- 1School of Psychology & Public Health, La Trobe University, Melbourne, VIC, Australia
- 2Department of Neurology, Sunshine Hospital, St Albans, VIC, Australia
- 3Department of Medicine, Faculty of Medicine and Allied Health, University of Rajarata, Anuradhapura, Sri Lanka
- 4Department of Optometry & Vision Sciences, The University of Melbourne, Parkville, VIC, Australia
Background: Vision is rarely appraised either acutely or during recovery, following acute ischemic stroke. Our previous study found significant deficits in visual function after 2 to 3 days in ~68% of hospitalized mild-to-moderate acute ischemic stroke (AIS) patients with no comorbid eye disease. The purpose of this study was to evaluate recovery in vision after 2–6 months in a subgroup of the original participants.
Methods: Visual assessments were performed within the first week of admission and 2–6 months later. Testing was achieved on an iPad and included visual acuity (VA), VA-in-noise, visual field, visual neglect, and time to complete an eye–hand coordination (EHC) task. All cases were radiologically confirmed, and 10 had left hemisphere lesions. The outcomes were compared to 20 age-matched healthy controls who were tested and retested over a similar duration using the same vision tests. The testing took 12 min.
Results: During the first week of admission, 19/20 (95%) AIS patients returned normal visual acuity (>6/12 VA, p = 0.11), yet 11/20 (55%) had reduced VA-in-noise (p < 0.000).Visual neglect was present in 2/20 cases. Visual field defects were present in 16/20 (80%, p < 0.001), with 7/16 (44%) being unaware of their visual field loss. All of the patients chose to use their dominant right hand despite 10 having left hemisphere lesions and 13/20 (65%, p < 0.001) returning longer times to complete the EHC tracing tasks. After 2–6 months of recovery, all stroke patients returned normal visual attention, although 3/20 (15%) continued to show reduced VA in the presence of noise masks. Seven out of 20 (35%) retained visual field defects, and 8/20 (40%, three right and five left hemisphere lesions) had visuomotor impairment. Posterior circulation territory strokes and left hemisphere lesions were more likely to result in a persistent visual field loss and visuomotor deficit.
Conclusion: Given that stroke is the leading cause of neurological disability affecting over 110 million people, our findings highlight the necessity for both acute and longitudinal vision assessments subsequent to mild stroke. Exposing the persistent limitations in visual functions could aid in identifying suitability for driving and the visuomotor rehabilitation of stroke survivors.
Clinical Trial Registration: https://www.ANZCTR.org.au/ACTRN12618001111268.aspx, identifier ACTRN12618001111268.
1 Introduction
Stroke is a leading cause of neurological disability and the second leading cause of death worldwide (1–4). Yet while vision is the primary sensory information channel to the brain (5–8), driving attention (9), cognition (10), and most activities of daily living (10, 11), objective visual function is seldom routinely assessed in either acute or post-stroke settings. This is despite the seminal works of Rowe and colleagues (12, 13) in the UK, who have reported that up to 2/3 of many unselected cohorts of patients, with varying degrees of stroke severity, retain visual field loss, eye movement disorders, and perceptual deficits such as losses to contrast sensitivity for at least 12 months after the event. We have also found impaired visual acuity-in-noise, visual fields, and eye–hand coordination in greater than 68% of our previously published cohort of mild-to-moderate hospitalized acute ischemic stroke (AIS) patients who had no prior history of visual problems (14). Surprisingly, 44% of this cohort were unaware of these visual field limitations (14).
Currently, standard neurological practice in Australia typically allows stroke survivors to return to daily life and driving after 4 weeks of acute stroke recovery, provided that their neurological examination is normal based on functional assessments (15). Objective vision evaluations are limited primarily to those with observable hemianopia. Given that many of the mild-to-moderate ischemic stroke patients (14, 16) are unaware of their visual limitations, the aim of this study was to investigate recovery in visual capacity in a smaller cohort of the original group to consider the extent of recovery of visual acuity, visual fields, visual neglect, and eye–hand coordination occurring within 2–6 months of cases with mild-to-moderate stroke. We have investigated whether the change is comparable to that reported for other sensory (17) and motor functions (18–20). We expected that it is this group of mild-to-moderate stroke survivors who are likely to be discharged from the hospital without a recommendation for ophthalmic care after the acute AIS event, and they are likely to present for more regular eye care in the community.
To date, most studies (12, 21) that have performed visual evaluations in emergency departments (ED) for stroke have been restricted to visual acuity (22, 23) that is seldom impaired (14, 24) and qualitative tests (such as confrontation) that have limited sensitivity for identifying functional defects (12, 25).
The gold standard ophthalmic methodologies for assessments such as visual fields using bowl perimetry are cumbersome and expensive and require trained clinical assistants. Therefore, we have circumvented the limitations of testing by assessing visual fields, visual acuity (with and without noise masks), visual neglect, and eye–hand coordination using novel and validated (26, 27) modern technology (28). These tests are able to be presented on an iPad tablet both acutely and at retest and can be used by the patient’s bedside. Visual acuity-in-noise was included as a measure of visually driven attention and sensory processing of visual stimuli (29) following research that has indicated that noise masking is a useful biomarker for impaired visual perception in macaques with lesions in the extrastriate areas and humans with neurological conditions such as stroke (29), migraine (30), visual snow (31), and amblyopia (32).
2 Materials and methods
2.1 Participants
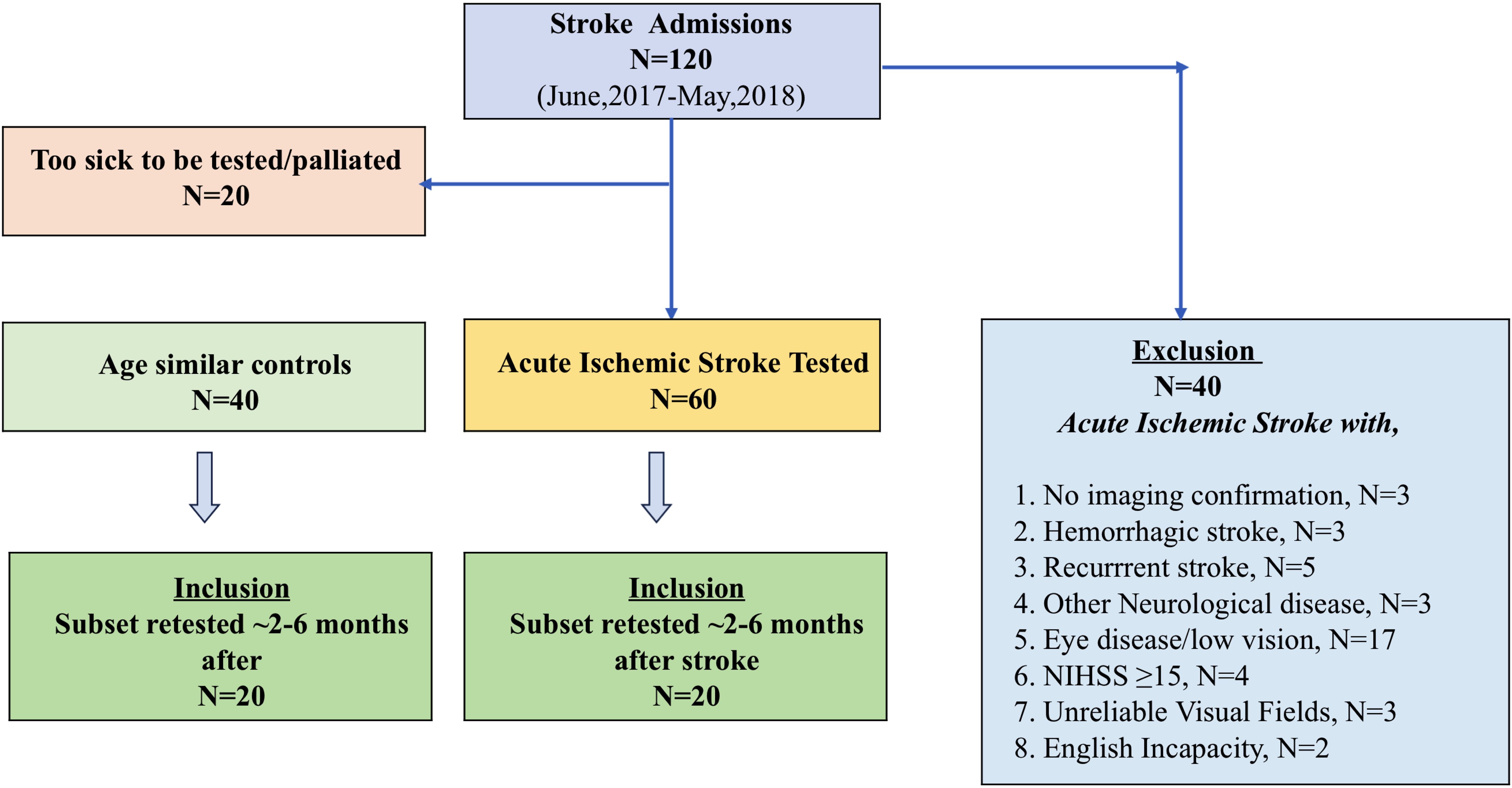
A total of 120 consecutive cases of acute ischemic stroke (AIS) presenting to the Sunshine Hospital Emergency Department in Melbourne between May 2018 and June 2019 were recruited for vision testing as detailed elsewhere (14). The clinical inclusion criteria required neurologist-determined, radiologically confirmed, hospitalized, first-episode acute mild-to-moderate stroke: i.e., National Institute of Health Stroke Scale >5 and <15 (13, 33–35) consistent with the proposal of Fischer et al. in 2010 (33). Mild-to-moderate AIS cases involved mild non-disabling motor symptoms at the time of the event without loss of consciousness or general attentiveness. The site of the infarction and the vascular territory affected were established and confirmed in all of our cases using brain computer tomography (CT/CT angiography) or magnetic resonance imaging (MRI/MR angiography) (34–36) as required for patient management and is reported in Wijesundera et al. in 2020 (14).
