- John F. Hardesty Department of Ophthalmology and Visual Sciences, Washington University in St.Louis School of Medicine, St. Louis, MO, United States
Myelin oligodendrocyte glycoprotein-associated optic neuritis (MOG-ON) is a sight-threatening demyelinating disorder that can present with various ocular manifestations. Here, we describe a unique case of bilateral MOG-ON with unilateral retinal hemorrhages and Roth spots. We present the case of a 48-year-old man with acute-onset painful, severe vision loss in both eyes. Initial fundoscopic examination revealed bilateral optic nerve edema with unilateral retinal hemorrhages and Roth spots. Imaging was notable for perineural enhancement along both optic nerves. Serological testing revealed elevated MOG antibodies. The patient was treated with high-dose intravenous steroids followed by plasmapheresis, which resulted in substantial clinical improvement. We conducted a literature review of all available studies published before March 30, 2025, using PubMed, including the keywords “myelin oligodendrocyte glycoprotein-associated optic neuritis,” “myelin oligodendrocyte glycoprotein,” “optic neuritis,” “Roth spots,” and “retinal hemorrhage.” We found that this is the first reported case in a male patient—and only the third reported case overall—of retinal hemorrhages and Roth spots occurring in the context of MOG-ON. While retinal hemorrhage and Roth spots have not historically been associated with MOG-ON, recognizing the spectrum of fundoscopic findings is crucial for the early diagnosis and management of this potentially sight-threatening disease.
1 Introduction
