- 1Ophthalmology Department, University Medical Center Utrecht, Utrecht, Netherlands
- 2Department of Ophthalmology, Bergman Clinics, Naarden, Netherlands
- 3Amsterdam University Medical Center (UMC), Vrije Universiteit Amsterdam, Ophthalmology, Amsterdam, Netherlands
- 4Amsterdam Public Health Research Institute, Amsterdam, Netherlands
- 5Easee BV, Amsterdam, Netherlands
Purpose: This study was aimed at identifying barriers and opportunities to use a self-administered online refractive eye test by various stakeholders of a pediatric vision screening program.
Methods: This qualitative study performed semi-structured interviews with myopic children and their parents, eye care professionals, and policymakers. Three topic lists were developed, delineating themes to identify gaps, barriers, and opportunities. Interviews were anonymously recorded, transcribed, and coded using thematic analysis. Quantitative data was acquired from a concomitant clinical validation study.
Results: In total, 14 interviews were conducted, of which seven were with children and their parents, four with eye care professionals, and three with policymakers. The patients and parents were positive about the instructions and age appropriateness. They noted that the test could be designed as more child-friendly and preferred receiving feedback during the test. Eye care professionals and policymakers saw potential for using the test in children aged ≥12 without high refractive errors, yet they also underlined the false-positives rates, impacting care demand and costs. The population refraining from participation was expected to have higher health gains, yet including them was expected to be challenging without facilitating awareness.
Conclusions: This qualitative study shows the perspectives for an online pediatric refractive screening. The patients and parents were open to self-administered screening and suggested improvements. The eye care professionals and policymakers were receptive to screening but also cautious, highlighting costs and scientific reliability. For better implementation, the policymakers underlined the relevance of the screening criteria, while the eye care professionals recommended targeting a specific population at risk that benefits most rather than screening the whole population.
Introduction
Recently, a web-based test for self-assessing visual acuity and refractive errors in myopic children was evaluated for its reliability (1). The study demonstrated that children could successfully complete the test with parental supervision and highlighted areas for future improvement in accuracy. However, to understand the broader societal value of such a tool, it is crucial to explore the barriers and opportunities identified by stakeholders directly involved (2). To address this, we conducted a follow-up study to gather insights from stakeholders in the context of an online screening program.
Screening for myopia in children could be relevant as it increases awareness and potentially facilitates early detection (3). Thereby, myopic progression could be delayed, retaining as much sight and reducing as much complications as possible (4–6). Traditionally, this is achieved through physical screening programs in schools or community settings. However, these programs are demanding on resources and healthcare personnel. A digital remote self-assessment could serve as an auxiliary scalable and scalable tool.
This study explores the perspectives of patients (children and their parents), eye care professionals, and policymakers to use a digital test as a screening tool to detect myopia in a pediatric population aged 6 years or older. In this context, policymakers are defined as public decision-makers at governmental or organizational levels who are responsible for designing, implementing, and overseeing health programs, including those related to vision screening. Their role is essential in establishing guidelines, securing funding, integrating screening tools into national or regional health strategies, and promoting equitable access to care. By understanding their perspectives—alongside those of clinicians and end-users—this study aims to identify barriers and opportunities for the adoption of a remote screening program for refractive errors using an online eye test in The Netherlands. Thereby, it assesses stakeholders’ perspectives on how a digital screening could contribute to support early detection and increased awareness of myopia.
Methods
This qualitative interview study originated from a clinical method comparison study evaluating an independent digital visual acuity and refractive error assessment in myopic children. The Medical Ethics Review Committee Erasmus MC approved this study (MEC-2021-0816). This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participating children (called patients in this paper) and from their parent(s) or caregiver(s). The method of the comparison study was previously described in depth (1). Besides the methodology, we described the validation and limitations of using this application as a way to measure refractive error. In summary, in the comparison study, we invited children ≥6 years to perform web-based eye tests twice at home, assisted by a parent. Data collection occurred between September 1, 2022 and June 30, 2023. The participants were invited for the second test 5 days after their first home test. To perform the test, the participants were in possession of a smartphone and tablet or computer (Figure 1). The patients were recruited from the myopia control clinic at the Erasmus Medical Centre and Radboud Medical Centre; both university clinics are located in The Netherlands. This study gave rise to qualitative research questions on whether the test could be used as a screening program. Thus, patients and their parents, eye care professionals, and policymakers were invited to expand on their perspectives in the current study. Interviews were performed between August 1 and November 1, 2023.