Infarcts that involved the blood supply to the posterior regions of the brain such as occipital lobe strokes and posterior cerebral artery, branches of middle cerebral, cerebellar, and basilar arteries, were defined as “posterior strokes”, whereas those involving the frontal lobe, frontoparietal regions, and the anterior branches of middle cerebral artery were defined as “anterior strokes”.
The exclusion criteria were as follows: stroke complicated by other neurological diseases, recurrent stroke, hemorrhagic stroke, clinically diagnosed ischemic stroke without radiological imaging, or ischemic stroke with NIHSS score ≥15. Two patients who had difficulty in understanding test instructions due to limited understanding of the English language and three patients who repeatedly performed unreliably (>35% false positives) on visual field assessment were also excluded (Figure 1). A 35% false positive rate was used to define our reliability criterion as it returns a clinically acceptable tolerance for MD error (≤1 dB from the true MD) (37).
A total of 60 “mild-to-moderate” AIS cases (age: 65 ± 14 years, male: 32/60) who had no history of eye disease were recruited and tested at their bedside following admission and diagnosis of acute ischemic stroke (days 2 to 3), and their findings have been published elsewhere (14, 37). Moreover, 45 out of 60 were classified as mild stroke (NIHSS score of ≤5). Furthermore, 20 (age: 66 ± 9 years, 12 male) of our original cohort of 60 AIS patients and 20 (age: 66 ± 7 years, 10 male) control agreed to return for retest after an average of 3.5 month (range: 2–6 months) duration. Our controls had no neurological or eye disease and were recruited following a routine optometry eye examination. We chose to recruit healthy controls who attended for clinical eye care for comparison of visual outcomes so that our data would not be confounded by other psychological (anxiety) or systemic conditions as would be expected if a hospitalized control group were used. It is this cohort of 40 participants (20 AIS and 20 controls) who are reported in the current manuscript.
All ischemic stroke patients were actively managed by the neurology team in line with hospital guidelines and specified treatments (thrombolysis/thrombectomy) for their presentation, and all patients received standard rehabilitative therapy in the period after the stroke.
2.2 Assessment procedure
All acute ischemic stroke patients were wearing their habitual reading glasses and had initial assessment within 5 days of admission at their hospital bedside. Testing was performed on an Apple iPad (9.7 inch) using the Melbourne Rapid Field-Neural (MRFn) (26) and UNSW (Department of Optometry, University of New South Wales, Australia) SLURP Eye–Hand Coordination applications (27). The test instructions and a practice trial required about 1 min per patient, whereas full testing of both eyes took ~12 min.
The Melbourne Rapid Fields-Neural (MRFn) application (Glance Optical Pty Ltd, https://www.visiondata.net.au) was administered monocularly at 33 cm (13 inches) starting with the right eye (RE) and high-contrast visual acuity, followed by visual acuity-in-noise (acuity-in-noise) and visual field thresholds out to ±20 degrees using a modified 24–2 pattern (Figure 2C) (14, 26). The EHC test was undertaken using binocular viewing after both vision tests were completed. In testing visual acuity (VA), MRFn presents single Landolt C targets shown in a box outline at one of four randomly chosen orientations. The patient’s task was to match the correct orientation (see Figure 2A) by screen touch or nominate the “?” symbol if unsure (see Figure 2A). The software identifies the lowest level (logMAR) achieved by the patient at a two of three correct criteria. We required a visual acuity of 6/12 (logMAR 0.3), being the current Australian driving license requirement, or better to indicate adequacy of reading glasses. The visual acuity-in-noise test uses the same Landolt C target shown in the presence of a dynamic luminance noise mask (Figure 2A shows a static image). For the visual field, a reduced and modified 24-2 (20° × 15°) test grid (see Figure 2C) was used to return local thresholds (in dB). These were compared to a normative database by the software to give a global mean deviation (MD, dB difference to age-similar norm). A qualitative description of the visual field loss was also determined by the researcher. The reduced 24–2 visual field test grid was chosen in our study because it does not require any fixational eye movements and is completed in 2 to 3 minutes. Patients who gave reliable visual field results (≤35% false positives) were included in our analysis; three unreliable individuals have been excluded (Figure 1). These vision tests were coupled with the SLURP Eye–Hand Coordination test performed binocularly (Figure 2B) using an iPad stylus for tracking (27, 37). The evaluation of eye–hand coordination required the participants to trace three shapes (see Figure 2B). If the stylus is maintained within a tolerance zone of the shape outline (± 2.5 mm) during tracing, the shape changes color (from blue to pink, Figure 2B), but if the participant deviates beyond the tolerance limit, the color change stops and the patient is asked to return to the location of the color border in the shape as rapidly as possible and resume tracing from the last correct location (Figure 2B). Detailed procedures have been reported in our previous publications (14, 37). The SLURP test was chosen for our purpose as it requires goal-directed attention and eye movements to focus on the shape and accurately plan, control, trace, and correct any eye–hand deviation errors which have been reported as having potential for change following stroke (10, 38). Our previous analysis of the number of deviations, magnitude of deviation, and completion time finds that completion time is the best index of performance (37); hence, timing is the only measure considered in the present study. Recovery was evaluated using the same tests presented in the same order at retest (~3.5 months post-stroke) following a comprehensive optometry examination. All of the patients chose to use their previously dominant right hand for tracing despite the fact that 10/20 (50%) of our first-episode stroke patients had experienced a radiologically confirmed left hemisphere lesion (39), and our data show that they do not give normal tracing performance.
