- 1Department of Psychological Sciences, University of Connecticut, Storrs, CT, United States
- 2Institute for Systems Research, University of Maryland, College Park, College Park, MD, United States
- 3Department of Electrical and Computer Engineering, University of Maryland, College Park, College Park, MD, United States
- 4Department of Biology, University of Maryland, College Park, College Park, MD, United States
Voice pitch carries linguistic and non-linguistic information. Previous studies have described cortical tracking of voice pitch in clean speech, with responses reflecting both pitch strength and pitch value. However, pitch is also a powerful cue for auditory stream segregation, especially when competing streams have pitch differing in fundamental frequency, as is the case when multiple speakers talk simultaneously. We therefore investigated how cortical speech pitch tracking is affected in the presence of a second, task-irrelevant speaker. We analyzed human magnetoencephalography (MEG) responses to continuous narrative speech, presented either as a single talker in a quiet background or as a two-talker mixture of a male and a female speaker. In clean speech, voice pitch was associated with a right-dominant response, peaking at a latency of around 100 ms, consistent with previous electroencephalography and electrocorticography results. The response tracked both the presence of pitch and the relative value of the speaker’s fundamental frequency. In the two-talker mixture, the pitch of the attended speaker was tracked bilaterally, regardless of whether or not there was simultaneously present pitch in the speech of the irrelevant speaker. Pitch tracking for the irrelevant speaker was reduced: only the right hemisphere still significantly tracked pitch of the unattended speaker, and only during intervals in which no pitch was present in the attended talker’s speech. Taken together, these results suggest that pitch-based segregation of multiple speakers, at least as measured by macroscopic cortical tracking, is not entirely automatic but strongly dependent on selective attention.
Introduction
Pitch is a function of temporal periodicity and spectral order in acoustic waveforms (de Cheveigné, 2005). The cochlea transforms temporal periodicity into a spatial code by mapping different frequencies in the signal to different spatial locations along the basilar membrane. Subcortical responses retain the periodicity in ranges critical for speech and thus represent pitch temporally and spatially (Skoe and Kraus, 2010; Maddox and Lee, 2018). However, phase locking at faster frequencies gradually declines in the ascending auditory system (Joris et al., 2004). Extracranial recordings of cortical responses have observed population-level phase locking to periodicity in speech only up to approximately 110 Hz (Coffey et al., 2016; Kulasingham et al., 2020). This is insufficient for encoding most voice pitches as voices often exhibit a fundamental frequency above 100 Hz. Instead, the auditory cortex is abundant with frequency-selective receptive fields (Saenz and Langers, 2014), and pitch features are encoded through a combination of place and rate code (Fishman et al., 2013).
Cortical pitch tracking has primarily been analyzed with higher level representations of pitch. In speech, pitch is present intermittently, forming the basis of voiced segments and interrupted for unvoiced segments. In total, two aspects of pitch can thus be described separately that are relevant for cortical tracking: pitch strength, that is, the extent to which pitch is present at each moment in the speech signal, and pitch value, that is, the height of the perceived pitch, generally corresponding to the fundamental frequency. Both these features are tracked by scalp electroencephalography (EEG) responses to continuous narrative speech (Teoh et al., 2019). Intracranial recordings suggest that representations of relative pitch, corresponding to speaker-independent intonation contours, are more prominent than representations of absolute pitch (Tang et al., 2017) and that the pitch of speech is associated with a prominent neural response at around 100 ms latency (Li et al., 2021).
In this study, we investigate how pitch tracking is affected when listening to multiple simultaneous speakers. When sound from two speakers is mixed, the sound waveforms combine additively. For simplicity, we will consider the case of a single audio channel mixed signal presented diotically, that is, the two source waveforms are mixed into a single mixed waveform presented to both ears. The problem of stream segregation is segregating the spectro-temporal elements of the heard sound into those associated with either of the sources (Bregman, 1990). Pitch can be a strong cue for stream segregation (Bregman, 1990; Micheyl and Oxenham, 2010). For example, pitch tracking can aid segregation by grouping together the different harmonics of a shared fundamental frequency (Popham et al., 2018). The spatial code in A1 provides sufficient information to distinguish two concurrent vowels that differ in fundamental frequency by four semitones, consistent with human perceptual judgments (Fishman et al., 2014, 2016). Non-primary areas might thus reconstruct pitch from this representation (Bendor and Wang, 2005), for example, using harmonic templates (Fishman et al., 2014). This would potentially allow the auditory cortex to segregate the pitch of two speakers, especially if those two streams differ substantially in pitch (e.g., a male and a female speaker). A pitch-sensitive region in the anterior portion of the superior temporal plane (Norman-Haignere et al., 2013) could be the potential locus for such pitch-based segregation.
If pitch extraction is automatic for each of several sources in a mixture, it could then be used as bottom-up cue in stream segregation. This would be consistent with suggestions that the subcortical representation of voice pitch (Maddox and Lee, 2018; Van Canneyt et al., 2021a,b) is affected by attention (Forte et al., 2017; Etard et al., 2019; Saiz-Alía et al., 2019). Cortical responses might then be expected to simultaneously track the pitch in the attended and the ignored speakers. Pitch tracking might still be affected by overlapping pitch to the extent that the overlap imposes additional demands for segregation. On the other hand, pitch tracking might reflect a secondary representation constructed during attentive speech processing, for example, for linguistic prosody. In this case, pitch tracking might depend on selective attention, possibly without demonstrating pitch tracking for the ignored speaker at all.
To investigate this, we analyzed a previously studied dataset of magnetoencephalography (MEG) responses to audiobooks in two conditions: speech from a single speaker in a quiet background and speech from two speakers, one male and one female, reading different audiobooks mixed together and presented diotically, with the task of listening to one speaker and ignoring the other (Brodbeck et al., 2020). In the original study, we analyzed responses as a function of spectrogram representations and found that listeners segregate acoustic features even of the ignored speaker from the acoustic mixture. In this study, we ask to what degree listeners additionally track pitch in the attended and the ignored speaker. In this analysis of pitch tracking, all predictors used in the original analysis are also controlled for Brodbeck et al. (2020). For clean speech, we model pitch through two separate time-dependent predictors, pitch strength and pitch value (Figure 1A). For the two-speaker mixture, we additionally distinguish (1) between pitch in the attended and the ignored talker, and (2) between pitch when it is overt, that is, when only one of the two speakers exhibits pitch at a time, and when it is masked, that is, when both speech signals contain pitch simultaneously (Figure 1B). Masked pitch deserves special attention because the two sources of pitch may interfere with each other, such that naive pitch detection algorithms would fail without considering the effect of that interference through some kinds of segregation mechanism (Micheyl and Oxenham, 2010).