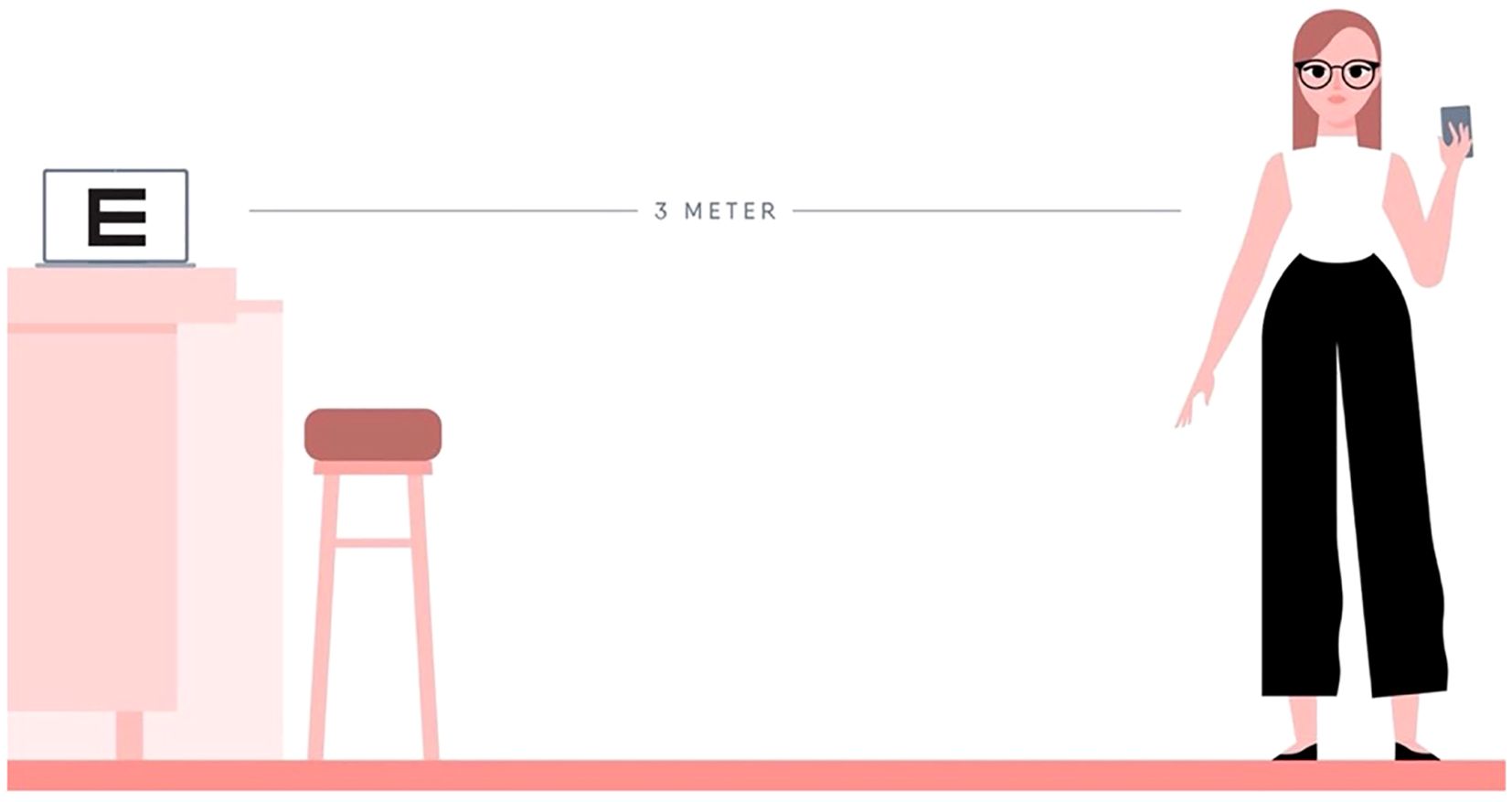
Figure 1. Online test set-up. A computer, distance of 3 m, and a phone are required to conduct the test.
Online eye test
The online eye test was developed by Easee BV, Amsterdam, The Netherlands. The self-administered test measured the visual acuity and refractive error at home by using a phone and a laptop. In minors, the test was supervised by a parent or caregiver. The preparation of the test included several steps (Figure 1). First, the computer screen was calibrated so that optotypes were shown in the correct size. Then, the laptop was positioned at a 3-m distance and connected to a mobile phone. The patients were instructed to use a measuring tape or, alternatively, they could provide their shoe size and the number of heel-to-toe steps from the screen until a distance of 3 m was measured by the test. The patients were instructed to place a chair at that spot to ensure that they remained at the correct distance. Afterward, the optotypes and astigmatism dials were displayed on the screen. The phone functions as a remote control. Visual, written, and audio instructions were given before, during, and after the test. As a reference, the test time including set-up phase was previously assessed in an elderly population to be, on average, 18 min (SD ± 4) for both eyes (up to 93 years old; mean, 71 years) (7).