Myelin oligodendrocyte glycoprotein (MOG) is essential for the myelination of the nerves within the central nervous system. Antibodies against MOG are a recognized cause of optic neuritis. We report a case of bilateral MOG-associated optic neuritis (MOG-ON) presenting with unilateral peripheral retinal hemorrhages and Roth spots. A 48-year-old man was referred to our academic center for further evaluation of severe, painful bilateral vision loss. His medical history was notable for hereditary hearing loss, dyslipidemia, and well-controlled hypertension. His ocular history was unremarkable, except for color vision deficiency. Written consent to publish this case report was obtained from the patient.
2 Case presentation
Prior to the development of vision loss, the patient had an upper respiratory infection and was prescribed methylprednisolone and a 6-day course of azithromycin. After completing the course of medications, he experienced blurred vision in both eyes, headache, and eye pain that was exacerbated by extraocular movements. A day after symptom onset, he presented to an ophthalmologist. During this examination, his visual acuity was count fingers in the right eye (OD) and hand motion in the left eye (OS). The examination revealed optic disc edema and a white-centered retinal hemorrhage (Roth spot) in the mid-periphery OS. Optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) showed an average RNFL thickness of 111 μm OD and 118 μm OS. Magnetic resonance imaging (MRI) of the brain, performed the morning after symptom onset, was interpreted as normal. MRI of the orbits was not included in the initial imaging studies.
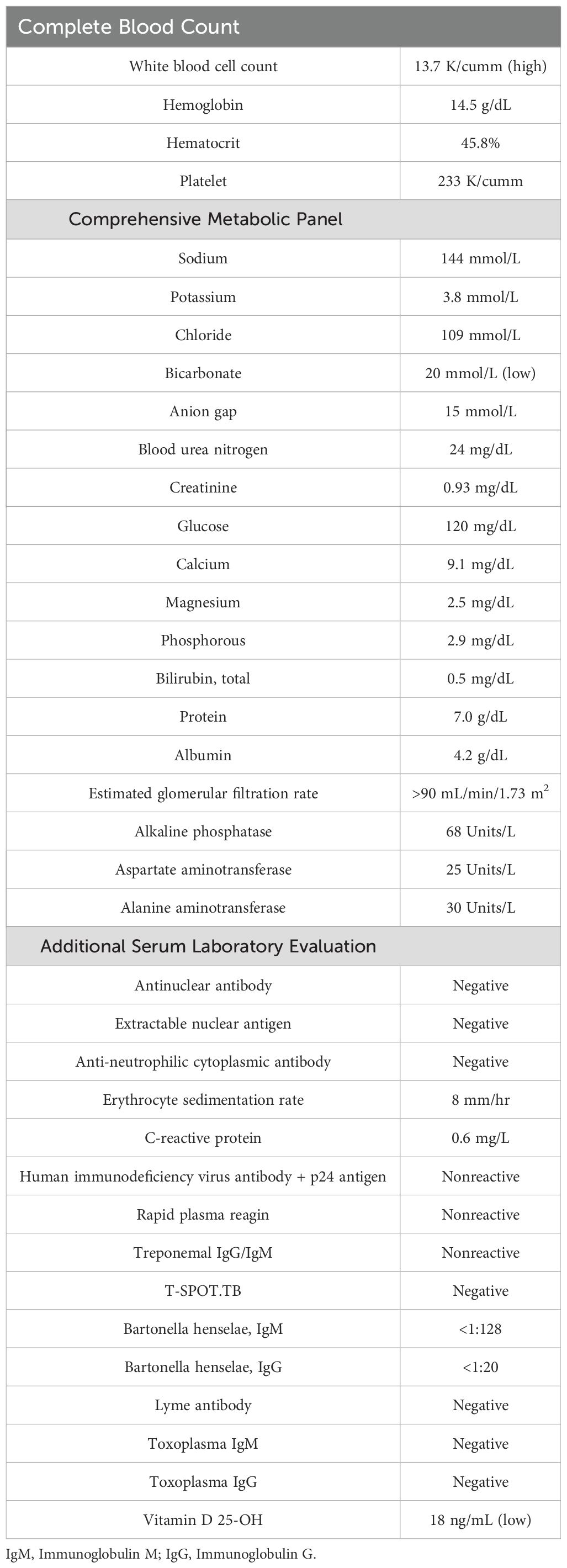