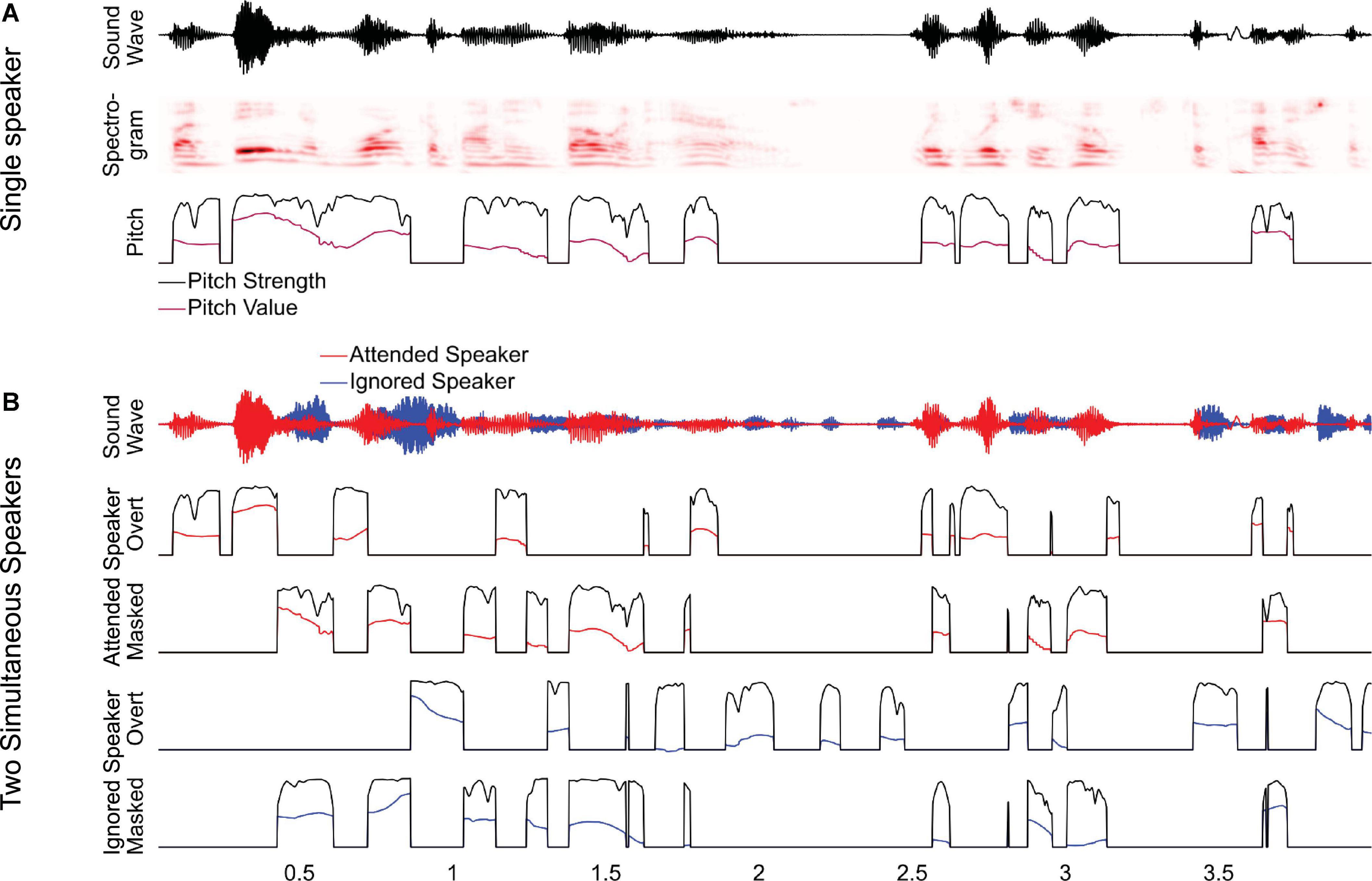
Figure 1. Predictors for analyzing pitch tracking. (A) For a single speaker, pitch tracking was estimated using two predictors: pitch strength, reflecting the degree to which a distinctive pitch is present in the sound signal, and pitch value, reflecting the fundamental frequency of the pitch, relative to the baseline. For moments when pitch strength is 0, the pitch value is set to the default baseline value. (B) For two-speaker stimuli, pitch strength and value were estimated separately for each speaker and then split into two separate predictors, reflecting overt pitch (i.e., pitch is present only in a single speaker) and masked pitch (i.e., pitch is present in both speakers). Note that, as a consequence of this definition, the two masked pitch predictors are always simultaneous, whereas the overt pitch predictors are mutually exclusive.
Methods
We reanalyzed MEG responses from 26 native speakers of English (8 females, 18 males; age mean 45.2 years, range 22–61 years), listening to multiple 1-min duration audiobook segments, in quiet and in a two-talker mixture (8 and 16 min, respectively). Mixtures always consisted of a male and a female speaker, clearly separable by pitch. Most of the analysis followed essentially the same procedures as the original study (Brodbeck et al., 2020) but with additional predictors to isolate representations of pitch.
MEG recordings were pre-processed in MNE-Python (Gramfort et al., 2014) with temporal signal-space separation (Taulu and Simola, 2006) and a 1- to 40-Hz band-pass filter (zero-phase FIR filter, MNE-Python 0.15 default settings). Independent component analysis (Bell and Sejnowski, 1995) was used to remove known biological artifacts such as eye blinks and heart beat (on average, 4.7 components were removed per subject). An additional 20-Hz low-pass filter (same details as the band-pass filter) was applied to increase analysis power because consistent phase-locked responses occur predominantly at lower frequencies (Ding and Simon, 2013). Response epochs related to the stimuli were then extracted and downsampled to 100 Hz. For two-talker stimuli, the first second contained only the target talker, and data corresponding to this second were discarded. Responses were then projected to current dipoles oriented perpendicularly to the white matter surface (fourfold icosahedral subdivision) using distributed minimum ℓ2 norm source current estimates, using a noise covariance estimate from empty room data and regularization λ = 1/6 (no depth weighting).