Data collection
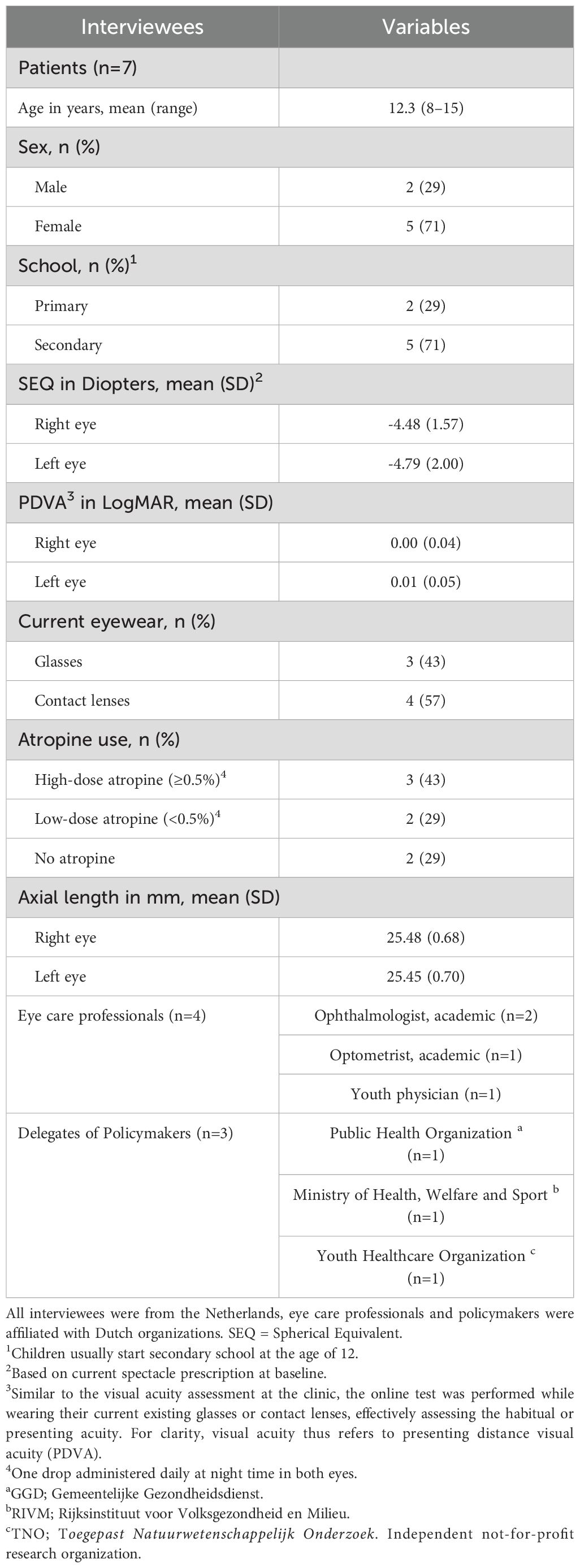
The identified stakeholder groups were divided into two groups (1): patients and parents and (2) eye care professionals and policymakers. All participants from the concomitant study who performed the test within the last 3 months were invited via email for an interview. Similar to the visual acuity assessment at the clinic, the online test was performed while wearing their current existing glasses or contact lenses, effectively assessing the habitual or presenting acuity. For clarity, visual acuity thus refers to presenting distance visual acuity (PDVA). Data on the demographics (Table 1) of all interviewees were collected. For patients, these were age, sex, school (primary, secondary), the spherical equivalent (diopters), the presenting distance visual acuity (LogMAR), current eyewear, use of atropine medication, and axial length of the eyes. For eye care professionals and delegates of policymakers, these were with regard to their profession and relevant background. The interviewees were interviewed in Dutch, and the interview took place within 6 months after performing the online test.
The eye care professionals and policymakers were selected based on their status as key opinion leaders in the field or their affiliation with relevant organizations, as identified by the research team or other key opinion leaders who were interviewed. Three topic lists were developed, delineating themes and corresponding questions designed to identify gaps, barriers, and opportunities from their respective perspectives. The included topics were general perspectives, user experiences and social influences, trust in telemedicine and screening, clinical perspectives, and pitfalls, challenges, and effects of the screening program. This was subsequently supervised by experts in qualitative research from Amsterdam UMC (RN, HA, PR). Data was collected through semi-structured interviews to allow leeway for the interviewee to accentuate and elaborate on topics that they found important. This also gave the interviewees the possibility to introduce their own topics, if they were so inclined. All interviews were conducted via secured video calls (CZ and JC, both medical doctors) and direct supervision was provided (PR, a PhD–epidemiologist, with experience in qualitative research). All have experience in the field of ophthalmology. At the beginning of the interviews, the interviewees were provided with a presentation as a reminder of how the online eye test and the screening program worked. Quotes exemplifying relevant viewpoints of the interviewees were reported. These quotes were carefully translated into English by JC and CZ and subsequently supervised to ensure translational accuracy.
Data analysis
Interviews were anonymously recorded, edited transcription was applied, and transcripts were imported into NVivo (release 14.23.0). The transcripts were analyzed thematically per stakeholder group (CZ and JC). A codebook was developed in advance of transcription based on the topic lists. Coding insights that emerged during transcription were added to the code book. The first transcript from each stakeholder was coded by both researchers (CZ and JC) to identify coding discrepancies, which were resolved through consultation with a third researcher if applicable (RW—for the codebook, see Supplementary Table S1). Descriptive statistics were analyzed using Microsoft Excel Version 16.89.1. The Consolidated Criteria for Reporting Qualitative Research (COREQ) was used to ensure a comprehensive and transparent description of the processes and details involved in both data collection and data analysis (8).
Results
A total of 14 interviews were conducted, including myopic children and their parents (n = 7 children with nine parents), eye care professionals (n = 4), and policymakers (n = 3). The children were all Dutch and, on average, 12 years old (see Table 1 for the descriptions). The identified themes were divided by (1) perspectives of patients and parents and (2) perspectives of eye care professionals and policymakers.
