- 1University of Southern California (USC) Roski Eye Institute, Keck School of Medicine of the University of Southern California, Los Angeles, CA, United States
- 2Eyesthetica, Oculofacial and Cosmetic Surgery Associates, Los Angeles, CA, United States
We report a case of thyroid eye disease (TED) reactivation, review the literature, and raise awareness of severe ophthalmopathy and myositis following the initiation of cancer treatment with an immune checkpoint inhibitor (ICI). In this case, after starting nivolumab, a 68-year-old woman with a past medical history of stage 2C uterine carcinoma status post-hysterectomy and past ocular history of thyroid eye disease developed ophthalmoplegia, proptosis, decreased color vision, optic disc hemorrhage, and ocular inflammation. Halting nivolumab and starting 2 weeks of intravenous steroids, one dose of teprotumumab, and low-dose orbital radiation resulted in an improvement in her orbitopathy. ICIs have become popular in oncologic treatment regimens, but they can have serious adverse effects including thyroiditis, Hashimoto’s hypothyroidism, or Graves’ disease complicated by TED. Multiple cases have been reported in the literature of both the reactivation of TED and the presentation of a TED-like orbital inflammation, which we summarize here. Awareness of orbital inflammation due to ICI use is critical, and ICIs should be used with caution in patients with a history of thyroid disease due to the risk of TED reactivation.
Introduction
In recent years, immune checkpoint inhibitors (ICIs) have become a mainstay of cancer management. ICIs are currently used as adjuvant and neo-adjuvant therapies as well as primary chemotherapy for metastatic cancers and have been approved to treat over 20 types of tumors thus far (1). Like all immunotherapies, ICIs like ipilimumab and tremelimumab, which target cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and nivolumab and pembrolizumab, which target programmed cell death protein 1 (PD-1), are associated with adverse effects, including hypothyroidism, Graves’ disease, and painless thyroiditis (2–4). Herein, we describe a case of severe ophthalmopathy following initiation of cancer treatment with nivolumab and outline previous cases of similar orbital inflammation that coincided with ICI use. Written informed consent was obtained from the patient for the publication of this information in accordance with the Declaration of Helsinki.
Case report
The patient is a 68-year-old woman with a past medical history of stage 2C uterine carcinoma status post-hysterectomy and lymph node dissection 3 months prior and past ocular history of thyroid eye disease (TED) s/p bilateral transantral orbital decompression (2004) (Figure 1A) and teprotumumab infusion (complete cycle of eight infusions in 2023). She presented with 1 week of double vision, proptosis, and increasingly severe bilateral orbital pain after two cycles of chemotherapy with nivolumab for endometrial cancer management. On exam, her visual acuity was 20/25 on the right and 20/20 on the left, intraocular pressure was normal, and pupils were round and reactive bilaterally with no afferent pupillary defect. She had right-sided color desaturation, measured using Ishihara color plates. Her extraocular movements were −4 in all directions, and she exhibited proptosis (Hertel exophthalmometry base 100; eye protrusion from orbital rim 30 mm OU) and restrictive right upper eyelid ptosis (Figure 1B). The external exam was remarkable for brow fullness and edema, eyelid swelling, lid lag, conjunctival injection, caruncle inflammation, and erythema [Clinical Activity Score (CAS) 5]. A dilated funduscopic exam revealed a right-sided inferior splinter disc hemorrhage. Computed tomography of the head revealed enlargement of extraocular muscle bellies sparing the muscle tendons bilaterally, as well as taut optic nerves without globe tenting bilaterally, consistent with thyroid eye disease rather than infection or metastasis (Figure 1D). Her chemotherapy was stopped, and she received multiple doses of intravenous methylprednisolone of 1.25 g and was referred to radiation oncology for low-dose orbital radiation. On a follow-up visit, she presented with dysphagia of unclear etiology and was admitted to the hospital for a higher level of care where she received one dose of teprotumumab for new-onset left optic neuropathy. At 1 month after presentation, the combination of 2 weeks of IV steroids, one dose of teprotumumab, and two doses of 2-Gy photonic orbital radiation led to improvement in her eyelid swelling and erythema, decreased lid lagophthalmos, reduced conjunctival injection, disappearance of the disc hemorrhage, resolution of her orbital pain, and improvement in extraocular movements. Her proptosis decreased (Hertel exophthalmometry base 100; eye protrusion from orbital rim 25 mm OD and 26 mm OS; Clinical Activity Score 1; Figure 1C). She opted to forgo any additional chemotherapy for her uterine cancer.