Predictors
Pitch was extracted from each stimulus using Praat (Boersma and Weenink, 2017). Pitch strength was taken directly from the Praat estimate, which quantifies the degree of periodicity in the signal, taking values between 0 and 1. The pitch value, reflecting the frequency in hertz, was log-transformed, and sections without pitch (pitch strength of zero) were set to the 5th percentile value of sections with pitch (see Figure 1A). This baseline correction was performed for each 1-min segment separately, to derive relative pitch, regardless of the specific speaker’s fundamental frequency. The pitch value was oriented relative to the lower end of the scale to account for the observation in electrocorticography that native English speakers show selective responses to higher relative pitch (Li et al., 2021). Across all stimuli, pitch strength and value were moderately correlated (r = 0.66). To control for spectro-temporal acoustic processing, we included the acoustic spectrogram and onset spectrogram predictors from the original study (eight bands each).
We also considered a pitch onset predictor (Krumbholz et al., 2003), based on the half-wave rectified derivative of the pitch strength. We reasoned that this predictor might be able to isolate responses related to the initial detection of pitch. However, the predictor did not improve predictive power beyond pitch strength and value [tmax = 2.28, p = 0.385, when restricted to the superior temporal gyrus (STG)], and we consequently dropped it from further analysis. A reason for this might be that pitch onset in speech almost always coincides with a sound onset in the spectrogram, which our analysis always controlled for.
For the two-speaker condition, we first generated pitch predictors for each of the two source segments in the mixture. Masking was operationalized as a binary distinction: The ignored speaker was considered masked where the pitch strength of the attended speaker exceeded 0.5, and vice versa. Based on this, both speaker’s pitch predictors were split into two different sets, one reflecting overt pitch and the other masked pitch (Figure 1B). On average, overt speech predictors were non-zero at 24.6% of time points and masked speech predictors at 29.9%. To control for spectro-temporal processing, we included all predictors from the two-talker condition of the original study, including overt and masked onsets (Brodbeck et al., 2020, second equation on p. 17).
Model tests
Multivariate temporal response function (mTRF) models were estimated separately for each subject and source dipole with Eelbrain (Brodbeck et al., 2021). As in the original study, models with a latency range 0–500 ms were estimated and tested on held-out data using fourfold cross-validation. Predictive power was quantified as the proportion of the variability in the source-localized MEG responses explained by the model. Each predictor was evaluated by comparing the predictive power of the complete model (all predictors) with a model that was estimated while excluding the to-be-tested predictor. Importantly, these tests assess the unique predictive power of the predictor under investigation, after controlling for all other predictors (i.e., a significant result indicates that this predictor contains information about the brain responses that is not present in any of the other predictors). This is important because different speech features are often correlated, and a spurious predictor by itself might derive some predictive power simply from being correlated with a neurally meaningful speech feature (e.g., Gillis et al., 2021).
We defined anatomical areas for mass-univariate tests (based on “aparc” labels; Desikan et al., 2006): For pitch representations of clean speech, we initially tested in the whole cortex, with the exception of the occipital lobe, insula, and cingulate cortex (i.e., regions in which we did not expect a substantive auditory response, excluded in order to expedite these numerically intensive computations). Based on these results, we performed tests for the two-speaker condition in more restricted areas in the STG (transverse and superior temporal gyrus labels) and the inferior frontal gyrus (IFG; pars opercularis, pars triangularis, and pars orbitalis labels). Anatomical maps of predictive power were smoothed (SD = 5 mm) and compared with mass univariate related measures t-tests, correcting for multiple comparisons with threshold-free cluster enhancement (Smith and Nichols, 2009) and a permutation distribution based on 10,000 random permutations of condition labels. Tests of whether a given predictor improved predictive power were one-tailed, and all other comparisons were two-tailed. Even though we sometimes report results separately for the left and right hemispheres, multiple comparisons correction was always based on a permutation distribution estimated from the combination of both hemispheres.
To express model predictive power as a meaningful quantity, the predictive power of different predictors is expressed as % of the explanatory power of the most complete model (separately for the single speaker and the two-speaker conditions).
There is no standard measure of effect size for mass univariate tests. As a compromise, we report tmax for mass univariate tests, that is, the largest t-value in the significant area (or the whole tested area for non-significant results). However, to provide a more traditional measure of effect size, we also defined a functional region of interest (ROI). This ROI was defined based on the intersection of significant activation in the single speaker condition (to either of the two pitch predictors) and the STG anatomical area. We used this ROI to extract the average explained variability attributable to pitch strength and value combined (for swarm plots) or each predictor individually (for univariate ROI tests).
ANOVA for difference in localization
Localization differences in MEG should be interpreted with caution (Lütkenhöner, 2003; Bourguignon et al., 2018). However, the question whether two localizations are based on the same underlying source configuration can be tested in a straightforward manner, based on the linearity of the forward and inverse models. Two brain activation patterns with the same underlying source configuration should produce the same relative measurements at the sensor level (McCarthy and Wood, 1985) and, consequently, in the source-localized responses. Based on this, we test the null hypothesis that two predictors are represented in the same neural sources by first normalizing the two respective maps and subtracting one from the other. If the two underlying maps have the same shape, the sources should now only contain random noise. We thus used a one-way repeated measures ANOVA with factor source dipole to test whether there is a systematic pattern left after the subtraction (in other words, whether there is a systematic difference between the patterns of localization of the two predictors).
Temporal response functions
To analyze temporal response functions (TRFs), mTRF models were re-estimated using a latency range from −100 to 500 ms and without held-out data (but still using early stopping based on cross-validation). For TRF estimation, predictors and MEG responses were normalized, and TRFs were analyzed at this normalized scale. Note that TRFs are equivalent to coefficients in a regression problem and, unlike the model tests, may thus be sensitive to contamination from correlated predictors.
Results
Pitch strength and value are tracked in single-talker speech
In single-talker clean speech, pitch strength and pitch value were both represented neurally (Figure 2). This was assessed by comparing the predictive power of a full model, containing pitch strength and value along with an auditory spectrogram and an acoustic onset spectrogram, with the predictive power of two control models which were fit either without the pitch strength or without the pitch value predictor. This indicated that both predictors contributed unique predictive power to the full model (strength: tmax = 5.58, p < 0.001; value: tmax = 6.00, p < 0.001). Overall, source localization is consistent with the majority of sources being in the vicinity of the auditory cortex in Heschl’s gyrus and the superior temporal gyrus (see Figure 2A). Pitch strength was significantly right-lateralized (Figure 2B; tmax = 4.90, p < 0.001), with no significant tracking in the left hemisphere (tmax = 3.24, p = 0.670).