Perspectives of patients and parents
Theme 1.1: Usability of the online test
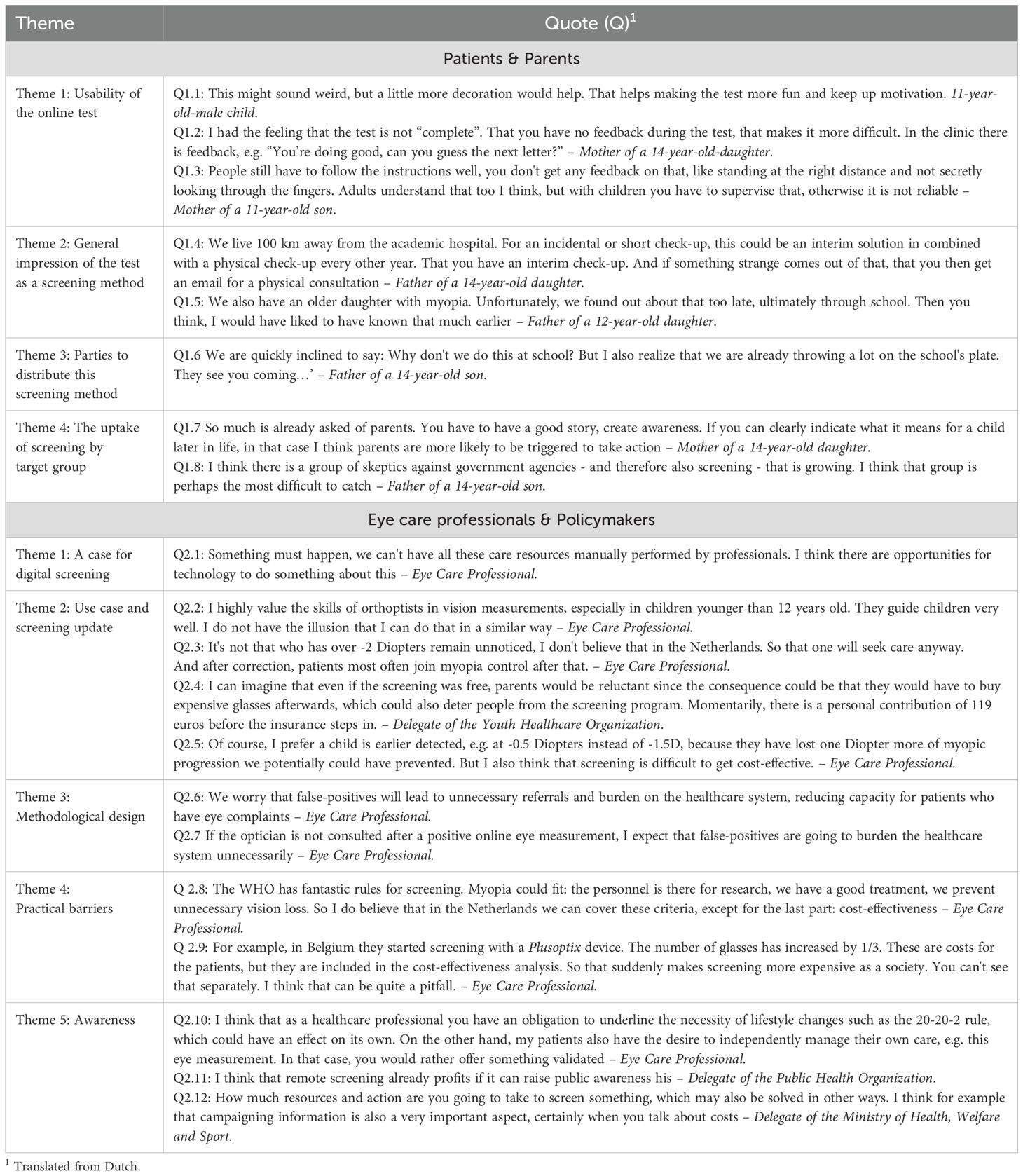
The users thought that the instructions were clear. Most thought that the test was age appropriate, yet some noted that the test could be more child-friendly (Table 2, quote 1.1). All children finished the test with adequate motivation according to the parents, and all parents mentioned that supervision by them was necessary. The children needed help in setting up the test, e.g., finding the website link and measuring the 3-m distance. A possible improvement mentioned by one parent was that feedback could be given on how well the participants adhere to the test instructions, e.g., distance and staying motivated (quotes 1.2 and 1.3). None had problems with the fact that an eye care professional was not physically present during the test.
Theme 1.2: The test as a screening method
All parents had a positive first impression about using the test as a screening method. Some mentioned it to be a relief in such a way that they might have to travel less to the hospital (quote 1.4). Others noted how difficult it can be to recognize vision loss in children and how their child possibly could have benefitted from earlier detection (quote 1.5). All parents mentioned that they trusted the outcome of the test, even if the outcome would be different from what they would have expected.
Theme 1.3: Parties that could distribute the screening method
Most parents named the Dutch Municipal Health Services (MHS; Gemeentelijke Gezondheidsdienst) and schools as best options to supervise and distribute the screening program. The MHS was said to be trustworthy, transparent, and well known. The parents were accustomed to receiving information from the MHS. Both parents and patients named schools as a relevant location for supervision and distribution as well since they claimed that teachers are among the first to notice vision loss in children (e.g., difficulties reading the blackboard). Moreover, some children mentioned that school would be the most fun place to take the test, yet most parents also noted that primary schools already are under pressure due to a lack of personnel and might not be able to safekeep medical data (quote 1.6).
Theme 1.4: Use case and the uptake of screening by target group
All parents underlined that a part of the target group was expected to refrain from screening, further defined as “non-participators”. They found it important that extra focus and resources would be spent on reaching these non-participators. The reasons for non-participation in their opinion were either practical or motivational. The anticipated practical reasons mentioned were language barriers, low socioeconomic status, or simply being preoccupied. The anticipated motivational reasons were limited awareness on the consequences of myopia, health beliefs that differ from evidence-based medicine, and distrust in safekeeping of medical data (quotes 1.7 and 1.8). The parents noted that increasing awareness might reduce the number of non-participants.
