. KG: Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. EW: Writing – original draft, Writing – review & editing. MDM: Writing – original draft, Writing – review & editing. HH: Writing – original draft, Writing – review & editing. PB: Writing – original draft, Writing – review & editing. SB: Writing – review & editing, Writing – original draft. BS: Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing. RU: Writing – original draft, Writing – review & editing. SP-P: Writing – original draft, Writing – review & editing. JG: Investigation, Methodology, Writing – original draft, Writing – review & editing. PM: Investigation, Methodology, Writing – original draft, Writing – review & editing. KA: Data curation, Validation, Writing – original draft, Writing – review & editing. MH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to the whole Bernese Stroke team, which contributed to data acquisition for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1293905/full#supplementary-material
Abbreviations
AIS, acute ischaemic stroke; CT, computed tomography; EO, excellent outcome; FI, functional independence; LVO, large vessel occlusion; MRI, magnetic resonance imaging; mRS, modified Rankin scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; RCT, randomized controlled trial; sICH, symptomatic intracranial hemorrhage; SR, successful reperfusion; TIA, transient ischemic attack; TICI, Thrombolysis In Cerebral Infarction.
References
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. American Heart Association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
2. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. ESC Scientific Document Group. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J. (2018) 39:763–816. doi: 10.1093/eurheartj/ehx095
3. Heldner MR, Li L, Lovett NG, Kubiak MM, Lyons S, Rothwell PM. Long-term prognosis of patients with transient ischemic attack or stroke and symptomatic vascular disease in multiple arterial beds. Stroke. (2018) 49:1639–46. doi: 10.1161/STROKEAHA.118.020913
4. Boulanger M, Li L, Lyons S, Lovett NG, Kubiak MM, Silver L, et al. Effect of coexisting vascular disease on long-term risk of recurrent events after TIA or stroke. Neurology. (2019) 93:e695–707. doi: 10.1212/WNL.0000000000007935
5. Röther J, Alberts MJ, Touzé E, Mas JL, Hill MD, Michel P, et al. Risk factor profile and management of cerebrovascular patients in the REACH registry. Cerebrovasc Dis. (2008) 25:366–74. doi: 10.1159/000120687
6. Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Rother J, et al. Three-year follow-up and event rates in the international REduction of atherothrombosis for continued health registry. Eur Heart J. (2009) 30:2318–26. doi: 10.1093/eurheartj/ehp355
7. Purroy F, Vicente-Pascual M, Arque G, Baraldes-Rovira M, Begue R, Gallego Y, et al. Sex-related differences in clinical features, neuroimaging, and long-term prognosis after transient ischemic attack. Stroke. (2021) 52:424–33. doi: 10.1161/STROKEAHA.120.032814
8. Cordonnier C, Sprigg N, Sandset EC, Pavlovic A, Sunnerhagen KS, Caso V, et al. Women initiative for stroke in Europe (WISE) group. Stroke in women - from evidence to inequalities. Nat Rev Neurol. (2017) 13:521–32. doi: 10.1038/nrneurol.2017.95
9. Strong B, Lisabeth LD, Reeves M. Sex differences in IV thrombolysis treatment for acute ischemic stroke: A systematic review and meta-analysis. Neurology. (2020) 95:e11–22. doi: 10.1212/WNL.0000000000009733
10. Purroy F, Vena A, Forné C, de Arce AM, Dávalos A, Fuentes B, et al. Age- and sex-specific risk profiles and in-hospital mortality in 13,932 Spanish stroke patients. Cerebrovasc Dis. (2019) 47:151–64. doi: 10.1159/000500205
11. Liu M, Li G, Tang J, Liao Y, Li L, Zheng Y, et al. The influence of sex in stroke thrombolysis: a systematic review and meta-analysis. J Clin Neurol. (2018) 14:141–52. doi: 10.3988/jcn.2018.14.2.141