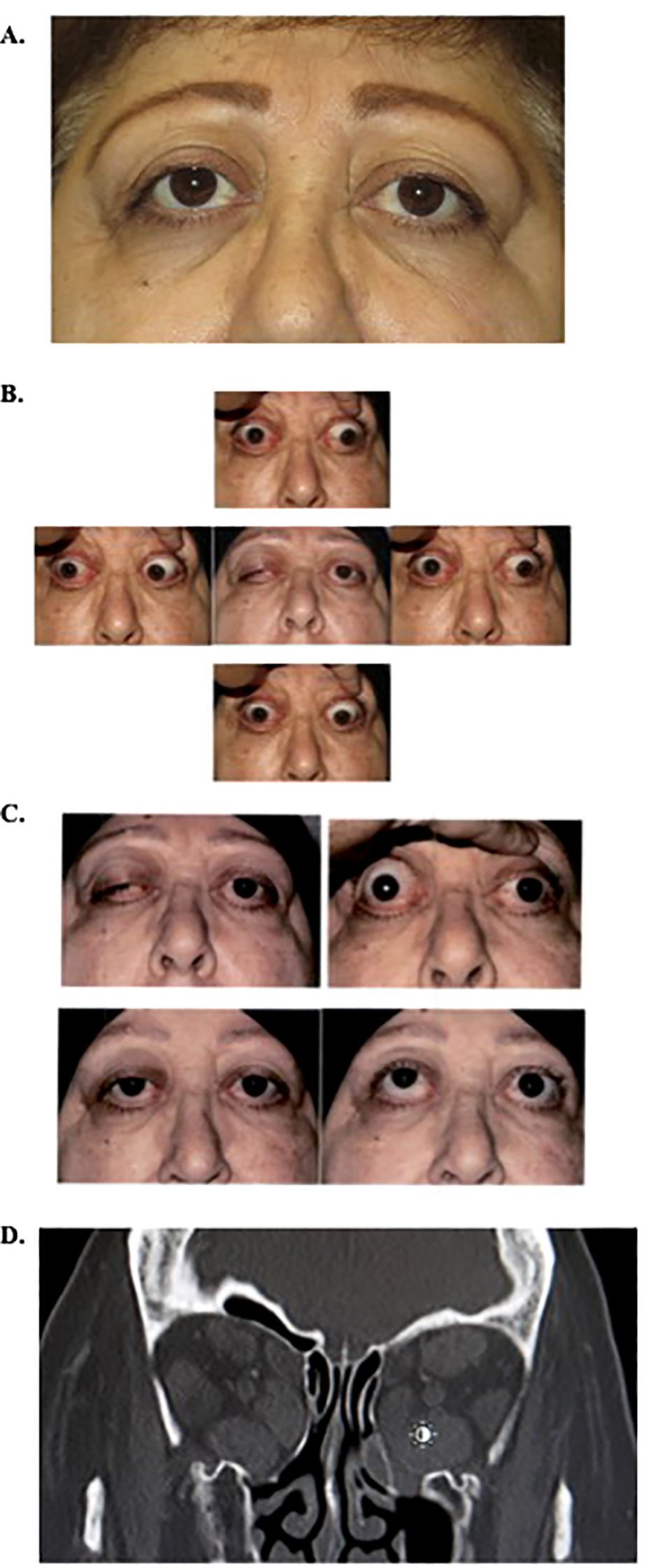
Figure 1. (A) External photo prior to the current episode of TED reactivation, after bilateral graded transantral decompression in 2004, Clinical Activity Score of 0. (B) External photos on initial presentation showing reactivation of TED demonstrating ptosis, proptosis, and decreased extraocular motility in all fields of gaze. (C) External photos before and 4 weeks after initial presentation following IV methylprednisolone, one dose of teprotumumab, and two doses of low-dose orbital radiation. Photos in primary and upgaze. (D). Coronal image, computed tomography of orbits on current presentation, demonstrating enlargement of extra-ocular muscles. Note previous graded transantral orbital decompression with infracture of medial wall and orbital floor removal. TED, thyroid eye disease.
Previous reports of orbital inflammation
Along with this case, there are rare case reports of a direct link between ICI use and the reactivation of TED, including those resulting in vision loss (Table 1). Similar to our reported case, multiple patients experienced severe reactivation of thyroid eye disease with nivolumab, a PD-1 inhibitor. While one patient developed eyelid retraction and ophthalmoplegia with minimal inflammation amounting to a CAS of 1 and thyroid function testing consistent with a hyperthyroid state, other patients developed more active inflammatory orbitopathies without any abnormalities in thyroid function testing. None of these patients had a prior history of thyroid eye disease, but the majority demonstrated diffuse enlargement of all extraocular muscles on imaging (5–7).