Perspectives of eye care professionals and policymakers
Theme 2.1: The test as a screening method
All stakeholders were supportive of digital screening (quote 2.1). If scientifically proven reliable in children, all eye care professionals mentioned to have trust in the online test being performed by patients and their parents independently of the support of a healthcare professional. They noted that the screening flow was accessible (i.e., steps taken to complete the test), and it especially might have potential in places with a constraint access to care, such as low-income countries.
Theme 2.2: Use case and the uptake of screening by target group
All eye care professionals underlined age and the refractive errors as important factors to determine the use case. First, some noted that it is difficult to measure vision in young children (<12 years) due to a shorter attention span, stronger accommodative reflex, and an increased risk of amblyopia (quote 2.2). They suggest that patients ≥12 years were more feasible for screening. Second, patients with higher refractive errors (>2 diopters, D) were expected to have visual complaints and are therefore expected to seek care by themselves, making screening less effective (quote 2.3). The eye care professionals feared that the detection of small refractive errors (e.g., 0.5 D) could result in the unnecessary prescription of glasses, yet they also noted that early detection prevents myopia progression (quotes 2.4 and 2.5). It was additionally suggested that screening might be more effective if solely patients with risk factors were screened, e.g., patients with myopic parents. Lastly, they found it important to identify non-participators, as they argued that this might be a group with higher potential health gains. In addition to the previous arguments given by children and their parents, they expected that unfamiliarity of the Dutch healthcare system could result in non-participation.
Theme 2.3: Methodological challenges
All eye care professionals and policymakers underlined the risk for high false-positives (being incorrectly classified as in need of new glasses), potentially resulting in more referrals, unnecessary new glasses, increased costs, and a higher demand for healthcare (quotes 2.6, 2.8, and 2.9). To reduce this effect, the eye care professionals advised that positive tests should be re-evaluated by an optician before being referred (quote 2.7).
Theme 2.4: Practical barriers and parties that could distribute the screening method
The identified barriers were the costs of screening and the supervising organization. First, when aligning the screening with the World Health Organization (WHO) and Wilson & Junger screening criteria (9, 10), this program was expected to comply with most criteria, though costs were expected to be more challenging (quote 2.8). The eye care professionals and policymakers anticipated a rise in costs if the false-positive rates were not adequately controlled. The reasons mentioned were increased demand for care and the unnecessary need for patients to purchase glasses (quote 2.9). Second, the eye care professionals and policymakers consistently mentioned two options on how the participants could best be invited for screening: via the MHS and schools. The MHS was preferred, as it is considered trustworthy and safe and has contact information of most children. The schools were advised to be complementary.
Theme 2.5: Awareness
Creating awareness was frequently mentioned to be relevant since myopia is significantly impacted by lifestyle choices (quote 2.10). The stakeholders mentioned that the online screening was expected to result in a complementary increased awareness effect (quote 2.11), yet they also underline that a comparable awareness could also be created with a campaign to inform people about the consequences of myopia instead of screening (quote 2.12).
Discussion
In this qualitative interview study, the barriers and opportunities of using a remote digital screening program for pediatric myopia in The Netherlands, as experienced by myopic patients (aged ≥6 years), their parents, eye care professionals, and policymakers, were analyzed. The test is self-administered and thereby detects refractive errors at home. The results were thematically reported.
Regarding usability, the patients and parents were positive about the instructions and age appropriateness of the test. As improvements, they suggested that the layout of the test could be made more fun for children and they preferred receiving feedback during the test on how well the test instructions were followed (e.g., maintaining correct distance). In line with literature, making a vision test more fun with sounds, cartoons, and other elements is known as “gamification” and can positively impact a child’s motivation, without affecting outcomes (11, 12). Interestingly, none of the interviewees saw it as a barrier that the online test is self-administered (in the direct physical absence of a healthcare professional) as opposed to measuring refractive errors in the clinic. Although this was anticipated to be a barrier, our findings align with literature, concluding that patients are not reluctant to the increased responsibilities due to telemonitoring, which are shifted from healthcare professionals to patients (13).
All interviewees were supportive of the online test as a screening method. The parents noted how difficult it can be to recognize vision loss in children and how their child could have possibly benefitted from earlier detection. Moreover, the patients specifically reported it to be a relief that they might have to travel less to the hospital. High patient satisfaction and acceptance rates are often reported in studies driven by increased accessibility and reduced travel costs and time (14). The eye care professionals and policymakers were also supportive due to the high demand for eye care, highlighting the need to explore alternative options. However, they stress the importance of clearly shown scientific reliability and feasibility. In addition, literature reports that healthcare professionals repeatedly mention other barriers to telemedicine, including the need for trained staff, resistance to change, and concerns about the perception of impersonal care (15).
Regarding the ideal party to distribute the screening method, the MHS was found to be the most reliable partner for the supervision of the screening program by all interviewees since this institution is generally considered trustworthy, transparent, and safe and has contact information of most children. Though all interviewees mentioned schools as a good secondary option, literature reports that telemedicine delivered via schools are much more common, with its popularity steadily increasing (16). This suggests that both options are a valid approach for distribution.