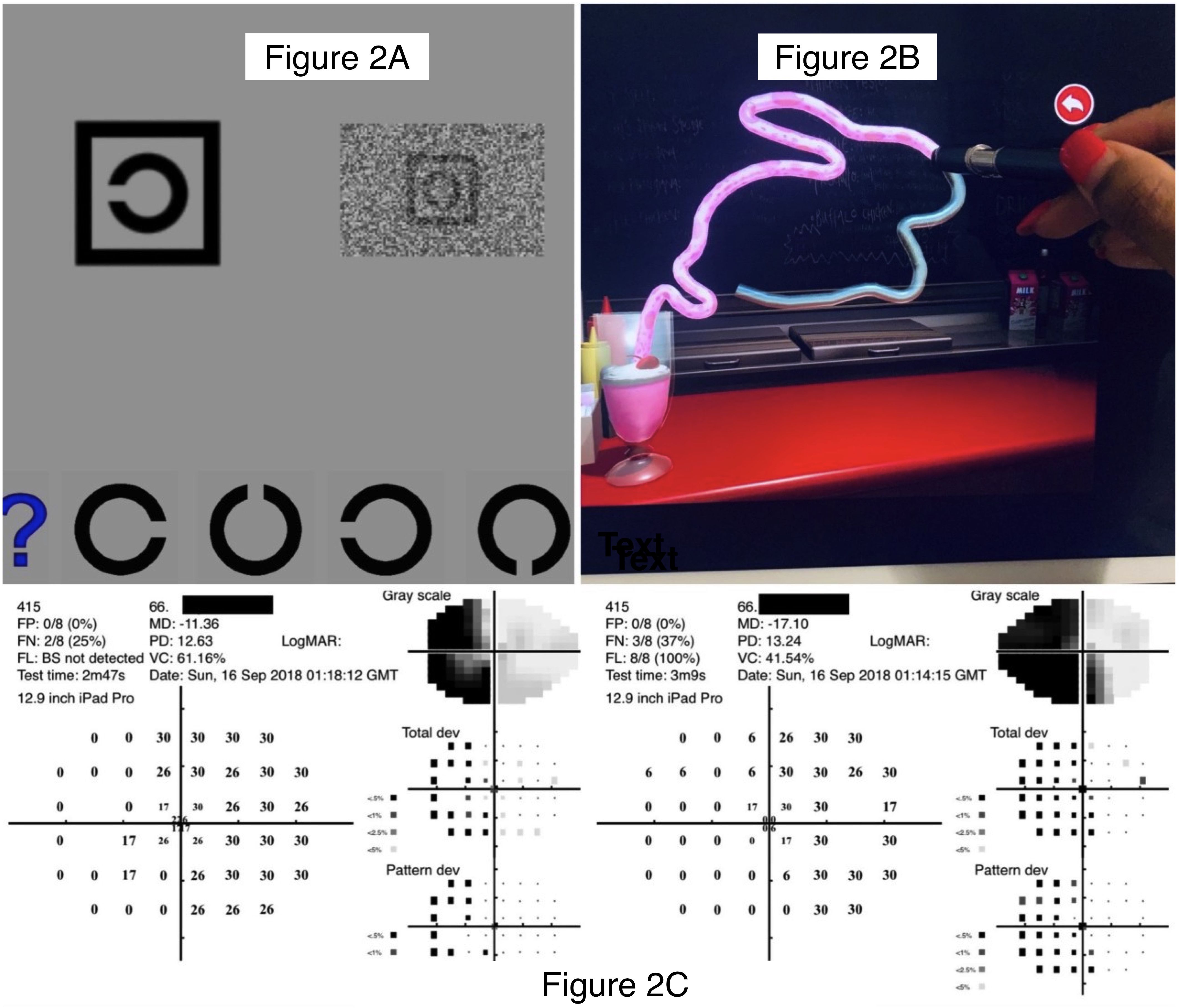
Figure 2. Images of the test suite used on the iPad Tablet. (A): Optotype letter “C” shown with the MRFn acuity test: in high contrast (left) and dynamic noise mask (right). The participants’ task is to match the orientation of the “C” in the box at the top of the panel with the appropriate option at the bottom. High-contrast optotypes are shown first during visual acuity assessment, followed by the visual acuity-in-noise optotype. (B): Top right: Tracing of the shape “Rabbit” using the SLURP Eye-hand Coordination App showing the stylus used for tracking, the completed track (pink), and the blueish white track remaining to be done. (C): Outcome for a left hemianopia in a 66-year-old patient tested with the MRFn test grid which took about 3 min in both eyes. This modified grid has reduced vertical and horizontal extent (15° Å~ 21°) and four test points added near the fovea (0.8°)..
Hemi-spatial neglect (HSN) was considered in all cases of stroke recovery using a line bisection test and the MRF cancellation test. The MRF cancellation test requires the patients to “tap” on 40 frowning faces (20 in each hemi-field) and change them to “smiley” faces. We consider the presence of HSN when the difference in L and R hemifield identifications exceeds 4.
Ethics approval was provided by Sunshine Hospital (Western Health Ethics Committee HREC/16/WH/1) review board, and the study was conducted in accordance with the tenets of the Declaration of Helsinki with all of the participants (or their carers) giving signed informed consent to participate. Our study was registered as a clinical trial at ACTRN12618001111268.
3 Data analysis
Non-parametric statistical tests were used to compare the stroke and control groups given the non-gaussian data distributions. Kruskal–Wallis tests were used to identify differences between group medians, with Dunn’s multiple comparison applied to identify significant differences between groups. Although each eye was tested, the eye ipsilateral to the CT/MRI defined lesion was analyzed in the stroke group as each eye is driven by 5/6 ipsilateral eye muscles, and hence the visual function of the eye ipsilateral to the infarct is more likely to be affected in the AIS than the contralateral eye (40). We compared this eye to the RE of controls as the visual acuity of the two eyes of the controls was similar. Analysis of the fellow eye in our data set does (stroke or normal groups) not alter the general findings.
The performance of our worst control (one of 20) was used to define a non-parametric 95th percentile to define “abnormal” outcomes. Group performance is specified with 95% confidence intervals. Statistical analysis was conducted using GraphPad Prism v7.00 for Windows (www.graphpad.com), and a p-value of 0.05 was used to indicate statistical significance in our results.
4 Results
4.1 Vision deficits in the acute phase of ischemic stroke
Normal visual acuity (>6/12) was the consistent finding in 19 of our 20 (95%) AIS patients in the acute phase, although around half (11/20, 55%) of the participants demonstrated significant deficits for visual acuity-in-noise, visual field loss (16/20, 80%) and had a prolonged tracing time on the eye–hand coordination task (13/20, 65%) compared to controls. The acute visual field losses involved 9/16 patients with hemianopia, 4/16 quadrantanopias, and 3/16 altitudinal defects. Despite these limitations to vision and visuomotor capacity, 7/16 (44%) patients with these severe visual field defects were unaware of their visual limitation.
4.2 Recovery in visual deficits
4.3 Visual acuity
Only one patient with a large right posterior cerebellar artery stroke had abnormal visual acuity (6/12) at the initial assessment. This patient returned near normal visual acuity of 6/7.5 after 3 months of recovery. On the other hand, 10 of the 11 of our AIS group who initially had abnormal (<6/12) visual acuity-in-noise showed a significant improvement in their visual acuity-in-noise at retest (mean, test: 6/12, retest: 6/7.5, p < 0.001) with eight of the 11 (72%) returning normal outcomes for this test (Figures 3, 4). The three patients who continued to show abnormal visual acuity-in-noise at retest involved a right frontal lobe and two occipital lobe lesions. No substantial changes were found in controls for visual acuity-in-noise at retest (controls: mean, test: 6/7.5; retest: 6/6, p = 0.60).