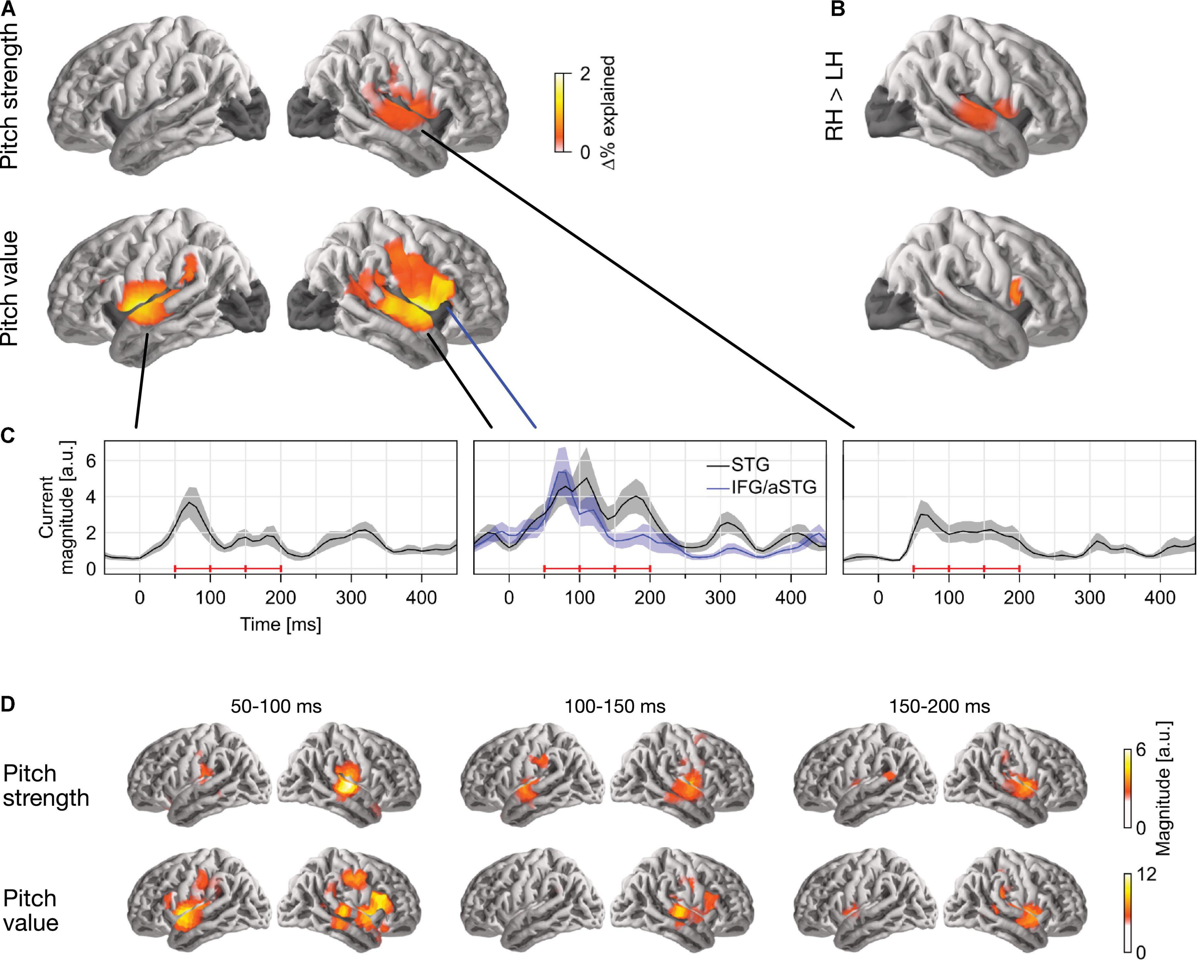
Figure 2. Separable tracking of pitch strength and pitch value of a single talker. (A) Pitch strength and pitch value both improved model predictions independently, when controlling for acoustic envelope and onset spectrograms (p ≤ 0.05, corrected; darkened areas excluded from analysis). The color scale reflects the explained variability in MEG responses, expressed as % of the complete model. (B) Both pitch predictors showed some right lateralization. The plots show the right–left hemisphere predictive power difference, same scale as (A). (C) Temporal response functions (TRFs) showed dominant responses at latencies between 50 and 200 ms. TRF magnitude is shown for regions of significant model prediction. The three horizontal red bars indicate time windows used in (D). (D) Anatomical distribution of TRFs in 50 ms time windows. LH, left hemisphere; RH, right hemisphere; STG, superior temporal gyrus; IFG, inferior frontal gyrus; aSTG, anterior STG.
The localization of pitch value was more complex: when tested in the whole brain, it was significantly right-lateralized (tmax = 4.90, p < 0.001). However, the region of significant difference coincided with the anatomical label of the pars opercularis of the IFG. When repeated in the STG only, lateralization was not significant (tmax = 2.28, p = 0.385). To confirm that pitch strength and value are tracked by non-identical sources, we applied a one-way ANOVA with factor source dipole to the difference between the two normalized activation patterns in the right hemisphere (see section “Methods”, “ANOVA for difference in localization”). This indicated that the distribution of sources tracking the two predictors was indeed different (F(117, 2925) = 1.85, p < 0.001). Together, these results suggest that pitch value tracking engages additional, more anterior sources than pitch strength tracking. The source localization raises the possibility that pitch value specifically engages the right IFG, although due to the proximity to the anterior temporal lobe, it is impossible to exclude the possibility of an anterior temporal source with dispersion into IFG due to imperfect source localization (cf. Bourguignon et al., 2018).
Response to pitch peaks around 100 ms latency
The temporal response functions (TRFs) are the estimated impulse responses to elementary pitch features, shown in Figures 2C,D. Figure 2C shows the response magnitude, summed across source dipoles, as a function of time. Responses are shown in functional ROIs, based on combining the region of significant model predictions (union across the two predictors) with anatomical STG and IFG labels. Most of the response power is concentrated in the first 50–200 ms, with a clear response peak to pitch value around 100 ms. Comparison of responses to pitch value in the STG and IFG ROIs suggests that the relative involvement of the anterior peak is stronger at the shorter latencies. The anatomical distribution of the response magnitude is consistent with this, showing a stronger response at the anterior source in the early time window (Figure 2D).
In two simultaneous talkers, pitch-tracking depends on selective attention
To test how pitch is tracked when listening to one of two concurrent talkers, we generated four versions of each predictor: first, we generated separate versions for pitch of the attended speaker and that of the ignored speaker; second, for each of those, we separated each time point into overt or masked pitch, based on whether pitch was simultaneously present in the other talker or not (see Figure 1B). First, we tested for pitch tracking in the STG by combining pitch strength and value in each of the four categories (Figure 3A). The results indicated significant pitch tracking for overt pitch, regardless of whether pitch originated from the attended (tmax = 4.38, p < 0.001) or the ignored speaker (tmax = 3.74, p = 0.008). In contrast to this, masked pitch was tracked only in the attended speaker (tmax = 3.57, p = 0.003), whereas we did not find evidence for tracking of masked pitch in the unattended speaker (tmax = 2.42, p = 0.432). None of the effects were significantly lateralized. Although tracking of overt pitch in the ignored speaker was only significant in the right hemisphere, the lateralization of this effect was not significant either (tmax = 2.47, p = 0.054; other ps ≥ 0.199).