Some eye care professionals noted that it is difficult to measure myopia in young children (<12 years) and therefore this age should be considered an exclusion criterion. Moreover, they expected that screening would be less effective on higher refractive errors, as patients would seek care independently of the screening program due to visual complaints. The experts feared that the detection of small refractive errors (e.g., 0.5 D) could result in the prescription of unnecessary glasses, yet they also argued that early detection combined with, e.g., lifestyle changes could prevent myopic progression compared to no detection or treatment. In addition, all stakeholders had remarks on the fact that they expected that a part of the target population was expected to refrain from screening (i.e., non-participators) even though this is expected to be a group with high potential health gains. Interestingly, an association between digital exclusion and social deprivation with monitoring to smartphone-based self-monitoring has not been found in other studies, suggesting that vision self-monitoring is accessible (17). Nevertheless, the reasons mentioned by the interviewees for non-participation were categorized into those facing structural barriers and those opting out due to personal beliefs and attitudes. This is in line with the study of Vongsachang et al. (18), where the authors examined factors related to decreasing participation in school-based vision programs. Categorizing non-participators helps in finding targeted solutions. Regarding structural barriers, a lower socioeconomic status has long been associated with decreased health, access to healthcare, and attendance to screening programs, even when the financial barriers are minimized (19–23). Though digital health is known to positively influence health equity when implemented properly, recognizing non-participators who are vulnerable remains essential (24, 25). Non-participation due to personal beliefs is often reported to occur due to mistrust in evidence-based medicine, as corroborated in several studies, in which the parents think that glasses weaken the eyes (18, 26, 27). Parental and children’s knowledge of eye health is associated with children undergoing eye examinations and leads to wearing of glasses due to less stigmatization (26, 27). This highlights the importance of awareness in boosting participation. In a qualitative study exploring e-health in adults, it is recommended to focus on building trust, and that access to the healthcare professional is to be retained when indicated or deemed necessary by the patient (28). The strategies suggested are communicating the results clearly and consistently, providing education, and offering logistic support for access to care (26, 27, 29).
Both stakeholder groups expected the costs to be a challenge due to the potential of high false-positive rates not being in balance due to the increased demand for care and patients needing to buy glasses they would not need. This concern underscores the importance of sensitivity and specificity in evaluating digital screening tools. A test with suboptimal specificity may lead to unnecessary referrals and overtreatment, which could undermine trust in the system and result in increased healthcare expenditures. Similarly, insufficient sensitivity may cause missed cases of refractive error, reducing the purpose of early detection. Therefore, while this study focused on stakeholder perspectives, future implementation efforts must carefully consider the diagnostic accuracy of the tool to ensure clinical and economic feasibility.
This occurred previously in Flanders with the PlusOptix screening program, leading to uncompensable high societal costs due to high rates of false-positives (30). Therefore, the stakeholders involved in that project suggested a re-evaluation of the positive tests by a local optician before referring to an ophthalmologist to prevent these adverse effects. An alternative solution could be to re-test positive cases by the digital test itself before approaching opticians, as this reduces the false-positives and is considered cost-neutral. It was also proposed to focus screening on patients with risk factors for myopia to increase the effectiveness of the program (e.g., children with myopic parents). Vice versa, when screening programs are performed in children, these have the potential to have a major contribution in the light of lifetime costs as is repeatedly reported in interventions for overweight children (31). In turn, apart from the perspective of screening, costs and reimbursements have been most frequently mentioned as barriers for the adoption of telemedicine (15).
Lastly, several stakeholders suggested that raising awareness about myopia through an informative campaign could have a similar effect on screening in terms of prompting parental action and increasing early detection.
Awareness campaigns primarily aim to educate the public about the risks and long-term consequences of myopia, thereby increasing general understanding and prompting a health-seeking behavior. These programs offer several advantages: they are cheap, have the potential to reach a broad audience—including underserved populations—and can empower parents through education. Additionally, they can address common misconceptions (e.g., that glasses weaken the eyes), reduce stigma, and increase the uptake of existing vision services. However, awareness alone lacks objective assessment, making it dependent on parental initiative and the presence of noticeable symptoms. It also introduces risks such as false reassurance, delayed diagnosis, and unequal engagement—especially in asymptomatic children or populations with limited health literacy. In contrast, programs incorporating self-administered tools provide a direct and measurable method for detecting refractive errors that might enable a more timely identification of myopia, regardless of symptom awareness. However, they may come with higher resource demands, risks of false positives, and accessibility challenges in digitally excluded groups. As such, combining awareness efforts with accessible self-screening may create a synergistic effect—raising motivation to engage while simultaneously lowering the barriers to detection and care.
The main idea of early detection to prevent disease in essence is simple and admirable in theory, yet Wilson and Jungner (1968) showed in their landmark study that for a successful implementation, a screening method should uphold all proposed screening criteria. This is in line with suggestions by the interviewed policymakers. Although several adaptations have been made to the criteria since, decades later these criteria stood well against the test of time and are still interpreted as the gold standard of screening assessments. Therefore, barriers identified by the interviewees such as non-participation, false positives, and costs should be assessed carefully before the program is implemented.
Strengths and considerations
This study benefited from having semi-structured interviews, thus allowing the participants to accentuate, elaborate, and introduce topics. Moreover, we chose to widen the scope by including multiple relevant stakeholder groups, ensuring that barriers and opportunities were validated from different perspectives. All interviewees also had experience in eye healthcare and so had background knowledge to draw upon. With regards to limitations, this study could be prone to both confirmation and selection bias. Confirmation bias should be considered since thematic analysis is subjective and relies on the researchers’ judgment, even though we explicitly asked the interviewees for improvements and pitfalls. Selection bias should be considered as well since patients accepting invitations to participate in research are often more highly educated than those who do not participate. Finally, all patients were treated in a tertiary myopia center, and therefore this selected population might be more positive about eye screening compared to the average population.