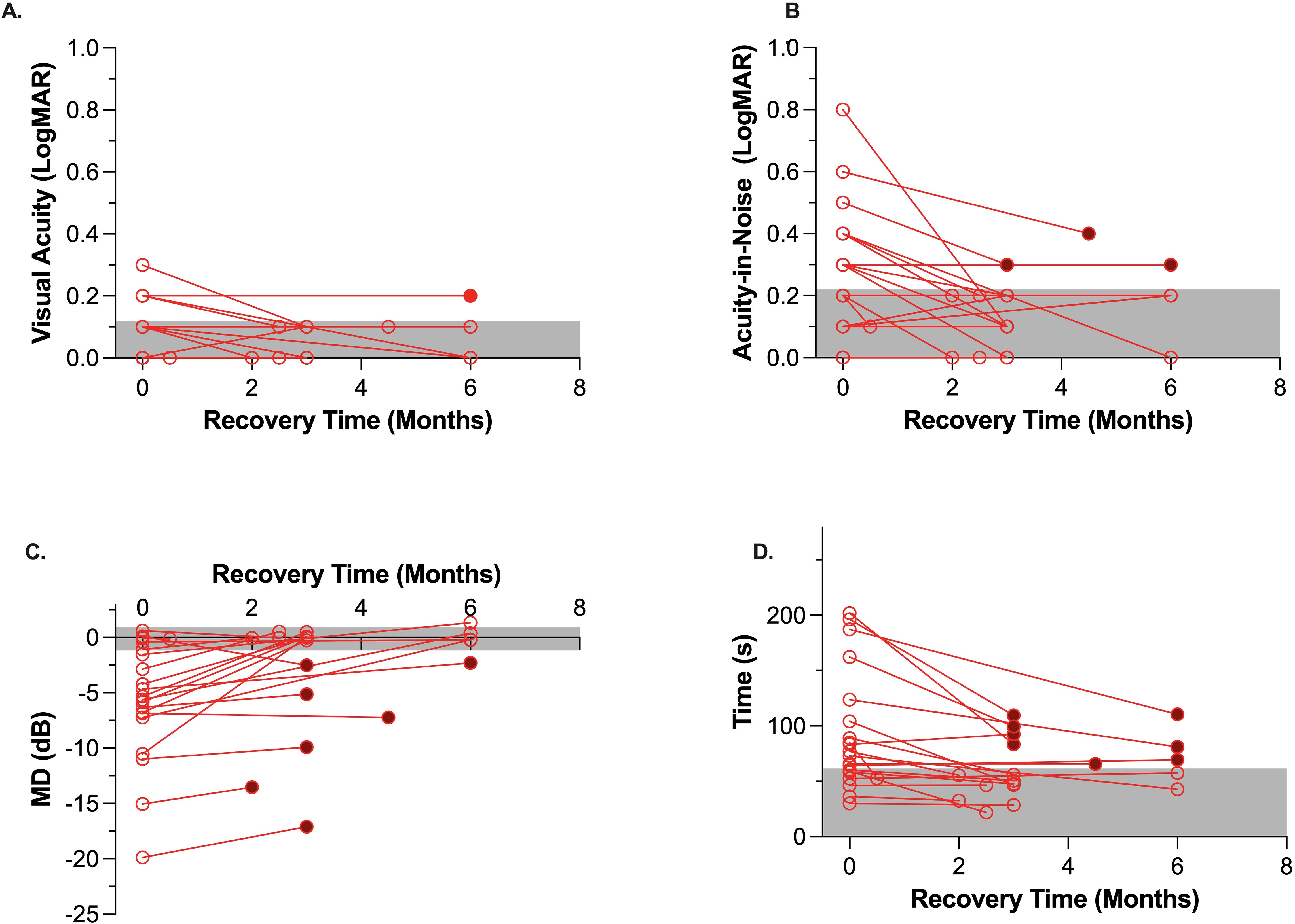
Figure 3. Change in visual function of the stroke cohort (n = 20) between acute assessment in hospital (i.e., unfilled circles on left) and at the exact retest time shown on the x-axis. Filled symbols at retest time indicate cases which remained beyond the worst control (gray area). The gray zone indicates the 95% confidence interval for age-similar controls. (A): Visual Acuity, (B): Visual acuity-in-noise, (C): Visual Field Mean Deviation (MD-dB), 3D: Eye-hand coordination time (s).

Figure 4. (A) An example of a 65-year-old stroke patient with a right inferior quadrantanopia that resolved by the time of retest. Top: Visual field measured during the acute phase of stroke on admission to the hospital (September 19, 2018). Bottom: Visual field measured at retest (December 12, 2018). (B) Stable visual fields measured from a patient showing a right superior quadrantic defect. This patient shows no change in their visual field 2.5 months after stroke as evident qualitatively and in the MD values. Top: Visual field measured during the acute phase of stroke on admission to the hospital; MD LE -10.98 and RE -6.66. Bottom: Visual fields measured at 2.5 months later return MD of LE -9.92 and RE -6.70.
4.4 Visual fields
Our recovery analyses (Figure 3) indicates that visual field thresholds for mild-to-moderate stroke patients can improve in both the global MD index (AIS: -5.7 ± 5.5 dB, retest: -2.8 ± 5.4 dB, p < 0.001) and in the manifest form of visual field scotoma after an average recovery time of 3.5 months (Figures 3A, 4, 5C). Indeed of the 16 AIS participants who initially showed abnormal visual fields, 9/16 (56%) showed a substantial improvement at retest (Figure 3), with normalization of their initial quadrantanopic, hemianopic, or altitudinal visual field loss, whereas 4/16 (25%) had partial recovery and 3/16 (38%) remained unchanged and abnormal at retest. Overall, 7/20 (35%) stroke participants showed significant and persistent visual field defects at retest.
We found that two of our cases (10%) had parietal stroke with visual neglect. They failed the line bisection test and the MRF cancellation test (Figure 5) contralateral to the site of lesion. Both patients correctly identified all 20 faces on the side of lesion, but only 13/20 on the contralateral side. The visual field losses found acutely in these patients recovered in both cases.
4.5 Visuomotor function: eye–hand coordination
Acutely, 13 out of the 20 (65%) stroke patients returned significantly slower eye–hand tracing times. Eight of 13 (62%) had left hemisphere lesions, and five (38%) had right hemisphere lesions, but all chose to use the iPad stylus in their previously dominant right hand. At retest, eight of the 13 had maintained this visuomotor defect, whereas the other five (38%) showed significant recovery and normal eye–hand coordination tracing time (Figure 3). The lesions of the eight individuals whose visuomotor function did not improve were posterior infarcts, with 5/8 experiencing left hemisphere (Figure 4) lesions.
4.6 Recovery of visual deficits post-stroke in relation to brain blood supply
Although many patients initially showed significant impairment in visual acuity-in-noise, these cases did not show any particular association with anatomical location of the lesion or blood supply (Figure 4). On the other hand, in the 16/20 AIS lesions that initially resulted in visual field defects, posterior right-sided lesions give significantly worse outcomes compared with anterior or left-sided lesions (Figure 6). A significant increase in eye–hand coordination tracing time is likewise associated with left-sided lesions.