A day after his initial ophthalmology visit, the patient was admitted to an outside hospital. Repeat MRI of the brain and orbits revealed thickening and perineural enhancement along both optic nerves, which were more prominent on the left than on the right. These findings were suggestive of optic neuritis (Figure 1). He was started on broad-spectrum systemic antibiotics, acetazolamide 250 mg three times daily, and intravenous (IV) methylprednisolone 500 mg every 12 h. The patient was transferred to our facility 3 days later for further evaluation.
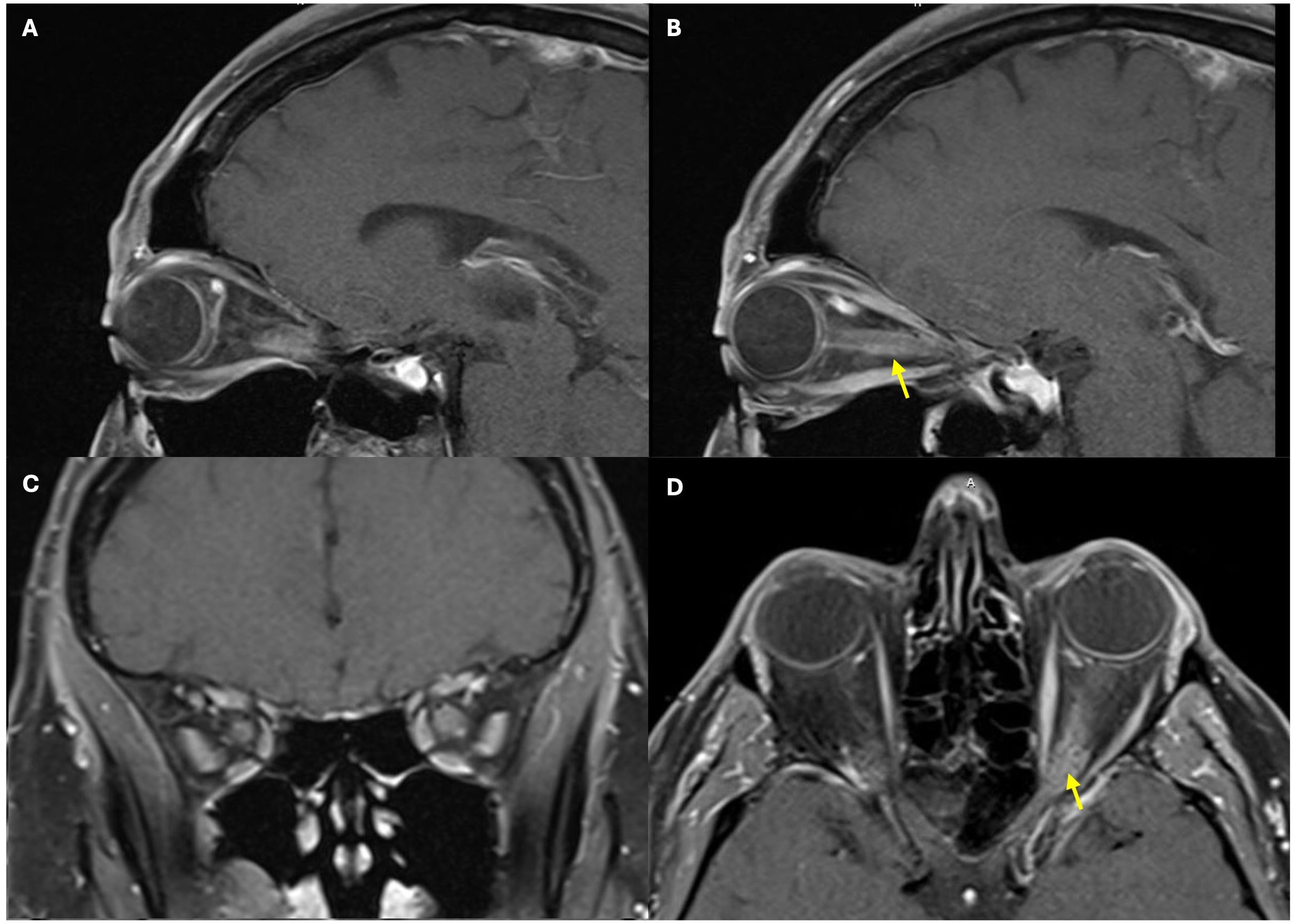
Figure 1. Magnetic resonance imaging of the brain and orbits demonstrating thickening and perineural enhancement along both optic nerves, the left greater than the right, with findings suggestive of optic neuritis. The figure includes sagittal views of the left optic nerve (A, B), coronal view of the bilateral optic nerves (C), and axial view of the bilateral optic nerves (D). Yellow arrows highlight the significant thickening and perineural enhancement along the left optic nerve.
Upon admission, the patient reported subjective improvement in his vision and a reduction in pain during extraocular movements. Examination revealed a visual acuity of 20/25 OD and count fingers OS. A relative afferent pupillary defect was present in the left eye. The anterior segment appeared unremarkable in both eyes (OU). The patient was unable to read any Ishihara color plate, including the control plate, using either eye. Fundoscopy showed bilateral optic disc edema that was worse OS. Peripapillary hemorrhages were identified OS (Figure 2). Several intraretinal hemorrhages and Roth spots were located inferiorly (Figure 3) and temporally.