Conclusions
This qualitative stakeholder analysis identified the barriers and opportunities as experienced by patients, eye care professionals, and policymakers of a pediatric vision screening program using a self-administered online eye test. The patients and parents were supportive of screening and suggested improvements. The eye care professionals and policymakers were receptive to screening but also cautious, highlighting concerns about costs and scientific reliability. The policymakers underlined the relevance of fulfilling all screening criteria before implementation, and the eye care professionals recommended to focus on a specific population at risk that benefits most as opposed to screening the whole population. They saw potential in children from an age of 12 years and older without high refractive errors. If the test would be implemented, they recommended to facilitate awareness as a complementary strategy and to re-evaluate positive tests before referring the patients to opticians or eye clinics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Review Committee Erasmus MC. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
CZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. JC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PR-K: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SI: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. RN: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. RW: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HA: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We express our gratitude to B. Renkema, BSc, for her support during interviews with patients and parents during her 6-week internship.
Conflict of interest
RW is shareholder and consultant of Easee BV. CZ, JC, PR-K, SI, RN, and HA are independent researchers from the University Medical Center Utrecht or Amsterdam University Medical Centers. The authors have no additional financial or proprietary interest in the materials presented herein.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2025.1585320/full#supplementary-material
References
1. Claessens JLJ, Janssen MA, van der Zee C, Polling JR, Meester-Smoor MA, Klaver CCW, et al. Evaluating a web-based visual acuity and refractive error self-assessment tool in myopic children. Ophthalmic Physiol Optics. (2024) 00:1. doi: 10.1111/OPO.13370
2. Heijsters F, Santema J, Mullender M, Bouman M-B, de Bruijne M, and van Nassau F. Stakeholders barriers and facilitators for the implementation of a personalised digital care pathway: a qualitative study. BMJ Open. (2022) 12:e065778. doi: 10.1136/bmjopen-2022-065778
3. Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith EL, Zhou X, et al. Myopia. Nat Rev Dis Primers. (2020) 6:1–20. doi: 10.1038/s41572-020-00231-4
4. Lam CSY, Tang WC, Tse DYY, Lee RPK, Chun RKM, Hasegawa K, et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. (2020) 104:363–8. doi: 10.1136/BJOPHTHALMOL-2018-313739
5. Sun Y, Xu F, Zhang T, Liu M, Wang D, Chen Y, et al. Orthokeratology to control myopia progression: a meta-analysis. PloS One. (2015) 10. doi: 10.1371/JOURNAL.PONE.0124535
6. Iyer V, Enthoven CA, van Dommelen P, van Samkar A, Groenewoud JH, Jaddoe VVW, et al. Rates of spectacle wear in early childhood in the Netherlands. BMC Pediatr. (2022) 22:1–7. doi: 10.1186/S12887-022-03467-Z/FIGURES/1
7. van der Zee C, Huynh LDH, Imhof SM, Ossewaarde-van Norel J, van Leeuwen R, and Wisse RPL. Remote web-based self-assessment of visual acuity versus ETDRS in patients with macular diseases: a method comparison study. Int J Retina Vitreous. (2025) 11:29. doi: 10.1186/s40942-025-00656-7
8. Tong A, Sainsbury P, and Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. (2007) 19:349–57. doi: 10.1093/intqhc/mzm042
9. Andermann A, Blancquaert I, Beauchamp S, and Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. (2008) 86:317. doi: 10.2471/BLT.07.050112