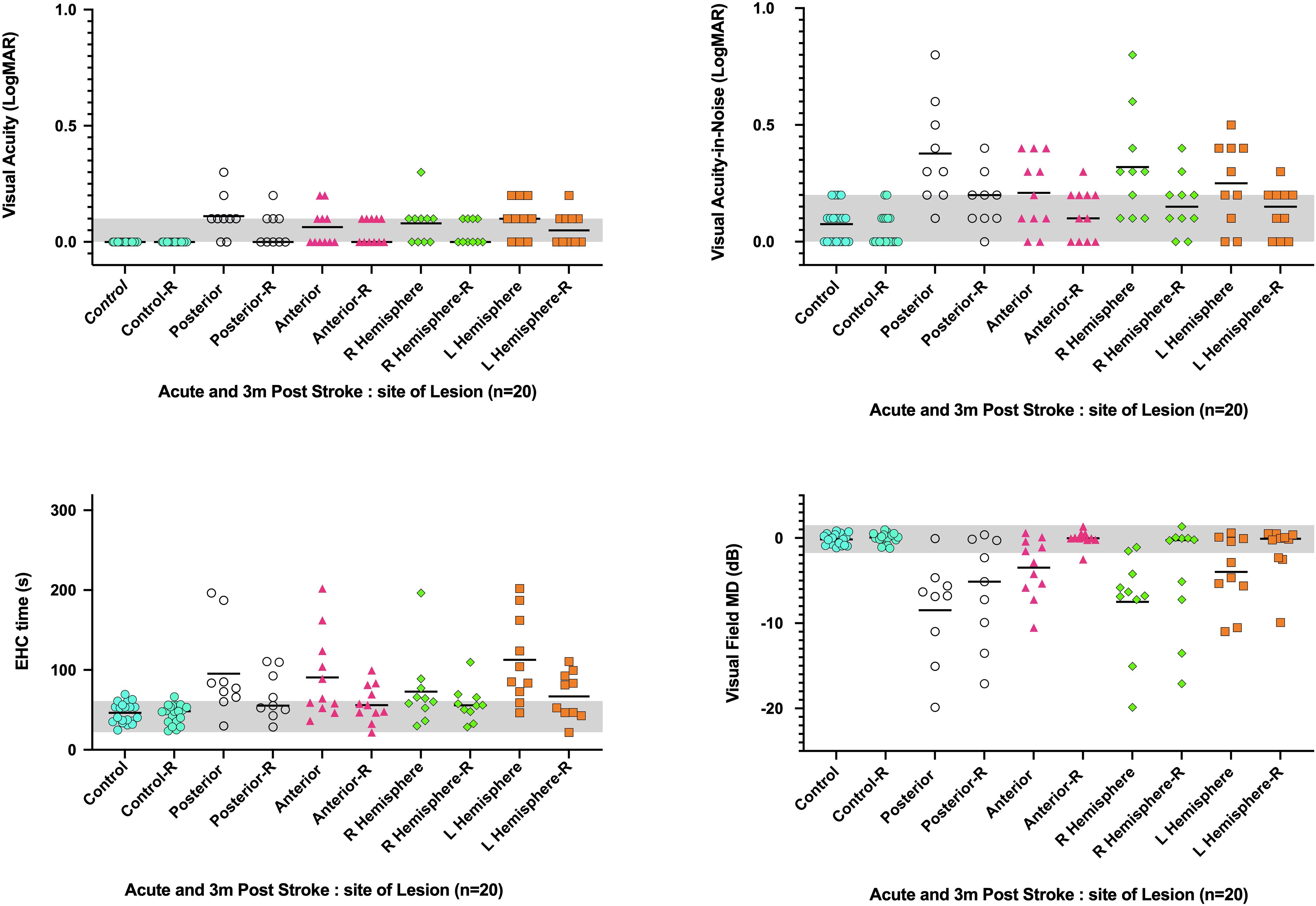
Figure 6. Visual abnormalities observed acutely and after 3.5 months average recovery time (-R) in relation to the site of stroke lesion (i.e., right or left hemisphere and anterior or posterior blood supply). Y axis: visual function parameter, X axis: Acute and recovery site of lesion. The bar identifies the cohort mean.
4.7 Nature of vision loss
As shown in the Venn analysis, Figure 4 summarizes impaired visual functions assessed during the acute and recovery phases. Notably, 19/20 (95%) of our radiologically confirmed AIS cases showed (Figure 7, left) at least one aspect of impaired vision immediately on presentation for stroke. The fact that 4/20 (20%) show impairment in all three key aspects of vision, i.e., visual acuity-in-noise, visual field thresholds, and eye–hand coordination tracing time, indicates that each aspect of this test battery is identifying unique deficits associated by the stroke. Significant improvement was found in all aspects of vision after 2–6 months, with 9/20 (45%) returning normal visual capacity and none showing impairment in all aspects of vision (Figure 7, right). Persistent visual acuity-in-noise deficits were present in 3/20 (15%) cases and visual field loss in 7/20 (35%), and 8/20 (40%) retained eye–hand coordination delays.
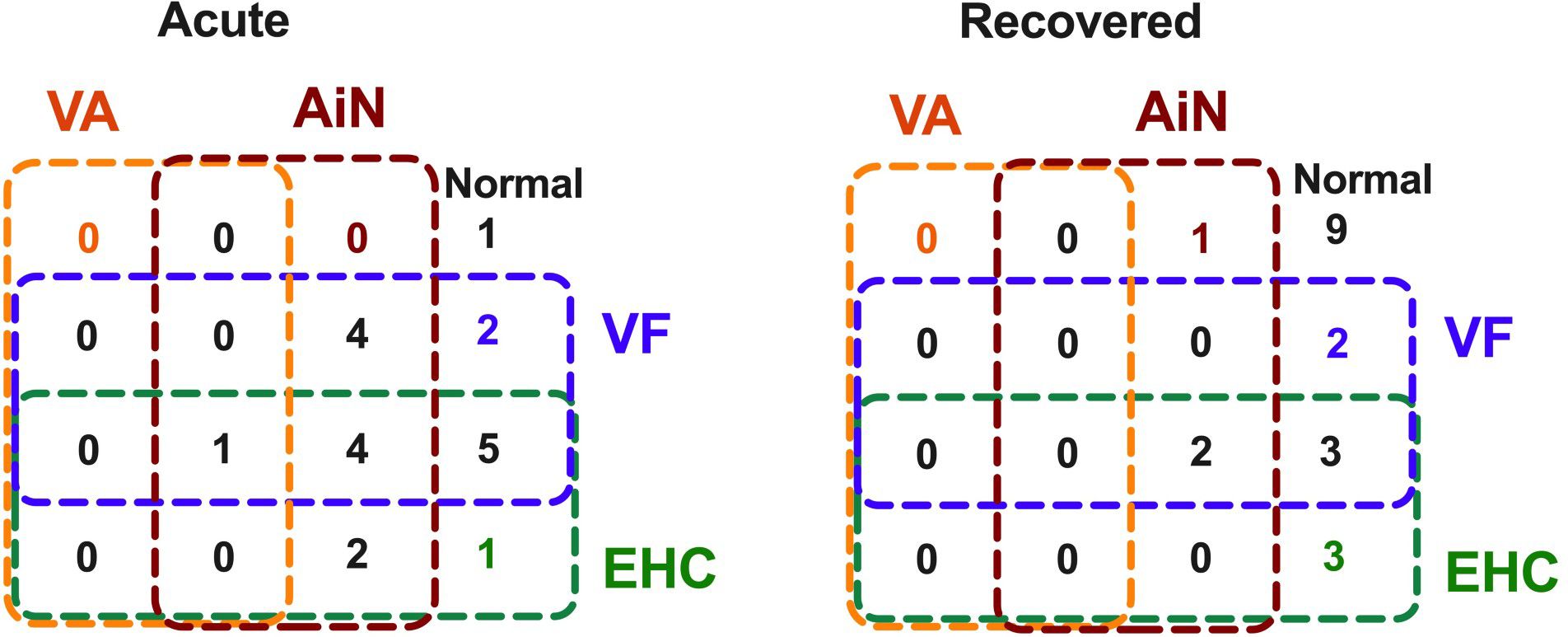
Figure 7. Venn diagram: Association of vision changes at acute onset and on recovery in 20 cases of mild-to-moderate acute ischemic stroke.
5 Discussion
The aim of this study was to explore the persistence of abnormal vision following mild-to-moderate ischemic stroke. A subset of 20 of the original cohort of 60 radiologically confirmed mild-to-moderate AIS patients (14, 37), of whom 44% of the original cohort were unaware of their visual field deficits (14, 37) were recruited to this trial as mild-to-moderate stroke survivors. The average age of our sample was 66 ± 8 years, who are likely to be mobile, driving, and visiting ophthalmic practices and already receiving age-related vision management. Hence, they are likely to benefit from comprehensive functional vision assessment following recovery from stroke and to consider any limitations to mobility and daily activities.