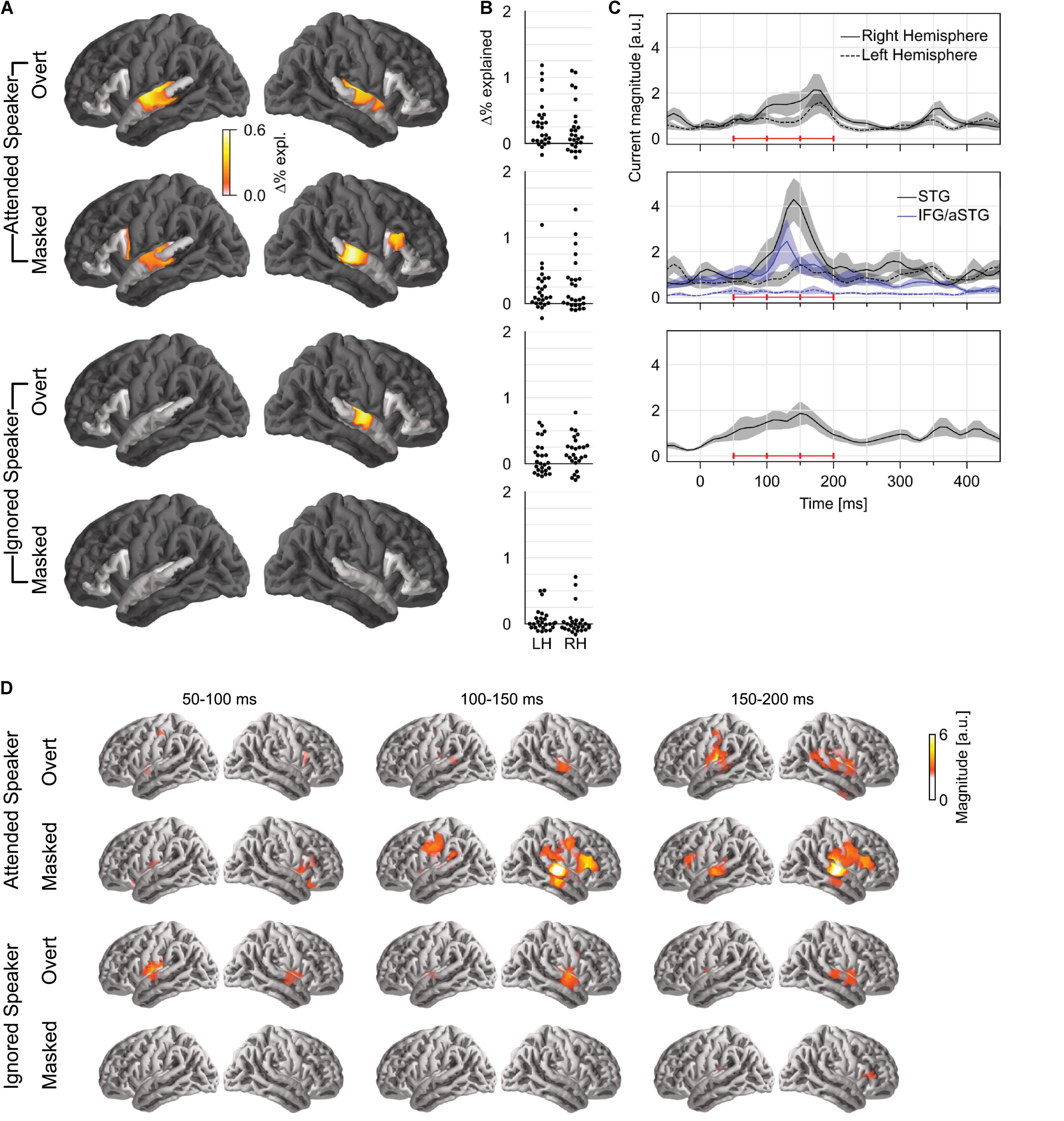
Figure 3. Pitch tracking in two simultaneous speakers depends on selective attention. (A) Significance tests of pitch tracking for overt and masked pitch in the attended and ignored speakers. STG and IFG were separately tested (darkened area excluded from tests). (B) Individual subject data (% variability explained) in a region of interest, defined as the intersection of the region of significant activity in the single speaker condition and the STG anatomical label. (C) Temporal response function (TRF) magnitude with dominant response at 100–200 ms latency. The three horizontal red bars indicate time windows used in (D). (D) TRF activity localized mainly to the auditory cortex, with involvement of a more anterior region for masked pitch in the attended speaker. LH, left hemisphere; RH, right hemisphere; STG, superior temporal gyrus; IFG, inferior frontal gyrus; aSTG, anterior STG.
A direct comparison in the STG confirmed effects of selective attention for tracking of overt and masked pitches. For masked pitch, tracking was significantly stronger in the attended speaker than in the unattended speaker in both hemispheres (one-tailed test: right: tmax = 3.88, p = 0.005; left: tmax = 3.07, p = 0.042). For overt pitch, tracking of the attended speaker was stronger in the left hemisphere only (tmax = 3.26, p = 0.031; right: tmax = 2.48, p = 0.415), again without significant lateralization of the effect. While these results appear more robust for masked pitch than for overt pitch, there were somewhat more data for masked pitch than for overt pitch (29.9 vs. 24.6%), suggesting that the former test might be somewhat more powerful, and this difference should be interpreted with care.
Only attended masked pitch was also significant in the IFG area (tmax = 3.20, p = 0.025), and in this area, attended masked pitch was significantly stronger than ignored masked pitch (tmax = 3.54, p = 0.017). Representation of attended masked pitch was also stronger than that of attended overt pitch (tmax = 3.40, p = 0.035) (subject to the same caveat that there were slightly more data for masked pitch).
Next, we asked whether pitch strength and pitch value were independently tracked for concurrent speakers in each of the significant categories. Surprisingly, in mass univariate tests in the STG, none of the pitch strength predictors were significant, while all three pitch value predictors were significant. To derive proper measures of effect size, we also performed univariate tests in an ROI based on significant STG activation in the one speaker condition (Table 1). While these results are largely consistent with the mass univariate test results, they do suggest marginally significant tracking of overt pitch strength in the left hemisphere. The univariate tests are likely less conservative than the mass univariate tests, by not correcting for multiple comparisons in the two hemispheres. Nevertheless, the result suggests that some pitch strength tracking might exist, although with a much weaker effect size than pitch value tracking.