10. Wilson JM and Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam. (1968) 65:281–393.
11. Bodduluri L, Boon MY, Ryan M, and Dain SJ. Impact of gamification of vision tests on the user experience. Games Health J. (2017) 6:229–36. doi: 10.1089/G4H.2016.0100
12. Asghar I, Egaji OA, and Griffiths MG. (2021). Digital applications for eye-health screening of children: challenges, opportunities and solutions, in: 7th International Conference on Engineering and Emerging Technologies, ICEET 2021, . doi: 10.1109/ICEET53442.2021.9659695
13. Oudshoorn N. Diagnosis at a distance: The invisible work of patients and healthcare professionals in cardiac telemonitoring technology. Sociol Health Illn. (2008) 30:272–88. doi: 10.1111/J.1467-9566.2007.01032.X
14. Sreelatha OK and Ramesh SVS. Teleophthalmology: improving patient outcomes? Clin Ophthalmol. (2016) 10:285–95. doi: 10.2147/OPTH.S80487
15. Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, and Brooks M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J Telemed Telecare. (2018) 24:4–12. doi: 10.1177/1357633X16674087
16. Komisarow S and Hemelt SW. School-based health care and absenteeism: evidence from telemedicine. Educ Finance Policy. (2024) 19:252–82. doi: 10.1162/edfp_a_00398
17. Mendall J, Islam M, Wong K, Sansome S, Sim DA, Bachmann LM, et al. Digital exclusion, social deprivation, and clinical outcomes of patients undergoing hyperacuity home monitoring. Ophthalmol Ther. (2024) 13:2759–69. doi: 10.1007/s40123-024-01020-y
18. Vongsachang H, Friedman DS, Inns A, Kretz AM, Mukherjee MR, Callan J, et al. Parent and teacher perspectives on factors decreasing participation in school-based vision programs. Ophthalmic Epidemiol. (2020) 27:226–36. doi: 10.1080/09286586.2020.1730910
19. Wang J and Geng L. Effects of socioeconomic status on physical and psychological health: lifestyle as a mediator. Int J Environ Res Public Health. (2019) 16:1–9. doi: 10.3390/IJERPH16020281
20. McMaughan DJ, Oloruntoba O, and Smith ML. Socioeconomic status and access to healthcare: interrelated drivers for healthy aging. Front Public Health. (2020) 8:231. doi: 10.3389/FPUBH.2020.00231
21. Williamson TH, Andrews R, Dutton GN, Murray G, and Graham N. Assessment of an inner city visual screening programme for preschool children. Br J Ophthalmol. (1995) 79:1068–73. doi: 10.1136/BJO.79.12.1068
22. Majeed M, Williams C, Northstone K, and Ben-Shlomo Y. Are there inequities in the utilisation of childhood eye-care services in relation to socio-economic status? Evidence from the ALSPAC cohort. Br J Ophthalmol. (2008) 92:965–9. doi: 10.1136/BJO.2007.134841
23. Aarts MJ, Voogd AC, Duijm LEM, Coebergh JWW, and Louwman WJ. Socioeconomic inequalities in attending the mass screening for breast cancer in the south of the Netherlands–associations with stage at diagnosis and survival. Breast Cancer Res Treat. (2011) 128:517–25. doi: 10.1007/S10549-011-1363-Z
24. Koehle H, Kronk C, and Lee YJ. Digital health equity: addressing power. Usability Trust to Strengthen Health Syst. (2022). doi: 10.1055/s-0042-1742512
25. Brewer LPC, Fortuna KL, Jones C, Walker R, Hayes SN, Patten CA, et al. Back to the future: achieving health equity through health informatics and digital health. JMIR Mhealth Uhealth. (2020) 8. doi: 10.2196/14512
26. Masarwa D, Niazov Y, Ben Natan M, and Mostovoy D. The role of parental health beliefs in seeking an eye examination for their child. BMC Ophthalmol. (2023) 23. doi: 10.1186/S12886-023-02994-2
27. Bruce A, Sanders T, and Sheldon TA. Qualitative study investigating the perceptions of parents of children who failed vision screening at the age of 4–5 years. BMJ Paediatr Open. (2018) 2. doi: 10.1136/BMJPO-2018-000307
28. Claessens JLJ, Maats EPE, Iacob ME, Wisse RPL, and Jongsma KR. Introducing e-health technology to routine cataract care: patient perspectives on web-based eye test for postoperative telemonitoring. J Cataract Refract Surg. (2023) 49:659–65. doi: 10.1097/J.JCRS.0000000000001189
29. Su Z, Marvin EK, Wang BQ, Van Zyl T, Elia MD, Garza EN, et al. Identifying barriers to follow-up eye care for children after failed vision screening in a primary care setting. J AAPOS. (2013) 17:385–90. doi: 10.1016/J.JAAPOS.2013.05.008
30. Van Der Ploeg CPB, Grevinga M, Eekhout I, Vlasblom E, Lanting CI, Van Minderhout HME, et al. Costs and effects of conventional vision screening and photoscreening in the Dutch preventive child health care system. Eur J Public Health. (2021) 31:193–9. doi: 10.1093/EURPUB/CKAA098
Keywords: stakeholder analysis, online myopia screening, children, Dutch health care system, refractive screening, telemedicine, qualitative study, health policy
Citation: van der Zee C, Claessens JLJ, Rausch-Koster PT, Imhof SM, van Nispen RMA, Wisse RPL and van der Aa HPA (2025) Stakeholders’ perspectives on a digital myopia screening program in children: a qualitative analysis. Front. Ophthalmol. 5:1585320. doi: 10.3389/fopht.2025.1585320
Received: 28 February 2025; Accepted: 16 June 2025;
Published: 08 July 2025.
Edited by:
Rohit Saxena, All India Institute of Medical Sciences, IndiaReviewed by:
Siti Nurliyana Abdullah, Raja Isteri Pengiran Anak Saleha Hospital, BruneiNatali Gutierrez, Universidad Antonio Nariño, Colombia
Copyright © 2025 van der Zee, Claessens, Rausch-Koster, Imhof, van Nispen, Wisse and van der Aa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Casper van der Zee, Yy52YW5kZXJ6ZWUtM0B1bWN1dHJlY2h0Lm5s
†ORCID: Casper van der Zee, orcid.org/0000-0002-2795-2322
Janneau L. J. Claessens, orcid.org/0000-0001-9586-9080
Petra T. Rausch-Koster, orcid.org/0000-0001-8558-5260
Saskia M. Imhof, orcid.org/0000-0001-9332-0377
Ruth M. A. van Nispen, orcid.org/0000-0003-1227-1177
Robert P. L. Wisse, orcid.org/0000-0002-2844-9868
Hilde P. A. van der Aa, orcid.org/0000-0002-1853-5674
 Casper van der Zee
Casper van der Zee Janneau L. J. Claessens1†
Janneau L. J. Claessens1† Ruth M. A. van Nispen
Ruth M. A. van Nispen Robert P. L. Wisse
Robert P. L. Wisse