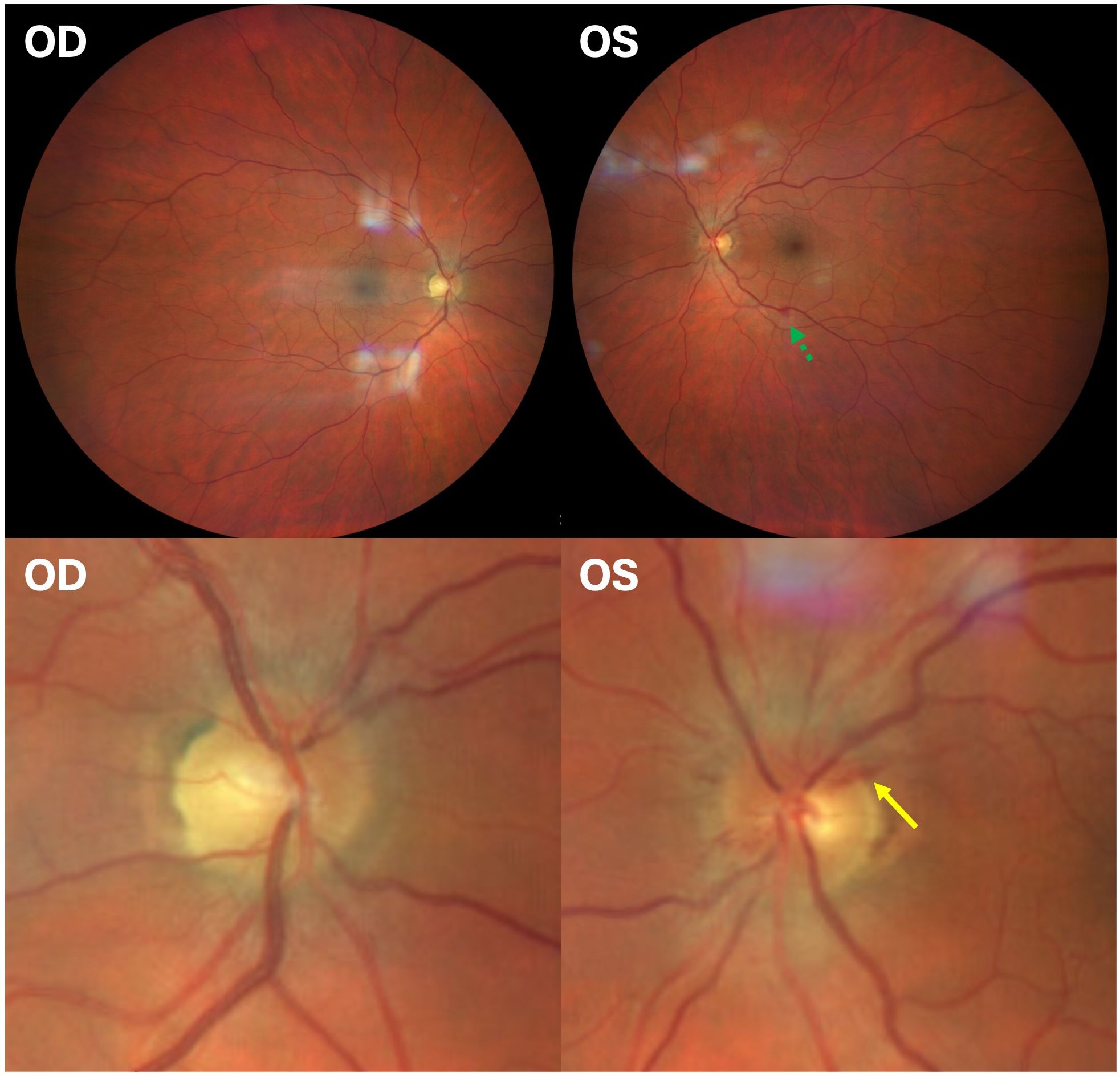
Figure 2. Fundus photographs of the right and the left eye. A retinal hemorrhage adjacent to an inferior retinal vein is noted by a green dotted arrow. The lower images show an enlarged view, demonstrating optic nerve edema and peripapillary hemorrhage (yellow solid arrow). The inferior and temporal Roth spots are not pictured in the left eye.
Figure 3. Fundus photograph of the left eye depicting intraretinal hemorrhages and Roth spots. Roth spots are highlighted with yellow arrows. The lower image shows an enlarged view of the concentrated hemorrhages along the inferior peripheral retina. Temporal Roth spot not pictured.