12. Casetta I, Fainardi E, Pracucci G, et al. Sex differences in outcome after thrombectomy for acute ischemic stroke. A propensity score-matched study. Eur Stroke J. (2022) 7:151–7. doi: 10.1177/23969873221091648
13. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STROKEAHA.119.026917
14. Kennedy SA, Baerlocher MO, Baerlocher F, Socko D, Sacks D, Nikolic B, et al. Meta-analysis of local endovascular therapy for acute ischemic stroke. J Vasc Interv Radiol. (2016) 27:307–21. doi: 10.1016/j.jvir.2015.11.053
15. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
16. Jung S, Mattle H, Horvath T, Seiffge D, Heldner M, Meinel T, et al. Stroke Guidelines of the Bern Stroke Network Physicians. (2024). Available online at: https://neurologie.insel.ch/de/aerzte-und-zuweiser/richtlinien
17. Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. J Vasc Interv Radiol. (2018) 29:441–53. doi: 10.1016/j.jvir.2017.11.026
18. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos R, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
19. Van Swieten JC, Koudstaal PK, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
20. Bhatt DL, Peterson ED, Harrington RA, Ou FS, Cannon CP, Gibson CM, et al. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J. (2009) 30:1195–202. doi: 10.1093/eurheartj/ehp099
21. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. (2017) 136:583–96. doi: 10.1161/CIRCULATIONAHA.116.023163
22. Miller JD, Aronis KN, Chrispin J, Patil KD, Marine JE, Martin SS, et al. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J Am Coll Cardiol. (2015) 66:2899–906. doi: 10.1016/j.jacc.2015.10.047
23. Demel SL, Kittner S, Ley SH, McDermott M, Rexrode KM. Stroke risk factors unique to Women. Stroke. (2018) 49:518–23. doi: 10.1161/STROKEAHA.117.018415
24. Manwani B, Finger C, Lisabeth L. Strategies for maintaining brain health: the role of stroke risk factors unique to elderly women. Stroke. (2022) 53:2662–72. doi: 10.1161/STROKEAHA.121.036894
25. Carbajo-García AM, Cortés J, Arboix A, Massons J, Díez L, Vergés E, et al. Predictive clinical features of cardioembolic infarction in patients aged 85 years and older. J Geriatr Cardiol. (2019) 16:793–9. doi: 10.11909/j.issn.1671-5411.2019.11.008
26. Zotter M, Piechowiak EI, Balasubramaniam R, Von Martial R, Genceviciute K, Blanquet M, et al. Endovascular therapy in patients with large vessel occlusion due to cardioembolism versus large-artery atherosclerosis. Ther Adv Neurol Disord. (2021) 14:1756286421999017. doi: 10.1177/1756286421999017
27. Guglielmi V, LeCouffe NE, Zinkstok SM, Compagne KCJ, Eker R, Treurniet KM, et al. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke. (2019) 50:3360–8. doi: 10.1161/STROKEAHA.119.026299
28. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. (2018) 391:1775–82. doi: 10.1016/S0140-6736(18)30668-8
29. Pinho J, Küppers C, Nikoubashman O, Wiesmann M, Schulz JB, Reich A, et al. Frailty is an outcome predictor in patients with acute ischemic stroke receiving endovascular treatment. Age Ageing. (2021) 50:1785–91. doi: 10.1093/ageing/afab092
30. Pilotto A, Brass C, Fassbender K, Merzou F, Morotti A, Kämpfer N, et al. Premorbid frailty predicts short- and long-term outcomes of reperfusion treatment in acute stroke. J Neurol. (2022) 269:3338–42. doi: 10.1007/s00415-022-10966-7
31. Arnold M, Kappeler L, Nedeltchev K, Brekenfeld C, Fischer U, Keserue B, et al. Recanalization and outcome after intra-arterial thrombolysis in middle cerebral artery and internal carotid artery occlusion: does sex matter? Stroke. (2007) 38:1281–5. doi: 10.1161/01.STR.0000259711.13490.23
32. de Ridder IR, Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Wermer MJ, et al. Is intra-arterial treatment for acute ischemic stroke less effective in women than in men? Interv Neurol. (2016) 5:174–8. doi: 10.1159/000447331
33. Carvalho A, Cunha A, Gregório T, Paredes L, Costa H, Veloso M, et al. Is the efficacy of endovascular treatment for acute ischemic stroke sex-related. Interv Neurol. (2018) 7:42–7. doi: 10.1159/000484098
34. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. (2010) 376:112–23. doi: 10.1016/S0140-6736(10)60834-3
35. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
36. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. (2017) 377:1217–27. doi: 10.1056/NEJMoa1706444
37. Carolei A, Chamorro A, Laloux P, Leys D, Rother J, Sander D, et al. Identification and management of polyvascular disease in patients with noncardioembolic ischaemic stroke. Int J Stroke. (2008) 3:237–48. doi: 10.1111/j.1747-4949.2008.00220.x
38. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. (2015) 65:2638–51. doi: 10.1016/j.jacc.2015.05.001
39. Dayoub EJ, Eberly LA, Nathan AS, Khatana SAM, Adusumalli S, Navar AM, et al. Adoption of PCSK9 inhibitors among patients with atherosclerotic disease. J Am Heart Assoc. (2021) 10:e019331. doi: 10.1161/JAHA.120.019331
40. Ruotsalainen AK, Mäkinen P, Ylä-Herttuala S. Novel RNAi-based therapies for atherosclerosis. Curr Atheroscler Rep. (2021) 23:45. doi: 10.1007/s11883-021-00938-z
41. Kosmas CE, Bousvarou MD, Sourlas A, Papakonstantinou EJ, Peña Genao E, Echavarria Uceta R, et al. Angiopoietin-like protein 3 (ANGPTL3) inhibitors in the management of refractory hypercholesterolemia. Clin Pharmacol. (2022) 14:49–59. doi: 10.2147/CPAA.S345072
42. Gutierrez JA, Aday AW, Patel MR, Jones WS. Polyvascular disease: reappraisal of the current clinical landscape. Circ Cardiovasc Interv. (2019) 12:e007385. doi: 10.1161/CIRCINTERVENTIONS.119.007385
43. Hirsh J, Bhatt DL. Comparative benefits of clopidogrel and aspirin in high-risk patient populations: lessons from the CAPRIE and CURE studies. Arch Intern Med. (2004) 164:2106–10. doi: 10.1001/archinte.164.19.2106
44. Bonaca MP, Creager MA, Olin J, Scirica BM, Bohula EA, Murphy SA, et al. Vorapaxar reduces peripheral revascularization regardless of number of diseased territories: insights from the TRA2°P-TIMI 50 trial. Am College Cardiol Sci Sessions. (2013) 22:18. doi: 10.1016/S0735-1097(13)62018-5
45. Gutierrez JA, Mulder H, Jones WS, Rockhold FW, Baumgartner I, Berger JS, et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: A secondary analysis of the EUCLID trial. JAMA Netw Open. (2018) 1:e185239. doi: 10.1001/jamanetworkopen.2018.5239
46. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. (2018) 391:219–29. doi: 10.1016/S0140-6736(17)32409-1
Keywords: cerebrovascular disease/stroke, acute stroke therapy, atherosclerosis, endovascular treatment, large vessel occlusion
Citation: Peycheva M, Padlina G, Genceviciute K, Krasteva MP, Boronylo A, Goeldlin MB, Müller M, Wenz ES, Müller MD, Hammer H, Bücke P, Bigi S, Simonetti BG, Hoffmann A, Umarova RM, Pilgram-Pastor S, Gralla J, Mordasini P, Antonenko K and Heldner MR (2024) Baseline characteristics and outcome of stroke patients after endovascular therapy according to previous symptomatic vascular disease and sex. Front. Neurol. 15:1293905. doi: 10.3389/fneur.2024.1293905
Received: 22 September 2023; Accepted: 02 April 2024;
Published: 17 April 2024.
Edited by:
Ana Catarina Fonseca, University of Lisbon, PortugalReviewed by:
Clara M. Barreira, University of São Paulo, BrazilCristina Duque, Hospital Pedro Hispano, Portugal
Copyright © 2024 Peycheva, Padlina, Genceviciute, Krasteva, Boronylo, Goeldlin, Müller, Wenz, Müller, Hammer, Bücke, Bigi, Simonetti, Hoffmann, Umarova, Pilgram-Pastor, Gralla, Mordasini, Antonenko and Heldner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirjam R. Heldner, mirjam.heldner@insel.ch
†These authors have contributed equally to this work
 Marieta Peycheva
Marieta Peycheva Giovanna Padlina1,3†
Giovanna Padlina1,3† Marina P. Krasteva
Marina P. Krasteva Mandy D. Müller
Mandy D. Müller Helly Hammer
Helly Hammer Philipp Bücke
Philipp Bücke Roza M. Umarova
Roza M. Umarova Sara Pilgram-Pastor
Sara Pilgram-Pastor Kateryna Antonenko
Kateryna Antonenko Mirjam R. Heldner
Mirjam R. Heldner