Our most noteworthy finding is that only half of the stroke cases recovered normal visual function over the 3- to 4-month period despite most having normal central visual acuity immediately after the acute cerebrovascular event. Sparing of central VA was expected given the dual foveal projection that projects to both hemispheres, resulting in macular sparing (41–43). We adopted visual acuity measurement in our trial as VA is often needed in diagnosing acute neuro-ophthalmologic presentations. Furthermore, previous clinical trials on acute stroke which included patients with vision problems and more severe expression of stroke found the visual acuity to be reduced in up to 56% (23, 44, 45) of the cohort. In our trial, we excluded 16 stroke patients with a past record of eye disease from our test cohort. The post-hoc analysis of all of our data (including these 16 cases) finds that having poor visual acuity (≤6/12) is a good indicator of the presence of co-morbid eye disease in a stroke patient, yielding a likelihood ratio of 5.7 for ocular co-morbidity. Our data find that a loss of VA does not often occur in mild stroke. Indeed previous trials reported by Rowe et al. (12, 44, 46) suggest that reduced visual acuity can be associated with severe expressions of stroke.
Visuomotor capacity and visual field thresholds show the least recovery, leaving nearly half of the stroke survivors with induced visual defects (Figure 7) and 25% (5/20) of them with concomitant visuomotor and visual field defects.
Abnormal visual fields (mean deviation) were found in 16/20 (80%) of our test cohort during the acute phase. Residual visual field defects remained in 7/20 (35%) at retest. Six out of the seven stroke patients whose visual fields did not normalize included four occipital lobe and two posterior cerebellar artery infarcts with contralateral hemianopic and quadranatopic defects showing partial improvement in the form of developing incongruous hemianopias (47). Notably, all four occipital lobe patients showed no improvement in their hemianopia or quadrantanopia at retest, meaning that in terms of recovery, our findings are similar to those previously reported in the literature (48) where 39% of the patients show partial recovery of visual field defects, 7.5% showed full recovery, and 52% retain the loss in visual field sensitivity. Our observations suggest that acutely impaired visual function following stroke is a more generalized response to vascular dysfunction than infarct location per se. Many cases can recover from this acute event, but those who do not may fail to do so because their lesion-specific locations affect their vision as reported by Hayes and Merigan in macaques and humans a long time after their stroke (29).
A consistent improvement of ≥1 dB in mean deviation on successive visual field tests (L, R, L, R) has been suggested as due to a “learning effect” (49). We investigated our data for such trends and find that three of our 20 acute ischemic stroke test/retest patients (15%) met this criterion, indicating that this potential confound arises from learning rather than recovery in cortical function and needs to be considered by clinicians in evaluating stroke cases especially in considering recovery.
Significantly increased eye–hand coordination time was initially required by 13/20 (65%) of our cohort, with only 5/13 (38%) showing recovery. Of the 8/20 of the retest cases who continued to show abnormal EHC, five had left-sided lesions, suggesting that the increase in visuomotor “timing” (37) is likely related to the patient’s impaired visually driven motor capacity.All of our results confirm the large body of data previously published by Rowe and colleagues (12, 44) in the UK, highlighting the fact that AIS contributes significantly to many persistent impairments in vision, eye movement dysfunction, and visual field defects (50, 51) and probably also to binocular dysfunction (52) and mis-coordination between ocular and motor function and have a negative impact on the quality of life. Quality of life is also likely to be further compromised by persistent visual deficiencies that result in an increased need for additional conscious visual attention to simple visuomotor activities [such as lifting a hand or walking (53, 54) or reading, watching television, holding a cup of water, grasping for an object, or driving (55, 56)]. The need for such conscious additional attention has previously been reported to induce increased fatigue (57), concentration, higher risk of falls, and anxiety and negatively affect mood (58). Importantly, for the 7/20 (35%) persistent quadrantanopic or hemianopic defects, it would also be expected to impact negatively on their mobility and capacity to drive as well as reading and tracking. It is vital for eye care practitioners to understand the impact of left hemianopias causing trouble in picking up the start of a line when reading versus right hemianopias causing asthenopia when reading from left to right and to implement thorough binocular vision assessment as per the patient’s daily activities.
Impaired visual acuity-in-noise that is usually associated with impaired visual attention (59) in other neurological conditions (30, 32, 60) such as migraine (30), amblyopia (32), schizophrenia (60), and autism spectrum disorder (61) has not previously received much consideration either acutely nor early in recovery in stroke (29). Indeed in our retest subset, 11/20 (55%) were acutely affected, and eight improved over time, with deficits only persisting in 3/20 (15%) patients 2–6 months later. Two out of the three patients with persistent visual acuity-in-noise deficits at retest had occipital lobe lesions, whereas the third had a right frontal lobe lesion and returned 6/12 visual acuity-in-noise at both test and retest, implying that visual processing and the ability of vision to drive conscious attention, memory, and visuomotor areas (41, 62) remain impaired if the parieto-frontal network (63) is permanently damaged (9, 64, 65). Our findings highlight the importance of acute visual assessment following stroke in patients, particularly in those with posterior stroke who are likely to benefit from early visual rehabilitation to deal with edema-induced impairments in visual attention and contrast sensitivity that are likely to affect mobility and daily life.
6 Limitations
Although the generalizability of this study could be expected to be limited by the size of the sample of mild-to-moderate stroke patients without eye disease and in terms of limited diversity in age, ethnicity, and comorbid conditions in a hospitalized environment that is usually associated with severe strokes, the similarities of our retest results to previous less stringently selected patient samples where such cases were included (12, 44, 46) increase its likely importance as an early biomarker of visual dysfunction immediately following stroke as well as on recovery and indicate the need for incorporation of such visual assessment into stroke rehabilitation programs. More constraining to long-term understanding of the temporal trajectory of AIS pathophysiology for our work is having only one time point of short-term recovery, namely, 3 months, that limits understanding of the duration of time needed for recovery of different visual functions. It is also possible that early treatment with thrombolysis in ischemic stroke patients may have produced a lessened functional impact than has previously been reported following AIS (66, 67).
7 Conclusion
Our findings demonstrate the presence of acute and persistent vision deficits after 2–6 months in ~50% of mild-to-moderate AIS survivors of whom 44% were initially unaware of their visual deficits. Our results highlight the consequences on visual function of a mild AIS event and the need for comprehensive regular monocular and binocular visual examinations and the significant implications for each patient’s recovery and rehabilitation as undiagnosed visual impairments may hinder full functional recovery and quality of life.
Our Venn diagram also highlights that 95% of acute AIS cases can be correctly identified using three vision tests (acuity-in-noise, visual field, and EHC) in 10 min of testing. This information should be useful for first responders in the early diagnosis of stroke and later for eye care providers diagnosing and managing stroke and post-stroke patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Sunshine Hospital (Western Health Ethics Committee HREC/16/WH/1) review board and the study was conducted in accordance with the tenets of the Declaration of Helsinki with all participants (or their carers) giving signed informed consent to participate. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. TW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. AV: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. • AV partly funded by a seeking grant from Graeme Clark Institute HealthTech Innovation Challenge • SC had internal funding from La Trobe University.
Acknowledgments
Glance Optical Pty Ltd provided software and data support for the project. Specsavers Keilor Downs helped to recruit the voluntary controls following an eye examination.
Conflict of interest
AV is a founding director of Glance Optical Pty Ltd, the maker of Melbourne Rapid Field-Neural MRFn App.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2025.1505836/full#supplementary-material
Abbreviations
NIHSS, National Institute of Health Stroke Scale; MRFn, Melbourne Rapid Field-Neural; VA, visual acuity; VA-in-noise, visual acuity-in-noise; EHC, eye–hand coordination; AIS, acute ischemic stroke; RE, right eye.