The patient underwent an extensive workup. Notable laboratory findings included a positive MOG immunoglobulin G (IgG) antibody with a serum titer of 1:1,000, which was obtained via fluorescence-activated cell sorting. The initial complete blood count and the complete metabolic panel were unremarkable (Table 1). The serum antinuclear antibody, extractable nuclear antigen, and anti-neutrophilic cytoplasmic antibody tests were negative. The erythrocyte sedimentation rate and the C-reactive protein levels were normal. Further serum laboratory testing for human immunodeficiency virus, tuberculosis, syphilis, Bartonella, Lyme disease, and Toxoplasma gondii was negative. His vitamin D levels were low at 18 ng/mL. Transthoracic echocardiogram showed no evidence of endocarditis. Magnetic resonance angiography and venography of the brain, as well as MRI of the spine, were normal. Cerebrospinal fluid (CSF) analysis showed an elevated nucleated cell count (31/mm3) with 95% lymphocytes and an elevated CD8 count (894 cells/μL). CSF studies including cryptococcal antigen, T. gondii polymerase chain reaction, bacterial culture, and fungal culture were negative. CSF analysis revealed no oligoclonal bands and no significant B-cell population. Opening pressure on lumbar puncture was not recorded.
Positron emission tomography/computed tomography (PET/CT) showed mild 18F-fludeoxyglucose uptake along the optic nerves, consistent with optic neuritis. Whole-body PET/CT imaging and CT imaging of the chest, abdomen, and pelvis were negative for occult malignancy. The patient underwent repeat OCT of the RNFL, which showed stable RNFL thickness in the right eye (111 μm) and an increased RNFL thickness in the left eye (135 μm). OCT of the macula was normal, and analysis of the ganglion cell complex (GCC) revealed normal average thickness in both eyes: 75 μm OD and 80 μm OS (Figure 4).
During his admission, he was treated with an additional 3 days of 1 g of IV methylprednisolone daily, followed by an oral prednisone taper. The patient had a gradual recovery of vision in the left eye and was subsequently treated with five sessions of plasmapheresis. After completion of the plasmapheresis regimen, he showed improvement in both subjective symptoms and objective visual acuity. The patient was subsequently discharged with a visual acuity of 20/20 OD and 20/30 OS. Repeat fundoscopy at discharge revealed stable intraretinal hemorrhages and Roth spots in the temporal and inferior posterior pole OS.
At 6 weeks after hospital discharge, the patient was evaluated in the outpatient setting. His visual acuity was 20/20 OU. The patient had a persistent relative afferent pupillary defect OS. OCT revealed RNFL thickness of 82 μm OD and 87 μm OS, while OCT of the GCC showed mild bilateral thinning, measuring 64 μm OD and 65 μm OS. Fundoscopy revealed resolution of the previously identified retinal hemorrhages and Roth spots. At an outpatient visit 3 months after the initial hospitalization, the patient reported a mild recurrence of symptoms, including color vision abnormalities and increased light sensitivity, following a recent decrease in prednisone dosage. Due to the continued symptoms, the patient’s steroid taper was slowed. Despite continued symptoms, repeat testing at the 3-month follow-up visit showed a decrease in the serum MOG IgG titer to 1:100, down from the initial 1:1,000 at admission. At the 8-month follow-up appointment, the patient had finished the prednisone taper and was largely symptom-free. OCT revealed progressive thinning of the RNFL (66 μm OD and 67 μm OS) and the GCC (59 μm OD and 58 μm OS) (Figure 4).