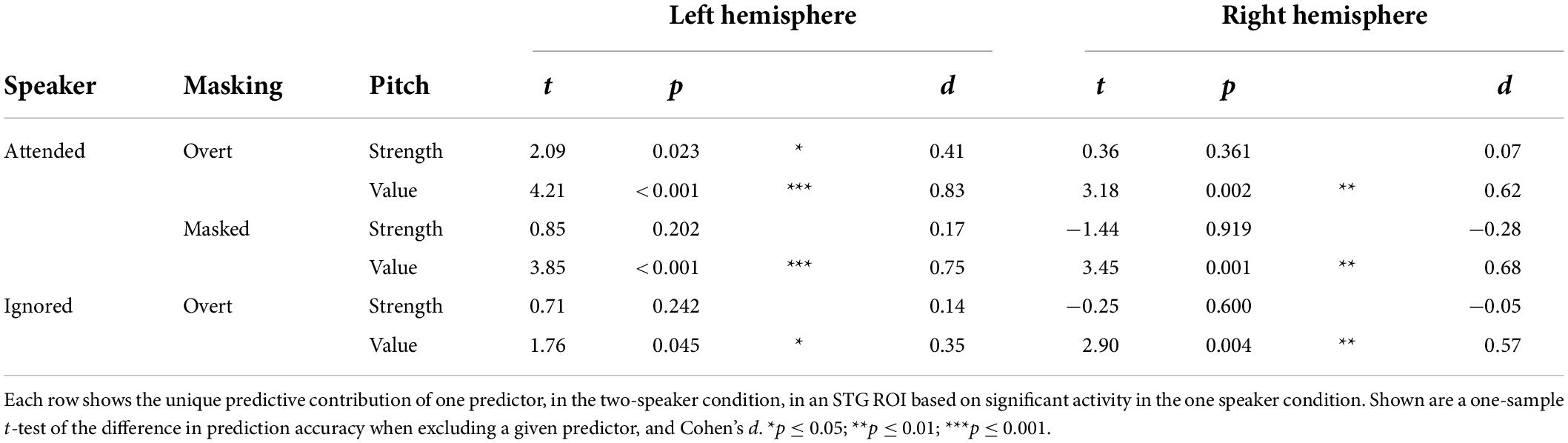
Table 1. In the two-talker mixture, pitch tracking is dominant due to pitch value, and not pitch strength.
Based on the aforementioned results, we only analyzed TRFs to pitch value. The TRFs to overt pitch in the attended and the ignored speaker were qualitatively similar (Figures 3B,C). By contrast, masked pitch in the attended speaker was associated with a large peak in the right STG around 140 ms. Figure 3D shows the anatomical distribution of the TRFs between 50 and 200 ms.
Discussion
Our analysis of responses to clean speech confirms a previous report of separate cortical pitch strength and pitch value tracking seen with EEG (Teoh et al., 2019). In addition, source localization suggested a differentiation between the two representations, with a right-lateralized STG representation of pitch strength and a bilateral STG representation of pitch value, with potential additional involvement of a more anterior region. While source localization suggests that this more anterior region lies in the IFG, we cannot exclude the possibility of this being an artifact of imperfect source localization (Bourguignon et al., 2018) as a source in the anterior STG would be more consistent with fMRI reports of a pitch representation in the anterior STG (Norman-Haignere et al., 2013).
In the presence of two simultaneous speakers, pitch tracking depends on selective attention, but not exclusively. Overt pitch was similarly represented, regardless of whether that pitch was in the attended or the ignored speaker. This suggests that overt pitch extraction occurs without a need for selective attention and might form part of an auditory background representation. On the other hand, when pitch was present in both speakers simultaneously, selective attention had a strong effect: pitch in the attended speaker was tracked very robustly, with recruitment of additional, more anterior neural sources, possibly reflecting additional resources recruited for speaker segregation. At the same time, we found no evidence for the representation of pitch in the ignored speaker when pitch was simultaneously present in the attended speaker.
Stream segregation of a monaural mixture is cortical and depends on selective attention
A long-standing question on cocktail party speech processing is whether segregation of multiple speakers occurs pre-attentively, with selective attention merely selecting one of multiple input streams, or post-attentively, with selective attention actively contributing to the segregation. Recent evidence supports the latter view, at least when the speech signals are mixed together monophonically, that is, without spatial separation cues (Puvvada and Simon, 2017; O’Sullivan et al., 2019; Brodbeck et al., 2020). Our new results are consistent with this. On the one hand, significant tracking of overt pitch in the ignored speaker suggests that pitch tracking itself does not require selective attention, as long as the pitch is easily extracted through the periodicity of the signal. However, in masked pitch, we found a strong effect of selective attention, with no evidence of tracking of ignored pitch at all. Consistent with the previous reports, the present results do not provide evidence for pre-attentive pitch-based segregation, but do suggest enhanced pitch processing in a selectively attended speaker.
Cortical pitch tracking reflects acoustic and possibly linguistic processes
Our results also have implications for determining the neural processes that give rise to cortical pitch tracking. Cortical voice pitch tracking might reflect purely acoustic processing, that is, the extraction of pitch and the pitch trajectory (e.g., Andermann et al., 2021). However, pitch also carries linguistic information (Stevens, 1998), and neural responses to linguistic events may be confounded with responses to pitch per se. For instance, the presence of pitch normally coincides with voiced segments, predominantly vowels, and thus, responses related to pitch might also reflect extraction of vowel features. Furthermore, the pitch contour is a prosodic cue that relates to the information structure and phrase structure of an utterance. Pitch tracking could thus also reflect processing of such higher level properties such as pitch accents (Llanos et al., 2021).
Our data suggest that pitch tracking at least partially reflects relatively low-level, auditory processing. We here observed robust tracking of overt pitch in the ignored talker during times when the attended talker did not also produce pitch. Previous work suggests that linguistic processing of ignored speech in selective listening tasks is very limited (Brodbeck et al., 2018; Broderick et al., 2018). In particular, in the same dataset as used here, we found no evidence for time-locked processing of words in the ignored speaker (Brodbeck et al., 2018). The tracking of pitch in the ignored speaker thus likely reflects acoustic processing at a pre-lexical stage. The sources of pitch tracking in the attended speaker thus likely include similar acoustic processing but might in addition include higher level, linguistic processes. Engagement of higher level processing might also explain the enhanced tracking of overt pitch in the attended speaker vs. ignored speaker in the left STG.