References
1. Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/S1474-4422(18)30499-X
2. Foerch C, Misselwitz B, Sitzer M, Steinmetz H, and Neumann-Haefelin T. The projected burden of stroke in the German federal state of Hesse up to the year 2050. Dtsch Arztebl Int. (2008) 105:467–73. doi: 10.3238/arztebl.2008.0467
3. Grisold W, Dodick DW, Guekht A, Lewis S, and Wijeratne T. The world federation of neurology world brain day 2023. Lancet Neurol. (2023) 22(8):652. doi: 10.1016/S1474-4422(23)00240-5
4. Wijeratne T, Dodick DW, Lewis SL, Guekht A, Pochigaeva K, and Grisold W. World Brain Day 2023 - Brain Health and Disability: “Leave no one behind. J Neurol Sci. (2023) 451:120720. doi: 10.1016/j.jns.2023.120720
5. Vater C, Wolfe B, and Rosenholtz R. Peripheral vision in real-world tasks: A systematic review. Psychonomic Bull Review. (2022) 29:1531–57. doi: 10.3758/s13423-022-02117-w
6. Duncan PW, Samsa GP, Weinberger M, Goldstein LB, Bonito A, Witter DM, et al. Health status of individuals with mild stroke. Stroke. (1997) 28:740–45. doi: 10.1161/01.STR.28.4.740
7. Hodson T, Gustafsson L, and Cornwell P. The lived experience of supporting people with mild stroke. Scand J Occup Ther. (2020) 27:184–93. doi: 10.1080/11038128.2019.1633401
8. Wei WE, De Silva DA, Chang HM, Yao J, Matchar DB, Young SHY, et al. Post-stroke patients with moderate function have the greatest risk of falls: a National Cohort Study. BMC Geriatrics. (2019) 19:373. doi: 10.1186/s12877-019-1377-7
9. Goodale MA and Milner AD. Separate visual pathways for perception and action. Trends Neurosci. (1992) 15:20–5. doi: 10.1016/0166-2236(92)90344-8
10. Corbetta M and Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. (2002) 3:201–15. doi: 10.1038/nrn755
11. Ahlström C, Kircher K, Nyström M, and Wolfe B. Eye tracking in driver attention research—How gaze data interpretations influence what we learn. Front Neuroergonomics. (2021) 2:778043. doi: 10.3389/fnrgo.2021.778043
12. Rowe F, Brand D, Jackson CA, Price A, Walker L, Harrison S, et al. Visual impairment following stroke: do stroke patients require vision assessment? Age Ageing. (2009) 38:188–93. doi: 10.1093/ageing/afn230
13. Patterson G, Howard C, Hepworth L, and Rowe F. The impact of visual field loss on driving skills: A systematic narrative review. Br Ir Orthopt J. (2019) 15:53–63. doi: 10.22599/bioj.129
14. Wijesundera C, Vingrys AJ, Wijeratne T, and Crewther SG. Acquired visual deficits independent of lesion site in acute stroke. Front Neurol. (2020) 11:705. doi: 10.3389/fneur.2020.00705
15. Wijeratne T, Sales C, Wijeratne C, Karimi L, and Jakovljevic M. Systematic review of existing stroke guidelines: case for a change. BioMed Res Int. (2022) 2022:5514793. doi: 10.1155/2022/5514793
16. Rowe F. Symptoms of stroke-related visual impairment. Strabismus. (2013) 21:150–4. doi: 10.3109/09273972.2013.786742
17. Lee KB, Lim SH, Kim KH, Kim KJ, Kim YR, Chang WN, et al. Six-month functional recovery of stroke patients: a multi-time-point study. Int J Rehabil Res. (2015) 38:173–80. doi: 10.1097/mrr.0000000000000108
18. Cortes JC, Goldsmith J, Harran MD, Xu J, Kim N, Schambra HM, et al. A short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair. (2017) 31:552–60. doi: 10.1177/1545968317697034
19. Branco JP, Oliveira S, Sargento-Freitas J, Laíns J, and Pinheiro J. Assessing functional recovery in the first six months after acute ischemic stroke: a prospective, observational study. Eur J Phys Rehabil Med. (2019) 55:1–7. doi: 10.23736/s1973-9087.18.05161-4
20. Ballester BR, Maier M, Duff A, Cameirão M, Bermúdez S, Duarte E, et al. A critical time window for recovery extends beyond one-year post-stroke. J Neurophysiol. (2019) 122:350–57. doi: 10.1152/jn.00762.2018
21. Falkenberg HK, Mathisen TS, Ormstad H, and Eilertsen G. Invisible” visual impairments. A qualitative study of stroke survivors` experience of vision symptoms, health services and impact of visual impairments. BMC Health Serv Res. (2020) 20:302. doi: 10.1186/s12913-020-05176-8
22. Naeem Z. The prevalence of visual problems in stroke patients and the effectiveness of the current screening tool used. Br Irish Orthoptic J. (2012) 9:55. doi: 10.22599/bioj.56
23. Rowe FJ, Hepworth LR, Howard C, Hanna KL, and Currie J. Impact of visual impairment following stroke (IVIS study): a prospective clinical profile of central and peripheral visual deficits, eye movement abnormalities and visual perceptual deficits. Disabil Rehabil. (2022) 44:3139–53. doi: 10.1080/09638288.2020.1859631
24. Rowe FJ, Hepworth LR, Howard C, Hanna KL, and Currie J. Impact of visual impairment following stroke (IVIS study): a prospective clinical profile of central and peripheral visual deficits, eye movement abnormalities and visual perceptual deficits. Disabil Rehabil. (2020) 44(13):3139–3153. doi: 10.1080/09638288.2020.1859631
25. Quinn TJ, Livingstone I, Weir A, Shaw R, Breckenridge A, McAlpine C, et al. Accuracy and feasibility of an android-based digital assessment tool for post stroke visual disorders-the strokeVision app. Front Neurol. (2018) 9:146. doi: 10.3389/fneur.2018.00146
26. Vingrys AJ, Healey JK, Liew S, Saharinen V, Tran M, Wu W, et al. Validation of a tablet as a tangent perimeter. Transl Vis Sci Technol. (2016) 5:3. doi: 10.1167/tvst.5.4.3
27. Lee K, Junghans BM, Ryan M, Khuu S, and Suttle CM. Development of a novel approach to the assessment of eye-hand coordination. J Neurosci Methods. (2014) 228:50–6. doi: 10.1016/j.jneumeth.2014.02.012
28. Nesaratnam N, Thomas PBM, Kirollos R, Vingrys AJ, Kong GYX, and Martin KR. Tablets at the bedside - iPad-based visual field test used in the diagnosis of Intrasellar Haemangiopericytoma: a case report. BMC Ophthalmol. (2017) 17:53. doi: 10.1186/s12886-017-0445-z
29. Hayes RD and Merigan WH. Mechanisms of sensitivity loss due to visual cortex lesions in humans and macaques. Cereb Cortex. (2007) 17:1117–28. doi: 10.1093/cercor/bhl021
30. Webster KE, Dickinson JE, Battista J, McKendrick AM, and Badcock DR. Evidence for increased internal noise in migraineurs for contrast and shape processing. Cephalalgia. (2012) 32:125–39. doi: 10.1177/0333102411432725
31. McKendrick AM, Chan YM, Tien M, Millist L, Clough M, Mack H, et al. Behavioral measures of cortical hyperexcitability assessed in people who experience visual snow. Neurol. (2017) 88:1243–49. doi: 10.1212/WNL.0000000000003784
32. Pelli DG, Levi DM, and Chung ST. Using visual noise to characterize amblyopic letter identification. J Vis. (2004) 4:904–20. doi: 10.1167/4.10.6
33. Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, Marchis GMD, et al. What is a minor stroke? Stroke. (2010) 41:661–66. doi: 10.1161/STROKEAHA.109.572883
34. Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. (1999) 30:1174–80. doi: 10.1161/01.str.30.6.1174
35. Allen LM, Hasso AN, Handwerker J, and Farid H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. RadioGraphics. (2012) 32:1285–97. doi: 10.1148/rg.325115760