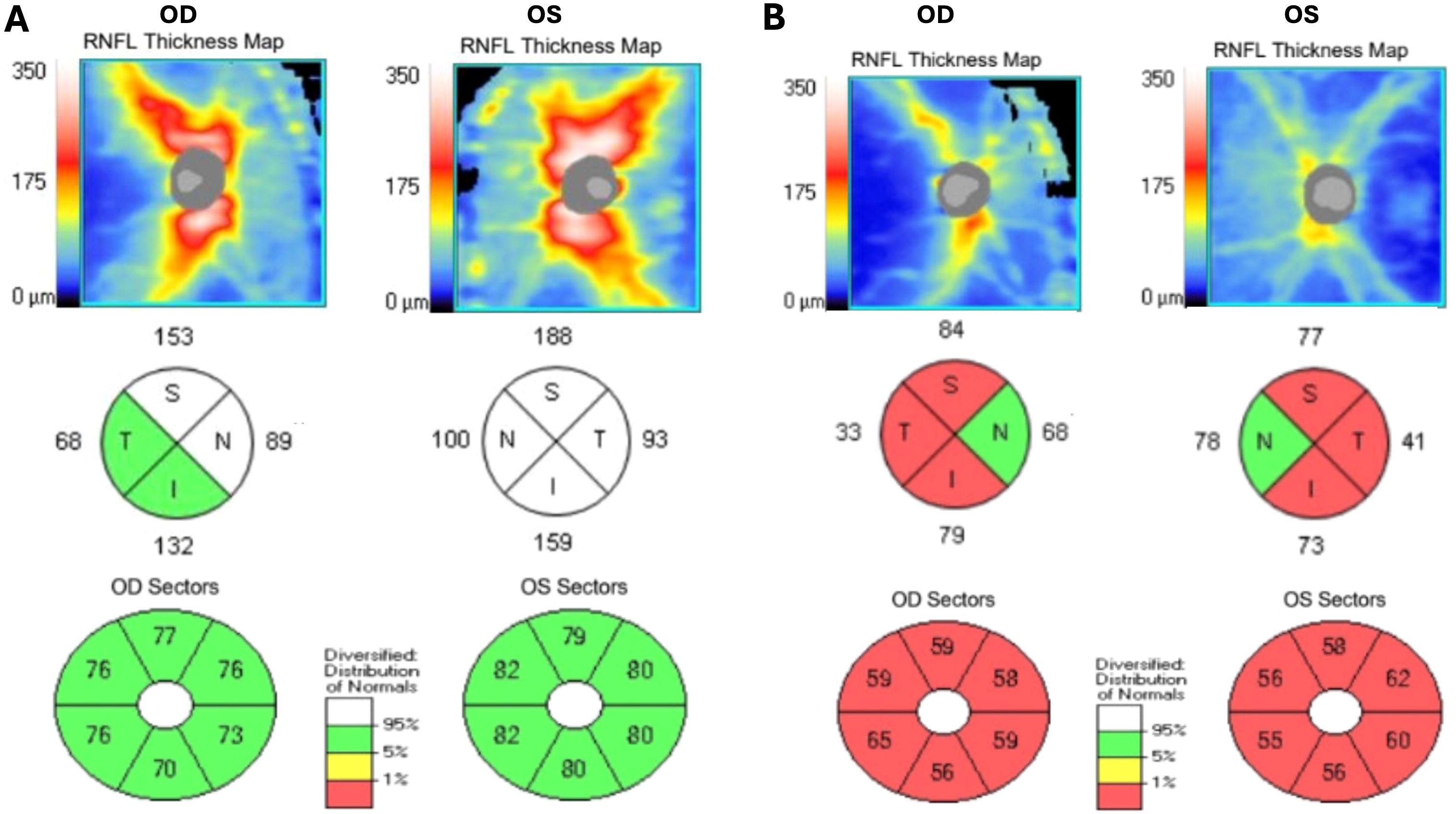
Figure 4. Spectral domain optical coherence tomography demonstrating retinal nerve fiber layer thickening in two quadrants in the right eye and all four quadrants in the left eye on initial presentation (A), with subsequent retinal nerve fiber layer and ganglion cell complex thinning at 8-month follow-up (B).
3 Discussion
Retinal hemorrhages and Roth spots have not traditionally been associated with MOG-ON, and their presence may obscure or delay diagnosis. We presented a rare case of MOG-ON with unilateral retinal hemorrhages and Roth spots, expanding the clinical spectrum of fundus findings linked to this condition.
The diagnosis of MOG-ON in this case was supported by imaging that demonstrated perineural enhancement along the optic nerves, a finding highly suggestive of an inflammatory etiology (1). The patient had an elevated serum MOG IgG titer. His clinical course included a modest improvement in vision after high-dose steroids, followed by near-complete recovery in visual acuity after plasmapheresis, which is consistent with a typical recovery trajectory of MOG-ON. While retinal hemorrhages and Roth spots are nonspecific findings seen in infectious, inflammatory, and oncologic conditions, the patient’s well-controlled systemic diseases and unrevealing systemic workup argue against alternative etiologies. Moreover, the resolution of the intraretinal hemorrhages and Roth spots following treatment, along with the absence of retinal hemorrhages in the less affected contralateral eye, provides both spatial and temporal support for an association with the patient’s MOG-ON.