Pembrolizumab is an additional immune checkpoint inhibitor potentially implicated in the activation of TED, and its mechanism of action entails blocking PD-1. One case of pembrolizumab use was associated with rapid development of Graves’ orbitopathy within 24 hours of its initiation, combined with elevated thyroid-stimulating hormone receptor antibodies, in a patient who had actually developed a similar presentation with ipilimumab use in the past (8).
The majority of reported cases of inflammatory orbitopathies in the setting of ICI use involved ipilimumab, a CTLA-4 inhibitor. One case involved complete ophthalmoplegia without other inflammatory signs, while thyroid function tests remained normal (9). In another patient, ipilimumab infusions triggered diffuse periorbital edema and ophthalmoplegia correlated with enlargement of extraocular muscles on imaging, with both lateral recti being most enlarged, distinct from the patterns of muscle enlargement seen most commonly in thyroid eye disease (10). Another patient who started ipilimumab as part of her cancer treatment developed proptosis, conjunctival chemosis and injection, and ophthalmoplegia along with extensive muscle and tendon enlargement on computed tomography imaging but was actually found to be hypothyroid (11). In many cases involving ipilimumab, imaging also revealed tendon-involving muscle enlargement, as opposed to the tendon-sparing form classically seen in TED. Case reports of inflammatory orbitopathies after ipilimumab use were associated with both TED activation and orbital syndromes that did not coincide with elevated thyroid function and antibody tests (12–14).
Finally, some reports of thyroid eye disease activation have been described in patients who underwent treatment with tremelimumab. One patient developed a hyperthyroid state, confirmed by serum testing, and orbital inflammation with a CAS of 6 (6). Another patient developed periorbital edema and proptosis associated with enlargement of extraocular muscles on imaging, correlating with elevated thyroid function tests consistent with hyperthyroidism (15).
Discussion
This case report and literature review highlights the risk of developing orbital inflammation secondary to the use of immune checkpoint inhibitors. Previous studies have suggested that CTLA-4 is implicated in the pathogenesis of Graves’ disease and that PD-L1 expression is elevated in Hashimoto’s hypothyroidism (1, 16, 17). (18) Inhibiting PD-1 can also result in thyroid antibody production by B cells (2). Other studies have determined that there may be a direct connection between the increased T-cell activity caused by ICIs and the development of orbital myositis, while some papers theorize that there may be a process similar to that of IgG-4-related disease (14). The mechanism underlying thyroid dysfunction, thyroid eye disease, or any inflammatory orbitopathies after ICI use is not yet well understood. Newer research has demonstrated that patients with thyroiditis triggered by ICI use have significantly larger cell counts of T lymphocytes (19). Similar studies have shown that other cell lines tend to proliferate in the setting of ICI-associated thyroiditis as well, including monocytes and natural killer cells, although their role in the pathogenesis has not yet been specifically delineated (20). We suggest caution in using ICIs in patients with a history of thyroid eye disease as well as multi-disciplinary collaboration across oculofacial plastic surgery, endocrinology, and oncology to co-manage these patients. Further research should investigate the pathophysiology and mechanisms that underlie this phenomenon to better understand how to counsel patients who may be starting an ICI.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
Author contributions
SM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AP: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. KH: Methodology, Writing – original draft, Writing – review & editing. MB: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan S, Day D, Nicholls SJ, Segelov E. Immune checkpoint inhibitor therapy in oncology. JACC Cardio Oncol. (2022) 4:579–97. doi: 10.1016/j.jaccao.2022.09.004