Lateralization
Cortical pitch processing has sometimes been specifically associated with the right hemisphere. For example, pitch judgments engage the right prefrontal cortex (Zatorre et al., 1992), and the right auditory cortex might play a causal role in pitch discrimination learning (Matsushita et al., 2021). Our results provide additional evidence for a tendency toward right lateralization of at least some aspects of pitch tracking in speech as we found evidence for stronger pitch representations of clean speech in the right hemisphere. However, we did not find significant lateralization effects in the two-speaker condition. This suggests that pitch processing might become more bilateral in the more demanding condition, possibly through recruitment of additional (left hemispheric) higher level processes.
Conclusion
The central finding of this study is that cortical pitch tracking is modulated by selective attention. While listeners represent overt pitch similarly in an attended or an ignored speaker, they do not seem to track pitch of an ignored speaker that is masked by pitch in the attended speaker. By contrast, tracking of masked pitch is robust for an attended speaker, suggesting that this pitch is selectively extracted and processed.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: http://hdl.handle.net/1903/26370.
Ethics statement
The study involving human participants was reviewed and approved by the University of Maryland Baltimore Institutional Review Board. The participants provided their written informed consent to participate in this study.
Author contributions
CB and JZS conceived of the analysis. CB implemented the analysis and wrote the first draft of the manuscript. Both authors contributed to manuscript revisions and approved the submitted version.
Funding
This work was supported by the National Institutes of Health (R01-DC014085 and R01- DC019394 for JZS) and the National Science Foundation [SMA-1734892 for JZS and 1754284 for CB (J. Magnuson, PI)].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andermann, M., Günther, M., Patterson, R. D., and Rupp, A. (2021). Early cortical processing of pitch height and the role of adaptation and musicality. NeuroImage 225:117501. doi: 10.1016/j.neuroimage.2020.117501
Bell, A. J., and Sejnowski, T. J. (1995). An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural. Comput. 7, 1129–1159. doi: 10.1162/neco.1995.7.6.1129
Bendor, D., and Wang, X. (2005). The neuronal representation of pitch in primate auditory cortex. Nature 436, 1161–1165. doi: 10.1038/nature03867
Boersma, P., and Weenink, D. (2017). Praat: Doing Phonetics by Computer [Computer program]. Available online at: http://www.praat.org/ (aceessed April 2021).
Bourguignon, M., Molinaro, N., and Wens, V. (2018). Contrasting functional imaging parametric maps: the mislocation problem and alternative solutions. NeuroImage 169, 200–211. doi: 10.1016/j.neuroimage.2017.12.033
Bregman, A. S. (1990). Auditory Scene Analysis: the Perceptual Organization of Sound. Cambridge: MIT Press.
Brodbeck, C., Das, P., Kulasingham, J. P., Bhattasali, S., Gaston, P., Resnik, P., et al. (2021). Eelbrain: a Python toolkit for time-continuous analysis with temporal response functions. bioRxiv [Preprint]. doi: 10.1101/2021.08.01.454687
Brodbeck, C., Hong, L. E., and Simon, J. Z. (2018). Rapid Transformation from Auditory to Linguistic Representations of Continuous Speech. Curr. Biol. 28:3976–3983.e5. doi: 10.1016/j.cub.2018.10.042
Brodbeck, C., Jiao, A., Hong, L. E., and Simon, J. Z. (2020). Neural speech restoration at the cocktail party: auditory cortex recovers masked speech of both attended and ignored speakers. PLoS Biol. 18:e3000883. doi: 10.1371/journal.pbio.3000883
Broderick, M. P., Anderson, A. J., Liberto, G. M. D., Crosse, M. J., and Lalor, E. C. (2018). Electrophysiological Correlates of Semantic Dissimilarity Reflect the Comprehension of Natural, Narrative Speech. Curr. Biol. 28:803–809.e3. doi: 10.1016/j.cub.2018.01.080
Coffey, E. B. J., Herholz, S. C., Chepesiuk, A. M. P., Baillet, S., and Zatorre, R. J. (2016). Cortical contributions to the auditory frequency-following response revealed by MEG. Nat. Commun. 7:11070. doi: 10.1038/ncomms11070
de Cheveigné, A. (2005). “Pitch Perception Models,” in Pitch: Neural Coding and Perception Springer Handbook of Auditory Research, eds C. J. Plack, R. R. Fay, A. J. Oxenham, and A. N. Popper (New York, NY: Springer), 169–233. doi: 10.1007/0-387-28958-5_6
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Ding, N., and Simon, J. Z. (2013). Adaptive Temporal Encoding Leads to a Background-Insensitive Cortical Representation of Speech. J. Neurosci. 33, 5728–5735. doi: 10.1523/JNEUROSCI.5297-12.2013
Etard, O., Kegler, M., Braiman, C., Forte, A. E., and Reichenbach, T. (2019). Decoding of selective attention to continuous speech from the human auditory brainstem response. NeuroImage 200, 1–11. doi: 10.1016/j.neuroimage.2019.06.029
Fishman, Y. I., Micheyl, C., and Steinschneider, M. (2013). Neural Representation of Harmonic Complex Tones in Primary Auditory Cortex of the Awake Monkey. J. Neurosci. 33, 10312–10323. doi: 10.1523/JNEUROSCI.0020-13.2013
Fishman, Y. I., Micheyl, C., and Steinschneider, M. (2016). Neural Representation of Concurrent Vowels in Macaque Primary Auditory Cortex. eneuro 3:ENEURO.71–ENEURO.16. doi: 10.1523/ENEURO.0071-16.2016
Fishman, Y. I., Steinschneider, M., and Micheyl, C. (2014). Neural Representation of Concurrent Harmonic Sounds in Monkey Primary Auditory Cortex: Implications for Models of Auditory Scene Analysis. J. Neurosci. 34, 12425–12443. doi: 10.1523/JNEUROSCI.0025-14.2014
Forte, A. E., Etard, O., and Reichenbach, T. (2017). The human auditory brainstem response to running speech reveals a subcortical mechanism for selective attention. eLife 6:e27203. doi: 10.7554/eLife.27203