To our knowledge, this is only the third case of MOG-ON with concurrent retinal hemorrhages and Roth spots. Until recently, neither retinal hemorrhages nor Roth spots have been recognized features of MOG-ON, and fundus findings beyond optic nerve edema and peripapillary hemorrhages are rare. Retinal hemorrhages in MOG-ON may be underreported in the literature given their lack of specificity. A PubMed literature review identifying cases of MOG-ON associated with retinal hemorrhages or Roth spots before 30 March 2025 yielded 107 results. Among these, only nine cases (8.4%) documented hemorrhages outside of the peripapillary region. Notably, five of these nine cases had systemic comorbidities, including diabetes, hypertension, and coronavirus disease 2019 (COVID-19) (Table 2). Only two cases (1.8%) documented Roth spots in patients with MOG-ON, both in female patients aged 27 and 49 years, respectively (2, 3). Similar to these reports, the presentation of our patient prompted an extensive systemic evaluation, underscoring the diagnostic challenge posed by the fundoscopic findings.
Table 2. Initial episodes of MOG-ON - clinical features and fundus findings beyond optic disc edema and peripapillary hemorrhage.
The etiology of retinal hemorrhages and Roth spots in MOG-ON remains largely unknown, although several mechanisms have been proposed. One proposed mechanism is impaired retinal venous outflow secondary to optic disc edema, as supported by previous reports (2–4). In this case, the patient demonstrated perineural enhancement on MRI, raising the possibility of vascular occlusion as a rare downstream consequence of optic nerve sheath involvement, as seen in optic perineuritis (5). These mechanisms suggest vascular congestion as a contributing factor rather than a direct implication of the pathogenesis of MOG antibody-associated disease (MOGAD). Notably, MOGAD-related inflammation involves a complex interplay between humoral and cellular immune responses (6, 7). Perivascular inflammation has been observed in MOGAD and shows phenotypic and pathologic overlap with central nervous system vasculitis (8, 9). Thus, vascular inflammation may underlie the development of Roth spots in this patient. Although fluorescein angiography was not performed, it might have provided additional evidence to clarify the mechanism for the retinal findings. OCT did not reveal evidence of intraretinal inflammation.
4 Conclusions
The final diagnosis was MOG-ON with associated intraretinal hemorrhages and Roth spots. The diagnosis of MOG-ON was supported by a classic clinical course, characteristic imaging findings, and a high MOG IgG serum titer. While earlier descriptions of MOG-ON fundus findings highlighted optic nerve edema and peripapillary hemorrhage, retinal hemorrhages have now emerged as a documented clinical finding in more recent reports. To our knowledge, this is the first reported case of retinal hemorrhages and Roth spots associated with MOG-ON in a male patient, and only the third reported case of any patient with these findings. This case helps to better differentiate the fundus findings associated with MOG-ON, identify intricacies in its presentation, and aid in the recognition of a disease that can have potentially devastating ocular sequelae.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical approval was not required for the studies involving humans because this is a case report and as such does not count as research. Ethical approval was not obtained. Patient consent was obtained in writing. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JW: Data curation, Conceptualization, Visualization, Investigation, Writing – review & editing, Writing – original draft. OC: Writing – original draft, Writing – review & editing, Data curation, Visualization. ME: Writing – review & editing, Conceptualization, Supervision, Investigation. GV: Writing – review & editing, Supervision, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We would like to acknowledge the patient for allowing us to share his story for the benefit of future patients suffering from myelin oligodendrocyte glycoprotein-associated optic neuritis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A review of clinical and MRI features, diagnosis, and management. Front Neurol. (2022) 13:885218. doi: 10.3389/fneur.2022.885218