2. Chera A, Stancu AL, Bucur O. Thyroid-related adverse events induced by immune checkpoint inhibitors. Front Endocrinol. (2022) 13. doi: 10.3389/fendo.2022.1010279
3. Baek HS, Jeong C, Shin K, Lee J, Suh H, Lim DJ, et al. Association between the type of thyroid dysfunction induced by immune checkpoint inhibitors and prognosis in cancer patients. BMC Endocr Disord. (2022) 22:89. doi: 10.1186/s12902-022-01004-8
4. Chau CYC, Shih KC, Chow LLW, Lee VHF. Considerations for use of immune checkpoint inhibitors in cancer therapy for patients with co-existing thyroid eye disease. Ophthalmol Ther. (2021) 10:5–12. doi: 10.1007/s40123-020-00317-y
5. Campredon P, Imbert P, Mouly C, Grunenwald S, Mazières J, Caron P. Severe inflammatory ophthalmopathy in a euthyroid patient during nivolumab treatment. Eur Thyroid J. (2018) 7:84–7. doi: 10.1159/000485742
6. Sagiv O, Kandl TJ, Thakar S, Thuro B, Busaidy N, Cabanillas M, et al. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthal Plast Reconstr Surg. (2019) 35:50–2. doi: 10.1097/IOP.0000000000001161
7. Park BJ, Warning AW, Akella SS. Checkpoint inhibitor-related myasthenia-myocarditis-myositis overlap syndrome in the orbit. Orbit Amst Neth. (2024) 2:1–7. doi: 10.1080/01676830.2024.2351519
8. Rhea L, Yoon JW, Jang S. Rapid development of graves’ Ophthalmopathy after treatment with ipilimumab and recurrence with pembrolizumab in a patient with previously treated graves’ Disease. J Oncol Pract. (2018) 14:747–9. doi: 10.1200/JOP.18.00442
9. McElnea E, Ní Mhéalóid A, Moran S, Kelly R, Fulcher T. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit Amst Neth. (2014) 33:424–7. doi: 10.3109/01676830.2014.949792
10. Jebaraj AP, Etheridge TJ, Winegar BA, Marx DP. Ipilimumab-related orbitopathy: a case report. Orbit. (2024) 43:100–4. doi: 10.1080/01676830.2022.2074049
11. Sheldon CA, Kharlip J, Tamhankar MA. Inflammatory orbitopathy associated with ipilimumab. Ophthal Plast Reconstr Surg. (2017) 33:S155–8. doi: 10.1097/IOP.0000000000000509
12. Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. (2011) 27:e87–88. doi: 10.1097/IOP.0b013e3181ef72a1
13. Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biologic therapy. Eur J Endocrinol Eur Fed Endocr Soc. (2011) 164:303–7. doi: 10.1530/EJE-10-0833
14. Hassanzadeh B, DeSanto J, Kattah JC. Ipilimumab-induced adenohypophysitis and orbital apex syndrome: importance of early diagnosis and management. Neuro-Ophthalmol. (2017) 42:176–81. doi: 10.1080/01658107.2017.1368090
15. Camacho LH. Novel therapies targeting the immune system: CTLA4 blockade with tremelimumab (CP-675,206), a fully human monoclonal antibody. Expert Opin Invest Drugs. (2008) 17:371–85. doi: 10.1517/13543784.17.3.371
16. Shu X, Shao Y, Chen Y, Zeng C, Huang X, Wei R. Immune checkpoints: new insights into the pathogenesis of thyroid eye disease. Front Immunol. (2024) 15:1392956. doi: 10.3389/fimmu.2024.1392956
17. Yu CW, Yau M, Mezey N, Joarder I, Micieli JA. Neuro-ophthalmic complications of immune checkpoint inhibitors: A systematic review. Eye Brain. (2020) 12:139–67. doi: 10.2147/EB.S277760
18. Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol. (2022) 187:G1–G21. doi: 10.1530/EJE-22-0689
19. Kotwal A, Gustafson MP, Bornschlegl S, Kottschade L, Delivanis DA, Dietz AB, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid. (2020) 30:1440–50. doi: 10.1089/thy.2020.0075
20. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. (2017) 102:2770–80. doi: 10.1210/jc.2017-00448
Keywords: immune checkpoint inhibitor, inflammatory orbital disease, thyroid eye disease (TED), thyroid eye disease treatment, PD-1 inhibitor, CTLA-4 inhibitor
Citation: Madala S, Parikh A, Hong K and Burnstine M (2025) Case Report: Development of severe inflammatory orbitopathy after immune checkpoint inhibitor initiation. Front. Ophthalmol. 5:1574643. doi: 10.3389/fopht.2025.1574643
Received: 11 February 2025; Accepted: 03 April 2025;
Published: 03 June 2025.
Edited by:
Alon Kahana, Oakland University William Beaumont School of Medicine, United StatesReviewed by:
Olivia Cheng, Beaumont Hospital, Beaumont Health, United StatesCopyright © 2025 Madala, Parikh, Hong and Burnstine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Burnstine, bWljaGFlbEBleWVzdGhldGljYS5jb20=
 Samantha Madala
Samantha Madala Alomi Parikh1,2
Alomi Parikh1,2