36. Birenbaum D, Bancroft LW, and Felsberg GJ. Imaging in acute stroke. West J Emerg Med. (2011) 12:67–76.
37. Wijesundera C, Crewther SG, Wijeratne T, and Vingrys AJ. Vision and visuomotor performance following acute ischemic stroke. Front Neurol. (2022) 13. doi: 10.3389/fneur.2022.757431
38. Moore RT, Piitz MA, Singh N, Dukelow SP, and Cluff T. Assessing impairments in visuomotor adaptation after stroke. Neurorehabil Neural Repair. (2022) 36:415–25. doi: 10.1177/15459683221095166
39. Ocklenburg S, Garland A, Ströckens F, and Uber Reinert A. Investigating the neural architecture of handedness. Front Psychol. (2015) 6:148. doi: 10.3389/fpsyg.2015.00148
40. Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, and Martin A. Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci U S A. (2013) 110:E3435–44. doi: 10.1073/pnas.1302581110
41. Horton JC, Economides JR, and Adams DL. The mechanism of macular sparing. Annu Rev Vis Sci. (2021) 7:155–79. doi: 10.1146/annurev-vision-100119-125406
42. Leventhal AG, Ault SJ, and Vitek DJ. The nasotemporal division in primate retina: the neural bases of macular sparing and splitting. Science. (1988) 240:66–7. doi: 10.1126/science.3353708
43. Crewther DP, Crewther SG, and Cleland BG. Is the retina sensitive to the effects of prolonged blur? Exp Brain Res. (1985) 58:427–34. doi: 10.1007/bf00235859
44. Rowe FJ, Hepworth LR, Howard C, Hanna KL, Cheyne CP, and Currie J. High incidence and prevalence of visual problems after acute stroke: An epidemiology study with implications for service delivery. PloS One. (2019) 14:e0213035. doi: 10.1371/journal.pone.0213035
45. Rowe FJ and Hepworth LR. The impact of visual impairment in stroke (IVIS) study - evidence of reproducibility. Neuroophthalmol. (2021) 45:165–71. doi: 10.1080/01658107.2020.1849317
46. Rowe FJ, Wright D, Brand D, Jackson C, Harrison S, Maan T, et al. A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. BioMed Res Int. (2013) 2013:719096. doi: 10.1155/2013/719096
47. Goodwin D. Homonymous hemianopia: challenges and solutions. Clin Ophthalmol. (2014) 8:1919–27. doi: 10.2147/OPTH.S59452
48. Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, et al. Interventions for disorders of eye movement in patients with stroke. Cochrane Database Syst Rev. (2011) 10:Cd008389. doi: 10.1002/14651858.CD008389.pub2
49. Heijl A, Lindgren A, and Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. (1989) 108:130–35. doi: 10.1016/0002-9394(89)90006-8
50. Niehorster DC, Peli E, Haun A, and Li L. Influence of hemianopic visual field loss on visual motor control. PloS One. (2013) 8:e56615. doi: 10.1371/journal.pone.0056615
51. Zhang X, Kedar S, Lynn MJ, Newman NJ, and Biousse V. Homonymous hemianopia in stroke. J Neuroophthalmol. (2006) 26:180–3. doi: 10.1097/01.wno.0000235587.41040.39
52. Schow T, Harris P, Teasdale T, and Rasmussen M. Evaluation of a four month rehabilitation program for stroke patients with balance problems and binocular visual dysfunction. NeuroRehabilitation. (2016) 38:331–41. doi: 10.3233/NRE-161324
53. Singh T, Perry CM, Fritz SL, Fridriksson J, and Herter TM. Eye movements interfere with limb motor control in stroke survivors. Neurorehabil Neural Repair. (2018) 32:724–34. doi: 10.1177/1545968318790016
54. Ali M, Hazelton C, Lyden P, Pollock A, and Brady M. Recovery from poststroke visual impairment: evidence from a clinical trials resource. Neurorehabil Neural Repair. (2013) 27:133–41. doi: 10.1177/1545968312454683
55. Motta K, Lee H, and Falkmer T. Post-stroke driving: examining the effect of executive dysfunction. J Saf Res. (2014) 49:33–8. doi: 10.1016/j.jsr.2014.02.005
56. Devos H, Hawley CA, Conn AM, Marshall SC, and Akinwuntan AE. Driving after stroke. In: Platz T, editor. Clinical Pathways in Stroke Rehabilitation: Evidence-based Clinical Practice Recommendations. Springer, Cham (CH (2021). p. 243–60.
57. Pedersen SG, Løkholm M, Friborg O, Halvorsen MB, Kirkevold M, Heiberg G, et al. Visual problems are associated with long-term fatigue after stroke. J Rehabil Med. (2023) 55:jrm00374. doi: 10.2340/jrm.v55.4813
58. Pascoe MC, Crewther SG, Carey LM, and Crewther DP. Inflammation and depression: why poststroke depression may be the norm and not the exception. Int J Stroke. (2011) 6:128–35. doi: 10.1111/j.1747-4949.2010.00565.x
59. Hall CM and McAnany JJ. Luminance noise as a novel approach for measuring contrast sensitivity within the magnocellular and parvocellular pathways. J Vis. (2017) 17:5. doi: 10.1167/17.8.5
60. Shoshina I, Isajeva E, Mukhitova Y, Tregubenko I, Khan’ko A, Limankin O, et al. The internal noise of the visual system and cognitive functions in schizophrenia. Proc Comput Sci. (2020) 169:813–20. doi: 10.1016/j.procs.2020.02.158
61. Park WJ, Schauder KB, Zhang R, Bennetto L, and Tadin D. High internal noise and poor external noise filtering characterize perception in autism spectrum disorder. Sci Rep. (2017) 7:17584. doi: 10.1038/s41598-017-17676-5
62. Backhaus W, Braaß H, Higgen FL, Gerloff C, and Schulz R. Early parietofrontal network upregulation relates to future persistent deficits after severe stroke-a prospective cohort study. Brain Commun. (2021) 3:fcab097. doi: 10.1093/braincomms/fcab097
63. Lee J-I, Gemerzki L, Weise M, Boerker L, Graf J, Jansen L, et al. Retinal layers and visual conductivity changes in a case series of microangiopathic ischemic stroke patients. BMC Neurol. (2020) 20:333. doi: 10.1186/s12883-020-01894-y
64. Pitcher D and Ungerleider LG. Evidence for a third visual pathway specialized for social perception. Trends Cogn Sci. (2021) 25:100–10. doi: 10.1016/j.tics.2020.11.006
65. Mangiardi M, Bonura A, Iaccarino G, Alessiani M, Bravi MC, Crupi D, et al. The pathophysiology of collateral circulation in acute ischemic stroke. Diagn (Basel). (2023) 13(14):2425. doi: 10.3390/diagnostics13142425
66. Robinson T, Zaheer Z, and Mistri AK. Thrombolysis in acute ischaemic stroke: an update. Ther Adv Chronic Dis. (2011) 2:119–31. doi: 10.1177/2040622310394032
Keywords: acute ischemic stroke, eye-hand coordination, visual field, visual acuity-in-noise, vision, visuomotor function, Melbourne rapid field-neural (MRFn), UNSW Lee-Ryan Eye-Hand Coordination Test (SLURP)
Citation: Wijesundera CN, Crewther SG, Wijeratne T and Vingrys AJ (2025) Persistent visual impairments following mild-to-moderate ischemic stroke. Front. Ophthalmol. 5:1505836. doi: 10.3389/fopht.2025.1505836
Received: 03 October 2024; Accepted: 21 April 2025;
Published: 26 May 2025.
Edited by:
Nasif Zaman, Smith-Kettlewell Eye Research Institute, United StatesReviewed by:
Christopher Charles Glisson, Michigan State University, United StatesAbhishek Mahesh Appaji, BMS College of Engineering, India
Copyright © 2025 Wijesundera, Crewther, Wijeratne and Vingrys. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheila Gillard Crewther, cy5jcmV3dGhlckBsYXRyb2JlLmVkdS5hdQ==
 Chamini Niroshika Wijesundera
Chamini Niroshika Wijesundera Sheila Gillard Crewther
Sheila Gillard Crewther Tissa Wijeratne
Tissa Wijeratne Algis J. Vingrys
Algis J. Vingrys