Gillis, M., Vanthornhout, J., Simon, J. Z., Francart, T., and Brodbeck, C. (2021). Neural markers of speech comprehension: measuring EEG tracking of linguistic speech representations, controlling the speech acoustics. J. Neurosci. 41, 10316–10329. doi: 10.1523/JNEUROSCI.0812-21.2021
Gramfort, A., Luessi, M., Larson, E., Engemann, D. A., Strohmeier, D., Brodbeck, C., et al. (2014). MNE software for processing MEG and EEG data. NeuroImage 86, 446–460. doi: 10.1016/j.neuroimage.2013.10.027
Joris, P. X., Schreiner, C. E., and Rees, A. (2004). Neural Processing of Amplitude-Modulated Sounds. Physiol. Rev. 84, 541–577. doi: 10.1152/physrev.00029.2003
Krumbholz, K., Patterson, R. D., Seither-Preisler, A., Lammertmann, C., and Lütkenhöner, B. (2003). Neuromagnetic Evidence for a Pitch Processing Center in Heschl’s Gyrus. Cereb. Cortex 13, 765–772. doi: 10.1093/cercor/13.7.765
Kulasingham, J. P., Brodbeck, C., Presacco, A., Kuchinsky, S. E., Anderson, S., and Simon, J. Z. (2020). High gamma cortical processing of continuous speech in younger and older listeners. NeuroImage 222:117291. doi: 10.1016/j.neuroimage.2020.117291
Li, Y., Tang, C., Lu, J., Wu, J., and Chang, E. F. (2021). Human cortical encoding of pitch in tonal and non-tonal languages. Nat. Commun. 12:1161. doi: 10.1038/s41467-021-21430-x
Llanos, F., German, J. S., Gnanateja, G. N., and Chandrasekaran, B. (2021). The neural processing of pitch accents in continuous speech. Neuropsychologia 158:107883. doi: 10.1016/j.neuropsychologia.2021.107883
Lütkenhöner, B. (2003). Magnetoencephalography and its Achilles’ heel. J. Physiol.-Paris 97, 641–658. doi: 10.1016/j.jphysparis.2004.01.020
Maddox, R. K., and Lee, A. K. C. (2018). Auditory Brainstem Responses to Continuous Natural Speech in Human Listeners. eneuro 5:ENEURO.441–ENEURO.417. doi: 10.1523/ENEURO.0441-17.2018
Matsushita, R., Puschmann, S., Baillet, S., and Zatorre, R. J. (2021). Inhibitory effect of tDCS on auditory evoked response: simultaneous MEG-tDCS reveals causal role of right auditory cortex in pitch learning. NeuroImage 233:117915. doi: 10.1016/j.neuroimage.2021.117915
McCarthy, G., and Wood, C. C. (1985). Scalp Distributions of Event-Related Potentials - an Ambiguity Associated with Analysis of Variance Models. Electroencephalogr. Clin. Neurophysiol. 61:S226–S227. doi: 10.1016/0013-4694(85)90858-2
Micheyl, C., and Oxenham, A. J. (2010). Pitch, harmonicity and concurrent sound segregation: psychoacoustical and neurophysiological findings. Hear. Res. 266, 36–51. doi: 10.1016/j.heares.2009.09.012
Norman-Haignere, S., Kanwisher, N., and McDermott, J. H. (2013). Cortical Pitch Regions in Humans Respond Primarily to Resolved Harmonics and Are Located in Specific Tonotopic Regions of Anterior Auditory Cortex. J. Neurosci. 33, 19451–19469. doi: 10.1523/JNEUROSCI.2880-13.2013
O’Sullivan, J., Herrero, J., Smith, E., Schevon, C., McKhann, G. M., Sheth, S. A., et al. (2019). Hierarchical Encoding of Attended Auditory Objects in Multi-talker Speech Perception. Neuron 104, 1195–1209. doi: 10.1016/j.neuron.2019.09.007
Popham, S., Boebinger, D., Ellis, D. P. W., Kawahara, H., and McDermott, J. H. (2018). Inharmonic speech reveals the role of harmonicity in the cocktail party problem. Nat. Commun. 9:2122. doi: 10.1038/s41467-018-04551-8
Puvvada, K. C., and Simon, J. Z. (2017). Cortical Representations of Speech in a Multitalker Auditory Scene. J. Neurosci. 37, 9189–9196. doi: 10.1523/JNEUROSCI.0938-17.2017
Saenz, M., and Langers, D. R. M. (2014). Tonotopic mapping of human auditory cortex. Hear. Res. 307, 42–52. doi: 10.1016/j.heares.2013.07.016
Saiz-Alía, M., Forte, A. E., and Reichenbach, T. (2019). Individual differences in the attentional modulation of the human auditory brainstem response to speech inform on speech-in-noise deficits. Sci. Rep. 9:14131. doi: 10.1038/s41598-019-50773-1
Skoe, E., and Kraus, N. (2010). Auditory Brain Stem Response to Complex Sounds: A Tutorial. Ear Hear. 31, 302–324. doi: 10.1097/AUD.0b013e3181cdb272
Smith, S. M., and Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061
Tang, C., Hamilton, L. S., and Chang, E. F. (2017). Intonational speech prosody encoding in the human auditory cortex. Science 357, 797–801. doi: 10.1126/science.aam8577
Taulu, S., and Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 51:1759. doi: 10.1088/0031-9155/51/7/008
Teoh, E. S., Cappelloni, M. S., and Lalor, E. C. (2019). Prosodic pitch processing is represented in delta-band EEG and is dissociable from the cortical tracking of other acoustic and phonetic features. Eur. J. Neurosci. 50, 3831–3842. doi: 10.1111/ejn.14510
Van Canneyt, J., Wouters, J., and Francart, T. (2021a). Enhanced Neural Tracking of the Fundamental Frequency of the Voice. IEEE Trans. Biomed. Eng. 68, 3612–3619. doi: 10.1109/TBME.2021.3080123
Van Canneyt, J., Wouters, J., and Francart, T. (2021b). Neural tracking of the fundamental frequency of the voice: the effect of voice characteristics. Eur. J. Neurosci. 53, 3640–3653. doi: 10.1111/ejn.15229
Keywords: temporal response functions, TRF, mTRF, auditory cortex, cocktail party
Citation: Brodbeck C and Simon JZ (2022) Cortical tracking of voice pitch in the presence of multiple speakers depends on selective attention. Front. Neurosci. 16:828546. doi: 10.3389/fnins.2022.828546
Received: 03 December 2021; Accepted: 08 July 2022;
Published: 08 August 2022.
Edited by:
Andre Rupp, University Hospital Heidelberg, GermanyReviewed by:
Inyong Choi, The University of Iowa, United StatesTobias Reichenbach, University of Erlangen Nuremberg, Germany
Copyright © 2022 Brodbeck and Simon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Brodbeck, christianbrodbeck@me.com
 Christian Brodbeck
Christian Brodbeck Jonathan Z. Simon
Jonathan Z. Simon