2. Srimanan W and Ngathaweesuk Y. A rare presentation of myelin oligodendrocyte glycoprotein-associated optic neuritis with venous stasis retinopathy and premacular hemorrhage: A case report. Case Rep Ophthalmol. (2024) 15:669–77. doi: 10.1159/000540776
3. Aboab J, Errera MH, Espinoza S, Girmens JF, and Héron E. Atypical case of MOG antibody-associated optic neuritis with roth spots. Ocul Immunol Inflamm. (2023) 31:1068–72. doi: 10.1080/09273948.2022.2062386
4. Wilke GA, Spencer M, Gilman CA, Guerriero R, Bohm P, Van Stavern GP, et al. Peripheral retinal haemorrhages in a patient with MOG-associated optic neuritis. Neuroophthalmology. (2024) 48:176–82. doi: 10.1080/01658107.2023.2290540
5. Purvin V, Kawasaki A, and Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol. (2001) 119:1299–306. doi: 10.1001/archopht.119.9.1299
6. Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. (2020) 143:1431–46. doi: 10.1093/brain/awaa102
7. Mader S, Ho S, Wong HK, Baier S, Winklmeier S, Riemer C, et al. Dissection of complement and Fc-receptor-mediated pathomechanisms of autoantibodies to myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci U S A. (2023) 120:e2300648120. doi: 10.1073/pnas.2300648120
8. Gilani A and Kleinschmidt-DeMasters BK. Childhood small-vessel primary angiitis of the central nervous system: overlap with MOG-associated disease. Pediatr Dev Pathol. (2023) 26:18–29. doi: 10.1177/10935266221121445
9. Stredny CM, Blessing MM, Yi V, Ryan ME, Zhang B, Solomon IH, et al. Mimics of pediatric small vessel primary angiitis of the central nervous system. Ann Neurol. (2023) 93:109–19. doi: 10.1002/ana.26531
10. Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, and Patel VR. Myelin oligodendrocyte glycoprotein antibody–associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. (2020) 40(3):398–402. doi: 10.1097/WNO.0000000000001049
11. Mittal A, Baig IF, Merchant AG, Chen JJ, Choi JJ, Goldberg A, et al. Sjögren disease and myelin oligodendrocyte glycoprotein antibody–associated optic neuritis. J Neuroophthalmol. (2021) 41(1):e48–50. doi: 10.1097/WNO.0000000000000945
12. Lukewich MK and Micieli JA. Venous stasis retinopathy secondary to myelin oligodendrocyte glycoprotein antibody-positive optic neuritis. Retin Cases Brief Rep. (2022) 16(3):305–7. doi: 10.1097/ICB.0000000000000977
13. Schichtel LT, Ibrahim R, Scoville N, Wagle B, Kalavar M, Yanoga F, et al. Myelin oligodendrocyte glycoprotein-associated disease optic neuritis with concurrent combined central retinal artery and vein occlusion. J Neuroophthalmol. (2024) 45(3):e177–9. doi: 10.1097/WNO.0000000000002196
14. Teru SS, Dogiparthi J, Bonitz TJ, and Buzas C. Myelin oligodendrocyte glycoprotein antibody-associated disease: A case report. Cureus. (2024) 16(3):e55652. doi: 10.7759/cureus.55652
Keywords: myelin oligodendrocyte glycoprotein-associated optic neuritis, MOGAD, Roth spots, retinal hemorrhage, atypical optic neuritis, peripapillary hemorrhage, acute vision loss
Citation: Wilson JR, Cummings OW, Elitt MS and Van Stavern GP (2025) Case Report: Fundus findings in myelin oligodendrocyte glycoprotein-associated optic neuritis. Front. Ophthalmol. 5:1620614. doi: 10.3389/fopht.2025.1620614
Received: 29 April 2025; Accepted: 18 August 2025;
Published: 22 September 2025.
Edited by:
Sachin Kedar, Emory University, United StatesReviewed by:
Swati Phuljhele, All India Institute of Medical Sciences, IndiaShikha Talwar Bassi, Sankara Nethralaya, India
Copyright © 2025 Wilson, Cummings, Elitt and Van Stavern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John R. Wilson, d2lsc29uLmpvaG5Ad3VzdGwuZWR1
 John R. Wilson
John R. Wilson Olivia W. Cummings
Olivia W. Cummings Matthew S. Elitt
Matthew S. Elitt Gregory P. Van Stavern
Gregory P. Van Stavern